Abstract
Bats can host viruses of pandemic concern without developing disease. The mechanisms underlying their exceptional resilience to viral infections are largely unresolved, necessitating the development of physiologically relevant and genetically tractable research models. Here, we developed respiratory and intestinal organoids that recapitulated the cellular diversity of the in vivo epithelium present in Rousettus aegyptiacus, the natural reservoir for the highly pathogenic Marburg virus (MARV). In contrast to human counterparts, bat organoids and mucosal tissue exhibited elevated constitutive expression of innate immune effectors, including type I interferon-ε (IFNε) and IFN-stimulated genes (ISGs). Upon infection with diverse zoonotic viruses, including MARV, bat organoids strongly induced type I and III IFN responses, which conferred robust antiviral protection. Type III IFNλ3 additionally displayed virus-independent self-amplification, acting as an ISG to enhance antiviral immunity. Our organoid platform reveals key features of bat epithelial antiviral immunity that may inform therapeutic strategies for viral disease resilience.
Subject terms: Mucosal immunology, Viral infection
Kellner et al. develop respiratory and intestinal organoids from Rousettus aegyptiacus to show elevated basal expression of interferon-ε (IFNε) and IFN-stimulated genes, along with robust, self-amplifying type III IFN responses that drive antiviral defense against zoonotic RNA viruses.
Main
Bats possess a unique ability to host and tolerate pathogens that are highly virulent to humans and nonhuman primates1. Insights from comparative genomic studies in bats have suggested a genetic basis for their exceptional immunity, supported by positive selection or loss of genes that could enhance innate immune responses and limit overt inflammation2–5. However, functional genetic studies in bats remain a challenging task owing to their unique lifestyle, protected status and the limited molecular tools developed and optimized for these non-model organisms6. Pioneering research on bat antiviral immunity has largely focused on peripheral immune responses in infected bats or immortalized cell lines, which have provided crucial insights into their immune defense mechanisms7–9. However, mucosal surfaces, which serve as primary sites for viral entry and form the first line of antiviral defense against both local and systemic infections, have not been thoroughly studied in bats10.
In this study, we developed a sustainable organoid platform that accurately models the respiratory and small intestinal (SI) epithelia of Rousettus aegyptiacus (Egyptian fruit bat), a natural reservoir for several human pathogens, including the highly lethal Marburg virus (MARV)7,11–13. Through single-cell RNA sequencing (scRNA-seq), viral infection and genetic perturbation experiments, we uncovered a heightened constitutive expression of innate immune effector genes and enhanced IFN responses to zoonotic viruses in R. aegyptiacus epithelial organoids compared to human counterparts. We further delineated the role of type I and III IFNs in providing robust and long-lasting antiviral protection. These findings establish a valuable resource for studying antiviral immunity at bat epithelial surfaces and reveal species-specific immune adaptations that may underlie bat resilience to emerging zoonotic viruses.
Results
R. aegyptiacus airway organoids contain diverse cell types
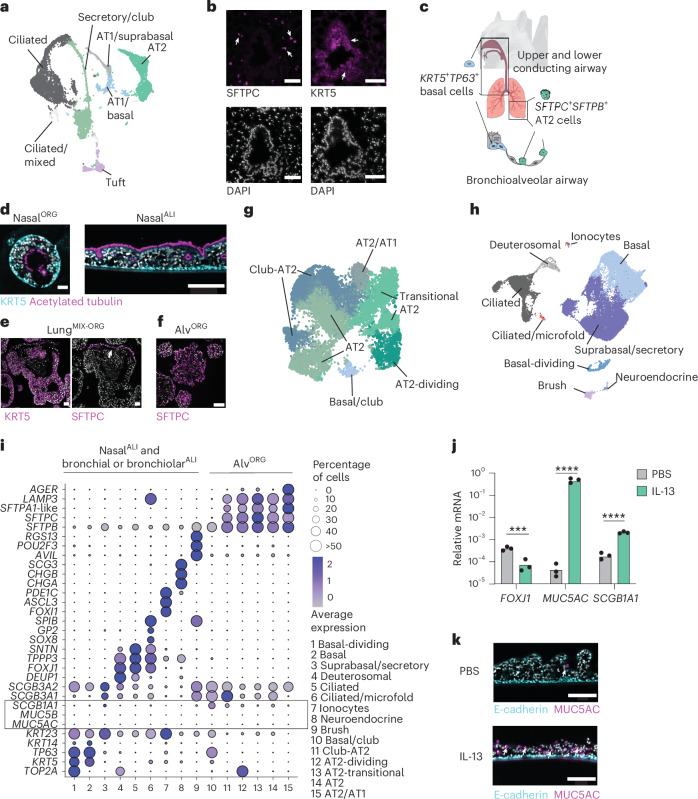
The mammalian respiratory epithelium has a central role in orchestrating immune responses to viral infections14. We aimed to generate bat adult stem cell-derived epithelial organoids from the upper and lower respiratory tract of R. aegyptiacus as the model species. To establish a tissue reference dataset, we performed integrative scRNA-seq on whole trachea and lung tissue fragments from a captive-bred R. aegyptiacus and identified distinct clusters of immune, stromal and epithelial cell (EC) lineages (Extended Data Fig. 1a,b and Supplementary Table 1). Among EC clusters, we identified two progenitor stem cell types, namely KRT5+TP63+ basal cells, predominantly found in the trachea, and SFTPC+SFTPB+ alveolar type 2 (AT2) cells, which were exclusively present in the lung (Fig. 1a and Extended Data Fig. 1c). Differentiated epithelial cell lineages included MUC5AC+MUC5B+ secretory goblet and club cells (SCGB1A1, SCGB3A1 and SCGB3A2), ciliated cells (FOXJ1, SNTN and TPPP3), brush cells (POU2F3, AVIL and RGS13) and alveolar type I (AT1) cells (AGER, HOPX and CAV1) (Fig. 1a and Extended Data Fig. 1c). Immunofluorescence staining of bat lung showed that KRT5+ basal cells localized to conducting bronchial and bronchiolar airway structures, while SFTPC⁺ AT2 cells were distributed throughout the lung parenchyma (Fig. 1b). The Egyptian fruit bat respiratory airway epithelium thus contains at least two different progenitor cell types, KRT5+TP63+ basal cells of the upper and lower conducting airway and SFTPC+SFTPB+ AT2 cells of the lung (Fig. 1c).
Extended Data Fig. 1. Establishment and characterization of R. aegyptiacus airway organoids.
a, UMAP of R. aegyptiacus airway cell types resolved by scRNA-seq of lung and trachea tissue from a captive-bred bat. Immune, stromal, and epithelial cell lineages are labeled and color-coded. EC, Endothelial cells, LC, Lymphatic cells, DC, Dendritic cells. b, Seurat DotPlot showing scaled average expression of marker genes for cell clusters in (a), grouped by major tissue-resident lineages. Dot sizes were set to a maximum percentage of 50% (genes expressed by more than 50% of cells have the same dot size). Color intensity represents expression level. c, Seurat DotPlot showing marker expression across epithelial cell types. Dot sizes were set to a maximum percentage of 50%. d, Heatmap showing RT-qPCR analysis of KRT5, SCGB1A1, SFTPC, or SFTPB in mixed lung progenitor organoids (LungMIX-ORG) cultured with indicated factors. Expression normalized to EEF1A1 and shown as % of maximum. Each value represents the average relative expression (n = 3). e, Representative immunofluorescence of KRT5 in bat nasalORG grown in basal cell organoid medium (EGF, FGF10, Rspondin1, Noggin) (n = 3). Composite image with DAPI nuclei counter stain. Scale, 50 µm. f, Representative brightfield images of nasalORG, tracheaORG, or bronchialORG at day four following single-cell passage (n = 3). Scale, 100 µm. g, Representative brightfield (left) and fluorescence image (right) of bat lungMIX-ORG grown in complete alveolar medium (-Noggin) stained with LysoTracker-Red for 2 hours (n = 3 each). Scale, 100 µm. h, Mean Fluorescence Intensity (MFI) of LysoTracker Red staining in mixed lungMIX-ORG (n = 3) cultured with indicated factors. Dots, image intensity per single organoid analyzed in Fiji. Two-sided unpaired Mann-Whitney tests were performed to compare MFI distribution of lungMIX-ORG cultured in different media (**** P < 0.0001). i, Schematic showing alvORG derivation from LysoTracker Red+ AT2 FACS-sorted cells from lungMIX-ORG. j, FACS plots showing LysoTracker Red intensity in single gated cells from nasalORG or lungMIX-ORG cultured in complete alveolar medium. Percentages of LysoTracker+ cells are shown. k, Representative brightfield image of bat alvORG established from FACS-sorted AT2 cells. Scale, 100 µm.
Fig. 1. R. aegyptiacus nasal, bronchial and alveolar airway organoids contain diverse cell types.
a, Uniform manifold approximation and projection (UMAP) of EC types of lung and tracheal tissue, identified using scRNA-seq from a captive-bred R. aegyptiacus. Clusters labeled with ‘/’ indicate mixed identities: AT1/basal (AT1 lung or basal cells from the lung or trachea); AT1/suprabasal (AT1 lung or suprabasal trachea); secretory/club (goblet or club cells); ciliated/mixed (cells with ambiguous identity expressing a ciliated marker). b, Representative KRT5 or SFTPC immunofluorescence staining (top) and 4′,6-diamidino-2-phenylindole (DAPI) nucleus counterstaining (bottom) in R. aegyptiacus lung sections (n = 3). Arrowheads indicate KRT5+ basal or SFTPC+ AT2 cells. Scale bars, 50 µm. c, Schematic of the main adult respiratory epithelial stem cell types: KRT5+TP63+ basal cells in the upper and lower conducting airways and SFTPC+SFTPB+ AT2 cells in the alveolar epithelium. d, Representative KRT5 and acetylated tubulin immunofluorescence staining in differentiated nasalORG (left) and nasalALI (right) cultures derived from R. aegyptiacus. Scale bars, 50 µm (left) or 150 µm (right). e, Representative KRT5 (left) or SFTPC (right) immunofluorescence staining in lungMIX-ORG derived from R. aegyptiacus and grown in complete alveolar medium (Methods). The arrowhead highlights SFTPC+ cells. Scale bars, 50 µm. f, Representative SFTPC immunofluorescence staining in alvORG derived from R. aegyptiacus. Scale bar, 100 µm. g, UMAP of the R. aegyptiacus-derived alvORG scRNA-seq dataset showing the cell-type clusters. h, UMAP of the integrated R. aegyptiacus-derived nasalALI and bronchialALI scRNA-seq dataset showing the cell-type clusters. i, Seurat DotPlot showing the average expression of the markers for each cell cluster in a merged dataset of R. aegyptiacus-derived nasalALI + bronchialALI and alvORG. The dot size represents the percentage of a cell type expressing a given marker. Color intensity represents the average expression value. Dot size was set to a maximum percentage of 50%. (Genes expressed by more than 50% of cells have the same dot size.) The box highlights the low-to-absent expression of the club and goblet cell markers MUC5AC and MUC5B. j, RT–qPCR analysis of FOXJ1, MUC5AC or SCGB1A1 expression (n = 3 each; normalized to EEF1A1, 2−ΔCt) in R. aegyptiacus-derived nasalALI treated with 10 ng ml−1 recombinant human IL-13 or PBS from days 10 to 25 of the ALI culture. k, Representative immunofluorescence staining of MUC5AC and E-cadherin in sections from R. aegyptiacus-derived nasalALI treated with IL-13 or PBS as in i. Scale bars, 150 µm. Representative images in d–f,k were derived from n = 3. DAPI was used as a nuclear counterstain in immunofluorescence imaging.
Because a major bottleneck was access to fresh bat tissue, we established a protocol for effective cryopreservation of primary bat tissue, enabling shipping and subsequent use as the starting material for organoid derivation (Methods). Through empirical testing of growth factors known to support the proliferation of adult airway stem cells in vitro15,16, we identified serum-free medium compositions that promoted long-term expansion of basal cell-derived and alveolar cell-derived organoids for at least 6 months (Extended Data Fig. 1d). KRT5+ basal cell organoids derived from nasal, tracheal or bronchial and bronchiolar lung tissue of R. aegyptiacus (designated nasalORG, trachealORG and bronchialORG) exhibited compact morphology when grown in expansion medium (Extended Data Fig. 1e) and formed a lumen with inward-facing, beating cilia on switching to differentiation medium (Fig. 1d). To obtain well-differentiated organotypic cultures, we also differentiated nasalORG-derived or bronchialORG-derived cells at the air–liquid interface (ALI) (nasalALI or bronchialALI) (Fig. 1d). Bat lung alveolar organoids (alvORG) were established from sorted alveolar AT2 cells of early-passage mixed progenitor lung organoids (lungMIX-ORG), containing bronchial and bronchiolar basal and AT2 cells; Fig. 1e) using LysoTracker Red, a cell-permeable dye that labels lamellar bodies in AT2 cells17 (Extended Data Fig. 1g–j). Bat alvORG exhibited a saccular morphology consisting of SFTPC+ AT2 cells (Fig. 1f and Extended Data Fig. 1k) and could be serially passaged for at least 6 months. Notably, we successfully established and characterized organoids from independently frozen tissue samples from three different R. aegyptiacus bats with similar results.
To evaluate the cellular diversity of organoids, we subjected bat alvORG, or bat nasalALI and bronchialALI, to scRNA-seq. Integrative scRNA-seq analyses of bat alvORG or bat nasalALI and bronchialALI showed that cells clustered according to cell type rather than culture model or individual bat (Fig. 1g,h, Extended Data Fig. 2a,b and Supplementary Table 2). scRNA-seq further revealed that bat alvORG, nasalALI and bronchialALI retained the expression patterns of prototypical regional homeobox transcription factors, such as a gradient of increasing expression of IRX2 (ref. 18) from nasalALI to bronchialALI to alvORG, or the exclusive expression of SIX3 (ref. 19) in bat nasalALI (Extended Data Fig. 2c), highlighting the preservation of positional memory after extended in vitro culture. In alvORG, we observed an expected enrichment of AT2 cell lineages (SFTPC, SFTPB, SFTPA1-like (LOC107509426)), annotated as AT2, dividing AT2 (coexpressing TOP2A) and transitional AT2 (AGERlo, HOPXlo), over TP63+ basal cells (Fig. 1g,i). AlvORG also consisted of SFTPC+SFTPB+ AT2 cells that additionally expressed secretory or club cell genes (SCGB3A2, MUC20; Club/AT2) or markers of AT1 cells (AGERhiHOPXhi,CAV1hi; AT2/AT1) (Fig. 1g,i and Extended Data Fig. 2d). Among cell-type clusters from bat nasalALI or bronchialALI, we identified basal cells characterized by the expression of TP63 and KRT5, most of which also coexpresssed KRT14 (encoded by LOC107513879) in nasalALI or SCGB3A2 in bronchialALI (Fig. 1h,i and Extended Data Fig. 2e). Further classification enabled us to distinguish suprabasal and secretory cells in nasalALI (KRT5lo, TP63lo, KRT23, PIGR) or bronchialALI (KRT5lo, TP63lo, KRT23, SCGB3A1, SCGB3A2), deuterosomal (TOP2A, DEUP1, FOXJ1) and ciliated cells (FOXJ1, TPPP3, SNTN) (Fig. 1h,i and Extended Data Fig. 2e). We also identified rare brush and tuft cells (AVIL, RGS13, POU2F3) in bat nasalALI or bronchialALI (Fig. 1h,i and Extended Data Fig. 2e). These cells have a crucial role in innate immunity by detecting pathogens and producing cytokines such as interleukin-25, as well as lipid inflammatory mediators like cysteinyl leukotrienes20. Additionally, we uncovered cells expressing markers of ionocytes (ASLC3, FOXI1, PDE1C) and neuroendocrine cells (CHGA, CHGB, SCG3) in nasalALI (Fig. 1h,i and Extended Data Fig. 2e). KRT13+IL1A+KRT5− nasal immune-interacting floor-epithelial-like cells, which were recently described in mice21 but are absent in human nasal respiratory tissue22, were not identified in the bat nasalALI scRNA-seq dataset (Extended Data Fig. 2f).
Extended Data Fig. 2. Single-cell RNA sequencing of bat and human airway organoids.
a, UMAP of alvORG cell types resolved by scRNA-seq, colored by bat donor (n = 3). b, UMAP of bat nasalALI and bronchialALI cell types by sample group (left, nasalALI or bronchialALI) or donor (right, n = 3). c, Seurat VlnPlots showing IRX2 (top) and SIX3 (bottom) expression in bat nasalALI, bronchialALI, or alvORG. Wilcoxon Rank Sum Test used (**** P < 0.0001). d, Seurat DotPlot showing marker expression in alvORG clusters. Dot sizes were set to a maximum percentage of 50% (genes expressed by more than 50% of cells have the same dot size). Dot color intensity represents scaled expression level. e, Same as (d) for bat nasalALI and bronchialALI. f, Seurat VlnPlot showing KNIIFE-cell markers in KRT13+ vs. KRT13− cells in the NasalALI scRNA-seq dataset. g, UMAP of human nasalALI (top) or bronchialALI (bottom) airway cell types resolved by scRNA-seq. Cell types are labeled and color-coded. h, Seurat DotPlot marker gene expression human nasalALI (left) or bronchialALI (right). Dot sizes were set to a maximum percentage of 50%. Dot color intensity represents scaled expression level. i, Bar plots showing the percentages of rare cell types relative to all cells in the bat nasalALI (top) or bronchialALI (bottom) scRNA-seq dataset.
We observed little to no secretory MUC5B+MUC5AC+ goblet cells in bat nasalALI or bronchialALI (Fig. 1i and Extended Data Fig. 2e), in contrast to human nasalALI or bronchialALI, prepared in parallel using identical culture conditions (Extended Data Fig. 2g,h and Supplementary Table 3). To test whether R. aegyptiacus basal cells had the intrinsic capacity to differentiate into goblet cells in vitro, we stimulated bat nasalALI cultures with the type 2 immunity-associated cytokine interleukin-13 (IL-13), which in humans drives goblet cell metaplasia resulting in an altered ciliated:secretory cell ratio23. IL-13 treatment of bat nasalALI triggered significantly increased expression of the secretory club cell marker SCGB1A1 mRNA and a more than 1,000-fold increase in the goblet cell marker MUC5AC, whereas expression of the ciliated cell marker FOXJ1 was decreased (Fig. 1j). Immunofluorescence staining of IL-13-treated bat nasalALI indicated substantially more abundant MUC5AC+ goblet cells compared to mock-treated bat nasalALI cultures (Fig. 1k). These data are consistent with a report on tracheal organoids from Eonycteris spelaea (cave nectar bath) and suggesting that environmental factors present in vivo may control airway goblet cell formation and maintenance24.
Lastly, we identified a cell cluster expressing both ciliated cell (FOXJ1) and microfold cell markers (SPIB, CCL20 and TNFAIP2) in bat bronchialALI (Fig. 2a,b). Microfold cells are rare antigen-sampling cells found in the innate lymphoid tissue of the nasal and intestinal tract (Peyer’s patch) that require the RANKL–RANK signaling axis for expansion and differentiation25. These cells are also present at a frequency of less than 0.1% in the mouse lung26, making them the rarest epithelial lung cell type identified to date. A subset of cells in the ciliated and microfold cell cluster expressed high levels of the master regulators of microfold cell fate SOX8 and SPIB, in addition to the bacterial uptake anchor protein encoded by GP2, and RANK (also known as TNFRSF11A) (Fig. 2b). These GP2+ microfold cells lacked the expression of the ciliated cell marker FOXJ1 and comprised only 0.2% of all sequenced cells in bat bronchialALI (Extended Data Fig. 2i). Expression of RANKL (also known as TNFSF11) was largely restricted to a subset of basal cells (Fig. 2c), whereas the RANKL decoy receptor OPG (TNFRSF11B) was almost exclusively expressed in GP2+ cells (Fig. 2c), potentially limiting the continuous formation of microfold cells in bat bronchialALI. In summary, our data demonstrated that organoids derived from the frozen airway tissue of R. aegyptiacus recapitulated multiple distinct cell lineages.
Fig. 2. Microfold cells endogenously arise in bat bronchial organoid-derived ALI cultures.
a, UMAP of the integrated R. aegyptiacus-derived nasalALI and bronchialALI scRNA-seq dataset showing the ciliated, and mixed ciliated and microfold, cell clusters. b, Seurat VlnPlot analysis of the microfold cell markers SOX8, SPIB, GP2, TNFAPI2, TNFRSF11A, CCL20, TNFAPI2 and AIF1, and the ciliated cell marker FOXJ1 in ciliated cell, mixed ciliated and microfold cells, and GP2+ microfold cells of R. aegyptiacus-derived bronchialALI. The expression distribution was derived from individual cells. c, Seurat DotPlot showing the average expression of receptor–ligand pair markers for the RANK–RANKL signaling axis (TNFSF11, TNFRSF11A and TNFRSF11B) for clusters 1–10 in the R. aegyptiacus-derived bronchialALI scRNA-seq dataset. The dot size represents the percentage of an individual cell type expressing a given marker. Color intensity represents the average expression value. Dot size was set to a maximum percentage of 50%. (Genes expressed by more than 50% of cells have the same dot size.)
Bat SIORG recapitulate native SI epithelial differentiation
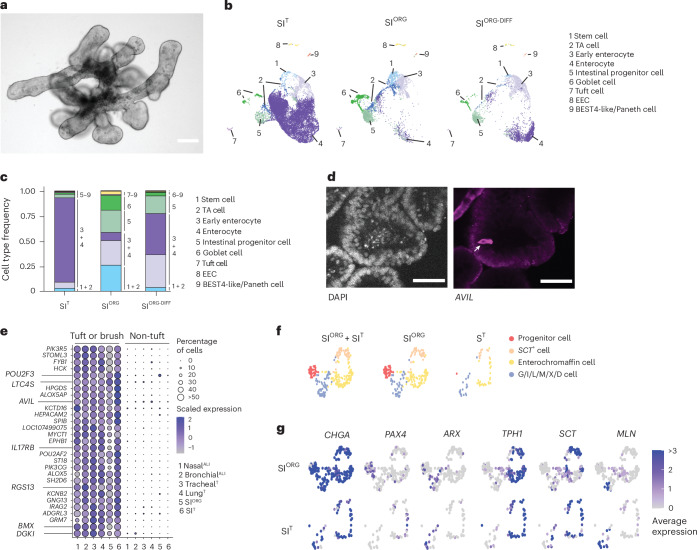
We further derived organoids from the frozen SI tissue of R. aegyptiacus, which could be serially passaged for at least 6 months in a niche-inspired human organoid medium27 (Methods). These bat SI organoids (hereafter referred to as SIORG) exhibited a budded morphology with visible interspersed granular cells (Fig. 3a). To benchmark in vitro cell-type diversity, we generated an SI reference dataset by performing scRNA-seq on whole SI fragments from a captive-bred R. aegyptiacus, identifying distinct clusters of immune, stromal and epithelial lineages (Extended Data Fig. 3a–c and Supplementary Table 4).
Fig. 3. R. aegyptiacus SI organoids recapitulate the cellular diversity of the native bat intestinal epithelium.
a, Representative bright-field image of R. aegyptiacus-derived SIORG (n = 3). b, UMAP of the integrated R. aegyptiacus-derived SIT, SIORG and differentiated SIORG-DIFF showing the individual cell types. c, Stacked bar plot showing the relative cell-type proportions of cell types in R. aegyptiacus-derived SIT, SIORG and differentiated SIORG-DIFF. d, Immunofluoresence of DAPI nucleus counterstaining (left) and AVIL antibody staining (right) in SIORG from R. aegyptiacus. The arrow points to an individual AVIL+ tuft cell. e, Seurat DotPlot showing the scaled average expression of markers enriched in tuft and brush cells for tuft/brush, and non-tuft/brush, ECs in the R. aegyptiacus-derived nasalALI, bronchialALI, trachealT, lungT, SIORG and SIT scRNA-seq dataset. The dot size represents the percentage of an individual cell type expressing a given marker. Color intensity represents the average expression value. Dot size was set to a maximum percentage of 50%. (Genes expressed by more than 50% of cells have the same dot size.) f, UMAP of EEC subtypes from the integrated R. aegyptiacus SIT + SIORG (left), SIORG (middle) or SIT (right). Cells with an EEC sublineage are color-coded. g, Seurat FeaturePlot showing the average expression of EEC (CHGA) and EEC sublineage marker genes in individual cells of SIORG (top) or SIT (bottom). ARX, differentiated G/I/L/M/X/D EECs; TPH1, enterochromaffin cells; MLN, M cells; PAX4, EEC progenitor cells; SCT, SCT+ S-like cells. The maximum color cutoff for the average expression was set to 3. Cells with an average expression of 3 or greater for a given marker have the same color. Scale bars, 50 µm.
Extended Data Fig. 3. Single-cell RNA sequencing of R. aegypticus SIT and SIORG.
a, UMAP of R. aegyptiacus single cell transcriptomes resolved by scRNA-seq of whole SI tissue from a captive-bred bat. Immune, stromal, and epithelial cell lineages are labeled and color-coded. b, UMAP of R. aegyptiacus SIT cell types in the scRNA-seq dataset. Individual cell types are color-coded and epithelial cell types (hereafter designated as SIT) are additionally highlighted by text labels. c, Seurat DotPlot showing scaled average expression of marker genes for cell clusters in R. aegyptiacus whole SI tissue scRNA-seq dataset. Dot sizes were set to a maximum percentage of 50% (genes expressed by more than 50% of cells have the same dot size). Dot color intensity represents scaled expression. d, Seurat DotPlot showing the scaled average expression of marker genes for cell clusters in the integrated scRNA-seq data of R. aegyptiacus SIT epithelial cells (left) and SIORG (middle) or differentiated SIORG (SIORG-DIFF) (right). Dot sizes were set to a maximum percentage of 50% (genes expressed by more than 50% of cells have the same dot size). Dot color intensity represents scaled expression. e, Seurat FeaturePlot showing the average expression of Enteroendocrine cells (EECs expressing CHGA) and EEC sublineage marker genes in individual EECs of SIORG (top) or SIT (bottom). PAX4, EEC progenitor; ARX, differentiated G/I/L/M/X/D EEC; TPH1, Enterochromaffin cells; SCT, SCT+, S-like cells; SST, D cells; CCK, I cells; GAST, G cells; GHRL, X cells; MLN, M cells. The maximum expression color cutoff set to 3 (cells with an average expression ≥ 3 of a given marker have the same color).
ECs from the SI tissue dataset (hereafter designated SIT) were subsequently used for integrative analysis with bat SI organoids cultured under expansion (SIORG) or differentiation (SIORG-DIFF) (SIORG culture medium without WNT3A and Noggin) conditions. This analysis revealed a high concordance of cell-type diversity between SIORG and SIT (Fig. 3b and Supplementary Table 4). Among the major cell types, we identified stem (LGR5, SMOC2 and CD44) and transit-amplifying cells (HELLS, PCNA), intestinal progenitor cells (PLK2, SOX4 and DLL4), early and mature goblet cells (ATOH1, MUC2 and SPINK4), early enterocytes (KRT19hi, FABP1hi and FABP3hi) and mature enterocytes (SLC2A2, APOA1, KRT20 and ACE2) (Fig. 3b and Extended Data Fig. 3d). While SIORG were enriched in progenitor cell types (LGR5, SMOC2, CD44, HELLS and SOX4), more than 70% of all cells in SIORG-DIFF expressed markers of enterocytes (FABP1, FABP3, SLC2A2, APOA1, KRT20 and ACE2), which were also the dominant cell type in SIT (Fig. 3c and Extended Data Fig. 3d). scRNA-seq analyses further uncovered rare EC types in SIORG or SIT, namely solitary intestinal tuft cells (POU2F3, AVIL and RGS13) (Fig. 3d and Extended Data Fig. 3d), enteroendocrine cells (EECs) (CHGA and CHGB) and cells expressing markers of recently characterized human BEST4-like28 (OTOP2 and CA7) or Paneth cells (DEFA5 (also known as LOC107504266) and SPIB) (Fig. 3b,c and Extended Data Fig. 3d). Intestinal tuft cells and the related airway brush cells of bat organoids and tissue exclusively expressed a set of core genes (AVIL, POU2F3, LT4C4S, RGS13, IRAG2, ALOX5AP, SH2D6, HCK, IL17RB, PIK3CG, BMX and DGKI), distinguishing them from other non-tuft EC types present in these samples (Fig. 3e). Detailed assessment of CHGA+CHGB+ EECs in SIORG further revealed sublineages representing early EECs (PAX4), enterochromaffin cells (TPH1), SCT+ cells and various differentiated, hormone-producing EECs29 (M cells (MLN), X cells (GHRL), G cells (GAST), N cells (NTS), D cells (SST) or I cells (CCK)), which clustered together with EECs from SIT (Fig. 3f,g and Extended Data Fig. 3e). These observations indicated that R. aegyptiacus SIORG closely replicated the EC type diversity observed in vivo.
Bat organoids constitutively express innate immune effectors
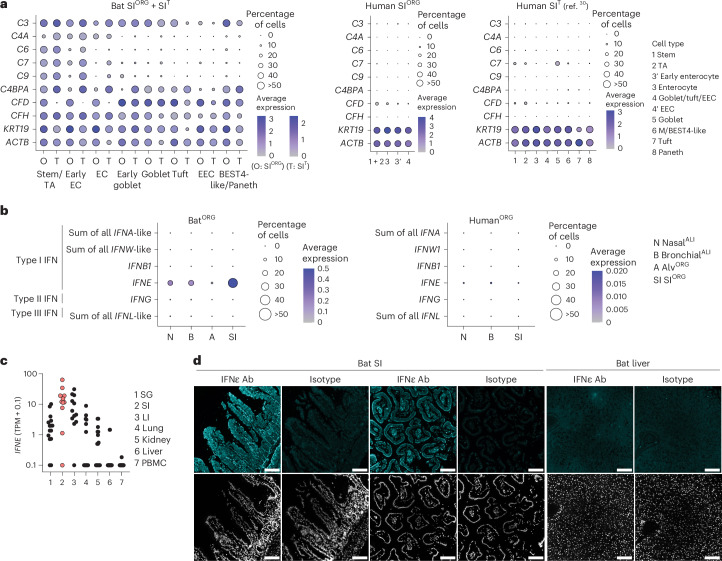
Next, we performed comparative RNA expression analysis of bat SIORG, nasalALI or bronchialALI to human nasalALI, bronchialALI or SIORG, prepared in parallel under identical conditions to bat counterparts. We detected a heightened expression of genes with innate immune effector functions (IFN-stimulated genes (ISGs) and complement genes) in bat over human organoid models (Extended Data Fig. 4a). For example, bat SIORG exhibited high and exclusive expression of several genes associated with the classical and alternative complement system (that is, C2, C3, C6, C7, C9, C4BPA, CFD and CFH (also known as LOC107520841)) compared to human SIORG or to a public scRNA-seq dataset from the human SI epithelium30 (Fig. 4a and Extended Data Fig. 4b,c), which is consistent with a report on the expression of complement genes in barrier tissues in R. aegyptiacus5.
Extended Data Fig. 4. R. aegyptiacus SIORG and SIT express heightened levels of complement system genes compared to human SIORG or human SIT.
a, Seurat FindMarker differential gene expression analysis comparing bat to human SIORG (left), bat to human nasalALI (middle) or bat to human bronchialALI, cultured side-by-side and analyzed by scRNA-seq. Each dot represents the log2 transformed sum of Seurat SCT normalized expression gene counts (bat + human) of differentially regulated genes (x axis) and the log2 fold changes (logFC) from the analysis between the two species (y axis). Positive logFC values indicate upregulated in bat organoids compared to human organoids. Selected complement system genes or interferon stimulated genes (ISGs) are labeled and colored in red. Upregulated markers in human compared to bat organoids are labeled and colored in blue. b, Seurat DotPlot showing the average expression of complement system genes in cell types in the R. aegypticus SIORG (O) or SIT (T) scRNA-seq dataset. Dot size, the percentage of an individual cell type expressing a given marker. Color intensity, the average expression value. Dot sizes were set to a maximum percentage of 50% (genes expressed by more than 50% of cells have the same dot size). c, same as in (b) but in the human SIORG scRNA-seq (left) or in a published human SIT scRNA-seq datasets (ref. 30).
Fig. 4. Bat organoids show heightened expression of innate immune genes, including complement system genes, IFNε and ISGs in comparison to human organoids.
a, Seurat DotPlot showing the average expression of genes associated with the complement system and β-actin (ACTB) in cell types in the R. aegyptiacus (bat) SIORG (O) or SIT (T) scRNA-seq dataset (left), human SIORG (middle) or from a published human SIT scRNA-seq dataset30. The dot size represents the percentage of an individual cell type expressing a given marker. Color intensity represents the average expression value. Dot size was set to a maximum percentage of 50%. (Genes expressed by more than 50% of cells have the same dot size.) b, Seurat DotPlot showing the average expression of type I IFNα (sum of all annotated IFNA-like genes), IFNω (sum of all annotated IFNW-like genes), IFNβ (IFNB1), IFNε (IFNE), type II IFNγ (IFNG) and type III IFNλ (sum of all annotated IFNL-like genes) in the R. aegyptiacus (left) and human (right) nasalALI (N), bronchialALI (B), alvORG (A) or SIORG (SI) scRNA-seq dataset. Dot size represents the percentage of an individual cell type expressing a given marker. Color intensity represents the average expression value. Dot size was set to a maximum percentage of 50%. c, IFNE mRNA expression (in transcripts per million (TPM) + 0.1, pseudocount) in published bulk RNA data32 of R. aegyptiacus salivary gland (SG), SI, large intestine (LI), lung, kidney, liver and PBMCs. Each dot represents an individual bat (n = 11–17). Samples from the SI are highlighted in red. d, Representative immunofluorescence of IFNε or isotype control staining (top) and DAPI nucleus counterstaining (bottom) in R. aegyptiacus SI or liver sections (n = 3). Scale bars, 100 µm.
We also observed high expression of IFNε mRNA (encoded by IFNE) in bat SIORG, nasalALI or bronchialALI compared to human counterparts (Fig. 4b). IFNε is a noncanonical type I IFN with antiviral and immune-regulatory functions, typically restricted to the female reproductive tract in humans and mice31. IFNE was the only IFN gene (among type I IFNA-like subtypes, IFNB1, IFNW-like subtypes, type II IFNG or type III IFNL-like) expressed at robustly detectable levels (more than 5% in any given organoid model) in the bat SIORG, nasalALI or bronchialALI scRNA-seq dataset (Fig. 4b). Increased expression of IFNE was particularly prominent in the enterocytes of SIORG and the basal cells of nasalALI or bronchialALI (Extended Data Fig. 5a). The highest expression of IFNE was also detected in the intestine in a public bulk RNA-seq dataset32 that evaluated several nonreproductive tissues (salivary gland, intestine, liver, lung, spleen and peripheral blood mononuclear cells (PBMCs)) of R. aegyptiacus (Fig. 4c). Immunofluorescence analysis in R. aegyptiacus tissue sections showed strong IFNε staining along the SI villi, but not in the liver or isotype controls (Fig. 4d). The basal expression of IFNE was associated with a heightened basal expression of conserved ISGs33 in nasalALI, bronchialALI or SIORG (for example, MX1, MX2, IRF7, PSMB9, DTX3L, OASL, LGALS9 and ISG15) (Extended Data Fig. 5b), suggesting a potential role for IFNε in constitutive antiviral immunity, consistent with its reported function in mouse reproductive tissue31. Of note, we observed higher mRNA expression of ISGs in both human and bat nasalALI cultures compared to bronchialALI or SIORG (Extended Data Fig. 5c), indicating that additional factors beyond IFNε contribute to this effect. Nevertheless, bat nasalALI, bronchialALI or SIORG exhibited significantly higher expression of conserved ISGs compared to their human counterparts cultured in parallel (Extended Data Fig. 5c).
Extended Data Fig. 5. Comparison of IFNE or ISG expression in R. aegyptiacus and human organoids, and genetic perturbation of IFNE in bat SIORG.
a, Seurat DotPlot showing the average expression of IFNE and cell type-specific marker genes in the integrated bat nasalALI + bronchialALI (left), or bat SIORG scRNA-seq dataset. Dot size, the percentage of an individual cell type expressing a given marker. Color intensity, the average expression value. Dot sizes were set to a maximum percentage of 50% (genes expressed by more than 50% of cells have the same dot size). b, Seurat DotPlot showing the average expression of ISGs in the bat nasalALI, bronchialALI, alvORG or SIORG scRNA-seq dataset (left), or human nasalALI, bronchialALI or SIORG scRNA-seq dataset (right). Dot size, the percentage of an individual cell type expressing a given marker. Color intensity, the average expression value. c, Violin plots showing the ISG module enrichment score distribution for conserved ISGs (ref. 33) in scRNA-seq of bat or human nasalALI, bronchialALI or SIORG. The distribution was derived from the enrichment scores of individual cells. Median shown as solid line. A positive score indicates enrichment of ISGs in a culture model. Two-sided Mann-Whitney tests were performed to compare human to bat nasalALI, bronchialALI or SIORG (****: P-value < 0.0001). d, Sanger sequencing trace of PCR amplicons spanning the bat IFNE gene in bat SIORG expressing Cas9 and a guide RNA targeting IFNE (sgIFNE) or a non-targeting control guide RNA (sgScrambled). The guide RNA spacer sequence and expected cut site is shown above. e, RT-qPCR analysis of ISGs, normalized to EEF1A1 (2–ΔCT), in Cas9-sgRNA expressing bat SIORG (n = 3). Two-sided unpaired Student’s t-tests were performed to compare ISG expression between sgIFNE-SIORG and sgScrambled-SIORG (**** P < 0.0001). f, VSV titer measured from the culture supernatant of infected sgIFNE (n = 3) or sgScrambled bat SIORG (n = 3) after 8 or 24 hours post infection (hpi). The titer was derived from TCID50 assays performed in VeroE6 cells with five replicates per sample supernatant.
To investigate the role of IFNε in bat epithelial antiviral immunity, we disrupted the IFNE gene in bat SIORG using lentivirus delivery for CRISPR–Cas9 and single-guide RNAs (sg RNAs) (Extended Data Fig. 5d). Quantitative PCR with reverse transcription (RT–qPCR) analysis of selected ISGs (IFIT1 (also known as LOC107501624), IRF7 and ISG15) revealed a modest but significant reduction in the mRNA expression of these genes in IFNε-perturbed organoids (sgIFNE) compared to organoids transduced with a nontargeting control guide RNA (sgScrambled) (Extended Data Fig. 5e). Vesicular stomatitis virus encoding the enhanced green fluorescent protein (VSV-eGFP), which is sensitive to bat IFNs4, replicated to higher titers in infected sgIFNE-SIORG compared to sgScrambled-SIORG (Extended Data Fig. 5f), indicating increased susceptibility to viral infection. Together, these findings revealed differences in gene expression between bat and human respiratory and intestinal ECs, particularly in genes linked to constitutive innate immunity, which may contribute to enhanced resistance to pathogens at bat epithelial surfaces.
MARV infection triggers an IFN response in bat organoids
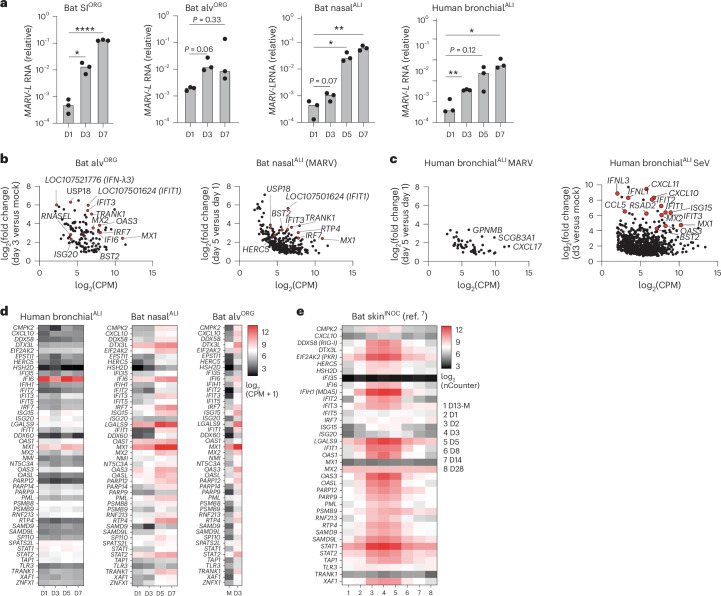
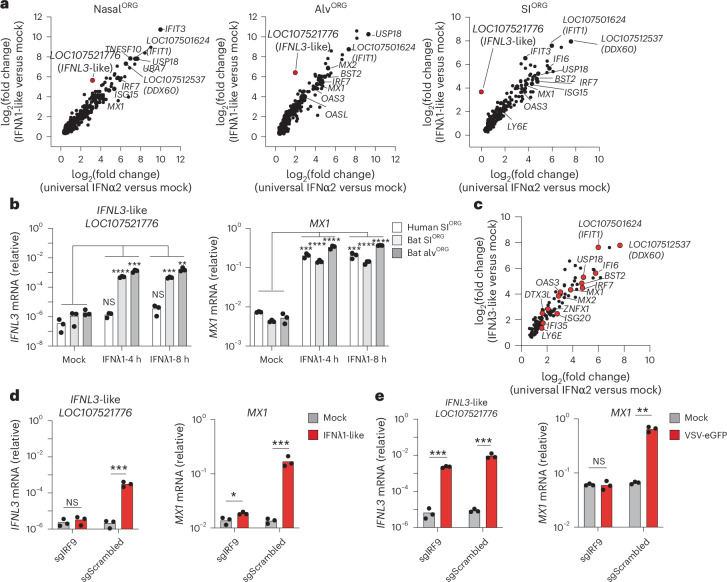
Organoids hold great potential as in vitro models to investigate host–virus interactions at near physiological levels. scRNA-seq analysis of bat nasalALI, bronchialALI, alvORG or SIORG revealed the expression of viral entry factors for multiple zoonotic viruses of human concern, including NPC1, the entry receptor for Ebola virus (EBOV) and MARV, ACE2 (for SARS-CoV-1 and SARS-CoV-2), DPP4 (for Middle East respiratory syndrome coronavirus (MERS-CoV)), EFNB2 (for the Hendra and Nipah viruses) and TMPRSS2/TMPRSS4 (serine proteases used by SARS-CoV-2, MERS-CoV or the influenza A virus (IAV) subtypes H1N1 and H7N9) (Extended Data Fig. 6a). Given the disparate disease outcomes of MARV infection in R. aegyptiacus bats7 compared to humans34, together with a suspected infection route via mucosal surfaces in these species35, we investigated the susceptibility and cellular responses to MARV infection in bat SIORG, nasalALI or alvORG, and well-differentiated human bronchialALI cultures. RT–qPCR indicated a tenfold to 1,000-fold increase in the MARV-L RNA (Musoke isolate) in SIORG, nasalALI or alvORG between day 3 and day 7 after infection compared to day 1 (Fig. 5a), while human bronchialALI cultures infected with MARV also displayed a significant increase in MARV-L RNA expression over a 7-day time course experiment (Fig. 5a), indicating that differentiated R. aegyptiacus and human ECs supported entry and replication of MARV. Notably, we detected higher release of infectious MARV in human bronchialALI compared to bat nasalALI at day 5 and day 7 after infection (Fig. 5a and Extended Data Fig. 6b). Next, we used pooled 3′-end sample-barcoded bulk RNA-seq (hereafter bulk RNA-seq) to investigate the genome-wide transcriptional landscape in nasalALI, alvORG or human bronchialALI infected with MARV (Fig. 5b–d and Supplementary Table 5). We found a significant temporal induction of ISGs (for example, MX1, RTP4, IRF7, USP18, TRANK1) in alvORG at day 3 after infection compared to mock-infected samples or nasalALI at day 5 and day 7 compared to day 1 after infection (Fig. 5b,d and Supplementary Table 5). Gene Ontology (GO) enrichment analysis of upregulated genes in MARV-infected bat alvORG or nasalALI revealed a strong enrichment of pathways associated with viral defense and IFN signaling (for example, defense response to virus (accession GO:0051607), negative regulation of viral process (accession GO:0048525), positive regulation of type I IFN production (accession GO:0032481)) (Extended Data Fig. 6c). Among IFNs, we detected the mRNA of an IFNL1-like gene (also known as LOC107521777) and two distinct IFNL3-like genes (also known as LOC107521776 or LOC107520938) as the most robustly induced IFNs in MARV-infected versus uninfected bat alvORG (Extended Data Fig. 6d). By contrast, MARV-infected human bronchialALI showed little to no significant ISG induction (Fig. 5c,d and Supplementary Table 5), but triggered a strong IFN response (for example, IFNL1, IFNL3, IFIT3, MX1, RSAD2, BST2 and ISG15) when challenged with murine Sendai virus (SeV).
Extended Data Fig. 6. Characterization of virus entry factor expression and antiviral responses to MARV infection in R. aegyptiacus organoids and animals.
a, Seurat DotPlot analysis showing the average expression of virus entry factors of cell types in the bat nasalALI, bronchialALI, alvORG or SIORG scRNA-seq dataset. Dot size, the percentage of an individual cell type expressing a given marker. Color intensity, the scaled average expression value. Dot sizes were set to a maximum percentage of 50% (top) or 25% (bottom). Genes expressed by more than 50% or 25% of cells have the same dot size. b, MARV (Musoke) titer (in focus forming units per ml (FFU/ml)) measured from combined solutions of apical washes and basal chamber medium of infected human BronchialALI (n = 3) or bat NasalALI (n = 3) after one-, three- or seven-days post infection. c, Gene ontology (GO) enrichment analysis, performed using clusterProfiler, revealed biological processes significantly enriched among genes upregulated in MARV-infected vs. mock-infected bat alvORG, or in bat nasalALI at day 5 vs. day 1 post-infection. The top 10 enriched biological processes are shown. The x axis indicates the ratio of enriched to total genes within each GO term. Dot size reflects the number of enriched genes per pathway. d, Heatmap showing normalized gene expression (log2-counts per million + 1) from bulk RNA-seq for IFN subtypes in MARV-infected or mock-infected bat alvORG. Each mRNA value represents the average of three biological replicates. Days post-infection are indicated (D3: day 3 post infection, M: day 3 mock-infected). e, Heatmap displaying log₂-normalized gene expression of MARV NP from reanalyzed public nCounter NanoString data of skin, liver, and colon samples from MARV-infected R. aegyptiacus (n = 4–6). Each mRNA value represents the average of four to six animals present in the dataset. Days post-infection are indicated (D1–D28, D13-M: day 13 mock-infected). f, Heatmap displaying log₂-normalized gene expression of ISGs from reanalyzed public nCounter NanoString data of liver and colon samples from MARV-infected R. aegyptiacus (n = 4–6). Each mRNA value represents the average of four to six animals present in the dataset. Days post-infection are indicated (D1–D28, D13-M: day 13 mock-infected).
Fig. 5. MARV infection in bat organoids triggers an IFN response.
a, RT–qPCR analysis of intracellular MARV-L expression (normalized to EEF1A1, 2-ΔCt) in bat SIORG(n = 3), alvORG(n = 3), nasalALI (n = 3) or human bronchialALI (n = 3) infected with MARV (Musoke strain) at an estimated multiplicity of infection (MOI) of 0.5–1 (50,000 plaque forming units (PFU) for bat SIORG and alvORG; 100,000 PFU for bat nasalALI or human bronchialALI) from day 1 to day 7 (D1–D7) after infection. A two-sided Student’s t-test was used to compare the average intracellular MARV-L mRNA level at each time point to the first analyzed time point (D1) for each condition. P > 0.05, no significant difference, *P < 0.05, **P < 0.01,, ****P < 0.0001. b, EdgeR differential gene expression analysis of bat alvORG (n = 3) infected with MARV for 72 h compared to mock-infected (left) or bat nasalALI (n = 3) at day 5 after infection compared to day 1 after infection with MARV (right). Each dot represents the expression (in log2-transformed counts per million (CPM)) and log2-transformed fold change of a differentially expressed gene (DEG) between two comparisons. Selected ISGs are highlighted in red and labeled. c, Human bronchialALI (n = 3) at day 5 after infection compared to day 1 after infection with MARV (left) or 72 h after infection with SeV compared to mock infection (right). Each dot represents the expression (in log2(CPM)) and log2(fold change) of a DEG between two comparisons. Selected genes are labeled and highlighted in red for ISGs. d, Heatmap showing normalized gene expression (log2(CPM + 1)) from bulk RNA-seq for ISGs in human bronchialALI (left), bat nasalALI (middle) or bat alvORG (right) from day 1 to day 7 (D1–D7) after infection with MARV or day 3 in mock infection (M). Each mRNA value represents the average of three biological replicates. e, Heatmap displaying log2-normalized gene expression of ISGs from reanalyzed public nCounter NanoString data of skin samples (inoculation site) from R. aegyptiacus7 (bat skinINOC, n = 4–6) from day 1 to day 28 (D1–D28) after infection with MARV or day 13 in mock infection (D13-M). Each mRNA value represents the average of 4–6 bats in the published dataset.
The induction of ISGs in MARV-infected bat nasalALI or alvORG, but not human bronchialALI, was also observed in reanalyzed public mRNA expression data from R. aegyptiacus7 infected subcutaneously with MARV (Fig. 5e). There, ISG expression peaked at day 3–5 after infection at the skin inoculation site and to a lesser extent in other tissues, such as the liver and colon, where viral MARV-NP mRNA levels remained low, before returning to baseline by day 28 (Fig. 5e and Extended Data Fig. 6e,f). Thus, MARV-infected bat respiratory epithelial organoids recapitulated antiviral gene expression changes at barrier tissues in MARV-infected bats, contrasting with the blunted IFN responses observed in MARV-infected differentiated human airway EC cultures.
Bat organoids predominantly induce type III IFNs after virus infection
Next, we extended our findings on the antiviral responses to MARV infection in bat organoids to other zoonotic RNA viruses not naturally found in R. aegyptiacus. RT–qPCR analysis of bat nasalORG, alvORG or SIORG infected with murine SeV, VSV-eGFP, MERS-CoV or human IAV (H1N1, cell-culture-adapted A/WS/33 strain), showed the induction of IFNL1-like (LOC107521777), IFNL3-like (LOC107521776 and LOC107520938) and IFNB1, as well as downstream ISGs, such as IFIT1 and CCL5 (Fig. 6a). Poly(I:C) double-stranded RNA (dsRNA) treatment of nasalORG, alvORG and SIORG induced these genes to similar levels compared to viral infection (Fig. 6a).
Fig. 6. Type III IFNs are predominantly induced in bat organoids upon zoonotic virus infection.
a, RT–qPCR analysis of type I IFNB1, type III IFNL1-like (LOC107521777), IFNL3-like (LOC107521776), IFIT1 (LOC107501624) and CCL5 (normalized to EEF1A1, 2-ΔCt) in virus-infected (SeV, H1N1, VSV-eGFP or MERS-CoV), poly(I:C)-transfected or mock-treated bat nasalORG, SIORG or alvORG. Each mRNA value represents the average of three biological replicates. SeV (S), H1N1 (I) and VSV (V) infections were performed at an MOI of 0.5 for 16 h; poly(I:C) (R) was transfected at 1 µg ml−1 for 16 h and MERS-CoV infections were conducted for 24 (C1), 48 (C2) or 72 h (C3) or mock (MC). b, Heatmap showing normalized gene expression (log2(CPM + 1)) from bulk RNA-seq in SeV-infected bat SIORG (left), SeV-infected bat alvORG (middle) or MERS-CoV-infected bat alvORG (right) for IFNA-like (sum of all IFNA-like genes), IFNW-like (sum of all IFNW-like genes), IFNK, IFNB1, IFNG, IFNL-like (sum of all IFNL-like genes) (top) and ISGs or the epithelial marker EPCAM (bottom) at D1–D3 after infection. Each mRNA value represents the average of three biological replicates. The single asterisk indicates IFN or ISGs expressed in at least two of three biological replicates. c, Normalized gene expression (CPM + 1) from bulk RNA-seq for IFNE and the ISGs IRF7, MX2 or RTP4 in SeV-infected or mock-infected bat SIORG (n = 3) or bat alvORG (n = 3). Biological replicates are represented by the dots, with the bar height indicating the average expression. A two-sided Student’s t-test was used to compare the average mRNA expression levels of each gene in SeV-infected organoids versus uninfected organoids at day 3 after infection. NS, not significant; ***P < 0.001, ****P < 0.0001.
To broaden our analysis from selected genes to all annotated IFNs and ISGs, we performed bulk RNA-seq of SeV-infected bat alvORG or SIORG, and MERS-CoV-infected alvORG. We found that, in addition to a broad induction of ISGs (for example, DTX3L, OAS3, RTP4, IRF7, MX2, IFIT3, IFIH1), only IFNB1 and, more robustly, IFNL1-like and IFNL3-like were induced in virus-infected organoids at 1 and 3 days after infection compared to the uninfected controls (Fig. 6b and Supplementary Table 5). IFNE, which was constitutively expressed, was not further increased by SeV infection in bat SIORG or alvORG (Fig. 6c). This is consistent with reports in mice, where IFNε is not induced by classical pattern recognition receptor ligands associated with RNA viruses31. These observations indicated that IFNλ genes were the predominant and most robustly induced IFN produced after viral infection in R. aegyptiacus ECs.
IFNλs drive robust antiviral gene responses in bat organoids
In mice and humans, type I and III IFNs induce a largely overlapping set of genes that perform common antiviral functions36. To compare type III IFN-mediated and type I IFN-mediated gene induction in R. aegyptiacus ECs, we performed bulk RNA-seq on bat nasalORG, alvORG, or SIORG treated for 8 h with recombinant homemade R. aegyptiacus type III IFNλ1-like (see Methods), universal type I IFNα2 or PBS (mock-treated). Universal type I IFNα2 is a recombinant IFNα hybrid protein that exhibits bioactivity across multiple species33, including R. aegyptiacus37. In parallel, we treated human SIORG or bronchialALI with recombinant human IFNλ1 or universal type I IFNα2 for 8 h to establish a cross-species reference dataset. Bulk RNA-seq revealed significant induction of classical ISGs (for example, ADAR, IFIT1, IFIT2, IFIT3, IFI6, IFIH1, OAS3, OASL, IRF7, USP18 and ZNFX1) in bat nasalORG, alvORG or SIORG incubated with universal IFNα2 or bat IFNλ1-like compared to mock-treated organoids (Fig. 7a and Supplementary Table 6). We identified 124 genes that were significantly upregulated by both IFN treatments in all bat organoid models (for example, ADAR, IRF7, ISG15, LGALS9, MX2, PSMB9 and ZNFX1) (Supplementary Table 6). Notably, IFNλ1-like treatment significantly upregulated more genes relative to mock treatment than universal IFNα2, across all bat organoid models (Extended Data Fig. 7a and Supplementary Table 6). Co-treatment with the Janus kinase (JAK) inhibitor ruxolitinib abolished the induction of the ISGs IFIT3 or ISG15 by universal IFNα2 or bat IFNλ1-like in nasalORG (Extended Data Fig. 7b), indicating that both type I and type III IFNs signal through the JAK–signal transducer and activator of transcription (STAT) signaling pathway in R. aegyptiacus ECs. GO terms related to antiviral defense and IFN responses (defense response to virus (accession GO:0051607), negative regulation of viral process (accession GO:0048525), response to type I IFN (accession GO:0034340)) were the top enriched biological processes in response to both bat IFNλ1-like and universal IFNα2 treatment (Extended Data Fig. 7c,d). Similarly, universal IFNα2 or human IFNλ1 stimulation of human SIORG or human bronchialALI induced the expression of antiviral response genes (for example, IFIT1, IFIT2, IFIT3, OAS1, OAS2, OAS3, IRF7 and RSAD2) (Extended Data Fig. 7e–h and Supplementary Table 6). However, we observed a stronger induction of pro-inflammatory chemokines (for example, CXCL1, CXCL2, CXCL9, CXCL11, CX3CL1 and CCL22) in human SIORG or bronchialALI treated with universal IFNα2 compared to human IFNλ1, aligning with previous results published in mice8 (Extended Data Fig. 7e–h and Extended Data Fig. 8a). By contrast, universal IFNα2 or bat IFNλ1-like-treatment or virus-infection of bat nasalORG (MARV), alvORG (MARV, MERS, SeV) or SIORG (SeV), resulted in minimal or no changes in the expression of these pro-inflammatory genes (Extended Data Fig. 8b,c).
Fig. 7. Bat type III IFNλ drive self-amplified antiviral responses in bat organoids.
a, Scatter plots showing the log2-transformed fold changes in gene expression from bulk RNA-seq comparing nasalORG (left), alvORG (middle) or SIORG (right) treated with universal IFNα2 (x axis) or IFNλ1-like (y axis) to mock-treated controls after 8 h. Individual ISGs are highlighted. IFNL3-like (LOC107521776) is highlighted in red. b, RT–qPCR analysis of IFNL3-like (LOC107521776) in bat SIORG or alvORG; IFNL3 in human SIORG) normalized to EEF1A1 (2−ΔCt) after treatment with bat IFNλ1-like (bat organoids) or human IFNλ1 (human SIORG) for 4 and 8 h. A two-sided Student’s t-test was used to compare IFNL3-like mRNA levels at 4 or 8 h after treatment to mock-treated controls. c, Scatter plot showing the log2-transformed fold changes in gene expression from bulk RNA-seq comparing SIORG treated with universal IFNα2 (x axis) or IFNλ3-like (derived from LOC107521776) (y axis) to mock-treated controls after 8 h. Individual ISGs are highlighted in red. d, RT–qPCR analysis of IFNL3-like (LOC107521776) or MX1 (normalized to EEF1A1, 2−ΔCt) in bat SIORG engineered with Cas9 and IRF9 targeting (sgIRF9) or control (sgScrambled) RNA, treated with or without bat IFNλ1-like for 8 h. A two-sided Student’s t-test was used to compare IFNL3-like mRNA levels between sgIRF9 and sgScrambled SIORG at 8 h after treatment. e, As in d but comparing sgIRF9 and sgScrambled SIORG after infection with VSV-eGFP at an MOI of 0.05 for 72 h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Extended Data Fig. 7. Type I/III IFNs drive antiviral gene responses in bat and human organoids.
a, Venn diagrams (generated using eulerr.co) showing the number of significantly upregulated genes determined by bulk RNA-seq in bat nasalORG, alvORG, or SIORG (n = 3 each) following treatment with universal IFNα2 (uIFNα2) or IFNλ1-like compared to mock-treated. The total number of upregulated genes per treatment are indicated. Overlapping regions represent genes commonly induced by both IFNs. b, RT-qPCR analysis of IFIT3 and ISG15 expression, normalized to EEF1A1 (2–ΔCT), in bat nasalORG (n = 3) treated with uIFNα2 or IFNλ1-like for 8 h, with or without ruxolitinib (n = 3). Two-sided unpaired Student’s t-tests were used to compare mRNA levels between ruxolitinib-treated and mock-treated, interferon-stimulated nasalORG. (ns: not significant; *** P < 0.001, **** P < 0.0001). c, Gene ontology (GO) enrichment analysis of bulk RNA-seq data, performed with clusterProfiler, revealed biological processes significantly enriched among genes upregulated in uIFNα2 vs. mock-infected bat nasalORG, alvORG, or SIORG after 8 h (n = 3 each). The top 10 enriched biological processes are shown. The x axis indicates the ratio of enriched to total genes within each GO term. Dot size reflects the number of enriched genes per pathway. d, Same as in (c) but for IFNλ1-like vs. mock-treated bat nasalORG, alvORG, or SIORG. e, Scatter plot showing log2-fold changes in gene expression from bulk RNA-seq comparing human SIORG treated with uIFNα2 (x axis) or IFNλ1-like (y axis) to mock-treated controls after 8 h (n = 3). Individual ISGs and selected pro-inflammatory cytokines (red) are highlighted and labeled. f, same as in (c) but for uIFNα2 (left) or IFNλ1 (right) vs. mock-treated human SIORG. g, Same as in (e) but for human bronchialALI (n = 3). h, Same as in (f) but for GO enrichment analysis of uIFNα2 (left) or IFNλ1 (right) vs. mock-treated human bronchialALI.
Extended Data Fig. 8. Pro-inflammatory genes are not broadly induced in bat organoids treated with IFN or infected with RNA viruses.
a, Heatmap showing gene expression log2-fold changes (from EdgeR bulk RNA-seq differential gene expression analysis) of selected pro-inflammatory genes and selected ISGs in universal IFNα2 (uIFNα2) or IFNλ1 vs. mock-treated human SIORG or human BronchialALI (n = 3 each). b, Same as for (a) but for IFN-treated bat SIORG (n = 3), bat alvORG (n = 3), or bat nasalORG (n = 3). c, Same as for (a) but for MARV-, MERS-CoV- or SeV-infected bat alvORG (n = 3 each), SeV-infected bat SIORG (n = 3), or MARV-infected bat nasalALI (n = 3). Comparisons used for calculation of edgeR log2-fold change are indicated in the legend.
Among R. aegyptiacus ISGs that piqued our interest was the putative IFNL3-like gene (LOC107521776), which was induced by IFN in nasalORG, alvORG or SIORG, particularly after 8 h of stimulation with bat IFNλ1-like compared to mock-treated control cultures (Fig. 7a). RT–qPCR analysis confirmed the bulk RNA-seq data and indicated that IFNL3-like was significantly upregulated at 4 and 8 h after treatment with IFNλ1-like in bat SIORG or bat alvORG, but not in human SIORG, despite an equally strong induction of the ISG MX1 (Fig. 7b). Treatment of bat SIORG with home-made recombinant bat IFNλ3-like (Methods) for 8 h induced the expression of ISGs (for example, BST2, IFIT1, DDX60, IFI6, IRF7, OAS3, ZNFX1) to a similar extent as universal-IFNα2-treated SIORG (Fig. 7c and Extended Data Fig. 9a,b), confirming its function as IFN. Notably, recombinant IFNλ3-like, derived from the second annotated IFNL3-like gene in R. aegyptiacus (LOC107520938), also triggered ISG expression after 8 h of treatment in SIORG compared to mock-treated controls (Extended Data Fig. 9c,d).
Extended Data Fig. 9. Bat type III IFNλ3-like drive self-regulated antiviral responses in bat SIORG.
a, EdgeR differential gene expression analysis of bat SIORG (n = 3) treated with IFNλ3-like (LOC107521776) (dose equivalent to 1000 U/ml universal IFNα2) for 8 h compared to mock-treated (n = 3). Each dot represents the average expression (in log2-count per million, x axis) and log2-fold change (y axis) of a differentially expressed gene between IFNλ3-like vs. mock-treated SIORG. Selected ISGs are highlighted in red and labeled. b, Gene ontology (GO) enrichment analysis of bulk RNA-seq data, performed with clusterProfiler, revealed biological processes significantly enriched among genes upregulated in IFNλ3-like vs. mock-infected bat SIORG after 8 h (n = 3 each). The top 10 enriched biological processes are shown. The x-axis indicates the ratio of enriched to total genes within each GO term. Dot size reflects the number of enriched genes per pathway. c, Same as in (a) but for IFNλ3-like (LOC107520938) vs. mock-treated SIORG (n = 3). d, Same as in (b) but for IFNλ3-like (LOC107520938) vs. mock-treated SIORG. e, RT–qPCR analysis of IFNL3-like (LOC107521776) mRNA, normalized to EEF1A1 (2–ΔCT) in bat SIORG or alvORG treated with or without recombinant bat IFNλ3-like (equivalent to 1000 U/ml universal IFNα2) for 8 h. A two-sided Student’s t-test was used to compare IFNL3-like mRNA levels 8 h after treatment to mock-treated controls. ***P<0.001, ****P<0.0001.
To determine whether IFNL3-like (LOC107521776) was a canonical ISG, we performed bulk CRISPR–Cas9 editing of IRF9, the key transcription factor that mediates signaling by both type I and type III IFNs36. IRF9 gene perturbation in bat SIORG resulted in an almost complete loss of the IFNλ1-like-dependent (Fig. 7d), but not VSV-eGFP-dependent (Fig. 7e), induction of IFNL3-like. By contrast, MX1 gene expression in bat SIORG was largely abolished after IRF9 perturbation, both in response to IFNλ1-like and VSV infection (Fig. 7d,e), indicating both virus-dependent and virus-independent pathways for the induction of IFNL3-like. Additionally, we observed that recombinant IFNλ3-like protein robustly induced its own expression in bat SIORG and alvORG (Extended Data Fig. 9e), indicating that IFNλ3-like might control a positive autoregulatory feedback loop. In summary, we characterized the functional capacity of three type III IFNs to induce antiviral gene responses in R. aegyptiacus epithelial organoids and identified a unique role for IFNL3-like (LOC107521776), which can also function as an ISG itself.
Type I and type III IFNs protect bat organoids from viral infection
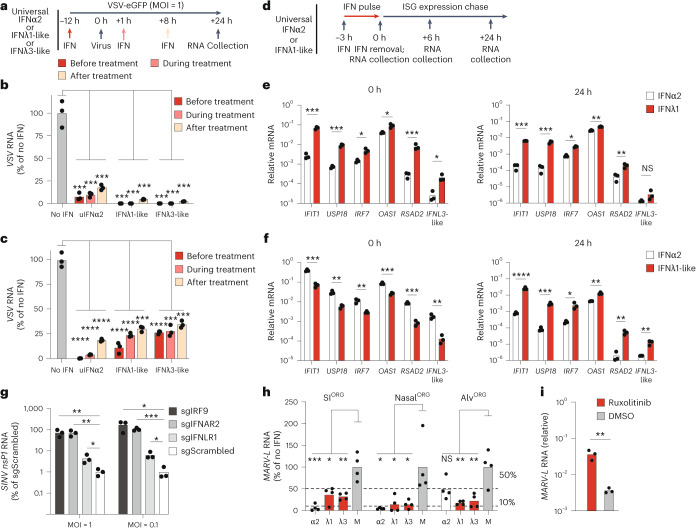
To assess the antiviral potential and kinetic differences of type I and type III IFN responses in bat organoids, we infected bat nasalORG or SIORG with VSV-eGFP, a fast-replicating virus sensitive to bat IFN4, and treated them with recombinant universal IFNα2, bat IFNλ1-like or bat IFNλ3-like, 12 h before infection (before treatment), during infection (co-treatment) or 8 h after infection (after treatment) (Fig. 8a). RT–qPCR measurement of intracellular viral RNA indicated that all three IFNs strongly protected both bat nasalORG and SIORG from viral infection in all treatment schemes (Fig. 8b,c). IFN treatment before infection reduced intracellular viral RNA to less than 1–10%, whereas treatment after infection reduced it to less than 20–30% of the levels observed in infected, untreated organoids (Fig. 8b,c).
Fig. 8. Type I and type III IFNs protect bat organoids from zoonotic virus infection.
a, Experimental workflow showing the administration of universal IFNα2, IFNλ1-like or IFNλ3-like 12 h before, during or 8 h after treatment with VSV infection of bat nasalORG or SIORG. Intracellular VSV viral RNA was measured 24 h after infection. b,c, RT–qPCR analysis of intracellular VSV-NP viral RNA in bat SIORG (n = 3) (b) or nasalORG (n = 3) (c), normalized to EEF1A1 (2−ΔCt) and expressed as a percentage relative to no IFN control. A two-sided Student’s t-test was used to compare VSV viral RNA levels across before, during and after treatment, and no IFN conditions, comparing each IFN treatment to no IFN control. d, Experimental workflow showing bat nasalORG or SIORG treated with universal IFNα2 or bat IFNλ1-like for 3 h, followed by washout and incubation in IFN-free medium for 24 h and RNA collection at 0, 3 and 24 h after IFN washout. e,f, RT–qPCR analysis of ISGs (normalized to EEF1A1) in bat SIORG (n = 3) (e) or nasalORG (n = 3) (f) treated with universal IFNα2 or bat IFNλ1-like at 3 h (left) or 24 h (right) after IFN removal. A two-sided Student’s t-test was used to compare ISG mRNA levels between treatments. g, RT–qPCR analysis of intracellular SINV-eGFPnsP2-P726G nsP1 RNA (normalized to EEF1A1 (2−ΔCt) and expressed as fold change relative to the mean of the sgScrambled control) in bat SIORG engineered with Cas9 and targeted guide RNA (sgIRF9, sgIFNAR2, sgIFNLR1) or control (sgScrambled), infected with SINV-eGFPnsP2-P726G. A two-sided Student’s t-test was used to compare SINV nsP1 viral RNA levels between treatment groups. nsP1, nonstructural protein 1. h, RT–qPCR analysis of intracellular MARV-L viral RNA (normalized to EEF1A1) in bat SIORG, nasalORG or alvORG (n = 4) treated with or without IFNs during a 3-day MARV infection. A two-sided Student’s t-test was used to compare MARV viral RNA levels between IFN-treated and untreated controls. i, RT–qPCR analysis of MARV-L viral RNA in bat alvORG treated with or without 5 µM ruxolitinib during a 3-day infection. A two-sided Student’s t-test was used to compare MARV viral RNA levels between ruxolitinib and dimethylsulfoxide (DMSO) mock-treated alvORG and MARV-infected alvORG. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To examine the difference in antiviral response kinetics between universal IFNα2 and bat IFNλ1-like in bat nasalORG or SIORG, we measured the induction and persistence of ISG expression (IFIT1, USP18, IRF7, OAS1, RSAD2 and IFNL3-like) over a 24-h period using a pulse-chase experiment in which organoids were treated with universal IFNα2 or bat IFNλ1-like for 3 h, followed by washout and incubation in IFN-free culture medium (Fig. 8d). We observed stronger expression of all measured ISGs in bat SIORG treated with bat IFNλ1-like compared to universal IFNα2, while the reverse was true in bat nasalORG (Extended Data Fig. 10a–c). Despite this difference, treatment with bat IFNλ1-like resulted in significantly longer-lasting expression of all tested ISGs (except IFNL3-like), maintaining increased levels up to 24 h after IFN removal in both organoid types, in contrast to universal IFNα2 treatment, which led to ISG expression returning to basal levels by the 24-h time point (Fig. 6e,f and Extended Data Fig. 10b,c). These data suggested differences in the sensitivity and timing of the response of R. aegyptiacus intestinal and respiratory ECs to type I and type III IFNs.
Extended Data Fig. 10. 10: Bat type I/III IFN drive antiviral responses with different kinetic profiles in bat SIORG or NasalORG.
a, Experimental workflow to assess the temporal induction and maintenance of ISG expression. Bat NasalORG or SIORG are treated with universal IFNα2 (uIFNα2) or bat IFNλ1-like for 3 hours, followed by washout and incubation in IFN-free medium for 24 hours. b, Heatmaps showing RT-qPCR analysis of selected ISGs in bat SIORG (n = 3), with expression values calculated using the 2 -ΔΔCT method: first normalized to EEF1A1 (2–ΔCT), then to baseline levels at −3 h (before treatment). Timepoints shown include before IFN treatment (−3 h), immediately after treatment (0 h), and various hours post-interferon removal (+6h, +24h). Each heatmap value represents the average normalized ISG expression across three biological replicates. Left, uIFNα2. Right, IFNλ1-like treatment. c, same as in (b) but for bat nasalORG. d, RT-qPCR analysis of IFIT1 (normalized to EEF1A1(2–ΔCT), then expressed as a percentage relative to the average sgScrambled control in bat nasalORG engineered with Cas9 and a targeted guide RNA (sgIRF9, sgIFNAR2 or sgIFNLR1) or control guide RNA (sgScrambled) (n = 3 each), treated or not for 8 h with uIFNα2 or bat IFNλ1-like.
To further assess the requirement for endogenous IFN signaling in cell-intrinsic viral restriction, we used bulk CRISPR–Cas9 editing to target IRF9, the type I IFN receptor IFNAR2 and the type III IFN receptor IFNLR1 in bat nasalORG (knockout (KO) organoids). Gene editing significantly reduced the sensitivity of knockout organoids to exogenous IFNs, but did not completely abolish signaling, a limitation inherent in bulk CRISPR–Cas9 editing (Extended Data Fig. 10d). IRF9, IFNAR2 and IFNLR1 knockout organoids challenged with SINV-eGFP-nsP2-P726G, an attenuated variant of the alphavirus Sindbis virus (SINV) carrying a point mutation in the nsP2 gene and eGFP38, accumulated significantly more intracellular viral RNA compared to scrambled guide control nasalORG (Fig. 8g), suggesting that both type I and III IFN receptor signaling were important for viral restriction.
Lastly, we evaluated the ability of type I and type III IFNs to restrict MARV replication in bat ECs. Treatment with universal IFNα2, bat IFNλ1-like or bat IFNλ3-like at the time of MARV infection significantly reduced intracellular viral RNA in bat nasalORG, alvORG or SIORG compared to mock-treated organoids (Fig. 8h). Moreover, treating MARV-infected bat alvORG with the JAK–STAT signaling inhibitor ruxolitinib during infection significantly increased intracellular viral RNA (Fig. 8i), indicating that endogenous IFN signaling was required to limit MARV replication. In conclusion, our findings identified distinct antiviral roles for type I and type III IFNs in R. aegyptiacus ECs, supporting both rapid induction and prolonged antiviral protection.
Discussion
In this study, we developed a sustainable organoid platform to model epithelial innate immunity and enable functional genetic studies in R. aegyptiacus. Our findings demonstrate that bat adult stem cells retain their full differentiation potential in vitro, faithfully recapitulating the cellular diversity and gene expression profiles found in the native epithelium. Cross-species comparisons with human organoids revealed an enhanced antiviral gene expression signature in bat organoids both at baseline and after infection. By delineating type I and type III IFN responses, we identified their critical roles in restricting viral infection through both rapid induction and sustained antiviral activity.
Based on our findings, we propose a model for epithelial antiviral immune responses in R. aegyptiacus in which basal expression of ISGs, partially driven by type I IFNε, provides a constitutive level of defense and primes ECs against infection, with complement gene expression potentially contributing to this baseline protection. Upon viral sensing, the induction of type I IFNβ, type III IFNλ1-like and IFNλ3-like confers robust antiviral protection. Notably, the consistent and strong induction of type III IFNs, irrespective of viral species, coupled with their long-lasting protective effects and unique virus-independent regulation of IFNλ3-like, underscores the pivotal role of IFNλ in limiting viral replication at epithelial surfaces in R. aegyptiacus.
Therefore, asymptomatic MARV infection in R. aegyptiacus7,35 could result from the strong induction of innate antiviral immunity at infected mucosal barrier tissues, which would restrict virus dissemination and concurrently limit excessive inflammation. Supporting this hypothesis, pharmacological inhibition of innate immune responses using dexamethasone enhances MARV replication in R. aegyptiacus, causing MARV-like disease in infected animals39. On the other hand, an inability of humans and nonhuman primates to mount a potent early antiviral response at mucosal surfaces against MARV infection may permit systemic virus dissemination, leading to the observed uncontrolled virus replication, widespread inflammation and lethal disease40. The broad cellular distribution of the major filovirus host entry factor NPC1 probably contributes to the detrimental disease outcome when local virus restriction is impaired. A mechanistic explanation for the observed species-specific differences in antiviral responses may lie in the ability of bats to overcome innate immune antagonists encoded by filoviruses41. Two such viral proteins, VP35, a dsRNA-binding protein and RIG-I antagonist, and VP40, which inhibits JAK–STAT signaling, may function differently in R. aegyptiacus ECs compared to human cells or may fail to block type I and type III IFN signaling altogether42,43. The elevated basal expression of ISGs observed in this study and shown previously1,33,44, including pattern recognition receptors and downstream signaling proteins, may further lower the intracellular threshold for viral sensing in R. aegyptiacus ECs, enhancing their capacity to detect and respond to viral infection. While most of our experiments focused on the antiviral effects of IFN against VSV, we also observed that type III IFNλ1-like and IFNλ3-like effectively inhibited MARV replication in bat organoids. This finding suggests that IFNλ could be explored as a therapeutic strategy to prevent lethal filovirus infections in humans and nonhuman primates.
This study has limitations. Mucociliary differentiation is a hallmark of the respiratory epithelium; while our bat organoid models successfully formed ciliated and specialized ECs (for example, microfold cells, tuft and brush cells, and EECs), goblet cells were rare unless stimulated with IL-13. Similar to the absence of Paneth cells in human SI organoids45, we speculate that in vivo microbial cues may trigger cytokine production that is absent or not supported by the commercial human differentiation media used. To address this, an optimized differentiation cocktail, probably requiring species-specific growth factors and tailored culture conditions, needs to be developed. Additionally, in vitro models require careful interpretation when extrapolating findings to in vivo contexts, particularly given the absence of classical immune cells, which have a crucial role in systemic antiviral immunity. Incorporating immune cells into organoid cultures could provide valuable insights into epithelial immune crosstalk during viral infections. Moreover, direct exposure of epithelial organoids may not fully replicate the natural infection dynamics at epithelial surfaces in vivo. Future studies should validate the role of epithelial immune responses in viral clearance through mucosal inoculation rather than systemic injection, potentially also combining these experiments with pharmacological inhibition or exogenous stimulation of the bat mucosa with type I or type III IFN to further explore their roles during infection in Egyptian fruit bats.
Our bat organoid platform provides a valuable tool for investigating genetic pathways and complements the limited in vivo studies possible in bats. Despite its limitations, this organoid-based approach bridges in silico4, in vitro46 and in vivo7 research, advancing our understanding of bat antiviral immunity and how bats can harbor pathogenic zoonotic viruses without disease. It also enables direct comparison with human and animal organoids, providing insight into cross-species viral dynamics and epithelial responses to infection. Together, these advances provide a foundation for future mechanistic studies using complex, non-immortalized in vitro models of R. aegyptiacus.
Methods
Bat tissue collection and human primary cell isolation
R. aegyptiacus bat tissue for organoid generation was derived from a breeding colony at the Friedrich-Loeffler-Institut in Germany. Collection was carried out in accordance with European and national animal welfare regulations applicable in the federal state of Mecklenburg-Western Pomerania, Germany. Freshly collected organ tissue from male and female bats was washed in PBS and cut into 3–5-mm pieces before placing up to four individual pieces into a 1-ml cryostorage tube containing 0.5 ml of CryoStor CS10 freezing medium (STEMCELL Technologies). Vials were immediately transferred to a cryostorage container (Mr. Frosty) and frozen overnight at −70 °C, followed by liquid nitrogen storage the following day. In total, nasal respiratory, lung and small intestinal tissue from three individual bats (males, n = 2; females, n = 1) and tracheal tissue from two individual bats (males, n = 2) were obtained and included in this study.
Human nasal ECs were collected from a brush biopsy of the middle turbinate section of a healthy female donor and placed into Advanced DMEM/F-12 (Gibco). After collection of cells via centrifugation, individual cells were obtained using TrypLE Express (Gibco) enzyme dissociation, followed by plating onto PureCol-treated (Advanced BioMatrix, diluted 1:30 in PBS or Advanced DMEM/F-12) cell culture dishes in PneumaCult-Ex Plus Medium (STEMCELL Technologies). Nasal ECs were expanded for up to two additional passages in PneumaCult-Ex Plus Medium and cryopreserved in liquid nitrogen in CryoStor CS10 freezing medium. Experiments were approved by the ethics commission of the Medical University of Vienna (no. ECS 2234/2021). ALI quality-controlled human bronchial ECs were purchased from PromoCell (cat. no. C-12640) and were initially recovered in PromoCell airway expansion medium. Cells were further expanded for up to two additional passages in PromoCell airway expansion medium or PneumaCult-Ex Plus Medium and cryopreserved in CryoStor CS10 freezing medium.
Establishment and maintenance of bat airway organoids
Frozen tissue pieces were quickly thawed in a water bath before transferring to a gentleMACS dissociation tube (Miltenyi Biotec) containing Advanced DMEM/F-12, supplemented with 1:100 GlutaMAX (Gibco), 10 mM HEPES (Gibco), DNase I (Roche) and 1.25 µg ml−1 collagenase from Hathewaya histolytica (Collagenase from Clostridium histolyticum, Sigma-Aldrich). A homogenous cell suspension was obtained by initial disruption of bulk tissue pieces using program m_lung_01, followed by dissociation using protocol 37C_mLIDK_01 in a gentleMACS dissociator (Miltenyi Biotec). Dissociation was stopped by adding an equal volume of 10% FBS (Heat-inactivated, Gibco, A5670801) in Advanced DMEM/F-12. The cell suspension was strained through a 70-µm filter and cells were collected by centrifugation at 300g for 5 min. To remove red blood cells, the cell pellet was resuspended and incubated in 1 ml of red blood cell lysis buffer (Roche) for 5 min. Cells were resuspended in Advanced DMEM/F-12 and counted using Trypan Blue to estimate the live and dead cell number percentages. If cell viability was below 60%, dead cells were removed using annexin V-based magnetic bead selection according to the manufacturer’s instructions (STEMCELL Technologies). Briefly, cells were collected using centrifugation, resuspended in 1 ml PBS, supplemented with 2% FCS and 1 mM CaCl2 (Sigma-Aldrich), and transferred to a 5-ml fluorescence-activated cell sorting (FACS) tube. Then, 50 µl of annexin V biotin beads were added and incubated for 5 min; 50 µl of magnetic RapidSpheres were added for 3 min. Then, 1.5 ml of PBS, supplemented with 2% FCS and 1 mM CaCl2, was added and the tube was subsequently placed into a magnet. After a 3-min separation, unbound viable cells were decanted, collected using centrifugation (300g for 5 min) and resuspended in 1 ml Advanced DMEM/F-12 before counting.
For nasal and tracheal epithelial tissues, cells were either expanded two-dimensionally using the PneumaCult-Ex Plus Medium as described for human airway ECs (see above) or expanded under three-dimensional culture conditions (expanding organoids). To establish expanding basal cell nasalORG, tracheaORG or bronchialORG, 25,000 viable cells were resuspended in 50 µl ice-cold Matrigel (cat. no. 356255, Corning) and plated onto prewarmed 24-well plates. After 15–20 min of Matrigel solidification, basal cell expansion medium (Advanced DMEM/F-12 + 1:100 GlutaMAX + 10 mM HEPES + 1.25 mM N-acetyl-l-cysteine (Sigma-Aldrich) + 1:50 B-27 Supplement (Gibco) + 1:100 N-2 Supplement (Gibco) + 50 ng ml−1 human EGF (Gibco) + 25 ng ml−1 human FGF10 (STEMCELL Technologies) + 25 ng ml−1 human Noggin (STEMCELL Technologies) + 10 vol% conditioned medium containing R-spondin 1 (produced from the HA-R-Spondin 1-Fc 293T cell line, Trevigen) + 500 nM A-83-01 (Selleck Chemicals) + 1:500 Primocin (InvivoGen) + 10 µM Y-27632 ROCK inhibitor (STEMCELL Technologies)) was added. (The ROCK inhibitor was used only during the first 2 days.) The medium was changed every 2–3 days. Organoids were split every 7–9 days by collecting them in 1 ml cold Advanced DMEM/F-12 and quick centrifugation (short acceleration to 5,000g, ~3–4 s), followed by removal of the supernatant containing dead cells and residual Matrigel and resuspension in 500 µl of TrypLE Express enzyme. After single-cell dissociation at 37 °C for 5 min, 500–1,000 µl of Advanced DMEM/F-12 was added and cells were collected by centrifugation at 300g for 4 min, followed by cell resuspension in 1,000 µl Advanced DMEM/F-12 and centrifugation at 300g for 4 min. Five thousand viable single cells were reseeded in 50 µl Matrigel droplets in 24-well plates. Expanding organoids with fewer than ten passages were used for the differentiation experiments or cryopreserved in CryoStor CS10 freezing medium. For differentiation at the ALI, 100,000 nasalORG or bronchialORG derived ECs were seeded in 200 µl basal cell expansion medium or PneumaCult-Ex Plus Medium supplemented with 10 µM Y-27632 onto PureCol-treated Transwell inserts (0.4 µm pore size, 24-well plate, Corning). The apical and basal chamber medium was replaced every 2–3 days until cells reached confluency, after which they were air-lifted by removing medium in the apical chamber and replacing the basal chamber medium with complete PneumaCult ALI medium (STEMCELL Technologies) + 1:500 Plasmocin. Cultures were differentiated at the ALI for 30–40 days with medium changes every 3–4 days and weekly apical PBS washes to remove mucus or dead cells. Alternatively, organoids were differentiated three-dimensionally by replacing the expansion medium with PneumaCult ALI medium supplemented with 10 µM DAPT (Sigma-Aldrich) at day 4 or 5 after initial expansion, for 15–20 days. To prevent loss of differentiating three-dimensional organoids because of attachment to the plastic dish, organoids were collected in Cell Recovery Solution (Corning) and left on ice for 30 min to remove Matrigel. Organoids were then reseeded into fresh Matrigel and overlayed with PneumaCult ALI medium supplemented with 10 µM DAPT. This step was necessary after a total incubation period of ~10 days.
To determine the optimal expansion medium for lung organoids containing bronchial or bronchiolar basal and alveolar AT2 progenitor cells, 25,000 dissociated viable lung cells were seeded per 50 µl Matrigel droplet, followed by the addition of lung basal medium (Advanced DMEM/F-12 with 1:100 GlutaMAX, 10 mM HEPES, 1.25 mM N-acetyl-l-cysteine, 1:50 B-27 Supplement, 1:100 N-2 Supplement, 50 ng ml−1 human EGF, 1:500 Primocin and 0.002% heparin (Sigma-Aldrich)) supplemented with 10 µM Y-27632 (until the first medium change after 3 days) and additional growth factors (shown in Extended Data Fig. 1c). These factors included 50 vol% conditioned medium containing human WNT3A (the l-Wnt3a cell line was a gift from H. Clevers), 10 vol% conditioned medium containing R-spondin 1, human FGF10 (25 ng ml−1), human Noggin (25 ng ml−1), CHIR9902 (3 µM, STEMCELL Technologies), SB431542 (10 µM, STEMCELL Technologies) and 1 µM BIRB796 (1 µM, STEMCELL Technologies). Lung bronchialORG were cultured in the basal cell expansion medium described for nasalORG and tracheaORG. To generate bat alveolar organoids (alvORG), mixed lung progenitor organoids (lungMIX-ORG) were established from 25,000 dissociated viable lung cells grown in 50 µl Matrigel droplets and alveolar expansion medium (lung basal medium supplemented with 25 ng ml−1 human FGF10 + 25 ng ml−1 human Noggin + 3 µM CHIR9902 + 10 µM SB-431542 (STEMCELL Technologies) + 1 µM BIRB796 + 10 µM Y-27632 for the first 3 days after passaging) and expanded at a ratio of 1:3 as described above for nasalORG, tracheaORG or bronchialORG for one to three passages. AT2 and non-AT2 progenitor cells (bronchiolar basal cells) were separated using FACS with LysoTracker Red DND-99 dye (Thermo Fisher Scientific) for specific staining of alveolar AT2 cells. To accomplish this, LysoTracker dye (1:10,000 dilution) was added to lungMIX-ORG cultures for 2 h, followed by single-cell dissociation using TrypLE Express enzyme at 37 °C and sorting of LysoTracker+ (AT2 cells for alvORG) and LysoTracker− (basal cells for bronchialORG) lung epithelial progenitor cells. Sorted cells were placed in basal cell expansion medium (LysoTracker− cells, bronchialORG) or complete alveolar expansion medium (LysoTracker+, alvORG), supplemented with 10 µM Y-27632 for the first 3 days. Lung bronchialORG were split using single-cell dissociation as described for nasalORG and tracheaORG AlvORG were passaged every 10–14 days by collecting them in cold Advanced DMEM/F-12, followed by resuspension in TrypLE Express and fragment dissociation for 3–5 min at room temperature. Fragments were collected in Advanced DMEM/F-12 and passaged at a split ratio of 1:4–1:8 and reseeded in 50 µl Matrigel droplets into new 24-well cell culture plates, followed by the addition of complete alveolar expansion medium.
Establishment and maintenance of bat SI organoids
Bat SI cells were obtained from frozen tissue as described above for airway cells. To establish SIORG, 25,000 cells were seeded in 50 µl Matrigel and overlayed with SI organoid expansion medium containing Advanced DMEM/F-12, 10 vol% conditioned medium containing R-spondin 1, 50 vol% conditioned medium containing human WNT3A (the l-Wnt3a cell line was a gift from H. Clevers), 1:100 GlutaMAX, 10 mM HEPES, 1 mM N-acetyl-l-cysteine, 1:50 B-27 Supplement, 50 ng ml−1 human EGF, 100 ng ml−1 human Noggin, 100 ng ml−1 human IGF1 (STEMCELL Technologies), 50 ng ml−1 human FGF2 (STEMCELL Technologies), 500 nM A 83-01, 1:500 Primocin and 10 µM Y-27632 (only for the first 2 days). A full medium change was done after 2–3 days. Passaging was done by collecting SIORG grown in Matrigel droplets in cold Advanced DMEM/F-12 and quick centrifugation (short acceleration to 5,000g, ~3–4 s). The supernatant containing dead cells and Matrigel was removed and the cell pellet resuspended in 500 µl of TrypLE Express. After 3–5-min TrypLE Express assisted fragmentation of SIORG at room temperature, 500 µl of cold Advanced DMEM/F-12 was added to the suspension and organoids were fragmented using manual pipetting (five to ten times up and down pipetting using a P1000 pipette). After quick centrifugal collection (short acceleration to 5,000g, ~3–4 s) of organoid fragments, the supernatant was removed and 1,000 µl Advanced DMEM/F-12 was added. To obtain the correct split ratio, an aliquot of the organoid fragment suspension was transferred to a new tube and cells were collected using quick centrifugation (short acceleration to 5,000g, ~3–4 s). Finally, organoid fragments were seeded in ice-cold Matrigel. We typically split SIORG once a week at a 1:6–1:12 split ratio.
Organoid differentiation was initiated by replacing the culture medium 3 days after passaging with differentiation medium containing Advanced DMEM/F-12, 10 vol% conditioned medium containing R-spondin 1, 1:100 GlutaMAX, 10 mM HEPES, 1 mM N-acetyl-l-cysteine, 1:50 B-27 Supplement, 50 ng ml−1 human EGF, 100 ng ml−1 human IGF1, 50 ng ml−1 human FGF2, 500 nM A 83-01 and 1:500 Primocin. SIORG differentiation was carried out for another 2–4 days before using the organoids for the experiments. Human SIORG were established from healthy duodenal biopsy specimens by isolating intestinal crypts (full ethical approval: REC-12/EE/0482). We received a vial of frozen organoids47 and cultured them alongside bat organoids in expansion or differentiation medium as detailed above.
Production of Egyptian fruit bat IFNλ
The coding sequences lacking the signal peptide of R. aegyptiacus IFNL1-like (LOC107521777) and IFNL3-like (LOC107521776 or LOC107520938) were obtained using RT–qPCR from RNA of poly(I:C)-stimulated (InvivoGen) bat nasal ECs. Stimulation was carried out by transfecting 30,000 cells with 100 ng of high-molecular-weight poly(I:C) in 10 µl Opti-MEM I (Gibco) + 0.15 µl Lipofectamine 3000 (Thermo Fisher Scientific) for 8 h before collecting RNA. The PCR fragments were cloned into a PiggyBac mammalian expression vector (cat. no. PB210PA-1, System Biosciences), modified to harbor a Secrecon-AA signal peptide48 for secretion at the N terminus and 6× His-tag at the C terminus. The expression vector was further modified to contain an internal ribosome entry site-puromycin selection cassette. A stable IFNλ-expressing cell line was obtained by transfecting one million Lenti-X cells (293T, Takara Bio) with 500 ng Super PiggyBac Transposase vector and 1,250 ng of PiggyBac Transposase mammalian expression vector using Lipofectamine 3000 according to the manufacturer’s instructions. Stable cells were selected with 1 µg ml−1 Puromycin and expanded for 2 weeks. For the collection of bat IFNλ, cells were seeded at high density (1.2 million cells per one well of a six-well plate) and incubated for 3 days. The supernatant was cleared using centrifugation, filtered through a 0.22-µm syringe filter and stored in aliquots at −70 °C. Biological activity was determined by adding diluted amounts of bat IFNλ supernatant to primary bat nasal ECs and measuring the induction of ISGs (IFIT1, OAS1, RTP4) using RT–qPCR, 8 h after stimulation. Universal IFNα2 (cat. no. 11200-1, PBL Assay Science) at 1,000 U ml−1 was used as the positive control; unmodified Lenti-X culture supernatant and ruxolitinib (1 µM, InvivoGen) inhibition were used as negative controls. For the stimulation experiments with bat IFNλ, we used bat IFNλ1-like or bat IFNλ3-like containing supernatants at amounts needed to reach IFIT1 mRNA induction, equivalent to induction with universal IFNα2 (~1:50–1:100 IFNλ dilution equivalent to 1,000 U ml−1 universal IFNα2).
IFN stimulation assays
Bat nasalORG, alvORG or SIORG, and human SIORG, were grown in expansion medium, collected in cold Advanced DMEM/F-12 using quick centrifugation (short acceleration to 5,000g, ~3–4 s), which was followed by resuspension of the organoid pellet in 500 µl TrypLE Express and incubation for 2 min at room temperature; this was enough to remove excess Matrigel and produce large organoid fragments. Equivalent amounts of Advanced DMEM/F-12 were then added and organoid fragments collected using quick centrifugation. The pellet containing the organoid fragments was resuspended in organoid expansion medium (bat alvORG without the mitogen-activated protein kinase inhibitor BIRB796) and seeded onto Matrigel-coated wells of a 96-well plate (1:50 dilution of Matrigel in cold Advanced DMEM/F-12 for 1 h at 37 °C). One 50-µl Matrigel droplet (one well of a 24-well plate) of a 7–10-day-old (3–4 days for SIORG) organoid culture was used per six wells of a 96-well plate and resuspended at 100 µl of medium per condition (600 µl of medium per Matrigel droplet containing organoids, approximately 300,000 cells or 50,000 cells per well in a 96-well plate). After 1–2 days after seeding, diluted amounts of recombinant bat IFNλ (dose equivalent to 1,000 U ml−1 of universal IFNα2), 1,000 U ml−1 of universal IFNα2 or 100 ng ml−1 of recombinant human IFNλ1 (PeproTech) were added for 8 h unless otherwise indicated. RNA was collected by removing culture medium and adding 200 µl of KingFisher RNA lysis buffer (Thermo Fisher Scientific, supplemented with 1 M dithiothreitol (DTT) (Roche)). Lysates were stored at −70 °C for up to 2 weeks before RNA extraction. To stimulate human bronchialALI, recombinant IFN was diluted in Gibco OptiPRO serum-free medium (100 ng ml−1 human IFNλ1 or 1,000 U ml−1 universal IFNα2) and added to the apical chamber of the culture insert for the indicated amount of time (8 h unless otherwise indicated). RNA was collected by removal of culture medium from the apical and basal chamber, followed by the addition of 400 µl DTT-supplemented KingFisher RNA lysis buffer and subsequently transferred to collection tubes for storage at −70 °C.
Viruses and viral infection
All experiments involving MERS-CoV and MARV infection were done in compliance with the Swedish Public Health Agency guidelines in the appropriate biosafety level 3 (MERS-CoV) and biosafety level 4 (MARV) laboratories. All experiments involving SeV (murine respirovirus), IAV subtype H1N1 or VSV-eGFP infections were performed in a biosafety level 2 laboratory in compliance with the health and biosafety committee of the Vienna BioCenter. Virus strains used were MARV Musoke strain (GenBank accession DQ217792), MERS-CoV (EMC/2012), IAV H1N1 (A/WS/33, VR-1520, ATCC), SeV (cat. no. NR-3227, BEI Resources) and VSV-eGFP (Indiana strain modified to express eGFP, gift from M. Hein). SINV encoding eGFP and harboring a mutation in the nsP2 gene (P726G) was constructed using PCR site-directed mutagenesis of the pBG218 rescue plasmid (Kerafast) encoding a replication-competent SINV genome. Virus rescue was carried out by transfecting 70% confluent BHK-21 cells (ATCC) with the pBG218-nsP2-P726G plasmid using Lipofectamine 3000 (120 ng per 1 × 106 cells) in standard D10A cell culture medium (DMEM + 10% FCS + 1× GlutaMAX + penicillin-streptomycin (100 U ml−1)). Four hours after transfection, the medium was changed to D5A (as D10A but with 5% FCS) and cells were incubated for 48 h before the supernatant was collected and titrated on Vero E6 cells using the 50% tissue culture infectious dose assay. Virus stocks were generated by one more round of passaging on Vero E6 (MOI = 0.1) cells before using for the infection experiments (passage two used for the experiments).
For the infection of bat nasalORG, alvORG or SIORG, organoid fragments were seeded on Matrigel-coated 96-well plates as described above for IFN stimulation for 2 days before infection. For infection, the medium was removed and replaced with absorption medium (Gibco OptiPRO serum-free medium, 1:100 GlutaMAX, 10 mM HEPES) containing virus at various doses (estimated MOI range = 0.1–1). After 2–5 h of absorption (1 h for SeV, SINV, H1N1 and VSV-eGFP), the inoculum was removed and replaced with fresh organoid growth medium (that is, differentiation medium for SI organoids or alveolar growth medium without BIRB796 mitogen-activated protein kinase inhibitor for alvORG). The culture medium was further supplemented with IFNs or inhibitors for the specific experimental variations used in the study (for example, IFN protection assays or JAK inhibition using ruxolitinib). RNA was collected at the indicated time points by removing the supernatant and adding TRIzol. TRIzol lysates were transferred into separate tubes and handled according to the respective biosafety protocols before storage at −70 °C and RNA extraction.
For 24-well Transwell-grown ALI cultures, the apical side was first washed once with PBS to remove excess mucus and dead cells. Infection was initiated by adding 100 µl of virus inoculum containing 100,000 PFU (MOI range estimate = 0.5–1) in virus absorption medium (OptiPRO serum-free medium, 1:100 GlutaMAX, 10 mM HEPES) to the apical chamber of the ALI cultures. After incubation for 5 h in a tissue culture incubator, the inoculum was removed and fresh ALI medium was added to the basal chamber. At the indicated time points after infection, the medium was removed and RNA was collected by adding TRIzol, and transferred to separate collection tubes for storage at −70 °C and RNA extraction.
CRISPR–Cas9 gene editing in bat organoids
Oligonucleotides containing guide RNA spacer sequences with overhangs were ordered (‘CAAC’ for the top oligonucleotides, ‘AAAC’ for the bottom oligonucleotides) and cloned into the lentiCRISPRv2 using Golden Gate cloning. In brief, top and bottom strand oligonucleotides (1 µl of 100 µM each) were phosphorylated with T4 polynucleotide kinase (New England Biolabs) for 30 min at 37 °C, denatured for 5 min at 95 °C and slowly cooled to 25 °C (0.1 °C per second ramp rate) for annealing. One microliter of 1:100 diluted product was used for Golden Gate cloning into lentiCRISPRv2 puro plasmid (plasmid no. 52961, Addgene) using 11 cycles of 5 min T4 ligase ligation at 16 °C and 5 min of BsmbI digestion at 37 °C, followed by a final digestion of 15 min at 37 °C. Two microliters of the reaction was transformed into Stbl3 Escherichia Coli cells and grown at 34 °C. After plasmid isolation, lentivirus was produced by transfecting 95% confluent Lenti-X cells grown in the wells of six-well plates with 1,000 ng psPAX2 (plasmid no. 12260, Addgene) packaging plasmid, 500 ng of pCMV-VSV-G (plasmid no. 8454, Addgene) and 1,000 ng of cloned lentiCRISPRv2 puro plasmid using Lipofectamine (5 µl P3000, 7 µl L3000). After 6 h, the medium was changed to virus production medium containing Opti-MEM I + 5% FCS + 1:100 GlutaMAX + 1:100 MEM-nonessential amino acids (Gibco) + 1:100 sodium pyruvate + 1:100 penicillin-streptomycin solution. Virus was collected 24 and 48 h after transfection, filtered through a 0.22-µm syringe filter and stored in aliquots at −70 °C. For editing, organoid fragments were prepared as described above for passaging, resuspended in 50 µl of undiluted lentivirus supernatant + 50 µl expansion medium + 5 µM cyclosporin A (Sigma-Aldrich) + 5 µg ml−1 Polybrene (Sigma-Aldrich) and spun at 800g for 1.5 h at 32 °C in a microcentrifuge. The supernatant was removed and organoid fragments were resuspended in 50 µl of cold Matrigel and seeded as droplets into 24-well plates. Expansion medium containing 5 µM cyclosporin A + 10 µM Y-27632 was added for 24 h, then replaced with standard expansion medium. Puromycin selection (1 µg ml−1) was started 60 h after transfection. Organoids were passaged two more times in puromycin selection medium before they were used in the experiments. Loss of function was determined by ISG induction using RT–qPCR (IRF9, IFNLR1 and IFNAR2) in response to IFN stimulation. Targeting of IFNE was confirmed using PCR amplification of the IFNE gene and Sanger sequencing of the amplicon.
RNA extraction
For RNA extraction involving noninfected material, a semiautomated KingFisher magnetic bead-assisted protocol was used, including DNase I (RNase-free, New England Biolabs) digestion to remove genomic DNA. For virus-inactivated samples in TRIzol, a magnetic bead-assisted semiautomated purification workflow was performed using Direct-zol-96 RNA Kits (Zymo Research), including DNase I digestion to remove genomic DNA. RNA was eluted in 50 µl nuclease-free water and stored at −70 °C for further use.
RT–qPCR and analysis
RT–qPCR on total RNA (100–500 ng) was performed with random hexamer/oligo(dT) primers containing LunaScript RT SuperMix (New England Biolabs) according to the manufacturer’s instructions. First-strand complementary DNA was diluted 1:6 in nuclease-free water. Two microliters were subsequently used in real-time qPCR reactions containing SYBR Green-based Luna universal dye qPCR mix (New England Biolabs) and gene-specific PCR primers, following the fast cycling protocol outlined in the manufacturer’s instructions. RT–qPCR primers were designed using the Integrated DNA Technologies PrimeQuest tool, using the NCBI transcript ID of interest and qPCR primer and intercalating dye as input options. For the data analysis, ΔCt values were first calculated by subtracting the Ct value measured for the reference gene EEF1A1 from the Ct value of a given gene measured from the same sample cDNA (for example, ΔCt for gene 1 in sample 1 was defined by calculating Ct gene 1 (sample 1) minus Ct EEF1A1 (sample 1)). Normalized relative expression values were then calculated by raising the negative ΔCt value of each sample to the power of two (2−ΔCt). This represents the relative expression value of a given gene in each sample in non-logarithmic space. Alternative normalization methods are described in the respective figure legends. Normalized relative expression values were used for visualization and statistical analysis in Prism (GraphPad Software). Primer pairs can be found in Supplementary Table 7.
Immunofluorescence staining
Organoids grown in Matrigel were collected in Corning Cell Recovery Solution and subsequently fixed in 4% paraformaldehyde (PFA) (Sigma-Aldrich) in PBS for 45 min to 2 h at room temperature, followed by three washes with PBS and storage in PBS at 2–8 °C. Organs were fixed by placing 3–5-mm tissue pieces in 4% PFA solution in PBS for 1 h at room temperature before transferring them to 2–8 °C for overnight fixation. Transwell insert membranes from ALI cultures were washed in PBS and fixed in 4% PFA for 45 min to 2 h at room temperature, followed by three washes with PBS and storage in PBS at 2–8 °C. Next, fixed samples were embedded in paraffin and sectioned at 2-µm thickness. Sample sections were dewaxed before a 30-min citrate buffer (10 mM sodium citrate, pH 6, 0.05% Tween-20) antigen removal step in a water simmer. After allowing sections to cool to room temperature, samples were blocked in PBS buffer containing 5% Donkey Serum (Sigma-Aldrich) (or 2% BSA) and 0.25% Triton X-100 (Sigma-Aldrich) for 30 min. Primary antibody dilutions in PBS containing 3% Donkey Serum (or 1% BSA) and 0.05% Triton X-100 were then added to the samples overnight at 2–8 °C in a humidified chamber. The following day, sections were washed three times in PBS before adding secondary Abs diluted in PBS containing 3% Donkey Serum (or 1% BSA) and 0.05% Triton X-100 or 2 h at room temperature in a humidified dark chamber. Sections were washed three times with PBS, DNA was counterstained with DAPI, and specimens were mounted in mounting medium. The following antibodies were used: anti-Keratin 5 (rabbit, 1:200 dilution, cat. no. SAB4501651, Sigma-Aldrich); anti- acetylated alpha Tubulin (clone 6-11B-1, 1:500 dilution, Santa Cruz Biotechnology); anti-AVIL (rabbit, 1:200 dilution, cat. no. PA5-90703, Thermo Fisher Scientific), anti-SFTPC (rabbit, 1:200 dilution, cat. no. PA5-71680, Thermo Fisher Scientific); anti-E-Cadherin (mouse, 1:200 dilution, cat. no. 610182, BD Biosciences); and anti-IFN-epsilon (mouse monoclonal, 1:200 dilution, cat. no. MAB9147-100, R&D Systems).
scRNA-seq library preparation and sequencing
Single-cell suspensions of bat tissue were obtained as detailed above for the generation of organoids. After red blood cell removal, cells were collected using centrifugation and viable cells were enriched using an annexin V magnetic bead dead cell removal step according to the manufacturer’s instructions. Recovered viable cells were pelleted using centrifugation, resuspended in Advanced DMEM/F-12 and counted. The single-cell suspension was submitted to an in-house facility to generate 3′ gene expression libraries using the 10x Genomics Chromium Single Cell 3′ Reagent Kit v4.
Single-cell suspensions of organoids were obtained by dissociating organoids or ALI membranes containing cells in TrypLE Express, supplemented with DNase I, for 15–30 min at 37 °C using manual pipetting at 5-min intervals. Cells were collected using centrifugation at 300g; viable cells were enriched using an annexin V magnetic bead dead cell removal step according to the manufacturer’s instructions. Recovered viable cells were pelleted using centrifugation at 300g, resuspended in Advanced DMEM/F-12 and counted. Single-cell suspensions from identical organoid formats, but different animals or samples, were pooled and submitted to an in-house facility to generate the 3′ gene expression libraries using the 10x Genomics Chromium Single Cell 3′ Reagent Kit v3. Sample pooling was performed to reduce batch variability and cost. For sequencing, a barnyard approach was chosen, where cells from individual samples were mixed and bioinformatically separated downstream using species (human versus bat) or single-nucleotide polymorphism information (different animals). Libraries were sequenced on Illumina instruments using a 150-bp pair-end sequencing mode aiming at 50,000 reads per cell. Individual FASTQ files for downstream analysis were generated by demultiplexing raw reads using Illumina sample indices.
scRNA-seq analysis
For R. aegyptiacus lung, trachea and SI libraries, sequencing reads from 10x Genomics libraries were processed using Cell Ranger (v.7.1). A custom genome index was created using cellranger mkref with the R. aegyptiacus reference genome (assembly mRouAeg1.p). Gene expression count matrices were generated using cellranger count. Next, filtered and de-noised count matrices were produced using CellBender49 (cellbender remove-background), with the following parameters: --expected-cells 20,000--total-droplets-included 30,000--fpr 0.01--epochs 150. Doublets were identified using Scrublet50 on the filtered gene expression matrix (expected doublet rate = 0.1). Downstream analysis was conducted in R using Seurat51,52 (v.4.2.1). Gene expression matrices in .h5 format were imported using Read10X_h5 to generate individual Seurat objects. Doublets identified by Scrublet were removed. Seurat objects were filtered to retain cells with more than 300 and fewer than 40,000 detected genes per cell; data were log-normalized (NormalizeData). Cell cycle scores (G2/M and S phase via CellCycleScoring) were computed and regressed out during scaling (ScaleData). The top 2,000 variable features per sample were identified (FindVariableFeatures), and integration anchors were calculated using FindIntegrationAnchors. Samples were integrated with Seurat canonical correlation analysis (CCA) using IntegrateData, followed by data scaling and principal component analysis (PCA) (RunPCA). The number of principal components for UMAP dimensionality reduction was selected using ElbowPlot. Cell clusters were identified using FindNeighbours and FindClusters (resolution = 0.5–1). Marker genes were identified with FindAllMarkers (parameters: min.pct = 0.25, log.fc = 0.25, only.pos = TRUE). Clusters were annotated based on known marker genes28,53,54 (clustering results in Supplementary Tables 1 and 4). A subset containing EC types was extracted to obtain ECs only. For bat lung and trachea, the clustering and annotation pipeline was repeated to generate a reference dataset. The SI epithelial dataset (SIT) was further integrated together with the bat SIORG (see below).
For the organoid libraries (bat and human nasalALI, bat and human bronchialALI, bat alvORG, and bat and human SIORG), sequencing reads were processed using Cell Ranger (v.7.1) with a custom reference index built from the R. aegyptiacus (mRouAeg1.p) and Homo sapiens (GRCh38.90) genomes, generated using cellranger mkref. For the bat alvORG samples, a custom index containing only the R. aegyptiacus (mRouAeg1.p) genome was used. CellBender remove-background was applied with the same parameters as described above for the tissue samples. Souporcell55 (souporcell_pipeline.py) was used to cluster cells according to single-nucleotide polymorphisms using aligned BAM files (possorted_genome_bam.bam) with the following parameters: -k X, -t 8,--skip_remap True,--ignore True. For downstream analysis, individual Seurat objects were created per 10x library (for example, human + bat SIORG, human + bat nasalALI, human + bat bronchialALI or bat alvORG). Meta-data from Cell Ranger and Souporcell were added; multiplets and unassigned cells were excluded. For downstream batch correction and sample integration, Seurat objects of individual donor samples (n = 3 for bats) were generated using the Souporcell meta-data and SplitObject; next, lists of individual Seurat objects were made. In total, three independent integration sets were analyzed: bat nasalALI + bronchialALI (three donors each, six samples); bat alvORG (three donors); and bat SIORG (two donors) + SIORG-DIFF + SIT (four samples). Each integration dataset was processed as follows: For each sample, cells with more than 500 detected genes were retained, followed by log-normalization (NormalizeData). Cell cycle scores were regressed out during data scaling. The top 2,000 variable genes were selected. Integration anchors were identified using FindIntegrationAnchors and samples were integrated with IntegrateData. PCA was performed and UMAP dimensions were chosen based on ElbowPlot (typically 1–30 principal components). Clusters were identified (FindNeighbours, FindClusters) and marker genes were computed (FindAllMarkers). Cell cluster annotation was performed manually using well-established marker genes for the mammalian airway and intestinal epithelium28,53,54 (clustering results in Supplementary Tables 2 and 4).
To refine rare cell types in bat airway organoids, GP2⁺ microfold cells were identified in the ciliated and microfold cluster based on nonzero expression of GP2. Ionocytes were annotated based on the expression of FOXI1, ASCL3 or PDE1C (only in nasalALI; bronchialALI counterparts lacked expression and were therefore reassigned as suprabasal and secretory cells). Neuroendocrine cells expressing CHGA, CHGB or SCG3 were detected in nasalALI only, while cells from bronchialALI in that cluster that did not were similarly reassigned to the suprabasal and secretory lineage. In the SIORG and SIT datasets, EECs identified by CHGA and CHGB expression were subset and reclustered according to the Seurat’s guided clustering vignette.
Human datasets (nasalALI, bronchialALI and SIORG) were analyzed separately according to the Seurat’s online guided clustering vignette; clusters were annotated based on established marker genes27,28,54 (clustering results in Supplementary Table 3).
To compare gene expression across different bat organoid models, Seurat objects were merged into a single object after count normalization using SCTransform. For cross-species comparisons between human and bat organoids, a list of 1:1 orthologs was generated; merged Seurat objects were filtered to retain orthologs only. This involved extracting raw counts using GetAssayData, filtering for orthologs, and reconstructing the Seurat object with the filtered count matrix and associated meta-data. Expression data were then normalized using SCTransform and cell identities were assigned based on species. Differentially expressed genes (DEGs) were identified using FindMarkers (ident.1 = bat, ident.2 = human, min.pct = 0.25, log.fc = 0.25). Plots were generated in Prism (v.9). ISG scores were calculated using Seurat’s AddModuleScore function, with a published list of conserved mammalian ISGs33 as input.
One external scRNA-seq dataset was used: small intestinal 10x tissue single-cell sequencing data were downloaded from the Gene Expression Omnibus (accession GSE185224)30. ECs from the ileum, duodenum and jejunum of three human donors each were included.
Pooled 3′-end bulk RNA-seq library preparation
Libraries for 3′-end RNA-seq were generated according to the pooled library amplification for transcriptome expression sequencing protocol (PLATEseq) with modifications56. Varying amounts of total RNA were mixed with 1 µl of 10 µM unique molecular identifier (UMI)-containing barcoded anchored oligo(dT) primers (5′ Adapter-BC(8)-UMI(12)-dT(35)VN-3′, consisting of a 5′ adapter sequence, an 8-nucleotide barcode (BC) for sample indexing, a 12-nucleotide unique molecular identifier (UMI) for molecule counting, followed by a 35-mer oligo(dT) stretch to anneal to the poly(A) tail, and a VN anchor to reduce non-specific priming; Supplementary Table 7), 250 nM deoxynucleoside triphosphate mix and heated at 72 °C for 3 min. A reverse transcription mix containing 1x first-strand buffer, 10 mM DTT, 0.15 µl murine RNase inhibitor (New England Biolabs) and 0.2 µl SuperScript III Reverse Transcriptase (Thermo Fisher Scientific) was added to get a total volume of 20 µl. For 48 samples, 20 ng of RNA were used for the reverse transcription reaction and scaled accordingly. Reverse transcription was performed at 50 °C for 30 min, followed by heat inactivation at 85 °C for 10 min. Then, 10 μl of barcoded reverse transcription reaction were pooled into a single tube; 1 µl per 50 µl pool volume of Thermolabile Exonuclease I (New England Biolabs) was then added and incubated for 4 min at 37 °C and 1 min at 80 °C. The cDNA from the pool was purified using in-house AMPure XP-like beads at a 1:1.4 sample-to-bead ratio and eluted in 50 µl nuclease-free water. Then 10 µl each of 1 M NaOH and 0.5 M EDTA, pH 8, were added to the purified sample and heated at 65 °C for 15 min to hydrolyze RNA in the RNA–cDNA hybrids. The reaction was purified using a Zymo Research Oligo Clean & Concentrator kit according to the manufacturer’s instructions for cDNA cleanup and eluted in 17 µl. A 23-µl reaction containing purified first-strand cDNA, 2.5 µl of Buffer 2 (New England Biolabs), 1 µl of 100 µM adapter-random hexamer primer and 200 nM deoxynucleoside triphosphate mix was heated to 95 °C for 1 min, followed by slow cooling to room temperature (0.1 °C s−1) in a PCR thermocycler. Second-strand synthesis was performed by adding 2 µl Klenow-Fragment DNA Polymerase (New England Biolabs) and incubating for 15 min at 30 °C. The reaction products were purified with a Zymo Research DNA Clean & Concentrator-5 Kit and eluted in 20 µl nuclease-free water. A final limited cycling PCR was performed using dual-indexing Illumina Primers (5 µM for each primer) and the Q5 NEBNext Ultra II Q5 Master Mix (New England Biolabs) for 10–14 cycles. The reaction was gel-purified, excising fragments between 200 and 900 bp and collected in 20 µl of elution buffer (10 mM TrisCl, pH 8). Libraries were sequenced on an Illumina NovaSeq S4 lane using a 150-bp pair-end mode.
Pooled 3′-end bulk RNA sequencing analysis
For pooled 3′-end bulk RNA-seq, FASTQ read files were processed using the BRB-seqTools57 pipeline (https://github.com/DeplanckeLab/BRB-seqTools). First, read 1 was trimmed to 25 nucleotides using Cutadapt. Read 2 was then aligned to either the R. aegyptiacus reference genome (assembly mRouAeg1.p) or the human reference genome (assembly GRCh38.90) using STAR with the following parameters: --runMode alignReads,--genomeDir /path/to/STAR_index,--outFilterMultimapNmax 1,--outSAMtype BAM Unsorted,--outFileNamePrefix /path/to/output_folder, and --readFilesIn /path/to/read2.fastq. UMI-based gene count matrices were generated using the BRBSeq-CreateDGEMatrix tool, which combines the aligned read 2 BAM file with the trimmed read 1 FASTQ file containing the UMI and sample barcode information. The command included input files for trimmed read 1, BAM alignment, the sample barcode sheet and the gene annotation file (GTF), with parameters specifying UMI length and the output directory. The resulting gene expression matrix was used for downstream differential gene expression analysis in R using the edgeR package58. Genes with low expression were excluded from the analysis, specifically those with less than one CPM in at least two of three replicates in a given sample group (for example, mock-treated, IFN-treated or virus-infected). Trimmed mean of M-values normalization (TMM) was applied to raw counts. A gene-wise negative binomial generalized linear model was then fitted to the data using the glmQLFit function, incorporating sample group information. DEGs were identified using glmQLFTest and filtered for significance based on an absolute log fold change greater than 0.25 and a P < 0.05. DEG tables were used for visualization and statistical analysis in Prism (v.9). GO enrichment analysis of upregulated genes was performed using the clusterProfiler59 package in R. Exclusive and overlapping sets of DEGs were visualized with eulerr (https://eulerr.co/). TMM-normalized CPM were calculated using the edgeR cpm function with TMM-normalized counts as input.
The bulk and scRNA-seq data analysis is fully described in the Methods. Interested researchers are encouraged to contact the corresponding author(s) for additional details.
Reanalyses of in vivo MARV Egyptian fruit bat infection data
Gene expression data from MARV-infected Egyptian fruit bats were reanalyzed by downloading the published nCounter-normalized dataset from ref. 7. A subset of the data was generated, focusing on the expression of ISGs and MARV genes in selected tissue samples, including the skin at the inoculation site, liver and colon.
Statistical analysis
The statistical tests used are described in the corresponding figure legends. Data collection and analysis were not performed blind to the experimental conditions. Data distribution was assumed to be normal, but this assumption was not formally tested.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41590-025-02155-1.
Supplementary information
scRNA-seq cluster information for R. aegyptiacus lung and tracheal samples
scRNA-seq cluster information for R. aegyptiacus nasal and bronchial ALI cultures or bat alveolar organoids.
scRNA-seq cluster information for human nasal and bronchial ALI cultures.
scRNA-seq cluster information for R. aegyptiacus small intestinal tissue and small intestinal organoids.
Differential gene expression analysis for bat and human organoids stimulated with type I or III interferon.
Differential gene expression analysis for bat and human organoids infected with virus
Primer sequences used in this study.
Acknowledgements
We thank all members of the Penninger laboratory, J. Zuber (Research Institute of Molecular Pathology, Vienna) and S. Knapp (Medical University of Vienna) for their support and critical feedback on the presented work. We also thank the Vienna Biocenter Core Facilities (VBCF) for providing excellent scientific infrastructure and services, especially the BioOptics facility, the Molecular Biology Service, the VBCF Histology Facility, the next-generation sequencing facility (T. Grentzinger for preparing the 10x scRNA-seq libraries) and bioinformatic support (M. Novatchkova for providing help regarding the Souporcell pipeline). The laboratory of J.M.P. received funding from the Austrian Academy of Sciences, the Medical University of Vienna, the Vienna Science and Technology Fund (no. 10.47379/EICOV20002), the Austrian Science Fund Wittgenstein award (no. Z 271-B19), the Swedish Research Council (no. 2018-05766), the Fundació La Marató de TV3 (no. 202125-31), the T. Von Zastrow Foundation, the Canada 150 Research Chairs Program (no. F18-01336), the Canadian Institutes of Health Research COVID-19 grant nos. F20-02343 and F20-02015, and the Innovative Medicines Initiative 2 Joint Undertaking under grant no. 101005026. This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations. We also gratefully acknowledge funding from the German Federal Ministry of Education and Research under the ‘Microbial Stargazing – Erforschung von Resilienzmechanismen von Mikroben und Menschen’ project (ref. 01KX2324).
Extended data
Author contributions
M.J.K. conceived the study. M.J.K. and J.M.P. designed the study. M.J.K. established bat organoids and performed characterization, BSL-2 virus infection and IFN stimulation experiments. V.M.M. performed MARV and MERS-CoV infection experiments under the supervision of A.M. P.Z. produced recombinant bat IFNs. J.J. prepared the scRNA-seq libraries from the bat tissue. M.J.K. performed the data analysis and visualization. G.P. and A.B-B. procured the bat tissue and isolated bat nasal ECs under the supervision of A.D. K.N. generated the human SI organoids under the supervision of M.Z. M.O. established human SI organoid cultures in the laboratory of J.M.P. and produced the R-spondin 1 and WNT3A conditioned media. A.B-B. maintains the Egyptian fruit bat colony at the Friedrich-Loeffler-Institut and oversees all animal experimentation. M.J.K. and J.M.P. wrote the manuscript with editing help from all other authors. J.M.P. and A.M. acquired the funding for the project.
Peer review
Peer review information
Nature Immunology thanks Ivan Zanoni, Jane Zhou, and Michael Gale Jr for their contribution to the peer review of this work. Ioana Staicu was the primary editor and managed its editorial process and peer review in collaboration with the rest of the Nature Immunology editorial team.
Funding
Open access funding provided by Helmholtz-Zentrum für Infektionsforschung GmbH (HZI).
Data availability
Raw and processed sequencing data are available at the Gene Expression Omnibus (GEO) under accession GSE291815. The scRNA-seq samples, including the Seurat objects presented in the study, can be accessed under the following accession numbers: bat and human SI organoids (GSM8842800); bat and human bronchial ALI cultures (GSM8842801); bat alveolar organoids (GSM8842802); bat and human nasal ALI cultures (GSM8842803); bat SI tissue (GSM8842804, GSM8842805, GSM8842806); bat lung tissue (GSM8842807, GSM8842808, GSM8842809, GSM8842810); bat trachea tissue (GSM8842811); pooled 3′-end bulk RNA-seq data, including demultiplexed raw UMI count matrices for bat SI organoids (GSM8842812); bat alveolar organoids (GSM8842813); bat nasal and intestinal organoids infected with MARV (GSM8842814); bat nasal organoids treated with IFN (GSM8842815); human SI organoids (GSM8842816); and human bronchial ALI cultures (GSM8842817). Reagents are available upon reasonable request and after signing a materials transfer agreement.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Max J. Kellner, Email: maxjosef.kellner@helmholtz-hzi.de
Josef M. Penninger, Email: josef.penninger@helmholtz-hzi.de
Extended data
is available for this paper at 10.1038/s41590-025-02155-1.
Supplementary information
The online version contains supplementary material available at 10.1038/s41590-025-02155-1.
References
- 1.Irving, A. T., Ahn, M., Goh, G., Anderson, D. E. & Wang, L.-F. Lessons from the host defences of bats, a unique viral reservoir. Nature589, 363–370 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Jebb, D. et al. Six reference-quality genomes reveal evolution of bat adaptations. Nature583, 578–584 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang, G. et al. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science339, 456–460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlovich, S. S. et al. The Egyptian rousette genome reveals unexpected features of bat antiviral immunity. Cell173, 1098–1110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morales, A. E. et al. Bat genomes illuminate adaptations to viral tolerance and disease resistance. Nature638, 449–458 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, L.-F., Gamage, A. M., Chan, W. O. Y., Hiller, M. & Teeling, E. C. Decoding bat immunity: the need for a coordinated research approach. Nat. Rev. Immunol.21, 269–271 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guito, J. C. et al. Asymptomatic infection of Marburg virus reservoir bats is explained by a strategy of immunoprotective disease tolerance. Curr. Biol.31, 257–270 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Forero, A. et al. Differential activation of the transcription factor IRF1 underlies the distinct immune responses elicited by type I and Type III interferons. Immunity51, 451–464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gamage, A. M. et al. Single-cell transcriptome analysis of the in vivo response to viral infection in the cave nectar bat Eonycteris spelaea. Immunity55, 2187–2205 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Whitsett, J. A. & Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol.16, 27–35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuh, A. J. et al. Natural reservoir Rousettus aegyptiacus bat host model of orthonairovirus infection identifies potential zoonotic spillover mechanisms. Sci. Rep.12, 20936 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halwe, N. J. et al. Egyptian fruit bats (Rousettus aegyptiacus) were resistant to experimental inoculation with avian-origin influenza A virus of subtype H9N2, but are susceptible to experimental infection with bat-borne H9N2 virus. Viruses13, 672 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amman, B. R. et al. Experimental infection of Egyptian rousette bats (Rousettus aegyptiacus) with Sosuga virus demonstrates potential transmission routes for a bat-borne human pathogenic paramyxovirus. PLoS Negl. Trop. Dis.14, e0008092 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt, R. J. & Lloyd, C. M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol.21, 347–362 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs, N. et al. Long-term expanding human airway organoids for disease modeling. EMBO J.38, e100300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konishi, S., Tata, A. & Tata, P. R. Defined conditions for long-term expansion of murine and human alveolar epithelial stem cells in three-dimensional cultures. STAR Protoc.3, 101447 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Velden, J. L., Bertoncello, I. & McQualter, J. L. LysoTracker is a marker of differentiated alveolar type II cells. Respir. Res.14, 123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker, M. B., Zülch, A., Bosse, A. & Gruss, P. Irx1 and Irx2 expression in early lung development. Mech. Dev.106, 155–158 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Oliver, G. et al. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development121, 4045–4055 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Ualiyeva, S. et al. Tuft cell-produced cysteinyl leukotrienes and IL-25 synergistically initiate lung type 2 inflammation. Science6, eabj0474 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kazer, S. W. et al. Primary nasal influenza infection rewires tissue-scale memory response dynamics. Immunity57, 1955–1974 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deprez, M. et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med.202, 1636–1645 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Kanoh, S., Tanabe, T. & Rubin, B. K. IL-13-induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin. Exp. Allergy41, 1747–1756 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Chan, L. L. Y. et al. Generation of self-replicating airway organoids from the cave nectar bat Eonycteris spelaea as a model system for studying host–pathogen interactions in the bat airway epithelium. Emerg. Microbes Infect.12, e2148561 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabbott, N. A., Donaldson, D. S., Ohno, H., Williams, I. R. & Mahajan, A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol.6, 666–677 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surve, M. V. et al. Single-cell transcriptomes, lineage, and differentiation of functional airway microfold cells. Am. J. Respir. Cell Mol. Biol.69, 698–701 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujii, M. et al. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell23, 787–793 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Elmentaite, R. et al. Cells of the human intestinal tract mapped across space and time. Nature597, 250–255 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beumer, J. et al. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell181, 1291–1306 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Burclaff, J. et al. A proximal-to-distal survey of healthy adult human small intestine and colon epithelium by single-cell transcriptomics. Cell. Mol. Gastroenterol. Hepatol.13, 1554–1589 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung, K. Y. et al. Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science339, 1088–1092 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaprakash, A. D. et al. Marburg and Ebola virus infections elicit a complex, muted inflammatory state in bats. Viruses15, 350 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw, A. E. et al. Fundamental properties of the mammalian innate immune system revealed by multispecies comparison of type I interferon responses. PLoS Biol.15, e2004086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuomo-Dannenburg, G. et al. Marburg virus disease outbreaks, mathematical models, and disease parameters: a systematic review. Lancet Infect. Dis.24, e307–e317 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuh, A. J. et al. Modelling filovirus maintenance in nature by experimental transmission of Marburg virus between Egyptian rousette bats. Nat. Commun.8, 14446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazear, H. M., Schoggins, J. W. & Diamond, M. S. Shared and distinct functions of type I and type III interferons. Immunity50, 907–923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrichs, V., Balkema-Buschmann, A., Dorhoi, A. & Pei, G. Selection and stability validation of reference gene candidates for transcriptional analysis in Rousettus aegyptiacus. Sci. Rep.11, 21662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frolova, E. I. et al. Roles of nonstructural protein nsP2 and Alpha/Beta interferons in determining the outcome of Sindbis virus infection. J. Virol.76, 11254–11264 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guito, J. C. et al. Coordinated inflammatory responses dictate Marburg virus control by reservoir bats. Nat. Commun.15, 1826 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, K. L. et al. Temporal characterization of Marburg virus Angola infection following aerosol challenge in rhesus macaques. J. Virol.89, 9875–9885 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messaoudi, I., Amarasinghe, G. K. & Basler, C. F. Filovirus pathogenesis and immune evasion: insights from Ebola virus and Marburg virus. Nat. Rev. Microbiol.13, 663–676 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valmas, C. et al. Marburg virus evades interferon responses by a mechanism distinct from Ebola virus. PLoS Pathog.6, e1000721 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edwards, M. R. et al. Differential regulation of interferon responses by Ebola and Marburg virus VP35 proteins. Cell Rep.14, 1632–1640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irving, A. T. et al. Interferon regulatory factors IRF1 and IRF7 directly regulate gene expression in bats in response to viral infection. Cell Rep.33, 108345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He, G.-W. et al. Optimized human intestinal organoid model reveals interleukin-22-dependency of Paneth cell formation. Cell Stem Cell29, 1333–1345 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold, C. E. et al. Transcriptomics reveal antiviral gene induction in the Egyptian rousette bat is antagonized in vitro by Marburg virus infection. Viruses10, 607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraiczy, J. et al. DNA methylation defines regional identity of human intestinal epithelial organoids and undergoes dynamic changes during development. Gut68, 49–61 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Güler-Gane, G. et al. Overcoming the refractory expression of secreted recombinant proteins in mammalian cells through modification of the signal peptide and adjacent amino acids. PLoS ONE11, e0155340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleming, S. J. et al. Unsupervised removal of systematic background noise from droplet-based single-cell experiments using CellBender. Nat. Methods20, 1323–1335 (2023). [DOI] [PubMed] [Google Scholar]
- 50.Wolock, S. L., Lopez, R. & Klein, A. M. Scrublet: computational identification of cell doublets in single-cell transcriptomic data. Cell Syst.8, 281–291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuart, T. et al. Comprehensive integration of single-cell data. Cell177, 1888–1902 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol.36, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levinger, R. et al. Single-cell and spatial transcriptomics illuminate bat immunity and barrier tissue evolution. Mol. Biol. Evol.42, msaf017 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sikkema, L. et al. An integrated cell atlas of the lung in health and disease. Nat. Med.29, 1563–1577 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heaton, H. et al. Souporcell: robust clustering of single-cell RNA-seq data by genotype without reference genotypes. Nat. Methods17, 615–620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bush, E. C. et al. PLATE-Seq for genome-wide regulatory network analysis of high-throughput screens. Nat. Commun.8, 105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alpern, D. et al. BRB-seq: ultra-affordable high-throughput transcriptomics enabled by bulk RNA barcoding and sequencing. Genome Biol.20, 71 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
scRNA-seq cluster information for R. aegyptiacus lung and tracheal samples
scRNA-seq cluster information for R. aegyptiacus nasal and bronchial ALI cultures or bat alveolar organoids.
scRNA-seq cluster information for human nasal and bronchial ALI cultures.
scRNA-seq cluster information for R. aegyptiacus small intestinal tissue and small intestinal organoids.
Differential gene expression analysis for bat and human organoids stimulated with type I or III interferon.
Differential gene expression analysis for bat and human organoids infected with virus
Primer sequences used in this study.
Data Availability Statement
Raw and processed sequencing data are available at the Gene Expression Omnibus (GEO) under accession GSE291815. The scRNA-seq samples, including the Seurat objects presented in the study, can be accessed under the following accession numbers: bat and human SI organoids (GSM8842800); bat and human bronchial ALI cultures (GSM8842801); bat alveolar organoids (GSM8842802); bat and human nasal ALI cultures (GSM8842803); bat SI tissue (GSM8842804, GSM8842805, GSM8842806); bat lung tissue (GSM8842807, GSM8842808, GSM8842809, GSM8842810); bat trachea tissue (GSM8842811); pooled 3′-end bulk RNA-seq data, including demultiplexed raw UMI count matrices for bat SI organoids (GSM8842812); bat alveolar organoids (GSM8842813); bat nasal and intestinal organoids infected with MARV (GSM8842814); bat nasal organoids treated with IFN (GSM8842815); human SI organoids (GSM8842816); and human bronchial ALI cultures (GSM8842817). Reagents are available upon reasonable request and after signing a materials transfer agreement.