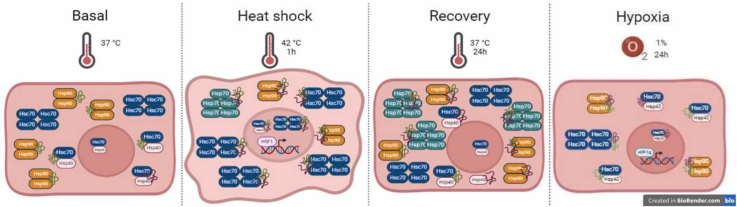
Abstract
Heat shock proteins (HSPs) play crucial roles in human endothelial cell functions such as migration and angiogenesis. However, human HSP dynamics under stress conditions such as heat shock (HS) and hypoxia in human endothelial cells (ECs) are enigmatic, and the characteristics of HSPs in ECs after exposure to thermal stress and a low-oxygen environment are unknown. We hypothesized that ECs adapt to HS and hypoxia by modulating chaperome oligomerization and that HSP70 is a major determinant of the endothelial phenotype. HSP70 inhibition with VER-155008 or YM-1 in primary human endothelial cells decreases EC proliferation, migration, and angiogenesis at baseline and after heat shock recovery. We showed that vascular-independent HSC/P70 multimeric complexes in primary human veins (HUVEC) and coronary artery ECs (HCAEC) accumulate after HS and are decreased by hypoxia. HS recovery increases the number of HSP90 dimers, inducible HSP70, and HSP40 macromolecular complexes, whereas HSC70 returns to baseline. We demonstrated that the HS response and hypoxia regulate HSPs through a new layer of complexity, oligomerization, in addition to classical cochaperone/NEF interactions. The biphasic temporal oligomerization of molecular chaperones in the recovery phase provides a novel face of the heat shock response. In addition, shifts in the subcellular location and upregulation of HSP70 were also observed here. The decrease in HSP expression caused by hypoxia raises the possibility that decreased chaperone power contributes to the endothelial dysfunction found in atherosclerosis, thrombosis, and cancer. Together, these results show that HSP70 is pivotal to the healthy endothelial response in veins and coronary arteries, and we revealed human HSP dynamics in the vascular response to proteotoxic stress.
Keywords: Endothelial cell, HSP70, Heat-shock response, Hypoxia, Oligomerization
Graphical abstract
Introduction
Heat shock proteins (HSPs) perform central functions in maintaining proteostasis by supporting productive multidomain protein folding and assembly.1 An ancient endogenous mechanism involves the upregulation of HSP70 by environmental stresses, such as heat, low-oxygen pressure (hypoxia), oxidative stress, shear stress, and infection.2, 3 HSP70 and HSP90 represent 5.5% of the total mass of human protein and, together with other proteins, participate in cellular quality control and compose the chaperome.4 HSP70 and HSP90 are downregulated in the endothelial cells (ECs) of patients with chronic thromboembolic pulmonary hypertension and in human EC monolayers and mouse aortae exposed to proatherogenic flow.2 HSP90 regulates angiogenic EC function and endothelial nitric oxide synthase (eNOS)-dependent vasorelaxation stimulated by acetylcholine.5 Loss of function of HSP70 impairs EC migration,6, 7, 8 IL-5-stimulated eNOS phosphorylation8, 9, nucleolin surface translocation,6 basal expression of phosphatidylinositol 3-kinase p110 subunit expression,10 and vascular endothelial growth factor-mediated or asymmetric dimethylarginine-mediated AKT phosphorylation.10, 11
HSP70, a stress-inducible protein, and HSC70, a constitutive protein, are classically described as monomers. HSP70 self-associates, from bacteria to mammals, and is stabilized by ADP and disrupted by ATP.12 The HSP70-ATP dimer is also found in nonstressed HEK293 cells.13 Heat shock (HS) enhances HSP70 oligomerization in Escherichia coli Hsp70,14 DnaK, and inducible form-HSP70 from monkey kidney cells but barely affects HSC70 oligomers naturally present in normal cells.15 Due to contradictory observations, the activity of HSP70 oligomers is unclear. DnaK and HSC70 oligomers lost luciferase refolding capacity, whereas DnaK maintained holdase activity.14 Human HSP70 dimerization in vitro allows client loading on HSP90 and facilitates interaction with HSP40, which leads to luciferase refolding and the binding of the cochaperone E3 ubiquitin ligase CHIP.16 Furthermore, trimeric HSC70 uncoats clathrin, resulting in context-dependent HSP70 oligomeric activity.12 HSP90 acts as a homodimer under physiological conditions, resulting in conformational changes linked with dimerization of the C-terminal and N-terminal domains promoted by ATP binding and hydrolysis.17 Despite their importance in vascular functions18, 12, 3 and clear evidence for HSP70 and HSP90 heteromultimeric complexes,18, 17 the status of HSP oligomerization and its dynamics under physiological stress, such as HS and hypoxia, in vascular cells remain enigmatic. Here, we showed the dynamic oligomerization of HSPs after two different stresses, HS and hypoxia, which are involved in the biogenesis of several cardiovascular pathologies. We first demonstrated that endogenous HSP endothelial regulation by stress, proliferation, and coronary artery EC basal angiogenesis is mediated by HSP70.
Results
Heat shock culminates in a classical response in vascular cells
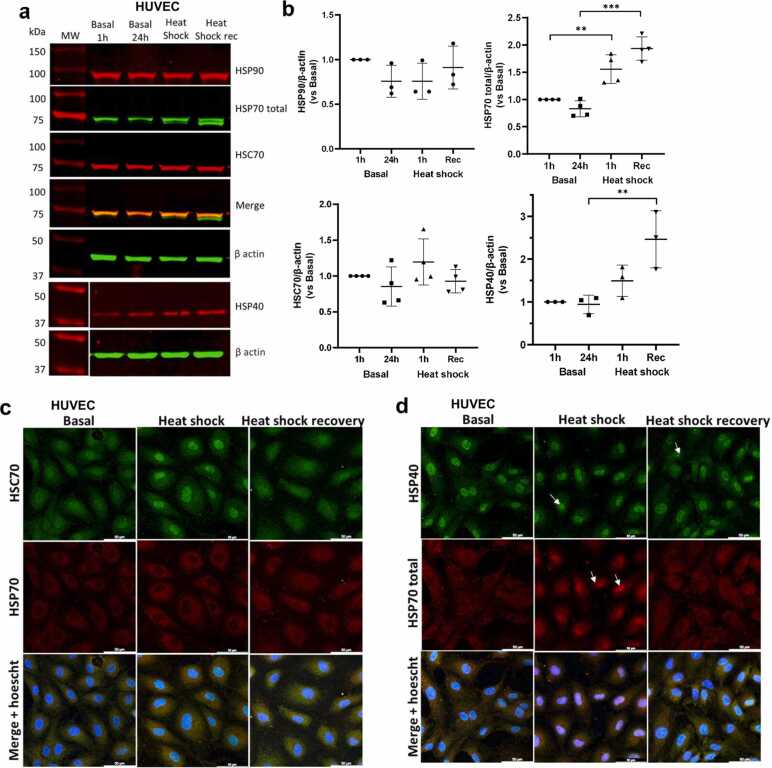
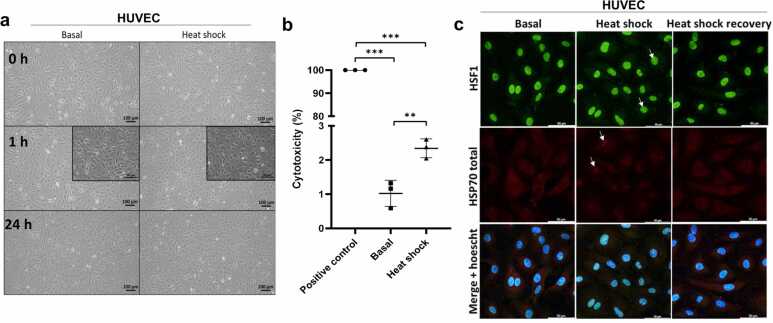
Heat shock (HS) is crucial to organism survival and directly increases HSP expression to address toxic cell effects and proteostasis challenges.19 To the best of our knowledge, we are the first to screen for the effects of HS, specifically in primary human ECs, as full characterization of HS in vascular cells is lacking. ECs upregulate HSP70 mRNA, whereas HSP90 levels are not affected after heat shock20; however, data concerning appropriate Western blotting controls, such as housekeeping genes, and their quantification are lacking. Assessment of protein expression after HS and HS recovery revealed the upregulation of HSP70 and HSP40/Hdj1, whereas HSP90 and HSC70 levels remained unchanged in human umbilical veins (HUVECs) (Figure 1(a) and (b)). HS led to HSC70 nuclear accumulation, strong cytosolic staining of inducible HSP70 (Figure 1(c) and (d)), and HSP40/Hdj1 nuclear cluster formation (Figure 1(d)). Thermal stress changed EC morphology to a rounded morphology with <3% death after thermal stress (Figure 2(a) and (b)) and induced nuclear foci of heat shock transcription factor 1 (HSF1) (Figure 2(c)).
Fig. 1.
Screening of the heat shock (HS) response in primary human endothelial cells. (a) Whole HUVEC lysates after HS for 1 h and recovery for 24 h or basal conditions were subjected to Western blot analysis for HSP40 (CST, 4868S), total HSP70 (Invitrogen, MA3-006), HSC70 (Invitrogen, PA5-27337), and HSP90 (CST, 4877S) expression. The data are representative of n = 3–4 independent experiments with cells from different lots (19TL127368, 19TL028325, 19TL023264, and 21TL195720). (b) Data normalized to the loading control β-actin are presented as the means ± SEMs; one-way ANOVA, with Tukey’s post hoc test; **P < 0.01, ***P < 0.001 versus the respective control; HSP40 and HSP90, n = 3; HSP70 total and HSC70, n = 4. (c) Immunofluorescence staining of HSC70 (green, Invitrogen PA5-27337) and HSP70 (red, Invitrogen MA3-009) and (d) HSP40 (green, CST 4356S) and total HSP70 (red, Invitrogen MA3-006) in HUVECs exposed to HS and recovery or basal conditions. Nuclei were stained with Hoechst (blue). Representative images of n = 3 independent experiments using cells from lots 19TL028324 and 19TL023264 (Lonza). The arrows indicate the nuclear focus of the proteins in ECs after HS. Scale bar: 50 µm. Abbreviation used: HUVEC, human vein.
Fig. 2.
Morphological characterization of thermal stress in human endothelial cells. (a) Primary HUVECs were exposed to heat shock (HS) at 42 °C for 1 h and allowed to recover at 37 °C for 24 h. Images were acquired using optical microscopy (ZEISS Primovert) before HS (0 h), immediately after HS (1 h), and after recovery (24 h). Scale bar: 100 µm. Inset scale bar: 50 µm. Representative images of n = 8 independent experiments using cells from different lots (19TL028325, 19TL023264, 19TL127368, and 21TL195720, Lonza). (b) Supernatants from these cells were collected, and HS cytotoxicity was measured by quantifying lactate dehydrogenase (LDH) activity. The data are shown as the means ± SEMs of 3 independent experiments (lot 19TL127368 and 19TL028325), ordinary one-way ANOVA, with Tukey’s multiple comparisons test, **P < 0.01, ***P < 0.001 versus positive control. HUVECs were treated with Triton X-100 to achieve maximum LDH release from the supernatant. (c) HSF1 (green, CST 4356S) and total HSP70 (red, Invitrogen MA3-006). Abbreviation used: HUVEC, human vein.
Heat shock and hypoxia control the stability of HSP multimers independent of the human endothelial vascular bed
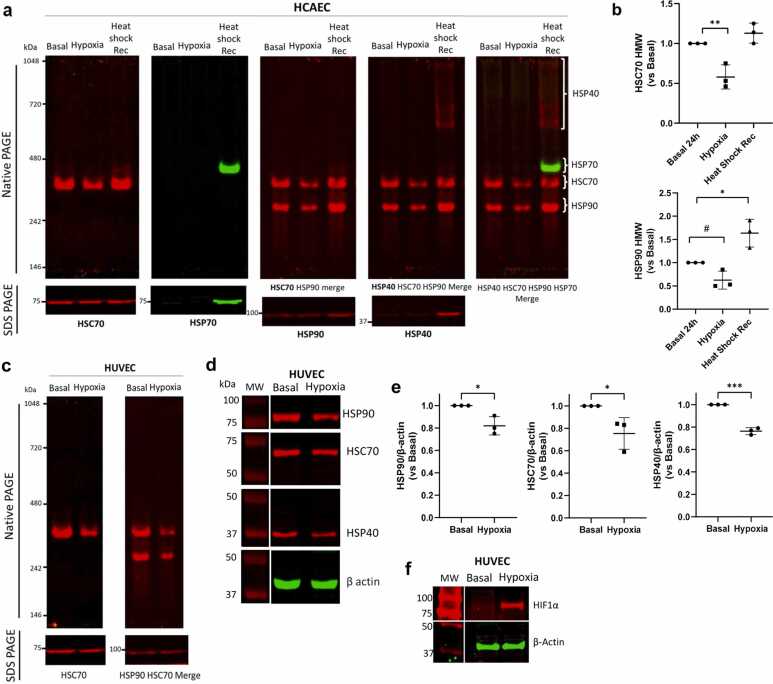
To shed light on another possible mechanism that contributes to the HS response in human ECs, we hypothesized that HS in ECs could also induce the formation of high-molecular-weight complexes of HSPs, as reported previously in different in vitro14, 16, 21 and in vivo stresses.12, 15, 22, 23 These complexes are well-known hallmarks of the proteotoxic response found in tumors and Alzheimer’s disease, named the epichaperome, which is centralized in HSC70, a constitutively expressed HSP70 family member, and HSP90.22, 23 Native PAGE is the gold standard methodology for evaluating chaperone high-molecular-weight complexes.24 Our results revealed that HSC70 oligomerization was a feature of primary human ECs under basal conditions, was increased by HS, and returned to basal levels after recovery. HSP70 expression was further increased after HS recovery compared with that after HS recovery (Figure 3(a)–(d)). In contrast to HSC70, the amount of the HSP90 dimer decreased in response to HS and increased after HSR. HSP40 high-molecular-weight accumulated only after the HSR and remained monomeric under all conditions (Figure 3(c) and (d)). Sequential membrane staining with specific antibodies and its quantification are shown in Figure 3(b). We took advantage of coupling Native gel electrophoresis with near-infrared fluorescent LICOR Odyssey imaging system. To evaluate migration profiles and quantify HSPs within the same assay, we incubated the same membrane with specific antibodies, followed by appropriate mouse or rabbit secondary antibodies (Figure 3(e)). This was done sequentially for each HSP analyzed, without removing previous staining, thereby maintaining membrane integrity and enabling direct comparison of migration profiles through image merging. This original approach allowed us to compare migration patterns across proteins. Notably, the quantification presented in the native gel in graphs was carried out only after each individual primary antibody staining, as shown in the separate panels, to ensure maximum accuracy and specificity in detecting each protein. EC behavior was notably different from that of cancer cells,24 as oligomerization did not occur in the presence of other HSPs.
Fig. 3.
Temporal modulation of human HSP high-molecular-weight complexes by heat shock in primary ECs. (a) Invitrogen’s NativeMark™ was subjected to Native PAGE, stained with Coomassie blue, and used as a standard to identify high-molecular-weight complexes in native PAGE. (b) Whole-cell lysates from HUVECs exposed to heat shock, recovery, or basal conditions were subjected to native PAGE and sequentially stained for HSP40 (CST, 4868S), HSC70 (Invitrogen PA5-27337), HSP70 (Invitrogen MA3-009), and HSP90 (CST, 4877S). Below, sodium dodecyl sulfate-PAGE for each protein is displayed. The data are representative of n = 3–6 independent experiments with cells from different lots (19TL127368, 19TL028325, 19TL023264, and 21TL195720). (c) Merged image of all the stained samples shown in (b). The data are representative of n = 3–6 independent experiments in cells from different lots (19TL127368, 19TL028325, 19TL023264, and 21TL195720). (d) Quantification of the high-molecular-weight complex intensities from specific proteins from (b) versus Basal n. The data are presented as one-way ANOVA, with Tukey’s post hoc test; **P < 0.01, ***P < 0.001 versus the respective control; HSP40, n = 3; HSP70, n = 4; HSC70, n = 5; and HSP90, n = 6. (E) Schematic representation of the native PAGE procedure. Proteins in their native state from cell lysates were separated on a 6% polyacrylamide gel and transferred to a nitrocellulose membrane overnight. The membrane was blocked with nonfat milk, incubated with primary antibody overnight, and then incubated with secondary antibody. The membrane was scanned using the LI-COR Odyssey® 2-channel near-infrared fluorescence imaging system, and the fluorescence levels of the last HSP probed were quantified. The same membrane was then incubated overnight with a primary antibody against another chaperone, followed by secondary antibody incubation, scanning with the near-infrared fluorescence imager, and quantification. This process was repeated daily until all chaperones were stained on the same membrane, in this order: day 1 anti-HSP40; day 2 anti-HSC70; day 3 anti-HSP90; day 4 anti-HSP70. Abbreviations used: EC, endothelial cell; HSP, heat-shock protein; HUVEC, human vein.
Next, we investigated whether this human EC oligomerization is vascular bed dependent and specific to HS. First, we showed that human coronary artery endothelial cells (HCAECs) also present an oligomeric response that is quite similar to a venous response (Figure 4(a) and (b)). We also evaluated the effects of hypoxic conditions on the amount and oligomerization of heat shock proteins in ECs. The intersection between cancer and cardiovascular diseases is a low-oxygen-pressure environment,25 with HSP upregulation-like protective mechanisms well documented in several cell types.26 Hypoxic ECs succeeded in changing oxidative phosphorylation toward the glycolytic pathway and downregulated HSP7027; however, the data failed to support protein quantification, precluding solid conclusions. Human lung fibroblast, alveolar, and pulmonary smooth muscle cells have been shown to upregulate proteins with a molecular weight corresponding to HSPs.28 Interestingly, the stability of the HSP70 oligomer and HSP90 dimers was greatly diminished in hypoxic HCAECs (Figure 4(a) and (b)) and HUVECs (Figure 4(c)). The hypoxia assays were performed in a HYPO 856 chamber under 1% oxygen gas atmosphere. A major transcription factor activated by hypoxia is the hypoxia-inducible factor α (HIF-1α), which is not hydroxylated under hypoxic conditions and thus avoids being degraded. In response to this stress, HIF-1α accumulates inside the cell. These results mirrored the decreased intracellular HSP90 and HSC70 expression (Figure 4(d) and (e)), and as expected, hypoxia increased HIF-1α stability (Figure 4(f)). Taken together, these results indicate the opposite picture, which is dependent on the type of stress to which ECs are exposed.
Fig. 4.
Hypoxia induces decreases in multimeric HSPs in human coronary arteries (HCAECs) and vein endothelial cells (HUVECs). (a) Whole-cell lysates from HCAECs exposed to hypoxia, heat-shock recovery, or basal conditions were subjected to native PAGE and sequentially stained for HSC70 (Invitrogen PA5-27337), HSP70 (Invitrogen MA3-009), HSP90 (CST 4877S), and HSP40 (CST 4868S), as shown in the top panels. Below, sodium dodecyl sulfate-PAGE results for each protein are displayed. Data are representative of n = 3 independent experiments using two different lots of cells (20TL365545 and 20TL064651, Lonza). Both native PAGE and Western blot experiments were performed using two different lots of cells (20TL365545 and 20TL064651). (b) Quantification of HSC70, HSP70, and HSP90 high-molecular-weight complexes in (a). Data are presented as the means ± SEMs, one-way ANOVA, with Tukey’s post hoc n = 3, *P < 0.05, **P < 0.01 versus basal conditions. Unpaired t test (#P < 0.05) was used to compare only the HSP90 hypoxic and basal groups. (c) Whole-cell lysates from HUVECs exposed to hypoxia or basal conditions were subjected to native PAGE and sequentially stained for HSC70 (Invitrogen PA5-27337) and HSP90 (CST 4877S), as shown in the top panels. Below, sodium dodecyl sulfate-PAGE for each protein is displayed. The data are representative of n = 2 independent experiments using cells from lot 19TL028324. (d) Whole-cell lysates of HUVECs exposed to hypoxia or basal conditions were subjected to Western blot analysis for HSP40 (CST 4868S), total HSP70 (Invitrogen MA3-006), HSC70 (Invitrogen PA5-27337), and HSP90 (CST 4877S). The data are representative of n = 3 independent experiments using cells from lots 19TL028324 and 23TL086130. (e) Data from (d) are presented as the mean ± SEM, unpaired Student’s t test, n = 3 with *P < 0.05 and ***P < 0.001. β-actin, loading control. (f) HUVECs subjected to basal or hypoxic 1% O2 (94% N2, 5% CO2) for 24 h were subjected to Western blot analysis for HIF1α (CST, #48085) with β-actin (Sigma, A5441) loading control. The assay was performed three times using two different lots of cells (21TL195720 and 23TL086130).
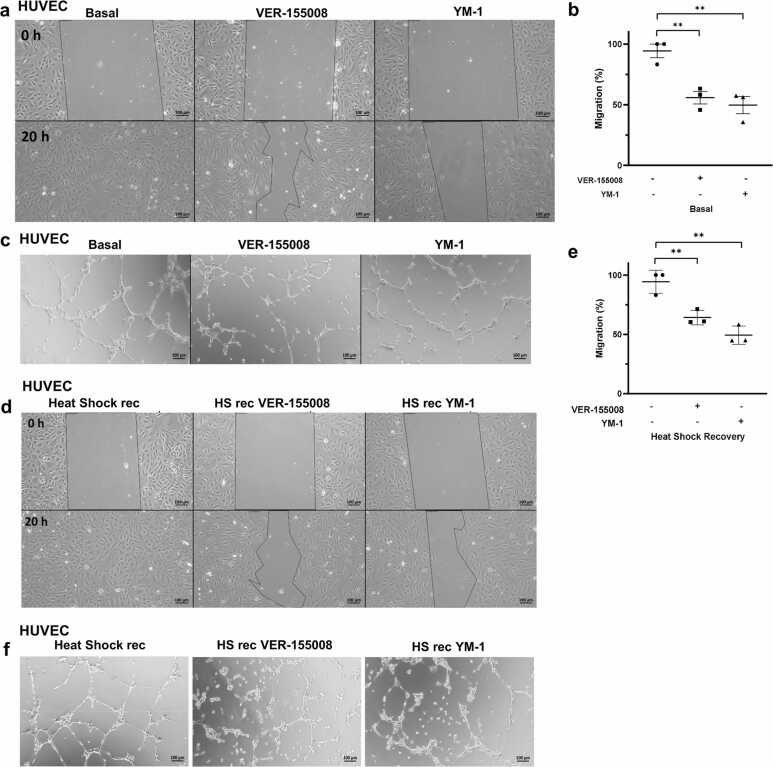
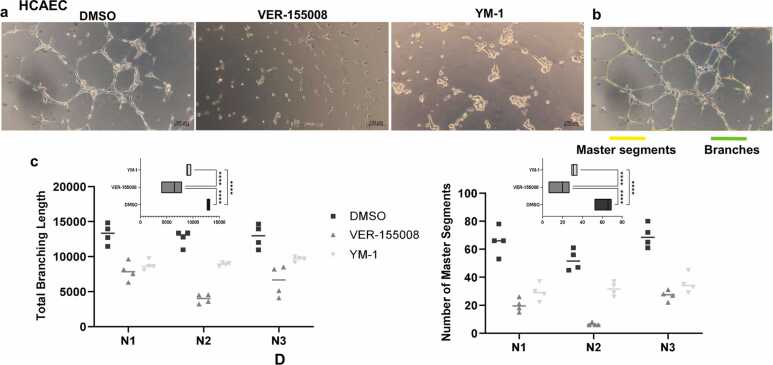
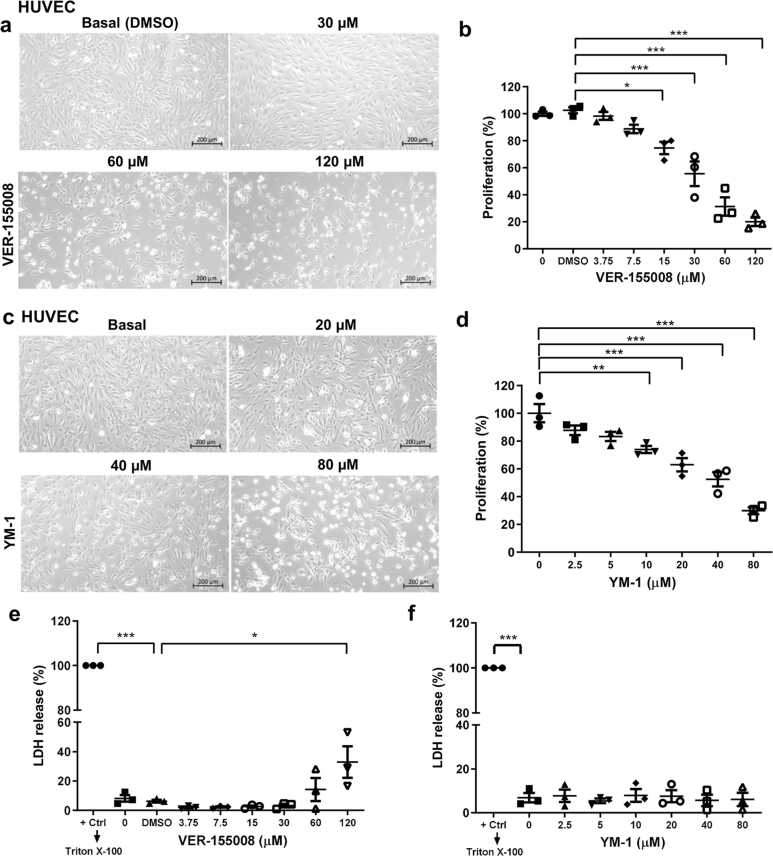
To understand HSP70 function in this context, we probed the role of HSP70 in primary human ECs after HS and recovery using specific cytosolic HSP70 inhibitors, VER-15500829, 30 and YM-1,31, 32 which affect ATPase activity and stabilize the ADP-HSP70 conformation, respectively. Both inhibitors impaired EC migration at normal levels (Figure 5(a) and (b)) and after HS recovery (Figure 5(d) and (e)). We also performed similar HSP70 inhibition in angiogenesis assays in vitro and observed a negative impact of HSP70 loss of function on tube formation, which was independent of stressed EC behavior (Figure 5(c) and (f)) and EC type (Figure 6(a)). VER-155008 and YM-1 reduce the total branching length and number of master segments in HCAEC (Figure 6(b) and (c)). HSP70 inhibitors can promote a decrease in the cellular proliferation rate.33 Recently, it was demonstrated that HSP70 performs mitotic functions.34 Proliferation is a well-described EC feature in angiogenesis and cardiovascular diseases. Therefore, we investigated whether HSP70 contributes to the EC cell cycle through proliferation assays. Finally, we showed that endothelial cell proliferation was reduced by HSP70 inhibition (Figure 7(a)–(d)). Furthermore, we confirmed that the two inhibitors were not cytotoxic to ECs (Figure 7(e) and (f)). These results demonstrate that, despite the general upregulation of HSPs, which is thermally stress dependent through heat-shock transcription factor 1 (HSF1) activation,19 HSP70 governs the majority of the EC phenotype.
Fig. 5.
HSP70 plays a central role in the primary human endothelial cell (EC) phenotype. (a) Migration was evaluated in a wound healing assay in HUVECs cultured for 1 h in EBM-2 under basal conditions or in the presence of 30 µM VER-155008 or 20 µM YM-1. After scratching, the medium was changed to EGM-2 with the respective inhibitors, and the cells were allowed to migrate for 20 h. Representative images of n = 3 independent experiments using two different lots of cells (21TL195720 and 19TL028324) are shown. Scale bar: 100 µm. (b) The area of each scratch at 0 and 20 h was measured, and the percentage of migration was calculated as follows: migration = (original wound area − area after healing)/original wound area × 100. The data from (a) are presented as the means ± SEMs; ordinary one-way ANOVA with Tukey’s multiple comparisons test, **P < 0.01 versus the control; n = 3. (c) HUVECs were cultured under basal conditions and then plated in Matrigel in the presence of the inhibitors VER-155008 (50 µM) or YM-1 (40 µM). After 5 h of incubation, images were acquired. Representative images of n = 3 independent experiments using cells from lot 21TL195720. Scale bar: 100 µm. (d) HUVEC migration after heat-shock recovery, followed by 30 µM VER-155008 or 20 µM YM-1 treatment, was evaluated in a wound healing assay. Representative images of three independent experiments using two different cell lots (21TL195720 and 19TL028324) are shown. Scale bar: 100 µm. (e) The area of each scratch at 0 and 20 h was measured from (a). Data are presented as the mean ± SEM; one-way ANOVA with Tukey’s post hoc test, n = 3; **P < 0.01 versus conditions. (f) HUVECs exposed to heat shock for 1 h and allowed to recover for 24 h, as in (d), were treated with 50 µM VER-155008 or 40 µM YM-1 and subjected to 5 h of tube formation. Representative images of three independent experiments using cells from lot 21TL195720. Scale bar: 100 µm. Abbreviation used: HUVEC, human vein.
Fig. 6.
HSP70 loss of function impairs coronary artery angiogenesis in vitro (a) HCAECs were cultured under basal conditions and then plated in Matrigel in the presence of the inhibitors VER-155008 (50 µM) or YM-1 (40 µM). After 5 h of incubation, images were acquired. Representative images of triplicate independent experiments are shown. (b) Representative image of angiogenesis analysis with a map of selected parameters. (c) Quantification of the total branching length (left) and number of master segments (right). Each point represents repeated measurements taken in three independent experiments (N) for each treatment (symbol). The insets show the results of two-way ANOVA with Tukey’s post hoc test; ****P < 0.0001 indicates significant differences among means. Abbreviations used: HCAEC, human coronary artery endothelial cell; HUVEC, human vein.
Fig. 7.
Characterization of VER-155008 and YM-1 in primary human endothelial cells. (a) Phase-contrast images (ZEISS Primovert) of primary HUVECs treated with 30, 60, or 120 µM VER-155008 for 24 h and (c) 2.5, 5, 10, 20, 40, or 80 µM YM-1. The volume of DMSO used corresponded to the highest concentration of VER-155008 (120 µM). Scale bar: 200 µm. (b) Cell proliferation was measured by counting the number of cells after treatment with 3.75, 7.5, 15, 30, 60, or 120 µM VER-155008 for 24 h and (d) 2.5, 5, 10, 20, 40, or 80 µM YM-1. (e) and (f) A cytotoxicity assay was performed by quantifying the activity of lactate dehydrogenase in the supernatants. The data are presented as the means ± SEMs. Statistical significance was determined by ordinary one-way ANOVA with Tukey’s multiple comparisons test; *P < 0.05, **P < 0.01, ***P < 0.001 versus the respective control. All experiments were independently performed three times using two different lots of cells (21TL195720 and 19TL028324). Abbreviation used: HUVEC, human vein.
Discussion
A major mechanism of the ancient and highly conserved HS response involves the handling of HSPs at the transcriptional and translational levels to guarantee organism survival.19 Our results shed light on a new layer of this complex response: dynamic HSP oligomerization during HS, recovery, and hypoxia conditions preserved in both primary human vein and coronary artery ECs (Fig. 3, Fig. 4). We demonstrated that HSP70-linked angiogenesis occurs during HS recovery (Figure 5). Overall, cytosolic human HSP70 has been shown to govern three EC responses: migration, angiogenesis, and proliferation (Fig. 5, Fig. 6, Fig. 7). These results suggest that HSP70 performs a major function in preventing EC dysfunction, as we demonstrated in type IV hypertension8 and in cardiovascular disease.18, 3 We and others previously reported that HSP70 plays roles in migration and angiogenesis,8, 10, 35 as also confirmed here.
Notably, intracellular and extracellular HSP70 pools can elicit opposite cardiovascular outcomes,36, 2 and intracellular HSP70 protects cardiomyocytes under ischemic conditions induced by thermal stress.37 A full analysis with a loading control and quantification of HSPs in ECs has not been reported in the literature. We observed a healthy endothelial cell response through the induction of HSPs (Figure 1) and HSC70 nuclear translocation (Figure 1(c)), as observed in HeLa cells,38 whereas inducible HSP70 remained in the cytosol (Figure 1(c), (d)). HSP40/Hdj1/DNAJB1 was also detected in the nucleus, even in nonstressed cells, and was upregulated by thermal stress in ECs (Figure 1). The HSP40 family delivers and stimulates low ATPase HSP70 activity.39 The unchanged levels of pan-HSP90 were unexpected (Figure 1(a)) since the antibody also recognizes HSP90α, and we could not detect increases in cytosolic inducible HSP90.
Furthermore, we showed that hypoxic ECs, which are also characterized by HSF1 activation,40 unexpectedly reduced HSP70/40/90 levels (Figure 4(d) and (e)). This is not due to a failure of the HS response because we observed that, in ECs, HSP upregulation likely occurred through HSF1 (Figure 2(c)), as expected.41 HSF1 is usually found as a monomer in the cytoplasm and is translocated to the nucleus in the HS response.19 We were curious to observe HSF1 in the endothelial nucleus even under normal conditions (Figure 2(c)). These data deserve further investigation. Overexpressing HSF1 in human aortic ECs upregulated HSP70, eNOS, and thrombomodulin and decreased the expression of plasminogen activator maintain inhibitor-1 (PAI-1) and endothelin-1 secretion.42 Furthermore, we showed that HS increases the expression of HSP70 and HSP40 (Figure 1(a) and (b)).
HS promotes HSC70 and HSP70 oligomer accumulation in ECs as the amount of HSP70 increases, whereas HSC70 presents biphasic behavior that returns to normal levels parallel to the detection of high-molecular-weight HSP40 complexes (Figure 3(b) and (c)). HSC70 helps fold and maintain proteins close to native or refoldable conformations in stress granules.19 This oligomerization, stimulated by HS, is found in the HSP70 family in other cell types,12 suggesting a conserved evolutionary phenomenon related to function.13 Together, these data support a protective mechanism independent of the origin of vascular cells elucidated by thermal stress in the vessel and likely crucial to EC thermotolerance. Here, we present another example, in addition to overlapping arterial and vein network responses to shear stress,43 in which HS and hypoxia responses activate signaling pathways, culminating in opposite HSP dynamic oligomerization in HCAECs and HUVECs.
We discovered that low-oxygen pressure alters the equilibrium stability of HSC/P 70 oligomers toward monomers and prevents the further oligomerization of HSP70 and HSP40 (Figure 4(a)–(c)) in a concentration-dependent manner. Importantly, HIF1α was shown to be an HSP70 client in HeLa cells.44 Although both HS and hypoxia are proteotoxic stresses, opposite oligomerization profiles coincide with thermotolerance, which is beneficial to the endothelium.40, 3 The chronic hypoxic environment promotes endothelial mesenchymal transition,45 and we propose that the low intracellular HSP expression that we observed contributed to this phenomenon. The downregulation of HSPs under hypoxia is curious, as HSF1 activation in ECs40 indicates that posttranslational mechanisms could be involved in HSP stability.
Conclusions
From a phenotypic perspective, several diseases, including restenosis, atherosclerosis, hypertension, thrombosis, stroke, and diabetes, are caused by angiogenesis failure.46 We showed that during recovery from thermal stress (HSR), HSP70 continues to be important for migration and in vitro angiogenesis (Figure 5(c) and (f) and Figure 6(a) and (c)). Our results challenge the classical view of the modulation of HSP functions through unique upregulated expression, possibly contributing to cellular defense mechanisms. In addition to the well-known HSP70–HSP90 heterocomplex, our work highlights a new structural composition that supports temporal molecular chaperone dynamics modulated by thermal and hypoxia stresses. Oligomerization is the hallmark of human ECs independent of the vascular bed, indicating that it could be an important therapeutic target in cardiovascular disease by increasing their formation or stimulating their stability. Although ubiquitous molecular chaperones differ in terms of oligomerization, HSP40 forms high-molecular-weight complexes, and the self-oligomerization of HSP70 and HSC70 and the formation of the HSP90 dimer appear to be related to subcompartmentalization in the crowded environment, which deserves further investigation. Our work in primary human ECs from veins and coronary arteries supports the central role of HSP70 in the human vasculature under normoxia and hypoxia and advances our knowledge of how molecular chaperones, particularly HSPs, support the phenotypic plasticity underlying cardiovascular disease biogenesis.
Materials and methods
Cell culture
Primary human ECs obtained from Lonza were cultured in a humidified incubator at 37 °C and 5% CO2 (Thermo Scientific and CELLXPERT®C170I, Eppendorf) in adherent culture flasks with EBM-2 (CC-3156, Lonza) supplemented with the EGM-2 BulletKit (CC-3162, Lonza) for HUVECs (passages 2–5, C2519A, Lonza) 1 or the EGM-2MV BulletKit for HCAECs (passages 4–7, CC-2585, Lonza) 2. Confluent adherent cells were washed with 25 mM HEPES buffer (pH 7.5), detached with 0.25% trypsin/EDTA, and subcultured. All experiments were performed in EBM-2 medium supplemented with EGM-2 or EGM-2MV unless otherwise specified, using the different lots of cells described in Table 1. All materials and reagents listed in Table 2
Table 1.
HUVEC and HCAEC lots.
| Cell | Brand | Lot number | Assays |
|---|---|---|---|
| HUVEC | Lonza | 19TL028324 | Migration, IF |
| HUVEC | Lonza | 19TL023264 | Western blot Native PAGE Heat shock, IF |
| HUVEC | Lonza | 19TL127368 | Western blot Native PAGE LDHGlo Heat shock |
| HUVEC | Lonza | 19TL028325 | Western blot Native PAGE LDHGlo Heat shock |
| HUVEC | Lonza | 21TL195720 | Migration Tube formation Western blot Native PAGE Heat shock |
| HUVEC | Lonza | 23TL086130 | Hypoxia Western blot |
| HUVEC | Lonza | 21TL95720 23TL104314 23TL135742 |
WB |
| HCAEC | Lonza | 20TL365545 | Western blot Native PAGE Hypoxia Heat shock |
| HCAEC | Lonza | 20TL064651 | Western blot Native PAGE Hypoxia Heat shock |
| HCAEC | Lonza | 21TL316169 | Tube formation |
Abbreviations used: HCAEC, human coronary artery endothelial cell; HUVEC, human umbilical vein endothelial cell.
Table 2.
Materials and reagents.
| Material or reagent | Brand | Catalog number |
|---|---|---|
| Pierce™ BCA Protein Assay Kits | Thermo Fisher Scientific | 23227 |
| Acrylamide/Bis-acrylamide, 30% | Sigma-Aldrich | A3574 |
| Acrylamide/Bis-acrylamide, 40% | Sigma-Aldrich | A7802 |
| Lonza Endothelial Basal Medium-2 (EBM-2) | Lonza | CC-3156 |
| BulletKit EGM-2 | Lonza | CC-3162 |
| Halt™ Protease Inhibitor Cocktail (100X) | Thermo Fisher Scientific | 78429 |
| Halt™ Phosphatase Inhibitor Cocktail (100X) | Thermo Fisher Scientific | 78420 |
| 75 cm² U-shaped canted neck cell culture flask | Corning | 3290 |
| 100 mm Culture Dish | Corning | 430167 |
| 12-well Cell Culture Plate | Corning | 3513 |
| Fetal Bovine Serum, Qualified, Brazil (SFB) | Gibco | 12657029 |
| Matrigel® Matrix | Corning | 354230 |
| Pierce™ Protein A/G Agarose | Thermo Scientific | 20421 |
| Millicell® Cell Culture Insert | Sigma-Aldrich | PIHP01250 |
| Glycerol | Sigma-Aldrich | G5516 |
| HEPES | Sigma-Aldrich | H3375 |
| Nitrocellulose Membrane | Bio-Rad | 1620115 |
| Methanol | Merck | 106007 |
| Fetal Bovine Serum | Gibco | 12657-029 |
| Trizma Base | Sigma-Aldrich | 93352 |
| VER-155008 | Sigma-Aldrich | SML0271 |
| YM-1 | Sigma-Aldrich | SML0943 |
| EZ Slide Millicell | Sigma-Aldrich | PEZGS0816 |
| NP-40 | Sigma-Aldrich | I8896 |
| Bovine Serum Albumin | Sigma-Aldrich | A7906 |
| Hoechst | Sigma-Aldrich | B2261 |
| ProLong™ Diamond Antifade Mountant | Invitrogen | P36961 |
| Triton™ X-100 | Sigma-Aldrich | T8787 |
| Sodium Chloride (NaCl) | Sigma-Aldrich | 59888 |
| Dibasic Sodium Phosphate (Na2HPO4) | Synth | F103201 |
| Monobasic Potassium Phosphate (KH2PO4) | Sigma-Aldrich | P9791 |
| Potassium Chloride (KCl) | Sigma-Aldrich | 104936 |
| Glycine | Sigma-Aldrich | G8898 |
| Sodium Dodecyl Sulfate (SDS) | Sigma-Aldrich | L3771 |
| Tween-20 | Sigma-Aldrich | P1379 |
| LDH-Glo™ Cytotoxicity Assay | Promega | J2381 |
| DMSO | Sigma-Aldrich | D8418 |
Heat Shock
A total of 2 × 106 cells (passages 4–6) were plated in 100 mm culture dishes and cultured for 24 h in an incubator at 37 °C and 5% CO2. Four plates of cells were washed with 25 mM HEPES pH 7.5 and incubated for 1 h at 37 °C (basal condition) or 42 °C in EBM-2 (CC-3156, Lonza), followed by lysis for Native PAGE. Under basal recovery and recovery conditions, the medium was changed to supplemented EBM-2, and the mixture was incubated for 24 h in an incubator at 37 °C and 5% CO2. A total of 8 × 106 cells were used for each condition.
Hypoxia
Cells at passages 4–6 were seeded in four 100 mm culture dishes at 2 × 106 cells/plate, totaling 8 × 106 cells. They were cultured for 24 h in an incubator at 37 °C with 5% CO2 in supplemented culture medium. The culture medium was changed to fresh medium, and the cells were kept in a Glove Box HYPO 856 Plas Labs hypoxia chamber at 1% O2 (by adding 94% N2 and 5% CO2) and 37 °C for 24 h.
Native PAGE
After HS or hypoxia, primary HUVECs or HCAECs were lysed according to the methods of Rodina et al.24 After the treatments, the cells were washed once with ice-cold 25 mM HEPES (pH 7.5), removed from the plates, centrifuged at 1500 rpm and 10 °C for 5 min and lysed in lysis buffer containing 0.01% NP40 and 20 mM Tris (pH 7.4), 20 mM KCl, 5 mM MgCl2 and 10% glycerol with protease inhibitors (diluted 1:100) and phosphatase (diluted 1:100) for 30 min on ice, followed by 5 cycles of freezing on dry ice and thawing. The lysates were centrifuged at 11,000 rpm and 4 °C for 1 min, the lysate supernatants were collected, and the protein concentrations were quantified using a Pierce™ BCA protein assay kit. Native PAGE was performed with 30 μg of lysate protein mixed with 6× sample buffer (37.5% Tris 1 M pH 8, 60% glycerol, 0.06% bromophenol blue) and subjected to electrophoresis on a 6% polyacrylamide gel (acrylamide/bisacrylamide 40%) with running buffer (25 mM Tris-base, 192 mM glycine, pH 8.3) at 100 V and 4 °C for 4 h on a Bio-Rad electrophoresis system. Proteins were transferred to nitrocellulose membranes in transfer buffer (25 mM Tris-base, 192 mM glycine, 20% methanol, 0.1% sodium dodecyl sulfate (SDS)) at 30 V overnight (18 h) on a Bio-Rad system in a refrigerator. Next, the membranes were blocked with 5% milk solution in TBS-T buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 1 h at room temperature with agitation and stained with primary antibodies diluted in TBS-T in an overnight incubation at 4 °C with agitation. The membranes were then incubated with anti-mouse IRDye 800CW or anti-rabbit IRDye 680RD secondary antibodies diluted 1:4000 in TBS-T for 1 h at room temperature and washed again three times with TBS-T for 10 min. Fluorescence was detected using ChemiDoc (Bio-Rad) or Odyssey (LI-COR) instruments. The fluorescence intensity of the labels was quantified using Image Studio 5.2 (LI-COR) (Table 3).
Table 3.
Antibodies used in the experimental assays.
| Target/Antibody | Brand | Catalog number | Assay |
|---|---|---|---|
| HSP70 | Invitrogen | MA3-006 | WB 1:1000, IF 1:400 |
| Inducible HSP70 | Invitrogen | MA3-009 | NP 1:1000, WB 1:1000, IF 1:400 |
| HSP90 | Cell signaling | 4877 | NP 1:1000, WB 1:1000 |
| HSP40 | Cell signaling | 4868 | NP 1:500, WB 1:500 IF 1:200 |
| HSC70 | Invitrogen | PA5-27337 | NP 1:1000, WB 1:1000 IF 1:200 |
| HSF1 | Cell signaling | 4356S | IF 1:200 |
| HIF1α | Cell signaling | 48085 | WB 1:1000 |
| β-actin | Sigma—Aldrich | A5441 | WB 1:10.000 |
| Anti-mouse IgG secondary antibody | LI-COR | 926-32212 | NP 1:4000, WB 1:10.000 |
| Anti-rabbit IgG secondary antibody | LI-COR | 926-68073 | NP 1:4000, WB 1:10.000 |
| Alexa Fluor 488 | Invitrogen | 2110499 | IF 1:400 |
| Alexa Fluor 546 | Invitrogen | A11030 | IF 1:400 |
NP, native PAGE; WB, Western blot; IF, immunofluorescence.
Tube formation
As previously described by Salibe-Filho et al.8 each well of a precooled 48-well plate was coated with 150 µL of Matrigel® Matrix and polymerized for 5 min at room temperature, followed by 30 min at 37 °C. A total of 2 × 104 HUVECs and 3 × 104 HCAECs exposed to basal conditions (24 h) or HS recovery were plated per well in EGM-2 medium containing 50 μM VER-155008 or 40 μM YM-1 and DMSO (VER vehicle) and incubated for 5 h in a humidified oven at 37 °C and 5% CO2. Images were obtained using a Carl Zeiss Primovert inverted microscope (491206-0005-000) coupled to an Axiocam 208 camera with a 10X objective and ZEN 3.1 software.
Migration (wound healing) assay
As previously described for HPAECs in Salibe-Filho et al.,8 0.7 × 105 HUVECs per well were plated in a 12-well plate (Corning, 3513) in EGM-2 medium and incubated in a humidified oven at 37 °C and 5% CO2 for 24 h. The heat-shock protocol was then performed with 24-h recovery as described above. The basal and heat-shocked cells at 23 h of recovery were incubated for 1 h in EBM-2 supplemented with the inhibitors 30 μM VER-155008 or 20 μM YM-1 or DMSO (control). A longitudinal scratch with a sterile P200 tip was made in the well center, and the cells were maintained in EBM-2 supplemented with EGM-2 and cultivated in an incubator at 37 °C and 5% CO2 for 20 h. Images were captured using a Carl Zeiss Primovert inverted microscope (491206-0005-000) coupled to an Axiocam 208 camera with a 10X objective at 0 h and 20 h after the scratch and migration had ended. The wound area was determined using the ZEN 3.6 program (Zeiss), and the migration rate was calculated using the equation %Migration = (original wound area - wound area after healing)/original wound area × 100.
Western blotting
Cells were lysed in different buffer compositions as specified in each assay, and all the steps described below were performed using the Bio-Rad system (mini-PROTEAN Tetra Cell, Mini Trans-Blot Module Core, and PowerPac Basic Power Supply). Total protein lysates (20–40 µg) were subjected to SDS-PAGE in a 12% bis-acrylamide gel at 100 V at room temperature in running buffer (25 mM Tris-base, 192 mM glycine, and 1% SDS, pH 8.3). The gel was then transferred to a membrane using a wet transfer protocol at 100 V at 4 °C with transfer buffer containing 48 mM Tris-base, 39 mM glycine, 0.037% SDS, and 20% methanol (pH 8.3). The nitrocellulose membrane was blocked with 5% nonfat milk (in TBS-T (20 mM Tris-Base, 150 mM NaCl, and 0.1% Tween-20, pH 7.4)) for 1 h at room temperature and then incubated with primary antibody solution in TBS-T overnight at 4 °C. The following day, the membrane was washed three times with TBS-T for 10 min and incubated with the following secondary antibodies: IRDye® 800CW donkey anti-mouse IgG or (most)/and IRDye® 680RD donkey anti-rabbit IgG diluted 1:10,000 in TBS-T for 1.5 h at room temperature. This protocol is based on previous work47. The membrane was washed three times with TBS-T for 10 min before it was scanned using a LI-COR OdysseyR 2-channel near-infrared fluorescence imaging system (Odyssey DL-x), and the protein bands were visualized and quantified according to the pixel density with Empiria Studio or ImageJ as recommended by the manufacturer. The specifications of the antibodies used are listed in Table 3
Immunofluorescence
Primary HUVECs were plated at 2.5 × 104 cells per well in EZ Slide Millicell 8-well chambers. The cells were treated after 24 h of growth with 30 µM VER-155008 and 20 µM YM-1 in a humidified incubator (37 °C, 5% CO2) under normoxic or hypoxic conditions (Hypoxia Chamber 856-Series, PLAS LABS, INC). After treatment, the cells were rinsed in PBS (7.78 mM Na2HPO4, 2.20 mM KH2PO4, 140 mM NaCl, and 2.73 mM KCl; pH 7.2) at 37 °C, fixed in 200 µL of 4% paraformaldehyde at 25 °C for 20 min, washed twice with 400 µL of PBS, permeabilized with 100 µL of 0.1% NP-40 in PBS for 30 min at 37 °C, and blocked with 2% bovine serum albumin (BSA) in PBS for 30 min at 37 °C Finally, the cells were incubated overnight at 4 °C with primary antibodies diluted in 1% BSA in PBS. The next day, the cells were washed three times with 400 µL of PBS and stained with Alexa Fluor 488 or Alexa Fluor 546 (1:400 dilution) for 1.5 h. Nuclei were stained with 20 µg/mL Hoechst along with secondary antibodies. The cells were washed three times with 400 µL of PBS, and the coverslips were mounted with 1 ProLong Diamond Antifade Mountant (Invitrogen): 1 PBS. The cells were visualized through an inverted laser confocal microscope (SP8 Leica) at 63× magnification from INFAR-UNIFESP facilities. Images were processed using Leica LasX software. The fluorescence intensities of the green and red channels were analyzed using ImageJ with the “RGB plot profile” tool. The specifications of the antibodies used are listed in .
Cell proliferation
Primary HUVECs were plated at 1.5 × 105 cells/well in a 12-well plate for 24 h. The cells were treated with 3.75, 7.5, 15, 30, 60, or 120 µM VER-155008 and 2.5, 5, 10, 20, 40, or 80 µM YM-1 for 24 h. Two controls were used: an equivalent volume of DMSO used in 120 µM VER-155008 and another without DMSO. For the YM-1 assay, no vehicle was added. The following day, the cells were washed with 1X PBS, detached from the plate with 200 µL of trypsin, and incubated at 37 °C for 5 min. After detachment, the cells were resuspended in 300 µL of supplemented EGM-2 medium. The viable cells were counted in a Neubauer counting chamber (1:1 cell and trypan blue). The number of cells was converted into a percentage, and the values were plotted using GraphPad Prism software. Images of the cells were captured using a microscope (Carl Zeiss) coupled with an Axiocam 208 color camera. The data were analyzed using statistical analyses as described in the legends.
Extracellular lactate dehydrogenase cytotoxicity assay
Primary HUVECs were plated at 1.5 × 105 cells per well in a 12-well plate, and the cells were treated with VER-155008 (3.75, 7.5, 15, 30, 60, or 120 µM) or YM-1 (2.5, 5, 10, 20, 40, or 80 µM) for 24 h or a DMSO equivalent volume of 120 µM VER-155008 as a specific control. The next day, the positive control (maximum lactate dehydrogenase (LDH) release) was obtained by treating the cells with 0.2% Triton X-100 diluted in EGM-2 medium for 20 min. The supernatants were subsequently collected and diluted in LDH storage buffer (200 mM Tris pH 7.3, 10% glycerol, and 1% BSA) at a 1:100 ratio. Fifty microliters of the diluted supernatant was added to 96-well plates, followed by the addition of 50 µL of LDH detection reagent, which contained lactate, NAD+, reductase, reductase substrate, and Ultra-Glo™ rLuciferase. The plate was incubated at room temperature for 1 h. The percentage of cytotoxicity was calculated as follows: LDH release from samples/maximum LDH release from the positive control × 100. Both were previously subtracted from the background LDH level in the culture medium. An LDH-Glo™ Cytotoxicity Assay Kit was used to measure LDH levels in the cell culture supernatants.
Statistics
Graphs were generated and statistical analyses were performed using GraphPad Prism software. For comparisons involving multiple groups, one-way Analysis of variance (ANOVA) followed by Tukey’s post hoc test was used, and for comparisons of two groups, t test was used. A P-value of less than 0.05 was considered statistically significant, with significance described as *P < 0.05, **P < 0.01, and ***P < 0.001. All the results are shown as error bars indicating the mean ± SEM and were validated through a minimum of three independent experiments.
Funding and support
This work is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant no. 2018/13739-8), T.L.S.A. fellowship 2019/20435-8, L.B.C.T.C. fellowship 2020/11249-3, I.C.B.P. fellowship 2023/06938-2, A.P.M. fellowship 2024/20224-5, E.G.M. fellowship 2019/25503-1 and Capes. We are grateful to Prof Francisco Laurindo (InCor/FMUSP) and Prof Júlio Borges (IQSC-USP) for their help. We thank Iolanda Cuccovia, Flavia Meotti, Maurício Baptista from IQ-USP, and Carlos Gun from IDPC for providing infrastructure. We thank Marcia da Silva from IQ-USP and Elizabeth and Caroline for their technical support at the confocal UNIFESP facility (Laboratório Multiusuários-INFAR).
CRediT authorship contribution statement
Luiza B. C. T. Coimbra: Visualization, Methodology, Formal analysis, Data curation. Andrea Pinto-Martinez: Visualization, Validation, Methodology, Formal analysis, Data curation. Isadora C.B. Pavan: Visualization, Validation, Methodology, Formal analysis, Data curation. Everton G. Melo: Validation, Methodology, Data curation. Thaís L.S. Araujo: Writing – review and editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declarations of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- 1.Balchin D., Hayer-Hartl M., Hartl F.U. In vivo aspects of protein folding and quality control. Science. 2016;353 doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- 2.Araujo T.L.S., Venturini G., Moretti A.I.S., et al. Cell-surface HSP70 associates with thrombomodulin in endothelial cells. Cell Stress Chaperones. 2019;24:273–282. doi: 10.1007/s12192-018-00964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin I.J., McMillan D.R. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.RES.83.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Finka A., Goloubinoff P. Proteomic data from human cell cultures refine mechanisms of chaperone-mediated protein homeostasis. Cell Stress Chaperones. 2013;18:591–605. doi: 10.1007/s12192-013-0413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Cardeña G., Fan R., Shah V., et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y., Song N., Liu C., et al. Heat shock cognate 70 regulates the translocation and angiogenic function of nucleolin. Arterioscler Thromb Vasc Biol. 2012;32:126–134. doi: 10.1161/ATVBAHA.112.247502. [DOI] [PubMed] [Google Scholar]
- 7.Kim T.-K., Na H.J., Lee W.R., et al. Heat shock protein 70-1A is a novel angiogenic regulator. Biochem Biophys Res Commun. 2016;469:222–228. doi: 10.1016/j.bbrc.2015.11.125. [DOI] [PubMed] [Google Scholar]
- 8.Salibe-Filho W., Araujo T.L.S., Melo E.G., et al. Shear stress-exposed pulmonary artery endothelial cells fail to upregulate HSP70 in chronic thromboembolic pulmonary hypertension. PLoS One. 2020;15 doi: 10.1371/journal.pone.0242960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S.L., et al. HSP70-1 is required for interleukin-5-induced angiogenic responses through eNOS pathway. Sci Rep. 2017;7:44687. doi: 10.1038/srep44687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiota M., Kusakabe H., Izumi Y., et al. Heat shock cognate protein 70 is essential for Akt signaling in endothelial function. Arterioscler Thromb Vasc Biol. 2010;30:491–497. doi: 10.1161/ATVBAHA.109.193631. [DOI] [PubMed] [Google Scholar]
- 11.Sun X., et al. Asymmetric Dimethylarginine Stimulates Akt1 Phosphorylation via Heat Shock Protein 70-Facilitated Carboxyl-Terminal Modulator Protein Degradation in Pulmonary Arterial Endothelial Cells. Am J Respir Cell Mol Biol. 2016;55:275–287. doi: 10.1165/rcmb.2015-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takakuwa J.E., Nitika, Knighton L.E., Truman A.W. Oligomerization of Hsp70: current perspectives on regulation and function. Front Mol Biosci. 2019;6:81. doi: 10.3389/fmolb.2019.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trcka F., Durech M., Vankova P., et al. Human stress-inducible Hsp70 has a high propensity to form ATP-dependent antiparallel dimers that are differentially regulated by cochaperone binding. Mol Cell Proteomics. 2019;18:320–337. doi: 10.1074/mcp.RA118.001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson A.D., Bernard S.M., Skiniotis G., Gestwicki J.E. Visualization and functional analysis of the oligomeric states of Escherichia coli heat shock protein 70 (Hsp70/DnaK) Cell Stress Chaperones. 2012;17:313–327. doi: 10.1007/s12192-011-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelidis C.E., Lazaridis I., Pagoulatos G.N. Aggregation of hsp70 and hsc70 in vivo is distinct and temperature-dependent and their chaperone function is directly related to non-aggregated forms. Eur J Biochem. 1999;259:505–512. doi: 10.1046/j.1432-1327.1999.00078.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgner N., Schmidt C., Beilsten-Edmands V., et al. Hsp70 forms antiparallel dimers stabilized by post-translational modifications to position clients for transfer to Hsp90. Cell Rep. 2015;11:759–769. doi: 10.1016/j.celrep.2015.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiosis G., Digwal C.S., Trepel J.B., Neckers L. Structural and functional complexity of HSP90 in cellular homeostasis and disease. Nat Rev Mol Cell Biol. 2023;24:797–815. doi: 10.1038/s41580-023-00640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diteepeng T., Del Monte F., Luciani M. The long and winding road to target protein misfolding in cardiovascular diseases. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13504. [DOI] [PubMed] [Google Scholar]
- 19.Richter K., Haslbeck M., Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Wagner M., Hermanns I., Bittinger F., Kirkpatrick C.J. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- 21.Araujo T.L.S., Borges J.C., Ramos C.H., et al. Conformational changes in human Hsp70 induced by high hydrostatic pressure produce oligomers with ATPase activity but without chaperone activity. Biochemistry. 2014;53:2884–2889. doi: 10.1021/bi500004q. [DOI] [PubMed] [Google Scholar]
- 22.Ginsberg S.D., Sharma S., Norton L., Chiosis G. Targeting stressor-induced dysfunctions in protein–protein interaction networks via epichaperomes. Trends Pharmacol Sci. 2023;44:20–33. doi: 10.1016/j.tips.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi S., Wang T., Araujo T.L.S., et al. Adapting to stress — chaperome networks in cancer. Nat Rev Cancer. 2018;18:562–575. doi: 10.1038/s41568-018-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodina A., Wang T., Yan P., et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature. 2016;538:397–401. doi: 10.1038/nature19807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong B.W., Marsch E., Treps L., et al. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36:2187–2203. doi: 10.15252/embj.201696150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K., Sawa Y., Kaneda Y., et al. Overexpressed heat shock protein 70 attenuates hypoxic injury in coronary endothelial cells. J Mol Cell Cardiol. 1998;30:1129–1136. doi: 10.1006/jmcc.1998.0678. [DOI] [PubMed] [Google Scholar]
- 27.Oehler R., Schmierer B., Zellner M., et al. Endothelial cells downregulate expression of the 70 kDa heat shock protein during hypoxia. Biochem Biophys Res Commun. 2000;274:542–547. doi: 10.1006/bbrc.2000.3184. [DOI] [PubMed] [Google Scholar]
- 28.Graven K.K., Zimmerman L.H., Dickson E.W., et al. Endothelial cell hypoxia associated proteins are cell and stress specific. J Cell Physiol. 1993;157:544–554. doi: 10.1002/jcp.1041570314. [DOI] [PubMed] [Google Scholar]
- 29.Schlecht R., Scholz S.R., Dahmen H., et al. Functional analysis of Hsp70 inhibitors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson D.S., Borgognoni J., Clay A., et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem. 2009;52:1510–1513. doi: 10.1021/jm801627a. [DOI] [PubMed] [Google Scholar]
- 31.Colvin T.A., Gabai V.L., Gong J., et al. Hsp70–Bag3 interactions regulate cancer-related signaling networks. Cancer Res. 2014;74:4731–4740. doi: 10.1158/0008-5472.CAN-14-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang A.M., Miyata Y., Klinedinst S., et al. Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat Chem Biol. 2013;9:112–118. doi: 10.1038/nchembio.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gestwicki J.E., Shao H. Inhibitors and chemical probes for molecular chaperone networks. J Biol Chem. 2019;294:2151–2161. doi: 10.1074/jbc.TM118.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodina A., Xu C., Digwal C.S., et al. Systems-level analyses of protein-protein interaction network dysfunctions via epichaperomics identify cancer-specific mechanisms of stress adaptation. Nat Commun. 2023;14:3742. doi: 10.1038/s41467-023-39241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jang J., Kim M.R., Kim T.-K., et al. CLEC14a-HSP70-1A interaction regulates HSP70-1A-induced angiogenesis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-11118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krause M., Heck T.G., Bittencourt A., et al. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/249205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano M., Mann D.L., Knowlton A.A. Blocking the endogenous increase in HSP 72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation. 1997;95:1523–1531. doi: 10.1161/01.CIR.95.6.1523. [DOI] [PubMed] [Google Scholar]
- 38.Chu A., Matusiewicz N., Stochaj U. Heat-induced nuclear accumulation of hsc70 proteins is regulated by phosphorylation and inhibited in confluent cells. FASEB J. 2001;15:1478–1480. doi: 10.1096/fj.00-0680fje. [DOI] [PubMed] [Google Scholar]
- 39.Rosenzweig R., Nillegoda N.B., Mayer M.P., Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019;20:665–680. doi: 10.1038/s41580-019-0133-3. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin I.J., Kröger B., Williams R.S. Activation of the heat shock transcription factor by hypoxia in mammalian cells. Proc Natl Acad Sci USA. 1990;87:6263–6267. doi: 10.1073/pnas.87.16.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portig I., Pankuweit S., Lottspeich F., Maisch B. Identification of stress proteins in endothelial cells. Electrophoresis. 1996;17:803–808. doi: 10.1002/elps.1150170431. [DOI] [PubMed] [Google Scholar]
- 42.Uchiyama T., Atsuta H., Utsugi T., et al. HSF1 and constitutively active HSF1 improve vascular endothelial function (heat shock proteins improve vascular endothelial function) Atherosclerosis. 2007;190:321–329. doi: 10.1016/j.atherosclerosis.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 43.Maurya M.R., Gupta S., Li J.Y.-S., et al. Longitudinal shear stress response in human endothelial cells to atheroprone and atheroprotective conditions. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2023236118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo W., Zhong J., Chang R., et al. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1α but not HIF-2α. J Biol Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu X., Tan X., Tampe B., et al. Snail is a direct target of hypoxia-inducible factor 1α (HIF1α) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells. J Biol Chem. 2015;290:16653–16664. doi: 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 47.Araujo T.L., et al. Protein disulfide isomerase externalization in endothelial cells follows classical and unconventional routes. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.