Abstract
Increasing evidence has shown that programmed cell death (PCD) plays a crucial role in tumorigenesis and cancer progression. The components of PCD are complex and include various mechanisms such as apoptosis, necroptosis, alkaliptosis, oxeiptosis, and anoikis, all of which are interrelated in their functions and regulatory pathways. Given the significance of these processes, it is essential to conduct a comprehensive study on PCD to elucidate its multifaceted nature. Key signaling pathways, particularly the caspase signaling pathway, the RIPK1/RIPK3/MLKL pathway, and the mTOR signaling pathway, are pivotal in regulating PCD and influencing tumor progression. In this review, we briefly describe the generation mechanisms of different PCD components and focus on the regulatory mechanisms of these three major signaling pathways within the context of global PCD. Furthermore, we discuss various tumor therapeutic compounds that target different signaling axes of these pathways, which may provide novel strategies for effective tumor therapy and help improve patient outcomes in cancer treatment.
Keywords: Tumor, Caspase, MTOR, RIPK1, RIPK3, MLKL, Programmed cell death
Introduction
Cell death can be defined as the programmed cell death (PCD) and accidental cell death (ACD) [1]. The former is manipulated by signaling cascades and molecular effectors, occurring autonomously and in an orderly manner [1–3]. PCD includes apoptosis, necroptosis, ferroptosis, autophagy, pyroptosis, etc. It is characterized by cell contour shrinkage, nuclear condensation, abnormal mitochondrial cristae morphology, the formation of membrane bubbles, surface pores, apoptotic body, etc. [4, 5]. ACD, like necrosis, showed mainly the inflammatory response that is critically associated with ischemia–reperfusion damage. It’s triggered by cell swelling, loss of biofilm integrity, overflow of cell contents, and dissipation of ion gradients [6]. Cell death is closely associated with tumors and has been the research point of tumor research [7, 8]. Recently research indicated that PCD affected tumor progression and metastasis by regulating immunogenicity of the tumor microenvironment (TME). Cell death shows significant potential in cancer treatment. Different forms of cell death have an overlap in mechanism, thus it’s necessary to systematically analyze cell death as a whole. They will promote the conjunction utilization of PCD modulators in cancer treatment [9].
The signaling pathway has an essential role in the occurrence of PCD. Cysteinyl aspartate protease (caspase) mediates apoptosis, necroptosis and other PCD, thereby interfering with a variety of definite tumor progression [10]. PI3 K/AKT/mTOR signaling pathways mediated apoptosis in melanocyte tumors [11]. Receptor-Interacting Protein Kinase 3 (RIPK3) and Mixed Lineage Kinase Like (MLKL)-dependent necroptosis creates an immunosuppressive tumor microenvironment. It stimulates the immune system and induces cytotoxic CD8 + T lymphocytes to exert a strong adaptive immune response, and finally prevents tumor progression [12]. Previous studies summarized the mechanism of various signaling pathways which mediated the different cell death. However, it remained unknown how the signaling pathway works when considering the cell death as a whole. This will provide insight into the search for common signaling molecules in different cell death.
In this review, we first systematically characterized the components and depicted molecular mechanisms of PCD. Then we screened three signaling pathways that are significant in PCD, namely the caspase signaling pathway, RIPK1/RIPK3/MLKL signaling pathway and mTOR signaling pathway. We also reviewed the molecular mechanisms of four signaling pathways regulating PCD in the tumor microenvironment and elaborated on their essential roles in tumor progression. Finally, combined with the latest research progress, we summarize the targeted therapy of the three signaling pathways involved in PCD. This provides new ideas for the treatment of tumors and guide the study of combined drugs.
The components and mechanism of PCD in the TME
Apoptosis
Apoptosis can be categorized into exogenous and endogenous apoptosis, both of which involve intricate mechanisms encompassing multiple [13]. The extrinsic apoptotic pathway is initiated by the interaction of death receptors (belonging to the tumor necrosis factor receptor superfamily, TNFR) with their ligand, which activate pro-caspase 8, transforming it to caspase 8 [14]. Death receptor ligands encompass TRAILR2 (DR5)-TRAIL, TRAILR1 (DR4)-TRAIL, FAS (CD95, APO-1)-FasL, TNFR1-TNFα [15]. Adaptor proteins FADD and TRAD sequester caspase-8 and/or −10, resulting in the formation of the Death-Inducing Signaling Complex (DISC), which amplifies local pro-caspase concentrations, facilitating mutual autoactivation [16]. Activation of the initiator caspase leads to the processing of the downstream effector protein caspase-3, −6, and −7, resulting in the cleavage of vital substrates for cell survival, ultimately inducing cell death [17]. On the one hand, the endogenous apoptotsis (mitochondria-dependent) is triggered by intracellular signals such as irradiation and chemotherapeutic drug treatment [17]. Inner stimuli such as irreparable genetic damage, hypoxia, high intracellular calcium ion concentration, and severe oxidative stress initiate the endogenous mitochondrial pathways [18]. It has been established that pro-apoptotic proteins, such as Bak and Bax from the Bcl-2 family, are activated, disrupting the outer mitochondrial membrane permeability (MOMP), which allows the diffusion of apoptotic factors, like cytochrome c, normally confined to the mitochondrial membrane space, into the cytoplasm [19]. The pro-apoptotic proteins BH3-only members of the Bcl-2 family (Bak and Bax) and the anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 are activated, leading to disruption of mitochondrial outer-membrane permeabilization (MOMP), thereby allowing the diffusion of apoptotic factors such as cytochrome c, which are normally confined to the membrane space, into the cytoplasm [19]. Cytochrome c binds to cytoplasmic Apaf-1(Apoptotic protease activating factor 1) and initiates the formation of a complex called apoptosome, which recruits the promoter pro-caspase-9 to its caspase recruitment domain (CARD) for autoactivation and proteolysis. Caspase 9 started the caspase cascade and activated the chain reaction of caspase-3/−6/−7/−9/−10 to lead to apoptosis [20–22].
Apoptosis is closely linked to genomic damage and tumorigenesis, influenced by interconnected signaling pathways and immune responses [23, 24]. During chemoradiotherapy, apoptosis can stimulate adaptive anti-tumor or antiviral immune responses by activating NF-κB signaling and cGAS/STING pathways [25–27]. Apoptosis triggered by drugs or cytotoxic immune cells also aids in eliminating cancer cells within the TME. However, although antineoplastic drugs exert potent in vitro anti-tumor effects, they often develop in vivo resistance, leading to weakened apoptosis [28].
Necroptosis
Necroptosis plays a role in lysing infected or damaged cells in inflammatory diseases. Various innate immune signaling pathways induce necroptosis, including RIGI-like receptor stimulation, Toll-like receptors (TLR), death receptor pathway, and ZDNA binding protein 1 (ZBP1). Current evidence suggests that RIPK1 kinase activity prompts RIPK3/MLKL-dependent necroptosis. Upon TNF-α binding to TNFR1 at the plasma membrane surface, RIPK1 is recruited intracellularly. When caspase-8 is absent or inhibited, RIPK3 assembles into RIPK1-RIPK3 necroplasts, leading to RIPK3 phosphorylation and MLKL recruitment, resulting in complex IIb formation and necroptosis induction. The phosphorylated RIPK1 and RIPK3 complex constitute the necrosome in this pathway. Auto-phosphorylated RIPK1 exposes the RIP Homotypic Interaction Motif (RHIM), prompting self-oligomerization and RIPK3 recruitment. RIPK3 phosphorylates MLKL, which further phosphorylates and oligomerizes to target the plasma membrane, rupturing it and causing necroptosis [29]. Moreover, ZBP1 participates in necroptosis by sensing intracellular nucleic acid changes. It has been shown that RIPK1 inhibits ZBP1's induction function, preventing RIPK3/MLKL-associated necroptosis. The conserved RHIM of RIPK1 hampers ZBP1 from combining and activating RIPK3 [30]. Interestingly, necroptosis holds both pro-tumor and anti-tumor effects in the TME [31]. On the one hand, low RIPK3 and MLKL expression in early tumor stages predict poor prognosis in various solid tumors, promotes dendritic cell maturation while suppressing CD8+ T cell anti-tumor immunity through RIPK1 and NF-κB signaling [31–34]. Notably, necrotic cells exhibit a limited ability to efficiently activate CD8+ T cells in vivo, distinguishing necroptosis from necrosis [34]. Additionally, RIPK3 depletion diminishes NKT cell cytotoxicity against tumors. Studies have shown that necroptotic cancer cell vaccines trigger dendritic cell maturation, CD8+ T cell cross-priming, and IFN-γ production, enhancing anti-tumor immunity. Hence, inducing tumor cell necroptosis while activating cytotoxic T cells effectively inhibits tumor progression [35]. On the other hand, it has been shown that the protein expression level of MLKL and RIPK3 is increased in the advanced stage of breast cancer (BRCA), promoting tumor metastasis and macrophage-induced T cell suppression [35, 36].
Ferroptosis
Ferroptosis is an iron-dependent programmed cell death sparked by uncontrollable lipid peroxidation and plasma membrane rupture, characterized by the accumulation of reactive oxygen species (ROS) [37, 38]. The regulatory mechanism of ferroptosis includes extrinsic and intrinsic pathways. The extrinsic pathway regulates effector ferroptosis inducers'interaction with different transporters. It has been shown that erastin inhibits the amino acid antiporter system (System xc−), hindering cysteine (GSH) metabolism and leading to lipid peroxide accumulation, damaging membrane protein and the membrane itself. Iron transporters, serotransferrin, and lactotransferrin, are activated to promote iron transportation, raising intracellular labile iron levels and triggering ferroptosis [39]. Intrinsic pathways are closely related to GSH and GPX4. GSH can transactivate GPX4 to perform its catalytic activity of reducing lipid peroxidation toxicity and maintaining membrane lipid bilayer homeostasis [40]. GPX4 inhibitors, Squalene synthetase, and HMG-CoA can directly counteract GPX4. Glutathione deficiency triggers GSH deficiency, deactivating GPX4 and causing ferroptosis [41]. Ferroptosis inflicts cellular damage and stimulates inflammation-linked immunosuppression in the tumor microenvironment, supporting tumor growth. Current evidence suggests that mutations in cancer-associated genes (RAS and TP53) and stress response pathways (NFE2L2 signaling) in ferroptosis are closely tied to tumor progression [42]. Ferroptosis also influences T cell anti-tumor effects in the TME. Glutathione peroxidase 4 (GPX4) deficiency in CD8+ and CD4+ T cells induces ferroptosis, impairing protection against infections and promoting cancer development [43].
Autophagy
It is now understood that autophagy initiation involves the ATG1/ULK1 (UNC-51-like kinase 1) complex and the VPS34/PI3 K C III (phosphatidylinositol 3-kinase III) complex. The ATG1/ULK1 complex, an upstream regulator of the PI3 K C III complex, stimulates the activated ULK1 complex to relocate the PI3 K C III complex from the dynein motor complex to the endoplasmic reticulum (ER) by phosphorylating autophagy/Beclin 1 regulator 1 (AMBRA1), a PI3 K C III complex component. The VPS34 complex in the ER, analogous to the PI3 K C III complex, phosphorylates PI to PI (3,4,5) P3, launching autophagy nucleation [44, 45]. Through acetylation, core elements of the ULK1 complex, the Beclin-1-PIK3 C3 complex, and the LC3 lipidation system foster expansion and closure, forming double-membrane autophagosomes [46–49]. These autophagosomes encase and subsequently ferry intracellular materials to lysosomes, where they undergo degradation [49]. ULK1 also activates the VPS34 complex, phosphorylating Beclin 1 in the VPS34 complex and inducing autophagy [50]. Interestingly, autophagy can be suppressed by mTORC1, which directly phosphorylates ULK1 and ATG13 within the ULK1 complex, as well as ATG14 within the PI3 K C III complex containing ATG14. In an inactive state, mTORC1 sequesters the PI3 K C III complexes containing ULK1 and ATG14. AMP-activated protein kinase (AMPK), a pivotal energy susceptor governing cellular metabolism for energy equilibrium, actively promotes autophagy. Both mTORC1 and AMPK exert control over autophagy by directly phosphorylating ULK1 [51]. In recent years, significant emphasis has been placed on the role of autophagy in cancer, spanning from the biology of tumor cells to clinical trials [52]. It is widely thought that autophagy holds a tumor-suppressive impact during tumorigenesis yet can exhibit a tumor-promoting effect in established tumors [53]. In addition, autophagy is widely believed to autonomously regulate CD8+ T cell metabolism, carrying implications for anti-tumor immunity [54].
Pyroptosis
Pyroptosis is a novel PCD process orchestrated by specific inflammatory caspases, including caspase-1/4/5/11 [55, 56]. The pyroptosis process is performed by gasdermin D (GSDMD) and manifests as continuous cell volume expansion until the cytomembrane ruptures, releasing cellular contents that activate a robust inflammatory response [57, 58]. Caspase-1/11/4/5 cleave and unleash the N-terminal domains, which interact with cell membrane lipids and perforate the membrane. This event disrupts cell osmotic pressure and triggers gradual swelling, resulting in cell membrane rupture [59–61]. Indeed, an inflammatory environment is associated with increased probability of tumor onset in cells and tissues subject to chronic exposure [62].
Pyroptosis shares similarities with apoptosis and ferroptosis in terms of critical signaling pathways, morphological transformations, and cell release [62]. In the context of regulating anti-tumor immunity within the tumor microenvironment, pyroptosis yields both pro-tumor and anti-tumor effects. In this regard, pyroptosis yields cytokines to promote inflammation such as IL-18 and IL-1β, which facilitate the infiltration of immune cells into the immunosuppressive tumor microenvironment, indicating its anti-tumor effect [63]. Conversely, pyroptotic cells release proinflammatory cytokines such as IL-1β, IL-6, and IL-18, promoting tumor progression and immune evasion [63]. Therefore, when employing pyroptosis-inducing chemotherapy agents, it is crucial to assess the impact of pyroptosis on tumor cells to select the optimal therapeutic strategy.
Other types of pan-cell death
Parthanatos is triggered by oxidative stress-induced DNA damage and chromatin lysis which is a poly (ADP-ribose) polymerase 1 (PARP1) -dependent form of non-apoptotic cell death [3, 64]. Under pathophysiological conditions, excessive PARP1 activation by DNA damage leads to the accumulation of PAR multimers and nuclear translocation of apoptosis-inducing factor (AIF). Eventually, this process leads to parthanatos [65]. Parthanatos has been found to play an increasingly important role in tumor progression [66, 67]. Therefore, the association between Parthanatos and tumors will become an essential field for PCD treatment of tumors, and the parthanatos-related pathways will also become a research hotspot.
NETosis is a cell death caused by neutrophils releasing their nuclear and mitochondrial DNA and being modified by cytoplasmic and granule proteins to form neutrophil extracellular traps (NETs) [68–71]. Increasing evidence suggests that NETs are associated with cancer metastasis [72–75]. NETosis affects the proliferation of tumor cells through proteases or activation signals, especially BRCA, lung cancer cells and chronic myeloid leukemia, by inducing neutrophils [76, 77].
Alkaliptosis, a PH-dependent form of regulatory cell death, was identified as a compelling novel anticancer strategy in pancreatic cancer (PCA) [78]. Oxeiptosis is a ROS-induced apoptosis-like cell death pathway that is non-caspase-dependent and non-inflammatory and does not depend on caspase [79]. Oxeiptosis was shown to be a novel tumor suppressor, but it is not well studied [80]. Anoikis is a kind of programmed cell death that occurs after detachment of cells from the original extracellular matrix, and disrupts integrin junctions sequentially. It is a critical mechanism that prevents dysplastic cells from growing or attaching to inappropriate substrates. Bcl-2 family proteins are critical factors involved in this process [81]. Disulfidptosis, a cell death triggered by metabolic therapy with glucose transporter (GLUT) inhibitors, has inhibited tumor growth [82]. Cuproptosis is a copper-dependent mode of death that can be distinguished from other cell death [83]. PANoptosis is a distinct pattern of inflammatory PCD regulated by the multifaceted PANoptosome complex [84].
Three signaling pathways and the target therapies
Caspase signaling pathway
The mechanism of caspase signaling pathway regulated PCD in tumor microenvironment
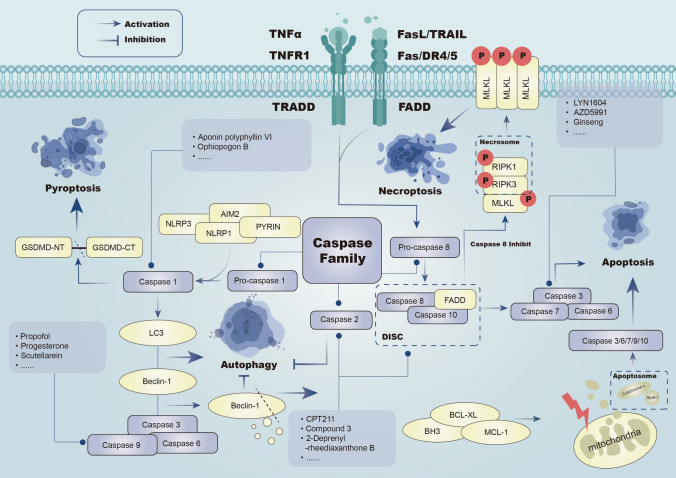
Caspases serve as central players in several critical signaling pathways. Within the context of apoptosis, caspases can be categorized into initiator caspases (− 2, − 8, − 9, − 10) and effector caspases (− 3, − 6, and − 7) [56, 85]. The extrinsic apoptosis pathway is initiated by initiator caspases 8 and 10, activated by the death-inducing signaling complex [86]. Within the intrinsic apoptosis pathway, initiator caspase-9 becomes part of the apoptosome with the assistance of CARD and undergoes proximity-induced autoactivation [87, 88]. RIPK1 stimulates the conversion of inactive pro-caspase 8 into activated caspase 8, triggering extrinsic apoptosis [89, 90]. In cases where caspase 8’s expression is blocked or inhibited in tumors, RIPK1 mediated the RIPK3/MLKL signaling in an active state, forming necroptotic bodies that ultimately lead to necroptosis [91].
The inflammatory caspase family includes caspases 1, 4, 5, 11, and 12, which are closely associated with pyroptosis [92]. In the realm of cancer, pyroptosis pathways linked to caspases can be categorized into canonical and non-canonical pathways. For the former, inactive pro-caspase 1 is activated by various inflammasomes (NLRP3, AIM2, NLRP1, PYRIN, NLRC4) [93–95]. Mechanistically, caspase 1 and caspase 11 cleave the linker region between the N- and C-terminal domains of GSDMD, releasing its N-terminal (p30) and C-terminal (p20) fragments, sustaining pyroptosis [59, 60, 95]. In the nonclassical pathway, lipopolysaccharide (LPS) activates Caspases 4/5/11 to cleave GSDMD to induce pyroptosis. Beyond these two pathways, caspase 3 cleaves GSDME, releasing its N-terminal fragment and triggering pore formation, which is crucial for pyroptosis [96, 97]. PD-L1 induces pyroptosis in BRCA through the caspase 8/GSDMC signaling pathway, shifting TNF-α or chemotherapeutic-induced apoptosis towards pyroptosis.
It is now understood that autophagy and apoptosis intricately regulate each other, largely through interactions between autophagy-related proteins (ATGs) and caspases. Caspase 1 fosters cytoprotective autophagy and eliminates damaged mitochondria following redox changes by activating LC3 and Beclin-1 [98]. Generally, caspase 2 exerts a negative regulatory effect on the autophagic process. This phenomenon was observed in model mouse embryonic fibroblasts (MEFs) and young adult mouse cortical neurons, where early-stage caspase 2 knockdown prompted mobilization of cytoprotective autophagy [99, 100]. In the absence of DISC, pro-caspase 8 can be activated in interaction with autophagosomes, which induces the occurrence of apoptosis [101]. Besides, Caspase 3/9 cleaves and partially inactivates ATG5 and Beclin-1 through interactions with autophagy-related proteins in various tumor cells.In murine bone marrow cells undergoing extended starvation, caspase 3 cleaves Beclin-1 to produce c-fragments that reduce autophagic fluxes enhancing apoptosis.
Blocking caspase activity does not stop parthanatos because caspase activation happens later in the process [102] (Fig. 1).
Fig. 1.
Caspase signaling can induce endogenous apoptosis through caspase 8,10,7,3 and exogenous apoptosis through the cascade of caspase3,6,7,9,10. Inhibition of Caspase 8 can induce necroptosis. Pyroptosis and autophagy can be induced through caspase 1. Caspase3,6,9 -mediated Beclin-1 degradation and Caspase 2 both inhibit autophagy
The targeted therapy of the caspase signaling pathway related to PCD
Targeting at caspases 3
Targeting genes with low expression in tumors, such as ULK1 in BRCA tissues, offers a viable strategy for cancer treatment. The ULK1 activator LYN1604 prompts autophagy by interacting with activator of RAD21 Recombinant Protein, caspase 3, and transcription 3 (ATF3) [103]. Besides, AZD5991 displays stable anti-tumor activity in hematologic malignancies such as multiple myeloma and AML. AZD5991 binds to Mcl-1, triggering the Bak-dependent intrinsic apoptotic pathway and activating caspase 3, ultimately inducing apoptosis [104]. Following AZD5991 monotherapy, about three-fifths of the patients experienced symptomatic relief. In addition, all patients were associated with varying degrees of elevated troponin levels [104]. Moreover, the 3',5'-degraded chalcone (C10) induces pyroptosis through the PKCδ/JNK pathway by activating caspase 3, leading to PARP and GSDME-dependent pyroptosis [105]. Interestingly, a therapeutic approach against metastatic triple-negative breast cancer (mTNBC) involving the conversion of a peptide-doxorubicin conjugate (PDC) mediated by tumor caspase 3 utilizes two separate caspase 3-cleaving PDCs: EMC-KGDEVD-DOX (MPD1) and RGDEVD-DOX (TPD1). These PDCs were conceived to target either tumor cells or vasculature associated with the aggressive advancement of mTNBC and exhibited notable effectiveness in inducing tumor cell death [106].
It is widely acknowledged that Ginsenosides, a traditional Chinese medicine, exhibits diverse pharmacological activities. The introduction of the piperazine group into ginsenoside upregulates Cl-Caspase 3, Cl-Caspase 9 and Cl-PARP expression was found to result in cellular apoptosis [107]. In patients with advanced hepatocellular carcinoma (HCC), Ginsenosides combined with catheter-based arterial chemoembolization (TACE) resulted in an overall survival of 13.2 months compared to 3.1 months with TACE alone [108]. Dihydroartemisinin (DHA), an artemisinin derivative found in artemisia annua, a traditional Chinese medicine, inhibits the proliferation and tumorigenicity of BRCA cells by activating caspase 3, upregulating the expression level of gasdermin E and melanoma 2, and inducing pyroptosis [109]. Adverse reactions such as anemia, nausea, and vomiting are 60% likely to occur during the treatment of solid tumors with Dihydroartemisinin. Notably, Dihydroartemisinin has a one in four success rate in controlling progression of solid tumors [110].
Besides, current evidence suggests that cisplatin and paclitaxel both induce significant apoptosis in lung cancer cells. However, cisplatin-induced caspase 3/GSDME-dependent pyroptosis yields a more sustained response than paclitaxel-induced pyroptosis, suggesting that cisplatin may offer extra benefits in treating lung cancer with GSDME overexpression [111–113]. In the phase 2 NeoSAC trial, the 36-month disease-free survival rate of apatinib and sindilizumab in combination with carboplatin and albumin-conjugated paclitaxel chemotherapy for early-stage triple-negative breast cancer was 94.1%, with a median follow-up time of 39.1 months. This demonstrates that this therapy shows promising prospects and a manageable safety profile for early TNBC [114]. Alantolactone (ATL), a terpenoid derived from the traditional Chinese herb Inula helenium, was documented to demonstrate remarkable anti-inflammatory, antibacterial, and anti-tumor properties. It was demonstrated that ATL triggers ROS-mediated activation of mitochondria-dependent caspases 3, 7, and 9, inducing apoptosis and GSDME-dependent pyroptosis [115]. BI 905711, a novel quadrivalent bispecific antibody targeting TRAILR2 and Cadherin-17 (CDH17), exhibits anti-tumor efficacy in CDH17-positive colorectal cancer (CRC) xenografts, activating caspases 3, 7, and 8, both in tumor tissue and plasma [116].
Green tea extract Polyphenon E (Poly E) halts the G0 and G1 checkpoint in PNT1a cells, activating caspases 3, 7 as well as 9. After autophagy, Polyphenon E® prompts anoikis, a form of cell death [117]. In a phase II trial of Poly E for the treatment of patients at high risk for recurrent colorectal cancer, Poly E did not significantly reduce the number of abnormal crypt foci of the rectum, despite demonstrating good tolerability [118]. Checkpoint nano-proteolysis-targeting chimeras (nano-PROTACs) incorporate photosensitizer protoporphyrin IX (PpIX) and SHP2-targeting PROTAC peptide (aPRO), joined by caspase 3 cleavable fragments. Moreover, light-activated aPRO triggers SHP2 degradation via the ubiquitin–proteasome system due to increased caspase 3 expression in tumor cells post-light exposure. Persistent SHP2 deletion hinders immunosuppressive checkpoint signaling (CD47/SIRPα and PD-1/PD-L1), reactivating anti-tumor macrophages and T cells. This approach can work synergistically with immunogenic phototherapy, augmenting anti-tumor immune responses [119]. Dihydrotanshinone I (DHTS), a potent tanshinone, has been reported to exhibit strong anti-colon cancer activity. DHTS-induced cytotoxicity relies on ROS and induces activation of caspases 3, 2, and 9, promoting apoptosis and autophagy through the mitochondrial pathway in COAD cells. Accordingly, DHTS holds promise as a lead compound for anti-tumor drug development or as an adjuvant for COAD treatment [120].
Targeting caspase 3 holds significant potential in inducing the death of various tumor cells and effectively delaying tumor progression. This has positioned caspase 3 as a crucial target within the caspase pathway, with drugs directed toward it showing promising therapeutic effects against tumors.
Targeting at caspases 8, 9 and 10
It has been shown that the activation of caspase 8 by progesterone leads to apoptosis of cancer cells. By combining these two effects, the intrinsic and extrinsic apoptotic pathways can be simultaneously activated, effectively inhibiting tumorigenesis [121, 122]. A study revealed that ABBV-621, a TRAIL agonist, enhances caspase 8 aggregation and the formation of death signaling complexes, leading to the death of solid cancer cells at subnanomolar concentrations [123]. Besides, scutellarein (SCU), derived from Scutellaria baicalensis, activates caspases 8 and 3 by regulating Fas and FasL to overexpressed, inducing extrinsic apoptosis in Hep3B cells and exerting an anti-tumor effect [124]. Imipramine, an antidepressant, triggers extrinsic apoptosis in glioblastoma cells by upregulating FasL and activating caspases 8 and 3 [125]. CPT211, a novel camptothecin derivative, effectively suppresses BRCA cell proliferation by activating Fas/FADD/caspase 8 signaling and eventually inducing apoptosis [126]. Propofol triggers apoptosis in Leydig tumor cells by activating caspase 8, caspase 3, and caspase 9 through Akt pathway inhibition [127]. However, a multicenter randomized trial demonstrated that propofol management of elderly patients after major cancer surgery did not increase their overall or cancer-specific survival [128].
Targeting at caspases 1, 4, 5 and 11
Long non-coding RNA GAS5 enhance the assembly and expression of inflammasome by hinder the bioactivity of glucocorticoid receptors, thus increasing IL-1β and IL-18 inflammatory mediators through caspase 1, inducing pyroptosis [129]. It has been shown that aponin polyphyllin VI (PPVI) impedes non-small cell lung cancer progression by activating caspase 1 through the ROS/NF-κB/NLRP3/GSDMD signaling axis, prompting pyroptosis in lung cancer cells [130]. Simvastatin has been reported to mitigate NSCLC tumor growth by driving pyroptosis via Caspase 1, NLRP3, IL-18 and IL-1β activation [131]. However, a randomized phase II study showed no significant difference in survival between afatinib alone and afatinib combined with simvastatin in patients with non-small cell lung cancer [132].
Targeting at other caspases
Although additional exploration is needed for drugs that target different caspases, specific targeted drugs for certain caspases are already available. For instance, during the treatment of leukemia using the antibody conjugate gemtuzumab ozogamicin (GO; Mylotarg®), Caspase 2 can facilitate apoptotic signal transmission mediated by the cleavage triggered by GO in human AML cells, subsequently initiating the apoptotic process. This finding could potentially lay the groundwork for future leukemia treatments [133]. In summary, the caspase signaling pathway plays a role in various forms of cell death, including apoptosis, pyroptosis, autophagy, and necroptosis. Nevertheless, the involvement of the caspase pathway in cell death processes such as Alkaliptosis and Oxeiptosis remains unclear. Notably, drugs targeting the caspase signaling pathway are well-developed, with a primary focus on caspases 3, 1, and 8. However, the exploration of targeted drugs for caspases 2, 12, and 14 is still in its preliminary stages. Caspase 2, known for cleaving specific substrates, acts as a tumor suppressor in adenocarcinoma, melanoma, and COAD [134]. The development of targeted drugs to enhance the caspase signaling pathway holds promise for introducing novel chemotherapy strategies for the prevention and treatment of tumors (Table 1).
Table 1.
Compounds targeting of the caspase signaling pathway related to PCD in cancer: ↓ represents decrease or inhibition. ↑ represents increase or activation
| Compound name | Target | Current stage | Mechanism in PCD | Tumor type | Refs |
|---|---|---|---|---|---|
| LYN1604 | ULKL, ATF3, RAD21 and caspase 3↑ | Preclinical study | Induce autophagy | Breast cancer | [103] |
| AZD5991 | Mcl-1↓ Bak and caspase 3↑ | Phase 1/2 clinical trial | Induce apoptosis | Multiple myeloma, acute myeloid leukemia | [104] |
| 3',5'-degraded chalcone (C10) | PKCδ/JNK, caspase 3↑ | Preclinical study | Induce pyroptosis | Prostate cancer | [105] |
| RGDEVD-DOX (TPD1) | Caspase 3↑ | Preclinical study | Induce apoptosis | Triple-negative breast cancer | [106] |
| EMC-KGDEVD-DOX (MPD1) | Caspase 3↑ | Preclinical study | Induce apoptosis | Triple-negative breast cancer | [106] |
| Ginsenosides | Cl-Caspase 3, Cl-Caspase 9 and Cl-PARP↑ | Phase 2/3 clinical trial | Induce apoptosis | Hepatocellular carcinoma | [107, 108] |
| Dihydroartemisinin | Caspase 3↑ | Phase 1 clinical trial | Induce pyroptosis | Breast cancer | [110] |
| Paclitaxel | Caspase 3, caspase 8↑ | Phase 2/3 clinical trial | Induce apoptosis | Lung cancer | [111–113] |
| Cisplatin | Caspase 3/GSDME↑ | Phase 2/3 clinical trial | Induce apoptosis and pyroptosis | Lung cancer | clinicaltrials.gov |
| Alantolactone (ATL) | ROS, caspase 3, caspase 7 and caspase 9↑ | Preclinical study | Induce apoptosis and pyroptosis | Breast cancer | [115] |
| BI 905711 | Caspase 3, caspase 7, caspase 8↑ | Phase 1 clinical trial | Induce apoptosis | Colorectal cancer | clinicaltrials.gov |
| Green tea extract Polyphenon E | Caspase 3, caspase 7 and caspase 9, cleaved poly (ADP-ribose) polymerase 1↑ | Phase 1/2 clinical trial | Induce autophagy and anoikis | colorectal cancer | [117], clinicaltrials.gov |
| Nano-PROTACs | Caspase 3↑SHP2↓ | Preclinical study | Induce apoptosis | Breast cancer | [119] |
| Dihydrotanshinone I (DHTS) | ROS, caspase 3, caspase 2 and caspase 9↑ | Preclinical study | Induce apoptosis and autophagy | Colon cancer | [120] |
| Progesterone | Caspase 8↑ | Phase 1/2 clinical trial | Induce apoptosis | Endometrial cancer | [121, 122] |
| ABBV-621 | TRAIL, caspase 8↑ | Preclinical study | Induce apoptosis | Colorectal cancer | [123] |
| Scutellarein (SCU) | Fas/FasL, caspase 8 and caspase 3↑ | Preclinical study | Induce apoptosis | Hepatocellular carcinoma | [124] |
| Imipramine | FasL, caspase 8 and caspase 3↑ | Preclinical study | Induce apoptosis | Glioblastoma | [125] |
| CPT211 | Fas/FADD/caspase 8↑ | Preclinical study | Induce apoptosis | Breast cancer | [126] |
| Propofol | Akt, caspase 8, caspase 3, and caspase 9↑ | Phase 1 clinical trial | Induce apoptosis | Leydig tumor | clinicaltrials.gov, [127] |
| lncRNA GAS5 | caspase 1, IL-1β and IL18↑ | Preclinical study | Induce pyroptosis | Ovarian cancer | [129] |
| Aponin polyphyllin VI (PPVI) | ROS/NF-κB/NLRP3/GSDMD and caspase 1↑ | Preclinical study | Induce pyroptosis | Lung cancer | [130] |
| Simvastatin | NLRP3, Caspase 1, IL-1β, and IL-18↑ | Phase 2 clinical trial | Induce pyroptosis | Non-small cell lung cancer | [131], clinicaltrials.gov |
| Mylotarg® | Cleaved caspase 2↑ | Post-Marketing Surveillance | Induce apoptosis | Leukemia | [133] |
RIPK1/RIPK3/MLKL signaling pathway
The mechanism of RIPK1/RIPK3/MLKL signaling pathway regulated PCD in tumor microenvironment
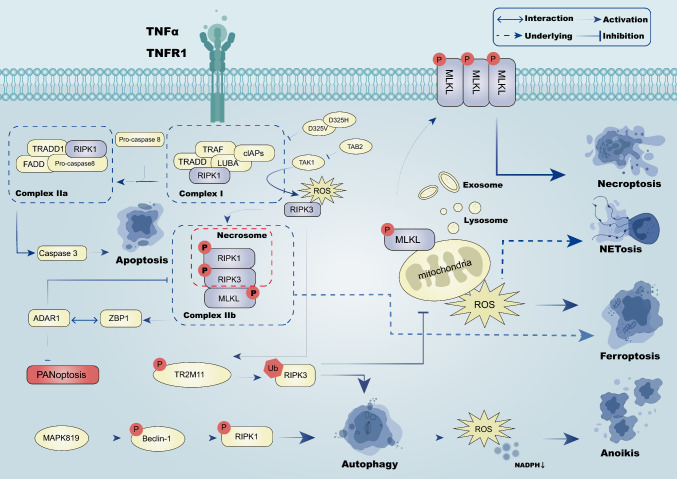
RIPK1 is a TNF-induced pleiotropic adaptor protein, and phosphorylated RIPK1 contributes to RIPK1 self-oligomerization and RIPK3 recruitment. In the context of the cancer cell microenvironment, complex I could lead to necroptosis and apoptosis under the regulation of RIPK1. Consequently, it is feasible to prove the occurrence of complex I disruption during apoptosis by monitoring RIPK1 [135]. Following TNF-α binding to TNFR1 on the plasma membrane, RIPK1 begins to function in cytoplasm as a receptor, forming complex I [136]. Prolonged cancer cell survival is facilitated by the polyubiquitinated RIPK1 Lys63 domain, promoting the recruitment of the IĸB kinase (IKK) complex and the growth factor β-activated kinase growth factor β-activated kinase (TAK) complex, ultimately activating NF-κB through the IKKα/IKKβ comple [137]. Additionally, TANK-binding kinase 1 (TBK1), IKKε, and IKKα/IKKβ contribute to phosphorylated RIPK1’s dephosphorylation, impeding its translocation to complex II and consequently preventing RIPK1-dependent cell death which is non-NF-κB-dependent [138]. In the context of AML driver mutations, RIPK3 knockout significantly accelerates leukemia development in mice which is transplanted with bone marrow cells. This may be attributed due to reduced MLKL protein expression downstream of RIPK3, potentially resulting in the inhibition of necroptosis caused by the failure to form inflammasomes [139].
TGF-β-activated kinase 1 (TAK1) kinase functions as an inhibitory phosphatase. An increasing body of evidence suggests that RIPK1 can be suppressed by TAK1-mediated inhibitory phosphorylation and TAK1-activated kinases (MK2 and IKKs) [140–142]. Cells may directly trigger RIPK1-dependent apoptosis (RDA) in response to TNFα stimulation overcoming the inhibitory effect of TAK1 on RIPK1 kinase. [143–145]. Functioning as a crucial upstream regulator, RIPK1 governs various downstream signaling pathways of TNFR1 [136]. Upon TNF-α be joint to TNFR1 on the, protein molecules like RIPK1, LUBA, cIAPs, TRAF, and TRADD are recruited to form complex I. RIPK1 initiates apoptosis by associating with caspase 8-adaptor protein FADD, with the apoptotic process sustained through mitochondrial damage induction and cleavage of downstream caspase proteins like caspase 3 [141]. Current evidence suggests that TBK1, an endogenous inhibitor, mitigates RIPK1 expression in inflammatory apoptosis. Inhibition of TBK1 activates RIPK1, triggering apoptosis via direct phosphorylation [146]. Mutants of TGF-β-activated kinase 1 binding protein 2 (TAB2) impede the activation of RIPK1 kinase and the creation of the apoptotic complex (RIPK1, FADD, and caspase 8) through the TAK1-dependent pathway [147].
In the context of ferroptosis, Ferrostatin-1(Inhibitor of ferroptosis) inhibits the elevation of kidney RIPK3 and MLKL mRNA expression, suggesting that ferroptosis might augment susceptibility to necroptosis by amplifying RIPK3 and MLKL levels. In human fibroblasts, RIPK1 mutants D325 V or D325H mitigate RIPK1 expression, rendering them resistant to ROS generation and ferroptosis prevention [148]. A dual-active compound known as Nec-1f was reported to mitigate RIPK1 kinase activity and halt downstream MLKL phosphorylation, ultimately inhibiting ferroptosis progression [149].
During autophagy, RIPK1 is recruited to the early autophagosomes, leading to the activation of caspase 8 [150]. ULK1, an autophagy-initiating kinase, phosphorylates RIPK1 in a manner that hinders complex IIb and necrosome assembly, thus preventing cell death [151]. Under glucose starvation, AMPK induces autophagy by directly activating ULK1 through Ser 317 and Ser 777 phosphorylation [152]. Recent studies have identified RIPK3 as another member of the AMPK-activating kinase family, suggesting that RIPK3-dependent AMPK activation represents a reciprocal interaction between necroptosis and autophagy [152]. Moreover, RIPK3 undergoes selective autophagic degradation via tripartite motif containing 11 (TRIM11)-mediated ubiquitination, suppressing necroptosis [153]. RIPK1 upregulation can reportedly induce autophagy due to Beclin-1 activation upon dissociation from BCL2L11, itself phosphorylated by MAPK8/9 [154].
Neutrophil extracellular traps (NETs) induced by programmed necrosis-associated stimuli constitute a network of extracellular DNA and microbial-killing proteins that trap and destroy invading pathogens, which is dependent on the production of ROS, potentially linking the mechanisms of NETosis and RIPK1 at the molecular level [164]. Notably, activated MLKL may also translocate to various subcellular compartments, including mitochondria, exosomes, and lysosomes, where it contributes to diverse functions like aerobic respiration, ROS generation, extracellular vesicle generation, cytokine release, endocytosis, and autophagy [155–158]. Current evidence suggests the E3 subunit of the pyruvate dehydrogenase complex is directly phosphorylated and activated by RIPK3, promoting aerobic respiration and mitochondrial ROS production [155].
In the context of cancer, RIPK1-mediated mitophagy induction could prove effective for eliminating ECM-shed cancer cells. RIPK1 activation during ECM detachment stimulates mitophagy through a PGAM5-dependent mitochondrial phosphatase mechanism. Moreover, mitophagy-induced NADPH reduction in isolated-cell mitochondria leads to anoikis and nonapoptotic cell death due to increased ROS levels. Moreover, epigenetic changes repress RIPK1 to inhibit anoikis and enhance tumor cell metastatic potential, further contributing to tumorigenesis in head and neck squamous cell carcinoma (HNSCC) [159]. The antagonism of RIPK1/PGAM5 accentuates tumor formation in vivo [160]. However, it has been established that parthanatos does not lead to RIPK1 activation [1]. However, adenosine deaminase acting on RNA 1 (ADAR1) inhibits PANoptosis by interacting with the Zα2 domain of ZBP1, limiting the interaction between ZBP1 and RIPK3, promoting tumorigenesis [161] (Fig. 2).
Fig. 2.
The RIPK1/RIPK3/MLKL signaling pathway is involved in tumor cells through Complex I (TRADD, TRAF, cIAPs, LUBA and RIPK1) and Complex IIa(TRADD1, RIPK1,FADD, and pro-caspase 8) induces apoptosis, necroptosis and prevents PANoptosis through Complex IIb (RIPK1, RIPK3, MLKL). Phosphorylated MLKL can promote mitochondrial ROS generation, which induces NETosis and ferroptosis. Ubiquitinated RIPK3 can inhibit this process while inducing autophagy. Phosphorylated RIPK1 can also induce autophagy, which can promote the reduction of ROS production and NADPH production, thereby inducing anoikis
The target therapy of RIPK1/RIPK3/MLKL signaling related to PCD
Targeting at RIPK1
Ophiopogonin D’ (OPD’), derived from Ophiopon japonicas, triggers necroptosis in androgen-dependent PCA by enhancing FasL-dependent RIPK1 protein expression, offering therapeutic potential for tumors [162]. Moreover, Inula viscosa (L.) Aiton ethanolic extract hampers the growth of human AGS and human non-small-cell lung cancer (A549) cell lines. Studies have revealed that RIPK1 role in necroptosis pathway is essential in the toxicity induced by Enterobacteriaceae extracts [163]. NP-ALT, a therapeutic liposome peptide was shown to induce ROS and RIPK1-dependent necroptosis in xenograft models and endocrine therapy-resistant BRCA cells, which blocked tyrosine phosphorylation of p27kip1 (CDKN1B) as well as activation of CDK4 and CDK2 [164]. Resistance to apoptosis induction is closely linked to tumor drug resistance. RETRA, a small molecule that reactivates p53-regulated gene expression in mutant (MT) p53 cells, exerts its effect by affecting the phosphorylation level of the RIPK1/RIPK3/MLKL pathway in cervical cancer (CESC) cells. This leads to cell cycle arrest in the S phase, elevated p21 expression, reduced cyclin-d3 levels, mitochondrial hyperpolarization, ROS production, and ultimately selective necroptosis induction in the absence of p53. Co-administration of RETRA and Necrostatin-1 was found to reverse the impact of RETRA and prevent CESC cell death. RIPK1 inhibition with Nec-1 increases the survival of adrenocortical cancer (ACC) and anaplastic thyroid cancer (ATC) cells during radiotherapy, suggesting RIPK1's vital role in procedural necrosis induction [165]. The HSP90 inhibitor, 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) treatment reduced the RIPK1 [166, 167]. In a phase I dose-escalation study, the combination of 17-DMAG and trastuzumab was shown to be effective in improving the efficacy of the treatment in ovarian cancer, and the appropriate weekly dose of 17-DMAG was found to be 80 mg/m [168]. As a robust third-generation platinum drug, lobaplatin has fewer side reactions and less cross-resistance to platinum. Lobaplatin promoted the proteasomal degradation of cell inhibitor of apoptosis protein-1/2 (cIAP1/2). This synergistic pyroptosis effect was inhibited by the cleavage of ROS and caspase 3 by inhibition of Ripoptosome (RIPK1/Caspase 8/FADD) with the cIAP1/2 antagonist birinapant [169]. The use of birinapant in the treatment of ovarian cancer revealed that although birinapant theoretically has a clear in vivo target inhibition, however, there was no significant efficacy in the ovarian cancer patients in the study [170]. In the context of cancer, as cancer cells resist anoikis, signaling via adhesion-related molecules is indispensable for cancer growth and metastasis. Focal adhesion kinase (FAK) is activated in focal adhesion, conveying survival signals dependent anchorage. In certain malignancies or malignant cell subsets, reduced FAK levels bolster cIAP2:RIPK1 complex formation, leading to decreased TPL2 expression levels and impeding tumor progression. When FAK inhibitors VS-4718, PF-573228, defactinib, GSK2256098 and IN10018 acted on cancer, cIAP2:RIPK1 complex formation was elevated, decreasing TPL2 expression level to induce anoikis and suppressed the tumor progression [171–174].
Targeting at RIPK3
It has been documented that resibufagenin induces programmed necrosis of CRC cells by activating glutamate dehydrogenase (GLUDL), glutamine synthetase (GLUL), and glycogen phosphorylase (PYGL), mediated by RIPK3 and consequently inhibiting tumor growth [175]. In recent years, curcumin has emerged as a potent RIPK3 inhibitor by upregulating necroptosis blockade, inhibiting the phosphorylation of MLKL and RIPK3 while sparing RIPK1. In PCA PC-3 AcT cells, proteins like p-RIP3 and p-MLKL contribute to necroptosis and apoptosis of PCA, eventually diminishing the viability of PC-3 AcT cells [176, 177]. There was no difference in the mean number or size of intestinal adenomas in the curcumin-treated group compared with the placebo group, but there were fewer adverse effects [178]. Neoalbuminol (NA), extracted from the fungus Albatrellus confluens, triggers autocrine TNFα production by modulating the RIPK/NF-κB signaling pathway and instigating ROS production dependent on RIPK3 in tumor cells, initiating necroptosis [215]. Studies have shown that the ubiquitin–proteasome system owns the possibility to regulate RIPK3-dependent necroptosis, and proteasome inhibitors acted as anticancer drugs to target the necroptosis pathway. It is widely thought that the ubiquitin–proteasome system might regulate RIPK3-dependent necroptosis, and proteasome inhibitors could serve as anticancer agents targeting the necroptosis pathway. The proteasome inhibitors MG132 and bortezomib can evoke RIPK3 and MLKL-dependent necroptosis human leukemia cells [179]. Polyinosinic: polycytidylic acid (PolyI: C), a viral dsRNA analog, has been convinced to trigger programmed necrosis in CESC cells, contingent upon RIPK3 expression [180]. Moreover, B-Raf(V600E) inhibitors like dabrafenib, essential anticancer agents for metastatic melanoma, may competitively bind with ATP of the RIPK3 enzyme to inhibit its activity in vitro [181, 182]. Polyphyllin D, a significant component of traditional herbal medicine, elicits RIPK3 expression and instigates various forms of programmed cell necrosis, exerting anti-tumor effects on neuroblastoma in vitro and in vivo [183]. Chloroquine (CQ) substantially enhances receptor-interacting RIPK3 expression involving CQ-related autophagy [184, 185].
Targeting at MLKL
MLKL, a pivotal regulatory gene in necrosis, can be significantly inhibited by gene deletion or low-dose chemical inhibitors, suppressing tumor recurrence and even reducing tumorigenicity both in vivo and in vitro. Staurosporine (STS), an alkaloid isolated from Streptomyces staurosporeus, induces RIPK1/MLKL-dependent necroptosis in leukemic cells when caspase activation is compromised [186, 187]. It has been established that IMB5036, a novel pyridazinone compound with potent cytotoxicity, exerts a lethal effect on pancreatic ductal adenocarcinoma (PDAC) cells. This compound triggers the translocation of MLKL and p-MLKL from the cytoplasm to the cell membrane, upregulating p-RIP1, p-RIP3, and p-MLKL, and partially inducing apoptosis and pyroptosis. Ultimately, IMB5036 impedes pancreatic transplantation tumor growth, augments the tumor necrosis area, and restrains human PDAC cell proliferation [188]. Sorafenib, a multikinase inhibitor and first-line treatment for advanced HCC, can be combined with the HSP90 inhibitor ganetespib. Notably, LAMP2 aids in MLKL degradation via the chaperone-mediated autophagy pathway. The synergistic use of ganetespib and sorafenib potentially engenders an anti-angiogenic impact by triggering necroptosis, suppressing macroautophagy, and offering a novel approach for HCC treatment [189–191]. In addition, ganetespib did not improve survival in patients with advanced lung adenocarcinoma treated with salvage therapy, along with adverse effects such as neutropenia time [192]. Clinical trials involving HSP90 inhibitors such as 17 AAG and Alvespimycin (IPI-504, MAG, and 17-D) have been conducted with cancer patients, potentially and indirectly inhibiting necroptosis [193–195].
Targeting at RIPK1 and RIPK3
Necrostatin-1 (NEC-1) functions as a traditional inducer of necroptosis, effectively blocking the interaction between RIPK1 and RIPK3. It inhibits programmed necrosis, providing a targeted approach for almost all common cancer types, particularly CRC and hematopoietic system malignancies. Notably, NEC-1 selectively suppresses programmed necrosis without affecting normal cellular functions or apoptosis [196]. Shikonin, a naturally occurring naphthoquinone present in traditional Chinese medicine, circumvents resistance regulated by drug transporters or anti-apoptotic Bcl-2 proteins by triggering necroptosis in human leukemia cell lines [197–199]. In PCA, shikonin induces necroptosis by modulating the expression of RIPK1 and RIPK3 [200]. Additionally, a study revealed that shikonin could stimulate mitochondrial ROS synthesis in TNBC cells, thereby initiating necroptosis or apoptotic elimination of BRCA cells [201]. Shikonin was also found to effectively inhibit primary tumor growth in an in vivo osteosarcoma model, primarily through RIPK1 and RIPK3-dependent necroptosis, and notably reduce lung metastasis [202]. Shikonin triggers RIPK1 and RIPK3-dependent necroptosis along with augmented autophagy, which, in turn, potentiates the upregulation of damage-associated molecular patterns (DAMPs). Importantly, the combined application of shikonin and chloroquine could potentially boost immunogenicity and vaccine efficacy, offering a novel strategy for cancer vaccine development [203]. Chelerythrine (CHE) orchestrates the formation of the RIPK1-RIPK3-Drp1 complex via mitochondrial ROS (mtROS), facilitating the mitochondrial translocation of Drp1 and promoting necroptosis [204].
Targeting at RIPK1, RIPK3 and MLKL
In the context of colorectal cancer, elaidic acid, a primary active component of the Active Fraction of Polyrhachis vicina Roger (AFPR), triggers necroptosis by activating ERK/RIPK1/RIPK3/MLKL. This compound offers a promising alternative for CRC [205]. Paeoniflorigenone (Paeo), an active substance extracted from the root bark of P. suffruticosa, significantly inhibits the migration and invasion of human YD-10B HNSCC cells. Paeo exerts this anti-metastatic effect by suppressing programmed cell apoptosis through the dephosphorylation of key programmed apoptosis proteins, including RIPK1, RIPK3, and MLKL [206]. Paeo's anti-metastatic action is attributed to the dephosphorylation of key programmed apoptosis proteins, namely RIPK1, RIPK3, and MLKL, leading to a substantial reduction in migration and invasion of human YD-10B HNSCC cells [206].
Targeting at producing ROS
The mitochondrial complex I inhibitor arctigenin induces necroptosis in PCA cells by inducing ROS-mediated mitochondrial damage and elevating CCN1 levels. This cascade eventually culminates in heightened levels of p-RIP3 and p-MLKL, contributing to the reduced viability of PCA cells [207]. Doramectin (DRM), an avermectin drug, exhibits anti-tumor effects by inducing ROS overproduction in glioma cells. This surge in ROS triggers necroptosis through the RIPK1/RIPK3/MLKL pathway, with mitochondria bridging these two pathways. Moreover, DRM holds huge promise as a potential therapeutic agent for inducing apoptosis and necroptosis in cancer treatment [208].
In conclusion, we summarized the mechanism of RIPK1/RIPK3/MLKL signaling in necroptosis, apoptosis, PANoptosis, autophagy, NETosis, and anoikis. In the occurrence and progression of tumors, there will be various PCD combined effects and unknown changes. Therefore, we provide a dynamic and holistic idea for studying the mechanism of tumor development and treatment. In addition, we summarized the chemotherapeutic agents targeting the RIPK1/RIPK3/MLKL signaling pathway, but we found that studies on drug combinations were imperfect. Therefore, we provide a reference for the combination of drugs targeting the RIPK1/RIPK3/MLKL signaling pathway to treat tumors (Table 2).
Table 2.
Compounds targeting of the RIPK1/RIPK/MLKL signaling pathway related to PCD in cancer: ↓ represents decrease or inhibition. ↑ represents increase or activation
| Compound name | Target | Current stage | Mechanism in PCD | Tumor type | Refs |
|---|---|---|---|---|---|
| Ophiopogonin D’ (OPD’) | FasL and RIPK1↑ | Preclinical study | Induce necroptosis | prostate cancer | [162] |
| Inula viscosa (L.) Aiton Ethanolic Extract | RIPK1 ↑ | Preclinical study | Induce necroptosis | Lung cancer | [163] |
| NP-ALT | CDK4, CDK2, ROS and RIPK1↑ | Preclinical study | Induce necroptosis | Breast cancer | [164] |
| RETRA | RIPK1/RIPK3/MLKL, p21, ROS↑cyclin-d3, ROS↓ | Preclinical study | Induce necroptosis | Cervical cancer | [165] |
| 17-DMAG | Hsp90, RIPK1↓ | Phase 1 clinical trial | Inhibit necroptosis, induce apoptosis | ovarian cancer | [166, 167], clinicaltrials.gov |
| Birinapant | cIAP1/2, RIPK1/caspase 8/FADD, ROS, caspase 3↓ | Phase 1/2 clinical trial | Inhibit pyroptosis | Solid tumor/ovarian cancer | [169, 170] |
| Defactinib | FAK↓ cIAP2:RIPK1↑ | Phase 1/2 clinical trial | Induce anoikis | Pleural mesothelioma/Non-small cell lung cancer | clinicaltrials.gov |
| IN10018 | FAK↓ cIAP2:RIPK1↑ | Phase 1/2 clinical trial | Induce anoikis | Solid tumor | clinicaltrials.gov |
| Resibufagenin | RIPK3↑ | Preclinical study | Induce necroptosis | Colorectal cancer | [175] |
| Curcumin | P-RIPK3 and p-MLKL↑ | Phase 1/2 clinical trial | Inhibit necroptosis | Intestinal adenoma | [178] |
| Neoalbuminol (NA) | RIPK/NF-Κb, ROS↑ | Preclinical study | Induce necroptosis and apoptosis | Cervical cancer, colorectal cancer, osteosarcoma | [215] |
| MG132 | RIPK3 and MLKL↑ | Preclinical study | Induce necroptosis | Leukemia | [179] |
| Bortezomib | RIPK3 and MLKL↑ | Phase 1/2/3 clinical trial | Induce necroptosis | Leukemia/Refractory prostate cancer | clinicaltrials.gov, [179] |
| Polyinosinic: polycytidylic acid | RIPK3↑ | Preclinical study | Induce necroptosis | Cervical cancer | [180] |
| Dabrafenib | RIPK3↓ | Phase 1/2/3 clinical trial | Inhibit necroptosis | Metastatic melanoma | [181, 182] |
| Polyphyllin D | RIPK3↑ | Preclinical study | Induce necroptosis | Neuroblastoma | [183] |
| Chloroquine | RIPK3↑ | Phase ½ clinical trial | Induce autophagy | Colon cancer/Small cell lung cancer | [184, 185], clinicaltrials.gov |
| Staurosporine | MLKL↑ | Preclinical study | Induce necroptosis | Leukemia | [186, 187] |
| IMB5036 | p-RIPK1, p-RIPK3 and p-MLKL↑ | Preclinical study | Induce apoptosis and pyroptosis | Pancreatic ductal adenocarcinoma | [188] |
| 17 AAG | MLKL↓ | Phase 1/2 clinical trial | Induce necroptosis | Colon cancer | [193–195] |
| IPI-504 | MLKL↓ | Phase 1/2 clinical trial | Induce necroptosis | Breast cancer | [193–195] |
| Ganetespib | MLKL↓ | Phase 1/2/3 clinical trial | Induce necroptosis, inhibit autophagy | Hepatocellular carcinoma/ | [193–195] |
| Necrostatin-1 | RIPK1 and RIPK3↓ | Preclinical study | Inhibit necroptosis | Colorectal cancer | [196] |
| Shikonin | RIPK1 and RIPK3↓ | Preclinical study | Induce necroptosis and autophagy | Osteosarcoma, leukemia, pancreatic cancer and breast cancer | [197–199] |
| Chelerythrine | RIPK1-RIPK3-Drp1 and mtROS ↑ | Preclinical study | Induce necroptosis | Glioma | [204] |
| Elaidic acid | ERK/RIPK1/RIPK3/MLKL↑ | Preclinical study | Induce necroptosis | Colorectal cancer | [205] |
| Paeoniflorigenone (Paeo) | p-RIPK1, p-RIPK3 and p-MLKL↓ | Preclinical study | Inhibit apoptosis | Head and neck squamous cell carcinomas | [206] |
| Arctigenin | ROS, CCN1, p-RIP3 and p-MLKL ↑ | Phase 1 clinical trial | Induce necroptosis | Prostate cancer | [207] |
| Doramectin (DRM) | ROS and RIPK1/RIPK3/MLKL ↑ | Preclinical study | Induce necroptosis and apoptosis | Glioma | [208] |
mTOR signaling pathway
The mechanism of mTOR signaling pathway regulated PCD in tumor microenvironment
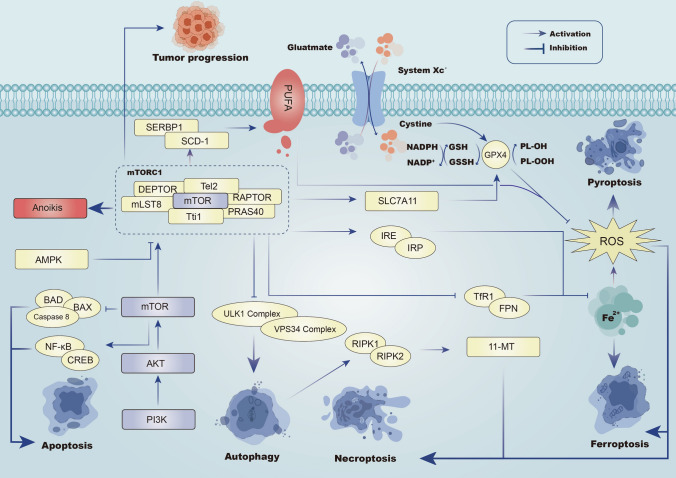
The atypical serine/threonine kinase mTOR acts as a significant role in mediating cell growth and metabolism. Indeed, mTOR signaling is activated in tumors generally, altering the expression levels or activities of critical metabolic factors in the pathway [209]. mTOR orchestrates tumor-related anabolic processes such as fatty acid, lipid synthesis, protein, and nucleotide while also governing PCD processes such as autophagy and apoptosis. mTOR is formed by two structurally and functionally distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1, containing mTOR, is sensitive to rapamycin, whereas mTORC2 remains insensitive to rapamycin [210].
The PI3 K/AKT/mTOR signaling pathway is closely intertwined with angiogenesis, contributing to tumor growth, metastasis, and lethality. Both normal and tumor-related angiogenesis are influenced by the PI3 K/AKT/mTOR pathway [211]. Futhermore, The PI3 K/AKT/mTOR signaling pathway inhibits apoptosis. This pathway also exerts inhibition on apoptosis-related genes, including BAX, BAD, Forkhead Box Protein O1 (FOXO1), glycogen synthase kinase 3 (GSK-3), and caspase 9. Instead, it promotes the expression of anti-apoptotic proteins like NF-κB and cAMP response element-binding protein (CREB), thereby playing a crucial role in promoting cell survival [212, 213].
The PI3 K/AKT/mTOR signaling pathway inhibits cellular autophagic fluxes.During the initiation of autophagy, mTORC1 and AMPK work synergistically to inhibit the synthesis of ULK1 and VPS34 complexes. mTOR can counteract the inhibition of RIPK1/RIPK3 interaction through autophagy, thus enhancing 11-methoxytabersonine (11-MT)-mediated necroptosis [44, 214, 215]. In addition, inhibition of mtor-mediated mitochondrial autophagy resulted in limited ROS production, ultimately limiting ROS production and preventing necroptosis [216].
Interestingly, inflammasomes can serve as specialized executors of pyroptosis. The intricate interplay between pyroptosis and autophagy is linked to the characteristic activation of the pyrin domain-containing protein 3 (NLRP3) inflammasome [130]. Growing literature suggests that mTOR, by inhibiting autophagy, promotes ROS accumulation and exacerbates pyroptosis. Suppression of MTOR-induced mitophagy removes damaged mitochondria, thus decreasing ROS generation, alleviating oxidative stress-related damage, and inhibiting pyroptosis by suppressing the activation of NLRP3 inflammasomes [217–220].
The PI3 K/AKT/mTOR signaling pathway inhibits ferroptosis by reducing cellular iron loading and enhancing the intracellular oxidative system xc—glutathione (GSH)-GPX4.Current evidence suggests that mTORC1 promotes stearoyl-CoA via the PI3 K-AKT-mTORC1 axis through Sterol regulatory element-binding protein 1 (SERBP1) and stearoyl-CoA desaturase-1 (SCD-1), resulting in the production of monounsaturated fatty acids, which inhibits lipid peroxidation and, finally, ferroptosis [221, 222]. In addition, mTORC1 regulates the body's iron load, as its overactivation increases the levels of transferrin receptor 1 (TfR1) and ferroportin (FPN), resulting in reduced iron load and suppressed ferroptosis [223]. Ferroptosis can be restrained by targeting mTOR signaling pathways, especially mTORC1, which hinders the iron regulatory protein/iron response element (IRE/IRP) pathway, consequently reducing iron load and inhibiting ferroptosis [224]. In addition, mTORC1 mitigates ferroptosis by regulating the major antioxidant system Xc-glutathione (GSH)-GPX4. Additionally, mTORC1 inhibition-induced autophagy-mediated GPX4 degradation further suppresses ferroptosis [225].
Inhibition of anoikis in cancer cells after detachment from the extracellular matrix is a critical step in the metastasis process. In BRCA cells, the activation of AMPK-mediated mTORC1 inhibition and protein synthesis contributes to anti-anoikis effects [226]. F FDX1, a gene linked to cuproptosis, may obstruct PI3 K/AKT/mTOR signaling, playing a role in multiple chemoresistance scenarios [227]. The cuproptosis prognostic predictor PCA-associated transcript 6 (PCAT6) has been reported to trigger activation of the PI3 K/Akt/mTOR signaling pathway [228] (Fig. 3).
Fig. 3.
In the mTOR signaling pathway, PI3 K/AKT/mTOR axis can induce the occurrence of apoptosis. mTORC1 complex (mLST8, RAPTOR, DEPTOR, Tti1, Tel2, PRAS40) can induce anoikis. mTORC1 can promote autophagy through ULK1 complex and VPS34 complex, and can promote necroptosis through autophagy. mTORC1 can inhibit Fe2 + and thus ferroptosis. AMPK/mTOR axis inhibits ferroptosis and necroptosis by regulating the system Xc-glutathione (GSH)-GPX4 axis to inhibit ROS generation
Targeted therapies related to mTOR signaling pathways
Targeting at PI3 K/AKT/mTOR
ATP-competitive mTOR inhibitors represent an effective strategy to inhibit the PI3 K/AKT/mTOR axis. One such inhibitor is MLN0128, a pan-mTOR inhibitor that induces apoptosis and exhibits potent antitumor effects in vitro and in vivo, targeting tumors like bone and soft tissue sarcoma, BRCA, and primary effusion lymphoma [229–231]. The combination of sapanisertib and paclitaxel demonstrated significant clinical activity in the treatment of metastatic uroepithelial carcinoma (mUC) [232]. AZD8055, an ATP-competitive mTOR inhibitor, selectively inhibits class I PI3 K isoforms and other PI3 K-like kinase members. This leads to the induction of autophagy in conditions such as leukemia and BRCA [233–236]. Additionally, AZD6482, a specific inhibitor of pi3kβ, holds potential in inhibiting proliferation and inducing apoptosis in glioma cells [237]. SC66 is an AKT inhibitor that blocks the induction of apoptosis in CESC cell and glioblastoma cells by arresting cells in the G1 phase and inducing apoptosis by inhibiting mTOR activity and down-regulating Akt/β-catenin signaling pathway, respectively [238, 239]. The PI3 K/mTOR inhibitor VS-5584 can inhibit cell proliferation and promote apoptosis in acute lymphoblastic leukemia and glioblastoma [240, 241].
Melatonin combined with rapamycin treatment was found to block the negative feedback loop from the specific downstream effects of mTOR activation of S6 K1 to the Akt signaling pathway, thereby reducing cell viability, proliferation, and colony formation ability [242]. Apatinib is a vascular endothelial growth factor receptor-2 inhibitor that induces apoptosis and autophagy of ATC cells by downregulating p-mTOR and p-AKT signaling through the AKT/mTOR signaling pathway. In addition, apatinib promoted autophagy and elevated apoptosis in ATC cells, while the combined use of apatinib and autophagy inhibitor chloroquine significantly inhibited tumor growth both in vitro and in vivo. Therefore, molecular-targeted therapy combined with autophagy inhibitors is expected to advance the treatment of ATC patients [243]. Apatinib in combination with sindilizumab and carboplatin paclitaxel chemotherapy for treatment-phase early TNBC resulted in complete remission in 61.8% after imaging assessment, in addition to a disease-free survival rate of 94.1% at 36 months. This demonstrates the high level of clinical activity and manageable safety profile of apatinib [114]. Baohuoside-1, Mahanine, and Genipin inhibition can induce p-PI3 K, p-AKT, and p-mTOR, respectively, to induce apoptosis of oral cancer epithelial-mesenchymal stem cells, apoptosis of glioma cells, and autophagy of oral squamous cell carcinoma cells [244–246]. Isoglycyrrhizin (ISL) is a flavonoid extracted from licorice that inhibits mTOR by docking with its ATP-binding pocket (i.e., competing with ATP). It can effectively inhibit the proliferation and induce apoptosis of HCC and AGS cells by inducing autophagy in vivo and in vitro [247]. Pectin (PEC), geniposide (GJ), and baicalin are all flavonoids. The former induces autophagy in AGS cells, and the latter can enhance the induced apoptosis and autophagy in glioblastoma cells [246, 248, 249]. Tanshinone I (Tan—I) is isolated from the Chinese herbal medicine salvia miltiorrhiza, a critical fat-soluble monomer compound. Tan-I induces autophagy by inhibiting PI3 K/AKT/mTOR pathway in ovarian cancer (OV) [250].
Brusato, a natural quassinoid isolated from traditional Bruceae Fructus, can block PI3 K/Akt/mTOR to induce autophagy in HCC cells. Thus, it can effectively inhibit the proliferation and induce apoptosis of HCC cells and inhibit the invasion and migration of tumors in vitro and in vivo [251]. Gynostemma pentaphyllum (Thunb.) Makino is a traditional medicine commonly used in China, East Asia, and Southeast Asia. Importantly, gypenosides can induce apoptosis of renal cancer cells by regulating the PI3 K/Akt/mTOR signaling pathway by reducing the phosphorylation levels of Akt and mTOR [252]. Sinomenine (SIN), isolated from the traditional Chinese medicine Sinomenum, activates autophagy in renal cancer by inactivating the PI3 K/AKT/mTOR pathway [253]. Ricolinostat (ACY-1215) inhibits the proliferation and promotes apoptosis of esophageal squamous cell carcinoma through miR-30 d/PI3 K/AKT/mTOR and ERK pathways. ACY-1215 may be a promising antitumor drug for esophageal squamous cell carcinoma [254]. Novel quinazolinone MJ-33 induces AKT/mTOR-mediated autophagy-related apoptosis in 5 FU-resistant CRC cells [255]. Treatment of schwannoma cells with LiCl can lead to the generation of reactive oxygen species and the activation of the AKT/mTOR pathway, consequently inducing necroptosis. This effect was counteracted by the application of necrostatin-1, suggesting a potentially innovative mechanism of LiCl-triggered tumor cell [256]. Lorlatinib enhances the susceptibility of melanoma to ferroptosis by targeting the IGF1R-mediated PI3 K/AKT/mTOR signaling cascade. Consequently, the combination of lorlatinib significantly broadens the applicability of GPX4 inhibition in melanoma patients exhibiting elevated IGFR1 expression [257]. Loratinib was superior to crizotinib in the treatment of patients with ALK-positive non-small cell lung cancer, characterized by more durable benefits. However, the incidence of grade 3–4 adverse reactions with loratinib was 76%, leading to discontinuation of treatment in some patients [258]. In HCC cells, miR-21-5p functions to suppress ferroptosis by regulating MELK, which in turn modulates the AKT/mTOR signaling pathway [259].
Targeting at AMPK/mTOR
In non-small cell lung cancer A549 cell carcinoma, Resveratrol (RSV) enhances nerve growth factor receptor (NGFR) by increasing NGFR mRNA expression and prolonging the lifetime of NGFR mRNA and protein. It can induce the activation of AMPK/mTOR downstream and induce autophagy and apoptosis [260]. β-elemene is a potent anti-cancer compound extracted from Curcuma SPP. Moreover, β-elemene can induce apoptosis and autophagy of CRC cells by mediating ROS/AMPK/mTOR pathway, increasing ROS level, promoting AMPK protein phosphorylation, and inhibiting mTOR protein phosphorylation [261]. Ellagic acid induces apoptosis and autophagy in COAD cells through the AMPK/mTOR pathway. These results suggest that EA inhibits COAD growth and induces apoptosis and protective autophagy through the AMPK/mTOR pathway [262]. Besides, Periplocin inhibits the proliferation and induces apoptosis of PANC1 and CFPAC1 cells by activating the AMPK/mTOR pathway and inhibiting p70 S6 K [263]. It has been reported that Trifolirhizin induces autophagy-dependent apoptosis of COAD cells through AMPK/mTOR signaling pathway [264]. Chaga mushroom extract induces autophagy in BRCA cells by activating AMPK and inhibiting the mTOR signaling pathway, which involves activation of AMPK and inhibition of mTOR signaling pathway [265].
Targeting at MET/AKT/mTOR
The MET/AKT/mTOR signaling pathway regulates many biological processes, including cell apoptosis, autophagy, proliferation, tumorigenesis, and invasion. Cynaroside (Cy), a traditional Chinese medicine monomer, is a flavonoid glycoside compound which is widely present in plants. Cy can block the MET/AKT/mTOR axis by reducing the phosphorylation of AKT, mTOR, and P70S6 K. Therefore, the MET/AKT/mTOR axis can be a vital target of Cy, with anticancer efficacy and is expected to be a promising drug for treating AGS [266].
Targeting at EGFR/mTOR
Physakengose G (PG), a novel compound from the traditional Chinese medicine Physalis alkekengi var. franchetii, has shown promising anti-tumor effects in human osteosarcoma cells by inducing apoptosis through the EGFR/mTOR signaling axis. PG blocks the phosphorylation of EGFR and inhibits epidermal growth factor (EGF)-induced activation of downstream signaling molecules such as AKT and mTOR. In addition, PG induces apoptosis through the mitochondrial pathway [267]. GALNT14 regulates apoptosis and ferroptosis in OV through the EGFR/mTOR pathway. Importantly, GALNT14 inhibits the activity of the mTOR pathway by modifying the O-glycosylation of EGFR, providing a potantial method to overcome cisplatin resistance in OV patients [268]. The abnormal expression of GALNT14 can change the neogenesis, proliferation, migration, metastasis and drug resistance of tumor cells, and can be used as an important target for chemotherapy [269].
Targeting at other signaling pathway axis
Circ_0004585 plays a cancer-promoting role in the invasion and metastasis of PCA by targeting the miR-1248/TM9SF/mTOR axis, which provides a new idea for the development of therapeutic strategies for metastatic PCA [270]. The mTOR/S6 K1 signaling axis is known to regulate cell growth and exhibits a hyperactivated state in various cancers. miR-224 targets the 3'-UTR of mTOR, leading to the inactivation of apoptotic signaling and activation of cell proliferation [271]. Homoharringtonine (HHT) is a natural alkaloid from Taxus capitis with well-established anti-cancer effects on hematological malignancies. HHT inhibits the growth and promotes apoptosis of BRCA cells by regulating the miR-18a-3p/AKT/mTOR signaling pathway, and HHT may be a promising anti-tumor drug [272]. Sorafenib in combination with homoharringtonine in relapsed or refractory acute myeloid leukemia (R/R AML) demonstrated a highly active and manageable safety profile with an overall survival of 18.1 months, with fewer patients experiencing grade 3–4 adverse events [273]. Mitogen-activated protein/extracellular signal-regulated kinase (MEK) inhibitor U0126 induces BRCA cells (MDA-MB231 and S6 K) to undergo anoikis by simultaneously blocking extracellular signal-regulated kinase (ERK) and mammalian target of rapamycin (mTOR)/p70(S6 K) pathways, which may provide a therapeutic strategy for selectively targeting tumors that proliferate in the ectopic region [274]. GO induces autophagy and apoptosis in CRC cells through the ROS-dependent AMPK/mTOR/ULK-1 pathway [275]. The polyphyllin I ROS/AKT/mTOR pathway promotes autophagic death and apoptosis of COAD cells [276]. A recent study revealed that active metabolite of ginsenoside Compound K (CK) inhibited AKT/mTOR/c-Myc signaling pathway and its downstream hexokinase 2 (HK2) and pyruvate kinase isoenzyme M2 (PKM2) to promote the apoptosis of HepG2 and Huh7 human HCC cells [277].
Chrysin is a flavonoid that is abundant in honey and phytochemicals with an ERK/MTOR-mediated autophagy effect on oral cancer cells [278]. Chitooligosaccharide (COS) is used to trigger apoptosis and autophagy in osteosarcoma cells through the p53/mTOR signaling pathway [279]. Artematrolide A (AR—A) is a compound derived from Artemisia atrovirens that activates the ROS/ERK/mTOR signaling pathways. It orchestrates the transition of cell metabolism from aerobic glycolysis to mitochondrial respiration and prompts G2/M phase arrest and apoptosis in CESC cells. These findings suggest its potential as a therapeutic agent for CESC treatment [280]. Last but not least, in human oral squamous cell carcinoma, falcarinol inhibits cell proliferation, division, and metastasis by inducing apoptotic and autophagic cell death through the PI3 K/AKT/mTOR/p70S6 K pathway [281]. Novel conjugates of endoperoxide and 4-anilinoquinazoline inhibits the IGF1—R/AKT/mTOR signaling pathways and induces myeloma cell apoptosis [282]. Icariin and curcumol synergize to regulate miR-7/mTOR/SREBP1 pathway to induce autophagy and ferroptosis in PCA cells and affect lipid metabolism [283]. The natural product osthole exerts its anticancer effect in KRAS-mutated CRC cells by inducing ferroptosis by inhibiting the AMPK/Akt/mTOR signaling pathway [284].
We summarize our understanding of how mTOR signaling modulates the tumor microenvironment in PCD and summarize drugs targeting multiple mTOR signaling axes. mTOR signaling pathway has been used to study the relationship between apoptosis and autophagy. However, we found that autophagy, apoptosis, pyroptosis, anoikis, ferroptosis, and cuprotosis are closely related to the mTOR signaling pathway in the process of tumor development. Therefore, mTOR is closely related to regulating PCD and tumorigenesis. In addition, targeted drugs of the mTOR signaling pathway are abundant. Therefore, mTOR can be used as an essential target for the treatment of tumors (Table 3).
Table 3.
Compounds targeting of the mTOR signaling pathway related to PCD in cancer: ↓ represents decrease or inhibition. ↑ represents increase or activation
| Compound name | Target | Current stage | Mechanism in PCD | Tumor type | Refs |
|---|---|---|---|---|---|
| MLN0128(Sapanisertib) | PI3 K/AKT/mTOR↓ | Phase ½ clinical trial | Induce apoptosis | Soft tissue sarcoma, breast cancer, and primary effusion lymphoma | [229–231], clinicaltrials.gov |
| AZD8055 | PI3 K↓ | Phase 1 clinical trial | Induce autophagy | Leukemia and breast cancer | [233–236] |
| AZD6482 | pi3kβ↓ | Preclinical study | Induce apoptosis | Glioma | [237] |
| SC66 | Akt/β-catenin and mTOR↓ | Preclinical study | Induce apoptosis | Cervical cancer and glioblastoma | [238, 239] |
| VS-5584 | PI3 K/mTOR↓ | Phase 1 clinical trial | Induce apoptosis | Acute lymphoblastic leukemia and glioblastoma | clinicaltrials.gov, [240, 241] |
| Apatinib | p-AKT and p-mTOR ↓ | Phase 2/3 clinical trial | Induce apoptosis and autophagy | Anaplastic thyroid cancer | [243], clinicaltrials.gov |
| Baohuoside-1 | p-PI3 K, p-AKT and p-mTOR↓ | Preclinical study | Induce apoptosis | Oral cancer | [244–246] |
| Mahanine | p-PI4 K, p-AKT and p-mTOR↓ | Preclinical study | Induce apoptosis | Glioma | [244–246] |
| Genipin | p-PI5 K, p-AKT and p-mTOR↓ | Preclinical study | Induce autophagy | Oral squamous cell carcinoma | [244–246] |
| Isoglycyrrhizin | ATP and mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Hepatoma and gastric cancer | [246, 248, 249] |
| Tanshinone I | PI3 K/AKT/mTOR↓ | Preclinical study | Induce autophagy | Ovarian cancer | [250] |
| Brusato | PI3 K/AKT/mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Hepatocellular cancer | [251] |
| Gynostemma pentaphyllum (Thunb.) | PI3 K/AKT/mTOR↓ | Preclinical study | Induce apoptosis | Renal cancer | [252] |
| Sinomenine | PI3 K/AKT/mTOR↓ | Preclinical study | Induce autophagy | Renal cancer | [253] |
| Ricolinostat | miR-30 d/PI3 K/AKT/mTOR↓ | Phase 1 clinical trial | Induce apoptosis | Esophageal squamous cell carcinoma | clinicaltrials.gov, [254] |
| Novel quinazolinone MJ-33 | PI3 K/AKT/mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Colorectal cancer | [255] |
| LiCl | ROS and AKT/mTOR↑ | Preclinical study | Induce necroptosis | Schwannoma | [256] |
| Lorlatinib | IGF1R-mediated PI3 K/AKT/mTOR and GPX4↓ | Phase 2/3 clinical trial | Induce ferroptosis | Melanoma/Non-small cell lung cancer | [257], clinicaltrials.gov |
| miR-21-5p | GPX4 and AKT/mTOR↑ | Preclinical study | Inhibit ferroptosis | Hepatocellular carcinoma | [259] |
| Resveratrol | AMPK↑, mTOR↓ | Phase 1/2 clinical trial | Induce apoptosis and autophagy | Non-small cell lung cancer | [260] |
| β-elemene | ROS and p-AMPK↑, mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Colorectal cancer | [261] |
| Ellagic acid | AMPK↑, mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Colon cancer | [262] |
| Periplocin | AMPK/mTOR↑, p70S6 K↓ | Preclinical study | Induce apoptosis | Pancreatic cancer | [263] |
| Trifolirhizin | AMPK↑, mTOR↓ | Preclinical study | Induce apoptosis | Colon cancer | [264] |
| Cynaroside | MET/AKT/mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Gastric cancer | [266] |
| Physakengose G | EGFR/mTOR↓ | Preclinical study | Induce apoptosis | Osteosarcoma | [267] |
| GALNT14 | EGFR/mTOR↓ | Phase 2 clinical trial | Induce apoptosis and ferroptosis | Hepatocellular carcinoma | [268], clinicaltrials.gov |
| circ_0004585 | miR-1248/TM9SF/mTOR↓ | Preclinical study | Induce autophagy and inhibit anoikis | Metastatic prostate cancer | [270] |
| Homoharringtonine | miR-18a-3p/AKT/mTOR↓ | Phase 2 clinical trial | Induce apoptosis | Breast cancer/leukaemia | [272, 273] |
| U0126 | mTOR/p70S6 K↓ | Preclinical study | Induce anoikis | Breast cancer | [274] |
| Gemtuzumab ozogamicin | AMPK/mTOR/ULK-1↓ | Phase 1/2/3 clinical trial | Induce apoptosis and autophagy | leukaemia | clinicaltrials.gov |
| Polyphyllin I | ROS/AKT/mTOR and CIP2 A/AKT/mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Colon cancer | [276] |
| Compound K | AKT/mTOR/c-Myc↓ | Preclinical study | Induce apoptosis | Hepatocellular carcinoma | [277] |
| Chrysin | ERK/mTOR↓ | Preclinical study | Induce autophagy | Oral cancer | [278] |
| Chitooligosaccharide | p53/mTOR↓ | Preclinical study | Induce apoptosis and autophagy | Osteosarcoma | [279] |
| Artematrolide A | ROS/ERK/mTOR↑ | Preclinical study | Induce apoptosis | Cervical cancer | [280] |
| Falcarino | PI3 K/AKT/mTOR/p70S6 K↓ | Preclinical study | Induce apoptosis and autophagy | Oral squamous cell carcinoma | [281] |
| Novel conjugates of endoperoxide and 4-anilinoquinazoline | IGF1—R/AKT/mTOR↓ | Preclinical study | Induce apoptosis | Myeloma | [282] |
| Icariin | miR-7/mTOR/SREBP1↓ | Preclinical study | induce autophagy and ferroptosis | Prostate cancer | [283] |
| Curcumol | miR-7/mTOR/SREBP1↓ | Preclinical study | induce autophagy and ferroptosis | Prostate cancer | [283] |
| Osthole | AMPK/Akt/mTOR ↓ | Osthole | Induce ferroptosis | Colorectal cancer | [284] |
Limitations of targeting three signaling pathways
These three signaling pathways have different pathway activities as well as differences in activation modes in different types of tumors. For example, in lung cancer, aberrant aggregation of FRMD6 activates mTOR expression and enhances the interaction between mTOR and S6 K to promote lung cancer progression [285]. However, in breast cancer, aberrant activation of mTOR is mainly associated with an increased flux of HIF-1α and a lack of the upstream gene, PTEN [286]. This is probably one of the reasons why Rapamycin has efficacy in specific types of cancer only. It is worth noting that the functions played by caspase 3 in different types of tumors are also highly variable. caspase-3 activation promotes oncogene-induced malignant transformation and accelerates breast cancer progression by triggering translocation of mitochondrial endonuclease G (EndoG), which activates the Src-STAT3 signaling pathway [287]. In colorectal cancer, the Caspase-3 generates the short form of IL-18 by cleaving interleukin 18 (IL-18), alters the phosphorylation state of STAT1, and promotes the secretion of ISG15, thereby strengthening the anti-tumor function of natural killer cells [288]. In addition, the upstream and downstream regulatory transduction of these three signaling pathways as well as the associated signaling pathways have not been fully revealed.
Targeting these three signaling pathways can lead to drug resistance, which will limit the use of targeted drugs. The apoptotic pathway is characterized by complexity and redundancy. Thus, when drugs targeting the caspase signaling pathway activate caspase 3 activity to induce apoptosis, aberrant activation of endogenous anti-apoptotic proteins prevents this task from proceeding [289]. It is worth noting that apoptosis-induced cell proliferation (AiP) remains a phenomenon that cannot be ignored by targeting the caspase signaling pathway, and a large number of tumor cells undergo apoptosis may lead to further tumor deterioration and progression [290]. In addition, apoptosis with compensatory activation of the necrotic pathway is an important mechanism for the development of drug resistance to drugs targeting RIPK1/RIPK3/MLKL. Specifically, inhibition of the RIPK1/RIPK3/MLKL pathway initiates the onset of other programmed cell deaths, including apoptosis and autophagy [291]. Interestingly, the O-GlcNAc glycosylation modification of RIPK1 was able to lead to resistance to sunitinib by inhibiting the RIPK1-dependent apoptosis (RDA) pathway [292]. When targeting the mTOR signaling pathway with mTOR inhibitors, cancer cells aberrantly activate the PI3 K/AKT pathway and the MAPK pathway to bypass the action of mTOR inhibitors [293]. Studies have shown that some cancer cells may upregulate the expression of the transporter proteins ABCB1 and ABCG2, which expel the mTOR inhibitors outside the cell, reducing intracellular concentrations and leading to resistance [294]. Therefore, how to solve the resistance of targeted drugs is an important direction for small molecule targeted therapy of PCD. Finally, targeting these three signaling pathways faces the problem of a large gap between preclinical studies and clinical applications. In our previous discussion, most of the drugs targeting these three signaling pathways are still in the preclinical research stage and have not entered the clinical research stage. Further, although some small molecule drugs have achieved good results in in vitro experiments, they still face significant problems when entering clinical studies. In conclusion, although targeting these three signaling pathways associated with PCD for the treatment of tumors is promising, there are still some limitations that restrict its application and development.
Novel targeted therapies associated with PCD
Novel therapeutic approaches such as the CRISPR/Cas gene editing system, immune checkpoint inhibitors and gene therapy have shown great potential in cancer treatment. These approaches can not only directly target cancer cells, but also regulate immune cells and signaling pathways in the tumor microenvironment. CRISPR/Cas can induce apoptosis and inhibit tumor growth by precisely editing key genes in cancer cells [295]. For example, editing of apoptosis-suppressing genes or activation of key proteins in apoptosis pathway can effectively induce apoptosis in cancer cells. Moreover, CRISPR/Cas can be used to repair or activate tumor suppressor genes, such as p53, thereby restoring the normal regulatory mechanisms of cells [296].
Immune checkpoint inhibitors restore or enhance the immune response of T cells against cancer cells by blocking immune-suppressing signals [297]. Studies have shown that immune checkpoint inhibitors not only enhance the cytotoxicity of T cells, but also exert anti-tumor effects by inducing PCD in cancer cells [298]. Gene therapy achieves targeted treatment of tumors by introducing exogenous genes into cancer cells or immune cells [299]. For example, the introduction of apoptosis-related genes into cancer cells via viral vectors can directly induce apoptosis. Although the CRISPR/Cas system, immune checkpoint inhibitors, and gene therapies have shown great potential in cancer treatment, single therapeutic approaches often struggle to overcome the complexity and heterogeneity of tumors. Therefore, an integrated therapeutic strategy combining PCD signaling pathways may be an important direction for future cancer treatment.
Conclusion
Different types of PCD play essential roles in tumor progression. The components of PCD can interact and transform each other, and the combined study of components in PCD can carry out more in-depth research on tumors and treatment. Signaling pathways play a massive role in inducing PCD. When signaling pathways induce multiple PCDS to occur, the nature and outcome of PCD may be altered. This review focuses on the mechanism and targeted drugs of the caspase signaling pathway, RIPK1/RIPK3/MKLK signaling pathway and mTOR signaling pathway in PCD. In this review, 32 drugs mainly target the caspase signaling pathway, 34 drugs primarily target the RIPK1/RIPK3/MKLK signaling pathway and 49 drugs principally target the mTOR signaling pathway. In addition, since the regulation of components in PCD in tumor immunity is a complex and holistic role, the role of PCD may even be reversed in different stages of tumor development, and the specific targets will be different due to the complexity of tumor components and types, so the use of targeted drugs needs more in-depth research. Combination of drugs, development of new drugs and avoidance of chemoresistance may provide new strategies for the treatment of cancer. In addition, the immune microenvironment plays a vital role in maintaining cell homeostasis and signaling pathways, and inflammation also has a causal relationship with PCD such as pyroptosis. The interaction between the immune microenvironment and PCD may be a new direction for tumor treatment in the future.
Drug development targeting the PCD pathway is driving a paradigm shift in tumor therapy. By constructing a dynamic model of the apoptosis signaling network, the imbalance in the regulation of Bcl-2 family proteins in the tumor microenvironment has been precisely addressed. Based on the structural analysis of caspase complexes, researchers successfully designed small molecules with specific binding sites, breaking through the bottleneck of death receptor signaling regulation. In addition, for the off-target risk of apoptosis inducers, the novel early warning system can identify the molecular features of Smac mimics that accidentally activate the NF-κB pathway, thus improving the efficiency of drug safety assessment. The current challenge lies in the heterogeneity of tumor cell death leading to biased pathway modeling, which needs to be combined with single-cell sequencing technology to address the signal crosstalk between necrosis and apoptosis in different subpopulations. Therefore, with the breakthrough of computational simulation technology, it will be a promising research direction to promote more drugs targeting PCD into clinical studies.
Acknowledgements
The authors sincerely appreciated Pubmed, Web of Science, and Google Scholar.
Abbreviations
- 11-MT
11-Methoxytabersonine
- XB
2-Deprenyl-rheediaxanthone B
- ACD
Accidental cell
- AFPR
Active Fraction of Polyrhachis vicina Roger
- ACC
Adrenocortical cancer
- AGS
Advanced gastric cancer
- ATL
Alantolactone
- AMPK
AMP-activated protein kinase
- ATC
Anaplastic thyroid cancer
- AIF
Apoptosis-inducing factor
- Apaf-1
Apoptotic protease activating factor 1
- AR-A
Artematrolide A
- AMBRA1
Autophagy/Beclin 1 regulator 1
- ATGs
Autophagy-related proteins
- CDH17
Cadherin-17
- CREB
CAMP response element-binding protein
- CARD
Caspase recruitment domain
- cIAP1/2
Cell inhibitor of apoptosis protein-1/2
- CESC
Cervical cancer
- C10
Chalcone 3',5'-degraded
- CHE
Chelerythrine
- COS
Chitooligosaccharide
- CQ
Chloroquine
- COAD
Colon cancer
- CRC
Colorectal cancer
- CK
Compound K
- Cy
Cynaroside
- GSH
Cysteine
- DAMPs
Damage-associated molecular patterns
- DISC
Death-Inducing Signaling Complex
- DHA
Dihydroartemisinin
- DHTS
Dihydrotanshinone I
- DRM
Doramectin
- MPD1
EMC-KGDEVD-DOX
- ER
Endoplasmic reticulum
- EGF
Epidermal growth factor
- ERK
Extracellular signal-regulated kinase
- FADD
Fas-associated with death domain protein
- FPN
Ferroportin
- FAK
Focal adhesion kinase
- FOXO1
Forkhead Box Protein O1
- GSDMD
Gasdermin D
- GO
Gemtuzumab ozogamicin
- GJ
Geniposide
- GLUT
Glucose transporter
- GLUDL
Glutamate dehydrogenase
- GLUL
Glutamine synthetase
- GPX4
Glutathione peroxidase 4
- PYGL
Glycogen phosphorylase
- GSK-3
Glycogen synthase kinase 3
- IMs
GSH/ROS dual response nanogel system
- HNSCC
Head and neck squamous cell carcinoma
- HCC
Hepatocellular carcinoma
- HK2
Hexokinase 2
- HHT
Highharringtonine
- HDAC
Histone deacetylase
- IKK
IĸB kinase
- IRE/IRP
Iron regulatory protein/iron response element
- ISL
Isoglycyrrhizin
- MR
Mannose receptors
- mTNBC
Metastatic triple-negative breast cancer
- MOMP
Mitochondrial membrane permeability
- mtROS
Mitochondrial ROS
- MEK
Mitogen-activated protein/extracellular signal-regulated kinase
- MLKL
Mixed Lineage Kinase Like
- MEFs
Mouse embryonic fibroblasts
- mTORC1
MTOR complex 1
- mTORC2
MTOR complex 2
- MT
Mutant
- nano-PROTACs
Nano-proteolysis-targeting chimeras
- NEC-1
Necrostatin-1
- NA
Neoalbuminol
- NGFR
Nerve growth factor receptor
- NETs
Neutrophil extracellular traps
- OPD’
Ophiopogonin D’
- OV
Ovarian cancer
- Paeo
Paeoniflorigenone
- PCA
Pancreatic cancer
- PDAC
Pancreatic ductal adenocarcinoma
- PCAT6
PCA-associated transcript 6
- PEC
Pectin
- PDC
Peptide-doxorubicin conjugate:
- PI3 K C III
Phosphatidylinositol 3-kinase III
- PG
Physakengose G
- PARP1
Poly(ADP-ribose)polymerase
- PolyI: C
Polyinosinic: polycytidylic acid
- PPVI
Polyphyllin VI
- polyU
Poly-ubiquitinated
- PCD
Programmed cell death
- PCA
Proliferation of prostate cancer
- PpIX
Protoporphyrin IX
- NLRP3
Pyrin domain-containing protein 3
- RICTOR
Rapamycin-insensitive mTOR chaperone
- ROS
Reactive oxygen species
- RIPK3
Receptor-Interacting Protein Kinase 3
- RSV
Resveratrol
- TPD1
RGDEVD-DOX
- RHIM
RIP homotypic interaction motif
- RDA
RIPK1-dependent apoptosis
- SCU
Scutellarein
- aPRO
SHP2-targeting PROTAC peptide
- SIN
Sinomenine
- SHP2
Src homology2 (SH2) domain-containg protein tyrosine phosphatase (PTPase)
- STS
Staurosporine
- SCD-1
Stearoyl-CoA desaturase-1
- SERBP1
Sterol regulatory element-binding protein 1
- TBK1
TANK-binding kinase 1
- Tan–I
Tanshinone I
- TAK1
TGF-β-activated kinase 1
- TAB2
TGF-β-activated kinase 1 binding protein 2
- TAK
The growth factor β-activated kinase growth factor β-activated kinase
- TRAIL
TNFSF10
- TLR
Toll-like receptors
- ATF3
Transcription 3
- TfR1
Transferrin receptor 1
- TRIM11
Tripartite motif containing 11
- TNBC
Triple-negative breast cancer
- TME
Tumor microenvironment
- TNFR
Tumor necrosis factor receptor
- Ub
Ubiquitin
- ULK1
UNC-51-like kinase 1
- ZBP1
ZDNA binding protein 1
Author contributions
XGZ and KH conceived this study. CPS wrote the manuscript. YLS and JWG made the figures and tables. LH, JWG and YLS performed literature searching. All authors have read and approved the article.
Funding
The research project is supported by National Natural Science Foundation of China (Grant no. 82172989, 82273068 and 82260524); Key Research and Development projects in Jiangxi (Grant no. 20212BBG73021); Jiangxi Training Program for academic and technical leaders of major disciplines,Young talents program (Grant no. 20212BCJ23023); Key project of Science and Technology Innovation of Health Commission (Grant no. 2023ZD003); Jiangxi Provincial Natural Science Foundation(grant no. 20232BAB206101); Jiangxi Province Department of Education Science and technology research project, China (Grant no. GJJ210177); Project Fund of Jiangxi Provincial Health Commission (20204343, 202130873, 202210044).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xingen Zhu, Email: ndefy89006@ncu.edu.cn.
Kai Huang, Email: kaihuang@ncu.edu.cn.
References
- 1.Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, Chen Y, Han B. Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther. 2022;7(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nirmala JG, Lopus M. Cell death mechanisms in eukaryotes. Cell Biol Toxicol. 2020;36(2):145–64. [DOI] [PubMed] [Google Scholar]
- 3.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdel Wahab SI, Abdul AB, Alzubairi AS, Mohamed Elhassan M, Mohan S. In vitro ultramorphological assessment of apoptosis induced by zerumbone on (HeLa). J Biomed Biotechnol. 2009;2009:769568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28(7):2029–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9(4):539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strasser A, Vaux DL. Cell death in the origin and treatment of cancer. Mol Cell. 2020;78(6):1045–54. [DOI] [PubMed] [Google Scholar]
- 9.Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Populo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13(2):1886–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong RSY. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23(6):1625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulda S, Debatin K-M. Death receptor signaling in cancer therapy. Curr Med Chem Anticancer Agents. 2003;3(4):253–62. [DOI] [PubMed] [Google Scholar]
- 16.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15(6):725–31. [DOI] [PubMed] [Google Scholar]
- 17.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22(53):8543–67. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007. 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 19.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–19. [DOI] [PubMed] [Google Scholar]
- 20.Shiozaki EN, Shi Y. Caspases, IAPs and Smac/DIABLO: mechanisms from structural biology. Trends Biochem Sci. 2004;29(9):486–94. [DOI] [PubMed] [Google Scholar]
- 21.Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6(11):1067–74. [DOI] [PubMed] [Google Scholar]
- 22.Kuribayashi K, Mayes PA, El-Deiry WS. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol Ther. 2006;5(7):763–5. [DOI] [PubMed] [Google Scholar]
- 23.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9(4):367–93. [DOI] [PubMed] [Google Scholar]
- 24.Deets KA, Vance RE. Inflammasomes and adaptive immune responses. Nat Immunol. 2021;22(4):412–22. [DOI] [PubMed] [Google Scholar]
- 25.Giampazolias E, Zunino B, Dhayade S, Bock F, Cloix C, Cao K, Roca A, Lopez J, Ichim G, Proïcs E, et al. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat Cell Biol. 2017;19(9):1116–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rongvaux A, Jackson R, Harman CCD, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan C-Y, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159(7):1563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, van Delft MF, Bedoui S, Lessene G, Ritchie ME, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159(7):1549–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 29.Green DR. The coming decade of cell death research: five riddles. Cell. 2019;177(5):1094–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, Pasparakis M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540(7631):124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin X, Ma D, Tan Y-X, Wang H-Y, Cai Z. The role of necroptosis in cancer: a double-edged sword? Biochim Biophys Acta Rev Cancer. 2019;1871(2):259–66. [DOI] [PubMed] [Google Scholar]
- 32.He L, Peng K, Liu Y, Xiong J, Zhu F-F. Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients. Onco Targets Ther. 2013;6:1539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidt SV, Seibert S, Walch-Rückheim B, Vicinus B, Kamionka E-M, Pahne-Zeppenfeld J, Solomayer E-F, Kim Y-J, Bohle RM, Smola S. RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1α release, and efficient paracrine dendritic cell activation. Oncotarget. 2015;6(11):8635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, da Barreira Silva R, Reise Sousa C, Green DR, Oberst A, Albert ML. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8⁺ T cells. Science. 2015;350(6258):328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, Delrue I, Taminau J, Wiernicki B, De Groote P, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15(2):274–87. [DOI] [PubMed] [Google Scholar]
- 36.Jiao D, Cai Z, Choksi S, Ma D, Choe M, Kwon H-J, Baik JY, Rowan BG, Liu C, Liu Z-G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018;28(8):868–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li D, Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct Target Ther. 2020;5(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang D, Kroemer G. Ferroptosis. Curr Biol. 2020;30(21):R1292–7. [DOI] [PubMed] [Google Scholar]
- 40.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shu X, Wu J, Zhang T, Ma X, Du Z, Xu J, You J, Wang L, Chen N, Luo M, et al. Statin-induced geranylgeranyl pyrophosphate depletion promotes ferroptosis-related senescence in adipose tissue. Nutrients. 2022. 10.3390/nu14204365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18(5):280–96. [DOI] [PubMed] [Google Scholar]
- 43.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212(4):555–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganley IG, Lam DH, Wang J, Ding X, Chen S, Jiang X. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. J Biol Chem. 2009;284(18):12297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, Pagliarini V, Matteoni S, Fuoco C, Giunta L, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191(1):155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24(1):24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21(8):439–58. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Wan W. Acetylation in the regulation of autophagy. Autophagy. 2023;19(2):379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22(11):733–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell RC, Tian Y, Yuan H, Park HW, Chang Y-Y, Kim J, Kim H, Neufeld TP, Dillin A, Guan K-L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15(7):741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Kundu M, Viollet B, Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Towers CG, Wodetzki D, Thorburn A. Autophagy and cancer: modulation of cell death pathways and cancer cell adaptations. J Cell Biol. 2020. 10.1083/jcb.201909033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14(2):177–85. [DOI] [PubMed] [Google Scholar]
- 54.DeVorkin L, Pavey N, Carleton G, Comber A, Ho C, Lim J, McNamara E, Huang H, Kim P, Zacharias LG, et al. Autophagy regulation of metabolism is required for CD8+ T cell anti-tumor immunity. Cell Rep. 2019. 10.1016/j.celrep.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 55.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277(1):61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, Hu J. The role of pyroptosis in cancer: pro-cancer or pro-"host"? Cell Death Dis. 2019;10(9):650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ge X, Li W, Huang S, Yin Z, Xu X, Chen F, Kong X, Wang H, Zhang J, Lei P. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Res. 2018;1697:10–20. [DOI] [PubMed] [Google Scholar]
- 59.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–5. [DOI] [PubMed] [Google Scholar]
- 60.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–54. [DOI] [PubMed] [Google Scholar]
- 61.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25(12):1285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei X, Xie F, Zhou X, Wu Y, Yan H, Liu T, Huang J, Wang F, Zhou F, Zhang L. Role of pyroptosis in inflammation and cancer. Cell Mol Immunol. 2022;19(9):971–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan Y, Chen Q, Li X, Zeng Z, Xiong W, Li G, Li X, Yang J, Xiang B, Yi M. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Y, Liu L, Tao S, Yao Y, Wang Y, Wei Q, Shao A, Deng Y. Parthanatos and its associated components: Promising therapeutic targets for cancer. Pharmacol Res. 2021;163:105299. [DOI] [PubMed] [Google Scholar]
- 65.Andrabi SA, Dawson TM, Dawson VL. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann N Y Acad Sci. 2008;1147:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao N, Mao Y, Han G, Ju Q, Zhou L, Liu F, Xu Y, Zhao X. YM155, a survivin suppressant, triggers PARP-dependent cell death (parthanatos) and inhibits esophageal squamous-cell carcinoma xenografts in mice. Oncotarget. 2015;6(21):18445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fatokun AA, Dawson VL, Dawson TM. Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol. 2014;171(8):2000–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176(2):231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–25. [DOI] [PubMed] [Google Scholar]
- 70.Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- 72.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123(8):3446–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saffarzadeh M. Neutrophil extracellular traps as a drug target to counteract chronic and acute inflammation. Curr Pharm Biotechnol. 2018;19(15):1196–202. [DOI] [PubMed] [Google Scholar]
- 74.Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109(32):13076–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grayson PC, Kaplan MJ. At the bench: neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukoc Biol. 2016;99(2):253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sangaletti S, Tripodo C, Vitali C, Portararo P, Guarnotta C, Casalini P, Cappetti B, Miotti S, Pinciroli P, Fuligni F, et al. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer Discov. 2014;4(1):110–29. [DOI] [PubMed] [Google Scholar]
- 77.Demers M, Wagner DD. Neutrophil extracellular traps: a new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology. 2013;2(2):e22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Kuang F, Kang R, Tang D. Alkaliptosis: a new weapon for cancer therapy. Cancer Gene Ther. 2020;27(5):267–9. [DOI] [PubMed] [Google Scholar]
- 79.Holze C, Michaudel C, Mackowiak C, Haas DA, Benda C, Hubel P, Pennemann FL, Schnepf D, Wettmarshausen J, Braun M, et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat Immunol. 2018;19(2):130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pallichankandy S, Thayyullathil F, Cheratta AR, Subburayan K, Alakkal A, Sultana M, Drou N, Arshad M, Tariq S, Galadari S. Targeting oxeiptosis-mediated tumor suppression: a novel approach to treat colorectal cancers by sanguinarine. Cell Death Discov. 2023;9(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taddei ML, Giannoni E, Fiaschi T, Chiarugi P. Anoikis: an emerging hallmark in health and diseases. J Pathol. 2012;226(2):380–93. [DOI] [PubMed] [Google Scholar]
- 82.Zheng P, Zhou C, Ding Y, Duan S. Disulfidptosis: a new target for metabolic cancer therapy. J Exp Clin Cancer Res. 2023;42(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pandian N, Kanneganti T-D. PANoptosis: a unique innate immune inflammatory cell death modality. J Immunol. 2022;209(9):1625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kesavardhana S, Kanneganti TD. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol. 2017;29(5):201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramirez MLG, Salvesen GS. A primer on caspase mechanisms. Semin Cell Dev Biol. 2018;82:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue-Yamauchi A, Jeng PS, Kim K, Chen HC, Han S, Ganesan YT, Ishizawa K, Jebiwott S, Dong Y, Pietanza MC, et al. Targeting the differential addiction to anti-apoptotic BCL-2 family for cancer therapy. Nat Commun. 2017;8:16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Derakhshan A, Chen Z, Van Waes C. Therapeutic small molecules target inhibitor of apoptosis proteins in cancers with deregulation of extrinsic and intrinsic cell death pathways. Clin Cancer Res. 2017;23(6):1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1(5):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133(4):693–703. [DOI] [PubMed] [Google Scholar]
- 91.Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, Komuves L, Webster JD, Dixit VM. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574(7778):428–31. [DOI] [PubMed] [Google Scholar]
- 92.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–32. [DOI] [PubMed] [Google Scholar]
- 93.Rathinam VAK, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. [DOI] [PubMed] [Google Scholar]
- 95.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. [DOI] [PubMed] [Google Scholar]
- 97.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Norman JM, Cohen GM, Bampton ET. The in vitro cleavage of the hAtg proteins by cell death proteases. Autophagy. 2010;6(8):1042–56. [DOI] [PubMed] [Google Scholar]
- 99.Tiwari M, Sharma LK, Vanegas D, Callaway DA, Bai Y, Lechleiter JD, Herman B. A nonapoptotic role for CASP2/caspase 2: modulation of autophagy. Autophagy. 2014;10(6):1054–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiwari M, Lopez-Cruzan M, Morgan WW, Herman B. Loss of caspase-2-dependent apoptosis induces autophagy after mitochondrial oxidative stress in primary cultures of young adult cortical neurons. J Biol Chem. 2011;286(10):8493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171(4):603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Luo W, Wang Y. PARP-1 and its associated nucleases in DNA damage response. DNA Repair. 2019;81:102651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang L, Fu L, Zhang S, Zhang J, Zhao Y, Zheng Y, He G, Yang S, Ouyang L, Liu B. Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chem Sci. 2017;8(4):2687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Desai P, Lonial S, Cashen A, Kamdar M, Flinn I, O’Brien S, Garcia JS, Korde N, Moslehi J, Wey M, et al. A phase 1 first-in-human study of the MCL-1 inhibitor AZD5991 in patients with relapsed/refractory hematologic malignancies. Clin Cancer Res. 2024;30(21):4844–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y, Yang J, Wen Z, Chen X, Yu J, Yuan D, Xu B, Luo H, Zhu J. A novel 3’,5’-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCdelta/JNK signal in prostate cancer. Aging. 2020;12(10):9103–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim HR, Cho YS, Chung SW, Choi JU, Ko YG, Park SJ, Kim SY, Byun Y. Caspase-3 mediated switch therapy of self-triggered and long-acting prodrugs for metastatic TNBC. J Control Release. 2022;346:136–47. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y-Y, Li M-Z, Shen H-H, Abudukeyoumu A, Xie F, Ye J-F, Xu F-Y, Sun J-S, Li M-Q. Ginsenosides in endometrium-related diseases: emerging roles and mechanisms. Biomed Pharmacother. 2023;166:115340. [DOI] [PubMed] [Google Scholar]
- 108.Zhou B, Yan Z, Liu R, Shi P, Qian S, Qu X, Zhu L, Zhang W, Wang J. Prospective study of transcatheter arterial chemoembolization (TACE) with Ginsenoside Rg3 versus TACE alone for the treatment of patients with advanced hepatocellular carcinoma. Radiology. 2016;280(2):630–9. [DOI] [PubMed] [Google Scholar]
- 109.Li Y, Wang W, Li A, Huang W, Chen S, Han F, Wang L. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chem Biol Interact. 2021;340:109434. [DOI] [PubMed] [Google Scholar]
- 110.Deeken JF, Wang H, Hartley M, Cheema AK, Smaglo B, Hwang JJ, He AR, Weiner LM, Marshall JL, Giaccone G, et al. A phase I study of intravenous artesunate in patients with advanced solid tumor malignancies. Cancer Chemother Pharmacol. 2018;81(3):587–96. [DOI] [PubMed] [Google Scholar]
- 111.Mielgo A, Torres VA, Clair K, Barbero S, Stupack DG. Paclitaxel promotes a caspase 8-mediated apoptosis through death effector domain association with microtubules. Oncogene. 2009;28(40):3551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu KH, Lue KH, Chou MC, Chung JG. Paclitaxel induces apoptosis via caspase-3 activation in human osteogenic sarcoma cells (U-2 OS). J Orthop Res. 2005;23(5):988–94. [DOI] [PubMed] [Google Scholar]
- 113.Zhang C-C, Li C-G, Wang Y-F, Xu L-H, He X-H, Zeng Q-Z, Zeng C-Y, Mai F-Y, Hu B, Ouyang D-Y. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24(3–4):312–25. [DOI] [PubMed] [Google Scholar]
- 114.Shen G, Liu Z, Wang M, Zhao Y, Liu X, Hou Y, Ma W, Han J, Zhou X, Ren D, et al. Neoadjuvant apatinib addition to sintilimab and carboplatin-taxane based chemotherapy in patients with early triple-negative breast cancer: the phase 2 NeoSAC trial. Signal Transduct Target Ther. 2025;10(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu Y, Wen Q, Cai Y, Liu Y, Ma W, Li Q, Song F, Guo Y, Zhu L, Ge J, et al. Alantolactone induces concurrent apoptosis and GSDME-dependent pyroptosis of anaplastic thyroid cancer through ROS mitochondria-dependent caspase pathway. Phytomedicine. 2023;108:154528. [DOI] [PubMed] [Google Scholar]
- 116.García-Martínez JM, Wang S, Weishaeupl C, Wernitznig A, Chetta P, Pinto C, Ho J, Dutcher D, Gorman PN, Kroe-Barrett R, et al. Selective tumor cell apoptosis and tumor regression in CDH17-positive colorectal cancer models using BI 905711, a novel liver-sparing TRAILR2 agonist. Mol Cancer Ther. 2021;20(1):96–108. [DOI] [PubMed] [Google Scholar]
- 117.Rizzi F, Naponelli V, Silva A, Modernelli A, Ramazzina I, Bonacini M, Tardito S, Gatti R, Uggeri J, Bettuzzi S. Polyphenon E(R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis. 2014;35(4):828–39. [DOI] [PubMed] [Google Scholar]
- 118.Sinicrope FA, Viggiano TR, Buttar NS, Song L, Schroeder KW, Kraichely RE, Larson MV, Sedlack RE, Kisiel JB, Gostout CJ, et al. Randomized phase II trial of polyphenon E versus placebo in patients at high risk of recurrent colonic neoplasia. Cancer Prev Res. 2021;14(5):573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang C, Xu M, He S, Huang J, Xu C, Pu K. Checkpoint nano-PROTACs for activatable cancer photo-immunotherapy. Adv Mater. 2023;35(6):e2208553. [DOI] [PubMed] [Google Scholar]
- 120.Wang L, Hu T, Shen J, Zhang L, Chan RL, Lu L, Li M, Cho CH, Wu WK. Dihydrotanshinone I induced apoptosis and autophagy through caspase dependent pathway in colon cancer. Phytomedicine. 2015;22(12):1079–87. [DOI] [PubMed] [Google Scholar]
- 121.Konstantinopoulos PA, Lee EK, Xiong N, Krasner C, Campos S, Kolin DL, Liu JF, Horowitz N, Wright AA, Bouberhan S, et al. A phase II, two-stage study of letrozole and abemaciclib in estrogen receptor-positive recurrent endometrial cancer. J Clin Oncol. 2023;41(3):599–608. [DOI] [PubMed] [Google Scholar]
- 122.McGlorthan L, Paucarmayta A, Casablanca Y, Maxwell GL, Syed V. Progesterone induces apoptosis by activation of caspase-8 and calcitriol via activation of caspase-9 pathways in ovarian and endometrial cancer cells in vitro. Apoptosis. 2021;26(3–4):184–94. [DOI] [PubMed] [Google Scholar]
- 123.Phillips DC, Buchanan FG, Cheng D, Solomon LR, Xiao Y, Xue J, Tahir SK, Smith ML, Zhang H, Widomski D, et al. Hexavalent TRAIL fusion protein eftozanermin alfa optimally clusters apoptosis-inducing TRAIL receptors to induce on-target antitumor activity in solid tumors. Cancer Res. 2021;81(12):3402–14. [DOI] [PubMed] [Google Scholar]
- 124.Sang Eun H, Seong Min K, Ho Jeong L, Vetrivel P, Venkatarame V, Jeong Doo H, Eun Hee K, Sang Joon L, Gon Sup K. Scutellarein induces fas-mediated extrinsic apoptosis and G2/M cell cycle arrest in Hep3B hepatocellular carcinoma cells. Nutrients. 2019. 10.3390/nu11020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsu FT, Chiang IT, Wang WS. Induction of apoptosis through extrinsic/intrinsic pathways and suppression of ERK/NF-kappaB signalling participate in anti-glioblastoma of imipramine. J Cell Mol Med. 2020;24(7):3982–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chiu CF, Lin YQ, Park JM, Chen YC, Hung SW, Chiu CC, Chang CF. The novel camptothecin derivative, CPT211, induces cell cycle arrest and apoptosis in models of human breast cancer. Biomed Pharmacother. 2020;128:110309. [DOI] [PubMed] [Google Scholar]
- 127.Kang FC, Wang SC, So EC, Chang MM, Wong KL, Cheng KS, Chen YC, Huang BM. Propofol may increase caspase and MAPK pathways, and suppress the Akt pathway to induce apoptosis in MA-10 mouse leydig tumor cells. Oncol Rep. 2019;41(6):3565–74. [DOI] [PubMed] [Google Scholar]
- 128.Cao SJ, Zhang Y, Zhang YX, Zhao W, Pan LH, Sun XD, Jia Z, Ouyang W, Ye QS, Zhang FX, et al. Long-term survival in older patients given propofol or sevoflurane anaesthesia for major cancer surgery: follow-up of a multicentre randomised trial. Br J Anaesth. 2023;131(2):266–75. [DOI] [PubMed] [Google Scholar]
- 129.Li J, Yang C, Li Y, Chen A, Li L, You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. 2018. Biosci Rep. 10.1042/BSR20171150. [DOI] [PMC free article] [PubMed]
- 130.Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R, Qiu WQ, Pan R, Law BY, Wong VK, Yu CL, et al. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-kappaB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers. 2020. 10.3390/cancers12010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang F, Liu W, Ning J, Wang J, Lang Y, Jin X, Zhu K, Wang X, Li X, Yang F, et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int J Biol Sci. 2018;14(4):406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee Y, Lee KH, Lee GK, Lee SH, Lim KY, Joo J, Go YJ, Lee JS, Han JY. Randomized phase II study of afatinib plus simvastatin versus afatinib alone in previously treated patients with advanced nonadenocarcinomatous non-small cell lung cancer. Cancer Res Treat. 2017;49(4):1001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hååg P, Olsson M, Forsberg J, Lindberg ML, Stenerlöw B, Zong D, Kanter L, Lewensohn R, Viktorsson K, Zhivotovsky B, et al. Caspase-2 is a mediator of apoptotic signaling in response to gemtuzumab ozogamicin in acute myeloid leukemia. Cell Death Discov. 2022;8(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eichler M, Distler U, Nasrullah U, Krishnan A, Kaulich M, Husnjak K, Eberhardt W, Rajalingam K, Tenzer S, Pfeilschifter J, et al. The caspase-2 substrate p54nrb exhibits a multifaceted role in tumor cell death susceptibility via gene regulatory functions. Cell Death Dis. 2022;13(4):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amin P, Florez M, Najafov A, Pan H, Geng J, Ofengeim D, Dziedzic SA, Wang H, Barrett VJ, Ito Y, et al. Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFalpha-mediated apoptosis. Proc Natl Acad Sci U S A. 2018;115(26):E5944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14(11):727–36. [DOI] [PubMed] [Google Scholar]
- 137.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60(1):63–76. [DOI] [PubMed] [Google Scholar]
- 138.Lafont E, Draber P, Rieser E, Reichert M, Kupka S, de Miguel D, Draberova H, von Mässenhausen A, Bhamra A, Henderson S, et al. TBK1 and IKKε prevent TNF-induced cell death by RIPK1 phosphorylation. Nat Cell Biol. 2018;20(12):1389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Höckendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger F, Heuser M, et al. RIPK3 restricts myeloid leukemogenesis by promoting cell death and differentiation of leukemia initiating cells. Cancer Cell. 2016;30(1):75–91. [DOI] [PubMed] [Google Scholar]
- 140.Bianchi S, Scaranari V, Tomatis R. Synthesis and biological activity of a cyclic hexapeptide related to peptide T. Farmaco. 1989;44(12):1233–7. [PubMed] [Google Scholar]
- 141.Geng J, Ito Y, Shi L, Amin P, Chu J, Ouchida AT, Mookhtiar AK, Zhao H, Xu D, Shan B, et al. Regulation of RIPK1 activation by TAK1-mediated phosphorylation dictates apoptosis and necroptosis. Nat Commun. 2017;8(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menon MB, Gropengiesser J, Fischer J, Novikova L, Deuretzbacher A, Lafera J, Schimmeck H, Czymmeck N, Ronkina N, Kotlyarov A, et al. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat Cell Biol. 2017;19(10):1248–59. [DOI] [PubMed] [Google Scholar]
- 143.Dondelinger Y, Jouan-Lanhouet S, Divert T, Theatre E, Bertin J, Gough PJ, Giansanti P, Heck AJ, Dejardin E, Vandenabeele P, et al. NF-kappaB-independent role of IKKalpha/IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol Cell. 2015;60(1):63–76. [DOI] [PubMed] [Google Scholar]
- 144.Mihaly SR, Ninomiya-Tsuji J, Morioka S. TAK1 control of cell death. Cell Death Differ. 2014;21(11):1667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morioka S, Broglie P, Omori E, Ikeda Y, Takaesu G, Matsumoto K, Ninomiya-Tsuji J. TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J Cell Biol. 2014;204(4):607–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xu D, Jin T, Zhu H, Chen H, Ofengeim D, Zou C, Mifflin L, Pan L, Amin P, Li W, et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell. 2018;174(6):1477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sessler T, Healy S, Samali A, Szegezdi E. Structural determinants of DISC function: new insights into death receptor-mediated apoptosis signalling. Pharmacol Ther. 2013;140(2):186–99. [DOI] [PubMed] [Google Scholar]
- 148.Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, Pan H, Bai R, Zhang J, Wang Y, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. 2020;577(7788):109–14. [DOI] [PubMed] [Google Scholar]
- 149.Tonnus W, Meyer C, Steinebach C, Belavgeni A, von Massenhausen A, Gonzalez NZ, Maremonti F, Gembardt F, Himmerkus N, Latk M, et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat Commun. 2021;12(1):4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bell BD, Walsh CM. Coordinate regulation of autophagy and apoptosis in T cells by death effectors: FADD or foundation. Autophagy. 2009;5(2):238–40. [DOI] [PubMed] [Google Scholar]
- 151.Wu W, Wang X, Berleth N, Deitersen J, Wallot-Hieke N, Bohler P, Schlutermann D, Stuhldreier F, Cox J, Schmitz K, et al. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31(3):107547. [DOI] [PubMed] [Google Scholar]
- 152.Wu W, Wang X, Sun Y, Berleth N, Deitersen J, Schlutermann D, Stuhldreier F, Wallot-Hieke N, Jose Mendiburo M, Cox J, et al. TNF-induced necroptosis initiates early autophagy events via RIPK3-dependent AMPK activation, but inhibits late autophagy. Autophagy. 2021;17(12):3992–4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xie Y, Zhao Y, Shi L, Li W, Chen K, Li M, Chen X, Zhang H, Li T, Matsuzawa-Ishimoto Y, et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J Clin Invest. 2020;130(4):2111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luan Q, Jin L, Jiang CC, Tay KH, Lai F, Liu XY, Liu YL, Guo ST, Li CY, Yan XG, et al. RIPK1 regulates survival of human melanoma cells upon endoplasmic reticulum stress through autophagy. Autophagy. 2015;11(7):975–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, Chen X, Liang Y, Wu J, Zhao S, et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 2018;20(2):186–97. [DOI] [PubMed] [Google Scholar]
- 156.Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity. 2017;47(1):51–65. [DOI] [PubMed] [Google Scholar]
- 157.Frank D, Vaux DL, Murphy JM, Vince JE, Lindqvist LM. Activated MLKL attenuates autophagy following its translocation to intracellular membranes. J Cell Sci. 2019. 10.1242/jcs.220996. [DOI] [PubMed] [Google Scholar]
- 158.Guo FX, Wu Q, Li P, Zheng L, Ye S, Dai XY, Kang CM, Lu JB, Xu BM, Xu YJ, et al. The role of the LncRNA-FA2H-2-MLKL pathway in atherosclerosis by regulation of autophagy flux and inflammation through mTOR-dependent signaling. Cell Death Differ. 2019;26(9):1670–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McCormick KD, Ghosh A, Trivedi S, Wang L, Coyne CB, Ferris RL, Sarkar SN. Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma. Carcinogenesis. 2016;37(5):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hawk MA, Gorsuch CL, Fagan P, Lee C, Kim SE, Hamann JC, Mason JA, Weigel KJ, Tsegaye MA, Shen L, et al. RIPK1-mediated induction of mitophagy compromises the viability of extracellular-matrix-detached cells. Nat Cell Biol. 2018;20(3):272–84. [DOI] [PubMed] [Google Scholar]
- 161.Karki R, Sundaram B, Sharma BR, Lee S, Malireddi RKS, Nguyen LN, Christgen S, Zheng M, Wang Y, Samir P, et al. ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep. 2021;37(3):109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lu Z, Wu C, Zhu M, Song W, Wang H, Wang J, Guo J, Li N, Liu J, Li Y, et al. Ophiopogonin D’ induces RIPK1-dependent necroptosis in androgen-dependent LNCaP prostate cancer cells. Int J Oncol. 2020;56(2):439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rechek H, Haouat A, Hamaidia K, Pinto D, Boudiar T, Valega M, Pereira DM, Pereira RB, Silva AMS. Inula viscosa (L.) aiton ethanolic extract inhibits the growth of human AGS and A549 cancer cell lines. Chem Biodivers. 2023;20(3):e202200890. [DOI] [PubMed] [Google Scholar]
- 164.Jilishitz I, Quinones JL, Patel P, Chen G, Pasetsky J, VanInwegen A, Schoninger S, Jogalekar MP, Tsiperson V, Yan L, et al. NP-ALT, a liposomal: peptide drug, blocks p27Kip1 phosphorylation to induce oxidative stress, necroptosis, and regression in therapy-resistant breast cancer cells. Mol Cancer Res. 2021;19(11):1929–45. [DOI] [PubMed] [Google Scholar]
- 165.Nehs MA, Lin C-I, Kozono DE, Whang EE, Cho NL, Zhu K, Moalem J, Moore FD, Ruan DT. Necroptosis is a novel mechanism of radiation-induced cell death in anaplastic thyroid and adrenocortical cancers. Surgery. 2011;150(6):1032–9. [DOI] [PubMed] [Google Scholar]
- 166.Chen L, Fan H, Chu H, Du F, Chen Y, Hu L, Li Z, Wang W, Hou X, Yang L. The HSP90 inhibitor 17-DMAG alleviates primary biliary cholangitis via cholangiocyte necroptosis prevention. J Cell Biochem. 2022;123(11):1857–72. [DOI] [PubMed] [Google Scholar]
- 167.Hertlein E, Wagner AJ, Jones J, Lin TS, Maddocks KJ, Towns WH, Goettl VM, Zhang X, Jarjoura D, Raymond CA, et al. 17-DMAG targets the nuclear factor-kappaB family of proteins to induce apoptosis in chronic lymphocytic leukemia: clinical implications of HSP90 inhibition. Blood. 2010;116(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jhaveri K, Miller K, Rosen L, Schneider B, Chap L, Hannah A, Zhong Z, Ma W, Hudis C, Modi S. A phase I dose-escalation trial of trastuzumab and alvespimycin hydrochloride (KOS-1022; 17 DMAG) in the treatment of advanced solid tumors. Clin Cancer Res. 2012;18(18):5090–8. [DOI] [PubMed] [Google Scholar]
- 169.Chen Z, Xu G, Wu D, Wu S, Gong L, Li Z, Luo G, Hu J, Chen J, Huang X, et al. Lobaplatin induces pyroptosis through regulating cIAP1/2, ripoptosome and ROS in nasopharyngeal carcinoma. Biochem Pharmacol. 2020;177:114023. [DOI] [PubMed] [Google Scholar]
- 170.Amaravadi RK, Schilder RJ, Martin LP, Levin M, Graham MA, Weng DE, Adjei AA. A phase I study of the SMAC-mimetic birinapant in adults with refractory solid tumors or lymphoma. Mol Cancer Ther. 2015;14(11):2569–75. [DOI] [PubMed] [Google Scholar]
- 171.Pan HC, Lai DW, Lan KH, Shen CC, Wu SM, Chiu CS, Wang KB, Sheu ML. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis. 2013;34(11):2568–79. [DOI] [PubMed] [Google Scholar]
- 172.Vougioukalaki M, Georgila K, Athanasiadis EI, Eliopoulos AG. Cell adhesion tunes inflammatory TPL2 kinase signal transduction. Cell Mol Life Sci: CMLS. 2022;79(3):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–49. [DOI] [PubMed] [Google Scholar]
- 174.Frame MC, Patel H, Serrels B, Lietha D, Eck MJ. The FERM domain: organizing the structure and function of FAK. Nat Rev Mol Cell Biol. 2010;11(11):802–14. [DOI] [PubMed] [Google Scholar]
- 175.Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y, Jing L, Sun X. Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis. J Transl Med. 2018;16(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee YJ, Park KS, Lee SH. Curcumin targets both apoptosis and necroptosis in acidity-tolerant prostate carcinoma cells. Biomed Res Int. 2021;2021:8859181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhong Y, Tu Y, Ma Q, Chen L, Zhang W, Lu X, Yang S, Wang Z, Zhang L. Curcumin alleviates experimental colitis in mice by suppressing necroptosis of intestinal epithelial cells. Front Pharmacol. 2023;14:1170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cruz-Correa M, Hylind LM, Marrero JH, Zahurak ML, Murray-Stewart T, Casero RA Jr, Montgomery EA, Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, et al. Efficacy and safety of curcumin in treatment of intestinal adenomas in patients with familial adenomatous polyposis. Gastroenterology. 2018;155(3):668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moriwaki K, Chan FK. Regulation of RIPK3- and RHIM-dependent necroptosis by the proteasome. J Biol Chem. 2016;291(11):5948–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schmidt SV, Seibert S, Walch-Ruckheim B, Vicinus B, Kamionka EM, Pahne-Zeppenfeld J, Solomayer EF, Kim YJ, Bohle RM, Smola S. RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1alpha release, and efficient paracrine dendritic cell activation. Oncotarget. 2015;6(11):8635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Long GV, Hauschild A, Santinami M, Kirkwood JM, Atkinson V, Mandala M, Merelli B, Sileni VC, Nyakas M, Haydon A, et al. Final results for adjuvant dabrafenib plus trametinib in stage III melanoma. N Engl J Med. 2024;391(18):1709–20. [DOI] [PubMed] [Google Scholar]
- 182.Awada G, Dirven I, Schwarze JK, Tijtgat J, Fasolino G, Kockx M, Neyns B. Phase II clinical trial of trametinib and low-dose dabrafenib in advanced, previously treated BRAF(V600)/NRAS(Q61) wild-type melanoma (TraMel-WT). JCO Precis Oncol. 2024;8:e2300493. [DOI] [PubMed] [Google Scholar]
- 183.Watanabe S, Inoue M, Suzuki T, Kondo Y, Murayama M. Polyphyllin D induces necroptosis in neuroblastoma cells (IMR-32 and LA-N-2) in mice. Pediatr Surg Int. 2023;39(1):196. [DOI] [PubMed] [Google Scholar]
- 184.Hou X, Yang C, Zhang L, Hu T, Sun D, Cao H, Yang F, Guo G, Gong C, Zhang X, et al. Killing colon cancer cells through PCD pathways by a novel hyaluronic acid-modified shell-core nanoparticle loaded with RIP3 in combination with chloroquine. Biomaterials. 2017;124:195–210. [DOI] [PubMed] [Google Scholar]
- 185.Lee SM, Hewish M, Ahmed S, Papadatos-Pastos D, Karapanagiotou E, Blackhall F, Ford A, Young R, Garcia A, Arora A, et al. Hydroxychloroquine in combination with platinum doublet chemotherapy as first-line treatment for extensive-stage small cell lung cancer (study 15): a randomised phase II multicentre trial. Eur J Cancer. 2025;215:115162. [DOI] [PubMed] [Google Scholar]
- 186.Hatzimichael E, Georgiou G, Benetatos L, Briasoulis E. Gene mutations and molecularly targeted therapies in acute myeloid leukemia. Am J Blood Res. 2013;3(1):29–51. [PMC free article] [PubMed] [Google Scholar]
- 187.Ōmura S, Asami Y, Crump A. Staurosporine: new lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J Antibiot. 2018;71(8):688–701. [DOI] [PubMed] [Google Scholar]
- 188.Zhao Q, Zheng Y, Lv X, Gong J, Yang L. IMB5036 inhibits human pancreatic cancer growth primarily through activating necroptosis. Basic Clin Pharmacol Toxicol. 2022;130(3):375–84. [DOI] [PubMed] [Google Scholar]
- 189.Jacobsen AV, Lowes KN, Tanzer MC, Lucet IS, Hildebrand JM, Petrie EJ, van Delft MF, Liu Z, Conos SA, Zhang JG, et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7(1):e2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marunouchi T, Nishiumi C, Iinuma S, Yano E, Tanonaka K. Effects of Hsp90 inhibitor on the RIP1-RIP3-MLKL pathway during the development of heart failure in mice. Eur J Pharmacol. 2021;898:173987. [DOI] [PubMed] [Google Scholar]
- 191.Saber S, Hasan AM, Mohammed OA, Saleh LA, Hashish AA, Alamri MMS, Al-Ameer AY, Alfaifi J, Senbel A, Aboregela AM, et al. Ganetespib (STA-9090) augments sorafenib efficacy via necroptosis induction in hepatocellular carcinoma: implications from preclinical data for a novel therapeutic approach. Biomed Pharmacother. 2023;164:114918. [DOI] [PubMed] [Google Scholar]
- 192.Pillai RN, Fennell DA, Kovcin V, Ciuleanu TE, Ramlau R, Kowalski D, Schenker M, Yalcin I, Teofilovici F, Vukovic VM, et al. Randomized phase III study of ganetespib, a heat shock protein 90 inhibitor, with docetaxel versus docetaxel in advanced non-small-cell lung cancer (GALAXY-2). J Clin Oncol. 2020;38(6):613–22. [DOI] [PubMed] [Google Scholar]
- 193.Scaltriti M, Serra V, Normant E, Guzman M, Rodriguez O, Lim AR, Slocum KL, West KA, Rodriguez V, Prudkin L, et al. Antitumor activity of the Hsp90 inhibitor IPI-504 in HER2-positive trastuzumab-resistant breast cancer. Mol Cancer Ther. 2011;10(5):817–24. [DOI] [PubMed] [Google Scholar]
- 194.Wagner AJ, Chugh R, Rosen LS, Morgan JA, George S, Gordon M, Dunbar J, Normant E, Grayzel D, Demetri GD. A phase I study of the HSP90 inhibitor retaspimycin hydrochloride (IPI-504) in patients with gastrointestinal stromal tumors or soft-tissue sarcomas. Clin Cancer Res. 2013;19(21):6020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li G, Zhang C, Liang W, Zhang Y, Shen Y, Tian X. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells. Pharm Biol. 2021;59(1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu BQ, Li JH, Jing L, Jiang JL, Tang J, et al. Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice. Am J Cancer Res. 2015;5(10):3174–85. [PMC free article] [PubMed] [Google Scholar]
- 197.Todorovic Z, Milovanovic J, Arsenijevic D, Vukovic N, Vukic M, Arsenijevic A, Djurdjevic P, Milovanovic M, Arsenijevic N. Shikonin derivatives from onsoma visianii decrease expression of phosphorylated STAT3 in leukemia cells and exert antitumor activity. Nutrients. 2021. 10.3390/nu13041147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hsu P-C, Huang Y-T, Tsai M-L, Wang Y-J, Lin J-K, Pan M-H. Induction of apoptosis by shikonin through coordinative modulation of the Bcl-2 family, p27, and p53, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J Agric Food Chem. 2004;52(20):6330–7. [DOI] [PubMed] [Google Scholar]
- 199.Huang X, Chen Z, Ni F, Ye X, Qian W. Shikonin overcomes drug resistance and induces necroptosis by regulating the miR-92a-1-5p/MLKL axis in chronic myeloid leukemia. Aging. 2020;12(17):17662–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.James AD, Richardson DA, Oh I-W, Sritangos P, Attard T, Barrett L, Bruce JIE. Cutting off the fuel supply to calcium pumps in pancreatic cancer cells: role of pyruvate kinase-M2 (PKM2). Br J Cancer. 2020;122(2):266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shahsavari Z, Karami-Tehrani F, Salami S, Ghasemzadeh M. RIP1K and RIP3K provoked by shikonin induce cell cycle arrest in the triple negative breast cancer cell line, MDA-MB-468: necroptosis as a desperate programmed suicide pathway. Tumour Biol. 2016;37(4):4479–91. [DOI] [PubMed] [Google Scholar]
- 202.Fu Z, Deng B, Liao Y, Shan L, Yin F, Wang Z, Zeng H, Zuo D, Hua Y, Cai Z. The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer. 2013;13:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lin SY, Hsieh SY, Fan YT, Wei WC, Hsiao PW, Tsai DH, Wu TS, Yang NS. Necroptosis promotes autophagy-dependent upregulation of DAMP and results in immunosurveillance. Autophagy. 2018;14(5):778–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang P, Zheng SY, Jiang RL, Wu HD, Li YA, Lu JL, Xiong Y, Han B, Lin L. Necroptosis signaling and mitochondrial dysfunction cross-talking facilitate cell death mediated by chelerythrine in glioma. Free Radic Biol Med. 2023;202:76–96. [DOI] [PubMed] [Google Scholar]
- 205.Li DM, Zhu FC, Wei J, Xie JX, He JH, Wei DM, Li Y, Lai KD, Liu LM, Su QB, et al. The active fraction of polyrhachis vicina roger (AFPR) activates ERK to cause necroptosis in colorectal cancer. J Ethnopharmacol. 2023;312:116454. [DOI] [PubMed] [Google Scholar]
- 206.Park KR, Lee H, Kim SH, Yun HM. Paeoniflorigenone regulates apoptosis, autophagy, and necroptosis to induce anti-cancer bioactivities in human head and neck squamous cell carcinomas. J Ethnopharmacol. 2022;288:115000. [DOI] [PubMed] [Google Scholar]
- 207.Lee YJ, Nam HS, Cho MK, Lee SH. Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis. Mol Cell Biochem. 2020;467(1–2):45–56. [DOI] [PubMed] [Google Scholar]
- 208.Du S, Liang H, Zhou L, Chen C, Sun R, Zhang J, Meng X, Gao A. Effect of doramectin on programmed cell death pathway in glioma cells. Clin Transl Oncol. 2023. 10.1007/s12094-023-03147-z. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Y, Kwok-Shing Ng P, Kucherlapati M, Chen F, Liu Y, Tsang YH, de Velasco G, Jeong KJ, Akbani R, Hadjipanayis A, et al. A pan-cancer proteogenomic atlas of PI3K/AKT/mTOR pathway alterations. Cancer Cell. 2017;31(6):820–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu Y, Cao GF, Xue J, Wan J, Wan Y, Jiang Q, Yao J. Tumor necrosis factor-alpha (TNF-alpha)-mediated in vitro human retinal pigment epithelial (RPE) cell migration mainly requires Akt/mTOR complex 1 (mTORC1), but not mTOR complex 2 (mTORC2) signaling. Eur J Cell Biol. 2012;91(9):728–37. [DOI] [PubMed] [Google Scholar]
- 211.Zhou Y, Li S, Li J, Wang D, Li Q. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem. 2017;42(4):1431–46. [DOI] [PubMed] [Google Scholar]
- 212.Jafari M, Ghadami E, Dadkhah T, Akhavan-Niaki H. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol. 2019;234(3):2373–85. [DOI] [PubMed] [Google Scholar]
- 213.Abraham J. PI3K/AKT/mTOR pathway inhibitors: the ideal combination partners for breast cancer therapies? Expert Rev Anticancer Ther. 2015;15(1):51–68. [DOI] [PubMed] [Google Scholar]
- 214.Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, Kundu M, Kim D-H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20(7):1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ge D, Tao HR, Fang L, Kong XQ, Han LN, Li N, Xu YX, Li LY, Yu M, Zhang H. 11-Methoxytabersonine induces necroptosis with autophagy through AMPK/mTOR and JNK pathways in human lung cancer cells. Chem Pharm Bull. 2020;68(3):244–50. [DOI] [PubMed] [Google Scholar]
- 216.Xie Y, Lei X, Zhao G, Guo R, Cui N. mTOR in programmed cell death and its therapeutic implications. Cytokine Growth Factor Rev. 2023. 10.1016/j.cytogfr.2023.06.002. [DOI] [PubMed] [Google Scholar]
- 217.Zhang XJ, Shang K, Pu YK, Wang Q, Wang TT, Zou Y, Wang YM, Xu YJ, Li XL, Zhang RH, et al. Leojaponin inhibits NLRP3 inflammasome activation through restoration of autophagy via upregulating RAPTOR phosphorylation. J Ethnopharmacol. 2021;278:114322. [DOI] [PubMed] [Google Scholar]
- 218.Li MY, Zhu XL, Zhao BX, Shi L, Wang W, Hu W, Qin SL, Chen BH, Zhou PH, Qiu B, et al. Adrenomedullin alleviates the pyroptosis of leydig cells by promoting autophagy via the ROS-AMPK-mTOR axis. Cell Death Dis. 2019;10(7):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wu C, Chen H, Zhuang R, Zhang H, Wang Y, Hu X, Xu Y, Li J, Li Y, Wang X, et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int J Biol Sci. 2021;17(4):1138–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yao T, Chen JM, Shen LE, Yu YS, Tang ZH, Zang GQ, Zhang Y, Chen XH. Astragalus polysaccharide alleviated hepatocyte senescence via autophagy pathway. Kaohsiung J Med Sci. 2022;38(5):457–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci USA. 2020;117(49):31189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cheng Q, Chen M, Liu M, Chen X, Zhu L, Xu J, Xue J, Wu H, Du Y. Semaphorin 5A suppresses ferroptosis through activation of PI3K-AKT-mTOR signaling in rheumatoid arthritis. Cell Death Dis. 2022;13(7):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H, et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314(3):H659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.La P, Yang G, Dennery PA. Mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation stabilizes ISCU protein: implications for iron metabolism. J Biol Chem. 2013;288(18):12901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang Y, Swanda RV, Nie L, Liu X, Wang C, Lee H, Lei G, Mao C, Koppula P, Cheng W, et al. mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat Commun. 2021;12(1):1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ng TL, Leprivier G, Robertson MD, Chow C, Martin MJ, Laderoute KR, Davicioni E, Triche TJ, Sorensen PH. The AMPK stress response pathway mediates anoikis resistance through inhibition of mTOR and suppression of protein synthesis. Cell Death Differ. 2012;19(3):501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hu H, Yin Y, Jiang B, Feng Z, Cai T, Wu S. Cuproptosis signature and PLCD3 predicts immune infiltration and drug responses in osteosarcoma. Front Oncol. 2023;13:1156455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ma YL, Yang YF, Wang HC, Yang CC, Yan LJ, Ding ZN, Tian BW, Liu H, Xue JS, Han CL, et al. A novel prognostic scoring model based on copper homeostasis and cuproptosis which indicates changes in tumor microenvironment and affects treatment response. Front Pharmacol. 2023;14:1101749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo J, Hu X. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6(5):1641–9. [DOI] [PubMed] [Google Scholar]
- 231.Slotkin EK, Patwardhan PP, Vasudeva SD, de Stanchina E, Tap WD, Schwartz GK. MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol Cancer Ther. 2015;14(2):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bellmunt J, Maroto P, Bonfill T, Vazquez F, Perez-Gracia JL, Juanpere N, Hernandez-Prat A, Hernandez-Llodra S, Rovira A, Juan O, et al. Dual mTOR1/2 inhibitor sapanisertib (FTH-003/TAK-228) in combination with weekly paclitaxel in patients with previously treated metastatic urothelial carcinoma: a phase II Open-label study. Clin Genitourin Cancer. 2024;22(5):102123. [DOI] [PubMed] [Google Scholar]
- 233.Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE, Vincent JP, Ellston R, Jones D, Sini P, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70(1):288–98. [DOI] [PubMed] [Google Scholar]
- 234.Sini P, James D, Chresta C, Guichard S. Simultaneous inhibition of mTORC1 and mTORC2 by mTOR kinase inhibitor AZD8055 induces autophagy and cell death in cancer cells. Autophagy. 2010;6(4):553–4. [DOI] [PubMed] [Google Scholar]
- 235.Willems L, Chapuis N, Puissant A, Maciel TT, Green AS, Jacque N, Vignon C, Park S, Guichard S, Herault O, et al. The dual mTORC1 and mTORC2 inhibitor AZD8055 has anti-tumor activity in acute myeloid leukemia. Leukemia. 2012;26(6):1195–202. [DOI] [PubMed] [Google Scholar]
- 236.Jordan NJ, Dutkowski CM, Barrow D, Mottram HJ, Hutcheson IR, Nicholson RI, Guichard SM, Gee JM. Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res. 2014;16(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xu PF, Yang JA, Liu JH, Yang X, Liao JM, Yuan FE, Liu BH, Chen QX. PI3Kbeta inhibitor AZD6482 exerts antiproliferative activity and induces apoptosis in human glioblastoma cells. Oncol Rep. 2019;41(1):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rashmi R, DeSelm C, Helms C, Bowcock A, Rogers BE, Rader JL, Rader J, Grigsby PW, Schwarz JK. AKT inhibitors promote cell death in cervical cancer through disruption of mTOR signaling and glucose uptake. PLoS ONE. 2014;9(4):e92948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gao L, Liu J, Xu P, Deng G, Liu B, Yuan F, Tan Y, Sun Q, Xu Y, Zhang H, et al. AKT inhibitor SC66 inhibits proliferation and induces apoptosis in human glioblastoma through down-regulating AKT/beta-catenin pathway. Front Pharmacol. 2020;11:1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McKinnon T, Venier R, Yohe M, Sindiri S, Gryder BE, Shern JF, Kabaroff L, Dickson B, Schleicher K, Chouinard-Pelletier G, et al. Functional screening of FGFR4-driven tumorigenesis identifies PI3K/mTOR inhibition as a therapeutic strategy in rhabdomyosarcoma. Oncogene. 2018;37(20):2630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen Y, Tsai HW, Tsai YH, Tseng SH. VS-5584, a PI3K/mTOR dual inhibitor, exerts antitumor effects on neuroblastomas in vitro and in vivo. J Pediatr Surg. 2021;56(8):1441–8. [DOI] [PubMed] [Google Scholar]
- 242.Shen YQ, Guerra-Librero A, Fernandez-Gil BI, Florido J, Garcia-Lopez S, Martinez-Ruiz L, Mendivil-Perez M, Soto-Mercado V, Acuna-Castroviejo D, Ortega-Arellano H, et al. Combination of melatonin and rapamycin for head and neck cancer therapy: suppression of AKT/mTOR pathway activation, and activation of mitophagy and apoptosis via mitochondrial function regulation. J Pineal Res. 2018. 10.1111/jpi.12461. [DOI] [PubMed] [Google Scholar]
- 243.Feng H, Cheng X, Kuang J, Chen L, Yuen S, Shi M, Liang J, Shen B, Jin Z, Yan J, et al. Apatinib-induced protective autophagy and apoptosis through the AKT-mTOR pathway in anaplastic thyroid cancer. Cell Death Dis. 2018;9(10):1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang R, Lu X, Yu R. Lycopene inhibits epithelial-mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR signal pathway. Drug Des Devel Ther. 2020;14:2461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chen M, Yin X, Lu C, Chen X, Ba H, Cai J, Sun J. Mahanine induces apoptosis, cell cycle arrest, inhibition of cell migration, invasion and PI3K/AKT/mTOR signalling pathway in glioma cells and inhibits tumor growth in vivo. Chem Biol Interact. 2019;299:1–7. [DOI] [PubMed] [Google Scholar]
- 246.Wei M, Wu Y, Liu H, Xie C. Genipin induces autophagy and suppresses cell growth of oral squamous cell carcinoma via PI3K/AKT/MTOR pathway. Drug Des Devel Ther. 2020;14:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Song L, Luo Y, Li S, Hong M, Wang Q, Chi X, Yang C. ISL induces apoptosis and autophagy in hepatocellular carcinoma via downregulation of PI3K/AKT/mTOR pathway in vivo and in vitro. Drug Des Devel Ther. 2020;14:4363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee HJ, Venkatarame Gowda Saralamma V, Kim SM, Ha SE, Raha S, Lee WS, Kim EH, Lee SJ, Heo JD, Kim GS. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients. 2018. 10.3390/nu10081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhu Y, Fang J, Wang H, Fei M, Tang T, Liu K, Niu W, Zhou Y. Baicalin suppresses proliferation, migration, and invasion in human glioblastoma cells via Ca(2+)-dependent pathway. Drug Des Devel Ther. 2018;12:3247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhou J, Jiang YY, Chen H, Wu YC, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2):e12739. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 251.Ye R, Dai N, He Q, Guo P, Xiang Y, Zhang Q, Hong Z, Zhang Q. Comprehensive anti-tumor effect of brusatol through inhibition of cell viability and promotion of apoptosis caused by autophagy via the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;105:962–73. [DOI] [PubMed] [Google Scholar]
- 252.Liu H, Li X, Duan Y, Xie JB, Piao XL. Mechanism of gypenosides of gynostemma pentaphyllum inducing apoptosis of renal cell carcinoma by PI3K/AKT/mTOR pathway. J Ethnopharmacol. 2021;271:113907. [DOI] [PubMed] [Google Scholar]
- 253.Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2018;97:1269–74. [DOI] [PubMed] [Google Scholar]
- 254.Cao J, Lv W, Wang L, Xu J, Yuan P, Huang S, He Z, Hu J. Ricolinostat (ACY-1215) suppresses proliferation and promotes apoptosis in esophageal squamous cell carcinoma via miR-30d/PI3K/AKT/mTOR and ERK pathways. Cell Death Dis. 2018;9(8):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang C, Wang Q, Zhou X, Zhang L, Yao Y, Gu J, Chen H, Qian J, Luo C, Bai Q, et al. MicroRNA-138 modulates glioma cell growth, apoptosis and invasion through the suppression of the AKT/mTOR signalling pathway by targeting CREB1. Oncol Rep. 2020;44(6):2559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wang Y, Zhang Q, Wang B, Li P, Liu P. LiCl treatment induces programmed cell death of schwannoma cells through AKT- and MTOR-mediated necroptosis. Neurochem Res. 2017;42(8):2363–71. [DOI] [PubMed] [Google Scholar]
- 257.Zeng F, Ye L, Zhou Q, He Y, Zhang Y, Deng G, Chen X, Liu H. Inhibiting SCD expression by IGF1R during lorlatinib therapy sensitizes melanoma to ferroptosis. Redox Biol. 2023;61:102653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Solomon BJ, Bauer TM, Mok TSK, Liu G, Mazieres J, de Marinis F, Goto Y, Kim DW, Wu YL, Jassem J, et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023;11(4):354–66. [DOI] [PubMed] [Google Scholar]
- 259.Hu Z, Li L, Li M, Zhang X, Zhang Y, Ran J, Li L. miR-21-5p inhibits ferroptosis in hepatocellular carcinoma cells by regulating the AKT/mTOR signaling pathway through MELK. J Immunol Res. 2023;2023:8929525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li J, Fan Y, Zhang Y, Liu Y, Yu Y, Ma M. Resveratrol induces autophagy and apoptosis in non-small-cell lung cancer cells by activating the NGFR-AMPK-mTOR pathway. Nutrients. 2022. 10.3390/nu14122413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wang GY, Zhang L, Geng YD, Wang B, Feng XJ, Chen ZL, Wei W, Jiang L. beta-Elemene induces apoptosis and autophagy in colorectal cancer cells through regulating the ROS/AMPK/mTOR pathway. Chin J Nat Med. 2022;20(1):9–21. [DOI] [PubMed] [Google Scholar]
- 262.Ni X, Shang FS, Wang TF, Wu DJ, Chen DG, Zhuang B. Ellagic acid induces apoptosis and autophagy in colon cancer through the AMPK/mTOR pathway. Tissue Cell. 2023;81:102032. [DOI] [PubMed] [Google Scholar]
- 263.Xie G, Sun L, Li Y, Chen B, Wang C. Periplocin inhibits the growth of pancreatic cancer by inducing apoptosis via AMPK-mTOR signaling. Cancer Med. 2021;10(1):325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sun D, Tao W, Zhang F, Shen W, Tan J, Li L, Meng Q, Chen Y, Yang Y, Cheng H. Trifolirhizin induces autophagy-dependent apoptosis in colon cancer via AMPK/mTOR signaling. Signal Transduct Target Ther. 2020;5(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lee MG, Kwon YS, Nam KS, Kim SY, Hwang IH, Kim S, Jang H. Chaga mushroom extract induces autophagy via the AMPK-mTOR signaling pathway in breast cancer cells. J Ethnopharmacol. 2021;274:114081. [DOI] [PubMed] [Google Scholar]
- 266.You M, Savaraj N, Kuo MT, Wangpaichitr M, Varona-Santos J, Wu C, Nguyen DM, Feun L. TRAIL induces autophagic protein cleavage through caspase activation in melanoma cell lines under arginine deprivation. Mol Cell Biochem. 2013;374(1–2):181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lin H, Zhang C, Zhang H, Xia YZ, Zhang CY, Luo J, Yang L, Kong LY. Physakengose G induces apoptosis via EGFR/mTOR signaling and inhibits autophagic flux in human osteosarcoma cells. Phytomedicine. 2018;42:190–8. [DOI] [PubMed] [Google Scholar]
- 268.Li HW, Liu MB, Jiang X, Song T, Feng SX, Wu JY, Deng PF, Wang XY. GALNT14 regulates ferroptosis and apoptosis of ovarian cancer through the EGFR/mTOR pathway. Future Oncol. 2022;18(2):149–61. [DOI] [PubMed] [Google Scholar]
- 269.Chen WT, Lin SM, Lee WC, Wu TJ, Lin CC, Shen CH, Chang ML, Lin CL, Yeh CT. GALNT14 genotype-guided chemoembolization plus sorafenib therapy in hepatocellular carcinoma: a randomized trial. Hep Intl. 2022;16(1):148–58. [DOI] [PubMed] [Google Scholar]
- 270.Yu Y, Song Y, Cheng L, Chen L, Liu B, Lu D, Li X, Li Y, Lv F, Xing Y. CircCEMIP promotes anoikis-resistance by enhancing protective autophagy in prostate cancer cells. J Exp Clin Cancer Res. 2022;41(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhang Y, Li CF, Ma LJ, Ding M, Zhang B. MicroRNA-224 aggrevates tumor growth and progression by targeting mTOR in gastric cancer. Int J Oncol. 2016;49(3):1068–80. [DOI] [PubMed] [Google Scholar]
- 272.Wang LB, Wang DN, Wu LG, Cao J, Tian JH, Liu R, Ma R, Yu JJ, Wang J, Huang Q, et al. Homoharringtonine inhibited breast cancer cells growth via miR-18a-3p/AKT/mTOR signaling pathway. Int J Biol Sci. 2021;17(4):995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yu S, Zhang Y, Yu G, Wang Y, Shao R, Du X, Xu N, Lin D, Zhao W, Zhang X, et al. Sorafenib plus triplet therapy with venetoclax, azacitidine and homoharringtonine for refractory/relapsed acute myeloid leukemia with FLT3-ITD: A multicenter phase 2 study. J Intern Med. 2024;295(2):216–28. [DOI] [PubMed] [Google Scholar]
- 274.Fukazawa H, Noguchi K, Murakami Y, Uehara Y. Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway. Mol Cancer Ther. 2002;1(5):303–9. [PubMed] [Google Scholar]
- 275.Shen J, Dong J, Shao F, Zhao J, Gong L, Wang H, Chen W, Zhang Y, Cai Y. Graphene oxide induces autophagy and apoptosis via the ROS-dependent AMPK/mTOR/ULK-1 pathway in colorectal cancer cells. Nanomedicine (Lond). 2022;17(9):591–605. [DOI] [PubMed] [Google Scholar]
- 276.Luo Q, Jia L, Huang C, Qi Q, Jahangir A, Xia Y, Liu W, Shi R, Tang L, Chen Z. Polyphyllin I promotes autophagic cell death and apoptosis of colon cancer cells via the ROS-inhibited AKT/mTOR pathway. Int J Mol Sci. 2022. 10.3390/ijms23169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Shin N, Lee HJ, Sim DY, Im E, Park JE, Park WY, Cho AR, Shim BS, Kim SH. Apoptotic effect of compound K in hepatocellular carcinoma cells via inhibition of glycolysis and Akt/mTOR/c-Myc signaling. Phytother Res. 2021;35(7):3812–20. [DOI] [PubMed] [Google Scholar]
- 278.Jung GH, Lee JH, Han SH, Woo JS, Choi EY, Jeon SJ, Han EJ, Jung SH, Park YS, Park BK, et al. Chrysin induces apoptosis via the MAPK pathway and regulates ERK/mTOR-mediated autophagy in MC-3 cells. Int J Mol Sci. 2022. 10.3390/ijms232415747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pan Z, Cheng DD, Wei XJ, Li SJ, Guo H, Yang QC. Chitooligosaccharides inhibit tumor progression and induce autophagy through the activation of the p53/mTOR pathway in osteosarcoma. Carbohydr Polym. 2021;258:117596. [DOI] [PubMed] [Google Scholar]
- 280.Zhang XT, Hu J, Su LH, Geng CA, Chen JJ. Artematrolide A inhibited cervical cancer cell proliferation via ROS/ERK/mTOR pathway and metabolic shift. Phytomedicine. 2021;91:153707. [DOI] [PubMed] [Google Scholar]
- 281.Guo Y, Zhu H, Weng M, Chen B, Wang C, Sun L. Baohuoside-1 targeting mTOR inducing apoptsis to inhibit hepatocellular carcinoma proliferation, invasion and migration. Biomed Pharmacother. 2020;128:110366. [DOI] [PubMed] [Google Scholar]
- 282.Xu Y, Zeng K, Wang X, Zhang J, Cao B, Zhang Z, Qiao C, Xu X, Wang Q, Zeng Y, et al. Novel conjugates of endoperoxide and 4-anilinoquinazoline induce myeloma cell apoptosis by inhibiting the IGF1-R/AKT/mTOR signaling pathway. Biosci Trends. 2020;14(2):96–103. [DOI] [PubMed] [Google Scholar]
- 283.Xu W, Ding J, Li B, Sun T, You X, He Q, Sheng W. Effects of icariin and curcumol on autophagy, ferroptosis, and lipid metabolism based on miR-7/m-TOR/SREBP1 pathway on prostate cancer. BioFactors. 2023;49(2):438–56. [DOI] [PubMed] [Google Scholar]
- 284.Zhou X, Kang J, Zhang L, Cheng Y. Osthole inhibits malignant phenotypes and induces ferroptosis in KRAS-mutant colorectal cancer cells via suppressing AMPK/Akt signaling. Cancer Chemother Pharmacol. 2023;92(2):119–34. [DOI] [PubMed] [Google Scholar]
- 285.Wang T, Guo H, Zhang L, Yu M, Li Q, Zhang J, Tang Y, Zhang H, Zhan J. FERM domain-containing protein FRMD6 activates the mTOR signaling pathway and promotes lung cancer progression. Front Med. 2023;17(4):714–28. [DOI] [PubMed] [Google Scholar]
- 286.Chen C, Chen Z, Zhao J, Wen X, Yao H, Weng Z, Xiong H, Zheng Z, Wu J. TMEM45A enhances palbociclib resistance and cellular glycolysis by activating AKT/mTOR signaling pathway in HR+ breast cancer. Cell death discovery. 2025;11(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhu C, Fan F, Li CY, Xiong Y, Liu X. Caspase-3 promotes oncogene-induced malignant transformation via EndoG-dependent Src-STAT3 phosphorylation. Cell Death Dis. 2024;15(7):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Shen J, Zhang Y, Tang W, Yang M, Cheng T, Chen Y, Yu S, Guo Q, Cao L, Wang X, et al. Short IL-18 generated by caspase-3 cleavage mobilizes NK cells to suppress tumor growth. Nat Immunol. 2025. 10.1038/s41590-024-02074-7. [DOI] [PubMed] [Google Scholar]
- 289.Su L, Chen Y, Huang C, Wu S, Wang X, Zhao X, Xu Q, Sun R, Kong X, Jiang X, et al. Targeting Src reactivates pyroptosis to reverse chemoresistance in lung and pancreatic cancer models. Sci Transl Med. 2023;15(678):eab17895. [DOI] [PubMed] [Google Scholar]
- 290.Diwanji N, Bergmann A. An unexpected friend—ROS in apoptosis-induced compensatory proliferation: implications for regeneration and cancer. Semin Cell Dev Biol. 2018;80:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhang Z, Zhang F, Xie W, Niu Y, Wang H, Li G, Zhao L, Wang X, Xie W. Induced necroptosis and its role in cancer immunotherapy. Int J Mol Sci. 2024. 10.3390/ijms251910760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zeng X, Chen Z, Zhu Y, Liu L, Zhang Z, Xiao Y, Wang Q, Pang S, Zhao F, Xu B, et al. O-GlcNAcylation regulation of RIPK1-dependent apoptosis dictates sensitivity to sunitinib in renal cell carcinoma. Drug Resist Updat. 2024;77:101150. [DOI] [PubMed] [Google Scholar]
- 293.Burke JE, Triscott J, Emerling BM, Hammond GRV. Beyond PI3Ks: targeting phosphoinositide kinases in disease. Nat Rev Drug Discover. 2023;22(5):357–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, Sengupta S, Kumar D, Garg M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023;8(1):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10(10):957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang H, La Russa M, Qi LS. CRISPR/Cas9 in genome editing and beyond. Annu Rev Biochem. 2016;85:227–64. [DOI] [PubMed] [Google Scholar]
- 297.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sun LL, Linghu DL, Hung MC. Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res. 2021;11(12):5856–63. [PMC free article] [PubMed] [Google Scholar]
- 299.Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Li J, Yang C, Li Y, Chen A, Li L, You Z. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. 2018. Biosci Rep. 10.1042/BSR20171150. [DOI] [PMC free article] [PubMed]
Data Availability Statement
No datasets were generated or analysed during the current study.