Abstract
Camptothesome, a sphingomyelin (SM)-conjugated camptothecin (CPT) vesicular nanotherapeutic, addresses the poor solubility and lactone instability of CPT while enhancing drug loading, pharmacokinetics, and tumor distribution compared to CPT physically entrapped in conventional liposomes. Despite these improvements, the tumor uptake remains limited. To further enhance the tumor delivery efficiency and minimize the off-target distribution, we functionalize Camptothesome with the LinTT1 peptide, a CendR motif, which binds to overexpressed p32 proteins on tumor cell surface, initiating effective transcytosis for deep tumor penetration. Via systematic screening, the optimal peptide ratio on Camptothesome is identified. LinTT1/Camptothesome significantly increases cancer cell uptake without affecting normal cell internalization, resulting in enhanced anti-colorectal cancer cells activity. Additionally, decorating Camptothesome with the LinTT1 cell-penetrating peptide enables effective transcytosis via a Golgi-dependent intracellular trafficking mechanism, significantly improving the intratumoral delivery while reducing distribution to normal tissues. In a human HCT116 xenograft colorectal cancer (CRC) mouse model, LinTT1/Camptothesome demonstrates superior antitumor efficacy compared to both Camptothesome and Onivyde by upregulating cleaved caspase-3 and γH2AX. Our study substantiates the potential of leveraging a tumor-penetrating peptide to enhance the tumor delivery efficiency of Camptothesome, maximizing its therapeutic index for improved treatment of human CRC.
Keywords: Nanotherapeutics, Camptothesome, Tumor-homing LinTT1 Peptide, Colorectal Cancer, p32
Introduction
Lipid-derived nanotechnology has made significant strides in drug development, particularly by improving pharmacokinetics and enhancing therapeutic delivery while minimizing systemic side effects [1–6]. Liposome, known for its biocompatibility and biodegradability, is one of the most effective drug delivery platforms evidenced by over a dozen FDA-approved liposomal nanomedicines [1, 7]. Inspired by liposome, Camptothesome, an innovative nano-vesicular delivery platform comprising sphingomyelin (SM)-conjugated camptothecin (CPT) within the lipid bilayer, addresses the poor solubility, lactone instability, and severe non-specific toxicities associated with free CPT [3, 6, 8, 9]. Compared to conventional liposomes with physically loaded CPT, Camptothesome significantly improved the drug loading capacity and formulation stability. Furthermore, Camptothesome outperformed free CPT on pharmacokinetics and tumor delivery, demonstrating superior therapeutic effects to free CPT and Onivyde against CRC and triple negative breast cancer mouse models [3, 6, 8, 9].
Despite the improved tumor drug delivery by Camptothesome compared to free CPT, the overall tumor distribution and penetration remain limited. While the enhanced permeability and retention (EPR) effect facilitates the accumulation of nanotherapeutics at the periphery of diseased sites (e.g., tumors) through leaky vasculature, achieving efficient intracellular internalization and deep tissue penetration continues to pose a challenge. This is primarily due to the dense extracellular matrix and high interstitial fluid pressure, which hinder effective drug distribution and compromise the therapeutic outcomes. Therefore, developing an approach to further enhance tumor delivery is crucial to maximizing the therapeutic potential of Camptothesome in cancer therapy.
The C-end Rule (CendR) motif-neuropilin (NRP) receptor system has shown great potential in facilitating tumor-homing peptides to bind to the cellular surface receptor and trigger endocytic internalization [10, 11] (Fig. 2c). Upon cleavage of the CendR motif, R/KXXXR/K by furin-like proprotein convertase (PC), the cleaved peptide is recognized by the cognate surface receptors NRP-1 and NRP-2, which play a crucial role in mediating endocytosis, transcytosis, and intercellular trafficking [12–14]. LinTT1, a peptide belonging to the CendR motif family [15, 16], binds to p32 protein (also known as gC1qR), which is overexpressed on many cancer cells, including CRC cells, while remaining predominantly intracellular (mitochondrial protein) in normal cells [11, 17]. For example, LinTT1-coated iron oxide nanoworms targeting the p32 protein were used for treating peritoneal carcinomatosis [16], demonstrating the potential of leveraging the interaction between the LinTT1 peptide and NRP-1 to trigger the trans-tissue transport pathway, as confirmed by the optical imaging and Magnetic Resonance Imaging (MRI). Additionally, LinTT1 was proven to be the most potent CendR peptide among other CendR motifs in augmenting the accumulation of the nanoworms within breast cancer tumors [15]. Therefore, the use of LinTT1 peptide to enhance the tumor-homing ability of nanoparticles is both attractive and promising.
Figure 2. Development of peptide-decorated SM-derived CPT liposomal nanovesicle (LinTT1/Camptothesome).
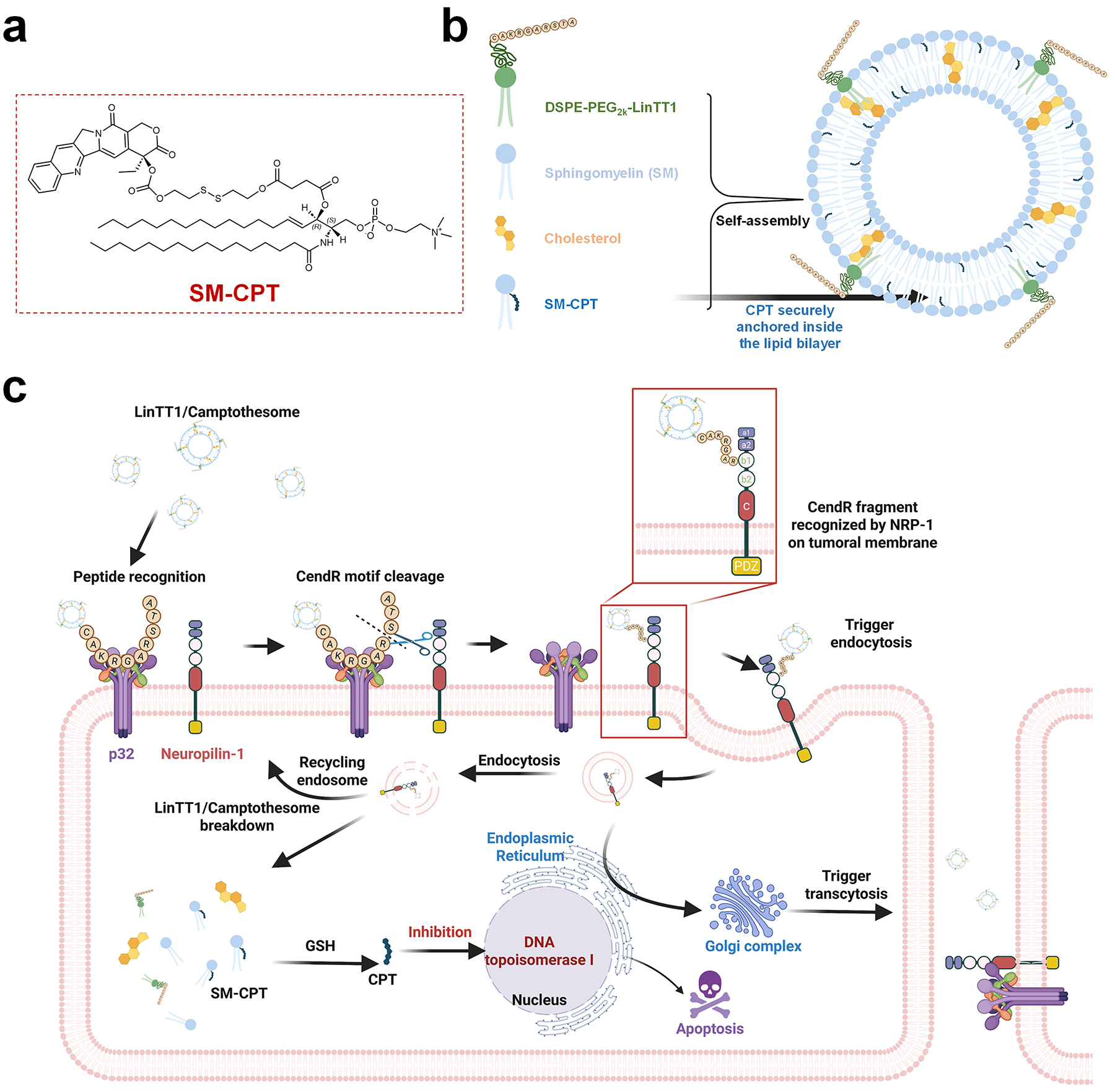
a, Chemical structure of SM-CPT [3]. b, Self-assembly into LinTT1/Camptothesome. c, Schematic mechanism of peptide-mediated Camptothesome transcytosis.
In this study, we successfully functionalized Camptothesome nanovesicles with the CendR motif via thiol-maleimide chemistry. The addition of a cysteine (C) at the N-terminus of the LinTT1 peptide enabled its chemical conjugation with DSPE-PEG2K-Maleimide, which can serve as an anchor to insert into the lipid bilayer (Fig. 2b). Through monitoring the formulation stability and intracellular uptake, we screened various peptide ratios on Camptothesome and identified that 4.3% molar ratio of LinTT1 provided the most stable nanovesicle with the greatest uptake improvement in HCT116 and CT26 CRC cells. No uptake difference was observed between Camptothesome and peptide/Camptothesome in normal Beas-2b cells, highlighting the selective targeting of LinTT1 to cancer cells. LinTT1 functionalization significantly enhanced the anticancer activity of Camptothesome, as evidenced by a lower IC50 in CRC cells compared to Camptothesome alone. The decoration of LinTT1 also improved tumor delivery and penetration by facilitating effective transcytosis via a Golgi-dependent intracellular trafficking machinery, while reducing off-target distribution to healthy tissues. In vivo, LinTT1/Camptothesome demonstrated markedly better anti-CRC efficacy compared to both Camptothesome and Onivyde in a human HCT116 xenograft CRC tumor mouse model, which was attributed to the increased levels of cleaved caspase-3 (CC-3) and phosphorylated histone H2AX (γH2AX). These findings corroborate that effectively functionalizing Camptothesome with a tumor-homing peptide can unlock its full therapeutic potential.
Materials and methods
Materials
Sphingomyelin (egg, 98%) and DSPE-PEG2K (99%) were purchased from Lipoid (Germany). (S)-(+)-camptothecin (CPT, 98%) and DSPE-PEG2K-Maleimide were purchased from BLDpharm (Shanghai, China). DSPE-PEG-Cy5.5 (99%), and cholesterol (ovine, 99%) were purchased from Avanti (Alabama, USA). Onivyde® (Ispsen Biopharmaceuticals, Inc) was acquired from Pharmacy Department, Banner-University Medical Center Tucson. DRAQ5 was purchased from Novus Biologicals. Wheat Germ Agglutinin 488 and ProLong™ Glass Antifade Mountant were purchased from ThermoFisher Scientific. Anti-CD31 antibody (PECAM-1) and Donkey Anti-Rabbit IgG H&L (Alexa Fluor® 488) were purchased from Abcam.
Cell lines
Beas-2b (ATCC, CRL-3588), CT26 (ATCC, CRL-2638) and HCT116 (ATCC, CCL-247) cell lines were materialsed from UACC; Beas-2b cells were cultured in complete DMEM medium (GIBCO,11995–065); CT26 cells were cultured in complete RPMI medium (GIBCO, 11875–093); HCT116 cells were cultured in complete McCoy 5A medium (GIBCO, 16600–082). All culture mediums were complemented with 10% FBS (GIBCO, A5256801), 100 U/mL penicillin streptomycin (GIBCO, 15140–122), and 0.5 μg/mL Amphotericin B (FisherScientific, 15-290-026), and the cell lines were cultured at 37°C in a CO2 incubator.
Animal assay
NU/J mice (The Jackson Laboratory, 5 weeks old, female) were used in this study. To ensure gender uniformity, all mice used in this study were female. The mice were housed in the Standard Individually Ventilated Caging (IVC) system to ensure a pathogen-free environment. The animal facility followed the National Institutes of Health (NIH, United States) Guide for the Care and Use of Laboratory Animals. Tumor measurements were taken using a digital caliper, with tumor size calculated using the formula: 0.5 × length × width2. According to the animal ethics guidelines of The Institutional Animal Care and Use Committee (IACUC) and animal welfare regulations, the maximum permitted tumor size was 2000 mm3, and the mice were sacrificed once the tumor volume reached to ≥ 2000 mm3, or if animals showed significant weight loss, extreme weakness, or inactivity.
The synthesis of LinTT1 peptide
The synthesis of the peptide fragments was based on solid-phase peptide synthesis through the standard Fmoc solid-phase protocols, using 2-cholotrityl chloride resin (0.5 mmol g−1 loading) at a 0.35 mmol synthesis scale. To initiate the synthesis of LinTT1, 2-cholotrityl chloride resin was initially swollen in DMF for 2h, followed by the coupling of all the amino acids (CAKRGARSTA) beginning from the C-terminus (Alaine) coupling onto the resin with amino acids/PyBOP/DIPEA at a ratio of 3:3:6 (mmol:mmol:mmol) in DMF for 4 hours. The coupling reaction was repeated and followed by Fmoc removal with 20% piperidine. Each coupling and Fmoc-removal step were tested by Kaiser test kit (Sigma-Aldrich, 60017), using manufacturer’s protocol, to confirm the addition/removal of Fmoc. Finally, LinTT1 was cleaved from the resin using a mixture of TFA/TIS/H2O at a ratio of 95:2.5:2.5 (v:v:v). The crude final product was obtained as precipitates in cold diethyl ether. The pure final product was separated by reverse-phase HPLC purification and confirmed by High-resolution mass spectrometry (Supplementary Fig. 1a), and the pure LinTT1 was lyophilized and stored at 4°C for future use.
The synthesis of DSPE-PEG2K-LinTT1
The LinTT1 peptide was chemically conjugated with DSPE-PEG2K-Maleimide (BLDpharm, BD01110439) by a thiol-maleimide reaction. LinTT1 peptide was initially dissolved in water and reduced with TCEP (10 eq.) and then mixed with DSPE-PEG2K-Maleimide (5 eq.) under a nitrogen atmosphere. The reaction was stirred at room temperature for 4 hours and at 4°C for 20 hours, followed by dialysis to remove excess DSPE-PEG2K-Maleimide, and the final product was confirmed by MALDI-FTICR (Supplementary Fig. 1b). The pure DSPE-PEG2K-LinTT1 was lyophilized and stored at 4°C for future use.
Preparation of LinTT1/Camptothesome
The synthesis of SM-CPT was conducted according to our previous methods [3]. The preparation of LinTT1/Camptothesome was followed by the thin-film hydration technique. Briefly, SM, SM-CPT, and cholesterol were dissolved in ethanol, and DSPE-PEG2K-LinTT1 was dissolved in chloroform, as shown in the molar ratio in Fig. 3b, and the mixture was added into a round-bottom flask. The mixture was evaporated under ultra-high-pressure vacuum (MaximaDry, Fisherband) with a rotary evaporator (RV 10 digital, IKA®) overnight to completely remove organic residues, leaving a thin layer of lipid film in the round-bottom flask. A 5% Dextrose was added into the round-bottom flask to hydrate the film at 50°C for 30 mins. The solution was then sonicated under a 3/2s on/off pulse at a power output of 60 W (VCX130, Sonics & Materials Inc.) for 12 mins. The size, zeta potential, and PDI of LinTT1/Camptothesome were determined by dynamic light scatter (DLS).
Figure 3. Development and optimization of peptide-decorated Camptothesome.
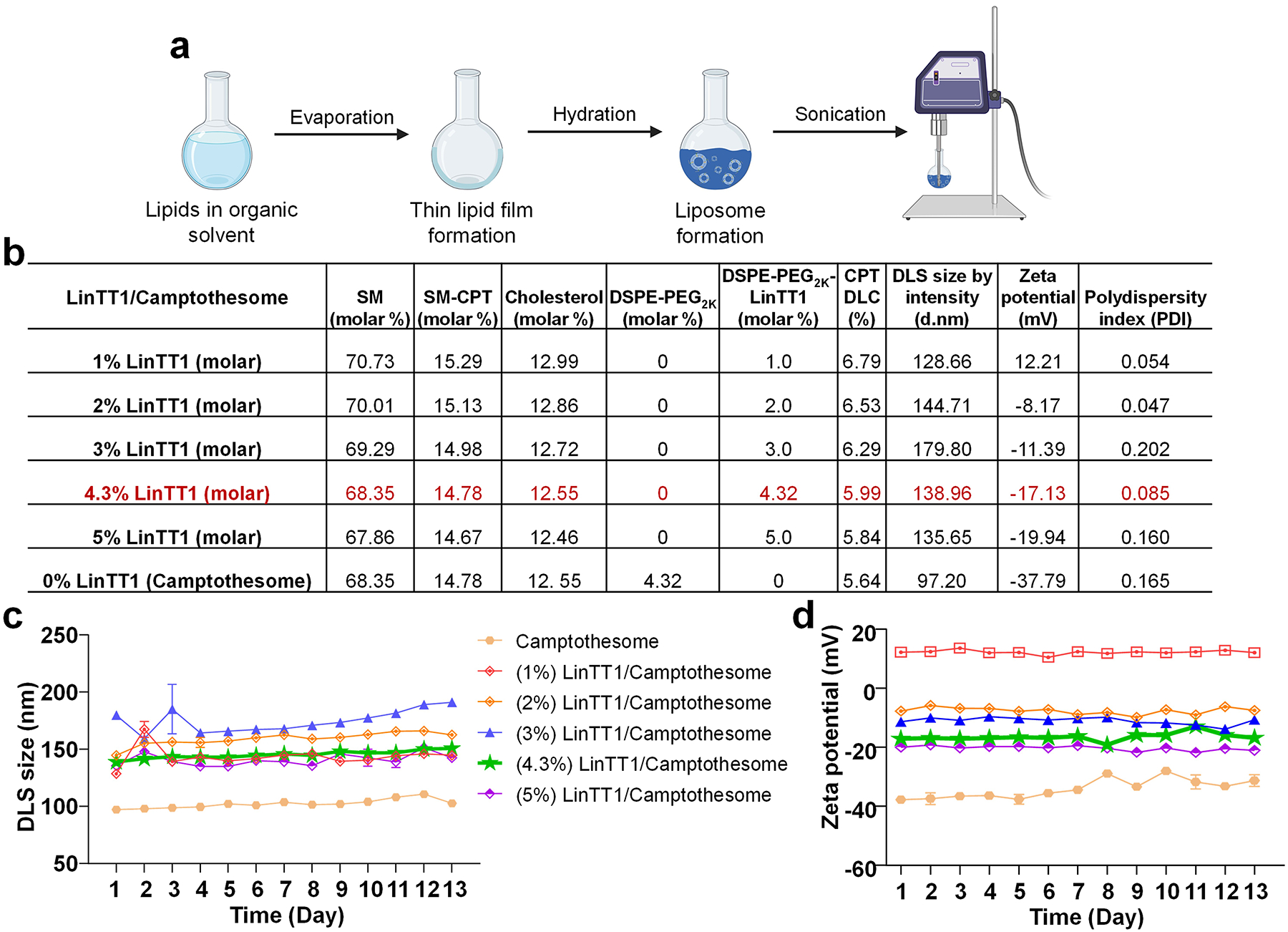
a, LinTT1/Camptothesome is synthesized by thin-film hydration method, followed with probe sonication, as previously established [3, 6]. b, A table listing the physicochemical characterizations of LinTT1/Camptothesome comprised of SM/SM-CPT/Cholesterol/DSPE-PEG2K/DSPE-PEG2K-LinTT1 with different molar ratios. DLS, dynamic light scattering; d.nm, diameter value in nanometers. Data in DLS size by intensity, zeta potential, and polydispersity index are expressed as mean (n=3). Monitoring the DLS size (c) and zeta potential (d) of Camptothesome with different molar ratios of peptide in 5% Dextrose at 4°C. Data in c and d are expressed as mean ± SD (n=3).
In vitro cytotoxicity
3×103 Beas-2b, CT26, and HCT116 cells were plated per well in a 96-well plate and incubated overnight. The medium was then aspirated and exchanged for fresh complete medium containing Camptothesome or LinTT1/Camptothesome (in CT26, HCT116, and Beas-2b cells, drug concentrations: 100, 50, 25, 5, 1, 0.5, 0.1, 0.05, 0.01 μM (in eq. to Log(drug concentration): 2, 1.69897, 1.39794, 0.69897, 0, −0.30103, −0.60206, −1.30103, −2, respectively. 10 μL of MTT solution was added to each well, and after 4 hours of incubation, absorbance in each well was read at 490 nm using a SpectraMax M3 reader (SoftMax Pro (v.7.1.0), Molecular Devices).
In vitro flow cytometry
1×105 HCT116 cells were plated per well in a 12-well plate and incubated overnight. The medium was then aspirated and exchanged for fresh complete medium containing Camptothesome or LinTT1/Camptothesome at 50 μg CPT/mL for 3 hours. The cells were trypsinized and washed with chilled PBS twice, and 400 μL of staining buffer (eBioscience, #00-4222-26) was added to resuspend the cells. Finally, intracellular uptake was analyzed using a BD FACSCanto™ II flow cytometer (BD Biosciences).
Intracellular uptake
1×106 HCT116 cells were seeded on coverslips and incubated overnight. The medium was then aspirated and exchanged for fresh complete medium containing Camptothesome or LinTT1/Camptothesome at eq. dose of CPT (50 μg/mL) for 3 hours. Coverslips were washed with chilled PBS twice and stained with DRAQ5 (Novus Biologicals, NBP2–81125) and Wheat Germ Agglutinin 488 (ThermoFisher Sci, W11261) for 30 mins at room temperature. Finally, the cells were fixed with 4% paraformaldehyde for 30 mins at room temperature, and the coverslips were mounted onto the slide with ProLong™ Glass Antifade Mountant (ThermoFisher Sci, P36982). The coverslips were visualized with a Zeiss LSM880 inverted microscope (Zen Blue software (v. 3.4.91.00000)) at the University of Arizona Imaging Cores – Optical Core Facility (RRID:SCR_023355).
Intercellular transcytosis
1×106 HCT116 cells were seeded on coverslips (i), (ii), (iii) and incubated overnight. The medium was then aspirated and exchanged for fresh complete medium containing Camptothesome or LinTT1/Camptothesome at eq. dose of CPT (50 μg/mL) for 3 hours. Coverslip (i) was washed with chilled PBS twice and stained with DRAQ5 for 30 mins at room temperature, and coverslip (i) was transferred to a plate with fresh complete medium along with coverslip (ii) for 12 hours. The same steps were repeated for coverslip (iii). Finally, the cells were fixed with 4% paraformaldehyde for 30 mins at room temperature, and the coverslips were mounted onto the slide with ProLong™ Glass Antifade Mountant. The coverslips were visualized with a Zeiss LSM880 inverted microscope.
Intracellular trafficking
1×106 HCT116 cells were seeded on coverslips and incubated overnight. Cells were pretreated with 50 μM of Exo1 (MedChemExpress, HY-112670) for 1 hour at 37 °C and then exchanged with fresh complete medium containing Camptothesome or LinTT1/Camptothesome at eq. dose of CPT (50 μg/mL) for 3 hours. Coverslips were washed with chilled PBS twice and stained with DRAQ5 for 30 mins at room temperature, and applied Golgi-ID Green assay kit (Enzo, #ENZ-51028-K100) according to the manufacturer’s protocol. Finally, the cells were fixed with 4% paraformaldehyde for 30 mins at room temperature, and the coverslips were mounted onto the slide with ProLong™ Glass Antifade Mountant. The coverslips were visualized with a Zeiss LSM880 inverted confocal microscope.
Efficacy study
HCT116 cells (5×106) in 100 μL of serum-free medium were subcutaneously injected into NU/J mice (n=5). When tumors grew to ~100 mm3 in size on day 6, the mice received a single intravenous injection of 5% dextrose (vehicle control), free CPT (5 mg/kg at maximum tolerated dose(MTD), in a solution of 10% Tween 80/0.9% NaCl (9:1) under sonication for 20 mins), Onivyde (33.7 mg irinotecan/kg), Camptothesome or LinTT1/Camptothesome at equivalent 20 mg CPT/kg. Mice were photographed on day 33, and all mice were sacrificed; livers and kidneys were collected for haematoxylin & eosin (HE) staining; and intestines were collected to assess mucositis in GI tract by periodic acid-Schiff (PAS) reaction and counterstained with haematoxylin; and tumors were collected for immunohistochemical (IHC) staining with γ-H2AX (DNA damage) and cleaved caspase-3 (CC-3, apoptosis), respectively. The slides were imaged using a Leica DMI6000 fluorescence microscope (Leica Application Suite X (v. 4.2.0.23645)) at the University of Arizona Imaging Cores – Optical Core Facility.
Biodistribution study
HCT116 cells (5×106) in 100 μL of serum-free medium were subcutaneously injected into NU/J mice (n=3). When tumors grew to ~300 mm3 in size, the mice received a single intravenous injection of Onivyde (33.7 mg irinotecan/kg), Camptothesome or LinTT1/Camptothesome at equivalent 20 mg CPT/kg. 48 hours after drug administration, tumors and major organs (heart, liver, spleen, lung, and kidneys) were collected and homogenized in acidified methanol (0.075 M HCl, 900 μL/100 mg tissue). Supernatants from organ samples were analyzed for their drug content via HPLC.
Visualization of tumor distribution and deep-tumor penetration
To visualize LinTT1/Camptothesome tumor delivery, DSPE-PEG-Cy5.5 (vehicle control), Cy5.5/Camptothesome (equivalent of 20 mg CPT/kg), or Cy5.5/LinTT1/Camptothesome (equivalent of 20 mg CPT/kg) were intravenously injected into the subcutaneous HCT116 tumor-bearing mice (n=3, tumor size: ~300mm3). At 48 hours, tumors and major organs (Heart, liver, spleen, lung, and kidney) were dissected and imaged under fluorescence channel via the Lago optical imager. The results were analyzed by Aura 64-bit analysis software (v. 4.0.8). To investigate deep tumor penetration, tumors were sectioned and processed for immunofluorescence staining. The blood vessels were stained with PECAM1 antibody (ab28364, Abcam), followed by an Alexa Flour 488-conjugated secondary antibody (ab150073, Abcam). Tumor cell nuclei were stained with DAPI. The slides were visualized using a Zeiss LSM880 inverted confocal microscope.
Statistical analysis
The level of significance in all statistical analyses was set at P<0.05. Data are presented as a mean ± s.d. and were analyzed using one-way ANOVA (for 3 or more groups) followed by Tukey’s multiple comparisons test, and two-tailed, unpaired Student’s t-test (for 2 groups) using Prism 8.0 (GraphPad Software).
Results and discussion
Synthesis of tumor penetrating peptide, LinTT1
To obtain the tumor-homing CendR motif, LinTT1 [15, 16], we utilized the standard solid-phase peptide synthesis (SPPS) method (Fig. 1a). Briefly, the designed peptide sequence was sequentially coupled onto 2-cholortrityl chloride resin starting from the C-terminus, with a Kaiser test performed after each deprotection and amino acid coupling step. The successful synthesis of LinTT1 was confirmed using high-resolution electrospray ionization-liquid chromatography mass spectrometry (HR ESI-LCMS, Supplementary Fig. 1a). To further construct DSPE-PEG2K-LinTT1 for efficient anchoring into the lipid bilayer, LinTT1 was chemically modified using DSPE-PEG2K-Maleimide via the cysteine residue through thiol-maleimide chemistry (Fig. 1b). The DSPE-PEG2K-LinTT1 was verified by MALDI-FTICR (Supplementary Fig. 1b).
Figure 1. Synthesis mechanism of DSPE-PEG2K-LinTT1.
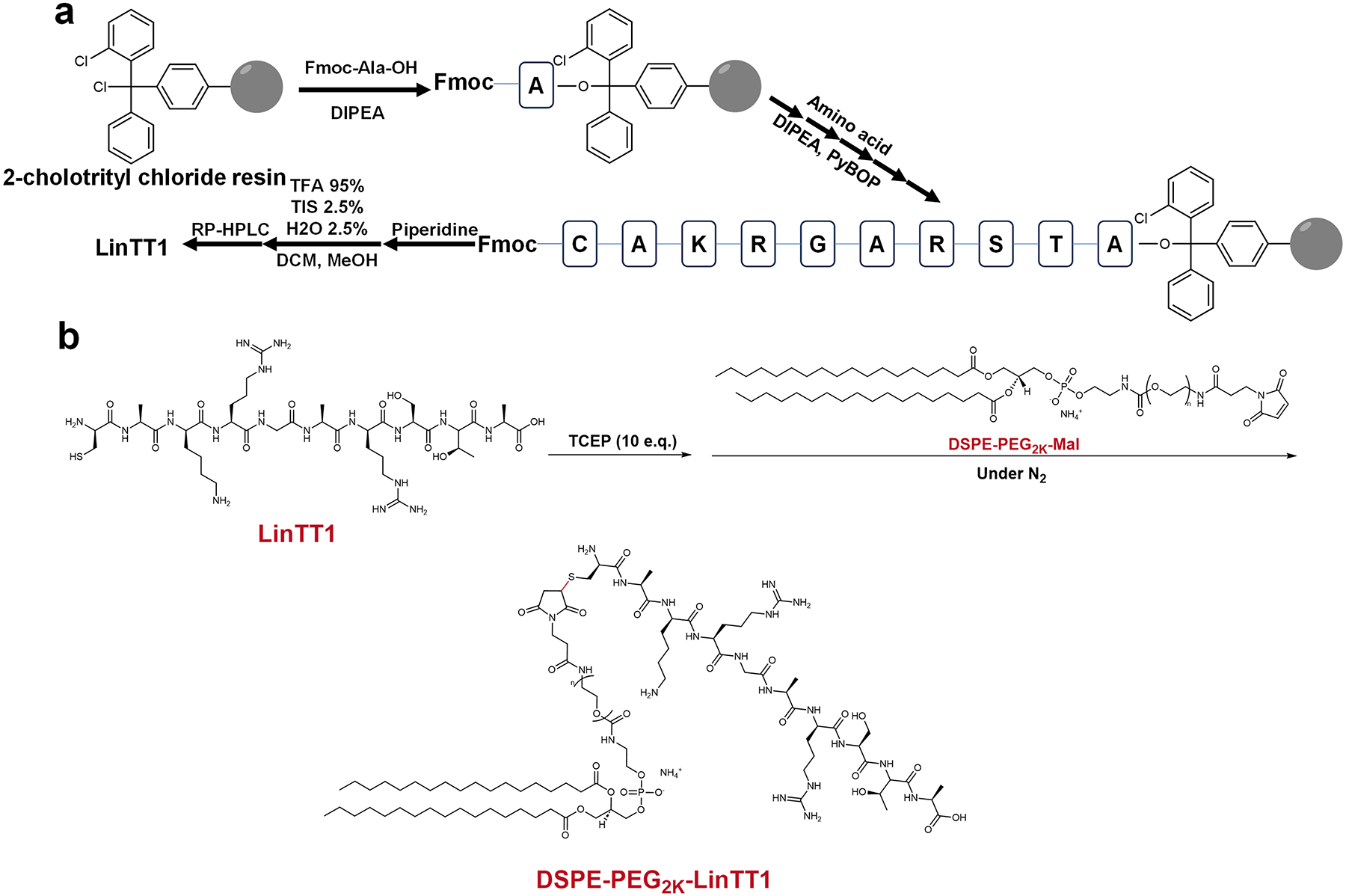
Synthesis schematic of LinTT1 (a) and DSPE-PEG2K-LinTT1 (b).
Development of tumor penetrating peptide coated Camptothesome
SM-derived CPT (Fig. 2a), featuring a disulfide link and a longer link, was synthesized following our published method [8, 9]. LinTT1/Camptothesome was prepared using the thin-film hydration method as established [2, 3, 5, 6, 8, 9] (Fig. 3a). To identify the optimal composition ratio of LinTT1 on Camptothesome, various molar ratios of LinTT1 (1%, 2%, 3%, 4.3% and 5%) were tested and coated onto Camptothesome. We conducted comprehensive physicochemical characterizations, including DLS size, zeta potential, polydispersity index (PDI), and formulation stability, comparing the peptide-modified formulations to the original Camptothesome (Fig. 3b,c,d).
The size of Camptothesome with 1% and 3% peptide fluctuated significantly within the first few days, stabilizing after approximately 5 days and 10 days, respectively. In contrast, the 2%, 4.3%, and 5% peptide-decorated formulations maintained good stability throughout the observation period, as indicated by minimal variation in size and zeta potential. Based on the exact ratio of DSPE-PEG2K used in Camptothesome, we chose 4.3 molar % of DSPE-PEG2K-LinTT1 [9]. Notably, as the ratio of DSPE-PEG2K-LinTT1 decreased from 5% to 1%, the zeta potential shifted from negative to positive due to overcompensation effect [18].
Based on the ionization properties of the amino acids in the peptide sequence, LinTT1 has a net charge of +3 at physiological pH [19]. The zeta potential of liposomes is influenced by both lipid composition and the attached LinTT1 peptide. At low molar ratios, the positively charged peptide interacts with the negatively charged lipids, resulting in a positive zeta potential [20]. However, as the molar ratio increases, the peptide-lipid interaction becomes more pronounced, leading to an overcompensation effect and a shift towards a more negative zeta potential [21]. This occurs due to peptide saturation on the liposome surface, charge redistribution, and steric hindrance at higher concentrations, despite LinTT1 being positively charged [22, 23].
LinTT1/Camptothesome markedly enhances the intracellular delivery
To determine the optimal peptide concentration in nanovesicles, we first assessed the cellular uptake in HCT116 human CRC cells (Fig. 4a,b). Flow cytometry analysis showed that while Camptothesome with 1–3% and 5% of peptide enhanced CPT delivery compared to the original Camptothesome, the most significant improvement was observed with (4.3%) LinTT1 decoration. Coupled with better stability performance (Fig. 3c,d), (4.3%) LinTT1/Camptothesome was selected as the optimum candidate for further studies.
Figure 4. LinTT1 peptide enhanced the cellular uptake of Camptothesome in CRC cancer cells but not in normal cells.
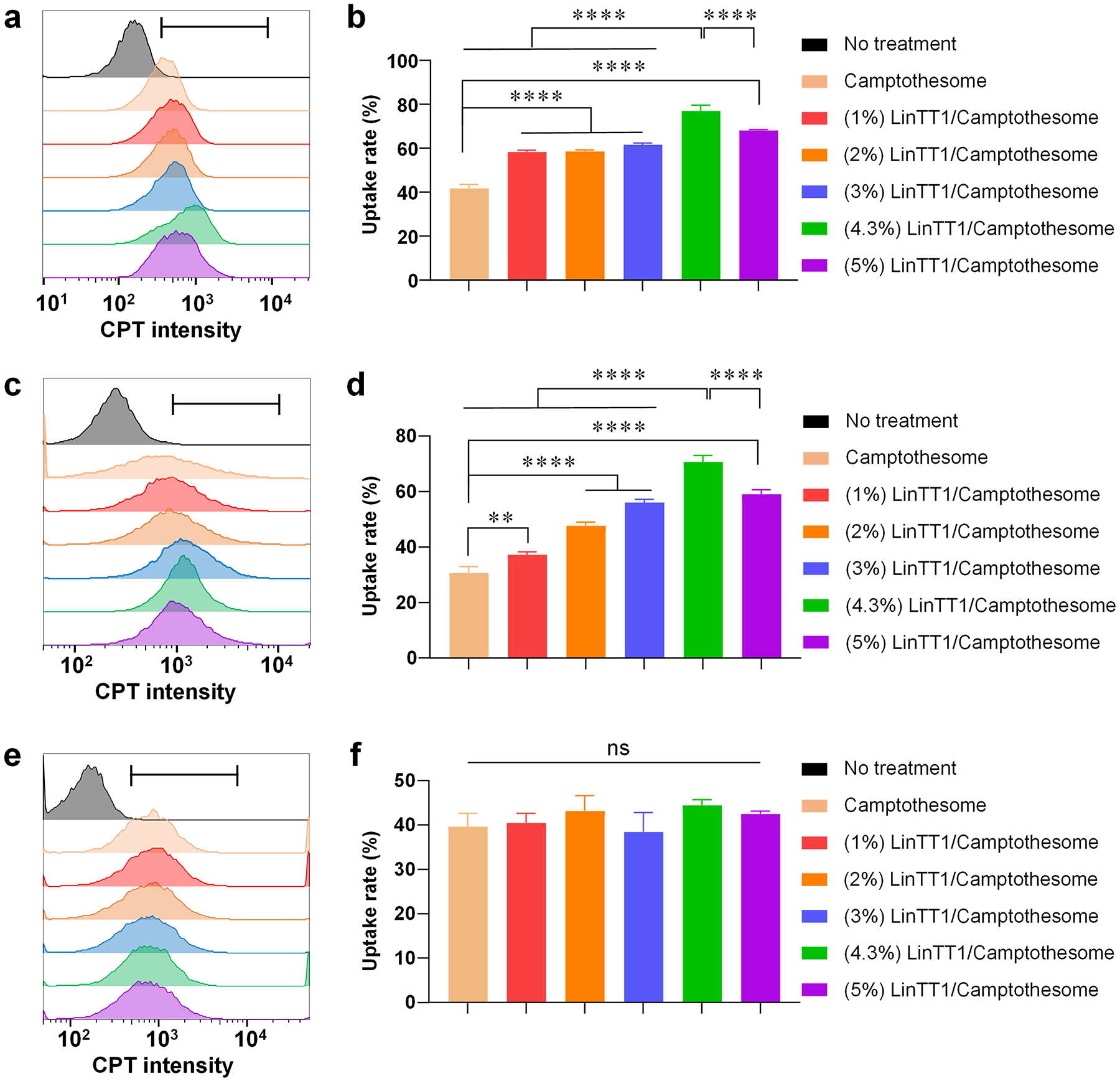
Representative flow cytometry histogram of CPT intensity in HCT116 (a), CT26 (c), and Beas-2b (e) cells treated by Camptothesome or Camptothesome with different peptide ratios at 50μg CPT/mL for 3 hours. The black gate indicates the CPT subset analyzed by FlowJo. b, d, f, Quantitative cellular uptake analyzed by GraphPad. Data in b, d, and f are expressed as mean ± SD (n=3). Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test. ns not significant, **P<0.01, ****P<0.0001.
In addition to HCT116, we also evaluated the uptake efficiency in mouse CT26 CRC cells (Fig. 4c,d) and confirmed that (4.3%) LinTT1/Camptothesome drastically outperformed other ratios and the plain Camptothesome, further validating the advantage of using a tumor-penetrating peptide. To ensure the enhanced cellular uptake was due to the peptide-induced endocytosis, we tested the uptake in Beas-2b normal epithelial cells, where the p32 protein receptor is primarily intracellular [24]. No significant difference was observed between Camptothesome with LinTT1-decorated and plain Camptothesome (Fig. 4e,f), ruling out off-target effects and confirming that the improved uptake is dependent on LinTT1/p32 receptor interaction.
Improved anticancer activity in vitro
To evaluate the effectiveness of peptide-decorated Camptothesome in killing cancer cells, we performed cytotoxicity assays in human HCT116 and mouse CT26 CRC cells (Fig. 5a,b). MTT assays revealed that (4.3%) LinTT1/Camptothesome exhibited significantly enhanced anticancer activity compared to plain Camptothesome, as indicated by the drastically reduced IC50 levels (0.28 vs 0.87 μM in HCT116, and 1.29 vs 4.58 μM in CT26). In contrast, no difference was observed between (4.3%) LinTT1/Camptothesome and plain Camptothesome in normal Beas-2b cells (16.13 vs 17.06 μM) (Fig. 5c), further demonstrating the cancer-specific targeting achieved through LinTT1 peptide functionalization.
Figure 5. Coating LinTT1 onto Camptothesome fortified the killing effects in cancer cells but not in normal cells.
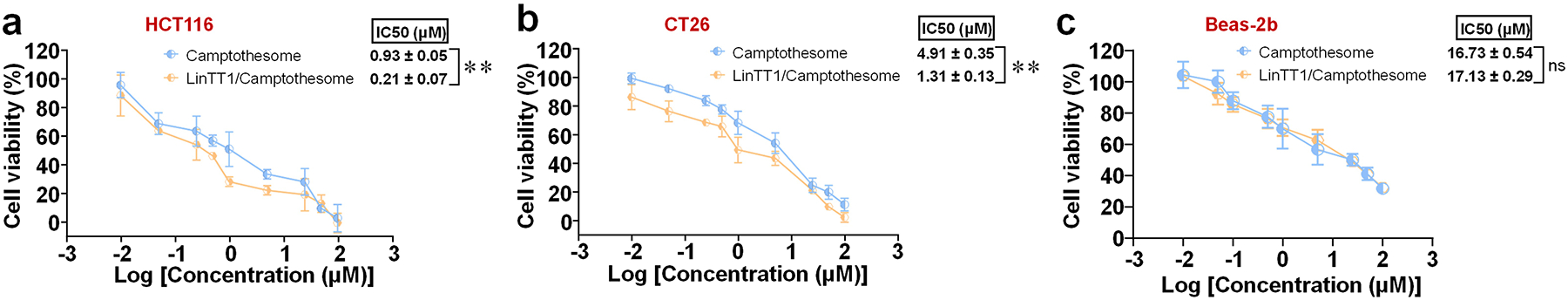
Cytotoxicity study of (4.3%) LinTT1/Camptothesome in (a) HCT116, (b) CT26, and (c) Beas-2b cells at 48 hours by MTT assay. Data are expressed as mean ± SD (n=3). Statistical significance was determined by two-tailed, unpaired Student’s t-test. ns not significant, **P<0.01.
LinTT1-mediated intercellular transcytosis in tumor cells
CendR peptides are known for enhancing vascular permeability through NRP-1 binding-induced endocytosis and enabling effective transcytosis for deep tumor penetration [25, 26]. To assess whether LinTT1/Camptothesome could trigger transcytosis in vitro, we conducted a co-incubation assay with three batches of HCT116 cancer cells and used fluorescence microscopy to evaluate the transcellular delivery efficiency (Fig. 6a,b). Camptothesome showed observable CPT fluorescence in the cancer cells on coverslips (ii) and (iii), indicating some transcellular delivery. However, LinTT1/Camptothesome significantly enhanced the transcytotic effect, delivering more Camptothesome to the cancer cells on coverslips (ii) and (iii) (Fig. 6b). We further investigated the intracellular trafficking mechanism involved in facilitating exocytosis after endocytosis, focusing on the Golgi apparatus, which plays a key role in exporting proteins and lipid molecules from the cell [27, 28].
Figure 6. LinT11/Camptothesome triggers transcytosis via intracellular trafficking to Golgi.
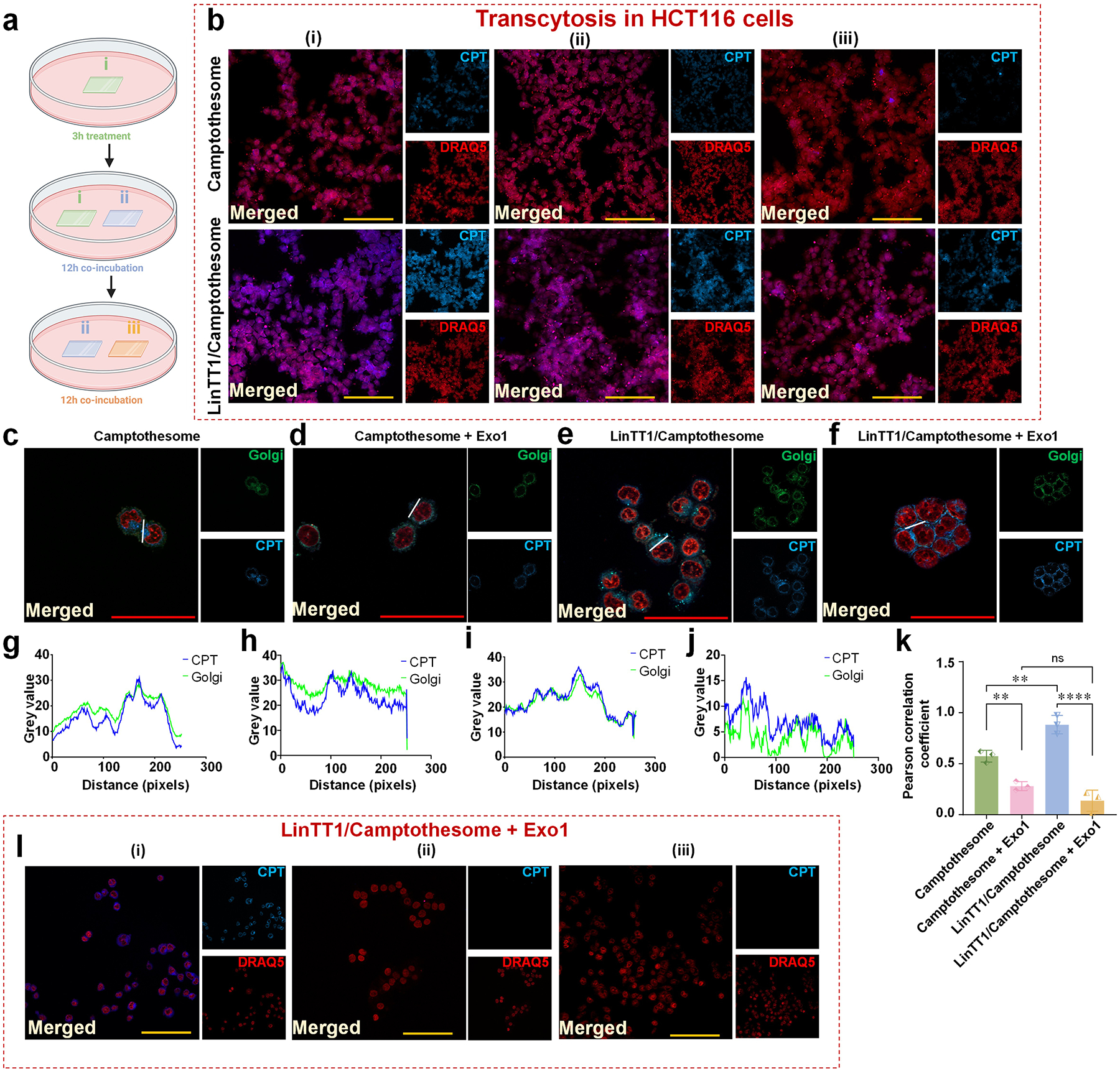
a, Illustration to show the experimental procedure to investigate transcytosis mechanism. b, Microscopic fluorescence imaging of Camptothesome (upper panel) and LinTT1/Camptothesome (lower panel). Scale bar: 100 μm. c-f, Colocalization of Golgi and CPT signal in HCT116 cells treated with Camptothesome or LinTT1/Camptothesome for 3 hours at 50 μg CPT/mL, with or without pretreatment of the exocytosis blocker (Exo1). Scale bar: 50 μm. g-j, Fluorescence intensity profile of CPT signal and Golgi across cells along the randomly selected white line in c-f were analyzed by pixel intensity with ImageJ. k, Pearson correlation coefficient analysis of CPT and Golgi from c-f. l, Transcytosis of LinTT1/Camptothesome (3 hours, 50 μg CPT/mL) was blocked after being pretreated with Exo1 for 1 hour. The experiment procedures were the same as a. Scale bar: 100 μm. Data in k is expressed as mean ± SD (n=3). Statistical significance was determined by two-tailed, unpaired Student’s t-test. ns not significant, **P<0.01, ****P<0.0001.
To decipher whether Golgi played a role, we performed the colocalization analysis for the nanovesicles (CPT signal) with the Golgi in HCT116 cells (Fig. 6c–k). Golgi was stained by Golgi-ID green assay kit (green) and CPT had the blue fluorescence, while the nuclei was stained by DRAQ5 (red) in Fig. 6c–f. Herein, we used the microscopic fluorescence imaging to determine the distribution overlap of the CPT signal (in Camptothesome and LinTT1/Camptothesome) with Golgi (Fig. 6c,e). Their colocalization pattern across cells along the randomly selected white line was analyzed by pixel intensity with ImageJ in Fig. 6g,i, which was quantified by Pearson correlation coefficient (Fig. 6k). Our data revealed that while Camptothesome had certain level of colocalization with Golgi, the degree of which was markedly enhanced in LinTT1/Camptothesome. To further verify that the Golgi was indeed involved in exocytosis, we pretreated cells with the Exo1 (Fig. 6d,f,h,j,l), a reversible inhibitor of vesicular trafficking between the endoplasmic reticulum (ER) and the Golgi apparatus. After the Exo1 treatment, the Pearson correlation coefficient for both modified and unmodified Camptothesome decreased drastically (Fig. 6k), demonstrating the indispensability of the Golgi for exocytosis. Thus, we postulated that LinTT1/Camptothesome-enabled transcytosis was mediated via LinTT1/p32 >>> CendR motif/NRP-1>>> ER >>> Golgi trafficking pathway. Previously, we reported that Camptothesome entered cells via clathrin-mediated endocytosis [9]. Collectively, we posited that Camptothesome-induced transcellular delivery occurred through clathrin >>> ER >>> Golgi vesicular trafficking machinery.
LinTT1-decorated Camptothesome enhances tumor delivery, enables deeper tumor penetration, and reduces the non-specific tissue distribution in a xenograft CRC mouse model
To evaluate the tissue distribution of LinTT1-engineered nanovesicles, we performed ex vivo tissue optical imaging 48 hours after IV administration of free dye or dye-labeled nanovesicles in a human HCT116 xenograft CRC mouse model (Fig. 7a). Free dye was quickly eliminated from circulation and did not show detectable signal. Consistent with our prior study in another tumor model [3], dye-labeled Camptothesome exhibited strong fluorescence intensity in tumor, confirming its good tumor targeting ability. However, Cy5.5/nanovesicles also showed significant distribution to other healthy tissues such as liver, spleen, lung, and kidneys. In contrast, LinTT1/Camptothesome was able to further boost the tumor delivery efficiency as reflected by the greater fluorescence intensity and reduced distribution to normal organs (especially in liver), highlighting the advantage of functionalizing Camptothesome with a tumor-homing peptide for more selective targeting. Additionally, a quantitative biodistribution study was conducted to validate the imaging results (Fig. 7b). Onivyde, the FDA-approved liposomal irinotecan (CPT analogue), was used as a control alongside the original Camptothesome. HPLC analysis revealed that while approximately 5% of the injected Camptothesome reached tumor at 48h, this was markedly enhanced by peptide functionalization, which increased tumor delivery to ~7%, representing a 40% improvement. More importantly, in accordance with the ex vivo imaging findings, LinTT1/Camptothesome exhibited reduced distribution to healthy tissues compared to plain Camptothesome. Notably, Camptothesome, especially with peptide decoration, outperformed Onivyde on tumor targeting.
Figure 7. CendR motif, LinTT1, extends circulation time and enhances tumor delivery of Camptothesome.
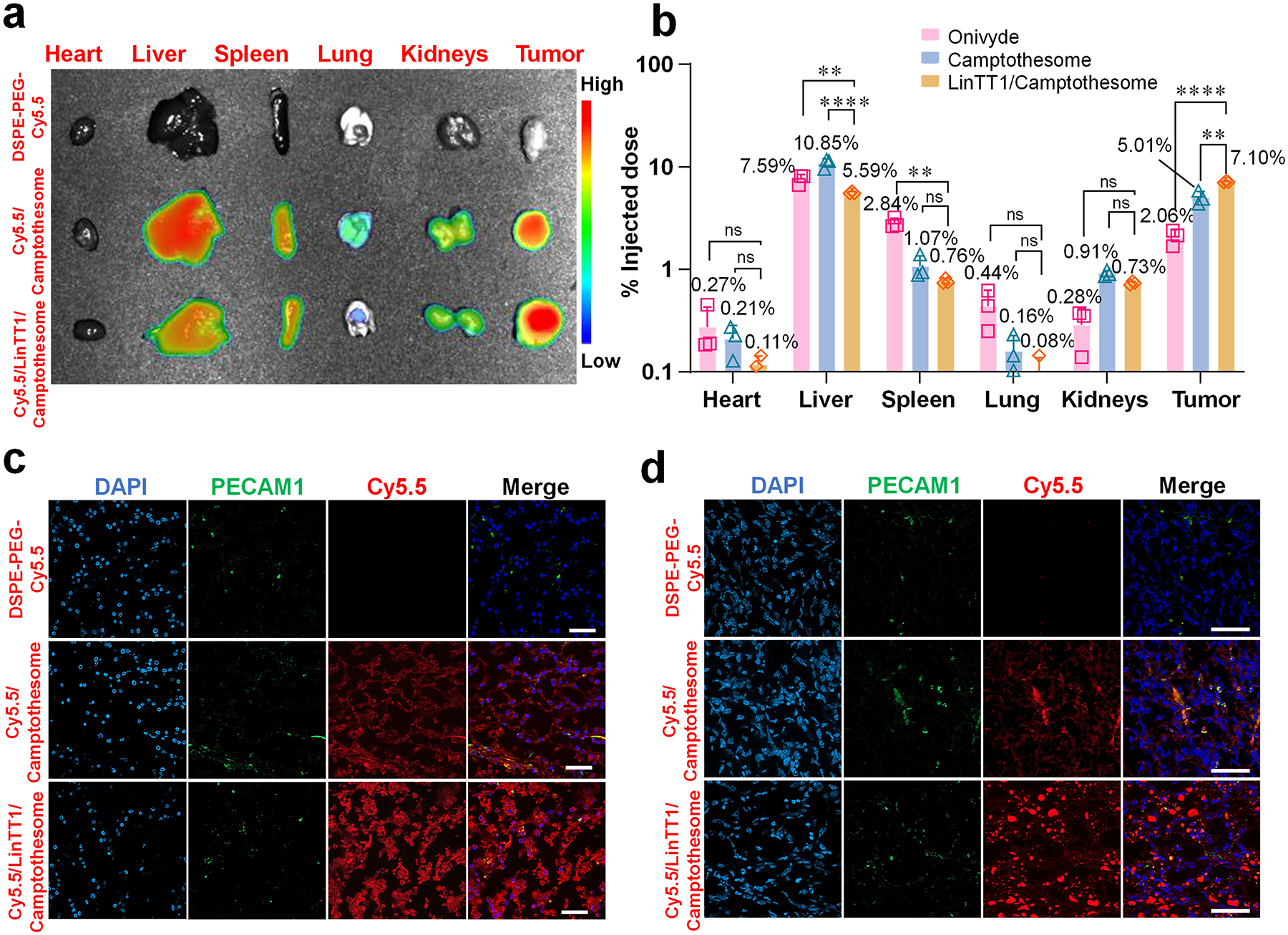
a, Mouse Lago optical imaging of ex-vivo imaging of different organs at 48 hours after an I.V. injection of DSPE-PEG-Cy5.5, Cy5.5/Camptothesome (20 mg CPT/kg), and Cy5.5/LinTT1/Camptothesome (20 mg CPT/kg) in subcutaneous HCT116 tumor mice (n=3, tumors: ~300 mm3). b, Tissue biodistribution at 48 hours in subcutaneous HCT116 tumor mice (n=3, tumors: ~300 mm3). Mice received a single I.V. injection for Onivyde (33.7 mg irinotecan/kg), Camptothesome and LinTT1/Camptothesome at e.q. 20 mg CPT/kg. c, Investigate the ability of LinTT1/Camptothesome to enable deep tumor penetration in the HCT116 tumor model. Scale bar: 50 μm. d, Investigate the ability of LinTT1/Camptothesome to enable deep tumor penetration in the 4T1 tumor model. Scale bar: 50 μm. Data in c is expressed as mean ± SD (n=3). Statistical significance was determined by one-way ANOVA followed by Tukey’s multiple comparisons test. ns not significant, **P<0.01, ****P<0.0001.
To prove that the in vitro transcellular transport observed with LinTT1/Camptothesome translated to deep tumor penetration, we examined tumor sections via microscopic imaging in HCT116 tumor model (Fig. 7c). The blood vessels were stained with PECAM1 (green), and the nuclei were doped by DAPI (blue). As observed in our previous study [3], Cy5.5/Camptothesome showed a strong red (Cy5.5) signal across the tumor tissue, indicating its good tumor penetration potential. However, Cy5.5/LinTT1/Camptothesome demonstrated markedly deeper tumor infiltration and travelled far more distance from the blood vessels, leading to a more uniform distribution within the HCT116 tumor compared to the Cy5.5/Camptothesome. In addition, we further explored the tumor penetration in a hypopermeable 4T1 triple negative breast cancer model (Fig. 7d) [29]. This data revealed a consistent trend, where Cy5.5/LinTT1/Camptothesome exhibited much deeper penetration and travelled significantly farther from the blood vessels in 4T1 tumors compared to Cy5.5/Camptothesome. Taken together, these results underscore the potential of LinTT1/Camptothesome to significantly enhance antitumor efficacy.
LinTT1/Camptothesome beats Camptothesome on therapeutic efficacy in a human xenograft CRC carcinoma mouse model
The antitumor efficacy of LinTT1/Camptothesome was evaluated in a HCT116 human xenograft subcutaneous mouse model (Fig. 8). The control groups included free CPT (MTD dose, 5 mg/kg [3, 9]), Onivyde, and Camptothesome (eq. 20 mg CPT/kg). When tumors reached ~100mm3 on day 6, mice were intravenously injected with various treatments. Tumors in the vehicle control group grew rapidly, highlighting the aggressive nature of this tumor type (Fig. 8a). Both free CPT and Onivyde significantly delayed tumor growth, and Camptothesome further strengthen tumor reduction. Notably, LinTT1/Camptothesome demonstrated superior efficacy compared to Camptothesome, achieving the greatest level of tumor inhibitory effect and completely eradicating the tumor in 1 out of 5 mice (Fig. 8a–d). No significant weight loss was observed in all groups throughout the study period (Fig. 8b). Moreover, tumor tissue from the LinTT1/Camptothesome showed the highest level of apoptosis, as indicated by the increased level of cleaved caspase-3 (CC-3) expression, and significantly DNA damage, as evidenced by elevated γH2AX levels (Fig. 8e). The enhanced therapeutic efficacy observed in the LinTT1/Camptothesome against the HCT116 CRC carcinoma model could be ascribed to the improved tumor delivery and penetration effectiveness (Fig. 7).
Figure 8. LinTT1/Camptothesome was superior to Camptothesome in anti-CRC efficacy.
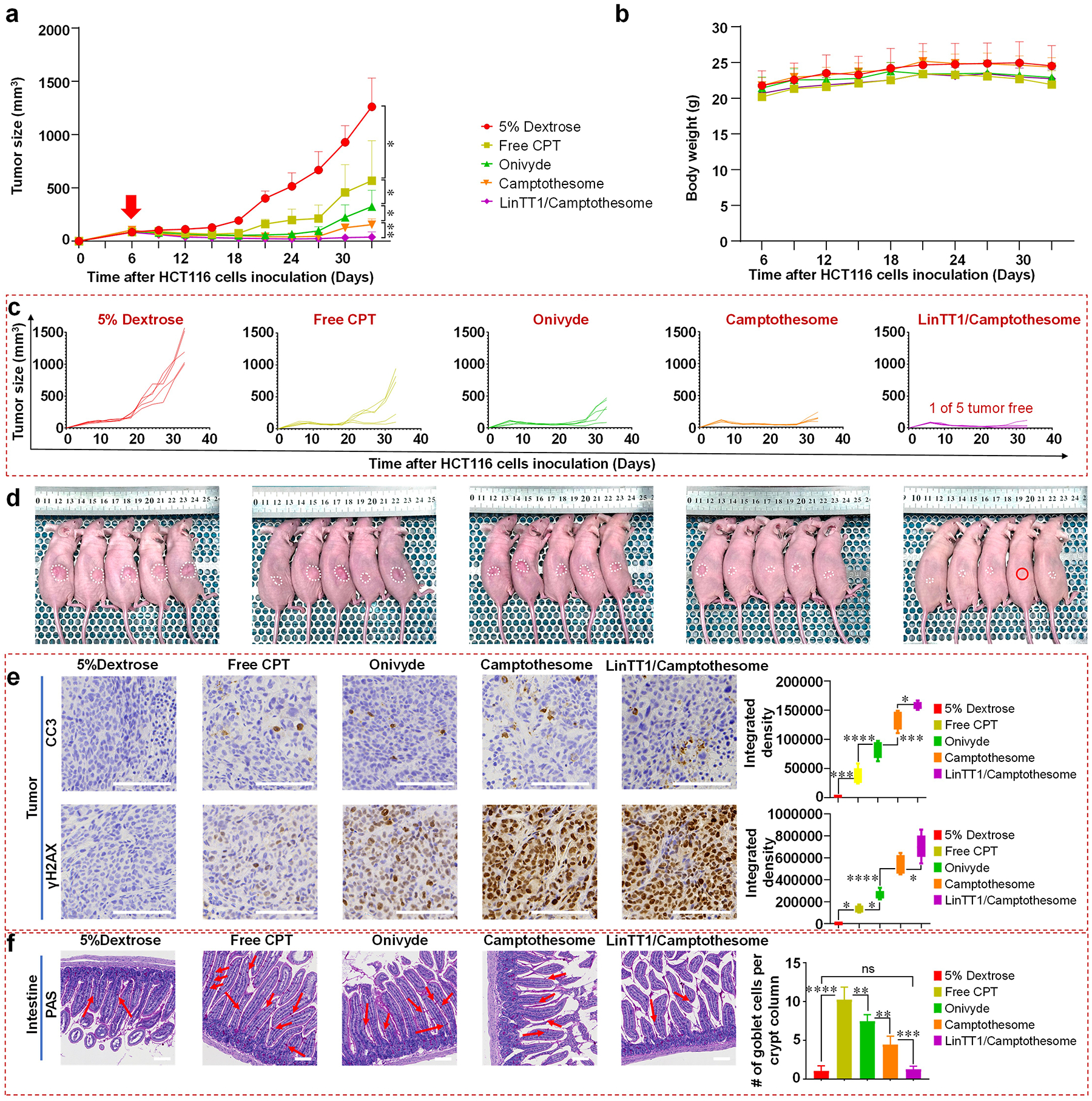
a, Tumor growth curves in subcutaneous HCT116 xenograft CRC tumor model (tumors: ~100 mm3). Mice were intravenously injected with a single dose of 5% dextrose, free CPT (5 mg CPT/kg, MTD), Onivyde (33.4 mg irinotecan/kg, eq. 20 mg CPT/kg), Camptothesome and LinTT1/Camptothesome at 20 mg CPT/kg on day 6. The red arrow indicates the date that mice received the single I.V. injection. b, Mouse body weight. c, Individual tumor growth curves in subcutaneous HCT116 xenograft CRC tumor model. d, Tumor-bearing mice images on day 33. Red circle indicates tumor free mouse, white dash line circles tumor volume on each mouse. e, Representative IHC staining of tumors for CC-3 (upper panel) and γH2AX (lower panel) from d and respective quantitative analysis. f, Representative histologic sections of the intestine mucosa stained by periodic acid-Schiff (PAS) reaction and counterstained with haematoxylin from d and respective quantitative analysis. Red arrow indicates the mucin-containing goblet cells. Scale bar: 100 μm. Data in a, b, and data in (f, right panel) are presented as a mean ± SD (n=5), and data in (e, right panel) are presented as box-and-whisker plots (n=5). Statistical significance was analyzed by two-tailed, unpaired Student’s t-test. ns not significant, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Furthermore, as gastrointestinal (GI) toxicity is the primary clinical side effect associated with CPT [9, 30], we measured the levels of GI mucositis in treated mice using periodic acid-Schiff (PAS) staining, as shown in Fig. 8a. Consistent with our previous study [9], free CPT elicited severe intestinal damage, characterized by a significant increase in mucin-containing goblet cells. Onivyde can mitigate mucositis to a certain degree compared to free CPT, but Camptothesome demonstrated the reduction in GI toxicity, especially with LinTT1 decoration (Fig. 8f). These results suggest that LinTT1/Camptothesome alleviates off-target effects, contributing to minimized GI toxicity (Fig. 7).
Conclusions
Through systematic screening of the molar ratios of the p32 surface protein-binding LinTT1 on Camptothesome, we determined the optimal amount of the peptide on nanovesicles that can enable the highest cancer cell uptake, markedly decreasing the IC50 in CRC cancer cells without affecting the normal cells. LinTT1 coating further enhanced the transcytotic potential of Camptothesome mechanistically, through the Golgi-mediated exocytosis. Importantly, optical imaging and biodistribution studies corroborated that LinTT1/Camptothesome markedly boosted the tumor targeting and minimized the distribution to healthy tissues with deeper tumor infiltration. Apart from the syngeneic CRC mouse model reported in our prior work, for the first time, we showed that Camptothesome outperformed free CPT and Onivyde in therapeutic efficacy in a human HCT116 xenograft CRC model. The antitumor effects were further strengthened when Camptothesome was decorated with the LinTT1 peptide by increasing the CC-3-mediated apoptosis and γH2AX-enabled DNA damage. In summary, our findings demonstrate that LinTT1/Camptothesome nanotherapeutic portends significant promise to improve the Camptothesome-based cancer therapy.
Supplementary Material
Acknowledgements
This work was supported in part by Startup and Retention Funds from the R. Ken Coit College of Pharmacy at The University of Arizona (UArizona), a PhRMA Foundation Faculty Starter Grant in Drug Delivery, UArizona Comprehensive Cancer Center (UACCC) Internal Pilot Award, and the National Institutes of Health (NIH) grants (R35GM147002 and R01CA272487). We acknowledge the use of Mass Spectrometry in Analytical and Biological Mass Spectrometry Core Facility at the UArizona BIO5 Institute; the UArizona Translational Bioimaging Resource Core for the Lago live animal imaging; Tissue Acquisition and Cellular/Molecular Analysis Shared Resource (TACMASR) at UACCC for the PAS and H&E staining. We thank Doug Cromey, the co-manager of the University of Arizona Imaging Cores – Optical Core Facility, for providing trainings and supports; and the Flow Cytometry Immune Monitoring Shared Resource (FCIMSR) core for flow cytometry studies at UACCC, which are supported by NIEHS P30 ES006694 and NCI P30 CA023074.
Footnotes
CRediT authorship contribution statement
Yanhao Jiang: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Visualization. Zhiren Wang: Investigation. Wenpan Li: Investigation. Teng Ma: Investigation. Mengwen Li: Investigation. Shuang Wu: Investigation. Ethan Lin: Investigation. Karlie Elizabeth Flader: Investigation. Mengjiao Ma and Hongmin Li: Assistance in immunofluorescence staining. Mengyang Chang and Wei Wang: Assistance in peptide synthesis and RP-HPLC. Jianqin Lu: Conceptualization, Supervision, Formal analysis, Writing – original draft, Funding acquisition.
Declaration of Competing Interest
J.L. has applied for patents related to the Camptothesome technology. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
The data supporting the findings of this study are available within the article and the Supplementary Information. All relevant data are available from the authors upon reasonable request.
References
- [1].Jiang Y, Li W, Wang Z, Lu J, Lipid-Based Nanotechnology: Liposome, Pharmaceutics 16(1) (2024) 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang Z, Li W, Jiang Y, Park J, Gonzalez KM, Wu X, Zhang QY, Lu J, Cholesterol-modified sphingomyelin chimeric lipid bilayer for improved therapeutic delivery, Nature Communications 15(1) (2024) 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang Z, Little N, Chen J, Lambesis KT, Le KT, Han W, Scott AJ, Lu J, Immunogenic camptothesome nanovesicles comprising sphingomyelin-derived camptothecin bilayers for safe and synergistic cancer immunochemotherapy, Nature Nanotechnology 16(10) (2021) 1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang X, Zhao W, Nguyen GN, Zhang C, Zeng C, Yan J, Du S, Hou X, Li W, Jiang J, Functionalized lipid-like nanoparticles for in vivo mRNA delivery and base editing, Science advances 6(34) (2020) eabc2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Z, Li W, Jiang Y, Tran TB, Cordova LE, Chung J, Kim M, Wondrak G, Erdrich J, Lu J, Sphingomyelin-derived nanovesicles for the delivery of the IDO1 inhibitor epacadostat enhance metastatic and post-surgical melanoma immunotherapy, Nature Communications 14(1) (2023) 7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang Z, Li W, Jiang Y, Tran TB, Chung J, Kim M, Scott AJ, Lu J, Camptothesome-based combination nanotherapeutic regimen for improved colorectal cancer immunochemotherapy, Biomaterials (2024) 122477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Milano G, Innocenti F, Minami H, Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs, Cancer Science 113(7) (2022) 2224–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Z, Cordova LE, Chalasani P, Lu J, Camptothesome Potentiates PD-L1 Immune Checkpoint Blockade for Improved Metastatic Triple-Negative Breast Cancer Immunochemotherapy, Molecular Pharmaceutics 19(12) (2022) 4665–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Z, Li W, Park J, Gonzalez KM, Scott AJ, Lu J, Camptothesome elicits immunogenic cell death to boost colorectal cancer immune checkpoint blockade, Journal of Controlled Release 349 (2022) 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E, C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration, Proceedings of the National Academy of Sciences of the United States of America 106(38) (2009) 16157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Agemy L, Kotamraju VR, Friedmann-Morvinski D, Sharma S, Sugahara KN, Ruoslahti E, Proapoptotic peptide-mediated cancer therapy targeted to cell surface p32, Molecular Therapy 21(12) (2013) 2195–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pang H-B, Braun GB, Friman T, Aza-Blanc P, Ruidiaz ME, Sugahara KN, Teesalu T, Ruoslahti E, An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability, Nature communications 5(1) (2014) 4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E, Tissue-penetrating delivery of compounds and nanoparticles into tumors, Cancer cell 16(6) (2009) 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balistreri G, Yamauchi Y, Teesalu T, A widespread viral entry mechanism: The C-end Rule motif-neuropilin receptor interaction, Proceedings of the National Academy of Sciences of the United States of America 118(49) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sharma S, Kotamraju VR, Molder T, Tobi A, Teesalu T, Ruoslahti E, Tumor-penetrating nanosystem strongly suppresses breast tumor growth, Nano letters 17(3) (2017) 1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hunt H, Simón-Gracia L, Tobi A, Kotamraju VR, Sharma S, Nigul M, Sugahara KN, Ruoslahti E, Teesalu T, Targeting of p32 in peritoneal carcinomatosis with intraperitoneal linTT1 peptide-guided pro-apoptotic nanoparticles, Journal of Controlled Release 260 (2017) 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fogal V, Zhang L, Krajewski S, Ruoslahti E, Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma, Cancer research 68(17) (2008) 7210–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sabín J, Vázquez-Vázquez C, Prieto G, Bordi F, Sarmiento F, Double Charge Inversion in Polyethylenimine-Decorated Liposomes, Langmuir 28(28) (2012) 10534–10542. [DOI] [PubMed] [Google Scholar]
- [19].Boehnke N, Dolph KJ, Juarez VM, Lanoha JM, Hammond PT, Electrostatic Conjugation of Nanoparticle Surfaces with Functional Peptide Motifs, Bioconjugate Chemistry 31(9) (2020) 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tunuguntla R, Bangar M, Kim K, Stroeve P, Ajo-Franklin CM, Noy A, Lipid bilayer composition can influence the orientation of proteorhodopsin in artificial membranes, Biophysical Journal 105(6) (2013) 1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Munye MM, Ravi J, Tagalakis AD, McCarthy D, Ryadnov MG, Hart SL, Role of liposome and peptide in the synergistic enhancement of transfection with a lipopolyplex vector, Scientific Reports 5(1) (2015) 9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Orozco-Alcaraz R, Kuhl TL, Impact of membrane fluidity on steric stabilization by lipopolymers, Langmuir 28(19) (2012) 7470–7475. [DOI] [PubMed] [Google Scholar]
- [23].Vu TQ, Sant’Anna LE, Kamat NP, Enhancing functionalized liposome avidity to cells via lipid phase separation, bioRxiv (2022) 2022.10. 18.512758. [Google Scholar]
- [24].Muta T, Kang D, Kitajima S, Fujiwara T, Hamasaki N, p32 protein, a splicing factor 2-associated protein, is localized in mitochondrial matrix and is functionally important in maintaining oxidative phosphorylation, Journal of Biological Chemistry 272(39) (1997) 24363–24370. [DOI] [PubMed] [Google Scholar]
- [25].Li Y-X, Pang H-B, Macropinocytosis as a cell entry route for peptide-functionalized and bystander nanoparticles, Journal of Controlled Release 329 (2021) 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu X, Lin P, Perrett I, Lin J, Liao YP, Chang CH, Jiang J, Wu N, Donahue T, Wainberg Z, Nel AE, Meng H, Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer, Journal of Clinical Investigation 127(5) (2017) 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P, Transport from the Trans Golgi Network to the cell exterior: Exocytosis, Molecular Biology of the Cell. 4th edition, Garland Science; 2002. [Google Scholar]
- [28].Cooper GM, Ganem D, The cell: a molecular approach, Nature Medicine 3(9) (1997) 1042–1042. [Google Scholar]
- [29].Ke W, Yin W, Zha Z, Mukerabigwi JF, Chen W, Wang Y, He C, Ge Z, A robust strategy for preparation of sequential stimuli-responsive block copolymer prodrugs via thiolactone chemistry to overcome multiple anticancer drug delivery barriers, Biomaterials 154 (2018) 261–274. [DOI] [PubMed] [Google Scholar]
- [30].Guabiraba R, Besnard A-G, Menezes GB, Secher T, Jabir M, Amaral S, Braun H, Lima-Junior RC, Ribeiro R, Cunha F.d.Q., IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice, Mucosal immunology 7(5) (2014) 1079–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and the Supplementary Information. All relevant data are available from the authors upon reasonable request.


