Abstract
Background
Targeted therapy has significantly transformed the treatment landscape of colorectal cancer (CRC), enabling personalized treatment approaches and improving patient prognosis. This study employs bibliometric analysis to explore the research hotspots and development trends in the field of CRC-targeted therapy from 2000 to 2023.
Methods
Based on the Web of Science Core Collection, this study collected literature related to CRC-targeted therapy published between 2000 and 2023. CiteSpace and VOSviewer were used for data analysis, with a focus on publication trends, key contributors, and keyword co-occurrence patterns.
Results
A total of 2252 relevant articles were included, demonstrating a steady growth trend in research output. China ranked first in terms of the number of publications, while the University of Texas MD Anderson Cancer Center was identified as the institution with the highest research output. Josep Tabernero was the most prolific author in this field. Among journals, Cancers had the highest impact, while Clinical Cancer Research held a significant advantage in citation frequency. Keyword co-occurrence and clustering analysis indicated that research primarily focused on treatment strategies and precision medicine, with emerging technologies such as cell therapy and liquid biopsy garnering increasing attention.
Conclusion
This study reveals the research trends, core hotspots, and emerging directions in the field of CRC-targeted therapy, providing valuable insights for future research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02632-x.
Keywords: Colorectal cancer, Targeted therapy, Bibliometric analysis, Data visualization
Introduction
Colorectal cancer (CRC) is a major global public health concern, ranking third in cancer incidence and second in cancer-related mortality worldwide [1, 2]. While conventional treatments, including surgery, chemotherapy, and radiation therapy, have demonstrated efficacy, the prognosis for advanced-stage CRC remains poor due to high mortality rates and limited therapeutic options [3–6]. This highlights the urgent need for more precise and effective treatment strategies.
In recent decades, targeted therapies have transformed CRC management by facilitating personalized treatment approaches that selectively disrupt molecular pathways driving tumor progression. Agents targeting the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) pathways have significantly improved both survival outcomes and quality of life for CRC patients [7, 8]. Furthermore, the advent of precision oncology has expanded therapeutic options through strategies tailored to specific genetic alterations, such as RAS, BRAF, and HER2 mutations, as well as the use of immune checkpoint inhibitors for microsatellite instability-high (MSI-H) CRC [9–14].
Despite these advancements, several challenges remain. Tumor heterogeneity contributes to diverse therapeutic responses and the emergence of resistance, complicating treatment effectiveness. Moreover, metastatic CRC remains particularly difficult to manage due to its aggressive nature and limited curative options [15, 16]. The rapid expansion of CRC-targeted therapy research has also led to a highly fragmented knowledge base, posing challenges for synthesizing findings and identifying overarching trends. Key areas requiring further investigation include the mechanisms of drug resistance, the role of the tumor microenvironment in treatment response, and advances in biomarker development, such as liquid biopsies.
To address these gaps, this study employs a bibliometric analysis to systematically map the intellectual landscape of CRC-targeted therapy research from 2000 to 2023. The study aims to: (1) identify influential publications, researchers, and institutions shaping the field; (2) elucidate key research hotspots, including resistance mechanisms, novel molecular targets, and combination therapies; and (3) highlight emerging trends, such as advances in immunotherapy and innovative therapeutic strategies. Through an in-depth analysis of publication patterns, citation networks, and keyword co-occurrence, this study seeks to provide comprehensive insights into the evolution of CRC-targeted therapy and offer guidance for future research directions.
Materials and methods
Data collection and search strategy
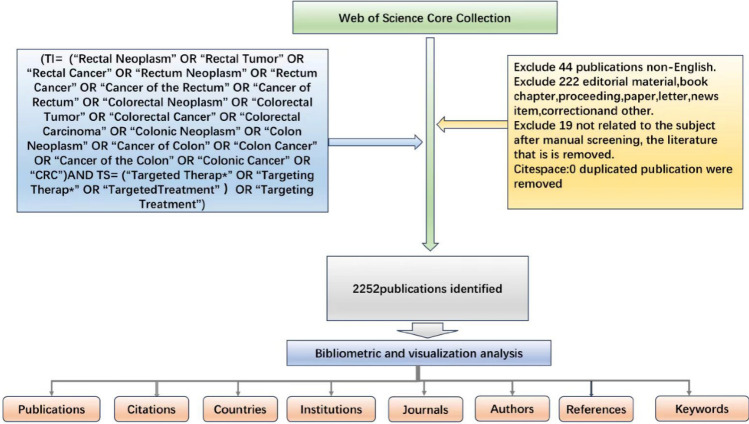
This study utilized the Web of Science Core Collection (WoSCC) database, a globally recognized authoritative platform for bibliometric research, which covers a vast amount of peer-reviewed literature [17]. To ensure comprehensive literature retrieval, we incorporated Medical Subject Headings (MeSH) into our search strategy. MeSH is a controlled vocabulary widely used in PubMed for indexing and retrieving biomedical literature, enhancing the accuracy and coverage of literature searches. In addition, we included Entry Terms associated with MeSH terms. These Entry Terms are synonyms or closely related terms that further optimize the search process. In this study, we queried the MeSH database (https://www.ncbi.nlm.nih.gov/MeSH/) using the keywords “colorectal cancer” and “targeted therapies.” The results identified 15 Entry Terms related to colorectal cancer (CRC) and 7 Entry Terms related to targeted therapies. Based on these MeSH terms, Entry Terms, and our research objectives, we developed a comprehensive search formula to ensure thoroughness and accuracy. The search strategy and formula are outlined in Fig. 1.
Fig. 1.
Detailed flowchart steps of the search strategy in screening publications
Literature retrieval was performed using the Topic Search (TS) and Title (TI) functions in WoSCC, focusing on English-language articles related to colorectal cancer and targeted therapies published between January 1, 2000, and December 31, 2023. This study selects the period from 2000 to 2023 for the following reasons: First, after 2000, the clinical application of targeted drugs such as cetuximab and bevacizumab significantly transformed the treatment paradigm for colorectal cancer. Second, advancements in genomics and precision medicine during this period accelerated the development of targeted therapies. Finally, the research data was downloaded in November 2024. Considering the publication lag in academic research, data from 2024 may still be incomplete. Limiting the study period to 2023 ensures a more comprehensive and reliable dataset. The document types were restricted to research articles and review articles, explicitly excluding conference abstracts, editorials, conference papers, early access publications, letters, corrections, and book chapters. The search was conducted on November 1, 2024, yielding a total of 2271 articles. To ensure relevance and data quality, a manual screening process was applied based on content and quality criteria outlined in Supplementary Table 1. This process excluded 19 irrelevant articles, and redundant entries were removed using CiteSpace software [18, 19]. After these refinements, a total of 2252 articles were confirmed for inclusion in the analysis
Data analysis
A variety of analytical techniques and software tools were employed to explore the dataset and visualize trends in CRC-targeted therapy research. The analysis focused on identifying patterns in publication trends, journal distributions, co-authorship networks, and research hotspots, providing a foundational understanding of the research landscape. The temporal distribution of publications and journal contributions was visualized using Origin Pro 2023, which generated graphs illustrating annual publication volumes, journal output, and other key metrics. These visualizations offered a preliminary overview of the field's growth and evolution over time.
VOSviewer (version 1.6.20) and CiteSpace (version 6.1R6) were used for bibliometric analysis to explore the intellectual structure and collaborative networks in the field. VOSviewer was utilized in both Overlay Visualization and Network Visualization modes to map co-authorship, co-citation, and keyword co-occurrence networks. Parameters such as Resolution (set to 1.00) and Minimum Cluster Size (set to 1) were dynamically adjusted to optimize graphical clarity and enhance interpretability. These network maps revealed relationships between key authors, institutions, and topics, highlighting significant areas of collaboration and research focus.
Simultaneously, CiteSpace was used for a more detailed bibliometric network analysis. Data were input using the"Full Record with Cited References"option, and time slicing was implemented with one-year granularity for precise temporal analysis of research trends [20]. The cosine algorithm was applied to calculate the strength of connections between nodes, and the top 10% of nodes in each time slice were extracted to focus on the most influential contributions. Advanced pruning techniques, such as Pathfinder Pruning, were employed to refine network structures and eliminate irrelevant connections, ensuring a clear and concise representation of the data. Additionally, keyword clustering was performed using the Latent Semantic Indexing (LSI) algorithm, which grouped keywords into meaningful semantic clusters and assigned descriptive labels based on their contextual relationships. These clusters provided insights into prominent research areas and emerging trends, such as advancements in combination therapies, resistance mechanisms, and immunotherapy integration.
Quality control and visualization
To enhance the quality of visualizations, parameters such as scale, labels, and lines were dynamically adjusted. Final plots were reviewed to ensure clarity and aesthetic appeal, supporting the interpretability of results.
Results
Database search and data overview
This study retrieved a total of 2252 publications authored by 13,853 researchers from 88 countries, affiliated with 3400 institutions. These publications were disseminated across 596 journals and collectively cited 78,385 articles from 6183 distinct journals. This dataset provides a comprehensive foundation for analyzing the current research landscape on targeted therapy for colorectal cancer (CRC).
Analysis of publication and citation trends
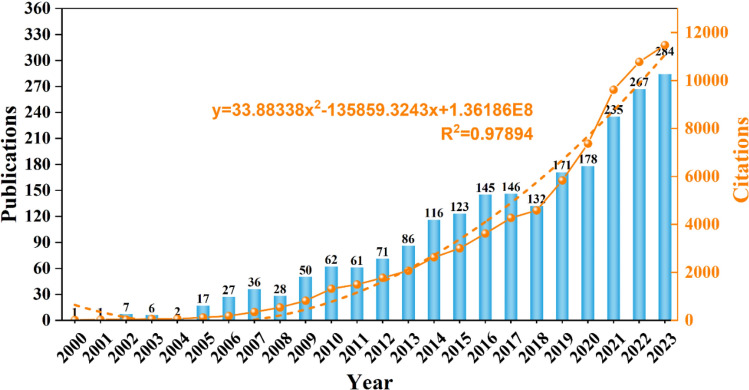
The annual publication volume and citation trends are illustrated in Fig. 2. Research in this field dates back to the year 2000, with the number of annual publications first exceeding double digits in 2005. This year marked a turning point, coinciding with the emergence of targeted therapy as a promising treatment strategy for colorectal cancer. The publication count peaked in 2023, reaching 284 articles. Regarding citation trends, the number of citations increased from just 17 in 2000 to 11,472 in 2022, with a particularly sharp rise since 2018. A polynomial fitting analysis demonstrated a strong correlation between citation frequency and publication year (R2 = 0.9615), indicating that targeted therapy for colorectal cancer has increasingly become a focal point of research over the past two decades.
Fig. 2.
Annual publication and citation trends of targeted therapy for colorectal cancer from 2000 to 2023. Analysis of contributions by countries
Analysis of contributions by countries
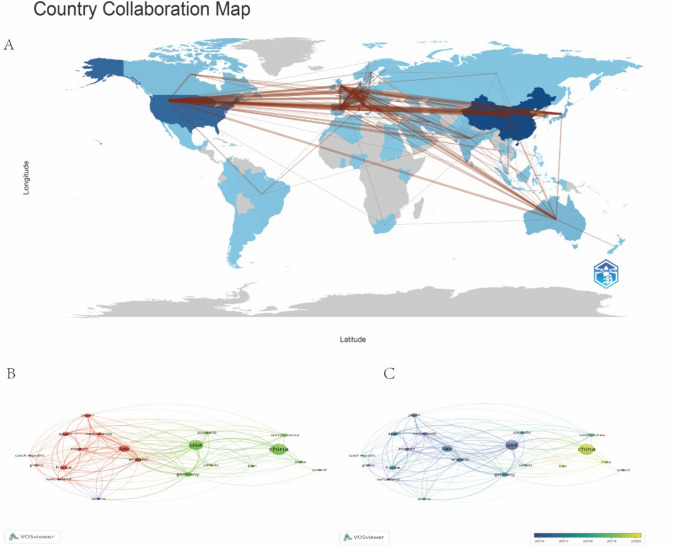
Research on targeted therapy for colorectal cancer (CRC) is characterized by extensive global collaboration. The retrieved literature spans 88 countries and regions, highlighting the strong cooperative relationships among them. Figure 3A presents the publication statistics and collaboration patterns between different countries/regions. The United States and China are the leading contributors to this field, with 503 and 618 publications, respectively. However, a notable disparity exists in citation counts: the United States has accumulated 28,450 citations, whereas China has received 13,808. Figure 3B illustrates the extensive collaboration among the top 20 countries/regions in terms of publication volume. Major partnerships are observed among the United States, Europe, and East Asia, with China increasingly strengthening its ties with these regions. Figure 3C further highlights China's expanding influence in this field, particularly in recent years, as advancements in molecular diagnostics and translational research have significantly enhanced its impact. The interplay between research quantity and quality underscores the necessity of fostering cross-regional collaborations to maximize the scientific and clinical impact of CRC targeted therapy research.
Fig. 3.
Global publication and collaboration in targeted therapy for CRC. A Geographic distribution of global publications on targeted therapy for CRC, illustrated on a world map. B Network visualization map showing collaboration among the top 20 countries in the field. C Overlay visualization map highlighting trends and temporal evolution in research collaboration
Analysis of contributions by institutions
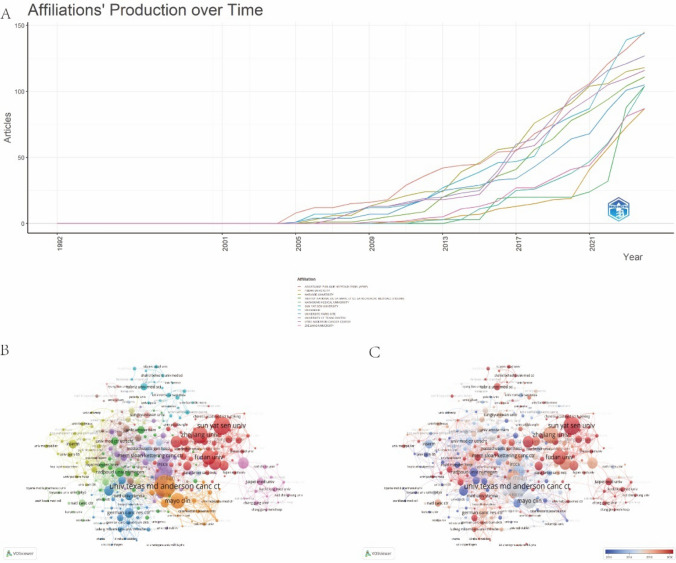
As shown in Fig. 4A, the number of publications by global institutions in the field of colorectal cancer (CRC) targeted therapy has steadily increased since 2000. Notably, European institutions, such as Paris Public Hospitals and the French Comprehensive Cancer Center, have demonstrated the most rapid growth. Figure 4B presents a collaboration network comprising 292 institutions, where the size of each node represents the number of publications, and the width of each connecting line indicates the strength of collaboration. Fourteen distinct clusters, represented in different colors, illustrate the cooperative relationships among these institutions. Among them, the University of Texas MD Anderson Cancer Center and Mayo Clinic in the United States, along with Sun Yat-sen University and Zhejiang University in China, lead in publication volume and maintain extensive collaborations with other institutions. Figure 4C further highlights the increasing influence of Chinese institutions in this field, particularly Sun Yat-sen University and Zhejiang University. These institutions not only rank highly in terms of publication output but have also significantly strengthened their collaborative networks in recent years.
Fig. 4.
Analysis of institutions in the field of targeted therapy for CRC. A Trend of the number of articles in the top 10 most productive institutions. B A network visualization map. C A overlay visualization map
Analysis of contributions by prolific and co-cited authors
The literature retrieval analysis indicates that 13,853 authors and 50,342 co-cited authors have contributed to research on colorectal cancer (CRC) targeted therapy. Table 1 presents the top ten most prolific authors and the most frequently co-cited authors in this field. Josep Tabernero stands out as the most productive author, with 30 published papers. Salvatore Siena ranks first in citation count, with 3015 citations, and his influential research has significantly impacted clinical practice in CRC treatment. Federica Di Nicolantonio has also made substantial contributions, with an impressive average citation count of 140.313 per publication. The co-citation analysis (see Table 1) highlights the critical role of foundational figures in the field. Notably, Eric Van Cutsem is the most frequently co-cited author, with 1510 co-citations, underscoring his significant influence on CRC research.
Table 1.
The top 10 authors and co-citation authors related to targeted therapy for colorectal cancer
| Rank | Author | Documents | Citayions | Average citations | H-index | Co-cited authors | Citations |
|---|---|---|---|---|---|---|---|
| 1 | Josep Tabernero | 30 | 2270 | 75.667 | 116 | Van Cutsem, et al. | 1510 |
| 2 | Bardelli, Alberto | 27 | 2782 | 103.037 | 12 | Douillard JY | 586 |
| 3 | Siena, Salvatore | 24 | 3015 | 125.625 | 93 | Saltz, LB | 493 |
| 4 | Ciardiello, Fortunato | 24 | 1898 | 79.083 | 55 | Sartore, Bianchi, A | 482 |
| 5 | Tejpar, Sabine | 20 | 2673 | 133.65 | 74 | Kopetz, S | 470 |
| 6 | Scott Kopetz | 20 | 1528 | 76.4 | 91 | De, Rock, W | 467 |
| 7 | Andrea Sartore-Bianchi | 19 | 2368 | 124.632 | 63 | Cunningham, D | 457 |
| 8 | Elena Élez | 17 | 723 | 42.529 | 37 | Grothey, A | 423 |
| 9 | Federica Di Nicolantonio | 16 | 2245 | 140.313 | 56 | Siegel, RL | 398 |
| 10 | Punt, Cornelis J | 16 | 1219 | 76.188 | 54 | Bokemeyer, C | 386 |
Analysis of contributions by journals
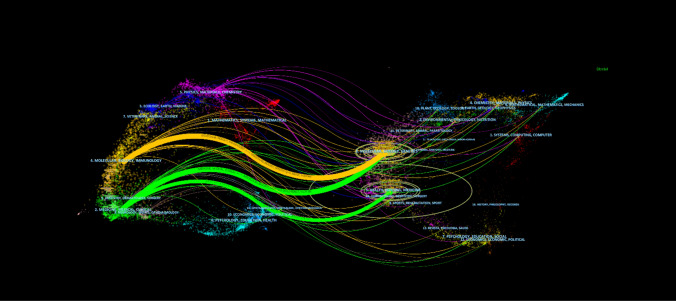
From 2000 to 2023, research on colorectal cancer (CRC) targeted therapy was published in 596 journals. Table 2 presents the top ten journals with the highest number of articles on this topic. Among them, Cancers ranks first, publishing 95 articles (impact factor: 4.5). Although Clinical Cancer Research holds the highest impact factor (10.4), it has fewer publications, with only 37 articles. In contrast, the Journal of Clinical Oncology leads in citation count, with 11,950 citations (impact factor: 42.1). To further analyze the development trends in CRC targeted therapy research, a dual-map overlay method was employed to analyze the citation flow of literature published from 2000 to 2023 (Fig. 5). This figure illustrates the citation relationships between journals: those on the left represent applied fields, while those on the right represent research foundations. Curves between them show citation connections. Three main citation paths emerge from the analysis: the orange path links molecular biology/genetics to molecular biology/immunology, highlighting the translational nature of CRC research. The two green paths emphasize the prominence of clinical and medical journals, underscoring the strong focus on patient-centered innovations and therapeutic applications. Based on these trends, the dual-map overlay method visually predicts a gradual shift in the focus of CRC targeted therapy research toward medical, healthcare, and clinical fields
Table 2.
The top 10 journals with publications and top 10 co-cited journals with citations in the field of targeted therapy for colorectal cancer
| Rank | Journal | Documents | Citations | Average citations | JCR | Impact factor (2023) | Co-cited journal | Citations | JCR | Impact factor (2023) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cancers | 95 | 1457 | 15.3368 | 1 | 4.5 | Journal of Clinical Oncology | 11,950 | 1 | 42.1 |
| 2 | Clinical Colorectal Cancer | 70 | 1663 | 23.7571 | 2 | 3.3 | The New England Journal of Medicine | 4379 | 1 | 96.3 |
| 3 | Frontiers in Oncology | 65 | 911 | 14.0154 | 2 | 3.5 | Clinical CancerResearch | 4305 | 1 | 10.4 |
| 4 | Oncotarget | 44 | 1335 | 30.3409 | 2 | 5.168 | Ccancer Research | 4135 | 1 | 12.5 |
| 5 | International Journal of Molecular Sciences | 40 | 938 | 23.45 | 1 | 4.9 | Annals of Oncology | 3921 | 1 | 56.7 |
| 6 | Plos One | 40 | 1557 | 38.925 | 1 | 2.9 | Nature | 3062 | 1 | 50.5 |
| 7 | Clinical Cancer Research | 37 | 2579 | 69.7027 | 1 | 10.4 | Lancet Oncology | 2609 | 1 | 41.6 |
| 8 | World Journal of Gastroenterology | 37 | 2032 | 54.9189 | 1 | 4.3 | British Journal of Cancer | 2598 | 1 | 6.4 |
| 9 | 0ncology Letters | 32 | 608 | 19 | 3 | 2.5 | European Journal of Cancer | 1696 | 1 | 7.6 |
| 10 | BMC Cancer | 30 | 588 | 19.6 | 2 | 3.4 | PNAS | 1682 | 1 | 9.4 |
Fig. 5.
The dual-map overlay of journals related to targeted therapy for CRC
Analysis of references and co-cited references
Co-cited literature refers to documents that are frequently cited together by multiple studies within a given research domain. To investigate the background and foundational knowledge of colorectal cancer (CRC) targeted therapy, Table 3 presents the ten most co-cited documents. Among them, the study titled"Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer"ranks first, with 307 co-citations, followed by"Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer,"which has been co-cited 304 times. Both papers were published in The New England Journal of Medicine in 2004. Research on bevacizumab played a pivotal role in establishing anti-angiogenesis therapy as a cornerstone of CRC treatment, contributing to the standardization of combination regimens involving targeted therapy and chemotherapy. Additionally, this research stimulated further exploration of tumor angiogenesis and the tumor microenvironment.
Table 3.
The top 10 co-cited references involved in research on neoadjuvant therapy for triple negative breast cancer
| Rank | Co-cited reference | Citations | Journal | Types |
|---|---|---|---|---|
| 1 | Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–42. doi: 10.1056/NEJMoa032691 | 307 | New England Journal of Medicine | Clinical Trial |
| 2 | Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004 Jul 22;351(4):337–45. doi: 10.1056/NEJMoa033025 | 304 | New England Journal of Medicine | Clinical Trial |
| 3 | Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009 Apr 2;360(14):1408–17. doi: 10.1056/NEJMoa0805019 | 276 | New England Journal of Medicine | Clinical Trial |
| 4 | Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008 Apr 1;26(10):1626–34. doi: 10.1200/JCO.2007.14.7116 | 269 | Journal of Clinical Oncology | Clinical Trial |
| 5 | K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008 Oct 23;359(17):1757–65. doi: 10.1056/NEJMoa0804385 | 261 | New England Journal of Medicine | Clinical Trial |
| 6 | Effects of KRAS, BRAF, NRAS, and PIK3 CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010 Aug;11(8):753–62. doi: 10.1016/S1470-2045(10)70130-3 | 234 | Lancet Oncology | Clinical Trial |
| 7 | Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013 Sep 12;369(11):1023–34. doi: 10.1056/NEJMoa1305275 | 233 | New England Journal of Medicine | Clinical Trial |
| 8 | Global cancer statistics., 61(2), 69–90. doi: 10.3322/caac.20107 | 228 | Original Article | |
| 9 | Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008 Dec 10;26(35):5705–12. doi: 10.1200/JCO.2008.18.0786 | 194 | Journal of Clinical Oncology | Clinical Trial |
| 10 | The consensus molecular subtypes of colorectal cancer. Nat Med. 2015 Nov;21(11):1350–6. doi: 10.1038/nm.3967 | 194 | Nature Medicine | Original Article |
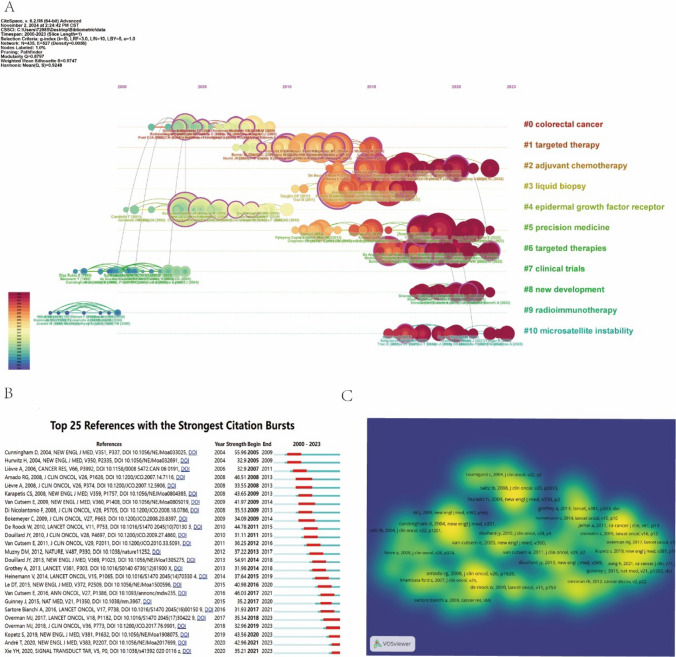
Figure 6A illustrates the timeline view of co-cited literature, as identified by CiteSpace. Nodes of varying colors along the same line represent references from different years, with those positioned further to the right indicating more recent citations. The ten largest co-citation clusters are as follows:"Colorectal Cancer"(Cluster 0),"Targeted Therapy"(Cluster 1),"Adjuvant Chemotherapy"(Cluster 2),"Liquid Biopsy"(Cluster 3),"Epidermal Growth Factor Receptor"(Cluster 4),"Precision Medicine"(Cluster 5),"Targeted Therapy"(Cluster 6),"Clinical Trials"(Cluster 7),"New Developments"(Cluster 8),"Radiation Immunotherapy"(Cluster 9), and"Microsatellite Instability"(Cluster 10). Among these, Cluster #4 is the largest, while Cluster #10 represents the most recent research focus. The evolution of these clusters over time highlights a progressive shift toward molecular biomarkers, novel treatment strategies (such as immunotherapy and targeted therapy), and the growing role of precision medicine. These developments reflect an increasing emphasis on genomic analysis in clinical decision-making.
Fig. 6.
Analysis of co-cited references and their visualizations. A The largest 10 clusters of co-cited references identified through cluster analysis. B Visual analysis of reference bursts, highlighting periods of heightened citation activity. C Density visualization map of the top 50 co-cited references, emphasizing the centrality and impact of key references
Citation bursts refer to documents that experience a significant surge in citation frequency within a specific period, indicating their role in shaping research trends. Such analysis provides valuable insights into the evolution of research hotspots. Figure 6B highlights the top 25 documents with the strongest citation bursts, where red bars denote periods of high citation frequency, and blue bars indicate lower frequencies. Furthermore, the density distribution in Fig. 6C underscores the central role of key documents, which serve as foundational pillars for ongoing CRC targeted therapy research.
Analysis of keywords
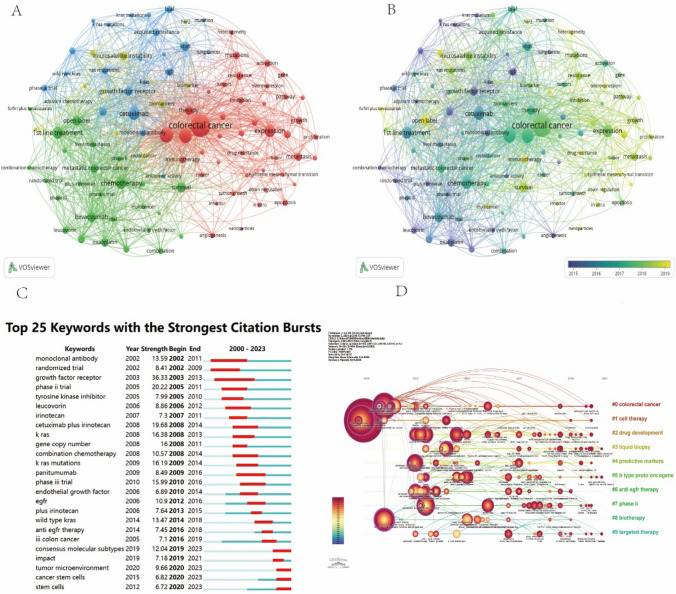
Keyword clustering involves grouping keywords with similar themes into multiple clusters, while co-occurrence analysis identifies relationships between different topics within a discipline. Figure 7A presents 102 keywords with a frequency exceeding 40, categorized into four distinct clusters, each representing a major research theme. Cluster 1 (Red): This cluster focuses on the molecular mechanisms underlying colorectal cancer (CRC), including gene expression, apoptosis, angiogenesis, and genetic mutations. Understanding alterations in gene expression is essential for elucidating tumor development. Additionally, investigating how tumor cells evade immune surveillance and apoptosis is critical for designing novel therapies. Angiogenesis, which facilitates tumor growth and metastasis, along with mutations in key genes such as APC, TP53, and KRAS, significantly influences CRC onset and treatment response. Research in this domain lays the theoretical foundation for molecular-targeted therapies and plays a crucial role in advancing precision medicine for CRC. Cluster 2 (Green): This cluster highlights chemotherapy and targeted therapy strategies, encompassing traditional chemotherapy, emerging targeted therapies, combination treatments, and clinical trials. While chemotherapy remains a standard treatment for CRC, its clinical application is often constrained by severe side effects. Consequently, research efforts are directed toward optimizing therapeutic strategies to enhance efficacy and reduce toxicity. Targeted therapies that inhibit key molecules such as EGFR, VEGF, and BRAF offer more precise cancer cell suppression. Combination therapies, which integrate chemotherapy with targeted agents, have demonstrated improved treatment outcomes. Additionally, clinical trials drive the development of novel drugs and personalized treatment approaches, further diversifying and refining therapeutic interventions. Cluster 3 (Yellow): This cluster emphasizes biomarkers and MSI, which offer valuable insights into personalized therapy and immunotherapy. Biomarker research is instrumental in early diagnosis, prognosis assessment, and treatment monitoring for CRC. MSI represents a key molecular characteristic, particularly in hereditary nonpolyposis colorectal cancer (HNPCC). Studies indicate that patients with high MSI (MSI-H) exhibit favorable responses to immune checkpoint inhibitors such as PD-1/PD-L1 inhibitors. Thus, MSI research is crucial for guiding the clinical application of immunotherapy in precision oncology. Cluster 4 (Blue): This cluster focuses on EGFR, BRAF, KRAS, and monoclonal antibodies, which are pivotal in personalized CRC treatment. EGFR serves as a crucial therapeutic target, while mutations in BRAF and KRAS not only influence disease progression but also affect patient responses to targeted therapies such as cetuximab and panitumumab. These monoclonal antibodies are particularly effective in KRAS wild-type patients. Investigating these genetic alterations is vital for developing personalized treatment strategies, optimizing therapy selection, and minimizing adverse effects.
Fig. 7.
Visualizations of network, overlay, and keyword analyses in CRC-targeted therapy. A Visualizations of network, overlay, and keyword analyses in CRC-targeted therapy. B An overlay visualization map displaying the temporal evolution of research focus areas. C The top 25 terms with the most significant citation bursts, highlighting periods of intensive focus. D A timeline and keyword clustering display showing the temporal trends and clustering of research topics
Figure 7B highlights recent research hotspots, including drug resistance, the tumor microenvironment, immunotherapy, and novel therapeutic targets, which have remained relevant from 2000 to the present. Burst word analysis offers valuable insights into emerging research trends and their temporal dynamics. Figure 7C illustrates keyword bursts in CRC targeted therapy over the past two decades. Notably, keywords still experiencing bursts include molecular profiling, tumor microenvironment, cancer stem cells, and stem cell mapping, suggesting potential future research directions. Figure 7D presents a keyword clustering timeline, delineating temporal trends and research cycles. Keywords such as colorectal cancer, cell therapy, drug treatment, and liquid biopsy have remained prominent research areas since 2000, indicating their enduring significance in the field.
Discussion
Publication overview
This study, based on the WoSCC database, retrieved 2252 relevant articles published between January 1, 2000, and December 31, 2023, spanning 86 countries, 3400 institutions, and 13,853 authors. The findings indicate a notable upward trend in research publications in the field of colorectal cancer (CRC) targeted therapy, with particularly rapid growth in recent years. This surge is primarily driven by breakthroughs in immunotherapy, precision medicine, and the development of new targeted drugs. It is anticipated that research in this area will continue to increase in the coming years.
Among the top contributing countries, China ranks first in publication volume, followed by the United States. However, the total citation count for the United States is more than double that of China, reflecting its greater academic influence. This disparity is closely linked to the United States'sustained investment in scientific research, its well-established research infrastructure, and its extensive international collaborations. For instance, research output from the University of Texas MD Anderson Cancer Center has shown consistent growth in recent years, benefiting from robust funding support and interdisciplinary collaboration. While China has made substantial progress in cancer research, its academic influence is still developing. With continued government investment in biomedical research and enhanced international cooperation, China is expected to bridge this gap and play an increasingly prominent role in global CRC targeted therapy research.
It is noteworthy that five of the ten most productive institutions globally are based in China. For example, Sun Yat-sen University Cancer Center developed an innovative triple-targeted immunotherapy regimen, which achieved breakthrough results in the CAPability-01 study, significantly improving patient clinical response rates (nearly doubling) and progression-free survival (extended fivefold) [21]. Additionally, the Institute of Pharmaceutical Sciences at Zhejiang University optimized cetuximab through a structure-guided and phage-assisted evolution (SGAPAE) approach, successfully overcoming resistance issues caused by mutations in the EGFR extracellular domain (ECD) [22].
In terms of author contributions, Josep Tabernero from the Vall d'Hebron Institute of Oncology (VHIO) is recognized as one of the leading scholars in this field. His research contributed to the establishment of standardized treatment strategies for CRC patients with the BRAF V600E mutation, involving a combination of BRAF inhibitors (encorafenib), MEK inhibitors (binimetinib), and EGFR inhibitors (cetuximab) [23]. Salvatore Siena, with 3,015 citations, leads in citation count and has made significant contributions to the development of anti-EGFR therapies, particularly through research on K-RAS and B-RAF mutations [24].
Regarding journal impact, the top 11 journals published 21.8% of the relevant articles, underscoring their academic dominance in the field of CRC targeted therapy. Cancer published 95 papers (impact factor 4.5), covering basic research, clinical trials, and translational medicine, and its high publication volume has bolstered its academic influence. Clinical Cancer Research (impact factor 10.4), though publishing fewer papers (37), is renowned for its rigorous peer review process and high-quality clinical translational research, making it particularly suitable for innovative and clinically relevant studies. Journal of Clinical Oncology (JCO), with 11,950 citations (impact factor 42.1), ranks first among cancer journals globally and prioritizes research with direct clinical applications. Researchers should select journals based on their focus, review standards, and alignment with research goals to enhance the visibility and academic impact of their work.
Research foundation
This study analyzed the ten most-cited papers in the field of colorectal cancer (CRC) targeted therapy and identified two landmark articles published in The New England Journal of Medicine in 2004. These studies played a pivotal role in shaping the development of CRC targeted therapy. The first article, titled Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer (cited 307 times), evaluated the efficacy of the anti-angiogenesis drug bevacizumab in combination with irinotecan, fluorouracil, and leucovorin (the IFL regimen) for treating metastatic colorectal cancer (mCRC) [25]. The results demonstrated that, compared to the IFL regimen alone, the addition of bevacizumab significantly prolonged median survival (20.3 vs. 15.6 months) and improved progression-free survival (PFS). This study established the central role of anti-angiogenesis therapy in CRC targeted treatment, positioning bevacizumab as the standard therapy for mCRC and spurring further research into the tumor microenvironment and angiogenesis mechanisms. The second article, Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer (cited 304 times), examined the efficacy of the epidermal growth factor receptor (EGFR) monoclonal antibody cetuximab as monotherapy and in combination with irinotecan for mCRC patients resistant to irinotecan [26]. The study revealed that the combination therapy significantly increased the objective response rate (22.9 vs. 10.8%) and disease control rate, demonstrating superior efficacy compared to monotherapy. Moreover, this research clarified the critical role of EGFR-targeted therapy in mCRC treatment and laid the foundation for precision medicine. It also identified RAS gene mutations as predictive biomarkers for the efficacy of EGFR inhibitors, leading to the widespread adoption of genetic testing in clinical decision-making.
These two studies have profoundly influenced CRC treatment strategies and research directions. First, they contributed to the widespread adoption of anti-angiogenesis therapy and EGFR-targeted therapy in CRC, forming the basis for standardized combination treatment regimens. Second, they established RAS mutation testing as a crucial factor in determining the suitability of EGFR-targeted therapy, advancing the application of precision medicine in CRC. Finally, they initiated extensive research into resistance mechanisms associated with anti-angiogenesis and EGFR-targeted therapies, providing a theoretical framework for the development of next-generation targeted drugs.
Evolution of research trends and emerging topics
Co-citation analysis of research hotspots and emerging topics
This study uses CiteSpace’s co-citation analysis to reveal the evolutionary trajectory of CRC research through timeline visualization. The results indicate that from 2000 to 2023, CRC research has gone through three major stages: (1) Initiation of Basic Research and Targeted Therapy (2000–2010): Research during this period mainly focused on fundamental studies, chemotherapy, and epidermal growth factor receptor (EGFR)-targeted therapy (Cluster #4). Clinical trials on EGFR inhibitors, such as cetuximab, laid the groundwork for CRC-targeted therapy, signaling a shift from traditional chemotherapy to molecular-targeted therapies. (2) Maturation of Precision Medicine and Multi-Target Therapy(2010–2020): With advancements in genetic testing technologies, precision medicine (Cluster #5) became the central focus during this period. Targeted therapies (Clusters #1, #6) matured, particularly with the widespread use of anti-angiogenesis therapies like bevacizumab, which provided diversified treatment options for CRC. Additionally, clinical trials (Cluster #7) optimized various treatment strategies, highlighting the significance of personalized therapy. Molecular testing plays a critical role in the early diagnosis, treatment decision-making, and prognostic evaluation of colorectal cancer. Below are several common molecular testing methods: RAS gene mutation testing (KRAS/NRAS), BRAF V600E mutation testing, MSI-H/dMMR (Microsatellite Instability/Deficient Mismatch Repair) testing, NTRK fusion gene testing ctDNA (circulating tumor DNA) testing. Although molecular testing technologies have advanced rapidly, there are still several challenges in their clinical application, including: High detection costs, which hinder widespread use, issues with the quality of tissue samples, heterogeneity of biomarkers and their therapeutic efficacy, andl ack of standardized protocols for biomarker testing. To address these issues, the following solutions are needed to promote the inclusion of molecular testing in health insurance and commercial insurance coverage, and encourage the development of low-cost, rapid testing technologies, develop and promote standardized sample collection and processing procedures to enhance the reliability of test results, combine multi-omics data (genomics, proteomics, metabolomics, etc.) to optimize individualized treatment strategies and improve the level of precision medicine, and establish a unified standardized testing process and promote global consistency in molecular testing guidelines. (3) Emergence of Immunotherapy and Cutting-edge Technologies (2020–Present): In recent years, immunotherapy (Cluster #9) and microsatellite instability (MSI-H, Cluster #10) have emerged as frontiers in CRC research. Immune checkpoint inhibitors (e.g., PD-1/PD-L1 antibodies) combined with targeted therapies have shown significant clinical promise. Furthermore, the exploration of radioimmunotherapy is pushing the boundaries of CRC treatment, establishing it as a crucial direction for future research.
An in-depth analysis of research clusters reveals three main focal points in CRC research: (1) Precision Medicine and Liquid Biopsy (Clusters #5 & #3) Advancements in genetic testing and non-invasive detection technologies have made precision medicine and liquid biopsy central to CRC research. Liquid biopsy holds substantial promise in real-time monitoring of tumor heterogeneity, minimal residual disease (MRD), and resistance mechanisms, paving the way for early diagnosis, efficacy assessment, and personalized treatment. (2) Microsatellite Instability and Immunotherapy (Cluster #10) Microsatellite instability (MSI-H) has emerged as a vital biomarker for predicting the efficacy of immune checkpoint inhibitors. Immunotherapy for MSI-H and mismatch repair-deficient (dMMR) CRC patients has become a prominent research area. Future studies will explore immune resistance mechanisms and their combination with other therapeutic strategies. (3) Radioimmunotherapy Combination Strategies (Cluster #9) Radioimmunotherapy, which combines the localized control of radiotherapy with the systemic anti-tumor effects of immunotherapy, shows considerable potential in CRC treatment. Future research will focus on optimizing the combinations of radioimmunotherapy and investigating its biological mechanisms, particularly in synergy with novel targeted drugs like KRAS inhibitors.
Based on the evolution of research hotspots, future CRC research will focus on the following key directions: (1) Advancements in Precision Therapy and Genetic Testing Technologies Precision medicine will play an increasingly significant role in CRC early diagnosis, efficacy evaluation, and resistance mechanism studies. Emerging technologies such as liquid biopsy and single-cell sequencing will drive the development of personalized treatments and facilitate the integration of multi-omics data analysis. (2) Exploration of Immunotherapy and Resistance Mechanisms.
Future research will delve into the role of MSI-H and other biomarkers in immunotherapy, especially exploring mechanisms of immune resistance and their reversal strategies. The combination of immunotherapy with targeted therapy and chemotherapy will continue to be a major research focus. (3) Integration of Radioimmunotherapy with Novel Therapeutics.
Combining radioimmunotherapy with novel targeted drugs such as KRAS inhibitors could potentially overcome resistance and improve treatment efficacy. Future studies will aim to elucidate its biological mechanisms and optimize the best treatment strategies to enhance CRC therapeutic outcomes.
Keyword analysis of research hotspots and emerging topics
Keyword co-occurrence network analysis reveals that CRC research keywords are grouped into several major clusters: Cluster 1 (Red): Focuses on"colorectal cancer"itself, along with terms related to"tumor growth"and"cancer."Cluster 2 (Green): Primarily includes terms related to chemotherapy, such as"bevacizumab"and the"FOLFIRI"regimen. Cluster 3 (Yellow): Covers immune checkpoint inhibitors (e.g., PD-1/PD-L1), tumor microenvironment, inflammatory responses, and cell therapies. Recently, immunotherapy has emerged as a frontier in CRC research, with topics like T-cell infiltration, cancer-associated fibroblasts (CAF), and immune evasion mechanisms becoming central. Additionally, tumor stem cells (CSC) are recognized as key mechanisms for drug resistance and recurrence, making them potential therapeutic targets. Cluster 4 (Blue): Focuses on targeted therapy, with an emphasis on monoclonal antibodies like EGFR, BRAF, KRAS, and HER2 in precision medicine.
Keyword burst analysis identifies the top 25 keywords that have seen a sharp increase in academic attention from 2000 to 2023. Keywords such as"monoclonal antibody"and"randomized trial"began gaining attention around 2002 and remain prominent. Other keywords like"biomarker"and"KRAS mutations"also showed early bursts of academic interest. Recently, terms like"tumor microenvironment"and"cancer stem cells"have gained attention, reflecting the recent trends in immunotherapy and precision treatment research.
The keyword clustering timeline trend, based on the LSI algorithm, illustrates the development and evolution of research themes. From 2000 to 2010, research focused on keywords related to traditional chemotherapy and targeted therapy. After 2010, advancements in technology and treatment methods shifted the focus toward precision medicine and immunotherapy. CRC has remained a central theme in research, while cell therapy and liquid biopsy have emerged as new directions, signaling advancements in early cancer diagnosis and treatment.
Clinical application and progress of targeted therapy and immunotherapy
The timeline view of the references indicates that targeted therapy for colorectal cancer (CRC) began in 2000, with EGFR inhibitors and VEGFR inhibitors as the representative therapies. After 2010, with advancements in genetic testing technologies, new targets such as BRAF, KRAS, and HER2 were identified, further expanding treatment options. Since 2020, immunotherapy research has emerged as a hot topic in CRC treatment. Co-occurrence analysis and keyword clustering have also identified key research hotspots in this field. The following discussion will focus on important clinical trials related to targeted therapy in CRC and the latest advancements in immunotherapy.
Epidermal growth factor receptor (EGFR) and targeted therapy in CRC
Epidermal growth factor receptor (EGFR) and its targeted therapies play a pivotal role in the treatment of colorectal cancer (CRC). Cetuximab, when combined with FOLFIRI or FOLFOX, significantly improves progression-free survival (PFS) and overall survival (OS) in KRAS wild-type patients. However, these effects are less pronounced in KRAS-mutant patients. The TAILOR trial demonstrated that cetuximab combined with FOLFOX significantly improved PFS (20.7 months) and OS (17.8 months) in KRAS wild-type patients. Panitumumab, also suitable for KRAS wild-type patients, improves PFS when combined with FOLFIRI, although its impact on OS is minimal [27]. The PRIME trial showed that while panitumumab combined with FOLFOX improved PFS, it did not significantly increase OS. The ASPECCT trial revealed that the efficacy of panitumumab is comparable to cetuximab, with similar incidences of grade 3–4 toxicities between both groups [28].
Vascular endothelial growth factor receptor (VEGFR) targeted therapy in CRC
VEGFR-targeted therapies play a significant role in metastatic colorectal cancer (mCRC). Bevacizumab, when combined with chemotherapy, has been shown to significantly increase both OS and PFS in patients. In the IFL + bevacizumab group, the median OS was 20.3 months, significantly higher than the 15.6 months observed in the IFL group. Aflibercept, when combined with FOLFIRI, significantly improves OS (13.5 vs. 12.1 months) and PFS (6.9 vs. 4.7 months) [25]. Ramucirumab combined with FOLFIRI also significantly improves OS (13.3 vs. 11.7 months), with better effects seen in patients with CEA ≤ 10 [29]. Regorafenib, in the CONCUR trial, significantly improved OS (6.4 vs. 5.0 months). In the CONCUR trial, regorafenib significantly improved survival in Asian patients (8.8 vs. 6.3 months) [30]. In the FRESCO trial, fukutinib significantly improved OS (9.3 vs. 6.6 months) and PFS (3.7 vs. 1.8 months) [31]. The FRESCO-2 trial showed that in refractory patients, OS increased to 7.4 months, significantly higher than the placebo group (4.8 months) [32]. These findings indicate that various VEGFR-targeted therapies have demonstrated excellent efficacy, especially in refractory patients.
BRAF, MEK, and KRAS inhibition in targeted therapy for CRC
BRAF V600E mutations are closely associated with CRC, and MEK and BRAF inhibitors have been extensively studied in this context. Encorafenib (a BRAF inhibitor) and binimetinib (a MEK inhibitor), when used together, effectively inhibit tumor growth. The BEACON trial showed that triplet therapy (encorafenib + binimetinib + cetuximab) significantly improved OS (9.0 months) and PFS compared to doublet therapy (5.4 months) and the control group [33]. KRAS and NRAS mutations are among the most common driver mutations in CRC, affecting approximately 30–40% of patients. Studies have shown that KRAS-mutant CRC patients have significantly lower survival rates compared to KRAS wild-type patients. RAS mutations are closely associated with resistance to EGFR inhibitors, primarily due to the persistent activation of downstream RAS-RAF-MEK-ERK and PI3 K-AKT pathways, which bypass EGFR inhibition and lead to treatment failure. Mutations in EGFR, particularly the T790M mutation in exon 20, can alter the drug-binding site, reducing the efficacy of EGFR inhibitors. Additionally, RAS mutations activate the PI3 K/Akt pathway, further promoting resistance. Among these, the KRAS G12 C mutation occurs in about 4% of CRC patients and is associated with a poor prognosis. Sotorasib, in combination with panitumumab, showed a median PFS of 5.6 months in a Phase III trial, significantly higher than the standard treatment (2.2 months) [34]. Adagrasib, when combined with cetuximab, demonstrated a 46% response rate and a median PFS of 6.9 months, with Phase III trials still ongoing. Although KRAS-mutant patients show poor responses to traditional EGFR inhibitors, combining KRAS inhibitors with EGFR inhibitors significantly improves treatment outcomes, with most adverse reactions being mild or moderate, including rash and diarrhea [35].
HER2 pathway and its targeted therapy
HER2 (human epidermal growth factor receptor 2) is a receptor tyrosine kinase encoded by the ERBB2 gene and is associated with multiple malignancies. Although HER2 amplification is relatively rare in colorectal cancer (CRC) (approximately 3–5%), targeted therapies against this pathway have made significant progress. Tucatinib: The MOUNTAINEER Phase II trial evaluated tucatinib combined with trastuzumab in HER2-amplified metastatic colorectal cancer (mCRC), showing an objective response rate (ORR) of 46% [36], and it has received FDA accelerated approval. The MOUNTAINEER-03 trial is further evaluating its efficacy in combination with mFOLFOX. Pertuzumab: The MyPathway trial showed an ORR of 32% for pertuzumab combined with trastuzumab in HER2-amplified mCRC, with good treatment tolerance [37]. Lapatinib: The HERACLES trial evaluated the combination of lapatinib with trastuzumab, showing an ORR of 30%, with a median overall survival (OS) of 10 months, no grade 4 or 5 adverse events, and one patient remaining in complete remission after 7 years of treatment [38].Trastuzumab Deruxtecan: The DESTINY-CRC01 trial showed an ORR of 45.3% and an OS of 15.5 months in HER2-positive mCRC patients, with a progression-free survival (PFS) of 6.9 months. Currently, the DESTINY-CRC02 trial is evaluating its multi-dose safety and efficacy [39].
Dual HER2-targeted therapy is considered the most promising strategy, including combinations of tucatinib or pertuzumab with trastuzumab, as well as trastuzumab deruxtecan. The NCCN guidelines recommend that HER2-amplified mCRC patients receive combinations of trastuzumab with pertuzumab, lapatinib, or tucatinib, or trastuzumab deruxtecan alone.
Multi-target combination therapy
BRAF V600E mutations account for about 8–10% of colorectal cancer patients and are usually associated with more aggressive disease and poorer prognosis. First-line treatment typically includes chemotherapy combined with bevacizumab or a three-drug combination, but efficacy and safety remain controversial. Targeted combination therapies have made significant progress. The BREAKWATER study evaluated the effect of encorafenib (a BRAF inhibitor) combined with cetuximab (an anti-EGFR drug) and FOLFIRI chemotherapy in BRAF V600E-mutant metastatic colorectal cancer (mCRC) patients [40]. In the first-line treatment, the objective response rate (ORR) was 83.3%, with the median progression-free survival (PFS) and overall survival (OS) not yet reached, indicating durable efficacy. In second-line treatment, the ORR was 44.4%, with a median PFS of 12.6 months and median OS of 19.7 months, showing significant improvements over historical data. The incidence of adverse events was similar between the two groups, with nausea, anemia, diarrhea, and appetite loss as the main side effects. The EC + FOLFIRI regimen had good tolerance, with no new safety concerns. This study offers new hope for the treatment of BRAF V600E-mutant mCRC patients.
The KRAS G12 C mutation is a significant therapeutic challenge in colorectal cancer, leading to resistance to EGFR inhibitors. To overcome this issue, researchers have proposed a dual-target strategy combining KRAS inhibitors with EGFR monoclonal antibodies. The CodeBreaK 100 trial assessed the efficacy and safety of the KRAS G12 C inhibitor sotorasib combined with EGFR inhibitors in metastatic colorectal cancer patients [41]. The combination therapy showed an ORR of 33.3%, significantly higher than the 10.6% for monotherapy. The median PFS in the combination therapy group was 4.1 months, with an OS of 11.3 months, both superior to the monotherapy group (PFS 2.8 months, OS not reached). Although adverse reactions such as rash and diarrhea were more common in the combination therapy group, most were mild or moderate and manageable, with treatment adjustments or pauses necessary in some cases to alleviate adverse effects.
The main adverse reactions of multi-target combination therapy include nausea, vomiting, bone marrow suppression, rash, diarrhea, and decreased appetite. In clinical practice, managing the side effects of multi-target combination therapy is crucial for improving patient tolerance and enhancing treatment outcomes. The management strategies for side effects typically include: dose adjustments, symptomatic treatment, monitoring and early intervention, and supportive care.
Advances in immunotherapy research
In recent years, immune checkpoint inhibitors (ICIs), such as PD-1 and PD-L1 inhibitors, have become an essential part of colorectal cancer (CRC) treatment, especially in patients with microsatellite instability (MSI-H) or mismatch repair deficiency (dMMR). However, the efficacy of ICIs is limited in patients with microsatellite stable (MSS) or low MSI colorectal cancer, and these patients constitute the majority. Despite this, immunotherapy still shows potential in MSS CRC, particularly when used in combination therapies.
The KEYNOTE-177 study is a Phase III clinical trial designed to evaluate the clinical benefits of pembrolizumab monotherapy compared to standard treatment (FOLFOX or FOLFIRI chemotherapy ± bevacizumab or cetuximab) in first-line treatment for MSI-H/dMMR metastatic colorectal cancer (mCRC) patients [42]. The results showed that the monotherapy group had a median progression-free survival (PFS) of 16.5 months, significantly higher than the 8.2 months in the standard treatment group, with a three-year PFS rate of 42 vs. 11%. The objective response rate (ORR) for the monotherapy group was 45.1%, higher than the 33.1% for the standard treatment group. Furthermore, more than one-third of patients in the monotherapy group were able to tolerate fewer grade 3 or higher adverse events (AEs), with a lower incidence of AEs compared to the standard treatment group. Based on its high efficacy and low toxicity, pembrolizumab was approved by the FDA in 2020 for the treatment of unresectable or metastatic MSI-H/dMMR CRC. The KEYNOTE-164 study is a global, open-label, non-randomized, multicenter Phase II clinical trial designed to evaluate the efficacy and safety of pembrolizumab in previously treated advanced or metastatic MSI-H/dMMR CRC patients [43]. The results showed that the ORR in both cohorts exceeded 30%, with a three-year PFS rate exceeding 30% and a three-year overall survival (OS) rate approaching 50%. Furthermore, efficacy was sustained after two years of treatment. Compared to the KEYNOTE-177 and KEYNOTE-164 results, pembrolizumab exhibited more significant efficacy in first-line treatment for MSI-H/dMMR mCRC patients and also showed good efficacy in second-line and later-line treatments.
The METIMMOX study is a Phase II clinical trial aimed at comparing first-line treatment efficacy in MSS metastatic colorectal cancer patients [44]. In the experimental group, patients were treated with the FLOX regimen followed by two cycles of nivolumab (FLOX for 2 cycles, then nivolumab for 2 cycles, totaling 8 cycles), while the control group received the FLOX regimen alone. The primary endpoint was PFS. After an average follow-up of 6.4 months (range: 0.5–20 months), the median PFS in the FLOX + nivolumab group was 6.6 months, slightly higher than the 5.6 months in the FLOX group. In the FLOX + nivolumab group, 16% of patients with RAS or BRAF mutations achieved complete remission after 8 months, and 32% maintained objective responses. The FLOX group showed no complete remissions, with 23% maintaining objective responses. This study suggests that short-term oxaliplatin chemotherapy in MSS metastatic colorectal cancer patients may alter tumor immunogenicity and trigger responses to immune checkpoint inhibitors.
Immune checkpoint inhibitors (ICIs) have demonstrated remarkable efficacy in microsatellite instability-high (MSI-H) colorectal cancer (CRC), whereas their effectiveness in microsatellite-stable (MSS) CRC remains limited. This restriction may be attributed to the following mechanisms: Low Tumor Mutational Burden (TMB): MSS CRC typically exhibits a lower TMB, leading to a reduced generation of immunogenic neoantigens. This, in turn, diminishes the ability of the immune system to recognize and eliminate tumor cells. Lack of Immune Cell Infiltration in the Immune Microenvironment: MSS CRC is often classified as a"cold tumor,"characterized by insufficient T-cell infiltration in its immune microenvironment. This deficiency weakens the efficacy of immunotherapy. Immune Evasion Mechanisms: MSS CRC tumors may evade immune surveillance by upregulating immunosuppressive factors or altering immune cell functions, thereby reducing sensitivity to immunotherapy. This process may involve high expression of immune checkpoint molecules, recruitment of immunosuppressive cells such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and loss of tumor antigens. In recent years, the gut microbiota has been recognized as playing a crucial role in regulating host immune responses and immune evasion mechanisms. It may influence the efficacy of immunotherapy through the following mechanisms: Promotion of T-cell Activation: Specific gut microbiota can enhance the function of CD8+ T cells, improving their ability to recognize and eliminate tumor cells. Regulation of the Immunosuppressive Microenvironment: Certain bacterial populations may influence the number or function of Treg cells, promoting the formation of an immunosuppressive microenvironment and thereby weakening the anti-tumor immune response. Impact on Immune Tolerance: The gut microbiota may modulate antigen presentation, the release of inflammatory factors, and the expression of co-stimulatory signals, further affecting immune tolerance mechanisms and ultimately influencing the efficacy of immunotherapy.
To improve the response rate of MSS CRC to immunotherapy, the following strategies can be employed: Combination Therapy: Combining ICIs with other treatment modalities, such as chemotherapy, targeted therapy, or novel immunotherapies. For example, anti-angiogenic agents can improve tumor vascular structure, enhance T-cell infiltration, and increase the effectiveness of immunotherapy. Additionally, oncolytic viruses can promote tumor cell lysis and antigen release, thereby strengthening immune activation. Personalized Treatment: Identifying predictive biomarkers for immunotherapy response in MSS CRC patients, such as PD-L1 expression levels or T-cell exhaustion markers, can aid in selecting suitable patients and optimizing combination therapy strategies. Modulation of the Tumor Microenvironment: Targeting immunosuppressive cells (such as Tregs and MDSCs) or relevant immunosuppressive molecules within the tumor microenvironment may help create a more favorable immune landscape for immunotherapy.
In terms of immune-targeted combination therapies, the efficacy of regorafenib as a commonly used drug has been demonstrated in several studies. For example, in the REGONIVO study, the combination of regorafenib with nivolumab achieved an ORR of 33.3% in MSS CRC patients, compared to 7% in a similar study conducted in the United States [45]. Additionally, in the REGONIVO study, the response rate for lung-target lesions was 63.6%, while liver-target lesions had a response rate of 8.3%. In contrast, in the REGOMUNE study, the combination of regorafenib with Avelumab in 48 patients showed no responses [46]. The median PFS in immune-targeted combination therapies ranged from 2.3 months in the LEAP-005 study to 7.9 months in the REGONIVO study, with the incidence of grade 3 or higher adverse events reaching up to 90% in combination treatments.
In conclusion, immune checkpoint inhibitors show significant efficacy in MSI-H/dMMR CRC patients, particularly with pembrolizumab in first-line treatment compared to traditional chemotherapy. Immune-targeted combination therapies also demonstrate potential in MSS CRC patients, but their efficacy and the incidence of adverse events need further evaluation and optimization.
Conclusion
This study utilized bibliometric visualization software, CiteSpace and VOSviewer, to analyze and visualize publications in the field of targeted therapy for colorectal cancer (CRC) over the past two decades. It provides a comprehensive overview of the research hotspots, evolution pathways, and development trends in this field. The results indicate that both China and the United States have made significant contributions to this area. Moving forward, it is essential to strengthen collaboration between countries, regions, institutions, and authors. Furthermore, the development trends reveal a notable shift in CRC research focus, from traditional chemotherapy to targeted therapy, followed by precision medicine and immunotherapy. In the future, precision medicine, liquid biopsy, immunotherapy, and their combination strategies will be core areas of CRC research, particularly in exploring resistance mechanisms and integrated treatment approaches, which hold significant clinical application potential. With the integration of multidisciplinary collaboration and new technologies, personalized and precise treatments for CRC will continue to evolve, offering new opportunities for improving patient prognosis.
Limitations
Our bibliometric study has some limitations. First, only English literature was included in this study, important literature in other languages was not included in the analysis. Second, recently published high-quality articles cannot attract sufficient attention because of their short publication time and low citation frequency.
Supplementary Information
Acknowledgements
Thanks to the reviewers and editors for their sincere comments.
Author contributions
Xiangnv Meng and Zhongting Lu contributed equally to the study, with Meng leading the study design, data collection, analysis, and manuscript drafting, and Lu assisting in data analysis, visualization, literature review, and critical revisions. Fu Mi supported data collection, visualization, and methodology writing, while Sha Sha provided technical assistance, language editing, and input on the discussion section. Tao Li supervised the project, provided academic guidance, reviewed and revised the manuscript, and handled journal communications as the corresponding author.
Funding
None.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Declarations
Ethics approval and consent to participate
All data used in this study were downloaded from public databases; therefore, no ethical approval or informed consent was required.
Consent for publication
Not applicable.
Patient consent
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiangnv Meng and Zhongting Lu contributed equally.
References
- 1.Lopes SR, Martins C, Santos IC, Teixeira M, Gamito É, Alves AL. Colorectal cancer screening: a review of current knowledge and progress in research. World J Gastrointest Oncol. 2024;16(4):1119–33. 10.4251/wjgo.v16.i4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4(12):913–33. 10.1016/S2468-1253(19)30345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony T, Jones C, Antoine J, Sivess-Franks S, Turnage R. The effect of treatment for colorectal cancer on long-term health-related quality of life. Ann Surg Oncol. 2001;8(1):44–9. 10.1007/s10434-001-0044-2. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Vignoud J, Tournigand C, Louvet C, André T, Varette C, et al. Oxaliplatin with high-dose leucovorin and 5-fluorouracil 48-hour continuous infusion in pretreated metastatic colorectal cancer. Eur J Cancer. 1997;33(2):214–9. 10.1016/s0959-8049(96)00370-x. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Dong X, Yan S, Liu B, Li X, Li S, et al. Comparison of the long-term survival outcome of surgery versus stereotactic body radiation therapy as initial local treatment for pulmonary oligometastases from colorectal cancer: a propensity score analysis. Int J Radiat Oncol Biol Phys. 2024. 10.1016/j.ijrobp.2024.07.2324. [DOI] [PubMed] [Google Scholar]
- 6.Feria A, Times M. Effectiveness of standard treatment for stage 4 colorectal cancer: traditional management with surgery, radiation, and chemotherapy. Clin Colon Rectal Surg. 2024;37(2):62–5. 10.1055/s-0043-1761420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24(34):3834–48. 10.3748/wjg.v24.i34.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Can Res. 2015;75(2):245–9. 10.1158/0008-5472.Can-14-2240. [DOI] [PubMed] [Google Scholar]
- 9.Francoual M, Etienne-Grimaldi MC, Formento JL, Benchimol D, Bourgeon A, Chazal M, et al. EGFR in colorectal cancer: more than a simple receptor. Ann Oncol. 2006;17(6):962–7. 10.1093/annonc/mdl037. [DOI] [PubMed] [Google Scholar]
- 10.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raponi M, Winkler H, Dracopoli NC. KRAS mutations predict response to EGFR inhibitors. Curr Opin Pharmacol. 2008;8(4):413–8. 10.1016/j.coph.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Kundu S, Ali MA, Handin N, Conway LP, Rendo V, Artursson P, et al. Common and mutation specific phenotypes of KRAS and BRAF mutations in colorectal cancer cells revealed by integrative -omics analysis. J Exp Clin Cancer Res. 2021;40(1):225. 10.1186/s13046-021-02025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med. 2021;27(11):1899–903. 10.1038/s41591-021-01553-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wankhede D, Yuan T, Kloor M, Halama N, Brenner H, Hoffmeister M. Clinical significance of combined tumour-infiltrating lymphocytes and microsatellite instability status in colorectal cancer: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2024;9(7):609–19. 10.1016/s2468-1253(24)00091-8. [DOI] [PubMed] [Google Scholar]
- 15.Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6(2):147–53. 10.1158/2159-8290.Cd-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, et al. Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 2017;23(10):2414–22. 10.1158/1078-0432.Ccr-16-1863. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, Weng L, Hu Y, Mao Y, Zhang Y, Lu Z, et al. Exercise for stroke rehabilitation: a bibliometric analysis of global research from 2001 to 2021. Front Aging Neurosci. 2022;14: 876954. 10.3389/fnagi.2022.876954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1(Suppl 1):5303–10. 10.1073/pnas.0307513100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724–8. [PMC free article] [PubMed] [Google Scholar]
- 20.van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38. 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Jin Y, Wang M, Luo HY, Fang WJ, Wang YN, et al. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med. 2024;30(4):1035–43. 10.1038/s41591-024-02813-1. [DOI] [PubMed] [Google Scholar]
- 22.Zhuang X, Wang Z, Fan J, Bai X, Xu Y, Chou JJ, et al. Structure-guided and phage-assisted evolution of a therapeutic anti-EGFR antibody to reverse acquired resistance. Nat Commun. 2022;13(1):4431. 10.1038/s41467-022-32159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAF(V600E)-mutant colorectal cancer. Cancer Discov. 2018;8(4):428–43. 10.1158/2159-8290.Cd-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101(19):1308–24. 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–45. 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol. 2018;36(30):3031–9. 10.1200/jco.2018.78.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15(6):569–79. 10.1016/s1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 29.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. 10.1016/s1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29. 10.1016/s1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. JAMA. 2018;319(24):2486–96. 10.1001/jama.2018.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dasari A, Sobrero A, Yao J, Yoshino T, Schelman W, Yang Z, et al. FRESCO-2: a global Phase III study investigating the efficacy and safety of fruquintinib in metastatic colorectal cancer. Future Oncol. 2021;17(24):3151–62. 10.2217/fon-2021-0202. [DOI] [PubMed] [Google Scholar]
- 33.Van Cutsem E, Huijberts S, Grothey A, Yaeger R, Cuyle PJ, Elez E, et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol. 2019;37(17):1460–9. 10.1200/jco.18.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fakih MG, Salvatore L, Esaki T, Modest DP, Lopez-Bravo DP, Taieb J, et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023;389(23):2125–39. 10.1056/NEJMoa2308795. [DOI] [PubMed] [Google Scholar]
- 35.Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med. 2023;388(1):44–54. 10.1056/NEJMoa2212419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer E, Cervera P, André T, Cohen R. Targeting HER2 in colorectal cancer. Bull Cancer. 2023;110(4):402–11. 10.1016/j.bulcan.2023.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20(4):518–30. 10.1016/s1470-2045(18)30904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738–46. 10.1016/s1470-2045(16)00150-9. [DOI] [PubMed] [Google Scholar]
- 39.Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22(6):779–89. 10.1016/s1470-2045(21)00086-3. [DOI] [PubMed] [Google Scholar]
- 40.Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–43. 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 41.Dy GK, Govindan R, Velcheti V, Falchook GS, Italiano A, Wolf J, et al. Long-term outcomes and molecular correlates of sotorasib efficacy in patients with pretreated KRAS G12C-mutated non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J Clin Oncol. 2023;41(18):3311–7. 10.1200/jco.22.02524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–18. 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 43.Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–9. 10.1200/jco.19.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ree AH, Šaltytė Benth J, Hamre HM, Kersten C, Hofsli E, Guren MG, et al. First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer-the randomised METIMMOX trial. Br J Cancer. 2024;130(12):1921–8. 10.1038/s41416-024-02696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61. 10.1200/jco.19.03296. [DOI] [PubMed] [Google Scholar]
- 46.Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, et al. Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): a single-arm, open-label, phase II trial. Clin Cancer Res. 2021;27(8):2139–47. 10.1158/1078-0432.Ccr-20-3416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.