Abstract
Two dogs with patent ductus arteriosus and severe pulmonary hypertension were presented to our veterinary teaching hospital. In both dogs, sildenafil was initiated to treat pulmonary hypertension, and surgery was supposed to be scheduled after dose titration. Although no obvious improvement in pulmonary hypertension was observed, no dog had polycythaemia or an increased haematocrit level. The dogs underwent ductal occlusion, and treatment with beraprost sodium was subsequently initiated. Thereafter, severe pulmonary hypertension dramatically improved. No dog showed any clinical sign of right heart failure or adverse drug reaction postoperatively, thereby demonstrating the successful treatment of two dogs with patent ductus arteriosus and severe pulmonary hypertension, using sildenafil and beraprost sodium.
Keywords: bidirectional shunt, ductus arteriosus, ductus closure, polycythaemia, right heart failure
Patent ductus arteriosus (PDA) is a common congenital heart disease in dogs [18]. Pulmonary hypertension (PH) rarely occurs in dogs with PDA [13]. Increased endothelial shear stress owing to augmented pulmonary blood flow can result in reactive vasoconstriction, progressive medial hypertrophy, and intimal proliferation of the pulmonary vasculature. The shunt eventually proceeds bidirectionally or from the pulmonary artery to the aorta (right-to-left) in patients with severe PH [3]. In cases with large shunt blood flow from the pulmonary artery to the aorta, polycythaemia occurs to compensate hypoxaemia. In such cases, if the ductus is occluded, severe PH can cause acute right-sided heart failure [17]. This is because complete attenuation of the shunt prevents right ventricular blood from entering the systemic circulation, raising the right ventricular afterload and increasing the risk of shock and possible death [17]. Therefore, in such cases, shunt closure is not usually recommended, and conservative treatment is preferred [17].
In a bidirectional or right-to-left shunt with polycythaemia, PH and polycythaemia treatments are needed. Sildenafil is the most common drug for treating PH [12]. Beraprost sodium is also used for PH in human medicine [23], although there are limited reports of its use in veterinary medicine. Treatment for polycythaemia involves phlebotomy or medication comprising hydroxyurea; however, these treatments may be poorly tolerated by animals and can affect their quality of life, resulting in poor prognosis [6].
There are few reports on dogs with PDA and severe PH, and even fewer reports on ductal occlusion [6, 19, 24, 25]. However, no medical treatment has been established for cases with PH without any clinical sign of polycythaemia. Herein, we describe the use of sildenafil and beraprost sodium in the successful treatment of ductus closure in two dogs with PDA and severe PH, without any clinical sign of polycythaemia.
Case 1: A 2-year-old mixed-breed dog weighing 1.7 kg was presented to the Okayama University of Science Veterinary Teaching Hospital for evaluation of exercise intolerance and tachypnoea (heart rate: 150 beats/min, rectal temperature: 37.2 C, respiratory rate: 40 breaths/min, and systolic blood pressure: 155 mmHg). Grade II/VI diastolic murmur at the heart base, mild effort respiration, and jugular vein distension were observed on physical examination. Complete blood counts and serum biochemical analyses were within normal reference ranges. Thoracic radiography revealed severe pleural effusion (Fig. 2A and 2B). Echocardiography revealed severe enlargement of the right atrium and ventricle, ventricular septal flattening, and enlargement of the main pulmonary artery (Fig. 3A–C). An enlarged ductus arteriosus (DA) was observed, and bidirectional blood flow was detected through the DA (Fig. 1A and 1B). The tricuspid valve had severe insufficiency (peak velocity: 5.5 m/sec). Abdominal ultrasonography revealed a dilated hepatic vein and mild ascites. Based on these findings, the dog was diagnosed with a bidirectional shunting PDA, severe PH, and secondary right-sided congestive heart failure. Although the owner requested surgery, treatment with sildenafil (1.0 mg/kg PO q8 hr) and furosemide (1.5 mg/kg PO q12 hr) was initiated to improve PH and right heart failure, as surgery may have posed a high risk. Three days after the initial presentation, pleural effusion and ascites resolved. The medication was continued for 1 month. One month after the initial presentation, the peak velocity of the tricuspid valve insufficiency was 5.8 m/sec, although the pleural effusion and ascites had improved. The sildenafil dosage was gradually increased to 3.0 mg/kg PO q8 hr for surgery [16]. At the 2-month follow-up, the systolic blood pressure was 140 mmHg, and the peak velocity of the tricuspid valve insufficiency was 5.5 m/sec; however, the dog showed no signs of polycythaemia, an increased haematocrit level, or right heart failure. Bidirectional blood flow through the ductus persisted. Due to concerns about the prospect for additional recovery, multiple phlebotomies, and medication side effects, the owner elected to pursue surgery despite the risks.
Fig. 2.
Thoracic radiography (Case 1). Pleural effusion was suspected during the first examination (A and B). Three days postoperatively, no obvious changes were observed in the cardiac silhouette (C and D). One month postoperatively, enlargement of the right heart and bulging of the main pulmonary artery had improved (E and F).
Fig. 3.
Echocardiography (Case 1). During the first examination, enlargement of the right atrium and ventricle and ventricular septal flattening were observed (A, B, and C). Three days postoperatively, complete occlusion was confirmed, although enlargement of the right atrium and ventricle and ventricular septal flattening persisted (D, E, and F). Both findings had improved 1 month postoperatively (G, H, and I).
Fig. 1.
Bidirectional shunt (Cases 1 and 2). In case 1, an enlarged ductus arteriosus was observed, and bidirectional blood flow was detected through the ductus arteriosus (A, B, and C). In case 2, an enlarged ductus arteriosus was observed, and trivial blood flow was detected through the ductus arteriosus (D, E, and F).
Transarterial coil embolisation of the PDA was performed. The patient was premedicated with midazolam (0.3 mg/kg IV) and fentanyl (5 µg/kg IV), induced with ketamine (5.0 mg/kg IV) and alfaxalone (1.4 mg/kg IV), and maintained on sevoflurane. Additionally, the patient received a continuous-rate infusion (CRI) of fentanyl, CRI of dopamine, and intravenous atropine. A cutdown was used to access the right femoral artery, and a catheter was inserted. The invasive blood pressures in the aorta and pulmonary artery were 122/64/88 mmHg and 88/50/65 mmHg (systolic/diastolic/mean blood pressure), respectively. On angiography, the pulmonary ostium of the DA measured 2.5 mm, and the ductal ampulla measured 5.6 mm, with type IIA morphology, according to the Miller classification system [11]. An MReye Flipper Detachable Embolisation Coil with 5-mm diameter and 5 loops was implanted, and immediate complete occlusion of the DA was achieved under intraoperative angiography. Postoperatively, treatment with beraprost sodium (15 µg/kg PO q12 hr) was initiated.
Three days postoperatively, complete occlusion was confirmed, although enlargement of the right atrium and ventricle and ventricular septal flattening persisted, and the peak velocity of the tricuspid valve insufficiency was 5.0 m/sec (Figs. 2C and 2D, and 3D, 3E, and 3F). One month postoperatively, the enlargement of the right atrium and ventricle and ventricular septal flattening had improved, and no pleural effusion was observed (Figs. 2E and 1F, and 3G, 3H, and 3I). Also, tricuspid valve insufficiency had disappeared. The furosemide dosage was gradually decreased, whereas those of sildenafil (3 mg/kg PO q8 hr) and beraprost sodium (15 µg/kg PO q12 hr) were maintained. Twenty months postoperatively and after initiating beraprost sodium, the dog had gained weight (2.2 kg), showed no clinical signs of right heart failure and no adverse drug reaction, and exhibited improved PH.
Case 2: A 3-month-old Pembroke Welsh Corgi weighing 5.0 kg was presented to the veterinary teaching hospital with a cardiac enlargement (heart rate: 140 beats/min, rectal temperature: 38.7°C, respiratory rate: 42 breaths/min, and systolic blood pressure: 110 mm Hg). No cardiac murmur at the heart base was observed. Complete blood counts and serum biochemical analyses were within normal reference ranges. Thoracic radiography revealed pulmonary artery dilation (Fig. 4A and 4B). Echocardiography revealed enlargement of the right atrium and ventricle, ventricular septal flattening, and enlargement of the main pulmonary artery (Fig. 4A–C). An enlarged DA was observed, and trivial blood flow was detected through the DA during diastole (Fig. 1C and 1D). The tricuspid valve had severe insufficiency (peak velocity: 5.4 m/sec). Contrast echocardiography revealed no evidence of an intracardiac shunt; however, within seconds of peripheral venous injection of agitated saline, microbubbles were observed in the abdominal portion of the aorta. Based on these findings, the dog was diagnosed with bidirectional shunting through the PDA. The owner wanted surgery; however, surgery could have been high risk. Therefore, treatment with sildenafil (1.0 mg/kg PO q12 hr) was first initiated to slightly improve PH and decrease the risk of right heart failure before and after surgery, after which surgery was reconsidered. Sildenafil dosage was gradually increased to 3.0 mg/kg PO q12 hr for surgery [16]. Three months after the initial presentation, the systolic blood pressure was 105 mmHg, and the peak velocity of the tricuspid valve insufficiency was 4.7 m/sec. Blood flow through the ductus remained bidirectional. The dog showed no signs of polycythaemia or an increased haematocrit level. Due to concerns such as the prospect for additional recovery, multiple phlebotomies, and medication side effects, the owner elected to pursue surgery despite the risks.
Fig. 4.
Thoracic radiography (Case 2). During the first examination, enlargement of the right heart and bulging of the main pulmonary artery were observed (A and B). Three days postoperatively, no obvious changes were observed in the cardiac silhouette (C and D). These findings had improved 3 months postoperatively (E and F).
Three months after the initial diagnosis, transarterial embolisation of the PDA was performed by implanting an Amplatz Canine Duct Occluder (ACDO). The patient was premedicated with fentanyl (5 µg/kg IV), induced using propofol (6 mg/kg IV), and maintained on propofol. Additionally, the patient received a CRI of fentanyl, CRI of dopamine, and intravenous phenylephrine. A surgical cutdown was used to access the right femoral artery. The invasive blood pressures in the aorta and pulmonary artery were 104/65/78 mmHg and 100/60/74 mmHg, respectively. On angiography, the pulmonary ostium of the DA measured 4.5 mm, and the ductal ampulla measured 8.8 mm, with type IIA morphology [11]. An ACDO with a waist diameter of 7 mm was implanted according to the manufacturer’s recommendations. Postoperatively, treatment with beraprost sodium (15 µg/kg PO q12 hr) was initiated.
Three days postoperatively, complete occlusion was confirmed, although enlargement of the right atrium and ventricle and septal flattening persisted (Figs. 4C and 4D, and 5D, 5E, and 5F). The peak velocity of the tricuspid valve insufficiency was 4.9 m/sec. Three months postoperatively, the right atrial and ventricular enlargement and septal flattening had improved (Figs. 4E and 4F, and 5G, 5H, and 5I). Also, tricuspid valve insufficiency had disappeared. Eight months postoperatively and after initiating beraprost sodium, the dog had no clinical signs of right heart failure, no adverse drug reaction, and showed improvement in PH.
Fig. 5.
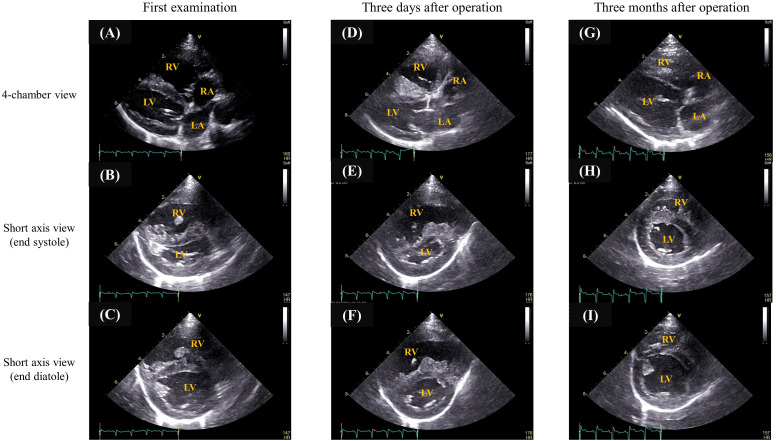
Echocardiography (Case 2). During the first examination, enlargement of the right atrium and ventricle and severe ventricular septal flattening were observed (A, B, and C). Three days postoperatively, complete occlusion was confirmed, although enlargement of the right atrium and ventricle and ventricular septal flattening persisted (D, E, and F). Both findings had improved 3 months postoperatively (G, H, and I).
The best time for surgery is when patients with PDA have a left-to-right shunt. Although there are some reports on ductus closure in canine PDA with PH [6, 8, 25], closure is generally not recommended in PDA patients with severe PH ductus. However, in our patients, PH dramatically improved after surgery. No dog had polycythaemia and an increased haematocrit level before surgery, sildenafil was initiated and increased preoperatively, and beraprost sodium was initiated postoperatively; however, the effective factor remains unclear. We postulated several possible explanations. The most likely reason is the postoperative administration of beraprost sodium. Although beraprost sodium is a chemically stable prostaglandin I2 analogue used to treat PH in humans [5], no previous study has reported its use for the treatment of PDA with severe PH. It protects vascular endothelial cells, inhibits inflammatory cytokine production, and has antiplatelet effects [9, 20]. In vivo, beraprost sodium reportedly relieved hypoxia by inducing neovascularisation and reducing thrombosis [2]. In canine models of chronic PH, beraprost sodium decreases pulmonary vascular impedance and improves PH but decreases systemic vascular impedance more than pulmonary vascular impedance, depending on the dose [21]. It has been reported that sildenafil decreases pulmonary artery pressure and vascular resistance without notable changes in systemic arterial pressure or systemic vascular resistance [1, 10]. Both cases showed no signs of polycythaemia or an increased haematocrit level, indicating that the shunt blood flow from the pulmonary artery to the aorta may have been minimal, despite severe PH and bidirectional blood flow through the ductus. Since these changes due to treatment may have led to the deterioration of the right-to-left shunt, sildenafil treatment was initiated and gradually increased preoperatively to slightly improve PH and decrease the risk of right heart failure before and after surgery, while beraprost sodium treatment was initiated after ductal occlusion in our patients. Although few reports have examined the effects of beraprost sodium in dogs with severe PH [22], both cases had no adverse drug reactions (e.g., shock, haemostatic abnormality, and hypotension [4]) and the drug may be considered effective. While we believe that PDA caused irreversible PH in these patients, other possibilities include a reactive PH. In patients with PDA, blood flow through the DA increases the pulmonary artery pressure, which damages smaller blood vessels in the lungs, resulting in PH [15]. PH of Grades 1–3 are potentially reversible; however, Grades 4–6 are usually irreversible [7]. Pulmonary disease and idiopathic factors also affect PH. Since lung biopsies were not performed in these patients, the degree of damage to the lung vessels was unclear, and the true grades also remained unclear.
Here, we describe the successful treatment of two dogs with PDA and severe PH. Both dogs had a bidirectional shunt, but showed no signs of polycythaemia, an increased haematocrit level or right heart failure preoperatively. PH improved dramatically with ductus occlusion as well as sildenafil and beraprost sodium treatment, and no dog showed any clinical sign of right heart failure or adverse drug reaction postoperatively. Although the postoperative administration of beraprost sodium might be effective, further research involving larger sample sizes and action mechanism of beraprost sodium are warranted to evaluate treatment methods in dogs with PDA and severe PH. Additionally, beraprost sodium was initiated after PDA occlusion in our cases. Long-term administration of beraprost sodium has been reported to ameliorate the degree of pulmonary arterial hypertension in a human patient with PDA, ventricular septal defect, atrial septal defect, and severe PH [14]. Further research is needed to investigate the start time of beraprost sodium. In both cases, sildenafil was initiated for PH treatment, but PH showed no improvement. Both cases had severe PH but showed no signs of polycythaemia or an increased haematocrit level preoperatively. The best time and condition for surgery should also be reconsidered.
CONFLICTS OF INTEREST
No conflicts of interest have been declared.
REFERENCES
- 1.Akabane R, Sakatani A, Ogawa M, Nagakawa M, Miyakawa H, Miyagawa Y, Takemura N. 2020. The effect of sildenafil on pulmonary haemodynamics in a canine model of chronic embolic pulmonary hypertension. Res Vet Sci 133: 106–110. doi: 10.1016/j.rvsc.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 2.Atsuta H, Uchiyama T, Kanai H, Iso T, Tanaka T, Suga T, Maeno T, Arai M, Nagai R, Kurabayashi M. 2009. Effects of a stable prostacyclin analogue beraprost sodium on VEGF and PAI-1 gene expression in vascular smooth muscle cells. Int J Cardiol 132: 411–418. doi: 10.1016/j.ijcard.2007.12.119 [DOI] [PubMed] [Google Scholar]
- 3.de Campos FPF, Benvenuti LA. 2017. Eisenmenger syndrome. Autops Case Rep 7: 5–7. doi: 10.4322/acr.2017.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox LE. 2007. 17−Laryngeal tumors. p. 173–175. In: Canine Internal Medicine Secrets (Rubin SI, Carr AP edrs.), Mosby St. Louis. [Google Scholar]
- 5.Fukuda K, Date H, Doi S, Fukumoto Y, Fukushima N, Hatano M, Ito H, Kuwana M, Matsubara H, Momomura SI, Nishimura M, Ogino H, Satoh T, Shimokawa H, Yamauchi-Takihara K, Tatsumi K, Ishibashi-Ueda H, Yamada N, Yoshida S, Abe K, Ogawa A, Ogo T, Kasai T, Kataoka M, Kawakami T, Kogaki S, Nakamura M, Nakayama T, Nishizaki M, Sugimura K, Tanabe N, Tsujino I, Yao A, Akasaka T, Ando M, Kimura T, Kuriyama T, Nakanishi N, Nakanishi T, Tsutsui H. Japanese Circulation Society and the Japanese Pulmonary Circulation and Pulmonary Hypertension Society Joint Working Group. 2019. Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017). Circ J 83: 842–945. doi: 10.1253/circj.CJ-66-0158 [DOI] [PubMed] [Google Scholar]
- 6.Greet V, Bode EF, Dukes-McEwan J, Oliveira P, Connolly DJ, Sargent J. 2021. Clinical features and outcome of dogs and cats with bidirectional and continuous right-to-left shunting patent ductus arteriosus. J Vet Intern Med 35: 780–788. doi: 10.1111/jvim.16072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath D, Edwards JE. 1958. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 18: 533–547. doi: 10.1161/01.CIR.18.4.533 [DOI] [PubMed] [Google Scholar]
- 8.Iizuka T, Hoshi K, Ohmaki M, Sakata I. 2010. Surgical ligation of patent ductus arterisus suspected severe pulmonary hypertension in a dog. Abv Anim Cardiol 42: 43–48. [Google Scholar]
- 9.Kurihara J, Sahara T, Kato H. 1990. Protective effect of beraprost sodium, a new chemically stable prostacyclin analogue, against the deterioration of baroreceptor reflex following transient global cerebral ischaemia in dogs. Br J Pharmacol 99: 91–96. doi: 10.1111/j.1476-5381.1990.tb14659.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michelakis ED. 2003. The role of the NO axis and its therapeutic implications in pulmonary arterial hypertension. Heart Fail Rev 8: 5–21. doi: 10.1023/A:1022150819223 [DOI] [PubMed] [Google Scholar]
- 11.Miller MW, Gordon SG, Saunders AB, Arsenault WG, Meurs KM, Lehmkuhl LB, Bonagura JD, Fox PR. 2006. Angiographic classification of patent ductus arteriosus morphology in the dog. J Vet Cardiol 8: 109–114. doi: 10.1016/j.jvc.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Yamasaki M, Ohta H, Sasaki N, Murakami M, Bandula Kumara WR, Takiguchi M. 2011. Effects of sildenafil citrate on five dogs with Eisenmenger’s syndrome. J Small Anim Pract 52: 595–598. doi: 10.1111/j.1748-5827.2011.01127.x [DOI] [PubMed] [Google Scholar]
- 13.Oswald GP, Orton EC. 1993. Patent ductus arteriosus and pulmonary hypertension in related Pembroke Welsh corgis. J Am Vet Med Assoc 202: 761–764. doi: 10.2460/javma.1993.202.05.761 [DOI] [PubMed] [Google Scholar]
- 14.Oyamada J, Toyono M, Shimada S, Aoki-Okazaki M, Tamura M, Takahashi T. 2009. Long-term administration of beraprost sodium for pulmonary arterial hypertension associated with congenital heart disease. Intern Med 48: 1531–1534. doi: 10.2169/internalmedicine.48.2251 [DOI] [PubMed] [Google Scholar]
- 15.Philip R, Nathaniel Johnson J, Naik R, Kimura D, Boston U, Chilakala S, Hendrickson B, Rush Waller B, Sathanandam S. 2019. Effect of patent ductus arteriosus on pulmonary vascular disease. Congenit Heart Dis 14: 37–41. doi: 10.1111/chd.12702 [DOI] [PubMed] [Google Scholar]
- 16.Plumb DC. 2018. Plumb’s Veterinary Drug Handbook. Ninth ed., Pharma Vet Inc., Stockholm. [Google Scholar]
- 17.Pyle RL, Park RD, Alexander AF, Hill BL. 1981. Patent ductus arteriosus with pulmonary hypertension in the dog. J Am Vet Med Assoc 178: 565–571. doi: 10.2460/javma.1981.178.06.565 [DOI] [PubMed] [Google Scholar]
- 18.Schrope DP. 2015. Prevalence of congenital heart disease in 76,301 mixed-breed dogs and 57,025 mixed-breed cats. J Vet Cardiol 17: 192–202. doi: 10.1016/j.jvc.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 19.Seibert RL, Maisenbacher HW, 3rd, Prosek R, Adin DB, Arsenault WG, Estrada AH. 2010. Successful closure of left-to-right patent ductus arteriosus in three dogs with concurrent pulmonary hypertension. J Vet Cardiol 12: 67–73. doi: 10.1016/j.jvc.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 20.Seiji K, Tsuda M, Matsuhashi T, Takase K, Miyachi H, Yamada T, Ishibashi T, Higano S, Takahashi S. 2009. Treatment of in-stent restenosis with beraprost sodium: an experimental study of short- and intermediate-term effects in dogs. Clin Exp Pharmacol Physiol 36: 1164–1169. doi: 10.1111/j.1440-1681.2009.05209.x [DOI] [PubMed] [Google Scholar]
- 21.Suzuki R, Yuchi Y, Saito T, Teshima T, Matsumoto H, Koyama H. 2022. Investigation of beraprost sodium on cardiac function and hemodynamics in canine models of chronic pulmonary hypertension. Front Vet Sci 9: 876178. doi: 10.3389/fvets.2022.876178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki R, Yuchi Y, Saito T, Yasumura Y, Teshima T, Matsumoto H, Koyama H. 2022. Beraprost Sodium for Pulmonary Hypertension in Dogs: Effect on Hemodynamics and Cardiac Function. Animals (Basel) 12: 2078. doi: 10.3390/ani12162078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velayati A, Valerio MG, Shen M, Tariq S, Lanier GM, Aronow WS. 2016. Update on pulmonary arterial hypertension pharmacotherapy. Postgrad Med 128: 460–473. doi: 10.1080/00325481.2016.1188664 [DOI] [PubMed] [Google Scholar]
- 24.Winter RL, Remaks JD, Newhard DK. 2020. Development of spontaneous echocardiographic contrast after transarterial occlusion of a patent ductus arteriosus in an adult dog with concurrent pulmonary hypertension. Front Vet Sci 7: 103. doi: 10.3389/fvets.2020.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita M, Shibazaki A. 2008. Surgical ligation of patent ductus arteriosus with severe pulmonary hypertension in three dogs. Nippon Juishikai Zasshi 61: 459–462. [Google Scholar]