Abstract
Background and Objectives
Disturbed sleep is common after stroke, yet its relationship with cerebral small vessel disease (SVD) and cognitive performance in the stroke population, particularly patients with TIA/mild stroke who are on the milder end of the cerebrovascular spectrum, remains understudied. We aim to examine the associations of self-reported sleep metrics with neuroimaging markers of SVD and cognitive performance in patients with TIA/mild stroke from 2 prospective stroke cohorts.
Methods
We studied adult patients with TIA/mild stroke (NIH Stroke Scale [NIHSS] score <7) who were consecutively recruited from Mild Stroke Study 3 (MSS3, University of Edinburgh) and the stroke cohort (the University of Hong Kong, HKU) during 2018–2022. Both MSS3 (N = 211) and HKU (N = 211) cohorts assessed SVD burden visually on brain MRI, cognitive performance using Montreal Cognitive Assessment (MoCA), and sleep quality using a structured sleep questionnaire at baseline visit. The primary outcomes were SVD markers, and the secondary outcome was total MoCA score. The associations of sleep metrics with SVD markers and cognitive performance were assessed using regression models, adjusted for demographics, vascular risk factors, history of depression and stroke, and study sites.
Results
In 422 patients (65.6 ± 11.8 years, 67% male, median NIHSS score 1.0), longer in-bed time was independently associated with greater global SVD and Fazekas periventricular white matter hyperintensity (WMH) burden: odds ratio (OR)summary SVD score = 1.27 per 1-SD increase (95% CI 1.05–1.53), false discovery rate (FDR)–adjusted p = 0.04; ORperiventricular WMH = 1.53 per 1-SD increase (95% CI 1.18–2.00), p = 0.003. Longer sleep duration was independently associated with presence of cerebral microbleeds: OR = 1.42 per 1-SD increase (95% CI 1.09–1.87), p = 0.04. Longer in-bed time was associated with a lower total MoCA score after covariate adjustment: standardized β = −0.58 (95% CI −0.99 to −0.16), p = 0.02.
Discussion
Disturbed sleep, including longer in-bed time and longer sleep duration, was cross-sectionally associated with greater SVD burden and worse cognitive performance in patients with TIA/mild stroke. Future longitudinal studies are warranted to validate our findings.
Introduction
Disturbed sleep is common among patients with stroke and often presents with prolonged time in bed, insomnia, hypersomnia, and excessive daytime sleepiness.1 It is estimated that up to 38% of stroke patients have insomnia2 and 27% have sleep needs longer than 10 hours per day.1 Disturbed sleep may be linked to adverse brain health through worsening vascular disease or disrupting glymphatic function, according to a Scientific Statement from the American Heart Association.3 Yet, its role in brain health in the stroke population, particularly patients with TIA/mild stroke who are on the milder end of the cerebrovascular spectrum, remains understudied.
Cerebral small vessel disease (SVD), characterized by abnormal small perforating arterioles and capillaries, is a major cause of stroke and vascular dementia worldwide.4 SVD imaging markers include recent small subcortical infarcts, white matter hyperintensities (WMHs), lacunes, enlarged perivascular spaces (PVSs), cerebral microbleeds, and brain atrophy.5 In middle-aged and older adults, poor sleep quality has been associated with worse WMHs,6 brain atrophy,7 and more PVSs,8-10 but not with cerebral microbleeds or lacunes,6 and longitudinally with increasing WMHs in older people.11 Nonetheless, these studies reported inconsistent findings or comprised stroke-free individuals with small sample sizes in single cohorts. Moreover, despite the gender difference in sleep pattern,12 SVD burden,13 and vascular cognitive impairment,14 research on gender differences in the relationships between sleep and brain health in stroke populations is limited.
In this study, we explored the associations of self-reported sleep metrics with a full range of SVD markers and cognitive performance in patients with TIA/mild stroke, from 2 prospective stroke cohorts reflecting different ethnic backgrounds. We hypothesize that disturbed sleep is cross-sectionally associated with greater SVD burden and worse cognitive performance across 2 stroke cohorts, with sex as a potential moderator.
Methods
Study Participants
We used data from 2 prospective stroke cohorts: Mild Stroke Study 3 (MSS3)15 (N = 211) at the University of Edinburgh and the TIA/mild stroke cohort at the University of Hong Kong (HKU) (N = 211). Both studies recruited consecutive adult patients with TIA or mild ischemic stroke (NIH Stroke Scale [NIHSS] score <7) from 2018 to 2022, diagnosed based on clinical stroke syndromes and imaging findings, from the NHS Lothian clinical stroke services, Edinburgh, United Kingdom, or the Acute Stroke Unit/TIA Clinic of Queen Mary Hospital, Hong Kong, respectively. Patients were excluded if they were diagnosed with major stroke (NIHSS score ≥7) or were not available to complete a sleep questionnaire or brain MRI. All participants had baseline assessment at 1–3 months after stroke including demographics, medical history, 3T brain MRI, sleep, and cognitive and NIHSS assessments.
Standard Protocol Approvals, Registrations, and Patient Consents
MSS3 was approved by the South East Scotland Research Ethics Committee (reference 18/SS/0044). The HKU cohort study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW18-361). All study participants gave written informed consent.
Sleep Metric Analysis
We extracted several self-reported sleep metrics from an adapted version of the Pittsburgh Sleep Quality Index at baseline visit,16 a well-validated sleep questionnaire assessing sleep quality using subjective ratings among patients with stroke within a one-month time interval.17 Several quantitative sleep metrics including in-bed time (hours), nighttime sleep duration (hours), sleep latency (minutes), and sleep efficiency (sleep duration/in-bed time × 100%) were calculated for further analysis.
MRI Acquisitions and Analysis
All participants underwent brain MRI at baseline. MRI sequences included 3D T1-weighted, T2-weighted, fluid-attenuated inversion recovery, susceptibility-weighted, and diffusion-weighted imaging, as described previously.15,18 An experienced team, supervised by a neuroradiologist (J.M.W.) and neurologist (K.K.L.), visually assessed neuroimaging markers of SVD using STRIVE criteria,5 including presence of lacunes, WMH burden (Fazekas scores 0–3) in deep and periventricular regions, PVSs in basal ganglia and centrum semiovale regions (BG-PVS and CSO-PVS, categorized as ≤10, 11–20, >20), and presence of cerebral microbleeds using validated visual rating scales.19-21 The sum of SVD score was calculated based on WMHs, PVSs, cerebral microbleeds, and lacunes as previously described.22 We also assessed brain atrophy in the deep (ventricular enlargement) and superficial (gyral enlargement) brain regions against a validated normal aging reference template, ranging from 1 (normal) to 6 (severe atrophy).23
Cognitive Assessment
All the participants underwent the Montreal Cognitive Assessment (MoCA)24 at baseline, a validated screening tool for vascular cognitive impairment after acute stroke,25 to evaluate global cognition.
Covariates
The following covariates were preselected, based on known confounders of sleep and SVD burden or cognition in literature review: age (years), sex (male and female), educational level (categorized as 0–6, 7–9, 10–12, and >12 years), vascular risk score (a composite sum score comprising presence of hypertension, hyperlipidemia, diabetes, and ever-smoking), history of depression, history of stroke, and study site (MSS3 or HKU cohort).
Statistical Analysis
We described the study population using mean and SDs or median (interquartile range [IQR]) for continuous variables, or relative frequencies for categorical variables, respectively. We compared the difference in demographic, sleep, and imaging characteristics between 2 stroke cohorts using the Student t test, Wilcoxon rank-sum test, χ2 test, or Fisher exact test, as appropriate. We also performed correlation analysis to explore the linear relationship between sleep duration and other sleep metrics.
To examine the associations between sleep metrics and SVD burden, we used multivariable ordinal (summary SVD score [0–4], WMH [0, 1–2, 3] or PVS [≥10, 11–20, >20] burden, tertiles of the total brain atrophy score [sum of brain atrophy score in deep and superficial regions: 0–4, 5–8, 9–12]) or binomial (presence of lacunes or cerebral microbleeds) logistic regression analysis, with each SVD marker as the dependent variable and each sleep metric as the independent variable. We also used a linear regression model to examine the associations between each sleep metric and total MoCA score, respectively. All the sleep metrics and MoCA score were converted into z-scores. We applied 2 multivariable models: model 1 corrected for age, sex, and study site; model 2 further corrected for vascular risk score, education, history of depression, and history of stroke. We further examined whether sex moderates the associations between sleep and SVD burden or cognitive performance by including an interaction term for sleep metric × sex, respectively.
All statistical analyses were performed using R (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria) and Python (version 3.11.3, Python Software Foundation, Wilmington, Delaware). In the regression models, we reported odds ratios (ORs) or standardized beta coefficients with its 95% CIs and p values. We computed a false discovery rate (FDR) to account for multiple comparisons and used q = 0.05 as the significance threshold.
Data Availability
The anonymized data that support the findings of this study are available from the corresponding authors on reasonable request.
Results
Study Sample Characteristics
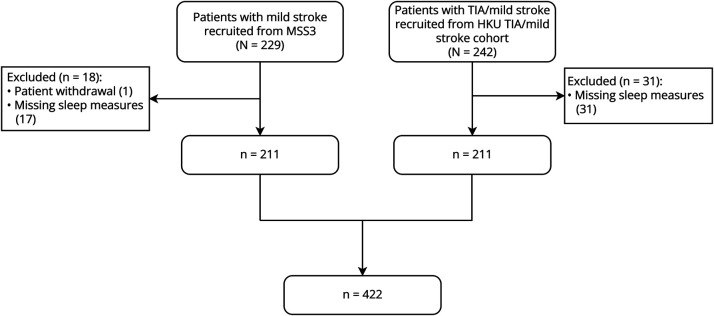
There were 422 participants included in the final analysis (Figure 1). The study sample had a mean age of 65.4 ± 11.8 years, 66% were male, and the median NIHSS score was 1.0 (IQR 2.0). The MSS3 cohort was predominantly White British while the HKU cohort was predominantly Chinese. Compared with participants in the HKU cohort, MSS3 participants had similar mean age and were more likely to have higher educational level, history of depression, smoking history, hyperlipidemia, presence of lacunes, higher deep WMH and PVS burden, and worse brain atrophy, but less likely to have diabetes (all p < 0.05, Table 1). In addition, MSS3 participants had longer total in-bed time, longer sleep duration, and lower sleep efficiency (all p < 0.05, Table 1).
Figure 1. Study Flowchart.
HKU = University of Hong Kong; MSS3 = Mild Stroke Study 3.
Table 1.
Baseline Demographic, Clinical, and Imaging Characteristics of the 2 Stroke Cohorts
| Characteristic | Overall (N = 422) | HKU TIA/mild stroke cohort, Hong Kong (N = 211) | MSS3, Edinburgh, United Kingdom (N = 211) | p Value |
| Demographics | ||||
| Age, y | 65.4 (11.8) | 64.9 (12.4) | 65.9 (11.2) | 0.43 |
| Male | 278 (66) | 140 (66) | 138 (65) | 0.92 |
| Education years | <0.001 | |||
| 0–6 y | 68 (16) | 68 (32) | 0 (0) | |
| 7–9 y | 94 (22) | 40 (19) | 54 (26) | |
| 10–12 y | 134 (32) | 63 (30) | 71 (34) | |
| >12 y | 126 (30) | 40 (19) | 86 (41) | |
| Clinical | ||||
| NIHSS score | 1.0 (2.0) | 1.0 (2.0) | 1.0 (2.0) | 0.42 |
| Hypertension | 109 (26) | 64 (30) | 45 (21) | 0.045 |
| Hyperlipidemia | 267 (63) | 124 (59) | 143 (68) | 0.07 |
| Diabetes | 230 (55) | 74 (35) | 156 (74) | <0.001 |
| Previous TIA/stroke | 52 (12) | 19 (9.0) | 33 (16) | 0.05 |
| Atrial fibrillation | 38 (9.0) | 19 (9.0) | 19 (9.0) | 1 |
| Depression | 50 (12) | 3 (1.4) | 47 (22) | <0.001 |
| Ever-smoker | 185 (44) | 65 (31) | 120 (57) | <0.001 |
| Alcohol drinker | 104 (25) | 45 (21) | 59 (28) | 0.14 |
| MoCA score | 23.7 (5.0) | 22.9 (6.1) | 24.5 (3.6) | 0.18 |
| Imaging | ||||
| Summary SVD score | 0.18 | |||
| 0 | 118 (28) | 65 (31) | 53 (25) | |
| 1 | 99 (23) | 55 (26) | 44 (21) | |
| 2 | 94 (22) | 38 (18) | 56 (27) | |
| 3 | 69 (16) | 34 (16) | 35 (17) | |
| 4 | 42 (10) | 19 (9.0) | 23 (11) | |
| Presence of lacunes | 183 (43) | 80 (38) | 103 (49) | 0.024 |
| Presence of microbleeds | 75 (18) | 37 (18) | 38 (18) | 0.90 |
| Deep WMH | 0.05 | |||
| Fazekas score 0 | 50 (12) | 32 (15) | 18 (8.5) | |
| Fazekas scores 1–2 | 316 (75) | 148 (70) | 168 (80) | |
| Fazekas score 3 | 56 (13) | 31 (15) | 25 (12) | |
| Periventricular WMHs | 0.15 | |||
| Fazekas score 0 | 10 (2.4) | 2 (0.9) | 8 (3.8) | |
| Fazekas scores 1–2 | 319 (76) | 163 (77) | 156 (74) | |
| Fazekas score 3 | 93 (22) | 46 (22) | 47 (22) | |
| Basal ganglia PVSs | <0.001 | |||
| ≤10 | 182 (43) | 102 (48) | 80 (38) | |
| 11–20 | 165 (39) | 88 (42) | 77 (36) | |
| >20 | 75 (18) | 21 (10) | 54 (26) | |
| Central semiovale PVSs | <0.001 | |||
| ≤10 | 174 (41) | 94 (45) | 80 (38) | |
| 11–20 | 132 (31) | 86 (41) | 46 (22) | |
| >20 | 116 (27) | 31 (15) | 85 (40) | |
| Tertile of total brain atrophy score | 0.019 | |||
| 0–4 | 179 (42) | 102 (48) | 77 (36) | |
| 5–8 | 165 (39) | 69 (33) | 96 (45) | |
| 9–12 | 78 (18) | 40 (19) | 38 (18) | |
| Sleep | ||||
| Total in-bed time, h | 8.5 (1.7) | 8.0 (1.6) | 9.1 (1.6) | <0.001 |
| Sleep duration, h | 6.8 (1.5) | 6.7 (1.4) | 7.0 (1.6) | 0.04 |
| Sleep latency, min | 26.0 (29.9) | 26.9 (35.1) | 25.2 (23.5) | 0.11 |
| Sleep efficiency, % | 81.4 (15.8) | 85.4 (15.2) | 77.5 (15.5) | <0.001 |
Abbreviations: IQR = interquartile range; MoCA = Montreal Cognitive Assessment; NIHSS = NIH Stroke Scale; PVSs = perivascular spaces; SVD = small vessel disease; WMH = white matter hyperintensity.
Data are presented as mean (SD), median (IQR), or n (%).
Correlation of Sleep Metrics
Both in-bed time and sleep efficiency were positively correlated with sleep duration: in-bed time: r = 0.52, p < 0.001; sleep efficiency: r = 0.56, p < 0.001. Sleep latency was negatively correlated with sleep duration (r = −0.24, p < 0.001).
Sleep and SVD Markers
In the pooled analysis, in-bed time was associated with all SVD markers except for deep WMHs and presence of cerebral microbleeds, when adjusting for age, sex, and study site (Figure 2, model 1). The associations remained significant after full adjustment for in-bed time with summary SVD score and periventricular WMHs (ORsummary SVD score = 1.27 per 1-SD increase [95% CI 1.05–1.53], FDR-adjusted p = 0.04; ORperiventricular WMHs = 1.53 per 1-SD increase [95% CI 1.18–2.00], p = 0.003) while did not reach statistical significance with presence of lacunes or brain atrophy (ORpresence of lacunes = 1.30 per 1-SD increase [95% CI 1.03–1.64], p = 0.06; ORtertile of total brain atrophy score = 1.25 per 1-SD increase [95% CI 1.01–1.57], p = 0.07; Figure 2, model 2).
Figure 2. Adjusted Odds Ratios With 95% CI for In-Bed Time and SVD Markers.
Model 1 adjusted for age, sex, and study site. Model 2 further adjusted for vascular risk score, education, history of depression, and history of stroke. Data are presented as odds ratio (95% CI) with FDR-adjusted p value. *FDR-adjusted p < 0.05; ** FDR-adjusted p < 0.01. BG = basal ganglia; CSO = central semiovale; FDR = false discovery rate; PVS = perivascular space; SVD = small vessel disease; WMH = white matter hyperintensity.
Sleep duration was associated with presence of lacunes and cerebral microbleeds in model 1 (Figure 3, model 1). The association only remained significant for presence of cerebral microbleeds after full adjustment (ORpresence of cerebral microbleeds = 1.42 per 1-SD increase [95% CI 1.09–1.87], p = 0.04; ORpresence of lacunes = 1.27 per 1-SD increase [95% CI 1.03–1.58], p = 0.07; Figure 3, model 2). Similar to the patterns observed for in-bed time, a positive association was found between sleep duration and both summary SVD score and brain atrophy, although these associations did not reach statistical significance. No significant association was found between sleep duration and other SVD markers (Figure 3, model 2).
Figure 3. Adjusted Odds Ratios With 95% CI for Sleep Duration and SVD Markers.
Model 1 adjusted for age, sex, and study site. Model 2 further adjusted for vascular risk score, education, history of depression, and history of stroke. Data are presented as odds ratio (95% CI) with FDR-adjusted p value. *FDR-adjusted p < 0.05. BG = basal ganglia; CSO = central semiovale; FDR = false discovery rate; PVS = perivascular space; SVD = small vessel disease; WMH = white matter hyperintensity.
In addition, we did not find any association between sleep latency or sleep efficiency and SVD markers, respectively (eTable 1).
Sleep and Cognitive Performance
Pooled estimates revealed that longer in-bed time, rather than longer sleep duration, was associated with a lower total MoCA score after covariate adjustment (standardized βtotal time in bed = −0.58 [95% CI −0.99 to −0.16], p = 0.02; standardized βsleep duration = −0.22 [95% CI −0.61 to 0.17], p = 0.48; Table 2, model 2). No significant associations were detected between sleep latency or sleep efficiency and total MoCA score (all p > 0.05) (Table 2, model 2).
Table 2.
Association Between Sleep Metrics and Total MoCA Score
| Sleep metrics (z-score) | Model 1 | Model 2 |
| In-bed time, h | −0.15 (−0.24 to −0.05)** | −0.58 (−0.99 to −0.16)* |
| Sleep duration, h | −0.02 (−0.11 to 0.07) | −0.22 (−0.61 to 0.17) |
| Sleep efficiency, % | 0.12 (0.03 to 0.21)* | 0.32 (−0.09 to 0.73) |
| Sleep latency, min | −0.12 (−0.21 to −0.04)* | −0.44 (−0.82 to −0.05) |
Abbreviations: FDR = false discovery rate; MoCA = Montreal Cognitive Assessment.
Data are presented as standardized β (95% CI) with FDR-adjusted p value. Model 1 adjusted for age, sex, and study site; model 2 further adjusted for vascular risk score, education, history of depression, and history of stroke.
*FDR-adjusted p < 0.05; ** FDR-adjusted p < 0.01.
Moderation Analysis
There was no moderating effect of sex on the associations between self-reported sleep metrics and SVD burden (eTable 2) or total MoCA score (eTable 3).
Discussion
In this pooled analysis of 2 prospective stroke cohorts, we examined cross-sectional associations between self-reported sleep metrics, SVD markers, and cognitive performance at 1–3 months after TIA/mild stroke. Our results indicated that longer in-bed time was associated with greater global SVD and periventricular WMH burden, and worse cognitive performance. Longer sleep duration was associated with presence of cerebral microbleeds, but not with cognitive performance.
Increasing evidence has linked poor sleep quality to SVD burden, mainly WMH load in community-dwelling populations,10,11,26-29 as well as PVS burden9,11 and brain atrophy7 in older adults. In the Northern Manhattan Study, long sleep (≥9 hours) was independently associated with a greater WMH volume.26 In the Atahualpa Project, poor sleep was associated with severity of WMHs, but not with lacunes or cerebral microbleeds.6 Furthermore, a U-shape relationship between sleep duration and WMHs was recently revealed in the UK Biobank analysis.29,30 While short sleep duration has been thought of as potentially leading to or a marker of adverse brain health, other markers of impaired sleep quality may be more sensitive to SVD burden in patients suffering from cerebrovascular disease. Our findings suggest that longer in-bed time is associated with greater global SVD and periventricular WMH burden in patients with TIA or mild stroke. Similar trends were observed for associations of both in-bed time and nighttime sleep duration with presence of lacunes and brain atrophy, but these did not reach statistical significance. In addition, longer sleep duration was associated with an increased risk of cerebral microbleeds. Discrepancies may stem from differences in demographics (age, sex, ethnicity), sleep patterns, vascular risk factors, and SVD burden between community populations and stroke patients because community-dwelling individuals are generally healthier. Future prospective studies are needed to explore the longitudinal effects of prolonged sleep on brain health in community-dwelling vs stroke population.
Poor sleep quality, as measured with actigraphy or a sleep questionnaire, was cross-sectionally and longitudinally associated with cognitive impairment after stroke.31,32 Consistent with these findings, our results indicate that longer in-bed time is associated with worse cognitive performance after stroke. Future large longitudinal studies are needed to confirm whether a consistent pattern exists between longer sleep pattern and worse cognitive performance after stroke.
The mechanism linking prolonged sleep patterns to brain health is not clear. Disturbed sleep may disrupt waste clearance including β-amyloid clearance, accentuating endothelial blood-brain barrier dysfunction and neuroinflammation,33,34 and eventually lead to SVD development and cognitive impairment. Excessive sleep patterns may be a marker of circadian dysfunction, which has recently been shown to affect blood pressure regulation and the onset or progression of hypertension.35 This, in turn, could ultimately contribute to SVD burden.36 In addition, by disrupting brain connections or through strategically placed lesions, SVD lesions themselves could contribute to sleep impairment and potentially worsen brain health. Moreover, white matter changes are associated with apathy and gait decline,37,38 which may similarly contribute to prolonged in-bed time. Longer in-bed time, in turn, could be a symptom of greater SVD burden. Future studies are needed to clarify the specific pathophysiologic processes linking longer sleep pattern to distinct SVD pathologies and vice versa.
Gender difference has been reported in sleep patterns, SVD burden, and vascular cognitive impairment. Women tend to have better polysomnography-defined sleep than men.12 Moreover, women more often had pronounced WMH burden while men more often had presence of lacunes among patients with vascular cognitive impairment.14 Only 1 study reported that longer sleep latency was associated with poor cognitive performance in men, but not in women.39 Although we did not find any significant moderating effect of sex, our results should be validated in future longitudinal studies.
The strengths of our study include the pooled analysis of Asian and European stroke populations, comprehensive assessment of SVD markers, and availability of information on potential confounders. Limitations include the cross-sectional design and the use of visual assessments rather than computational measures to describe WMH burden and brain atrophy. In addition, we did not exclude patients with a history of sleep apnea or track potential changes in sleep quality before and after stroke because of a lack of clinical data. Our results should be interpreted with caution because we only examined baseline cross-sectional data, which cannot establish causal relationships. Some relationships between sleep, SVD, and cognition may differ in a longitudinal context and at different time points after stroke.
In conclusion, 2 markers of disturbed sleep, longer in-bed time and longer sleep duration, were cross-sectionally associated with greater SVD burden and worse cognitive performance in patients with TIA/mild stroke.
Acknowledgment
The authors thank Ms. Roxanna Liu, Ms. Siu Wah Hui, Dr. Carlin Chang, Dr. Kay Cheong Teo, Dr. Yuan Gao, and Dr. William Leung of the Neurology Team, Queen Mary Hospital for assisting with patient recruitment, and Ms. Debbie Wong for database management in the HKU cohort. The authors thank Dr. Stewart Wiseman, Dr. Olivia Hamilton, Mr. Will Hewin, and Mrs. Rachel Locherty for assistance with patient assessment, and Mrs. Charlotte Jardine, Mrs. Gayle Barclay, Mrs. Iona Hamilton, and Mrs. Donna McIntyre for performing the MRI scanning of the MSS3 cohort.
Glossary
- BG
basal ganglia
- CSO
central semiovale
- FDR
false discovery rate
- HKU
University of Hong Kong
- IQR
interquartile range
- MoCA
Montreal Cognitive Assessment
- MSS3
Mild Stroke Study 3
- OR
odds ratio
- PVS
perivascular space
- SVD
small vessel disease
- WMH
white matter hyperintensity
Author Contributions
D.X. Liu: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. M.S.-M. Ip: analysis or interpretation of data. D.C.-L. Lam: analysis or interpretation of data. F.M. Chappell: analysis or interpretation of data. U. Clancy: major role in the acquisition of data. D. Jaime Garcia: major role in the acquisition of data. C. Arteaga-Reyes: major role in the acquisition of data. M.D.C. Valdés Hernández: major role in the acquisition of data. M. Thrippleton: major role in the acquisition of data. M.S. Stringer: major role in the acquisition of data. Y. Cheng: major role in the acquisition of data. J. Zhang: major role in the acquisition of data. F. Doubal: major role in the acquisition of data; study concept or design. G.K.K. Lau: study concept or design; analysis or interpretation of data. J.M. Wardlaw: drafting/revision of the manuscript for content, including medical writing for content; study concept or design.
Study Funding
This work received funding from the UK Dementia Research Institute at the University of Edinburgh (award number UK DRI-4002) through UK DRI Ltd., principally funded by the UK Medical Research Council; European Union Horizon 2020 (PHC-03-15, project no. 666881 “SVDs@Target”); Foundation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease (ref. no. 16CVD 05); Row Fogo Charitable Trust (grant no. BRO-D.FID3668413); the Chief Scientist Office of Scotland (UC; CAF/18/08); the British Heart Foundation Research Excellence Award III (RE/18/5/34216); The Mexican National Council of Science and Technology (CAR; 2021-000007-01EXTF-00234); HKU Foundation Postgraduate Fellowship (DXDL); HKU Research Postgraduate Exchange Scheme (DXDL); Stroke Association Research Development Fellowship 2018 (UC), SVD-SOS project (SA PG 19\100068) Senior Lectureship (FD: TSA LECT 2015/04), and Postdoctoral Fellowships (SA PDF 18\100026, SAPDF 23/100007, MSS); and the Wellcome Trust (DJG, Grant number 224912/Z/21/Z, Translational Neuroscience PhD, University of Edinburgh).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73(16):1313-1322. doi: 10.1212/wnl.0b013e3181bd137c [DOI] [PubMed] [Google Scholar]
- 2.Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101222. doi: 10.1016/j.smrv.2019.101222 [DOI] [PubMed] [Google Scholar]
- 3.Gottesman RF, Lutsey PL, Benveniste H, et al. Impact of sleep disorders and disturbed sleep on brain health: a scientific statement from the American Heart Association. Stroke. 2024;55(3):e61-e76. doi: 10.1161/str.0000000000000453 [DOI] [PubMed] [Google Scholar]
- 4.Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: a clinical review. Neurology. 2019;92(24):1146-1156. doi: 10.1212/wnl.0000000000007654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822-838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Brutto OH, Mera RM, Zambrano M, Lama J, Del Brutto VJ, Castillo PR. Poor sleep quality and silent markers of cerebral small vessel disease: a population-based study in community-dwelling older adults (The Atahualpa Project). Sleep Med. 2015;16(3):428-431. doi: 10.1016/j.sleep.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH, Mera RM, Zambrano M, Castillo PR. The association between poor sleep quality and global cortical atrophy is related to age. Results from the Atahualpa Project. Sleep Sci. 2016;9(3):147-150. doi: 10.1016/j.slsci.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo JC, Loh KK, Zheng H, Sim SKY, Chee MWL. Sleep duration and age-related changes in brain structure and cognitive performance. Sleep. 2014;37(7):1171-1178. doi: 10.5665/sleep.3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Brutto OH, Mera RM, Del Brutto VJ, Castillo PR. Enlarged basal ganglia perivascular spaces and sleep parameters. A population-based study. Clin Neurol Neurosurg. 2019;182:53-57. doi: 10.1016/j.clineuro.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Aribisala BS, Riha RL, Valdes Hernandez M, et al. Sleep and brain morphological changes in the eighth decade of life. Sleep Med. 2020;65:152-158. doi: 10.1016/j.sleep.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 11.Aribisala BS, Valdés Hernández MDC, Okely JA, et al. Sleep quality, perivascular spaces and brain health markers in ageing: a longitudinal study in the Lothian Birth Cohort 1936. Sleep Med. 2023;106:123-131. doi: 10.1016/j.sleep.2023.03.016 [DOI] [PubMed] [Google Scholar]
- 12.Mong JA, Cusmano DM. Sex differences in sleep: impact of biological sex and sex steroids. Philos Trans R Soc Lond B Biol Sci. 2016;371(1688):20150110. doi: 10.1098/rstb.2015.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Sánchez L, Hamilton OKL, Clancy U, et al. Sex differences in cerebral small vessel disease: a systematic review and meta-analysis. Front Neurol. 2021;12:756887. doi: 10.3389/fneur.2021.756887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exalto LG, Boomsma JMF, Babapour Mofrad R, et al. Sex differences in memory clinic patients with possible vascular cognitive impairment. Alzheimers Dement. 2020;12(1):e12090. doi: 10.1002/dad2.12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy U, Garcia DJ, Stringer MS, et al. Rationale and design of a longitudinal study of cerebral small vessel diseases, clinical and imaging outcomes in patients presenting with mild ischaemic stroke: Mild Stroke Study 3. Eur Stroke J. 2021;6(1):81-88. doi: 10.1177/2396987320929617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 17.Luo Y, Yu G, Liu Y, Zhuge C, Zhu Y. Sleep quality after stroke: a systematic review and meta-analysis. Medicine (Baltimore). 2023;102(20):e33777. doi: 10.1097/md.0000000000033777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau KK, Li L, Schulz U, et al. Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology. 2017;88(24):2260-2267. doi: 10.1212/wnl.0000000000004042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fazekas F, Chawluk J, Alavi A, Hurtig H, Zimmerman R. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351-356. doi: 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- 20.Cordonnier C, Potter GM, Jackson CA, et al. Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. 2009;40(1):94-99. doi: 10.1161/strokeaha.108.526996 [DOI] [PubMed] [Google Scholar]
- 21.Doubal FN, MacLullich AMJ, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010;41(3):450-454. doi: 10.1161/strokeaha.109.564914 [DOI] [PubMed] [Google Scholar]
- 22.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36(10):2806-2811. doi: 10.1016/j.neurobiolaging.2015.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrell C, Chappell F, Armitage PA, et al. Development and initial testing of normal reference MR images for the brain at ages 65-70 and 75-80 years. Eur Radiol. 2009;19(1):177-183. doi: 10.1007/s00330-008-1119-2 [DOI] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 25.Ghafar MZAA, Miptah HN, O'Caoimh R. Cognitive screening instruments to identify vascular cognitive impairment: a systematic review. Int J Geriatr Psychiatry. 2019;34(8):1114-1127. doi: 10.1002/gps.5136 [DOI] [PubMed] [Google Scholar]
- 26.Ramos AR, Dong C, Rundek T, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study. J Sleep Res. 2014;23(5):524-530. doi: 10.1111/jsr.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Nasrallah I, Hoang TD, et al. Sleep duration and white matter quality in middle-aged adults. Sleep. 2016;39(9):1743-1747. doi: 10.5665/sleep.6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sexton CE, Zsoldos E, Filippini N, et al. Associations between self-reported sleep quality and white matter in community-dwelling older adults: a prospective cohort study. Hum Brain Mapp. 2017;38(11):5465-5473. doi: 10.1002/hbm.23739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning J, Zhang W, Chen SF, et al. Association of sleep behaviors with white matter hyperintensities and microstructural injury: a cross-sectional and longitudinal analysis of 26 354 participants. Sleep. 2023;46(5):zsad020. doi: 10.1093/sleep/zsad020 [DOI] [PubMed] [Google Scholar]
- 30.Clocchiatti-Tuozzo S, Rivier CA, Renedo D, et al. Suboptimal sleep duration is associated with poorer neuroimaging brain health profiles in middle-aged individuals without stroke or dementia. J Am Heart Assoc. 2024;13(1):e031514. doi: 10.1161/jaha.123.031514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falck RS, Best JR, Davis JC, et al. Sleep and cognitive function in chronic stroke: a comparative cross-sectional study. Sleep. 2019;42(5):zsz040. doi: 10.1093/sleep/zsz040 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Xia X, Zhang T, et al. Relation between sleep disorders and post-stroke cognitive impairment. Front Aging Neurosci. 2023;15:1036994. doi: 10.3389/fnagi.2023.1036994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348-355. doi: 10.1016/j.nbd.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J, Hsuchou H, He Y, Kastin AJ, Wang Y, Pan W. Sleep restriction impairs blood-brain barrier function. J Neurosci. 2014;34(44):14697-14706. doi: 10.1523/jneurosci.2111-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faraci FM, Scheer FAJL. Hypertension: causes and consequences of circadian rhythms in blood pressure. Circ Res. 2024;134(6):810-832. doi: 10.1161/circresaha.124.323515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chokesuwattanaskul A, Cheungpasitporn W, Thongprayoon C, et al. Impact of circadian blood pressure pattern on silent cerebral small vessel disease: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(12):e016299. doi: 10.1161/jaha.119.016299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollocks MJ, Lawrence AJ, Brookes RL, et al. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain. 2015;138(pt 12):3803-3815. doi: 10.1093/brain/awv304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Holst HM, Tuladhar AM, Zerbi V, et al. White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin. 2018;17:731-738. doi: 10.1016/j.nicl.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medeiros LdS, Santos FH, Almeida AP, et al. Sex differences in the cognitive performance in adults: role of impaired sleep. Sleep Sci. 2022;15(1):17-25. doi: 10.5935/1984-0063.20210022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The anonymized data that support the findings of this study are available from the corresponding authors on reasonable request.