Abstract
Background
PTEN hamartoma tumour syndrome (PHTS) patients have a high hereditary risk of cancer, especially breast (BC), endometrial (EC), and thyroid cancer (TC). However, the prognosis of PHTS-related cancers is unknown.
Methods
This European cohort study included adult PHTS patients with data from medical files, registries, and/or questionnaires. Overall survival (OS) was assessed using Kaplan-Meier analyses and were compared with sporadic cancer and the general population using standardized mortality (SMR) and relative survival rates (RSR). Survival bias was addressed using left-truncation.
Results
Overall, 147 BC patients were included. The 10y-OS was 77% (95%CI = 66–90), decreasing with increasing stage from 90% (95%CI = 73–100) for stage 0 to 0% (95%CI = 0–0) for stage IV. BC relative survival was comparable to sporadic BC in the first two years (2y-RSR = 1.1; 95%CI = 1.1–1.1) and increasing thereafter (5y-RSR = 1.7; 95%CI = 1.6–1.7). For TC (N = 56) and EC (N = 35), 10y-OS was 87% (95%CI = 74–100) and 64% (95%CI = 38–100), respectively. Overall and cancer-specific mortality in female PHTS patients exceeded general population rates (SMR = 3.7; 95%CI = 2.6–5.0 and SMR = 2.7; 95%CI = 1.6–4.4).
Conclusions
The prognosis of PHTS-related cancers was comparable to the general population. The higher overall mortality in PHTS patients is presumably related to their higher cancer incidence. These findings, and the high survival observed in early-stage cancer, emphasise the importance of recognising PHTS early to facilitate cancer surveillance.
Background
PTEN hamartoma tumour syndrome (PHTS) is caused by a pathogenic germline variant in the PTEN gene. Individuals with PHTS have a high hereditary risk of developing cancer, especially female breast cancer (BC). This BC risk ranges from 54% to 76%, a risk comparable to that conferred by BRCA1/2 pathogenic variants [1–4]. PHTS patients also have a high risk of endometrial cancer (EC; 6% to 22%) and thyroid cancer (TC; 9% to 21%), and a moderately increased risk of colorectal cancer (CRC), renal cancer (RC) and melanoma (< 10%) [5].
PHTS patients with cancer currently receive standard treatment. However, a range of research suggests that the prognosis and treatment response of PHTS-related cancers might differ from that of sporadic cancers (i.e. without genetic predisposition). For example, somatic PTEN loss has been associated with tumour proliferation in EC and triple-negative BC, tumour recurrence and shortened survival in BC patients, and poor RC-specific survival [6–10]. Aberrant somatic PTEN signalling has been associated with BC and RC metastasis and decreased survival [11, 12]. Activation of the somatic PI3K pathway (which includes PTEN) has been shown to contribute to radiotherapy resistance in various cancer types [13, 14], and PTEN activity in stromal breast tissue has been associated with diminished radiotherapy and chemotherapy responses [15, 16]. Furthermore, patients with metastatic BC and somatic PTEN loss have a shortened survival after trastuzumab treatment [17].
The prognosis and outcomes of treatment in PHTS patients with cancer are unknown, leading to uncertainty whether standard cancer treatment is an appropriate choice for these patients. Therefore, we explored the prognosis of PHTS-related cancers in PHTS patients and the effects of different cancer characteristics and treatments.
Methods
Patient selection
Adult PHTS patients were retrospectively recruited via genetic centres, PHTS expert centres, and self-recruitment in Europe during 2019–2023 (Supplementary methods). Patients with a pathogenic or likely pathogenic PTEN germline variant, self-reported or reported by the genetic centre (n = 512), were included. One additional untested patient who met the genetic testing criteria from the National Comprehensive Cancer Network and had PHTS in first-degree relatives was included [18].
In total, 513 adult PHTS patients were included in mortality analyses, and 249 PHTS patients with cancer were included in survival analyses (Fig. 1).
Fig. 1. Schematic presentation of the total PHTS cohort and cancer patient cohort.
The total PHTS cohort consisted of patients who were recruited based on their PHTS diagnosis, with or without (a history of) cancer (n = 513). The PHTS cancer patient cohort consisted of PHTS patients with a history of cancer (n = 249). Data for patients only included in the cancer patient cohort were obtained from centres that only provided data on PHTS patients with cancer (n = 25).
This cohort study was approved by the institutional ethics committees, and written informed consent was obtained when indicated by the ethical committee.
Patient data
Data on cancer characteristics, treatment, follow-up, vital status, PHTS diagnosis, and index status (i.e. an index patient is the first PHTS patient identified in a family; Supplementary methods) were collected from medical files using standardized data dictionaries and patient questionnaires. Data on cause of death were collected from medical files, and death due to cancer was regarded as cancer-specific. For Dutch patients, additional information on vital status, cancers, and treatments was collected via the Dutch Nationwide Pathology Databank (PALGA) and Cancer Registry.
Statistical analysis
Standardized mortality rates were calculated to compare observed mortality in the total PHTS population with expected mortality using sex, calendar-year, age, and country-standardized population mortality rates (SMR). Observed cancer-specific mortality was compared with expected mortality using cancer-specific population mortality data (SMRca). SMR were stratified because of the different cancer spectra and cancer risks in male and female PHTS patients. As patients develop cancer early in life, the mortality rates were also presented by age group to explore the shape of the cancer risk curves [19].
Survival for the first primary cancer of that specific type was assessed using Kaplan-Meier analyses for PHTS-related cancers (BC, EC, TC, CRC, RC, and melanoma). Overall survival (OS) was calculated from cancer diagnosis to death from any cause or last follow-up, whichever occurred first. For the OS of a specific cancer type, no censoring was applied for subsequent cancer diagnoses. Cancer-specific survival (CSS) was calculated from cancer diagnosis to cancer-specific death or last follow-up, whichever occurred first. Censoring was applied for deaths from other causes. Metastasis-free survival (MFS) was assessed for patients without metastasis at primary cancer diagnosis and was calculated from cancer diagnosis to metastasis, recurrence, death, or last follow-up, whichever occurred first. Disease-free survival (DFS) was assessed for cancer-free patients at the end of treatment and was calculated from end of treatment until diagnosis of metastasis, recurrence, or last known disease-free moment, whichever occurred first. Survival probabilities were only assessed when numbers were sufficient and/or events occurred. To address survival bias, left-truncation was applied using delayed entry times for patients with cancer pre-dating PHTS diagnosis. This decreases the follow-up time after cancer diagnosis for a subset of the population of interest.
Relative risks associated with cancer stage, age, and treatment on survival were analysed using multivariable Cox regression. The proportionality assumption was verified by assessing log-minus-log plots and Schoenfeld residuals.
Relative survival rates were assessed to compare observed survival in PHTS cancer patients with expected survival in the general population using sex, calendar-year, and age-specific reference data (RSR) [19]. Using cancer-specific reference data, the observed survival was compared with expected survival of sporadic cancer (RSRca). Dutch and Belgian cancer-specific reference data were available (46% of cohort), and Dutch reference data were used for other countries [20, 21]. Sensitivity analyses indicated that the Dutch sub-cohort resembled the total cohort (data not shown).
Analyses were performed using RStudio (V.4.1.1). Statistical significance was set at p < 0.05. For SMR, relative risks and RSR, a rate of 1.0 indicates no difference between the reference population or reference category and PHTS population. A rate above (or below) 1.0 indicates a difference between the reference population or reference category and PHTS population. The higher (or lower) the rate, the greater the difference. Statistical significance is reached when the 95% confidence interval (95%CI) does not include 1.0.
Results
Overall mortality
Of the 513 patients, including 65% female and 55% index patients, 61 died at median age of 55 years (interquartile range (IQR) 45–63). Of these deaths, 27 were reported as cancer-specific. Female patients had a significantly higher overall mortality than the general population (SMR = 3.7, 95%CI = 2.6–5.0), with the greatest significant increase observed between 50 and 59 years of age (SMR = 5.2, 95%CI = 2.9–8.5) (Table 1). Cancer-specific mortality was also significantly higher in female PHTS patients compared to the general population (SMRca = 2.7, 95%CI = 1.6–4.4).
Table 1.
Overall and cancer-specific standardized mortality ratesa.
| Standardized mortality rate (95%CI), n events | |||||
|---|---|---|---|---|---|
| Age category (y) | Overall | Any cancer | BC | ||
| Female | Male | Female | Male | Female | |
| 20–29 | 2.1 (0.2–7.5), 2 | 2.4 (0.5–7.1), 3 | n/a, 0 | 12.4 (1.4–44.7), 2 | n/a, 0 |
| 30–39 | 4.2 (1.5–9.1), 6 | n/a, 0 | 5.4 (1.4–13.8), 4 | n/a, 0 | 20.5 (5.5–52.6), 4 |
| 40–49 | 2.2 (0.7–5.2), 5 | 1.5 (0.3–4.5), 3 | n/a, 0 | 1.3 (0.0–7.1), 1 | n/a, 0 |
| 50–59 | 5.2 (2.9–8.5), 15 | 0.7 (0.1–2.7), 2 | 3.4 (1.4–7.0), 7 | 0.8 (0.0–4.2), 1 | 11.9 (4.3–25.9), 6 |
| 60–69 | 3.5 (1.6–6.7), 9 | 1.6 (0.4–4.1), 4 | 3.9 (1.4–8.4), 6 | 0.8 (0.0–4.6), 1 | 7.4 (0.8–26.8), 2 |
| 20–69 | 3.7 (2.6–5.0), 37 | 1.2 (0.6–2.2), 12 | 2.7 (1.6–4.4), 17 | 1.3 (0.4–3.1), 5 | 8.1 (4.2–14.2), 12 |
an/a not applicable, BC breast cancer.
Standardized mortality rates (SMRs) are presented with corresponding 95% confidence intervals (95%CI) by age groups and sex for the overall PHTS cohort, PHTS cohort with any cancer, and PHTS cohort with breast cancer (BC) (compared to the general population). The number of events is presented for each age category.
Cancer prognosis, treatment, and mortality
In total, 482 primary cancers were diagnosed in 249 patients (82% female, 66% index). Most patients (89%) had PHTS-related cancers, of which 24% had two different types of cancer and 5% had three types. Females often had BC (147/204) and males often had TC (15/45). PHTS was diagnosed at a median age of 45 years (IQR 34–54) in females and at 50 years (IQR 41–56) in males. Median age at last follow-up was 50 years (IQR 41–59) in females and 58 years (IQR 50–64) in males.
Breast cancer
A total of 147 females were included in BC analyses (73% index; Table 2). In 31%, the first primary BC was diagnosed after the PHTS diagnosis (i.e. incident cancer diagnosis). BC was diagnosed at a median age of 41 years (IQR 35–49). The BC stage at diagnosis was mostly stage 0 (33%), stage I (27%), and stage II (23%). Histology was largely carcinoma of no special type (56%) and carcinoma in situ (29%) (Supplementary Table 1). Twenty-five patients died (52% BC-specific) at a median age of 55 years (IQR 50–60).
Table 2.
Cancer characteristics and treatment characteristics of cancer patient cohortsa.
| BC | EC | TC | CRC | RC | Melanoma | |
|---|---|---|---|---|---|---|
| Cancer characteristics | Female | Female | Total | Total | Total | Total |
| Population, No. | 147 | 35 | 56 | 22 | 12 | 19 |
| Sex, No. (%) female | 147 (100) | 35 (100) | 41 (73) | 11 (50) | 8 (67) | 12 (63) |
| Sex, No. (%) male | – | – | 15 (27) | 11 (50) | 4 (33) | 7 (37) |
| Index, No. (%) | 108 (73) | 26 (74) | 37 (66) | 14 (64) | 7 (58) | 11 (58) |
| Incident cancer diagnosis, No. (%)b | 45 (31) | 9 (26) | 20 (36) | 6 (27) | 7 (58) | 5 (26) |
| Ages, median (IQR) | ||||||
| Cancer diagnosis | 41 (35–49) | 46 (36–55) | 37 (26–46) | 60 (53–64) | 53 (51–58) | 35 (30–42) |
| PHTS diagnosis | 45 (35–53) | 51 (41–62) | 40 (32–55) | 60 (50–65) | 51 (45–54) | 45 (36–52) |
| Last follow-up | 51 (42–59) | 57 (50–66) | 51 (41–61) | 65 (58–72) | 60 (52–64) | 51 (45–62) |
| Follow-up after cancer, median (IQR) | 7.2 (3.2–12.9) | 6.0 (3.0–16.3) | 11.3 (4.5–17.7) | 4.2 (2.0–10.0) | 2.6 (1.7–7.3) | 16.5 (6.2–17.3) |
| Follow-up after cancer left-truncated analysis, median (IQR) | 4.0 (1.6–7.0) | 2.3 (1.2–4.8) | 5.2 (1.8–9.7) | 2.6 (1.0–6.0) | 1.9 (0.9–3.6) | 5.5 (2.8–10.2) |
| Death, No. (%) | 25 (17) | 5 (14) | 5 (9) | 5 (23) | 1 (7) | 2 (11) |
| Cancer-specific, No. (%) | 13 (52) | 2 (40) | 3 (60) | 2 (40) | 0 (0) | 0 (0) |
| Death age, median (IQR) | 55 (50–61) | 62 (55–67) | 62 (57–64) | 73 (59–75) | 72 | 55, 70 |
| Stage at diagnosis, No. (%) | ||||||
| 0 | 43 (33) | – | – | – | – | – |
| I | 35 (27) | 22 (79) | 45 (87) | 3 (16) | 8 (80) | 10 (91) |
| II | 30 (23) | 4 (14) | 3 (6) | 5 (26) | 1 (10) | 1 (9) |
| III | 17 (13) | 0 (0) | 0 (0) | 7 (37) | 0 (0) | 0 (0) |
| IV | 5 (4) | 2 (7) | 4 (8) | 4 (21) | 1 (10) | 0 (0) |
| Unknownc | 17 | 7 | 4 | 3 | 2 | 8 |
| Grade, No. (%)d | 115 (78) | 27 (77) | – | 15 (68) | 5 (42) | 2 (11) |
| Grade 1 | 27 (24) | 20 (74) | – | 0 (0) | 2 (40) | 2 (100) |
| Grade 2 | 53 (46) | 6 (22) | – | 14 (93) | 3 (60) | 0 (0) |
| Grade 3 | 35 (30) | 1 (4) | – | 1 (7) | 0 (0) | 0 (0) |
| Hormone receptor status, No. (%)d | 100 (68) | – | – | – | – | – |
| Hormone receptor ER/PR+ | 85 (85) | – | – | – | – | – |
| HER2 status, No. (%)d | 84 (57) | – | – | – | – | – |
| HER2+ | 5 (6) | – | – | – | – | – |
| Triple-negative (ER/PR/HER2-), No. (%) | 12 (14) | – | – | – | – | – |
| Node-status+ (N1/2/3), No. (%) | 42 (29) | 1 (3) | 10 (18) | 8 (36) | 1 (8) | 0 (0) |
| Metastasis-status+ (M1), No. (%) | 5 (3) | 2 (6) | 3 (5) | 4 (18) | 0 (0) | 0 (0) |
| Treatment characteristics, No. (%)e | ||||||
|---|---|---|---|---|---|---|
| Surgeryd | 141 (97) | 34 (97) | 56 (100) | 20 (91) | 12 (100) | 18 (95) |
| No | 3 (2) | 2 (9) | 2 (4) | 2 (10) | 2 (17) | 0 (0) |
| Yes | 138 (98) | 32 (91) | 54 (96) | 18 (90) | 10 (83) | 18 (100) |
| Mastectomy | 95 (65) | – | – | – | – | – |
| Mastectomy bilateral | 66 (69) | – | – | – | – | – |
| Mastectomy unilateral | 29 (31) | – | – | – | – | – |
| Lumpectomy | 44 (31) | – | – | – | – | – |
| Hysterectomy | – | 29 (90) | – | – | – | – |
| Total thyroidectomy | – | – | 42 (78) | – | – | – |
| Hemithyroidectomy | – | – | 10 (19) | – | – | – |
| Total colectomy | – | – | – | 2 (11) | – | – |
| Hemicolectomy | – | – | – | 9 (50) | – | – |
| Nephrectomy unilateral | – | – | – | – | 6 (60) | – |
| Partial nephrectomy | – | – | – | – | 4 (40) | – |
| Skin resection | – | – | – | – | – | 18 (100) |
| Chemotherapyd | 137 (93) | 30 (86) | 54 (96) | 20 (91) | 11 (92) | 17 (89) |
| No | 75 (55) | 29 (97) | 53 (98) | 12 (60) | 10 (91) | 17 (100) |
| Yes | 62 (45) | 1 (3) | 1 (2) | 8 (40) | 1 (9) | 0 (0) |
| Neo-adjuvant | 24 (39) | 0 (0) | 0 (0) | 2 (25) | 0 (0) | 0 (0) |
| Adjuvant | 28 (45) | 1 (100) | 1 (100) | 6 (75) | 1 (100) | 0 (0) |
| Unknown | 10 | 0 | 0 | 0 | 0 | 0 |
| Agents used | ||||||
| Alkaloids | 62 (100) | 1 (100) | 0 (0) | 3 (38) | 1 (100) | – |
| Antitumour antibiotics | 45 (73) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Antimetabolites | 25 (40) | 0 (0) | 0 (0) | 8 (100) | 1 (100) | – |
| Platinum-containing | 3 (5) | 1 (100) | 1 (100) | 5 (63) | 0 (0) | – |
| Topoisomerase inhibitors | 0 (0) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | – |
| Other | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | – |
| Radiotherapyd | 137 (93) | 31 (89) | 53 (95) | 20 (91) | 11 (92) | 17 (89) |
| No | 73 (53) | 22 (71) | 52 (96) | 20 (100) | 11 (100) | 17 (100) |
| Yes | 64 (47) | 9 (29) | 2 (4) | 0 (0) | 0 (0) | 0 (0) |
| Radioiodine therapyd | – | – | 34 (61) | – | – | – |
| No | – | – | 2 (6) | – | – | – |
| Yes | – | – | 32 (94) | – | – | – |
| Hormone therapyd | 134 (91) | 31 (89) | 53 (95) | 20 (91) | 11 (92) | 18 (95) |
| No | 69 (51) | 30 (97) | 53 (100) | 20 (100) | 11 (100) | 18 (100) |
| Yes | 65 (49) | 1 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Agents used | ||||||
| Progestogen | 0 (0) | 1 (100) | – | – | – | – |
| Antioestrogen | 40 (62) | 0 (0) | – | – | – | – |
| Aromatase-inhibitor | 16 (25) | 0 (0) | – | – | – | – |
| LHRH-agonist | 2 (3) | 0 (0) | – | – | – | – |
| Other | 7 (11) | 0 (0) | – | – | – | – |
| Immune therapyd | 130 (88) | 31 (89) | 54 (96) | 20 (91) | 11 (92) | 18 (95) |
| No | 122 (94) | 31 (100) | 53 (98) | 20 (100) | 11 (100) | 18 (100) |
| Yes | 8 (6) | 0 (0) | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Agents used | ||||||
| Trastuzumab | 6 (75) | – | – | – | – | – |
| Atezolizumab | 2 (25) | – | – | – | – | – |
| Lenvatinib | 0 (0) | – | 1 (100) | – | – | – |
aBC breast cancer, EC endometrial cancer, TC thyroid cancer, CRC colorectal cancer, RC renal cancer, IQR interquartile range.
bCancer diagnosed after DNA diagnosis of PHTS is an incident cancer diagnosis. BC was diagnosed a median of 0.9 year (IQR 0.0–6.4) before PHTS. EC was diagnosed 1.4 years (IQR 0.0–9.9) before PHTS. TC was diagnosed 1.2 years (IQR 0.0–12.0) before PHTS. CRC was diagnosed 0.0 years (IQR 0.0–1.4) before PHTS.
c‘Unknown’ stage is not included in the percentages.
dAvailability of information.
eThe frequencies for treatment are not mutually exclusive as participants could have multiple treatments and multiple agents used per treatment type.
All patients, except three with stage IV BC, underwent surgical treatment (96%; Table 2) including bilateral mastectomy in 47% of patients (35% of patients with an incident BC diagnosis), unilateral mastectomy in 21% (7% in incident BC), and lumpectomy in 31% (24% in incident BC). Overall, 58% received both systemic and non-systemic treatments, and 31% only non-systemic. Treatment initiation was related to tumour characteristics, e.g. 0%–39% received chemotherapy for stages 0–I versus 77%–80% for stages II–IV.
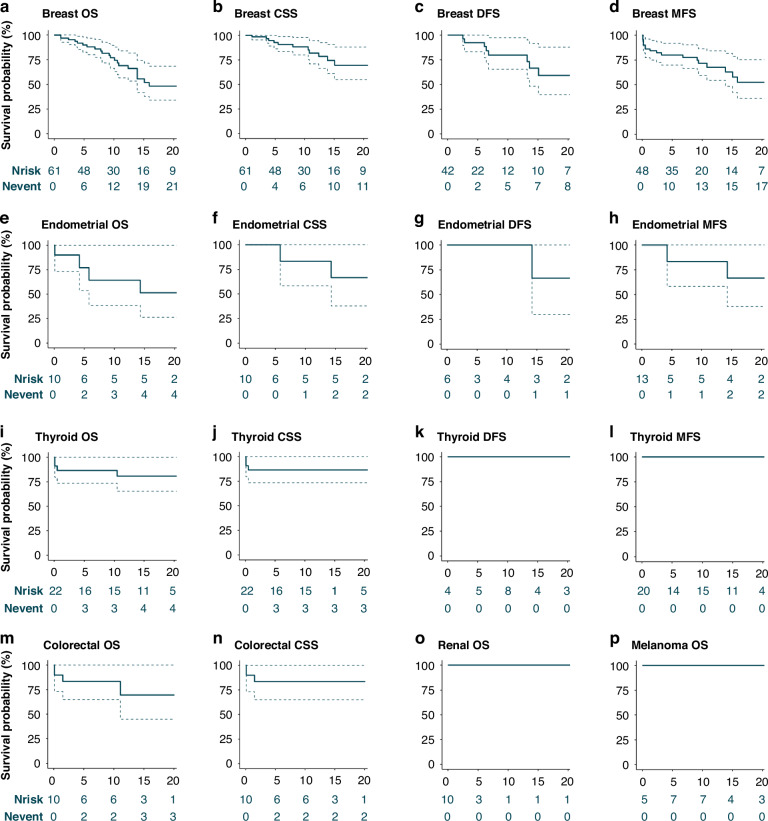
The 5y- and 10y-OS were 90.0% (95%CI = 82.7–98.0) and 77.1% (95%CI = 66.3–89.6), respectively. The median OS was 21 years. For invasive BC, the 5y- and 10y-OS were 83.6% (95%CI = 73.7–94.8) and 67.3% (95%CI = 54.3–83.4), respectively. For stages 0–IV the 5y-OS was 100.0% (95%CI = 100.0–100.0), 93.3% (95%CI = 81.5–100.0), 88.9% (95%CI = 70.6–100.0), 80.0% (95%CI = 51.6–100.0), and 33.3% (95%CI = 6.7–100.0), respectively. The 10y-OS by stage was 90.0% (95%CI = 73.2–100.0), 83.0% (95%CI = 63.5–100.0), 74.1% (95%CI = 48.4–100.0), 51.4% (95%CI = 26.4–100.0) and 0.0% (95%CI = 0.0–0.0), respectively. CSS and DFS were higher, whereas MFS was lower than OS (Fig. 2; Supplementary Table 2).
Fig. 2. Survival per cancer type.
The survival probability per cancer type in percentages (%) on the y-axis is presented for the left-truncated analyses. The x-axis represents the number of years after cancer diagnosis. The dashed lines represent 95% confidence intervals. The number of patients at risk (Nrisk) and the cumulative number of events (Nevent) are presented in the risk tables. Survival for breast and endometrial cancer is presented only for females. Overall survival (OS) is presented for female breast, endometrial, thyroid, colorectal, renal cancer, and melanoma (a, e, i, m, o, p). The cancer-specific survival (CSS) is presented for female breast, endometrial, thyroid, and colorectal cancer (b, f, j, n). Disease-free survival (DFS) is presented for female breast, endometrial, and thyroid cancer (c, g, k). The metastasis-free survival (MFS) is presented for breast, endometrial, and thyroid cancer (d, h, l). The 5- and 10-year survival per cancer type is presented in Supplementary Table 2.
BC patients who underwent mastectomy appeared to have better survival compared to those who underwent breast-conserving surgery (HRunilateral = 0.8, 95%CI = 0.2–3.7 and HRbilateral = 0.2, 95%CI = 0.03–0.9; Supplementary Fig. 1). Poorer survival was observed in patients treated with chemotherapy or radiotherapy compared to those treated without chemotherapy or radiotherapy (HR = 5.4, 95%CI = 1.1–25.3 and HR = 3.1, 95%CI = 0.8–11.9), respectively. All patients with hormone receptor-positive BC who did not receive hormone therapy survived ten years after diagnosis. Overall, patients who received systemic therapy had lower survival rates compared to those receiving non-systemic treatment (HR = 7.6, 95%CI = 1.0–59.7). Results of multifactorial analyses of hormone therapy, mastectomy, and systemic treatment, including nodal-status or age at diagnosis, were similar (data not shown). Multifactorial analyses of chemotherapy were not possible.
Compared to the general population, BC-specific mortality was significantly higher in PHTS patients (SMRca = 8.1, 95%CI = 4.2–14.2) and a trend towards stronger increase at younger age was observed (Table 1).
For patients with PHTS, relative survival after a BC diagnosis was comparable to survival in the general population (Fig. 3a). Until two years post-diagnosis, relative survival was also comparable to sporadic BC (1y-RSRca = 1.0, 95%CI = 1.0–1.0 and 2y-RSRca = 1.1, 95%CI = 1.1–1.1). Thereafter, it gradually increased to 1.7 (95%CI = 1.6–1.7) at five years post-diagnosis (Fig. 3a). For stage 0 (carcinoma in situ), relative survival was comparable up to five years post-diagnosis (RSRca = 1.0, 95%CI = 1.0–1.0). Comparable relative survival was observed for stage I-II BC until three years post-diagnosis and for stage III-IV BC until one year post-diagnosis, and increased thereafter (Fig. 3b).
Fig. 3. Relative survival rates for breast cancer, endometrial cancer, and thyroid cancer.
Comparison of observed survival in PHTS cancer patients with expected survival in the general population is presented as the relative survival rates (RSR). Comparison of observed survival in PHTS cancer patients with expected survival in sporadic cancer is presented as RSRca. RSR is presented on the y-axis for 1 to 5 years after cancer diagnosis (x-axis). Error bars represent the 95% confidence intervals. For breast and endometrial cancer, the RSRca is additionally presented by stage (b, d). Breast cancer stage 0 refers to carcinoma in situ (b).
Endometrial cancer
Thirty-five females with EC were included (74% index; Table 2), of whom 20% previously used tamoxifen. Of EC diagnoses, 26% were incident. EC was diagnosed at a median age of 46 years (IQR 36–55), PHTS at 51 years (IQR 41–62), and last follow-up was at 57 years (IQR 50–66). EC was mostly stage I (79% of patients) and of endometrioid histology (69%; Supplementary Table 1). Most patients underwent surgical treatment, including hysterectomy in 90%. Five patients died at a median age of 62 years (IQR 55–67), of which two EC-specific deaths occurred between 60 and 69 years.
The 5y- and 10y-OS were 77.1% (95%CI = 53.5–100.0) and 64.3% (95%CI = 38.5–100.0), respectively. The 5y- and 10y-OS were 80.0% (95%CI = 51.6–100.0) for patients with stage I EC. Numbers were too small for specific stage II-IV analyses. Two EC-specific deaths were observed, reflected in the high 5y- and 10y-CSS (Fig. 2f; Supplementary Table 2). None of the patients without EC after treatment (n = 6) had metastasis or recurrence after ten years of follow-up (DFS = 100%, 95%CI = 100–100). The 5y- and 10y-MFS were both 83.3% (95%CI = 58.3–100.0).
Relative survival after EC was comparable to the general population (Fig. 3c). Relative survival after EC was also comparable to sporadic EC until one year post-diagnosis (1y-RSRca = 1.0, 95%CI = 0.9–1.1) and gradually increased to 2.6 (95%CI = 2.3–2.9) five years post-diagnosis (Fig. 3c). Stage I-specific relative survival was comparable to sporadic EC (1y-RSRca = 1.0, 95%CI = 0.9–1.1 to 5y-RSRca = 1.3, 95%CI = 1.1–1.5) (Fig. 3d).
Thyroid cancer
Fifty-six patients with TC were included (66% index, 73% female; Table 2). Of TC diagnoses, 36% were incident. TC was diagnosed at a median age of 37 years (IQR 26–46). TC was mostly stage I (87%) and follicular (36%) or papillary histology (41%) (Supplementary Table 1). Most patients underwent surgical treatment including total (78%) or hemithyroidectomy (19%) and received additional radioiodine therapy (94%). Five patients died (3 TC-specific) at a median age of 62 years (IQR 57–64). All three TC-deaths occurred within one year of an initial stage IV TC diagnosis.
The 5y- and 10y-OS for TC were both 86.6% (95%CI = 73.5–100.0) and were comparable by sex (data not shown). Stage I-specific OS was 100% after ten years. No recurrences or metastases were observed after the TC diagnosis and treatment (Fig. 2j–l; Supplementary Table 2).
Relative survival after TC was comparable to the general population for males and females (Fig. 3e, f). Relative survival after TC was also comparable to sporadic TC until three years post-diagnosis for males (1-3y-RSRca = 1.0, 95%CI = 0.9–1.2) and until one year post-diagnosis for females (1y-RSRca = 1.1, 95%CI = 1.0–1.2). Thereafter, the RSRca gradually increased to 1.9 (95%CI = 1.7–2.2) for males and to 2.9 (95%CI = 2.7–3.1) for females five years post-diagnosis. Stage-specific analyses were not possible due to small numbers.
Colorectal cancer
Twenty-two patients with CRC were included (64% index, 50% female; Table 2). Of CRC diagnoses, 27% were incident. CRC was diagnosed at a median age of 60 years (IQR 53–64). Stage at diagnosis was mostly stage III (37%). Most patients underwent surgical treatment, consisting of hemicolectomy in 50% of patients. Eight patients received additional chemotherapy (75% adjuvant), mostly for stage III CRC (75%). Five patients died (2 CRC-specific) at a median age of 73 years (IQR 59–75). Both CRC-specific deaths occurred within five years of diagnosis. The 5y- and 10y-OS were both 83.6% (95%CI = 64.9–100.0) (Fig. 2n; Supplementary Table 2).
Renal cancer
Twelve patients with RC were included (58% index, 63% female; Table 2). Of RC diagnoses, 58% were incident. RC was diagnosed at a median age of 53 years (IQR 51–58). Most patients had stage I RC (80%). Most patients underwent surgical treatment (83%), including unilateral (60%) and partial nephrectomy (40%). One patient died, which was not RC-specific.
Melanoma
Nineteen patients with melanoma were included (58% index, 63% female; Table 2). Of melanoma diagnoses, 26% were incident. Melanoma was diagnosed at a median age of 35 years (IQR 30–42). Most patients had stage I melanoma (91%) and all (100%) received surgical treatment only. Two patients died from other causes.
Discussion
Rare hereditary syndromes associated with predisposition to cancer can offer valuable insights into sporadic cancers which often have somatic (i.e. non-germline) variants in the gene associated with hereditary predisposition. Somatic variants of the PTEN tumour suppressor gene are common in sporadic cancer, and the effect of these variants on the prognosis of sporadic cancers has been debated for years [22, 23]. Unlike well described hereditary cancer risk syndromes such as BRCA1/2 [24–26], this is the first study to evaluate the prognosis of cancer in patients with germline pathogenic variants in PTEN (i.e. in individuals with PHTS). Unlike findings from previous studies on somatic PTEN variants in sporadic cancer [6–17], this study found no indication of germline pathogenic variants in PTEN adversely affecting cancer prognosis.
Breast cancer
In our study, the 5y- and 10y-OS of PHTS patients with BC was 90.0% and 77.1%, respectively. This is comparable to outcomes for sporadic BC (75%–92% and 66%–80%) [21, 27]. Consistent with sporadic BC, survival decreased with increasing stage in PHTS patients with BC. The 5y-OS was 93.3%, 88.9%, 80.0%, 33.3%; and the 10y-OS was 83.0%, 74.1%, 51.4%, 0.0% for stage I-IV BC in PHTS patients, compared to 5y-OS: 99%, 92%, 77%, 31%; and 10y-OS: 96%, 85%, 64%, 12% for BC in the general population [21].
Relative survival for PHTS patients with BC was comparable to patients with sporadic BC for the first two years after diagnosis. Thereafter, it gradually increased to 1.7 (95%CI = 1.6–1.7) at five years post-diagnosis, indicating relatively favourable outcomes compared to sporadic BC. Comparable relative survival initially followed by slightly increased relative survival compared to sporadic BC was observed for both stage I-II and stage III-IV BC in PHTS patients. In these analyses, relative survival may have been overestimated due to survival bias. Furthermore, more aggressive treatment, cancer prevention strategies, and healthy screener effects might have influenced these results, which requires cautious interpretation of these results [28–30].
Of note, (bilateral and unilateral) mastectomy rates were higher in PHTS patients with BC than in patients with sporadic BC (65% vs. 15–30%) and were comparable to rates in patients with a BRCA1/2 pathogenic variant (59%) [31–33]. Amongst PHTS patients with BC, better survival was observed with unilateral and bilateral mastectomy than with breast-conserving surgery (HRunilateral = 0.8, 95%CI = 0.2–3.7 and HRbilateral = 0.2, 95%CI = 0.03–0.9), consistent with some BRCA1/2 reports while other reports demonstrated no difference in survival [34, 35]. However, survival after unilateral mastectomy in PHTS patients with BC was not statistically significantly increased, and the intent of surgery was not always known. Furthermore, this effect could be related to the high risk of second primary BC in PHTS patients [36]. Thus, further confirmation using a larger cohort is desirable. While awaiting such results, our findings support counselling of female PHTS patients on the possibility of bilateral mastectomy as cancer treatment for the affected breast and as risk-reducing surgery for the contralateral breast.
Our results suggest lower survival in PHTS patients with BC who received chemotherapy or radiotherapy compared to patients who did not (HR = 5.4, 95%CI = 1.1–25.3 and HR = 3.1, 95%CI = 0.8–11.9, respectively). However, this is most likely related to underlying prognostic factors which indicated a need for these treatment modalities [37]. Therefore, additional data are needed and no new recommendations can be made on use of chemotherapy or radiotherapy for BC treatment in PHTS patients.
Although survival (i.e. percentage of people alive at selected time-points after diagnosis) in PHTS patients with BC was no worse than in sporadic BC patients, BC mortality (i.e. number of deaths from BC in the PHTS population) and overall mortality (i.e. number of deaths from any cause in the PHTS population) in female PHTS patients was increased compared to the general population (SMRca = 3.7, 95%CI = 2.6–5.0), and was to an extent comparable to that in female BRCA1/2 patients (SMRca = 3.2, 96%CI 2.0–5.3) [24]. This presumably relates to the higher BC incidence in PHTS patients and might be overestimated due to ascertainment bias [5]. More PHTS patients develop cancer due to a high risk of cancer compared to the general population. Despite the comparable prognosis compared to sporadic cancer, the high cancer incidence presumably contributes to increased mortality, which likely results in excess mortality in PHTS patients. Numbers for SMR subgroup analyses by age were low and should therefore be interpreted with caution.
Other PHTS-related cancers
Our findings in other PHTS-related cancers were similar to findings in BC. Most patients with EC, TC, RC, and melanoma had early-stage disease (stage I) whereas half of the patients with CRC had stage III-IV disease at diagnosis. Histology, grade, and hormone status distribution across cancer types is similar to the general population, except for RC which is potentially due to low number of events [21]. Treatments for these PHTS-related cancers were generally comparable to treatments for sporadic cancers. Most PHTS patients with EC underwent hysterectomy (90% vs. 97% of patients with sporadic EC), and a minority underwent radiotherapy (29% vs. 31%) [38]. TC treatment in PHTS patients was comparable to sporadic TC for total thyroidectomy (78% vs. 85%), with a higher frequency of radioiodine therapy in PHTS patients (94% vs. 72%) [39]. CRC treatment in PHTS patients was comparable for chemotherapy (40% vs. 35%), but more PHTS patients than sporadic CRC patients underwent surgery (90% vs. 75%) [40]. The frequency of surgery in PHTS patients with RC was higher although less extensive than in patients with sporadic RC (unilateral nephrectomy 60% vs. 82%; partial nephrectomy 40% vs. 14%), probably reflecting the early stage at diagnosis. The frequency of surgery was comparable for PHTS patients with melanoma and sporadic melanoma (100% vs. 95%) [41, 42].
As seen for PHTS patients with BC, OS was no worse in PHTS-related cancers than in sporadic cancers. The 5y-OS and 10y-OS for EC (77.1% and 64.3%) and TC (both 86.6%) were comparable to sporadic cancer (EC: 5y-OS 79%–82% and 10y-OS 76%; TC: 5y-OS 85% and 10y-OS 80%) [21, 43, 44], with no indication of worse relative survival. The 5y- and 10y-OS for CRC (both 83.6%) were higher than reported for sporadic CRC (5y-OS 67% and 10y-OS 61%), potentially due to a lower proportion of advanced CRC in PHTS patients, who may undergo regular colonoscopies for polyps from an early age [21, 45, 46]. However, stage-specific analyses could not be performed due to low numbers of PHTS patients with CRC. For RC and melanoma, no deaths occurred within ten years, suggesting favourable prognoses. Although death from any cause was taken into account, no censoring was applied for diagnosis of other cancers in the follow-up, which might have led to underestimation of these OS estimates.
Limitations
The rarity of PHTS makes collecting sufficient patient data challenging. Potential survival bias was addressed by applying left-truncated analyses and excluding patients from institutes where information sharing from deceased patients was impossible. Although we collected a substantial cohort of PHTS cancer patients, including patients from sixteen countries, the number remained limited for less prevalent cancers and multifactor analyses of cancer characteristics. While lymph node status was assessed for BC in our study, other factors (including hormone and HER2 receptor status, physical activity, comorbidities, and other primary cancers) that could influence survival outcomes were not sufficiently available or not collected [47–49]. A matched case-control study could potentially provide more statistical power to assess combinations of prognostic factors in PHTS compared to cancer in the general population [27]. Although a follow-up study using a larger prospective cohort is desirable, short-term realization is not feasible due to the rarity of PHTS.
Significance
For high hereditary cancer risk syndromes, it is crucial to understand cancer prognosis, treatment, and mortality to optimize patient care. Our study shows that the prognosis of cancer in PHTS patients is no worse than for comparable cancers in the general population (sporadic cancers). Thus, unlike previous studies on somatic PTEN variants in sporadic cancer [6–17], our findings suggest that pathogenic germline PTEN variants do not worsen cancer prognosis. Our results also support the view that standard cancer treatment is appropriate for PHTS patients with cancer. These findings may provide some reassurance to PHTS patients. For PHTS patients with BC, bilateral mastectomy may result in a higher survival compared to breast-conserving surgery, but further evaluation is required.
Although the prognosis of PHTS-associated cancers (BC, EC, TC, CRC, RC, and melanoma) was comparable to sporadic cancers, overall and cancer-specific mortality was higher in female PHTS patients than the general population. This is presumably related to the high incidence of cancer in female PHTS patients. These observations and the favourable outcomes of early-stage cancer in PHTS patients highlight the importance of a timely diagnosis of PHTS and initiation of appropriate surveillance and/or risk-reducing surgery in patients with PHTS.
Supplementary information
Acknowledgements
This research was supported (not financially) by the European Reference Network on Genetic Tumour Risk Syndromes (ERN GENTURIS)—Project ID No. 739547. ERN GENTURIS is funded by the European Union. D.G.E. was supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (IS-BRC-1215-20007). M.T. was supported by the NIHR Cambridge Biomedical Research Centre (NIHR203312). We thank the Dutch Nationwide Pathology Databank (PALGA) for retrieving the pathology data and contributing to this study, the Belgian Cancer Registry for providing us with reference data, the Netherlands Comprehensive Cancer Organization (IKNL) for the collection of data for the Netherlands Cancer Registry, and Claire Barton of the PTEN Research Foundation for editorial support.
Author contributions
N.H., J.R.V., L.A.J.H., and K.C.J.V contributed to study conception and design. L.A.J.H., K.C.J.V., J.H.M.S.H., R.D.P., H.B., S.H.V.D., V.C.A., L.F., P.R.B., A.G., C.C., M.C.V., C.H., M.B., R.H., S.A., A.J., V.S.L., G.I., D.T., V.B., M.G., A.P., M.B., A.I., M.M.D.J., T.P.L., E.M.L., D.G.M.B., S.H.D., R.S.V.D.P., A.R.M., H.W., H.H.V., M.T.H., K.J., L.M., S.B., J.D.G., A.B., J.B., M.T., J.B., R.L.P., E.T., M.T., D.G.E., Z.H., N.H., and J.R.V. contributed to the data collection. L.A.J.H., K.C.J.V., and J.R.V. performed the data analysis. L.A.J.H., K.C.J.V., J.H.M.S.H., J.R.V., and N.H. wrote the initial version of the manuscript. L.A.J.H., K.C.J.V., J.H.M.S.H., R.D.P., H.B., S.H.V.D., V.C.A., L.F., P.R.B., A.G., C.C., M.C.V., C.H., M.B., R.H., S.A., A.J., V.S.L., G.I., D.T., V.B., M.G., A.P., M.B., A.I., M.M.D.J., T.P.L., E.M.L., D.G.M.B., S.H.D., R.S.V.D.P., A.R.M., H.W., H.H.V., M.T.H., K.J., L.M., S.B., J.D.G., A.B., J.B., M.T., J.B., R.L.P., E.T., M.T., D.G.E., Z.H., N.H., and J.R.V. reviewed and approved the final manuscript.
Funding
This work has been funded, in part, by the PTEN Research Foundation, a charity governed by English law (charity number 117358) to L.A.J.H. and J.R.V. under grant number RUM 17-002; and Horizon Europe to K.C.J.V. under grant number 101095483.
Data availability
The individual patient data that support the findings of this study are not openly available due to privacy and ethical reasons. Aggregate study data are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Radboud university medical centre.
Competing interests
A.R.M. received funds from AstraZeneca for contribution to sponsored quality assessment and variant interpretation of VUS in BRCA1 and BRCA2. This funding was not related to this study. J.B. received funding support from GSK for an educational activity unrelated to this study. MT is the Editor-in-Chief of BJC Reports, he was not involved in any aspect of the handling of this manuscript or any editorial decisions. The remaining authors have no competing interests to declare.
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. The Research Ethics Committee of the Radboud university medical centre (file number 2018-5056) approved this study and the institutional ethics committees approved this study. Written informed consent was obtained when indicated by the ethics committee.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Linda A. J. Hendricks, Katja C. J. Verbeek.
Supplementary information
The online version contains supplementary material available at 10.1038/s44276-025-00157-y.
References
- 1.Brohet RM, Velthuizen ME, Hogervorst FB, Meijers-Heijboer HE, Seynaeve C, Collée MJ, et al. Breast and ovarian cancer risks in a large series of clinically ascertained families with a high proportion of BRCA1 and BRCA2 Dutch founder mutations. J Med Genet. 2014;51:98–107. [DOI] [PubMed] [Google Scholar]
- 2.Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. Jama. 2017;317:2402–16. [DOI] [PubMed] [Google Scholar]
- 3.Nelen MR, Kremer H, Konings IB, Schoute F, van Essen AJ, Koch R, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet. 1999;7:267–73. [DOI] [PubMed] [Google Scholar]
- 4.Pilarski R PTEN Hamartoma Tumor Syndrome: A Clinical Overview. Cancers (Basel). 2019;11. [DOI] [PMC free article] [PubMed]
- 5.Hendricks LAJ, Hoogerbrugge N, Mensenkamp AR, Brunet J, Lleuger-Pujol R, Høberg-Vetti H, et al. Cancer risks by sex and variant type in PTEN hamartoma tumor syndrome. J Natl Cancer Inst. 2023;115:93–103. [DOI] [PubMed] [Google Scholar]
- 6.Malentacchi F, Turrini I, Sorbi F, Projetto E, Castiglione F, Fambrini M, et al. Pilot investigation of the mutation profile of PIK3CA/PTEN genes (PI3K pathway) in grade 3 endometrial cancer. Oncol Rep. 2019;41:1560–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith IN, Briggs JM. Structural mutation analysis of PTEN and its genotype-phenotype correlations in endometriosis and cancer. Proteins. 2016;84:1625–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebok P, Kopperschmidt V, Kluth M, Hube-Magg C, Özden C, B T, et al. Partial PTEN deletion is linked to poor prognosis in breast cancer. BMC Cancer. 2015;15:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beg S, Siraj AK, Prabhakaran S, Jehan Z, Ajarim D, Al-Dayel F, et al. Loss of PTEN expression is associated with aggressive behavior and poor prognosis in Middle Eastern triple-negative breast cancer. Breast Cancer Res Treatment. 2015;151:541–53. [DOI] [PubMed] [Google Scholar]
- 10.Tang L, Li X, Gao Y, Chen L, Gu L, Chen J, et al. Phosphatase and tensin homolog (PTEN) expression on oncologic outcome in renal cell carcinoma: A systematic review and meta-analysis. PLoS One. 2017;12:e0179437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saal LH, Johansson P, Holm K, Gruvberger-Saal SK, She QB, Maurer M, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci USA. 2007;104:7564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2018;97:1269–74. [DOI] [PubMed] [Google Scholar]
- 13.Burrows N, Williams J, Telfer BA, Resch J, Valentine HR, Fitzmaurice RJ, et al. Phosphatidylinositide 3-kinase (PI3K) and PI3K-related kinase (PIKK) activity contributes to radioresistance in thyroid carcinomas. Oncotarget. 2016;7:63106–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Xu J, Fu J, Yuan D, Guo F, Zhou C, et al. MiR-29a regulates radiosensitivity in human intestinal cells by targeting PTEN gene. Radiat Res. 2016;186:292–301. [DOI] [PubMed] [Google Scholar]
- 15.Sizemore GM, Balakrishnan S, Thies KA, Hammer AM, Sizemore ST, Trimboli AJ, et al. Stromal PTEN determines mammary epithelial response to radiotherapy. Nat Commun. 2018;9:2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brana I, Pham NA, Kim L, Sakashita S, Li M, Ng C, et al. Novel combinations of PI3K-mTOR inhibitors with dacomitinib or chemotherapy in PTEN-deficient patient-derived tumor xenografts. Oncotarget. 2017;8:84659–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treatment. 2011;128:447–56. [DOI] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic (Version 1.2020) [Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. [DOI] [PubMed]
- 19.Di Carlo VRB, Bannon F, Woods LM, Maringe C, Bonaventure A, Coleman MP, et al. Life tables for the CONCORD programme. Available from: http://csg.lshtm.ac.uk/life-tables, downloaded on March 2023.
- 20.Belgian Cancer Registry, Brussels, 2023.
- 21.Dutch Cancer Registry. Dutch Cancer Figures 2023. www.cijfersoverkanker.nl.
- 22.Carbognin L, Miglietta F, Paris I, Dieci MV. Prognostic and Predictive Implications of PTEN in Breast Cancer: Unfulfilled Promises but Intriguing Perspectives. Cancers (Basel). 2019;11:1401. [DOI] [PMC free article] [PubMed]
- 23.Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15:222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Öfverholm A, Einbeigi Z, Wigermo A, Holmberg E, Karsson P. Increased overall mortality even after risk reducing surgery for BRCA-positive women in Western Sweden. Genes (Basel). 2019;10:1046. [DOI] [PMC free article] [PubMed]
- 25.Huszno J, Kołosza Z, Grzybowska E. BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. Oncol Lett. 2019;17:1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt MK, van den Broek AJ, Tollenaar RA, Smit VT, Westenend PJ, Brinkhuis M, et al. Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst. 2017;109. 10.1093/jnci/djw329. [DOI] [PubMed]
- 27.Lee EH, Park SK, Park B, Kim SW, Lee MH, Ahn SH, et al. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122:11–25. [DOI] [PubMed] [Google Scholar]
- 28.Forjaz de Lacerda G, Howlader N, Mariotto AB. Differences in cancer survival with relative versus cause-specific approaches: an update using more accurate life tables. Cancer Epidemiol Biomarkers Prev. 2019;28:1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Withrow DR, Pole JD, Nishri ED, Tjepkema M, Marrett LD. Choice of relative or cause-specific approach to cancer survival analysis impacts estimates differentially by cancer type, population, and application: evidence from a Canadian population-based cohort study. Popul Health Metr. 2017;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–74. [DOI] [PubMed] [Google Scholar]
- 32.Corradini S, Reitz D, Pazos M, Schönecker S, Braun M, Harbeck N, et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: outcome comparison of 7565 cases. Cancers (Basel). 2019;11:160. [DOI] [PMC free article] [PubMed]
- 33.Emiroglu SÖE, Cabıoglu N, Igci A, Saip P, Yazici H, Ozmen T, et al. Is breast conserving surgery efficacious in breast cancer patients with BRCA1 or BRCA2 germline mutation? Breast Cancer (Dove Med Press). 2023;15:163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. Bmj. 2014;348:g226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Oldenburg HSA, Rutgers EJ, Russell NS, et al. Prognostic impact of breast-conserving therapy versus mastectomy of BRCA1/2 mutation carriers compared with noncarriers in a consecutive series of young breast cancer patients. Ann Surg. 2019;270:364–72. [DOI] [PubMed] [Google Scholar]
- 36.Ngeow J, Stanuch K, Mester JL, Barnholtz-Sloan JS, Eng C. Second malignant neoplasms in patients with Cowden syndrome with underlying germline PTEN mutations. J Clin Oncol. 2014;32:1818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1194–220. [DOI] [PubMed] [Google Scholar]
- 38.Friedenreich CM, Cook LS, Wang Q, Kokts-Porietis RL, McNeil J, Ryder-Burbidge C, et al. Prospective cohort study of pre- and postdiagnosis physical activity and endometrial cancer survival. J Clin Oncol. 2020;38:4107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer S, Husson O, Tomaszewska IM, Locati LD, Kiyota N, Scheidemann-Wesp U, et al. Quality-of-life priorities in patients with thyroid cancer: a multinational European Organisation for Research and Treatment of Cancer Phase I Study. Thyroid®. 2016;26:1605–13. [DOI] [PubMed] [Google Scholar]
- 40.Steele SR, Park GE, Johnson EK, Martin MJ, Stojadinovic A, Maykel JA, et al. The impact of age on colorectal cancer incidence, treatment, and outcomes in an equal-access health care system. Dis Colon & Rectum. 2014;57:303–10. [DOI] [PubMed]
- 41.Thorstenson A, Bergman M, Scherman-Plogell A-H, Hosseinnia S, Ljungberg B, Adolfsson J, et al. Tumour characteristics and surgical treatment of renal cell carcinoma in Sweden 2005–2010: a population-based study from the National Swedish Kidney Cancer Register. Scandinavian Journal of Urology. 2014;48:231–8. [DOI] [PubMed] [Google Scholar]
- 42.Plym A, Ullenhag GJ, Breivald M, Lambe M, Berglund A. Clinical characteristics, management and survival in young adults diagnosed with malignant melanoma: A population-based cohort study. Acta Oncologica. 2014;53:688–96. [DOI] [PubMed] [Google Scholar]
- 43.Tejerizo-García A, Jiménez-López JS, Muñoz-González JL, Bartolomé-Sotillos S, Marqueta-Marqués L, López-González G, et al. Overall survival and disease-free survival in endometrial cancer: prognostic factors in 276 patients. Onco Targets Ther. 2013;9:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felix AS, Brinton LA. Cancer progress and priorities: uterine cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tischkowitz M, Colas C, Pouwels S, Hoogerbrugge N. Cancer Surveillance Guideline for individuals with PTEN hamartoma tumour syndrome. Eur J Hum Genet. 2020;28:1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drissen M, Vos JR, van der Biessen-van Beek DTJ, van der Post RS, Nagtegaal ID, van Kouwen MCA, et al. Detection and yield of colorectal cancer surveillance in adults with PTEN hamartoma tumour syndrome. Cancers (Basel). 2022;14:4005. [DOI] [PMC free article] [PubMed]
- 47.Smolarz B, Nowak AZ, Romanowicz H. Breast cancer-epidemiology, classification, pathogenesis and treatment (Review of Literature). Cancers (Basel). 2022;14:2569. [DOI] [PMC free article] [PubMed]
- 48.Vaccarella S, Georges D, Bray F, Ginsburg O, Charvat H, Martikainen P, et al. Socioeconomic inequalities in cancer mortality between and within countries in Europe: a population-based study. Lancet Reg Health Eur. 2023;25:100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual patient data that support the findings of this study are not openly available due to privacy and ethical reasons. Aggregate study data are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Radboud university medical centre.