Abstract
Background
Bronchiectasis is associated with psychological comorbidity and poor quality of life (QoL), yet guidelines lack focus on psychological morbidity. Using data obtained from the BronchUK database (1341 patients), we examined the link between anxiety/depression and physical disease severity, QoL and long-term outcomes in bronchiectasis.
Methods
Computed tomography-confirmed bronchiectasis patients enrolled in the BronchUK study with Hospital Anxiety and Depression Scale (HADS-A/D) data were studied. HADS-A/D scores ≥8 indicated anxiety/depression. QoL was measured by the St George's Respiratory Questionnaire and QoL-Bronchiectasis Questionnaire. Exacerbations during annual follow-up were analysed by negative binomial regression with time in study as an offset adjusted for age, body mass index, sex, Pseudomonas infection, diabetes and forced expiratory volume in 1 s (FEV1). Cox regression determined probability of hospitalisation using time to first exacerbation.
Results
1341 patients were included; 418 had anxiety (31%), 269 (20%) had depression and 201 (15%) had both conditions. HADS-A/D ≥8 was associated with worse QoL (p<0.0001) and clinical severity (e.g. Bronchiectasis Severity Index, FEV1 and Medical Research Council dyspnoea score (all p<0.01). HADS-A/D ≥8 each was associated with exacerbation (rate ratio (RR) 1.42, 95% CI 1.32–1.52 for HADS-A; RR 1.45, 95% CI 1.34–1.56 for HADS-D, both p<0.0001) and hospitalisation risk (RR 1.58, 95% CI 1.29–1.92 for HADS-A; RR 1.76, 95% CI 1.43–2.17 for HADS-D, both p<0.001). HADS-A/D ≥8 each predicted future hospitalisation (HR 1.30, 95% CI 0.98–1.72, p=0.067 for HADS-A; HR 1.40 95% CI 1.04–1.88, p=0.027 for HADS-D).
Interpretation
Anxiety and depression are common in bronchiectasis, correlate with disease severity and predict poor outcomes. Consideration of psychological comorbidities should be evaluated in routine bronchiectasis care.
Shareable abstract
Anxiety and depression are common in bronchiectasis, correlate with disease severity, and predict poor outcomes. Psychological support is important in bronchiectasis care. https://bit.ly/4gEP6ez
Introduction
Bronchiectasis is characterised by abnormally dilated bronchi susceptible to recurrent infection [1]. It is increasingly recognised [2] and is significantly associated with increased mortality compared with the general population [3]. The Bronchiectasis Severity Index (BSI) [4] comprises different measures of microbiological, clinical and radiological characteristics to generate three severity categories predicting hospital admissions, exacerbations and mortality [5].
In more severe bronchiectasis, Pseudomonas aeruginosa is associated with increased morbidity [6], mortality [7] and anxiety and depression [8]. Single-centre data suggest that anxiety and depression are common in bronchiectasis, with high levels of prevalence ranging 5–55% [9, 10] and 30–39.9% [11, 12], respectively, which are significantly associated with poorer quality of life (QoL) [13]. Bronchiectasis guidelines often focus on physical symptoms and medical management without discussing psychological morbidity [14, 15]. The European Respiratory Society guidelines highlight health-related QoL (HRQoL) improvements when pulmonary rehabilitation (PR) programs are completed [16, 17]. Additionally, completion of PR is associated with reduced depression and anxiety in bronchiectasis [18], suggesting psychological comorbidity is a treatable trait.
We sought to define how common anxiety and depression are in bronchiectasis and investigate the relationships between disease severity, HRQoL and exacerbations.
Methods
Participants
Participants were recruited from across 14 UK centres into the BronchUK longitudinal prospective cohort study, following the BronchUK protocol [19], requiring adults (age ≥18 years) with computed tomography (CT)-proven bronchiectasis from 25 November 2015 to 28 August 2019. Any aetiology of bronchiectasis was eligible with recruitment and data capture in a stable nonexacerbated state at recruitment. Ethical approval was granted by the UK National Research Ethics Service (15/NE/0172). The dataset, downloaded from the study database (www.Bronch.ac.uk), contained 1521 patients but our analysis was limited to 1341 bronchiectasis patients (836 females and 505 males) where Hospital Anxiety and Depression Scale (HADS) data were available. Full medication histories, including medications for nonrespiratory indications were not part of the original case report form.
Parameters for analysis
The database contains BSI, lung function (forced expiratory volume in 1 s (FEV1) recorded and % predicted), number of exacerbations, respiratory-related emergency department visits and hospital admissions before the study in preceding 12 months, Pseudomonas status, dyspnoea and sputum volume. HADS and HRQoL scores, information on comorbidities, including a prior diagnosis of anxiety and depression and comorbidities [19]. Comorbidities included “known anxiety or depression” were checked during baseline visits from both patient reports and prior medical records. At baseline, the HADS was collected; it is a simple one-page questionnaire self-administered that was checked for completeness by the research team after patient completion in a nonexacerbating stable state. There are seven questions per domain and the maximum score for each domain is 21. In this study HADS-anxiety/depression (A/D) scores ≥8 indicated that anxiety or depression symptoms were present and ≥11 indicated moderate-to-severe symptoms. A clinically significant threshold of ≥8 was used to dichotomise the population after initial descriptive statistics as this is supported by previous work in the field [20, 21].
We recorded the St Georges Respiratory questionnaire (SGRQ) where the minimal clinically important difference (MCID) is four points [22]. High SGRQ scores indicate poorer HRQoL. QoL-Bronchiectasis (QoL-B) provides eight scores from 0–100 but not a total score. Lower QoL-B scores demonstrate a higher number of symptoms, worse functioning and poorer HRQoL. Dependent on domains, the MCID ranges from 7–10 [23].
Analyses
We used the Statistical Package for the Social Sciences (SPSS). Continuous variables were found to be non-normally distributed, so nonparametric methods were used. Medians and interquartile ranges are presented. Chi-squared tests were used for categorical variables. Mann–Whitney U-tests and Kruskal–Wallis tests were used to test for differences between distributions of continuous variables. Due to the use of multiple testing, the critical value for statistical significance was taken to be p<0.01. Logistic regression was used to model the association between dichotomised HADS-A/D scores and dependent variables, including QoL-B and SGRQ, with adjustments made for age, sex, body mass index (BMI) category and number of comorbidities.
Longitudinal analyses following the BronchUK protocol [19] were conducted on 1127 patients where data were available for at least one follow-up time point [19]. Negative binomial regression was conducted with adjustment made for BMI category, age, sex, Pseudomonas infection, and having comorbid diabetes and lung function impairment groups. Cox proportional regression was used to determine the probability of hospitalisation using data for time to first severe exacerbation.
Subgroup analyses
The cohort was categorised based on number of exacerbations (0, 1, 2 and ≥3) with ≥3 considered to be clinically significant, as predictive of mortality in the BSI [24] and having the greatest effect on HRQoL [25]. The number of respiratory-related emergency department visits and hospital admissions were also compared in two groups (0 and ≥1) in line with these categorisations within the BSI [24]. Medical Research Council (MRC) dyspnoea scores ≥3 were deemed important [24, 26]. For statistical analysis of FEV1 % predicted, participants were split into groups that are prognostically important in the BSI: <30% and ≥30% [24].
Results
1521 patients’ data were available in the database but 180 were excluded due to incomplete/missing HADS data. There were no significant differences between the reported HRQoL scores and/or BSI/ BSI components such as Pseudomonas, age and FEV1 % predicted for the 180 patients excluded from the total database of 1521 due to missing HADS data (all p>0.05). Of the 1341 participants with complete HADS, 62.3% were female with a median age of 67 years (table 1). Aetiologies were typical of a bronchiectasis cohort with idiopathic (41.1%) and post-infective (32.3%) being the most common. Of the 145 participants enrolled with a previous history of anxiety, 96 (66.2%) showed anxiety with a HADS-A ≥8, whereas the remaining 49 participants (33.8%) had HADS-A scores <8. Notably, of the 1196 participants with no previous history anxiety, 322 (26.9%) had a HADS-A ≥8, suggesting unrecognised anxiety symptoms in these individuals. In the 180 with a previous depression diagnosis, only 83 (46.1%) had a HADS-D ≥8 and 97 (53.9%) had HADS-D scores <8. Additionally, in 1161 with no previous depression history, 186 (16.0%) had a HADS-D ≥8, suggesting unrecognised depression symptoms. Coexistent anxiety and depression were seen in 201 participants (see table 1 for full breakdown of the above). Following interrogation of electronic health care records from 166 patients across three centres to assess the clinical use of antidepressant/anxiolytic medications, we observed 12 (8.6%) of those with a history of anxiety and/or depression were on antidepressants/anxiolytics. Of these, 43% and 57% had HADS-A or HADS-D score ≥8, respectively.
TABLE 1.
Demographics of patients enrolled in BronchUK with complete HADS-A/D questionnaire, including clinical status
| Variable | Patients with complete HADS-A/D | |
|---|---|---|
| n | 1341 | |
| Patient demographics | ||
| Age, years | 67 (59–73) | |
| Female | 836 (62.3) | |
| BMI category, kg·m−2 | <18.5 | 54 (4.0) |
| 18.5–24.9 | 559 (41.7) | |
| 25–29.9 | 396 (29.5) | |
| >30 | 281 (21.0) | |
| Missing | 51 (3.8) | |
| HADS-A | ||
| HADS-A score | 5.0 (3.0–9.0) | |
| HADS-A score ≥8 | 418 (31.2) | |
| HADS-A score ≥11 (moderate–severe anxiety) | 211 (15.7) | |
| Previous anxiety diagnosis | HADS-A<8 | 49 (5.3) |
| HADS-A≥8 | 96 (23.0) | |
| No previous anxiety diagnosis | HADS-A<8 | 874 (94.7) |
| HADS-A≥8 | 322 (77.0) | |
| HADS-D | ||
| HADS-D score | 4.0 (2.0–7.0) | |
| HADS-D score ≥8 | 269 (20.1) | |
| HADS-D score ≥11 (moderate–severe depression) | 94 (7.0) | |
| Previous depression diagnosis | HADS-D<8 | 97 (9.0) |
| HADS-D≥8 | 83 (30.9) | |
| No previous depression diagnosis | HADS-D<8 | 975 (91.0) |
| HADS-D≥8 | 186 (69.1%) | |
| HADS-A and HADS-D | ||
| Both ≥8 | 201 (14.9) | |
| Both ≥11 | 65 (4.8) | |
| Clinical status | ||
| BSI | 7 (5–10) | |
| BSI severity category | ≤4 (mild) | 334 (24.9) |
| 5–8 (moderate) | 563 (42.0) | |
| ≥9 (severe) | 444 (33.1) | |
| FEV1 % predicted group | ≥80% (mild) | 564 (42.1) |
| <80 to ≥50% (moderate) | 478 (35.6) | |
| <50 to ≥30% (severe) | 157 (11.7) | |
| <30% (very severe) | 47 (3.5) | |
| Missing | 95 (7.1) | |
| Exacerbations in previous year | 0 | 314 (23.4) |
| 1 | 255 (19.0) | |
| 2 | 210 (15.7) | |
| ≥3 | 562 (41.9) | |
| Respiratory-related emergency room visits in previous year | 0 | 1180 (88.0) |
| 1 | 108 (8.1) | |
| 2 | 31 (2.3) | |
| ≥3 | 22 (1.6) | |
| Hospitalisations in previous year | 0 | 1086 (81.0) |
| 1 | 159 (11.9) | |
| 2 | 51 (3.8) | |
| ≥3 | 45 (3.3) | |
| MRC Dyspnoea Score | 0 | 461 (34.4) |
| 1 | 468 (34.9) | |
| 2 | 232 (17.3) | |
| 3 | 123 (9.2) | |
| 4 | 57 (4.3) | |
| Median sputum vol (mL·day−1) | 7 (0–30) | |
Data are presented as n (%) or median (interquartile range). HADS-A/D: Hospital Anxiety and Depression Scale – Anxiety/Depression Score; BMI: body mass index; BSI: Bronchiectasis Severity Index; FEV1: forced expiratory volume in 1 s; MRC: Medical Research Council.
Table 1 shows the clinical status and HRQoL of the overall cohort. Briefly, within the last year, 19% were hospitalised at least once, 41.9% had ≥3 exacerbations and 12% had ≥1 respiratory-related emergency department visit. Overall, the median BSI score was 7. The median HADS-A score was 5 and the median HADS-D score was 4. Participants had moderately impaired HRQoL with a median SGRQ total score of 37.6 (25% percentiles 20.8–55.7). Microbiology data for the cohort are reported in supplementary table 1.
We determined the associations between the HADS-A/D groups (<8 and ≥8) and various categorical variable groups using chi-squared tests. We observed statistically significant associations (p≤0.001) between HADS-A/D (χ2=297.475, p<0.001), BSI, exacerbations, hospitalisations, MRC dyspnoea score, recorded history of anxiety and depression, comorbid COPD and smoking status (see supplementary tables 2 and 3).
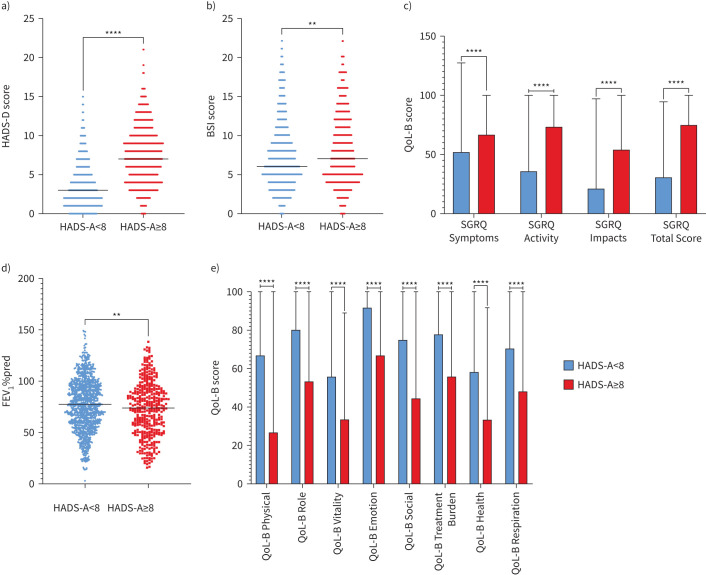
Mann–Whitney U-tests were used to determine differences in continuous variables between HADS-A/D groups. All differences for the HADS-A groups were significant (supplementary table 4). Participants with HADS-A scores ≥8 had significantly higher HADS-D, BSI and SGRQ scores, as well as lower lung function and QoL-B scores (figure 1). We also observed that those with HADS-A scores ≥8 tended to be younger than those with scores <8 (p<0.001).
FIGURE 1.
Results of Mann–Whitney U-tests comparing HADS-A groups (<8 or ≥8) with a) HADS-A scores, b) BSI, c) SGRQ scores, d) FEV1 % predicted and e) QoL-B questionnaire scores. Blue represents those with HADS-D scores <8 (n=923) and red represents those with HADS-D scores ≥8 (n=418). HADS-A: Hospital Anxiety and Depression Scale – Anxiety Score; HADS-D: Hospital Anxiety and Depression Scale – Depression Score; BSI: Bronchiectasis Severity Index; FEV1: forced expiratory volume in 1 s; SGRQ: St George's Respiratory Questionnaire; QoL-B: Quality of Life in Bronchiectasis. **: p<0.01; ****: p<0.0001.
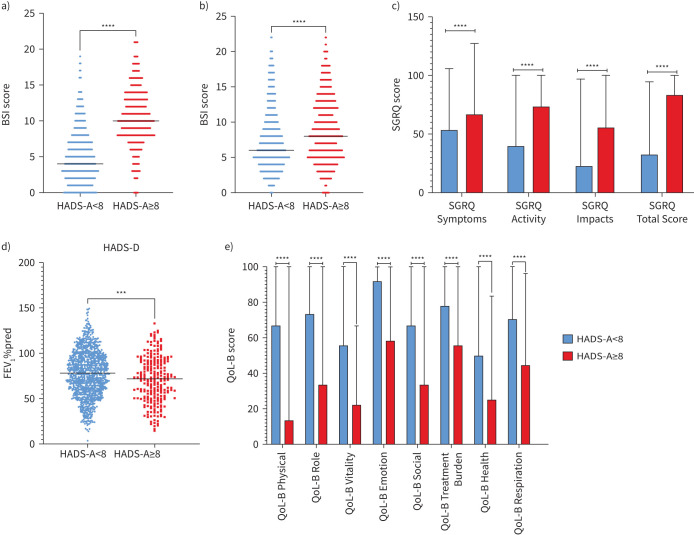
All differences for the HADS-D groups were significant (supplementary table 5), with participants with a HADS-D score ≥8 having significantly higher HADS-A (p<0.0001), BSI (p<0.001) and SGRQ scores (p<0.0001) compared with those with scores <8 (figure 2a–c). Additionally, participants with HADS-D scores ≥8 had a lower FEV1 (p<0.001) and scored significantly lower on the QoL-B questionnaire (p<0.0001) compared with those with HADS-D scores <8 (figure 2d, e). Those with HADS-D scores ≥8 were significantly younger than those with a score <8 (p=0.006).
FIGURE 2.
Results of Mann–Whitney U-tests comparing HADS-D groups (<8 or ≥8) with a) HADS-A scores, b) BSI, c) SGRQ scores, d) FEV1 % predicted and e) QoL-B questionnaire scores. Blue represents those with HADS-D scores <8 (n=1072). Red represents those with HADS-D scores ≥8 (n=269). HADS-A: Hospital Anxiety and Depression Scale – Anxiety Score; HADS-D: Hospital Anxiety and Depression Scale – Depression Score; BSI: Bronchiectasis Severity Index; FEV1: forced expiratory volume in 1 s; SGRQ: St George's Respiratory Questionnaire; QoL-B: Quality of Life in Bronchiectasis. ***: p<0.001; ****: p<0.0001.
We then carried out logistic regression to ascertain the effects of significant variables on the likelihood of HADS-A/D ≥8. Logistic regression analysis for HADS-A showed that domains of the QoL-B questionnaire could predict HADS-A score (table 2). Most notable of the QoL-B scores was Emotion, where the likelihood of anxiety increases by 8% per one unit decrease (p<0.001). Subscales of the SGRQ questionnaire, including Symptoms (p=0.037) and Impacts scores (p=0.011), were also independent predictors of HADS-A score, where the likelihood of anxiety increases by 1% and 4% per one unit increase, respectively.
TABLE 2.
Logistic regression analysis for elevated symptoms for anxiety (HADS-A scores) in patients with bronchiectasis
| Factors | OR (95% CI) | p-value |
|---|---|---|
| QoL-B Vitality | 1.02 (1.00–1.03) | p=0.013 |
| QoL-B Emotion | 0.92 (0.91–1.01) | p<0.001 |
| QoL-B Treatment Burden | 1.02 (1.00–1.03) | p=0.008 |
| QoL-B Health | 0.98 (0.96–0.99) | p=0.002 |
| SGRQ Symptoms | 1.01 (1.00–1.03) | p=0.037 |
| SGRQ Impacts | 1.04 (1.01–1.08) | p=0.011 |
Adjustments have been made for age, sex, body mass index and total comorbidities. HADS-A: Hospital Anxiety and Depression Scale – Anxiety Score; OR: odds ratio; CI: confidence intervals; QoL-B: Quality of Life Bronchiectasis Questionnaire; SGRQ: St George's Respiratory Questionnaire.
Similarly, a logistic regression analysis for depression (HADS-D) variables showed that, QoL-B questionnaire scores on Role (OR 0.97, 95% CI 0.96–0.99; p<0.001), Emotion (OR 0.95, 95% CI 0.94–0.96; p<0.001) and Health (OR 0.98; 95% CI 0.97–1.00; p=0.010) were significant for predicting HADS-D scores. The likelihood of depression decreases by 3%, 5% and 2%, per one unit increase in the QoL-B subscales of Role, Emotion and Health, respectively after adjustments were made for age, sex, BMI and total number of comorbidities.
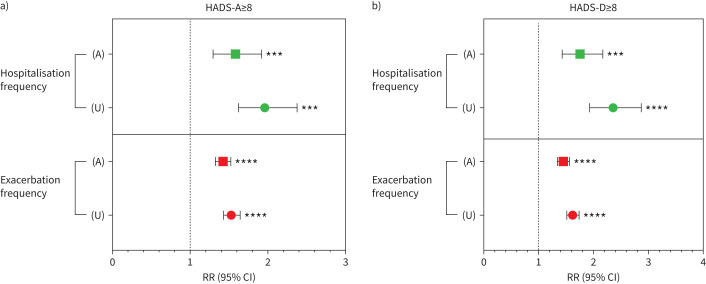
Finally, looking further at the long-term clinical outcomes associated with elevated HADS-A/D scores, we observed that those with HADS-A scores ≥8 had an increased risk of exacerbation (RR 1.53, 95% CI 1.43–1.64; p<0.0001 for unadjusted versus RR 1.42, 95% CI 1.32–1.52; p<0.0001 for adjusted analysis; figure 3a) and hospitalisation (RR 1.96, 95% CI 1.62–2.37; p<0.001 for unadjusted versus RR 1.58, 95% CI 1.29–1.92; p<0.001 for adjusted analysis; figure 3a). Similarly, those with HADS-D scores ≥8 had a significantly increased risk of exacerbation (RR 1.62, 95% CI 1.51–1.74; p<0.0001 for unadjusted versus RR 1.45, 95% CI 1.34–1.56; p<0.0001 for adjusted analysis; figure 3b) and hospitalisation (RR 2.36, 95% CI 1.93–2.87; p< 0.0001 for unadjusted versus RR 1.76, 95% CI 1.43–2.17; p<0.001 for adjusted analysis; figure 2b).
FIGURE 3.
a) Long-term outcomes of those with elevated HADS-A scores (≥8) and b) elevated HADS-D scores (≥8). Data are shown as incident rate ratio (RR) with 95% confidence intervals (CIs). Reference line (x=1.0) represents those individuals with HADS-D/A scores <8. A represents adjusted analysis and U represents unadjusted analysis. Data shown for frequency of exacerbation and frequency of hospitalisation have undergone adjustment for age, sex, body mass index category, diabetes, Pseudomonas aeruginosa status and bronchiectasis disease severity in the form of forced expiratory volume in 1 s Global Initiative for Chronic Obstructive Lung Disease group classification. HADS-A/D: Hospital Anxiety and Depression Scale – Anxiety/Depression Score. ***: p<0.001; ****: p<0.0001.
The association of HADS-A/D score with mortality was not reported due to very few deaths occurring over long-term follow-up (<50).
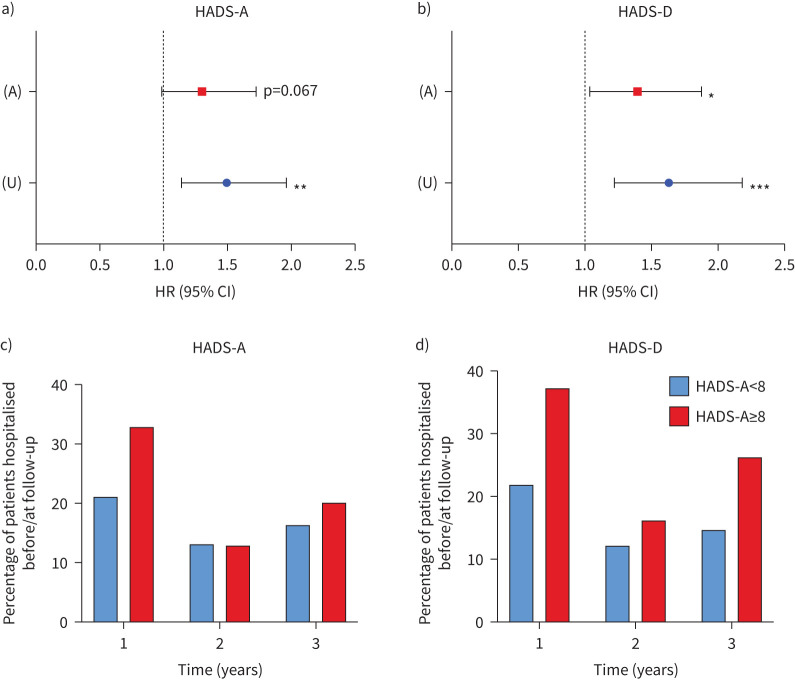
Following adjustment for various confounders, we observed that HADS-A scores ≥8 predicted time to first hospitalisation, but this effect was not statistically significant (HR 1.30, 95% CI 0.98–1.72; p=0.067; figure 4a). There was a trend for those with HADS-A scores ≥8 and a higher percentage of patients with HADS-A scores ≥8 being hospitalised at the 1- and 3-year time points but not at the 2-year time point (figure 4c).
FIGURE 4.
a) Time to first severe exacerbation requiring hospitalisation of those with elevated HADS-A scores (≥8) and b) elevated HADS-D scores (≥8), c, d) including percentage of patients hospitalised at each follow-up time point. Data are shown as hazard ratio (HR) with 95% confidence intervals (CIs). Reference line (x=1.0) represents those individuals with HADS-D/A scores <8. U represents unadjusted analysis, and A represents analysis that has undergone adjustment for age, sex, BMI category, diabetes, Pseudomonas aeruginosa status and bronchiectasis disease severity in the form of forced expiratory volume in 1 s (FEV1) % predicted classification. HADS-A/D: Hospital Anxiety and Depression Scale–Anxiety/Depression Score. *: p<0.05; **: p<0.01; ***: p<0.001.
However, following adjustment for various confounders, we observed that HADS-D scores ≥8 could predict time to first severe exacerbation requiring hospitalisation (HR 1.40, 95% CI 1.04–1.88; p=0.027; figure 4b). We also observed a higher percentage of patients with a HADS-D score ≥8 hospitalised before/at each follow-up time point compared with those with HADS-D scores<8, with most of those hospitalisations (37%) occurring within the first year of follow-up (figure 4d).
Discussion
The study aimed to explore the association between physical health parameters, QoL measures, risk of exacerbations and hospitalisations with anxiety and/or depression in a large multicentre bronchiectasis cohort.
While 65% of participants reported no symptoms suggestive of psychological comorbidity, the prevalence of anxiety (31%) exceeded estimates of anxiety in the general population of 5.9% (National Health Service (NHS) England, 2016 [27]). This higher prevalence of anxiety is generally consistent with an increased risk as shown in other chronic respiratory diseases [10], as well as previous studies in bronchiectasis. One recent study similarly found that 30% of bronchiectasis patients were clinically anxious [11].
Prevalence of depression (20%) from this cohort also aligned with previous studies of depression in bronchiectasis [9, 10, 13, 28, 29]. Again, patients demonstrated a higher prevalence of depression than the general population (9.7% in the United Kingdom) and was comparable with other serious physical diseases, including asthma and coronary heart disease [30, 31]. Notably our data correlated well with the rates of anxiety and depression noted in a recent multicentre study of 434 patients from China; this study also prospectively collected anxiety and depression data [32]. The prevalence defined again as HADS-A >8 for anxiety was 23% and HADS-D >8 for depression was 29%. The study similar to our data found that depression, rather than anxiety, was independently associated with an increased risk of exacerbations and hospitalisations, and reduced time to the first exacerbation in bronchiectasis. Both this study and our study had much higher rates of psychological comorbidity than recent reported within the European registry of nearly 17 000 patients where anxiety and depression were recorded at 13 and 14%; these large differences in prevalence reflect the importance of using structured validated questionnaires compared with using previous medical history.
Similarly, data in cystic fibrosis (CF) from the 6000+ participant CF TIDES study showed that both anxiety and depression were common [33]. Similar data have been reported for primary ciliary dyskinesia [34]. These high symptom burdens are likely to impact daily functioning and could contribute to difficulties with treatment adherence in CF [35]. Higher mortality has been reported in CF when depression symptoms are present [36]. In our study, although there was a good correlation between previous history of psychological comorbidity and current symptoms, many patients had significant psychological symptoms in the absence of a previous psychological disease history. The corollary was also true; many with a previous history of psychological disease reported no active symptoms.
There were significant associations between both anxiety and depression and disease severity based on BSI and HRQoL measures such as the SGRQ and QoL-B, supporting the hypothesis that those with a higher BSI score, and worse HRQoL scores, had significantly higher psychological comorbidity.
HADS-A and HADS-D scores were significantly associated with previous exacerbations, respiratory-related emergency department visits and hospital admissions. Those with anxiety and depression were more likely to have ≥3 exacerbations, ≥1 emergency department visits and ≥1 hospitalisations, compared with those without. On average, the HADS-A ≥8 group experienced 2.5 exacerbations, whereas the <8 group experienced one exacerbation. Similarly, the ≥8 HADS-D group experienced three exacerbations before the study, compared with the <8 group with two. This difference is both statistically and clinically significantly and aligns with findings that the number of exacerbations in the previous year exert the largest effect on total SGRQ score [25]. Therefore, those with ≥3 exacerbations are not only likely to experience a poor QoL and have an increased risk of mortality [24] but also have greater psychological comorbidity.
Importantly, both anxiety and depression were associated with an increased risk of exacerbations and hospitalisations. HADS-A/D ≥8 predicted future hospitalisation though this did not reach statistical significance. The association between psychological comorbidity and poorer outcomes needs further study. The associations are likely to be complex but may include different perception of physical disease symptoms, different levels of adherence [37, 38] and potentially more complex biological mechanisms [39] driving disease worsening through physical and psychological health.
The strengths of our study include the large sample size and the use of prospective data from multiple centres across the United Kingdom making these results more generalisable to the UK population. Much of the existing research employs small sample sizes from single centres. We used measures validated for this population and has been validated for use in bronchiectasis patients [25, 39, 40] and can be applied routinely. We used SGRQ, which is validated for use in bronchiectasis patients [25], alongside QoL-B, a bespoke questionnaire designed for bronchiectasis [23].
Limitations of our work include that there was no age-matched control population as the cohort focussed only on bronchiectasis patients. Therefore, comparisons of prevalence rely on population estimates collected in other studies. Although data were collected prospectively, there was a recording of past medical history from medical notes rather than an interview-based checklist; thus, some past diagnoses of anxiety may have been missed. This study has limitations, which include the potential for selection bias; patients were prospectively recruited but the psychological comorbidities of interest may themselves lend to variable engagement rates. In the present study we used HADS with a clinical cut-off ≥8 suggesting a probable case of anxiety; however, future studies may choose to use a cut-off ≥11, which may indicate moderate or severe anxiety [30]. The threshold of ≥8 used herein aligns to previous literature and is clinically relevant. Recent evidence showed that cognitive behavioural therapy was successful in improving anxiety in COPD patients with HADS-A or D scores ≥8 [21].
We have not exhaustively assessed the differences in baseline bronchiectasis therapies used in this cohort and, for example, compared rates of inhaled antibiotics or long-term macrolides between the groups studied.
In the United Kingdom, NICE guidance (2011) [41] recommends screening for psychological comorbidity but noted uncertainty on the best and/or most pragmatic tool: notably the CF TIDES study led to a consensus statement on integrating psychological and physical healthcare [42]. Work arising from this study led to international guidelines, recommending the Patient Health Questionnaire-9 (PHQ-9) and General Anxiety Disorder-7 (GAD-7) as the best evidence-based screening tools in CF [43]. This was, however, published after our study had commenced. Successful screening for psychological comorbidity was facilitated by mental healthcare coordinators at CF centres. We have previously published the beneficial effects of respiratory nurse-delivered cognitive behavioural therapy in COPD showing that it was both clinically and cost-effective [21]. Such approaches are increasingly available and need to be tested in bronchiectasis.
We have established that psychological comorbidity is common in patients with bronchiectasis. It is associated with poorer HRQoL. It is associated with more severe physical health parameters and clinical course but many patients with milder physical disease suffer from anxiety and/or depression. These psychological comorbidities are associated with increased future risks of exacerbations and, to a lesser extent, hospitalisations. Consideration of annual screening for anxiety and depression and appropriate management should be recommended within current bronchiectasis guidelines.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00348-2024.SUPPLEMENT (1.3MB, pdf)
Acknowledgements
We acknowledge the support of the EMBARC project and sharing of the data platform kindly adapted by colleagues at Health Informatics Centre (HIC), University of Dundee. Anthony De Soyza is Chief Investigator for BronchUK. Jennifer Pollock, Tess Saunders, Georgina Wild and Richard McNally supported with data analysis and drafting this manuscript. We acknowledge support from Henil Upadhyay with additional data retrieval at the lead NHS site. Martin Kelly, Adam Hill, Tim Gatheral, Anita Sullivan, Charles Haworth, John Hurst, Jeremy Brown, Mary Carroll, Michael Loebinger, Gareth Davies, Just Bradley, Paul Walker, John Steer, Jamie Duckers, Megan Crichton and James D. Chalmers are Principal Investigators and lead research staff at participating hospitals in BronchUK contributing patient recruitment and data used in this manuscript. Stuart Elborn and Vidya Navaratnam are collaborators on BronchUK and were consulted regarding this manuscript. Phil Mawson is a research project manager for BronchUK and collated data from study database and formatted this manuscript for submission. All named authors reviewed the manuscript.
Provenance: Submitted article, peer reviewed.
Ethics statement: Ethical approval was granted by the UK National Research Ethics Service (15/NE/0172).
Conflict of interest: T. Saunders, J. Brown, A. Sullivan, M. Carroll, P. Mawson, T. Gatheral, A.T. Hill, G. Davies, J. Pollock, M. Kelly, R. McNally, G. Wild, V. Navarantnam and H. Upadhyay declare no conflict of interest in relation to this manuscript.
Conflict of interest: A. De Soyza reports receiving funds from AstraZeneca, Byers, Forest, Gilead, Insmed, GSK and Novartis outside of work for this manuscript.
Conflict of interest: S. Elborn reports research grants from iABC outside of work for this manuscript.
Conflict of interest: C. Haworth reports consulting fees from 30 Technology, Aradigm, CSL Behring, Chiesi, Gilead, Grifols, GSK, Insmed, Janssen, LifeArc, Meiji, Mylan, Novartis, Pneumagen, Shionogi, Teva, Vertex and Zambon; payment from Chiesi, Grifols, GSK, Insmed, Mylan, Novartis, Teva, Vertex and Zambon; and support for attending meetings and/or travel from Zambon outside of work for this manuscript.
Conflict of interest: J.R. Hurst reports consulting fees from AstraZeneca and GSK; payments from Boehringer Ingelheim, Chiesi, Sanofi and Takeda; support for attending meetings and travel from AstraZeneca; participation on an advisory board AstraZeneca; and receipt of equipment from Nonin outside of work for this manuscript.
Conflict of interest: M. Loebinger reports consulting fees from Armata, 30T, AstraZeneca, Insmed, Cheisi, Zambon, Electromed, Recode, Boehringer Ingelheim, Ethris, Mannkind and AN2 Therapeutics; and received payment or honoraria for lectures or presentations from Insmed outside of work for this manuscript.
Conflict of interest: J. Bradley reports research grants from Health and Social Care (Northern Ireland), iABC, NIHR and DfE; and receipt of equipment from PARI outside of work for this manuscript.
Conflict of interest: P.P. Walker reports unpaid work as Chair of the Board, British Thoracic Society, outside of work for this manuscript.
Conflict of interest: J. Steer reports research grants from Chiesi and Menarini Pharmaceutica; and speaker fees and conference and travel fees from AstraZeneca outside of work for this manuscript.
Conflict of interest: J. Duckers reports consulting fees from Insmed; payment received from Vertex, Chiesi and Insmed; participation on a data safety monitoring board for NOMAB study; Chair of British Thoracic Society CF Specialist advisory Group, CF Trust Registry Steering group and South-East Wales Research Ethics Committee; and receipt of medical writing from IQVIA outside of work for this manuscript.
Conflict of interest: M. Crichton declares consulting fees received from Boxer Capital LLC.
Conflict of interest: J.D. Chalmers reports research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Gilead Sciences, Novartis and Insmed; and received consultancy or speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Insmed, Janssen, Novartis and Zambon outside of work for this manuscript; and is an associate editor of this journal.
Support statement: We acknowledge the original funding obtained from the MRC (MR/L011263/1) and ongoing financial support from the COPD Foundation. BronchUK was further supported by educational grants for network activities from Bayer, Chiesi, Forest, Teva and GSK. BronchUK is sponsored by the Newcastle upon Tyne NHS Foundation Trust. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Barker AF. Bronchiectasis. N Engl J Med 2002; 346: 1383–1393. doi: 10.1056/NEJMra012519 [DOI] [PubMed] [Google Scholar]
- 2.Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47: 186–193. doi: 10.1183/13993003.01033-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finklea JD, Khan G, Thomas S, et al. Predictors of mortality in hospitalized patients with acute exacerbation of bronchiectasis. Respir Med 2010; 104: 816–821. doi: 10.1016/j.rmed.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 4.Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J 2015; 45: 1446–1462. doi: 10.1183/09031936.00119114 [DOI] [PubMed] [Google Scholar]
- 5.McDonnell MJ, Aliberti S, Goeminne PC, et al. Multimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016; 71: 1110–1118. doi: 10.1136/thoraxjnl-2016-208481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014; 189: 975–982. doi: 10.1164/rccm.201312-2208OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12: 1602–1611. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Ji X-B, Mao B, et al. Pseudomonas aeruginosa isolation in patients with non-cystic fibrosis bronchiectasis: a retrospective study. BMJ Open 2018; 8: e014613. doi: 10.1136/bmjopen-2016-014613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Leary CJ, Wilson CB, Hansell DM, et al. Relationship between psychological well-being and lung health status in patients with bronchiectasis. Respir Med 2002; 96: 686–692. doi: 10.1053/rmed.2002.1330 [DOI] [PubMed] [Google Scholar]
- 10.Ryu YJ, Chun EM, Lee JH, et al. Prevalence of depression and anxiety in outpatients with chronic airway lung disease. Korean J Intern Med 2010; 25: 51–57. doi: 10.3904/kjim.2010.25.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bekir M, Kocakaya D, Balcan B, et al. Clinical impact of depression and anxiety in patients with non-cystic fibrosis bronchiectasis. Tuberk Toraks 2020; 68: 103–111. doi: 10.5578/tt.69348 [DOI] [PubMed] [Google Scholar]
- 12.Gao YH, Guan WJ, Zhu YN, et al. Anxiety and depression in adult outpatients with bronchiectasis: associations with disease severity and health-related quality of life. Clin Respir J 2018; 12: 1485–1494. doi: 10.1111/crj.12695 [DOI] [PubMed] [Google Scholar]
- 13.Olveira C, Olveira G, Gaspar I, et al. Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res 2013; 22: 597–605. doi: 10.1007/s11136-012-0188-5 [DOI] [PubMed] [Google Scholar]
- 14.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019; 74: 1–69. [DOI] [PubMed] [Google Scholar]
- 15.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Resp J 2017; 50. 1–23. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 16.Mandal P, Sidhu MK, Kope L, et al. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Respir Med 2012; 106: 1647–1654. doi: 10.1016/j.rmed.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 17.Zanini A, Aiello M, Adamo D, et al. Effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: a retrospective analysis of clinical and functional predictors of efficacy. Respiration 2015; 89: 525–533. doi: 10.1159/000380771 [DOI] [PubMed] [Google Scholar]
- 18.Wynne SC, Patel S, Barker RE, et al. Anxiety and depression in bronchiectasis: response to pulmonary rehabilitation and minimal clinically important difference of the Hospital Anxiety and Depression Scale. Chron Respir Dis 2020; 17. doi: 10.1177/1479973120933292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Soyza A, Mawson P, Hill A, et al. BronchUK: protocol for an observational cohort study and biobank in bronchiectasis. ERJ Open Res 2021; 7: 00775-2020. doi: 10.1183/23120541.00775-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Actapsychiatrica Scandinavia 1983; 67: 361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 21.Heslop-Marshall K, Baker C, Carrick-Sen D, et al. Randomised controlled trial of cognitive behavioural therapy in COPD. ERJ Open Res 2018; 4: 00094-2018. doi: 10.1183/23120541.00094-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones PW. St. George's respiratory questionnaire: MCID. COPD 2005; 2: 75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 23.Quittner AL, Marciel KK, Salathe MA, et al. Quality of Life Questionnaire- Bronchiectasis: final psychometric analyses and determination of minimal important difference scores. Thorax 2015; 70: 12–20. doi: 10.1136/thoraxjnl-2014-205918 [DOI] [PubMed] [Google Scholar]
- 24.Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. doi: 10.1164/rccm.201309-1575OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson CB, Jones PW, O'Leary CJ, et al. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997; 156: 536–541. doi: 10.1164/ajrccm.156.2.9607083 [DOI] [PubMed] [Google Scholar]
- 26.NHS England. Adult psychiatric morbidity survey: survey of mental health and wellbeing, England, 2014. Date last updated: 29 September 2016. https://digital.nhs.uk/data-and-information/publications/statistical/adult-psychiatric-morbidity-survey/adult-psychiatric-morbidity-survey-survey-of-mental-health-and-wellbeing-england-2014
- 27.Martínez-García MÁ, Perpina-Tordera M, Roman-Sanchez P, et al. Quality-of-life determinants in patients with clinically stable bronchiectasis. Chest 2005; 128: 739–745. doi: 10.1378/chest.128.2.739 [DOI] [PubMed] [Google Scholar]
- 28.Boussoffara L, Boudawara N, Gharsallaoui Z, et al. Anxiety-depressive disorders and bronchiectasis. Rev Mal Respir 2014; 31: 230–236. doi: 10.1016/j.rmr.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 29.Cacopardo G, Cumbo F, Spicuzza L, et al. Depression in patients with non-cystic fibrosis bronchiectasis. Eur Respir J 2019; 59: PA2683. 10.1183/13993003.congress-2019.pa2683 [DOI] [Google Scholar]
- 30.Office for National Statistics . Coronavirus and depression in adults, Great Britain: June 2020. Date last accessed: Nov 2023. Date last updated: 2020. www.ons.gov.uk/peoplepopulationandcommunity/wellbeing/articles/coronavirusanddepressioninadultsgreatbritain/june2020
- 31.Waraich P, Goldner EM, Somers JM, et al. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry 2004; 49: 124–138. doi: 10.1177/070674370404900208 [DOI] [PubMed] [Google Scholar]
- 32.Carney RM, Freedland KE. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med 2009; 76: 13–17. doi: 10.3949/ccjm.76.s2.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao YH, Zheng HZ, Lu HW, et al. The impact of depression and anxiety on the risk of exacerbation in adults with bronchiectasis: a prospective cohort study. Eur Respir J 2023; 61: 2201695. doi: 10.1183/13993003.01695-2022 [DOI] [PubMed] [Google Scholar]
- 34.Quittner AL, Abbott J, Georgiopoulos AM, et al. International committee on mental health in cystic fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax 2016; 71: 26–34. doi: 10.1136/thoraxjnl-2015-207488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graziano S, Ullmann N, Rusciano R, et al. Comparison of mental health in individuals with primary ciliary dyskinesia, cystic fibrosis, and parent caregivers. Resp Med 2023; 207: 107095. doi: 10.1016/j.rmed.2022.107095 [DOI] [PubMed] [Google Scholar]
- 36.Knudsen KB, Pressler T, Mortensen LH, et al. Associations between adherence, depressive symptoms and health-related quality of life in young adults with cystic fibrosis. Springerplus eCollection 2016; 5: 1216. doi: 10.1186/s40064-016-2862-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schechter MS, Ostrenga JS, Fink AK, et al. Decreased survival in cystic fibrosis patients with a positive screen for depression. J Cyst Fibros 2021; 20: 120–126. doi: 10.1016/j.jcf.2020.07.020 [DOI] [PubMed] [Google Scholar]
- 38.DiMatteo MR, Giordani PJ, Lepper HS, et al. Patient adherence and medical treatment outcomes a meta-analysis. Med Care 2002; 40: 794–811. doi: 10.1097/00005650-200209000-00009 [DOI] [PubMed] [Google Scholar]
- 39.Shi L, Xia Z, Guo J, et al. Maresin-1 improves LPS-induced depressive-like behavior by inhibiting hippocampal microglial activation. J Affect Disord 2023; 328: 261–272. doi: 10.1016/j.jad.2023.02.016 [DOI] [PubMed] [Google Scholar]
- 40.Colomo N, Olveira C, Hernández-Pedrosa J, et al. Validity of self-rating screening scales for the diagnosis of depression and anxiety in adult patients with bronchiectasis. Arch. Bronconeumol. (Engl. Ed.) 2021; 57: 179–185. doi: 10.1016/j.arbr.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 41.NICE. Generalised anxiety disorder and panic disorder in adults: management. Clinical guideline [CG113]. Date last updated: 15 June 2020. Date last accessed: August 2024. https://www.nice.org.uk/guidance/CG113/guidance/cg113
- 42.Spinou A, Fragkos KC, Lee KK, et al. The validity of health-related quality of life questionnaires in bronchiectasis: a systematic review and meta-analysis. Thorax 2016; 71: 683–694. doi: 10.1136/thoraxjnl-2015-207315 [DOI] [PubMed] [Google Scholar]
- 43.Quittner AL, Barker D, Graziano S, et al. National integration of mental health screening and treatment into specialized care for cystic fibrosis: what predicts success? Pediatr Pulmonol 2023; 58: 1768–1776. doi: 10.1002/ppul.26400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00348-2024.SUPPLEMENT (1.3MB, pdf)