Abstract
Pancreatic neuroendocrine tumors (pNETs) often overexpress somatostatin receptor type 2 (SSTR2), making them ideal targets for theranostics, which integrates molecular imaging with targeted radionuclide therapy. 177Lu-DOTATATE significantly extends progression-free survival (22.8 vs. 8.5 months) compared to octreotide LAR. Despite these advances, challenges remain, including treatment resistance and long-term toxicities. In this review, we explore advancements in specialized imaging techniques, rationale combination strategies, and exploring next-generation radiopharmaceuticals.
Subject terms: Cancer imaging, Radiotherapy, Pancreatic cancer, Targeted therapies
Introduction
Pancreatic neuroendocrine tumors (pNETs) are the second most common type of neuroendocrine tumor (NET)1,2, accounting for approximately 8-10% of all pancreatic neoplasms3. The incidence of pNETs has more than doubled over the past three decades, largely due to advances in radiographic detection, although the contributions of genetic and environmental factors are still under investigation1,4. Despite diagnostic advancements, managing pNETs remains challenging due to their heterogeneous behavior, and limited systemic treatment options. Systemic therapy for pNETs has traditionally focused on somatostatin analogs (SSA) due to the high expression of somatostatin receptor type 2 (SSTR2) on tumor cells. While SSAs are effective at controlling hormonal symptoms and stabilizing disease, they rarely induce significant tumor regression, highlighting a critical unmet clinical need for therapies that actively reduce tumor burden. The development of peptide receptor radionuclide therapy (PRRT) has introduced a promising therapeutic strategy by leveraging SSTR2 expression to enable highly sensitive imaging of small tumors and the delivery of targeted radiation directly to tumor cells. This review explores recent advances in specialized imaging techniques, PRRT-based combination therapies, and emerging radiopharmaceutical agents for the treatment of pNETs.
Biology of pNETs and the role of SSTR
pNETs originate from pancreatic islet cells and exhibit diverse biological behaviors, from indolent to highly aggressive. Approximately 75–90% of pNETs are non-functional, meaning they do not secrete metabolically active hormones, leading to asymptomatic progression and delaying diagnosis until after metastasis has occurred1,5. In contrast, functional tumors can secrete hormones, such as glucagon, insulin, and gastrin, causing distinct clinical syndromes that can prompt earlier detection6. Somatostatin, a hormone produced by neuroendocrine cells, regulates glucagon and insulin secretion and influences pancreatic cell growth through multiple signaling pathways7,8. A key biological characteristic of pNETs is the overexpression of somatostatin receptors (SSTRs), particularly SSTR2, which is found in around 80% of non-functional pNETS9. The high expression of SSTR2 in well-differentiated pNETs, while poorly differentiated tumors tend to exhibit lower levels of this receptor10. This differential expression of SSTR2 not only serves as a diagnostic marker but also presents a valuable therapeutic target. High SSTR2 expression is associated with a favorable response to SSAs due to their preferential binding to SSTR211. Consequently, SSAs are widely employed to control tumor growth and alleviate hormonal symptoms in patients12. The limited efficacy of SSAs in causing significant tumor regression combined treatment resistance, underscores the necessity for more targeted and effective treatment strategies. The availability of a tumor-specific target (SSTR2) combined with advances in nuclear medicine has paved the way for theranostics, an approach that integrates molecular imaging and targeted radionuclide therapy to offer a more precise and effective treatment strategy.
Specialized imaging
While traditional imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) are commonly used for pNET detection, their sensitivity is often limited, especially for small or well-differentiated lesions. In contrast, molecular imaging techniques utilizing radiolabeled somatostatin analogs have significantly enhanced the ability to localize and assess tumor activity with higher precision. The Octreoscan (Mallinckrodt, St. Louis, MO), the first Food and Drug Administration (FDA)-approved imaging modality for pNETs, utilizes planar and SPECT/CT imaging performed 24 and 36 h after the intravenous administration of 111In-pentetreotide, a gamma-emitting radiotracer targeting SSTR2. However, since the FDA approval of 68Ga-DOTATATE Positron Emission Tomography-Computed Tomography (PET/CT) in June 2016, this tracer has largely replaced Octreoscan due to its superior sensitivity (96–97%) which is significantly higher than 111In-pentetreotide SPECT/CT (65–72%), and comparable specificity (93%) in detecting SSTR2-expressing tumors13. This shift is also driven by reduced radiation exposure and a shorter imaging protocol, as 68Ga-DOTATATE scans are completed within 1–2 h14–20.
68Ga is a positron-emitting isotope chelated to 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), which is conjugated to peptides with high affinity for SSTRs. Common 68Ga compounds include DOTATOC, DOTANOC, and DOTATATE21. While these tracers show minor differences in binding affinities to specific SSTR subtypes, they exhibit comparable diagnostic accuracy for detecting neuroendocrine tumors (NETs)22,23. 68Ga-DOTATATE PET/CT is the most widely used in the United States, while DOTATOC and DOTANOC are more commonly utilized in Europe. 68Ga-DOTATATE PET/CT is particularly effective for imaging well-differentiated, low-grade pNETs due to their high SSTR2 expression, whereas poorly differentiated, grade 3 pNETs, with reduced SSTR expression and increased glycolytic activity, are better evaluated using 18F-fluorodeoxyglucose (18F-FDG) PET/CT imaging23.
Emerging radiotracers
Among newer agents, 64Cu-DOTATATE, approved in the U.S. since 2020, offers significant advantages over 68Ga-based tracers, including a longer half-life of 12.7 h, compared to 68 min for 68Ga24–26.This extended half-life allows for greater flexibility in imaging schedules and facilitates centralized production and distribution to sites without on-site cyclotron facilities27. 64Cu-DOTATATE also provides lower radiation exposure and improved lesion detection25. A comparative study revealed that 64Cu-DOTATATE detected 42 additional lesions compared to and 68Ga-DOTATOC, 33 of which were confirmed as true positives (p < 0.001), demonstrating its superior sensitivity28. Despite these benefits, 64Cu-DOTATATE has limitations, including higher production costs and longer imaging times (1–3 h) compared to 68Ga-DOTATATE28. While these factors may limit widespread adoption, current evidence is inconclusive regarding the superiority of one over another, and both remain valuable diagnostic options in clinical practice.
Current SSTR-mediated imaging relies on the internalization of radiolabeled agonists into tumor cells. However, preclinical studies suggest that radiolabeled SSTR antagonists may offer superior tumor uptake and detection due to enhanced receptor recognition29,30. For instance, 68Ga-DOTA-JR11, an SSTR antagonist, demonstrated 1.3 times higher tumor uptake compared to 68Ga-DOTATATE, despite having 150 times lower affinity for SSTR229. Early human studies using 68Ga-DOTA-JR11 have shown favorable safety profiles, optimal biodistribution, and the ability to detect lesions as small as 1.4 mL17. SSTR antagonists may provide a more comprehensive assessment of tumor burden in pNET patients.
PET imaging techniques: dual-tracer PET and hybrid imaging
Dual-tracer PET imaging has emerged as a valuable approach for staging and grading pNETs, particularly those with heterogeneous features. This technique combines the high sensitivity of 68Ga-DOTATATE for detecting well-differentiated, SSTR-positive tumors with the ability of 18F-FDG PET/CT to identify glycolytically active, poorly differentiated tumors31. In a recent study involving 124 patients with Grade 1 and Grade 2 pNETs (49.2%/50.8%, respectively), 68Ga-DOTATOC PET/CT detected disease in 122 patients (98.4%), while 18F-FDG PET/CT identified lesions in 64 patients (51.6%), resulting in a combined sensitivity of 99.2%32. This dual-tracer approach not only enhances diagnostic accuracy but also improves treatment stratification by distinguishing SSTR-positive, indolent tumors from highly glycolytic, aggressive subtypes, allowing for targeted treatment planning31,33–35. Similarly, hybrid imaging techniques such as PET/MRI can further refine staging accuracy by integrating the functional insights of PET with the high-resolution anatomical detail of MRI36. This combination is particularly effective for detecting small liver metastases and peritoneal implants that might be missed by conventional imaging36,37. For example, PET/MRI has shown superior performance in identifying sub-centimeter liver lesions and evaluating vascular invasion, thereby providing more precise information for surgical planning37.
Collectively, advanced imaging techniques address the limitations of traditional imaging by offering more precise staging and a deeper understanding of tumor heterogeneity, thereby enabling more personalized treatment approaches.
Current treatment approaches for pNETS
Surgical resection for localized or limited metastatic disease remains the primary potentially curative option for patients with pNETs. However, the majority of patients (60.2%) present with metastatic disease, while an additional 20.7% are diagnosed with regionally advanced tumors38, necessitating a focus on reducing tumor burden, and managing symptoms through multimodal strategies. These strategies often include a combination of surgery, liver-directed treatments, and systemic therapies. The following sections explore key systemic therapies, highlighting their roles in the comprehensive management of pNETs (see Table 1).
Table 1.
Landmark Clinical Trials for the Treatment of GEP-NETs
| Trial Name/ID/Study design | Primary endpoint | Result | Dates of Enrollment | Number Enrolled | Status |
|---|---|---|---|---|---|
|
PROMID /NCT00171873/Randomized, Double blind Phase III |
Time to Tumor Progression |
Time to tumor progression: Octreotide cohort- 14.3 mo (CI 95% 11.0 to 28.8) Placebo cohort- 6 mo (CI 95% 3.7 to 9.4) |
2001–2013 | 85 | Completed |
| NCT00353496/ CLARINET/Phase III, Randomised, Double-blind | PFS |
Median PFS: Lanreotide cohort- was not reached Placebo cohort- 72 weeks (CI 95% 48.6 to 96.0) |
2006–2013 | 264 | Completed |
| NCT00842348/Phase III Open Label Extension Study of Lanreotide Autogel 120 mg | AE |
AE: 86 out of 89 patients with treatment emergent AEs PFS: Lanreotide-Lanreotide cohort: 154.14 weeks (CI 95%, 123.57 to 237.43) Placebo-Lanreotide cohort: 72.00 weeks (CI 95%, 48.43 to 84.57) |
2009–2015 | 89 | Completed |
| NCT01824875/Randomized Phase II | PFS |
PFS: Temozolomide cohort- 15.1 months (CI 95% 10.5 to 21.0) Temozolomide and Capecitabine (CAPTEM) cohort- 23.2 months (CI 95% 16.6 to 32.2) |
2013–2023 | 144 | Active, not recruiting |
| NCT01578239/ NETTER-1/Randomized Phase III | PFS |
PFS: 177Lu-DOTATATE cohort- at month 20 was 65.2% (95% CI, 50.0 to 76.8) LAR Octreotide cohort- at month 20, 10.8% (95% CI, 3.5 to 23.0) P = 0.004 ORR: 177Lu-DOTATATE- 18% (CI 95% 7.8 to 21.6) LAR Octreotide cohort- 3% (CI 95% 0.2 to 7.8) OS: 177Lu-DOTATATE cohort- 48 months (CI 95% 37.4 to 55.2) LAR Octreotide cohort- 36.3 months (CI 95% 25.9 to 51.7) (HR 0.84, 95% CI 0.60-1.17. p value 0.3039) |
2012–2021 | 231 | Completed |
Somatostatin analogs
SSAs are a widely used first-line treatment for patients with advanced NETs that express SSTRs, due to their anti-proliferative effects and efficacy in symptom management39. Common SSAs include octreotide long-acting release (LAR) and lanreotide, both of which have demonstrated significant improvements in progression-free survival (PFS) in separate Phase III clinical trials40,41. The CLARINET trial (NCT00353496), which evaluated the efficacy of lanreotide in patients with gastroenteropancreatic NETs (GEP-NETs), reported a median PFS that was not reached in the lanreotide group compared to 18.0 months in the placebo group (HR 0.47; 95% CI, 0.30–0.73; p < 0.001)42. This trial also included a subgroup analysis focused specifically on pNET patients, which demonstrated a favorable trend for disease control in those receiving lanreotide (HR = 0.58; 95% CI, 0.32–1.04)43. While SSAs are effective for disease stabilization, they have limitations, including treatment resistance, incomplete tumor control, and gastrointestinal side effects.
Peptide receptor radionucleotide therapy (PRRT)
PRRT (Fig. 1) utilizes radiolabeled somatostatin analogs by linking the β-emitting radioisotope 177Lutetium (177Lu) to the somatostatin analog octreotate through a DOTA chelator, enabling targeted radiation delivery to tumors with high SSTR expression44. The NETTER-1 trial (NCT01578239)was the first phase III trial to assess the efficacy and safety of 177Lu-DOTATATE in patients with metastatic or inoperable, well-differentiated SSTR positive midgut NETs that had progressed despite prior SSA therapy45. The trial demonstrated that 177Lu-DOTATATE reduced the risk of progression or death by 79% compared to high-dose octreotide LAR, with a 20-month progression-free survival (PFS) rate of 65.2% for the 177Lu-DOTATATE group versus 10.8% for the control group45. In addition, the objective response rate (ORR) was significantly higher in the 177Lu-DOTATATE group (18%) compared to 3% in the control group (P < 0.001)45. Although OS was longer in the PRRT group, the difference between the two groups did not reach statistical significance46. Given the favorable PFS, and significant objective response rate observed in patients with midgut neuroendocrine tumors in the NETTER-1 trial, the FDA extended 177Lu-DOTATATE approval to encompass all gastrointestinal and pancreatic neuroendocrine tumors47. 177Lu-DOTATATE is generally well tolerated, but grade 3 or 4 nephrotoxicity has been reported in 2–3% of patients, grade 3 or 4 bone marrow toxicity in 9% as well as Myelodysplastic syndrome (MDS), leukemia at 2% and 3%, respectively48. To mitigate nephrotoxicity, co-infusion of positively charged amino acids (e.g., L-lysine and L-arginine) can reduce renal radiation dose by up to 65%48. Regular monitoring of renal function and blood counts is essential for early detection of complications49.
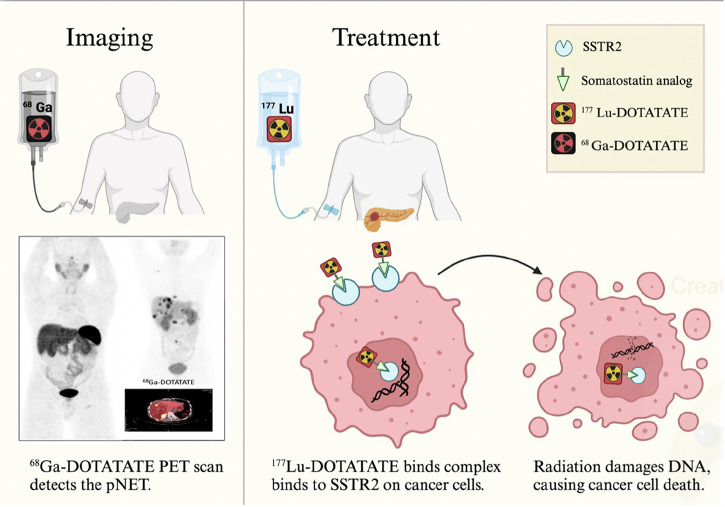
Fig. 1. Radionuclide Detection and Treatment of pNETs.
This figure illustrates the radionuclide-based approach for detecting and treating pancreatic neuroendocrine tumors (pNETs). The process begins with the intravenous injection of 68Ga-DOTATATE, a somatostatin receptor type 2 (SSTR2) agonist that binds to receptors expressed on the surface of neuroendocrine tumor (NET) cells. A subsequent PET/CT scan detects NETs by visualizing this receptor-ligand interaction. For treatment, patients receive 177Lu-DOTATATE intravenously as part of peptide receptor radionuclide therapy (PRRT). The 177Lu-DOTATATE binds specifically to SSTR2 receptors on neuroendocrine cancer cells, is internalized, and delivers targeted radiation directly to the tumor. This radiation induces DNA damage, leading to cancer cell death while sparing surrounding healthy tissues. Created in Biorender. Karimi, A. (2025).
The follow-up Phase III NETTER-2 trial (NCT03972488) is the first randomized study to assess radioligand therapy as a first-line treatment50. Results from the trial indicate that combining 177Lu-DOTATATE with octreotide LAR substantially improved PFS (22.8 months, 95% CI: 19.4–not estimated) compared to octreotide LAR alone (8.5 months, 95% CI: 7.7–13.8, p < 0.0001)50. A subgroup analysis further emphasized the benefit of 177Lu-DOTATATE in pNETs, showing a median PFS of 19.4 months versus 8.5 months in the control arm, and an objective response rate (ORR) of 51.2% compared to 12.2%51. The NETTER-2 trial demonstrated a higher ORR compared to NETTER-1, despite enrolling patients with higher-grade (Grade 2 and 3) GEP-NETs—a population characterized by more aggressive tumor biology. In contrast, NETTER-1 focused on patients with Grade 1 and 2 midgut NETs, which are generally considered less aggressive. This discrepancy in ORR between the two trials highlights the enhanced efficacy of the PRRT in a treatment naïve population.
The promising outcomes of the NETTER-2 trial underscore the expanding role of PRRT in managing pNETs. Because of this, there has been further exploration of additional PRRT administration beyond the conventional 3–4 dose course52,53. Vaughan et al. found that retreatment with PRRT was safe and effective, with a median PFS of 17.5 months (95% CI, 11–23.8) and median OS of 71 months (95% CI, 57-89)54. A meta-analysis by Strosberg et al. of seven studies involving 414 patients showed a median pooled PFS of 12.52 months (95% CI, 9.82–15.22) and OS of 26.78 months (95% CI, 18.73–34.83) following PRRT retreatment, with grade 3/4 adverse events (AEs) occurring in 5% of patients, with hematologic events particularly noteworthy55,56. Despite the increase in AEs compared to initial PRRT, these findings support PRRT retreatment as a viable option for select patients with tumors that retain SSTR2 expression after initial PRRT treatment53,56. Further research is required to address key challenges, including strategies to address hematologic toxicity, and developing standardized protocols for optimal imaging timing for tumor response assessment57.
Combination therapy with 177Lu-DOTATATE: enhancing efficacy while managing toxicity
Ongoing research is exploring the potential of combination therapies to enhance therapeutic efficacy of PRRT. Since 177Lu-DOTATATE induces DNA damage through radiation, combining it with agents that target DNA repair mechanisms may amplify its therapeutic effects. In addition to the studies highlighted below, numerous other agents are being investigated, as detailed in Table 2.
Table 2.
Ongoing clinical trials and combination therapies
| Clinical trial registry number/phase | Description | Therapeutic Target | Primary Endpoint | Duration | Number Enrolled | Status |
|---|---|---|---|---|---|---|
| NCT03972488/ NETTER-2/ Randomized Phase III | Evaluate the Efficacy and Safety of 177-Lu Dotatate | N/A | PFS | 2020–2027 | 2226 | Active |
| NCT05459844/Randomized Phase III | Comparing 177-Lu Oxodotreotide Injection to Octreotide LAR | N/A | PFS | 2022–2028 | 196 | recruiting |
| NCT05884255/Randomized Phase III | Study of 177Lu-Oxodotreotide Injection | N/A | PFS | 2023–2030 | 220 | Not yet recruiting |
|
NCT05247905/ A022001 Phase II Randomized Prospective Trial/ |
Comparing Capecitabine and Temozolomide in Addition to Lu 177 Dotatate vs Lu 177 Dotatate | Chemotherapeutic agent | PFS | 2022–2033 | 198 | Recruiting |
| NCT04234568/ETCTN 10388/Phase I | Addition of Triapine to 177-Lu Dotatate vs 177-Lu Dotatate | Ribonucleotide reductase inhibitor |
MTD & DLT |
2020–2024 | 31 | Active,not recruiting |
| NCT05724108/ETCTN 10558/Randomized Phase II/ | Addition of Triapine to Lu 177 Dotatate vs Lu 177 Dotatate | Ribonucleotide reductase inhibitor | ORR | 2023–2025 | 94 | Recruiting |
| NCT04750954/ETCTN 10450/ | Addition of Peposertib with 177-Lu Dotatate | DNA dependent protein kinase (DNA-PK) inhibitor |
RP2D & DLT |
2021–2024 | 29 | Recruiting |
| NCT05687123/ ETCTN 10479/Phase I | Addition of Sunitinib Malate to 177-Lu Dotatate | Tyrosine kinase inhibitor (activity against VEGF) | AE | 2023–2025 | 24 | Recruiting |
| NCT04086485/ Phase I/II Study | 177-Lu Dotatate in Combination With Olaparib | PARP Inhibitor |
Phase I: MTD Phase II: ORR |
2023–2026 | 37 | Recruiting |
| NCT03478358/Phase I | Treatment Using Long-lasting Radiolabeled Somatostatin Analog 177-Lu-DOTA-EB-TATE | N/A |
Change of SUV of 68Ga-DOTA-TATE & safety of 3.7GBq of 177-Lu-DOTA-EB-TATE with and without amino acid infusion |
2017–2023 | 60 | Recruiting |
| NCT05475210/Phase I Open-Label Study | Safety and Dosimetry of 3-Dose Regimen of Escalating Doses 177-Lu-DOTA-EB-TATE in untreated patients | N/A |
Safety & DLT & MTD |
2022–2024 | 9 | Recruiting |
| NCT05477576/ ACTION-1/ phase Ib/III Global, Randomized, Controlled, Open-label Trial | Safety, pharmacokinetics and recommended Phase 3 dose (RP3D) of RYZ101 | N/A |
Phase Ib: RP3D & Phase III: PFS |
2022–2028 | 218 | Recruiting |
| NCT02609737/ Phase I | Theranostics of Radiolabeled Somatostatin Antagonists 68-Ga-DOTA-JR11 vs177-Lu-DOTA-JR11 | N/A |
ORR & AE |
2015–2020 | 20 | c |
| NCT04919226/ COMPOSE trial /Randomized, Open-labeled phase III | Efficacy and safety of 177-Lu-edotreotide vs the best standard care | N/A | PFS | 2021–2026 | 202 | Recruiting |
| NCT03049189/COMPETE Trial/ Randomized, Open-label, Multicentre Phase III study | Safety of PRRT With 177-Lu-Edotreotide Compared to Targeted Molecular Therapy With Everolimus | N/A | PFS | 2017–2029 | 309 | Active, not recruiting |
| NCT03590119/ LUTIA/ Randomized Phase II/III trial | Intra-arterial 177-Lu Dotatate adminestration | N/A | Difference in post treatment tumor-to-non-tumor (T/N) activity concentration ratio on SPECT/CT | 2018–2022 | 26 | c |
| NCT04544098/Early phase I clinical trial | Evaluate safety and dosimetry of 177-Lu Dotatate | N/A |
number of patients who successfully complete 2 IA injections & ORR |
2020–2029 | 10 | Recruiting |
| NCT04837885/LUTARTERIAL | Intra-arterial Hepatic (IAH) Infusion of Radiolabelled Somatostatin Analogs | N/A | Standardized uptake value (SUVmax) on liver metastases | 2021–2024 | 20 | Recruiting |
Combining PRRT with conventional chemotherapy agents
The combination of 177Lu-DOTATATE with standard chemotherapeutic agents, such as the antimetabolite capecitabine and the alkylating agent temozolomide (or both, as in the CAPTEM regimen), is being explored as a strategy to improve tumor response58,59. Chemotherapeutic agents can inhibit DNA repair pathways, thereby prolonging radiation-induced DNA breaks and increasing apoptosis60. Ongoing Phase II trials (NCT027500 and NCT02358356) are investigating the efficacy of these combinations. The E2211 Phase II trial (NCT01824875) provided compelling evidence supporting this approach, demonstrating that CAPTEM significantly improved median PFS to 22.7 months compared to 14.4 months with temozolomide alone (HR = 0.58, P = 0.022) in 144 patients. The final analysis showed a median OS of 58.7 months in the CAPTEM arm compared to 53.8 months with temozolomide alone, although the difference was not statistically significant (HR = 0.82, P = 0.42)61. Additional evidence from the NCT02358356 trial further supports the efficacy of CAPTEM in combination with PRRT. In patients with pNETs, the 12-month PFS was 77% with PRRT + CAPTEM versus 60% in the control arm, suggesting an added benefit of chemotherapy in this subset62. However, combining these treatments resulted in a higher incidence of grade 3/4 hematologic toxicities, particularly in patients with midgut NETs, where such events were nearly twice as frequent with PRRT + CAPTEM (88% vs. 46%)63,64. To mitigate these risks, careful patient selection, close monitoring, and dose adjustments are essential. Approaches such as prophylactic growth factor support may help reduce the risk of severe neutropenia and thrombocytopenia65.
Combining PRRT with targeted therapy
177Lu-DOTATATE exerts its therapeutic effects primarily through β-particle radiation, which causes single-strand DNA breaks in SSTR-positive tumor cells. However, the efficacy of this approach can be limited by intrinsic DNA repair mechanisms that allow tumor cells to recover from radiation-induced damage66. Targeting DNA repair proteins such as PARP, HSP90, and checkpoint kinase 1 (CHEK1) holds promise for enhancing the efficacy of radioligand therapy.
Poly ADP-ribose polymerase (PARP) inhibitors
PARP enzymes play a critical role in DNA repair by addressing single- and double-strand breaks67. Inhibiting PARP can enhance the sensitivity of tumor cells to 177Lu-DOTATATE by prolonging DNA damage, promoting cell cycle arrest and apoptosis68–72. Early results from the Phase I/II study (NCT04086485) combining the PARP inhibitor olaparib with 177Lu-DOTATATE indicate the combination is well tolerated with minimal hematologic toxicity, with only grade 1 fatigue and alopecia reported73,74. Since PARP inhibitors can also exacerbate bone marrow suppression, close monitoring of hematologic parameters and the use of dose modifications may be necessary75. Although PARP inhibitors are FDA-approved for treating pancreatic adenocarcinoma in patients with germline BRCA1/2 mutations, the low incidence of BRCA mutations in pNETS, significantly limits the potential therapeutic benefit of using a mutation-specific patient selection approach76.
DNA repair pathway inhibitors
DNA-PK, a key enzyme in the non-homologous end joining pathway of DNA repair, may prevent the repair of DNA damage caused by 177Lu-DOTATATE, thereby enhancing tumor control77. Peposertib, a potent radiosensitizer78, has been evaluated in combination with radiation and cisplatin chemotherapy in patients with thoracic and head and neck cancers, where it demonstrated acceptable tolerability in early human trials79. Maculopapular rash and nausea were the most common Grade 3 AEs80. The Experimental Therapeutics Clinical Trials Network (ETCTN) 10450 trial (NCT04750954) is currently evaluating peposertib in combination with 177Lu-DOTATATE in patients with well-differentiated, PRRT-naïve GEP-NETs who have progressed on somatostatin analogs. However, this approach may also increase gastrointestinal and hematologic toxicity, highlighting the need for careful patient selection and supportive care measures81.
Ribonucleotide reductase, a rate-limiting enzyme in DNA synthesis and repair, can also contribute to resistance against 177Lu-DOTATATE by repairing radiation-induced DNA damage. The ETCTN 10388 Phase I trial (NCT04234568) is investigating the combination of the RR inhibitor, Triapine, with 177Lu-DOTATATE in SSTR-positive GEP-NETs. Preliminary findings revealed that 22 out of 28 patients remained progression-free at 12 months, indicating the potential of Triapine to overcome PRRT resistance82. A Phase II study (ETCTN 10558, NCT05724108) is currently recruiting patients to directly compare the efficacy of Triapine in combination with 177Lu-DOTATATE versus 177Lu-DOTATATE alone in well-differentiated SSTR-positive NETs83.
Mammalian target of rapamycin (mTOR) inhibitors
The mTOR pathway, which regulates cell growth, metabolism, and DNA damage response, has emerged as a target for enhancing PRRT efficacy84,85. Everolimus, an mTOR inhibitor, is FDA approved for the treatment of progressive pNETs, with the RADIANT-3 trial demonstrating a median PFS of 11.0 months compared to 4.6 months with placebo (HR 0.35; P < 0.001)86. Grade 3 or 4 adverse events (AEs), including anemia (6%) and hyperglycemia (5%), have been reported, with hyperglycemia potentially becoming more severe in patients with preexisting glucose intolerance86. The rationale for combining everolimus with 177Lu-DOTATATE lies in its ability to inhibit cell proliferation and reduce angiogenesis, which can enhance the cytotoxic effects of radiation87. However, conflicting results from preclinical studies also demonstrated increased metastasis in a pancreatic tumor mouse model88. Ongoing trials, such as the Phase I/II study (NCT03629847), aim to assess the efficacy of this combination in patients with unresectable Grade 1 and 2 NETs from the gastrointestinal tract, lungs, and pancreas89.
Tyrosine kinase inhibitors
Sunitinib, a tyrosine kinase inhibitor (TKI) targeting multiple enzymes involved in tumor cell proliferation and angiogenesis, improved median PFS (11.4 vs. 5.5 months, HR 0.42, P < 0.001) and OS (HR 0.41, P = 0.02) in patients with pNETs (NCT00428597)90. The rationale for combining sunitinib with 177Lu-DOTATATE is based on its ability to enhance the tumor vasculature and oxygenation, possibly increasing radiation sensitivity91,92. The study was stopped early due to higher deaths in the placebo group (25% vs. 10%), reinforcing Sunitinib’s benefit90. The ongoing ETCTN 10479 Phase I trial (NCT04919226) is assessing the maximum tolerated dose (MTD) of this combination and its impact on AEs90.
Structural modifications of 177Lu-DOTATATE
177Lu-DOTA-EB-TATE: reducing renal toxicity
Efforts to improve the efficacy of 177Lu-DOTATATE while minimizing toxicities have led to the development of structural modifications aimed at addressing its limitations. One significant challenge with 177Lu-DOTATATE has been its rapid renal cleance, which contributes to increased renal toxicity and reduced tumor retention. To overcome this issue, 177Lu-DOTA-EB-TATE, an albumin-binding variant that incorporates Evans blue has been developed. This modification extends the half-life of the radionuclide in the bloodstream, while reducing renal toxicity93–96.A Phase I trial (NCT03478358) reported a mPFS of 36 months, an improvement over the 28.4 months observed with conventional 177Lu-DOTATATE97,98. The toxicity profile was favorable, with no Grade 4 events and limited Grade 3 hematotoxicity (13.3% thrombocytopenia, 3.3% anemia) and Grade 3 hepatotoxicity (3.3%). Notably, no Grade 2/3/4 nephrotoxicity was observed and no cases of leukemia or myelodysplastic syndrome were reported during follow-up98. An ongoing Phase I trial (NCT05475210) continues to evaluate its safety and optimal dosage in patients with untreated advanced GEP-NETs99.
177Lu-DOTATOC: an alternative with unique advantages
177Lu-DOTATOC, containing the modified somatostatin analog edotreotide, serves as an alternative to 177Lu-DOTATATE, which may be particularly valuable during pharmaceutical shortages64. A Phase II study investigating 177Lu-DOTATOC in patients with GEP-NETs reported a mPFS of 29 months and OS of 47 months100. The ORR was 16%, with a disease control rate of 82%100. Adverse events were mostly mild (Grade 1 or 2), with Grade 3 or 4 toxicity occurring in less than 10% of patients, primarily hematological, with no Grade 3 or 4 renal toxicity101.The ongoing Phase III COMPOSE trial (NCT04919226) aims to compare 177Lu-DOTATOC to standard care options such as CAPTEM, everolimus, and FOLFOX in patients with aggressive Grade 2/3 SSTR-positive GEP-NETs102. Results from this trial will be critical in determining cost-effectiveness and clinical utility, especially given the lower production costs and greater availability of 177Lu-DOTATOC compared to 177Lu-DOTATATE64.The Phase III COMPETE trial (NCT03049189), is evaluating 177Lu-DOTATOC versus everolimus in SSTR-positive GEP-NETs, and has met its primary objective of prolonging median PFS to 23.9 months compared to 14.1 months on everolimus (p value = 0.022)103. While OS data is still maturing, the trial marks the first instance of a targeted radiopharmaceutical therapy outperforming a molecular therapy in this setting, with plans for a U.S. New Drug Application submission in 2025104,105.
Targeted alpha therapy
Targeted Alpha Therapy (TAT) presents a compelling alternative to beta-emitting PRRT by utilizing alpha particles, that cause highly localized, irreparable DNA double-strand breaks due to their large size and short travel range than beta particles106. In contrast, beta emitters predominantly induce single-strand DNA breaks, that are more easily repaired, potentially contributing to tumor resistance107. Due to their limited tissue penetration, alpha particles provide precise tumor targeting with minimal off-target effects108,109, makes TAT particularly attractive for treating liver metastases (Fig. 2)110. Although preliminary results are encouraging, TAT remains in the early stages of clinical development111. Ongoing trials are exploring the safety and efficacy of these agents (Table 3).
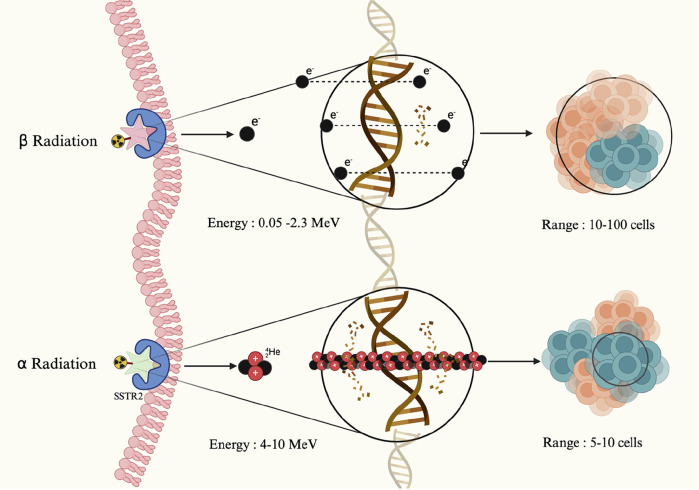
Fig. 2. Beta and alpha radiation in targeted radionuclide therapy.
This figure illustrates the mechanisms of targeted radionuclide therapy via somatostatin receptor type 2 (SSTR2). The top pathway depicts beta radiation, which affects a broader area, targeting 10-100 cells and causing single-stranded DNA damage through the emission of electrons with a longer range. In contrast, the bottom pathway highlights alpha radiation, which impacts a much smaller area, targeting only 5-10 cells. Alpha particles deliver higher energy with a shorter range, resulting in highly localized and concentrated DNA damage, leading to irreparable double-strand breaks in tumor cells. Created in Biorender Karimi, A. (2025).
Table 3.
Ongoing TAT trials
| Clinical trial registry number/phase | Radiopharmaceutical | Number enrolled | Duration | Primary endpoint |
|---|---|---|---|---|
| NCT05153772/Phase II trial | 212Pb-DOTAMTATE | 69 | 2021–2026 | Assess Safety and efficiency |
| NCT05636618/phase 1/2a | 212Pb-VMT-α-NET | 10 | 2022–2027 |
Assess safety & RP2D |
| NCT05477576/ACTION-1/Phase 3/Randomized Open-label | 225Ac -DOTATATE (RYZ101) | 288 |
TEAEs & AE |
213-bismuth (213Bi)
213Bi-DOTATOC has shown considerable promise in preclinical studies112, significantly reducing tumor growth without causing chronic kidney damage or hematologic toxicity113. In a Phase I trial involving seven patients with pNETs that progressed after 177Lu-DOTATOC therapy, 213Bi-DOTATOC induced sustained tumor responses with lower acute hematotoxicity and moderate chronic kidney toxicity, suggesting it may offer a favorable safety profile for tumors resistant to beta radiation114. In a related clinical study, Giesel et al. demonstrated that contrast-enhanced ultrasound is a reliable modality for assessing treatment response in patients treated with 213Bi-DOTATOC by detecting early changes in tumor microcirculation perfusion115. The study found that 66% of patients treated with 213Bi-DOTATOC showed a significant reduction in enhancement, with more pronounced declines observed during short-term follow-up, compared to 33% of patients treated with 177Lu/90Y-DOTATOC115.
225-actinium (225Ac)
225Ac, an alpha-emitter with a longer half-life of 9.9 days, has become a focal point in TAT research. Preclinical studies in mouse models of liver metastases from pancreatic NETs demonstrated that 225Ac-DOTATOC significantly improved survival compared to non-radioactive DOTATOC116. In a study by Ballal et al., patients who had achieved disease control with prior 177Lu-DOTATOC therapy and were subsequently retreated with 225Ac-DOTATATE showed the best outcomes, with a 24-month OS rate of 95%117. The ongoing ACTION-1 Phase III clinical trial (NCT05477576) is evaluating 225Ac -DOTATATE in patients with well-differentiated GEP-NETs that have progressed following 177Lu-DOTATATE therapy118. Preliminary data from the Phase 1b portion of the trial reported promising efficacy, with an ORR of 29.4%, including a complete response in one patient, partial responses in four patientsand stable disease in 41.2% of participants. While the median PFS was not yet estimable, early results suggest durable responses119. A notable case report further demonstrated the potential of 225Ac in tandem-PRRT approaches. A patient with rapidly progressing pNET and extensive metastases demonstrated an exceptional response to tandem-PRRT using 177Lu-DOTA-LM3 and 225Ac-DOTA-LM3 after exhausting prior chemotherapy and 177Lu-DOTATATE PRRT options120. This combination therapy led to significant improvements across all metastatic sites, particularly in the liver, highlighting the synergistic effects of alpha and beta emitters120.
212-lead (212Pb)
212Pb is another alpha-emitter under investigation in combination with various chelating agents. In a study by Delpasand et al, GEP-NET patients receiving 212Pb-DOTAMTATE exhibited an 80% objective radiologic response, with the treatment being well tolerated and no severe AEs reported121. The ongoing ALPHAMEDIX02 Phase II trial (NCT05153772) is investigating ²¹²Pb-DOTAMTATE in SSTR-positive, PRRT-naïve NET patients. Pooled results from Phase I/II trials demonstrated a high ORR of 56.8%, with the median response duration of 14 months (range: 5–22 months), indicating a promising efficacy profile122. Additionally, a Phase I/IIa study (NCT05636618) is evaluating 212Pb VMT-alpha-NET, which employs a novel polyethylene linker to conjugate octreotide to 212Pb for advanced SSTR2-positive, PRRT-naïve NET patients123. In this small cohort, 9 out of 10 patients (90%) who completed all treatment cycles remained progression-free at the last follow-up, with a median follow-up duration of 17.4 months (range: 9–26 months)124. Common AEs included nausea (31%) and alopecia (25%), while Grade 3 toxicities occurred in 5% of patients, with no reported Grade 4 events124.
While TAT offers significant advantages over beta-emitting PRRT—including higher linear energy transfer, superior tumor control, and the ability to eradicate targeted metastatic lesions—its widespread clinical application faces substantial challenges125,126. Radionuclide availability and high production costs is a major hurdle, as alpha emitters require complex production processes and are limited to a few specialized facilities worldwide127. From a logistical perspective, the short half-lives of many alpha emitters complicate transport, storage, and coordination between production sites and clinical centers128. Moreover, the short path length of alpha particles necessitates precise targeting to minimize off-target effects, requiring advanced imaging techniques to ensure optimal therapeutic index129,130. To overcome these barriers, strategies such as streamlined cost-effective production processes and establishing international guidelines for the safe and efficient use of TAT will be crucial for its integration into standard clinical practice130.
SSTR2 antagonists: advancing imaging and therapy
Historically, it was believed that internalization of radiotracers was necessary for effective SSTR-targeted therapy and imaging. However, research by Ginj et al. challenged this paradigm by demonstrating that SSTR2 antagonists could offer superior efficacy compared to agonists131. Antagonists bind to a greater number of receptor sites on the tumor surface without internalizing, thereby providing higher binding affinity and specificity131,132. Several SSTR2 antagonists have been developed and tested in preclinical and clinical studies.
177Lu-DOTA-JR11
177Lu-DOTA-JR11, an SSTR2 antagonist, has emerged as a leading candidate for clinical translation due to its ability to deliver high radiation doses selectively to SSTR-positive tumors29. Its companion imaging agent, 68Ga-NODAGA-JR11, has demonstrated superior diagnostic accuracy compared to 68Ga-DOTATATE133,134. In two human studies, 68Ga-NODAGA-JR11 outperformed 68Ga-DOTATATE in sensitivity (91.7% vs. 77.2%) and lesion detection (1095 vs. 1003 lesions, P = 0.007), providing better image contrast, particularly in patients with low to intermediate-grade GEP-NETs. Moreover, 68Ga-NODAGA-JR11 exhibited a significantly higher target-to-background ratio in liver lesions (6.4 ± 8.7 vs. 3.1 ± 2.6, P = 0.000), thereby enhancing its diagnostic accuracy and potentially enabling more accurate staging and treatment planning133,134.
LM3,4
SSTR2 antagonists LM3 and LM4 have demonstrated significant potential in both imaging and therapeutic applications. In clinical settings, 177Lu-DOTA-LM3 PRRT achieved a disease control rate of 85.1%, with 36.2% of patients experiencing a partial response135. The treatment was well tolerated, with only mild nausea (9.8%) and thrombocytopenia (5.9%) reported, and no cases of severe nephrotoxicity, hepatotoxicity, or hematologic toxicity135. In diagnostic applications, 68Ga-NODAGA-LM3 and 68Ga-DOTA-LM3 have demonstrated significant accuracy for NET detection compared to 68Ga-DOTATATE136. 68Ga-NODAGA-LM3 showed significantly higher tumor uptake (SUVmax: 29.1 vs. 21.6, P < 0.05) and an improved tumor-to-liver ratio (5.0 vs. 2.9, P < 0.05), thereby enhancing its diagnostic accuracy. Similarly, 68Ga-DOTA-LM3 demonstrated a higher tumor-to-liver ratio (5.2 vs. 2.1, P < 0.05) while maintaining lower uptake in normal organs, thus improving image contrast and lesion detection136. LM4, a modified version of LM3, has shown enhanced tumor retention and reduced kidney uptake in preclinical studies137. In human studies, 68Ga-DATA5m-LM4 demonstrated high tumor uptake with SUVmax reaching 167.93 (mean ± SD: 44.47 ± 36.22). When compared directly to 68Ga-DOTA-TATE, 68Ga-DATA5m-LM4 exhibited significantly lower uptake in normal liver parenchyma (SUVmean: 3.90 ± 0.88 vs. 9.12 ± 3.64, P < 0.000001) as well as in the thyroid, pancreas, and spleen (P < 0.05). This favorable biodistribution, characterized by high tumor contrast and minimal background uptake, underscores the potential of 68Ga-DATA5m-LM4 as an enhanced imaging agent for NET staging138.
Challenges and future directions
Theranostics has significantly transformed the management of pNETs by integrating molecular imaging with targeted therapy, thereby enhancing both diagnostic precision and therapeutic efficacy. Beta-emitting PRRT, particularly 177Lu-DOTATATE, is becoming a cornerstone treatment with positive results in both first- and second-line settings. However, the limited response rates and development of treatment resistance have highlighted the urgent need for alternative strategies. In this review, we have explored combination approaches involving other systemic therapies as well as emerging radiopharmaceuticals including TATs and SSTR2 antagonists. While these innovative therapies offer substantial potential, their widespread clinical implementation faces several barriers, including combined toxicities, challenges in radionuclide production and transport, high costs, and the necessity for specialized infrastructure.
Biomarker-driven patient selection represents a critical area for future research to ensure that theranostic approaches are targeted to the most suitable candidates. Currently, patient selection is primarily based on the intensity of SSTR expression assessed through PET imaging and the exclusion of patients with preexisting glucose intolerance, anemia, thrombocytopenia, or renal disease. However, emerging blood-based biomarkers offer promising alternatives for predicting PRRT sensitivity and treatment efficacy. For instance, the NETest, a 51-multigene assay utilizing PCR analysis of specific NET circulating transcripts, generates a score reflecting real-time tumor activity and has shown potential in predicting PRRT outcomes130,139. Similarly, an inflammation-based index, derived from serum C-reactive protein and albumin levels is being investigated as a prognostic tool for assessing survival and treatment response in patients with metastatic NETs140. The development of such biomarkers could significantly enhance patient stratification.
Another critical challenge that demands attention is the long-term safety of these therapies, particularly regarding hematologic and renal toxicity48,141. The cumulative effects of radiation-induced toxicity remain inadequately explored, necessitating prolonged follow-up studies and the development of risk mitigation strategies57. Advances in personalized dosimetry could play a pivotal role in this regard by allowing for individualized radiation doses that maximize therapeutic benefits while minimizing toxicity risks97. Optimizing dosimetric approaches could enable the safe administration of higher radiation doses to patients with aggressive tumor phenotypes or poor prognostic indicators .
In conclusion, the continued integration of molecular imaging and novel radiopharmaceuticals holds the potential to advance pNET treatment by creating personalized therapeutic strategies. The path forward will require a multidisciplinary approach, but the promising clinical outcomes to date underscore the transformative potential of these innovative theranostic strategies.
Acknowledgements
Biorender was used to create the schematics.
Author contributions
D.S. conceived the study, conducted literature reviews, edited figures and tables, wrote, and edited the manuscript; A.K. conducted literature reviews, edited figures and tables, wrote, and edited the manuscript. C.B. conducted literature reviews, edited figures and tables, wrote, and edited the manuscript, E.O. edited the manuscript.
Data availability
No datasets were generated or analysed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McKenna, L. R. & Edil, B. H. Update on pancreatic neuroendocrine tumors. Gland Surg.3, 258–275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill, J. S. et al. Pancreatic neuroendocrine tumors: The impact of surgical resection on survival. Cancer115, 741–751 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria, K. Y. et al. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J. Gastrointest. Surg.11, 1460–1467 (2007). Novdiscussion 1467-9. [DOI] [PubMed] [Google Scholar]
- 4.Pathak, S., Starr, J. S., Halfdanarson, T., Sonbol, M. B. Understanding the increasing incidence of neuroendocrine tumors. 10.1080/17446651.2023.2237593 (2023). [DOI] [PubMed]
- 5.Cloyd, J. M. Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J. Gastroenterol.21, 9512–9525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liaquat M. T., Kasi A. Pancreatic Islet Cell Cancer. StatPearls. StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC.; (2023). [PubMed]
- 7.Ampofo, E., Nalbach, L., Menger, M. D. & Laschke, M. W. Regulatory mechanisms of somatostatin expression. Int. J. Mol. Sci.21, 4170 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian, Z. R. et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas45, 1386–1393 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu, L. M. et al. Differences and similarities in the clinicopathological features of pancreatic neuroendocrine tumors in China and the United States: A multicenter study. Med. (Baltim.)95, e2836 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaemmerer, D. et al. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget6, 27566–27579 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomes-Porras M., Cárdenas-Salas, J., Álvarez-Escolá, C. Somatostatin analogs in clinical practice: A review. Int. J. Mol. Sci. 29;21 10.3390/ijms21051682 (2020). [DOI] [PMC free article] [PubMed]
- 12.Mizutani, G. et al. Expression of somatostatin receptor (SSTR) subtypes (SSTR-1, 2A, 3, 4 and 5) in neuroendocrine tumors using real-time RT-PCR method and immunohistochemistry. Acta Histochem Cytochem45, 167–176 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanli, Y. et al. Neuroendocrine tumor diagnosis and management: 68Ga-DOTATATE PET/CT. Am. J. Roentgenol.211, 267–277 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Khanna, L. et al. Pancreatic neuroendocrine neoplasms: 2020 update on pathologic and imaging findings and classification. Radiographics40, 1240–1262 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Hu, Y. et al. Role of somatostatin receptor in pancreatic neuroendocrine tumor development, diagnosis, and therapy. Front Endocrinol. (Lausanne)12, 679000 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, L., Ito, T. & Jensen, R. T. Imaging of pancreatic neuroendocrine tumors: Recent advances, current status, and controversies. Expert Rev. Anticancer Ther.18, 837–860 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krebs, S. et al. Biodistribution and radiation dose estimates for 68Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging46, 677–685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker, R. C. et al. Measured human dosimetry of68Ga-DOTATATE. J. Nucl. Med.54, 855–860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, I. et al. Comparison of diagnostic sensitivity and quantitative indices between 68Ga-DOTATOC PET/CT and 111In-pentetreotide SPECT/CT in neuroendocrine tumors: A preliminary report. Nucl. Med. Mol. Imaging49, 284–290 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deppen, S. A. et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J. Nucl. Med.57, 708–714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malan, N., & Vangu, M.-D.-T. Normal variants, pitfalls and artifacts in Ga-68 DOTATATE PET/CT imaging. Front. Nuclear Med. 2022;2 10.3389/fnume.2022.825486 (2022). [DOI] [PMC free article] [PubMed]
- 22.Poeppel, T. D. et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med.52, 1864–1870 (2011). [DOI] [PubMed] [Google Scholar]
- 23.Deroose, C. M. et al. Molecular imaging of gastroenteropancreatic neuroendocrine tumors: Current status and future directions. J. Nucl. Med57, 1949–1956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeifer, A. et al. 64Cu-DOTATATE PET for neuroendocrine tumors: A prospective head-to-head comparison with 111In-DTPA-Octreotide in 112 patients. J. Nucl. Med56, 847–854 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Delpassand, E. S. et al. (64)Cu-DOTATATE PET/CT for imaging patients with known or suspected somatostatin receptor-positive neuroendocrine tumors: Results of the first U.S. prospective, reader-masked clinical trial. J. Nucl. Med. 61, 890–896 (2020). [DOI] [PubMed]
- 26.Johnbeck, C. B., Knigge, U. & Kjær, A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: Current status and review of the literature. Future Oncol.10, 2259–2277 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Leupe, H. et al. (18)F-labeled somatostatin analogs as PET tracers for the somatostatin receptor: Ready for clinical use. J. Nucl. Med. 64, 835–841 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Johnbeck, C. B. et al. Head-to-head comparison of (64)Cu-DOTATATE and (68)Ga-DOTATOC PET/CT: A prospective study of 59 patients with neuroendocrine tumors. J. Nucl. Med.58, 451–457 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Fani, M., Nicolas, G. P. & Wild, D. Somatostatin receptor antagonists for imaging and therapy. J. Nucl. Med58, 61s–66s (2017). [DOI] [PubMed] [Google Scholar]
- 30.Zhu, W. et al. Head-to-head comparison of 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT in patients with metastatic, well-differentiated neuroendocrine tumors: A prospective study. J. Nucl. Med.61, 897–903 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majala, S. et al. Prediction of the aggressiveness of non-functional pancreatic neuroendocrine tumors based on the dual-tracer PET/CT. EJNMMI Res.9, 116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paiella, S. et al. Dual-Tracer (68Ga-DOTATOC and 18F-FDG-)-PET/CT Scan and G1-G2 nonfunctioning pancreatic neuroendocrine tumors: A single-center retrospective evaluation of 124 nonmetastatic resected cases. Neuroendocrinology112, 143–152 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Mapelli, P. et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET improve preoperative evaluation of aggressiveness in resectable pancreatic neuroendocrine neoplasms. Diagnostics (Basel). 28;11 10.3390/diagnostics11020192 (2021). [DOI] [PMC free article] [PubMed]
- 34.Mapelli, P. et al. <strong>Dual tracer 68Ga-DOTATOC and 18F-FDG PET/CT for preoperative risk evaluation in pancreatic neuroendocrine tumours: explorative texture analysis</strong>. J. Nucl. Med.60, 476–476 (2019). [Google Scholar]
- 35.Zhou, Y. et al. Heterogeneous Uptake of 68 Ga-DOTATATE and 18 F-FDG in initial diagnosed neuroendocrine tumors patients : Which patients are suitable for dual-tracer PET imaging? Clin. Nucl. Med49, 516–520 (2024). [DOI] [PubMed] [Google Scholar]
- 36.Gao, J., Xu, S., Ju, H., Pan, Y. & Zhang, Y. The potential application of MR-derived ADCmin values from 68Ga-DOTATATE and 18F-FDG dual tracer PET/MR as replacements for FDG PET in assessment of grade and stage of pancreatic neuroendocrine tumors. EJNMMI Res.13, 10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jawlakh, H., Velikyan, I., Welin, S. & Sundin, A. (68) Ga-DOTATOC-PET/MRI and (11) C-5-HTP-PET/MRI are superior to (68) Ga-DOTATOC-PET/CT for neuroendocrine tumour imaging. J Neuroendocrinol33, e12981 (2021). [DOI] [PubMed]
- 38.Halfdanarson, T. R., Rabe, K. G., Rubin, J. & Petersen, G. M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol.19, 1727–1733 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma, Z. Y. et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol.26, 2305–2322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito, T. et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: Diagnosis, treatment, and follow-up: a synopsis. J. Gastroenterol.56, 1033–1044 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rinke, A. et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results of long-term survival. Neuroendocrinology104, 26–32 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Caplin, M. E. et al. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: Final results of the CLARINET open-label extension study. Endocrine71, 502–513 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caplin, M. E. et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 371, 224–233 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Das, S., Al-Toubah, T., El-Haddad, G. & Strosberg, J. (177)Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol.13, 1023–1031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strosberg, J. R. et al. 177Lu-Dotatate plus long-acting octreotide versus high‑dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol.22, 1752–1763 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Strosberg, J. R. et al. (177)Lu-Dotatate plus long-acting octreotide versus high‑dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol.22, 1752–1763 (2021). [DOI] [PubMed] [Google Scholar]
- 47.(FDA) FaDA. FDA approves lutetium Lu 177 dotatate for treatment of GEP-NETS. Accessed 01/26/2018, 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lutetium-lu-177-dotatate-treatment-gep-nets.
- 48.Alsadik, S. et al. Safety of peptide receptor radionuclide therapy with (177)Lu-DOTATATE in neuroendocrine tumor patients with chronic kidney disease. J. Nucl. Med63, 1503–1508 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brabander, T. et al. Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin. Cancer Res. 23, 4617–4624 (2017). [DOI] [PubMed]
- 50.Singh, S. et al. [177Lu]Lu-DOTA-TATE plus long-acting octreotide versus high‑dose long-acting octreotide for the treatment of newly diagnosed, advanced grade 2–3, well-differentiated, gastroenteropancreatic neuroendocrine tumours (NETTER-2): An open-label, randomised, phase 3 study. Lancet403, 2807–2817 (2024). [DOI] [PubMed] [Google Scholar]
- 51.Scott, R. Lutetium Lu 177 dotatate maintains PFS, ORR benefits across subgroups in SSTR+ GEP-NETs. Onclive. Accessed 6/26, 2024. https://www.onclive.com/view/lutetium-lu-177-dotatate-maintains-pfs-orr-benefits-across-subgroups-in-sstr-gep-nets.
- 52.Strosberg, J. et al. Phase 3 trial of (177)Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med.376, 125–135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodrigues, M. et al. Long-term survival and value of (18)F-FDG PET/CT in patients with gastroenteropancreatic neuroendocrine tumors treated with second peptide receptor radionuclide therapy course with (177)Lu-DOTATATE. Life (Basel). 4;11 10.3390/life11030198 (2021). [DOI] [PMC free article] [PubMed]
- 54.Vaughan, E. et al. Retreatment with peptide receptor radionuclide therapy in patients with progressing neuroendocrine tumours: Efficacy and prognostic factors for response. Br. J. Radiol.91, 20180041 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strosberg, J., Leeuwenkamp, O. & Siddiqui, M. K. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat. Rev.93, 102141 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Puliani, G. et al. New insights in PRRT: Lessons From 2021. Mini review. Front. Endocrinol. 2022;13 10.3389/fendo.2022.861434 (2022). [DOI] [PMC free article] [PubMed]
- 57.Liberini, V. et al. The challenge of evaluating response to peptide receptor radionuclide therapy in gastroenteropancreatic neuroendocrine tumors: The present and the future. Diagnostics10, 1083 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ballal, S., Yadav, M. P., Damle, N. A., Sahoo, R. K. & Bal, C. Concomitant 177Lu-DOTATATE and capecitabine therapy in patients with advanced neuroendocrine tumors: A long-term-outcome, toxicity, survival, and quality-of-life study. Clin. Nucl. Med. 42, e457–e466 (2017). [DOI] [PubMed] [Google Scholar]
- 59.van Essen, M. et al. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging35, 743–748 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li, L. Y., Guan, Y. D., Chen, X. S., Yang, J. M. & Cheng, Y. DNA repair pathways in cancer therapy and resistance. Front Pharm.11, 629266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kunz, P. L. et al. Randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors (ECOG-ACRIN E2211). J. Clin. Oncol.41, 1359–1369 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pavlakis N. Capecitabine ON temozolomide radionuclide therapy octreotate lutetium-177 NeuroEndocrine Tumours Study (CONTROL NETS). clinicaltrials.gov. Accessed 5/7, 2022. https://clinicaltrials.gov/study/NCT02358356.
- 63.Group AG-IT. Capecitabine ON Temozolomide Radionuclide Therapy Octreotate Lutetium-177 NeuroEndocrine Tumours Study. My Cancer Genome. 2017. Accessed 11/22/2017, 2017. https://clinicaltrials.gov/show/NCT02358356
- 64.https://clin.larvol.com/trial-detail/NCT02358356.
- 65.Yordanova A., & Ahmadzadehfar, H. Combination therapies with PRRT. Pharmaceuticals (Basel). 30;14 10.3390/ph14101005 (2021). [DOI] [PMC free article] [PubMed]
- 66.O’Neill, E. et al. Imaging DNA damage repair in vivo after (177)Lu-DOTATATE therapy. J. Nucl. Med61, 743–750 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ray Chaudhuri, A. & Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol.18, 610–621 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camero, S. et al. PARP inhibitors affect growth, survival and radiation susceptibility of human alveolar and embryonal rhabdomyosarcoma cell lines. J. Cancer Res Clin. Oncol.145, 137–152 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lesueur, P. et al. Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies. Oncotarget8, 69105–69124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Purohit, N. K. et al. Potentiation of (177)Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget9, 24693–24706 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nonnekens, J. et al. Potentiation of peptide receptor radionuclide therapy by the PARP inhibitor olaparib. Theranostics6, 1821–1832 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cullinane, C. et al. Enhancing the anti-tumour activity of (177)Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci. Rep.10, 10196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin, F. et al. <strong>Phase 2 study of Lu-177-DOTATATE in combination with olaparib in patients with metastatic or inoperable GI neuroendocrine tumors - first results on safety and efficacy</strong>. J. Nucl. Med.64, P1299–P1299 (2023). (supplement 1). [Google Scholar]
- 74.Hallqvist, A. et al. Optimizing the Schedule of PARP Inhibitors in Combination with (177)Lu-DOTATATE: A dosimetry rationale. Biomedicines. Oct 29;9 10.3390/biomedicines9111570 (2021). [DOI] [PMC free article] [PubMed]
- 75.Zhou, J. X., Feng, L. J. & Zhang, X. Risk of severe hematologic toxicities in cancer patients treated with PARP inhibitors: A meta-analysis of randomized controlled trials. Drug Des. Devel Ther.11, 3009–3017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohindroo, C. et al. Prevalence of germline variants in patients with pancreatic neuroendocrine tumors. J. Clin. Oncol.41, 4135–4135 (2023). [Google Scholar]
- 77.Chauhan, A. et al. ETCTN 10450: A phase I trial of peposertib and lutetium 177 DOTATATE in well-differentiated somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol.41, TPS658–TPS658 (2023). [Google Scholar]
- 78.Romesser, P. B. et al. A phase Ib study of the DNA-PK inhibitor peposertib combined with neoadjuvant chemoradiation in patients with locally advanced rectal cancer. Clin. Cancer Res.30, 695–702 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samuels, M. et al. A phase 1 study of the DNA-PK inhibitor peposertib in combination with radiation therapy with or without cisplatin in patients with advanced head and neck tumors. Int. J. Radiat. Oncol. Biol. Phys.118, 743–756 (2024). [DOI] [PubMed] [Google Scholar]
- 80.van Bussel, M. T. J. et al. A first-in-man phase 1 study of the DNA-dependent protein kinase inhibitor peposertib (formerly M3814) in patients with advanced solid tumours. Br. J. Cancer124, 728–735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Testing the Addition of An Anti-cancer Drug, M3814 (Peposertib), to the Usual Radiation-Based Treatment (Lutetium Lu 177 Dotatate) for Pancreatic Neuroendocrine Tumors. Clinicaltrials.gov. Accessed 3/6, 2025. https://clinicaltrials.gov/study/NCT04750954.
- 82.Chauhan, A. et al. Abstract CT194: ETCTN 10388: a first in human phase I trial of triapine and lutetium Lu 177 DOTATATE in well-differentiated somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Cancer Res.83, CT194–CT194 (2023). [Google Scholar]
- 83.Chauhan, A. et al. Pharmacokinetics and RP2D analysis from ETCTN 10388: A phase I trial of triapine and lutetium Lu-177 dotatate in well-differentiated somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs). J. Clin. Oncol.41, 648 (2023). [Google Scholar]
- 84.Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell168, 960–976 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma, Y., Vassetzky, Y. & Dokudovskaya, S. mTORC1 pathway in DNA damage response. Biochim Biophys. Acta Mol. Cell Res1865, 1293–1311 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Yao, J. C. et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 364, 514–523 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnbeck, C. B. et al. 18F-FDG and 18F-FLT-PET imaging for monitoring everolimus effect on tumor-growth in neuroendocrine tumors: studies in human tumor xenografts in mice. PLoS One9, e91387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pool, S. E. et al. mTOR inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res73, 12–18 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Aljubran, A. H. et al. Combination of everolimus and lu-177 PRRT in treatment of G1-2 neuroendocrine tumors (NET): Phase 1-2 study. J. Clin. Oncol.37, 386–386 (2019).30589600 [Google Scholar]
- 90.Raymond, E. et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med.364, 501–513 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Aggarwal, P. et al. Sunitinib in tandem with 177 Lu-DOTATATE therapy in advanced pancreatic neuroendocrine tumor : A new treatment approach. Clin. Nucl. Med49, e85–e86 (2024). [DOI] [PubMed] [Google Scholar]
- 92.Melincovici, C. S. et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol.59, 455–467 (2018). [PubMed] [Google Scholar]
- 93.Wang, H. et al. Response to single low-dose (177)Lu-DOTA-EB-TATE treatment in patients with advanced neuroendocrine neoplasm: A prospective pilot study. Theranostics8, 3308–3316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tian, R. et al. Evans blue attachment enhances somatostatin receptor subtype-2 imaging and radiotherapy. Theranostics8, 735–745 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hänscheid, H., Hartrampf, P. E., Schirbel, A., Buck, A. K. & Lapa, C. Intraindividual comparison of [177Lu]Lu-DOTA-EB-TATE and [177Lu]Lu-DOTA-TOC. Eur. J. Nucl. Med. Mol. Imaging48, 2566–2572 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu, Q. et al. Dose escalation of an Evans blue-modified radiolabeled somatostatin analog (177)Lu-DOTA-EB-TATE in the treatment of metastatic neuroendocrine tumors. Eur. J. Nucl. Med Mol. Imaging47, 947–957 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harris, P. E. & Zhernosekov, K. The evolution of PRRT for the treatment of neuroendocrine tumors; What comes next? Front Endocrinol. (Lausanne)13, 941832 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang, Y. et al. Safety and efficacy of peptide receptor radionuclide therapy with (177)Lu-DOTA-EB-TATE in patients with metastatic neuroendocrine tumors. Theranostics12, 6437–6445 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bodei L. A Phase I Study of 177Lu-DOTA-EB-TATE in people with advanced neuroendocrine cancers. Phase 1 study. https://www.mskcc.org/cancer-care/clinical-trials/21-362.
- 100.Sundlöv, A. et al. Phase II trial demonstrates the efficacy and safety of individualized, dosimetry-based (177)Lu-DOTATATE treatment of NET patients. Eur. J. Nucl. Med Mol. Imaging49, 3830–3840 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sundlöv, A. et al. Phase II trial demonstrates the efficacy and safety of individualized, dosimetry-based 177Lu-DOTATATE treatment of NET patients. Eur. J. Nucl. Med. Mol. Imaging49, 3830–3840 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Halfdanarson, T.R. et al. COMPOSE: Pivotal Phase III Trial for Well-Differentiated Aggressive Grade 2/3 Gastroenteropancreatic Neuroendocrine Tumors Comparing 177Lu-edotreotide with Best Standard of Care. Endocrine Abstracts. https://www.endocrine-abstracts.org/ea/0089/ea0089t2.
- 103.ITM Presents Positive Topline Phase 3 COMPETE Trial Data with n.c.a. 177Lu-edotreotide (ITM-11), a targeted radiopharmaceutical therapy, in patients with grade 1 or 2 gastroenteropancreatic neuroendocrine tumors at the ENETS 2025 Conference. biospace. https://www.biospace.com/press-releases/itm-presents-positive-topline-phase-3-compete-trial-data-with-n-c-a-177lu-edotreotide-itm-11-a-targeted-radiopharmaceutical-therapy-in-patients-with-grade-1-or-2-gastroenteropancreatic-neuroendocrine-tumors-at-the-enets-2025-conference.
- 104.Hijioka, S. et al. Current status of medical treatment for gastroenteropancreatic neuroendocrine neoplasms and future perspectives. Jpn J. Clin. Oncol.51, 1185–1196 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.La Salvia, A., Espinosa-Olarte, P., Riesco-Martinez, M. D. C., Anton-Pascual, B., Garcia-Carbonero, R. Targeted cancer therapy: What’s new in the field of neuroendocrine neoplasms? Cancers (Basel). 3;13 10.3390/cancers13071701 (2021). [DOI] [PMC free article] [PubMed]
- 106.Poty, S., Francesconi, L. C., McDevitt, M. R., Morris, M. J. & Lewis, J. S. α-emitters for radiotherapy: From basic radiochemistry to clinical studies-part 2. J. Nucl. Med59, 1020–1027 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khazaei Monfared, Y. et al. DNA damage by radiopharmaceuticals and mechanisms of cellular repair. Pharmaceutics. 15 10.3390/pharmaceutics15122761 (2023). [DOI] [PMC free article] [PubMed]
- 108.Kassis, A. I. & Adelstein, S. J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 46, 4s–12s (2005). [PubMed] [Google Scholar]
- 109.Morgenstern, A. et al. An overview of targeted alpha therapy with (225)actinium and (213)bismuth. Curr. Radiopharm.11, 200–208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sgouros, G. et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med51, 311–328 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Makvandi, M. et al. Alpha-emitters and targeted alpha therapy in oncology: From basic science to clinical investigations. Target Oncol.13, 189–203 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Nayak, T. K. et al. Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(-)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl. Med Biol.34, 185–193 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Norenberg, J. P. et al. 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal Model. Clin. Cancer Res.12, 897–903 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Kratochwil, C. et al. ²¹³Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur. J. Nucl. Med Mol. Imaging41, 2106–2119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giesel, F. L. et al. Contrast-enhanced ultrasound monitoring of perfusion changes in hepatic neuroendocrine metastases after systemic versus selective arterial 177Lu/90Y-DOTATOC and 213Bi-DOTATOC radiopeptide therapy. Exp. Oncol.35, 122–126 (2013). [PubMed] [Google Scholar]
- 116.Lugat, A. et al. Survival impact of [(225)Ac]Ac-DOTATOC alpha-therapy in a preclinical model of pancreatic neuroendocrine tumor liver micrometastases. Eur. J. Nucl. Med. Mol. Imaging. 13;10.1007/s00259-024-06918-0 (2024). [DOI] [PubMed]
- 117.Ballal, S., Yadav, M. P., Tripathi, M., Sahoo, R. K., Bal, C. Survival outcomes in metastatic gastroenteropancreatic neuroendocrine tumor patients receiving concomitant (225)Ac-DOTATATE targeted alpha therapy and capecitabine: A real-world scenario management based long-term outcome study. J. Nucl. Med. 21;10.2967/jnumed.122.264043 (2022). [DOI] [PubMed]
- 118.Morris, M. et al. ACTION-1 phase Ib/3 trial of RYZ101 in somatostatin receptor subtype 2–expressing (SSTR2+) gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu somatostatin analogue (SSA) therapy: Initial safety analysis. J. Clin. Oncol.41, 4132–4132 (2023). [Google Scholar]
- 119.Strosberg, J. Phase 1b portion of the ACTION-1 phase 1b/3 trial of RYZ101 in gastroenteropancreatic neuroendocrine tumors (GEP-NET) progressing after 177Lu somatostatin analogue (SSA) therapy: Safety and efficacy findings. https://meetings.asco.org/abstracts-presentations/241453.
- 120.Perrone, E. et al. Impressive response to TANDEM peptide receptor radionuclide therapy with (177)Lu/(225)AcDOTA-LM3 somatostatin receptor antagonist in a patient with therapy-refractory, rapidly progressive neuroendocrine neoplasm of the pancreas. Diagnostics (Basel). 26;14 10.3390/diagnostics14090907 (2024). [DOI] [PMC free article] [PubMed]
- 121.Delpassand, E. S. et al. Targeted α-emitter therapy with (212)Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: First-in-humans dose-escalation clinical trial. J. Nucl. Med.63, 1326–1333 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strosberg, J. R. et al. Safety, tolerability and efficacy of 212Pb-DOTAMTATE as a targeted alpha therapy for subjects with unresectable or metastatic somatostatin receptor-expressing gastroenteropancreatic neuroendocrine tumors (SSTR+ GEP-NETs): A phase 2 study. J. Clin. Oncol.42, 4020–4020 (2024). [Google Scholar]
- 123.Prasad, V. et al. A Phase I/IIa of [212Pb]VMT-a-NET targeted alpha-particle therapy for advanced SSTR2 positive neuroendocrine tumors. J. Nucl. Med. 65, 242430 (2024).
- 124.Sen, I., Malik, D., Thakral, P. & Schultz, M. 212Pb-VMT-α-NET targeted alpha therapy in metastatic neuroendocrine tumors: First in human study on safety and efficacy. J. Nucl. Med. 65, 242556 (2024). [DOI] [PubMed]
- 125.Hooijman, E. L. et al. Implementing Ac-225 labelled radiopharmaceuticals: practical considerations and (pre-)clinical perspectives. EJNMMI Radiopharm. Chem.9, 9 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poty, S., Francesconi, L. C., McDevitt, M. R., Morris, M. J. & Lewis, J. S. α-emitters for radiotherapy: From basic radiochemistry to clinical studies-Part 1. J. Nucl. Med59, 878–884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tosato, M. et al. Alpha Atlas: Mapping global production of α-emitting radionuclides for targeted alpha therapy. Nucl. Med. Biol.142-143, 108990 (2025). [DOI] [PubMed] [Google Scholar]
- 128.Shober, M. Regulating alpha-emitting radioisotopes and specific considerations for actinium-225 containing actinium-227. Appl. Radiat. Isotopes187, 110337 (2022). [DOI] [PubMed] [Google Scholar]
- 129.Miederer, M. et al. Alpha-emitting radionuclides: Current status and future perspectives. Pharmaceuticals17, 76 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elgqvist, J., Frost, S., Pouget, J. P. & Albertsson, P. The potential and hurdles of targeted alpha therapy - clinical trials and beyond. Front Oncol.3, 324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ginj, M. et al. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA103, 16436–16441 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Minczeles, N. S., Hofland, J., de Herder, W. W. & Brabander, T. Strategies towards improving clinical outcomes of peptide receptor radionuclide therapy. Curr. Oncol. Rep.23, 46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin, Z. et al. Head-to-head comparison of (68)Ga-NODAGA-JR11 and (68)Ga-DOTATATE PET/CT in patients with metastatic, well-differentiated neuroendocrine tumors: Interim analysis of a prospective bicenter study. J. Nucl. Med64, 1406–1411 (2023). [DOI] [PubMed] [Google Scholar]
- 134.Nicolas, G. P. et al. Sensitivity comparison of (68)Ga-OPS202 and (68)Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: A prospective phase II imaging study. J. Nucl. Med. 59, 915–921 (2018). [DOI] [PubMed] [Google Scholar]
- 135.Baum, R. P., Zhang, J., Schuchardt, C., Müller, D. & Mäcke, H. First-in-humans study of the SSTR antagonist (177)Lu-DOTA-LM3 for peptide receptor radionuclide therapy in patients with metastatic neuroendocrine neoplasms: Dosimetry, safety, and efficacy. J. Nucl. Med. 62, 1571–1581 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu, W. et al. A prospective randomized, double-blind study to evaluate the diagnostic efficacy of (68)Ga-NODAGA-LM3 and (68)Ga-DOTA-LM3 in patients with well-differentiated neuroendocrine tumors: compared with (68)Ga-DOTATATE. Eur. J. Nucl. Med Mol. Imaging49, 1613–1622 (2022). [DOI] [PubMed] [Google Scholar]
- 137.Viswanathan, R. et al. Head-to-Head Comparison of SSTR Antagonist [(68)Ga]Ga-DATA(5m)-LM4 with SSTR Agonist [(68)Ga]Ga-DOTANOC PET/CT in patients with well differentiated gastroenteropancreatic neuroendocrine tumors: A prospective imaging study. Pharmaceuticals (Basel). 22;17 10.3390/ph17030275 (2024). [DOI] [PMC free article] [PubMed]
- 138.Zhang, J. et al. First-in-human study of an optimized, potential kit-type, SSTR Antagonist 68Ga-DATA5m-LM4 in patients with metastatic neuroendocrine tumors. J. Nucl. Med.15, 2510–2522 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bodei, L. et al. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: The NETest. Eur. J. Nucl. Med Mol. Imaging47, 895–906 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.CORRIGENDUM FOR “The inflammation-based index can predict response and improve patient selection in NETs treated with PRRT: A pilot study”. J. Clin. Endocrinol. Metab.104:2151-2151. 10.1210/jc.2019-00844 (2019). [DOI] [PubMed]
- 141.Bergsma, H. et al. Persistent hematologic dysfunction after peptide receptor radionuclide therapy with (177)Lu-DOTATATE: Incidence, course, and predicting factors in patients with gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 59, 452–458 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.