Significance
Koh et al. show that 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is pathologically elevated in human and mouse Alzheimer’s disease (AD), traumatic brain injury (TBI), and aging, with 15-PGDH localized to myeloid cells of the blood–brain barrier (BBB). They further show that pharmacologic and genetic inhibition of 15-PGDH protects the BBB, blocks production of reactive oxygen species, prevents downstream neurodegeneration, and preserves cognition in mouse models of AD and TBI. Thus, 15-PGDH inhibition represents a therapeutic approach for AD and TBI by protecting the BBB.
Keywords: neuroprotection, Alzheimer’s disease, traumatic brain injury, 15-PGDH, blood–brain barrier
Abstract
Alzheimer’s disease (AD) and traumatic brain injury (TBI) are currently untreatable neurodegenerative disorders afflicting millions of people worldwide. These conditions are pathologically related, and TBI is one of the greatest risk factors for AD. Although blood–brain barrier (BBB) disruption drives progression of both AD and TBI, strategies to preserve BBB integrity have been hindered by lack of actionable targets. Here, we identify 15-hydroxyprostaglandin dehydrogenase (15-PGDH), an enzyme that catabolizes eicosanoids and other anti-inflammatory mediators, as a therapeutic candidate that protects the BBB. We demonstrate that 15-PGDH is enriched in BBB-associated myeloid cells and becomes markedly elevated in human and mouse models of AD and TBI, as well as aging, another major risk factor for AD. Pathological increase in 15-PGDH correlates with pronounced oxidative stress, neuroinflammation, and neurodegeneration, alongside profound BBB structural degeneration characterized by astrocytic endfeet swelling and functional impairment. Pharmacologic inhibition or genetic reduction of 15-PGDH in AD and TBI models strikingly mitigates oxidative damage, suppresses neuroinflammation, and restores BBB integrity. Most notably, inhibiting 15-PGDH not only halts neurodegeneration but also preserves cognitive function at levels indistinguishable from healthy controls. Remarkably, these neuroprotective effects in AD are achieved without affecting amyloid pathology, underscoring a noncanonical mechanism for treating AD. In a murine microglia cell line exposed to amyloid beta oligomer, major protection was demonstrated by multiple anti-inflammatory substrates that 15-PGDH degrades. Thus, our findings position 15-PGDH inhibition as a broad-spectrum strategy to protect the BBB and thereby preserve brain health and cognition in AD and TBI.
Over 55 million people worldwide currently suffer from dementia due to neurodegenerative conditions, such as Alzheimer’s disease (AD) and traumatic brain injury (TBI) (1, 2). Historically, efforts to discover neuroprotective treatments for these conditions have focused mostly on directly protecting neurons in the brain. Unfortunately, this has not led to disease-modifying treatments for patients. Thus, it is important to consider other modifiable aspects of brain health in the pursuit of effective neuroprotective therapies.
A prominent and early feature of AD is deterioration in blood–brain barrier (BBB) structure and function (3–5), which impairs neurovascular coupling and increases permeability of blood-borne substances and peripheral immune cells into the brain parenchyma, triggering perivascular inflammation (6–9). These events induce oxidative stress and lead to neurodegeneration and cognitive impairment (10, 11). Importantly, BBB dysfunction occurs in the earliest pathological stages of AD (12–15) and has been proposed as an early biomarker for the disease (16, 17). Aging further contributes to BBB deterioration, especially in the hippocampus (2), a region essential for learning and memory that is particularly vulnerable in AD (18–22).
In addition to aging, another major risk factor for AD is TBI, defined as any injury resulting from an external force that disrupts normal brain function (23). Common causes of TBI include falls, motor vehicle accidents, explosions, interpersonal violence, and sports injuries. Although the mechanistic links between TBI and AD remain unclear, TBI-induced vascular dysfunction at the BBB has been proposed as a contributing factor (14). Given the central role of BBB deterioration in declining brain health associated with aging, TBI, and AD, protecting the BBB represents a new direction that merits therapeutic exploration.
In this context, we report that inhibiting the enzyme 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is a neuroprotective approach by virtue of protecting the BBB. We find that 15-PGDH is enriched in myeloid cells associated with the BBB and that its elevated expression and activity are tightly associated with BBB disruption in both AD and TBI. Importantly, inhibiting 15-PGDH confers neuroprotection and preserves cognitive function in preclinical models of both conditions. Encoded by the Hpgd gene, 15-PGDH is a short-chain dehydrogenase/reductase that catalyzes nicotinamide adenine dinucleotide (NAD+)-dependent oxidative degradation of various substrates throughout the body, including prostaglandin E2 (PGE2), PGF2α, resolvin D1 (RvD1), lipoxin A4 (LXA4), and 15-hydroxyeicosatetraenoic acid (15-HETE) (24, 25). Although studies using the small molecule 15-PGDH inhibitor (+)-SW033291 and Hpgd knockout mice have demonstrated that inhibition of 15-PGDH promotes tissue regeneration and repair in models of peripheral disease and ischemic damage (26–34), investigation of 15-PGDH in the brain remains limited (35).
Results
Elevated 15-PGDH Expression and Activity in the Brains of Mice and People with AD, TBI, and Aging, with Notable Enrichment in Microglia and Perivascular Macrophages.
In human AD brains, we identified a significant 75% increase in 15-PGDH mRNA compared to controls (Fig. 1A). This finding was corroborated by RNA sequencing data from the Allen brain atlas (36, 37), which revealed elevated 15-PGDH mRNA levels in the hippocampus of human AD subjects (SI Appendix, Fig. S1A). The amyloid beta (Aβ)-driven 5xFAD mouse model of AD (38) recapitulated these human findings, displaying increased brain expression of 15-PGDH mRNA (Fig. 1B). Notably, 6-mo-old symptomatic 5xFAD mice exhibited a twofold increase in brain 15-PGDH enzymatic activity relative to wild-type (WT) littermates, escalating to over threefold by 12-mo of age and paralleling disease progression (Fig. 1C). Similarly, symptomatic 6-mo-old TgF-344 AD rats, an additional preclinical model of AD (39, 40), showed elevated brain 15-PGDH activity relative to WT littermates (SI Appendix, Fig. S1B).
Fig. 1.
Brain 15-PGDH increases in human and mouse AD, TBI, and aging and is enriched in brain myeloid cells. (A) qPCR data from the human cortex reveal that 15-PGDH mRNA (HPGD expression) is increased in subjects with AD, compared to control subjects without dementia. HPGD was normalized to the geometric mean of ACTB, POLR1B, and LDHA (*P < 0.05, unpaired t test). (B) Mouse whole-brain qPCR data reveal that 15-PGDH mRNA (Hpgd expression) is increased in symptomatic (6 mo old) 5xFAD mice, compared to WT littermates. Hpgd was normalized to Actb (**P < 0.01, unpaired t test). (C) Mouse 15-PGDH enzymatic activity is increased in symptomatic (6 and 12 mo old) 5xFAD mice, compared to WT littermates (5xFAD versus WT genotype effect P < 0.001, 6 versus 12 mo time effect P = 0.0533, ***P < 0.001, two-way ANOVA and Tukey’s post hoc analysis). (D) Human brain RNA seq data from Seo et al. (ERP015139) (41) reveals that 15-PGDH mRNA (HPGD expression) is increased in subjects with chronic traumatic encephalopathy (CTE), compared to control subjects (*P < 0.05, unpaired t test). Data are shown from both the superior parietal cortex and posterior visual cortex. (E) Mouse hippocampal 15-PGDH enzymatic activity is increased 3 wk after TBI, relative to sham-injury (****P < 0.0001, unpaired t test). (F) Fluorescent in situ hybridization (FISH) for Hpgd mRNA (red) in the mouse brain shows more 15-PGDH positive cells in the cortex and hippocampus of symptomatic (6 mo old) 5xFAD mice, compared to WT littermates (*P < 0.05, ***P < 0.001, unpaired t test; 2 to 7 brain sections/mouse). (G) CD11b+ myeloid cells show increased 15-PGDH protein expression in the brains of 6-mo-old 5xFAD mice, compared to WT littermates (Interaction**P < 0.01, ****P < 0.0001, two-way ANOVA). (H) Single-cell RNA sequencing data analyzed from Yao et al. (42) reveals that Hpgd-expressing myeloid cells are composed of Mrc1 positive PVMs and Tmem119 positive microglia. A heatmap of cortical myeloid cells depicting expression [log2(SCT)] of 15-PGDH mRNA (Hpgd), Mrc1 [perivascular macrophages (PVMs)], Lyve1 (PVMs), Aif1 (microglia and macrophages), and Tmem119 (microglia) is shown. (I) RNA FISH in the mouse brain shows an increase in Hpgd-expressing microglia [double positive for 15-PGDH mRNA, Hpgd (red) and Tmem119 mRNA (green)], in symptomatic 5xFAD mice (6 mo old), compared to WT littermates, in the cortex and hippocampus (**P < 0.01, unpaired t test; 4 to 8 brain sections/mouse). (J) RNA FISH shows an increase in Hpgd-expressing PVMs [double positive for 15-PGDH mRNA, Hpgd (red) and Mrc1 mRNA (green)] in symptomatic 5xFAD mice (6 mo old), compared to WT littermates, in the cortex and hippocampus (*P < 0.05, **P < 0.01, unpaired t test; 2 to 7 brain sections/mouse). Expanded view of the boxed region is shown at Lower Right. In all graphs (A–J), data points indicate values of individual human subjects or individual mice.
TBI is a major risk factor for AD, and these conditions also display various common pathological features (23). To interrogate a potential role for 15-PGDH in TBI, we next analyzed transcriptomic microarray data from human chronic traumatic encephalopathy (CTE) cases (41), a neurodegenerative condition of the brain caused by repetitive TBI. We identified a significant 30% elevation in 15-PGDH brain mRNA in CTE compared to non-CTE controls (Fig. 1D). Supporting these human findings, brain 15-PGDH activity was also comparably elevated in the well-established preclinical mouse model of multimodal TBI (43–51) (Fig. 1E).
Aging is another major risk factor for AD. To investigate whether 15-PGDH increases in the aging brain, we analyzed publicly available human brain microarray data from individuals aged 24 to 106 y (52). This analysis revealed a significant positive correlation between brain 15-PGDH mRNA levels and advancing age (SI Appendix, Fig. S1C). Mirroring these human findings, aged (22 to 24-mo-old) WT littermate mice also exhibited increased brain 15-PGDH mRNA and enzymatic activity levels compared to younger (5 to 7-mo-old) WT littermate mice (SI Appendix, Fig. S1 D and E). Thus, brain 15-PGDH is increased in human and mouse AD, as well as in the AD risk factors of aging and TBI.
These results motivated us to more deeply explore the possible role of 15-PGDH in AD pathogenesis. Through RNA-fluorescence in situ hybridization (RNA-FISH), we visualized Hpgd-expressing cells and quantified a two- to three-fold increase in both the cortex and the hippocampus of 5xFAD mice, compared to WT littermates (Fig. 1F). To explore the identity of these cells, we analyzed public single-cell RNA-sequencing (sc-RNAseq) data of the mouse brain cortex (42), which revealed that 15-PGDH expression was most prominent in a myeloid cell cluster annotated as microglial and peri-vascular macrophages (PVMs) (SI Appendix, Fig. S2A, annotated as micro-PVM), with 48% of these myeloid cells expressing Hpgd (average expression level of 0.64 log2(sctransform normalized units) [log2(SCT)] (53) SI Appendix, Fig. S2 B–G). By contrast, the next highest Hpgd-expressing population comprised a rare subset of Layer L2/3 glutamatergic cortical neurons, with only 9% of these neurons showing expression (average expression level of 0.1 log2(SCT) (SI Appendix, Fig. S2 B–E). Notably, 15-PGDH enrichment was similarly observed in the hippocampal myeloid cell (micro-PVM) cluster (SI Appendix, Fig. S2 F and G).
Bead fractionation of murine cortical cells into CD11b+ and CD11b− populations confirmed an approximately 200-fold enrichment of 15-PGDH enzyme activity in the CD11b+ myeloid cell population (SI Appendix, Fig. S3A). Visualization by dual RNA-FISH further confirmed prominent localization of cortical Hpgd mRNA expression to Itgam (encoding CD11b)-marked myeloid cells (SI Appendix, Fig. S3B). Furthermore, combination of dual RNA-FISH plus CD31+ immunofluorescence staining revealed that the 15-PGDH-expressing myeloid cells were localized near cerebral vasculature, with approximately 50% of 15-PGDH and CD11b double positive (Hpgd+:Itgam+) cells residing within 5 µm of CD31+ blood vessel endothelial cells (SI Appendix, Fig. S3B). Additionally, western blot analysis detected 15-PGDH protein expression in CD11b+ but not CD11b- cells (SI Appendix, Fig. S3C). Furthermore, consistent with elevation of 15-PGDH in AD, an ~ fivefold increase in 15-PGDH protein was observed in CD11b+ cells from 5xFAD versus WT mice as revealed by western blot analysis (Fig. 1G and SI Appendix, Fig. S3C),
Subcluster analysis of the sc-RNAseq-defined Hpgd+ microglial-PVM populations revealed Hpgd to be expressed by two distinct myeloid cell subsets: Mrc1+ (PVMs) (54) and Tmem119+ (brain microglia) (55) (Fig. 1H). Dual RNA-FISH confirmed 15-PGDH colocalization with both microglia (Hpgd+ red and Tmem119+ green, double positive) and PVMs (Hpgd+ red and Mrc1+ green, double positive) in both 5xFAD and WT mice (Fig. 1 I and J). Notably, 5xFAD mice exhibited a twofold increase in both 15-PGDH-expressing microglia and PVMs within both the cortical and hippocampal regions, as compared to WT littermates (Fig. 1 I and J). In sum, our findings demonstrate that 15-PGDH is predominantly enriched in brain myeloid cells tightly associated with the BBB, with 15-PGDH level and cellular prevalence markedly elevated in the AD mouse brain.
15-PGDH Inhibition Prevents Cognitive Impairment in AD without Reducing Amyloid Pathology.
Given the established roles of microglia and PVMs in AD pathogenesis (56, 57), we hypothesized that pharmacologic or genetic inhibition of elevated 15-PGDH activity would mitigate cognitive impairment in 5xFAD mice. To test our hypothesis, we administered the 15-PGDH inhibitor (+)-SW033291 (5 mg/kg twice daily via intraperitoneal (IP) injection, or vehicle) to 5xFAD mice and WT littermates from 2 to 6 mo of age (Fig. 2A). This regimen was selected based on the therapeutic efficacy of (+)-SW033291 in prior disease models (26–28, 33) and the onset of cognitive deficits in 5xFAD mice at 6 mo. Pharmacokinetic analysis confirmed sustained elevation of (+)-SW033291 in plasma and brain for up to 6 h postinjection (SI Appendix, Fig. S4 A and B), with brain concentrations sufficient to nearly abolish 15-PGDH enzymatic activity (Fig. 2B).
Fig. 2.
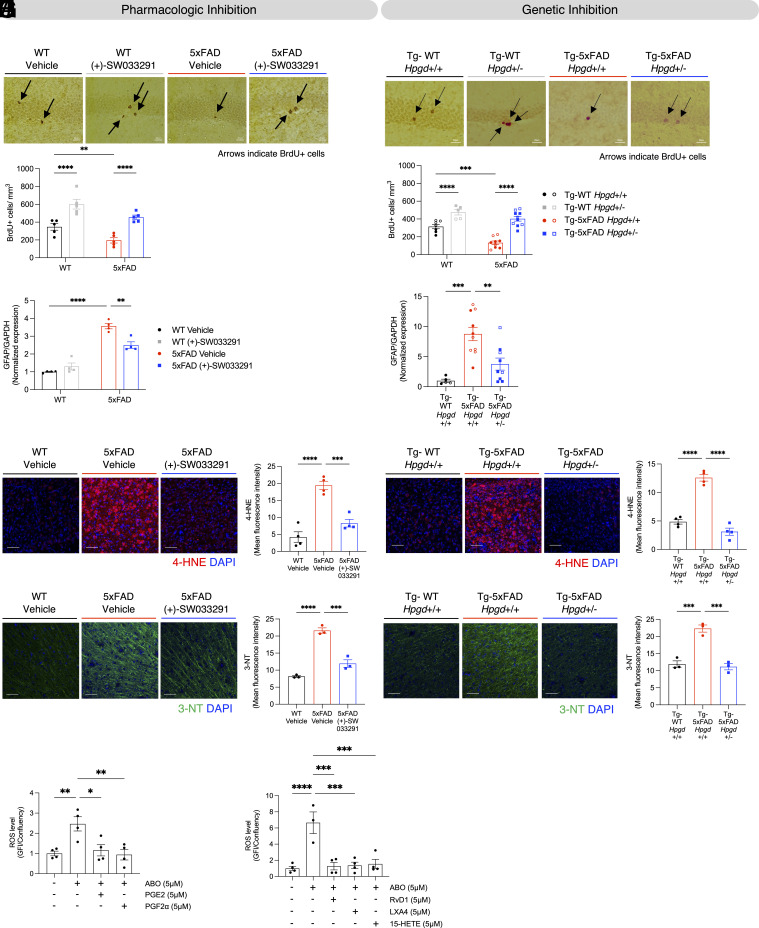
15-PGDH inhibition protects 5xFAD mice from cognitive impairment and BBB deterioration without reducing amyloid pathology. (A) Schematic diagram of experimental procedure for evaluating the protective efficacy of pharmacologic inhibition of 15-PGDH. Mice were intraperitoneally (IP) injected with (+)-SW033291 twice daily (5 mg/kg × 2 = 10 mg/kg/day) from 2 to 6 mo of age. At 5 mo of age, a bolus of bromodeoxyuridine (BrdU, 150 mg/kg) was administered to label newborn hippocampal neurons. Morris water maze (MWM) testing of cognition was conducted 1 wk before brain tissue was harvested for analysis at 6 mo of age. Males (shown as filled circles or filled squares in subsequent panels) and females (shown as open circles or open squares in subsequent panels) of all four combinations of 5xFAD genotype and treatment groups were housed under normal conditions. (B) Treatment with (+)-SW033291 for 4 mo markedly reduced brain 15-PGDH activity in both 5xFAD mice and WT littermates, as compared to vehicle-treated mice (Interaction P < 0.0001, **P < 0.01, ****P < 0.0001, two-way ANOVA and Tukey’s post hoc analysis). 15-PGDH activity was also higher in vehicle-treated 5xFAD mice than vehicle-treated WT littermates, consistent with the results shown in Fig. 1C. (C) (+) SW033291 fully prevents cognitive deficits in 5xFAD mice in the MWM test. In vehicle-treated animals, 5xFAD mice performed significantly worse than WT littermates, as shown by a significantly longer latency time to first cross the platform area. However, in 5xFAD mice treated with (+)-SW033291, performance (latency time) was preserved at the normal WT littermate vehicle levels. Performance was identical between WT mice treated with either vehicle or (+)-SW033291 (Interaction P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA and Tukey’s post hoc analysis). (D) Schematic diagram of experimental procedure for evaluating the protective efficacy of genetic haploinsufficiency of 15-PGDH (Hpgd+/-) in 5xFAD mice. Tg-WT and Tg-5xFAD refer to 5xFAD genotypes, respectively, designating the absence or presence of the 5xFAD transgene, and Hpgd+/+ and Hpgd+/- denote Hpgd genotype. Mice were injected with a bolus of BrdU (150 mg/kg) 1 mo before brains were harvested to label newborn hippocampal neurons. MWM testing of cognition was conducted 1 wk before brain tissue was harvested for analysis at 9 mo of age. Males (shown as filled circles or filled squares in subsequent panels) and females (shown as open circles or open squares in subsequent panels) of all four combinations of 5xFAD and 15-PGDH genotypes were housed under normal conditions. (E) Hpgd haploinsufficiency fully blocks the increase in 15-PGDH enzyme activity in symptomatic 5xFAD mice. Brain 15-PGDH enzyme activity is highly elevated in Tg-5xFAD Hpgd+/+ mice compared to control Tg-WT Hpgd+/+ mice and is preserved at control levels in Tg-5xFAD Hpgd+/- mice, with no difference detected between Tg-WT Hpgd+/+ and Tg-5xFAD Hpgd+/- mice (**P < 0.01, one-way ANOVA and Tukey’s post hoc analysis). (F) Hpgd haploinsufficiency prevents cognitive impairment in 5xFAD mice in the MWM. In the MWM probe test of memory, Tg-5xFAD Hpgd+/+ mice performed significantly worse than control Tg-WT Hpgd+/+ mice, as shown by significantly longer latency to first cross the platform area. Tg-5xFAD Hpgd+/- mice were protected from memory deficits, as evidenced by latency to first cross the platform area in the memory test being significantly lower than Tg-5xFAD Hpgd+/+ mice and not significantly different from Tg-WT Hpgd+/+ mice (Interaction P < 0.05, *P < 0.05, ****P < 0.0001, two-way ANOVA and Tukey’s post hoc analysis). (G) Aβ 4G8 staining shows that (+)-SW033291-mediated inhibition of 15-PGDH does not affect amyloid deposition in the 5xFAD mice hippocampus (**P < 0.001, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 50 µm) 3 to 4 images/mouse). (H) Aβ 4G8 staining shows that genetic inhibition of 15-PGDH does not affect amyloid deposition in the Tg-5xFAD mice hippocampus (**P < 0.01, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 50 µm) n = 3 to 4 images/mouse). (I) Transmission electron microscopy (TEM) shows structural damage of the BBB, as measured by the percentage of brain capillaries affected by astrocyte endfeet swelling in 5xFAD mice as compared to WT littermates. This damage was completely prevented by (+)-SW033291 treatment (****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 2 µm). Representative pictures are shown on the top (each graphed data point represents the average value from one mouse, with 50 images/mouse). (J) TEM shows structural damage of the BBB, as measured by the percentage of brain capillaries affected by astrocyte endfeet swelling in Tg-5xFAD Hpgd+/+ mice as compared to control Tg-WT Hpgd+/+ mice. Hpgd haploinsufficiency completely prevented this BBB damage (Tg-5xFAD Hpgd+/- versus Tg-5xFAD Hpgd+/+ comparison) (****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 2 µm). Representative pictures are shown on the Top (each graphed data point represents the average value from one mouse, with 50 images/mouse). (K) IgG staining shows functional impairment and increased permeability of the BBB as evidenced by IgG infiltration into the brain parenchyma in 5xFAD mice, compared to WT littermates. (+)-SW033291 treatment protects 5xFAD mice from this functional impairment of the BBB. (*P < 0.05, ***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 50 µm) 2 to 3 brain sections/mouse). (L) IgG staining shows functional impairment and increased permeability of the BBB as evidenced by IgG infiltration into the brain parenchyma in Tg-5xFAD Hpgd+/+ mice, compared to control Tg-WT Hpgd+/+ mice. Hpgd haploinsufficiency protects Tg-5xFAD Hpgd+/- mice from this impairment (Tg-5xFAD Hpgd+/+ compared to Tg-5xFAD Hpgd+/-) (*P < 0.05, **P < 0.01, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 50 µm) 2 to 3 brain sections/mouse). In all graphs (A–L), data points indicate values of individual mice.
Consistent with prior observations, 6-mo-old 5xFAD VEH (vehicle-treated) mice exhibited significant deficits in the MWM probe test as demonstrated by both prolonged latency and decreased number of platform crossings (Fig. 2C and SI Appendix, Fig. S4C), measures of hippocampal-dependent spatial memory. Strikingly, (+)-SW033291 treatment fully prevented this impairment (Fig. 2C and SI Appendix, Fig. S4C). No differences in learning and swim speed were observed between treatment or genotype groups, confirming the absence of confounding motor effects (SI Appendix, Fig. S4 D–F).
To independently validate these findings, we generated 5xFAD mice with genetic 15-PGDH reduction via Hpgd haploinsufficiency (heterozygous deletion, Fig. 2D). At 9 mo of age, when pathology in 5xFAD mice has greatly advanced, Hpgd haploinsufficiency suppressed elevated brain 15-PGDH enzymatic activity (Fig. 2E) and prevented 5xFAD mice from developing learning and memory deficits (Fig. 2F and SI Appendix, Fig. S4 G and H). Notably, cognitive protection occurred without alterations in swim speed (SI Appendix, Fig. S4 I and J). Additionally, both pharmacologic and genetic inhibition of 15-PGDH did not affect body weight gain over the treatment period or throughout the experimental period (SI Appendix, Fig. S5 A and B). These concordant pharmacologic and genetic results demonstrate that holding 15-PGDH activity to normal levels is sufficient to prevent AD-associated cognitive decline.
Critically, neither 15-PGDH inhibition strategy altered amyloid pathology. (+)-SW033291-treated and Hpgd-haploinsufficient 5xFAD mice both exhibited Aβ deposition equivalent to vehicle-treated AD mice (4G8 antibody; Fig. 2 G and H and SI Appendix, Fig. S5 C and D), with similar equivalency for Congo red-positive amyloid structures (SI Appendix, Fig. S5 E and F). These findings establish that inhibiting 15-PGDH effectively dissociates cognitive protection from amyloid pathology in AD, highlighting 15-PGDH as a therapeutic target for AD.
15-PGDH Inhibition Preserves BBB Integrity and Attenuates Neurodegeneration in AD.
Given the observed upregulation of 15-PGDH in AD and its prominent localization within brain myeloid cells at the BBB, we hypothesized that 15-PGDH inhibition could mitigate BBB dysfunction in AD. TEM analysis of symptomatic 5xFAD mice revealed major structural BBB impairments, most notably astrocytic endfeet swelling surrounding cerebral vasculature, a phenotype absent in age-matched WT littermates (Fig. 2 I and J). Notably, both pharmacologic and genetic inhibition of 15-PGDH robustly prevented BBB structural degradation in 5xFAD mice (Fig. 2 I and J).
The glial limitans barrier, formed through astrocytic endfeet interactions with endothelial cells, normally prevents infiltration of blood-derived components into brain tissue and represents a region of functional compromise associated with astrocytic endfeet swelling (58). To assess functional consequences, we confirmed that both pharmacologic and genetic 15-PGDH inhibition preserved effectual BBB integrity by blocking pathological leakage of immunoglobulin (IgG) demonstrated in the brain parenchyma of 5xFAD mice (Fig. 2 K and L). These findings demonstrate that inhibiting 15-PGDH rescues both BBB architecture and function in AD.
We next investigated whether preserving the BBB via 15-PGDH-inhibition also conferred neuroprotection. The hippocampus, a region exquisitely vulnerable to BBB disruption (2), exhibits early neurodegeneration in human AD and mouse models, characterized by progressively increased rate of loss of newborn hippocampal neurons. Survival of these neurons is critical for memory formation, and impairment in their survival is a hallmark of AD in humans and 5xFAD mice (59, 60). We therefore labeled newborn hippocampal neurons with a single bolus injection of bromodeoxyuridine (BrdU) and assessed their survival following genetic or pharmacologic 15-PGDH inhibition.
In non-AD mice, genetic deletion of Hpgd doubled the survival of BrdU-tagged newborn hippocampal neurons compared to WT littermates (SI Appendix, Fig. S6A). Similarly, pharmacological 15-PGDH inhibition in (+)-SW033291-treated WT mice enhanced survival of newborn hippocampal neurons by over twofold (SI Appendix, Fig. S6B), but showed no additional effect when given to 15-PGDH KO mice (SI Appendix, Fig. S6B), thereby confirming the on-target specificity of drug effect in WT mice. Notably, we observed that increased numbers of newborn hippocampal neurons were due to 15-PGDH inhibition improving neuronal survival, and not to increasing neural precursor cell proliferation, as 15-PGDH inhibition did not alter BrdU+ counts at 1 h postlabeling (SI Appendix, Fig. S6C). Furthermore, administering P7C3-A20, a validated prosurvival aminopropyl carbazole (47, 61–67), enhanced newborn neuron survival in 15-PGDH KO mice (SI Appendix, Fig. S6D), indicating that 15-PGDH KO does not simply saturate the capacity for further augmenting neuronal survival with a drug.
In 6-mo-old 5xFAD mice, newborn hippocampal neuron survival was reduced to approximately 50% that of WT mice (Fig. 3A). Remarkably, pharmacologic 15-PGDH inhibition fully rescued this neurodegeneration and attenuated axonal degeneration in the dorsal fornix, a major hippocampal output tract (Fig. 3A and SI Appendix, Fig. S6E). Genetic 15-PGDH haploinsufficiency (Hpgd+/-) similarly restored survival of adult-born neurons in 5xFAD mice to WT levels (Fig. 3B), showing that even partial 15-PGDH suppression suffices for neuroprotection.
Fig. 3.
15-PGDH inhibition protects 5xFAD mice from decreased survival of newborn hippocampal neurons, neuroinflammation, and oxidative stress. (A) 6-mo-old symptomatic 5xFAD mice have impaired survival of BrdU-labeled newborn hippocampal neurons (black arrows), compared to WT littermates, as assessed 28 d post–BrdU injection. (+)-SW033291 treatment preserves normal survival of newborn hippocampal neurons in 5xFAD mice (genotype P < 0.001, treatment P < 0.0001, **P < 0.01, ****P < 0.0001, two-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 20 µm). Each graphed dot represents the average value of BrdU-labeled cells from an individual mouse as determined from 5 to 6 sections spaced at least 400 µm apart across the hippocampus. (B) Tg-5xFAD Hpgd+/+ mice have impaired survival of BrdU-labeled newborn hippocampal neurons (black arrows), versus Tg-WT Hpgd+/+ littermates, as assessed 28 d post–BrdU injection. Genetic reduction of 15-PGDH in Tg-5xFAD Hpgd+/- mice restores newborn hippocampal neuron survival. (Interaction P < 0.05, genotype (Tg-WT vs Tg-5xFAD) P < 0.0001, genotype (Hpgd+/+ vs Hpgd+/-) P < 0.0001, ***P < 0.001, ****P < 0.0001, two-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 20 µm). Each dot represents the average value of BrdU-labeled cells from an individual mouse determined from 5 to 6 sections each spaced at least 400 µm apart across the hippocampus. (C) 5xFAD mice show increased hippocampal GFAP expression, which is significantly reduced by treatment with (+)-SW033291 (Interaction P < 0.001, **P < 0.01, ****P < 0.0001, two-way ANOVA and Tukey’s post hoc analysis). (D) Tg-5xFAD Hpgd+/+ mice show increased whole-brain GFAP expression compared to Tg-WT Hpgd+/+ mice, which is significantly prevented in Tg-5xFAD Hpgd+/- mice (**P < 0.01, ***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis). (E) 4-HNE staining analysis of the brain cortex and its quantification show increased 4-HNE in 5xFAD mice, relative to WT littermates, which is significantly prevented by (+)-SW033291 treatment (***P < 0.001, ****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis; 2 to 3 brain sections/mouse). (F) 4-HNE staining analysis of the brain cortex and its quantification show increased 4-HNE in Tg-5xFAD Hpgd+/+ mice compared to Tg-WT Hpgd+/+ mice, which is fully prevented in Tg-5xFAD Hpgd+/- mice (****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis; 2 to 3 brain sections/mouse). (G) 3-NT staining analysis of the brain cortex and its quantification show increased 3-NT in 5xFAD mice, relative to WT littermates, which is significantly prevented by (+)-SW033291 treatment (***P < 0.001, ****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis; 2 to 3 brain sections/mouse). (H) 3-NT staining analysis of the brain cortex and its quantification show increased 3-NT in Tg-5xFAD Hpgd+/+ mice compared to Tg-WT Hpgd+/+ mice, which is completely prevented in Tg-5xFAD Hpgd+/- mice (***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis; 2 to 3 brain sections/mouse). (I) Pretreatment with prostanoid substrates of 15-PGDH, either 5 µM PGE2 or 5 µM PGF2α, suppressed ROS induction assayed 30 min after 5 µM Aβ oligomer treatment (*P < 0.05, **P < 0.01, one-way ANOVA and Tukey’s post hoc analysis). (J) Pretreatment with 15-PGDH substrates, RvD1, LXA4, 15-HETE suppressed ROS induction assayed 30 min after 5 µM Aβ oligomer treatment (***P < 0.001, ****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis). In all graphs (A–J), data points indicate the value for an individual mouse or replicate of tested cells.
15-PGDH Inhibition Attenuates Astrocyte Reactivity and Microglial Oxidative Stress in AD.
Given the enrichment of 15-PGDH in myeloid cells, we hypothesized that in addition to protecting the BBB, inhibiting 15-PGDH would also reduce AD-associated neuroinflammation and oxidative stress. To evaluate this, we first measured protein levels of GFAP, a marker of astrocyte reactivity, and IBA1, a marker of microglia activation, (68, 69) in 5xFAD mice. Elevated GFAP and IBA1 expression in 5xFAD mice (Fig. 3 C and D and SI Appendix, Fig. S7 A–D) confirmed heightened neuroinflammatory responses. Strikingly, both pharmacologic and genetic 15-PGDH inhibition significantly reduced GFAP levels, indicating suppression of astrocyte-driven neuroinflammation, while IBA1 levels remained unchanged (Fig. 3 C and D and SI Appendix, Fig. S7 A–D).
Increased susceptibility to oxidative stress is a hallmark of the AD brain, driven by high oxygen demand, lipid-rich neuronal membranes prone to peroxidation (70), and increased generation of reactive oxygen species (ROS) from activated immune cells (71). We assessed 4-hydroxy-2-nonenal (4-HNE), a ROS-generated lipid peroxidation byproduct known to be elevated in human AD and 5xFAD brains (72, 73). 5xFAD mice exhibited approximately a 3-fold increase in 4-HNE, which could be fully prevented by pharmacologically and genetically inhibiting 15-PGDH (Fig. 3 E and F). Similarly, 3-nitrotyrosine (3-NT), a deleterious protein modification caused by peroxynitrite, which is formed by the reaction of superoxide radicals with nitric oxide, is also elevated in human AD and 5xFAD mice (74, 75), and we observed that 3-NT was reduced to baseline levels by pharmacologic and genetic 15-PGDH inhibition (Fig. 3 G and H).
To further investigate microglial involvement in mediating 15-PGDH inhibition’s protection from neuroinflammation, we studied mouse microglia BV2 cells treated with oligomerized Aβ, an in vitro model known to induce ROS (SI Appendix, Fig. S8 A) (76). Remarkably, ROS generation was significantly suppressed by treating BV2 cells with 5 uM concentrations of any five different substrates that 15-PGDH normally acts to limit and degrade: PGE2, PGF2α, RvD1, LXA4, and 15-HETE (Fig. 3 I and J). All these treatments had no effect on BV2 cell confluency (SI Appendix, Fig. S8 A–C).
Thus, our results show that 15-PGDH inhibition-mediated protection of the BBB in AD is tightly associated with attenuation of neuroinflammation and oxidative stress, likely through preservation of a family of anti-inflammatory lipid mediators whose degradation is directly catalyzed by 15-PGDH. These findings highlight 15-PGDH as a therapeutic target whose inhibition directly disrupts neuroinflammation and oxidative damage in AD.
15-PGDH Inhibition Preserves BBB Integrity and Mitigates Neurodegeneration in TBI.
As TBI is a major risk factor for AD and is associated with elevated 15-PGDH, we also investigated the neuroprotective potential of 15-PGDH inhibition in TBI. To begin, we administered the 15-PGDH inhibitor (+)-SW033291 (5 mg/kg twice daily, IP) beginning 24 h after TBI (Fig. 4A). We tested this time point because we reasoned that most patients experiencing a TBI could likely access medical care within a day of injury.
Fig. 4.
15-PGDH inhibition protects mice from TBI-induced cognitive impairment, oxidative stress, neurodegeneration, and BBB damage. (A) Schematic diagram of experimental procedure for evaluating the protective efficacy of (+)-SW033921-mediated 15-PGDH inhibition in TBI. 8-wk-old mice were administered multimodal TBI on day 0, and 24 h later started on a 21 d regimen of twice daily (+)-SW03391 (5 mg/kg × 2 = 10 mg/kg/day). MWM testing was conducted between days 14 and 18, and brain tissue was harvested for analysis on day 21. (B) Post–TBI administration of (+)-SW033291, initiated 24 h after injury and continued for 2 wk, inhibited brain 15-PGDH activity (****P < 0.0001, unpaired t test). (C) Vehicle-treated TBI-injured mice show significantly worse memory than sham-injury mice, as evidenced by significantly greater latency time to cross the platform area in the MWM probe test of memory. Treatment of TBI mice with (+)-SW033921 completely prevented post–TBI memory impairment (Interaction P < 0.01, ****P < 0.0001, two-way ANOVA and Tukey’s post hoc analysis). (D) Vehicle-treated TBI-injured mice showed significantly worse memory than sham-injury mice, as shown by a significantly lower number of platform area crossings on the MWM probe test of memory. Treatment of TBI mice with (+)-SW033921 prevented post–TBI memory impairment. (Interaction P < 0.05, **P < 0.01, two-way ANOVA and Tukey’s post hoc analysis). (E) TBI induces axonal degeneration, which was reversed by 15-PGDH pharmacologic inhibition with (+)-SW03329. Axonal degeneration was visualized and quantified by silver staining analysis, using percent positive area. (**P < 0.01, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 5 µm). (Each dot represents average value from one mouse as determined from 6 images/mouse). (F) TEM analysis and its quantification show that TBI-induced structural BBB damage, as evidenced by swelling of astrocyte endfeet, was blocked by 15-PGDH pharmacologic inhibition with (+)-SW033291 (****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis). (G) 3-NT staining and its quantification show that TBI-induced oxidative stress, as evidenced by increased 3-NT, was blocked by 15-PGDH pharmacologic inhibition with (+)-SW033291 (***P < 0.001, ****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis; 2 to 3 brain sections/mouse). (H) Schematic diagram of experimental procedure for evaluating the protective efficacy of genetic 15-PGDH inhibition in TBI. Control 8-wk-old Hpgd+/+ (WT) and Hpgd−/− (KO) mice were subjected to multimodal TBI. Brain tissue was harvested for analysis 3 wk after TBI. (I) TBI induces axonal degeneration, which was reversed by biallelic Hpgd gene deletion (Hpgd−/−). Axonal degeneration was visualized and quantified by silver staining analysis, using percent positive area. (**P < 0.01, ***P < 0.001, one-way ANOVA and Tukey’s post hoc analysis, (Scale bar, 5 µm). (Each dot represents average value from one mouse as determined from 6 images/mouse). (J) Electron microscopy analysis and its quantification show that TBI-induced structural BBB damage, as evidenced by swelling of astrocyte endfeet, was blocked by biallelic Hpgd gene deletion (Hpgd−/−) (****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis). (K) 3-NT staining and its quantification show that TBI-induced oxidative stress, as evidenced by increased 3-NT, was blocked by biallelic Hpgd gene deletion (Hpgd−/−) (****P < 0.0001, one-way ANOVA and Tukey’s post hoc analysis; 2 to 3 brain sections/mouse). In all graphs (A–K), data points indicate values of individual mice.
Pharmacologic inhibition with (+)-SW033291 robustly suppressed TBI-induced 15-PGDH enzymatic activity (Fig. 4B) and completely prevented cognitive deficits in the MWM (Fig. 4 C and D), without affecting swim speed or learning (SI Appendix, Fig. S9 A–C). Notably, severe axonal neurodegeneration induced 3 wk after TBI in WT mice was also fully prevented by (+)-SW033291 treatment (Fig. 4E), as was TBI-induced BBB disruption (Fig. 4F) and oxidative stress damage assayed by 3-NT (Fig. 4G) and 4-HNE (SI Appendix, Fig. S9D). As in AD, oxidative stress damage is a key pathway in the pathogenesis of TBI-induced neurodegeneration (77). In particular, lipid peroxidation is known to rapidly accumulate within hours of TBI, thereby driving the acute phase of pathology, and also to persist chronically and increase the risk of developing other forms of aging-related neurodegeneration, such as AD (23). Consistent with our previous reports in this model (43), we detected no changes in IBA1 or GFAP expression after TBI (SI Appendix, Fig. S9 E–G).
To validate target specificity, we further performed TBI studies in Hpgd+/+ and Hpgd−/− mice (Fig. 4H). Genetic ablation of 15-PGDH mirrored pharmacologic inhibition, conferring complete protection against axonal neurodegeneration (Fig. 4I), BBB damage (Fig. 4J), and oxidative stress (Fig. 4K and SI Appendix, Fig. S9H). Cognitive testing in Hpgd−/− mice was precluded due to their FVB strain background, which impairs visual acuity required for MWM, unlike the C57BL/6 J strain used in the AD studies.
Discussion
This study identifies 15-PGDH as a therapeutic target for AD and TBI, demonstrating that its inhibition preserves BBB integrity and cognitive function. We observed elevated 15-PGDH in aging, TBI, and AD in both human and mouse brains, with prominent localization to brain myeloid cells, including microglia and PVMs at the BBB. Genetic or pharmacologic inhibition of 15-PGDH in mouse models of AD and TBI mitigated perivascular inflammation, protected BBB integrity, and prevented downstream neuroinflammation, oxidative stress, and neurodegeneration. Notably, cognitive function was preserved in 5xFAD mice despite unchanged amyloid pathology, underscoring a therapeutic mechanism distinct from amyloid clearance, that has heretofore been the only target of clinically tested agents for attempting to slow AD progression in patients. Inhibiting 15-PGDH thus provides a route for AD treatment and prevention and addresses the clear and significant unmet need for new and effective therapies for patients with this disease.
Our findings align with prior evidence that depletion of PVMs leads to vascular damage, indicating a protective role for normal PVM physiology in BBB homeostasis (57). However, the interplay between myeloid cells, neuroinflammation, and vascular dysfunction in AD remains mechanistically unclear. We hypothesize that 15-PGDH inhibition is critically positioned to therapeutically interrupt the self-amplifying pathogenic cycle created as ROS generation and BBB degradation reciprocally drive and reinforce one another (78, 79). Future work with myeloid-specific elimination of 15-PGDH will enable rigorous investigation of this mechanism.
15-PGDH acts to catalyze the degradation of a diverse array of bioactive molecules with context-dependent pro- or anti-inflammatory effects (24–26). Our attempts to define global eicosanoid profile changes after 15-PGDH inhibition were confounded by the rapid induction and lability of these signaling molecules. However, our in vitro model of Aβ-mediated ROS induction in cultured microglia cells demonstrates that the protective effect of 15-PGDH inhibition strongly aligns with the resultant blocking of degradation of multiple anti-inflammatory 15-PGDH substrates, each of which is individually able to block ROS generation, and that in vivo are expected to act in combination. Furthermore, these individual 15-PGDH substrates act via multiple different receptors, some with opposing actions, such as raising versus lowering cyclic AMP (80). While our work establishes BBB protection and ROS inhibition as a central therapeutic mechanism of 15-PGDH inhibition, disentangling the contributions of individual 15-PGDH substrates and of their interconnected signaling pathways will be an important priority for future research, as will be applying methods that spatially resolve these signaling events, particularly at the BBB. Indeed, the potential importance of focal signaling events is exemplified by the discordance among different cell-specific knockouts of 2-arachidonoylglycerol (2-AG) degradation, whose efficacy in protecting from TBI runs opposite to their strength in increasing global brain substrate levels (81)
Other priorities for future studies will include interrogating protection from neurodegeneration in genetic models of myeloid-specific elimination of Hpgd, along with determining the respective contributions of inhibiting 15-PGDH in PVMs versus in microglia. Technologies such as dynamic contrast-enhanced MRI (DCE-MRI) or positron emission tomography imaging may assist by enabling direct visualization of BBB permeability in 5xFAD mice as a function of targeted 15-PGDH inhibition. It will also be of interest to test whether elimination of 15-PGDH is additionally protective in tau-based models related to AD.
Collectively, our findings establish 15-PGDH as a guardian of BBB integrity, a critical node in regulating brain ROS generation, and a compelling target for protection from neurodegenerative disease. The translational potential of 15-PGDH inhibition is underscored by the strong therapeutic efficacy in a mouse AD model of even the partial 15-PGDH inhibition achieved by genetic haploinsufficiency. Importantly, biotechnology and pharmaceutical companies have already identified small molecule 15-PGDH inhibitors as targets for development as therapeutics for peripheral disease indications (82). Now, our findings additionally position these agents as promising candidates for repurposing for treating neurodegenerative disorders. Given the enormous unmet need, 15-PGDH inhibition represents a paradigm-shifting therapeutic strategy for AD, TBI, and potentially other neurodegenerative conditions marked by BBB dysfunction.
Materials and Methods
Study design, details on animals used, human subjects, drug preparation, behavioral analysis, immunohistochemistry, in situ hybridization, TEM, microglia and nonmicroglia isolation for western blot, 15-PGDH enzymatic activity assay, in vivo multimodal TBI, microglia and nonmicroglia isolation for 15-PGDH activity assays, in situ hybridization combined with immunohistochemistry, quantification of immunohistochemistry, sample preparation for in situ hybridization, quantification of in situ hybridization, western blotting, quantitative real-time PCR, single-cell transcriptomic data reprocessing, Aβ peptide preparation, ROS measurement, and (+)-SW033291 pharmacokinetics are presented in SI Appendix.
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism, version 9.0.0 (GraphPad Software, Inc.) and R Studio. Distribution of residuals and model errors was visually inspected. For the analysis of two groups, unpaired two-tailed t test was performed. For multiple-group analysis, one-way ANOVA or two-way ANOVA was used depending on the study design. When interaction was not observed in two-way ANOVA, genotype, injury, and treatment effects were assessed. Tukey’s post hoc test was used to compare the means of different groups after ANOVA. For MWM learning data, sphericity of data was tested using the Mauchly test; then, repeated measures ANOVA was performed with Greenhouse–Geisser correction when assumption of sphericity was violated. For single-cell RNA sequencing data, Fisher’s exact test or t test was performed, depending on the hypothesis.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We acknowledge BioRender, a service we used to design our schematic figures. We also acknowledge Dr. Hsin-Hsiung Tai for his seminal contributions to studies of 15-PGDH. A.A.P., S.D.M., A.D.B., N.S.W., and J.M.R. were supported by NIH/NIGMS RM1 GM142002. A.A.P. was supported by The Valour Foundation, the Wick Foundation, the Meisel & Pesses Family Foundation, Department of Veterans Affairs Merit Award I01BX005976, and as the Rebecca E. Barchas, M.D., Professor in Translational Psychiatry of Case Western Reserve University and the Morley-Mather Chair in Neuropsychiatry of University Hospitals of Cleveland Medical Center. A.A.P. and B.D.P were supported by the American Heart Association and Paul Allen Foundation Initiative in Brain Health and Cognitive Impairment (19PABH134580006) and by NIH/NIA 1R01AG071512. A.A.P. also acknowledges support from NIH/NIA RO1AGs066707, NIH/NIA 1 U01 AG073323, the Louis Stokes VA Medical Center resources and facilities, the Mary Alice Smith Funds for Neuropsychiatry Research, the Lincoln Neurotherapeutics Research Fund, the Leonard Krieger Fund of the Cleveland Foundation, and an anonymous donor. B.D.P. acknowledges support from NIH/NIDA P50 DA044123, NIH/NIA 1R21AG073684-01, and RO1AG071512 and funding from the Solve-ME Foundation and the Catalyst Award from Johns Hopkins University. S.D.M. acknowledges support from NIH R35 CA197442, from the Markowitz-Ingalls Chair in Cancer Genetics at Case Western Reserve University, and from the Case Comprehensive Cancer Center. We acknowledge the resources and expertise of the University of Texas Southwestern Medical Center Preclinical Pharmacology Core in evaluating (+)-SW033291 brain and plasma levels. X.Z. was supported by NIH AGO83811, AG056363, and AG049479. M-K.S. was supported by the Research Education Component of Cleveland Alzheimer's Disease Research Center (P30AG072959) and also by the New Faculty Startup Fund (370C-20220110), Creative-Pioneering Researchers Program (370C-20230108), and a research grant (370C-20240120) from Seoul National University. M.-K.S. also acknowledges support from the National Research Foundation of Korea (RS-2023-00209597, RS-2024-00352229, RS-2024-00440679, and RS-2024-00466703), Seoul Research and Business Development program (BT240041), and donors of Alzheimer’s Disease Research, a program of BrightFocus Foundation (A2019551F). Y.K. was supported by NIH/NIA F99 AG083111. S.B. and E.M. were supported by the Alzheimer’s Disease Translational Data Science Training Program NIH T32 AG071474. E.V.-R. was supported by Department of Defense Peer-Reviewed Alzheimer’s Research Program Award AZ210092 (W81XWH-22-1-0129). T.G. was supported by National Institute on Aging P30 AG072977 (Northwestern ADRC), R56 AG075600, and the Karen Toffler Charitable Trust. T.L. was supported by NIH RO1CA217992 and RO1LM013067. F.G. was supported by the Functional Genomics Training Program NIH T32 GM135081. P.S.S. was supported by NIH/NIA F30AG076183 and NIH/NINDS T32NS077888. P.S.S. and S.B. were supported by Case Western Medical Scientist Training program NIH T32 GM007250. H.F. and E.B. were supported by the Case Western Reserve University Youth Engaged in Science/Scientific Enrichment Opportunity Program via NCI R25 CA221718. U.K. and R.A.L.-A. were supported by the University Hospitals of Cleveland Summer Undergraduate Research Experience Scholar Program of the American Heart Association. J.M.R was supported by the Welch Foundation (I-1612). Human brain samples were provided by Northwestern University (T.G and M.F. above) and X.Z. (Cleveland Alzheimer's Disease Research Center P30AG072959).
Author contributions
Y.K., E.V.-R., F.G., S.C., S.J.T., T.L., S.P.F., A.B.D., D.D., N.S.W., J.M.R., B.D.P., M.-K.S., S.D.M., and A.A.P. designed research; Y.K., E.V.-R., F.G., H.L., S.C., S.J.T., S.B., Z.B., A.B., U.P.K., R.A.L.-A., P.S.S., B.A.C., Y.Y., J.H., H. Fang, S.S., R.K., T.L., L.K., J.L., L.B., E.C., C.J.C.-P., K.F., M.F.F., E.M., V.I., K.L.N., M.D., F.B.T.P.L., E.B., H. Fujioka, S.P.F., A.B.D., D.D., N.S.W., Y.-K.K., J.M.R., B.D.P., M.-K.S., S.D.M., and A.A.P. performed research; K.A., C.Z., X.Z., M.E.F., and T.G. contributed new reagents/analytic tools; Y.K., E.V.-R., F.G., H.L., S.C., S.J.T., S.B., Z.B., A.B., U.P.K., R.A.L.-A., P.S.S., B.A.C., Y.Y., J.H., H. Fang, S.S., R.K., L.K., J.L., L.B., E.C., C.J.C.-P., K.F., M.F.F., E.M., M.D., F.B.T.P.L., E.B., B.W., H. Fujioka, S.P.F., A.B.D., D.D., N.S.W., J.M.R., B.D.P., M.-K.S., S.D.M., and A.A.P. analyzed data; and Y.K., E.V.-R., F.G., B.W., N.S.W., J.M.R., B.D.P., M.-K.S., S.D.M., and A.A.P. wrote the paper.
Competing interests
Y.K., E.V.-R., M.-K.S., J.M.R., N.S.W., S.D.M., and A.A.P. hold (+)-SW033291-related patents, some of which have been licensed and/or optioned to Amgen.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Min-Kyoo Shin, Email: minkyooshin@snu.ac.kr.
Sanford D. Markowitz, Email: sanford.markowitz@case.edu.
Andrew A. Pieper, Email: andrew.pieper@case.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Geneva: World Health Organization, Global Action Plan on The Public Health Response to Dementia 2017–2025 (World Health Organization, 2017). [Google Scholar]
- 2.Montagne A., et al. , Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Haar H. J., et al. , Blood-brain barrier leakage in patients with early alzheimer disease. Radiology 281, 527–535 (2016). [DOI] [PubMed] [Google Scholar]
- 4.van de Haar H. J., et al. , Neurovascular unit impairment in early Alzheimer’s disease measured with magnetic resonance imaging. Neurobiol. Aging. 45, 190–196 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Montagne A., et al. , Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 131, 687–707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultman K., Strickland S., Norris E. H., The APOE ε4/ε4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of alzheimer’s disease patients. J. Cereb. Blood Flow Metab. 33, 1251–1258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipser B. D., et al. , Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging. 28, 977–986 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Fiala M., et al. , Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. Eur. J. Clin. Investig. 32, 360–371 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Zenaro E., et al. , Neutrophils promote Alzheimer’s disease–like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Li J., et al. , Development of novel therapeutics targeting the blood-brain barrier: From barrier to carrier. Adv. Sci. 8, 2101090 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S., et al. , Targeting oxidative stress and inflammatory response for blood-brain barrier protection in intracerebral hemorrhage. Antioxid. Redox Signal. 37, 115–134 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Barisano G., et al. , Blood–brain barrier link to human cognitive impairment and Alzheimer’s disease. Nat. Cardiovasc. Res. 1, 108–115 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nation D. A., et al. , Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrahamson E. E., Ikonomovic M. D., Brain injury-induced dysfunction of the blood brain barrier as a risk for dementia. Exp. Neurol. 328, 113257 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Toledo J. B., et al. , Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136, 2697–2706 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padrela B., et al. , Developing blood-brain barrier arterial spin labelling as a non-invasive early biomarker of Alzheimer’s disease (DEBBIE-AD): A prospective observational multicohort study protocol. BMJ Open 14, e081635 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrall A. J., Wardlaw J. M., Blood–brain barrier: Ageing and microvascular disease – systematic review and meta-analysis. Neurobiol. Aging. 30, 337–352 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Braak H., Braak E., Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiol. Aging. 16, 271–278 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Tredici K. D., Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112, 389–404 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Flores R., Joie R. L., Chételat G., Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 309, 29–50 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Dennis E. L., Thompson P. M., Functional brain connectivity using fMRI in aging and alzheimer’s disease. Neuropsychol. Rev. 24, 49–62 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krajcovicova L., Marecek R., Mikl M., Rektorova I., Disruption of resting functional connectivity in alzheimer’s patients and at-risk subjects. Curr. Neurol. Neurosci. Rep. 14, 491 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Barker S., Paul B. D., Pieper A. A., Increased risk of aging-related neurodegenerative disease after traumatic brain injury. Biomedicines 11, 1154 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai H.-, Cho H., Tong M., Ding Y., NAD+-linked 15-hydroxyprostaglandin dehydrogenase: Structure and biological functions. Curr. Pharm. Des. 12, 955–962 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Wang D., DuBois R. N., Eicosanoids and cancer. Nat. Rev. Cancer 10, 181–193 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., et al. , Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science 348, aaa2340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho W. J., et al. , 15-PGDH regulates hematopoietic and gastrointestinal fitness during aging. PLoS ONE 17, e0268787 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palla A. R., et al. , Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science 371, eabc8059 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith J. N. P., et al. , Therapeutic targeting of 15-PGDH in murine pulmonary fibrosis. Sci. Rep. 10, 11657 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bärnthaler T., et al. , Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J. Allergy Clin. Immunol. 145, 818–833.e11 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Kim H. J., et al. , Inhibition of 15-PGDH prevents ischemic renal injury by the PGE2/EP4 signaling pathway mediating vasodilation, increased renal blood flow, and increased adenosine/A2A receptors. Am. J. Physiol.-Ren. Physiol. 319, F1054–F1066 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y., et al. , Inhibition of 15-hydroxyprostaglandin dehydrogenase protects neurons from ferroptosis in ischemic stroke. MedComm 5, e452 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakooshli M. A., et al. , Regeneration of neuromuscular synapses after acute and chronic denervation by inhibiting the gerozyme 15-prostaglandin dehydrogenase. Sci. Transl. Med. 15, eadg1485 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubino M., et al. , Inhibition of eicosanoid degradation mitigates fibrosis of the heart. Circ. Res. 132, 10–29 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Corwin C., et al. , Prostaglandin D2/J2 signaling pathway in a rat model of neuroinflammation displaying progressive parkinsonian-like pathology: Potential novel therapeutic targets. J. Neuroinflamm. 15, 272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen Institute for Brain Science, Aging, Dementia and TBI Study (Allen Institute for Brain Science, 2016), http://aging.brain-map.org/. [Google Scholar]
- 37.Miller J. A., et al. , Neuropathological and transcriptomic characteristics of the aged brain. eLife 6, e31126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oakley H., et al. , Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen R. M., et al. , A transgenic alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric Aβ, and frank neuronal loss. J. Neurosci. 33, 6245–6256 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voorhees J. R., et al. , −)-P7C3-S243 protects a rat model of alzheimer’s disease from neuropsychiatric deficits and neurodegeneration without altering amyloid deposition or reactive glia. Biol. Psychiatry 84, 488–498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seo J.-S., et al. , Transcriptome analyses of chronic traumatic encephalopathy show alterations in protein phosphatase expression associated with tauopathy. Exp. Mol. Med. 49, e333 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao Z., et al. , A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184, 3222–3241.e26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin T. C., et al. , P7C3 neuroprotective chemicals block axonal degeneration and preserve function after traumatic brain injury. Cell Rep. 8, 1731–1740 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper M. M., et al. , Identification of chronic brain protein changes and protein targets of serum auto-antibodies after blast-mediated traumatic brain injury. Heliyon 6, e03374 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin T. C., et al. , Acute axonal degeneration drives development of cognitive, motor, and visual deficits after blast-mediated traumatic brain injury in mice. eNeuro 3, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin M.-K., et al. , Reducing acetylated tau is neuroprotective in brain injury. Cell 184, 2715–2732.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vázquez-Rosa E., et al. , P7C3-A20 treatment one year after TBI in mice repairs the blood–brain barrier, arrests chronic neurodegeneration, and restores cognition. Proc. Natl. Acad. Sci. U.S.A. 117, 27667–27675 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wattiez A.-S., et al. , Different forms of traumatic brain injuries cause different tactile hypersensitivity profiles. Pain 162, 1163–1175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sridharan P. S., et al. , Acutely blocking excessive mitochondrial fission prevents chronic neurodegeneration after traumatic brain injury. Cell Rep. Med. 5, 101715 (2024), 10.1016/j.xcrm.2024.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chornyy S., et al. , Longitudinal in vivo monitoring of axonal degeneration after brain injury. Cell Rep. Methods 3, 100481 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin M.-K., et al. , Characterization of the jet-flow overpressure model of traumatic brain injury in mice. Neurotrauma Rep. 2, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu T., et al. , REST and stress resistance in ageing and Alzheimer’s disease. Nature 507, 448–454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hafemeister C., Satija R., Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galea I., et al. , Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia 49, 375–384 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Bennett M. L., et al. , New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U.S.A. 113, E1738–E1746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keren-Shaul H., et al. , A unique microglia type associated with restricting development of alzheimer’s disease. Cell 169, 1276–1290.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Park L., et al. , Brain perivascular macrophages initiate the neurovascular dysfunction of alzheimer Aβ peptides. Circ. Res. 121, 258–269 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yue Q., Hoi M. P. M., Emerging roles of astrocytes in blood-brain barrier disruption upon amyloid-beta insults in Alzheimer’s disease. Neural Regen. Res. 18, 1890–1902 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreno-Jiménez E. P., et al. , Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25, 554–560 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Farioli-Vecchioli S., Ricci V., Middei S., Adult hippocampal neurogenesis in Alzheimer’s Disease: An overview of human and animal studies with implications for therapeutic perspectives aimed at memory recovery. Neural Plast. 2022, 9959044 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sridharan P. S., Miller E., Pieper A. A., Application of P7C3 compounds to investigating and treating acute and chronic traumatic brain injury. Neurotherapeutics 20, 1616–1628 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pieper A. A., et al. , Discovery of a proneurogenic, neuroprotective chemical. Cell 142, 39–51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pieper A. A., McKnight S. L., Ready J. M., P7C3 and an unbiased approach to drug discovery for neurodegenerative diseases. Chem. Soc. Rev. 43, 6716–6726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bavley C. C., et al. , Dopamine D1R-neuron cacna1c deficiency: A new model of extinction therapy-resistant post-traumatic stress. Mol. Psychiatry 26, 2286–2298 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee A. S., et al. , The neuropsychiatric disease-associated gene cacna1c mediates survival of young hippocampal neurons 1,2,3. eNeuro 3, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaya M. O., et al. , Neurotherapeutic capacity of P7C3 agents for the treatment of Traumatic Brain Injury. Neuropharmacology 145, 268–282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker A. K., et al. , The P7C3 class of neuroprotective compounds exerts antidepressant efficacy in mice by increasing hippocampal neurogenesis. Mol. Psychiatry 20, 500–508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Escartin C., et al. , Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lull M. E., Block M. L., Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cermenati G., et al. , Lipids in the nervous system: From biochemistry and molecular biology to patho-physiology. Biochim. Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 1851, 51–60 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Simpson D. S. A., Oliver P. L., ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 9, 743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domenico F. D., Tramutola A., Butterfield D. A., Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med. 111, 253–261 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Shin S.-W., Kim D.-H., Jeon W. K., Han J.-S., 4-Hydroxynonenal Immunoreactivity Is Increased in the Frontal Cortex of 5XFAD Transgenic Mice. Biomedicines 8, 326 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sultana R., et al. , Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol. Dis. 22, 76–87 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Modi K. K., Roy A., Brahmachari S., Rangasamy S. B., Pahan K., Cinnamon and its metabolite sodium benzoate attenuate the activation of p21rac and protect memory and learning in an animal model of Alzheimer’s disease. PLoS ONE 10, e0130398 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geng L., Fan L. M., Liu F., Smith C., Li J.-M., Nox2 dependent redox-regulation of microglial response to amyloid-β stimulation and microgliosis in aging. Sci. Rep. 10, 1582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fesharaki-Zadeh A., Oxidative stress in traumatic brain injury. Int. J. Mol. Sci. 23, 13000 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pun P. B. L., Lu J., Moochhala S., Involvement of ROS in BBB dysfunction. Free Radic. Res. 43, 348–364 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Song K., et al. , Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Med. Cell. Longev. 2020, 1–27 (2020). [Google Scholar]
- 80.Sugimoto Y., Narumiya S., Prostaglandin E receptors*. J. Biol. Chem. 282, 11613–11617 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Hu M., et al. , Enhancing endocannabinoid signalling in astrocytes promotes recovery from traumatic brain injury. Brain: A J. Neurol. 145, 179–193 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gwaltney S. L., et al. , Compositions and methods of modulating short-chain dehydrogenase activity (2021). https://patents.google.com/patent/WO2021236779A1/en. Accessed 25 November 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.