Abstract
Objective
This study was aimed to investigate the relationship between neuron-specific enolase (NSE) and swallowing dysfunction in patients with acute ischemic stroke (AIS) and evaluate the impact of early enteral nutrition intervention on NSE levels.
Setting and participants
A retrospective study was conducted involving 445 AIS patients admitted to the neurology department of the Affiliated Hospital of Jiaxing University between September 2015 and August 2022. Data collected included gender, age, water-swallowing test (WST) score upon admission, and NSE examination results on admission, the 5th day, and the 10th day.
Results
Among 445 enrolled AIS patients, 42.0% (187/445) exhibited swallowing dysfunction. Key findings revealed: (1) Positive correlation between WST severity and serum NSE levels across all timepoints (P < 0.05). (2) Dysphagia patients demonstrated elevated NSE levels versus controls (P < 0.05). (3) Early enteral nutrition intervention (n = 98) significantly reduced NSE levels by day 10 compared to non-intervention group (P < 0.05), though no intergroup differences were observed at admission or day 5 (P > 0.05).
Conclusions
NSE measurement is a simple supplement to the WST. There existed a significant correlation between NSE and swallowing dysfunction, making NSE a potential preliminary screening indicator for evaluating in ischemic stroke patients. And early implementation of enteral nutrition intervention could effectively reduce NSE levels in patients with ischemic stroke.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-025-04236-y.
Keywords: Cerebral infarction, Neuronal specific enolase, Swallowing dysfunction, Oropharyngeal dysphagia, Enteral nutrition
Introduction
Oropharyngeal dysphagia (OD) is a dysfunction consisting of a difficulty in swallowing. It affects the proper transit of the bolus in the upper digestive tract, preventing a safe oral feeding process [1]. Ischemic stroke is the most prevalent type, accounting for 69.6–70.8% of all strokes [2]. The incidence of swallowing dysfunction in individuals during the acute phase of stroke ranges from 42 to 75% [3, 4]. Videofluoroscopy (VFS) is the gold standard in evaluating dysphagia and fibreoptic endoscopic evaluation of swallowing (FEES) are available [5], however, these methods have certain limitations when used for early identification of swallowing dysfunction in acute stages of stroke [6]. And the water-swallowing test (WST) is easy to incorporate as part of the clinical evaluation of swallowing due to low cost and quick administration [7]. Currently, WST is recommended for assessing swallowing dysfunction in ischemic stroke patients [6, 8]. However, due to cognitive dysfunction or mental abnormalities associated with stroke, patients often face difficulties in complying with the WST. Stroke patients with dysphagia frequently experience concurrent psychological challenges such as anxiety or depression, creating additional barriers to effective assessment participation [9, 10].
NSE, a glycolytic enzyme predominantly expressed in neurons and neuroendocrine cells, has emerged as a biomarker for neuronal damage across various neurological conditions [11, 12]. Elevated serum NSE levels correlate with the severity of traumatic brain injury and predict poor neurological outcomes [13, 14]. In Alzheimer’s disease, NSE levels in cerebrospinal fluid reflect synaptic degeneration and disease progression [14, 15]. Studies indicate serum NSE levels rise in correlation with brain injury severity, paralleling cerebral cell death [14, 16]. NSE is valuable for predicting stroke prognosis and is widely used in stroke research [13, 17]. In our clinical practice, we routinely measure NSE in acute stroke patients to monitor neuronal injury and prognosis dynamically. However, the relationship between NSE and swallowing dysfunction in ischemic stroke patients remains unclear.
This retrospective study analyzed existing clinical data to investigate this relationship by collecting and analyzing clinical data and examining changes in NSE levels following nutritional support therapy, so as to find a supplementary method to evaluate dysphagia in patients with acute ischemic stroke. By bridging the gap between serological biomarkers and functional swallowing outcomes, our findings may inform personalized rehabilitation strategies, such as combining WST with biomarker-guided interventions. Furthermore, our research findings aim to expand the clinical application of NSE and provide a basis for the routine detection of NSE to be included in future guidelines.
Materials and methods
Study design and patients
A total of 573 patients diagnosed with ischemic stroke between September 2015 and August 2022 were retrospectively included in this study at the Department of Neurology, Affiliated Hospital of Jiaxing University. After applying inclusion and exclusion criteria, 445 patients were finally included. Clinical data, including demographics, NIHSS scores, stroke location, and NSE measurements were systematically extracted from electronic medical records.
Inclusion criteria consisted of: (1) patients aged ≥ 18 years old; (2) patients with confirmed new ischemic stroke through diffusion-weighted magnetic resonance imaging of the brain; (3) conscious patients capable of cooperating with the WST; (4) patients with the absence of mental or emotional disorders or cognitive dysfunction; (5) patients with the onset-to-admission duration of ≤ 48 h.
Exclusion criteria were: (1) patients with acute ischemic stroke (AIS) combined with cerebral hemorrhage; (2) patients with ischemic stroke caused by intracranial venous system issues; (3) patients with other conditions affecting swallowing function, such as esophageal tumors, Guillain-Barre syndrome, or myasthenia gravis; (4) patients with AIS accompanied by serious diseases (e.g., cardiopulmonary function, liver and kidney failure, or malignant tumors); (5) unconscious or mentally/emotionally abnormal patients or those unable to cooperate with swallowing function assessment due to a mini-mental state examination (MMSE) score < 27 points; (6) patients hospitalized for less than 10 days; (7) incomplete clinical or laboratory data.
Grouping and data collection
For patients with dysphagia, follow the guidelines [18, 19] and fully respect the patient’s opinion after communication with the patient and family members to decide whether to perform enteral nutrition and data were collected retrospectively. The study divided patients into a swallowing dysfunction group and a non-swallowing dysfunction group. The dysphagia group was further categorized into the enteral nutrition group and non-enteral nutrition group. Clinical data including age, gender, WST results, and NSE levels were collected on admission, the 5th day, and the 10th day. Swallowing dysfunction was defined as a WST score ≥ 2 points [20]. Serum NSE levels were measured using Roche cobasE601 automatic electrochemiluminescence immunoassay (Roche Diagnostics, Basel, Switzerland).
Conventional therapy
All patients with AIS received treatment according to current guidelines [18, 19]. Upon admission, two neurologists assessed the patients, and a trained stroke treatment team initiated prompt therapy. Conventional medications included antiplatelet therapy with aspirin and/or clopidogrel, lipid regulation and plaque stabilization with atorvastatin calcium, as well as medications aiming to improve blood circulation and scavenge oxygen free radicals. Serial NSE assessments are routinely performed in stroke patients during hospitalization to dynamically monitor neuronal injury severity, evaluate therapeutic efficacy, and track disease progression. Swallowing rehabilitation was administered according to guidelines, with informed consent obtained from the patient and family members [18, 19].
WST
WST, widely employed for preliminary screening and risk assessment of swallowing problems [8, 21, 22], involved participants drinking 30 ml of water while seated. Observations were made regarding clinical behaviors (e.g., coughing and choking), quantity of water swallowed, and time taken. Outcomes are graded as shown in supplementary material 1. The clinical evaluation criteria are divided into normal, suspicious and abnormal by WST level: (1) normal means grade 1 in the WST; (2) suspicious means grade 1 with more than 5s or grade 2; (3) abnormal means grade 3 to 5. All assessments were performed by trained and clinically experienced neurologists. According to the guidelines, all patients underwent formalized screening for OD by WST within 24 h after admission and before taking their first sip of water and food.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 25.0 and GraphPad Prism version 9.0.0. Measurement data were presented as mean ± standard deviation (M ± SD), while count data were presented as numbers. Independent sample t-tests were used to compare two sample groups, and the Kruskal Wallis non-parametric test was used for multiple sample comparisons. The level of significance was set to a = 0.05 and P < 0.05.
Results
Between September 2015 and August 2022, a total of 573 patients diagnosed with AIS underwent three rounds of NSE testing. Ultimately, 445 patients met the inclusion and exclusion criteria and were enrolled in this study, comprising 226 males and 219 females. Among the participants, 187 patients (42.02%) were classified into the dysphagia group, while 258 patients (57.98%) were categorized into the non-dysphagia group. There were no significant differences in gender distribution (p = 0.251) or age (64.1 ± 10.05 vs. 65.6 ± 9.77 years, p = 0.103) between the two groups. The dysphagia group exhibited slightly higher NIHSS scores at admission (5.4 ± 2.02 vs. 5.1 ± 1.83, p = 0.064), although the difference was not statistically significant compared to the non-dysphagia group. The distribution of stroke locations did not differ significantly between the groups (p = 0.63). And, the WST scores were significantly higher in the dysphagia group (3.7 ± 1.15 vs. 0.3 ± 0.46, p < 0.001) (Table 1).
Table 1.
Clinical data of dysphagia group and non-dysphagia group
| dysphagia group | non-dysphagia group | P-value | |
|---|---|---|---|
| Number of people (n, %) | 187 (42.02%) | 258 (57.98%) | |
| Gender (male/ female) | 89/98 | 137/121 | 0.251 |
| Age (years) | 64.1 ± 10.05 | 65.6 ± 9.77 | 0.103 |
| WST (scores) | 3.7 ± 1.15 | 0.3 ± 0.46 | <0.001 |
| NIHSS (admission) | 5.4 ± 2.02 | 5.1 ± 1.83 | 0.064 |
| Stroke location(Anterior circulation/Posterior circulation/Anterior and posterior circulation) | 83/71/33 | 123/100/35 | 0.63 |
In the group of AIS patients with dysphagia, 98 patients received enteral nutrition support, while 89 patients did not receive such support. There were no significant differences in gender and age between the two groups (P > 0.05) (Table 2).
Table 2.
Clinical data of enteral nutrition group and non- enteral nutrition group
| enteral nutrition group | non- enteral nutrition | P-value | |
|---|---|---|---|
| Number of people | 98 | 89 | |
| Gender (male/ female) | 52/46 | 37/52 | 0.116 |
| Age (years) | 64.7 ± 10.07 | 63.4 ± 10.03 | 0.376 |
Based on the hospitalization records, three time points were selected: the day of admission, the 5th day, and the 10th day. Statistical analysis was performed on the WST scores and NSE levels at each time point. The findings indicate that in AIS patients with dysphagia, higher WST scores corresponded to higher NSE levels, consistent across all time points. Significantly different NSE values were observed between groups at different time points, indicating a correlation between WST results and NSE levels (Fig. 1).
Fig. 1.
Comparison of NSE levels in AIS patients with dysphagia at different time points according to the WST scores: (a-b) NSE levels in pre admission; (c-d) NSE levels in day 5; (e-f) NSE levels in day 10, NSE levels increase with the increase of WST score (a = 0.05)
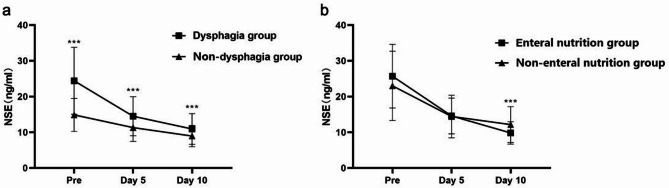
The AIS patients were categorized into dysphagia and non-dysphagia groups. The changes in NSE levels at different time points, specifically upon admission, on day 5, and on day 10, are depicted in Fig. 2a. It is evident that AIS patients with dysphagia exhibited significantly higher NSE levels compared to those without dysphagia during their hospital stay (P < 0.001), indicating an association between dysphagia and elevated NSE levels. Among the dysphagic patients, there was no significant difference in NSE levels between those who received enteral nutrition intervention and those who did not, upon admission and on day 5 (P > 0.05). However, the group receiving enteral nutrition intervention displayed lower NSE levels than the group without intervention (P < 0.001) (Fig. 2b). This suggested that the NSE levels of the two groups were similar upon admission, but after treatment, the enteral nutrition intervention group demonstrated a decrease in NSE levels, implying a favorable prognosis.
Fig. 2.
Differences in NSE levels in different groups: (a) grouping based on dysphagia; (b) grouping based on enteral nutrition intervention (***P < 0.001)
Discussion
Oropharyngeal dysphagia is an important factor contributing to increased mortality in AIS patients [3]. It is a common complication following stroke that not only affects nutrient intake and leads to malnutrition but also causes coughing during water consumption and increases the risk of aspiration pneumonia [4, 23]. Therefore, swallowing screening is critical for ensuring the safety and high-quality care of stroke patients. Given the prevalence of dysphagia in ischemic stroke patients, which has been associated with specific neurological impairment [24], NSE holds potential as a biochemical indicator for evaluating dysphagia in this population [25–27]. In this study, we conducted a comprehensive evaluation of dynamic changes in NSE levels in acute ischemic stroke patients, aiming to assess its potential utility as an adjunct to the WST for dysphagia assessment. Our findings revealed that AIS patients with dysphagia displayed higher NSE levels compared to those without dysphagia at different time points, and NSE levels increased alongside higher scores on the WST upon admission. These results suggest a correlation between NSE levels and dysphagia. Interestingly, no significant difference in NSE levels was observed between AIS patients who received enteral nutrition intervention upon admission and at day 5. However, NSE levels were lower on day 10 for patients receiving enteral nutrition compared to those without intervention, indicating the potential beneficial effect of active enteral nutrition support on neurological recovery in dysphagic patients.
Early assessment of swallowing dysfunction plays a key role in preventing stroke related deaths [28, 29]. Current clinical practice relies heavily on bedside screening tools such as the WST, yet its utility is constrained by patient compliance challenges, operator-dependent variability, and limited sensitivity in cognitively impaired individuals [7, 24, 30]. The integration of NSE into dysphagia screening protocols may address limitations inherent to functional assessments like WST. Unlike WST, NSE quantification is objective, and unaffected by patient cooperation, offering a pragmatic alternative for populations with cognitive deficits or indwelling feeding tubes. This dual-marker approach-combining serological and functional evaluations-could enhance early dysphagia detection, particularly in resource-limited settings. Although our study did not include patients with abnormal mental or emotional dysfunction or cognitive dysfunction due to their inability to undergo the WST, while, it is reasonable to infer that NSE can be used as a biochemical indicator for assessing swallowing function in ischemic stroke patients. Moreover, NSE offers advantages over WST, including greater convenience and the absence of experimental water consumption, making it an excellent screening tool for evaluating dysphagia in AIS patients, regardless of psychoemotional dysfunction or cognitive dysfunction. NSE can overcome the limitations of bedside examination, furthering our understanding of swallowing mechanisms. Additionally, it can assist in evaluating the feasibility of resuming oral diet, aiding clinical decision-making processes regarding the extension of indwelling interventions [31].
Expert consensus emphasizes addressing nutrition as a priority issue in dysphagic patients, recommending enteral nutrition for individuals without contraindications [32]. Early implementation of enteral nutrition support therapy has been shown to promote neurological function recovery in stroke patients with dysphagia [33]. Given that NSE can reflect neurological function damage, our study observed a greater decrease in NSE levels among AIS patients receiving enteral nutrition compared to those without enteral nutrition support, consistent with previous research. It is important to note the difficulty in repeatedly evaluating the WST in long-term care nursing institutions and homes. Nursing personnel may lack standardized training, leading to inaccurate results. In contrast, NSE testing avoids subjectivity and measurement errors since it involves blood-related tests, which is simpler and more feasible for patients with cognitive impairment or indwelling gastric tubes, aiding doctors in making more informed clinical decisions. Our findings highlight the potential of NSE as an objective adjunct to the WST, this biomarker-driven approach may enhance early dysphagia detection while complementing existing clinical protocols.
Despite these insights, our study bears limitations inherent to its retrospective design, including potential residual confounding and selection bias. The homogeneous cohort from a single center restricts generalizability, necessitating validation across diverse ethnic and geographic populations. Additionally, the exclusion of cognitively impaired patients, while methodologically necessary, limits applicability to the broader stroke population, wherein cognitive and swallowing deficits frequently coexist. External validation in diverse populations and healthcare settings is necessary to confirm the applicability of NSE as a universal biomarker for dysphagia. Although we balanced baseline characteristics between groups, unrecorded confounders (e.g., socioeconomic status, pre-stroke comorbidities) may still influence the results. Future multicenter prospective studies incorporating advanced neuroimaging and longitudinal biomarker tracking are warranted to elucidate causal pathways linking neural circuit integrity, and swallowing recovery. Such investigations could further refine risk stratification models and personalize rehabilitation strategies, ultimately improving functional outcomes in AIS patients.
Conclusions
In conclusion, NSE demonstrates significant potential as a biochemical screening indicator for oropharyngeal dysphagia in patients with AIS, serving as a valuable supplement to the WST. The correlation between elevated NSE levels and swallowing dysfunction highlights its utility in assessing dysphagia. Furthermore, early enteral nutrition intervention was associated with reduced NSE levels, suggesting a beneficial impact on neurological recovery. These findings underscore the importance of integrating biomarker-driven approaches, such as NSE measurement, into clinical practice to enhance early dysphagia detection and inform personalized rehabilitation strategies. Future multicenter prospective studies are warranted to validate these results and further explore the clinical applicability of NSE in diverse patient populations.
Limitations of this study
Limitations of this study include: (1) The retrospective design may introduce residual confounding and selection bias; (2) The single-center cohort with homogeneous Chinese participants limits generalizability to other populations; (3) External validation through multicenter prospective studies is needed to confirm NSE’s clinical utility.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AIS
Acute ischemic stroke
- WST
Water-swallowing test
- NSE
Neuron-specific enolase
- OD
Oropharyngeal dysphagia
- VFS
Videofluoroscopy
- FEES
Fibreoptic endoscopic evaluation of swallowing
Author contributions
JH designed and developed this study. DM and JH drafted and edited the manuscript. JH, YL and DM performed the analysis. YL and NC contributed to conception of the study. YL, BL, and JP collected the data. DM performed the interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical Science and Technology Project of Zhejiang Province (2023RC278), Supporting Discipline of Neurology in Jiaxing (2023-ZC-006), and Science and Technology Plan Project of Jiaxing(2016BY28007).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Human ethics and consent to participate declarations
It was a retrospective study. The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of the Affiliated Hospital of Jiaxing University, Jiaxing City (2023-KY-447). As this study was retrospective and did not contain any identifiable patient information and images, the Ethics Committee of Affiliated Hospital of Jiaxing University waived the participants’ written informed consent.
Clinical trial number
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Danyang Meng and Yanjing Lu contributed equally to this work.
15. References
- 1.Reber E, Gomes F, Dahn IA, Vasiloglou MF, Stanga Z. Management of dehydration in patients suffering swallowing difficulties. J Clin Med. 2019;8(11). 10.3390/jcm8111923. [DOI] [PMC free article] [PubMed]
- 2.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide Population-Based survey of 480 687 adults. 2017; 1524–4539 (Electronic). [DOI] [PubMed]
- 3.Banda KJ, Chu H, Kang XL, Liu D, Pien LC, Jen HJ, et al. Prevalence of dysphagia and risk of pneumonia and mortality in acute stroke patients: a meta-analysis. BMC Geriatr. 2022;22(1:420). 10.1186/s12877-022-02960-5. [DOI] [PMC free article] [PubMed]
- 4.Galovic M, Stauber AJ, Leisi N, Krammer W, Brugger F, Vehoff J, et al. Development and validation of a prognostic model of swallowing recovery and enteral tube feeding after ischemic stroke. JAMA Neurol. 2019;76 5:561–70. 10.1001/jamaneurol.2018.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuuskoski J, Vanhatalo J, Rekola J, Aaltonen LM, Jarvenpaa P. The water swallow test and EAT-10 as screening tools for referral to videofluoroscopy. Laryngoscope. 2023. 10.1002/lary.31038. [DOI] [PubMed] [Google Scholar]
- 6.Boaden E, Burnell J, Hives L, Dey P, Clegg A, Lyons MW, et al. Screening for aspiration risk associated with dysphagia in acute stroke. Cochrane Database Syst Rev. 2021;10 10:CD012679. 10.1002/14651858.CD012679.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sella-Weiss O. The test of mastication and swallowing solids and the timed water swallow test: reliability, associations, age and gender effects, and normative data. Int J Lang Commun Disord. 2023;58 1:67–81. 10.1111/1460-6984.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estupinan Artiles C, Regan J, Donnellan C. Dysphagia screening in residential care settings: A scoping review. Int J Nurs Stud. 2021;114:103813. 10.1016/j.ijnurstu.2020.103813. [DOI] [PubMed] [Google Scholar]
- 9.Horn JA-O, Simpson KN, Simpson AN, Bonilha LF, Bonilha HA-O. The relationship between poststroke dysphagia and poststroke depression and its risk factors. 1558–9110 (Electronic). [DOI] [PMC free article] [PubMed]
- 10.Karisik AA-O, Dejakum B, Moelgg K, Komarek SA-O, Toell TA-O, Mayer-Suess LA-O et al. Association between dysphagia and symptoms of depression and anxiety after ischemic stroke. 1468– 1331 (Electronic). [DOI] [PMC free article] [PubMed]
- 11.Lu K, Xu X, Cui S, Wang F, Zhang B, Zhao Y. Serum neuron specific enolase level as a predictor of prognosis in acute ischemic stroke patients after intravenous thrombolysis. 2015; 1878–5883 (Electronic). [DOI] [PubMed]
- 12.Kim BJ, Kim YJ, Ahn SH, Kim NY, Kang DW, Kim JS, et al. The second elevation of neuron-specific enolase peak after ischemic stroke is associated with hemorrhagic transformation. J Stroke Cerebrovasc Dis. 2014;23 9:2437–43. 10.1016/j.jstrokecerebrovasdis.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Yang P, Shi M, Liu Y, Mao X, Zhu Z, Wu X, et al. Neuron-Specific enolase levels and prognosis of ischemic stroke: two prospective cohort studies. Stroke. 2025;56(1):e1–2. 10.1161/STROKEAHA.124.048443. [DOI] [PubMed] [Google Scholar]
- 14.Haupt WF, Chopan G, Sobesky J, Liu WC, Dohmen C. Prognostic value of somatosensory evoked potentials, neuron-specific enolase, and S100 for short-term outcome in ischemic stroke. J Neurophysiol. 2016;115 3:1273–8. 10.1152/jn.01012.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama TA-O, Sawada J, Takahashi K, Yahara O, Hasebe N. Meta-analysis of cerebrospinal fluid neuron-specific enolase levels in Alzheimer’s disease, Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. 1758–9193 (Electronic). [DOI] [PMC free article] [PubMed]
- 16.Hoffmann J, Janowitz DA-O, Van der Auwera S, Wittfeld K, Nauck M, Friedrich N et al. Association between serum neuron-specific enolase, age, overweight, and structural MRI patterns in 901 subjects. 2017; 2158–3188 (Electronic). [DOI] [PMC free article] [PubMed]
- 17.Gao L, Xie J, Zhang H, Zheng H, Zheng W, Pang C, et al. Neuron-specific enolase in hypertension patients with acute ischemic stroke and its value forecasting long-term functional outcomes. BMC Geriatr. 2023;23(1:294). 10.1186/s12877-023-03986-z. [DOI] [PMC free article] [PubMed]
- 18.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American heart association/american stroke association. Stroke. 2014;45 7:2160–236. 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 19.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American heart association/american stroke association. Stroke. 2021;52 7:e364–467. 10.1161/STR.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 20.Ye T, Huang S, Dong Y, Dong Q. Comparison of two bedside evaluation methods of dysphagia in patients with acute stroke. Stroke Vasc Neurol. 2018;3 4:237–44. 10.1136/svn-2018-000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishiwaki K, Tsuji T, Liu M, Hase K, Tanaka N, Fujiwara T. Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia variables. J Rehabil Med. 2005;37 4:247–51. 10.1080/16501970510026999. [DOI] [PubMed] [Google Scholar]
- 22.Osawa A, Maeshima S, Tanahashi N. Water-swallowing test: screening for aspiration in stroke patients. Cerebrovasc Dis. 2013;35 3:276–81. 10.1159/000348683. [DOI] [PubMed] [Google Scholar]
- 23.Panjikaran ND, Iyer R, Sudevan R, Bhaskaran R. Utility of modified Mann assessment of swallowing ability (MMASA) in predicting aspiration risk and safe swallow in stroke patients. J Family Med Prim Care. 2022;11 9:5123–8. 10.4103/jfmpc.jfmpc_1628_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinhagen V, Grossmann A, Benecke R, Walter U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. 2009;40 5:1903–6. 10.1161/STROKEAHA.108.535468. [DOI] [PubMed] [Google Scholar]
- 25.Im S, Han YJ, Kim SH, Yoon MJ, Oh J, Kim Y. Role of bilateral corticobulbar tracts in dysphagia after middle cerebral artery stroke. Eur J Neurol. 2020;27 11:2158–67. 10.1111/ene.14387. [DOI] [PubMed] [Google Scholar]
- 26.Lee WH, Lim MH, Seo HG, Seong MY, Oh BM, Kim S. Development of a novel prognostic model to predict 6-Month swallowing recovery after ischemic stroke. Stroke. 2020;51(2):440–8. 10.1161/STROKEAHA.119.027439. [DOI] [PubMed] [Google Scholar]
- 27.Jang SH, Kwak SY, Chang CH, Jung YJ, Kim J, Kim SH, et al. Prognostic prediction of dysphagia by analyzing the corticobulbar tract in the early stage of intracerebral hemorrhage. Dysphagia. 2020;35 6:985–92. 10.1007/s00455-020-10093-3. [DOI] [PubMed] [Google Scholar]
- 28.Morone G, Iosa M, Paolucci T, Muzzioli L, Paolucci S. Relationship between body mass index and rehabilitation outcomes in subacute stroke with dysphagia. Am J Phys Med Rehabil. 2019;98(7):608–12. 10.1097/PHM.0000000000001159. [DOI] [PubMed]
- 29.Scrutinio D, Lanzillo B, Guida P, Passantino A, Spaccavento S, Battista P. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. 2020; 1532–X821 (Electronic). [DOI] [PubMed]
- 30.Imaizumi M, Murono S. Will levels of experience of examiners affect the diet provided for patients with swallowing impairment? Auris Nasus Larynx. 2023;50 5:765–9. 10.1016/j.anl.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Takeda M, Watanabe YA-O, Taira K, Miura K, Ohara YA-OX, Iwasaki MA-O et al. Association between death or hospitalization and observable variables of eating and swallowing function among elderly residents in Long-Term care facilities: A multicenter prospective cohort study. LID– 10.3390/healthcare11131827 [doi] LID– 1827. 2023; 2227–9032 (Print). [DOI] [PMC free article] [PubMed]
- 32.Middleton S, McElduff P, Drury P, D’Este C, Cadilhac DA, Dale S, et al. Vital sign monitoring following stroke associated with 90-day independence: A secondary analysis of the QASC cluster randomized trial. Int J Nurs Stud. 2019;89:72–9. 10.1016/j.ijnurstu.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Patejdl R, Kastner M, Kolbaske S, Wittstock M. Clinical nutrition and Gastrointestinal dysfunction in critically ill stroke patients. Neurol Res. 2017;39 11:959–64. 10.1080/01616412.2017.1367545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.