Abstract
Background/Objectives
Percutaneous coronary intervention (PCI) for isolated left anterior descending (LAD) ostial lesions remains challenging, with limited comparative data on stenting strategies. We aimed to evaluate the procedural and long-term outcomes of three single-stent techniques: precise ostial stenting (POS), floating stenting (FS), and crossover stenting (CS).
Methods
In this retrospective study, 116 patients with isolated LAD ostial disease underwent intravascular ultrasound (IVUS)-guided PCI using one of the three strategies. Baseline characteristics, procedural details, IVUS findings, and major adverse cardiac and cerebrovascular events (MACCEs) over two years were compared.
Results
Compared to POS and FS, CS resulted in larger minimal stent area at the ostium, and a higher rate of complete stent coverage (100% vs. 39.5% and 23.1%, p < 0.001). At 2-year follow-up, MACCE rates were significantly lower in the CS group (2.6%) compared to FS (13.5%) and POS (15.8%, p = 0.039), mainly due to reduced target lesion revascularization. FS showed improved coverage compared to POS, but inferior angiographic outcomes and higher event rates than CS.
Conclusions
In IVUS-guided PCI for isolated LAD ostial lesions, CS offers superior ostial coverage and clinical outcomes. FS may serve as a compromise when anatomical constraints limit crossover. These findings support a tailored strategy based on lesion characteristics and IVUS assessment.
Keywords: Ostial left anterior descending lesion, Percutaneous coronary intervention, Floating stent, Crossover stenting, Intravascular ultrasound
Introduction
The distribution of atherosclerotic plaque at the ostium of the left anterior descending (LAD) artery constitutes a distinctive and technically challenging subset of coronary bifurcation lesions. Percutaneous coronary intervention (PCI) in this region is associated with lower angiographic success rates and an increased risk of procedural complications [1, 2]. At present, the single-stent strategy is widely accepted as the default approach for managing bifurcation lesions [2]. However, for isolated ostial LAD stenosis—particularly cases classified as Medina 0,1,0 involving the left main coronary artery (LMCA) bifurcation—there remains no consensus regarding the most appropriate treatment strategy, as highlighted in the European Bifurcation Club (EBC) consensus document [3]. The development of coronary artery disease, including ostial lesions, is driven by complex interactions between endothelial dysfunction, lipid accumulation, inflammation, and abnormal shear stress at bifurcation zones. Ostial LAD lesions, in particular, are prone to high plaque burden due to turbulent flow and vessel geometry. Diagnostic modalities such as coronary angiography, intravascular ultrasound (IVUS), and fractional flow reserve (FFR) play a pivotal role in the accurate detection and assessment of ostial disease severity [4].
In clinical practice, three single-stent techniques have been proposed for addressing this complex lesion type: precise ostial stenting (POS), floating stenting (FS), and crossover stenting (CS). Each technique offers a different approach to stent positioning at the LAD ostium, yet evidence comparing their procedural effectiveness and long-term outcomes with modern drug-eluting stents remains limited. Several investigations have demonstrated the procedural feasibility and acceptable clinical outcomes associated with CS, rather than POS [1, 5, 6]. Nonetheless, CS may lead to ostial compromise of the left circumflex artery (LCX), even in the absence of baseline LCX stenosis [7]. This potential complication can increase procedural complexity and necessitate conversion to a two-stent strategy.
As an alternative to both POS and CS, the FS technique—defined by stent placement extending from the distal LMCA into the LAD—has been proposed as a viable approach with favorable clinical results [8]. Moreover, the use of IVUS can assist in optimizing stent placement and improving both procedural and long-term clinical outcomes in patients with ostial LAD involvement [9]. Despite these developments, the current body of evidence concerning isolated ostial LAD stenosis remains limited and often inconsistent across studies.
Accordingly, this study aimed to compare the two-year clinical outcomes of patients with angiographically confirmed isolated ostial LAD stenosis who underwent IVUS-guided PCI using one of three single-stent strategies: POS, FS, or CS.
Materials and methods
Study participants
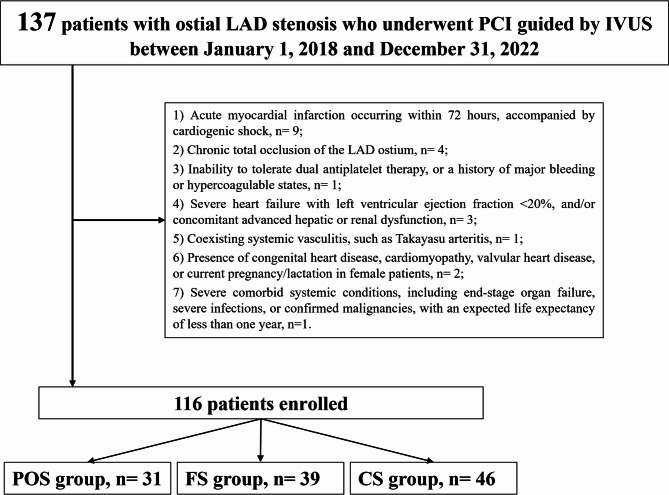
This retrospective, single-center study included patients treated at Xiangtan Central Hospital between January 1, 2018, and December 31, 2022. A total of 116 consecutive patients who underwent IVUS-guided PCI for isolated ostial stenosis of the LAD artery—defined as Medina 0,1,0 bifurcation lesions involving the LMCA [10] —were identified from hospital records. Demographic information, clinical presentation, angiographic features, procedural data, in-hospital management, and follow-up outcomes were retrospectively collected. Eligible patients were over 18 years of age, diagnosed with coronary artery disease, and presented with typical symptoms of myocardial ischemia. Ostial LAD lesions were defined as those within 3 mm of the LAD origin [11], and significant stenosis was confirmed by coronary angiography (CAG), showing a luminal narrowing exceeding 70%. Only patients with isolated LAD ostial disease classified as Medina 0,1,0 were included. All patients underwent IVUS evaluation before and after PCI to assess lesion morphology and guide intervention. Exclusion criteria included: (1) Acute myocardial infarction within 72 h, especially if complicated by cardiogenic shock; (2) Chronic total occlusion (CTO) of the LAD ostium; (3) Contraindications to dual antiplatelet therapy (DAPT), such as prior major bleeding or prothrombotic conditions; (4) Severe heart failure with left ventricular ejection fraction (LVEF) < 20%, or advanced hepatic/renal dysfunction; (5) Systemic vasculitis, such as Takayasu arteritis; (6) Structural heart diseases including congenital anomalies, cardiomyopathy, valvular pathology, or pregnancy/lactation in female patients; and (7) Major comorbidities such as end-stage organ failure, active infection, or malignancy with an expected survival of less than one year (Fig. 1). Patients were stratified into three groups based on the stenting technique employed. The POS group received stents confined to the LAD ostium without extension into the LMCA or LCX. The FS group underwent stenting from the LAD ostium with minimal protrusion into the LCX, without side branch intervention [12]. The CS group involved crossover stenting from the LMCA into the LAD. The study was approved by the Ethics Committee of Xiangtan Central Hospital and conducted in accordance with the Declaration of Helsinki (2013 revision). Written informed consent was obtained from all participants (Approval No. X201922314-1). This retrospective study did not require a clinical trial registration number. The medical records used in this retrospective study were accessed on 15/04/2019 for research purposes. During data collection, the authors had access to identifiable patient information, which was de-identified prior to statistical analysis.
Fig. 1.
Study Flow. POS, precise ostial stenting; FS, floating stenting; CS, crossover stenting; PCI, percutaneous coronary intervention; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex artery
Percutaneous coronary intervention procedure
All coronary angiography(CAG) and PCI were performed using standard techniques via either the femoral or radial artery approach. Prior to intervention, patients received a loading dose of aspirin (300 mg) and either clopidogrel (300 mg) or ticagrelor (180 mg). Intravenous unfractionated heparin was administered during the procedure at a dose of 70–100 IU/kg. Use of glycoprotein IIb/IIIa inhibitors was left to the discretion of the interventional cardiologist. Second-generation drug-eluting stents were implanted according to operator preference, including Resolute Integrity (Medtronic Inc., Galway, Ireland), Xience V and Xience Xpedition (Abbott Laboratories, USA), and Firebird2 (MicroPort Medical, Shanghai, China). The stenting strategy for ostial LAD lesions was selected based on anatomical and procedural assessments such as LMCA–LAD diameter mismatch, bifurcation angle between LAD and LCX, LCX ostial involvement, and carinal morphology as determined by IVUS. For the CS group, a stent was deployed from the LMCA into the LAD, in accordance with EBC recommendations [13]. The stent was extended from the distal LMCA into the LAD, following European Bifurcation Club recommendations. Pre-dilation was performed using a balloon sized 1:1 to the distal LAD. The proximal segment was optimized using POT. If the LCX showed impaired flow, pinching, or dissection after main vessel stenting, kissing balloon inflation or POT-side-POT technique was applied. In selected cases, a jailed balloon was used to protect the LCX during main vessel stenting [13]. In the FS group, a drug-eluting stent was deployed in the proximal LAD with minimal and intentional protrusion into the LMCA. The goal was to ensure adequate coverage of the LAD ostium without entering the LCX. No side-branch intervention was routinely performed [12]. This technique was chosen when IVUS findings excluded high-risk carinal features such as the “eyebrow sign” [12]. For the POS group, the stent was carefully aligned with the LAD ostium without extending into the LMCA. Angiography and IVUS were used to guide precise placement. The stent was deployed with a short distal landing zone, aiming to achieve full ostial coverage without geographic miss, and minimal post-dilation was performed to avoid disturbing the LMCA-LAD transition. The procedural distinctions among the three single-stent techniques (POS, FS, and CS) are summarized in Fig. 2 to illustrate key anatomic targets, technical steps, and potential risks. Following the procedure, patients were prescribed clopidogrel (75 mg daily) or ticagrelor (90 mg twice daily) for a minimum duration of 12 months, and aspirin (100 mg daily) was continued indefinitely [14]. Post-discharge, all patients were encouraged to adhere to guideline-recommended secondary prevention therapy, including statins, angiotensin-converting enzyme inhibitors, and β-blockers unless contraindicated. Routine coronary angiography was recommended between 9 and 12 months during follow-up [14]. Quantitative coronary angiography (QCA) was performed using the QAngio XA software (Medis Medical Imaging Systems, Leiden, the Netherlands), and lesion morphology was assessed based on established protocols [15]. All measurements were conducted offline by two experienced angiographers working independently and blinded to clinical information. Lesion visualization was acquired in at least two orthogonal views following intracoronary administration of 0.5 mg nitroglycerin.
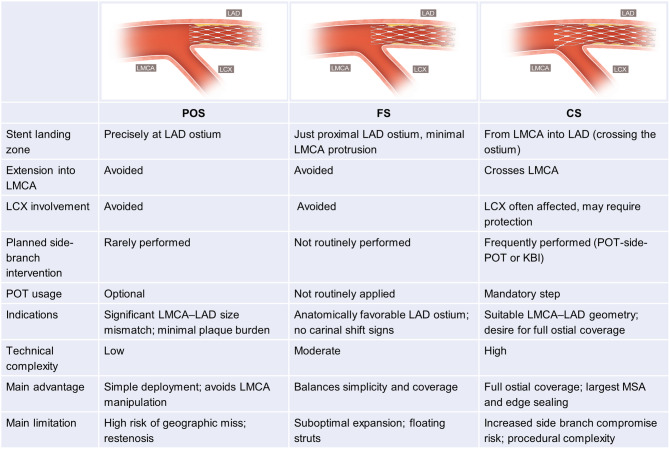
Fig. 2.
Schematic comparison of three single-stent techniques for ostial LAD lesions. This figure illustrates three IVUS-guided single-stent techniques for isolated ostial LAD lesions: precise ostial stenting (POS), floating stenting (FS), and crossover stenting (CS). Each panel shows stent position relative to the LMCA and LCX, and whether side-branch protection or optimization techniques were used. POS is deployed precisely at the LAD ostium without entering the LMCA or LCX, but may risk geographic miss. FS covers the ostium with minimal protrusion into the LMCA, balancing simplicity and coverage. CS extends from LMCA into LAD, offering full coverage but often requiring POT or kissing balloon inflation to manage side-branch compromise. Abbreviations: LAD, left anterior descending artery; LMCA, left main coronary artery; LCX, left circumflex artery; POT, proximal optimization technique; IVUS, intravascular ultrasound; FS, floating stenting; POS, precise ostial stenting; CS, crossover stenting; KBI. kissing balloon inflation; MSA, minimum stent area
IVUS image acquisition and assessment
IVUS examinations were conducted for all patients in accordance with contemporary clinical guidelines [16], utilizing a 40-MHz OptiCross™ catheter (Boston Scientific, Marlborough, MA, USA). After intracoronary administration of 0.1–0.2 mg nitroglycerin, automated transducer pullback was performed at a rate of 0.5 mm/s. The catheter was positioned to extend at least 10 mm beyond both the distal and proximal edges of the stent. Image interpretation was carried out using QIvus® software (Medis, Leiden, the Netherlands). For reference segment identification, the most normal-appearing cross-sections within 5 mm of each stent margin were selected [17]. Assessment of stent expansion and residual stenosis was based on the AVIO criteria (angiography versus intravascular ultrasound-optimized stent implantation) [18]. To minimize measurement variability, each IVUS parameter was recorded twice, and the mean of the two readings was used for final analysis. IVUS imaging was systematically performed before and after stent implantation in all cases, enabling evaluation of both baseline lesion characteristics and post-procedural stent outcomes. Coronary anatomical assessments from angiographic and IVUS images were independently performed by two experienced interventional cardiologists (X.W. and L.W.), who were blinded to clinical presentation and laboratory data. Inter-observer and intra-observer agreement was excellent, with κ values of 0.90 and 0.93, respectively.
Definitions
Stent thrombosis (ST) was characterized as a myocardial infarction attributable to the target vessel post-PCI, with angiographic evidence of either thrombotic occlusion or intraluminal thrombus within the stented segment [19]. On IVUS imaging, ST was identified by irregular, low-echogenic masses within the stent lumen, often exhibiting speckled or shimmering internal reflections. The stent expansion index was calculated as the ratio of the minimal stent cross-sectional area (CSA) to the CSA of the reference vessel lumen. Proximal optimization technique (POT) [20] involved post-deployment balloon inflation using a non-compliant balloon appropriately sized to the proximal main vessel. This step aimed to optimize stent apposition near the carina and improve access to the side branch when necessary. Balloon sizing for POT was guided by the AVIO criteria [18], which recommend using the average of the maximum and minimum media-to-media diameters obtained at the proximal, distal, or most severely narrowed segment of the treated vessel. The primary objective of POT was to ensure full stent expansion in the proximal main vessel and maintain the patency and accessibility of side branches when indicated.
Clinical follow-up and outcomes
All patients underwent scheduled clinical follow-up at 1, 6, and 12 months after the index PCI, followed by annual assessments. At each visit, clinical status, adverse events, and cardiac-related symptoms were systematically reviewed. In-hospital outcomes and complications were documented either upon discharge or at the time of in-hospital mortality. At 6 months post-procedure, non-invasive ischemia assessments were routinely conducted, including treadmill exercise electrocardiography, nuclear perfusion imaging, or stress echocardiography. Cardiac function was further evaluated using transthoracic echocardiography (TTE). Invasive CAG and IVUS were selectively performed in patients presenting with recurrent symptoms or objective evidence suggestive of myocardial ischemia, or in those requiring additional revascularization. Long-term follow-up data were retrospectively obtained via thorough review of electronic health records, direct patient contact by telephone, and verification of reported events through communication with referring physicians or institutions. Vital status was cross-checked using national mortality databases. The primary endpoint was the occurrence of major adverse cardiovascular and cerebrovascular events (MACCE), defined as a composite of cardiac death, target vessel myocardial infarction (MI), clinically indicated target lesion revascularization (TLR), ischemic stroke, or definite/probable ST occurring either during hospitalization or throughout follow-up. Clinically driven TLR was defined as any repeat PCI performed within the stented segment or 5 mm adjacent, in the presence of ischemic symptoms, objective ischemia (e.g., positive ECG or stress test), or angiographic diameter stenosis ≥ 50% with documented ischemia. Event definitions adhered to criteria established by the Academic Research Consortium [21].
Statistical analysis
All statistical computations were performed using SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). For continuous variables exhibiting normal distribution, data were summarized as mean ± standard deviation (SD) and compared using independent-sample t-tests or one-way analysis of variance (ANOVA). When the assumption of homogeneity of variance was satisfied, post hoc comparisons were conducted using the least significant difference (LSD) test; otherwise, Dunnett’s T3 test was applied. Non-normally distributed variables were described using medians with interquartile ranges and analyzed via the Kruskal–Wallis test. When appropriate, Bonferroni correction was employed for pairwise post hoc comparisons. Categorical variables were presented as frequencies and percentages, with between-group differences assessed using either the chi-square test or Fisher’s exact test, depending on expected cell counts. Univariate predictors of MACCE were identified and subsequently entered into a multivariate Cox proportional hazards model. Backward stepwise selection was used to determine independent risk factors associated with adverse clinical outcomes. The cumulative incidence of MACCE during follow-up was estimated using the Kaplan–Meier method, and survival curves were compared using the log-rank test. A two-sided P value of less than 0.05 was regarded as statistically significant.
Results
A total of 116 patients were analyzed, including 31 in the POS group, 39 in the FS group, and 46 in the CS group. As summarized in Table 1, no statistically significant differences were observed among the three groups in terms of baseline characteristics, including age, sex, body mass index (BMI), cardiovascular comorbidities (diabetes, hypertension, prior myocardial infarction, chronic kidney disease, atrial fibrillation), clinical presentation (chronic coronary syndrome [CCS], unstable angina, and non-ST elevation myocardial infarction [NSTEMI]), echocardiographic left ventricular ejection fraction (LVEF), oral anticoagulation use or prescribed medications (all P > 0.05).
Table 1.
Baseline clinical and angiographic characteristics
| Variable | POS group (n = 31) | FS group (n = 39) | CS group (n = 46) | F/χ²/t | P-value |
|---|---|---|---|---|---|
| Age | 63.88 ± 9.86 | 65.65 ± 13.74 | 64.58 ± 11.08 | 0.20 | 0.816 |
| Male, n (%) | 24 (77.4) | 27 (69.2) | 36 (78.3) | 1.05 | 0.592 |
| BMI | 25.27 ± 2.01 | 24.46 ± 2.85 | 24.42 ± 3.29 | 0.97 | 0.381 |
| Diabetes, n (%) | 10 (32.3) | 15 (38.5) | 18 (39.1) | 0.42 | 0.809 |
| Hypertension, n (%) | 15 (48.4) | 19 (48.7) | 23 (50.0) | 0.02 | 0.988 |
| Hyperlipidemia, n (%) | 17 (54.8) | 21 (53.8) | 25 (54.3) | 0.01 | 0.997 |
| CKD, n (%) | 8 (25.8) | 9 (23.1) | 10 (21.7) | 0.17 | 0.917 |
| Smoking, n (%) | 4 (12.9) | 12 (30.8) | 8 (17.4) | 3.87 | 0.145 |
| Previous MI, n (%) | 8 (25.8) | 3 (7.7) | 6 (13.0) | 4.69 | 0.096 |
| Previous PCI, n (%) | 2 (6.5) | 3 (7.7) | 4 (8.7) | 0.13 | 0.937 |
| Shock, n (%) | 1 (3.2) | 3 (7.7) | 3 (6.5) | 0.64 | 0.726 |
| Atrial fibrillation, n (%) | 2(6.5) | 3(7.7) | 3 (6.5) | 0.06 | 0.971 |
| Multivessel disease, n (%) | 2 (6.5) | 4 (10.3) | 7 (15.2) | 1.48 | 0.476 |
| Clinical presentation | |||||
| CCS, n (%) | 15 (48.4) | 20 (51.3) | 24 (52.2) | 0.11 | 0.946 |
| NSTEMI, n (%) | 10 (32.3) | 9 (23.1) | 13 (28.3) | 0.75 | 0.689 |
| USAP, n (%) | 6 (19.4) | 10 (25.6) | 9 (19.6) | 0.58 | 0.748 |
| LVEF, (%) | 57.98 ± 7.26 | 59.84 ± 7.31 | 59.45 ± 8.15 | 0.56 | 0.572 |
| Medication used | |||||
| Antiplatelet, n (%) | 31 (100.0) | 39 (100.0) | 46 (100.0) | - | - |
| Beta blockers, n (%) | 27 (87.1) | 31 (79.5) | 40 (87.0) | 1.12 | 0.572 |
| CCB, n (%) | 8 (25.8) | 10 (25.6) | 12 (26.1) | 0.0 | 0.998 |
| ACEI/ARB, n (%) | 28 (90.3) | 35 (89.7) | 43 (93.5) | 0.43 | 0.805 |
| Statin, n (%) | 31 (100.0) | 39 (100.0) | 45 (97.8) | - | - |
| Diuretics, n (%) | 3 (9.7) | 1 (2.6) | 1 (2.2) | 2.96 | 0.227 |
| Nitrate, n (%) | 11 (35.5) | 14 (35.9) | 19 (41.3) | 0.37 | 0.831 |
| Oral anticoagulation use, n (%) | 2(6.5) | 2(5.1) | 3 (6.5) | 0.06 | 0.958 |
Continuous variables were expressed as mean ± SD. Categorical variables were expressed as number (percentage)
Abbreviations: POS, precise ostial stenting; FS, floating stenting; CS, crossover stenting; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; NSTEMI, non-ST segment elevation myocardial infarction; USAP, unstable angina pectoris; CCS, chronic coronary syndrome; LVEF, left ventricle ejection fraction; CCB, calcium channel blocker; ACEI, angiotensin-converting inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease;
Procedural and angiographic data are detailed in Table 2. Patients in the CS group demonstrated significantly larger ostial LAD diameters (4.49 ± 0.28 mm) compared with POS (2.95 ± 0.26 mm) and FS (3.48 ± 0.32 mm) groups (P < 0.001). The CS group also had greater LCX diameters, stent diameters (3.54 ± 0.33 mm vs. 3.13 ± 0.25 mm in POS, P < 0.001), and post-dilation balloon sizes (P < 0.001). Use of adjunctive techniques such as kissing balloon inflation and POT-side-POT was significantly more frequent in the CS cohort (56.5% and 23.9%, respectively; both P < 0.01). No significant intergroup differences were found in other procedural metrics, including LMCA diameter, lesion severity, stent length, fluoroscopy time, and contrast volume.
Table 2.
Procedural parameters
| Variable | POS group (n = 31) | FS group (n = 39) | CS group (n = 46) | F/χ²/t | P-value |
|---|---|---|---|---|---|
| LMCA diameter, mm | 4.47 ± 0.32 | 4.55 ± 0.47 | 4.55 ± 0.71 | 0.22 | 0.8050 |
| Ostial LAD diameter, mm | 2.95 ± 0.26 | 3.48 ± 0.32 | 4.49 ± 0.28 | 294.09 | < 0.001 |
| Ostial LCx diameter, mm | 2.81 ± 0.35 | 3.09 ± 0.13 | 3.29 ± 0.36 | 23.31 | < 0.001 |
| Ostial LAD stenosis, mm | 95.90 ± 8.42 | 89.30 ± 14.64 | 94.01 ± 15.21 | 2.32 | 0.105 |
| Thrombus IVUS, n (%) | 9 (29.0) | 11 (28.2) | 7 (15.2) | 2.78 | 0.249 |
| Non target lesion, n (%) | 3 (9.7) | 4 (10.3) | 9 (19.6) | 2.14 | 0.343 |
| Rotablator, n (%) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1.53 | 0.464 |
| Thrombus aspiration, n (%) | 7 (22.6) | 5 (12.8) | 7 (15.2) | 1.28 | 0.528 |
| Direct stenting, n (%) | 2 (6.5) | 2 (5.1) | 2 (4.3) | 0.17 | 0.919 |
| Stent diameter, mm | 3.13 ± 0.25 | 3.34 ± 0.24 | 3.54 ± 0.33 | 19.33 | < 0.001 |
| Stent length, mm | 24.06 ± 7.47 | 23.13 ± 3.85 | 22.47 ± 4.15 | 0.88 | 0.418 |
| Post dilatation balloon diameter, mm | 3.50 ± 0.31 | 3.80 ± 0.29 | 4.32 ± 0.31 | 72.89 | < 0.001 |
| Procedure time, min | 48.53 ± 14.06 | 51.71 ± 12.04 | 50.60 ± 9.64 | 0.64 | 0.529 |
| Fluoroscopy time, min | 17.87 ± 7.05 | 19.90 ± 3.57 | 18.04 ± 4.01 | 2.03 | 0.136 |
| Side branch intervention | |||||
| Kissing balloon, n (%) | 4 (12.9) | 3 (7.7) | 26 (56.5) | 29.74 | < 0.001 |
| POT side POT, n (%) | 3 (9.7) | 0 (0.0) | 11 (23.9) | 11.6 | 0.003 |
| Failed balloon, n (%) | 0 (0.0) | 0 (0.0) | 1 (2.2) | 1.53 | 0.464 |
| Bailout stent, n (%) | 0 (0.0) | 0 (0.0) | 3 (6.5) | 4.69 | 0.096 |
| Contrast media volume, ml | 147.98 ± 47.20 | 119.98 ± 43.93 | 126.42 ± 68.25 | 2.35 | 0.100 |
Continuous variables were expressed as mean ± SD. Categorical variables were expressed as number (percentage)
Abbreviations: POS, precise ostial stenting; FS, floating stenting; CS, crossover stenting; IVUS, intravascular ultrasound; LAD, left anterior descending; LCx, left circumflex; LMCA, left main coronary artery; POT, proximal optimization technique;
IVUS analysis results are presented in Table 3. Baseline IVUS measurements showed comparable values across groups for minimum lumen area (MLA), reference segment dimensions, and plaque burden (all P > 0.05). However, post-procedural IVUS revealed a significantly larger ostial LAD stent area in the CS group (11.57 ± 1.69 mm²) relative to the POS group (9.07 ± 2.21 mm²; P < 0.001). Additionally, proximal stent edge plaque burden was lower in the CS group (34.05 ± 6.35%) compared with POS (41.01 ± 10.00%; P < 0.001), although stent expansion indices were similar among groups. Post-procedural plaque burden at the LAD ostium center was also assessed to evaluate the completeness of ostial coverage. No significant difference was observed among the POS (35.1 ± 4.5%), FS (33.3 ± 5.6%), and CS (33.1 ± 5.0%) groups (P = 0.25), suggesting similar ostial plaque coverage across all techniques under IVUS guidance.
Table 3.
Intravascular ultrasound findings
| Variable | POS group (n = 31) | FS group (n = 39) | CS group (n = 46) | F/χ²/t | P-value |
|---|---|---|---|---|---|
| Pre-procedure quantitative assessment | |||||
| MLA at ostial LAD, mm² | 5.66 ± 2.23 | 5.53 ± 0.94 | 4.94 ± 1.43 | 2.47 | 0.089 |
| Distal reference lumen area, mm² | 6.1 (5.8, 6.5) | 6.1 (5.8, 6.3) | 6.0 (5.8, 6.4) | 1.38 | 0.501 |
| Proximal reference lumen area, mm² | 15.6 (15.3, 16.0) | 15.7 (15.0, 16.3) | 15.5 (15.1, 16.5) | 0.09 | 0.955 |
| MLD at ostial LAD, mm | 2.0 (1.9, 2.1) | 2.0 (1.8, 2.2) | 2.0 (1.9, 2.2) | 0.26 | 0.879 |
| Plaque burden, % | 68.79 ± 10.75 | 66.62 ± 8.10 | 69.51 ± 9.94 | 1.01 | 0.370 |
| Post-procedure quantitative assessment | |||||
| MSA at ostial LAD, mm² | 9.07 ± 2.21 | 9.38 ± 2.95 | 11.57 ± 1.69 | 14.16 | < 0.001 |
| Plaque burden at the edge of the proximal LAD stent, % | 41.01 ± 10.00 | - | 34.05 ± 6.35 | 3.73 | < 0.001 |
| Minimum stent expansion, % | 79.65 ± 4.14 | 79.60 ± 3.35 | 80.35 ± 5.47 | 0.36 | 0.695 |
| Plaque burden at the LAD ostium, (%) | 35.12 ± 4.52 | 33.38 ± 5.64 | 33.13 ± 5.03 | 1.42 | 0.248 |
Continuous variables were expressed as mean ± SD or median (interquartile range). Categorical variables were expressed as number (percentage).
Abbreviations: POS, precise ostial stenting; FS, floating stenting; CS, crossover stenting; IVUS, intravascular ultrasound; LAD, left anterior descending; MLA, minimum lumen area; MLD, Minimum lumen diameter; MSA, minimum stent area;
At two-year follow-up, clinical outcome data (Table 4) indicated a significantly elevated rate of MACCE in the POS group (25.8%) versus FS (5.1%) and CS (2.2%) (P < 0.001), largely attributable to increased rates of clinically driven TLR (22.6% vs. 5.1% vs. 2.2%, respectively; P = 0.005). Incidences of cardiac death, myocardial infarction, stroke, and ST were low and did not differ significantly across groups (all P > 0.05). Among the seven patients in the POS group who experienced Clinically driven TLR, follow-up angiography or IVUS imaging was available in all cases. Of these, six showed restenosis localized to the proximal stent edge, consistent with geographic miss, while one case involved diffuse in-stent restenosis. No distal edge restenosis was observed. In the FS group, two TLR events occurred and were attributed to diffuse in-stent restenosis. In the CS group, one patient underwent repeat revascularization due to distal edge restenosis at the LAD. No proximal edge restenosis was observed in either FS or CS groups.
Table 4.
2-year clinical outcomes of study patients
| Variable | POS group (n = 31) | FS group (n = 39) | CS group (n = 46) | F/χ²/t | P-value |
|---|---|---|---|---|---|
| Primary end-point (MACCE), n (%) | 8(25.8) | 2(5.1) | 1(2.2) | 15.87 | < 0.001 |
| Cardiac death | 0(0) | 0(0) | 0(0) | - | - |
| Target vessel MI | 2(6.5) | 2(5.1) | 0(0.0) | 2.81 | 0.245 |
| Clinically driven TLR | 7(22.6) | 2(5.1) | 1(2.2) | 10.71 | 0.005 |
| Stroke | 1(3.2) | 0(0.0) | 1(2.2) | 1.15 | 0.562 |
| Stent thrombosis | 0(0) | 0(0) | 0(0) | - | - |
Categorical variables were expressed as number (percentage)
Abbreviations: POS, precise ostial stenting; FS, floating stenting; CS, crossover stenting; MI, myocardial infarction; TLR, target lesion revascularizatio; MACCE, major cardiovascular and cerebral events;
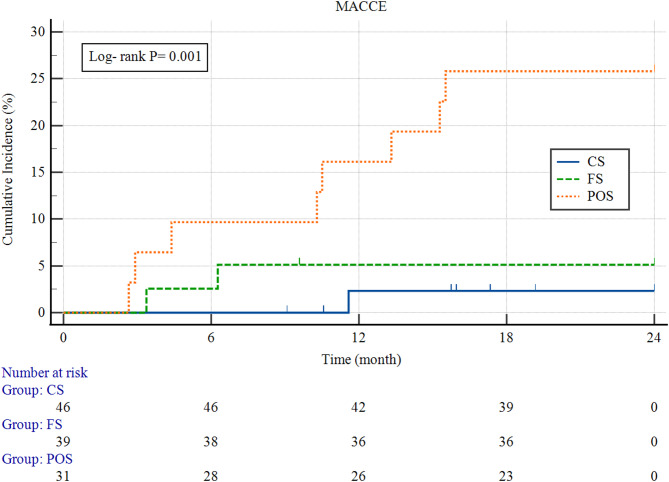
Kaplan–Meier survival curves (Fig. 3) demonstrated significantly lower MACCE-free survival in the POS group compared to the FS and CS groups (log-rank test, P = 0.001). Event-free survival analysis showed significant differences across groups. In multivariate Cox regression analysis, the POS strategy was associated with a significantly increased risk of MACCE at 2 years compared to both CS (HR = 12.93; 95% CI: 2.89–57.83; P = 0.001) and FS (HR = 5.41; 95% CI: 1.15–25.56; P = 0.001). Although the FS group had a numerically higher risk than the CS group, this difference did not reach statistical significance (HR = 2.39; 95% CI: 0.61–9.43; P = 0.206).
Fig. 3.
Kaplan–Meier Curves for MACCE Among the Three Groups. Kaplan–Meier analysis of cumulative MACCE during 2-year follow-up. The POS group exhibited a significantly higher incidence of MACCE compared to the FS and CS groups (log-rank P < 0.001). The CS group demonstrated the most favorable event-free survival, while the FS group showed intermediate outcomes. POS, precise ostial stenting; FS, floating stenting; CS, crossover stenting; MACCE, major adverse cardiovascular and cerebrovascular events
As shown in Table 5, multivariate Cox regression analysis identified the use of precise ostial stenting as an independent predictor of MACCE (HR: 2.351; 95% CI: 2.640–6.113; P < 0.001). While hypertension and baseline LVEF were significant in univariate analysis, they lost predictive value after multivariable adjustment.
Table 5.
Univariate and multivariate Cox regression analyses showing independent predictors of MACCE in patients with ostial LAD stenosis
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P value | HR | 95% CI | P value |
| Age | 0.952 | 0.529–1.527 | 0.843 | |||
| Hypertension | 1.847 | 1.155–2.953 | 0.018 | 1.17 | 0.721–1.926 | 0.543 |
| Diabetes | 1.029 | 0.452–2.203 | 0.821 | |||
| Precise ostial stenting | 3.526 | 2.256–6.037 | < 0.001 | 2.351 | 2.640–6.113 | < 0.001 |
| Thrombus identified by IVUS | 0.656 | 0.353–1.239 | 0.194 | |||
| LVEF at baseline | 1.662 | 1.024–2.72 | 0.041 | 1.422 | 0.845–2.418 | 0.195 |
Abbreviations: HR, hazard ratios; CI, confidence interval; IVUS, intravascular ultrasound; LVEF, left ventricle ejection fraction;
Discussion
This investigation assessed the comparative performance of three single-stent strategies for managing isolated ostial LAD lesions and produced the following principal observations: First, although baseline demographic, clinical, and angiographic parameters were similar across groups, the POS cohort experienced a significantly elevated rate of MACCE at two-year follow-up, primarily due to a higher incidence of clinically driven TLR. While the FS group exhibited fewer adverse events than POS, its outcomes remained inferior to those observed in the CS cohort. Second, from a technical standpoint, the CS approach resulted in the most extensive stent expansion at the LAD ostium, with superior plaque coverage, reflecting procedural efficiency and optimized lesion scaffolding. Conversely, POS was linked to suboptimal stent expansion and a greater likelihood of incomplete ostial coverage, underscoring its inherent limitations in treating this lesion subset. The FS technique provided moderate stent expansion while preserving the side branch ostium, indicating a procedural compromise between radial support and anatomic integrity. Third, multivariate analysis via Cox proportional hazards modeling identified POS as an independent risk factor for MACCE, suggesting that this technique may be less effective in ensuring durable long-term outcomes in patients undergoing PCI for ostial LAD involvement.
Mechanistic insights and therapeutic considerations in ostial LAD disease
Coronary bifurcation lesions constitute approximately 15–20% of all PCI, with ostial LAD involvement representing a common subtype of non-true bifurcations and accounting for nearly 20% of such cases [22]. The anatomical intricacy and hemodynamic environment at bifurcation sites—characterized by elevated shear stress at the flow divider and reduced shear stress along the lateral vessel walls—have been implicated in the localized development of atherosclerosis at the LAD ostium. Studies incorporating intravascular imaging and computational fluid dynamics have revealed an inverse correlation between local shear stress and plaque thickness at bifurcation points [23]. Zones exposed to low shear stress near the carina are particularly susceptible to the formation of rupture-prone lesions, such as thin-cap fibroatheromas (TCFAs) [24]. In addition, abnormal shear profiles at the bifurcation are associated with the development of heavy calcifications, further complicating procedural outcomes at the LAD ostium [25]. When compared to non-ostial LAD disease, ostial involvement has been linked to a greater incidence of post-procedural adverse cardiovascular events. Although advancements in stent technology and procedural techniques have improved clinical outcomes, the complexity of this anatomical region remains a procedural challenge. In this context, the integration of advanced intracoronary imaging—particularly IVUS—has proven valuable for pre-procedural assessment, accurate stent sizing, and precise lesion coverage. IVUS facilitates implementation of the POT, especially in the setting of vessel diameter mismatch between proximal and distal LAD segments. It also aids in the detection of stent-edge complications and guides additional corrective strategies, potentially reducing the incidence of complications such as vessel occlusion, in-stent restenosis (ISR), and TLR [2]. Nonetheless, despite these technological advancements, challenges such as ST and restenosis persist, highlighting the procedural difficulty of ostial LAD interventions. Therefore, proactive lesion assessment and judicious selection of single-stent techniques are essential for optimizing long-term outcomes in this high-risk lesion subset [26].
Comparative technical performance of Single-Stent strategies in ostial LAD lesions
Anatomically, coronary bifurcation lesions consist of a main vessel (MV) and a side branch (SB), with non-true bifurcation types—such as Medina 0,1,0 or 0,0,1—forming a distinct subset within complex bifurcation configurations [27]. For such lesions, a single-stent approach is typically favored, emphasizing complete lesion coverage to reduce the likelihood of carina shift, plaque redistribution, and subsequent ISR [28]. Accordingly, the selection of a stenting strategy is of paramount importance, as it directly affects procedural success and long-term clinical outcomes [26]. In LAD ostial interventions, the mechanical effects of stent deployment—such as carina displacement, plaque redistribution (e.g., the snowplow phenomenon) [29], and geometric alteration of the bifurcation angle [30]—can significantly impact SB patency [29]. These mechanical disturbances may result in SB narrowing or occlusion, potentially leading to myocardial infarction and adverse cardiovascular events [31]. The extent of SB compromise is closely associated with stent-induced carinal shift, which alters the anatomical structure of the SB ostium [32]. In our study, interventions targeting the side branch—such as kissing balloon inflation or the POT-side-POT technique—were predominantly performed in response to evident carina displacement following main vessel stenting. A significant intergroup difference in the frequency of SB compromise was observed, with both the POS and FS groups showing lower rates than the CS group. Notably, while baseline IVUS-derived lesion characteristics did not differ significantly among groups, post-intervention stent area at the LAD ostium was substantially smaller in the POS cohort, intermediate in the FS group, and greatest in the CS group. These findings imply that the crossover technique enables the most extensive ostial expansion but may be associated with more frequent SB encroachment. In contrast, POS carries a risk of under-expansion, whereas the FS strategy achieves adequate ostial scaffolding without substantial protrusion into the LMCA. Additionally, plaque burden at the stent edges was significantly lower in the CS group compared to POS, likely reflecting more complete lesion coverage. Taken together, these observations suggest that the FS technique offers procedural advantages by minimizing both edge-related plaque burden and side branch compromise, while ensuring sufficient stent expansion at the LAD ostium. Although the crossover approach provides optimal plaque coverage, it may inadvertently jeopardize SB integrity. Conversely, the POS technique may result in suboptimal scaffolding. The FS strategy appears to represent a procedural middle ground, balancing anatomical preservation with stent efficacy, and may thus be preferable in appropriately selected patients.
Clinical outcomes of POS, FS, and CS techniques
The results of our study complement existing literature by reaffirming the inferior clinical performance of POS compared to CS for treating LAD ostial lesions [1, 5, 33]. In our cohort, POS was associated with the highest 2-year MACCE rate (25.8%), largely driven by target TLR, whereas significantly lower event rates were observed in both the CS (2.2%) and FS (5.1%) groups. These outcomes are consistent with prior retrospective reports, which similarly indicated higher composite adverse event and TLR rates for POS relative to CS [33]. The suboptimal outcomes associated with POS are likely attributable to its inherent technical challenges. Achieving precise stent deployment at the LAD ostium is difficult, and even minor geographic miss—either proximally or distally—can result in incomplete coverage or unintentional protrusion into the LMCA or LCX [13, 26, 34]. Pathological and imaging studies have shown that plaque burden exceeding 50% at the stent edge strongly predicts restenosis, with diagnostic accuracy approaching 80% [35]. Further supporting the mechanistic basis for these outcome differences, follow-up imaging revealed distinct restenosis patterns across techniques. In the POS group, six out of seven TLR events were localized to the proximal stent edge, a finding strongly indicative of geographic miss and incomplete ostial coverage. This reinforces the known vulnerability of POS to suboptimal proximal scaffolding, particularly when IVUS is not systematically employed to confirm ostial edge alignment. In contrast, the two TLR cases in the FS group were attributable to diffuse in-stent restenosis, while the single CS group event involved the distal edge of the LAD. The absence of proximal edge restenosis in both FS and CS groups highlights the procedural advantage of achieving more complete lesion coverage with those techniques. Incomplete proximal coverage alone accounts for a substantial proportion of restenosis cases following POS [33], and strut protrusion into the LCX ostium may further elevate thrombotic risk due to impaired endothelialization and limited access for future revascularization [36]. In contrast, CS offers full lesion coverage and mitigates many of these limitations. Registry data support its clinical benefits; for instance, the LM-CROSSOVER study reported significantly lower rates of all-cause mortality and TLR among patients receiving CS compared to POS [37]. Similar findings were noted by Yang et al., who observed reduced MACE and TVR rates in the CS cohort [33]. Moreover, long-term prognosis following CS in ostial LAD lesions appears comparable to outcomes achieved with provisional LMCA bifurcation PCI [38]. Our data further support CS as the technique yielding the largest ostial stent area and the lowest edge plaque burden—both surrogate markers for favorable long-term patency. However, the technique is not without drawbacks. Increased side branch intervention rates (56.5% in our CS group) underscore the risk of carina shift and LCX encroachment, particularly in wide-angle bifurcations or those with disease-free LCX origins [39]. The FS strategy, although less extensively studied, demonstrated favorable procedural characteristics in our cohort. It achieved intermediate ostial stent expansion and significantly reduced need for side branch intervention relative to CS. IVUS confirmed effective ostial coverage without significant LCX protrusion or plaque burden. FS appears to balance technical simplicity with procedural safety—avoiding underexpansion associated with POS and excessive carinal distortion seen with CS. This makes it a viable middle-ground strategy, particularly in anatomically favorable cases. While CS remains the most extensively validated technique and is currently endorsed by expert consensus and recent guidelines (including the EBC MAIN trial and bifurcation PCI recommendations) [6, 38, 40], its use may be limited in cases of significant LMCA–LAD size mismatch or when LMCA manipulation should be minimized. In such settings, the FS approach may be particularly advantageous, especially under IVUS guidance to ensure optimal stent deployment.
Our findings—supported by contemporary registry and trial data [33, 37, 40, 41]—advocate for a tailored PCI strategy for LAD ostial lesions. While CS offers the most complete lesion coverage and best long-term outcomes, it increases procedural complexity and side branch risk. POS remains limited by technical constraints and a higher restenosis burden. The FS technique, by contrast, provides a technically straightforward and clinically effective alternative, and merits further prospective evaluation in larger, controlled studies.
Although our study did not directly compare different antiplatelet regimens, all patients received standard DAPT as per current guidelines. Recent meta-analyses have suggested that P2Y12 inhibitor monotherapy, particularly with ticagrelor, may reduce bleeding risk without increasing ischemic events in patients undergoing complex PCI, including bifurcation and ostial lesions. These findings [42] suggest that individualized antithrombotic approaches may improve safety profiles in such high-risk anatomical subsets. Patients with atrial fibrillation undergoing PCI present unique challenges due to the need for both oral anticoagulation and antiplatelet therapy, which heightens bleeding risk. Recent evidence emphasizes that procedural strategies—particularly the use of intravascular imaging (IVUS or optical coherence tomography)—can enhance stent optimization, reduce underexpansion and geographic miss, and potentially allow for de-escalation of antiplatelet therapy without compromising ischemic protection. As highlighted by Castiello et al. [43], combining imaging-guided PCI with individualized dual antithrombotic therapy (DAT) may improve net clinical outcomes, especially in complex PCI such as ostial LAD interventions. This integrative approach could enable safer application of abbreviated DAPT or DAT strategies, thereby reducing bleeding risks in this vulnerable population.
Limitation
Several limitations of this study should be acknowledged. First, the retrospective and observational nature of the study, combined with its single-center design and relatively small sample size, may restrict the external validity and generalizability of the findings. Moreover, operator discretion in selecting stenting techniques based on anatomical or clinical judgment may have introduced selection bias, as procedural decisions could not be randomized or fully standardized. Although our cohort represents a real-world patient population undergoing IVUS-guided PCI for isolated ostial LAD disease, caution is warranted in extrapolating the results. Further prospective, multicenter studies with larger sample sizes are warranted to validate and extend these findings. Second, the relatively small sample size and the exclusion of patients with LMCA stenosis > 20% or significant LCX ostial involvement may have led to underrepresentation of anatomical variations encountered in routine clinical practice. Third, although baseline characteristics appeared well balanced, the limited number of MACCE events constrained the multivariable model to only a few covariates, increasing the risk of residual confounding. Therefore, the influence of unmeasured clinical or anatomical variables cannot be fully excluded. Fourth, although atrial fibrillation and oral anticoagulant use were documented and showed no statistically significant differences among the three groups, we recognize that these variables may still exert a potential influence on long-term clinical outcomes. The small absolute number of patients receiving anticoagulation limits the statistical power to assess its independent effect, and future studies should carefully adjust for antithrombotic therapy as a possible confounder. Fifth, multiple drug-eluting stent (DES) platforms were used across patients, including Resolute Integrity, Xience, and Firebird2. While all are second-generation DES with favorable safety profiles, their differences in scaffold design, polymer composition, and deliverability may have influenced procedural parameters (e.g., expansion behavior) and long-term outcomes. Due to the limited sample size and retrospective design, subgroup analysis by stent type was not feasible in this study. Future prospective trials should further investigate whether device selection modifies outcomes in ostial LAD interventions. Lastly, the absence of physiological assessments—such as FFR, quantitative flow ratio, and three-dimensional QCA—limited the ability to evaluate functional lesion severity and may have reduced the precision of anatomical and outcome interpretation.
Conclusion
This study compared three single-stent strategies for treating ostial LAD lesions and found that CS achieved the most favorable procedural metrics and clinical outcomes, including greater ostial stent expansion and the lowest incidence of MACCE at two years. In contrast, POS was associated with a higher rate of target lesion revascularization, likely due to limited expansion and insufficient lesion coverage. The FS technique emerged as a procedural compromise, providing adequate ostial scaffolding while minimizing side branch involvement.
These results underscore the importance of individualized PCI approaches for ostial LAD disease, guided by anatomical complexity and intravascular imaging. While CS remains the most comprehensive method for lesion coverage and showed the best outcomes in our study, we do not suggest that it should be universally applied. Rather, its use should be limited to patients with suitable LMCA–LAD anatomy and when LMCA instrumentation is feasible and safe. For patients with vessel size mismatch or when LMCA access is to be avoided, the FS technique offers a technically simpler and clinically effective alternative. Future prospective, multicenter investigations are needed to further assess the long-term safety and efficacy of FS and to inform optimal strategy selection for this anatomically and technically complex lesion subset.
Acknowledgements
Not applicable.
Author contributions
X.W. and L.W. had the idea for the paper, reviewed and edited it critically for important intellectual content. H.B.H. and H.H. performed the literature search and analysis. X.W., M.X.W., L.W., Z.L. and H.H. substantially contributed to the conception of the paper, drafted and critically revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Funding
This work was supported by Natural Science Foundation of Hunan Province (No.2022JJ30575) and Health Research Project of Hunan Provincial Health Commission (No. 20233486).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The present research was carried out in accordance with the tenets mentioned in the Helsinki Declaration and was approved by the Ethical Board of Xiangtan Central Hospital (approval number: X201922314-1). Prior to the commencement of the research, our team obtained written informed consent from each patient.
Consent for publication
Not applicable. No individual patient data will be reported.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shi H, Hyasat K, Deshmukh T, Ada C, Chiha J, Asrress K, et al. Optimal percutaneous treatment approach to unprotected ostial left anterior descending artery disease: A Meta-Analysis and systematic review. Heart Lung Circ. 2024;33(8):1123–35. [DOI] [PubMed] [Google Scholar]
- 2.Lassen JF, Holm NR, Stankovic G, Lefèvre T, Chieffo A, Hildick-Smith D, et al. Percutaneous coronary intervention for coronary bifurcation disease: consensus from the first 10 years of the European bifurcation club meetings. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2014;10(5):545–60. [DOI] [PubMed] [Google Scholar]
- 3.Burzotta F, Lassen JF, Lefèvre T, Banning AP, Chatzizisis YS, Johnson TW et al. Percutaneous coronary intervention for bifurcation coronary lesions: the 15(th) consensus document from the European bifurcation club. EuroIntervention. 2021;16(16):1307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.López-Candales A, Sawalha K. Improving diagnostic assessments in the ever-changing landscape of atherosclerosis. J Cardiovasc Med (Hagerstown Md). 2023;24(4):221–9. [DOI] [PubMed] [Google Scholar]
- 5.Güner A, Akman C, Çiloğlu K, Gökçe K, Uzun F, Can C, et al. Long-Term evaluation of revascularization strategies for Medina 0.1.0 left main bifurcation lesions: the LM-CROSSOVER registry. Angiology. 2025;76(4):361–9. [DOI] [PubMed] [Google Scholar]
- 6.Rigatelli G, Zuin M, Baracca E, Galasso P, Carraro M, Mazza A, et al. Long-Term clinical outcomes of isolated ostial left anterior descending disease treatment: ostial stenting versus left main Cross-Over stenting. Cardiovasc Revascularization Medicine: Including Mol Interventions. 2019;20(12):1058–62. [DOI] [PubMed] [Google Scholar]
- 7.Burzotta F, Talarico GP, Trani C, De Maria GL, Pirozzolo G, Niccoli G, et al. Frequency-domain optical coherence tomography findings in patients with bifurcated lesions undergoing provisional stenting. Eur Heart J Cardiovasc Imaging. 2014;15(5):547–55. [DOI] [PubMed] [Google Scholar]
- 8.Zimarino M, Corazzini A, Ricci F, Di Nicola M, De Caterina R. Late thrombosis after double versus single drug-eluting stent in the treatment of coronary bifurcations: a meta-analysis of randomized and observational studies. JACC Cardiovasc Interventions. 2013;6(7):687–95. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Hong MK, Lee SW, Lee CW, Han KH, Kim JJ, et al. Randomized comparison of debulking followed by stenting versus stenting alone for ostial left anterior descending artery stenosis: intravascular ultrasound guidance. Am Heart J. 2004;148(4):663–9. [DOI] [PubMed] [Google Scholar]
- 10.Medina A, Suárez de Lezo J, Pan M. [A new classification of coronary bifurcation lesions]. Rev Esp Cardiol. 2006;59(2):183. [PubMed] [Google Scholar]
- 11.Kishi K, Kimura T, Morimoto T, Namura M, Muramatsu T, Nishikawa H, et al. Sirolimus-eluting stent implantation for ostial left anterior descending coronary artery lesions: three-year outcome from the j-Cypher registry. Circulation Cardiovasc Interventions. 2011;4(4):362–70. [DOI] [PubMed] [Google Scholar]
- 12.Medina A, Martín P, Suárez de Lezo J, Amador C, Suárez de Lezo J, Pan M, et al. Vulnerable carina anatomy and ostial lesions in the left anterior descending coronary artery after floating-stent treatment. Rev Esp Cardiol. 2009;62(11):1240–9. [DOI] [PubMed] [Google Scholar]
- 13.Burzotta F, Lassen JF, Banning AP, Lefèvre T, Hildick-Smith D, Chieffo A et al. Percutaneous coronary intervention in left main coronary artery disease: the 13th consensus document from the European bifurcation club. EuroIntervention. 2018;14(1):112–20. 10.4244/EIJ-D-18-00357 [DOI] [PubMed] [Google Scholar]
- 14.Neumann FJ, Sousa-Uva M. Ten commandments’ for the 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):79–80. [DOI] [PubMed] [Google Scholar]
- 15.Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel angioplasty prognosis study group. Circulation. 1990;82(4):1193–202. [DOI] [PubMed] [Google Scholar]
- 16.Lotfi A, Jeremias A, Fearon WF, Feldman MD, Mehran R, Messenger JC, et al. Expert consensus statement on the use of fractional flow reserve, intravascular ultrasound, and optical coherence tomography: a consensus statement of the society of cardiovascular angiography and interventions. Catheterization and cardiovascular interventions. Official J Soc Cardiac Angiography Interventions. 2014;83(4):509–18. [DOI] [PubMed] [Google Scholar]
- 17.Kang SJ, Lee JY, Ahn JM, Mintz GS, Kim WJ, Park DW, et al. Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circulation Cardiovasc Interventions. 2011;4(1):65–71. [DOI] [PubMed] [Google Scholar]
- 18.Chieffo A, Latib A, Caussin C, Presbitero P, Galli S, Menozzi A, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165(1):65–72. [DOI] [PubMed] [Google Scholar]
- 19.Alfonso F, Suárez A, Pérez-Vizcayno MJ, Moreno R, Escaned J, Bañuelos C, et al. Intravascular ultrasound findings during episodes of drug-eluting stent thrombosis. J Am Coll Cardiol. 2007;50(21):2095–7. [DOI] [PubMed] [Google Scholar]
- 20.Finet G, Derimay F, Motreff P, Guerin P, Pilet P, Ohayon J, et al. Comparative analysis of sequential proximal optimizing technique versus kissing balloon inflation technique in provisional bifurcation stenting: fractal coronary bifurcation bench test. JACC Cardiovasc Interventions. 2015;8(10):1308–17. [DOI] [PubMed] [Google Scholar]
- 21.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115(17):2344–51. [DOI] [PubMed] [Google Scholar]
- 22.GS Kassab G, Finet. Anatomy and function relation in the coronary tree: from bifurcations to myocardial flow and mass. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2015;11(Suppl V):V13–7. [DOI] [PubMed] [Google Scholar]
- 23.Papafaklis MI, Bourantas CV, Yonetsu T, Vergallo R, Kotsia A, Nakatani S et al. Anatomically correct three-dimensional coronary artery reconstruction using frequency domain optical coherence tomographic and angiographic data: head-to-head comparison with intravascular ultrasound for endothelial shear stress assessment in humans. EuroIntervention. 2015;11(4):407–15. 10.4244/EIJY14M06_11 [DOI] [PubMed] [Google Scholar]
- 24.Toutouzas K, Chatzizisis YS, Riga M, Giannopoulos A, Antoniadis AP, Tu S, et al. Accurate and reproducible reconstruction of coronary arteries and endothelial shear stress calculation using 3D OCT: comparative study to 3D IVUS and 3D QCA. Atherosclerosis. 2015;240(2):510–9. [DOI] [PubMed] [Google Scholar]
- 25.Park JK, Kim JY, Kwon HM, Kim TH, Oh SJ, Hong BK, et al. Multidetector computed tomography for the evaluation of coronary artery disease; the diagnostic accuracy in calcified coronary arteries, comparing with IVUS imaging. Yonsei Med J. 2014;55(3):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacevic M, Burzotta F, Srdanovic I, Petrovic M, Trani C. Percutaneous coronary intervention to treat unprotected left main: common (un-answered) challenges. Kardiologia Polska. 2022;80(4):417–28. [DOI] [PubMed] [Google Scholar]
- 27.Serruys PW. The treatment of coronary bifurcations: a true Art form. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2015;11 Suppl V:V7. [DOI] [PubMed] [Google Scholar]
- 28.Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet (London England). 2017;390(10096):793–809. [DOI] [PubMed] [Google Scholar]
- 29.Gao XF, Zhang YJ, Tian NL, Wu W, Li MH, Bourantas CV, et al. Stenting strategy for coronary artery bifurcation with drug-eluting stents: a meta-analysis of nine randomised trials and systematic review. EuroIntervention: J EuroPCR Collab Working Group Interventional Cardiol Eur Soc Cardiol. 2014;10(5):561–9. [DOI] [PubMed] [Google Scholar]
- 30.Girasis C, Onuma Y, Schuurbiers JC, Morel MA, van Es GA, van Geuns RJ, et al. Validity and variability in visual assessment of stenosis severity in Phantom bifurcation lesions: a survey in experts during the fifth meeting of the European bifurcation club. Catheterization Cardiovasc Interventions: Official J Soc Cardiac Angiography Interventions. 2012;79(3):361–8. [DOI] [PubMed] [Google Scholar]
- 31.Grundeken MJ, Wykrzykowska JJ, Ishibashi Y, Garg S, de Vries T, Garcia-Garcia HM, et al. First generation versus second generation drug-eluting stents for the treatment of bifurcations: 5-year follow-up of the LEADERS all-comers randomized trial. Catheterization Cardiovasc Interventions: Official J Soc Cardiac Angiography Interventions. 2016;87(7):E248–60. [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Hahn JY, Song YB, Choi SH, Choi JH, Lu C, et al. Carina shift versus plaque shift for aggravation of side branch ostial stenosis in bifurcation lesions: volumetric intravascular ultrasound analysis of both branches. Circulation Cardiovasc Interventions. 2012;5(5):657–62. [DOI] [PubMed] [Google Scholar]
- 33.Yang ZK, Hu J, Ding FH, Ni JW, Zhang RY, Shen WF. One-year outcome of single-stent crossover versus accurate ostial stenting for isolated left anterior descending ostial stenosis. Coron Artery Dis. 2022;31(1):e67–72. [DOI] [PubMed] [Google Scholar]
- 34.Calvert PA, Brown AJ, Hoole SP, Obaid DR, West NE, Bennett MR. Geographical miss is associated with vulnerable plaque and increased major adverse cardiovascular events in patients with myocardial infarction. Catheterization Cardiovasc Interventions: Official J Soc Cardiac Angiography Interventions. 2016;88(3):340–7. [DOI] [PubMed] [Google Scholar]
- 35.Kang SJ, Cho YR, Park GM, Ahn JM, Kim WJ, Lee JY, et al. Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am J Cardiol. 2013;111(10):1408–14. [DOI] [PubMed] [Google Scholar]
- 36.Milasinovic D, Tomasevic M, Vukcevic V, Stankovic G. OCT guidance for detection and treatment of Free-Floating struts following ostial LAD stenting. JACC Cardiovasc Interventions. 2021;14(12):1376–7. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto K, Sakakura K, Akashi N, Watanabe Y, Noguchi M, Taniguchi Y, et al. Clinical outcomes of left main crossover stenting for ostial left anterior descending artery acute myocardial infarction. Heart Vessels. 2018;33(1):33–40. [DOI] [PubMed] [Google Scholar]
- 38.Hildick-Smith D, Egred M, Banning A, Brunel P, Ferenc M, Hovasse T, et al. The European bifurcation club left main coronary stent study: a randomized comparison of Stepwise provisional vs. systematic dual stenting strategies (EBC MAIN). Eur Heart J. 2021;42(37):3829–39. [DOI] [PubMed] [Google Scholar]
- 39.Fujino Y, Attizzani GF, Tahara S, Naganuma T, Takagi K, Yabushita H, et al. Difference in vascular response between sirolimus-eluting- and everolimus-eluting stents in ostial left circumflex artery after unprotected left main as observed by optical coherence tomography. Int J Cardiol. 2017;230:284–92. [DOI] [PubMed] [Google Scholar]
- 40.Patel Y, Depta JP, Patel JS, Masrani SK, Novak E, Zajarias A, et al. Impact of intravascular ultrasound on the long-term clinical outcomes in the treatment of coronary ostial lesions. Catheterization Cardiovasc Interventions: Official J Soc Cardiac Angiography Interventions. 2016;87(2):232–40. [DOI] [PubMed] [Google Scholar]
- 41.Lee HM, Nam CW, Cho YK, Yoon HJ, Park HS, Kim H, et al. Long-term outcomes of simple crossover stenting from the left main to the left anterior descending coronary artery. Korean J Intern Med. 2014;29(5):597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gragnano F, Mehran R, Branca M, Franzone A, Baber U, Jang Y, et al. P2Y(12) inhibitor monotherapy or dual antiplatelet therapy after complex percutaneous coronary interventions. J Am Coll Cardiol. 2023;81(6):537–52. [DOI] [PubMed] [Google Scholar]
- 43.Castiello DS, Buongiorno F, Manzi L, Narciso V, Forzano I, Florimonte D et al. Procedural and antithrombotic therapy optimization in patients with atrial fibrillation undergoing percutaneous coronary intervention: A narrative review. J Cardiovasc Dev Disease. 2025;12(4):142. 10.3390/jcdd12040142 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.