Abstract
Delayed onset lactogenesis II has adverse effects on breastfeeding and neonatal health. This study aimed to determine the risk factors of DOL II, develop a predictive model for DOL II, and test its validity. This was a retrospective cohort study. A total of 450 pregnant women were involved in this study, including 112 cases with DOL II and 338 cases without DOL II, who were hospitalized between October 2023 and April 2024. Univariate and multivariate logistic regression analyses were conducted to determine the risk factors of DOL II, which were then included in the nomogram. The performance of the nomogram model was evaluated by the area under the receiver operating curve, calibration curve, and Hosmer–Lemeshow test. Decision curve analysis was used to evaluate the clinical efficacy of the nomogram. The results showed that maternal age, pre-pregnancy BMI, gestational age, parity, delivery mode, length of stage II labor, gestational diabetes, EPDS, maternal-infant separation, breastfeeding education during pregnancy, formula use, and onset of breastfeeding within the first hour were independent risk factors. In the derivation cohort, the AUC was 0.786. Youden index was 1.526, with a sensitivity of 0.781 and a specificity of 0.745. In the verification cohort, AUC was 0.748. The nomogram model has good consistency and performance, which can identify women who are at high risk of DOL II.
Keywords: Delayed onset of lactogenesis II (DOL II), Risk factors, Nomogram model, Breastfeeding, Pregnant woman
Subject terms: Risk factors, Pregnancy outcome
Introduction
Breastfeeding improves maternal and child health. The World Health Organization (WHO) has reported that breast milk is the ideal food for the growth of infants. WHO has recommended providing infants with exclusively breast milk for the first half a year of a newborn’s life. If possible, continuous breastfeeding should be maintained until at least 2 years of age and complementary food should be provided at 6 months after birth1–3. Breastfeeding benefits short- and long-term health results of maternal and infants. Breast milk is considered the ideal food source for newborns because it contains nutrients and bioactive substances, which are essential for infant growth and long-term development. It offers protection against infections and contributes to the development of a healthy immune system, intestines, and cognition in babies. Children who are breastfed have lower rates of infectious diseases, dental malocclusions, higher intelligence levels, and reduced risks of being overweight or developing diabetes later in life. Mothers also benefit from breastfeeding. They tend to come back to their weight before pregnancy faster and have a lower rate of obesity compared to those who do not breastfeed. Breastfeeding can help prevent ovarian and breast cancer, improve birth spacing, decrease the risk of type 2 diabetes, and lower the chances of cardiovascular disease4.
However, rates of breastfeeding in early life remain very low worldwide. In countries of low-income and middle-income, only 37% of newborns younger than 6 months of age are fed by exclusively breastfeeding. Generally, breastfeeding duration is shorter in developed countries than in undeveloped countries5. Breastfeeding practices are affected by various cultural, socioeconomic, and individual factors. These barriers influencing breastfeeding include health systems, workplaces, communities, and households6.
To increase the breastfeeding rate, it is required to understand the lactogenesis process and its influence factors. Lactogenesis mainly occurs in three stages, stage I, stage II, and stage III. Lactogenesis I, or secretory initiation, activates during pregnancy when mammary glands begin secreting a small quantity of colostrum. Lactogenesis II, or secretory activation, is defined as the beginning of secreting plenteous milk. It starts after childbirth and is the onset of secreting plenteous milk which normally occurs between 30 and 40 postnatal hours, increases rapidly and plateaus at approximately 96 postnatal hours. Lactogenesis III, also known as galactopoiesis, is the final period of lactogenesis. It is the producing and maintaining matured milk from day 9 after giving birth till weaning7,8. Delayed onset of lactogenesis II (DOL II) is diagnosed when the beginning of producing plentiful milk is delayed over 72 h after giving birth. It can contribute to early breastfeeding cessation. It also may increase the risks of excess newborns weight loss, early formula supplementation, and early milk weaning9,10. The incidence rates of DOL II range from 17 to 44%. Its incidence in the US is as high as 23–44%, and 26% in China11.
Numerous factors associated with DOL II have been extensively explored in the last decades12. Although multiple factors may contribute to insufficient lactation, the ability to predict insufficient lactation based on the presence of risk factors is limited. The social or cultural, psychological, and physical factors may differ among different populations. The model for predicting the risks of DOL II generally adopted in Western countries may not be suitable for Chinese women. Only some sporadic DOL II factors for Chinese women were investigated. Few models have been provided to predict the probability of developing a DOL II for Chinese women.
This study aimed to determine the risk factors of DOL II and develop a predictive model for DOL II, which can identify women who are at high risk of DOLII. It is helpful for medical staff to pay attention to the potential risk of DOL II and to intervene in high-risk women to reduce the incidence of DOL II, and improve successful breastfeeding.
Materials and methods
Participants of the derivation cohort
A retrospective study was conducted in this research. The pregnant women who were hospitalized at the affiliated hospital of Qingdao University for delivery from October 2023 to April 2024 were selected as the research subjects. Inclusion criteria: ① age ≥ 20 years, married; ② volunteer to participate in this survey ③ be willing to breastfeeding; ④ be able to complete the survey. Exclusion criteria: ① with an experience of breast surgery; ② taking any medication to promote or inhibit lactation after delivery; ③ with poor ability of communication;④ stillbirth.
Sample size
The sample size was determined according to the number of independent variables in the logistic regression, that was, at least 10 positive cases were required for each independent factor included in the final model. This study was expected to include 10 factors, and at least 100 cases of DOL II were required. Considering the estimated DOL II incidence of 25% and a sample-dismissing rate of 10%, the required number of samples was 445. The actual sample size was 450, showing that the statistical power was sufficient in this research.
Participants of the validation cohort
Pregnant women who gave birth in our hospital (a branch hospital different from the one for modeling) from May to June 2024 were selected. The inclusion and exclusion criteria of the study subjects were the same as those of the modeling group, and a total of 145 cases were used as the model validation group.
Definition of DOLII
DOL II is defined if the mother’s lactation perception appears after 72 h after giving birth. Maternal lactation perception onset is evaluated using the assessment of the onset of lactogenesis II, which is a valid index of the DOLII when comparing with the gold standard of test weights13. It is assessed by surveying women to recall the beginning of breast fullness, swelling, and milk leaking when these signs of lactation II first occur.
Design
DOL II risk questionnaire: Based on literature review and expert consultation, predictive variables were screened, and a self-designed DOL II risk questionnaire was developed. The collected data included maternal, infant, and delivery-related factors. (1) Maternal-related factors, including age, education level, employment, annual family income, drinking during pregnancy, pre-pregnancy body mass index (BMI), gestational weight gain (GWG), parity, conception mode, gestational age, gestational diabetes mellitus (GDM), Edinburgh Postnatal Depression Scale (EPDS) score, fatigue scale-14, and breastfeeding education during pregnancy. (2) Delivery-related factors including delivery mode (virginal delivery or cesarean section) and length of stage II labor. (3) Infant and feeding-related factors including 1-min Apgar score, maternal-infant separation, formula use, and onset of breastfeeding within the first hour.
Data collection
The maternal demographic data were collected from the hospital’s electronic medical record system. The puerpera was interviewed face-to-face by the researcher for the first postpartum visit 24 h after delivery. The main survey data included when the mother first breastfed after birth and there was any pain due to breastfeeding or not. Mothers were visited once a day to ask whether they felt breast fever, swelling, and hard lumps, whether there was obvious breast fullness, or milk volume increased significantly. For women discharged from the hospital before the onset of stage II lactation, the researchers contacted the women daily by telephone or wechat to determine when the women perceived the onset of lactation.
Data analysis
A statistical software of SPSS 19.0 was used for statistics analysis and data process. Counting data were presented by frequency and percentage, and χ2 test or non-parametric test was performed for comparison between groups. A multi-factor analysis was applied by logistic regression analysis. The scale discrimination was tested using a t-test for two independent samples. The prediction validity of the model was evaluated by specificity, sensitivity, Yuden index, and area under the receiver operating characteristic (ROC) curve. The Hosmer–Lemeshow test was used to determine the fitting degree of the model, and ROC curve was used to analyze the Yuden index and cutoff value of the model, and the accuracy of the prediction tool was evaluated. P < 0.05 was considered a statistically significant difference.
Ethical consideration
The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University, China (QYFY WZLL 29022). Before the study, all participants provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Results
Demographic characteristics of pregnant women
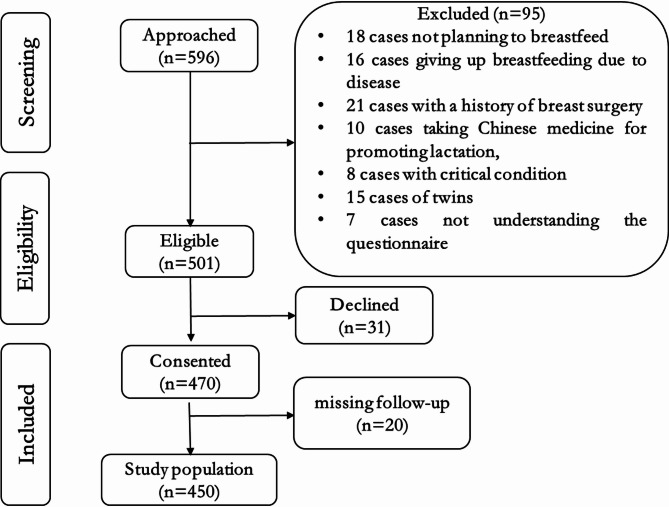
In this study, a total of 596 cases of data were collected, of which 146 were excluded. Those who were excluded included 18 cases of women who did not plan to breastfeed, 16 cases who gave up breastfeeding due to disease, 21 cases had a history of breast surgery, 10 cases of took Chinese medicine for promoting lactation, 8 cases of critical condition, 15 cases of twins, 7 cases did not understand the contents of the questionnaire, and 20 cases of missing follow-up and incomplete data, and 31 mothers resisting to take part in the study. A total of 450 patients were included, of which 112 (24.9%) developed DOL II. The age ranged from 23 to 45 (30.45 ± 4.55) years old. Education: 55 high school or below (12.2%), 286 university (63.6%), 109 postgraduate (24.2%). Prima flow diagram is shown in Fig. 1.
Fig. 1.
Prima flow diagram.
Univariate analysis of DOL II risk factors
The relevant factors were analyzed between the women with DOL II and those without DOL II in the modeling group. There were statistically significant differences between the two groups in maternal age, pre-pregnancy BMI, primiparity, gestational age, cesarean delivery, length of stage II labor, GDM, EPDS, maternal-infant separation, breastfeeding education during pregnancy, formula use, and onset of breastfeeding within the first hour (P < 0.05). There are no significant differences in education level, employment, annual family incomes, gestational weight gain, conception mode, and 1-min Apgar score, as shown in Table 1.
Table 1.
Single factor analysis for DOL II (n = 450).
| Predictor | DOL II group (n = 112) [n (%)] | Non DOL II group (n = 338) [n (%)] | χ2 | P |
|---|---|---|---|---|
| Age (years) | 7.806 | 0.005 | ||
| <35 | 50 (44.6) | 202 (59.8) | ||
| ≥ 35 | 62 (55.4) | 136 (40.2) | ||
| Education level | ||||
| High school or below | 17 (15.2) | 38 (11.2) | 1.315 | 0.518 |
| University | 70 (62.5) | 216 (63.9) | ||
| Post-graduate | 25 (22.3) | 84 (24.9) | ||
| Employment | 0.590 | 0.442 | ||
| Employed | 64 (57.1) | 207 (61.2) | ||
| Unemployed | 48 (42.9) | 131 (38.8) | ||
| Annual family incomes (CNY) | 1.207 | 0.547 | ||
| Low (< 100,000) | 33 (29.5) | 85 (25.1) | ||
| Middle (100,000-400,000) | 63 (56.3) | 193 (57.1) | ||
| High (>400, 000) | 16 (14.2) | 60 (17.8) | ||
| Drinking | 1.249 | 0.264 | ||
| Yes | 4 (3.6) | 6 (1.8) | ||
| No | 108 (96.4) | 332 (98.2) | ||
| Pre-pregnancy BMI | 13.646 | 0.003 | ||
| < 18.5 | 5 (4.4) | 34 (10.0) | ||
| 18.5–24.9 | 61 (54.4) | 223 (66.0) | ||
| 25-29.9 | 30 (26.8) | 55 (16.3) | ||
| ≥ 30 | 16 (14.4) | 26 (7.7) | ||
| Gestational weight gain (GWG) | 1.258 | 0.533 | ||
| Inadequate | 12 (10.7) | 28 (8.3) | ||
| Adequate | 38 (33.9) | 132 (39.1) | ||
| Excessive | 62 (55.4) | 178 (52.6) | ||
| Parity | 9.657 | 0.002 | ||
| Primiparous | 68 (60.7) | 148 (43.8) | ||
| Multiparous | 44 (39.3) | 190 (56.2) | ||
| Conception mode | 0.903 | 0.342 | ||
| Natural | 96 (85.7) | 301 (89.1) | ||
| Artificial | 16 (14.3) | 37 (10.9) | ||
| Gestational age (week) | 8.057 | 0.005 | ||
| ≥ 38 | 97 (88.4) | 320 (91.1) | ||
| < 37 | 15 (11.6) | 18 (8.9) | ||
| Delivery mode | 10.668 | 0.001 | ||
| Vaginal | 52 (46.4) | 216 (63.9) | ||
| Cesarean | 60 (53.6) | 122 (36.1) | ||
| Length of stage II labor | 6.439 | 0.011 | ||
| ≤ 1 h | 37 (33.0) | 158 (46.7) | ||
| > 1 h | 75 (67.0) | 180 (53.3) | ||
| Gestational diabetes | 10.733 | 0.001 | ||
| Yes | 28 (25.0) | 41 (12.1) | ||
| No | 84 (75.0) | 297 (87.9) | ||
| EPDS score | 4.324 | 0.038 | ||
| < 10 points | 71 (63.4) | 249 (73.7) | ||
| ≥ 10 points | 41 (36.6) | 89 (26.3) | ||
| Fatigue Scale-14 | 3.590 | 0.058 | ||
| < 9 points | 67 (60) | 235 (70) | ||
| ≥ 9 points | 45 (40) | 103 (30) | ||
| Apgar, 1-min | 2.194 | 0.139 | ||
| ≥ 7 points | 95 (84.8) | 304 (89.9) | ||
| < 7 points | 17 (15.2) | 34 (10.1) | ||
| Maternal-infant separation | 6.718 | 0.010 | ||
| No | 54 (48.2) | 210 (62.1) | ||
| Yes | 58 (51.8) | 128 (37.9) | ||
| Breastfeeding education during pregnancy | 8.345 | 0.004 | ||
| Yes | 44 (39.3) | 186 (55.0) | ||
| No | 68 (60.7) | 152 (45.0) | ||
| Formula use | 6.107 | 0.013 | ||
| No | 94 (83.9) | 311 (92) | ||
| Yes | 18 (16.1) | 27 (8) | ||
| Onset of breastfeeding in the first hour | 6.347 | 0.012 | ||
| Yes | 88 (78.6) | 298 (88.2) | ||
| No | 24 (21.4) | 40 (11.8) | ||
Assignment of predictive variables
The assignment of predictive variables was shown in Table 2. The score assignments for all factors were as following. Maternal age: 1 point = age ≥ 35, 0 points = age<35; pre-pregnancy BMI: 2 points for ≥ 30.0, 1 point for 25-29.9, and 0 points for 18.5.0-24.9; parity: 0 points = multipara, 1 point = primipara; prematurity: 0 points = no, 1 point = yes; delivery mode: 1 point for cesarean section, 0 points for virginal delivery; length of stage II labor: 0 points for ≤ 1 h, 1 point for > 1 h; GDM; 1 point = yes, 0 points = no; EPDS score: 0 points for EPDS < 10 points, 1 point for EPDS ≥ 10 points; maternal-infant separation: 0 = no, 1 = yes; breastfeeding education during pregnancy: 0 points = yes, 1 point = no; formula use: 0 = no, 1 = yes; onset of breastfeeding in the first hour: 0 point = yes, 1 = no.
Table 2.
Assignment of predictive variables.
| Predictor | 0 points | 1 point | 2 points |
|---|---|---|---|
| Maternal age | <35 | ≥ 35 | |
| Pre-pregnancy BMI | 18.5–24.9 | 25-29.9 | ≥ 30.0 |
| Parity | Multipara | Primipara | |
| Prematurity | No | Yes | |
| Delivery mode | Vaginal | Cesarean | |
| Length of stage II labor | ≤ 1 h | > 1 h | |
| GDM | No | Yes | |
| EPDS score | ≥ 10 | < 10 | |
| Maternal-infant separation | No | Yes | |
| Breastfeeding education during pregnancy | Yes | No | |
| Formula supplementation | No | Yes | |
| Onset of breastfeeding in the first hour | Yes | No |
Multivariate analysis of DOL II risk factors
A multi-factor analysis was conducted, and the variable with P < 0.05 as the independent variable. The predictive variables with statistically significant differences in the univariate analysis were selected and analyzed in the multivariate logistic regression. Logistic analysis showed that maternal age, pre-pregnancy BMI, parity, gestational age, delivery mode, length of stage II labor, GDM, EPDS, breastfeeding education during pregnancy, maternal-infant separation, formula use, and onset of breastfeeding in the first hour were independent risk factors for developing DOL II (P < 0.05). The results of logistic regression analysis were illustrated in Table 3.
Table 3.
Multivariate analysis of influencing factors of DOL II.
| Variable | β | SE | Wald χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Constant | − 1.658 | 0.620 | 30.045 | < 0.001 | 8.306 | – |
| Maternal age | 0.562 | 0.278 | 4.365 | 0.028 | 1.856 | 1.078–3.225 |
| Pre-pregnancy BMI | 1.398 | 0.625 | 5.052 | 0.025 | 4.038 | 1.185–12.650 |
| Prematurity | 0.795 | 0.345 | 5.402 | 0.021 | 2.213 | 1.125–4.355 |
| Parity | 0.625 | 0.280 | 4.980 | 0.026 | 1.858 | 1.078–3.230 |
| Cesarean section | 1.510 | 0.398 | 15.012 | <0.001 | 4.435 | 2.105–9.585 |
| Length of stage II labor | 0.965 | 0.389 | 6.210 | 0.013 | 2.624 | 1.235–5.622 |
| Gestational diabetes | 0.982 | 0.393 | 6.312 | 0.009 | 2.856 | 1.332–5.656 |
| EPDS score | 0.775 | 0.265 | 8.610 | 0.004 | 2.160 | 1.292–3.610 |
| Breastfeeding education during pregnancy | − 1.035 | 0.360 | 5.502 | 0.004 | 2.920 | 1.396–5.712 |
| Maternal-infant separation | 0.624 | 0.279 | 4.980 | 0.026 | 1.865 | 1.078–3.226 |
| Formula use | 1.078 | 0.391 | 7.660 | 0.006 | 2.960 | 1.385–6.402 |
| Onset of breastfeeding in the first hour | − 0.966 | 0.388 | 6.210 | 0.012 | 2.625 | 1.234–5.625 |
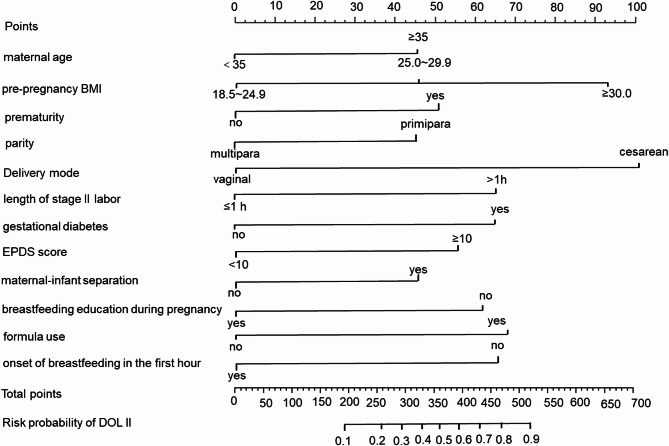
Construction of DOL II risk prediction model
The DOL II nomogram model was developed based on the above independent risk factors identified by multivariate logistic regression analysis (Fig. 2). Logit P=− 1.658 + 0.562×maternal age + 1.398×prenatal overweight or obesity + 0.795×prematurity + 0.625×primipara + 1.510×cesarean section + 0.965×length of stage II labor + 0.982× gestational diabetes + 0.77×EPDS score-1.035×breastfeeding education + 0.624 × maternal-infant separation + 1.078×formula use − 0.966 ×onset of breastfeeding in the first hour. The corresponding points of each factor were obtained, and the points of five factors were added together to obtain the total points. Then the probability of developing DOL II was determined according to the total points.
Fig. 2.
Risk prediction nomogram for DOL II.
Validation of the nomogram model
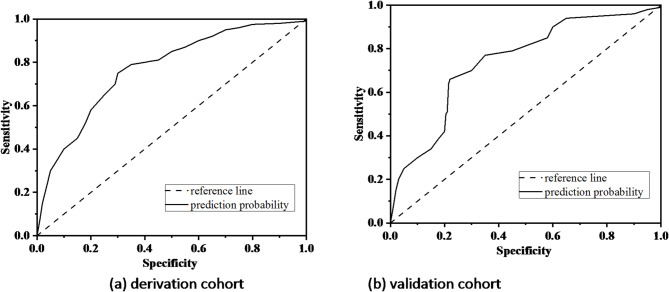
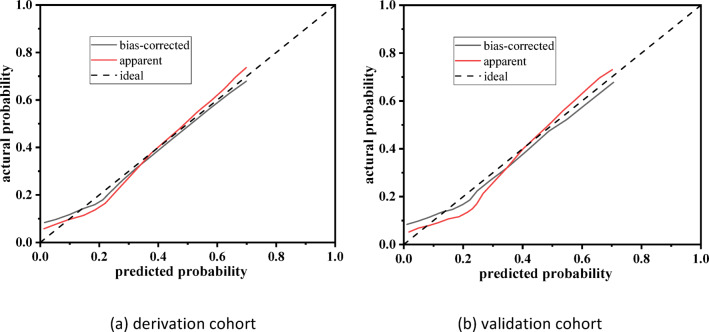
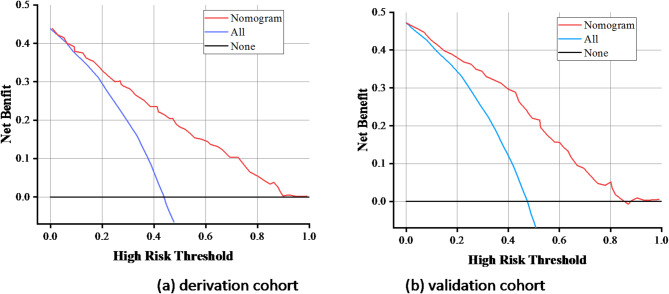
The area under ROC curve (AUC) was used to evaluate the predictive accuracy of the model. The AUC in the modeling group was 0.786 (95%CI: 0.715–0.825, P < 0.001), the specificity was 0.745, the sensitivity was 0.781, and the Yuden index was 1.526 (Fig. 3a). The AUC in the verification group was 0.748 (95%CI: 0.658–0.851, < 0.001), the specificity was 0.715, the sensitivity was 0.778, and the Yuden index was 1.493 (Fig. 3b). It indicated that that the model has a high predictive potential. The calibration cure shown in Fig. 4. There was a good agreement between the predicted probability and observed probability in the derivation cohort and validation cohort. The Brier Score for the calibration curve in the derivation cohort was 0.158, while in the validation cohort it was 0.165. DCA curve was used to evaluate the model’s clinical value14. The nomogram provides a higher net benefit across a range of threshold probability compared to the none and all curve, indicating that the nomogram offers better clinical benefit (Fig. 5). Hosmer–Lemeshow test showed that the P-values of the modeling group and the verification group were 0.825 and 0.256, respectively, indicating that the prediction deviation was not statistically significant.
Fig. 3.
ROC curve for the model. (a) Derivation cohort and (b) validation cohort.
Fig. 4.
Calibration curve for nomogram model. (a) Derivation cohort and (b) validation cohort.
Fig. 5.
DCA for the nomogram. (a) Derivation cohort and (b) validation cohort.
Discussion
This study comprehensively and systemically demonstrated the risk factors associated with DOL II. The results showed that the incidence rate of DOL II was 24.9%. The significantly independent factors include maternal age, pre-pregnancy BMI, prematurity, parity, cesarean section, length of stage II labor, GDM, EPDS, maternal-infant separation, breastfeeding education during pregnancy, formula use, and onset of breastfeeding in the first hour.
Our finding showed that maternal age (OR = 1.856, 95%CI: 1.078–3.225) was an independent risk factor for DOL II, which was consistent with the results of previous studies7,15. A prospective longitudinal observational cohort study in Brazil found that older mothers were at risk of delayed lactogenesis II onset11. A retrospective cohort study in Spain also reported that maternal age at birth was a predictive factor for DOL II3. Kitano in Japan found that older maternal age of ≥ 35 was a negative factor of success of breastfeeding16.
Pre-pregnancy BMI was a significantly independent factor for DOL II. Pregnant women with overweight or obese were at higher risk of DOLII. The pre-pregnancy BMI ≥ 25.0 kg/m2 was an independent risk factor for postpartum DOL II (OR = 4.845, 95%CI: 1.508–15.256). It may be related to the decrease of prolactin in overweight and obese women. Prolactin can increase the lactose concentration, which introduces body fluids into the breast cells, and the milk begins to be secreted in large quantities17. In addition, obesity also increases insulin resistance and insulin is necessary for lactation initiation and mature milk production. Insulin resistance delays the time required for milk to change from colostrum to mature milk. Pre-pregnancy obesity is a risk factor for postpartum DOL II. Therefore, maternal BMI ≥ 25.0 kg/m2 before pregnancy can be used as a predictor of postpartum DOLII.
Gestational age was an independent risk factor for DOLII, which was consistent with the results of previous studies18,19. Gestational age at delivery was strongly associated with timing of the onset of lactogenesis II. Most preterm infants need to be transferred to NICU after birth and therefore were separated from their mothers, which further deteriorated the adverse outcome of DOL II.
Primipartity was more likely to develop DOLII. The results showed that the risk of DOL II in primipara was nearly 2 times that of multipara and primiparity was an independent risk factor for DOL II. This was likely due to several reasons. First, the multiparous women had experienced the peak of prolactin during lactation, which increased the number of prolactin receptor in the mammary gland. Thereby it enhanced the effectiveness and sensitivity of lactation and reduced the risk of DOL II. Second, due to the lack of breastfeeding experience in primipara, postponement of postpartum milk opening time and incorrect feeding posture can lead to a decrease in the number of effective sucking times in newborns and increase the risk of DOL II20.
Cesarean section delivery was an important independent risk factor for DOL II, which was similar to the findings of other investigations. The incidence of women who underwent a cesarean section delivery was much higher (33.3%) than that (24.9%). This was associated with increased maternal stress levels induced by cesarean section. It was also reported that a significantly higher occurrence of DOL II in women who received emergency cesarean section than scheduled cesarean section. In this study, we only investigated the difference between cesarean section delivery and virginal delivery, and we did not separate emergency cesarean section from scheduled cesarean section. With appropriate guidance, mothers who have experienced cesarean section could generally conquer early breastfeeding problem and launch breastfeeding successfully21.
There was a significant association between a prolonged stage II labor duration and DOL II. The association with DOL II was similar to the finding of previous study. A prolonged duration of stage II labor was stressful to both mothers and infants, which resulted in a higher level of cortisol in both of them. A higher level of cortisol was associated with delayed onset of lactation.
Postpartum depression and anxiety remained significantly associated with DOL II. EPDS was applied to assess the depression and anxiety symptoms postpartum22. Postpartum depression, anxiety, and psychological distress had an important neuroendocrine influence on milk production, which was also reported in previous studies23. Mother with a higher degree of anxiety symptom tends to have a lower prolactin and a higher cortisol level. Depression probably played an important role in hormonal metabolism including lactogenesis II disruption etiology. Postpartum depression would also result in behaviors that would not benefit breastfeeding24.
The onset of breastfeeding in the first hour was an independent factor for DOL II. Early onset of breastfeeding in the first hour could prevent the occurrence of DOL II. The newborn sucking could increase the prolactin receptors on lactation cells and facilitate prolactin releases, which could effectively reduce DOL II and shorten the start time of lactation25. Therefore, healthcare workers should provide breastfeeding support in the early postpartum period, inform the importance of early breastfeeding, and guide mothers to breastfeed early to reduce the risk of DOL II.
The formula use may reduce the frequency and duration of breast sucking in newborns, leading to the occurrence of DOL II. Most mothers believe that the small amount of colostrum secreted in the first few days after delivery will not meet the physiological needs of the baby, and therefore add formula to the newborn. In the early sucking process of infants, the physiological reaction caused by milk empties may trigger the early start of lactation II, while creating a stable sucking pattern and milk production pattern and achieving long-term successful breastfeeding. Finally, it should be noticed that breastfeeding education during pregnancy were associated with DOL II. This was in line with the findings of other authors who have also described that educational interventions increased breastfeeding rates26,27.
There are several strengths and limitations in this study. The strength is the model’s strong ability to predict the risk for DOL II for Chinese pregnant women as the risk factors included are comprehensive and in line with clinical requirements. The limitation is that this study is a retrospective cohort study with an inherent risk of information bias, and further prospective studies are needed to verify the results. Secondly, the validation cohort is from a different branch hospital, but they are in the same city, potentially limiting generalizability to other populations with different demographic, clinical, or healthcare system characteristics.
Conclusions
This study developed a nomogram to predict the risk of DOL II for Chinese pregnant women. The independent risk factors included maternal age, pre-pregnancy BMI, prematurity, parity, cesarean section, length of stage II labor, GDM, EPDS, maternal-infant separation, breastfeeding education during pregnancy, formula use, and onset of breastfeeding in the first hour. This nomogram exhibits strong discriminatory and calibration capabilities, which can help identify women who are at high risk of DOL II.
Acknowledgements
We would like to thank all participants in this study.
Author contributions
Wenting Li: Investigation, original draft, Writing—review & editing; Guofang Kuang: Investigation, Methodology, Data curation; Yanqiu Chen: Investigation, Methodology, Data curation; Peng Yu: original draft, Investigation, Methodology, Data curation. Meng Zhang: Conceptualization, original draft, Writing—review & editing, Supervision.
Funding
This study was supported by the Clinical Medicine + X Project of the Affiliated Hospital of Qingdao University (QDFY + X 2023125).
Data availability
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. Breastfeeding 2021. https://www.whoint/health-topics/breastfeeding#tab=tab_1.
- 2.Pérez-Escamilla, R. et al. Breastfeeding: crucially important, but increasingly challenged in a market-driven world. Lancet401 (10375), 472–485 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Ballesta-Castillejos, A., Gómez-Salgado, J., Rodríguez-Almagro, J. & Hernández-Martínez, A. Development and validation of a predictive model of exclusive breastfeeding at hospital discharge: retrospective cohort study. Int. J. Nurs. Stud.117, 103898 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Xu, F., Qiu, L., Binns, C. W. & Liu, X. Breastfeeding in China: a review. Int. Breastfeed. J.4 (1), 1–15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Victora, C. G. et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet387 (10017), 475–490 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Rollins, N. C. et al. Why invest, and what it will take to improve breastfeeding practices? Lancet387 (10017), 491–504 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Miao, Y. et al. Prevalence and risk factors of delayed onset lactogenesis II in China: a systematic review and meta-analysis. J. Maternal-Fetal Neonatal Med.36 (1), 2214833 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Kelly, N. M., Smilowitz, J. T., Cagney, O., Flannery, R. L. & Tribe, R. M. Delayed onset of lactogenesis and reduced breastfeeding frequency in mothers who give birth by caesarean section. Proc. Nutr. Soc.79, E445 (2020).
- 9.Henkel, A. et al. Lactogenesis and breastfeeding after immediate vs delayed birth-hospitalization insertion of etonogestrel contraceptive implant: a noninferiority trial. Am. J. Obstet. Gynecol.228 (1), 55 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Farah, E., Barger, M. K., Klima, C., Rossman, B. & Hershberger, P. Impaired lactation: review of delayed lactogenesis and insufficient lactation. J. Midwifery Women’s Health. 66 (5), 631–640 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Rocha, B. O. et al. Risk factors for delayed onset of lactogenesis II among primiparous mothers from a Brazilian baby-friendly hospital. J. Hum. Lactation. 36 (1), 146–156 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Dong, D. et al. A prospective cohort study on lactation status and breastfeeding challenges in mothers giving birth to preterm infants. Int. Breastfeed. J.17 (1), 6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman, D. J. & Perez, E. R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J. Nutr.130 (12), 2972–2980 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Xiao, X., Feng, Z., Li, T., Qiao, H. & Zhu, Y. Predictive nomogram of ultrasound indicators for the termination outcome of caesarean Scar pregnancy. Sci. Rep.14 (1), 31378 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whipps, M. D. Education attainment and parity explain the relationship between maternal age and breastfeeding duration in US mothers. J. Hum. Lactation. 33 (1), 220–224 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Kitano, N. et al. Combined effects of maternal age and parity on successful initiation of exclusive breastfeeding. Prev. Med. Rep.3, 121–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadr Dadres, G. et al. Relationship of maternal weight status before, during, and after pregnancy with breast milk hormone concentrations. Obesity27 (4), 621–628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinoshita, M., White, M. J. & Doolan, A. Clinical assessment of breastfeeding in preterm infants. Eur. J. Clin. Nutr.1, 1–5 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu, X., Li, J., Lin, X. & Luan, D. Association between delayed lactogenesis II and early milk volume among mothers of preterm infants. Asian Nurs. Res.13 (2), 93–98 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Nommsen-Rivers, L. A., Chantry, C. J., Peerson, J. M., Cohen, R. J. & Dewey, K. G. Delayed onset of lactogenesis among first-time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. Am. J. Clin. Nutr.92 (3), 574–584 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Lian, W., Ding, J., Xiong, T., Liuding, J. & Nie, L. T. Determinants of delayed onset of lactogenesis II among women who delivered via Cesarean section at a tertiary hospital in China: a prospective cohort study. Int. Breastfeed. J.17 (1), 81 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathisen, S. E., Glavin, K., Lien, L. & Lagerløv, P. Prevalence and risk factors for postpartum depressive symptoms in Argentina: a cross-sectional study. Int. J. Women’s Health 787–793 (2013). [DOI] [PMC free article] [PubMed]
- 23.Lindau, J. F. et al. Determinants of exclusive breastfeeding cessation: identifying an at risk population for special support. Eur. J. Pediatrics. 174, 533–540 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Zhao, Y., Lin, Q. & Wang, J. An evaluation of a prenatal individualised mixed management intervention addressing breastfeeding outcomes and postpartum depression: a randomised controlled trial. J. Clin. Nurs.30 (9–10), 1347–1359 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Parker, L. A., Sullivan, S., Kruger, C. & Mueller, M. Timing of milk expression following delivery in mothers delivering preterm very low birth weight infants: a randomized trial. J. Perinatol.40 (8), 1236–1245 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Cole, J., Bhatt, A., Chapple, A. G., Buzhardt, S. & Sutton, E. F. Attitudes and barriers to breastfeeding among women at high-risk for not breastfeeding: a prospective observational study. BMC Pregnancy Childbirth. 24 (1), 81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan, M. Y., Ip, W. Y. & Choi, K. C. The effect of a self-efficacy-based educational programme on maternal breast feeding self-efficacy, breast feeding duration and exclusive breast feeding rates: A longitudinal study. Midwifery36, 92–98 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during and analyzed during the current study are available from the corresponding author on reasonable request.