Abstract
Recent discoveries concerning the network architecture of glioblastoma, including tumor microtubules and neuron–glioma synapses, have underscored critical pathways that sustain tumor growth, enhance resistance, and integrate glioblastoma with the surrounding neural environment. This review explores emerging therapeutic strategies targeting these pathways, including inhibitors of gap junctions, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, and glutamate signaling, which are currently being tested in clinical trials. By consolidating these advances, this review seeks to bridge the gap between neurobiology, cancer neuroscience, and oncology, proposing novel approaches to overcome resistance and improve patient outcomes. The insights derived from this comprehensive review hold the potential to significantly influence the future management of glioblastoma.
Introduction
Glioblastoma (GB), the most aggressive primary tumor of the central nervous system in adults, is associated with a dismal prognosis, with a median overall survival of only 17.1 months (1). The highly heterogeneous nature, the complex multicellular networks of GB, and the colonization of the entire brain (2, 3) make it particularly challenging to treat despite various treatment modalities, including surgical resection, radiotherapy, chemotherapy, and the potential use of tumor-treating fields. This heterogeneity encompasses genetic, epigenetic, transcriptional, metabolic, proteomic, morphologic, and functional factors (4–8) and is manifested in the tumor’s ability to form intricate intercellular networks (9–11), further complicating therapeutic efforts. Growth-associated protein 43 (GAP43; ref. 10), chitinase 3–like 1 (CHI3L1; ref. 12), and tweety-homolog 1 (TTYH1; ref. 13) play crucial roles in the growth and function of tumor microtubules (TM), therefore promoting the formation of the tumors’ malignant multicellular network.
Unraveling Tumor Cell Networks and Resistance-Promoting Biology of Glioblastoma
Recent research has highlighted the role of TMs in GB cell communication and resistance (10, 11, 14, 15). Glioma cells are interconnected through TMs, forming a highly organized network in which pacemaker cells drive tumor progression and network propagation (9, 10).
Additionally, glioma cells are synaptically integrated into surrounding neural circuitry (16, 17). Neurons contribute to tumor growth through both paracrine and direct electrochemical mechanisms, notably through glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (16, 17). The increased glutamate release in the tumor microenvironment, coupled with the loss of peritumoral GABAergic inhibitory interneurons, leads to network hyperexcitability that in itself further promotes tumor progression (16–20).
This review aims to summarize recent advances in the neurobiologic understanding of GB. We will explore how these findings can be used to develop or better understand the mechanism of novel therapeutic strategies that target the tumor’s complex network dynamics and neurodevelopmental mechanisms. Additionally, we will discuss ongoing clinical trials that are leveraging these discoveries, highlighting their potential to improve therapeutic outcomes.
TMs and Multicellular Networks
Most GB cells extend long, thin membrane protrusions known as TMs. These structures are not merely byproducts of tumor growth but integral components of a highly organized and functional multicellular network (9–11). Disrupting the tumor cell network has been shown to enhance the efficacy of radiotherapy and chemotherapy while inhibiting the malignant self-repair mechanisms that frequently drive resistance after surgical interventions (10, 11, 14). TMs have diameters of 0.5 to 2 microns and can span tens to hundreds of microns (in humans, probably millimeters or even centimeters) in length, forming physical bridges between GB cells. These protrusions contribute to various vital processes, such as tumor cell invasion, nuclear migration, and intercellular communication, effectively transforming GB into a connected syncytium (9–11, 13, 18, 21).
TMs mirror many characteristics of neuronal processes, such as neurites, observed during development, further reinforcing the notion that GB behaves, in many aspects, like a neural-derived structure (10, 18, 22–26). These TM structures display two distinct functional behaviors: they can either remain dynamically plastic, extending and retracting as they explore brain tissue, or they can stabilize to interconnect GB cells over extended periods (10, 18, 21). This dichotomy reflects an underlying cellular heterogeneity, in which some GB cells are equipped with no TMs, others with one or two TMs, and some with multiple TMs, enabling various levels of network integration and functional specialization (10, 13, 18, 21).
Molecular drivers such as GAP43 (10) and TTYH1 (13) have been identified to play a crucial role in regulating TM formation and function. GAP43 is expressed in all TMs and supports their role in cell communication and homeostasis (10), whereas TTYH1 is more specific to proinvasive and proliferative functions, particularly in cells with fewer TMs (13). This molecular diversity extends to the gene expression signatures of GB cells: cells with one or two TMs often exhibit oligodendrocyte progenitor cell–like or neural progenitor cell–like states, whereas cells with multiple TMs display astrocyte-like or mesenchymal-like (MES) characteristics (5, 12, 18, 21).
Importantly, the heterogeneity observed within individual tumors does not obscure the universal presence of TMs across glioma samples. Although the extent of TM connectivity can vary between patients (10, 12), all incurable glioma types studied so far exhibit these structures, reinforcing their classification as a hallmark of this cancer type. The network formation is not limited to GB but is also found in other diffuse gliomas (e.g., grade 2–4 astrocytomas and H3.K27M mutant diffuse midline gliomas; refs. 10, 16, 17). By contrast, oligodendrogliomas, which lack a relevant TM network, show greater sensitivity to treatments, further emphasizing the protective role of these multicellular structures (10).
Tumor Cell Communication and Resistance
At the core of GB’s resistance to therapy is its ability to form a robust, interconnected cellular network. TMs facilitate the formation of a syncytium in which GB cells communicate through gap junctions formed by connexin 43 (Cx43; ref. 10). The syncytium facilitates the distribution of small molecules, such as calcium, through TMs, thereby enabling the spread of signals throughout the tumor. This process is thought to prevent lethal intracellular concentrations and promote survival (14, 27, 28). The high expression of Cx43 in GB cells mirrors its role in nonmalignant astrocytes. In these cells, Cx43 forms intercellular connections that confer resistance to oxidative stress (29) and exhibit remarkable resistance to chemotherapy (30). The exchange of calcium waves, analogous to the processes seen in astrocytes, plays a critical role in maintaining cellular homeostasis within the tumor, shielding GB cells from environmental stressors, including cytotoxic treatments (10, 11, 14, 31).
Pacemaker-like Cells and Network Activation
The GB network is not uniformly structured but rather exhibits small-world and scale-free properties, which are characteristics of highly efficient biological systems, such as neural networks (9, 10, 32, 33). A small, specialized subset of GB cells serve as critical hubs within this network, exhibiting significantly higher levels of connectivity than the rest (9). Likewise, cells residing at these hub positions preferentially act in pacemaker-like function by autonomously generating rhythmic calcium oscillations with the expression of the KCa3.1 (KCNN4) potassium channel being a necessary and sufficient molecular requirement (9). Their activity drives the propagation of calcium transients to less-connected GB cells through TM-mediated gap junctions, orchestrating synchronized, network-wide responses to internal and external stimuli (9).
The pacemaker-like cells play a key role in network activation, promote proliferation throughout the tumor, and ultimately impair survival. The rhythmic calcium signals they generate occur at a frequency of 10 to 15 mHz and activate tumor-promoting pathways, including the MAPK and NF-κB signaling cascades, in a frequency-dependent manner (9, 34). This periodic activity is critical not only for maintaining cellular homeostasis but also for the growth and resilience of the entire GB cell network. Remarkably, these pacemaker cells are predominantly found in the MES-like GB subpopulation, which is known to be associated with poor prognosis and treatment resistance. The higher expression of KCa3.1 in MES-like cells correlates with worse survival, underscoring the importance of this cell type in driving tumor aggressiveness (9).
Mathematical network analysis revealed that the network architecture of GB is resistant to random damage due to its small-world, scale-free design. However, the network is highly vulnerable to perturbations at its key hubs, such as the pacemaker cells, which serve as critical nodes for network stability (9).
In addition, the plasticity of the GB network is a hallmark of its resilience. Even after the targeted destruction of pacemaker cells, the network can adapt by recruiting new cells into pacemaker-like roles, thereby restoring lost functionality (9). This dynamic ability to compensate for damage underscores the need to target KCa3.1 activity as a therapeutic approach. The vulnerability of the GB network to disruptions in periodic calcium activity offers a promising avenue for therapeutic intervention, with KCa3.1 inhibitors showing potential to target these key network nodes and weaken tumor resistance to conventional therapies in future research.
Glutamate as a Key Driver of GB Network Dynamics and Progression
Recent evidence suggests that excessive neuronal activity, particularly during seizures, may actively stimulate glioma progression (16, 17, 19, 20, 35). This causality implies that increased neuronal firing not only accompanies tumor development but also accelerates it through direct synaptic communication between neurons and glioma cells. The discovery of glutamatergic neuroglial synapses provides a morphologic and functional basis for this interaction with significant clinical implications (16, 17).
These synapses, located between presynaptic neurons and glioma cells, play a key role in promoting tumor growth by modulating calcium signaling within glioma networks. Glutamate release from neurons activates AMPA receptors on glioma cells, inducing synchronized calcium transients that propagate through the glioma network, ultimately promoting glioma cell invasion and proliferation. This communication mechanism hijacks normal neuronal–glial synaptic pathways, facilitating tumor expansion and increasing its malignant potential (16–19).
Recent insights into the role of electrochemically active neuroglial networks in GB progression reveal that glioma cells form networks via gap junctions and integrate into neuronal circuits, driving oncogenic activity (36, 37). GB networks are maintained by calcium oscillations derived from both hub cells and glutamatergic neuroglial synapses (9, 16, 17, 38). Remote neuronal remodeling and epileptic activity exacerbate this process, creating a vicious cycle that promotes tumor growth (19, 20, 39). Subclinical epileptic activity observed in patients with GB may contribute to reduced survival (40, 41).
GB cells secrete high levels of glutamate via the glutamate–cystine antiporter system xc (39, 42, 43). Furthermore, peritumoral reactive astrocytes exhibit a diminished capacity to uptake glutamate, resulting in elevated toxic glutamate levels (44). These elevated levels lead to the death of the inhibitory GABAergic neurons, thereby propagating a vicious cycle of elevated glutamate levels. This cycle promotes tumor invasion and neuronal hyperexcitability, and it amplifies the activity of neuroglial networks (43, 45, 46). The role of glutamate in glioma progression provides a strong rationale for targeting glutamate metabolism and signaling as a therapeutic approach (17, 47–49).
Gabapentin inhibits glutamate synthesis by blocking branched chain amino acid transaminase-1 (BCAT-1) and reduces neuroglial network connectivity by inhibiting the thrombospondin receptor α2δ-1 (48–50). Its anticonvulsant properties may be particularly beneficial given the role of epilepsy in GB progression (40, 41). Sulfasalazine inhibits glutamate release through the system xc antiporter, with early studies demonstrating reduced peritumoral glutamate levels following its administration (35, 51). Memantine blocks N-methyl-D-aspartate (NMDA) type glutamate receptors, which may prevent the formation of synapses between neurons and glioma cells, thereby reducing tumor cell invasion and neuroglial signaling (39, 52).
Therapeutic Strategies Targeting the GB Tumor Cell Network
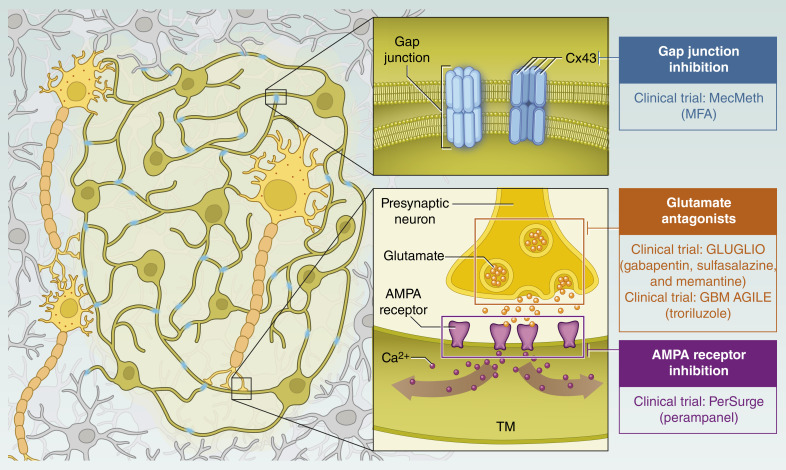
The evolving morphologic and structural understanding of GB is reshaping the approach of current clinical trials, providing a clearer basis for potential treatment strategies, and guiding future research (37, 53–55). TM-based networks underlie tumor resistance to cytotoxic treatments, and disruption of these networks is likely to improve the efficacy of chemotherapy and radiotherapy, as demonstrated in preclinical studies (10, 11, 56–58). Several therapeutic strategies are under investigation to target these networks, including meclofenamate (MFA), perampanel, and the glutamate inhibitors gabapentin, sulfasalazine, memantine, and troriluzole, each of which offers a unique approach to weakening the tumor cell network and thus a main mechanism of resistance (Fig. 1; Table 1).
Figure 1.
Conceptual basis for targeting GB tumor cell networks through therapeutic strategies currently being evaluated in clinical trials. MFA targets the structural integrity of GB networks by inhibiting Cx43, disrupting gap junction communication between glioma cells, and reducing TM formation. TMs are critical for GB resistance to therapy by providing essential metabolic and structural support. Perampanel disrupts the synaptic connections between glioma cells and neurons via AMPA receptors on TMs. This disruption is intended to affect neuronal signaling that promotes tumor growth and invasion. Troriluzole, gabapentin, sulfasalazine, and memantine target glutamate metabolism and signaling to address the high levels of glutamate secreted by GB cells. This approach seeks to alter neuroglial network activity that promotes tumor spread and neuronal hyperexcitability. (Adapted with permission from an illustration created by Sheena Gingerich.)
Table 1.
Current clinical trials investigating network-targeting therapies in GB.
| Trial name | Drug | Target | Phase | Diagnosis of eligible patients | Number of patients | Endpoint |
|---|---|---|---|---|---|---|
| MecMeth trial/NOA-24 | MFA | Gap junction |
|
GB with first relapse of MGMT-meth. | 6–122 × 30 |
|
| PerSurge trial/NOA-30 | Perampanel | AMPA receptor |
|
Progressive or recurrent GB | 2 × 33 | Single-cell transcriptomic analysis of resected tumor network connectivity; AI-based tumor growth assessment in preresection T2/FLAIR MRI |
| GLUGLIO trial | Gabapentin, sulfasalazine, and memantine | Glutamate signaling |
|
Newly diagnosed GB | 2 × 60 | Primary endpoint: PFS at 6 months; secondary endpoint: OS and seizure-free survival; QoL (patients and caregivers, symptom burden, and cognitive function) |
| GBM AGILE trial | Troriluzole | Glutamate signaling |
|
Newly diagnosed or recurrent GB | OS |
Summary of ongoing clinical trials investigating network-targeting therapies in GB, highlighting their therapeutic agents, molecular targets, trial phases, patient eligibility, and key endpoints.
Abbreviations: AI, artificial intelligence; MGMT-meth, O6-methylguanine-DNA methyltransferase–methylated; NOA, Neuro-Oncology Working Group; OS, overall survival; PFS, progression-free survival; QoL, quality of life.
Targeting TMs and Gap Junctions in GB Therapy
MFA, originally developed as an NSAID, has emerged as a promising candidate for TM-targeted therapy. Preclinical studies have shown that MFA disrupts gap junction–mediated communication by inhibiting Cx43 (59–61), which is critical for cytosolic exchange between GB tumor cells (47), and inhibits the formation of the neuron–glioma synapses (Fig. 1; ref. 17). By disrupting this communication, MFA undermines the tumor’s ability to resist treatment. In addition, MFA reduces the formation of TMs, compromising the structural network that supports tumor survival and repair mechanisms (47).
MFA’s ability to sensitize GB cells to temozolomide (TMZ) further enhances its therapeutic potential. In preclinical models, MFA enhances the efficacy of TMZ by weakening the tumor’s protective network architecture. As MFA is already approved for clinical use as an NSAID, its repurposing for the treatment of GB represents a viable and innovative strategy.
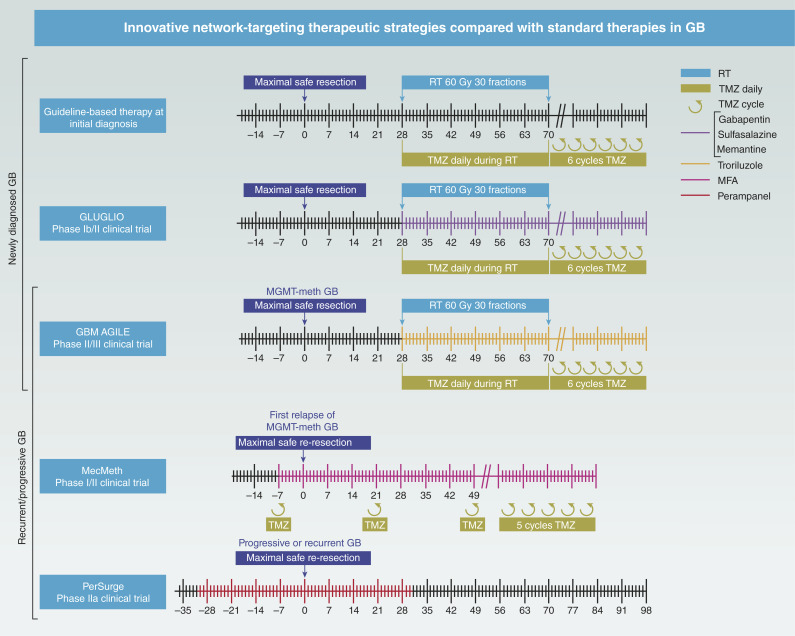
The ongoing MecMeth/NOA-24 trial is a phase I/II study designed to evaluate the safety and efficacy of combining MFA with the standard dose of TMZ in patients with first relapse of progressive O6-methylguanine-DNA methyltransferase–methylated GB (Fig. 2; Table 1; ref. 62). The trial started with a phase I component to evaluate the safety and feasibility of the combination therapy. In this phase, two dose levels of MFA were tested in combination with TMZ to determine the optimal dose for further evaluation. In the phase II component, patients are randomized into two study arms. This trial explores whether MFA’s inhibition of TM–mediated resistance mechanisms can improve patient outcomes by disrupting the tumor’s structural defenses (62).
Figure 2.
Timeline of current clinical network-targeting trials in GB therapy. This figure illustrates the timeline and design of major clinical trials investigating novel therapeutic strategies for GB. The standard-of-care regimen for newly diagnosed GB consists of fractionated radiotherapy (2 Gy/day, 5 days per week, for 6 weeks) combined with daily TMZ (75 mg/m2). Following radiotherapy, six cycles of adjuvant TMZ (150–200 mg/m2; days 1–5 of a 28-day cycle) are administered. The GLUGLIO trial compares chemoradiotherapy plus glutamate-modulating agents (gabapentin, sulfasalazine, and memantine) with chemoradiotherapy alone in patients with newly diagnosed GB. The study’s primary endpoint is progression-free survival at 6 months, with secondary endpoints including overall survival, seizure control, quality of life, and cognitive function. GBM AGILE is an adaptive global platform trial designed to evaluate multiple investigational therapies for newly diagnosed and recurrent GB, with the goal of identifying treatments that improve overall survival. The study continuously generates real-time evidence and facilitates collaboration between industry, academia, and healthcare systems. Among the therapeutic agents under investigation is troriluzole, an orally administered small molecule that modulates glutamate signaling. The MecMeth study investigates the use of MFA in patients diagnosed with GB. Patients receive MFA 7–10 days prior to surgery, allowing assessment of its blood–brain barrier permeability and tumor penetration. The trial also explores its impact on GB cellular dynamics and TM-based network connectivity. The PerSurge study is a controlled clinical trial that evaluates the perioperative use of perampanel in patients with progressive GB. Patients receive the drug or placebo before and after resection, with the aim of assessing its effect on tumor cell networks and imaging-based tumor progression. Control groups, clinical and instrumental diagnostics, and the exact drug dosages have been omitted for clarity. MGMT-meth, O6-methylguanine-DNA methyltransferase–methylated; RT, radiotherapy.
Targeting Neuron–Glioma Synapses in GB
The PerSurge trial is based on the discovery that AMPA receptor–mediated neuron–glioma synapses play a critical role in GB progression. GB cells form postsynaptic connections with neurons via AMPA receptors, primarily located on TMs, allowing neurons to transmit growth and invasion signals to the tumor (16–18, 36). In preclinical studies, inhibition of AMPA receptor activity with the FDA/European Medicines Agency–approved antiepileptic drug perampanel has been shown to reduce GB cell proliferation, invasion, and TM formation. Perampanel’s noncompetitive inhibition of AMPA receptors effectively disrupts the neuron–tumor synaptic connections that drive GB network connectivity and tumor spread (Fig. 1; refs. 16, 17, 36).
Perampanel has several pharmacokinetic advantages, including high blood–brain barrier penetration and a long half-life, making it a suitable candidate for the treatment of GB. Clinically, perampanel has already demonstrated efficacy in controlling seizures in patients with brain tumor–related epilepsy, further supporting its potential use in GB (63–65).
The PerSurge trial is a multicenter, phase IIa clinical and translational study evaluating the effects of perampanel in progressive or recurrent GB. It is a two-arm, double-blind, parallel-group superiority trial with patients randomized 1:1 to perampanel or placebo (Table 1). Each patient will undergo a 60-day treatment and observation period beginning 30 days prior to planned surgical resection (which is not part of the study procedures; Fig. 2). Only patients with a safe waiting period will be enrolled, and a safety MRI will be performed prior to resection. The PerSurge trial will evaluate the dual antitumor and antiepileptic effects of perampanel, potentially paving the way for a new treatment approach targeting neuron–tumor networks in GB (66).
Targeting Glutamatergic Signaling in GB
The GLUGLIO trial aims to evaluate the impact of combining gabapentin, sulfasalazine, and memantine with standard chemoradiotherapy on patient outcomes in GB (Fig. 1; Table 1; ref. 67). This approach is based on recent insights into the role of glutamate in GB progression.
Gabapentin inhibits glutamate synthesis by blocking BCAT-1 and reduces neuroglial network connectivity by inhibiting the thrombospondin receptor α2δ-1 (48–50). Its anticonvulsant properties may be particularly beneficial given the role of epilepsy in GB progression (40, 41). Sulfasalazine inhibits glutamate release through the system xc antiporter, and early studies have demonstrated reduced peritumoral glutamate levels following its administration (51). Memantine blocks NMDA-type glutamate receptors, which may prevent the formation of synapses between neurons and glioma cells, thereby reducing tumor cell invasion and neuroglial signaling (39, 52).
GLUGLIO is a multicenter, parallel-group, open-label, phase Ib/II trial comparing the combination of gabapentin, sulfasalazine, memantine, and chemoradiotherapy (arm A) with chemoradiotherapy alone (arm B) in patients with newly diagnosed GB. The trial uses 1:1 randomization (67). This study will evaluate whether pharmacologic disruption of glutamatergic pathways can inhibit glioma progression and improve patient outcomes. The combination of these three drugs targets different aspects of glutamate signaling and may provide a synergistic therapeutic effect (Fig. 1). The results of this study may inform future treatment strategies and clarify the potential role of antiglutamatergic therapies in GB.
The GBM AGILE trial, an adaptive phase II/III platform study, is evaluating innovative treatments for newly diagnosed and recurrent GB (Fig. 2; Table 1; ref. 68). Among these, troriluzole, a third-generation prodrug of riluzole, is being evaluated for its glutamate-modulating properties (Fig. 1). Troriluzole is given alongside standard radiotherapy and TMZ during a 6-week chemoradiotherapy phase, with dosing continued during a short rest period. Maintenance therapy includes TMZ for up to six cycles in combination with troriluzole, after which troriluzole monotherapy is continued (Fig. 2). Troriluzole reduces synaptic glutamate by enhancing astrocytic reuptake (69–71) and inhibiting release via sodium and calcium channel modulation (72–74).
By incorporating troriluzole into the treatment of GB, the trial aims to exploit these mechanisms to limit glioma progression and improve outcomes (Fig. 1). The results of GBM AGILE will help to clarify the role of glutamate modulation in GB therapy.
Repurposing Existing Drugs as a Fast-Track Strategy for GB Therapy
The process of repurposing existing drugs provides a streamlined pathway for developing novel GB therapies (75–77). This is due to the fact that repurposing allows for the leveraging of established safety profiles, pharmacokinetic properties, and, in many cases, the demonstrated ability to cross the blood–brain barrier. This significantly reduces both time and cost (78).
Nevertheless, the absence of financial incentives for off-patent drugs often precludes large-scale industry sponsorship. Trials investigating repurposed agents are predominantly investigator-initiated and rely on public funding sources. For instance, the PerSurge and MecMeth trials are supported by the German Federal Ministry of Education and Research, whereas the GLUGLIO trial receives funding from the Swiss National Science Foundation. GBM AGILE is conducted by the Global Coalition for Adaptive Research, a nonprofit organization initially supported by patient advocacy groups, including the National Foundation for Cancer Research, the Asian Fund for Cancer Research, and the National Brain Tumor Society. GBM AGILE collaborates with pharmaceutical partners such as Bayer, Kintara Therapeutics, Kazia Therapeutics, Vigeo Therapeutics, Biohaven Pharmaceuticals, and Polaris Pharmaceuticals.
Continued investment in investigator-led trials is essential to fully harness the therapeutic potential of repurposed drugs, offering new avenues for GB treatment.
Conclusion
Morphologic and structural insights into GB, particularly the role of TM-based networks, have provided a strong rationale for clinical trials investigating novel therapeutic strategies. MFA, perampanel, and the glutamate inhibitors troriluzole, gabapentin, sulfasalazine, and memantine each offer unique approaches to potentially disrupt GB network integrity, reduce treatment resistance, and improve patient outcomes. The ongoing trials will advance our understanding of GB biology.
In addition, the GB network provides additional targets for future therapeutic intervention. One promising concept is to use the network as a “Trojan horse” for drug delivery and propagation within the tumor cell network, thereby increasing tumor cell specificity and minimizing damage to healthy tissues. By exploiting the interconnectivity of tumor cells, this approach could improve drug delivery and efficacy.
In addition, emerging molecular targets within the GB network, such as KCa3.1, CHI3L1, and GAP43, are gaining attention for their potential role in maintaining tumor cell communication and network stability. Inhibition of these targets may further weaken tumor resistance and provide new avenues for treatment. Future research focusing on these and other network-interfering components may lead to the development of next-generation therapies aimed at dismantling the intricate and resistant architecture of GB.
Acknowledgments
M. Ratliff received funding from the Olympia Morata-Program, Heidelberg University. M. Ratliff’s research is also supported by funding from the 2024 American Association for Cancer Research–Novocure Cancer Research Grant, grant number 24-60-62-RATL. M. Ratliff and F. Winkler received funding from the German Research Foundation DFG (SFB 1389).
Authors’ Disclosures
M. Ratliff reports grants from Heidelberg University, Olympia Morata-Program, the American Association for Cancer Research–Novocure Tumor-Treating Fields Research Award (24-60–62-RATL), and the German Research Foundation DFG (SFB 1389) outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Ostrom QT, Shoaf ML, Cioffi G, Waite K, Kruchko C, Wen PY, et al. National-level overall survival patterns for molecularly-defined diffuse glioma types in the United States. Neuro Oncol 2023;25:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drumm MR, Dixit KS, Grimm S, Kumthekar P, Lukas RV, Raizer JJ, et al. Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas. Neuro Oncol 2020;22:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sahm F, Capper D, Jeibmann A, Habel A, Paulus W, Troost D, et al. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol 2012;69:523–6. [DOI] [PubMed] [Google Scholar]
- 4. Garofano L, Migliozzi S, Oh YT, D’Angelo F, Najac RD, Ko A, et al. Pathway-based classification of glioblastoma uncovers a mitochondrial subtype with therapeutic vulnerabilities. Nat Cancer 2021;2:141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 2019;178:835–49.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duhamel M, Drelich L, Wisztorski M, Aboulouard S, Gimeno J-P, Ogrinc N, et al. Spatial analysis of the glioblastoma proteome reveals specific molecular signatures and markers of survival. Nat Commun 2022;13:6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang L-B, Karpova A, Gritsenko MA, Kyle JE, Cao S, Li Y, et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 2021;39:509–28.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richards LM, Whitley OKN, MacLeod G, Cavalli FMG, Coutinho FJ, Jaramillo JE, et al. Gradient of Developmental and Injury Response transcriptional states defines functional vulnerabilities underpinning glioblastoma heterogeneity. Nat Cancer 2021;2:157–73. [DOI] [PubMed] [Google Scholar]
- 9. Hausmann D, Hoffmann DC, Venkataramani V, Jung E, Horschitz S, Tetzlaff SK, et al. Autonomous rhythmic activity in glioma networks drives brain tumour growth. Nature 2023;613:179–86. [DOI] [PubMed] [Google Scholar]
- 10. Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J, et al. Brain tumour cells interconnect to a functional and resistant network. Nature 2015;528:93–8. [DOI] [PubMed] [Google Scholar]
- 11. Weil S, Osswald M, Solecki G, Grosch J, Jung E, Lemke D, et al. Tumor microtubes convey resistance to surgical lesions and chemotherapy in gliomas. Neuro Oncol 2017;19:1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hai L, Hoffmann DC, Wagener RJ, Azorin DD, Hausmann D, Xie R, et al. A clinically applicable connectivity signature for glioblastoma includes the tumor network driver CHI3L1. Nat Commun 2024;15:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung E, Osswald M, Blaes J, Wiestler B, Sahm F, Schmenger T, et al. Tweety-homolog 1 drives brain colonization of gliomas. J Neurosci 2017;37:6837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osswald M, Solecki G, Wick W, Winkler F. A malignant cellular network in gliomas: potential clinical implications. Neuro Oncol 2016;18:479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sontheimer H. Brain cancer: tumour cells on neighbourhood watch. Nature 2015;528:49–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019;573:532–8. [DOI] [PubMed] [Google Scholar]
- 17. Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019;573:539–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venkataramani V, Yang Y, Schubert MC, Reyhan E, Tetzlaff SK, Wißmann N, et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022;185:2899–917.e31. [DOI] [PubMed] [Google Scholar]
- 19. Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, et al. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell 2015;161:803–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venkatesh HS, Tam LT, Woo PJ, Lennon J, Nagaraja S, Gillespie SM, et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 2017;549:533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ratliff M, Karimian-Jazi K, Hoffmann DC, Rauschenbach L, Simon M, Hai L, et al. Individual glioblastoma cells harbor both proliferative and invasive capabilities during tumor progression. Neuro Oncol 2023;25:2150–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cuddapah VA, Turner KL, Seifert S, Sontheimer H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J Neurosci 2013;33:1427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jung E, Alfonso J, Osswald M, Monyer H, Wick W, Winkler F. Emerging intersections between neuroscience and glioma biology. Nat Neurosci 2019;22:1951–60. [DOI] [PubMed] [Google Scholar]
- 24. Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol 2009;10:332–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marín O, Valiente M, Ge X, Tsai L-H. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol 2010;2:a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martini FJ, Valiente M, López Bendito G, Szabó G, Moya F, Valdeolmillos M, et al. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development 2009;136:41–50. [DOI] [PubMed] [Google Scholar]
- 27. McFerrin MB, Turner KL, Cuddapah VA, Sontheimer H. Differential role of Ik and BK potassium channels as mediators of intrinsic and extrinsic apoptotic cell death. Am J Physiol Cell Physiol 2012;303:C1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tombal B, Denmeade SR, Gillis J-M, Isaacs JT. A supramicromolar elevation of intracellular free calcium ([Ca(2+)](i)) is consistently required to induce the execution phase of apoptosis. Cell Death Differ 2002;9:561–73. [DOI] [PubMed] [Google Scholar]
- 29. Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, et al. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem 2014;289:1345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wick A, Wick W, Hirrlinger J, Gerhardt E, Dringen R, Dichgans J, et al. Chemotherapy-induced cell death in primary cerebellar granule neurons but not in astrocytes: in vitro paradigm of differential neurotoxicity. J Neurochem 2004;91:1067–74. [DOI] [PubMed] [Google Scholar]
- 31. Jung E, Osswald M, Ratliff M, Dogan H, Xie R, Weil S, et al. Tumor cell plasticity, heterogeneity, and resistance in crucial microenvironmental niches in glioma. Nat Commun 2021;12:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature 1998;393:440–2. [DOI] [PubMed] [Google Scholar]
- 33. Barabasi AL, Albert R. Emergence of scaling in random networks. Science 1999;286:509–12. [DOI] [PubMed] [Google Scholar]
- 34. Smedler E, Uhlén P. Frequency decoding of calcium oscillations. Biochim Biophys Acta 2014;1840:964–9. [DOI] [PubMed] [Google Scholar]
- 35. Robert SM, Buckingham SC, Campbell SL, Robel S, Holt KT, Ogunrinu-Babarinde T, et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med 2015;7:289ra86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Venkataramani V, Schneider M, Giordano FA, Kuner T, Wick W, Herrlinger U, et al. Disconnecting multicellular networks in brain tumours. Nat Rev Cancer 2022;22:481–91. [DOI] [PubMed] [Google Scholar]
- 37. Winkler F, Venkatesh HS, Amit M, Batchelor T, Demir IE, Deneen B, et al. Cancer neuroscience: state of the field, emerging directions. Cell 2023;186:1689–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weaver AK, Bomben VC, Sontheimer H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 2006;54:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med 2001;7:1010–5. [DOI] [PubMed] [Google Scholar]
- 40. Mastall M, Wolpert F, Gramatzki D, Imbach L, Becker D, Schmick A, et al. Survival of brain tumour patients with epilepsy. Brain 2021;144:3322–7. [DOI] [PubMed] [Google Scholar]
- 41. Tobochnik S, Dorotan MKC, Ghosh HS, Lapinskas E, Vogelzang J, Reardon DA, et al. Glioma genetic profiles associated with electrophysiologic hyperexcitability. Neuro Oncol 2024;26:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buckingham SC, Campbell SL, Haas BR, Montana V, Robel S, Ogunrinu T, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med 2011;17:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res 1999;59:4383–91. [PubMed] [Google Scholar]
- 44. Campbell SC, Muñoz-Ballester C, Chaunsali L, Mills WA 3rd, Yang JH, Sontheimer H, et al. Potassium and glutamate transport is impaired in scar-forming tumor-associated astrocytes. Neurochem Int 2020;133:104628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 2007;67:9463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci 1999;19:10767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schneider M, Vollmer L, Potthoff A-L, Ravi VM, Evert BO, Rahman MA, et al. Meclofenamate causes loss of cellular tethering and decoupling of functional networks in glioblastoma. Neuro Oncol 2021;23:1885–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krishna S, Choudhury A, Keough MB, Seo K, Ni L, Kakaizada S, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature 2023;617:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tönjes M, Barbus S, Park YJ, Wang W, Schlotter M, Lindroth AM, et al. BCAT1 promotes cell proliferation through amino acid catabolism in gliomas carrying wild-type IDH1. Nat Med 2013;19:901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009;139:380–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia 2001;15:1633–40. [DOI] [PubMed] [Google Scholar]
- 52. Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature 2019;573:526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monje M, Borniger JC, D’Silva NJ, Deneen B, Dirks PB, Fattahi F, et al. Roadmap for the emerging field of cancer neuroscience. Cell 2020;181:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pan C, Winkler F. Insights and opportunities at the crossroads of cancer and neuroscience. Nat Cell Biol 2022;24:1454–60. [DOI] [PubMed] [Google Scholar]
- 55. Venkataramani V, Yang Y, Ille S, Suchorska B, Loges S, Tost H, et al. Cancer neuroscience of brain tumors: from multicellular networks to neuroscience-instructed cancer therapies. Cancer Discov 2025;15:39–51. [DOI] [PubMed] [Google Scholar]
- 56. Murphy SF, Varghese RT, Lamouille S, Guo S, Pridham KJ, Kanabur P, et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res 2016;76:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Potthoff A-L, Heiland DH, Evert BO, Almeida FR, Behringer SP, Dolf A, et al. Inhibition of gap junctions sensitizes primary glioblastoma cells for temozolomide. Cancers (Basel) 2019;11:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schneider M, Potthoff A-L, Evert BO, Dicks M, Ehrentraut D, Dolf A, et al. Inhibition of intercellular cytosolic traffic via gap junctions reinforces lomustine-induced toxicity in glioblastoma independent of MGMT promoter methylation status. Pharmaceuticals (Basel) 2021;14:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harks EG, de Roos AD, Peters PH, de Haan LH, Brouwer A, Ypey DL, et al. Fenamates: a novel class of reversible gap junction blockers. J Pharmacol Exp Ther 2001;298:1033–41. [PubMed] [Google Scholar]
- 60. Manjarrez-Marmolejo J, Franco-Pérez J. Gap junction blockers: an overview of their effects on induced seizures in animal models. Curr Neuropharmacol 2016;14:759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ning N, Wen Y, Li Y, Li J. Meclofenamic acid blocks the gap junction communication between the retinal pigment epithelial cells. Hum Exp Toxicol 2013;32:1164–9. [DOI] [PubMed] [Google Scholar]
- 62. Zeyen T, Potthoff A-L, Nemeth R, Heiland DH, Burger MC, Steinbach JP, et al. Phase I/II trial of meclofenamate in progressive MGMT-methylated glioblastoma under temozolomide second-line therapy-the MecMeth/NOA-24 trial. Trials 2022;23:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. French JA, Krauss GL, Biton V, Squillacote D, Yang H, Laurenza A, et al. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology 2012;79:589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. French JA, Krauss GL, Steinhoff BJ, Squillacote D, Yang H, Kumar D, et al. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 2013;54:117–25. [DOI] [PubMed] [Google Scholar]
- 65. Krauss GL, Perucca E, Kwan P, Ben-Menachem E, Wang X-F, Shih JJ, et al. Final safety, tolerability, and seizure outcomes in patients with focal epilepsy treated with adjunctive perampanel for up to 4 years in an open-label extension of phase III randomized trials: study 307. Epilepsia 2018;59:866–76. [DOI] [PubMed] [Google Scholar]
- 66. Heuer S, Burghaus I, Gose M, Kessler T, Sahm F, Vollmuth P, et al. PerSurge (NOA-30) phase II trial of perampanel treatment around surgery in patients with progressive glioblastoma. BMC Cancer 2024;24:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mastall M, Roth P, Bink A, Fischer Maranta A, Läubli H, Hottinger AF, et al. A phase Ib/II randomized, open-label drug repurposing trial of glutamate signaling inhibitors in combination with chemoradiotherapy in patients with newly diagnosed glioblastoma: the GLUGLIO trial protocol. BMC Cancer 2024;24:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alexander BM, Ba S, Berger MS, Berry DA, Cavenee WK, Chang SM, et al. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin Cancer Res 2018;24:737–43. [DOI] [PubMed] [Google Scholar]
- 69. Brothers HM, Bardou I, Hopp SC, Kaercher RM, Corona AW, Fenn AM, et al. Riluzole partially rescues age-associated, but not LPS-induced, loss of glutamate transporters and spatial memory. J Neuroimmune Pharmacol 2013;8:1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carbone M, Duty S, Rattray M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem Int 2012;60:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu AY, Mathur R, Mei N, Langhammer CG, Babiarz B, Firestein BL. Neuroprotective drug riluzole amplifies the heat shock factor 1 (HSF1)- and glutamate transporter 1 (GLT1)-dependent cytoprotective mechanisms for neuronal survival. J Biol Chem 2011;286:2785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cheah BC, Vucic S, Krishnan AV, Kiernan MC. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr Med Chem 2010;17:1942–199. [DOI] [PubMed] [Google Scholar]
- 73. Machado-Vieira R, Manji HK, Zarate CA. The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders. Neuroscientist 2009;15:525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pittenger C, Coric V, Banasr M, Bloch M, Krystal JH, Sanacora G. Riluzole in the treatment of mood and anxiety disorders. CNS Drugs 2008;22:761–86. [DOI] [PubMed] [Google Scholar]
- 75. Abbruzzese C, Matteoni S, Signore M, Cardone L, Nath K, Glickson JD, et al. Drug repurposing for the treatment of glioblastoma multiforme. J Exp Clin Cancer Res 2017;36:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 2019;18:41–58. [DOI] [PubMed] [Google Scholar]
- 77. Corsello SM, Bittker JA, Liu Z, Gould J, McCarren P, Hirschman JE, et al. The Drug Repurposing Hub: a next-generation drug library and information resource. Nat Med 2017;23:405–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chong CR, Sullivan DJ Jr. New uses for old drugs. Nature 2007;448:645–6. [DOI] [PubMed] [Google Scholar]