Abstract
Nanoparticle-based drug delivery systems hold promise for tumor therapy; however, they frequently encounter challenges such as low delivery efficiency and suboptimal efficacy. Engineered living cells can redirect drug delivery systems to effectively reach targeted sites. Here, we used living macrophages as vehicles, attaching them with GeS nanosheets (GeSNSs) carrying β-elemene for transport to tumor sites. GeSNSs act as efficient sonosensitizers, enhancing ultrasound-induced reactive oxygen species generation for treating 4T1 breast tumors. Notably, macrophage hitchhiking delivery of β-elemene–loaded GeSNSs not only achieves high accumulation in tumor regions and suppresses tumor growth under ultrasound treatment, but also effectively remodels the immunosuppressive tumor microenvironment by improving M1-like macrophage polarization and enhancing the populations of mature dendritic cells, CD4+, and CD8+ lymphocytes, thereby facilitating enhanced sonodynamic chemoimmunotherapy. These findings underscore the potential of macrophage hitchhiking strategy for drug delivery and suggest broader applicability of engineered living materials–mediated delivery technologies in disease therapy.
β-elemene–loaded GeS nanosheets use macrophage hitchhiking and ultrasound synergy for enhanced cancer therapy.
INTRODUCTION
Cancer therapies pose substantial medical challenges, necessitating the adoption of innovative approaches to achieve targeted and effective interventions (1, 2). In recent years, nanoparticle-based drug delivery systems have emerged as promising strategies for cancer therapies. However, they still face challenges such as insufficient accumulation in lesions and suboptimal therapeutic efficacy following intravenous administration (3–5), primarily due to unwanted clearance by the reticuloendothelial system (RES). In contrast, naturally derived living cells, which have unique biological properties, offer opportunities for delivery technology and disease treatment (6, 7). Unlike conventional drug delivery systems, living cells exhibit the inherent capability to transverse through the animal body (8, 9), potentially serving as hitchhiking delivery systems by rerouting the nanoparticle-based drug delivery system. Therapeutic delivery systems employing living materials–based vectors, particularly immune cells such as macrophages, neutrophils, and lymphocytes, enable effective identification and targeting of tumor cells (10–12), rendering this strategy unique and particularly promising for enhanced drug accumulation in tumor areas.
Macrophages have the ability to sense and respond to various signals within their environment, such as chemical attractants released by tumor cells (13, 14). This chemotactic behavior enables them to actively migrate toward and infiltrate tumor sites, where they can accumulate and perform their therapeutic roles in modulating the tumor microenvironment and facilitating targeted drug delivery. Furthermore, macrophages can be chemically engineered to enhance their therapeutic efficacy and potentially activate anti-tumor immunity, rendering them promising “living materials drugs” (15–17). Despite their promise, a major challenge in using living macrophages as drug delivery vectors lies in achieving targeted drug delivery while preserving the carrier cells’ inherent functions. Now, living macrophage–mediated delivery primarily relies on using their intracellular compartments (18), yet premature drug leakage into the cytoplasm can reduce the biological activity and viability of engineered macrophages and reduce the bioavailability of therapeutics (19). Although directly loading approaches have been explored, challenges remain in complicated preparation and achieving optimal therapeutic effects (20–22). Consequently, we sought to explore an alternative approach, surface binding, to establish engineered macrophage-mediated delivery of nanotherapeutics that can enhance therapeutic accumulation in tumor areas and facilitate immune activation for improved therapeutic effects.
Ultrasound (US)–mediated sonodynamic therapy (SDT) has gained considerable attention due to its potential applications in targeted, deep penetration, and noninvasive treatments (5). In this paradigm, the development of innovative sonosensitizers capable of generating cytotoxic reactive oxygen species (ROS) upon US stimulation is particularly advantage for SDT. Germanium monosulfide (GeS), with its two-dimensional (2D) layered sheet-like structure materials and a narrow bandgap (~1.6 eV) (23), emerges as a suitable and innovative nanoparticle-based sonosensitizer for SDT and as a chemotherapeutic carrier for combination cancer therapy. However, a substantial challenge lies in overcoming the potential clearance of GeS nanosheets (GeSNSs) by the RES, particularly by monocytes and macrophages, to ensure optimal therapeutic efficacy.
In this study, instead of attempting to evade clearance by macrophages in vivo, we harness the “living materials” property of macrophages by engineering their surface to attach β-elemene–loaded GeSNSs (Fig. 1A). Leveraging the inherent tumor-homing ability of macrophages, the β-elemene–loaded GeSNSs use these cellular vehicles to evade host immune surveillance, ensuring efficient transit to the tumor microenvironment and enhanced accumulation at the tumor site for improved combination cancer therapy. These findings not only demonstrate the promise of this innovative macrophage hitchhiking strategy for improving drug delivery efficacy but also suggest its potential application in other living materials–based delivery technologies to enhance therapeutic outcomes in various disease settings.
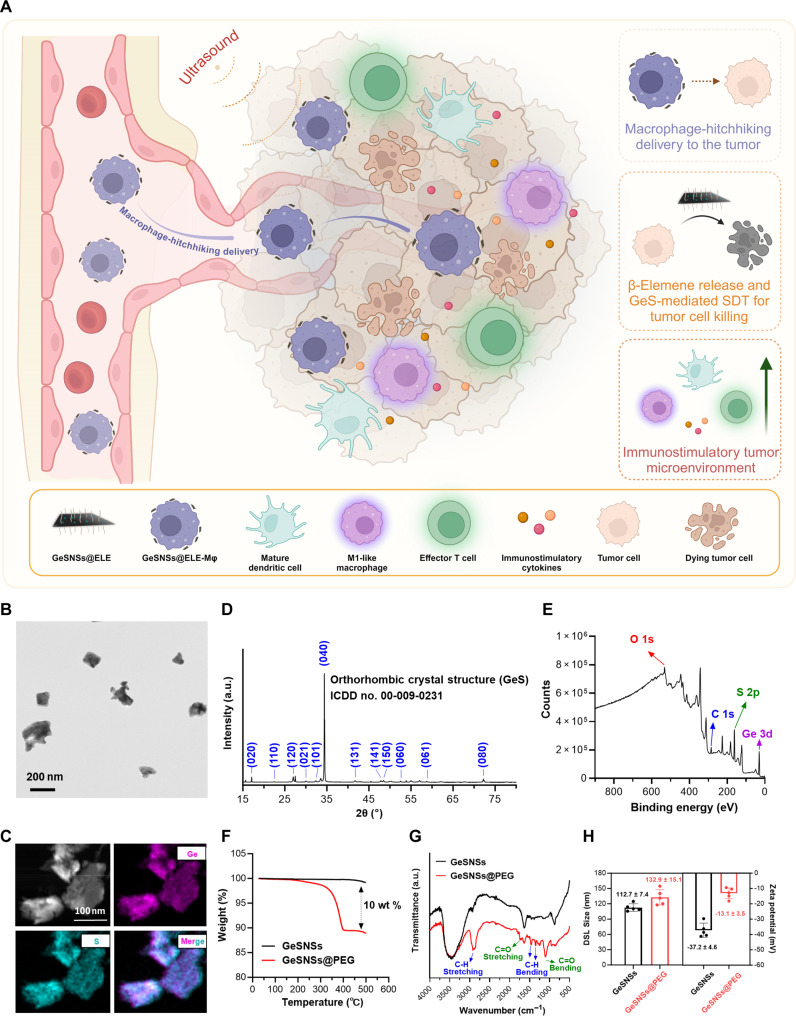
Fig. 1. Macrophage hitchhiking delivery of GeSNSs@ELE enhances tumor drug delivery and mediates effective sonodynamic immunotherapy.
(A) Schematic illustration of macrophages hitchhiking delivery of GeSNSs@ELE, where β-elemene–mediated chemotherapy and GeS-mediated SDT kill tumor cells, reprogramming the immunostimulatory tumor microenvironment. (B) Transmission electron microscopy (TEM) image of GeSNSs. (C) SEM with elemental mapping images of GeSNSs. (D) XRD pattern of GeSNSs. (E) XPS survey spectrum of GeSNSs. (F) TGA of GeSNSs and GeSNSs@PEG. (G) FTIR spectra of GeSNSs and GeSNSs@PEG. (H) DLS sizes and zeta potential values of GeSNSs and GeSNSs@PEG. Data are presented as means ± SD (n = 5).
RESULTS
Preparation and characterization of GeSNSs
GeSNSs were synthesized through ultrasonic treatment using a liquid-phase exfoliation method. Transmission electron microscope (TEM) images revealed an average lateral size of approximately 100 nm for obtained GeSNSs (Fig. 1B). Scanning electron microscopy (SEM)/energy-dispersive x-ray spectroscopy elemental mapping demonstrated a uniform distribution of Ge and S across the entire GeSNSs, further supporting the existence of Ge and S elements in the GeSNSs structure (Fig. 1C). X-ray diffraction (XRD) patterns showed that GeSNSs exhibited an orthorhombic crystal structure (International Centre for Diffraction Data, ICDD no. 00-009-0231) (Fig. 1D). X-ray photoelectron spectroscopy (XPS) confirmed the chemical composition of GeSNSs (Fig. 1E). The peaks at 531, 284, 161, and 30 eV in the XPS measurement spectrum of GeSNSs can be attributed to O 1s, C 1s, S 2p, and Ge 3d bands.
To improve the colloidal stability of GeSNSs in physiological environments, we coated their surface with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)] (DSPE-PEG), resulting in GeSNSs@PEG. Thermalgravimetric analysis (TGA), Fourier-transform infrared (FTIR) spectroscopy, dynamic light scattering (DLS) analysis, and zeta potential measurements verified the successful PEG coating on the surface of GeSNSs. First, when GeSNSs and GeSNSs@PEG were heated to 500°C, the weight loss difference between the two materials was 10 weight % (wt %) (Fig. 1F), indicating the efficient PEG coating on the surface of GeSNSs. Furthermore, the FTIR spectrum of GeSNSs@PEG showed absorption bands at 2917, 1738, 1457, 1349, and 1105 cm−1, corresponding to C─H stretching, C═O stretching, C─H bending, and C═O bending, respectively, indicating the existence of surface PEG (Fig. 1G). Moreover, the hydrodynamic average size of GeSNSs@PEG was 132.9 ± 15.1 nm (Fig. 1H), larger than that of GeSNSs (112.7 ± 7.4 nm). The zeta potential value of GeSNSs@PEG was −13.1 ± 3.5 mV, less negative than that of GeSNSs (−37.2 ± 4.6 mV) (Fig. 1H). DLS and zeta potential value measurements further confirmed the successful surface coating with PEG. We further investigated the stability of GeSNSs@PEG in phosphate-buffered saline (PBS), Dulbecco’s Modified Eagle Medium (DMEM), and fetal bovine serum (FBS). As shown in fig. S1 (A and B), GeSNSs@PEG exhibits good stability stored in biologically relevant environments (e.g., PBS solution, DMEM medium, or FBS), with no significant increase in size observed (fig. S1).
Sonodynamic performance of GeSNSs@PEG
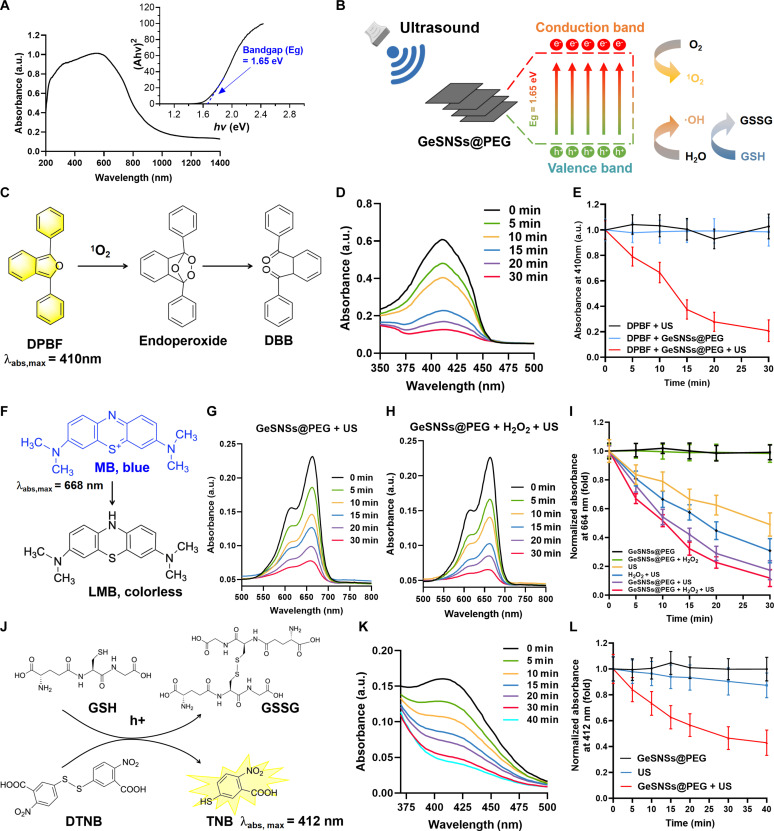
To investigate the potential of GeSNSs as sonosensitizers for SDT, we first measured their optical bandgap (Eg) using the absorption spectrum of solid GeSNSs@PEG and the Tauc plot of the Kubelka-Munk function. The optical bandgap of GeSNSs was calculated to be 1.65 eV (Fig. 2A), consistent with the previous studies (24, 25). The narrow bandgap of GeSNSs suggests that it may serve as an efficient sonosensitizer. As a sonosensitizer in SDT, it can effectively generate e− and h+ pairs to react with the surrounding O2 and H2O to generate cytotoxic 1O2, and ·OH ROS and consume glutathione (GSH) under US activation, thereby killing tumor cells (Fig. 2B). Next, we evaluated the sonodynamic performance of GeSNSs@PEG using three assays: (i) the production of 1O2, (ii) the production of ·OH, and (iii) the consumption of GSH under US activation. We first used 1,3-phenyldinitroformaldehyde (DPBF) as a probe to detect the production of 1O2 in the solution. DPBF acts as a receptor and undergoes a rapid oxidation reaction with 1O2, leading to the rapid fading of DPBF’s pale yellow color (Fig. 2C). We used US (1 MHz, 1 Wcm−2, 50% duty cycle) to trigger the mixed solution containing GeSNSs@PEG and DPBF for 0, 5, 10, 15, 20, and 30 min. The ultraviolet-visible (UV-vis) absorbance spectrum results showed that the absorbance of DPBF at 410 nm decreased with the increase of US exposure time, indicating that the 1O2 generated by US-triggered GeSNSs@PEG oxidized the yellowish DPBF into colorless 1,2-dibenzoylbenzene over time (Fig. 2D). In contrast, the US or GeSNSs@PEG alone did not produce 1O2 (Fig. 2E). This result demonstrated that the innovative GeSNSs@PEG sonosensitizer can effectively generate 1O2 under the trigger of US.
Fig. 2. Sonodynamic performance of GeSNSs@PEG.
(A) UV-Vis-NIR diffuse reflectance spectra of GeSNSs, with the optical bandgap (Eg) of GeSNSs calculated using the Kubelka-Munk equation depicted in the inset graph. a.u., arbitrary unit. (B) Proposed mechanism of sonodynamic performance of GeSNSs@PEG under US trigger. (C and D) Time-dependent oxidation of DPBF by 1O2 generated from US-triggered GeSNSs@PEG, with a US frequency of 1 MHz, power density of 1 W/cm2, and a 50% duty cycle. (E) A comparative analysis of the oxidation of DPBF under various conditions, including US alone, GeSNSs@PEG, and GeSNSs@PEG + US. Data are presented as means ± SD (n = 3). (F to H) Time-dependent degradation of MB by ·OH generated from US-triggered GeSNSs@PEG, with a US frequency of 1 MHz, power density of 1 W/cm2, and a 50% duty cycle. (I) Comparative analysis of the degradation of MB by GeSNSs@PEG under different treatments. Data are presented as means ± SD (n = 3). (J and K) Time-dependent degradation of GSH by h+ generated from US-triggered GeSNSs@PEG, with a US frequency of 1 MHz, power density of 2 W/cm2, and a 50% duty cycle. (L) Comparative analysis of the degradation of GSH by GeSNSs@PEG under different treatments. Data are presented as means ± SD (n = 3).
Next, we used methylene blue (MB) as probe to detect the generation of hydroxyl radical (·OH). The ·OH generated by the US-triggered sonosensitizers can react with the MB and produce Leuco Methylene Blue (LMB) (Fig. 2F). The results showed that the absorbance of MB at 668 nm gradually decreased as the US exposure time increased (Fig. 2G). Furthermore, this trend was more obvious after the addition of H2O2 to the mixed solution (Fig. 2H). This decrease in absorbance value with increasing US exposure time was not observed in the two control groups (GeSNSs@PEG and GeSNSs@PEG + H2O2) without US triggering (Fig. 2I), verifying the efficient generation of ·OH by US-triggered GeSNSs@PEG.
Glutathione is a pivotal antioxidant in maintaining intracellular redox balance to against oxidative damage. Therefore, the GSH depletion and its conversion to oxidized GSH (GSSG) caused by GeSNSs@PEG-mediated SDT can enhance the antitumor effect; we used 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) as a probe to detect GSH depletion. Colorless GSH can react with DTNB to form yellowish 5-thiodinitrophenol (TNB) and GSSG (Fig. 2J), with TNB serving as a key indicator. Results showed a gradual decrease in absorbance at 412 nm over increased US exposure time, demonstrating that US-triggered GeSNSs@PEG gradually depleted GSH in a time-dependent manner (Fig. 2K). Control tests using either US or GeSNSs@PEG alone showed no significant decrease in absorbance over time in the mixed solution, indicating that GeSNSs@PEG itself does not deplete GSH (Fig. 2L). In the absence of US, GeSNSs@PEG treatment did not significantly alter GSH levels. However, under US radiation, GeSNSs@PEG induced ROS generation, which was confirmed by increased cell apoptosis. The increase in apoptosis indicated that ROS caused oxidative stress and GSH depletion. Comparison between the US-only and GeSNSs@PEG + US group further confirmed that GeSNSs@PEG + US-induced ROS generation is responsible for GSH depletion, rather than a direct effect of GeSNSs@PEG itself. Collectively, these performance evaluations suggest that GeSNSs@PEG is a promising sonosensitizer for SDT.
Nanoformulation of GeSNSs@ELE for in vitro chemotherapy-enhanced SDT
2D nanomaterials are recognized for their exceptional potential as carriers in anticancer drug delivery due to their unique structures and properties (26). To evaluate the biological application potential of GeSNSs@PEG, we used an alarm blue assay to explore the biocompatibility of GeSNSs@PEG. GeSNSs@PEG has no cytotoxicity to normal cells (RAW264.7 macrophage) and cancer cells (4T1), even at very high concentrations (200 μg/ml) (fig. S2), which makes it an excellent nanomaterial for multimodal synergistic treatment of cancer.
To further assess GeSNSs@PEG’s suitability for drug delivery, we selected β-elemene, a promising anticancer drug known for its inhibition of tumor growth and metastasis (27). In this study, GeSNSs@PEG was added to β-elemene solutions of various concentrations, thoroughly mixed overnight, and subsequently washed to yield β-elemene-loaded GeSNSs@PEG (GeSNSs@ELE). High-performance liquid chromatography (HPLC) was used to quantify the loading capacity of β-elemene in GeSNSs@ELE (fig. S3A). Results indicated that at β-elemene concentrations of 2, 5, and 10 mg/ml, respectively, the loading capacities of β-elemene in GeSNSs@ELE were 1.4 ± 0.1, 4.3 ± 0.2, and 19.7 ± 0.2% (wt/wt), respectively (fig. S3B). Similar to the stability profile of GeSNSs@PEG, GeSNSs@ELE demonstrated stability in biological environments over a 1-week period, with an average hydrodynamic diameter of 131.8 nm and a polydispersity index of 0.307 (fig. S3, C and D). Furthermore, the release profile revealed a sustained release of β-elemene from the GeSNSs@ELE nanoformulation, reaching a plateau after 24 hours (fig. S4). These findings underscore the effective delivery capability of GeSNSs@ELE for β-elemene, alongside its superior colloidal stability.
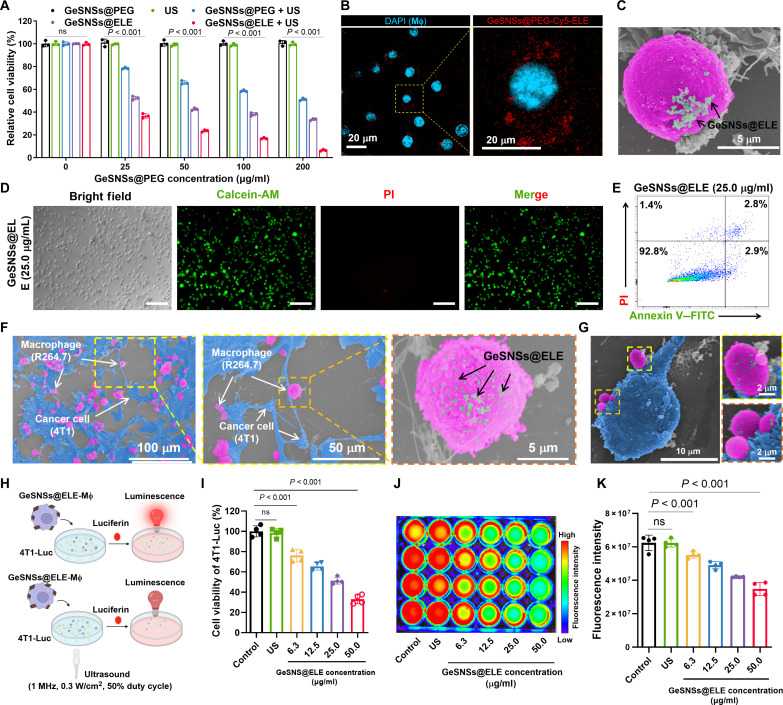
To evaluate the therapeutic potential of GeSNSs@ELE for chemoimmunotherapy and SDT, we conducted optical microscopy observations and an Alamar Blue assay to assess the impact of GeSNSs@ELE on the biological functions of 4T1 tumor cells. 4T1 cells were treated with various concentrations of GeSNSs@ELE and then subjected to US activation for a total of 3 min (1 min per cycle, three cycles). Our findings indicated that, compared to the GeSNSs@PEG group, there was no observed decrease in tumor cell viability in the group treated solely with US (Fig. 3A and fig. S5). This suggests that US treatment alone does not harm cells and is a biocompatible cancer treatment method. Conversely, when 4T1 cells were treated with GeSNSs@ELE at concentrations of 25, 50, 100, and 200 μg/ml and then subjected to US, the survival rates were 36.7 ± 2.0, 23.6 ± 1.0, 16.9 ± 0.7, and 6.4 ± 0.7%, respectively (Fig. 3A). These results demonstrate a significant decrease in 4T1 cell viability with increasing concentrations of GeSNSs@ELE, indicating effective, concentration-dependent SDT with GeSNSs@ELE.
Fig. 3. Construction and in vitro therapy of living materials (GeSNSs@ELE-Mφ).
(A) Cell viability of 4T1 cell following different treatments (n = 3). (B) Confocal microscope images of macrophages with GeSNSs@ELE attached to their surface. Scale bar, 20 μm. Blue: cell nuclei; Red: GeSNSs@ELE. (C) SEM image of a macrophage with GeSNSs@ELE attached to their surface. Scale bar, 5 μm. The macrophage (pink) and GeSNSs@ELE (as indicated by the arrow) are depicted using pseudo colors. (D) Fluorescence microscope images showing calcein-AM/PI costaining of macrophages after surface attachment of GeSNSs@ELE (one representative concentration is shown: 25.0 μg/ml; live cells: green; dead cells: red). Scale bars, 200 μm. (E) Flow cytometry apoptosis analysis of macrophages after surface attachment of GeSNSs@ELE (one representative concentration is shown: 25.0 μg/ml). (F and G) SEM images showing the interaction between tumor cells (4T1) and living materials (GeSNSs@ELE-Mφ). The tumor cells (blue), RAW264.7 macrophages (pink), and GeSNSs@ELE (as indicated by the arrow) are depicted using pseudo colors. (H) Schematic illustration showing the viability measurement of 4T1-Luc after the treatment of GeSNSs@ELE-Mφ and US via bioluminescence imaging. The bioluminescence signal intensity was used to quantify the viability of 4T1-Luc. (I) Viability of 4T1-Luc after the treatment of various concentration of GeSNSs@ELE-attached macrophages followed by US activation (n = 4). (J and K) Bioluminescence imaging and quantitative analysis of the bioluminescence intensity of 4T1-Luc after the treatment of various concentration of GeSNSs@ELE-attached macrophages (n = 4). PI, propidium iodide.
Construction of macrophages hitchhiking delivery platform for GeSNSs@ELE
Macrophages are pivotal immune cells known for their ability to target tumor areas and modulate the tumor immune microenvironment, thereby serving as potent hitchhiking delivery platforms for tumor therapy (28–30). Consequently, we used macrophages with tumor-targeting properties as hitchhiking delivery vehicles and loaded GeSNSs@ELE with SDT properties onto the surface of macrophages via a maleimide-thiol reaction.
To maximize the attachment of GeSNSs@ELE, we used tris(2-carboxyethyl) phosphine (TCEP) to selectively reduce disulfide bonds on the macrophage surface. This treatment exposed sulfhydryl (−SH) groups while preserving other functional groups. The increased terminal −SH groups on the macrophage surface were conjugated with maleimide-functionalized GeSNSs@PEG-MAL, forming GeSNSs@ELE-Mφ, which functioned as the macrophage-mediated drug delivery system. This reaction involves a highly specific chemical bond, unaffected by other components. DSPE-PEG-MAL (where MAL refers to maleimide) is a surfactant containing a phospholipid portion. Its phospholipid head group interacts with GeSNSs, mainly driven by two factors: hydrophobic interaction (the hydrophobic fatty chains in DSPE interact with the hydrophobic surface of GeSNSs to enhance adsorption stability) and van der Waals force (the planar structure of GeSNSs provides a larger contact area, generating van der Waals interactions with DSPE molecules, further enhancing stability). After modifying GeSNSs with DSPE-PEG-MAL using the thin-film hydration technique, the zeta potential of GeSNSs significantly decreases due to the shielding effect of the PEG chains (fig. S6). Next, we used Ellman’s assay (based on the reaction between thiol and DTNB) to quantify the exposed sulfhydryl (−SH) content on the macrophage surface after TCEP treatment. A standard curve of the thiol-DTNB reaction (fig. S7, A and B) revealed that increasing TCEP concentrations led to a higher concentration of −SH exposure on macrophage surface (fig. S7, C and D), without negatively affecting macrophage viability (figs. S8 and S9).
Following 2 mM TCEP treatment, confocal fluorescence microscopy and high-resolution SEM confirmed the successful loading of GeSNSs@ELE on macrophage surfaces (Fig. 3, B and C). This confirmed that maleimide groups reacted specifically with surface thiol groups, rather than being masked or covered by other components. Some of the GeSNS@PEG-ELE that enters the cells may be due to active cell uptake. Fluorescence intensity measurements of the washing supernatant (before centrifugation, after centrifugation, and after each PBS wash) showed that the fluorescence signal decreased with additional washes. After the third wash, the fluorescence signal stabilized near the detection limit (fig. S10), indicating the removal of unbound GeSNSs@PEG-Cy5. Moreover, we used inductively coupled plasma mass spectrometry (ICP-MS) to quantify the Ge content on the surface of macrophages (fig. S11). In the control group (without TCEP treatment), each macrophage had an average Ge content of 4.5 ± 0.4 pg. In contrast, macrophages treated with TCEP and incubated with GeS group exhibited a significantly higher Ge content of 28.9 ± 2.6 pg per cell. Similarly, the GeSNS-ELE group, which involved further drug loading, demonstrated a comparable Ge content of 27.7 ± 4.3 pg per cell. These results indicate that TCEP treatment effectively enhances the binding efficiency of GeS nanoparticles to macrophage surfaces. Moreover, various concentrations of GeSNSs@ELE (0, 3.1, 6.3, 12.5, 25.0, and 50.0 μg/ml) were tested to evaluate macrophage viability, apoptosis, and migration. Assays including Alamar Blue, live/dead cell staining [Calcein-AM/propidium iodide (PI)], flow cytometry, and Transwell confirmed no adverse effects on macrophage viability and migratory capabilities (Fig. 3, D and E, and figs. S12 to S15). Crucially, in vitro experiments demonstrated that macrophages loaded with GeSNSs@ELE retained their tumor-targeting ability and exerted a significant killing effect on 4T1-Luc breast cancer cells upon US stimulation (Fig. 3, F to K). The observed cytotoxicity in the in vitro experiments was attributed to localized ROS generation and enhanced ROS production induced by US activation.
Together, these findings demonstrate the successful construction of macrophage hitchhiking delivery system for GeSNSs@ELE. This platform effectively integrates both SDT and chemoimmunotherapy and has the ability to target and kill breast cancer cells in vitro, offering a promising approach for combination cancer therapy.
Biodistribution and antitumor effect of GeSNSs@ELE-Mφ
To verify whether this living materials-based delivery technology has a desirable tumor accumulation effect in vivo, we examined the biodistribution of GeSNSs@ELE-Mφ in a mouse model of 4T1 breast tumor. After intravenous injection, GeSNSs@Cy5-ELE-Mφ showed obvious accumulation in the tumor site with strong fluorescence, while free GeSNSs@PEG-Cy5 had weaker fluorescence intensity (fig. S16A). This further confirms that GeSNSs@ELE-Mφ, leveraging the tumor-targeting properties of macrophages, can efficiently accumulate at the tumor site. The fluorescence signal of GeSNSs@Cy5-ELE-Mφ peaked at 4 hours after injection (fig. S16B). Therefore, we selected 4 hours after intravenous injection as the critical time point for the following US treatment.
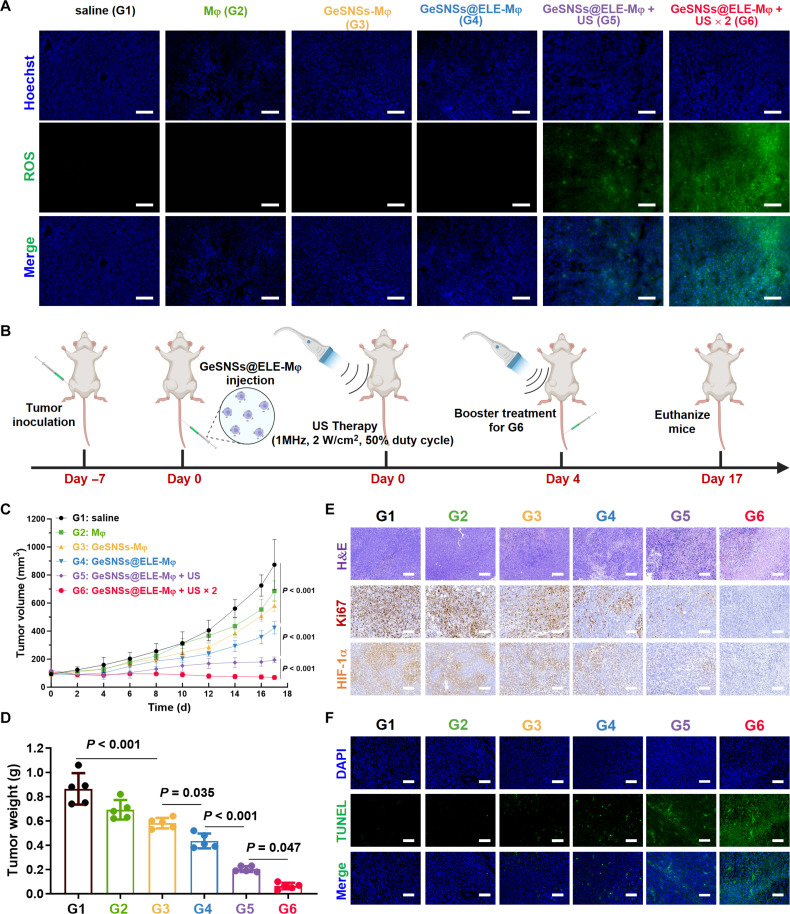
Next, we explored whether US radiation could induce the living materials–mediated delivery of GeSNSs@ELE-Mφ to effectively produce ROS at the tumor site. The results showed that, compared with the control group (saline) or the groups without US treatment (Mφ, GeSNSs-Mφ, and GeSNSs@ELE-Mφ), the ROS levels at the tumor site of the GeSNSs@ELE-Mφ group after US radiation (GeSNSs@ELE-Mφ + US) increased significantly, especially in the group subjected to two rounds of US irradiation (GeSNSs@ELE-Mφ + US × 2), where the ROS levels were the highest (Fig. 4A). This result further demonstrates that the living macrophage–based technology can effectively deliver sonosensitizers for improved ROS generation.
Fig. 4. Anti-tumor efficacy of GeSNSs@ELE-Mφ.
(A) Fluorescence microscope images of tumor sections depicting the levels of ROS (green) using the H2DCF-DA probe following different treatment regimens. Scale bars, 100 μm. (B) Timeline of the establishment of 4T1 tumor-bearing mice and treatment protocol on 4T1 tumor. (C) Tumor volume growth curve of 4T1 tumor-bearing mice in each group (n = 5). (D) The weight of tumors removed from 4T1 tumor-bearing mice at day 17 after various treatments (n = 5). (E) H&E staining and immunohistochemical staining for Ki67 and hypoxia-inducible factor-1α (HIF-1α) were performed on 4T1 tumors removed at day 17 after various treatments. Scale bars, 100 μm. (F) TUNEL fluorescence staining (green) was performed on 4T1 tumors removed at day 17 after various treatments. The cell nuclei were stained with DAPI. Scale bars, 100 μm. G1: saline; G2: Mφ; G3: GeSNSs-Mφ; G4: GeSNSs@ELE-Mφ; G5: GeSNSs@ELE-Mφ + US; G6: GeSNSs@ELE-Mφ + US × 2.
We next studied the therapeutic effect of GeSNSs@ELE-Mφ on 4T1 tumor-bearing mice (Fig. 4B). When the average tumor size reached approximately 80 to 100 mm3, we randomly divided the 4T1 tumor-bearing mice into six groups and performed the following treatments on day 0: (i) saline, (ii) Mφ, (iii) GeSNSs-Mφ, (iv) GeSNSs@ELE-Mφ, (v) GeSNSs@ELE-Mφ + US, and (vi) GeSNSs@ELE-Mφ + US × 2. The US radiation of the GeSNSs@ELE-Mφ + US and GeSNSs@ELE-Mφ + US × 2 groups was 4 hours after the tail vein injection of GeSNSs@ELE-Mφ, and the GeSNSs@ELE-Mφ +US × 2 group was given a second treatment on the 4th day. The purpose of secondary US treatment is to study whether repeat US exposure can enhance the therapeutic efficacy and provide a more durable treatment effect. On the 17th day of tumor-bearing mice, compared with the control group (G1), the use of Mφ (G2) or GeSNSs-Mφ (G3) alone did not show significant antitumor efficacy (Fig. 4C and figs. S17 and S18). In contrast, the groups treated with GeSNSs@ELE-Mφ or GeSNSs@ELE-Mφ + US irradiation (G4, G5, and G6) showed significantly lower tumor size than those in G1, G2, and G3. Especially after the second US treatment (G6), this tumor inhibition rate was even more obvious. Compared with G5, the tumor volume of G6 decreased by about 2.79 times (P < 0.001) (fig. S19). The tumor inhibition rate based on the average volume of tumors exhibited that the tumor inhibition rate of G6 was 92.04% (fig. S20). Tumor weight further supported this conclusion, showing similar results (Fig. 4D). Consistent with the trend in tumor volume changes, the results of hematoxylin and eosin (H&E) staining, Ki67 immunohistochemistry, hypoxia-inducible factor–1α immunohistochemistry, and TUNEL (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling) staining demonstrated that tumors treated with the G6 group exhibited the highest degree of tissue damage (Fig. 4, E and F). These results indicate that the macrophage-hitchhiking delivery of GeSNSs@ELE exhibits strong tumor-inhibiting capabilities. In addition, no significant weight loss was observed in mice during tumor treatment in each group (fig. S21). Although intravenously injected GeSNSs@ELE-Mφ partially accumulates in other organs, without direct US irradiation, H&E staining of major organs (heart, liver, spleen, lung, and kidney) in all groups did not show tissue damage (fig. S22). Furthermore, the size and weight of excised spleen showed no significant toxicity during treatment, further confirming the good biocompatibility (fig. S23).
GeSNSs@ELE-Mφ induces potent macrophage-mediated anti-tumor immune response in vivo
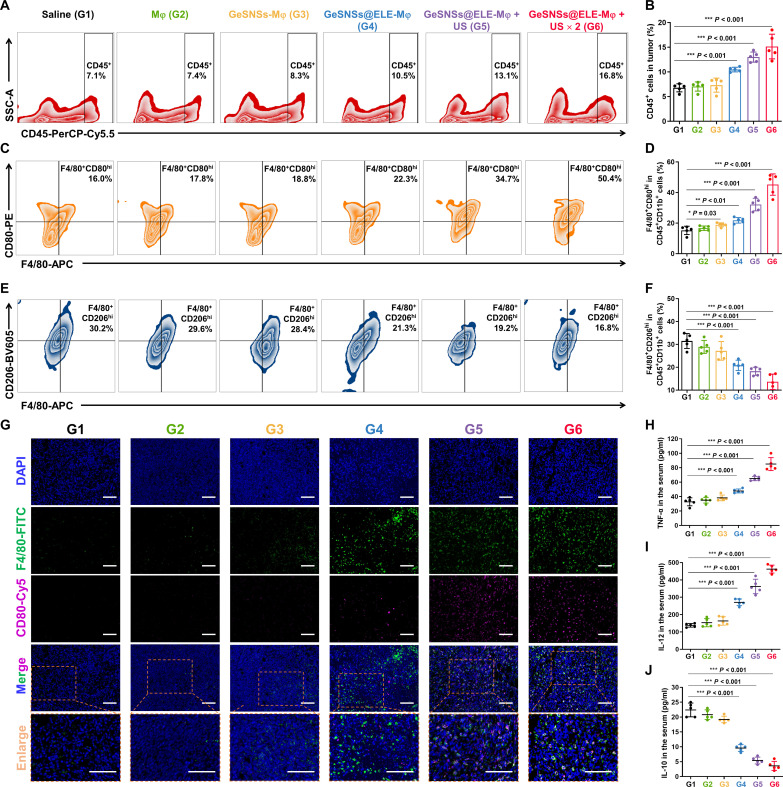
To investigate whether the combination of GeSNSs@ELE-Mφ and US-mediated SDT can induce macrophage-mediated antitumor immune response, we used flow cytometry and enzyme-linked immunosorbent assay (ELISA) methods to examine changes in immune cells and cytokines in the residual tumor tissues and serum of mice on the 17th day post-treatment (fig. S24). Quantitative flow cytometry results demonstrated a significant increase in the percentage of total infiltrating immune cells (CD45+ cell population) in tumors after treatment with US-activated GeSNSs@ELE-Mφ (from 7.1 to 16.8%) (Fig. 5, A and B). The proportion of immunostimulatory M1-like tumor-associated macrophages (TAMs; F4/80+CD80hi) within the CD45+CD11b+ cell population in tumor tissues markedly increased (from 16.0 to 50.4%) (Fig. 5, C and D), while the proportion of immunosuppressive M2-like TAM (F4/80+CD206hi) significantly decreased (from 30.2 to 16.8%) (Fig. 5, E and F). Moreover, to evaluate the effect of GeSNSs@ELE-Mφ on systemic antitumor immunity, we performed flow cytometry analysis of macrophage cell populations in spleens and lymph nodes (figs. S25 and S26). The percentages of CD45+CD11b+ and CD45+CD11b+F4/80+CD80hi (M1-like macrophage) cell populations in spleens and lymph nodes of G6 mice increased by 1.43 and 1.79 times and by 3.43 and 4.38 times, respectively, compared with those of G1 (fig. S27). Moreover, the percentages of CD45+CD11b+F4/80+CD206hi (M2-like macrophage) cell populations in the spleen and lymph nodes of G6 mice decreased by 0.83 and 0.55 times, respectively, compared with those of G1 (fig. S27).
Fig. 5. US-triggered GeSNSs@ELE-Mφ induces robust anti-tumor immune responses in 4T1 breast tumor-bearing mice.
(A and B) Representative flow cytometry and quantitative statistical results of CD45+ cells in mouse tumors from each treatment group. (C and D) Representative flow cytometry and quantitative statistical results of F4/80+CD80hi M1-like TAMs in mouse tumors from each treatment group. (E and F) Representative flow cytometry and quantitative statistical results of F4/80+CD206hi M1-like TAMs in mouse tumors from each treatment group. (G) Immunofluorescence images of tumor tissues after various treatments. Green: macrophages stained with anti-F4/80 antibody; purple: M1-like macrophage marker stained with anti-CD80 antibody; blue: cell nuclei stained with DAPI. Scale bars, 500 μm. (H to J) Serum levels of proinflammatory cytokines [TNF-α and IL-12 (p40)] and anti-inflammatory cytokine (IL-10) of 4T1 breast tumor-bearing mice in each treatment group. G1: saline; G2: Mφ; G3: GeSNSs-Mφ; G4: GeSNSs@ELE-Mφ; G5: GeSNSs@ELE-Mφ + US; G6: GeSNSs@ELE-Mφ + US × 2. Data are expressed as means ± SD (n = 5 mice per group). Statistical significance was calculated by one-way analysis of variance (ANOVA) test. *P < 0.05, **P < 0.01, ***P < 0.001, and P > 0.05 is not significant.
The expression of immunostimulatory M1-like TAMs and immunosuppressive M2-like TAMs in tumors after various treatments was further studied using immunofluorescence staining. Consistent with the flow cytometry results, combined treatment with GeSNSs@ELE-Mφ and US resulted in the enrichment of M1-like TAMs (CD80, purple; Fig. 5G) in tumors and the decrease of M2-like TAMs (CD206, yellow; fig. S28) in tumors, which were associated with macrophage markers (F4/80, green) overlap (Fig. 5G and fig. S28). This phenomenon is more obvious, especially after the second US treatment (G6).
Consistent with the changes in TAMs, the levels of immunostimulatory cytokines [tumor necrosis factor–α (TNF-α) and interleukin-12 (IL-12) (p40)] in serum significantly increased after GeSNSs@ELE-Mφ + US × 2 treatment. Specifically, TNF-α levels rose from 32.8 to 85.0 pg/ml (Fig. 5H), and IL-12 levels increased from 138.0 to 462.4 pg/ml (Fig. 5I). Conversely, the concentration of immunosuppressive cytokine IL-10 markedly decreased, from 22.4 to 3.6 pg/ml (Fig. 5J). These results confirm that, after US activation, GeSNSs@ELE-Mφ can significantly enhance the ratio of M1-/M2-like TAMs within tumors and effectively reverse the macrophage-associated immunosuppressive tumor microenvironment in vivo.
GeSNSs@ELE-Mφ reprograms immunosuppressive TME
Cytokines play a crucial role in regulating macrophage function and facilitating their mutual communication. We used immunohistochemistry (IHC) analysis to confirm that, consistent with the trend in serum, US-triggered GeSNSs@ELE-Mφ can promote the expression of immunostimulatory cytokines (TNF-α and IL-12) and reduce the expression of the immunosuppressive cytokine (IL-10) in tumors (Fig. 6A). We further quantified the cytokines in tumors in each group by ELISA. Consistent with the results of IHC, the US-triggered GeSNSs@ELE-Mφ treatment can significantly increase the secretion of immunostimulatory cytokines (TNF-α and IL-12) (Fig. 6, B and C) and reduce the secretion level of the immunosuppressive cytokine (IL-10) (Fig. 6D). The regulation of these cytokines and the polarization toward M1-like TAMs further confirm that this treatment strategy can effectively reverse the transformation of cold tumors intohot ones.
Fig. 6. US-triggered GeSNSs@ELE-Mφ reprograms the immunosuppressive TME of 4T1 tumors.
(A) Immunohistochemical staining images of TNF-α, IL-12, and IL-10 in tumor tissues after various treatments. Scale bars, 100 μm. (B to D) Secretion levels of TNF-α, IL-12 (p40), and IL-10 in tumor tissues after various treatments (n = 5). (E) Representative flow cytometry analysis images of CD45+CD11c+ cells in tumors after various treatments. (F) Representative flow cytometry analysis images of CD45+CD11c+CD103+ cells in tumors after various treatments. (G) Representative flow cytometry analysis images of CD45+CD11c+CD80+CD86+ cells in tumors after various treatments. (H) Quantification of CD45+CD11c+ cells in tumors after various treatments using flow cytometry. (I) Quantification of CD45+CD11c+CD103+ cells in tumors after various treatments using flow cytometry. (J) Quantification of CD45+CD11c+CD80+CD86+ cells in tumors after various treatments using flow cytometry. G1: saline; G2: Mφ; G3: GeSNSs-Mφ; G4: GeSNSs@ELE-Mφ; G5: GeSNSs@ELE-Mφ + US; G6: GeSNSs@ELE-Mφ + US × 2. Data are expressed as means ± SD (n = 5 mice per group). Statistical significance was calculated by one-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001, and P > 0.05 is not significant.
In addition to macrophages, dendritic cells (DCs) play a key role in the immune microenvironment. Mature DCs can present tumor antigens and activate T cells, which are pivotal in initiating and activating antitumor immune responses. We used flow cytometry to evaluate the expression levels of mature DCs within tumors (fig. S29). The results showed that GeSNSs@ELE-Mφ, when triggered by US × 2, led to a significant increase in the level of DCs (CD45+CD11c+) within the tumor (from 22.3 to 49.4%) (Fig. 6E). Compared with the saline control group, the GeSNS@ELE-Mφ +US × 2 group induced the highest levels of intratumoral mature DCs generation, including CD45+CD11c+CD103+ (increased from 38.0 to 70.5%) (Fig. 6F) and CD45+CD11c+CD80+CD86+ (increased from 29.7 to 48.7%) (Fig. 6G). Representative flow cytometry plots from each treatment group are illustrated (Fig. 6, E to G), and the corresponding statistical results are shown in Fig. 6 (H to J). Similarly, we evaluated changes in DCs in the spleen and lymph nodes of mice (figs. S30 and S31). The percentages of CD45+CD11c+, CD45+CD11c+CD103+, and CD45+CD11c+CD80+CD86+ cell populations in the spleen and lymph nodes of G6 mice increased by 2.2, 1.8, and 1.4 times and by 3.1, 5.0, and 3.9 times, respectively, compared with those of G1 (fig. S32). Collectively, these results demonstrate that GeSNS@ELE-Mφ, upon entering the tumor and being triggered by US, can transform the initially immunosuppressive tumor microenvironment through the polarization of M1/M2 macrophages, changes in cytokine, and the maturation of DCs. These changes shift the tumor microenvironment into an immunostimulatory state, thereby enhancing the immune system’s attack on tumors.
GeSNSs@ELE-Mφ stimulates effective T cell immune responses with high biocompatibility in vivo
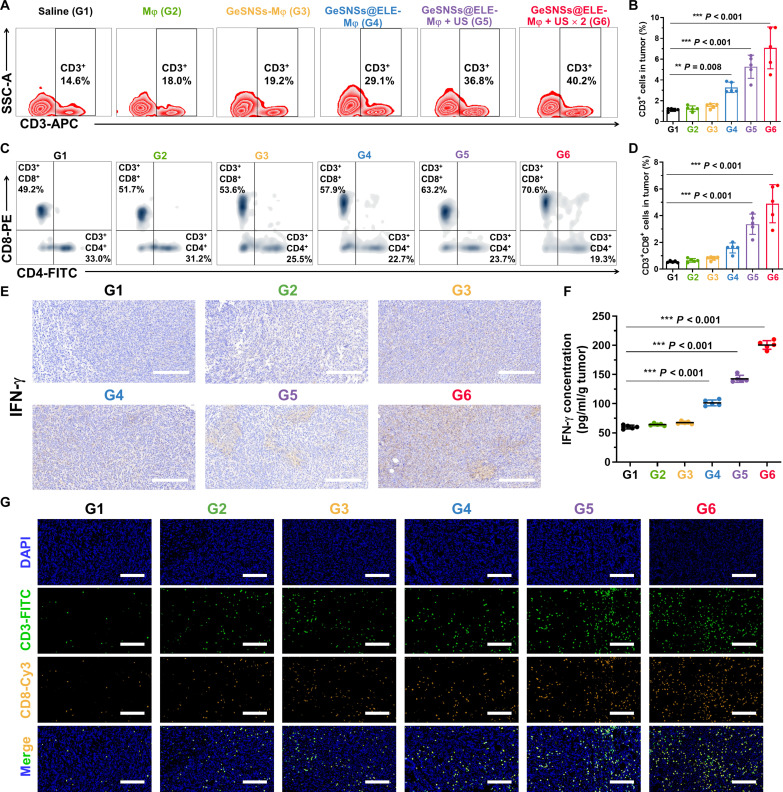
Inducing the increase and maturation of DCs can activate the antigen presentation function of these cells and stimulate the ability of T lymphocytes for enhancing antitumor immunity (31). We used flow cytometry to analyze the changes of T lymphocytes in tumors of each treatment group (fig. S33). The quantitative flow cytometry analysis results showed that compared with the saline control group, the group of GeSNSs@ELE-Mφ triggered by US could lead to a significant increase in T lymphocytes (CD45+CD3+, from 14.6 to 40.2% in CD45+ cells) within the tumor (Fig. 7, A and B). Moreover, CD8+ T cells are cytotoxic T cells that can directly recognize and kill tumor cells by releasing cytotoxins and activating apoptosis. Flow cytometry analysis showed that compared with the saline control group, the population of intratumoral cytotoxic T lymphocytes (CTLs; CD45+CD3+CD8+ cells) in the GeSNSs@ELE-Mφ + US × 2 group significantly increased (G1 is 0.55%, G2 is 0.66%, G3 is 0.81%, G4 is 1.59%, G5 is 3.36%, and G6 is 4.90%), and the population of helper T (TH) cells (TH cells, CD45+CD3+CD4+ cells) within the tumor also increased significantly (0.36% for G1, 0.35% for G2, 0.40% for G3, 0.79% for G4, 1.13% for G5, and 1.36% for G6) (Fig. 7, C and D). To evaluate the effect of this treatment strategy on systemic antitumor immunity, we analyzed the change of T cell populations in spleens and lymph nodes (figs. S34 and S35). The percentages of CD45+CD3+ T cells, CD45+CD3+CD4+ TH, and CD45+CD3+CD8+ CTL cell populations in the spleen and lymph nodes of G6 mice increased by 4.36, 1.27, and 1.68 times and by 1.85, 1.28 and 1.57 times, respectively, compared with those of G1 (fig. S36).
Fig. 7. US-triggered GeSNSs@ELE-Mφ induces potent T cell-mediated anti-tumor immune response in 4T1 breast tumor.
(A and B) Representative flow cytometry analysis images and quantitative analysis of CD45+CD3+ lymphocytes in tumor tissues after different treatments. (C and D) Representative flow cytometry analysis images and quantitative analysis of CD3+CD8+ CTLs and CD3+CD4+ helper T lymphocytes in tumor tissues after different treatments. (E) Immunohistochemistry (IHC) staining images of IFN-γ expression levels in tumors of mice after different treatments. Scale bars, 100 μm. (F) IFN-γ secretion levels in tumor tissues after different treatments. (G) Immunofluorescence staining images of CD3+ (green, FITC), and CD8+ (yellow, Cy3) T lymphocytes in tumor tissues after different treatments. Cell nuclei were stained with DAPI (blue). Scale bars, 100 μm. G1: saline; G2: Mφ; G3: GeSNSs-Mφ; G4: GeSNSs@ELE-Mφ; G5: GeSNSs@ELE-Mφ + US; G6: GeSNSs@ELE-Mφ + US × 2. Data are expressed as means ± SD (n = 5 mice per group). Statistical significance was calculated by one-way ANOVA test. **P < 0.01, ***P < 0.001, and P > 0.05 is not significant.
As a crucial cytokine secreted by CD8+ cells, interferon-γ (IFN-γ) plays a key regulatory role in promoting antitumor immune responses and modulating immune cell activity. Considering this, IHC results for IFN-γ confirmed its highest expression in the GeSNSs@ELE-Mφ + US × 2 group (Fig. 7E). Similarly, ELISA results revealed that, compared to the saline control group, the levels of IFN-γ in both the tumor and serum of the GeSNSs@ELE-Mφ + US × 2 group were increased by 3.3 and 10.4 times, respectively (Fig. 7F and fig. S37). Immunofluorescence staining further confirmed that the increase in tumor infiltrating CD3+ lymphocytes [CD3+, fluorescein isothiocyanate (FITC), green] and CD8+ CTLs (CD8+, Cy3, yellow) was most significant in the GeSNSs@ELE-Mφ + US × 2 group (Fig. 7G). Collectively, the study demonstrates that the macrophage-mediated delivery of GeSNSs@ELE-Mφ, when triggered by US, significantly enhances the antitumor immunity by increasing the infiltration and maturation of DCs and T lymphocytes and by boosting the production of the immunostimulatory cytokine IFN-γ. In addition, we evaluated the therapeutic effects of β-elemene (at the concentration used on the macrophages) + US (G7) and GeSNSs@ELE + US, i.e., minus the macrophages (G8). Our results showed that β-elemene (ELE) + US or GeSNSs@ELE +US treatment slightly slowed tumor progression (fig. S38) and had the moderate antitumor efficacy (fig. S39).
The in vivo toxicity and metabolic clearance of nanoparticles are critical factors in assessing their potential for clinical applications. An appropriate metabolic clearance rate minimizes the risk of toxicity from long-term accumulation, while low toxicity ensures their safety and efficacy. Therefore, addressing these aspects is essential for the successful transition of nanomedicines from laboratory research to clinical use. We systematically investigated the toxicity of GeSNSs@ELE-Mφ through histological examination and hematological analyses. As shown in fig. S16, the nanoparticles exhibited some distribution in major organs, including the liver, spleen, and lungs. H&E staining confirmed that GeSNSs@ELE-Mφ confirmed their good biocompatibility and safety for cancer treatment (fig. S22). In addition, to assess clearance, we analyzed the content of Ge in feces and urine using ICP-MS (fig. S40A). The results showed that both GeSNSs and GeSNSs@ELE-Mφ were gradually cleared, with less than 0.065% and 0.033% of the injected dose per gram of tissue (%ID/g) remaining after 14 and 30 days, respectively, indicating negligible long-term retention in the body. Moreover, we conducted hematological tests to evaluate the long-term systemic biosafety of GeSNSs@ELE-Mφ. The parameters measured included white blood cells (WBCs), red blood cells (RBCs), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelets (PLTs) (fig. S40B). No significant differences were observed between GeSNSs@ELE-Mφ–treated and control groups (saline) at days 1, 14, and 30 post-injections. In addition, we measured blood biochemical parameters, including aspartate aminotransferase (ALT), alanine aminotransferase (AST), albumin (ALB), gamma-glutamyl transferase (GGT), total protein (TP), blood urea nitrogen (UREA), creatinine (CREA), creatine kinase (CK), lactate dehydrogenase (LDH), and amylase (AMY) (fig. S40C). These results also showed no significant differences between treated and control groups at the same time points (1, 14, and 30 days). Collectively, these findings demonstrate that GeSNSs@ELE-Mφ exhibits high biocompatibility, efficient metabolic clearance, and excellent biosafety.
DISCUSSION
Despite major breakthroughs in nanoparticle-mediated tumor therapy in recent years, further research and optimization are essential to enhance therapeutic efficacy and provide safer treatment options (32, 33). In this study, we have developed an innovative type of macrophage-based living materials composed primarily of GeSNSs and chemotherapeutic drugs β-elemene. These living materials harness macrophages as a hitchhiking delivery vehicle, facilitating targeted delivery to tumor sites. This approach not only enhances the tumor immune microenvironment, enabling better identification and eradication of tumor cells, but also demonstrates potent SDT effects with GeSNSs. This reprogramming of cellular interactions transforms the cold tumor microenvironment into a hot tumor microenvironment, effectively inhibiting the growth of TNBC in mice.
Over the past decades, the development of nanoparticle-based drug delivery systems has been a primary focus for enhancing the bioavailability of active pharmaceutical ingredients. This nanomedicine-based technology has shown great promise in treating a wide range of diseases. However, the RES remains a critical challenge in the effective delivery of nanoparticle-based drug delivery systems, as it can lead to rapid clearance and reduced efficacy of therapeutic agents. With the rapid development of cell biology and nanomedicine technologies, an increasing number of studies are now attempting to use engineered living cell systems to revolutionize drug delivery and therapeutic interventions (34–37). Macrophages, as essential immune cells and antigen-presenting cells, have a long half-life in circulation and the ability to efficiently accumulate at tumor tissues (14). Therefore, applying macrophages in the delivery of nanotherapeutics significantly enhances drug accumulation in tumors (15).
In this study, we used the maleimide and thiol chemical conjugation strategy to successfully attach β-elemene–loaded GeSNSs onto the surface of macrophages, facilitating transportation via a macrophage “hitchhiking” mechanism (38). Further investigation revealed that GeSNSs has a narrow bandgap (1.65 eV) and, triggered by US, can generate potent ROS for cancer cell killing. This marks, to our knowledge, the first report of GeSNSs, obtained by a liquid-phase exfoliation strategy, being used as an innovative sonosensitizer in SDT. Under ultrasonic irradiation, the excitation of GeSNSs is primarily driven by the acoustic cavitation effect, a process involving the formation, growth, and collapse of bubbles, induced by ultrasonic waves in liquid media. The high-frequency vibration of these waves generates tiny bubbles that rapidly grow and collapse, releasing localized high temperature and high-pressure environments accompanying by strong mechanical shock waves (39–41). These extreme conditions provide the energy needed to excite GeSNSs. Mechanical energy is converted into thermal energy and compression energy through the cavitation effect, directly affecting the electronic structure of GeSNSs. Specifically, under the action of ultrasonic waves, the crystal structure of GeSNSs undergoes rapid mechanical vibrations and pressure wave shocks, causing the electrons in its band structure to jump from the valence band to the conduction band, forming electron-hole pairs (e−/h+) (42, 43). These electron-hole pairs can be efficiently generated under low-energy input conditions due to the narrow bandgap characteristics of GeSNSs. The excited electrons and holes react with surrounding water or oxygen molecules to generate highly active free radicals, such as hydroxyl radicals (·OH) and singlet oxygen (1O2), thereby promoting further oxidative reactions (5). Therefore, ultrasonic irradiation facilitates the conversion of mechanical energy into localized thermal energy and compression energy via the acoustic cavitation effect, exciting GeSNSs to produce electron-hole pairs. This process triggers the generation of ROS and improves the sonodynamic performance of GeSNSs under ultrasonic irradiation. In addition, β-elemene, extracted from traditional Chinese medicine, is a potent anticancer drug commonly used in the adjuvant treatment of various malignant tumors (44). However, because of poor water solubility and low bioavailability, β-elemene exhibits limited clinical efficacy (45). Therefore, there is a need to develop a suitable drug delivery system that overcomes these shortcomings to enhance its therapeutic effects. In addition to serving as sonosensitizers, we used GeSNSs as carriers for β-elemene loading. The drug β-elemene is encapsulated in the core of GeSNS@PEG-ELE and does not directly react with or shield the maleimide groups. This design ensures that GeSNS@DSPE-PEG-MAL remains stably bound to the macrophage surface while retaining the sustained release function of β-elemene. In vitro experiments confirmed that β-elemene–loaded GeSNSs did not exhibit toxicity to the host cell (macrophages), and its successful loading onto the surface of macrophages was verified through confocal laser microscopy and SEM. Such a macrophage hitchhiking delivery system, derived from living cells, inherently has excellent biocompatibility and offers high safety. It was confirmed that intravenous administration of GeSNSs@ELE-Mφ followed by US-mediated SDT can significantly inhibit tumor growth in a mouse model of 4T1 breast cancer.
The growth of tumors is closely associated with the tumor immune microenvironment, where immune cells and signaling molecules play a crucial role in influencing tumor development (46, 47). Macrophages, a key component of the immune system, play a pivotal role in maintaining tissue health and immune responses (48). This study successfully used macrophage as the critical component to modulate the tumor immune microenvironment. The results demonstrate that macrophages, loaded with GeSNSs@ELE, significantly accumulate at the tumor site, possibly correlated with the immune-inflammatory microenvironment in the tumor region (14).
Macrophages can be primarily categorized into M1 and M2 types, each exhibiting distinct functions at different stages of the immune response. M1-like macrophages typically participate in immunostimulatory immune response through producing immunostimulatory cytokines such as TNF-α and IL-12 to enhance immune responses (49). Conversely, M2-like macrophages are associated with immunosuppressive immune response and produce immunosuppressive cytokine such as IL-10 (50). Using various biological approaches, such as flow cytometry, immunofluorescence staining, immunohistochemistry, and ELISA, we found that after US trigger, GeSNSs@ELE-Mφ could significantly induce polarization of M2-like macrophages toward M1-like phenotype. This led to an increase in the expression levels of immunostimulatory cytokines (TNF-α and IL-12) and a decrease in the expression level of immunosuppressive cytokine (IL-10), successfully skewing the immunosuppressive tumor microenvironment toward a direction favorable for antitumor immunity. Moreover, this “living materials drug” plays a positive role in the maturation of DCs. The maturation of DC cells is a crucial step in the immune response (51), and this study observed the most significant maturation of DCs in the G6 group (GeSNSs@ELE-Mφ + US × 2) of tumor tissues. DC maturation can activate T cells by presenting antigens on their surface, guiding and regulating the initiation and development of cellular immune responses (52). Further analysis revealed that GeSNSs@ELE-Mφ indeed had a significant impact on T cells after US triggering. The substantial increase in T cells (CD3+ T cells, CD4+ T cells, and CD8+ T cells) and their secretion of cytokines could recognize and attack tumor cells, inhibiting tumor growth, and arouse antitumor immunity. Consistent with previous studies, secondary treatment can improve drug delivery or therapeutic efficacy in the system. Our results also showed that secondary US treatment further reduced tumor size and enhanced the antitumor effect by modulating the immunosuppressive tumor microenvironment. Therefore, leveraging the tumor-targeting properties of macrophages, the sonodynamic performance of GeSNSs, and the chemotherapeutic β-elemene, the living materials–mediated drug delivery system constructed in this study successfully reversed the immunosuppressive tumor microenvironment, achieving the transformation from cold tumors to hot ones for effective tumor therapy.
Our original design intention was to combine nanotechnology, chemotherapy, SDT, and immunotherapy to enhance antitumor immune response through macrophage hitchhiking. The key material GeSNSs@PEG used in our study showed good biocompatibility and nontoxicity, which provides safety assurance for its further clinical development. GeSNSs@PEG was prepared by the liquid phase exfoliation method, which is a mature and scalable technology with high reproducibility and production efficiency (3, 5, 27, 45). In addition, this study adopted the macrophage hitchhiking strategy to load the nanoparticles (GeSNSs@PEG-ELE) onto the surface of macrophages. The core steps (such as nanoparticle synthesis and macrophage surface modification) have been reported robust (10, 53), and our research shows that this method has high reproducibility and good safety. We are also optimizing the preparation process to further simplify the steps and reduce costs, paving the way for large-scale production in the future. In terms of clinical translation, SDT technology itself has been validated in multiple clinical trials (e.g., NCT04821284 and NCT06567301), highlighting its high translational potential. In addition, the drugs (β-elemene, ELE) used in this study is a typical Chinese Food and Drug Administration–approved chemodrug, and its efficacy and safety have been demonstrated in numerous studies (54–56). Here, we explored its combinational use with SDT to further improve the antitumor efficacy. Note that although the currently used macrophages have been proven to be useful for clinical applications, their source selection and nanoparticle loading capacity still need to be further optimized to meet clinical needs. These optimizations can not only improve the therapeutic effect but also improve the accessibility and scalability of the technology. Our study demonstrated that nanoparticles can effectively inhibit tumor growth without causing obvious toxic side effects, highlighting their promising potential for clinical applications. These characteristics make this strategy not only superior to traditional immunotherapy in terms of therapeutic effect but also more clinically feasible. In summary, this study not only explored the synergistic effects of nanotechnology, chemotherapy, SDT, and immunotherapy but also enhanced tumor targeting ability through the macrophage hitchhiking strategy. Combined with the high safety of the material, mature preparation technology, and good clinical convertibility, this strategy provides a new direction for future tumor immunotherapy and lays a solid foundation for further clinical development.
MATERIALS AND METHODS
Study design
This study aimed to evaluate the targeted delivery of germanium sulfide nanosheets as a nanosonosensitizer to tumor sites via macrophage hitchhiking, combined with chemotherapy and SDT to reverse the tumor immune microenvironment for the treatment of triple-negative breast cancer. We first synthesized and characterized GeSNSs by liquid phase exfoliation and coated them with PEG to enhance colloidal stability to form GeSNSs@PEG. Then, the anticancer drug β-elemene was loaded on GeSNSs@PEG by solution mixing to form stable GeSNSs@ELE. Then, we loaded GeSNSs@ELE onto the surface of macrophages (GeSNSs@ELE-Mφ) using maleimide-thiol reaction. In vitro studies evaluated ROS generation under US activation and cytotoxic effects on macrophages themselves and cancer cells. In vivo studies, the biodistribution of GeSNSs@ELE-Mφ in tumor-bearing mice was evaluated by fluorescence imaging.
The TNBC mouse model was used to evaluate the combined therapeutic effects of GeSNSs@ELE-Mφ–mediated chemotherapy and SDT, including the evaluation of tumor growth inhibition, ROS generation, and tumor immune microenvironment. The mice in this experiment were randomly assigned to each study group. The experimenters were not blinded during the study.
Materials
Germanium (II) monosulfide powder (99.99%), 1-methyl-2-pyrrolidinone (NMP; 99.5%), ethanol (99.5%), GSH (98.0%), DTNB (99%), DPBF (97%), MB, 2′,7′-dichlorofluorescin diacetate (97 + %), calcein-AM, PI, crystal violet solution (1%), and hematoxylin (1 mg/ml) and eosin stain solution (dye content 95%) were purchased from Sigma-Aldrich. DSPE-PEG-maleimide, molecular weight (Mw): 2000 Da [DSPE (PEG)-2000] (DSPE-PEG) was purchased from Nanosoft Polymers. DSPE-PEG-Cy5 (Mw: 5000 Da) was purchased from NANOCS.DMEM, FBS, penicillin-streptomycin (10,000 U/ml), 0.25% trypsin-EDTA, and PBS were obtained from Gibco Life Technologies (USA). AlamarBlue cell viability reagent was purchased from Thermo Fisher Scientific. TCEP (98%) was obtained from AMRESCO. β-Elemene (10 mg/ml in ethanol) was purchased from Cayman. RAW264.7, 4T1, and 4T1-Luc cell lines were purchased from the American Type Culture Collection. FITC–annexin V apoptosis detection kit was purchased from BD Biosciences. Transwell polycarbonate membrane cell culture inserts (8-μm pore size) was purchased from Corning. Paraformaldehyde solution (4% in PBS) was purchased from Santa Cruz Biotechnology.
Instruments and characterization
The size and morphology of GeSNSs were analyzed by TEM (Tecnai G2 F20, FEI, the Netherlands). The XRD pattern of GeSNSs was obtained using a MiniFlex600 diffractometer with Cu Kα radiation (λ = 1.5418 Å). The chemical composition of GeSNSs was determined by XPS (ESCALAB 250Xi, Thermo Scientific). TGA was conducted using a Synchronous DSC-TGA Thermal Analysis (SDT Q600) under a nitrogen atmosphere (flow rate of 50 ml/min) with a consistent heating rate of 10°C/min. The functional groups of GeSNSs and GeSNSs@PEG were identified by a FTIR spectrometer (iS50 ABX, USA) across the spectral range of 4000 to 400 cm−1. The DLS size and zeta potential of GeSNSs and GeSNSs@PEG were measured using a laser particle analyzer (ZetaPALS, Brookhaven Instruments). The diffuse reflectance spectrum of GeSNSs was recorded using a UV -vis–near infrared (NIR) spectrophotometer (Shimadzu UV-3600i Plus, Japan).
Synthesis of GeSNSs
A mixture of 100 mg of GeS powder and 20 ml of NMP solution was sonicated using an ultrasonic probe (FB505, Thermo Fisher Scientific) in an ice-water bath for 18 hours (10-s ON and 5-s OFF) with a power, intensity, and frequency of 500 W, 40% power output, and 20 kHz, respectively. Subsequently, the supernatant containing unexfoliated GeS was removed by centrifugation at a centrifugation speed of 1000 g for 10 min. Afterward, the pellets were redispersed in 10 ml of NMP. Next, centrifugation was performed at a speed of 14,800 rpm for 10 min to obtain GeSNSs. Last, the GeSNSs were washed three times with ethanol and stored in a −20°C refrigerator for further use.
Surface modification of GeSNSs using DSPE-PEG
Tqo improve the biocompatibility and dispersion stability of GeSNSs in the biological environments, we coated the surface of GeSNSs with DSPE-PEG-MAL or DSPE-PEG-Cy5. Our study used thin film hydration technology to modify DSPE-PEG-MAL and GeSNSs, offering several advantages: It enhances the self-assembly efficiency, allowing DSPE-PEG-MAL to uniformly cover the surface of GeSNSs, thereby improving water dispersibility and providing a stable biofunctionalized interface. In addition, this modification can effectively prevent the aggregation of the material in the aqueous phase. In general, 2 mg of GeSNSs and 10 mg of DSPE-PEG-MAL (or 2 mg of DSPE-PEG-Cy5) were mixed in 10 ml of ethanol. Afterward, the solution was under ultrasonic treatment in an ice-water bath for 30 min (15-s ON and 5-s OFF) using an ultrasonic probe (FB505, Thermo Fisher Scientific) at a power, intensity, and frequency of 500 W, 20% power, and 20 kHz, respectively. Subsequently, the ethanol was evaporated using a rotary evaporation method. The dry film of DSPE-PEG-MAL or DSPE-PEG-Cy5–coated GeSNSs (GeSNSs@PEG-MAL or GeSNSs@PEG-Cy5) was resuspended in 10 ml of ultrapure water and then centrifuged at 14,800 rpm for 10 min to remove the free DSPE-PEG-MAL or DSPE-PEG-Cy5. The wash procedure was repeated three times using ultrapure water. Last, the obtained GeSNSs@PEG-MAL or GeSNSs@PEG-Cy5 was stored in a dark environment at 4°C.
US-triggered generation of 1O2 from GeSNSs@PEG
To evaluate the generation of 1O2 from US (US)-triggered GeSNSs, we mixed 1 ml of PBS solution containing 200 μg of GeSNSs@PEG (200 μg ml-1) with 1 ml of ethanol containing 80 μg of DPBF (80 μg ml−1). After stirring for 5 min in the dark, the mixture was exposed to ultrasonic activation (1 MHz, 1 W/cm2, 50% duty cycle) (SoundCare Plus, 1 cm2 probe, Roscoe Medical) for 5, 10, 15, 20, and 30 min. Our previous experience (5, 45) and some other reports (57, 58) demonstrated 1 ~ 2 W/cm2 US power is sufficient for effectively activating the nanomaterials and exerting sonodynamic effects. This power can ensure accurate reflection of the kinetic process of the redox reaction. US power too high or too low may affect the SDT effect and bias the experimental results. This range of power can maintain the validity and accuracy of the experiment. The resulting solution was measured by a UV-vis-NIR spectrometer (350 ~ 550 nm) and the absorbance at 410 nm showing DPBF degradation was measured to quantify the 1O2 production. The groups include DPBF + US and DPBF + GeSNSs@PEG serve as the control.
US-triggered generation of ·OH from GeSNSs@PEG
We used MB as a probe to evaluate the generation of ·OH from US-triggered GeSNSs@PEG., In general we mix 1 ml of PBS solution containing 100 μg of GeSNSs@PEG (100 μg ml−1) and 1 ml of MB solution in PBS (5 μg ml−1), with or without the addition of H2O2 (50 μM). Then, the mixture was triggered by US (1 MHz, 2 W cm−2, 50% duty cycle) for different time periods (5, 10, 15, 20, and 30 min). The resulting solution was measured with a UV-vis-NIR spectrometer between 500 and 800 nm, and the absorbance change at 664 nm was measured to evaluate the generation of ·OH. The MB + GeSNSs@PEG, MB + GeSNSs@PEG + H2O2, MB + US, MB + H2O2 + US, and MB + GeSNSs@PEG + US groups served as the control group, and the MB + GeSNSs@PEG + H2O2 + US group served as the experimental group.
US-triggered GSH depletion of GeSNSs@PEG
The concentration of GSH was quantified using a DTNB molecule. PBS solution containing GeSNSs (200 μg ml−1) and GSH (30 μg ml−1) was exposed to US (1 MHz, 2 W cm−2, 50% duty cycle) for different time periods (5, 10, 15, 20, 30, and 40 min). After trigger, the GeSNSs@PEG in the solution was removed by centrifugation (14,800 rpm × 10 min), and the supernatant (190 μl) was mixed and stirred with 10 μl of dimethyl sulfoxide solution containing DTNB (0.5 mg ml−1). The absorbance of the resulting solution was measured with a UV-vis spectrometer (350 to 500 nm); the absorbance of the solution at 412 nm was measured to assess the depletion of GSH. The GSH + GeSNSs@PEG and GSH + US groups served as the control group, and the GSH + GeSNSs@PEG + US group served as the experimental group.
Loading of β-elemene onto GeSNSs@PEG
One mg of GeSNSs@PEG was mixed with 1.25 ml of ethanol solution containing 1.25 mg (β-elemene concentration: 1 mg/ml), 2.5 mg (β-elemene concentration: 2 mg/ml), 6.25 mg (β-elemene concentration: 5 mg/ml), or 12.5 mg (β-elemene concentration: 10 mg/ml). The mixture was stirred overnight at room temperature in a dark environment. The β-elemene–loaded GeSNSs@PEG (GeSNSs@ELE) was centrifuged at 14,800 rpm for 10 min, and the pellets were washed three times with PBS (2 ml for each) to remove excess ethanol and β-elemene. Last, the GeSNSs@ELE was resuspended in PBS for subsequent experiments.
To quantify the capacity of GeSNSs@PEG to load β-elemene, the β-elemene that had been loaded on GeSNSs@ELE through physical adsorption and van der Waals forces was removed and dissolved in ethanol. Briefly, 500 μg of GeSNSs@ELE was redispersed in 1 ml of ethanol. Afterward, the solution was centrifuged at 14,800 rpm, and the supernatant containing β-elemene was collected. The wash procedure for β-elemene collection was repeated three times. Last, the concentration of β-elemene soltion was measured using HPLC (Agilent 1260 Infinity II) connected to a C-18 column and its calibration curve (0, 3.125, 6.25, 12.5, 25, 50 and 100 μg/ml) The HPLC parameters are shown as follows: 20 μl of samples was injected into the column, with a flow rate of 1.0 ml/min. The peak elution time of β-elemene was about 3.5 min using a mobile phase of acetonitrile: deionized water (80:20, volume ratio). The signal of β-elemene was measured with a UV detector set at 210 nm.
Cell culture
Mouse macrophages (RAW264.7), triple-negative breast cancer cell line (4T1), and luciferase-expressing breast cancer cell line (4T1-Luc) were purchased from the American Type Culture Collection Center. We cultured macrophages and tumor cells in DMEM mixed with 10% FBS and 1% penicillin-streptomycin. The cells were then incubated in a standard cell culture incubator set at 37°C with 5% CO2.
Treatment of macrophages with TCEP
Macrophages (2 × 106 cells/ml) were suspended in 1 ml of PBS containing 0, 0.5, 1, 1.5, or 2 mM TCEP. Subsequently, the samples were incubated at 37°C for 30 min with shaking, followed by washing three times with PBS to remove TCEP. To assess the number of exposed thiols on the surface of the macrophages, the cysteine and DTNB chemical reaction were used. A total of 200 μl of l-cystine series standards of 1, 5, 10, 15, and 20 μM was prepared. An appropriate amount of DTNB reagent was added to each standard to a final concentration of 200 μM. After DTNB and l-cystine reacted in the mixed sample for 10 min, the absorbance value was measured at a wavelength of 412 nm. Moreover, to assess whether TCEP had toxicity effect on macrophages, the treated cells were stained with Calcein-AM (4 μg/ml) and PI (10 μg/ml) for 30 min followed by observing the ratio of live (green) to dead (red) cells under a fluorescence microscope. Furthermore, to quantify the cell viability, macrophages treated with various concentrations of TCEP for 30 min were washed three times with PBS. After 24 hours, cell viability was assessed using the alamar blue method. After the removal of medium, cells incubated with 100 μl of fresh culture medium containing 10% alamar blue reagent. After incubation at 37°C for 30 min, the fluorescence emission intensity was measured at 590 nm (excitation at 545 nm). In addition, cell apoptosis was detected using the annexin V–FITC/PI apoptosis detection kit. Cells in each group were collected, incubated with 5 μl of annexin V–FITC and 5 μl of PI for 30 min, and then tested on the flow cytometry.
Preparation of macrophages with GeSNSs@ELE loaded on their surface
The macrophages carrying GeSNSs@PEG-Mal-ELE or GeSNSs@PEG-MAL on their surface (GeSNSs@ELE-Mφ or GeSNSs-Mφ) was obtained through co-incubation of TCEP-treated macrophages with GeSNSs@ELE or GeSNSs@PEG-MAL. In brief, RAW264.7 cells (1 × 107 cells) were treated with 5 ml of TCEP (1 mM in PBS) for 30 min to expose more thiol groups on the macrophage surface. After removing TCEP through centrifuge, the thiol-maleimide reaction was utilized to conjugate the macrophages (1 × 107 cells/5 ml in PBS) with GeSNSs@PEG-MAL-ELE or GeSNSs@PEG-MAL at 37°C for 30 min. The mixed solution was centrifuged at 300g and 4°C and washed three times with 5 ml of PBS, and the supernatant was removed. This process effectively precipitated the macrophages and ensured the complete removal of free GeSNSs@PEG-MAL-ELE or GeSNSs@PEG-MAL from the washing solution. As a result, a purified macrophage suspension was obtained, with GeSNSs@ELE or GeSNSs@PEG-MAL bound to the surface (GeSNS@ELE-Mφ or GeSNSs-Mφ). We used fluorescence spectrophotometer to measure the fluorescence intensity (excitation wavelength of 649 nm and emission wavelength of 670 nm) in the supernatant after each time of washing (before centrifugation, after centrifugation, and after each PBS wash) to ensure complete purification.
To determine the loading amount of GeSNSs@PEG-MAL-ELE on the surface of macrophages, we used ICP-MS technology (NexION 300D, PerkinElmer) to quantify the content of GeS or GeSNSs@ELE on the surface of macrophages. Confocal laser scanning microscope (FV1000, Olympus) and SEM (S-4700, Hitachi) were used to observe the relative distribution of GeS or GeSNSs@ELE on the surface of macrophages.
In vitro cytotoxicity
In vitro cytotoxicity was assessed using the AlamarBlue method to determine the viability of cells in different groups. In general, cells were seeded in a 96-well plate at a density of 1 × 104 cells/100 μl of DMEM per well. After overnight adherence, the culture medium was removed, and cells were incubated for 24 hours in 100 μl of fresh culture medium containing various concentrations of GeSNSs@PEG or GeSNSs@ELE (0, 3.1, 6.3, 12.5, 25.0, and 50.0 μg/ml). Subsequently, the culture medium was removed, and cells were washed twice with 100 μl PBS before being incubated with 100 μl of fresh culture medium containing 10% AlamarBlue reagent. After 30 min of incubation at 37°C, the fluorescence emission intensity at 590 nm (excitation at 545 nm) was measured using a plate reader (Tecan Infinite M200PRO) to quantify the cell viability under each condition. A baseline group containing 100 μl of medium and 10 μl of AlamarBlue reagent was also included.
Calcein-AM/PI
Calcein-AM/PI costaining assay was performed to observe cell live/death. Specifically, cells were seeded in 96-well plates at a density of 1 × 105 cells/100 μl of medium per well. Then, cells were treated with different concentrations of TCEP (0, 0.5, 1, 1.5, and 2 mM) or GeSNS@ELE (0 μg/ml, 3.1, 6.3, 12.5, and 50.0 μg/ml). The treated macrophages were then incubated with cells that were stained with Calcein-AM (4 μg/ml) and PI (10 μg/ml) for 30 min at 37°C and washed three times with PBS. Last, Calcein-AM/PI costained cells were observed under a fluorescence microscope (Axio Vert.A1, ZEISS).
Cell apoptosis
Flow cytometry apoptosis staining kit was used to detect cell apoptosis. In genetal, cells were collected and prepared as a cell suspension. Subsequently, cells were washed with PBS and adjusted to a concentration of 1 × 106 cells/100 μl of binding buffer. Next, a mixture of 5 μl of annexin V and 5 μl of PI was added to the cell suspension, and the cells were stained for 30 min at room temperature. Subsequently, the cell suspension was analyzed using a flow cytometer (BD LSRFortessa).
The cytotoxic effects of GeSNSs@ELE-Mφ on 4T1-Luc tumor cells
A total of 5 × 105 4T1-Luc cells were seeded per well in a 24-well plate. After 12 hours, the cells were incubated with or without 5 × 105/500 μl of GeSNSs@ELE-Mφ for 24 hours. Subsequently, the cells were exposed to US irradiation (1 MHz, 0.3 W cm−2, 50% duty cycle) for 3 min, followed by a 5-min rest, and then another 3 min of US treatment. In in vitro cell treatments, we select a US power that would not cause cytotoxicity. No significant cell death or damage was observed at a US power of 0.3 W/cm2 in our cellular experiments, ensuring that the experimental results mainly reflect the sonodynamic effect rather than other nonspecific effects. The US power in similar studies (5, 45, 57, 58) is in the range of 0.1 to 3 W/cm2. The US power we selected is within that range, which strengthened the comparability to other studies.
The cells were then incubated in 500 μl of fresh culture medium containing d-luciferin (Promega, E2510) for 10 min to induce cell lysis. Afterward, luminescence was measured using a luminometer, and bioluminescence from each well was captured using the IVIS Spectrum/CT imaging system (PerkinElmer) to estimate the viability of 4T1 cells.
Animal
The animal protocol was approved by the Institutional Animal Care and Use Committee of Brigham and Women’s Hospital, Harvard Medical School (permit number: 2020 N000055). All in vivo studies were performed in accordance with the National Institutes of Health Animal Care Guidelines. Seven-week-old female BALB/C mice were purchased from the Jackson Laboratory. A total of 1 × 107 4T1-luc cells was inoculated into the left fourth mammary gland fat pad of mice to establish an orthotopic breast cancer model. The mouse tumor growth was monitored using an in vivo imaging system (IVIS, Lumina LT Series III, PerkinElmer), and the length and width of mouse orthotopic breast tumors were measured using vernier calipers. The volume size of mouse tumors was calculated using the formula: (Volume = width2 × Length/2).
In vivo biodistribution, clearance, and biosafety
The biodistribution of GeSNSs@PEG-Cy5-ELE-Mφ was assessed on 4T1 tumor-bearing mice. Mice were injected with GeSNSs@PEG-Cy5-ELE-Mφ (10 mg kg−1) via tail vein, and the imaging system (PXi 4 Touch, Syngene) was used to record the images at 0, 1, 2, 4, 6, 8, 10, 12, and 24 hours) for fluorescence observation. In addition, ex vivo biodistribution studies were performed 24 hours after injection. The GeSNSs@PEG-Cy5 group serves as a control.
To evaluate the clearance rate of GeSNSs or GeSNSs@PEG-ELE-Mφ in vivo, BALB/c mice were administered GeSNSs or GeSNSs@PEG-ELE-Mφ via intravenous injection. Feces and urine were collected at 0.5, 1, 3, 5, 7, 10, 14, 21, and 30 days post-injection, and the concentration of Ge in these excreta was quantified using ICP-MS (NexION 300D, PerkinElmer). For the biosafety assessment, seven-week-old female BALB/c mice received intravenous injections of either saline or GeSNSs@PEG-ELE-Mφ. Blood samples were collected on days 1, 14, and 30 post-injection to monitor changes in relevant hematological and biochemical parameters. These included WBC, RBC, HGB, HCT, MCV, MCH, MCHC, PLT, ALT, AST, ALB, GGT, TP, urea (blood urea nitrogen, UREA), CREA, CK, LDH, and AMY.
In vivo enhanced SDT
Mice bearing 4T1-luc tumor were randomly divided by six groups (N = 5). G1: saline; G2: Mφ; G3: GeSNSs-Mφ; G4: GeSNSs@ELE-Mφ; G5: GeSNSs@ELE-Mφ + US; G6: GeSNSs@ELE-Mφ + US × 2. For the G1: saline group, mice received intravenous injection of 100 μl of saline; for the G2: Mφ group, mice received intravenous injection of RAW264.7 cells (5 × 106) in 100 μl of PBS; for the G3: GeSNSs-Mφ group (GESNSs-Mφ refers to GeSNSs@PEG-MAL loaded onto the surface of macrophages but does not contain β-elemene), mice received intravenous injection of GeSNSs-Mφ (10 mg/kg) in 100 μl of PBS; for the G4: GeSNSs@ELE-Mφ group, mice received intravenous injection of GeSNSs@ELE-Mφ (10 mg/kg) in 100 μl of PBS; for the G5: GeSNSs@ELE-Mφ + US group, mice first received intravenous injection of GeSNSs@ELE-Mφ (10 mg/kg) in 100 μl of PBS. Four hours later, the tumor site was exposed to US irradiation (3 × 5 min with an interval of 5 min, 1 MHz, 2 W/cm2, 50% duty cycle). For the G6: GeSNSs@ELE-Mφ + US × 2, mice received two times of treatment with an interval of three days. US treatment was applied using the SoundCare Plus system (1 cm2 probe, Roscoe Medical), with US gel applied to the surface to ensure effective contact, thereby enhancing the treatment through cavitation and localized activation of the therapeutic agent. The US parameters were characterized using a 3D acoustic field scanning system (Precision Acoustics, SN: UMS3) equipped with a 1-mm high-frequency needle hydrophone (Precision Acoustics, SN: 2571) in deionized and degassed water (O2 < 3 parts per million, 22°C), and data were acquired using an oscilloscope (Tektronix DPO5034). Detailed acoustic parameters are summarized in table S1. The reported power density (2 W/cm2) corresponds to the spatial-peak temporal-average intensity measured at a depth of 3 mm using the hydrophone (59–62). According to the American Institute of Ultrasound in Medicine and related literature (5, 45, 63), US alone does not exhibit tumor-killing effects. Consistently, our in vitro experiments confirmed that US had no significant impact (Fig. 3A), the G2 and G3 groups were included as controls to assess the therapeutic effects of macrophages (G2) and macrophages loaded with GeSNSs (G3) without US activation. The current group design effectively isolates the effects of Mφ and GeS@Mφ, facilitating direct comparison with GeSNSs@ELE-Mφ and GeSNSs@ELE-Mφ + US. The body weights and tumor sizes of the mice were measured every 2 days. The inhibition rate was calculated using the following formula: Tumor growth inhibition ratio = [(tumor volume of mice in control group − tumor volume of mice in treatment group)/tumor volume of mice in control group] × 100%. After the treatments, mice were euthanized, and tumors, spleens and lymph nodes were harvested for photography and weighting.
For histological analysis, H&E staining, immunohistochemical staining, immunofluorescence (IF) staining, and TUNEL staining were performed. Tumors were collected 24 hours after different treatments; fixed in formalin; embedded in paraffin; and sectioned for H&E, IHC, and IF staining. In addition, for in situ ROS staining, tumor tissues were excised, embedded in Tissue-Tek O.C.T. (Sakura Finetek), and frozen. Sections of 15-μm thickness were cut, followed by staining with H2DCF-DA (20 μM) for 30 min. Afterward, the tissue slices were washed three times with PBS, stained with Hoechst, and washed again three times with PBS. The tumor sections were then observed under a fluorescence microscope to assess the ROS generation efficiency induced by the various treatments.
Preparation of single-cell suspension
On the 17th day of treatment, the mice were euthanized, and tumor tissues, spleens, and lymph nodes from each treatment group of mice were collected. Tissues were digested in DMEM containing 10% FBS, collagenase D (final concentration of 0.5 mg/ml; Sigma-Aldrich, 11088858001), and deoxyribonuclease I (final concentration of 10 μg/ml; Sigma-Aldrich, D4527) at 4°C for 12 hours. Subsequently, tumor tissues were filtered through a 70-μm mesh filter to isolate cells, while spleens and lymph nodes were filtered through 40-μm mesh filters. Afterward, the cells were washed twice with PBS containing 5% FBS (1200 rpm, 5 min). To remove RBCs, 1 ml of RBC lysis buffer (Thermo Fisher Scientific, 00433357) was added to the cell pellet, followed by gently mixing for 1 min. Afterward, the mixture was diluted with 5 ml of PBS and centrifuged at 1200 rpm for 5 min. The supernatant was then discarded. Last, cells were resuspended in PBS containing 5% FBS before staining.
Flow cytometry analysis of cell staining
The single-cell suspension was counted and diluted to 1 × 106 cells/100 μl. Cells were incubated with 0.5 μl of fixable viability dye (Thermo Fisher Scientific, 65086614) at room temperature in the dark for 30 min. Subsequently, cells were incubated with anti-mouse CD16/32 antibody (1 μl per sample, Miltenyi Biotec, catalog no. 130-092-575; 0.5 μg/ml) for 30 min to block Fc receptors. The following antibody cocktail was then used for analysis of mouse macrophages: PerCP/Cyanine5.5 anti-mouse CD45 (2.5 μg/ml; BioLegend, 109828), FITC anti-mouse/human CD11b (2.5 μg/ml; BioLegend, 101206), allophycocyanin (APC) anti-mouse F4/80 (2.5 μg/ml; BioLegend, 123116), phycoerythrin (PE) anti-mouse CD80 (5.0 μg/ml; BioLegend, 104708), and BV605 anti-mouse CD206 (MMR) (5.0 μg/ml; BioLegend, 141706). M1-like TAMs were identified as CD45+CD11b+F4/80+CD80hi cells, while M2-like TAMs were identified as CD45+CD11b+F4/80+CD206hi cells (table S2).
For DC analysis, the following antibody cocktail was used: PerCP/Cyanine5.5 anti-mouse CD45 (2.5 μg/ml; BioLegend, 109828), APC anti-mouse CD11c (2.5 μg/ml; BioLegend, 117310), APC/Cyanine7 anti-mouse CD103 (10.0 μg/ml; BioLegend, 121432), PE anti-mouse CD80 (5.0 μg/ml; BioLegend, 104708), and FITC anti-mouse CD86 (10.0 μg/ml; BioLegend, 105006). CD11c+ cells were identified as CD45+CD11c+ cells, CD103+ cells were identified as CD45+CD11c+CD103+ cells, and CD80+CD86+ cells were identified as CD45+CD11c+CD80+CD86+ cells (table S3).
For T cell analysis, the following antibody cocktail was used: PerCP/Cyanine5.5 anti-mouse CD45 (2 μg/ml; BioLegend, 109828), APC anti-mouse CD3 (2.5 μg/ml; BioLegend, 100236), FITC anti-mouse CD4 (1.5 μg/ml; BioLegend, 100406), and PE anti-mouse CD8 (1.5 μg/ml; BioLegend, 100708). CD3+ lymphocytes were identified as CD45+CD3+ cells, CD4+ T cells were identified as CD45+CD3+CD4+ cells, and CD8+ T cells were identified as CD45+CD3+CD8+ cells (table S4).
After 30 min of cell staining, cells were washed three times with PBS containing 5% FBS and centrifuge (1200 rpm, 5 min) to remove unbound antibody fluorescence dyes and resuspended in 500 μl of PBS containing 5% FBS. Flow cytometry analysis was performed using the BD LSRFortessa flow cytometer, and Flowjo software was used for further result analysis.
Quantification of cytokines in tumor tissues
On the 17th day, tumor-bearing mice were euthanized. Tumor tissues were then collected, homogenized, and processed with a digital tissue homogenizer (OMNI THQ 1356) in a mixture of cell lysis buffer (1×; Cell Signaling Technology, catalog no. 9803), phenylmethylsulfonyl fluoride (1 mM; Cell Signaling Technology, catalog no. 8553), and a protease/phosphatase inhibitor cocktail (1×; Cell Signaling Technology, catalog no. 5872) for 30 s on ice. After lysis, the solution was centrifuged at 14,800 rpm for 30 min at 4°C to collect the supernatant. Last, the concentrations of cytokines [TNF-α, IL-12 (p40), IL-10, and IFN-γ] in the supernatant were determined using the ELISA kit. The cytokine concentrations were expressed as picogram per milliliter per gram of tumor tissue, where the mass of the tumor tissue used for extraction was taken into account to standardize the results.
Quantification of inflammatory factor levels in serum
On the 17th day, blood was collected from the mice after various treatments. After standing for 30 to 60 min, the blood was centrifuged at 3500 rpm for 10 min, and the serum was collected and stored at −80°C for subsequent measurement. The serum concentrations of various cytokines [TNF-α, IL-12 (p40), IL-10, and IFN-γ] were quantified using ELISA kits (BioLegend, San Diego, CA, USA).
Statistical analysis
Data are presented as means ± SD. For parametric data, the two-tailed Student’s t test was used for intergroup comparisons. When comparing more than two groups, analysis of variance (ANOVA) was used for data analysis, followed by Brown-Forsythe and Welch ANOVA tests. Statistical analysis was performed using GraphPad Prism Version 10.0 software (GraphPad, USA). The significance level was set at *P < 0.05, **P < 0.01, and ***P < 0.001.
Acknowledgments
We appreciate the support provided by the animal welfare officers at Brigham and Women’s Hospital for the animal experiments.
Funding: This research was supported by the Harvard/Brigham Health & Technology Innovation Fund (2023A004452 to W.T.), the Nanotechnology Foundation (2022A002721 to W.T.), and Distinguished Chair Professorship Foundation (018129 to W.T.).
Author contributions: Conceptualization: S.C., Y.L., W.T., N.K., W.C., and T.X. Methodology: S.C., Y.L., Z.Z., Y.Z., M.M.K., X.J., and W.C. Validation: W.C. Formal analisis: S.C. Investigation: S.C., Y.L., Y.Z., M.M.K., and R.Q. Resources: Y.L., Y.Z., W.T., N.K., and T.X. Data curation: Y.L., W.C., and R.Q. Writing—original draft: S.C. Writing—review and editing: S.C., Y.L., Z.Z., Q.S., Y.Z., S.A., R.Q., W.T., N.K., W.C., and T.X. Visualization: S.C., Y.L., and W.C. Supervision: W.T., N.K., W.C., and T.X. Project administration: S.C. and W.C. Funding acquisition: W.T.
Competing interests: W.T. declares the following competing financial interest(s): W.T. receives consults (or on scientific advisory boards) for, lectured (and received a fee), or conducts sponsored research at Harvard Medical School/Brigham and Women’s Hospital for the following entities: Novo Nordisk A/S and Henlius USA Inc. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S40
Tables S1 to S4
REFERENCES AND NOTES
- 1.Brody H., Innovative cancer therapies offer new hope. Nature 629, S1 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L., Guilbaud E., Schmidt D., Kroemer G., Marincola F. M., Targeting immunogenic cell stress and death for cancer therapy. Nat. Rev. Drug Discov. 23, 445–460 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W., Li Y., Liu C., Kang Y., Qin D., Chen S., Zhou J., Liu H. J., Ferdows B. E., Patel D. N., Huang X., Koo S., Kong N., Ji X., Cao Y., Tao W., Xie T., In situ engineering of tumor-associated macrophages via a nanodrug-delivering-drug (β-Elemene@Stanene) strategy for enhanced cancer chemo-immunotherapy. Angew. Chem. Int. Ed. 62, e202308413 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Mitchell M. J., Billingsley M. M., Haley R. M., Wechsler M. E., Peppas N. A., Langer R., Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Chen W., Kang Y., Zhen X., Zhou Z., Liu C., Chen S., Huang X., Liu H. J., Koo S., Kong N., Ji X., Xie T., Tao W., Nanosensitizer-mediated augmentation of sonodynamic therapy efficacy and antitumor immunity. Nat. Commun. 14, 6973 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Qin D., Zou J., Li X., Guo X. D., Tang Y., Liu C., Chen W., Kong N., Zhang C. Y., Tao W., Living leukocyte-based drug delivery systems. Adv. Mater. 35, e2207787 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Zhu M. H., Zhu X. D., Long M., Lai X., Yuan Y., Huang Y., Zhang L., Gao Y., Shi J., Lu Q., Sun P., Lovell J. F., Chen H. Z., Fang C., Metal-coordinated adsorption of nanoparticles to macrophages for targeted cancer therapy. Adv. Funct. Mater. 33, 2214842 (2023). [Google Scholar]
- 8.Yang L., Zhang Y., Zhang Y., Xu Y., Li Y., Xie Z., Wang H., Lin Y., Lin Q., Gong T., Sun X., Zhang Z., Zhang L., Live macrophage-delivered doxorubicin-loaded liposomes effectively treat triple-negative breast cancer. ACS Nano 16, 9799–9809 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Vargason A. M., Anselmo A. C., Mitragotri S., The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 5, 951–967 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Zhou H., He H., Liang R., Pan H., Chen Z., Deng G., Zhang S., Ma Y., Liu L., Cai L., In situ poly I:C released from living cell drug nanocarriers for macrophage-mediated antitumor immunotherapy. Biomaterials 269, 120670 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Linde I. L., Prestwood T. R., Qiu J., Pilarowski G., Linde M. H., Zhang X., Shen L., Reticker-Flynn N. E., Chiu D. K., Sheu L. Y., Van Deursen S., Tolentino L. L., Song W. C., Engleman E. G., Neutrophil-activating therapy for the treatment of cancer. Cancer Cell 41, 356–372.e10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijkstra K. K., Cattaneo C. M., Weeber F., Chalabi M., van de Haar J., Fanchi L. F., Slagter M., van der Velden D. L., Kaing S., Kelderman S., van Rooij N., van Leerdam M. E., Depla A., Smit E. F., Hartemink K. J., de Groot R., Wolkers M. C., Sachs N., Snaebjornsson P., Monkhorst K., Haanen J., Clevers H., Schumacher T. N., Voest E. E., Generation of tumor-reactive t cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Mesquita S., Rua R., Brain border-associated macrophages: common denominators in infection, aging, and Alzheimer’s disease? Trends Immunol. 45, 346–357 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A., Allavena P., Marchesi F., Garlanda C., Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y., Rao L., Yao H., Wang Z., Ning P., Chen X., Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 32, e2002054 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Dooling L. J., Andrechak J. C., Hayes B. H., Kadu S., Zhang W., Pan R., Vashisth M., Irianto J., Alvey C. M., Ma L., Discher D. E., Cooperative phagocytosis of solid tumours by macrophages triggers durable anti-tumour responses. Nat. Biomed. Eng. 7, 1081–1096 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Li Z., Mo F., Chen-Mayfield T. J., Saini A., LaMere A. M., Hu Q., Chemically engineering cells for precision medicine. Chem. Soc. Rev. 52, 1068–1102 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Rennick J. J., Johnston A. P. R., Parton R. G., Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Chiu H. T., Su C. K., Sun Y. C., Chiang C. S., Huang Y. F., Albumin-gold nanorod nanoplatform for cell-mediated tumoritropic delivery with homogenous chemodrug distribution and enhanced retention ability. Theranostics 7, 3034–3052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao M., Hou S., Li W., Li K., Xue L., Hu Q., Zhu L., Chen Y., Sun H., Ju C., Zhang C., Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci. Transl. Med. 12, eaaz6667 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Doshi N., Swiston A. J., Gilbert J. B., Alcaraz M. L., Cohen R. E., Rubner M. F., Mitragotri S., Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv. Mater. 23, H105–H109 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Tang L., Zheng Y., Melo M. B., Mabardi L., Castaño A. P., Xie Y. Q., Li N., Kudchodkar S. B., Wong H. C., Jeng E. K., Maus M. V., Irvine D. J., Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 36, 707–716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutter E., Zhang B., Sun M., Sutter P., Few-layer to multilayer germanium(II) sulfide: Synthesis, structure, stability, and optoelectronics. ACS Nano 13, 9352–9362 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Sutter P., Argyropoulos C., Sutter E., Germanium sulfide nano-optics probed by STEM-cathodoluminescence spectroscopy. Nano Lett. 18, 4576–4583 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Tan D., Zhang W., Wang X., Koirala S., Miyauchi Y., Matsuda K., Polarization-sensitive and broadband germanium sulfide photodetectors with excellent high-temperature performance. Nanoscale 9, 12425–12431 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Tan C., Zhang H., Wang L., Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 44, 2681–2701 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Liu C., Sun S., Feng Q., Wu G., Wu Y., Kong N., Yu Z., Yao J., Zhang X., Chen W., Tang Z., Xiao Y., Huang X., Lv A., Yao C., Cheng H., Wu A., Xie T., Tao W., Arsenene nanodots with selective killing effects and their low-dose combination with ß-elemene for cancer therapy. Adv. Mater. 33, e2102054 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Xiang X., Wang J., Lu D., Xu X., Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct. Target. Ther. 6, 75 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao C., Cheng Q., Li J., Chen J., Wang Q., Wei J., Huang Q., Lee S. M., Gu D., Wang R., Supramolecular macrophage-liposome marriage for cell-hitchhiking delivery and immunotherapy of acute pneumonia and melanoma. Adv. Funct. Mater. 31, 2102440 (2021). [Google Scholar]
- 30.Li X., Zhang Y., Li S., Shi J., Liu C., Li X., Li Y., Luo S., Wang Y., Lai S., Li M., Zhang M., Sun L., Du X., Zhou M., Xing F., Zhang Q., Wu Z., Zheng T., Macrophage hitchhiking for systematic suppression in postablative multifocal HCC. Hepatology 81, 44–59 (2025). [DOI] [PubMed] [Google Scholar]
- 31.Albert M. L., Jegathesan M., Darnell R. B., Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat. Immunol. 2, 1010–1017 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Riley R. S., June C. H., Langer R., Mitchell M. J., Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy L. B., Salama A. K. S., A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70, 86–104 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Huang Y., Zucker B., Zhang S., Elias S., Zhu Y., Chen H., Ding T., Li Y., Sun Y., Lou J., Kozlov M. M., Yu L., Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat. Cell Biol. 21, 991–1002 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Liu A. A., Sun E. Z., Wang Z. G., Liu S. L., Pang D. W., Artificially regulated synthesis of nanocrystals in live cells. Natl. Sci. Rev. 9, nwab162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belluati A., Jimaja S., Chadwick R. J., Glynn C., Chami M., Happel D., Guo C., Kolmar H., Bruns N., Artificial cell synthesis using biocatalytic polymerization-induced self-assembly. Nat. Chem. 16, 564–574 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen M. E., Hindley J. W., Baxani D. K., Ces O., Elani Y., Hydrogels as functional components in artificial cell systems. Nat. Rev. Chem. 6, 562–578 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Huang W., Wu X., Gao X., Yu Y., Lei H., Zhu Z., Shi Y., Chen Y., Qin M., Wang W., Cao Y., Maleimide-thiol adducts stabilized through stretching. Nat. Chem. 11, 310–319 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Devos C. A.-O., Bampouli A. A.-O., Brozzi E. A.-O. X., Stefanidis G. A.-O., Dusselier M. A.-O., Van Gerven T. A.-O., Kuhn S. A.-O., Ultrasound mechanisms and their effect on solid synthesis and processing: A review. Chem. Soc. Rev. 54, 85–115 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H., Zheng Y., Chang M., Song J., Xia L., Wu C., Jia W., Ren H., Feng W., Chen Y., Ultrasound-based micro-/nanosystems for biomedical applications. Chem. Rev. 124, 8307–8472 (2024). [DOI] [PubMed] [Google Scholar]
- 41.Kumar A. R. S., Padmakumar A., Kalita U., Samanta S., Baral A., Singha N. K., Ashokkumar M., Qiao G. G., Ultrasonics in polymer science: Applications and challenges. Prog. Mater. Sci. 136, 101113 (2023). [Google Scholar]
- 42.Jeffries C. D., Electron-hole condensation in semiconductors: Electrons and holes condense into freely moving liquid metallic droplets, a plasma phase with novel properties. Science 189, 955–964 (1975). [DOI] [PubMed] [Google Scholar]
- 43.Mohanty R., Mansingh S., Parida K., Parida K., Boosting sluggish photocatalytic hydrogen evolution through piezo-stimulated polarization: a critical review. Mater. Horiz. 9, 1332–1355 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Ji X., Tang Z., Liu H., Kang Y., Chen L., Dong J., Chen W., Kong N., Tao W., Xie T., Nanoheterojunction-mediated thermoelectric strategy for cancer surgical adjuvant treatment and β-elemene combination therapy. Adv. Mater. 35, e2207391 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Chen W., Liu C., Ji X., Joseph J., Tang Z., Ouyang J., Xiao Y., Kong N., Joshi N., Farokhzad O. C., Tao W., Xie T., Stanene-based nanosheets for β-elemene delivery and ultrasound-mediated combination cancer therapy. Angew. Chem. Int. Ed. 60, 7155–7164 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Binnewies M., Roberts E. W., Kersten K., Chan V., Fearon D. F., Merad M., Coussens L. M., Gabrilovich D. I., Ostrand-Rosenberg S., Hedrick C. C., Vonderheide R. H., Pittet M. J., Jain R. K., Zou W., Howcroft T. K., Woodhouse E. C., Weinberg R. A., Krummel M. F., Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorin M., Rezanejad M., Karimi E., Fiset B., Desharnais L., Perus L. J. M., Milette S., Yu M. W., Maritan S. M., Doré S., Pichette É., Enlow W., Gagné A., Wei Y., Orain M., Manem V. S. K., Rayes R., Siegel P. M., Camilleri-Broët S., Fiset P. O., Desmeules P., Spicer J. D., Quail D. F., Joubert P., Walsh L. A., Single-cell spatial landscapes of the lung tumour immune microenvironment. Nature 614, 548–554 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Propper D. J., Balkwill F. R., Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Cao J., Ji L., Zhan Y., Shao X., Xu P., Wu B., Chen P., Cheng L., Zhuang X., Ou Y., Hua F., Sun L., Li F., Chen H., Zhou Z., Cheng Y., MST4 kinase regulates immune thrombocytopenia by phosphorylating STAT1-mediated M1 polarization of macrophages. Cell. Mol. Immunol. 20, 1413–1427 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West E. E., Merle N. S., Kamiński M. M., Palacios G., Kumar D., Wang L., Bibby J. A., Overdahl K., Jarmusch A. K., Freeley S., Lee D. Y., Thompson J. W., Yu Z. X., Taylor N., Sitbon M., Green D. R., Bohrer A., Mayer-Barber K. D., Afzali B., Kazemian M., Scholl-Buergi S., Karall D., Huemer M., Kemper C., Loss of CD4(+) T cell-intrinsic arginase 1 accelerates Th1 response kinetics and reduces lung pathology during influenza infection. Immunity 56, 2036–2053.e12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banchereau J., Steinman R. M., Dendritic cells and the control of immunity. Nature 392, 245–252 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Wculek S. K., Cueto F. J., Mujal A. M., Melero I., Krummel M. F., Sancho D., Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Lee E. S., Gao Z., Kim D., Park K., Kwon I. C., Bae Y. H., Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J. Control. Release 129, 228–236 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng Y., An Q., Zhao Z., Wu M., Yang C., Liang W., Xu X., Jiang T., Zhang G., Beta-elemene: A phytochemical with promise as a drug candidate for tumor therapy and adjuvant tumor therapy. Biomed. Pharmacother. 172, 116266 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Hu Q., Bai L., Zhu Z., Su Z., Bai P., Tang M., Dou C., Yan J., Tong R., Zhang W., Chen L., Cai L., β-Elemene-loaded polymeric micelles intensify anti-carcinoma efficacy and alleviate side effects. Chin. Chem. Lett. 31, 915–918 (2020). [Google Scholar]
- 56.Deng M., Liu B., Song H., Yu R., Zou D., Chen Y., Ma Y., Lv F., Xu L., Zhang Z., Lv Q., Yang X., Che X., Qu X., Liu Y., Zhang Y., Hu X., β-Elemene inhibits the metastasis of multidrug-resistant gastric cancer cells through miR-1323/Cbl-b/EGFR pathway. Phytomedicine 69, 153184 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Xu Q., Zhan G., Zhang Z., Yong T., Yang X., Gan L., Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics 11, 1937–1952 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding J., Su R., Yang R., Xu J., Liu X., Yao T., Li S., Wang C., Zhang H., Yue Q., Zhan C., Li C., Gao X., Enhancing the antitumor efficacy of oncolytic adenovirus through sonodynamic therapy-augmented virus replication. ACS Nano 18, 18282–18298 (2024). [DOI] [PubMed] [Google Scholar]
- 59.American Institute of Ultrasound in Medicine, Statement on data needed to characterize ultrasound system output (American Institute of Ultrasound in Medicine, 2022); www.aium.org/resources/official-statements/view/statement-on-data-needed-to-characterize-ultrasound-system-output.
- 60.ter Haar G., Shaw A., Pye S., Ward B., Bottomley F., Nolan R., Coady A. M., Guidance on reporting ultrasound exposure conditions for bio-effects studies. Ultrasound Med. Biol. 37, 177–183 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Padilla F., Ter Haar G., Recommendations for reporting therapeutic ultrasound treatment parameters. Ultrasound Med. Biol. 48, 1299–1308 (2022). [DOI] [PubMed] [Google Scholar]
- 62.American Institute of Ultrasound in Medicine, Statement on biological effects of therapeutic ultrasound (American Institute of Ultrasound in Medicine, 2023); www.aium.org/resources/official-statements/view/statement-on-biological-effects-of-therapeutic-ultrasound. [DOI] [PubMed]
- 63.American Institute of Ultrasound in Medicine, Statement on biological effects of ultrasound in vivo (American Institute of Ultrasound in Medicine, 2021); www.aium.org/resources/official-statements/view/statement-on-biological-effects-in-tissues-with-ultrasound-contrast-agents-2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S40
Tables S1 to S4