Abstract
Chronic renal allograft injury (CRAI) is a major cause of allograft loss in kidney transplant recipients (KTRs). The aim of this study was to evaluate the associations of urinary apolipoprotein A4 (ApoA-IV) levels with renal function and rapid renal function decline in KTRs. This study included 50 KTRs. Proteomic analysis via liquid chromatography‒mass spectrometry and tandem mass spectrometry (LC-MS/MS) was performed to identify potential urinary biomarkers. The SWATH (sequential window acquisition of all theoretical mass spectra) method was used for protein quantification. Urinary ApoA-IV levels were validated by enzyme-linked immunosorbent assay (ELISA). Rapid renal function decline was defined as an estimated glomerular filtration rate (GFR) decrease of >3 mL/min/1.73 m2 per year or initiation of dialysis. The log-transformed urinary ApoA-IV levels measured by ELISA had a significantly inverse correlation with the estimated GFR (r = -0.72, P < 0.001). Moreover, urinary ApoA-IV levels were higher in patients with rapid renal function decline than in those with stable renal function (215.4 ± 181.8 μg/mL vs. 42.5 ± 72.4 μg/mL, P = 0.001). Univariate logistic regression analysis revealed that log-transformed urinary ApoA-IV levels were significantly associated with rapid renal function decline (odds ratio [OR] 6.70, 95% confidence interval [CI] 2.56–22.83; P < 0.001). Multiple logistic regression showed urinary ApoA-IV levels remained a significant risk factor for rapid renal function decline (OR 4.10, 95% CI 1.10–19.55; P = 0.047). ROC curve analysis revealed the area under the curve (AUC) of 0.834 (95% CI 0.722–0.945, P < 0.001) for urinary ApoA-IV levels in predicting rapid renal function decline. Our results suggest that urinary ApoA-IV levels might be a potential biomarker for renal allograft function and could be used as a predictor for rapid renal function decline in KTRs.
Introduction
Although the short-term outcomes of kidney transplantation have improved remarkably over several decades with the introduction of potent immunosuppression, the long-term allograft survival rates of kidney transplantation remain similar [1]. Chronic renal allograft injury (CRAI) is a major cause of allograft loss in kidney transplant recipients (KTRs). The CRAI is a multifactorial clinical and pathological entity characterized by a progressive decline in the glomerular filtration rate (GFR), which is generally associated with several immunological and nonimmunological factors [2,3]. Immunological factors associated with the CRAI include sensitization, cellular and/or antibody-mediated rejection, and HLA mismatch, and nonimmunological factors include delayed graft function, recurrent infection episodes, arterial hypertension, recurrent glomerulonephritis, poor-quality donor kidney, ureteral stenosis, and calcineurin inhibitor nephrotoxicity. Histologically, CRAI is characterized by interstitial fibrosis and tubular atrophy [4].
Apolipoprotein A4 (ApoA-IV), which is known to have anti-atherogenic properties, is also known to be elevated in patients with chronic kidney disease [5]. In addition, several previous studies have reported that the serum ApoA-IV level could predict the progression of renal impairment in patients with nondiabetic primary kidney disease as well as in patients with type 2 diabetes mellitus (DM) [6–8]. Recent years have seen growing interest in identifying non-invasive urinary biomarkers for monitoring renal allograft function and predicting outcomes in KTRs. Several promising candidates have emerged, including neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and β2-microglobulin [9]. These biomarkers have shown potential in detecting acute kidney injury and predicting graft function, but their utility in CRAI remains under investigation [10].
Based on previous studies showing the association between serum ApoA-IV levels and renal function decline in various kidney diseases, we hypothesized that urinary ApoA-IV levels could serve as a potential biomarker for renal allograft function and predictor of rapid renal function decline in KTRs. We aimed to evaluate the associations between urinary ApoA-IV levels and both current renal function and future renal function decline in KTRs.
Materials and Methods
Ethics statement
The Institutional Review Board of Kangdong Sacred Heart Hospital approved this study (IRB no. 2015-07-006-001), which was conducted in accordance with the Declaration of Helsinki principles and the Istanbul Declaration. All participants provided written informed consent prior to the start of this study.
Selection of study subjects
A total of fifty KTRs who underwent follow-up at the Kangdong Sacred Heart Hospital transplant clinic between 30 November 2015 and 7 January 2016 were included in this study. The inclusion criteria were adult patients over 18 years of age who had undergone kidney transplantation for more than one year and who provided informed consent for this study. This study excluded patients on dialysis due to graft failure, acute rejection within 3 months, and active infections, including urinary tract infections. Patients on dialysis due to graft failure were excluded because their urinary protein profiles would be significantly altered and not representative of functioning kidney transplants. Patients with acute rejection within 3 months or active infections were excluded to avoid confounding effects on urinary protein levels that might not reflect CRAI. Patients were divided into two groups: a control group (n = 20) with normal renal function and a CRAI group (n = 30) with an estimated GFR of less than 60 mL/min/1.73 m2. Rapid renal function decline was defined as a decrease in the estimated GFR of more than 3 mL/min/1.73 m2 per year or initiation of dialysis. The definition of rapid renal function decline was referred to previous studies [11–13].
Sample preparation and proteomic analysis
Urine samples were collected from the enrolled KTRs and stored in a refrigerator at −70°C until proteomic analysis. Prior to proteomic analysis, samples were centrifuged at 3,300 × g for two minutes. The modified trypsin (Promega, USA) was used to digest the protein from the urine samples at 37°C overnight, and the peptides were recovered using a C18 cartridge (Sep-Pak, Waters, USA) and completely dried in a speed-vacuum. Proteomic analysis via liquid chromatography‒mass spectrometry and tandem mass spectrometry (LC‒MS/MS) was carried out via Triple-TOF-TM 5600+ (AB Sciex, Canada). The peptide separation was achieved using an Eksigent NanoLC-2D+ with Nanoflex cHiPLC system. Peptide samples were loaded at 1 μL/min onto a trap column (0.5 mm × 200 μm) and separated on an analytical column (15 cm × 75 μm) using a 2% to 35% gradient of acetonitrile with 0.1% formic acid over 30 minutes at a flow rate of 400 nL/min, followed by column regeneration by washing with 60% solvent for 50 min and equilibrating with 2% solvent for 10 min. For data-dependent acquisition, Triple-TOF-TM 5600 + mass spectrometer performed a 50-ms survey scan and a 50-ms automated MS/MS scan on the 15 most intense ions, with a precursor intensity of 150 counts, a charge state above 1, and a dynamic exclusion time option of 6 seconds to avoid repeated scans of the same ion. Ions were isolated at 0.7 Da resolution and fragmented with collision energy ramped from 15 to 45 eV over 50 ms for optimal fragmentation. The SWATH (sequential window acquisition of all theoretical mass spectra) mass spectrometry was used to ensure a comprehensive analysis, operating in looped product ion mode across 20 Da windows with a 1 Da overlap within the 400–1000 Da range. Each SWATH window received a customized collision energy for optimal fragmentation of doubly charged ions, and an accumulation time of 80 ms per fragment ion scan enabled high sensitivity across the entire duty cycle. This setup is designed to maximize peptide identification while also being sensitive to detect low-abundance proteins, providing a robust platform for deep proteome profiling. The SWATH approach is straightforward, reliable, and highly reproducible for proteomic analysis; thus, it could be applied to characterize proteomic analyses in large numbers of samples [14–16].
Verification by ELISA and data analysis
Through the SWATH method, the top 96 most highly expressed proteins were determined, along with quantification information for each protein after normalized to total protein abundances. These data were then compared between the CRAI group and the control group. The levels of several urinary proteins, including ApoA-IV, RBP4, SERPINF1, CA1, and B2M, were significantly greater in the CRAI group than in the control group. The list of 96 highly expressed proteins and the results of principal component analysis and volcano plot are shown in S1 Table, S1 and S2 Figures in S1 File. To verify the significant protein levels, including those of ApoA-IV, we conducted an enzyme-linked immunosorbent assay (ELISA) using the same urine samples. ELISA for ApoA-IV was performed using a commercial kit (Human ApoA4 ELISA Kit, Elabscience) according to the manufacturer’s instructions. A standard curve was generated for each assay using known concentrations of recombinant human ApoA-IV. The estimated GFR was calculated via the modification of diet in the renal disease study equation [17].
Statistical analysis
Continuous values are expressed as means ± standard deviations or median [interquartile range] as per Shapiro-Wilk normality test. Categorical values are expressed as frequencies (percentages) in the descriptive analysis. As appropriate, the chi-square test or the Fisher’s exact test was used to compare categorical data between groups, and Student’s t test was used to compare continuous variables. Urinary ApoA-IV levels and renal function were analyzed via Pearson correlation, and factors associated with rapid renal function decline in KTRs were analyzed via univariate and multiple logistic regression. The covariates selected for the multiple logistic regression model were chosen based on their established clinical relevance to renal allograft function and outcomes. Urinary ApoA-IV levels were also analyzed via a receiver operating characteristic (ROC) curve to determine its correlation with rapid decline in renal function. The optimal cut-off value in ROC curve analysis was determined by Youden’s J Statistic [18]. For the statistical analyses, SPSS version 18.0.0 (SPSS Inc., Chicago, IL) and R version 4.0.1 (www.r-project.org; The R Foundation for Statistical Computing, Vienna, Austria) software packages were utilized. A P value of less than 0.05 was considered significant.
Results
Demographic and clinical characteristics of the study subjects
In this study, 62.0% of the recipients were male, and the mean age at transplantation was 39.5 ± 10.3 years. The recipients’ mean estimated GFR and serum creatinine levels were 62.9 ± 25.8 mL/min/1.73 m2 and 1.3 ± 0.6 mg/dL, respectively, at the time of sampling. At transplantation, the mean age of the donors was 35.8 ± 15.7 years, with 70.0% being male. Table 1 shows a summary of the demographic and clinical characteristics of the subjects.
Table 1. Demographic and clinical characteristics of the study subjects.
| Parameters | Chronic renal allograft injury group (n = 30) |
Normal renal function group (n = 20) |
Total | P value a |
|---|---|---|---|---|
| Age at transplantation (year) | 37.3 ± 10.1 | 42.8 ± 9.9 | 39.5 ± 10.3 | 0.065 |
| Sex (%, male) | 20 (66.7%) | 11 (55.0%) | 31 (62.0%) | 0.592 |
| Diabetes (%) b | 9 (30.0%) | 5 (25.0%) | 14 (28.0%) | 0.949 |
| Pretransplant dialysis (%) | 0.342 | |||
| - Hemodialysis | 20 (66.7%) | 12 (60.0%) | 32 (64.0%) | |
| - Peritoneal dialysis | 7 (23.3%) | 7 (35.0%) | 14 (28.0%) | |
| - Preemptive | 3 (10.0%) | 1 (5.0%) | 4 (8.0%) | |
| Duration of dialysis (month) | 48.0 [22.5–84.0] | 32.0 [12.0–64.5] | 39.0 [13.0–72.0] | 0.315 |
| Cause of ESRD (%) | 0.244 | |||
| - Diabetes | 3 (10.0%) | 2 (10.0%) | 5 (10.0%) | |
| - Glomerulonephritis | 10 (33.3%) | 2 (10.0%) | 12 (24.0%) | |
| - Hypertension | 3 (10.0%) | 2 (10.0%) | 5 (10.0%) | |
| - Others | 1 (3.3%) | 4 (20.0%) | 5 (10.0%) | |
| - Unknown | 13 (43.3%) | 10 (50.0%) | 24 (46.0%) | |
| Months after transplantation at sampling (month) | 164.8 [102.1-229.2] | 147.9 [64.7-208.2] | 164.8 [81.0-222.4] | 0.151 |
| Serum creatinine at sampling (mg/dL) | 1.6 [1.4-1.7] | 0.8 [0.7-1.0] | 1.3 [0.9-1.7] | <0.001 |
| Estimated GFR at sampling (mL/min/1.73m2) | 44.0 ± 10.5 | 91.3 ± 11.7 | 62.9 ± 25.8 | <0.001 |
| Calcineurin inhibitor (%) | 0.434 | |||
| - Tacrolimus | 9 (30.0%) | 9 (45.0%) | 18 (36.0%) | |
| - Cyclosporine | 21 (70.0%) | 11 (55.0%) | 32 (64.0%) | |
| HLA mismatch (number) | 3.3 ± 1.3 | 3.1 ± 1.4 | 3.2 ± 1.3 | 0.636 |
| Donor type (%) | 0.513 | |||
| - Living donor | 13 (43.3%) | 6 (30.0%) | 19 (38.0%) | |
| - Deceased donor | 17 (56.7%) | 14 (70.0%) | 31 (62.0%) | |
| Acute rejection episode (%) | 11 (36.7%) | 0 (0.0%) | 11 (22.0%) | 0.002 |
| Donor age at transplantation (year) | 36.5 ± 16.1 | 34.8 ± 15.3 | 35.8 ± 15.7 | 0.716 |
| Donor sex (%, male) | 19 (63.3%) | 16 (80.0%) | 35 (70.0%) | 0.345 |
Continuous values are expressed as means ± standard deviations or median [interquartile range] as per Shapiro-Wilk normality test. Categorical values are expressed as frequencies (percentages). a Continuous variables were compared via t tests, and categorical variables were compared via the chi-square test or the Fisher’s exact test, as appropriate.
bDiabetes includes new-onset diabetes after transplantation
Conversion factors for units: serum creatinine in mg/dL to μmol/L, × 88.4.
ESRD, end-stage renal disease; GFR, glomerular filtration rate; HLA, human leukocyte antigen.
Association between urinary ApoA-IV and renal function in kidney transplant patients
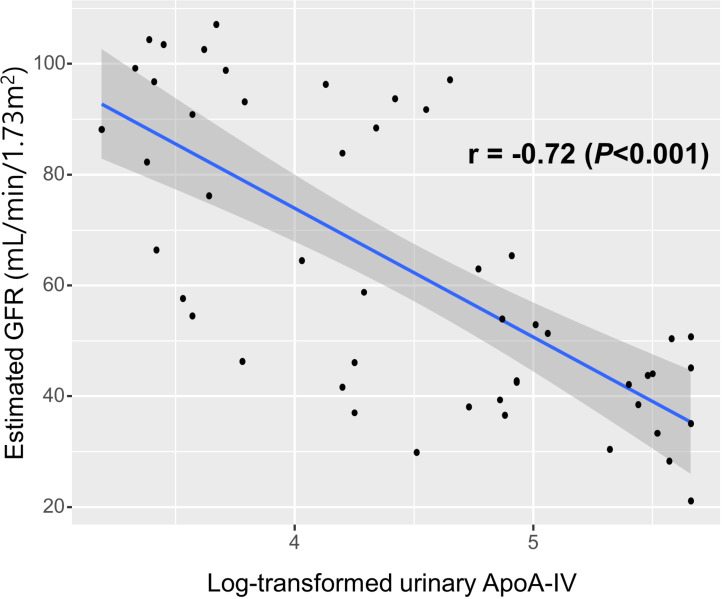
The levels of urinary ApoA-IV were inversely correlated with renal function in KTRs. The log-transformed urinary ApoA-IV levels measured by ELISA had a significantly inverse correlation with the estimated GFR at the time of initial sampling in KTRs (r = -0.72, P < 0.001) (Fig 1). In addition, among the 50 subjects, urinary ApoA-IV levels were significantly higher in the CRAI group than in the control group (170.9 ± 166.3 μg/mL vs. 14.2 ± 20.0 μg/mL, P < 0.001).
Fig 1. The correlation between urinary apolipoprotein A4 (ApoA-IV) levels and renal allograft function according to estimated glomerular filtration rate in kidney transplant recipients.
The log-transformed urinary ApoA-IV levels measured by ELISA had significantly inverse correlation with the estimated glomerular filtration rate in kidney transplant recipients.
Association between urinary ApoA-IV and rapid renal function decline in kidney transplant patients
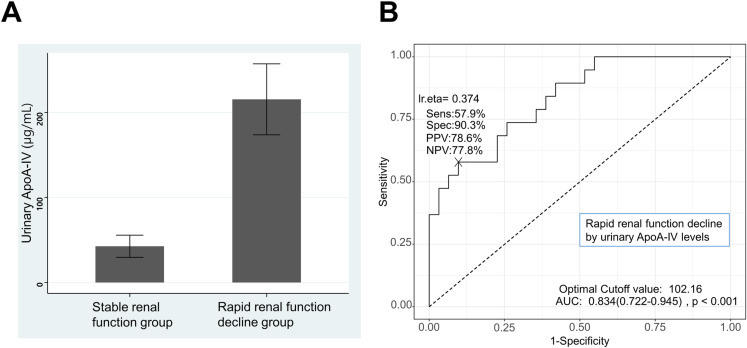
Among the 50 KTRs, 19 (38%) met the criteria for rapid renal function decline. Urinary ApoA-IV levels measured by ELISA were greater in patients with rapid renal function decline than in those with stable renal function (215.4 ± 181.8 μg/mL vs. 42.5 ± 72.4 μg/mL, P = 0.001) (Fig 2A). Univariate logistic regression analysis revealed that log-transformed urinary ApoA-IV levels were also significantly associated with rapid renal function decline (odds ratio [OR] 6.70, 95% confidence interval [CI] 2.56–22.83; P < 0.001). In the multiple logistic regression adjusted for recipient age, recipient sex, donor age, number of HLA mismatches, acute rejection episodes, and CRAI, urinary ApoA-IV levels remained a significant risk factor for rapid renal function decline (OR 4.10, 95% CI 1.10–19.55; P = 0.047) (Table 2). These results align with our initial hypothesis that urinary ApoA-IV levels would be associated with rapid renal function decline in KTRs. The significant odds ratio in the multiple logistic regression analysis supports the potential of urinary ApoA-IV as an independent predictor of renal function decline. We also performed simple and multiple linear regression analysis for factors related to annual declines of estimated GFR (mL/min/1.73m2 per year), and urinary ApoA-IV levels were significantly related to annual declines of estimated GFR (S2 Table in S1 File). In addition, the ROC curve analysis revealed that the area under the curve (AUC) of urinary ApoA-IV levels for rapid renal function decline was 0.834 (95% CI 0.722–0.945, P < 0.001) (Fig 2B). The optimal cut-off value of 102.16 μg/mL for urinary ApoA-IV was determined using Youden’s J Statistic, which maximizes the sum of sensitivity and specificity. This method provides an objective criterion for selecting the most appropriate threshold in ROC curve analysis [18]. With the 102.16 μg/mL cut-off value, the positive and negative predictive values were 78.6% and 77.8% for predicting rapid renal function decline, respectively. When the patients were stratified into two groups by the cut-off value (102.16 μg/mL) of urinary ApoA-IV levels, the upper group also had more rapid renal function decline (76.9% vs. 24.3%) and graft failure (23.1% vs. 5.4%) than did the lower group.
Fig 2. Association between urinary apolipoprotein A4 (ApoA-IV) levels and rapid renal function decline in kidney transplant recipients.
(A) Comparison of urinary ApoA-IV levels between the rapid renal function decline group and the stable renal function group in kidney transplant recipients. Boxplot showing significantly higher urinary ApoA-IV levels in patients with rapid renal function decline. (B) ROC curve of ApoA-IV for rapid renal function decline in kidney transplant recipients. ROC curve demonstrating the predictive value of urinary ApoA-IV for rapid renal function decline (AUC = 0.834). Urinary ApoA-IV levels above 102.16 μg/mL were associated with increased risk of rapid renal function decline.
Table 2. Logistic regression analysis of factors associated with rapid renal function decline in kidney transplant recipients.
| Variables | Univariate logistic regression | Multiple logistic regression a | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Recipient male sex (vs. female) | 2.31 | 0.69-8.59 | 0.187 | 2.93 | 0.49-22.14 | 0.255 |
| Recipient age | 0.96 | 0.90-1.02 | 0.169 | 1.01 | 0.92-1.13 | 0.767 |
| Donor age | 0.99 | 0.95-1.02 | 0.463 | 0.97 | 0.90-1.03 | 0.295 |
| Number of HLA mismatches | 1.19 | 0.76-1.92 | 0.461 | 1.22 | 0.67-2.43 | 0.529 |
| Acute rejection episodes | 2.40 | 0.61-9.81 | 0.207 | 1.17 | 0.17-9.47 | 0.878 |
| Chronic renal allograft injury b | 28.50 | 4.90-546.9 | 0.002 | 10.26 | 0.99-246.99 | 0.073 |
| Urinary ApoA-IV (log-transformed) | 6.70 | 2.56-22.83 | <0.001 | 4.10 | 1.10-19.55 | 0.047 |
a Adjusted for recipient age, recipient sex, donor age, number of HLA mismatches, acute rejection episodes, and chronic renal allograft injury.
b Chronic renal allograft injury was defined as an estimated GFR of less than 60 mL/min/1.73 m2.
OR, odds ratio; CI, confidence interval; HLA, human leukocyte antigen; ApoA-IV, apolipoprotein A4.
Discussion
In our study, the levels of urinary ApoA-IV were inversely correlated with renal allograft function in KTRs. Urinary ApoA-IV levels were significantly higher in the CRAI group than in the control group. Moreover, urinary ApoA-IV levels were also higher in patients with rapid renal function decline than in those with stable renal function and remained a significant factor for rapid renal function decline according to logistic regression analysis. The ROC curve analysis showed an AUC of 0.834, indicating good discriminatory ability. At the cut-off value of 102.16 μg/mL, the positive and negative predictive values were 78.6% and 77.8%, respectively. It suggests the clinical utility of ApoA-IV as a predictive biomarker for the risk of rapid renal function decline in KTRs.
ApoA-IV, a 46 kDa glycoprotein, is an apolipoprotein that is synthesized mainly in small intestinal enterocytes and is released as one of the structural proteins of chylomicrons, high-density lipoproteins, and very low-density lipoproteins, or it can be detected in its free form in plasma [19–21]. In vitro studies have indicated that ApoA-IV plays a role in reverse cholesterol transport by activating lipoprotein lipase, lecithin-cholesterol acyltransferase, and cholesteryl ester transfer protein, resulting in anti-atherogenic properties [22–25]. Studies in mice overexpressing human or mouse ApoA-IV revealed fewer atherosclerotic aortic lesions than in control mice [26,27]. Additionally, in human studies, lower levels of plasma ApoA-IV were found in patients with coronary heart disease than in control subjects [28,29].
In addition, plasma ApoA-IV levels are also known to be elevated in patients with chronic kidney disease or renal failure. High levels of plasma ApoA-IV are strongly associated with a low estimated GFR and are suggested as early markers of impaired kidney function [5,30,31]. Moreover, Boes E et al. [6] reported that ApoA-IV predicts the progression of chronic kidney disease in patients with nondiabetic primary kidney disease, and Cheng CW et al. [7] and Peters KE et al. [8] presented the role of serum ApoA-IV in predicting a rapid decline in renal function in patients with type 2 DM. The results of those studies are consistent with our findings that urinary ApoA-IV levels are associated with decreased renal function and could be a potential biomarker for rapid decline in renal allograft function in KTRs. The urinary ApoA-IV concentration remained a significant factor for rapid renal function decline in the multiple logistic regression analysis adjusted for multiple covariates, including the CRAI. ApoA-IV is filtered by the glomeruli and reabsorbed by proximal tubular cells, as shown by an immunohistochemical analysis of healthy human kidney tissue samples and studies of urine ApoA-IV in patients with proteinuria or Dent’s disease [32,33]. However, it is still unclear whether the increased levels of ApoA-IV in renal disease patients are only due to their impaired ability to filter or whether ApoA-IV is also involved in a defense mechanism against the disease [30].
While our study demonstrates a correlation between urinary ApoA-IV levels and renal allograft function, the specific mechanisms linking ApoA-IV to CRAI and long-term transplant outcomes remain to be fully elucidated. However, the elevated levels we observed in patients with declining renal function suggest that urinary ApoA-IV may also be a biomarker of ongoing allograft injury. It could allow for earlier identification of patients at risk of allograft dysfunction, enabling more timely interventions. Moreover, as a non-invasive test, it could reduce the need for frequent biopsies, thereby decreasing patient discomfort and potential complications. Our findings align with recent research on novel biomarkers for renal function decline. Peters et al. [8] identified several circulating biomarkers, including ApoA-IV, which predicted rapid decline in renal function in patients with type 2 diabetes. Their study, like ours, highlights the potential of ApoA-IV as a predictive biomarker for renal outcomes, suggesting its relevance across different patient populations with kidney disease. While our findings suggest that urinary ApoA-IV is a promising biomarker for CRAI, the pathophysiological mechanisms by which ApoA-IV influences renal function remain unclear. It is essential to determine whether ApoA-IV plays a causal role in renal dysfunction or merely serves as a marker of disease progression in future mechanistic studies.
While our study demonstrates the potential of urinary ApoA-IV as a biomarker for renal allograft function and predictor of rapid renal function decline, it is important to contextualize these findings with other established urinary biomarkers such as NGAL and KIM-1. Previous studies have shown that urinary NGAL and KIM-1 are effective in detecting acute kidney injury and predicting short-term graft function in KTRs [34–39]. Kielar et al. [35] reported that urinary NGAL measured after 1 year post-transplant predicts the relative and absolute changes in estimated GFR during the follow-up in 109 KTRs. The AUC of urinary NGAL in the prediction of >10% decrease in estimated GFR was 0.645 (95% CI 0.529–0.760) in ROC curve analysis [35]. Zhu et al. [39] assessed the predictive utility of urinary KIM-1 levels in 160 KTRs and found that elevated urinary KIM-1 on the first day after transplantation was associated with a 23.5% higher risk of delayed graft function and a 27.3% greater risk of prolonged renal allograft dysfunction. However, their utility in predicting long-term outcomes and chronic allograft nephropathy remains under investigation. While ApoA-IV is primarily synthesized in the intestine and filtered by the glomeruli, with altered levels potentially reflecting both systemic metabolic changes and kidney function [40], NGAL and KIM-1 reflect renal tubular cell injury so that they rise rapidly following acute kidney injury and remain elevated in chronic kidney disease [41,42]. Our findings suggest that urinary ApoA-IV may complement existing biomarkers by providing additional information on metabolic and inflammatory processes affecting the allograft. Combining ApoA-IV with NGAL and KIM-1 could offer a more comprehensive assessment of allograft health, particularly in the context of CRAI and long-term renal function decline. Further studies directly comparing the performance of ApoA-IV with NGAL and KIM-1 in the same cohort of KTRs are needed to fully elucidate their relative clinical utility. Such comparisons should assess not only their individual predictive values but also whether a panel combining these biomarkers could provide more accurate and clinically useful information.
This study has several limitations. First, the single-center design and a limited sample size may restrict the generalizability of our findings, despite the cohort size being comparable to those in other exploratory biomarker studies in kidney transplant populations [35,39]. Future multicenter studies with larger cohorts are needed to validate the predictive utility of urinary ApoA-IV across diverse demographic and clinical settings. In addition, the patient selection process may have introduced bias, as we excluded patients with graft failure, recent acute rejection, and active infections. This exclusion may have resulted in the selection of a healthier subset of KTRs, potentially underestimating the true association between ApoA-IV levels and renal function decline. Patients with severe complications, who were excluded from the study, might have exhibited higher ApoA-IV levels due to their more compromised graft status. To address this limitation, future studies should include a broader range of patients in multicenter cohorts to provide a more comprehensive understanding of the relationship between ApoA-IV levels and renal function across diverse clinical scenarios. Second, not all KTRs in our analysis had kidney biopsies used to make a histologic diagnosis of the CRAI. Third, the lack of serum ApoA-IV measurements is a significant limitation. Without serum levels, we cannot determine whether elevated urinary ApoA-IV reflects increased production, decreased renal clearance, or both. Future studies should include both serum and urinary ApoA-IV measurements to better understand the mechanisms underlying our observations and to potentially improve the predictive value of ApoA-IV as a biomarker. Nevertheless, in addition to examining the cross-sectional relationship between urinary ApoA-IV and renal allograft function, our study also prospectively assessed the significance of urinary ApoA-IV for the progression of renal function deterioration in KTRs. Fourth, another limitation of this study is the focus solely on GFR decline without consideration of proteinuria. Renal function physiologically includes both glomerular filtration and protein excretion. Our study did not assess proteinuria, which is an important marker of kidney damage and can provide additional information about allograft health. Future studies should incorporate both GFR and proteinuria measurements to provide a more comprehensive assessment of renal allograft function in KTRs. Lastly, several factors could potentially confound the relationship between urinary ApoA-IV levels and renal function decline. Immunosuppressive therapy, which all KTRs receive, may affect ApoA-IV metabolism or excretion. However, our study did not assess the impact of different immunosuppressive regimens on ApoA-IV levels. Additionally, factors such as proteinuria, lipid levels, and cardiovascular comorbidities could influence ApoA-IV levels and renal outcomes. Future studies should aim to control for these potential confounders to better elucidate the independent predictive value of urinary ApoA-IV.
In conclusion, our results suggest that urinary ApoA-IV levels might be a potential biomarker for renal allograft function and could be used as a predictor for rapid renal function decline in KTRs. These findings not only highlight the potential utility of ApoA-IV as a non-invasive biomarker but also provide new insights into the molecular mechanisms underlying CRAI. Further studies are warranted to elucidate the specific role of ApoA-IV in transplant outcomes and to explore its potential as a therapeutic target in managing CRAI. In addition, future research should focus on direct comparisons between urinary ApoA-IV and other established biomarkers such as NGAL and KIM-1 to better define its specific role in the clinical management of KTRs.
Supporting information
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was sponsored by Astellas Pharma, Inc. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Riella LV, Djamali A, Pascual J. Chronic allograft injury: Mechanisms and potential treatment targets. Transplant Rev (Orlando). 2017;31(1):1–9. doi: 10.1016/j.trre.2016.10.005 . [DOI] [PubMed] [Google Scholar]
- 2.Pascual J, Pérez-Sáez MJ, Mir M, Crespo M. Chronic renal allograft injury: early detection, accurate diagnosis and management. Transplant Rev (Orlando). 2012;26(4):280–90. doi: 10.1016/j.trre.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Ganji MR, Harririan A. Chronic allograft dysfunction: major contributing factors. Iran J Kidney Dis. 2012;6(2):88–93. [PubMed] [Google Scholar]
- 4.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, et al. Banff ’05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN’). Am J Transplant. 2007;7(3):518–26. doi: 10.1111/j.1600-6143.2006.01688.x [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg F, Kuen E, Ritz E, König P, Kraatz G, Lhotta K, et al. Apolipoprotein A-IV serum concentrations are elevated in patients with mild and moderate renal failure. J Am Soc Nephrol. 2002;13(2):461–9. doi: 10.1681/ASN.V132461 [DOI] [PubMed] [Google Scholar]
- 6.Boes E, Fliser D, Ritz E, König P, Lhotta K, Mann JFE, et al. Apolipoprotein A-IV predicts progression of chronic kidney disease: the mild to moderate kidney disease study. J Am Soc Nephrol. 2006;17(2):528–36. doi: 10.1681/ASN.2005070733 [DOI] [PubMed] [Google Scholar]
- 7.Cheng C-W, Chang C-C, Chen H-W, Lin C-Y, Chen J-S. Serum ApoA4 levels predicted the progression of renal impairment in T2DM. Eur J Clin Invest. 2018;48(6):e12937. doi: 10.1111/eci.12937 [DOI] [PubMed] [Google Scholar]
- 8.Peters KE, Davis WA, Ito J, Winfield K, Stoll T, Bringans SD, et al. Identification of Novel Circulating Biomarkers Predicting Rapid Decline in Renal Function in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. Diabetes Care. 2017;40(11):1548–55. doi: 10.2337/dc17-0911 [DOI] [PubMed] [Google Scholar]
- 9.Cassidy H, Slyne J, O’Kelly P, Traynor C, Conlon PJ, Johnston O, et al. Urinary biomarkers of chronic allograft nephropathy. Proteomics Clin Appl. 2015;9(5–6):574–85. doi: 10.1002/prca.201400200 [DOI] [PubMed] [Google Scholar]
- 10.Sigdel TK, Gao Y, He J, Wang A, Nicora CD, Fillmore TL, et al. Mining the human urine proteome for monitoring renal transplant injury. Kidney Int. 2016;89(6):1244–52. doi: 10.1016/j.kint.2015.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai C-W, Ting I-W, Yeh H-C, Kuo C-C. Longitudinal change in estimated GFR among CKD patients: A 10-year follow-up study of an integrated kidney disease care program in Taiwan. PLoS One. 2017;12(4):e0173843. doi: 10.1371/journal.pone.0173843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol. 2009;30(3):171–8. doi: 10.1159/000212381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali I, Chinnadurai R, Ibrahim ST, Kalra PA. Adverse outcomes associated with rapid linear and non-linear patterns of chronic kidney disease progression. BMC Nephrol. 2021;22(1):82. doi: 10.1186/s12882-021-02282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basak T, Bhat A, Malakar D, Pillai M, Sengupta S. In-depth comparative proteomic analysis of yeast proteome using iTRAQ and SWATH based MS. Mol Biosyst. 2015;11(8):2135–43. doi: 10.1039/c5mb00234f [DOI] [PubMed] [Google Scholar]
- 15.Gillet LC, Navarro P, Tate S, Röst H, Selevsek N, Reiter L, et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics. 2012;11(6):O111.016717. doi: 10.1074/mcp.O111.016717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee WK, Baek JH, Ryoo SW, Yu YG. SWATH-based Comparative Proteomic Analysis of the Mycobacterium bovis BCG-Korea Strain. Bulletin of the Korean Chemical Society. 2014;35(3):933–7. doi: 10.5012/BKCS.2014.35.3.933 [DOI] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004 [DOI] [PubMed] [Google Scholar]
- 18.Hassanzad M, Hajian-Tilaki K. Methods of determining optimal cut-point of diagnostic biomarkers with application of clinical data in ROC analysis: an update review. BMC Med Res Methodol. 2024;24(1):84. doi: 10.1186/s12874-024-02198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Utermann G, Beisiegel U. Apolipoprotein A-IV: a protein occurring in human mesenteric lymph chylomicrons and free in plasma. Isolation and quantification. Eur J Biochem. 1979;99(2):333–43. doi: 10.1111/j.1432-1033.1979.tb13261.x [DOI] [PubMed] [Google Scholar]
- 20.Green PH, Glickman RM, Riley JW, Quinet E. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J Clin Invest. 1980;65(4):911–9. doi: 10.1172/JCI109745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ezeh B, Haiman M, Alber HF, Kunz B, Paulweber B, Lingenhel A, et al. Plasma distribution of apoA-IV in patients with coronary artery disease and healthy controls. J Lipid Res. 2003;44(8):1523–9. doi: 10.1194/jlr.M300060-JLR200 [DOI] [PubMed] [Google Scholar]
- 22.Stein O, Stein Y, Lefevre M, Roheim PS. The role of apolipoprotein A-IV in reverse cholesterol transport studied with cultured cells and liposomes derived from an ether analog of phosphatidylcholine. Biochim Biophys Acta. 1986;878(1):7–13. doi: 10.1016/0005-2760(86)90337-1 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg IJ, Scheraldi CA, Yacoub LK, Saxena U, Bisgaier CL. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J Biol Chem. 1990;265(8):4266–72. doi: 10.1016/s0021-9258(19)39557-2 [DOI] [PubMed] [Google Scholar]
- 24.Steinmetz A, Utermann G. Activation of lecithin: cholesterol acyltransferase by human apolipoprotein A-IV. J Biol Chem. 1985;260(4):2258–64. doi: 10.1016/s0021-9258(18)89547-3 [DOI] [PubMed] [Google Scholar]
- 25.Guyard-Dangremont V, Lagrost L, Gambert P. Comparative effects of purified apolipoproteins A-I, A-II, and A-IV on cholesteryl ester transfer protein activity. J Lipid Res. 1994;35(6):982–92. doi: 10.1016/s0022-2275(20)40111-7 [DOI] [PubMed] [Google Scholar]
- 26.Duverger N, Tremp G, Caillaud JM, Emmanuel F, Castro G, Fruchart JC, et al. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 1996;273(5277):966–8. doi: 10.1126/science.273.5277.966 [DOI] [PubMed] [Google Scholar]
- 27.Cohen RD, Castellani LW, Qiao JH, Van Lenten BJ, Lusis AJ, Reue K. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J Clin Invest. 1997;99(8):1906–16. doi: 10.1172/JCI119358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronenberg F, Stühlinger M, Trenkwalder E, Geethanjali FS, Pachinger O, von Eckardstein A, et al. Low apolipoprotein A-IV plasma concentrations in men with coronary artery disease. J Am Coll Cardiol. 2000;36(3):751–7. doi: 10.1016/s0735-1097(00)00775-0 [DOI] [PubMed] [Google Scholar]
- 29.Manpuya MW, Guo J, Zhao Y. The relationship between plasma apolipoprotein A-IV levels and coronary heart disease. Chin Med J (Engl). 2001;114(3):275–9. [PubMed] [Google Scholar]
- 30.Stangl S, Kollerits B, Lamina C, Meisinger C, Huth C, Stöckl A, et al. Association between apolipoprotein A-IV concentrations and chronic kidney disease in two large population-based cohorts: results from the KORA studies. J Intern Med. 2015;278(4):410–23. doi: 10.1111/joim.12380 [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg F. Apolipoprotein L1 and apolipoprotein A-IV and their association with kidney function. Curr Opin Lipidol. 2017;28(1):39–45. doi: 10.1097/MOL.0000000000000371 [DOI] [PubMed] [Google Scholar]
- 32.Lingenhel A, Lhotta K, Neyer U, Heid IM, Rantner B, Kronenberg MF, et al. Role of the kidney in the metabolism of apolipoprotein A-IV: influence of the type of proteinuria. J Lipid Res. 2006;47(9):2071–9. doi: 10.1194/jlr.M600178-JLR200 [DOI] [PubMed] [Google Scholar]
- 33.Haiman M, Salvenmoser W, Scheiber K, Lingenhel A, Rudolph C, Schmitz G, et al. Immunohistochemical localization of apolipoprotein A-IV in human kidney tissue. Kidney Int. 2005;68(3):1130–6. doi: 10.1111/j.1523-1755.2005.00519.x [DOI] [PubMed] [Google Scholar]
- 34.Tabernero G, Pescador M, Ruiz Ferreras E, Morales AI, Prieto M. Evaluation of NAG, NGAL, and KIM-1 as Prognostic Markers of the Initial Evolution of Kidney Transplantation. Diagnostics (Basel). 2023;13(11):1843. doi: 10.3390/diagnostics13111843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kielar M, Dumnicka P, Gala-Błądzińska A, Będkowska-Prokop A, Ignacak E, Maziarz B, et al. Urinary NGAL Measured after the First Year Post Kidney Transplantation Predicts Changes in Glomerular Filtration over One-Year Follow-Up. J Clin Med. 2020;10(1):43. doi: 10.3390/jcm10010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca I, Oliveira JC, Almeida M, Cruz M, Malho A, Martins LS, et al. Neutrophil gelatinase-associated lipocalin in kidney transplantation is an early marker of graft dysfunction and is associated with one-year renal function. J Transplant. 2013;2013:650123. doi: 10.1155/2013/650123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qurashi S, Ghamdi G, Jaradat M, Tamim H, Aljumah A, Tamimi W, et al. Urinary neutrophil gelatinase-associated lipocalin and the occurrence of delayed graft function after kidney transplant. Exp Clin Transplant. 2014;12(5):396–400. doi: 10.6002/ect.2013.0300 [DOI] [PubMed] [Google Scholar]
- 38.Cappuccilli M, Capelli I, Comai G, Cianciolo G, La Manna G. Neutrophil Gelatinase-Associated Lipocalin as a Biomarker of Allograft Function After Renal Transplantation: Evaluation of the Current Status and Future Insights. Artif Organs. 2018;42(1):8–14. doi: 10.1111/aor.13039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu M, Chen Z, Wei Y, Yuan Y, Ying L, Zhou H, et al. The predictive value of urinary kidney injury molecular-1 for long-term graft function in kidney transplant patients: a prospective study. Ann Transl Med. 2021;9(3):244. doi: 10.21037/atm-20-2215a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong J, Yang H-C, Fogo AB. A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol. 2017;312(3):F375–84. doi: 10.1152/ajprenal.00266.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aldea PL, Rachisan AL, Stanciu BI, Picos A, Picos AM, Delean DI, et al. The Perspectives of Biomarkers in Predicting the Survival of the Renal Graft. Front Pediatr. 2022;10:869628. doi: 10.3389/fped.2022.869628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogulska K, Wojciechowska-Koszko I, Dołęgowska B, Kwiatkowska E, Roszkowska P, Kapczuk P, et al. The Most Promising Biomarkers of Allogeneic Kidney Transplant Rejection. J Immunol Res. 2022;2022:6572338. doi: 10.1155/2022/6572338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.