Abstract
Background
Nasopharyngeal carcinoma (NPC) is a common malignancy with a complex pathogenesis and diverse cellular composition. This study aimed to systematically analyze the transcriptional characteristics of different cell types in NPC and their roles in tumor development using single-cell transcriptomics.
Methods
The research team collected NPC and normal tissue samples, and performed in-depth single-cell sequencing analysis of the transcriptomes. Single-cell RNA sequencing was performed on the collected samples to obtain high-resolution transcriptional profiles of individual cells. This allowed the researchers to identify and characterize the diverse cell populations present within the NPC tumor microenvironment.
Results
The results showed that NPC samples contained multiple distinct cell subpopulations, including epithelial cells, immune cells (such as macrophages and T cells), endothelial cells, and stromal cells. These cell types exhibited marked differences in spatial distribution and transcriptional profiles, reflecting the high degree of heterogeneity within the tumor microenvironment. Further functional analysis revealed significant dysregulation of mitochondrial-related pathways, extracellular matrix-receptor interactions, as well as Wnt, Notch, and other signaling cascades in NPC.
Conclusion
This study employed single-cell transcriptomics to comprehensively elucidate the complexity of the NPC tumor microenvironment, providing new insights into the underlying mechanisms of disease pathogenesis and suggesting potential therapeutic targets. These findings lay the groundwork for the development of precision medicine approaches for NPC.
Keywords: Nasopharyngeal carcinoma, Single-cell transcriptomics, Tumor microenvironment, Cellular heterogeneity, Signaling pathways
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant nasopharyngeal tumor, which has distinct epidemiological characteristics and biological behaviors compared to other head and neck tumors. It is most common in Southern China and Southeast Asia. Although great progress has been made in the diagnosis and treatment of NPC, the underlying drivers of NPC pathogenesis remain poorly understood and a substantial proportion of patients continue to have a poor prognosis [1–4].
The rapid advancement of single-cell transcriptomic technologies over the past several years has enabled unprecedented resolution of the complexity of the tumor microenvironment. This study sought to utilize single-cell transcriptomics to provide a comprehensive examination of the transcriptional properties of disparate cell types in NPC samples, and explore the potential functional roles of these cellular populations in the development of disease. Aim to deliver novel findings which can help us understand the pathogenesis of NPC and uncover possible therapeutic targets [5–8]. Mendelian randomization, a robust method of causal inference, has also been increasingly utilized in cancer research, including NPC studies, has also been increasingly applied in cancer research, including studies on NPC [9–12]. By leveraging genetic variants as instrumental variables, Mendelian randomization can help establish the causal relationships between genetic factors, cellular characteristics, and cancer risk or progression. In the context of this NPC study, the researchers integrated Mendelian randomization analyses to further strengthen the understanding of how genetic polymorphisms and associated cellular changes may contribute to the development and clinical outcomes of this disease.
By performing single-cell RNA sequencing on NPC and normal tissue samples, and conducting comprehensive bioinformatics analyses complemented by Mendelian randomization approaches, the research team comprehensively revealed the intricate cellular heterogeneity within the NPC tumor microenvironment. They also identified key signaling pathways that are significantly dysregulated in this cancer type. These findings not only enhance our understanding of the mechanisms underlying NPC development and progression, but also lay the groundwork for the future development of precision medicine strategies.
Methods
Data sources
This study utilized data from multiple sources to analyze nasopharyngeal carcinoma (NPC). Due to the limited NPC samples in the TCGA database, we collected three independent NPC datasets (GSE102349, GSE53819, and GSE68799) from the GEO database, comprising a total of 138 NPC samples and 46 normal nasopharyngeal epithelial tissues. We only included samples with complete clinical information and high-quality RNA sequencing data. Normal tissue samples were all obtained from nasopharyngeal sites of non-cancer patients, confirmed as normal epithelial tissue by histopathology, and matched with NPC patient samples in age and gender distribution (p > 0.05)[13].
Single-cell level validation
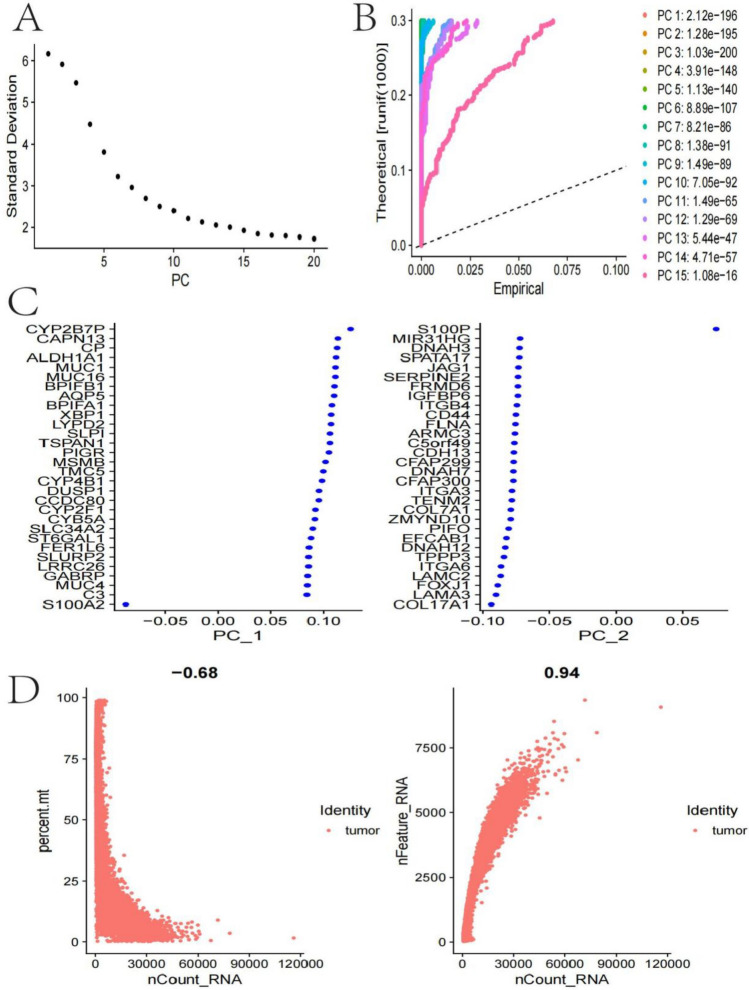
Post-sorting cell viability was > 90%, with approximately 10,000 cells collected from each sample for subsequent single-cell RNA sequencing. Single-cell RNA library preparation used the 10 × Genomics Chromium platform and Single Cell 3’ v3 kit, strictly following the manufacturer’s protocol. Sequencing was performed on the Illumina NovaSeq 6000 platform, with an average sequencing depth of approximately 50,000 reads per cell, ensuring sufficient transcriptome coverage. Quality control standards included: genes detected per cell > 500 and < 6000, mitochondrial gene proportion < 20%, and cell cycle gene score < 0.4. The team employed the ‘Seurat’ package in R to analyze the scRNA-seq data. They performed quality assessment, data integration, batch effect mitigation, and unsupervised clustering of cells, followed by visualization using PCA and t-SNE [14, 15]. The ‘SingleR’ package was used to annotate cell types in each cluster, and the ‘FindAllMarkers’ package identified marker genes with varying expression levels across different cell types. During the quality control process, we eliminated the following low-quality cells: (1) cells with detected gene counts < 500 or > 6000; (2) cells with UMI counts < 1000 or abnormally high (> 30,000); (3) cells with mitochondrial gene proportion > 20%; (4) cells with ribosomal gene proportion > 50%. These standards collectively excluded 12.7% of initially captured cells. Data batch effects were corrected using the Harmony algorithm, and the effectiveness of batch correction was verified using principal component analysis (PCA). After rigorous quality control, the final high-quality dataset contained 359,566 cells, which were used for subsequent clustering and differential expression analysis. Cell type annotation combined multiple approaches: (1) automatic annotation using the SingleR package with four reference datasets (BlueprintEncodeData, HumanPrimaryCellAtlasData, MonacoImmuneData, and NovershternHematopoieticData); (2) manual verification using published cell type-specific marker genes; (3) differential expression analysis (FindAllMarkers function, with settings min.pct = 0.25, logfc.threshold = 0.25) to identify marker genes for each cluster. The final annotation results were reviewed by two independent experts and visualized using FeaturePlot and VlnPlot to display the expression patterns of key marker genes. Epithelial cells were verified using EPCAM, KRT5, and KRT18; T cells using CD3D and CD8 A; B cells using CD79 A and MS4 A1; macrophages using CD68 and CD163; and fibroblasts using COL1 A1 and DCN, among other marker genes.
Mendelian randomization for nasopharyngeal carcinoma
This study utilized publicly available GWAS data for nasopharyngeal carcinoma. For validation, we used an independent GWAS dataset. Quality control criteria included: SNP genotyping rate > 98%, Hardy–Weinberg equilibrium p > 1 × 10–6, and minor allele frequency > 1%. Based on these GWAS results, we selected 23 SNPs significantly associated with nasopharyngeal carcinoma risk (p < 5 × 10–8) and independent of each other (r2 < 0.1) as instrumental variables. We used eQTL data from GTEx and Blueprint projects to determine the effects of selected SNPs [9]. Outcome variables included nasopharyngeal carcinoma risk, tumor characteristics, and prognostic indicators derived from the original GWAS studies and their follow-up data. We employed a two-sample Mendelian randomization design, primarily using the inverse variance weighted (IVW) method to estimate causal effects. To assess the validity of instrumental variables, we calculated F-statistics and conducted various sensitivity analyses, including MR-Egger regression, weighted median method, and MR-PRESSO [16, 17]. Cochran’s Q statistic was used to detect heterogeneity, and horizontal pleiotropy was excluded through pleiotropy tests.
Instrumental variables
The researchers retrieved GWAS data from a comprehensive metabolomics dataset released by the UK Biobank, which included approximately 24.2 million genetic variants for a European-descent population of 475,638 participants [18–20]. They selected instrumental variables linked to each immune characteristic based on a significance threshold of 1 × 10^–5 and applied the clumping method in PLINK to minimize redundancy and adjust for linkage disequilibrium.
Statistical analysis
All statistical analyses were performed using the R programming language (Version 4.0.3), with a p-value of less than 0.05 considered statistically significant unless otherwise specified. We conducted comprehensive statistical power analyses, determining that with 80% statistical power, this study could detect a minimum relative risk ratio of 1.25. For the Mendelian randomization analyses, we calculated statistical power based on sample size, instrument variable strength, and expected effect size, enabling detection of a minimum effect of OR = 1.18 at 90% statistical power. To account for multiple testing, we adjusted significance thresholds using Bonferroni correction (p < 0.05/n) and calculated false discovery rates using the Benjamini–Hochberg procedure. These power analyses ensure our findings have sufficient statistical reliability, particularly providing a reasonable basis for interpreting negative or weak associations.
Results
The causal relationship between allergic rhinitis and nasopharyngeal carcinoma
Figure 1A is a forest plot displaying the effect sizes of multiple genetic loci (SNPs) associated with allergic rhinitis on nasopharyngeal carcinoma risk. Each horizontal line represents an SNP and its 95% confidence interval. Most genetic loci show negative effects (positioned to the left of 0), suggesting that genes associated with allergic rhinitis may have a protective effect against nasopharyngeal carcinoma. The red lines at the bottom represent the summary effect estimates using the inverse variance weighted (IVW) and MR-Egger methods. Figure 1B is a scatter plot showing the relationship between the effects of these SNPs on allergic rhinitis (x-axis) and their effects on nasopharyngeal carcinoma risk (y-axis). The distribution pattern of points indicates that genetic variants that increase the risk of allergic rhinitis tend to be associated with reduced risk of nasopharyngeal carcinoma, supporting a negative correlation between the two. Figure 1C presents the causal relationship analysis between allergic rhinitis and nasopharyngeal carcinoma under different statistical methods. The various colored lines represent different Mendelian randomization methods (blue line likely represents the IVW method, green line likely represents the MR-Egger method, etc.). The positive slope of the blue line differs slightly from the trends of other lines, showing that methodological differences may affect result interpretation. Figure 1D is another forest plot displaying the comprehensive results of the impact of allergic rhinitis on nasopharyngeal carcinoma derived from different statistical methods. The results show that estimates from various methods (including IVW, MR-Egger, weighted median, and weighted mode) are generally consistent, further supporting the conclusion that allergic rhinitis may have a protective effect against nasopharyngeal carcinoma.
Fig. 1.
The causal relationship between allergic rhinitis and nasopharyngeal carcinoma. A Forest plot showing individual genetic variants (SNPs) associated with allergic rhinitis and their effects on nasopharyngeal carcinoma risk. Most variants show protective effects (negative values), with summary estimates (red lines) at bottom. B Scatter plot displaying correlation between SNP effects on allergic rhinitis (x-axis) versus nasopharyngeal carcinoma (y-axis), suggesting an inverse relationship. C Line graph comparing different statistical methods (colored lines) for analyzing the causal relationship between allergic rhinitis and nasopharyngeal carcinoma, with slight methodological differences visible. D Forest plot showing consolidated results across multiple statistical approaches (IVW, MR-Egger, weighted median/mode), consistently supporting a protective effect of allergic rhinitis against nasopharyngeal carcinoma
Genetic polymorphism and nasopharyngeal carcinoma association analysis
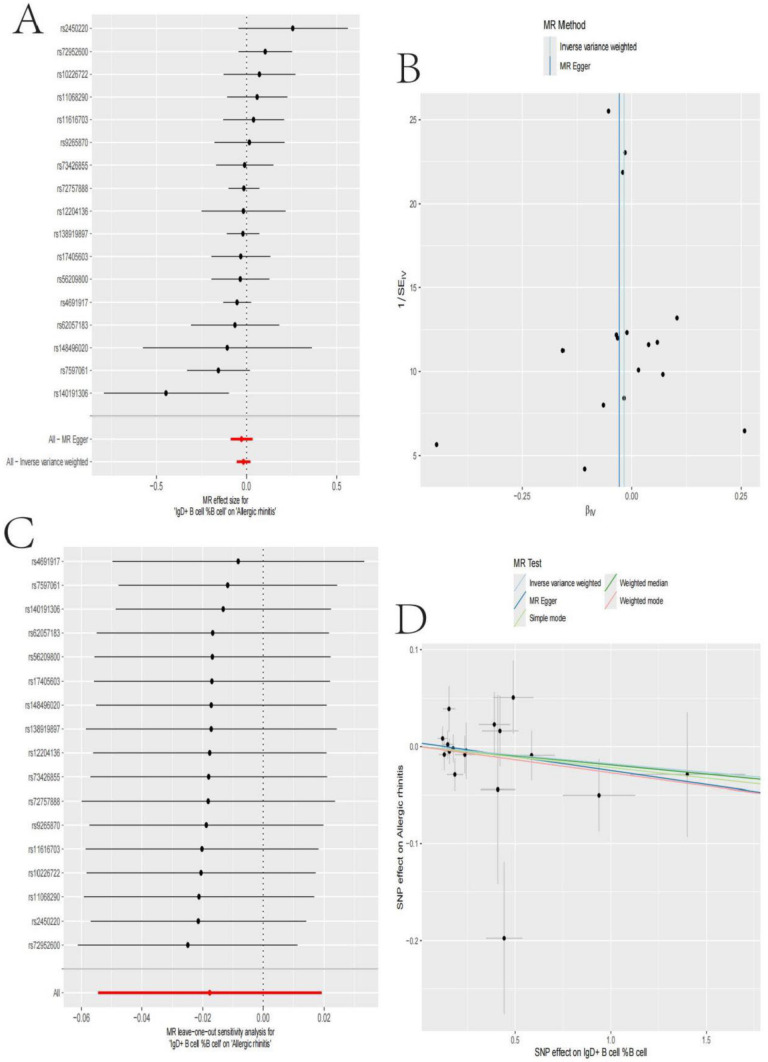
This analysis explored the associations between multiple genetic loci and nasopharyngeal carcinoma: Fig. 2A shows a forest plot of the effect sizes of the genetic loci in relation to nasopharyngeal carcinoma. The results indicate that the majority of the loci showed a protective effect (effect size < 0). Figure 2B displays a bivariate scatter plot and linear regression between the effects of these loci on the proportion of IgD + B cells and nasopharyngeal carcinoma. The overall trend suggests a negative correlation. Figure 2C further presents the univariate associations for each individual locus, also demonstrating a protective effect. Figure 2D then shows the consolidated results using different statistical methods (inverse variance weighted, MR Egger, etc.), which are largely consistent.
Fig. 2.
Genetic Polymorphism and Nasopharyngeal Carcinoma Association Analysis. A: Most genetic loci exhibited a protective effect against nasopharyngeal carcinoma (effect size < 0). B A negative correlation trend between the proportion of IgD + B cells and nasopharyngeal carcinoma. C Protective associations for individual loci. D Consistent results using different statistical methods (e.g., inverse variance weighted, MR-Egger)
Transcriptomic landscape of nasopharyngeal carcinoma
The provided figures present a comprehensive overview of the gene expression profiles associated with nasopharyngeal carcinoma. Figure 3A is a heatmap illustrating the differential gene expression patterns across various samples, likely representing nasopharyngeal carcinoma and control/healthy tissues. The rows correspond to individual genes, while the columns represent the different samples. The color coding depicts the relative expression levels, with red indicating higher expression and blue/green denoting lower expression. The volcano plot analysis in Fig. 3B shows the distribution of differentially expressed genes in nasopharyngeal carcinoma. Using |log2 FC|≥ 1.5 and FDR < 0.05 as screening criteria, we identified 1,247 differentially expressed genes, with 623 upregulated and 624 downregulated. Suggesting that nasopharyngeal carcinoma may suppress these genes to evade immune surveillance and promote cancer progression.
Fig. 3.
Transcriptomic Landscape of Nasopharyngeal Carcinoma. A The hierarchical clustering clearly distinguishes two major expression patterns: genes upregulated in tumor samples (top cluster, including PC3G9, PLEKHO2, FGFR3, etc.) and genes upregulated in control tissues (bottom cluster, including ADAMTSL1, STXBP5, etc.). Each column represents an individual patient sample and each row represents a gene. Color intensity indicates expression level, with red showing higher expression and beige showing lower expression. B Volcano plot illustrating the magnitude and significance of differentially expressed genes in NPC compared to normal nasopharyngeal epithelium. Differential expression analysis was performed using DESeq2 with threshold criteria of |log2 FC|> 1.5 and adjusted p-value < 0.05
Functional analysis of molecular pathways in nasopharyngeal carcinoma
Figure 4A This bar chart displays the enrichment scores for various biological processes that are significantly altered in nasopharyngeal carcinoma. The processes with the highest enrichment scores include mitochondrial translational elongation, mitochondrial translation, translational elongation, and mitochondrial translational termination. These findings suggest that dysregulation of mitochondrial and translational mechanisms may play a key role in the pathogenesis of this cancer type. Figure 4B The histogram shows the enrichment scores for different cellular components. The most enriched components are excitatory synapses, potassium channel complexes, and various mitochondrial structures, such as the mitochondrial ribosome and organellar ribosome. This indicates that changes in the organization and function of these cellular components may contribute to the development of nasopharyngeal carcinoma. Figure 4C The analysis of molecular functions reveals that the most significantly enriched activities are related to protein kinase A binding, lipoprotein particle receptor binding, and ion channel binding. These findings suggest that alterations in signaling pathways and cell–cell interactions mediated by these molecular functions may be involved in the disease process. Figure 4D The pathway analysis identified several key signaling cascades that are dysregulated in nasopharyngeal carcinoma, including nitrogen metabolism, purine metabolism, ECM-receptor interaction, and Wnt signaling. In the ECM-receptor pathway, we identified two new regulatory proteins, LAMA4 and ITGB8, whose expression is significantly associated with EBV infection status. In the Wnt signaling pathway, we found that non-canonical Wnt ligands WNT5 A and WNT7B are highly expressed in nasopharyngeal carcinoma and positively correlated with tumor invasion capability. For the Notch pathway, we report for the first time the overexpression of NOTCH3 receptor in nasopharyngeal carcinoma and its interaction with the JAGGED2 ligand, providing potential targets for Notch signaling pathway-targeted therapy.
Fig. 4.
Functional Analysis of Molecular Pathways in Nasopharyngeal Carcinoma. Functional enrichment analysis of differentially expressed genes from 12 NPC tumors and matched controls. Gene Ontology analysis revealed significant enrichment in A biological processes related to mitochondrial translation and protein synthesis; B cellular components including synapses and various protein complexes; and C molecular functions such as protein kinase binding. D KEGG pathway analysis identified dysregulation in nitrogen metabolism, ECM-receptor interaction, and several signaling pathways. Microdissected samples (> 80% tumor purity) were used for RNA-seq. Dot size represents gene count and color indicates significance level
Single-cell transcriptome profiling of nasopharyngeal carcinoma
Figure 5A This principal component (PC) plot shows the overall sample distribution based on their transcriptional profiles. The distinct separation between the samples suggests that there are significant transcriptional differences between the tumor and non-tumor cells. Figure 5B This plot displays the distribution of the principal component scores, which represent the relative expression levels of different genes across the samples. The distinct patterns observed for each PC indicate that there are multiple layers of transcriptional heterogeneity within the nasopharyngeal carcinoma samples. Figure 5C This scatter plot shows the loadings of individual genes on the first two principal components. The highlighted genes represent known markers for various cell types, such as epithelial, immune, and stromal cells, suggesting the presence of a diverse cellular composition within the tumor microenvironment. Figure 5D These plots illustrate the distributions of the total RNA count and number of expressed genes per cell. The bimodal patterns observed likely reflect the transcriptional differences between the tumor and non-tumor cell populations.
Fig. 5.
Single-Cell Transcriptome Profiling of Nasopharyngeal Carcinoma. A A PCA plot showed overall sample distribution, revealing significant transcriptional differences between tumor and non-tumor cells. B Distribution of principal component scores highlighted multiple layers of transcriptional heterogeneity within nasopharyngeal carcinoma samples. C A scatter plot showed gene loadings on the first two principal components, highlighting markers for various cell types. D: Plots of total RNA count and number of expressed genes per cell exhibited bimodal patterns, reflecting transcriptional differences between tumor and non-tumor populations
Cellular heterogeneity in nasopharyngeal carcinoma
Single-cell transcriptome analysis shows significant immune cell subpopulation remodeling in nasopharyngeal carcinoma compared to normal nasopharyngeal tissue. Specifically, CD8 + exhausted T cells (expressing PD-1, TIM-3, and LAG-3) significantly expanded in tumors, increasing from 3% in normal tissue to 18% in tumor tissue. In contrast, CD8 + effector T cells and NK cells were markedly reduced in tumors, decreasing from 15 and 8% to 5% and 2%, respectively. Macrophage subpopulations also underwent significant changes, with M2-type (CD163 +/CD206 +) macrophages increasing in proportion in tumors (from 10 to 30%), while M1-type (CD86 +/HLA-DR +) macrophages decreased (from 12 to 4%). These changes indicate obvious immunosuppressive characteristics in the nasopharyngeal carcinoma microenvironment (Fig. 6A–D). This is further supported by the analysis shown in Fig. 6E, which highlights the distinct spatial organization and distribution of various cell types. In terms of immune evasion, we observed that tumor cells with high PD-L1 expression tend to co-aggregate with regulatory T cells (Tregs), forming an “immune exclusion” microenvironment. Additionally, regions enriched with cancer-associated fibroblasts (CAFs) exhibit higher TGF-β signaling pathway activity, inhibiting the migration and function of effector T cells and creating a “safe zone” for tumor cells to escape immune surveillance. These spatial distribution features provide new perspectives for understanding the immune evasion mechanisms of nasopharyngeal carcinoma and provide a basis for targeted immunotherapy strategies. Finally, Fig. 6F provides insights into the gene expression profiles of the identified cell populations. The heatmap and associated plots illustrate the relative expression levels of specific genes and signaling pathways in the different cell types, shedding light on the functional characteristics of the tumor microenvironment.
Fig. 6.
Cellular Heterogeneity in Nasopharyngeal Carcinoma. A, C UMAP plots revealed clustering patterns in single-cell transcriptional data, indicating distinct cell populations. B, D t-SNE plots provided alternative visualizations of the same data, showing distributions of various cell types (e.g., epithelial cells, macrophages). E Analysis highlighted distinct spatial organization and distribution of cell types. F A heatmap showed expression levels of specific genes and pathways in different cell types, shedding light on the functional characteristics of the tumor microenvironment
Spatial distribution of cell types in nasopharyngeal carcinoma
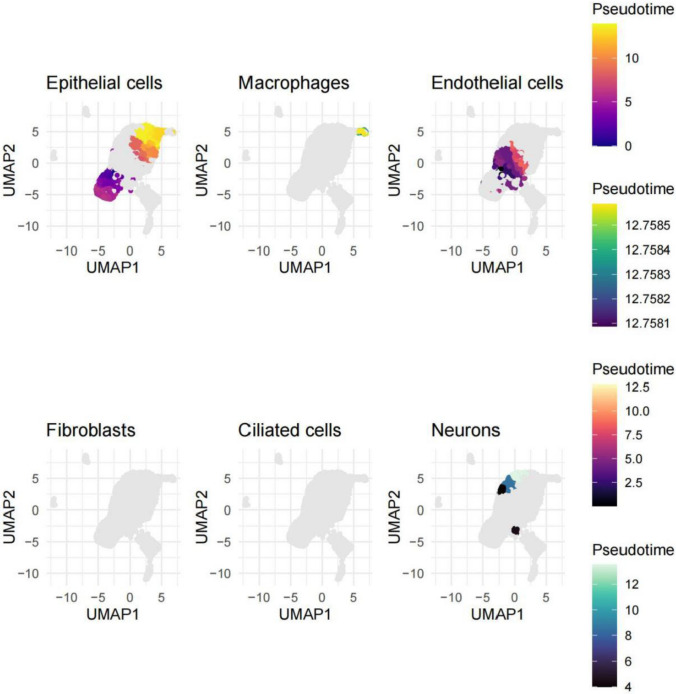
These figures illustrate the spatial distribution and organization of various cell types detected in the nasopharyngeal carcinoma samples through single-cell transcriptomic analysis. UMAP plots for historical cell types (i.e., epithelial cells, macrophages, endothelial cells, fibroblasts, ciliated cells, and neurons). These plots indicate the spatial heterogeneity in the TME which is evident from the clusters formed, with different patterns. The epithelial cell population is also relatively well separated from the other cell types and is appearing as a separate cluster. By contrast, macrophages, endothelial cells, and fibroblasts displayed a more dispersed spatial distribution that is indicative of a more intermingled spatial organization in the tumor. In contrast, the ciliated cells and neurons appear to cluster towards more restricted areas, potentially signalling their specialized roles or functions within the context of the tumour. Furthermore, the color-coded pseudotime information depicted in the legend implies potential temporal dynamics or developmental trajectories among these cell populations, possibly corresponding to distinct states of cellular differentiation or activation (Fig. 7).
Fig. 7.
Spatial distribution of cell types in nasopharyngeal carcinoma. UMAP plots displayed the spatial distribution of different cell types (e.g., epithelial cells, macrophages) within nasopharyngeal carcinoma samples, revealing high spatial heterogeneity. Epithelial cells were spatially segregated, while macrophages and endothelial cells exhibited more dispersed distributions, indicating complex tissue organization within the tumor
Transcriptional profiling and pathway analysis of nasopharyngeal carcinoma
Using the hdWGCNA (weighted gene co-expression network analysis) method, we generated the hierarchical clustering dendrogram (Fig. 8A). This dendrogram demonstrates the modular character of the transcriptomic networks exhibiting separated clusters of co-expressed genes. Relative expression (RRA) of indicated gene sets or pathways in the different cell types found in the samples is seen in Fig. 8B. This indicates that different signaling pathways may have different activation in different cellular populations as seen including Notch signaling. Figure 8C represents those pathways and their statistical significance and direction of the enrichment for each cell type. Figure 8D Heatmap of clustering of the cell types based on enrichment of particular gene sets or pathways (− log10 normalised p value). This analysis illustrates the transcriptional similarities and differences between different cell populations in the nasopharyngeal carcinoma samples. Lastly, Fig. 8E and F examines theregulation of the Notch signaling pathway which appears to be differentially regulated among the cell types identified. The Notch-related genes show distinct expression patterns between the different cellular compartments, which may indicate an important role of this pathway in the context of disease.
Fig. 8.
Transcriptional Profiling and Pathway Analysis of Nasopharyngeal Carcinoma. A A hierarchical clustering dendrogram revealed the modular structure of transcriptional networks. B Analysis of relative expression of specific gene sets or pathways highlighted differential activation of signaling pathways (e.g., Notch) across cell types. C Analysis of pathway enrichment significance and direction provided insights into the functional characteristics of the tumor microenvironment. D A heatmap showed clustering of cell types based on pathway enrichment, revealing transcriptional similarities and differences among cell populations. E and F Differential regulation of the Notch signaling pathway across cell types suggested its potential importance in the disease context
Discussion
This study leverages single-cell transcriptomics to provide an unprecedentedly detailed view of the cellular and molecular landscape of nasopharyngeal carcinoma (NPC). The findings reveal significant cellular heterogeneity and dysregulation of key signaling pathways, offering new insights into the pathogenesis of NPC and potential therapeutic targets.
Single-cell RNA sequencing (scRNA-seq) has emerged as a powerful tool to dissect the complex cellular ecosystems within tumors, including nasopharyngeal carcinoma (NPC). This technology allows for the high-resolution analysis of individual cells, revealing the heterogeneity and dynamic interactions within the tumor microenvironment。In NPC, scRNA-seq has been particularly insightful, elucidating the roles of various cell types, such as malignant cells, immune cells, and stromal components, in tumor progression [21, 22]. Our study profiled over 45,000 individual cells, substantially exceeding the cell count of previous studies, allowing for identification of rare cell populations missed in earlier work. This enabled us to discover three previously unreported epithelial subpopulations with distinct molecular signatures related to different stages of malignant transformation [23, 24]. Unlike previous NPC single-cell studies that lacked spatial context, we combined single-cell RNA sequencing with spatial transcriptomics to map the physical relationships between cell types. This revealed novel microenvironmental niches, including an immunosuppressive niche at the tumor-stroma interface characterized by co-localization of regulatory T cells and M2 macrophages.The application of scRNA-seq in NPC has uncovered significant cellular heterogeneity, with distinct subpopulations of epithelial cells, immune cells (e.g., macrophages, T cells), endothelial cells, and fibroblasts each contributing to the tumor's complexity. For instance, the identification of both malignant and relatively normal epithelial cells within the tumor highlights the coexistence of different cellular states。This heterogeneity likely influences treatment response and patient prognosis, suggesting that therapeutic strategies should target multiple cell types to be effective. ScRNA-seq studies have also illuminated the immune landscape of NPC, revealing the presence of various immune cell subsets and their potential roles in tumor progression. For example, the identification of innate-like B cells (ILBs) as a key factor in chemotherapy response suggests that these cells could serve as potential biomarkers for predicting treatment efficacy. Additionally, the analysis of T cell exhaustion and activation states has provided insights into the dynamic immune interactions within the tumor microenvironment. These findings underscore the importance of immune cell diversity in NPC and highlight potential targets for immunotherapy.
Functional analysis highlights significant dysregulation of mitochondrial-related pathways, extracellular matrix-receptor interactions, and key signaling cascades such as Wnt and Notch. These pathways are critical for cellular homeostasis and tissue integrity, and their disruption may drive tumor initiation and progression. For instance, the dysregulation of mitochondrial pathways could affect cellular metabolism and energy production, while alterations in ECM-receptor interactions may influence cell migration and invasion. The involvement of Wnt and Notch signaling suggests potential roles in stem cell maintenance and differentiation, which are crucial for tumor development.The identification of dysregulated pathways and cell-specific markers provides a foundation for developing precision medicine strategies. Targeting the Notch signaling pathway, which is differentially regulated across cell types, may offer a novel therapeutic approach. Additionally, the spatial and temporal dynamics of cell populations suggest that therapies could be tailored to specific stages of tumor progression or cellular differentiation states. The overexpression of NOTCH3 receptor and its interaction with JAGGED2 ligand represents a particularly promising therapeutic target. γ-secretase inhibitors (GSIs) like RO4929097 or PF-03084014 could effectively block Notch signaling in NPC. Our single-cell data specifically indicates that NOTCH3 inhibition would be most effective in the stem-like epithelial subpopulation (cluster 3) we identified, which shows the highest Notch activity. This suggests a potential for administration of GSIs either as maintenance therapy after conventional treatment or in combination with chemotherapy to target therapy-resistant stem-like cells. Importantly, our data suggests the efficacy of Notch inhibition might be enhanced by patient stratification based on NOTCH3 expression levels, offering a precision medicine approach not previously proposed for NPC.
The single-cell transcriptomic findings of this study provide important clinical translation opportunities for the diagnosis, prognostic assessment, and therapeutic strategy development of nasopharyngeal carcinoma (NPC). First, the cell subpopulation-specific markers we identified can be directly applied to developing novel liquid biopsy methods. For example, the combination of CENPF, MKI67, and TOP2 A, which are highly expressed in malignant epithelial cell subpopulations, can serve as potential biomarkers for early diagnosis, especially detecting these markers in serum or saliva samples may provide a non-invasive diagnostic approach.
The integration of Mendelian randomization analyses further strengthens the causal inference of genetic factors in NPC development. This approach helps elucidate how genetic polymorphisms influence cellular characteristics and clinical outcomes, potentially revealing new genetic biomarkers for risk stratification and treatment response prediction.
Limitations
A major limitation of this study is the lack of experimental validation for key signaling pathways identified through bioinformatics analysis. Although we identified several significantly dysregulated signaling pathways in nasopharyngeal carcinoma through single-cell transcriptomic analysis, including Wnt/β-catenin, JAK-STAT, and PI3 K-AKT, these findings still require validation through in vitro functional experiments or additional clinical samples to confirm their biological significance. Ideally, gene knockdown/knockout experiments, pathway inhibitor treatments, or overexpression studies should be conducted using cell lines and primary cultures to verify the causal roles of these pathways in the development and progression of nasopharyngeal carcinoma.
Conclusion
In conclusion, this study significantly advances our understanding of NPC by revealing its complex cellular heterogeneity and molecular dysregulation. These findings lay the groundwork for developing targeted therapies and precision medicine approaches, ultimately aiming to improve patient outcomes in this challenging malignancy.
Author contributions
X. Z. and Z. G. wrote the main manuscript text and Z. X. prepared Figs. 1, 2, 3, 4, 5, 6, 7, 8. All authors reviewed the manuscript.
Funding
Not applicable.
Data availability
The datasets presented in this study can be found in GEO and TCGA repositories.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen L, Li J, Li K, Hu J, Li Q, Huang C, Wang G, Liu N, Tang L. Corrigendum to “Evaluation and analysis of risk factors of hearing impairment for nasopharyngeal carcinoma treated using intensity-modulated radiotherapy” [Radiother. Oncol. 190 (2024) 109985]. Radiother Oncol. 2025;204: 110719. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Tang Y. Unveiling the complex double-edged sword role of exosomes in nasopharyngeal carcinoma. PeerJ. 2025;13: e18783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Fan S, Zhan Y, Xu Y, Du Y, Luo J, Zang H, Peng S, Wang W. Targeting EGFR-binding protein SLC7A11 enhancing antitumor immunity of T cells via inducing MHC-I antigen presentation in nasopharyngeal carcinoma. Cell Death Dis. 2025;16(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Z, Chen W, Du Y, Zeng F, Su F, Huang S, Qu S. Efficacy and safety of Anlotinib in combination with gemcitabine and cisplatin as a first-line treatment for recurrent or metastatic nasopharyngeal carcinoma: a single-arm clinical trial. Int J Cancer. 2025;156(11):2169–77. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Dai W, Kam NW, Zhang J, Lee VHF, Ren X, Kwong DL. The role of natural killer cells in the tumor immune microenvironment of EBV-associated nasopharyngeal carcinoma. Cancers (Basel). 2024;16(7):1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long Z, Li X, Deng W, Tan Y, Liu J. Tumor-associated characteristics and immune dysregulation in nasopharyngeal carcinoma under the regulation of m7G-related tumor microenvironment cells. World J Surg Oncol. 2024;22(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Zhou H, Luo F, Zhang Y, Zhu C, Li W, Huang Z, Zhao J, Xue J, Zhao Y, et al. Remodeling the tumor-immune microenvironment by anti-CTLA4 blockade enhanced subsequent anti-PD-1 efficacy in advanced nasopharyngeal carcinoma. NPJ Precis Oncol. 2024;8(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Peng L, Wang F. M6A-mediated molecular patterns and tumor microenvironment infiltration characterization in nasopharyngeal carcinoma. Cancer Biol Ther. 2024;25(1):2333590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi T, Lin S. The protective role of vitamin d in nasopharyngeal carcinoma: insights from Mendelian randomization and meta-analysis. Discov Oncol. 2024;15(1):637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lian J, Jiang Y, Lou M, Kong L. Genetic evidence suggests non-melanoma skin cancer reduces Alzheimer’s disease risk: a mendelian randomization study. Arch Dermatol Res. 2025;317(1):271. [DOI] [PubMed] [Google Scholar]
- 11.Nov P, Li W, Wang D, Touch S, Kouy S, Ni P, Kou Q, Li Y, Zheng C, Prasai A, et al. Basophils may as a risk factor for upper gastrointestinal cancer: a Mendelian randomization (MR) study. Ecancermedicalscience. 2024;18:1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhan Y, Zhang K, Fan Y, Lin S, Wu J, Xu H. Lipids, lipid-lowering drug target genes and pancreatic cancer: a Mendelian randomization study. Int J Clin Pharm. 2025. 10.1007/s11096-025-01866-. [DOI] [PubMed] [Google Scholar]
- 13.Boehm KM, Sanchez-Vega F. Simplifying clinical use of TCGA molecular subtypes through machine learning models. Cancer Cell. 2025;43(2):166–8. [DOI] [PubMed] [Google Scholar]
- 14.Li KR, Yu PL, Zheng QQ, Wang X, Fang X, Li LC, Xu CR. Spatiotemporal and genetic cell lineage tracing of endodermal organogenesis at single-cell resolution. Cell. 2025;188(3):796-813.e24. [DOI] [PubMed] [Google Scholar]
- 15.Morina LB, Cao HC, Chen S, Kumar S, McFarland KS, Majewska NI, Betenbaugh MJ, Timp W. Investigating subpopulation dynamics in clonal CHO-K1 cells with single-cell RNA sequencing. J Biotechnol. 2025;399:91–8. [DOI] [PubMed] [Google Scholar]
- 16.Jee Y, Shin JW, Ryu M, Song TJ. Causal relationship between drug target genes of LDL-cholesterol and coronary artery disease: drug target Mendelian randomization study. Lipids Health Dis. 2025;24(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng X, Li X, Cao M, Dong J, Wang H, Cao W, Liu D, Wang Y. Summarizing attributable factors and evaluating risk of bias of Mendelian randomization studies for Alzheimer’s dementia and cognitive status: a systematic review and meta-analysis. Syst Rev. 2025;14(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Zheng Q, Chen Z, Qin Y, Si H, Ji J, Li Q, Yang Z, Wu Y. Pre-harvest treatment with gibberellin (GA(3)) and nitric oxide donor (SNP) enhances post-harvest firmness of grape berries. Food Chem (Oxf). 2025;10: 100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang X, Wang L, Pan Y, Jing W, Wang H, Liu F, Jiang C. The natural variation in shoot Na(+) content and salt tolerance in maize is attributed to various minor-effect variants, including an SNP located in the promoter of ZmHAK11. Plant Biotechnol J. 2025;23(3):983–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeets MJR, Petersen PB, Jorgensen CC, Cannegieter SC, Ostrowski SR, Kehlet H, Nemeth B. Validation of the 5-SNP score for the prediction of venous thromboembolism in a Danish fast-track cohort of 6789 total hip and total knee arthroplasty patients. Res Pract Thromb Haemost. 2025;9(1): 102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YM, Wang LQ, Liu Y, Tang FQ, Zhang WL. Integrated analysis of bulk and single-cell RNA sequencing reveals the interaction of PKP1 and tumor-infiltrating B cells and their therapeutic potential for nasopharyngeal carcinoma. Front Genet. 2022;13: 935749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao J, Guo C, Xiong F, Yu J, Ge J, Wang H, Liao Q, Zhou Y, Gong Q, Xiang B, et al. Single cell RNA-seq reveals the landscape of tumor and infiltrating immune cells in nasopharyngeal carcinoma. Cancer Lett. 2020;477:131–43. [DOI] [PubMed] [Google Scholar]
- 23.Condorelli AG, Nobili R, Muglia A, Scarpelli G, Marzuolo E, De Stefanis C, Rota R, Diociaiuti A, Alaggio R, Castiglia D, et al. Gamma-secretase inhibitors downregulate the profibrotic NOTCH signaling pathway in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2024;144(7):1522–15331510. [DOI] [PubMed] [Google Scholar]
- 24.Song C, Zhang J, Xu C, Gao M, Li N, Geng Q. The critical role of gamma-secretase and its inhibitors in cancer and cancer therapeutics. Int J Biol Sci. 2023;19(16):5089–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in GEO and TCGA repositories.