Abstract
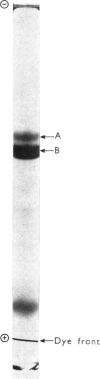
1. A stable, more highly purified, preparation of UDP-glucuronyltransferase was obtained than previously reported. 2. Enzyme activity towards o-aminophenyl and p-nitrophenyl was increased 43- and 46-fold respectively. 3. The final preparation contains only three staining polypeptide bands visible after sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. 4. The only known major accompanying protein appears to be epoxide hydratase. 5. The purified enzyme activity towards o-aminophenol can still be activated 3 fold by diethylnitrosamine. 6. On evidence from purification, o-aminophenol and p-nitrophenol appear to be glucuronidated by the same enzyme protein. The possible recognition of the UDP-glucuronyltransferase enzyme is discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airaksinen M. M., Miettinen T. A., Huttunen J. Glucuronidation of 5-hydroxyindole derivatives in vitro. Biochem Pharmacol. 1965 Jun;14(6):1019–1023. doi: 10.1016/0006-2952(65)90254-6. [DOI] [PubMed] [Google Scholar]
- Bentley P., Oesch F. Purification of rat liver epoxide hydratase to apparent homogeneity. FEBS Lett. 1975 Nov 15;59(2):291–295. doi: 10.1016/0014-5793(75)80395-4. [DOI] [PubMed] [Google Scholar]
- Blackburn G. R., Bornens M., Kasper C. B. Characterization of the membrane matrix derived from the microsomal fraction of rat hepatocytes. Biochim Biophys Acta. 1976 Jun 17;436(2):387–398. doi: 10.1016/0005-2736(76)90202-9. [DOI] [PubMed] [Google Scholar]
- Burchell B., Bentley P., Oesch F. Latency of epoxide hydratase and its relationship to that of UDPglucuronyltransferase. Biochim Biophys Acta. 1976 Sep 24;444(2):531–538. doi: 10.1016/0304-4165(76)90397-4. [DOI] [PubMed] [Google Scholar]
- Burchell B., Burchell A. Further purification of rat liver uridine diphosphate glucuronyltransferase. Biochem Soc Trans. 1976;4(3):521–522. doi: 10.1042/bst0040521. [DOI] [PubMed] [Google Scholar]
- Burchell B., Wishart G. J., Dutton G. J. Relation between the induction of hydroxylation and of glucuronidation in chick liver. FEBS Lett. 1974 Aug 1;43(3):323–326. doi: 10.1016/0014-5793(74)80671-x. [DOI] [PubMed] [Google Scholar]
- Del Villar E., Sanchez E., Autor A. P., Tephly T. R. Morphine metabolism. III. Solubilization and separation of morphine and p-nitrophenol uridine diphosphoglucuronyltransferases. Mol Pharmacol. 1975 Mar;11(2):236–240. [PubMed] [Google Scholar]
- Dignam J. D., Strobel H. W. Preparation of homogeneous NADPH-cytochrome P-450 reductase from rat liver. Biochem Biophys Res Commun. 1975 Apr 21;63(4):845–852. doi: 10.1016/0006-291x(75)90644-0. [DOI] [PubMed] [Google Scholar]
- Haugen D. A., van der Hoeven T. A., Coon M. J. Purified liver microsomal cytochrome P-450. Separation and characterization of multiple forms. J Biol Chem. 1975 May 10;250(9):3567–3570. [PubMed] [Google Scholar]
- Heirwegh K. P., Van de Vijver M., Fevery J. Assay and properties of dititonin-activated bilirubin uridine diphosphate glucuronyltransferase from rat liver. Biochem J. 1972 Sep;129(3):605–618. doi: 10.1042/bj1290605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland R. D., Burkhalter A., Trevor A. J., Hegeman S., Shirachi D. Y. Properties of lubrol-extracted uridine diphosphate glucuronyltransferase. Biochem J. 1971 Dec;125(4):991–997. doi: 10.1042/bj1250991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISSELBACHER K. J., CHRABAS M. F., QUINN R. C. The solubilization and partial purification of a glucuronyl transferase from rabbit liver microsomes. J Biol Chem. 1962 Oct;237:3033–3036. [PubMed] [Google Scholar]
- Kawalek J. C., Levin W., Ryan D., Thomas P. E., Lu A. Y. Purification of liver microsomal cytochrome P-448 from 3-methylcholanthrene-treated rabbits. Mol Pharmacol. 1975 Nov;11(6):874–878. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lu A. Y., Ryan D., Jerina D. M., Daly J. W., Levin W. Liver microsomal expoxide hydrase. Solubilization, purification, and characterization. J Biol Chem. 1975 Oct 25;250(20):8283–8288. [PubMed] [Google Scholar]
- Lucier G. W., McDaniel O. S., Hook G. E. Nature of the enhancement of hepatic uridine diphosphate glucuronyltransferase activity by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Biochem Pharmacol. 1975 Feb 1;24(3):325–334. doi: 10.1016/0006-2952(75)90213-0. [DOI] [PubMed] [Google Scholar]
- Mowat A. P., Arias I. M. Partial purification of hepatic UDP-glucuronyltransferase. Studies of some of its properties. Biochim Biophys Acta. 1970 Jul 15;212(1):65–78. doi: 10.1016/0005-2744(70)90179-8. [DOI] [PubMed] [Google Scholar]
- Nakata D., Zakim D., Vessey D. A. Modification of protein-lipid interactions in the Gunn rat by treatment of microsomal UDP-glucuronyltransferase with diethylnitrosamine. Biochem Pharmacol. 1975 Oct 1;24(19):1823–1825. doi: 10.1016/0006-2952(75)90466-9. [DOI] [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Ryan D., Lu Y. H., Kawalek J., West S. B., Levin L. Highly purified cytochrome P-448 and P=450 from rat liver microsomes. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1134–1141. doi: 10.1016/0006-291x(75)90812-8. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I., Greenwood D., McEwen J. Hepatic UDP-glucuronyltransferase in Wistar and Gunn rats--in vitro activation by diethylnitrosamine. Biochem Biophys Res Commun. 1968 Sep 6;32(5):866–872. doi: 10.1016/0006-291x(68)90321-5. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. XII. Electron microscopy of the enzyme from Escherichia coli. Biochemistry. 1968 Jun;7(6):2143–2152. doi: 10.1021/bi00846a017. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winsnes A. Studies on the activation in vitro of glucuronyltransferase. Biochim Biophys Acta. 1969 Nov 4;191(2):279–291. doi: 10.1016/0005-2744(69)90247-2. [DOI] [PubMed] [Google Scholar]