Abstract
Introduction
Renal cell carcinoma (RCC) is a common type of kidney cancer, and the prognosis for patients with advanced-stage disease remains poor. One major obstacle is the development of drug resistance, which severely limits the effectiveness of therapeutic interventions. This bibliometric study aims to provide a comprehensive overview of current research trends on drug resistance in RCC.
Methods
This study examines publications on drug resistance in RCC from 2000 to 2023, sourced from the Web of Science Core Collection (WoSCC). Detailed analyses were conducted to identify research hotspots, academic collaborations, and emerging trends. CiteSpace, SCImago Graphica, and VOSviewer were utilized to conduct these analyses comprehensively.
Results
This study analyzed a total of 2,804 publications from the WoSCC database. The number of annual publications showed a consistent upward trend, with an average annual growth rate of 8.12%. The United States had the highest number of publications, followed by China and Japan. The most productive institutions were the University of Texas System, Harvard University, and the National Institutes of Health (NIH). Alfred H. Schinkel emerged as the most prolific author, also having the highest H-index. The three most frequent research categories were oncology, pharmacology and pharmacy, and biochemistry and molecular biology. The evolution of research topics was assessed in 5-year intervals, revealing that recent themes such as ferroptosis and immunotherapy have gained increasing attention. Keyword analysis indicated a shift in research focus toward cell lipid metabolism, androgen receptor and specific molecular signatures.
Conclusion
This study offers the first comprehensive bibliometric analysis specifically focused on drug resistance in RCC. It identifies current research trends, highlights emerging hotspots, and provides insights into key contributors and ongoing challenges in the field. Our study provides a theoretical reference and guidance to guide future research efforts to address drug resistance in RCC more effectively.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02594-0.
Keywords: Bibliometric analysis, Renal cell carcinoma, Drug resistance, Targeted therapy, Visualization, Immunotherapy
Introduction
Renal cell carcinoma (RCC) is a common malignant tumor, ranking as the third most prevalent cancer in the urinary system. Advances in imaging technology have contributed to an increase in RCC diagnoses; however, the disease often remains asymptomatic in its early stages, complicating early detection. RCC is particularly challenging due to its high potential for early metastasis [1]. Surgery remains the primary treatment option for locally advanced RCC. Despite this, approximately 30% of RCC patients present with advanced or metastatic disease, resulting in a poor overall prognosis. Currently, the 5-year relative survival rate for RCC is approximately 77% [2]. RCC includes various subtypes with distinct clinical and epigenetic characteristics, with the clear cell subtype being the most common (80%), followed by papillary (15%) and chromophobe (3–5%) variants [3].
Surgical intervention, particularly partial or radical nephrectomy, has traditionally been the primary treatment for renal cell carcinoma [4]. However, RCC’s inherent resistance to chemotherapy and radiation therapy has necessitated the development of alternative treatments, making targeted therapies and immunotherapies crucial options [5, 6]. Initially, cytokine-based immunotherapies, such as interleukin-2 (IL-2) and interferon-alpha (IFN-α), were introduced [7, 8]. These were followed by the development of vascular endothelial growth factor (VEGF) inhibitors, including sunitinib, sorafenib, and bevacizumab, as well as mammalian target of rapamycin (mTOR) inhibitors like temsirolimus and everolimus [9–11]. Recently, a significant breakthrough was achieved with the introduction of immune checkpoint inhibitors (ICIs) such as nivolumab and pembrolizumab, which target immune checkpoints like programmed cell death protein 1 (PD-1) and its ligand (PD-L1), leading to improved survival rates for RCC patients [12]. Nevertheless, drug resistance remains a substantial challenge. Many patients develop resistance to sunitinib within six months of treatment, due to factors such as genetic mutations, alternative pathway activation, and changes in the tumor microenvironment [13, 14]. Tumor cells can evade immune detection by downregulating antigen presentation, while the tumor microenvironment often becomes immunosuppressive, inhibiting immune cell activity [15]. Consequently, research continues to delve into the molecular mechanisms underlying RCC and its drug resistance, aiming to improve patient outcomes and develop more effective, long-lasting treatment strategies.
Bibliometrics is a research method that uses quantitative analysis and statistical tools to assess scholarly publications and citation trends [16]. This approach sheds light on the impact, evolution, and dissemination of scientific knowledge. In medical research, especially in the field of cancer treatment, bibliometric analysis is particularly useful for identifying emerging trends, key researchers, influential institutions, and collaboration networks. Numerous studies have used bibliometric methods to uncover research hotspots across various scientific fields. Recently, a study reported the latest publishing trends and areas of intense activity of nutrition and gastric cancer, which help us better understand the impact of nutritional status on prognosis [17]. Another study about the bibliometric analysis of epigenetic therapy revealed that emerging themes in this field were drug resistance, immunotherapy and combination therapy [18]. However, the bibliometric analysis of drug therapy resistance in renal cell carcinoma remains unreported.
This study is the first to systematically analyze therapeutic drug resistance in renal cell carcinoma using bibliometric methods. Utilizing advanced bibliometric tools, we conducted a comprehensive analysis of research on RCC drug resistance from 2000 to 2023. By integrating bibliometric analysis with this topic, our study provides a broad overview of the current research landscape, helping clinicians and researchers understand present trends and future directions in RCC with drug resistance. Additionally, this study aims to facilitate progress in overcoming drug resistance in RCC, ultimately contributing to improved patient outcomes.
Methods
Data retrieval
The Web of Science Core Collection (WoSCC) database, recognized for its extensive global subject coverage, is widely used for bibliometric research. As one of the largest and most comprehensive electronic scientific literature databases in the world, WoSCC provides curated, high-impact publications with standardized citation indexing, ensuring data reliability for bibliometric analysis. The dataset acquired from WoSCC includes comprehensive cited reference information and data from the Science Citation Index Expanded (SCIE), ensuring the reliability of this study’s results. To identify relevant studies, we employed the search formula: TS = ((drug resistance) OR (medicine resistance) OR (agent resistance) OR (Antineoplastic agent resistance) OR (Antineoplastic drug resistance) OR (Antineoplastic resistance)) AND TS = ((renal cell carcinoma) OR (kidney cancer) OR (renal cancer) OR (renal carcinoma)). We downloaded the "full records and cited references" in text (".txt") format for further analysis.
Screening strategy
The publication time span for this study was from January 1, 2000, to December 31, 2023. We included only articles and reviews while excluding proceedings papers, book chapters, retracted publications, data papers, meeting abstracts, editorial materials, early access articles, letters, and corrections. Only English-language articles were considered, and those with content unrelated to our research topic were manually excluded by two independent researchers to ensure accuracy and consistency. The workflow for this process is illustrated in Fig. 1.
Fig. 1.
Flowchart depicting the publication screening strategy
Statistical analysis
Excel software was utilized to display the general data information of the publications, such as publication counts and publication types. VOSviewer (version 1.6.20) was employed to analyze the collaborative networks of countries/regions and institutions. Additionally, geographical maps depicting collaboration insights between countries/regions were created using both VOSviewer and SCImago Graphica (version 1.0.42). CiteSpace Advanced (version 6.3.R1), developed by Chaomei Chen, was used to explore author collaboration networks, visualize clusters, and detect bursts in categories, references, and keywords. R software (version 4.3.1) and the "bibliometrix" package served as essential tools for conducting general information analysis, journal analysis, category analysis, and research topic evolution paths [19]. In the analysis conducted with VOSviewer, the minimum number of documents for a country or organization was set at 20, with a minimum strength threshold of 0 and a clustering resolution of 1.00. Meanwhile, in the analysis using CiteSpace, the g-index filtering algorithm was employed for node selection. The parameters were set as follows: Linkage Range Factor (LRF) was 2.5, Link/Node Ratio (L/N) was 5.0, Label By (LBY) was set to 10, and the Exponential Growth Factor (e) was 1.0. In the visual graphs, nodes represent countries/regions, institutions, authors, categories, references, or keywords. The size of each node corresponds to the number of publications, while the thickness of the links between nodes indicates the strength of cooperation. Betweenness centrality (BC) is a network analysis metric that measures the extent to which a node acts as a bridge between other nodes. It quantifies the importance of a node in facilitating connections within a network by calculating how often it appears on the shortest paths between other nodes. When the value exceeded 0.1, the nodes were recognized as critical and central within the network. Average publication citations (APC) refer to the average number of times a set of publications has been cited by other works. It is calculated using the formula: Average Citations per Publication = Total Citations/Total Number of Publications. Furthermore, similar-meaning terms were merged to streamline the analysis (Table S1).
Results
General data analysis
In this study, we identified a total of 2,810 publications related to drug resistance in RCC treatment worldwide between 2000 and 2023 (Fig. 2A). The number of publications exceeded 100 in 2012 and reached 200 in 2021, with annual publications exhibiting an upward trend, indicating a gradual increase in focus on this field. The largest increase occurred in 2012, compared to the previous year. The annual growth rate was 8.12%, and the fitted curve of cumulative publications follows a quadratic polynomial (y = 4.9711x2–4.6428x + 62.859, R2 = 0.9998). On average, each publication received 4.65 citations per year from 2000 to 2023, peaking at 10.64 citations per publication in 2012 (Fig. 2B). Overall information on authors, author keywords, and references is shown in Fig. 2C.
Fig. 2.
Overview of publication data. A Annual and cumulative number of publications from 2000 to 2023, with dashed lines indicating growth trends using a non-linear fitting method. B Average citations per publication per year from 2000 to 2023. C Summary of key information related to drug resistance in the treatment of renal cell carcinoma
Countries/Regions, institutions and author analysis
A total of 82 countries/regions were included in our analysis of drug resistance publications. The top 10 countries/regions in terms of publication counts are listed in Table 1. Our analysis of publication counts revealed that the United States had the highest publication counts, followed by China and Japan (Fig. 3A). The closest cooperation was also observed between China and the United States. In terms of H-index, the United States still had the highest rank, followed by China. However, it is important to note that, regarding average publication citations (APC), China ranks lowest among the top 10 productive countries/regions. The central nodes identified were the United States (BC = 0.48), China (BC = 0.12), Italy (BC = 0.15), Germany (BC = 0.15), and France (BC = 0.12). Funding support is vital for countries/regions and institutions, especially in the field of drug resistance. The top 10 funding sources for research on drug resistance in RCC are shown in Table 2. The results indicated that the National Natural Science Foundation of China ranked first, while the National Institutes of Health ranked second. Significant gaps were evident between the first two funding sources and the third in terms of publication counts.
Table 1.
The Top 10 productive countries/regions, ranked by the number of publications
| Rank | Country/Region | Count | Percentage (%) | BC | H-index | APC |
|---|---|---|---|---|---|---|
| 1 | USA | 911 | 32.49 | 0.48 | 204 | 88.48 |
| 2 | China | 611 | 21.79 | 0.12 | 77 | 20.62 |
| 3 | Japan | 212 | 7.56 | 0.03 | 64 | 37.14 |
| 4 | Italy | 193 | 6.88 | 0.15 | 59 | 33.51 |
| 5 | Germany | 190 | 6.78 | 0.15 | 63 | 35.39 |
| 6 | United Kingdom | 157 | 5.60 | 0.09 | 67 | 53.05 |
| 7 | France | 141 | 5.03 | 0.12 | 69 | 49.65 |
| 8 | Netherlands | 108 | 3.85 | 0.06 | 69 | 97.1 |
| 9 | Canada | 106 | 3.78 | 0.04 | 55 | 54.91 |
| 10 | India | 94 | 3.35 | 0.08 | 36 | 22.52 |
BC Betweenness centrality, APC Average Publication Citations
Fig. 3.
Analysis of countries/regions, institutional and author collaboration. A In the network of national/regional cooperation, node size indicates the number of publications, while node color depth reflects the intensity of collaboration. B In the institutional collaboration network, node size represents the number of publications. Blue nodes indicate early-stage research activities, whereas yellow nodes indicate recent research efforts. C Co-authorship network map of authors. Node size indicates the number of publications, and the color depth reflects the publication year, with darker colors representing more recent publications. D Clustering analysis of authors, where nodes of the same color belong to the same cluster
Table 2.
The Top 10 Funds on the Research
| Rank | Fund | Publications | Percentage (%) |
|---|---|---|---|
| 1 | National Natural Science Foundation of China | 242 | 4.70 |
| 2 | National Institutes of Health | 217 | 4.22 |
| 3 | Grants-in-Aid for Scientific Research | 69 | 1.34 |
| 4 | Pfizer | 46 | 0.89 |
| 5 | Novartis | 39 | 0.76 |
| 6 | Cancer Research UK | 31 | 0.60 |
| 7 | Medical Research Council | 19 | 0.37 |
| 8 | Canadian Institutes of Health Research | 17 | 0.33 |
| 9 | AstraZeneca | 16 | 0.31 |
| 10 | GlaxoSmithKline | 16 | 0.31 |
The University of Texas System, Harvard University, and the National Institutes of Health (NIH) ranked as the top three productive institutions in terms of publication output (Table 3). Only Harvard University (BC = 0.11) and the University of California System (BC = 0.14) are identified as central nodes in the collaborative network. This network was generated using VOSviewer, providing a visual analysis of institutional collaborations (Fig. 3B). The University of California San Francisco remained at the center of the network, highlighting its prominence in research. Additionally, several institutions in China have recently initiated efforts in this field, indicating significant progress that has allowed China to achieve a competitive advantage in drug resistance research.
Table 3.
The Top 10 productive institutions, ranked by the number of publications
| Rank | Institution | Count | Percentage (%) | BC | H-index | APC |
|---|---|---|---|---|---|---|
| 1 | University of Texas System | 99 | 3.53 | 0.09 | 62 | 79.63 |
| 2 | Harvard University | 91 | 3.25 | 0.11 | 63 | 243.53 |
| 3 | National Institutes of Health (NIH) | 64 | 2.28 | 0.06 | 57 | 78.22 |
| 4 | UTMD Anderson Cancer Center | 63 | 2.25 | 0.06 | 50 | 83.66 |
| 5 | Institut National de la Sante et de la Recherche Medicale (Inserm) | 61 | 2.18 | 0.06 | 46 | 60.16 |
| 6 | University of California System | 60 | 2.14 | 0.14 | 46 | 60.16 |
| 7 | UNICANCER | 49 | 1.75 | 0.09 | 41 | 109.74 |
| 8 | Assistance Publique Hopitaux Paris (APHP) | 44 | 1.57 | 0.05 | 31 | 26.06 |
| 9 | University of London | 44 | 1.57 | 0.06 | 34 | 56.42 |
| 10 | Dana-Farber Cancer Institute | 40 | 1.42 | 0.06 | 28 | 312.42 |
BC Betweenness centrality, APC Average Publication Citations
The analysis of authors in publications related to drug resistance in RCC identified the top 10 most prolific contributors, as presented in Table 4. The three authors with the highest number of publications were Alfred H. Schinkel, Camillo Porta, and Michael B. Atkins. Notably, Michael B. Atkins had the highest APCs among them. The collaborative dynamics among researchers are visually represented in Fig. 3C. Clustering analysis further categorized high-productivity authors into 10 distinct clusters, with the three most active clusters being #0 immunotherapy, #1 angiogenesis, and #2 Wilms tumor (Fig. 3D).
Table 4.
The Top 10 productive authors, ranked by the number of publications
| Rank | Author | Count | Percentage (%) | H-index | APC |
|---|---|---|---|---|---|
| 1 | Schinkel, Alfred H | 11 | 0.39 | 16 | 141.78 |
| 2 | Porta, Camillo | 10 | 0.36 | 9 | 15.53 |
| 3 | Atkins, Michael B | 9 | 0.32 | 11 | 822.85 |
| 4 | Bates, SE | 9 | 0.32 | 13 | 159.82 |
| 5 | Ravaud, Alain | 8 | 0.29 | 8 | 25.08 |
| 6 | Escudier, Bernard | 8 | 0.29 | 13 | 102.88 |
| 7 | Rini, Brian I | 8 | 0.29 | 10 | 203.69 |
| 8 | Nowak-sliwinska, Patrycja | 8 | 0.29 | 9 | 21.33 |
| 9 | Czarnecka, Anna M | 8 | 0.29 | 10 | 22.00 |
| 10 | Szczylik, Cezary | 8 | 0.29 | 11 | 27.08 |
BC Betweenness centrality, APC Average Publication Citations
Journals and subjects categories
A total of 758 journals were involved in global publications related to drug resistance in RCC (Fig. 2C). Figure 4A presents the top 10 journals by publication counts. Figure 4B illustrates the publication trends of the top 5 journals over time, with most experiencing rapid growth since 2012. Among the top 5 journals, "Cancers" leads the ranking, followed by "Drug Metabolism and Disposition" and the "International Journal of Molecular Sciences." Bradford’s Law is a bibliometric principle that describes the distribution of articles across journals in a particular field. According to the law, a relatively small core of journals publishes the most significant number of articles on a specific topic, while a much larger number of journals publish fewer relevant articles [20]. According to Bradford’s Law, the 758 journals were classified into three zones, with 35 source journals falling into zone 1 (Fig. 4C, Table S2 and S3).
Fig. 4.
Visualization of journal analysis results. A Top 10 journals related to drug resistance in RCC ranked by publication counts. B Trends in publication output of the top 10 journals from 2000 to 2023. C Classification of core and non-core journals based on Bradford’s law
CiteSpace identified 92 research categories related to drug resistance in RCC (Fig. 5A). The three categories with the highest number of publications were oncology (n = 1,129), pharmacology and pharmacy (n = 651), and biochemistry and molecular biology (n = 368, Table 5). The network visualization revealed 10 central nodes, with the top three by centrality being pharmacology and pharmacy (BC = 0.43), oncology (BC = 0.33), and immunology (BC = 0.31). Fig. S1 illustrates the top 10 categories.
Fig. 5.
Research category and themes analysis. A Research category visualization. The size of the nodes indicates the number of publications, while the color gradient represents the publication years. B The progression of research themes using the R bibliometric thematic evolution tool. Time segmentation points were established in 2005, 2010, 2015, and 2020
Table 5.
Top 10 productive subject categories in drug resistance of RCC, ranked by the number of publications
| Rank | Subject category | Count | BC | Year |
|---|---|---|---|---|
| 1 | ONCOLOGY | 1129 | 0.33 | 2000 |
| 2 | PHARMACOLOGY & PHARMACY | 651 | 0.43 | 2000 |
| 3 | BIOCHEMISTRY & MOLECULAR BIOLOGY | 368 | 0.22 | 2000 |
| 4 | CELL BIOLOGY | 249 | 0.08 | 2000 |
| 5 | MEDICINE, RESEARCH & EXPERIMENTAL | 179 | 0.21 | 2000 |
| 6 | UROLOGY & NEPHROLOGY | 157 | 0.05 | 2000 |
| 7 | CHEMISTRY, MULTIDISCIPLINARY | 150 | 0.16 | 2001 |
| 8 | CHEMISTRY, MEDICINAL | 148 | 0.02 | 2000 |
| 9 | MULTIDISCIPLINARY SCIENCES | 86 | 0 | 2000 |
| 10 | GENETICS & HEREDITY | 70 | 0.01 | 2000 |
Research themes
The results are organized using the R bibliometrics-theme evolution tool, which facilitates data input for up to four time points. Figure 5B illustrates the progression of research topics over 23 years, divided into 5-year intervals. From 2000 to 2005, only three research themes were identified, chemoresistance, angiogenesis, and antitumor activity. The period from 2006 to 2010 saw the emergence of apoptosis and genetic variation. From 2011 to 2015, additional topics such as the cell cycle, clinical trials, and mTOR emerged. In the recent period of 2016–2020 and 2021–2023, new themes like ferroptosis and immunotherapy gained prominence, respectively. Fig. S2 further demonstrates the trends in these topics throughout the 23-year span.
Reference analysis
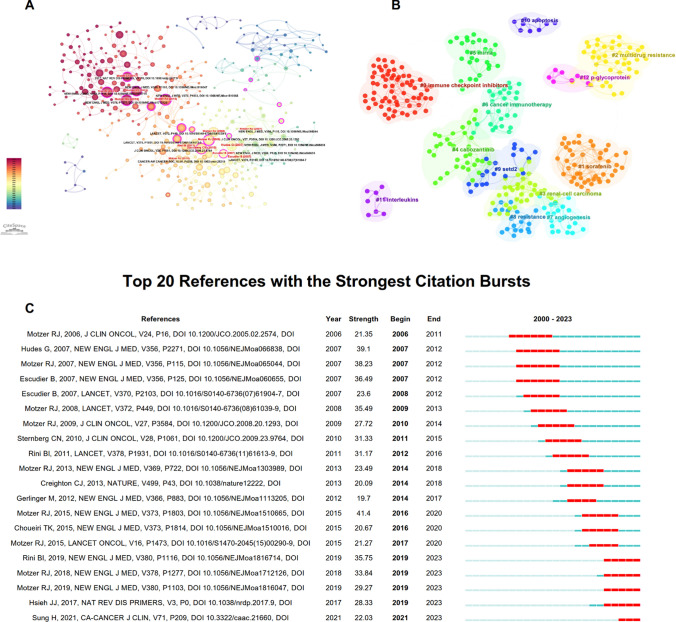
The 127,217 references represent the knowledge base of the current field (Fig. 2C). Figure 6A visualizes the highly cited references related to drug resistance in RCC. The publication with the highest citation intensity, titled “Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma” [21], was published in 2019. This study demonstrated that patients treated with pembrolizumab plus axitinib had significantly longer overall survival and progression-free survival compared to those treated with sunitinib. Clustering these highly cited references yielded a total of 10 clusters (Fig. 6B). The three most active clusters are: #0 immune checkpoint inhibitors, #1 sorafenib and #2 multidrug resistance. Some literature has been frequently cited over time, indicating new breakthroughs or hotspots in the field. The top 20 references with the strongest citation bursts are presented in Fig. 6C. The top three references in terms of burst strength are: Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma (strength = 41.40), Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma (strength = 39.10) and Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma (strength = 38.23) [22–24]. The most frequently cited references are predominantly clinical trials related to drug treatment for RCC.
Fig. 6.
Visualization results of references on drug resistance in RCC research. A Co-citation analysis of references. The size of the nodes represents the number of citations for each reference, and the depth of the color indicates the publication year of the references. B Clustering analysis of references, with the same color representing a cluster. C Burst analysis of references. The red portion of the blue line represents the burst duration
Keywords analysis
Given that keywords represent critical information in the articles, we conducted a keyword analysis of publications related to drug resistance in renal cell carcinoma (Fig. 7A). The three keywords with the highest frequency were renal cell carcinoma (n = 839), expression (n = 505), and cancer (n = 467, Table S4). In this network visualization, there are seven central nodes, with the top three by centrality being drug resistance (BC = 0.14), gene expression (BC = 0.13), and activation (BC = 0.12). The results of clustering analysis revealed that high-frequency keywords were categorized into 10 clusters (Fig. 7B and Table 6). The three most active clusters were #0 targeted therapy, #1 multidrug resistance, and #2 apoptosis, each containing more than 50 keywords. The timeline visualization of keywords illustrates the evolution of research focus over time (Fig. 7C). The focus shifted from tyrosine kinase inhibitor (TKI) to immune checkpoint inhibitors, tumor microenvironment, cell lipid metabolism, androgen receptor and signature. The top 20 keywords with the strongest citation bursts are presented in Fig. 7D. Multidrug resistance, P-glycoprotein, and in vivo were among the earliest keywords to appear. Additionally, induced apoptosis, endothelial growth factor, interferon alpha, TKI, and bevacizumab were significant during the development of drug resistance in RCC. Keywords such as open label, tumor microenvironment, clear cell renal cell carcinoma, progression, immunotherapy, and mechanisms continued to emerge through 2023, indicating their status as hot topics in drug resistance research in RCC.
Fig. 7.
Visualization results of keywords on drug resistance in RCC research. A Keyword network visualization. B Clustering analysis of keywords. C Keyword timeline visualization. D Burst analysis of keywords. The red portion of the blue line indicates the burst duration of the keywords
Table 6.
Information about all keyword clusters
| Cluster ID | Label (LLR) | Size | Silhouette | Mean (Year) |
|---|---|---|---|---|
| 0 | Targeted therapy | 68 | 0.675 | 2011 |
| 1 | Multidrug resistance | 62 | 0.6 | 2007 |
| 2 | Apoptosis | 52 | 0.617 | 2009 |
| 3 | miRNA | 38 | 0.629 | 2013 |
| 4 | Expression | 34 | 0.717 | 2008 |
| 5 | Renal cancer | 21 | 0.779 | 2015 |
| 6 | Drug resistance | 20 | 0.845 | 2006 |
| 7 | Phase III trial | 16 | 0.791 | 2010 |
| 8 | Antitumor activity | 16 | 0.841 | 2004 |
Discussion
General information on drug resistance in RCC
A total of 2,804 publications related to drug resistance in RCC from 2000 to 2023 were retrieved from the WoSCC database. Analyzing the cumulative number of publications, we observed a steady annual increase, particularly after 2018. The most notable surges in annual publication growth occurred in 2007 and 2012. The year 2012 also recorded the highest average number of citations per publication. Notably, the publication titled "Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer" had an exceptionally high citation count, reaching nearly 9000. This significantly influenced the average citation metric for 2012. This clinical trial reported a cumulative response rate of 27% in RCC patients treated with the anti–PD-1 antibody (nivolumab), making a substantial impact on the treatment landscape of RCC and contributing to advances in overcoming TKI drug resistance [25].
The United States has consistently maintained a leading position in the field of drug resistance in RCC, excelling in metrics such as the H-index, total publications, and average citations per paper. Although China ranks second in total publications, its average citations per paper are only 20.62, the lowest among the top ten publishing countries, suggesting a relatively lower quality of publications. Japan, despite being third in publication volume, does not hold a core position in this field. In contrast, Italy, Germany, and France are recognized as core countries contributing significantly to research on RCC drug resistance. Interestingly, we found that specific funding sources are closely associated with research trends, providing valuable insights into their impact on the overall research landscape. Public funding agencies, such as the National Natural Science Foundation of China and the National Institutes of Health, typically prioritize basic and translational research, fostering fundamental discoveries and mechanistic studies. In contrast, pharmaceutical companies are more likely to fund clinical and drug development studies, with a strong emphasis on translational applications and commercialization. Meanwhile, charitable organizations and research councils often serve as a bridge between basic and clinical research, supporting innovation and targeted therapies. A more detailed exploration of these funding patterns could reveal how financial support shapes research directions in renal cell carcinoma, influencing collaboration networks, the pace of innovation, and clinical translation.
In terms of the most productive research institutions, the University of Texas System, despite its high publication output, does not occupy a central position in the institutional network. In contrast, Harvard University and the University of California System serve as central nodes across all institutions, underscoring their pivotal role in fostering communication and integration within the research community. Notably, the Dana-Farber Cancer Institute exhibits the highest APC among the top ten institutions. This can be attributed to a combination of higher citation counts and a relatively lower volume of publications. A key contributing factor is their highly cited publication, previously mentioned, which has received 9470 citations [25]. Regarding the most prolific authors, Alfred H. Schinkel, Camillo Porta, and Michael B. Atkins are ranked as the top three. Similar to the institutional analysis, Michael B. Atkins attained the highest APC due to the substantial citations garnered by one of his key articles. Subsequently, we analyzed the work of Brian I. Rini, the author with the second highest APC, whose research identified IL-8 as a significant factor contributing to sunitinib resistance in clear cell RCC and proposed it as a potential therapeutic target to overcome both acquired and intrinsic resistance to sunitinib [26]. The H-index was used to evaluate the productivity and impact of researchers, aiming to reduce the bias caused by a single highly cited article. Harvard University and Alfred H. Schinkel were identified as the institution and author with the highest H-index, respectively.
In terms of the most influential journals, Cancers, Drug Metabolism and Disposition, International Journal of Molecular Sciences, Clinical Cancer Research, and Frontiers in Oncology are among the most prominent. The Journal Citation Reports (JCR) is a widely used metric for assessing the impact of journals, categorizing them into four quartiles (Q1–Q4) based on their impact factor. Of the top five most influential journals, four are ranked as Q1 journals, with the exception of Frontiers in Oncology. Journals with a high impact factor typically have greater academic influence and tend to attract more readers and citations. The high publication volume of these journals may also contribute to this trend. Clinical Cancer Research demonstrates a high impact factor and a lower annual publication volume, underscoring its role as a key platform for disseminating high-quality research on drug resistance in RCC. According to Bradford’s Law, there are 35 core source journals in Zone 1, which represent the foundational publications in the area of drug resistance in RCC. The latest research findings on drug resistance in RCC are primarily published in journals from disciplines such as oncology, pharmacology and pharmacy, biochemistry and molecular biology, cell biology, medicine, and experimental research. The field of drug resistance in RCC is characterized by its multidisciplinary and interdisciplinary nature. Advances in fundamental sciences, such as molecular biology and cell biology, have provided a robust theoretical foundation for understanding the mechanisms of drug resistance. Simultaneously, applied disciplines like oncology and medicine have effectively translated these fundamental research findings into clinical practice. The collaboration and integration of basic sciences with clinical application have driven the ongoing progress in RCC drug resistance research and offer promising avenues for future targeted and precision therapies.
Hotspots and frontiers
Analysis of research themes revealed several major key topics that emerged before 2015: chemoresistance, angiogenesis, mTOR signaling, and genetic variation. The inconsistent response of RCC to chemotherapy can be attributed to various mechanisms, including the overexpression of multidrug resistance protein P-glycoprotein [27, 28]. Anti-angiogenic drugs have significantly improved the objective response rate of RCC patients compared to chemotherapy or cytokine therapy. The efficacy, adverse reactions, and comparative effectiveness of bevacizumab [29], pazopanib [30], sorafenib, axitinib [31], and sunitinib [32] have become focal points of clinical trials and guidelines. With the widespread use of anti-angiogenic drugs and the increasing challenge of drug resistance, diverse clinical trials have become crucial for addressing resistance in RCC, helping to bridge the gap between basic research and clinical applications. Additionally, everolimus, an inhibitor of the mammalian target of rapamycin (mTOR), has shown promising efficacy and prolonged progression-free survival in patients with metastatic RCC [33]. Upregulation of Zinc Finger DHHC-Type Palmitoyltransferase 2 (ZDHHC2) has been linked to sunitinib resistance, mediating AGK S-palmitoylation to activate the PI3K-AKT-mTOR signaling pathway in RCC, which may enhance the antitumor efficacy of sunitinib [34]. The advancement of high-throughput sequencing technology has further facilitated the identification of genetic variants associated with drug resistance in RCC [35]. For instance, the homozygous deletion of CXXC Finger Protein 4 (CXXC4) at 4q24 leads to reduced expression of CXXC4 mRNA, promoting cell proliferation and inhibiting apoptosis after doxorubicin treatment in RCC via the Wnt signaling pathway [36].
From 2016 onwards, ferroptosis, cytotoxicity, and immunotherapy have gained prominence in the context of drug resistance in RCC. Changes in biological metabolites, including iron and lipids, support ferroptosis as a promising therapeutic strategy for RCC, with ferroptosis-related genes implicated in RCC progression [37]. Dipeptidyl peptidase 9 (DPP9) disrupts KEAP1-NRF2 binding in an enzyme-independent manner, driving NRF2-dependent transcription and suppressing ferroptosis, which contributes to sorafenib resistance in clear cell renal cell carcinoma (ccRCC) [38]. Ferroptosis is associated not only with the immune microenvironment but also influences the efficacy of immunotherapy in RCC, thereby impacting drug resistance. Inhibition of ubiquitin-specific protease 8 (USP8) sensitizes cancer cells to ferroptosis by destabilizing Glutathione Peroxidase 4 (GPX4), while also enhancing CD8 + T cell infiltration, thus boosting the tumor response to anti-PD-1 immunotherapy [39]. PD-1 and PD-L1 inhibitors, such as nivolumab, pembrolizumab, atezolizumab, and durvalumab, have become a significant group of immunotherapy agents, with their efficacy confirmed in multiple clinical trials [40]. Cytotoxicity is a critical mechanism not only in chemotherapy but also in immunotherapy, where immune cells directly attack and kill cancer cells. Current immune checkpoint blockade (ICB) therapies aim to enhance the activation of cytotoxic T cells, enabling them to target and eliminate cancer cells more effectively [41].
Keyword clustering analysis revealed a significant enrichment of miRNAs. miRNAs are known to downregulate gene expression by partially complementing one or more messenger RNAs, leading to translation inhibition. Recent studies indicate that miR-96-5p is expressed at lower levels in tumor tissues of sunitinib-resistant ccRCC patients. miR-96-5p represses Phosphatase and Tensin Homolog Deleted on Chromosome 10 (PTEN) expression by binding to its 3’ UTR site, thereby mediating sunitinib resistance in ccRCC [42]. The keyword timeline analysis showed a shift in the focus of drug resistance research in RCC from tyrosine kinase inhibitors to immune checkpoint inhibitors, lipid metabolism, androgen receptor (AR), and specific molecular signatures. Dysregulation of lipid metabolism, including decreased cholesterol biosynthesis, reduced fatty acid catabolism, and lipid droplet accumulation, is one of the most prominent changes observed in RCC, presenting new therapeutic opportunities [43]. For example, belzutifan may inhibit HIF-2α-driven lipid metabolism, and several clinical trials are currently underway to evaluate its effectiveness [44]. Furthermore, lncRNA HOTAIR has been found to interact with the AR, increasing the transcriptional activity of GLI2. HOTAIR and AR synergistically enhance VEGFA expression, promoting tumor angiogenesis and cancer stemness in RCC cells. These findings suggest that HOTAIR and GLI2 could serve as novel therapeutic targets in RCC [45]. Lastly, accurate prediction of genes and biomarkers associated with drug resistance in RCC remains a critical challenge. Recent studies have shown that cancer-associated fibroblasts contribute to treatment resistance in RCC and may represent a promising therapeutic target to overcome resistance [46].
Challenges and prospects
The current first-line treatments for RCC typically involve a combination of immunotherapy and targeted therapy. However, over time, factors such as the tumor microenvironment, pathway dysregulation, and genetic and epigenetic alterations can activate downstream signaling pathways, ultimately leading to drug resistance [47]. Despite these challenges, several promising therapeutic approaches are emerging. New strategies, including adoptive cell therapy, precision medicine, targeting the tumor microenvironment, and combination therapies, offer hope for more effective and personalized treatment options for RCC patients. Chimeric antigen receptor (CAR) T-cell therapies are a key area of interest, with ongoing investigations into CAR T-cells targeting carbonic anhydrase IX (CAIX) and CD70, as well as the development of bispecific antibodies. Radiopharmaceutical therapies, including agents targeting CAIX, prostate-specific membrane antigen (PSMA), and Radium-223, are also in early stages of development. Additionally, novel targeted therapies, such as poly (ADP-ribose) polymerase inhibitors (PARPi), cyclin-dependent kinase (CDK) inhibitors, and antibody–drug conjugates (ADCs), are being explored as potential new treatment options [48]. To overcome resistance, combinatory therapeutic strategies are being investigated. These include chemotherapy combined with immunotherapy, cytokine therapy plus immunotherapy, and targeted therapy plus immunotherapy, each aiming to address distinct molecular and cellular resistance mechanisms [49]. Various compounds and therapeutic targets focusing on mechanisms such as cell cycle arrest, apoptosis induction, autophagy activation, and cytotoxic effects are currently in preclinical evaluation [50]. In summary, tackling these challenges will require collaborative efforts among researchers and clinicians. Our research reveals potential collaborative relationships among key authors and institutions in both basic and clinical fields. This can facilitate our efforts to seek cooperation with experts in related areas, making interdisciplinary collaboration possible, which can integrate expertise from various fields and facilitate the development and translation of new therapies. Our bibliometric findings also can further inform research strategies, including drug discovery and development, biomarker development, and clinical trials. For instance, targeting the ferroptosis pathway shows promise. GPX4 protects cancer cells from ferroptosis by neutralizing lipid peroxides, while inhibitors such as RSL3 and ML210 can induce ferroptosis [51]. This form of cell death has also been shown to enhance anti-tumor immunity. Additionally, identifying biomarkers related to ferroptosis can aid in stratifying patients for personalized therapies. Elevated levels of GPX4 expression are associated with ferroptosis resistance in ccRCC and correlate with a poorer prognosis [52]. Combining ferroptosis inducers with ICIs may improve treatment efficacy in RCC patients. By deepening our understanding of the complex interplay between cancer cells and resistance mechanisms, the study provides crucial support for advancing progress and translational applications in RCC.
Limitations
Our study has some limitations that should be considered. First, we used only the WoSCC database as our data source. Although WoSCC is comprehensive and widely representative, it does not encompass all relevant literature in the field, particularly high-quality non-English studies. In future research, we will integrate multiple databases to enhance our analysis. Second, the wide time span of our analysis may lead to an overemphasis on articles published earlier, potentially downplaying the influence of recent, high-impact studies. Lastly, to ensure broad coverage of literature related to drug resistance in RCC, we opted for a topic-based search rather than a title-based search. Although we performed thorough manual screening afterward, a small number of unrelated studies might still have been included. Despite these limitations, the primary conclusions of our study remain strong and reliable.
Conclusions
Overall, this study presents the first comprehensive bibliometric analysis of drug resistance in RCC, providing a scientific, thorough, and systematic examination of the field. It identifies current research trends, highlights emerging hotspots, and offers insights into key contributors as well as ongoing challenges. The advancement of research on drug resistance in RCC requires collaborative efforts among researchers across different disciplines and countries. Our findings provide a theoretical reference and practical guidance for future research, better addressing drug resistance in RCC more effectively.
Supplementary Information
Abbreviations
- RCC
Renal cell carcinoma
- ccRCC
Clear cell renal cell carcinoma
- VEGF
Vascular endothelial growth factor
- mTOR
Mammalian target of rapamycin
- ICI
Immune checkpoint inhibitors
- PD-1
Programmed cell death protein 1
- WoSCC
Web of Science Core Collection
- NIH
National Institutes of Health
- BC
Betweenness centrality
- APC
Average publication citation
- TKI
Tyrosine kinase inhibitor
Author contributions
SY: Supervision, Project administration. SC: Investigation. ZZ: Methodology. XZ: Writing—review & editing, Supervision, Funding acquisition, Conceptualization. YZ: Writing—review & editing, Supervision, Resources, Conceptualization. KZ: Writing—review & editing, Writing—original draft, Methodology, Investigation, Data curation. All authors approved the final version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82473309, 82203862), Natural Science Foundation of Henan (252300421599), Science and Technology Research Project of Henan (242102311074), and Medical Science Technology Program of Henan (SBGJ202102140, LHGJ20200273).
Data availability
All data in this article is available through the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kenan Zhang, Shixu Chen, Yonghao Zhan and Xuepei Zhang have contributed equally to this work.
Contributor Information
Yonghao Zhan, Email: yonghao_zhan@163.com.
Xuepei Zhang, Email: zhangxuepei@263.net.
References
- 1.Li YD, Fu YX, Gong LL, Xie T, Tan W, Huang H, Zeng SJ, Liu C, Ren ZJ. Ultra-processed food consumption and renal cell carcinoma incidence and mortality: results from a large prospective cohort. BMC Med. 2024;22:459. 10.1186/s12916-024-03677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo M, Calio A, Brunelli M, Pezzicoli G, Ganini C, Martignoni G, Camillo P. Clinico-pathological implications of the 2022 WHO renal cell carcinoma classification. Cancer Treat Rev. 2023;116:102558. 10.1016/j.ctrv.2023.102558. [DOI] [PubMed] [Google Scholar]
- 4.Van Poppel H, Becker F, Cadeddu JA, Gill IS, Janetschek G, Jewett MA, Laguna MP, Marberger M, Montorsi F, Polascik TJ, Ukimura O, Zhu G. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60:662–72. 10.1016/j.eururo.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 5.Diamond E, Molina AM, Carbonaro M, Akhtar NH, Giannakakou P, Tagawa ST, Nanus DM. Cytotoxic chemotherapy in the treatment of advanced renal cell carcinoma in the era of targeted therapy. Crit Rev Oncol Hematol. 2015;96:518–26. 10.1016/j.critrevonc.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Siva S, Louie AV, Kotecha R, Barber MN, Ali M, Zhang Z, Guckenberger M, Kim MS, Scorsetti M, Tree AC, Slotman BJ, Sahgal A, Lo SS. Stereotactic body radiotherapy for primary renal cell carcinoma: a systematic review and practice guideline from the international society of stereotactic radiosurgery (ISRS). Lancet Oncol. 2024;25:e18–28. 10.1016/S1470-2045(23)00513-2. [DOI] [PubMed] [Google Scholar]
- 7.Bukowski RM. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer. 1997;80:1198–220. 10.1002/(sici)1097-0142(19971001)80:7%3c1198::aid-cncr3%3e3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Kawano Y, Takahashi W, Eto M, Kamba T, Miyake H, Fujisawa M, Kamai T, Uemura H, Tsukamoto T, Azuma H, Matsubara A, Nishimura K, Nakamura T, Ogawa O, Naito S. Prognosis of metastatic renal cell carcinoma with first-line interferon-alpha therapy in the era of molecular-targeted therapy. Cancer Sci. 2016;107:1013–7. 10.1111/cas.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Kaelin WG Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med. 2020;26:1519–30. 10.1038/s41591-020-1093-z. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch L, Flippot R, Escudier B, Albiges L. Immunomodulatory roles of VEGF pathway inhibitors in renal cell carcinoma. Drugs. 2020;80:1169–81. 10.1007/s40265-020-01327-7. [DOI] [PubMed] [Google Scholar]
- 11.Pal SK, Quinn DI. Differentiating mTOR inhibitors in renal cell carcinoma. Cancer Treat Rev. 2013;39:709–19. 10.1016/j.ctrv.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YW, Wang L, Panian J, Dhanji S, Derweesh I, Rose B, Bagrodia A, McKay RR. Treatment landscape of renal cell carcinoma. Curr Treat Options Oncol. 2023;24:1889–916. 10.1007/s11864-023-01161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol. 2009;10:992–1000. 10.1016/S1470-2045(09)70240-2. [DOI] [PubMed] [Google Scholar]
- 14.Sharma R, Kadife E, Myers M, Kannourakis G, Prithviraj P, Ahmed N. Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J Exp Clin Cancer Res. 2021;40:186. 10.1186/s13046-021-01961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Montero CM, Rini BI, Finke JH. The immunology of renal cell carcinoma. Nat Rev Nephrol. 2020;16:721–35. 10.1038/s41581-020-0316-3. [DOI] [PubMed] [Google Scholar]
- 16.Perrier L, Lightfoot D, Kealey MR, Straus SE, Tricco AC. Knowledge synthesis research: a bibliometric analysis. J Clin Epidemiol. 2016;73:50–7. 10.1016/j.jclinepi.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Li R, Zhao Z, Huang H, Yu J. Bibliometric analysis of nutrition in gastric cancer from 2013 to 2023. Front Nutr. 2024;11:1402307. 10.3389/fnut.2024.1402307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Liang X, Shao Q, Wang G, Huang Y, Wen P, Jiang D, Zeng X. Research hotspots and trends of epigenetic therapy in oncology: a bibliometric analysis from 2004 to 2023. Front Pharmacol. 2024;15:1465954. 10.3389/fphar.2024.1465954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arruda H, Silva ER, Lessa M, Proenca D Jr, Bartholo R. VOSviewer and bibliometrix. J Med Libr Assoc. 2022;110:392–5. 10.5195/jmla.2022.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venable GT, Shepherd BA, Loftis CM, McClatchy SG, Roberts ML, Fillinger ME, Tansey JB, Klimo P Jr. Bradford’s law: identification of the core journals for neurosurgery and its subspecialties. J Neurosurg. 2016;124:569–79. 10.3171/2015.3.JNS15149. [DOI] [PubMed] [Google Scholar]
- 21.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, Vynnychenko I, Kryzhanivska A, Bondarenko I, Azevedo SJ, Borchiellini D, Szczylik C, Markus M, McDermott RS, Bedke J, Tartas S, Chang YH, Tamada S, Shou Q, Perini RF, Chen M, Atkins MB, Powles T. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–27. 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ, Global AT. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, Kahnoski R, Futreal PA, Furge KA, Teh BT. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–71. 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naito S, Sakamoto N, Kotoh S, Goto K, Matsumoto T, Kumazawa J. Expression of P-glycoprotein and multidrug resistance in renal cell carcinoma. Eur Urol. 1993;24:156–60. 10.1159/000474284. [DOI] [PubMed] [Google Scholar]
- 28.Bhatt JR, Finelli A. Landmarks in the diagnosis and treatment of renal cell carcinoma. Nat Rev Urol. 2014;11:517–25. 10.1038/nrurol.2014.194. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N, investigators AT. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 30.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA, Kavina A, Zarba JJ, Chen M, McCann L, Pandite L, Roychowdhury DF, Hawkins RE. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 31.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 32.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Zhu L, Liu P, Zhang H, Guo F, Jin X. ZDHHC2-mediated AGK palmitoylation activates AKT-mTOR signaling to reduce sunitinib sensitivity in renal cell carcinoma. Cancer Res. 2023;83:2034–51. 10.1158/0008-5472.CAN-22-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong L, Zhang Y, Wang J, Yu M, Huang L, Hou Y, Li G, Wang L, Li Y. Novel small molecule inhibitors targeting renal cell carcinoma: status, challenges, future directions. Eur J Med Chem. 2024;267:116158. 10.1016/j.ejmech.2024.116158. [DOI] [PubMed] [Google Scholar]
- 36.Kojima T, Shimazui T, Hinotsu S, Joraku A, Oikawa T, Kawai K, Horie R, Suzuki H, Nagashima R, Yoshikawa K, Michiue T, Asashima M, Akaza H, Uchida K. Decreased expression of CXXC4 promotes a malignant phenotype in renal cell carcinoma by activating Wnt signaling. Oncogene. 2009;28:297–305. 10.1038/onc.2008.391. [DOI] [PubMed] [Google Scholar]
- 37.He C, Li Q, Wu W, Liu K, Li X, Zheng H, Lai Y. Ferroptosis-associated genes and compounds in renal cell carcinoma. Front Immunol. 2024;15:1473203. 10.3389/fimmu.2024.1473203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang K, Chen Y, Zhang X, Zhang W, Xu N, Zeng B, Wang Y, Feng T, Dai B, Xu F, Ye D, Wang C. DPP9 stabilizes NRF2 to suppress ferroptosis and induce sorafenib resistance in clear cell renal cell carcinoma. Cancer Res. 2023;83:3940–55. 10.1158/0008-5472.CAN-22-4001. [DOI] [PubMed] [Google Scholar]
- 39.Li H, Sun Y, Yao Y, Ke S, Zhang N, Xiong W, Shi J, He C, Xiao X, Yu H, Dai P, Xiang B, Xing X, Xu G, Song W, Song J, Zhang J. USP8-governed GPX4 homeostasis orchestrates ferroptosis and cancer immunotherapy. Proc Natl Acad Sci USA. 2024;121:e2315541121. 10.1073/pnas.2315541121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z, Jin Y, Zhou J, Chen F, Chen M, Gao Z, Hu L, Xuan J, Li X, Song Z, Guo X. PD1/PD-L1 blockade in clear cell renal cell carcinoma: mechanistic insights, clinical efficacy, and future perspectives. Mol Cancer. 2024;23:146. 10.1186/s12943-024-02059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf MM, Rathmell WK, de Cubas AA. Immunogenicity in renal cell carcinoma: shifting focus to alternative sources of tumour-specific antigens. Nat Rev Nephrol. 2023;19:440–50. 10.1038/s41581-023-00700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SE, Kim W, Hong JY, Kang D, Park S, Suh J, You D, Park YY, Suh N, Hwang JJ, Kim CS. miR-96-5p targets PTEN to mediate sunitinib resistance in clear cell renal cell carcinoma. Sci Rep. 2022;12:3537. 10.1038/s41598-022-07468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heravi G, Yazdanpanah O, Podgorski I, Matherly LH, Liu W. Lipid metabolism reprogramming in renal cell carcinoma. Cancer Metastasis Rev. 2022;41:17–31. 10.1007/s10555-021-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu MF, Zhang GD, Liu TT, Shen JH, Cheng JL, Shen J, Yang TY, Huang C, Zhang L. Hif-2alpha regulates lipid metabolism in alcoholic fatty liver disease through mitophagy. Cell Biosci. 2022;12:198. 10.1186/s13578-022-00889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai JY, Jin B, Ma JB, Liu TJ, Yang C, Chong Y, Wang X, He D, Guo P. HOTAIR and androgen receptor synergistically increase GLI2 transcription to promote tumor angiogenesis and cancer stemness in renal cell carcinoma. Cancer Lett. 2021;498:70–9. 10.1016/j.canlet.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 46.Ambrosetti D, Coutts M, Paoli C, Durand M, Borchiellini D, Montemagno C, Rastoin O, Borderie A, Grepin R, Rioux-Leclercq N, Bernhard JC, Pages G, Dufies M. Cancer-associated fibroblasts in renal cell carcinoma: implication in prognosis and resistance to anti-angiogenic therapy. BJU Int. 2022;129:80–92. 10.1111/bju.15506. [DOI] [PubMed] [Google Scholar]
- 47.Roy AM, George S. Emerging resistance vs. losing response to immune check point inhibitors in renal cell carcinoma: two differing phenomena. Cancer Drug Resist. 2023;6:642–55. 10.2051/cdr.2023.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi SH, Chen YW, Panian J, Yuen K, McKay RR. Emerging innovative treatment strategies for advanced clear cell renal cell carcinoma. Oncologist. 2024. 10.1093/oncolo/oyae276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berland L, Gabr Z, Chang M, Ilie M, Hofman V, Rignol G, Ghiringhelli F, Mograbi B, Rashidian M, Hofman P. Further knowledge and developments in resistance mechanisms to immune checkpoint inhibitors. Front Immunol. 2024;15:1384121. 10.3389/fimmu.2024.1384121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontes O, Oliveira-Pinto S, Baltazar F, Costa M. Renal cell carcinoma therapy: current and new drug candidates. Drug Discov Today. 2022;27:304–14. 10.1016/j.drudis.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Cheff DM, Huang C, Scholzen KC, Gencheva R, Ronzetti MH, Cheng Q, Hall MD, Arner ESJ. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023;62:102703. 10.1016/j.redox.2023.102703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648–57. 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this article is available through the corresponding author upon reasonable request.