Abstract
Background
Fabry disease is a rare lysosomal storage disorder. The genotypic and phenotypic heterogeneity of the disease complicates the prediction of disease activity. This study aimed to evaluate the association between multiple plasma biomarkers and disease activity in Fabry disease.
Methods
A cross-sectional analysis was conducted involving 87 Fabry patients, 46 chronic kidney disease (CKD) patients, and 41 healthy controls. Plasma levels of KIM-1, MCP-1, YKL-40, TNFR-1, TNFR-2, and cystatin-C were measured using ELISA. eGFR was calculated using creatinine and creatinine-cystatin C-based CKD-EPI formulas. Fabry patients on renal replacement therapy were analyzed as a subgroup. Primary analyses focused on 62 Fabry patients receiving enzyme replacement therapy.
Results
Although eGFR (cr) did not differ significantly between Fabry patients and healthy controls, eGFR(cr-cys) was significantly lower in Fabry patients. After adjusting for age, gender, and BMI, MCP-1 and TNFR-2 levels were significantly lower in Fabry patients than in CKD patients. Among Fabry patients, those with renal involvement, had significantly higher MCP-1 levels than those without. While KIM-1 and YKL-40 did not differ significantly between groups, both were significantly elevated in patients with Lyso-Gb3 > 4 ng/mL and positively correlated with Lyso-Gb3.
Conclusion
MCP-1, TNFR-2, YKL-40, and cystatin C may serve as potential biomarkers for different aspects of Fabry disease activity. Further investigation into the associated pathogenic pathways may support the development of novel diagnostic tools or targeted therapies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-025-04189-x.
Keywords: Fabry disease, Plasma biomarkers, Genetic kidney disease
Introduction
Fabry disease (FD) is a rare, systemic, genetic disorder caused by mutations in the GLA gene, which lead to deficiencies in the lysosomal enzyme alpha-galactosidase A (AGAL) [1, 2]. This enzyme deficiency results in the accumulation of glycosphingolipids, particularly globotriaosylceramide (Gb3), in various organs [3]. The accumulation of these substrates, combined with their immunogenic nature, triggers a cascade of harmful effects, including immune system activation, inflammation, and oxidative stress, ultimately leading to progressive tissue damage [4].
The genetic and clinical heterogeneity of FD presents a major challenge in predicting disease progression [5]. Therefore, identifying biomarkers that reflect organ-specific disease activity is of great clinical interest. Currently, Lyso-Gb3 is the most widely used biomarker to support the diagnosis of FD; however, its utility in assessing treatment response and predicting long-term outcomes remains limited and not fully standardized [6].
In this study, we selected six plasma biomarkers, each associated with different segments of the nephron and key pathological pathways: cystatin C as a marker of glomerular function; kidney Injury Molecule-1(KIM-1) for proximal tubular injury; tumor necrosis factor receptors 1 and 2 (TNFR-1 and TNFR-2) for inflammation and fibrosis; and monocyte chemoattractant protein-1(MCP-1) and YKL-40 as markers of tissue remodeling and fibrotic activity. Except for cystatin C, these biomarkers have been associated with the progression of diabetic kidney disease, as shown in the CRIC study [7]. KIM-1 is well established as a marker of acute kidney injury and has been linked to CKD progression in diabetic and polycystic kidney disease. TNFR-1 has been associated with mortality and end-stage renal disease in diabetes, and both TNFR-1 and TNFR-2 have shown longitudinal associations with CKD progression. MCP-1 plays a central role in monocyte recruitment and is involved in various inflammatory kidney diseases. YKL-40 is upregulated in renal macrophages following ischemia-reperfusion injury and may support tubular repair. Although these biomarkers have been studied in other renal conditions, their relevance in FD has not been fully explored [7].
This study aims to address this gap by assessing the relationship between these biomarkers and the clinical manifestations of FD, with a focus on their potential to provide insights into renal and cardiac involvement, as well as the efficacy of enzyme replacement therapy (ERT). By investigating the pathogenic pathways linked to these biomarkers, we aim to contribute to the development of more effective diagnostic and therapeutic strategies for FD.
Methods
Study design and participants
We performed a cross-sectional study between October 2021 and December 2022 in three different tertiary care nephrology centers located in different cities across Turkey. Participants were divided into three groups: [1] the Fabry disease (FD) group, consisting of 87 patients with a confirmed genetic diagnosis of FD; [2] the chronic kidney disease (CKD) group, comprising 46 patients with CKD under regular follow-up at nephrology clinics; and [3] the healthy control group, which included 41 participants without any known active or chronic diseases.
Inclusion criteria
Fabry patients aged 18 years or older with a confirmed genetic diagnosis.
CKD patients aged 18 years or older under regular nephrology follow-up.
Healthy controls aged 18 years or older without any known active or chronic diseases.
Exclusion criteria
Patients with active infections, current malignancies, or acute kidney injury.
Fabry patients undergoing renal replacement therapy (RRT) were included in the study but analyzed separately (n = 11). This left 76 patients, of whom 62 were receiving enzyme replacement therapy (ERT) and 14 were not. All primary analyses were conducted using only the 62 Fabry patients on ERT, including comparisons with CKD patients and healthy controls.
Data collection
Demographic and clinical data, including age, gender, body mass index (BMI), blood pressure, and smoking status, were collected from all participants. For Fabry patients, detailed medical histories, including mutation type, Lyso-Gb3 levels, and history of cardiac and renal involvement, were recorded. Data were collected through patient interviews and review of medical records, using a standardized form.
Biomarker analysis
Blood samples were collected from all participants into gel-containing biochemistry tubes (BD SST II 5 mL, 367953) and centrifuged at 3000 rpm for eight minutes. The serum was then separated and stored at -80 °C until further analysis. Plasma levels of kidney injury molecule-1 (KIM-1), monocyte chemoattractant protein-1 (MCP-1), YKL-40, tumor necrosis factor receptors 1 and 2 (TNFR-1 and TNFR-2), and cystatin C were measured using enzyme-linked immunoassay (ELISA) kits (Elabscience, catalog numbers: E-EL-H3643, E-EL-H0037, E-EL-H6029, E-EL-H0217, E-EL-H2436, E-EL-H6005). The intra- and inter-assay coefficients of variation (%CV) for all assays were maintained at less than 6.5%.
Renal function assessment
Estimated glomerular filtration rate (eGFR) was calculated using both creatinine-based CKD-EPI formula and creatinine-cystatin C-based CKD-EPI formulas. Fabry patients on RRT were analyzed separately to account for the potential impact of dialysis on biomarker levels.
Definitions
Fabry phenotypes were categorized based on mutation analysis and clinical presentation into classic, late-onset, and other variants (e.g., variants of uncertain significance). These classifications are described and detailed in Supplementary Table 1 [8]. ERT refers to the intravenous administration of recombinant alpha-galactosidase A or B, which is used in FD treatment. RRT includes hemodialysis and renal transplantation in our population. Cardiac involvement was defined by the presence of arrhythmia, septal hypertrophy (septum thickness > 9 mm in women and > 10 mm in men) on echocardiography, or Fabry related findings on cardiac MRI [9]. Arrhythmias were diagnosed by a cardiologist during patient interviews. Renal involvement was defined by eGFR(cr) < 90 and/or albuminuria (> 30 mg/day).
Statistical analysis
Descriptive statistics were reported as mean ± standard deviation or median and interquartile range (IQR) for continuous variables, and as counts and percentages for categorical variables. The Shapiro-Wilk test was used to assess the normality of continuous variables. For group comparisons, one-way ANOVA was used for normally distributed continuous variables, while the Kruskal-Wallis test was applied to non-normally distributed variables. The Chi-square or Fisher’s Exact test was used to compare categorical variables.
For comparisons between Fabry patients with and without renal or cardiac involvement, independent samples t-tests or Mann-Whitney U tests were used, depending on the distribution of the biomarkers. Multiple linear regression analysis was conducted to adjust for potential confounders, including age, gender, and BMI. To meet the assumptions of normality of errors and homoscedasticity, logarithmic transformations of the biomarker values were performed prior to their inclusion as dependent variables in the regression models.
All statistical analyses were conducted using IBM SPSS version 21 (IBM Corp., NY, USA) and R version 4.0.2. A p-value of less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
A total of 87 FD patients, 46 CKD patients, and 41 healthy controls were included in the study. The mean age of the Fabry patients was 40 ± 14 years, with 60.9% being female. Among the Fabry patients, 11 were undergoing RRT, including three who had received kidney transplants. The average body mass index (BMI) of Fabry patients was 25 ± 5.8 kg/m², and 35% were smokers. Consanguinity was reported by 26.4% of the Fabry patients. Sixty-seven patients had Lyso-Gb3 levels above the upper limit (< 1.3 ng/ml). Median Lyso-Gb3 level was four mg/ml. The mutations and the Lyso-Gb3 levels of the Fabry patients are detailed in Supplementary Table 1. Forty mutations were classified as pathogenic mutations with a classic phenotype, seven as pathogenic mutations with a late-onset phenotype, and thirty-nine as other (variants of uncertain significance and other variants). Of the 40 patients classified as classic phenotype; 27 (67.5%) had renal involvement, 37 (92.5%) had cardiac involvement, and 24 (60%) had both renal and cardiac involvement. We presented the mutation detected in Fabry patients, the phenotypic characteristic of the mutation, the presence of cardiac and renal involvement, Lyso-Gb3 level, the administration of ERT, and RRT in Supplementary Table 1.
Cranial MRI was performed in 61 patients, and 18 (29.5%) showed white matter hyperintensities consistent with FD. Seven patients (8.1%) had experienced a stroke. Additionally, among the 69 patients who underwent ophthalmologic evaluation, 66.6% had cornea verticillata. Documented findings also included sensorineural hearing loss in 25.9%, skin involvement in 17.4%, gastrointestinal symptoms in 10.5%, and peripheral neuropathy (based on EMG) in 14.7% of those evaluated. We presented non-Major Organ involvement and symptoms in Fabry patients in Supplementary Table 2.
The following analysis was conducted between Fabry patients not on RRT and receiving ERT (n = 62), CKD patients and healthy controls. The CKD group was predominantly female and significantly older, and exhibited higher rates of diabetes mellitus (DM) and hypertension (HT) than the Fabry group. There was no significant difference between Fabry patients, CKD group and healthy controls in terms of age, gender and BMI. Demographic, clinical and laboratory data of the groups are presented in Table 1.
Table 1.
Demographic, clinical and laboratory data between groups
| n (%) | Fabry (n:62) | CKD (n:46) | Healthy control (n:41) | p | |||
|---|---|---|---|---|---|---|---|
| Age | 37 (27–47.25) | 49 (34.8 − 56.3) | 36 (30–45) | 0.0041,2 | |||
|
Gender, n(%) Male Female |
25 (40.3) 37 (59.7) |
15 (32.6) 31 (67.4) |
24 (58.5) 17 (41.5) |
0.0181 | |||
| BMI, kg/m² | 23.74 (20.37–28.03) | 29.9 (24.7–33.6) | 24.8 (21.8–27.6) | < 0.0011,2 | |||
|
Smoking, n(%) No Yes |
35 (59.3) 24 (38.7) |
33 (78.6) 9 (21.4) |
29 (74.4) 10 (25.6) |
0.095 | |||
| DM, n (%) | 5 (8.1) | 14 (30.4) | 0 | 0.003 | |||
| HT, n (%) | 12 (19.4) | 29 (63) | 0 | < 0.001 | |||
| CAD, n (%) | 1 (1.6) | 2 (4.3) | 0 | 0.574 | |||
| ACEI/ARB, n (%) | 23 (37.1) | 30 (65.2) | 0 | 0.004 | |||
| BB, n (%) | 6 (9.7) | 12 (26.1) | 0 | 0.024 | |||
| OAD, n (%) | 2 (3.2) | 14 (30.4) | 0 | < 0.001 | |||
| Insulin, n (%) | 0 (0) | 5 (10.9) | 0 | 0.008 | |||
| SGLT2 Inhibitor, n (%) | 1 (1.6) | 7 (15.2) | 0 | 0.021 | |||
| Laboratory data of Fabry and CKD patients | |||||||
| Fabry (n:62) | CKD (n:46) | p | |||||
| Urea, mg/dl | 23 (18–29.4) | 34 (26.7–4.6) | < 0.001 | ||||
| Creatinine, mg/dl | 0.67 (0.59–0.83) | 0.95 (0.71–1.15) | < 0.001 | ||||
| eGFR(cr), ml/dk/1,73 m2 | 114.9 (95.7–126.4) | 88 (63.5–109) | 0.003 | ||||
| eGFR(cys-cr), ml/dk/1,73 m2 | 86.5 (52.7–118) | 63 (36,8–97,3) | 0.04 | ||||
| Albumin, g/dl | 4.7 (4.4–4.9) | 4.5 (4.3–4.8) | 0.018 | ||||
| CRP, mg/l | 1.9 (0.6–5.5) | 3.9 (1–6.4) | 0.179 | ||||
| Ferritin, ug/l | 37 (15.8–92.6) | 67.3 (37–122) | 0.044 | ||||
| Total cholesterol (mg/dl) | 165.27 ± 34.76 | 206.75 ± 53.46 | < 0.01 | ||||
| LDL, mg/dl | 95 (75.23-116.75) | 119 (92.25–148) | 0.003 | ||||
| TG, mg/dl | 85 (69–119) | 162 (118.5–205) | < 0.001 | ||||
| Hba1c, % | 5.5 (5.1–5.9) | 6.5 (5.7–8.2) | < 0.001 | ||||
| FBG, mg/dl | 88.8 (77–97) | 98.5 (88.8–119.8) | < 0.01 | ||||
| Hematuria in urinalysis | 11 (24.4) | 16 (35.6) | 0.25 | ||||
| Proteinuria in urinalysis | 10 (22.2) | 23 (51.5) | 0.004 | ||||
| 24/h urine protein, mg | 237.3 (145.8–557) | NA | NA | ||||
| 24/h urine microalbumin, mg | 26.5 (11.5–281) | NA | NA | ||||
| UPCR, mg/g | 145.5 (102.5–606.25) | 413.5 (121.5–1007) | 0.300 | ||||
| UACR, mg/g | 25(11.8–360.25) | 141 (30.2–335.5) | 0.114 | ||||
| 25 OH D vitamin, ng/ml | 14.9 (9.2–25) | 14.1 (8.7–19.8) | 0.327 | ||||
| Vitamin B12, ng/l | 374.5 (288–488.6) | 341.5 (232–523) | 0.311 | ||||
| Hgb, g/dl | 13.08 ± 1.68 | 13.51 ± 1.83 | 0.42 | ||||
| WBC, /µl | 7350 (5975–8600) | 6980 (5935–8400) | 0.637 | ||||
BMI: Body mass index, DM: Diabetes mellitus, HT: Hypertension, CAD: Coronary artery disease, ACEI/ARB: Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, BB: Beta-blocker, OAD: Oral antidiabetic, SGLT2: Sodium-glucose co-transporter 2, eGFR: Estimated glomerular filtration rate, Cys: Cystatin, LDL: Low-density lipoprotein, TG: Triglyceride, HbA1c: Hemoglobin A1c, FBG: Fasting blood glucose, 24 h: 24-hour, UPCR: Urine protein-creatinine ratio, UACR: Urine albumin-creatinine ratio, Hgb: Hemoglobin, WBC: White blood cell
1Healthy control-Patient control, 2Patient control-Fabry, 3Healthy control-Fabry
Renal function assessment and renal involvement
The median cr level in healthy controls was 0.78 mg/dL (IQR: 0.67–0.87), while in Fabry patients it was 0.67 mg/dL (IQR: 0.59–0.83). The median eGFR(cr) was 111 mL/min/1.73 m² (IQR: 100.5–117.5) in healthy controls and 114.9 mL/min/1.73 m² (IQR: 95.7–126.4) in Fabry patients. There was no significant difference between the two groups in terms of creatinine or eGFR(cr). However, the median eGFR(cr-cys) was significantly lower in Fabry patients (86.5 mL/min/1.73 m² (IQR: 52.7–118)) compared to healthy controls (111 mL/min/1.73 m² (IQR: 97–125.5)). In total, 30 (48.38%) out of 62 Fabry patients had renal involvement (Supplementary Table 1).
Plasma biomarker levels
Biomarker levels (Cystatin-C, KIM-1, MCP-1, YKL-40, TNFR-1 and 2) were analyzed in Fabry patients not on RRT and receiving ERT (n = 62), CKD patients, and healthy control groups as follows: Biomarker levels were compared across the Fabry patients and control groups (Fig. 1). Subsequently, Fabry patients were divided into two subgroups based on the presence or absence of renal involvement. Fabry patients with renal involvement (Fig. 2) and Fabry patients without renal involvement (Fig. 3) were compared with the control groups. Finally, Fabry patients were grouped according to the presence of renal (Fig. 4) and cardiac (Fig. 5) involvement, and biomarker levels were compared between those with and without organ involvement.
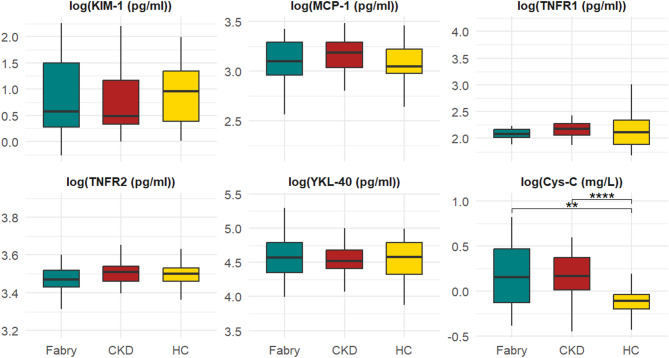
Fig. 1.
Comparison of biomarker levels in Fabry patients, CKD patients, and healthy control groups. HC: Healthy control CKD: Chronic kidney disease patients. ** MCP-1 p:0.062 ***Cystatin C p:<0.0011,3. 1HC-CKD, 2CKD-Fabry, 3HC-Fabry. The middle line represents the median value, the bottom and top edges of the box represent the 25th and 75th percentiles, and the whiskers indicate the minimum and maximum values
Fig. 2.
Comparison of biomarker levels in Fabry patients with renal involvement, patient control, and healthy control groups. HC: Healthy control CKD: Chronic kidney disease patients. ***Cystatin C p:<0.0011,3. 1HC-CKD, 2CKD-Fabry, 3HC-Fabry. The middle line represents the median value, the bottom and top edges of the box represent the 25th and 75th percentiles, and the whiskers indicate the minimum and maximum values
Fig. 3.
Comparison of biomarker levels in Fabry patients without renal involvement, patient control, and healthy control groups. HC: Healthy control CKD: Chronic kidney disease patients. TNFR-2 p:0.0292,3 ***MCP-1 p:0.0012 ***Cystatin C p:<0.0011,3. 1HC-CKD, 2CKD-Fabry, 3HC-Fabry. The middle line represents the median value, the bottom and top edges of the box represent the 25th and 75th percentiles, and the whiskers indicate the minimum and maximum values
Fig. 4.
Comparison of biomarker levels in Fabry disease subgroups with and without renal involvement. *MCP-1 p:0.019. The middle line represents the median value, the bottom and top edges of the box represent the 25th and 75th percentiles, and the whiskers indicate the minimum and maximum values
Fig. 5.
Comparison of biomarker levels in Fabry disease subgroups with and without cardiac involvement. The middle line represents the median value, the bottom and top edges of the box represent the 25th and 75th percentiles, and the whiskers indicate the minimum and maximum values
KIM-1 levels did not differ significantly between groups. MCP-1 levels were significantly lower in Fabry patients and Fabry patients without renal involvement compared to CKD patients (p = 0.006, p = 0.001, respectively) (Figs. 1 and 3). YKL-40 levels were lower in Fabry patients than in both control groups but it did not reach statistical significance. Cystatin C levels were significantly elevated in Fabry patients compared to healthy controls (p < 0.001), particularly in those with renal involvement (Figs. 1, 2 and 3). TNFR-2 levels were significantly lower in Fabry patients than CKD group (p = 0.016) (Fig. 1). TNFR-2 levels were also significantly lower in Fabry patients without renal involvement than in both control groups (p = 0.029) (Fig. 3).
After adjusting for age, gender and BMI, MCP-1 levels remained significantly lower in Fabry patients than in CKD patients (β = -0.110, p = 0.046). TNFR-2 levels were also significantly lower in Fabry patients (β = -0.040, p = 0.045). Cystatin C was significantly higher in Fabry and CKD groups compared to healthy controls (both p < 0.001). KIM-1 and TNFR-1 were not significantly associated with disease group after adjustment.
Organ-specific biomarker associations
The analysis of Fabry patients based on the presence of renal or cardiac involvement revealed the following:
Renal Involvement: Fabry patients with renal involvement had higher cystatin C levels compared to healthy controls (p < 0.001) (Fig. 2). MCP-1 levels were higher in Fabry patients with renal involvement than in those without renal involvement (p = 0.019).
Cardiac Involvement: Fabry patients with cardiac involvement exhibited higher MCP-1 levels than those without cardiac involvement but that did not reach statistical significance (p = 0.069).
Correlation of biomarkers with Lyso-Gb3
When comparing Fabry patients receiving ERT (n = 62) to those not receiving ERT (n = 14), no significant differences were observed in biomarker levels. However, when ERT-treated Fabry patients were stratified by median Lyso-Gb3 levels (cutoff: 4 ng/mL), patients with higher Lyso-Gb3 levels showed significantly elevated KIM-1 (p = 0.023) and YKL-40 (p = 0.001) levels. (Supplementary Table 3). There was a weak but statistically significant positive correlation between Lyso-Gb3 and KIM-1 (r = 0.296, p = 0.024). Additionally, a moderate positive correlation was observed between Lyso-Gb3 and YKL-40 (r = 0.345, p = 0.008). These findings may suggest that KIM-1 and YKL-40 might be useful in monitoring disease activity and response to ERT in FD. However, given that the clinical utility of Lyso-Gb3 in monitoring treatment response remains uncertain, these results should be interpreted with caution.
Subgroup analysis: patients undergoing renal replacement therapy
A subgroup analysis comparing Fabry patients on hemodialysis (HD, n = 8) with those not receiving RRT but having renal involvement (n = 30) is presented in Supplementary Table 4. Cystatin C levels were significantly higher in the HD group (p = 0.012). Additionally, YKL-40 levels were also significantly elevated in the HD group (p = 0.007), further supporting the hypothesis that YKL-40 may be associated with worsening renal function in FD.
Discussion
In this study, we investigated the relationship between multiple plasma biomarkers (Cystatin-C, KIM-1, MCP-1, YKL-40, TNFR-1 and 2) and disease activity in FD, focusing on their potential roles in reflecting renal and cardiac involvement as well as the effectiveness of ERT. Our findings indicate that MCP-1, YKL-40, TNFR-2 and cystatin C are valuable biomarkers that offer distinct insights into the pathophysiology and clinical progression of FD, though further validation is required before these biomarkers can be considered for routine clinical use.
Our findings indicate that Cystatin C is the most consistent marker of renal dysfunction in Fabry patients. Its levels were significantly higher in Fabry patients than in healthy controls. Our findings are consistent with previous studies, that demonstrated the utility of cystatin C as an early marker of renal dysfunction in Fabry patients [10–12]. Even after adjusting for potential confounders, elevated cystatin C levels remained consistently associated with renal involvement. This supports its clinical relevance for early detection, monitoring of kidney function, and timely initiation of treatment to help slow CKD progression in Fabry patients.
MCP-1 levels were significantly lower in Fabry patients without renal involvement than in CKD patients, even after adjusting for age, gender and BMI. Additionally, MCP-1 levels were significantly higher in Fabry patients with renal involvement compared to those without, supporting its potential utility as a marker of renal dysfunction in FD. A comparable pattern was noted in relation to cardiac involvement, with elevated MCP-1 levels observed in patients with cardiac manifestations; however, this trend did not reach statistical significance (p = 0.069). Despite the lack of significance, this finding aligns with existing evidence linking MCP-1 to inflammation and cardiac fibrosis, suggesting its possible contribution to multiorgan involvement in FD [13].
Additionally, MCP-1’s lack of association with Lyso-Gb3 levels implies it could serve as an independent marker for renal involvement, especially in cases of subclinical damage. MCP-1 has been linked to fibroblast infiltration and interstitial fibrosis in cell culture models [14]. In animal models, MCP-1 inhibition has demonstrated a protective effect in diabetic kidney disease and has been shown to inhibit hepatic monocyte/macrophage infiltration during chronic liver injury [15, 16]. Thus, MCP-1 inhibition may represent a promising new pharmacological approach for treating organ involvement in FD.
A previous study by Yogasundaram et al. reported elevated TNFR-1 and TNFR-2 levels in Fabry patients with left ventricular hypertrophy [17], however, we did not observe such elevations in our cohort. Differences in findings may stem from variations in ELISA kits or the antigenic properties of sphingolipids in FD [18]. Notably, TNFR-2 levels were significantly lower in ERT-treated Fabry patients without renal involvement compared to both CKD patients and healthy controls. This may reflect the anti-inflammatory effects of ERT and a more stable disease state. Clinically, TNFR-2 could serve as a potential marker for monitoring systemic inflammation and treatment response in FD. Further longitudinal research is warranted to clarify the role of TNFR-2 in FD and evaluate its potential as a marker of treatment response.
KIM-1 is known as a marker of tubular injury and, although it has primarily been reported as a biomarker for acute kidney injury, there are studies indicating its role in predicting CKD progression [19]. KIM-1 did not show significant differences across groups, however, the positive correlation between KIM-1 and Lyso-Gb3 levels, particularly in patients receiving ERT, highlights its potential role in monitoring disease activity or subclinical progression, though additional studies are necessary to confirm this.
YKL-40 did not differ significantly between Fabry patients and controls, yet it was significantly elevated in patients with high Lyso-Gb3 levels. Additionally, it was higher in patients with renal involvement than in those without, although this difference did not reach statistical significance. The significant correlation between YKL-40 and Lyso-Gb3 levels (r = 0.345, p = 0.008) supports the idea that YKL-40 could be used to monitor disease progression, especially in relation to renal involvement. Additionally, elevated YKL-40 levels in Fabry patients undergoing hemodialysis further indicate its potential as a marker of worsening renal function. A study found that plasma chitotriosidase levels are significantly higher in male Fabry patients, which is linked to the presence of lipid-filled macrophages [20]. It was suggested that these elevated levels can be detected even before symptoms appear at a very young age [20]. Particularly in female patients, where treatment decisions can be challenging, early initiation of ERT guided by YKL-40 levels may be considered even before symptom onset. Our study has some limitations. First, due to the genetic and phenotypic diversity of FD, further studies are needed to generalize our findings. Second, the patient control group was older and healthy controls had more males than the other groups, which may influence the results; however, we made statistical adjustments to account for these differences. Third, as this was a cross-sectional study, longitudinal follow-up would provide stronger evidence for the relationships we observed. Lastly, potential variability in sample collection and storage across centers may have impacted biomarker levels, but we addressed this by implementing a standardized protocol for blood collection and storage.
In conclusion, our findings support the notion that MCP-1, cystatin C, and YKL-40 may serve as organ-specific markers in FD. Cystatin C appears robust in reflecting renal impairment, while MCP-1 may help identify both renal and cardiac involvement. TNFR-2 may reflect the efficacy of ERT. YKL-40 and KIM-1, particularly when associated with elevated Lyso-Gb3 levels, may offer insight into residual disease activity despite therapy. These biomarkers could complement existing tools such as eGFR and Lyso-Gb3 in comprehensive disease monitoring. These biomarkers may play a crucial role in improving clinical management by facilitating early diagnosis and personalized treatment strategies for FD. Further research is warranted to validate these findings and explore the development of new therapeutic approaches targeting the pathways associated with these biomarkers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the laboratory and clinical staff of the participating centers for their valuable contributions.
Abbreviations
- FD
Fabry Disease
- ERT
Enzyme Replacement Therapy
- CKD
Chronic Kidney Disease
- eGFR
Estimated Glomerular Filtration Rate
- KIM-1
Kidney Injury Molecule-1
- MCP-1
Monocyte Chemoattractant Protein-1
- TNFR-1 and TNFR-2
Tumor Necrosis Factor Receptor-1 and - 2
- Lyso-Gb3
Globotriaosylsphingosine
- RRT
Renal Replacement Therapy
- BMI
Body Mass Index
- ELISA
Enzyme-Linked Immunosorbent Assay
- HD
Hemodialysis
Author contributions
SGO and NS conceptualized and designed the study. SGO, NE, MTD, ME, HO, KT, and ST contributed patient recruitment and data collection. MB conducted the biomarker analyses. ZA performed statistical analysis. SGO drafted the manuscript. NS and all other authors critically revised the manuscript for important intellectual content. All authors reviewed and approved the final version of the manuscript.
Funding
We conducted the study with the support of Istanbul University-Cerrahpasa Scientific Research Projects department (Project number: 36251, Project code: TTU-2022-36251).
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Istanbul Training and Research Hospital (approval no: 67) and conducted according to the Declaration of Helsinki. Informed consent was taken from all patients.
Consent for publication
All participants provided informed consent for participation and publication of anonymized data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Anderson W. A case of angeio-keratoma. Br J Dermatol. 1898;10(4):113–7. [Google Scholar]
- 2.Neisser A, Fabry J. Arch Dermatol Syph. 1898;43:187–200. [Google Scholar]
- 3.Desnick R, α-Galactosidase A. deficiency: Fabry disease. The metabolic and molecular bases of inherited disease. 1995:2741-84.
- 4.Mauhin W, Lidove O, Masat E, Mingozzi F, Mariampillai K, Ziza J-M et al. Innate and adaptive immune response in Fabry disease. JIMD Reports, Volume 22: Springer; 2015. pp. 1–10. [DOI] [PMC free article] [PubMed]
- 5.Ortiz A, Germain DP, Desnick RJ, Politei J, Mauer M, Burlina A, et al. Fabry disease revisited: management and treatment recommendations for adult patients. Mol Genet Metab. 2018;123(4):416–27. [DOI] [PubMed] [Google Scholar]
- 6.Ferraz MJ, Kallemeijn WW, Mirzaian M, Moro DH, Marques A, Wisse P, et al. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycosphingolipidoses. Biochim Et Biophys Acta (BBA)-Molecular Cell Biology Lipids. 2014;1841(5):811–25. [DOI] [PubMed] [Google Scholar]
- 7.Schrauben SJ, Shou H, Zhang X, Anderson AH, Bonventre JV, Chen J, et al. Association of multiple plasma biomarker concentrations with progression of prevalent diabetic kidney disease: findings from the chronic renal insufficiency cohort (CRIC) study. J Am Soc Nephrol. 2021;32(1):115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabry mutants list [Available from. http://fabry-database.org/mutants/
- 9.Barbieri A, Bursi F, Mantovani F, Valenti C, Quaglia M, Berti E, et al. Left ventricular hypertrophy reclassification and death: application of the recommendation of the American society of echocardiography/European association of echocardiography. Eur Heart Journal–Cardiovascular Imaging. 2012;13(1):109–17. [DOI] [PubMed] [Google Scholar]
- 10.Mehta A, Pastores GM, Pintos-Morell G. Cystatin C and NT-proBNP as prognostic biomarkers in Fabry disease. Curr Med Literature. 2012;10(2):51. [Google Scholar]
- 11.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum Cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16(5):1404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feriozzi S, Germain DP, Di Vito R, Legrand A, Ricci R, Barbey F. Cystatin C as a marker of early changes of renal function in Fabry nephropathy. J Nephrol. 2007;20(4):437–43. [PubMed] [Google Scholar]
- 13.Chen K-H, Chien Y, Wang K-L, Leu H-B, Hsiao C-Y, Lai Y-H, et al. Evaluation of Proinflammatory prognostic biomarkers for Fabry cardiomyopathy with enzyme replacement therapy. Can J Cardiol. 2016;32(10):1221. e1-. e9. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Niño MD, Carpio D, Sanz AB, Ruiz-Ortega M, Mezzano S, Ortiz A. Lyso-Gb3 activates Notch1 in human podocytes. Hum Mol Genet. 2015;24(20):5720–32. [DOI] [PubMed] [Google Scholar]
- 15.Xia Y, Frangogiannis NG. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm Allergy-Drug Targets (Formerly Curr Drug Targets-Inflammation Allergy)(Discontinued). 2007;6(2):101–7. [DOI] [PubMed] [Google Scholar]
- 16.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, et al. Pharmacological Inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61(3):416–26. [DOI] [PubMed] [Google Scholar]
- 17.Yogasundaram H, Nikhanj A, Putko BN, Boutin M, Jain-Ghai S, Khan A, et al. Elevated inflammatory plasma biomarkers in patients with Fabry disease: a critical link to heart failure with preserved ejection fraction. J Am Heart Association. 2018;7(21):e009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolter T, Sandhoff K. Sphingolipid metabolism diseases. Biochim Et Biophys Acta (BBA)-Biomembranes. 2006;1758(12):2057–79. [DOI] [PubMed] [Google Scholar]
- 19.Sandokji I, Greenberg JH, editors. Plasma and urine biomarkers of CKD: A review of findings in the CKiD study. Seminars in nephrology. Elsevier; 2021. [DOI] [PMC free article] [PubMed]
- 20.Vedder A, Cox-Brinkman J, Hollak C, Linthorst G, Groener J, Helmond M, et al. Plasma Chitotriosidase in male Fabry patients: a marker for monitoring lipid-laden macrophages and their correction by enzyme replacement therapy. Mol Genet Metab. 2006;89(3):239–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.