ABSTRACT
We previously reported that live, but not dead, virulent Mycobacterium tuberculosis (Mtb) H37Rv bacilli induce cell death in human lung fibroblast cell lines, MRC-5, MRC-9, and TIG-1. Here, using two distinct Mtb strains from two different lineages (HN878 lineage 2 and H37Rv lineage 4), we confirmed cell death at day 2 after infection with a device that measures cell growth/cytotoxicity in real time (Maestro-Z [AXION]). Mtb bacilli uptake by the fibroblast was confirmed with a transmission electron microscope on day 2. Expressions of inflammatory cytokines and interleukin (IL)−1β, IL-6, and IL-8 were observed when exposed to live, but not dead bacteria. The cell death of fibroblasts induced by both Mtb strains tested was prevented by caspase-1/4 and NLRP3 inflammasome inhibitors, but not by caspase-3 and caspase-9 inhibitors. Therefore, we classified the fibroblast cell death by Mtb infection as pyroptosis. To investigate the biological and pathological relevance of fibroblast cell death by Mtb infection, we performed dual RNA-Seq analysis on Mtb within fibroblasts and Mtb-infected fibroblasts at day 2. In Mtb bacilli tcrR, secE2, ahpD, and mazF8 genes were highly induced during infection. These genes play roles in survival in a hypoxic environment, production of a calcium-binding protein-inducing cytokine, and regulation of transcription in a toxin-antitoxin system. The gene expressions of IL-1β, IL-6, and IL-8, caspase-4, and NLRP3, but not of caspase-3 and caspase-9, were augmented in Mtb bacilli-infected fibroblasts. Taken together, our study suggests that Mtb bacilli attempt to survive in lung fibroblasts and that pyroptosis of the host fibroblasts activates the immune system against the infection.
IMPORTANCE
The role of “non-classical immune cells,” that is, fibroblasts, epithelial cells, adipocytes, etc., except for the “classical immune cells,” that is, macrophages and lymphoid cells, is not well known in the infection of Mtb bacilli. We have previously found that live, but not dead, Mtb bacilli induce cell death in human lung fibroblasts, except in human macrophages and monocytes. The present study reveals that fibroblasts ingest Mtb bacilli the same as macrophages and that in vivo Mtb bacilli within fibroblasts attempt to survive in the host cells, and pyroptosis, including the production of inflammatory cytokines, is induced in the Mtb-infected fibroblasts. Our results suggest that pyroptosis of the host fibroblasts activates the immune system against the infection.
KEYWORDS: Mycobacterium tuberculosis, pyroptosis, caspase, RNA-Seq, cytokine, fibroblasts
INTRODUCTION
Tuberculosis (TB), the world’s largest bacterial infectious disease, is caused by the Mycobacterium tuberculosis (Mtb) bacillus. We previously reported that live, but not dead, Mtb exhibits cytotoxicity to normal diploid fibroblasts from fetal lung cell lines MRC-5, MRC-9, and TIG-1 (1, 2). The cytotoxicity of the reported pathogenic Mycobacteria is stronger than the cytotoxicity of Mycobacterium avium, a pathogen of opportunistic infectious disease, and Mycobacterium bovis BCG Pasteur, a TB vaccine strain (1). In our previous studies, both live and dead Mtb induced cell death in other human-derived “classical immune cell” lines, that is, U937, THP-1, and HL-60.
Recently, many studies have evaluated the role of cell types other than macrophages in Mtb infection. Nevertheless, the role of fibroblasts in the progression or convergence of TB is not well understood. Several researchers reported that fibroblasts produce many inflammatory cytokines, including large quantities of IL-6 (3) and IL-8/CXCL8 (4), when infected with Mtb bacilli (5, 6). Furthermore, fibroblasts cooperate with other immune cells by interacting with an integral component of granuloma structures formed in response to Mtb bacilli infection, and this interaction activates immune regulatory functions to control the infection (6).
In recent years, pyroptosis has been recognized as the third type of cell death besides apoptosis and necrosis (7). In this study, we investigated whether the cytotoxicity of live Mtb is related to pyroptosis by analyzing inhibitors of caspase-1/4 and -3 and NLRP3 inflammasome. Furthermore, we used dual RNA-Seq analysis (8) to analyze both bacteria and host cell factors involved in cytotoxicity and examined gene expression in in vivo Mtb bacilli (i.e., within the host cell) and the role of fibroblast cell death in response to Mtb infection.
MATERIALS AND METHODS
Inhibitors
The inhibitors for caspase-1/4 (VX-765, Adooq BioScience LLC., Irvine, CA, USA), caspase-3 (PAC-1, Selleck Chemicals LLC, Houston, TX, USA), caspase-9 (z-LEHD-fmk, Selleck Chemicals LLC), and NLRP3 inflammasome (MCC950, Adooq BioScience LLC) were dissolved in dimethyl sulfoxide (DMSO) as 10 mg/mL stock and stored at −20°C until use.
Bacterial cultures and frozen stock, and preparation of dead bacteria
Mtb H37Rv (ATTC#27294) and Mtb HN878 (BEI Resources, NR-13647) were purchased from the American Type Culture Collection (Manassas, VA, USA) and obtained from NIH-supported BEI Resources, respectively. Bacterial cultures were prepared in Middlebrook 7H9 broth (Difco, Detroit, MI, USA) supplemented with 10% albumin dextrose catalase (5% bovine serum albumin [fraction V], 2% dextrose, and 0.004% bovine liver catalase; Difco) and 0.05% Tween 80 and were cultured at 37°C under static conditions. Bacteria were grown to an optical density of 0.6–0.8 at 530 nm. Then, the cultures were aliquoted and stored at −70°C until needed. The number of colony-forming units in the aliquots was determined by colony assays on Middlebrook 7H11 agar (Difco) supplemented with 10% oleic albumin dextrose catalase (0.05% oleic acid, 5% bovine serum albumin [fraction V], 2% dextrose, and 0.004% bovine liver catalase; Difco). The dead bacteria were prepared by boiling, 95°C for 30 min, or by treatment with antibiotics, 100 µg/mL isoniazid and 100 µg/mL streptomycin for 1 day.
Cell culture
The tissue culture medium for human embryonic lung fibroblast cell lines, MRC-5 (normal diploid fibroblasts from male), MRC-9 (normal diploid fibroblasts from female), and TIG-1 (normal diploid fibroblasts from female) (JCRB, Tsukuba, Ibaraki, Japan) were maintained, Dulbecco’s modified Eagle medium (D-MEM; low glucose) with L-glutamine and phenol red (Wako Pure Chemical Industries, Ltd., Osaka, Japan), 100 µg/mL streptomycin (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) and 100 units/mL penicillin G (Meiji Seika Pharma) and 5% heat-inactivated fetal bovine serum (FBS; Hyclone, GE Health Life Science, South Logan, UT, USA). The culture was maintained at 37°C in 5% CO2 in a 100 mm in diameter Falcon standard tissue culture dish (No. 353003, Thermo Fisher Scientific, Waltham, MA, USA).
Transmission electron microscopy
Fibroblasts (2 × 106 cells/dish) were cultured for 24 hours, and then incubated with 2 × 107 bacilli for the indicated period. After infection, the cells were washed with PBS (-) twice and scraped off with a cell scraper. Then, they were suspended in 1 mL of 2.5% glutaraldehyde (TAAB Laboratories Equipment Ltd, UK) in 100 mM phosphate buffer (PB) with pH 7.4 and fixed overnight at 4°C. After centrifugation and rinsing with PB, the cell pellets were post-fixed with 1% osmium tetroxide (Heraeus, South Africa) for 1 hour at 4 °C. After centrifugation, the osmium solution was discarded, and the cells were subjected to dehydration with a grading ethanol series with repeated centrifugation at room temperature (RT). Next, the cells were suspended in a solution of ethanol and Spurr’s resin (1:1) overnight at RT, and in complete Spurr’s resin for 12 hours twice at RT and then embedded with Spurr’s resin at 70 °C for 2 days. Ultrathin sections of 80 nm thickness were cut with ARTOS 3D (Leica Mikrosysteme GmbH, Vienna, Austria), mounted on a SiN window chip (Cat.# 783131836, JEOL, Tokyo, Japan) (9), and stained with uranyl acetate and lead citrate. Transmission electron microscopic examinations were performed with a JEM-2100 Plus microscope operated at 120 kV acceleration voltage and equipped with a complementary metal-oxide semiconductor (CMOS) camera with 2,048 × 2,048 pixels.
Acid-fast staining
Fibroblasts (2 × 105 cells/well) were cultured on an 8-well chamber microscope glass slide (SCS-No. 8, Matsunami Glass Ind., Ltd., Osaka, Japan) for 24 hours, and then incubated with 2 × 106 bacilli for the indicated period. Then, the cells were washed with 1 mL of PBS(−) twice and then fixed with 2.5% glutaraldehyde (TAAB Laboratories Equipment Ltd, UK) in PB at 4°C overnight. After rinsing with PB three times, the cells were stained with Ziehl-Neelsen.
Confocal laser microscopy
Fibroblasts (1 × 103 cell/mL) were cultured in a slide chamber (Matsunami Glass Ind. Ltd., Osaka, Japan) for 4 hours, and then enhanced green fluorescent protein expressing live Mycobacterium bovis BCG bacilli (1 × 104 cfu/mL), kindly provided by Dr. T. Mukai (National Institute of Infectious Disease Japan, Higashi Murayama, Japan), was added. The chamber was washed with PBS(−) twice, and fresh culture medium was added. Thirty minutes before examination with confocal laser microscopy (Zeiss LSM-410, Oberkochen, Germany), lysosomes and the nuclear membrane of the fibroblast were stained with 50 nM of LysoTracker Red DND-99 (Thermo Fisher Scientific, Waltham, MA, USA), and 5 µg/mL of Hoechst 33342 (Dojindo, Kumamoto, Japan), respectively.
Real-time measurement of cytotoxicity with Maestro-Z (AXION)
Before seeding fibroblasts on the 96-well plate (CytoView-Z, AXION, Atlanta, GA, USA) for Maestro-Z (AXION), the plate was coated with 100 µL of fibronectin (REF11051407001, Roche, Basel, Switzerland; 1 µg/mL) for 1 hour at RT. Then, the coating solution was removed, and 100 mL of the tissue culture medium was added, followed by adding 8 mL of sterile water on the plate reservoirs to increase the humidity. The plate was inserted into the chamber of the Maestro-Z to measure the media-only (MO) baseline. The cells were diluted with D-MEM 5% FBS containing 100 U/mL penicillin at 1.0 × 105 cells/mL. Once the MO baseline was obtained, the culture medium was removed from the plate, and then 100 µL of the cell suspension was seeded on the plate. For tissue culture, the fibroblast-seeded plate was incubated within a chamber of a Maestro-Z or a CO2 incubator.
Dual-RNA-Seq analysis
RNA was extracted according to the procedure described by Pisu et al. (8), using TRIzol reagents (Thermo Fisher Scientific, CA, USA) and mixing total RNA (eukaryotic + bacterial) from Mtb-infected MRC-5 fibroblasts at an optimal pathogen to host RNA ratio. As a reference, total RNA was extracted from cultured Mtb bacilli and uninfected MRC-5 fibroblasts. RNA-Seq and bioinformatics analysis were conducted by Macrogen (Kyoto, Japan).
Real-time quantitative RT-PCR (qRT-PCR) and RNase protection assay
Total RNA was extracted from the fibroblasts using TRIzol reagents (Thermo Fisher Scientific, CA, USA) according to the manufacturer’s instructions. The quantity and quality of the extracted RNA were determined using the NanoVue plus spectrophotometer (GE Healthcare, Chicago, IL, USA). The isolated RNA was digested with DNase1 (Takara Bio Inc., Shiga, Japan) at 37°C for 30 min. For cDNA synthesis, 100 ng of RNA was used with PrimeScript RT master mix (Takara Bio Inc.). Real-time PCRs were performed in an Applied Biosystems StepOnePlus real-time PCR system with real-time PCR software version 2.3 (Applied Biosystems, Thermo Fisher, Foster City, CA, USA). Each reaction was performed in a total volume of 10 µL on a 48-well optical reaction plate (Applied Biosystems) containing 5 µL of TB Green Fast qPCR Mix (Takara Bio Inc.), 100 to 1,000 ng of cDNA (1/10 dilution), and two gene-specific primers at a final concentration of 0.5 µM each. The real-time cycling conditions were as follows: (i) 95°C for 30 s and (ii) 40 cycles at 95°C for 5 s and 60°C for 10 s. Melting curve analysis verified that each reaction contained a single PCR product. Reported gene expression levels were normalized to transcripts of GAPDH. We used primer sets of human GAPDH (5′-TGCCAACGTGTCGGTTGT-3′, 5′-TGTCATCATATTTGGCAGGTTT-3′), human IL-1β (5′-GGGCATCAAGGGCTACAA-3′, 5′-CTGTCCAGGCGGTAGAAGAT-3′), human IL-6 (5′-AGAAATCCCTCCTCGCCAAT-3′, 5′- AAATAGCGAACGGCCCTCA-3′), and human IL-8 (5′- TCTGCAGCTCTGTGTGAAGG-3′, 5′- ACTTCTCCACAACCCTCTGC-3′).
The expression of mRNA of inflammatory cytokines was measured by RNase protection assay kits (BD Biosciences Pharmingen, San Diego, CA, USA).
Reporter gene assay
MRC-5 fibroblasts (3 × 105 cells/well) were cultured in a six-well plate and transfected with reporter plasmids by the calcium phosphate method. The plasmids contained the 5′ upstream region of IL-6 or IL-8 genes, including the NF-κB-binding site conjugated to the luciferase gene provided kindly by Prof. N. Mukaida (Kanazawa Univ., Japan). As a reference for the plasmid transfection, cells were also transfected with another plasmid-encoding β-galactosidase gene. After transfection with these plasmids, the fibroblasts were cultured with either live or dead Mtb bacilli (10 bacilli per fibroblast) up to day 3. The results were assessed as the ratio of luciferase to β-galactosidase activity.
Measurement of cytokine levels in the culture supernatant
The amounts of human interleukin-1β (IL-1β), IL-6, and IL-8 in the supernatants of fibroblasts were quantified by enzyme-linked immunosorbent assay (ELISA) using a BD OptEIA ELISA set (BD Bioscience, San Jose, CA, USA). IL-18 in the supernatant was quantified by human IL-18 ELISA kit (Medical & Biological Laboratories Co., LTD, Tokyo, Japan).
Statistical analysis
The statistical significance of the data sets, shown in Fig. 1, 3, 4, and 5; Fig. S3 and S5, was assessed by one-way analysis of variance with SigmaPlot (Systat Software, Inc., San Jose, CA, USA), and differences were considered significant at P < 0.05. Statistical analyses shown in Fig. 7 and 8 were performed by Macrogen (Kyoto, Japan) with Fold Change nbiomWorldTest using DESeq2 per comparison pair.
Fig 1.
Time course of cytotoxicity induced by live, but not Mycobacterium tuberculosis (Mtb) bacilli in fibroblasts. Cytotoxicity was measured in real time with Maestro-Z (AXION), which measures the electrical resistance of the fibroblasts adhering to the bottom of an electrode embedded well using CytoView-Z 96-well plate (AXION). Fibroblasts (1 × 104 cells/well) were incubated with live or heat-killed Mycobacterium tuberculosis (Mtb) H37Rv for 96 hours. Initially, 0–100 bacilli were added per fibroblast, (a) MRC-5, (b) MRC-9, and (c) TIG-1, respectively. (d) MRC-5 fibroblasts were incubated with live or heat-killed Mtb HN878 for 96 hours in the same manner. Solid and open shapes indicate the results of live and dead bacilli, respectively. Circle, triangle, and square indicate the number of bacteria added to the fibroblast: 100, 10, 1, respectively. Each experiment was conducted in triplicate. The statistical significance of the difference between the results of each experiment was analyzed by one-way analysis of variance, *P < 0.05, **P < 0.01, ***P < 0.001.
RESULTS
Early ingestion of Mtb H37Rv and Mtb HN878 by human fibroblast cell line induces cytotoxicity
We previously reported that the cytotoxic effect of live Mtb bacilli on MRC-5, MRC-9, and TIG-1, and lung epithelial cell A549 was observed 2 days after infection (1). In a previous study, we investigated live Mtb-induced cytotoxicity by measuring the clearance of an Mtb-infected cell line from a tissue culture plate; to do so, we assayed crystal violet staining and release of lactate dehydrogenase, a cytoplasmic enzyme from the infected cells (2). In the present study, we confirmed the cytotoxicity induced by live Mtb bacilli with the Maestro-Z (AXION), a device that assesses cytotoxicity in real time by measuring the electrical resistance of the fibroblasts adhering to the bottom of the well in a CytoView-Z 96-well plate (AXION), which is embedded in an electrode. Live, but not dead, Mtb, H37Rv lineage 4, induced cytotoxicity in MRC-5, MRC-9, and TIG-1 fibroblasts in a time- and bacterial number-dependent manner (Fig. 1a through c). The cytotoxicity by other strains of Mtb bacilli, HN878 lineage 2, was also induced in MRC-5 fibroblasts in the same manner (Fig. 1d).
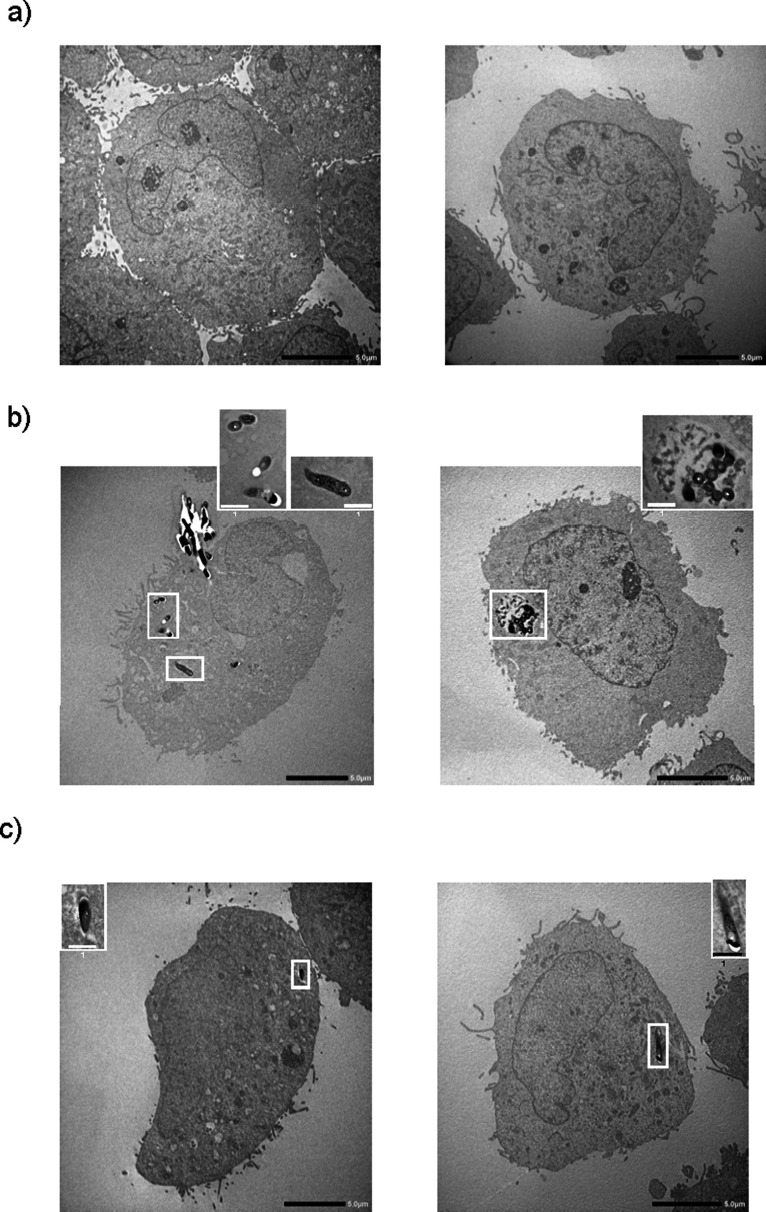
Mtb H37Rv bacilli were located inside fibroblasts at days 2 and 3 (Fig. 2b and c). We confirmed the result by acid-fast staining in the same preparation sample (data not shown). We further confirmed the phagocytosis of M. bovis BCG bacilli by fibroblasts with a confocal laser microscope (Fig. S1) and also observed that M. bovis BCG killed by heat or the anti-mycobacterial drugs, isoniazid and streptomycin, were also inside fibroblasts (Fig. S2). These data indicate that fibroblasts can ingest both live and dead bacilli, but that only live Mtb bacilli induce cytotoxicity in fibroblasts.
Fig 2.
Uptake of Mycobacterium tuberculosis H37Rv bacilli by MRC-5 fibroblasts. MRC-5 fibroblasts (2 × 106 cells/dish) were cultured in a 100 mm diameter tissue culture dish with live Mycobacterium tuberculosis (Mtb) H37Rv (2 × 107 colony-forming units per dish) for the indicated period. After the incubation period, the cells were fixed with glutaraldehyde, and the dehydration steps were performed. After samples were composed with Spurr’s resin, ultrathin sections of each sample were cut to 80 nm thickness with ARTOS 3D (Leica Mikrosysteme GmbH, Vienna, Austria) and stained with uranyl acetate and lead citrate. Transmission electron microscopic examinations were performed with JEM-2100 Plus equipped with a complementary metal-oxide semiconductor CMOS camera with 2,048 x 2,048 pixels. Panels a, b, and c indicate the incubation time of Mtb H37Rv bacilli and MRC-5 fibroblasts on days 1, 2, and 3, respectively. The black bar shows the scale (5.0 µm). The bacilli in the fibroblast are surrounded by a white frame. An enlarged image of the bacilli cut horizontally is shown in the top right corner of panel b. The white bar shows the scale (1.0 µm).
Effect of caspase and NLRP3 inflammasome inhibitors on the mycobacterial cytotoxicity against fibroblasts
Next, we investigated the type of cell death using selective protease inhibitors, that is, the caspase-1/4 inhibitor VX-765 (Ki = 0.8 nM), the caspase-3 inhibitor PAC-1 (EC50 = 0.22 µM), the caspase-9 inhibitor z-LEHD-fmk (IC50 = 0.12 µM), and the NLRP3 inflammasome inhibitor MCC950 (IC50 = 7.5 nM). We observed that the caspase 1/4 and NLRP3 inflammasome inhibitors, but not caspase-3 and the caspase-9 inhibitors, were able to inhibit the cytotoxicity induced by Mtb H37Rv and Mtb HN878 in MRC-5 (Fig. 3a; Fig. S3). VX-765 and MCC950, but not PAC-1 and z-LEHD-fmk, also inhibited the cytotoxicity in MRC-9 and TIG-1 fibroblasts in the same manner (Fig. 3b). These results suggest that live Mtb induced pyroptosis (7), but not apoptosis in fibroblasts.
Fig 3.
Effects of inhibitors for caspase-1/4, caspase-3, caspase-9, and NLRP3 inflammasome on live Mycobacterium tuberculosis (Mtb) bacilli-induced cytotoxicity to fibroblasts. (a) MRC-5 (1 × 104 cells/well) fibroblasts were seeded on a fibronectin pre-coated CytoView-Z 96-well plate 4 hours before the addition of the inhibitors of caspase-1/4 (VX-765), caspase-3 (PAC-1), caspase-9 (z-LEHD-fmk), and NLRP3 inflammasome (MCC950), and 30 minutes later, the cells were infected with (a) live Mycobacterium tuberculosis (Mtb) H37Rv and Mtb HN878 bacilli (1 × 106 CFU/well). Results are 2 days after incubation with the bacilli. The percentage of inhibition is shown in the panel, and row data are shown in Fig. S3. The error bars for negative numbers were excluded in panel b. MRC-9 and TIG-1 were infected with Mtb H37Rv, and the effects of inhibitors were investigated. Each experiment was performed in triplicates. The statistical significance of the difference between each experiment and the result of incubation with Mtb H37Rv or Mtb HN878 alone were analyzed by one-way analysis of variance, *P < 0.05, **P < 0.01, ***P < 0.001.
Induction of the inflammatory cytokines, IL-1β, IL-6, and IL-8, but not IL-18, by fibroblasts after Mtb infection
Inflammatory cytokines are produced by cells during pyroptosis. Fibroblasts were incubated with live or dead Mtb H37Rv for 3 days, and mRNA was extracted every day and evaluated by qRT-PCR. The mRNA expression of IL-1β (Fig. 4a), IL-6 (Fig. 4b), and IL-8 (Fig. 4c) was induced by live Mtb bacilli at day 2, and the inductions were sustained to day 3. Similar results were observed using RNase protection assay (Fig. S4).
Fig 4.
mRNA expression of IL-1β, IL-6, and IL-8 in MRC-5 fibroblasts induced by live, but not dead Mycobacterium tuberculosis H37Rv bacilli. MRC-5 fibroblasts (2 × 106 cell/dish) were cultured in a 100 mm diameter tissue culture dish with either live or heat-killed Mycobacterium tuberculosis H37Rv bacilli (2 × 107 bacilli/dish) for the indicated period. mRNA from MRC-5 fibroblasts was extracted with TRIzol reagents (Thermo Fisher Scientific). The mRNA expression of (a) IL-1β, (b) IL-6, and (c) IL-8 was measured by qRT-PCR using the specific primer sets. The statistical significance of the difference between each experiment and the result of incubation with dead bacilli at day 1 were analyzed by one-way analysis of variance, *P < 0.05, **P < 0.01, ***P < 0.001.
Because we believed that IL-1β, IL-6, and IL-8 were the biologically active cytokines produced by fibroblasts infected with Mtb, we measured the concentration of IL-1β, IL-6, and IL-8 proteins in supernatants of MRC-5, MRC-9, and TIG-1 fibroblasts by ELISA at day 3 (Fig. 5). We further investigated the gene expression of IL-6 and IL-8 by luciferase reporter assay and found that it was induced by live, but not dead, Mtb bacilli at day 2 and that the induction was sustained at day 3 (Fig. S5b and c, e, and f). The binding site of transcription factor NF-κB is located at 5′ upstream of inflammatory cytokine genes, such as IL-1β, IL-6, and IL-8, and NF-κB is known to activate the transcription of these genes during inflammation. The results of the reporter assay showed activation of NF-κB by live, but not dead Mtb bacilli infection at day 2 (Fig. S5f). These results suggest that in vivo Mtb bacilli-induced pyroptosis in fibroblasts was accompanied by the production of inflammatory cytokines.
Fig 5.
The production of IL-1β, IL-6, IL-8, and IL-18 from fibroblasts was induced by live, but not dead Mycobacterium tuberculosis (Mtb) bacilli. Fibroblasts (1 × 104 cells/well) indicated in each panel were cultured in a 96-well plate with either live or heat-killed Mycobacterium tuberculosis (Mtb) H37Rv and/or Mtb HN878 bacilli for 3 days. The amounts of human (a, b) IL-1β, (c, d) IL-6, (e, f) IL-8, and (g, h) IL-18 in the supernatants of fibroblasts were quantified by ELISA. The Med column shows the results without the bacteria. L and D indicated live and dead bacilli, and the numbers, 1, 10, and 100, indicate bacterial number per the fibroblast. Each experiment was conducted in triplicate. The statistical significance of the difference between each experiment and Med result was analyzed by one-way analysis of variance, *P < 0.05, **P < 0.01, ***P < 0.001.
IL-18, like IL-1β, is activated by caspase-1, but IL-18 was not detected in fibroblasts (Fig. 5; Table S2). These results suggest that IL-18 is not involved in cell death induced by live Mtb.
Dual-RNA-Seq analysis of live Mycobacterium tuberculosis bacilli induced cytotoxicity in fibroblasts
To perform a comprehensive analysis of mRNA expression in fibroblasts infected with live Mycobacterium tuberculosis (Mtb) H37Rv bacilli, on day 2 we extracted mRNAs from both in vitro Mtb H37Rv bacilli in the fibroblasts and the fibroblasts infected with the Mtb bacilli, and then performed dual RNA-Seq analysis to evaluate mRNA expression in both in vivo Mtb bacilli and Mtb-infected host cells (8).
First, we analyzed mRNA expression in in vivo Mtb bacilli. The gene ontology (GO) analysis of the biological processes of the in vivo bacilli showed that mRNAs from DNA-templated transcription were extensively upregulated compared with the in vitro cultured Mtb bacilli (Fig. 6a). The response to hypoxia and the positive regulation of growth were also augmented in the in vivo bacilli. The results of GO terms related to molecular function of the in vivo Mtb bacilli showed that oxidoreductase activity was highly upregulated in the in vivo bacilli compared with the in vitro bacilli (Fig. 6b). The expression of genes involved in iron binding and monooxygenase and methyltransferase activity was also augmented in the bacilli. These data suggest that Mtb bacilli incorporated into the fibroblasts survive in the hypoxic environment of the host cell.
Fig 6.
Dual RNA-Seq analysis of in vivo Mycobacterium tuberculosis (Mtb) bacilli. MRC-5 fibroblasts (2 × 106 cells/dish) were co-cultured with Mycobacterium tuberculosis (Mtb) H37Rv bacilli (2 × 107 CFU/dish) for 2 days. According to the reference method (8) for dual RNA-Seq analysis of the Mtb bacilli-infected macrophages, the host cells were solubilized with TRIsol solution. The lysate containing the particles of the in vivo Mtb bacilli was centrifuged, and the supernatant was used for RNA extraction and RNA-Seq analysis of the host cell. Fresh TRIsol solution and zirconia beads were added to the pellet, and bacterial bodies were broken up with a bead beater. After extracting the RNA fractions, the quality of nucleic acid was confirmed, and then RNA-Seq analysis was performed by Macrogen (Kyoto, Japan). (a) Gene ontology (GO) analysis of biological processes (BP), (b) GO analysis of molecular functions (MF), and (c) KEGG pathway analysis. Each experiment was performed in triplicates. The statistical significance of the difference between the result of each experiment and the result of in vitro cultured Mtb bacilli was analyzed by one-way analysis of variance, *P < 0.05, **P < 0.01.
Then, we further performed KEGG pathway analysis with the in vivo Mtb and found that the genes for benzoate, fatty acid, and steroid degradation were upregulated (Fig. 6c). Mycobacterium, Rhodococcus, and Pseudomonas are classified into steroid-degrading bacteria; thus, the augmentation of the gene expressions of steroid degradation was confirmed. The elevated gene expressions of benzoate and fatty acid degradation may correspond to the anaerobic biodegradation and acquisition of the carbon source in the hypoalimentation state inside the host cell.
Next, we performed a GO functional analysis of the host side, that is, we compared Mtb-infected and -uninfected fibroblasts. The analysis of biological processes showed upregulation of the genes for metabolic processes, including organic substances, cellular, primary, nitrogen compounds, and macromolecules (Fig. 7). Interestingly, the genes of binding and protein binding were strongly induced in the GO analysis of molecular function (Fig. 7b). Furthermore, the GO cellular component analysis showed that genes involved in intracellular anatomical structure, organelles, intercellular, membrane-bound organelles, and cytoplasm were highly induced in the Mtb-infected fibroblasts (Fig. 7c). These results were assumed to reflect changes in intracellular anatomical and subcellular organelle structure in the fibroblasts after incorporation of Mtb bacilli.
Fig 7.
Dual RNA-Seq analysis of Mycobacterium tuberculosis (Mtb) bacilli-infected fibroblasts. The preparation of the RNA fraction is described in the legend to Fig. 6. (a) Gene ontology (GO) analysis of biological processes, (b) GO analysis of molecular functions, (c) GO analysis of cellular components. Each experiment was performed in triplicates.
The top 50 differentially expressed genes in in vivo vs in vitro Mtb H37Rv are shown in Table 1. Transcriptional regulator TrcR (part of a two-component system), the protein translocase subunit SecE, alkyl hydroperoxide reductase AphD, NADH-quinone oxidoreductase subunit K, and toxin MazF8 genes were upregulated in the in vivo Mtb bacilli. These substances are important for survival in the fibroblasts, induction of cytotoxicity, and production of inflammatory cytokines by the host cell. The top 25 upregulated genes in Mtb H37Rv-infected fibroblasts are shown in Table 2. The dual RNA-Seq analysis confirmed that the IL-1β and IL-8/CXCL8 gene was also induced, with a 33.9- and 98.4-fold change (fc.) in the Mtb-infected vs -uninfected fibroblasts, respectively, at day 2 (Table 2). The IL-6 gene was also induced in the Mtb-infected fibroblasts (fc. 17.0; Table S2).
TABLE 1.
| Gene symbol | Description | Gene biotype | Protein ID | Locus tag | In vivo/in vitro Mtb H37Rv.fc |
|---|---|---|---|---|---|
| alaT | tRNA-Ala | tRNA | – | Rvnt02 | 24.8 |
| trcR | Two-component transcriptional regulator TrcR | protein_coding | NP_215549.1 | Rv1033c | 23.0 |
| leuU | tRNA-Leu | tRNA | – | Rvnt22 | 21.8 |
| secE2 | Protein translocase subunit SecE | protein_coding | YP_177722.1 | Rv0379 | 19.0 |
| – | Diterpene synthase | protein_coding | NP_217895.1 | Rv3378c | 17.2 |
| ahpD | Alkyl hydroperoxide reductase AphD | protein_coding | NP_216945.1 | Rv2429 | 17.1 |
| nuoK | NADH-quinone oxidoreductase subunit K | protein_coding | NP_217671.1 | Rv3155 | 15.6 |
| – | Hypothetical protein | protein_coding | NP_216787.1 | Rv2271 | 15.5 |
| – | Hypothetical protein | protein_coding | NP_216893.1 | Rv2377c | 14.5 |
| – | Hypothetical protein | protein_coding | NP_215867.1 | Rv1351 | 14.1 |
| – | Hypothetical protein | protein_coding | NP_216326.1 | Rv1810 | 13.9 |
| mazF8 | Toxin MazF8 | protein_coding | NP_216790.1 | Rv2274c | 13.6 |
| – | D-amino acid aminohydrolase | protein_coding | NP_217429.1 | Rv2913c | −135.3 |
| – | Transcriptional regulator | protein_coding | NP_215307.1 | Rv0792c | −92.3 |
| – | Hypothetical protein | protein_coding | NP_215306.1 | Rv0791c | −82.2 |
| – | Carotenoid cleavage oxygenase | protein_coding | NP_215168.1 | Rv0654 | −76.4 |
| alkB | Transmembrane alkane 1-monooxygenase AlkB | protein_coding | NP_217769.1 | Rv3252c | −76.3 |
| – | Hypothetical protein | protein_coding | NP_215305.1 | Rv0790c | −73.9 |
| – | TetR family HTH-type transcriptional regulator | protein_coding | NP_217428.1 | Rv2912c | −47.6 |
| – | Hypothetical protein | protein_coding | NP_215352.1 | Rv1813c | −45.8 |
| – | Hypothetical protein | protein_coding | NP_215352.1 | Rv0837c | −45.1 |
| narX | Nitrate reductase-like protein NarX | protein_coding | NP_216548.1 | Rv1736c | −37.8 |
| acg | NAD(P)H nitroreductase | protein_coding | NP_216548.1 | Rv2032 | −35.8 |
| – | Hypothetical protein | protein_coding | NP_217786.1 | Rv3269 | −30.8 |
| hsp | Heat shock protein | protein_coding | NP_214765.1 | Rv0251c | −30.5 |
| aldA | Aldehyde dehydrogenase AldA | protein_coding | NP_215282.1 | Rv0768 | −29.4 |
| ctpC | Manganese/zinc-exporting P-type ATPase | protein_coding | NP_217787.1 | Rv3270 | −27.6 |
| – | NAD(P)H nitroreductase | protein_coding | NP_217647.3 | Rv3131 | −26.2 |
| – | Hypothetical protein | protein_coding | NP_216982.1 | Rv2466c | −25.9 |
| cysD | Sulfate adenylyltransferase subunit 2 | protein_coding | NP_215801.1 | Rv1285 | −25.7 |
| hsaG | Acetaldehyde dehydrogenase | protein_coding | NP_218052.1 | Rv3535c | −24.7 |
| mmsA | Methylmalonate-semialdehyde dehydrogenase | protein_coding | NP_215267.1 | Rv0753c | −24.7 |
| – | Hypothetical protein | protein_coding | NP_214593.1 | Rv0079 | −22.4 |
| – | Oxidoreductase | protein_coding | NP_215279.1 | Rv0765c | −21.7 |
| cyp135A1 | Cytochrome P450 Cyp135A1 | protein_coding | NP_214841.1 | Rv0327c | −21.1 |
| mcr11 | Putative small regulatory RNA | ncRNA | – | RVnc0013 | −20.3 |
| – | Hypothetical protein | protein_coding | NP_218177.1 | Rv3660c | −19.3 |
| – | Hypothetical protein | protein_coding | NP_216546.1 | Rv2030c | −18.9 |
| tgs1 | Diacylglycerol O-acyltransferase | protein_coding | NP_217646.1 | Rv3130c | −18.5 |
| PPE17 | PPE family protein PPE17 | protein_coding | YP_177791.1 | Rv1168c | −18.4 |
| clpB | Chaperone protein ClpB | protein_coding | NP_214898.1 | Rv0384c | −18.0 |
| – | HTH-type transcriptional regulator | protein_coding | NP_215281.1 | Rv0767c | −17.7 |
| – | Phage integrase | protein_coding | NP_216102.1 | Rv1586c | −17.5 |
| – | Dioxygenase | protein_coding | NP_217923.1 | Rv3406 | −17.4 |
| – | Hypothetical protein | protein_coding | NP_218048.1 | Rv3531c | −17.1 |
| – | Oxidoreductase | protein_coding | NP_215283.1 | Rv0769 | −16.5 |
| – | Hypothetical protein | protein_coding | NP_217980.1 | Rv3463 | −16.1 |
| higB | Toxin HigB | protein_coding | NP_216471.2 | Rv1955 | −15.7 |
| cydD | Cytochrome biosynthesis ABC transporter ATP-binding protein/permease CydD | protein_coding | NP_216137.1 | Rv1621c | −14.9 |
| mymT | Metallothionein | protein_coding | YP_004837046.2 | Rv0186A | −14.3 |
DEG, differentially expressed gene; fc, fold change.
“–” represents genes that are nucleic acids and therefore do not have a protein ID.
TABLE 2.
Top 25 upregulated genes on Mtb H37Rv-infected MRC-5 fibroblasts by RNA seq analysis
| Gene symbol | Description | Gene biotype | Protein ID | Infected/un-infected MRC-5.fc |
|---|---|---|---|---|
| NEAT1 | Nuclear paraspeckle assembly transcript 1 | lncRNA | –a | 144.3 |
| ESM1 | Endothelial cell-specific molecule 1 | protein_coding | NP_001129076.1; NP_008967.1 | 130.9 |
| CXCL8 | C-X-C motif chemokine ligand 8 | protein_coding | NP_000575.1; NP_001341769.1 | 98.4 |
| LOC105369370 | Uncharacterized LOC105369370 | lncRNA | – | 64.5 |
| IFI44L | Interferon-induced protein 44 like | protein_coding | NP_001362575.1; NP_001362576.1; NP_001362577.1; NP_001362578.1; NP_001362579.1; NP_006811.2; XP_006710367.1 | 62.8 |
| LOC107987083 | Uncharacterized LOC107987083, transcript variant X2 | lncRNA | – | 48.6 |
| IL1A | Interleukin 1 alpha | protein_coding | NP_000566.3; NP_001358483.1 | 46.3 |
| MX2 | MX dynamin-like GTPase 2 | protein_coding | NP_002454.1; XP_005261040.1; XP_005261041.1; XP_011527873.1; XP_011527874.1; XP_011527875.1; XP_011527876.1; XP_024307848.1 | 45.1 |
| HMGB1P3 | High mobility group box 1 pseudogene 3 | pseudogene | – | 37.7 |
| LOC105375914 | Uncharacterized LOC105375914, transcript variant X6 | lncRNA | – | 34.5 |
| VTA1P2 | Vesicle trafficking 1 pseudogene 2 | pseudogene | – | 34.0 |
| IL1B | Interleukin 1 beta | protein_coding | NP_000567.1; XP_016859477.1 | 33.9 |
| MKRN5P | Makorin ring finger protein 5, pseudogene | pseudogene | – | 30.8 |
| OR6A2 | Olfactory receptor family 6 subfamily A member 2 | protein_coding | NP_003687.2 | 30.8 |
| HAS2 | Hyaluronan synthase 2 | protein_coding | NP_005319.1 | 30.7 |
| LOC105375247 | Uncharacterized LOC105375247 | lncRNA | – | 29.2 |
| RPL36AP42 | Ribosomal protein L36a pseudogene 42 | pseudogene | – | 28.2 |
| OR2AG2 | Olfactory receptor family 2 subfamily AG member 2 | protein_coding | NP_001004490.1 | 27.1 |
| LOC105374179 | Uncharacterized LOC105374179 | lncRNA | – | 27.0 |
| LOC100129577 | Mitochondrial carrier 1 pseudogene | pseudogene | – | 26.7 |
| ACTBP7 | ACTB pseudogene 7 | pseudogene | – | 26.6 |
| NUDCP1 | Nuclear distribution C pseudogene 1 | pseudogene | – | 26.1 |
| MIR8058 | MicroRNA 8058 | miRNA | – | 25.8 |
| RPL7AP42 | Ribosomal protein L7a pseudogene 42 | pseudogene | – | 25.7 |
| LOC105375310 | Uncharacterized LOC105375310 | lncRNA | – | 25.3 |
“–” represents genes that are nucleic acids and therefore do not have a protein ID.
Fold change (fc.) of caspase-1 mRNA was not observed in the fibroblasts, but caspase-4 mRNA was induced in the fibroblasts by Mtb infection (fc. 5.9; Table S2). Fold change of caspase-9 mRNA, an initiator of apoptosis (10, 11), but not that of caspase-3, was observed in the fibroblasts and downregulated by Mtb infection (fc. −2.1; Table S2). NLRP3 mRNA was upregulated by Mtb infection (fc. 2.3; Table S2). These results indicated that NLRP3 and caspase-4 contribute to the cell death of the fibroblasts caused by the infection with Mtb bacilli.
DISCUSSION
In this study, we first analyzed the model of host cell death induced by live Mtb bacilli with cell death analytic methods involving caspases and inflammasome inhibitors, and we discussed the role of fibroblasts in Mtb infection. We confirmed the cell death of three embryonic fibroblast cell lines derived from different origins, by live, but not dead Mtb bacilli, with a device for measuring cell growth/cytotoxicity in real time, Maestro-Z (AXION). Live Mtb bacilli induced cell death in fibroblasts in a time- and bacterial number-dependent manner; however, cell death was not observed on day 1 (Fig. 1), which was the same as in our previous study (2). Although we have reported the cytotoxicity of Mtb H37Rv, lineage 4, and clinical isolates of Mtb bacilli (1); in this study, we also confirmed that another Mtb strain, HN878 lineage 2, induced cytotoxicity in fibroblasts (Fig. 1D).
In their review, Randall et al. wrote the “non-classical immune cell” plays an important role in the host in case of Mtb infection (12). They summarized reports of interactions of Mtb with epithelial cells, endothelial cells, fibroblasts, adipocytes, glia, and neurons, which work with the “classical immune cells,” myeloid and lymphoid cells, to achieve an optimal immune outcome favoring the host. We previously reported that live Mtb induced marked cytotoxicity to the “non-classical immune cell,” human lung fibroblast cell lines MRC-5, MRC-9, and TIG-1 and the human lung epithelial cell line A549, compared with the “classical immune cell,” the human myeloid cell line HL-60 and lymphoid cell lines U937 and THP-1 (1, 2). Dead Mtb bacilli also induced cell death in HL-60, U937, and THP-1 cells, but not in fibroblasts and epithelial cells. In this study, we confirmed live, but not dead Mtb bacilli induced cytotoxicity to MRC-5, MRC-9, and TIG-1 fibroblasts (Fig. 1).
Fibroblasts also acted as macrophages and ingested both live and dead Mtb bacilli (Fig. 2; Fig. S1 and S2), and host cell death was observed 2 days after infection (Fig. 1). The expression of mRNA of the inflammatory cytokines, IL-1β, IL-6, and IL-8, was observed at day 2 (Fig. 4; Fig. S4). MRC-5, MRC-9, and TIG-1 fibroblast cell death induced by live Mtb bacilli 2 days after infection was specifically inhibited by VX-765, an inhibitor of caspase-1/4, and MCC950, an inhibitor of NLRP3 inflammasome, but not by PAC-1, a caspase-3 inhibitor, and z-LEHD-fmk, a caspase-9 inhibitor (Fig. 1 and 3). RNA-Seq analysis also revealed that mRNAs of NLRP3 and caspase-4, but not caspase-3, were induced in Mtb-infected fibroblasts. These results suggest that live, but not dead, Mtb bacilli induce fibroblast cell death by activating the inflammasome. Taken together, these results suggest that live Mtb bacilli induce pyroptosis (7) in fibroblasts and that pyroptosis due to fibroblast infection with Mtb bacilli promotes activation of immune system cells via signaling to “classical immune cells,” resulting in the pathogen being excluded by the host.
In this study, IL-18 was not detected (Fig. 5; Table S2), but other studies have shown that IL-18 was induced in pyroptosis in lipopolysaccharide-induced fibroblasts (13), suggesting that there may be another, as yet unknown, mechanism of cell death induced by live Mtb.
Other roles of fibroblasts in TB include acting as a reservoir of Mtb bacilli and supporting their active replication (14). Fibroblasts are also integrated into granulomas, where they control the Mtb infection by regulating immune responses (6). One study investigated the deposition of collagen produced by fibroblasts within granulomas in connection with the virulence of Mtb bacilli. Although H37Ra, an avirulent strain, induced collagen synthesis, H37Rv, a virulent strain, induced progressive collagen degradation via matrix metalloproteinases (MMPs), which promote cavity formation and diffusion of Mtb bacilli (15). Another study reported that Mtb bacilli upregulate MMP-1 in an NF-κb-dependent manner (16). In our study, dual RNA-Seq showed that NF-κB was activated (Fig. S5h) and MMP-1 was induced (fc. 3.0) by Mtb H37Rv infection (Table S2).
Next, we investigated the cyclopedic gene expression of Mtb in fibroblasts (Fig. 6) and Mtb-infected host cells (Fig. 7) at day 2 by the dual RNA-Seq method (8) because Mtb were ingested by fibroblasts, and host cell cytotoxicity appeared at day 2 after infection (Fig. 1 and 2). GO analysis of biological processes in in vivo Mtb bacilli in fibroblasts indicated that the expression of genes related to DNA-templated transcription and hypoxia response was highly induced compared with in vitro Mtb cultured in broth (Fig. 6a). GO analysis of molecular functions showed that the expressions of genes related to oxidoreductase activity, monooxygenase activity, and iron ion binding were augmented in in vivo Mtb (Fig. 6b). The KEGG pathway analysis showed that the expression of steroid degradation genes was induced in the in vivo bacilli, a finding that was supported by the classification into steroid-degrading bacteria, including Mycobacterium, Rhodococcus, and Pseudomonas (Fig. c). Expression of genes related to benzoate degradation was observed in anaerobic bacteria (Fig. 6c), which was related to the augmented expression of genes related to responses to hypoxia in the GO analysis of biological processes (Fig. 6a).
The bacillus, genus Mycobacterium, has a thick cell wall containing mycolic acid, which consists of a long-chain fatty acid. The bacilli are thought to obtain carbon by fatty acid degradation in the oligotrophic environment at the time of latent infection of the host cell. In addition, the bacilli require iron ions for a metabolic oxidation-reduction reaction. These results strongly suggest that in vivo Mtb survives within the host cell by adapting to the hypoxic oligotrophic environment (Fig. 6c).
The genes trcR, secE2, ahpD, and mazF8 were highly induced in in vivo Mtb bacilli according to top 50 differentially expressed gene analysis (Table 1). The T-cell response regulator (TrcR) encoded by tcrR belongs to a two-component system and has been reported to bind an AT-rich sequence upstream of Rv1057, which was expressed during early infection of human macrophages (17). The biological function of Rv1057 is not well known, but a knockout study of Rv1057 found that it regulated the mycobacterium ESAT-6 secretion system (18). In our study, Rv1057 was not listed in the dual RNA-Seq analysis, so we assumed that TrcR regulated other genes related to the survival of the bacilli in MRC-5 fibroblasts. SecE2 is a calcium-binding protein (19) and an abundant protein and mRNA in Mtb and stimulates cytokine production from human peripheral blood mononuclear cells (20). The enzyme alkylhydroperoxidase D (ahpD) contributes to survival in the host cell by resisting host cell oxidative stress during infection, not only with Mtb (21–25) but also with other Mycobacterium (26, 27) and with Streptococcus pneumoniae (28) and Legionella pneumophila (29). MazF and RelE play a role as regulators of translocation by cleaving mRNA as a toxin-antitoxin (TA) family under unfavorable growth conditions in E. coli (30, 31). TA loci are conserved widely in prokaryotes, and the Mtb genome includes many more TA loci than other bacterial species; indeed, Mtb H37Rv and CDC1551 genomes include 38 and 26 TA loci, respectively (32). In our study, the expressions of mazF and relE, encoding a toxin RelE, genes were enhanced in vivo (by fc. 13.6 and 5.9, respectively), which have contributed to the survival of the Mtb bacilli in the host cells (Table S1).
On the other hand, the GO analysis of biological processes showed that the expressions of genes related to metabolism were generally increased in the host cell (Fig. 7a). The results of the GO analysis of molecular function on the host side indicated that the expression of genes related to binding and protein binding was highly induced by Mtb infection (Fig. 7b). The GO analysis of cellular components clearly indicated that the expression of genes related to intracellular anatomical structure, organelles, intracellular and membrane-bound organelles, and cytoplasm was highly induced by Mtb infection. These results strongly suggest that the structure of the cell membrane and intracellular organelles is changed in fibroblasts that take up the Mtb bacilli (Fig. 7c); this hypothesis is supported by morphological observation (Fig. 2; Fig. S1 and S2).
The list of the top 25 upregulated cytokine genes included IL-8/CXCL8 and IL-1β (Table 2). In addition, the genes of chemokines, such as CXCL1, CXCL2, and CXCL3, were also upregulated (fc. 18.7, 19.8, and 24.2, respectively; Table S2). The gene expressions of IL-6, an inflammatory cytokine, and IRAK2 and IL-6ST, cytokine signaling molecules, were also induced in the infected cells (fc. 14.0, 3.2, and 2.3, respectively; Table S2). The gene expression of has2 was highly upregulated in the infected cells (Table 2). Interestingly, a study of the toxicity of antibiotic levofloxacin found that apoptosis of fibroblast-like synoviocytes induced caspase-3 in the study of toxicity of levofloxacin (33). During the cell death caused by the antibiotic, has2 gene expression and production of both IL-1 and IL-6 were downregulated. These results were opposite to our results but suggested that the cell death induced by Mtb bacilli infection is different from that caused by chemical compounds. Several researchers showed an anti-mycobacterial role of fibroblasts against Mtb infection in that the production of IL-8/CXCL8 by fibroblasts limited the growth of in vivo Mtb bacilli (5, 6). Our findings suggest that pyroptosis of fibroblasts contributes to host defenses against Mtb infection.
Findings about communication between fibroblasts and T cells through major histocompatibility complex (MHC-II) in Mtb infection are contradictory, that is, one study found augmentation of antigen presentation (34), whereas another found reduction of MHC-II expression (14). Because our RNA-Seq analysis showed that the MHC-II-related genes HLA-DPB1, encoding a MHC-II DP beta 1, and CIITA, encoding a MHC-II transactivator, were downregulated (fc. −2.0 and −2.5, respectively), it is unlikely that antigen presentation through MHC-II was not involved in the cell death of fibroblasts.
Mtb infection induced cell death in not only A549 but also HeLa cells, an epithelial cell line. The results of the experiments with the CRISPRi library (kindly provided by Prof. Yamasaki, Osaka University, Osaka, Japan) of HeLa cells suggested that caspase-3 contributes to host cell death by Mtb infection (unpublished data). Therefore, pyroptosis in fibroblasts may be different from apoptosis in other cells but may still play a role in defense against Mtb bacilli infection.
Limitations of this study
This study examined the biological response to bacterial infection at the cellular level, and the relationship with other cells in in vivo immune responses needs to be investigated using other methods.
ACKNOWLEDGMENTS
We appreciated Dr. T. Mukai, National Institute of Infectious Diseases, Japan, for kindly providing EGFP-expressing Mycobacterium bovis BCG Pasteur and also appreciate Prof. N. Mukaida, Kanazawa Univ., Japan, for kindly providing luciferase reporter plasmids of IL-8 and 4xNF-κB. We appreciated Naomi Yasuda and Mao Nakayama for their technical assistance.
This study was supported in part by Grant-in-Aid for Scientific Research (C) from the Japan Society for Promotion of Science (20K07125 and 24K09872), the Japan Agency for Medical Research and Development (JP21fk0108590 and JP23fk0108591), Japan BCG Laboratory, and the U.S.-Japan Cooperative Medical Sciences Program, Ministry of Health, Labour, and Welfare.
Contributor Information
Takemasa Takii, Email: t-takii@jata.or.jp.
Christina L. Stallings, Washington University in St. Louis School of Medicine, St. Louis, Missouri, USA
ETHICS APPROVAL
This study has been approved by an ethical committee (No. RIT/IRB 2024-24).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/msphere.00110-25.
Fibroblast uptakes Mycobacterium bacilli.
Fibroblast uptakes both live and dead Mycobacterium bacilli.
Effect of inhibitors for NLRP3 inflammasome, caspase-1/4, caspase-3, and caspase-9.
mRNA expression of inflammatory cytokines.
The activation of transcription of IL-6 and IL-8 genes.
DEG in in vivo vs in vitro Mycobacterium tuberculosis.
DEG in MRC-5 fibroblasts infected vs uninfected with Mycobacterium tuberculosis.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Takii T, Abe C, Tamura A, Ramayah S, Belisle JT, Brennan PJ, Onozaki K. 2001. Interleukin-1 or tumor necrosis factor-alpha augmented the cytotoxic effect of mycobacteria on human fibroblasts: application to evaluation of pathogenesis of clinical isolates of Mycobacterium tuberculosis and M. avium complex. J Interferon Cytokine Res 21:187–196. doi: 10.1089/107999001750133258 [DOI] [PubMed] [Google Scholar]
- 2. Takii T, Yamamoto Y, Chiba T, Abe C, Belisle JT, Brennan PJ, Onozaki K. 2002. Simple fibroblast-based assay for screening of new antimicrobial drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 46:2533–2539. doi: 10.1128/AAC.46.8.2533-2539.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yan F, Texter J. 2006. Capturing nanoscopic length scales and structures by polymerization in microemulsions. Soft Matter 2:109–118. doi: 10.1039/b513914g [DOI] [PubMed] [Google Scholar]
- 4. Tamura M, Tokuda M, Nagaoka S, Takada H. 1992. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun 60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rastogi N, Labrousse V, de Sousa JP. 1992. Mycobacterial growth and ultrastructure in mouse L-929 fibroblasts and bone marrow-derived macrophages: evidence that infected fibroblasts secrete mediators capable of modulating bacterial growth in macrophages. Curr Microbiol 25:203–213. doi: 10.1007/BF01570720 [DOI] [PubMed] [Google Scholar]
- 6. O’Kane CM, Boyle JJ, Horncastle DE, Elkington PT, Friedland JS. 2007. Monocyte-dependent fibroblast CXCL8 secretion occurs in tuberculosis and limits survival of mycobacteria within macrophages. J Immunol 178:3767–3776. doi: 10.4049/jimmunol.178.6.3767 [DOI] [PubMed] [Google Scholar]
- 7. Cookson BT, Brennan MA. 2001. Pro-inflammatory programmed cell death. Trends Microbiol 9:113–114. doi: 10.1016/s0966-842x(00)01936-3 [DOI] [PubMed] [Google Scholar]
- 8. Pisu D, Huang L, Grenier JK, Russell DG. 2020. Dual RNA-Seq of Mtb-infected macrophages in vivo reveals ontologically distinct host-pathogen interactions. Cell Rep 30:335–350. doi: 10.1016/j.celrep.2019.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konyuba Y, Haruta T, Ikeda Y, Fukuda T. 2018. Fabrication and characterization of sample-supporting film made of silicon nitride for large-area observation in transmission electron microscopy. Microscopy (Oxf) 67:367–370. doi: 10.1093/jmicro/dfy039 [DOI] [PubMed] [Google Scholar]
- 10. Kuida K. 2000. Caspase-9. Int J Biochem Cell Biol 32:121–124. doi: 10.1016/s1357-2725(99)00024-2 [DOI] [PubMed] [Google Scholar]
- 11. Würstle ML, Laussmann MA, Rehm M. 2012. The central role of initiator caspase-9 in apoptosis signal transduction and the regulation of its activation and activity on the apoptosome. Exp Cell Res 318:1213–1220. doi: 10.1016/j.yexcr.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 12. Randall PJ, Hsu NJ, Quesniaux V, Ryffel B, Jacobs M. 2015. Mycobacterium tuberculosis infection of the “non-classical immune cell”. Immunol Cell Biol 93:789–795. doi: 10.1038/icb.2015.43 [DOI] [PubMed] [Google Scholar]
- 13. Zhang X, Chen W, Liu W, Li D, Shen W. 2022. CITED2 alleviates lipopolysaccharide-induced inflammation and pyroptosis in - human lung fibroblast by inhibition of NF-κB pathway. Allergol Immunopathol 50:64–70. doi: 10.15586/aei.v50i4.628 [DOI] [PubMed] [Google Scholar]
- 14. Mariotti S, Sargentini V, Pardini M, Giannoni F, De Spirito M, Gagliardi MC, Greco E, Teloni R, Fraziano M, Nisini R. 2013. Mycobacterium tuberculosis may escape helper T cell recognition by infecting human fibroblasts. Hum Immunol 74:722–729. doi: 10.1016/j.humimm.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 15. González-Avila G, Sandoval C, Herrera MT, Ruiz V, Sommer B, Sada E, Ramos C, Sarabia MC. 2009. Mycobacterium tuberculosis effects on fibroblast collagen metabolism. Respiration 77:195–202. doi: 10.1159/000163064 [DOI] [PubMed] [Google Scholar]
- 16. O’Kane CM, Elkington PT, Jones MD, Caviedes L, Tovar M, Gilman RH, Stamp G, Friedland JS. 2010. STAT3, p38 MAPK, and NF-kappaB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am J Respir Cell Mol Biol 43:465–474. doi: 10.1165/rcmb.2009-0211OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haydel SE, Clark-Curtiss JE. 2006. The Mycobacterium tuberculosis TrcR response regulator represses transcription of the intracellularly expressed Rv1057 gene, encoding a seven-bladed beta-propeller. J Bacteriol 188:150–159. doi: 10.1128/JB.188.1.150-159.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu J, Zong G, Zhang P, Gu Y, Cao G. 2018. Deletion of the β-propeller protein gene Rv1057 reduces ESAT-6 secretion and intracellular growth of Mycobacterium tuberculosis. Curr Microbiol 75:401–409. doi: 10.1007/s00284-017-1394-8 [DOI] [PubMed] [Google Scholar]
- 19. Arockiasamy A, Aggarwal A, Savva CG, Holzenburg A, Sacchettini JC. 2011. Crystal structure of calcium dodecin (Rv0379), from Mycobacterium tuberculosis with a unique calcium-binding site. Protein Sci 20:827–833. doi: 10.1002/pro.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hadizadeh Tasbiti A, Yari S, Ghanei M, Siadat SD, Amanzadeh A, Tabarsi P, Saeedfar K, Bahrmand A. 2016. T cell cytokine responses in peripheral blood mononuclear cells from patients with multidrug-resistant tuberculosis following stimulation with proteins purified from Mycobacterium tuberculosis MDR clinical isolates. Int J Mycobacteriol 5 Suppl 1:S132–S133. doi: 10.1016/j.ijmyco.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 21. Hillas PJ, del Alba FS, Oyarzabal J, Wilks A, Ortiz De Montellano PR. 2000. The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. J Biol Chem 275:18801–18809. doi: 10.1074/jbc.M001001200 [DOI] [PubMed] [Google Scholar]
- 22. Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C. 2002. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295:1073–1077. doi: 10.1126/science.1067798 [DOI] [PubMed] [Google Scholar]
- 23. Koshkin A, Nunn CM, Djordjevic S, Ortiz de Montellano PR. 2003. The mechanism of Mycobacterium tuberculosis alkylhydroperoxidase AhpD as defined by mutagenesis, crystallography, and kinetics. J Biol Chem 278:29502–29508. doi: 10.1074/jbc.M303747200 [DOI] [PubMed] [Google Scholar]
- 24. Jaeger T, Budde H, Flohé L, Menge U, Singh M, Trujillo M, Radi R. 2004. Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis. Arch Biochem Biophys 423:182–191. doi: 10.1016/j.abb.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 25. Koshkin A, Knudsen GM, Ortiz De Montellano PR. 2004. Intermolecular interactions in the AhpC/AhpD antioxidant defense system of Mycobacterium tuberculosis. Arch Biochem Biophys 427:41–47. doi: 10.1016/j.abb.2004.04.017 [DOI] [PubMed] [Google Scholar]
- 26. Olsen I, Reitan LJ, Holstad G, Wiker HG. 2000. Alkyl hydroperoxide reductases C and D are major antigens constitutively expressed by Mycobacterium avium subsp. paratuberculosis. Infect Immun 68:801–808. doi: 10.1128/IAI.68.2.801-808.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farivar TN, Varnousfaderani PJ, Borji A. 2008. Mutation in alkylhydroperoxidase D gene dramatically decreases persistence of Mycobacterium bovis bacillus calmette-guerin in infected macrophage. Indian J Med Sci 62:275–282. [PubMed] [Google Scholar]
- 28. Meng Y, Sheen CR, Magon NJ, Hampton MB, Dobson RCJ. 2020. Structure-function analyses of alkylhydroperoxidase D from Streptococcus pneumoniae reveal an unusual three-cysteine active site architecture. J Biol Chem 295:2984–2999. doi: 10.1074/jbc.RA119.012226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeBlanc JJ, Davidson RJ, Hoffman PS. 2006. Compensatory functions of two alkyl hydroperoxide reductases in the oxidative defense system of Legionella pneumophila. J Bacteriol 188:6235–6244. doi: 10.1128/JB.00635-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christensen SK, Pedersen K, Hansen FG, Gerdes K. 2003. Toxin-antitoxin loci as stress-response-elements: ChpAK/MazF and ChpBK cleave translated RNAs and are counteracted by tmRNA. J Mol Biol 332:809–819. doi: 10.1016/s0022-2836(03)00922-7 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell 12:913–923. doi: 10.1016/s1097-2765(03)00402-7 [DOI] [PubMed] [Google Scholar]
- 32. Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33:966–976. doi: 10.1093/nar/gki201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tan Y, Lu K, Deng Y, Cao H, Chen B, Wang H, Magdalou J, Chen L. 2012. The effects of levofloxacin on rabbit fibroblast-like synoviocytes in vitro. Toxicol Appl Pharmacol 265:175–180. doi: 10.1016/j.taap.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 34. Boots AM, Wimmers-Bertens AJ, Rijnders AW. 1994. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology 82:268–274. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fibroblast uptakes Mycobacterium bacilli.
Fibroblast uptakes both live and dead Mycobacterium bacilli.
Effect of inhibitors for NLRP3 inflammasome, caspase-1/4, caspase-3, and caspase-9.
mRNA expression of inflammatory cytokines.
The activation of transcription of IL-6 and IL-8 genes.
DEG in in vivo vs in vitro Mycobacterium tuberculosis.
DEG in MRC-5 fibroblasts infected vs uninfected with Mycobacterium tuberculosis.