Abstract
Neurodegenerative diseases (NDs) pose a formidable challenge in modern healthcare and are characterized by progressive neuronal dysfunction and loss. Emerging research underscores the intricate interplay between neuroinflammation and mechanisms underlying ND pathogenesis. This review delves into the complex role of Krüppel-like factors (KLFs) in the context of neuroinflammation and major NDs. KLFs exert diverse effects in the brain on cellular processes such as blood-brain barrier integrity, neuronal cell cycle progression, and glial cell activation. Modulation of KLF expression and signaling emerges as a promising strategy to mitigate ND progression. By elucidating KLFs’ multifaceted implications across diverse pathways and cellular processes implicated in ND progression, this review offers valuable insights into their therapeutic potential as targets for NDs.
Keywords: Krüppel-like factor, neurodegenerative disease, neuroinflammation
Introduction
Krüppel-like Factors (KLFs) are a family of transcriptional factors that act as activators or/and repressors of gene transcription. They are zinc finger proteins that bind to CACCC, or a GT box, in target gene promoters. The C-terminal domain of KLFs contains the DNA-binding region and nuclear localization signals, and the N-terminal domain is the protein-interacting region. There are 18 members in the KLF family with various gene expression patterns [1]. KLFs are involved in the regulation of many cellular processes such as cell cycle progression, proliferation, migration, transformation and invasion [1]. Altered expression of KLFs is associated with a wide range of diseases, including metabolic abnormalities, heart failure, and cancer. Here we present KLFs’ involvement in the development of neurodegenerative diseases and the implications in the pathology behind the diseases.
Neurodegenerative Diseases (NDs) share common characteristics such as the propagation of aberrant protein aggregates, neuroinflammation, increased oxidative stress, impaired proteolysis, mitochondrial dysfunction, and ultimately neuronal cell death [2,3]. Currently, the focus on developing treatments for NDs has shifted from targeting cytoplasmic and extracellular proteins towards targeting the associated genes in the nucleus that encode for or regulate the proteins relevant to NDs [4,5].
Alzheimer’s Disease (AD), the most prevalent ND, is a progressive condition that causes the affected patients to present symptoms such as declining memory, aphasia, deteriorating cognitive impairment, and ultimately the development of dementia [6]. AD displays pathological changes in patients’ brains, such as neuronal loss in the hippocampal region, leading to defects in learning and memory [7]. Key characteristics of AD include the accumulation of extracellular amyloid-β (Aβ) protein plaques, intracellular neurofibrillary tangles of hyperphosphorylated tau protein, chronic neuroinflammation, and complex neuroimmune interactions that involve reactive microglia and astrocyte [8,9].
Parkinson’s Disease (PD), the second most common ND, presents protein aggregates the form of Lewy bodies. Lewy bodies are formed by accumulation of misfolded α-synuclein (α-syn) protein [10]. Tau pathology is also observed in PD and several other NDs. There is an overlap between the formation of Aβ plaques, tau tangles, and α-syn aggregates, suggesting that Aβ plaques contribute to α-syn spreading [11]. Furthermore, PD presents as the death of dopamine-producing neurons in the substantia nigra, resulting in the development of symptoms affecting the motor system such as bradykinesia, loss of balance, tremors, and stiffness [12,13].
Huntington’s disease (HD) is an autosomal dominant inherited condition. A CAG trinucleotide repeat in the huntingtin gene causes an aberrant protein phenotype. This aberrant protein disrupts a wide array of molecular and cellular processes including cellular homeostasis, neuronal transportation, gene expression, and function of mitochondria and synapsis. Consequently, the loss of corpus striatum GABAergic medium spiny neurons and cholinergic neurons occurs. Patients present a variety of symptoms disturbing motor and cognitive skills [3,14].
Roles of KLFs in the progression of NDs
ND progression begins with dysregulation of molecular signaling within the cellular network inside the brain. To understand the roles of KLFs in ND progression, we first looked at the peer-reviewed literature for the expression of KLFs in the brain. We found that all the 17 KLF family members are expressed in one or more CNS cell types and are relevant to NDs (Table 1). Notably, most of the data outlined in this table were obtained from experimental mice and more studies were done on some KLFs such as KLF4, KLF7, KLF9 and KLF11 than others considering the number of publications contributing to the studies. Nevertheless, analysis of data reported from the European Bioinformatics Institute’s database confirms that the KLF family members are indeed expressed in the human brain, in general and in NDs-associated regions such as the cerebral cortex and hippocampus, in particular (Table 2).
Table 1.
KLF family expression in the central nervous system
| Expression Time/Disease model | Model organism | Expression Location | Brain Cell Type | Reference | |
|
| |||||
| KLF1 | During development | Mice | Cerebral cortex | Retinal ganglion cells | [125] |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF2 | During development | Mice | Cerebral cortex | Retinal ganglion cells | [125] |
| Neural differentiation | - | In-vitro | Dental pulp-derived stem cells | [119] | |
| Alzheimer disease | C57/BL6 mice | Cerebral cortex | Endothelial | [25] | |
| Human | Temporal cortex | - | |||
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Cerebral ischemia | Mice | Cerebral cortex | Endothelial | [19] | |
| Alzheimer disease | Tg2576 Mice | Brain tissue | - | [26] | |
| Human brain endothelial cells | In-vitro | Endothelial | |||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
| Alzheimer disease | Kunming mice | Hippocampus | Neuronal | [128] | |
| Sciatic nerve injury | Sprague-Dawley rats | Dorsal root ganglia | Neuronal | [129] | |
| Hypoxic-ischemic brain damage | Sprague-Dawley rats | Hippocampus | Neuronal | [130] | |
| Cortex | |||||
| Spinal cord injury | Sprague-Dawley rats | Spinal cord | Neuronal | [131] | |
|
| |||||
| KLF3 | During development | Mice | Cerebral cortex | Retinal ganglion cells | [125] |
| - | Mice | Forebrain | Neural stem cells | [132] | |
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
|
| |||||
| KLF4 | During development | Mice | Cerebral cortex | Retinal ganglion cells | [125] |
| Glutamatergic stimulation | CD1 mice | Cerebral cortex | Neurons | [133] | |
| Developmental | Rats | Cerebral cortex | Neurons | [134] | |
| Neuroinflammation | BALB/c mice | Whole brain tissue | - | [87] | |
| - | In-vitro | BV-2 cells | |||
| Developmental (Ontogeny?) | C57BL/6 mice | Hypothalamus | Neurons | [135] | |
| Developmental | Mice | E13.5 forebrain | Neuronal stem cells | [132] | |
| E15.5 cortices | Neurons | ||||
| P0 cortices | Astrocytes | ||||
| Neuroinflammation | Mice | Brain tissue | Microglial | [136] | |
| - | In-vitro | BV-2 | |||
| Mice | Brain tissue | Astrocytes | |||
| Neuroinflammation | Mice | Brain tissue | Microglial | [137] | |
| - | In-vitro | BV-2 | |||
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Developmental | Mice | White matter | Neuronal stem cells | [138] | |
| Cerebral cortex | Glial | ||||
| Astrocytes | |||||
| Parkinson’s disease | - | In-vitro | M17 neuroblastoma cell line | [139] | |
| Neuroinflammation | BALB/c mice | Whole brain | Microglia | [140] | |
| - | In vitro | BV-2 cells | |||
| Neuronal regeneration | - | In-vitro | COS7 cells | [58] | |
| Mice | Retina | - | |||
| Optic nerve | Retinal ganglion cells | ||||
| Neuronal degeneration | C57BL/6NHSd mice | Hippocampus | Neurons | [141] | |
| - | In-vitro | PC12 cells | |||
| Traumatic brain injury | Sprague Dawley rats | Optic nerve | Retinal ganglion cells | [142] | |
| - | In-vitro | RGC-5 cells | |||
| Subarachnoid hemorrhage | Human subjects | Cerebrospinal fluid | - | [143] | |
| Psychological stress | Sprague Dawley rats | Cortex | In vivo: Tissue homogenate | [51] | |
| Hippocampus | In vitro: HT-22 cells | ||||
| Alzheimer disease | AD transgenic J20 mice | Brain tissue | Microglial cells | [86] | |
| - | In-vitro | BV-2 cells | |||
| Parkinson’s disease | - | In-vitro | SH-SY5Y cells | [144] | |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
| Cerebral edema | Sprague-Dawley rats | Cerebral cortex | Microglial cells | [145] | |
| Cerebral ischemia | Mice | Cerebral cortex | Brain microvascular endothelial cells | [146] | |
| - | In-vitro | bEnd.3 cells | |||
| Cerebral ischemia | C57BL/6 mice | Ischemic penumbra | Astrocytes | [40] | |
|
| |||||
| KLF5 | Chronic Schizophrenia | Human | Prefrontal cortex | - | [147] |
| Hippocampus | |||||
| During development | Mice | Cortex | Retinal ganglion cells | [125] | |
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
| Alzheimer disease | - | In-vitro | SH-SY5Y, and HT22 cells | [148] | |
| APP/PS1 mice | Hippocampus | Neurons | |||
| Cerebral cortex | |||||
| Human | Cerebrospinal fluid | - | |||
|
| |||||
| KLF6 | Developmental | Mice | Cortical plate | - | [149] |
| Hypothalamus | |||||
| Forebrain | |||||
| midbrain | |||||
| Developmental | Zebrafish | Optic nerve | Retinal ganglion cells | [150] | |
| - | Mice | Olfactory bulb | - | [151] | |
| Cerebral cortex | |||||
| Septum | |||||
| Hippocampus | |||||
| Basal ganglia | |||||
| Amygdala | |||||
| Thalamus | |||||
| Hypothalamus | |||||
| Developmental | Mice | Cortex | Retinal ganglion cells | [125] | |
| Developmental | Rats | Cerebral cortex | Neurons | [134] | |
| Status epilepticus | C57BL/6 mice | Hippocampus | Reactive astrocytes | [152] | |
| Active microglia | |||||
| Neurons | |||||
| Endothelial cells | |||||
| Neuronal regeneration | - | In-vitro | COS7 cells | [58] | |
| Mice | Retina | - | |||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF7 | Developmental differences | - | Ventral horn of the spinal cord | - | [77] |
| Dorsal root ganglia | |||||
| Sympathetic ganglia | |||||
| Cerebral cortex | |||||
| Cerebellum | |||||
| Dorsal root ganglia | |||||
| Developmental | Mice | Olfactory bulb | Neurons | [78] | |
| Optic nerve | Retinal ganglion cells | ||||
| Cerebral cortex | Neurons | ||||
| Developmental | Mice | Olfactory bulb | Neurons | [153] | |
| Nerve physical injury | Zebrafish | Optic nerve | Retinal ganglion cells | [150] | |
| Developmental | Mice | Cortex | Retinal ganglion cells | [125] | |
| Neural differentiation | - | In-vitro | PC12 cells | [154] | |
| Embryonic stem cells | |||||
| Neural stem cells | |||||
| Developmental | Mice | Olfactory bulb | - | [155] | |
| Pons | |||||
| Ventral midbrain | |||||
| Developmental | Mice | Cerebral cortex | Neurons | [156] | |
| Corticospinal tract | |||||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF8 | During development | Mice | Cortex | Retinal ganglion cells | [125] |
| Brain tumors | Human | Astrocytoma | - | [157] | |
| Glioblastoma | |||||
| Bain tissue | |||||
| Alzheimer’s disease | Wistar rats | Cerebral cortex | - | [46] | |
| Hippocampus | |||||
| Mice | Cerebral cortex | Glial cells | |||
| Hippocampus | Neuronal cells | ||||
| - | C57BL/6 mice | Cerebral cortex | Neurons | [45] | |
| Olfactory bulb | |||||
| Hypothalamus | |||||
| Pallidum | |||||
| Striatum | |||||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF9 | - | - | In vitro | N2a cells | [158] |
| Rats | Brain tissue | - | |||
| Stressor | Tadpoles | Brain tissue | - | [159] | |
| Developmental | C57BL/6 | Hippocampus | - | [160] | |
| cerebellum | |||||
| - | In-vitro | N2a cells | |||
| Developmental | Mice | Cortex | Retinal ganglion cells | [125] | |
| Neuronal maturation | Mice | Forebrain | - | [161] | |
| Hippocampus | |||||
| Cerebral cortex | |||||
| Developmental | Mice | cerebellum | Purkinje cells | [162] | |
| Developmental | - | In vitro | HT-22 cells | [163] | |
| C57/BL6J mice | Hippocampus | - | |||
| Differentiation and myelination | - | In-vitro | Oligodendrocyte precursor cells | [164] | |
| C57/Bl6 mice | cerebellum | - | |||
| Optic nerve | |||||
| Corpus callosum | |||||
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Developmental | Tadpoles | Middle brain region | - | [165] | |
| preoptic area | |||||
| diencephalon | |||||
| Induced oxidative stress | - | In vitro | Mes23.5, SH-SY5Y and N27 cell lines | [166] | |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
| Developmental | - | In-vitro | HT22 cells | [167] | |
| Parkinson’s disease | BL/6J mice | Substantia nigra | - | [168] | |
| - | In-vitro | SH-SY5Y cells | |||
|
| |||||
| KLF10 | During development | Mice | Cortex | Retinal ganglion cells | [125] |
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Alzheimer’s disease | C57BL/6J E16 mice | Cerebral cortex | Neurons | [169] | |
| Sprague Dawley E18 rat | |||||
| Human | Hippocampus | - | |||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF11 | During development | Mice | Cortex | Retinal ganglion cells | [125] |
| Focal cerebral ischemia | Mice | Cerebral microvessels | Cerebral vascular endothelial cells | [37] | |
| Nerve growth factor | Mice | Dorsal root ganglia | Neurons | [127] | |
| - | In-vitro | PC12 cells | |||
| Chronic Stress and Depressive Disorders | Human | Prefrontal cortex | - | [170] | |
| Chronic stress | Mice | Frontal cortex | - | ||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF12 | During development | Mice | Cortex | Retinal ganglion cells | [125] |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF13 | Developmental | Mice | Cortex | Retinal ganglion cells | [125] |
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
| Developmental | - | In-vitro | HT22 cells | [167] | |
|
| |||||
| KLF14 | Developmental | Mice | Cortex | Retinal ganglion cells | [125] |
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF15 | Developmental | Mice | Cortex | Retinal ganglion cells | [125] |
| Developmental | Mice | neocortical regions | Neural stem cells | [171] | |
| Nerve growth factor | - | In-vitro | PC12 cells | [127] | |
| Developmental | Mice | Cerebral cortex and Spinal cord white matter | Astrocyte | [172] | |
| Oligodendrocytes | |||||
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF16 | Developmental | Mice | Cortex | Retinal ganglion cells | [125] |
| Cerebral ischemia | Mice | Cerebral cortex | - | [126] | |
|
| |||||
| KLF17 | Developmental | Mice | Cortex | Retinal ganglion cells | [125] |
Table 2.
KLF family expression in the human brain as reported by the EMBL’s European bioinformatics institute
| KLF1 | KLF2 | KLF3 | KLF4 | KLF5 | KLF6 | KLF7 | KLF8 | KLF9 | KLF10 | KLF11 | KLF12 | KLF13 | KLF14 | KLF15 | KLF16 | KLF17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amygdala | VL | √ | √ | √ | √ | √ | √ | √ | √√ | √ | √ | √ | √√ | VL | √√ | √√√ | VL |
| Basal ganglia | √ | √√ | √√ | √ | √ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √√ | VL | √ | √√ | VL |
| Brain tissue | √ | √√ | √√√ | √√√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √ | √√ | √√√ | VL |
| Caudate nucleus | VL | √ | √ | √ | √ | √ | √ | √ | √√ | √ | √ | √ | √√ | VL | √√ | √√√ | VL |
| Cerebellum | √ | √√ | √√ | √ | √ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | VL | √√ | √√√ | VL |
| cerebral cortex | √ | √√ | √√ | √ | √ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | VL | √√ | √√ | VL |
| choroid plexus | √ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √ | √√ | √√ | √ |
| Diencephalon | √ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √√ | √ | √ | √√ | √ |
| Forebrain | √ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √√ | √ | √ | √√ | VL |
| Globus pallidus | VL | √ | √ | VL | VL | VL | √ | VL | √ | VL | VL | VL | √ | VL | VL | √ | VL |
| Hindbrain | √ | √ | √√ | √√ | √ | √√ | √ | √√ | √ | √√ | √√ | √√ | √√ | VL | √ | √√ | VL |
| Hippocampus | √ | √√ | √√ | √ | √ | √√ | √√ | √√ | √ | √√ | √√ | √ | √√ | VL | √√ | √√ | VL |
| Hypothalamus | VL | √√ | √ | √ | √ | √√ | √ | √ | √√ | √ | √ | √ | √√ | VL | √√ | √√ | VL |
| Medulla oblongata | √ | √√ | √√ | √ | √ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √√ | VL | √√ | √√ | VL |
| Midbrain | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √√ | VL | √ | √√ | √ |
| Pituitary gland | VL | √√ | √√√ | √√√ | √√√ | √√ | √ | √ | √√ | √√ | √ | √ | √√ | VL | √√ | √√√ | VL |
| Pons | √ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | √√ | VL | √√ | √√ | VL |
| Putamen | VL | √√ | √ | √ | VL | √ | √ | √ | √√ | √ | √ | √ | √√ | VL | √√ | √√ | VL |
| Substantia nigra | VL | √√ | √√ | √ | √ | √√ | √ | √ | √√ | √ | √ | √ | √√ | VL | √√ | √ | VL |
| Telencephalon | √ | √√ | √√ | √ | √ | √√ | √√ | √√ | √ | √√ | √√ | √√ | √√ | VL | √ | √√ | VL |
| Thalamus | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | √√√ | nda |
VL, very low expression; ü, √ü and √√ü, low, medium and high expression, respectively; nda, no data available.
KLFs and blood-brain barrier integrity
Dysregulated neurovascular units (NVUs) and the blood-brain barrier (BBB) are linked to NDs [15]. An NVU is a collection of cells composed of neurons, astrocytes, and endothelial cells of the BBB. These NVU cells work together to regulate neuroimmune response, brain blood flow, and waste clearance [16]. In NDs, aberrant Aβ and p-Tau protein aggregates present around cerebral blood vessels in the brain parenchyma can cause NVU dysfunction and loss of blood vessel integrity or BBB breakdown. BBB dysfunction is associated with increased vascular permeability, facilitated immune cell invasion, enhanced neuroinflammation, and ultimately degeneration of the NVU [17].
Several KLFs play a role in regulating BBB integrity (Figure 1). KLF2 may ameliorate BBB dysfunction by upregulating autophagic flux in endothelial cells. A study showed that KLF2 expression improves the blood-spinal cord barrier integrity and functional recovery from spinal cord injury by inducing tight junction (TJ) protein expression [18]. KLF2’s implication in cerebrovascular integrity is further emphasized by another study reporting that KLF2 expression reduces infarction size by improving BBB function in the focal cerebral ischemia mouse model [19]. In this study, KLF2 was found to induce the expression of several tight junction proteins, including occludin, claudin-12, and junction adhesion molecule-1 (JAM-1). These proteins play an important role in preserving endothelial barrier and vascular integrity [20,21]. KLF2 rescues TJ protein expression and stabilizes vasculature through mediating anti-inflammatory p53/KLF2 signaling and activating the angiopoietin-1/PI3K/Akt-myocyte enhancer factor-2 (MEF2)-KLF2 signaling in glia [22]. This signaling pathway counteracts the vascular endothelial growth factor (VEGF) inflammatory response [23,24]. Moreover, a study on mouse brain microvascular endothelial cells conveys the potential of KLF2 activation as a therapeutic strategy for cerebral vascular dysfunction in AD. In this study, KLF2 promotion leads to attenuated Aβ-induced oxidative stress, improved mitochondrial function, and reduced apoptosis, ultimately ameliorating AD progression [25]. Similarly, overexpressing KLF2 can rescue occludin expression that was priorly disrupted by Aβ [26]. Thus, KLF2 shows neuroprotective effects in the cerebrovascular system through various signaling pathways that seem to favor BBB integrity by ameliorating inflammation present in several NDs.
Figure 1.
KLFs play a role in blood-brain barrier integrity by modulating the expression of several tight junction proteins, enhancing mitochondrial function, and reducing apoptosis and oxidative stress (The arrow indicates activation, while the straight line with a “T-shape” end indicates inhibition. KLFs boxed in green indicate a neuroprotective effect. These labels apply to all the Figures that follow).
KLF4 is also suggested to have neuroprotective effects in the cerebral vascular system. KLF4 expression was found to increase with time in astrocytes after cerebral ischemia-reperfusion, and its activation modulates the nuclear-erythroid factor 2-related factor 2 (Nrf2)/thioredoxin 1 (Trx1) signaling and ameliorates BBB disruption [27]. Both KLF2 and KLF4 induce anti-inflammatory and vasoprotective phenotypes in endothelial cells by inhibiting the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) activation and induces endothelial nitric oxide synthase (eNOS) expression [28,29]. Upregulated NF-κB expression is also involved in BBB breakdown. NF-κB causes pericyte activation and matrix metalloproteinases (MMP) secretion, leading to basement membrane degradation and opening of the BBB. NF-κB activation may also induce TJ disruption, increasing the permeability of the endothelial cell layer [30]. Like KLF2 and KLF4, other KLFs such as KLF8 and KLF11 have been shown to regulate NF-κB activity associated with BBB integrity [31,32].
KLF11 presents neuroprotective properties by protecting the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) to repress the transcription of the pro-apoptotic miR-15a, resulting in cerebrovascular endothelial cell protection after ischemic insults [33,34]. PPAR-γ inhibits the inflammatory activation of the MAP kinases p38 and extracellular signal-regulated kinases (ERK)1/2 as well as NF-κB downstream of Toll-like receptors 2 and 4 (TLR2, TLR4) [33,34]. Genetic deletion of KLF11 resulted in increased infiltration of peripheral neutrophils and macrophages in mice with traumatic brain injury (TBI) and post-traumatic BBB disruption. Additionally, KLF11 seems to have a role in the prevention of astrocyte activation at the BBB [34]. Moreover, KLF11 increases the expression of TJ proteins including occludin and Zonula occludens-1 (ZO-1) [35]. These results suggest that KLF11 expression is important for the protection of the BBB integrity [36,37].
The effects of KLF10 on cerebral ischemia reperfusion were investigated in vitro [38]. Results revealed that the downregulation of KLF10 resulted in the suppression of apoptosis, and oxidative stress and ameliorated BBB dysfunction through activation of NRF-2/heme oxygenase (HO-1) signaling. This study suggests that KLF10 downregulation may reduce BBB permeability by modulating TJ proteins expression, including ZO-1, occludin and claudin-5 expression in endothelial cells under oxygen-glucose deprivation/reperfusion (OGD/R) conditions [38].
Clearly, several KLFs play a role in BBB stability and with more research, their related signaling pathways may be targeted to promote BBB reconstruction in NDs to help ameliorate neuroinflammation and reinstate homeostasis in the brain.
KLF signaling in neuroinflammation
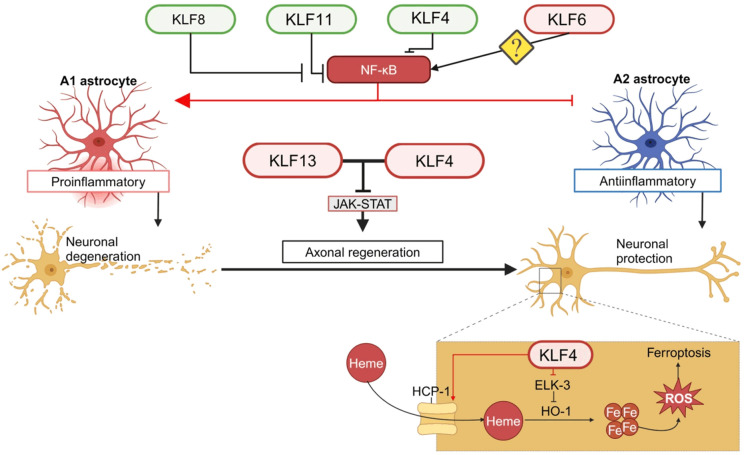
Several KLF signaling pathways including NF-κB, iron and JAK-STAT pathways have been implicated to play a role for neuroinflammation by regulating various cellular events such as glial polarization and ferroptosis in the brain (Figure 2).
Figure 2.
KLFs modulate neuroinflammation through glial polarization and ferroptosis by regulating various signaling pathways such as NF-κB, iron and JAK-STAT pathway.
Activation of NF-κB in microglia promotes neuronal degeneration while its expression in neurons is neuroprotective. NF-κB is found to be upregulated in the spinal cords of amyotrophic lateral sclerosis (ALS) patients, and inhibition of NF-κB signaling in microglia rescues motor neurons and extends survival in a mouse model [39]. Multiple KLFs have been found to play a role in neuroinflammation via NF-κB signaling. It was shown that after OGD/R, astrocytic KLF4 inhibited the activation of the A1 pro-inflammatory subtype of astrocytes and promoted the polarization of A2 anti-inflammatory subtype of astrocytes via modulation of NF-κB [40]. Like M1 microglia, A1 astrocytes are a source of neuroinflammation that is present with most NDs [41]. Additionally, M1 microglia play a role in astrocyte activation and NF-κB plays a critical role in the switch of microglia from M2 to M1 subtype. Likewise, KLF6 regulates NF-κB expression in coactivation of the NF-κB mediated inflammatory response, which is responsible for making ischemic-reperfusion injury more severe in the kidney [42]. KLF6 was also reported to promote inflammatory bowel disease by co-activating NF-κB and suppressing the STAT3 pathway in macrophages, which confers anti-inflammatory signaling [43,44]. There is not much research on KLF6 and its role in microglial polarization in the central nervous system (CNS), although there is much research linking KLF6 to macrophage polarization towards M1 phenotype [44]. More research is needed to evaluate whether KLF6 behaves similarly with microglial cells via NF-κB modulation. KLF11 was shown to play a BBB protective role through PPAR-γ-mediated inhibition of the inflammatory NF-κB pathway in microglial polarization [36,37]. KLF8 is highly expressed and active in the cerebral neurons in various regions such as the cerebral cortex, hippocampus, and hypothalamus [45]. Decreased expression of KLF8 was found in the brain of AD patients with disrupted Wnt/β-catenin signaling [46]. Indeed, KLF8 is a known regulator of the Wnt/β-catenin signaling [47], and β-catenin interaction with NF-κB is highly expressed in brains of patients with NDs such as PD and AD [48]. These results suggest that KLF8 in the brain may play a critical role in neuronal protection.
Iron plays a part in multiple cellular processes, such as oxygen transportation, mitochondrial respiration, DNA synthesis, neurotransmitter synthesis, and more. Dysregulated iron homeostasis can lead to oxidative damage and cause neurotoxicity [49]. Increased iron load in the brain is found to accelerate the formation of Aβ plaques and p-tau tangles and enhance oxidative stress production, which of course is associated with pathology in NDs [50]. A study showed that the activation of KLF4-heme carrier protein 1 (HCP1) signaling induced an increase in heme uptake under psychological stress. This leads to iron accumulation and promotes the release of reactive oxygen species (ROS) and subsequent neuronal damage due to ferroptosis [51]. KLF4 represses the transcription of ELK-3. ELK-3 is a transcription repressor of heme oxygenase 1 (HO-1) that degrades heme into bilirubin and frees iron. Thus, the KLF4-HO-1 signaling promotes iron deposition, resulting in exacerbated oxidative stress and cell damage [52,53]. Given that this KLF4 enhanced iron accumulation takes place in hippocampal neurons [51], it is plausible that aberrant KLF4 signaling like this can be harmful to these neurons critical for cognition and memory leading to NDs like AD.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling mediates many processes such as tissue repair, hemopoiesis, inflammation, and apoptosis. Disruption of the JAK/STAT signaling has been linked to neuroinflammation in AD [54]. Modulation of the JAK/STAT signaling has been demonstrated in mice to improve post-ischemic recovery from AD-like pathology such as aberrant protein accumulation, neuroinflammation, BBB damage and neuronal apoptosis [54-56]. Both KLF13 and KLF4 have been reported to inhibit JAK/STAT signaling essential for axon regeneration. In a study using the mouse hippocampus-derived cell line HT22 [57], KLF13 was shown to inhibit neurotrophic growth hormone induced JAK/STAT signaling by directly repressing the transcription of several genes in the pathway. Another report demonstrated that KLF4 physically binds phosphorylated STAT3 and prevents the STAT3 from DNA binding, resulting in the blockage of the JAK/STAT signaling downstream of axon regenerative cytokine [53,58]. Axon regeneration can be significantly enhanced by the cytokine treatment in KLF4 knockout mice [53,58]. These studies suggest that targeting KLF family members like KLF4 and KLF13 could help block neurodegenerative progression through JAK/STAT mediated axon regeneration.
KLFs and cell cycle regulation in the brain
Neuronal loss is linked to aberrant neuronal cell cycle progression with increased expression of cell-cycle related proteins found in pathologic areas in AD, HD and PD brains [59,60]. Half of the KLF family members are positive or negative regulators of cell cycle in brain cells including both neurons and non-neuronal cells [61,62] (Figure 3). KLF8 was originally identified as an activator of cyclin D1 transcription for cell cycle progression downstream of focal adhesion kinase (FAK) [63]. Research on KLF8 has been focused primarily on cancer [64-74]. However, recent studies have revealed that in the brain, KLF8 is predominantly expressed in the neurons [45] and its expression is significantly decreased in AD patient brains [46], suggesting a potential role for KLF8 in neuronal cell cycle progression. Other KLF members such as KLF2, KLF4, KLF6, KLF7, KLF12, KLF13, and KLF14 have also been shown to regulate cell cycle progression by regulating the expression of cyclins, cyclin-dependent kinase inhibitors [74-78], the PI3K/Akt/mTOR signaling activity [22,79]. Genetic deletion of KLF4 does not disrupt microglial cell proliferation during post-natal brain development in mouse models [80,81]. However, diminished expression of KLF4 seems to be responsible for the loss of expression of the rhythmic genes that are critical for aged microglial differentiation and reprogramming during protective immune responses [82].
Figure 3.
KLFs regulate cell cycle progression in neuronal and glial cells.
KLFs and cellular mechanisms of neuroinflammation
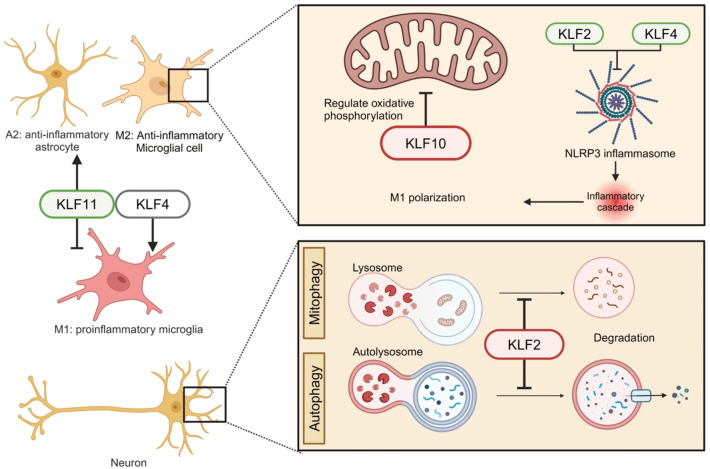
Neuroinflammation plays a critical role in NDs. Normally, it is carried out in a tight knit way with innate immune cells in the brain to protect neurons. However, an excessive neuroinflammatory response is a major contribution to NDs pathology [83]. KLFs play a role in neuroinflammatory progression through activation of inflammatory shifts of glial state, inflammasome formation and mitochondrial metabolism in microglia and autophagy or mitophagy in neurons (Figure 4).
Figure 4.
KLFs modulate inflammasome and mitochondrial oxidation in glial cells, and mitophagy/autophagy in neurons.
Microglia are innate immune cells in the CNS that present different phenotypes. Microglia in a classical activation state, known as M1, secrete proinflammatory cytokines, whereas microglia in alternative activation or acquired deactivation state, known as M2, secrete anti-inflammatory factors. M1 microglia are closely associated with the aggregation of misfolded proteins seen in PD, AD, HD and ALS [84]. Astrocytes play a big role in supporting neuronal function as they help regulate homeostasis and synaptic plasticity and may provide neuroprotection upon brain injury. However, the dysfunction of M2 astrocytes and their switch to A1 astrocytes are linked to NDs pathology [85]. KLF4 plays an essential role in the microglial M1/M2 switch. The switch of microglia from M1 to M2 can be achieved by inhibiting KLF4 interaction with histone deacetylase 1 and suppressing deacetylation. Moreover, oligomeric Aβ42 increases KLF4 expression in microglial BV2 cells. Conversely, overexpression of KLF4 exacerbates Aβ42-induced neuroinflammation [86]. KLF4 expression can be highly induced in activated microglia by lipopolysaccharides (LPS) stimulation, while KLF4 knockdown leads to significantly reduced production of the pro-inflammatory cytokines such as TNF-α and IL-6 as well as iNOS and Cox-2 [87]. A study using the BV2 microglial cell line investigating how the anti-neuroinflammatory agent, Agmatine, exerts its neuroprotective effect revealed that agmatine strongly binds to interferon regulatory factor 2 binding protein (IRF2BP2) in the cytoplasm. This interaction frees the IRF2 that enters the nucleus where it activates the transcription of KLF4 [88], suggesting an important role in activation of M2 microglia. Consistently, KLF4/STAT6 signaling was found to induce M2 macrophages as well [89]. A recent study showed that inhibition of KLF4 translation using miR-25802 resulted in activation of M1 microglia via NF-κB inflammatory signaling and AD pathology, which was reversed by overexpression of KLF4 [90]. KLF11 promotes TGF-β signaling [91] that is known to ameliorate AD pathology by targeting Aβ and Tau through decreasing the expression of pro-inflammatory cytokines and increasing neuronal survival factors [92]. Genetic deletion of KLF11 in mice enhances post-traumatic astrocyte activation, microglial polarization [37] and expression of various pro-inflammatory factors in a traumatic brain injury model of mice [34].
The nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3 (NLRP3) inflammasome is activated by the aggregation of misfolded proteins of Aβ, p-tau or α-syn, which causes initiation and promotion of the neuroinflammatory response in NDs such as AD [93]. Research on KLF4 and KLF2 suggests their potential role for the activation of NLRP3 inflammasome [94,95]. Overexpression of KLF4 increases the liver X receptor α (LXRα) and cholesterol 25-hydroxylase (CH25H) expression, resulting in the inhibition of NLRP3 inflammasome components and the promotion of microglia polarization from the M1 to M2 phenotype [96]. KLF2 was also shown to upregulate CH25H mRNA expression [96,97]. Treatment with simvastatin, a cholesterol lowering drug, causes an increase in the expression of KLF2 and inactivation of the NLRP3 inflammasome [98]. These results indicate the KLF family members such as KLF2 and KLF4 may play a part in neuroinflammation by regulating the NLRP3 inflammasome.
Microglial metabolism is dysregulated in AD with disrupted oxidative phosphorylation and lipid metabolism and a shift into glycolysis that is thought to decrease their ability to phagocytose Aβ and mediate AD pathology [99]. In vitro studies showed that microglia are affected by fluctuation in glucose concentration. A low-to-high glucose shift in BV-2 cells leads to an increase in the expression of the pro-inflammatory factors including the tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX2), while a high-to-low glucose shift promotes autophagy and apoptosis [99,100]. A KLF10 knockdown study suggested that KLF10 is a key regulator of energy metabolism in mitochondria in the cerebellum [101]. Consistently, KLF10 knockout, albeit in the liver, results in disrupted glucose metabolism [102]. Emerging research has shed light on the relationship between mitochondrial metabolic dysfunction and NDs. For example, aggregates of aberrant proteins like Aβ42 and p-tau may be the consequence of insulin resistance in the brain, and drug treatment against diabetes and insulin resistance has been shown to help preserve cognitive functions in AD patients [103,104]. These results suggest a neuroprotective role for KLF10 through regulation of glucose metabolism [105]. KLF9 induction in the neurons by the anti-oxidative stress factor NRF2 has been shown to promote cell survival against high levels of oxidative stress in animal models of brain damage [106]. This could serve as a neuroprotective mechanism of regulation of glutathione and cytochrome P450 against NDs such as PD [106-113].
Autophagy, including the mitophahy form, is important for the maintenance of homeostasis in the brain as it eliminates damaging protein aggregates, but aberrant autophagy can drive ND progression [106,114,115]. Although it is unclear how autophagic dysfunction accelerates ND pathology, some research suggests that the accumulation of Aβ protein is found in autophagic vacuoles, which eventually contributes to the formation of Aβ plaques [116]. Other research suggests that downregulation of autophagic activity leads to impaired clearance of aberrant protein aggregation by autophagy [117]. Consistent with positive regulation of autophagy by KLF2 during osteoblast differentiation and osteoclastogenesis [118], KLF2 deficiency is shown to negatively impact autophagy and mitophagy during neural differentiation of dental pulp-derived stem cell (DPSC), with downregulated expression markers for both autophagy (i.e., LC3B, ATG5, and LAMP1) and mitophagy (i.e., PINK1, Parkin, DRP1, FIS1) [119]. Interestingly, loss-of-function mutations in PINK1 and Parkin are associated with PD [120]. Aberrant activation of Wnt signaling was also observed in development of DPSC [119], suggesting the Wnt signaling regulator KLF8 in the brain [46] perhaps also plays a role in autophagy/mitophagy associated with the disease. A study on neurokinin-1 receptor (NK1R) signaling indicates that activation of this pathway can cause autophagy through the ERK5/KLF4/p62/Nrf2 signaling axis, resulting in the restoration of balanced redox signaling and the subsequent reduction of α-Syn aggregates [121]. Further investigation into how autophagy is regulated by KLFs in the brain is important for understanding mechanisms of progression of NDs.
Considering the critical role of KLF signaling for neuroinflammatory progression and aberrant protein aggregation in NDs [84,99,122-124], manipulating KLF expression could help balance the pro- vs. anti-inflammatory states of glia to ameliorate neuronal inflammation.
Conclusion
The exploration of cerebral expression and roles of KLFs within the intricate landscape of neuroinflammation and neurodegenerative diseases reveals a promising avenue for therapeutic intervention. The multifaceted roles of KLFs in modulating cellular processes such as BBB integrity and glial activation via critical signaling through JAK/STAT, NF-κB, and Wnt/β-catenin axis underscore their potential as key regulators of neuroprotection and neurodegeneration. The elucidation of these KLFs’ implications offer valuable insights into the pathophysiology of NDs, making KLFs potential therapeutic targets. By harnessing the regulatory power of KLFs in the brain, particularly using brain-specific gain/loss-of-function cellular and mouse models, we may unlock novel therapeutic strategies aimed at blocking or even reversing the progression of NDs, offering new hope for improved patient outcomes and quality of life.
Acknowledgements
This work was supported by a Florida Department of Health Ed and Ethel Moore Alzheimer’s Disease Research Program grant (23A07) and an institutional grant to J.Z. The figure images were created using BioRender under our institutional license.
Disclosure of conflict of interest
None.
Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ATG5
autophagy-related protein 5
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- BIG1
brefeldin A-inhibited guanine nucleotide-exchange protein 1
- CAG
cytosine-adenine-guanine
- CCM
cerebral cavernous malformations
- CH25H
cholesterol 25-hydroxylase
- CNS
central nervous system
- COX2
cyclooxygenase-2
- DPSC
dental pulp-derived stem cell
- DRP1
dynamin-related protein 1
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- FIS1
mitochondrial fission 1 protein
- GSH
glutathione
- HCP1
heme carrier protein
- HD
Huntington’s disease
- HO-1
heme oxygenase 1
- iNOS
nitric oxide synthase
- IRF2BP2
interferon regulatory factor 2 binding protein
- JAK/STAT
Janus kinase/signal transducer and activator of transcription
- JAM-1
junction adhesion molecule-1
- KLF
Krüppel-like factor
- LAMP1
lysosomal-associated membrane protein
- LC3B
microtubule-associated proteins 1A/1B light chain 3B
- LPS
lipopolysaccharide
- LXR
liver like receptor
- MEF2
myocyte enhancer factor 2
- MMP
matrix metalloproteinases
- MTP18
mitochondrial protein 18
- ND
neurodegenerative disease
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK1R
neurokinin 1 receptor
- NLRP3
nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3
- Nrf2/Trx1
nuclear factor erythroid 2-related factor 2/thioredoxin 1
- NVU
neurovascular unit
- OGD/R
oxygen-glucose deprivation/reperfusion
- PD
Parkinson’s disease
- PI3K/Akt
Phosphoinositide 3-kinase/Protein Kinase B
- PINK1
PTEN-induced putative kinase 1
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- ROS
reactive oxygen species
- TGF-β
transforming growth factor-beta
- TJ
tight junction
- TLR
toll like receptor
- TNF-α
tumor necrosis factor alpha
- TRIM59
tripartite motif containing 59
- VEGF
vascular endothelial growth factor
- Wnt
wingless/integrated
- ZO-1
zonula occludens-1
- α-syn
α-synuclein
References
- 1.Pollak NM, Hoffman M, Goldberg IJ, Drosatos K. Krüppel-like factors: crippling and un-crippling metabolic pathways. JACC Basic Transl Sci. 2018;3:132–156. doi: 10.1016/j.jacbts.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leuzy A, Chiotis K, Lemoine L, Gillberg PG, Almkvist O, Rodriguez-Vieitez E, Nordberg A. Tau PET imaging in neurodegenerative tauopathies-still a challenge. Mol Psychiatry. 2019;24:1112–1134. doi: 10.1038/s41380-018-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi H, Chang HY, Sang TK. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19:3082. doi: 10.3390/ijms19103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaquer-Alicea J, Diamond MI. Propagation of protein aggregation in neurodegenerative diseases. Annu Rev Biochem. 2019;88:785–810. doi: 10.1146/annurev-biochem-061516-045049. [DOI] [PubMed] [Google Scholar]
- 5.Finkbeiner S. Functional genomics, genetic risk profiling and cell phenotypes in neurodegenerative disease. Neurobiol Dis. 2020;146:105088. doi: 10.1016/j.nbd.2020.105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 7.Su JH, Anderson AJ, Cribbs DH, Tu C, Tong L, Kesslack P, Cotman CW. Fas and Fas ligand are associated with neuritic degeneration in the AD brain and participate in beta-amyloid-induced neuronal death. Neurobiol Dis. 2003;12:182–193. doi: 10.1016/s0969-9961(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 8.Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- 9.Yue Q, Hoi MPM. Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease. Neural Regen Res. 2023;18:1890–1902. doi: 10.4103/1673-5374.367832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pajares M, I Rojo A, Manda G, Boscá L, Cuadrado A. Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells. 2020;9:1687. doi: 10.3390/cells9071687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassil F, Brown HJ, Pattabhiraman S, Iwasyk JE, Maghames CM, Meymand ES, Cox TO, Riddle DM, Zhang B, Trojanowski JQ, Lee VM. Amyloid-Beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of lewy body disorders with Aβ pathology. Neuron. 2020;105:260–275. e266. doi: 10.1016/j.neuron.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 13.Domingues AV, Pereira IM, Vilaça-Faria H, Salgado AJ, Rodrigues AJ, Teixeira FG. Glial cells in Parkinson’s disease: protective or deleterious? Cell Mol Life Sci. 2020;77:5171–5188. doi: 10.1007/s00018-020-03584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McColgan P, Tabrizi SJ. Huntington’s disease: a clinical review. Eur J Neurol. 2018;25:24–34. doi: 10.1111/ene.13413. [DOI] [PubMed] [Google Scholar]
- 15.Al-Bachari S, Naish JH, Parker GJM, Emsley HCA, Parkes LM. Blood-brain barrier leakage is increased in Parkinson’s disease. Front Physiol. 2020;11:593026. doi: 10.3389/fphys.2020.593026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConnell HL, Mishra A. Cells of the blood-brain barrier: an overview of the neurovascular unit in health and disease. Methods Mol Biol. 2022;2492:3–24. doi: 10.1007/978-1-0716-2289-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soto-Rojas LO, Pacheco-Herrero M, Martínez-Gómez PA, Campa-Córdoba BB, Apátiga-Pérez R, Villegas-Rojas MM, Harrington CR, de la Cruz F, Garcés-Ramírez L, Luna-Muñoz J. The neurovascular unit dysfunction in Alzheimer’s disease. Int J Mol Sci. 2021;22:2022. doi: 10.3390/ijms22042022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Z, Du J, Zhang Y, Xu Y, Huang Q, Zhou Q, Wu M, Li Y, Zhang X, Zhang H, Cai Y, Ye K, Wang X, Zhang Y, Han Q, Xiao J. Kruppel-like factor 2 contributes to blood-spinal cord barrier integrity and functional recovery from spinal cord injury by augmenting autophagic flux. Theranostics. 2023;13:849–866. doi: 10.7150/thno.74324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Sheng B, Zhang F, Wu C, Zhang R, Zhu J, Xu K, Kuang Y, Jameson SC, Lin Z, Wang Y, Chen J, Jain MK, Atkins GB. Kruppel-like factor 2 protects against ischemic stroke by regulating endothelial blood brain barrier function. Am J Physiol Heart Circ Physiol. 2013;304:H796–805. doi: 10.1152/ajpheart.00712.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Lu H, Liang W, Hu W, Zhang J, Chen YE. Krüppel-like factors and vascular wall homeostasis. J Mol Cell Biol. 2017;9:352–363. doi: 10.1093/jmcb/mjx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin KJ, Hamblin M, Fan Y, Zhang J, Chen YE. Krüpple-like factors in the central nervous system: novel mediators in stroke. Metab Brain Dis. 2015;30:401–410. doi: 10.1007/s11011-013-9468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggan MR, Weaver M, Khalili K. PAM (PIK3/AKT/mTOR) signaling in glia: potential contributions to brain tumors in aging. Aging (Albany NY) 2021;13:1510–1527. doi: 10.18632/aging.202459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sako K, Fukuhara S, Minami T, Hamakubo T, Song H, Kodama T, Fukamizu A, Gutkind JS, Koh GY, Mochizuki N. Angiopoietin-1 induces Kruppel-like factor 2 expression through a phosphoinositide 3-kinase/AKT-dependent activation of myocyte enhancer factor 2. J Biol Chem. 2009;284:5592–5601. doi: 10.1074/jbc.M806928200. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Li F, Han G, Liu Z. Aβ(1-42) disrupts the expression and function of KLF2 in Alzheimer’s disease mediated by p53. Biochem Biophys Res Commun. 2013;431:141–145. doi: 10.1016/j.bbrc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Fang X, Zhong X, Yu G, Shao S, Yang Q. Vascular protective effects of KLF2 on Aβ-induced toxicity: implications for Alzheimer’s disease. Brain Res. 2017;1663:174–183. doi: 10.1016/j.brainres.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Li F, Han G, Liu Z. Aβ1-42 disrupts the expression and function of KLF2 in Alzheimer’s disease mediated by p53. Biochem Biophys Res Commun. 2013;431:141–145. doi: 10.1016/j.bbrc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Huang T, Yin J, Ren S, Zhang X. Protective effects of KLF4 on blood-brain barrier and oxidative stress after cerebral ischemia-reperfusion in rats through the Nrf2/Trx1 pathway. Cytokine. 2023;169:156288. doi: 10.1016/j.cyto.2023.156288. [DOI] [PubMed] [Google Scholar]
- 28.Cowan CE, Kohler EE, Dugan TA, Mirza MK, Malik AB, Wary KK. Kruppel-like factor-4 transcriptionally regulates VE-cadherin expression and endothelial barrier function. Circ Res. 2010;107:959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 30.Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweet DR, Fan L, Hsieh PN, Jain MK. Krüppel-like factors in vascular inflammation: mechanistic insights and therapeutic potential. Front Cardiovasc Med. 2018;5:6. doi: 10.3389/fcvm.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nath KA, Singh RD, Croatt AJ, Ackerman AW, Grande JP, Khazaie K, Chen YE, Zhang J. KLF11 is a novel endogenous protectant against renal ischemia-reperfusion injury. Kidney360. 2022;3:1417–1422. doi: 10.34067/KID.0002272022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 34.Zhou C, Sun P, Hamblin MH, Yin KJ. Genetic deletion of Krüppel-like factor 11 aggravates traumatic brain injury. J Neuroinflammation. 2022;19:281. doi: 10.1186/s12974-022-02638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, Tang X, Ma F, Fan Y, Sun P, Zhu T, Zhang J, Hamblin MH, Chen YE, Yin KJ. Endothelium-targeted overexpression of Krüppel-like factor 11 protects the blood-brain barrier function after ischemic brain injury. Brain Pathol. 2020;30:746–765. doi: 10.1111/bpa.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE. KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain. 2013;136:1274–1287. doi: 10.1093/brain/awt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Y, Xu Y, Pan Y, Guo H. KLF10 knockdown negatively regulates CTRP3 to improve OGD/R-induced brain microvascular endothelial cell injury and barrier dysfunction through Nrf2/HO-1 signaling pathway. Tissue Cell. 2023;82:102106. doi: 10.1016/j.tice.2023.102106. [DOI] [PubMed] [Google Scholar]
- 39.Swarup V, Phaneuf D, Dupré N, Petri S, Strong M, Kriz J, Julien JP. Deregulation of TDP-43 in amyotrophic lateral sclerosis triggers nuclear factor κB-mediated pathogenic pathways. J Exp Med. 2011;208:2429–2447. doi: 10.1084/jem.20111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Li L. The critical role of KLF4 in regulating the activation of A1/A2 reactive astrocytes following ischemic stroke. J Neuroinflammation. 2023;20:44. doi: 10.1186/s12974-023-02742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price BR, Johnson LA, Norris CM. Reactive astrocytes: the nexus of pathological and clinical hallmarks of Alzheimer’s disease. Ageing Res Rev. 2021;68:101335. doi: 10.1016/j.arr.2021.101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Li C, Guan C, Zhou B, Wang L, Yang C, Zhen L, Dai J, Zhao L, Jiang W, Xu Y. MiR-181d-5p Targets KLF6 to improve ischemia/reperfusion-induced AKI through effects on renal function, apoptosis, and inflammation. Front Physiol. 2020;11:510. doi: 10.3389/fphys.2020.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman WA, Omenetti S, Date D, Di Martino L, De Salvo C, Kim GD, Chowdhry S, Bamias G, Cominelli F, Pizarro TT, Mahabeleshwar GH. KLF6 contributes to myeloid cell plasticity in the pathogenesis of intestinal inflammation. Mucosal Immunol. 2016;9:1250–1262. doi: 10.1038/mi.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Syafruddin SE, Mohtar MA, Wan Mohamad Nazarie WF, Low TY. Two sides of the same coin: the roles of KLF6 in physiology and pathophysiology. Biomolecules. 2020;10:1378. doi: 10.3390/biom10101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobrivojević M, Habek N, Kapuralin K, Ćurlin M, Gajović S. Krüppel-like transcription factor 8 (Klf8) is expressed and active in the neurons of the mouse brain. Gene. 2015;570:132–140. doi: 10.1016/j.gene.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 46.Yi R, Chen B, Zhao J, Zhan X, Zhang L, Liu X, Dong Q. Krüppel-like factor 8 ameliorates Alzheimer’s disease by activating β-catenin. J Mol Neurosci. 2014;52:231–241. doi: 10.1007/s12031-013-0131-4. [DOI] [PubMed] [Google Scholar]
- 47.Yang T, Cai SY, Zhang J, Lu JH, Lin C, Zhai J, Wu MC, Shen F. Krüppel-like factor 8 is a new Wnt/beta-catenin signaling target gene and regulator in hepatocellular carcinoma. PLoS One. 2012;7:e39668. doi: 10.1371/journal.pone.0039668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun E, Motolani A, Campos L, Lu T. The pivotal role of NF-kB in the pathogenesis and therapeutics of Alzheimer’s disease. Int J Mol Sci. 2022;23:8972. doi: 10.3390/ijms23168972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters DG, Connor JR, Meadowcroft MD. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: two sides of the same coin. Neurobiol Dis. 2015;81:49–65. doi: 10.1016/j.nbd.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H, Zhang C, Shen H, Shen Z, Wu L, Mo F, Li M. Physiological stress-induced corticosterone increases heme uptake via KLF4-HCP1 signaling pathway in hippocampus neurons. SciRep. 2017;7:5745. doi: 10.1038/s41598-017-06058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schipper HM. Glial HO-1 expression, iron deposition and oxidative stress in neurodegenerative diseases. Neurotox Res. 1999;1:57–70. doi: 10.1007/BF03033339. [DOI] [PubMed] [Google Scholar]
- 53.Cheng Z, Zou X, Jin Y, Gao S, Lv J, Li B, Cui R. The role of KLF(4) in Alzheimer’s disease. Front Cell Neurosci. 2018;12:325. doi: 10.3389/fncel.2018.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rusek M, Smith J, El-Khatib K, Aikins K, Czuczwar SJ, Pluta R. The role of the JAK/STAT signaling pathway in the pathogenesis of Alzheimer’s disease: new potential treatment target. Int J Mol Sci. 2023;24:864. doi: 10.3390/ijms24010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong Y, Hu C, Huang C, Gao J, Niu W, Wang D, Wang Y, Niu C. Interleukin-22 plays a protective role by regulating the JAK2-STAT3 pathway to improve inflammation, oxidative stress, and neuronal apoptosis following cerebral ischemia-reperfusion injury. Mediators Inflamm. 2021;2021:6621296. doi: 10.1155/2021/6621296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu J, Wang J, Yu L, Cui R, Zhang Y, Ding H, Yan G. Shaoyao-Gancao decoction promoted microglia M2 polarization via the IL-13-mediated JAK2/STAT6 pathway to alleviate cerebral ischemia-reperfusion injury. Mediators of Inflammation. 2022;2022:1707122. doi: 10.1155/2022/1707122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ávila-Mendoza J, Delgado-Rueda K, Urban-Sosa VA, Carranza M, Luna M, Martínez-Moreno CG, Arámburo C. KLF13 regulates the activity of the GH-induced JAK/STAT signaling by targeting genes involved in the pathway. Int J Mol Sci. 2023;24:11187. doi: 10.3390/ijms241311187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin S, Zou Y, Zhang CL. Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat Commun. 2013;4:2633. doi: 10.1038/ncomms3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koseoglu MM, Norambuena A, Sharlow ER, Lazo JS, Bloom GS. Aberrant neuronal cell cycle re-entry: the pathological confluence of Alzheimer’s disease and brain insulin resistance, and its relation to cancer. J Alzheimers Dis. 2019;67:1–11. doi: 10.3233/JAD-180874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelegrí C, Duran-Vilaregut J, del Valle J, Crespo-Biel N, Ferrer I, Pallàs M, Camins A, Vilaplana J. Cell cycle activation in striatal neurons from Huntington’s disease patients and rats treated with 3-nitropropionic acid. Int J Dev Neurosci. 2008;26:665–671. doi: 10.1016/j.ijdevneu.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 61.Li ZY, Zhu YX, Chen JR, Chang X, Xie ZZ. The role of KLF transcription factor in the regulation of cancer progression. Biomed Pharmacother. 2023;162:114661. doi: 10.1016/j.biopha.2023.114661. [DOI] [PubMed] [Google Scholar]
- 62.Lu Y, Guo Z, Zhang Y, Li C, Zhang Y, Guo Q, Chen Q, Chen X, He X, Liu L, Ruan C, Sun T, Ji B, Lu W, Jiang C. Microenvironment remodeling micelles for Alzheimer’s disease therapy by early modulation of activated microglia. Adv Sci (Weinh) 2018;6:1801586. doi: 10.1002/advs.201801586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11:1503–1515. doi: 10.1016/s1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 64.Hao J, Lu H, Mukherjee D, Yu L, Zhao J. Role of kruppel-like factor 8 for therapeutic drug-resistant multi-organ metastasis of breast cancer. Am J Cancer Res. 2021;11:2188–2201. [PMC free article] [PubMed] [Google Scholar]
- 65.Mukherjee D, Hao J, Lu H, Lahiri SK, Yu L, Zhao J. KLF8 promotes invasive outgrowth of breast cancer by inducing filopodium-like protrusions via CXCR4. Am J Transl Res. 2022;14:1220–1233. [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherjee D, Lu H, Yu L, He C, Lahiri SK, Li T, Zhao J. Kruppel-like factor 8 activates the transcription of C-X-C cytokine receptor type 4 to promote breast cancer cell invasion, transendothelial migration and metastasis. Oncotarget. 2016;7:23552–23568. doi: 10.18632/oncotarget.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lahiri SK, Zhao J. Kruppel-like factor 8 emerges as an important regulator of cancer. Am J Transl Res. 2012;4:357–363. [PMC free article] [PubMed] [Google Scholar]
- 68.Li T, Lu H, Mukherjee D, Lahiri SK, Shen C, Yu L, Zhao J. Identification of epidermal growth factor receptor and its inhibitory microRNA141 as novel targets of Kruppel-like factor 8 in breast cancer. Oncotarget. 2015;6:21428–42. doi: 10.18632/oncotarget.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T, Lu H, Shen C, Lahiri SK, Wason MS, Mukherjee D, Yu L, Zhao J. Identification of epithelial stromal interaction 1 as a novel effector downstream of Kruppel-like factor 8 in breast cancer invasion and metastasis. Oncogene. 2014;33:4746–4755. doi: 10.1038/onc.2013.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu H, Hu L, Yu L, Wang X, Urvalek AM, Li T, Shen C, Mukherjee D, Lahiri SK, Wason MS, Zhao J. KLF8 and FAK cooperatively enrich the active MMP14 on the cell surface required for the metastatic progression of breast cancer. Oncogene. 2014;33:2909–2917. doi: 10.1038/onc.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu H, Wang X, Urvalek AM, Li T, Xie H, Yu L, Zhao J. Transformation of human ovarian surface epithelial cells by Kruppel-like factor 8. Oncogene. 2014;33:10–18. doi: 10.1038/onc.2012.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Lu H, Li T, Yu L, Liu G, Peng X, Zhao J. Kruppel-like factor 8 promotes tumorigenic mammary stem cell induction by targeting miR-146a. Am J Cancer Res. 2013;3:356–373. [PMC free article] [PubMed] [Google Scholar]
- 73.Wang X, Lu H, Urvalek AM, Li T, Yu L, Lamar J, DiPersio CM, Feustel PJ, Zhao J. KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene. 2011;30:1901–1911. doi: 10.1038/onc.2010.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26:456–461. doi: 10.1038/sj.onc.1209796. [DOI] [PubMed] [Google Scholar]
- 75.Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 76.Caltagarone J, Jing Z, Bowser R. Focal adhesions regulate Abeta signaling and cell death in Alzheimer’s disease. Biochim Biophys Acta. 2007;1772:438–445. doi: 10.1016/j.bbadis.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laub F, Aldabe R, Friedrich V Jr, Ohnishi S, Yoshida T, Ramirez F. Developmental expression of mouse Krüppel-like transcription factor KLF7 suggests a potential role in neurogenesis. Dev Biol. 2001;233:305–318. doi: 10.1006/dbio.2001.0243. [DOI] [PubMed] [Google Scholar]
- 78.Laub F, Lei L, Sumiyoshi H, Kajimura D, Dragomir C, Smaldone S, Puche AC, Petros TJ, Mason C, Parada LF, Ramirez F. Transcription factor KLF7 is important for neuronal morphogenesis in selected regions of the nervous system. Mol Cell Biol. 2005;25:5699–5711. doi: 10.1128/MCB.25.13.5699-5711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wezyk M, Spólnicka M, Pośpiech E, Pepłońska B, Zbieć-Piekarska R, Ilkowski J, Styczyńska M, Barczak A, Zboch M, Filipek-Gliszczynska A, Skrzypczak M, Ginalski K, Kabza M, Makałowska I, Barcikowska-Kotowicz M, Branicki W, Żekanowski C. Hypermethylation of TRIM59 and KLF14 influences cell death signaling in familial Alzheimer’s disease. Oxid Med Cell Longev. 2018;2018:6918797. doi: 10.1155/2018/6918797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Belhocine S, Machado Xavier A, Distéfano-Gagné F, Fiola S, Rivest S, Gosselin D. Context-dependent transcriptional regulation of microglial proliferation. Glia. 2022;70:572–589. doi: 10.1002/glia.24124. [DOI] [PubMed] [Google Scholar]
- 81.Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Hölscher C, Müller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu. 1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 82.Blacher E, Tsai C, Litichevskiy L, Shipony Z, Iweka CA, Schneider KM, Chuluun B, Heller HC, Menon V, Thaiss CA, Andreasson KI. Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol. 2022;23:229–236. doi: 10.1038/s41590-021-01083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rajesh Y, Kanneganti TD. Innate immune cell death in neuroinflammation and Alzheimer’s disease. Cells. 2022;11:1885. doi: 10.3390/cells11121885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 85.Li K, Li J, Zheng J, Qin S. Reactive astrocytes in neurodegenerative diseases. Aging Dis. 2019;10:664–675. doi: 10.14336/AD.2018.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li L, Zi X, Hou D, Tu Q. Krüppel-like factor 4 regulates amyloid-β (Aβ)-induced neuroinflammation in Alzheimer’s disease. Neurosci Lett. 2017;643:131–137. doi: 10.1016/j.neulet.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 87.Kaushik DK, Gupta M, Das S, Basu A. Krüppel-like factor 4, a novel transcription factor regulates microglial activation and subsequent neuroinflammation. J Neuroinflammation. 2010;7:68. doi: 10.1186/1742-2094-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim J, Sim AY, Barua S, Kim JY, Lee JE. Agmatine-IRF2BP2 interaction induces M2 phenotype of microglia by increasing IRF2-KLF4 signaling. Inflamm Res. 2023;72:1203–1213. doi: 10.1007/s00011-023-01741-z. [DOI] [PubMed] [Google Scholar]
- 89.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clément K, Jain MK. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao K, Liu J, Sun T, Zeng L, Cai Z, Li Z, Liu R. The miR-25802/KLF4/NF-κB signaling axis regulates microglia-mediated neuroinflammation in Alzheimer’s disease. Brain Behav Immun. 2024;118:31–48. doi: 10.1016/j.bbi.2024.02.016. [DOI] [PubMed] [Google Scholar]
- 91.Spittau B, Krieglstein K. Klf10 and Klf11 as mediators of TGF-beta superfamily signaling. Cell Tissue Res. 2012;347:65–72. doi: 10.1007/s00441-011-1186-6. [DOI] [PubMed] [Google Scholar]
- 92.von Bernhardi R, Cornejo F, Parada GE, Eugenín J. Role of TGFβ signaling in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci. 2015;9:426. doi: 10.3389/fncel.2015.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liang T, Zhang Y, Wu S, Chen Q, Wang L. The role of NLRP3 inflammasome in Alzheimer’s disease and potential therapeutic targets. Front Pharmacol. 2022;13:845185. doi: 10.3389/fphar.2022.845185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao J, Wang Z, Song W, Zhang Y. Targeting NLRP3 inflammasome for neurodegenerative disorders. Mol Psychiatry. 2023;28:4512–4527. doi: 10.1038/s41380-023-02239-0. [DOI] [PubMed] [Google Scholar]
- 95.Liu Y, Dai Y, Li Q, Chen C, Chen H, Song Y, Hua F, Zhang Z. Beta-amyloid activates NLRP3 inflammasome via TLR4 in mouse microglia. Neurosci Lett. 2020;736:135279. doi: 10.1016/j.neulet.2020.135279. [DOI] [PubMed] [Google Scholar]
- 96.Li Z, Martin M, Zhang J, Huang HY, Bai L, Zhang J, Kang J, He M, Li J, Maurya MR, Gupta S, Zhou G, Sangwung P, Xu YJ, Lei T, Huang HD, Jain M, Jain MK, Subramaniam S, Shyy JY. Krüppel-Like Factor 4 Regulation of cholesterol-25-hydroxylase and liver x receptor mitigates atherosclerosis susceptibility. Circulation. 2017;136:1315–1330. doi: 10.1161/CIRCULATIONAHA.117.027462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alatshan A, Benkő S. Nuclear receptors as multiple regulators of NLRP3 inflammasome function. Front Immunol. 2021;12:630569. doi: 10.3389/fimmu.2021.630569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang X, Huo R, Liang Z, Xu C, Chen T, Lin J, Li L, Lin W, Pan B, Fu X, Chen S. Simvastatin inhibits NLRP3 inflammasome activation and ameliorates lung injury in hyperoxia-induced bronchopulmonary dysplasia via the KLF2-mediated mechanism. Oxid Med Cell Longev. 2022;2022:8336070. doi: 10.1155/2022/8336070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lepiarz-Raba I, Gbadamosi I, Florea R, Paolicelli RC, Jawaid A. Metabolic regulation of microglial phagocytosis: implications for Alzheimer’s disease therapeutics. Transl Neurodegener. 2023;12:48. doi: 10.1186/s40035-023-00382-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep. 2019;9:840. doi: 10.1038/s41598-018-37215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kammoun M, Nadal-Desbarats L, Même S, Lafoux A, Huchet C, Meyer-Dilhet G, Courchet J, Montigny F, Szeremeta F, Même W, Veksler V, Piquereau J, Pouletaut P, Subramaniam M, Hawse JR, Constans JM, Bensamoun SF. Deciphering the role of Klf10 in the cerebellum. J Biomed Sci Eng. 2022;15:140–156. doi: 10.4236/jbise.2022.155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alvarez-Rodríguez R, Barzi M, Berenguer J, Pons S. Bone morphogenetic protein 2 opposes Shh-mediated proliferation in cerebellar granule cells through a TIEG-1-based regulation of Nmyc. J Biol Chem. 2007;282:37170–37180. doi: 10.1074/jbc.M705414200. [DOI] [PubMed] [Google Scholar]
- 103.de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs. 2017;77:47–65. doi: 10.1007/s40265-016-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer’s disease: from epidemiology to mechanism and treatment. Clin Interv Aging. 2015;10:549–560. doi: 10.2147/CIA.S74042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo HY, Zhu JY, Chen M, Mu WJ, Guo L. Krüppel-like factor 10 (KLF10) as a critical signaling mediator: versatile functions in physiological and pathophysiological processes. Genes Dis. 2022;10:915–930. doi: 10.1016/j.gendis.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parga JA, Rodriguez-Perez AI, Garcia-Garrote M, Rodriguez-Pallares J, Labandeira-Garcia JL. NRF2 activation and downstream effects: focus on Parkinson’s disease and brain angiotensin. Antioxidants (Basel) 2021;10:1649. doi: 10.3390/antiox10111649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang D, Lv Z, Zhang H, Liu B, Jiang H, Tan X, Lu J, Baiyun R, Zhang Z. Activation of the Nrf2 signaling pathway involving KLF9 plays a critical role in allicin resisting against arsenic trioxide-induced hepatotoxicity in rats. Biol Trace Elem Res. 2017;176:192–200. doi: 10.1007/s12011-016-0821-1. [DOI] [PubMed] [Google Scholar]
- 108.Ihara K, Oguro A, Imaishi H. Diagnosis of Parkinson’s disease by investigating the inhibitory effect of serum components on P450 inhibition assay. Sci Rep. 2022;12:6622. doi: 10.1038/s41598-022-10528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wójcikowski J, Daniel WA. The brain dopaminergic system as an important center regulating liver cytochrome P450 in the rat. Expert Opin Drug Metab Toxicol. 2009;5:631–645. doi: 10.1517/17425250902973703. [DOI] [PubMed] [Google Scholar]
- 110.Mandal PK, Goel A, Bush AI, Punjabi K, Joon S, Mishra R, Tripathi M, Garg A, Kumar NK, Sharma P, Shukla D, Ayton SJ, Fazlollahi A, Maroon JC, Dwivedi D, Samkaria A, Sandal K, Megha K, Shandilya S. Hippocampal glutathione depletion with enhanced iron level in patients with mild cognitive impairment and Alzheimer’s disease compared with healthy elderly participants. Brain Commun. 2022;4:fcac215. doi: 10.1093/braincomms/fcac215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mischley LK, Standish LJ, Weiss NS, Padowski JM, Kavanagh TJ, White CC, Rosenfeld ME. Glutathione as a biomarker in Parkinson’s disease: associations with aging and disease severity. Oxid Med Cell Longev. 2016;2016:9409363. doi: 10.1155/2016/9409363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ribeiro M, Rosenstock TR, Cunha-Oliveira T, Ferreira IL, Oliveira CR, Rego AC. Glutathione redox cycle dysregulation in Huntington’s disease knock-in striatal cells. Free Radic Biol Med. 2012;53:1857–1867. doi: 10.1016/j.freeradbiomed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 113.Ambani LM, Van Woert MH, Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch Neurol. 1975;32:114–118. doi: 10.1001/archneur.1975.00490440064010. [DOI] [PubMed] [Google Scholar]
- 114.Xu Y, Propson NE, Du S, Xiong W, Zheng H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc Natl Acad Sci U S A. 2021;118:e2023418118. doi: 10.1073/pnas.2023418118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schulte J, Littleton JT. The biological function of the huntingtin protein and its relevance to Huntington’s disease pathology. Curr Trends Neurol. 2011;5:65–78. [PMC free article] [PubMed] [Google Scholar]
- 116.Orr ME, Oddo S. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther. 2013;5:53. doi: 10.1186/alzrt217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang W, Xu C, Sun J, Shen HM, Wang J, Yang C. Impairment of the autophagy-lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm Sin B. 2022;12:1019–1040. doi: 10.1016/j.apsb.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Laha D, Deb M, Das H. KLF2 (kruppel-like factor 2 [lung] ) regulates osteoclastogenesis by modulating autophagy. Autophagy. 2019;15:2063–2075. doi: 10.1080/15548627.2019.1596491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Prateeksha P, Naidu P, Das M, Barthels D, Das H. KLF2 regulates neural differentiation of dental pulp-derived stem cells by modulating autophagy and mitophagy. Stem Cell Rev Rep. 2023;19:2886–2900. doi: 10.1007/s12015-023-10607-0. [DOI] [PubMed] [Google Scholar]
- 120.Sheng ZH. Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol. 2014;204:1087–1098. doi: 10.1083/jcb.201312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El-Deeb AM, Mohamed AF, El-Yamany MF, El-Tanbouly DM. Novel trajectories of the NK1R antagonist aprepitant in rotenone-induced Parkinsonism-like symptoms in rats: involvement of ERK5/KLF4/p62/Nrf2 signaling axis. Chem Biol Interact. 2023;380:110562. doi: 10.1016/j.cbi.2023.110562. [DOI] [PubMed] [Google Scholar]
- 122.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 123.Isik S, Yeman Kiyak B, Akbayir R, Seyhali R, Arpaci T. Microglia mediated neuroinflammation in Parkinson’s disease. Cells. 2023;12:1012. doi: 10.3390/cells12071012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koenigsknecht-Talboo J, Landreth GE. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J Neurosci. 2005;25:8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang X, Liu K, Hamblin MH, Xu Y, Yin KJ. Genetic deletion of Krüppel-like factor 11 aggravates ischemic brain injury. Mol Neurobiol. 2018;55:2911–2921. doi: 10.1007/s12035-017-0556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seo S, Lomberk G, Mathison A, Buttar N, Podratz J, Calvo E, Iovanna J, Brimijoin S, Windebank A, Urrutia R. Krüppel-like factor 11 differentially couples to histone acetyltransferase and histone methyltransferase chromatin remodeling pathways to transcriptionally regulate dopamine D2 receptor in neuronal cells. J Biol Chem. 2012;287:12723–12735. doi: 10.1074/jbc.M112.351395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duan Q, Si E. MicroRNA-25 aggravates Aβ1-42-induced hippocampal neuron injury in Alzheimer’s disease by downregulating KLF2 via the Nrf2 signaling pathway in a mouse model. J Cell Biochem. 2019;120:15891–15905. doi: 10.1002/jcb.28861. [DOI] [PubMed] [Google Scholar]
- 129.Wang Q, Gong L, Mao S, Yao C, Liu M, Wang Y, Yang J, Yu B, Chen G, Gu X. Klf2-Vav1-Rac1 axis promotes axon regeneration after peripheral nerve injury. Exp Neurol. 2021;343:113788. doi: 10.1016/j.expneurol.2021.113788. [DOI] [PubMed] [Google Scholar]
- 130.Wu F, Li C. KLF2 up-regulates IRF4/HDAC7 to protect neonatal rats from hypoxic-ischemic brain damage. Cell Death Discov. 2022;8:41. doi: 10.1038/s41420-022-00813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao K, Li R, Ruan Q, Meng C, Yin F, Zhu Q. microRNA-125b and its downstream Smurf1/KLF2/ATF2 axis as important promoters on neurological function recovery in rats with spinal cord injury. J Cell Mol Med. 2021;25:5924–5939. doi: 10.1111/jcmm.16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qin S, Liu M, Niu W, Zhang CL. Dysregulation of Kruppel-like factor 4 during brain development leads to hydrocephalus in mice. Proc Natl Acad Sci U S A. 2011;108:21117–21121. doi: 10.1073/pnas.1112351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu S, Tai C, MacVicar BA, Jia W, Cynader MS. Glutamatergic stimulation triggers rapid Krüpple-like factor 4 expression in neurons and the overexpression of KLF4 sensitizes neurons to NMDA-induced caspase-3 activity. Brain Res. 2009;1250:49–62. doi: 10.1016/j.brainres.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 134.Blackmore MG, Moore DL, Smith RP, Goldberg JL, Bixby JL, Lemmon VP. High content screening of cortical neurons identifies novel regulators of axon growth. Mol Cell Neurosci. 2010;44:43–54. doi: 10.1016/j.mcn.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pérez-Monter C, Martínez-Armenta M, Miquelajauregui A, Furlan-Magaril M, Varela-Echavarría A, Recillas-Targa F, May V, Charli JL, Pérez-Martínez L. The Krüppel-like factor 4 controls biosynthesis of thyrotropin-releasing hormone during hypothalamus development. Mol Cell Endocrinol. 2011;333:127–133. doi: 10.1016/j.mce.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 136.Ghosh A, Pahan K. Gemfibrozil, a Lipid-lowering drug, induces suppressor of cytokine signaling 3 in glial cells: implications for neurodegenerative disorders. J Biol Chem. 2012;287:27189–27203. doi: 10.1074/jbc.M112.346932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaushik DK, Mukhopadhyay R, Kumawat KL, Gupta M, Basu A. Therapeutic targeting of Krüppel-like factor 4 abrogates microglial activation. J Neuroinflammation. 2012;9:57. doi: 10.1186/1742-2094-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qin S, Zhang CL. Role of Kruppel-like factor 4 in neurogenesis and radial neuronal migration in the developing cerebral cortex. Mol Cell Biol. 2012;32:4297–4305. doi: 10.1128/MCB.00838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen J, Wang X, Yi X, Wang Y, Liu Q, Ge R. Induction of KLF4 contributes to the neurotoxicity of MPP + in M17 cells: a new implication in Parkinson’s disease. J Mol Neurosci. 2013;51:109–117. doi: 10.1007/s12031-013-9961-3. [DOI] [PubMed] [Google Scholar]
- 140.Kaushik DK, Thounaojam MC, Kumawat KL, Gupta M, Basu A. Interleukin-1β orchestrates underlying inflammatory responses in microglia via Krüppel-like factor 4. J Neurochem. 2013;127:233–244. doi: 10.1111/jnc.12382. [DOI] [PubMed] [Google Scholar]
- 141.Su C, Sun F, Cunningham RL, Rybalchenko N, Singh M. ERK5/KLF4 signaling as a common mediator of the neuroprotective effects of both nerve growth factor and hydrogen peroxide preconditioning. Age (Dordr) 2014;36:9685. doi: 10.1007/s11357-014-9685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cui DM, Zeng T, Ren J, Wang K, Jin Y, Zhou L, Gao L. KLF4 knockdown attenuates TBI-induced neuronal damage through p53 and JAK-STAT3 signaling. CNS Neurosci Ther. 2017;23:106–118. doi: 10.1111/cns.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kikkawa Y, Ogura T, Nakajima H, Ikeda T, Takeda R, Neki H, Kohyama S, Yamane F, Kurogi R, Amano T, Nakamizo A, Mizoguchi M, Kurita H. Altered expression of microRNA-15a and kruppel-like factor 4 in cerebrospinal fluid and plasma after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2017;108:909–916. e903. doi: 10.1016/j.wneu.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 144.Song Y, Liu Y, Chen X. MiR-212 attenuates MPP+-induced neuronal damage by targeting KLF4 in SH-SY5Y cells. Yonsei Med J. 2018;59:416–424. doi: 10.3349/ymj.2018.59.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wen M, Ye J, Han Y, Huang L, Yang H, Jiang W, Chen S, Zhong W, Zeng H, Li DY. Hypertonic saline regulates microglial M2 polarization via miR-200b/KLF4 in cerebral edema treatment. Biochem Biophys Res Commun. 2018;499:345–353. doi: 10.1016/j.bbrc.2018.03.161. [DOI] [PubMed] [Google Scholar]
- 146.Yang H, Xi X, Zhao B, Su Z, Wang Z. KLF4 protects brain microvascular endothelial cells from ischemic stroke induced apoptosis by transcriptionally activating MALAT1. Biochem Biophys Res Commun. 2018;495:2376–2382. doi: 10.1016/j.bbrc.2017.11.205. [DOI] [PubMed] [Google Scholar]
- 147.Yanagi M, Hashimoto T, Kitamura N, Fukutake M, Komure O, Nishiguchi N, Kawamata T, Maeda K, Shirakawa O. Expression of Kruppel-like factor 5 gene in human brain and association of the gene with the susceptibility to schizophrenia. Schizophr Res. 2008;100:291–301. doi: 10.1016/j.schres.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y, Cui Y, Liu J, Song Q, Cao M, Hou Y, Zhang X, Wang P. Krüppel-like factor 5 accelerates the pathogenesis of Alzheimer’s disease via BACE1-mediated APP processing. Alzheimers Res Ther. 2022;14:103. doi: 10.1186/s13195-022-01050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Laub F, Aldabe R, Ramirez F, Friedman S. Embryonic expression of Krüppel-like factor 6 in neural and non-neural tissues. Mech Dev. 2001;106:167–170. doi: 10.1016/s0925-4773(01)00419-1. [DOI] [PubMed] [Google Scholar]
- 150.Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 151.Jeong KH, Kim SK, Kim SY, Cho KO. Immunohistochemical localization of Krüppel-like factor 6 in the mouse forebrain. Neurosci Lett. 2009;453:16–20. doi: 10.1016/j.neulet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 152.Jeong KH, Lee KE, Kim SY, Cho KO. Upregulation of Krüppel-like factor 6 in the mouse hippocampus after pilocarpine-induced status epilepticus. Neuroscience. 2011;186:170–178. doi: 10.1016/j.neuroscience.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 153.Laub F, Dragomir C, Ramirez F. Mice without transcription factor KLF7 provide new insight into olfactory bulb development. Brain Res. 2006;1103:108–113. doi: 10.1016/j.brainres.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 154.Caiazzo M, Colucci-D’Amato L, Esposito MT, Parisi S, Stifani S, Ramirez F, di Porzio U. Transcription factor KLF7 regulates differentiation of neuroectodermal and mesodermal cell lineages. Exp Cell Res. 2010;316:2365–2376. doi: 10.1016/j.yexcr.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 155.Caiazzo M, Colucci-D’Amato L, Volpicelli F, Speranza L, Petrone C, Pastore L, Stifani S, Ramirez F, Bellenchi GC, di Porzio U. Krüppel-like factor 7 is required for olfactory bulb dopaminergic neuron development. Exp Cell Res. 2011;317:464–473. doi: 10.1016/j.yexcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 156.Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schnell O, Romagna A, Jaehnert I, Albrecht V, Eigenbrod S, Juerchott K, Kretzschmar H, Tonn JC, Schichor C. Krüppel-like factor 8 (KLF8) is expressed in gliomas of different WHO grades and is essential for tumor cell proliferation. PLoS One. 2012;7:e30429. doi: 10.1371/journal.pone.0030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Imataka H, Nakayama K, Yasumoto K, Mizuno A, Fujii-Kuriyama Y, Hayami M. Cell-specific translational control of transcription factor BTEB expression. The role of an upstream AUG in the 5’-untranslated region. J Biol Chem. 1994;269:20668–20673. [PubMed] [Google Scholar]
- 159.Bonett RM, Hu F, Bagamasbad P, Denver RJ. Stressor and glucocorticoid-dependent induction of the immediate early gene Krüppel-like factor 9: implications for neural development and plasticity. Endocrinology. 2009;150:1757–1765. doi: 10.1210/en.2008-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Denver RJ, Williamson KE. Identification of a thyroid hormone response element in the mouse Kruppel-like factor 9 gene to explain its postnatal expression in the brain. Endocrinology. 2009;150:3935–3943. doi: 10.1210/en.2009-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scobie KN, Hall BJ, Wilke SA, Klemenhagen KC, Fujii-Kuriyama Y, Ghosh A, Hen R, Sahay A. Krüppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci. 2009;29:9875–9887. doi: 10.1523/JNEUROSCI.2260-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Avci HX, Lebrun C, Wehrlé R, Doulazmi M, Chatonnet F, Morel MP, Ema M, Vodjdani G, Sotelo C, Flamant F, Dusart I. Thyroid hormone triggers the developmental loss of axonal regenerative capacity via thyroid hormone receptor α1 and krüppel-like factor 9 in Purkinje cells. Proc Natl Acad Sci U S A. 2012;109:14206–14211. doi: 10.1073/pnas.1119853109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bagamasbad P, Ziera T, Borden SA, Bonett RM, Rozeboom AM, Seasholtz A, Denver RJ. Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology. 2012;153:5334–5345. doi: 10.1210/en.2012-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dugas JC, Ibrahim A, Barres BA. The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci. 2012;50:45–57. doi: 10.1016/j.mcn.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu F, Knoedler JR, Denver RJ. A mechanism to enhance cellular responsivity to hormone action: Krüppel-like factor 9 promotes thyroid hormone receptor-β autoinduction during postembryonic brain development. Endocrinology. 2016;157:1683–1693. doi: 10.1210/en.2015-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parga JA, Rodriguez-Perez AI, Garcia-Garrote M, Rodriguez-Pallares J, Labandeira-Garcia JL. Data on the effect of angiotensin II and 6-hydroxydopamine on reactive oxygen species production, antioxidant gene expression and viability of different neuronal cell lines. Data Brief. 2018;21:934–942. doi: 10.1016/j.dib.2018.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Avila-Mendoza J, Subramani A, Denver RJ. OR01-04 Kruppel-like factors 9 and 13 cooperate to maintain mammalian neuronal differentiation. Journal of the Endocrine Society. 2020;4:OR01–04. [Google Scholar]
- 168.Lin D, Li Y, Huang K, Chen Y, Jing X, Liang Y, Bu L, Peng S, Zeng S, Asakawa T, Tao E. Exploration of the α-syn/T199678/miR-519-3p/KLF9 pathway in a PD-related α-syn pathology. Brain Res Bull. 2022;186:50–61. doi: 10.1016/j.brainresbull.2022.05.012. [DOI] [PubMed] [Google Scholar]
- 169.Killick R, Ribe EM, Al-Shawi R, Malik B, Hooper C, Fernandes C, Dobson R, Nolan PM, Lourdusamy A, Furney S, Lin K, Breen G, Wroe R, To AW, Leroy K, Causevic M, Usardi A, Robinson M, Noble W, Williamson R, Lunnon K, Kellie S, Reynolds CH, Bazenet C, Hodges A, Brion JP, Stephenson J, Simons JP, Lovestone S. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry. 2014;19:88–98. doi: 10.1038/mp.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Harris S, Johnson S, Duncan JW, Udemgba C, Meyer JH, Albert PR, Lomberk G, Urrutia R, Ou XM, Stockmeier CA, Wang JM. Evidence revealing deregulation of the KLF11-Mao a pathway in association with chronic stress and depressive disorders. Neuropsychopharmacology. 2015;40:1373–1382. doi: 10.1038/npp.2014.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohtsuka T, Shimojo H, Matsunaga M, Watanabe N, Kometani K, Minato N, Kageyama R. Gene expression profiling of neural stem cells and identification of regulators of neural differentiation during cortical development. Stem Cells. 2011;29:1817–1828. doi: 10.1002/stem.731. [DOI] [PubMed] [Google Scholar]
- 172.Fu H, Cai J, Clevers H, Fast E, Gray S, Greenberg R, Jain MK, Ma Q, Qiu M, Rowitch DH, Taylor CM, Stiles CD. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. J Neurosci. 2009;29:11399–11408. doi: 10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]