Abstract
RNA guanine (G)-quadruplexes (rG4s) are noncanonical structures formed by G-rich RNA sequences and have been demonstrated to play critical roles in various biological events, including translation, transcription, RNA processing, and other cellular functions. In contrast to DNA G-quadruplexes (dG4s), research on rG4s has been relatively limited until recently. Recent advances in targeting and imaging of rG4s have opened new avenues for understanding their functional significance and therapeutic potential. In this review, we summarize the innovative platforms and tools being developed to target rG4s, highlight the novel and important imaging probes that have been generated and applied for rG4 structure visualization in different biological contexts, and discuss the challenges and perspectives for further advancing these technologies and toolsets to facilitate rG4 targeting and imaging with greater precision and resolution across the Tree of Life. These scientific developments and breakthroughs will enable the discovery of new biological insights regarding rG4s and help decipher their molecular mechanisms and implications for health and disease.
Keywords: RNA, G-quadruplex structure, targeting, imaging, nucleic acids
INTRODUCTION
RNA, or ribonucleic acid, is a fundamental biomolecule characterized by its unique structure, which is pivotal to its diverse functions in biological systems. The presence of ribose in RNA, as opposed to the deoxyribose found in DNA, contributes to RNA's increased reactivity and flexibility (Sharp 2009; Elliott and Ladomery 2016; Cech et al. 2018). The single-stranded nature of RNA allows it to fold into intricate three-dimensional shapes, which are essential for its functionality (Assmann et al. 2023; Cao et al. 2024). These secondary structures, such as hairpins, pseudoknots, and G-quadruplexes, arise from base-pairing within the strand itself, enabling RNA to perform various regulatory roles beyond mere information transfer (Qian et al. 2019; Wang et al. 2021b; Spitale and Incarnato 2023; Cao et al. 2024). Understanding RNA structure is crucial for unraveling its multifaceted roles in cellular processes and its implications in health and disease (Bernat and Disney 2015; Spitale and Incarnato 2023).
Among the diverse RNA structure motifs, RNA Guanine (G)-quadruplexes (rG4s) are unique four-stranded nucleic acid structures formed primarily by G-rich sequences (Fig. 1A). These structures are stabilized by the formation of G-quartets (Fig. 1A), where four guanine bases interact through Hoogsteen hydrogen bonds (Kwok et al. 2018). While DNA G4s (dG4s) can adopt various topologies, including parallel and antiparallel arrangements, depending on the orientation of the strands and the loop lengths connecting them (Fig. 1A,B), most of the rG4s identified so far are of parallel topology, with a few exceptions reported recently (Varshney et al. 2020; Lyu et al. 2021). The structural diversities of rG4s can be exemplified by their many structural subtypes, including but not limited to the canonical (G3L1–7)4 (rG4s with four tracts of three consecutive Gs and loop length 1–7 nt), long loop rG4s, bulge-containing rG4s, and two-layer G-quartet rG4s, poly(UG) (pUG) fold that have been reported using transcript-specific and transcriptome-wide rG4 structure detection methods (Guo and Bartel 2016; Kwok et al. 2016; Lyu et al. 2021; Roschdi et al. 2022). Recent studies have indicated that rG4s are dynamic in cells, and their folding can be controlled by stress, developmental stages, proteins, and other factors (Yang et al. 2018, 2020, 2022; Chen et al. 2021a; Kharel et al. 2023; Lee et al. 2024).
FIGURE 1.
Structural diversities and biological functions of RNA G-quadruplexes. (A) The chemical structure of a G-quartet and the representative topologies of intermolecular and intramolecular G-quadruplex structures. (B) Representative three-dimensional structure of RNA G-quadruplexes. Protein Data Bank (PDB ID): 2KBP, 8X0S, and 6K84. (C) Representative biological roles of RNA G-quadruplexes in both coding mRNAs and noncoding RNAs (Dumas et al. 2021; Caterino and Paeschke 2022; Kharel et al. 2023; Sahayasheela and Sugiyama 2024; Shukla and Datta 2024).
Also, recent research findings suggested that besides intramolecular G4s, intermolecular G4 can be identified in cells, for example, the hybrid G4 structure formed by TERRA DNA (Moye et al. 2015). Specific proteins were characterized and demonstrated to show selectivity toward intermolecular G4s over intramolecular G4s, expanding the G4 binding proteins (G4-BP) list that has been summarized by a few recent comprehensive reviews (Dumas et al. 2021; Lyu et al. 2021; Frasson et al. 2022; Dai et al. 2023; Antariksa and Di Antonio 2024). For example, Cockayne Syndrome B (CSB) exhibits picomolar binding affinity toward intermolecular G4s in ribosomal DNA (rDNA) but negligible binding against intramolecular G4s via electrophoretic mobility shift assay (EMSA) (Liano et al. 2021). Many of these G4-BPs have been functionally characterized to carry out important regulatory roles in many biological processes including translation, splicing, transcription, and others for different classes of RNA (Fig. 1C; Fay et al. 2017; Dumas et al. 2021; Lyu et al. 2021; Bourdon et al. 2023; Dai et al. 2023). Moreover, these rG4s and G4-BPs were reported to be associated with diseases such as cancer and neurological disorders (Cammas and Millevoi 2017; Kharel et al. 2020; Kosiol et al. 2021; Wang et al. 2021a; Xu et al. 2021; Vijay Kumar et al. 2023); for example, the cancer-related gene TERRA rG4 shown to interact with several G4-BPs such as TRF2, and alter telomere maintenance (Cusanelli and Chartrand 2015). Also, the MALAT1 long noncoding RNAs (lncRNAs) interact with nucleolin within nuclear speckles and potentially influence pre-mRNA splicing (Ghosh et al. 2023). Besides, repeat rG4s, such as (GGGGCC)4 sequence, forming in C9orf72 gene is suggested to interact with cellular proteins in neurodegenerative diseases pathology (Reddy et al. 2013; Zamiri et al. 2014; Wortman et al. 2020).
Over the next few years, there will be a surge of interesting studies and exciting applications in the area of rG4 structure targeting and imaging, which will likely improve our understanding of the important roles of rG4s in cells and their potential to be promising targets for biomedical applications. This review aims to provide an extensive summary and critical analysis of the recent advances in the rG4 fields, in particular on rG4 targeting and imaging in different biological settings, which are currently missing in the latest reviews. We summarize the strategies and toolsets used to recognize rG4 targets, and also highlight the original imaging and tools developed and used to enable rG4 detection and biosensing in different cellular contexts. For each respective topic, we also discuss the current challenges and future perspectives to address the long-standing questions in these associated research areas in the rG4 field.
RNA G-QUADRUPLEX STRUCTURE TARGETING PLATFORMS AND TOOLS
Various rG4-targeting tools with distinct properties and applications, including small molecules, peptides, aptamers, and antibodies, have been developed (Banerjee et al. 2022), alongside rapidly advancing platforms for identifying rG4-binding ligands (Monsen and Trent 2018; Umar et al. 2022). In this part, we describe recent representative examples from each class, focusing on their interactions with diverse rG4 targets and regulatory effects on rG4-mediated cellular processes (Fig. 2; Table 1).
FIGURE 2.
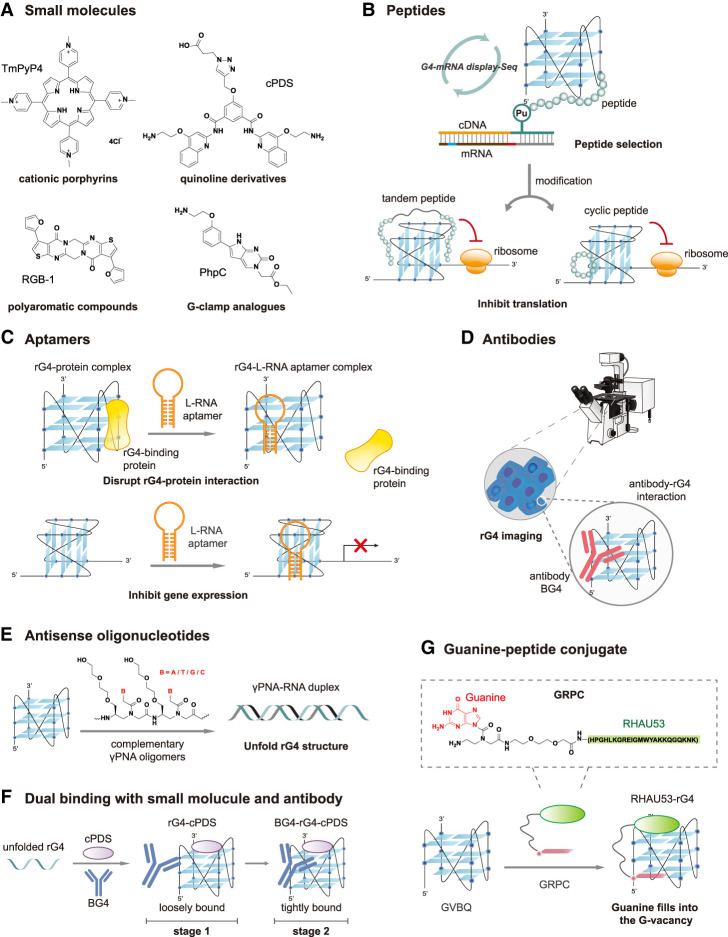
Diverse tools, including small molecules, peptides, aptamers, antibodies, and derived combinatorial tools, are used to target rG4s and regulate rG4-mediated gene expression. (A) Representative small molecules with different scaffolds for rG4 targeting, such as cationic porphyrins (TmPyP4), quinoline derivatives (cPDS), polyaromatic compounds (RGB-1), and guanine-clamp analogs (PhpC). (B) De novo peptides specifically target G4 structures selected by G4-mRNA display-seq. An mRNA library was linked to puromycin linker and translated in vitro to create the mRNA-peptide fusion library, followed by reverse-transcription and screening against an rG4 target to isolate functional sequences. The tandem and cyclic version of peptide showed enhanced binding to rG4 and downregulated rG4-mediated gene expression. (C) L-RNA aptamers bind to rG4, leading to disruption of rG4–protein interaction and inhibition of gene expression. (D) Antibody BG4 binding to rG4 for rG4 imaging in cells via confocal microscopy. Icon adapted from “confocal-scanning-laser-microscope-CSLM” by DBCLS (https://togotv.dbcls.jp/en/pics.html) is licensed under CC-BY 4.0 Unported (https://creativecommons.org/licenses/by/4.0/). (E) Antisense oligonucleotides composed of γPNA oligomers potently invade and destabilize rG4, resulted in formation of γPNA–RNA duplex. (F) Dual binding of an rG4 ligand cPDS and an antibody BG4 to TERRA rG4 in a two-stage manner. cPDS initially binds competitively to TERRA rG4, followed by a slow conformational rearrangement of ternary complex with BG4. (G) A bifunctional guanine-RHAU23 peptide conjugate (GRPC) for G-vacancy-bearing G-quadruplexes (GVBQs) targeting. The guanine group fills into the G-vacancy in a GVBQ, facilitating the binding of RHAU23 to the GVBQs.
TABLE 1.
Representative list of rG4 structure-targeting tools with their binding characteristics and biological effects
| Name | Target rG4 | Biophysical approaches | Biological effects | Reference |
|---|---|---|---|---|
| Small molecules | ||||
| TMPyP4 | NRAS rG4, KRAS rG4 | UV-Vis and fluorescence titrations, Job plot (target:ligand = 4:1), fluorescence spectroscopy (Kd = 301 ± 45 nM [NRAS]) | Cell lines used: Panc-1; inhibit KRAS and NRAS expression. | Ferino et al. 2020 |
| TERRA rG4 | UV-Vis absorption spectroscopy (redshift: 20 nm, hypochromicity: 35%), fluorescence spectroscopy (Kd = 1.92 × 106 M−1), induced circular dichroism (ICD), extensive triplet decay kinetics experiments, Job plot (1:1), NMR | NA | Qi et al. 2019 | |
| miRNA-1587 G4 | Circular dichroism (CD) | Cell lines used: HeLa; unwind G4 and enhances miRNA-1587 regulation of the target mRNA. | Li et al. 2019 | |
| Pre-miRNA-149 G4 | CD titrations, UV-Vis absorption spectroscopy (redshift: 20 nm, hypochromicity: 65%, Kd = 240 nM), Isothermal Titration Calorimetry (ITC), NMR | Cell lines used: MCF-7; restore endogenous miRNA-149 expression and inhibit cell proliferation. | Ghosh et al. 2019 | |
| FMR1 r(CGG) n G4 | Native gel electrophoresis | Cell lines used: HEK293; unwind G4 and enhance mRNA expression. | Ofer et al. 2009 | |
| MT3-MMP rG4 | UV-Vis absorption spectroscopy (redshift: 22 nm, hypochromicity: 75%), CD, NMR, native gel electrophoresis | Cell lines used: HeLa; unwind G4 and enhance reporter gene expression. | Morris et al. 2012 | |
| C9orf72 r(GGGGCC)8 repeat G4 | Native gel electrophoresis, visible absorption spectroscopy (redshift: 17 nm, hypochromicity: 64%), CD, UV melting | Unwind G4 and block interaction of RNA-binding proteins. | Zamiri et al. 2014 | |
| SARS-CoV-2 rG4 | CD melting (ΔTm = 23.8°C) | Cell lines used: Vero E6; inhibit SARS-CoV-2 RNA expression, SARS-CoV-2 infection (EC50 = 8.87 μM), and lung inflammation in virus-infected animal models. | Qin et al. 2022 | |
| Zaire ebolavirus L rG4 | CD melting (ΔTm = 11.9°C), competitive fluorescence spectroscopy, native gel electrophoresis | Cell lines used: BSR-T7; inhibit EBOV L-RNA expression. | Wang et al. 2016b | |
| HCV rG4 | CD melting, native gel electrophoresis | Cell lines used: HEK293, Huh-7.5.1; stabilize HCV rG4s and inhibit HCV C gene expression and HCV replication in host cells. | Wang et al. 2016a | |
| rG4 in 3′ UTR of immediate early gene (IE180) of PRV | CD, NMR | Cell lines used: HEK293, PK-15; destabilize IE180 rG4, inhibit IE180 expression and PRV replication. | Zhang et al. 2020b | |
| LANA rG4 of Kaposi's sarcoma-associated herpes virus (KSHV) | NA | Cell lines used: BCBL-1, BJAB-LYFP, B3Z, HEK293; inhibit LANA expression. | Dabral et al. 2020 | |
| ZIKV rG4 | ITC | Cell lines used: CCL-81; inhibit ZIKV growth, viral protein synthesis and genome replication in host cells. | Majee et al. 2021 | |
| TMPyP4-C14 | KRAS rG4 | UV-Vis titrations (Kd = 4.7 [±0.6] × 10−7 M [utr1], 3.4 [±0.1] × 10−7 M [utr2]), fluorescence quenching titrations (Kd = 2.8 [±0.5] × 10−7 M [utr2]) | Cell lines used: Panc-1; inhibit endogenous KRAS expression and cell growth. | Faudale et al. 2012 |
| Alkyl cationic porphyrins 2b/2d | KRAS rG4, NRAS rG4 | KRAS rG4: UV-Vis and fluorescence titrations (redshift: 13 nm, hypochromicity: ca. 50% [2b]), Job plot (target:ligand = 6:1 [2b], 9:1 [2d]); NRAS rG4: fluorescence spectroscopy (Kd = 107 ± 11 nM [2b], 32 ± 8 nM [2d]) | Cell lines used: Panc-1; inhibit KRAS and NRAS expression, metabolic activity of pancreatic cancer cells (EC50 (48 h) = 13.7 ± 0.7 nM [2b], 16.4 ± 2.1 nM [2d]), and the growth of a Panc-1 xenograft in SCID mice. | Ferino et al. 2020 |
| PDS | EWSR1 rG4 | Fluorescence titrations (Kd = 4.6 µM) | Cell lines used: TC32, SKNMC, HCT116, PC3, HEK-293T, RD-ES, TC71; block interaction of rG4-binding protein (IC50 = 7.7 μM) and inhibit cell viability (IC50 = 14 μM [TC32], 4.8 μM [SKNMC]). | Neckles et al. 2019 |
| HNF4a rG4 | NA | Inhibit HNF4a expression. | Guo and Lu 2017 | |
| EBNA1 rG4 | FRET melting | Cell lines used: HEK293, B3Z; stabilize EBNA1 rG4 and inhibit EBNA1 expression and antigen presentation. | Murat et al. 2014 | |
| ZIKV rG4 | Surface plasmon resonance (KA = 107–108 M−1), CD melting (ΔTm = 4.6°C–21°C), ITC, immunofluorescence assay (IFA) | Inhibit ZIKV infection (EC50 = 4.2 ± 0.4 μM), genome replication, and protein expression. | Zou et al. 2021 | |
| TMPRSS2 rG4 | FRET melting (ΔTm = 3.48°C), CD melting (ΔTm = 4.00°C), bio-layer interferometry (BLI) assay (Kd = 604.9 ± 2.84 nM), IFA | Cell lines used: H1299, HBE, LLC; inhibit TMPRSS2 expression and SARS-CoV-2 infection in mouse models. | Liu et al. 2022 | |
| cPDS | TERRA rG4 | FRET melting (ΔTm = 20.7 ± 0.1 K) | NA | Di Antonio et al. 2012 |
| PDP | HCV rG4 | FRET kinetic assay, native gel electrophoresis | Cell lines used: HEK-293, Huh-7.5.1; stabilize RNA G4s and inhibit HCV C gene expression and HCV replication in host cells. | Wang et al. 2016a |
| SARS-CoV-2 rG4 | CD melting (ΔTm = 13.9°C), native gel electrophoresis, IFA | Cell lines used: HeLa, HEK293T; inhibit SARS-CoV-2 RNA expression. | Zhao et al. 2021; Qin et al. 2022 | |
| 12459 | hTERT rG4 | Native gel electrophoresis | Alter hTERT alternative RNA splicing. | Gomez et al. 2004 |
| 360A | TRF2 rG4 | Competition FRET | Inhibit the reporter gene expression. | Gomez et al. 2010 |
| Phen-DC3 | TRF2 rG4 | Competition FRET | Inhibit the reporter gene expression. | Gomez et al. 2010 |
| EBNA1 rG4 | Pull-down assay | Cell lines used: H1299, Mutu-1, NPC-6661; block nucleolin–EBNA1 rG4 interaction and promote endogenous EBNA1 expression in EBV-infected cells. | Lista et al. 2017 | |
| HCV110-131 rG4 | FRET melting (ΔTm = 7.5°C), CD melting | Cell lines used: Huh7; inhibit HCV viral replication. | Jaubert et al. 2018 | |
| Pre-miRNA-149 G4 | Molecular docking simulations | NA | Carvalho et al. 2020 | |
| C8, C8-NH2, phenN2, phen2N4, PDS | Pre-miRNA-149 G4 | Molecular docking simulations | NA | Carvalho et al. 2020 |
| PyDH2 and PhenDH2 series | EBNA1 rG4 | Fluorescence melting, fluorescent indicator displacement (FID) assay (DC50 = 0.26 ± 0.05 μM [PyDH2], 0.30 ± 0.03 μM [PhenDH2]), pull-down assay | Cell lines used: H1299, Mutu-1; block nucleolin–EBNA1 rG4 interaction and enhance antigen presentation. | Reznichenko et al. 2019 |
| RR110 | NRAS rG4 | NMR, agarose gel electrophoresis | Inhibit NRAS expression. | Bugaut et al. 2010 |
| ZnAPC | NRAS rG4 | Visible absorption spectroscopy (Kd = 3.1 ± 0.3 μM), fluorescence spectroscopy, native gel electrophoresis | Cell lines used: MCF-7; photo-cleave NRAS rG4 and inhibit NRAS expression and cell viability. | Kawauchi et al. 2018 |
| Jatrorrhizine derivatives | miRNA-1587 G4 | Electrospray ionization mass spectrometry (ESI-MS) | Induce dimeric miRNA-1587 G4 formation. | Tan et al. 2018 |
| Pseudopalmatine | miRNA-1587 G4 | CD melting (ΔTm = 8°C) | Cell lines used: HeLa; inhibit miRNA-1587 regulation of the target mRNA. | Li et al. 2019 |
| RGB-1 | TERRA rG4 | Size exclusion chromatography (Kd = 5.9 μM, stoichiometry = 1:1.2), CD melting (ΔTm ≥ 5°C) | Cell lines used: HEK293; inhibit TERRA expression. | Katsuda et al. 2016 |
| NRAS rG4 | CD melting | Cell lines used: HEK293; inhibit endogenous NRAS expression. | Katsuda et al. 2016 | |
| PNADOTASQ | TERRA rG4, TRF2 rG4, VEGF rG4 | FRET melting (ΔTm = 21.2°C [TERRA], 10.1°C [TRF2], 12.0°C [VEGF]) | NA | Haudecoeur et al. 2013 |
| Octenidine | NRAS rG4 | FID (DC50 = 1.6 μM), fluorescence quenching (FQ) assay (Kd = 0.49 ± 0.03 μM), CD melting, native gel electrophoresis | Cell lines used: SK-MEL-2; block DHX36/NRAS rG4 interactions (IC50= 2.4 ± 0.4 μM), inhibit NRAS expression and cancer cells viability (IC50 = 2.34 ± 0.09 μM), and suppress tumor growth in mouse models. | Chen et al. 2023 |
| NMM | Influenza A Virus (IAV) rG4 | NMR (stoichiometry = 1:1), native gel electrophoresis | NA | Tomaszewska et al. 2021 |
| BRACO-19 | ZIKV rG4 | Fluorescence spectroscopy (>100-fold), ITC, CD melting (ΔTm = 3.6°C–13.1°C) | Cell lines used: CCL-81; inhibit ZIKV growth, viral protein synthesis, and genome replication in host cells. | Majee et al. 2021 |
| HIV-1 U3 rG4 | CD | Cell lines used: MT-4; inhibit the reverse transcription and block HIV-1 rG4–protein interaction. | Perrone et al. 2014; Butovskaya et al. 2019 | |
| EBNA1 rG4 | Native gel electrophoresis | Cell lines used: Raji, HeLa, BJAB, DG75; inhibit viability of EBV-positive cells and block functions of EBNA1. | Norseen et al. 2009 | |
| Topotecan (TPT) and Berbamine (BBM) | rG4s in multiple SARS-CoV-2 host factors, TERRA rG4 | In silico virtual docking (TERRA rG4), microscale thermophoresis (TPT: Kd = 5.20 nM [TERRA], 383.01 nM [Tmprss2], 29.81 nM [Ace2]; BBM: Kd = 11.55 nM [TERRA], 10.78 nM [Tmprss2], 264.76 nM [Ace2]) | Cell lines used: H1299, Vero-E6, hACE2-293T; inhibit the endogenous expression of SARS-CoV-2 host factors and block SARS-CoV-2 pseudovirus entry in cells and in mouse models. | Tong et al. 2023 |
| DANC | TERRA rG4 | CD titrations, fluorescence spectroscopy, FRET, native gel electrophoresis | Unwind rG4 and promote G4 nucleic acid processivity. | Liu et al. 2024 |
| PhpC | NRAS rG4 | Fluorescence quenching assay, competitive FRET melting (ΔΔTm = −0.9°C at 1:1 ratio) | Cell lines used: MCF7; unwind rG4 and enhance gene expression. | Mitteaux et al. 2024 |
| 2′-F C3 | rG4 | FRET, NMR | Cell lines used: HEK293FT; unwind rG4 and enhance gene expression. | Teng et al. 2024 |
| F1 | DHX15 rG4 | SPR (Kd = 12.6 ± 1.0 μM, stoichiometry [target:ligand] = 1:2), FID, CD melting (ΔTm = 3.6°C ± 0.3°C) | Inhibit DHX15 expression (IC50 = 22.9 ± 3.8 μM). | Prestwood et al. 2024 |
| Peptides | ||||
| RHAU | rG4 | Native gel electrophoresis (Kd = 14 nM [tetramolecular rAGA]) | Unwind rG4. | Lattmann et al. 2010; Truong et al. 2020 |
| RGG | sc1 rG4 | NMR (Kd = 3.8 nM) | NA | Phan et al. 2011 |
| TERRA rG4 | Native gel electrophoresis (Kd = 11 ± 1 nM) | NA | Yagi et al. 2018 | |
| Shank1a rG4, postsynaptic density protein 95 (PSD-95) rG4 | Native gel electrophoresis, fluorescence spectroscopy (Kd = 271 ± 21 nM [Shank1a], 92 ± 9 nM [PSD-95]), pull-down assay | NA | Imperatore et al. 2020 | |
| TLSRGG3Y | TERRA rG4 | Native gel electrophoresis, ITC (Kd = 10 ± 1 nM) | NA | Takahama and Oyoshi 2013 |
| MTD3/MTD14-RGG | rG4 | Native gel electrophoresis (Kd = 35 ± 12 nM), CD | Methylate G4-forming RNAs. | Yoshida et al. 2022 |
| TZIP | C2(G4C2)4 of C9orf72 | Native gel electrophoresis (Kd = 77 ± 12 nM), CD | Unwind rG4 and induce formation of high order structure. | Wortman et al. 2020 |
| Pep 11 | hTERC rG4 | MST (Kd = 1.37 ± 0.22 μM [pep11], 449 ± 62 nM [tandem pep11], 377 ± 35 nM [cyclic pep11]), fluorescence spectroscopy, filter binding assay, FRET melting | Cell lines used: HeLa; inhibit reporter gene expression. | Mou and Kwok 2023 |
| Aptamers | ||||
| L-Ap3-7 | rG4 | Native gel electrophoresis (Kd = 99.0 ± 20.3 nM [TERRA rG4]) | Block TERRA rG4–RHAU53 interaction (IC50 = 975.3 ± 1.1 nM). | Chan and Kwok 2020 |
| L-Apt.4-1c | rG4 | Native gel electrophoresis (Kd = 74.9 ± 7.5 nM [hTERC rG4]), MST (Kd = 59.1 ± 11.9 nM [hTERC rG4]) | Inhibit hTERC rG4–nucleolin interaction (IC50 = 1.69 ± 0.1 μM). | Umar and Kwok 2020 |
| L-Apt.8f | APP rG4 | Native gel electrophoresis (Kd = 24.06 ± 2.90 nM), MST (Kd = 12.76 ± 2.76 nM in 1 mM MgCl2) | Cell lines used: HeLa; inhibit APP expression. | Zhao et al. 2022 |
| L-Apt12-6 | parallel dG4 and rG4 | Native gel electrophoresis | NA | Ji et al. 2023 |
| L-Apt.1-1 | APP rG4 | Native gel electrophoresis (Kd = 12.48 ± 2.82 nM in 1 mM MgCl2) | Cell lines used: HeLa; inhibit endogenous APP expression. | Lau et al. 2024 |
| L-Apt.T8, L-Apt.T8-10D | HIV-1 U3-III rG4 | Native gel electrophoresis (Kd = 58.1 ± 4.9 nM [L-Apt.T8], 12.5 ± 5.17 nM [L-Apt.T8-10D]), MST (Kd = 54.0 ± 15.6 nM [L-Apt.T8], 8.5 ± 1.50 nM [L-Apt.T8-10D]) | Inhibit U3-III-tRNA3Lys segment interaction (IC50 = 87.9 ± 13.1 nM), U3-III–nucleocapsid interaction (IC50 = 124 ± 17.3 nM), and in vitro HIV-1 minus strand transfer (IC50 = 2.06 ± 0.50 μM). | Feng and Kwok 2024 |
| L-Apt1-12 | EBNA 1 rG4 | Native gel electrophoresis (Kd = 107.4 ± 10.4 nM), MST (Kd = 105.6 ± 30.6 nM) | Cell lines used: HEK293T, C666-1, NPC43, SNU719; inhibit endogenous EBNA1 expression and growth of EBV-positive cancer cells. | Ji et al. 2024 |
| L-apt3.1 | pUG fold | Native gel electrophoresis (Kd = 13.5 ± 2.8 nM) | Inhibit gene silencing in Caenorhabditis elegans. | Liew et al. 2025 |
| Antibody | ||||
| BG4 | TERRA rG4, NRAS rG4, BCL2 rG4 | Native gel electrophoresis (Kd = 18.0 ± 2.1 nM [TERRA], 5.5 ± 0.6 nM [NRAS], 6.5 ± 0.9 nM [BCL2]) | Cell lines used: HUVEC, MRC5, GM847, U2OS, HeLa; visualization of rG4. | Biffi et al. 2014 |
| Other tools | ||||
| dAS2 | EBNA 1 rG4 | Fluorescent trap assays, NMR, native gel electrophoresis, CD titrations | Cell lines used: HEK293E, HEK293KbC2, B3Z; unwind rG4 and enhance endogenous EBNA 1 expression and antigen presentation. | Murat et al. 2014 |
| Anti-H2G4 ASO | H2AFY rG4 | Native gel electrophoresis | Cell lines used: HEK293, Caco-2; unwind rG4 and enhance H2AFY expression. | Rouleau et al. 2015 |
| P7C and P7H | rG4 in aptamer RDQ | UV melting (ΔTm = ∼30°C [P7H]), CD, fluorescence spectroscopy (Kd = 8.1 × 108 M−1 [P7C]), Job plot (target:ligand = 1:1 [P7C], 1:2 [P7H]) | Affect G4 folding. | Marin and Armitage 2005 |
| γPNA | rG4 | SPR (Kd = 4.2 nM [γ5′], 1.9 nM [γ3′], 2.8 nM [γCen′]) | Inhibit reporter gene expression (IC50 [37°C)] = 15 nM [γ5′], 75 nM [γ3′], 90 nM [γCen′]). | Oyaghire et al. 2016 |
| WNV NS5B rG4 | CD, thermal difference spectroscopy (TDS), UV melting (ΔTm ≥ 37°C), fluorescence spectroscopy (Kd [37°C] = [2.1 ± 0.3] × 10−16 M) | Unwind rG4 | Sarkar and Armitage 2021 | |
| GRPC | G-vacancy-bearing rG4: MYOG-3332, MYOG-3333, ABTB2-3233 | CD, photo-cross-linking, FRET melting (ΔTm =41.2°C [MYOG-3332], 26°C [ABTB2-3233]), native gel electrophoresis (Kd = 40.6 ± 13.2 nM [MYOG-3333]) | Stabilize rG4 and inhibit in vitro RNA reverse transcription (IC50 = 0.14 μM [MYOG-3332], 0.065 μM [ABTB2-3233]) and RNA translation. | He et al. 2020 |
Small molecules that target RNA G-quadruplex structure
Cationic porphyrins are widely recognized as effective binders of nucleic acids (Pasternack et al. 1983; Pasternack and Gibbs 1996). The capacity of these compounds to bind DNA G4s was first identified in the 1990s (Wheelhouse et al. 1998). Cationic porphyrins, which possess large planar surfaces, are ideally suited for π–π stacking interactions with G-quartets at the terminal of G4s. For example, N-methyl mesoporphyrin IX (NMM) demonstrates favorable interactions with G4 structures compared to duplex DNAs and single-stranded DNAs through competitive dialysis assays (Ren and Chaires 1999). The significant interaction of cationic porphyrins with DNA G4s has led to their use in targeting RNA G-quadruplexes as well. To date, cationic porphyrins have been noted for their ability to regulate gene expression and treat diseases by binding and stabilizing various rG4 targets (Table 1; Bugaut and Balasubramanian 2012; Sanchez-Martin et al. 2021).
5,10,15,20-tetra-(N-methyl-4-pyridyl)porphine (TMPyP4) (Fig. 2A) is a well-characterized and representative G4 binder of cationic porphyrins (Ramos et al. 2021). Notably, counter ions significantly influence the selectivity of TMPyP4 in its interactions with human telomeric dG4s and rG4s. Studies using mass spectrometry indicated that TMPyP4 tetrachloride exhibits a binding strength to human telomeric rG4s, which is ∼10 times greater than that observed with the DNA counterpart (Bai et al. 2014). TMPyP4 is also a multifunctional G4 ligand, which has been used as a photosensitizer for cancer photodynamic therapy (Kawauchi et al. 2020). TMPyP4 and its alkyl-modified derivatives reportedly can effectively penetrate cell membranes and strongly bind to G4s in the 5′ UTR of KRAS and NRAS mRNA with nanomolar affinity, inducing reactive oxygen species (ROS) generation and repression of KRAS and NRAS expression upon photoactivation (Ferino et al. 2020). Among them, the designed alkyl porphyrin 2b effectively inhibited proliferation of pancreatic cancer cells in mouse xenograft models. TMPyP4 also binds rG4 motifs in microRNAs (miRNAs) such as miRNA-1587 and miRNA-149, exerting regulatory effects on miRNAs (Ghosh et al. 2019; Li et al. 2019, 2023). Additionally, TMPyP4 targets and binds various viral rG4s, including hepatitis C virus (HCV) rG4s (Wang et al. 2016a), Zika virus (ZIKV) rG4s (Majee et al. 2021), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rG4s (Qin et al. 2022), exhibiting potent antiviral activity.
The molecular recognition of monomeric and dimeric rG4s by TMPyP4 has been elucidated via X-ray crystallography, nuclear magnetic resonance (NMR), and molecular dynamics simulations to reveal the rationale for TMPyP4 binding and stabilization of rG4s (Mulholland et al. 2019; Qi et al. 2019; Zhang et al. 2020a). These studies demonstrated a binding mode similar to that of the TMPyP4–dG4 complex, with distinct binding preferences to different targets involving top stacking, bottom stacking, and side binding. In addition to performing its functions by binding and stabilizing rG4s, TMPyP4 also reportedly unfolds diverse rG4 structures in the 5′ UTR of MT3-MMP mRNA (Morris et al. 2012), the r(GGGGCC)8 repeat within the first intron of C9orf72 (Zamiri et al. 2014), miRNA-1587 (Li et al. 2019), and pre-miRNA-149 (Ghosh et al. 2019), thereby exerting G4-mediated functions. Haldar et al. revealed the mechanism of TMPyP4-induced unfolding of rG4s formed in pseudorabies virus (PRV), involving the transfer of TMPyP4 from the groove-bound to the top-face-bound state, followed by consecutive intercalation from top-to-bottom G-tetrads via disrupting Hoogsteen H-bonds between Gs (Haldar et al. 2022).
However, TMPyP4 has also been found to bind ssDNA, and its binding to dG4s could be completed by dsDNA, as verified by competitive dialysis assays and fluorescence resonance energy transfer (FRET) (Ren and Chaires 1999; De Cian et al. 2005; Johnson et al. 2024). Given its affinity to dG4s and insufficient selectivity against non-G4 targets, cautious application should be exercised in these small molecules, particularly in cellular contexts.
Quinoline derivatives, for example, pyridostatin, Phen-DC3, and 12459, featured scaffolds with multiple nonfused aromatic rings, and are extensively researched as dG4-binding ligands (Duarte et al. 2018). These designs maintain the planarity and rigidity of each component, while their adaptable structural conformations enable effective interactions with the loop and groove regions of G4, synergistically resulting in exceptional binding affinity and selectivity over duplex (Xiong et al. 2015; Andreeva et al. 2021). They also exhibit potential in rG4 targeting and regulation of various rG4-mediated cellular processes (Table 1; Sanchez-Martin et al. 2021; Zou et al. 2021). Phen-DC3, with a phenanthroline dicarboxamide core, was shown to bind and stabilize various rG4s, including the 5′ UTR of TRF2 mRNA (Gomez et al. 2010), pre-miRNA-149 (Carvalho et al. 2020), and the 3′-end of HCV110-131 (Jaubert et al. 2018). Bisquinolinium-triazine compound 12459 can impair the hTERT splicing machinery by stabilizing hTERT rG4s, thus downregulating telomerase activity (Gomez et al. 2004). Pyridostatin (PDS), another widely used G4-stabilizing quinoline-based ligand, effectively binds Epstein–Barr virus-encoded nuclear antigen 1 (EBNA1) rG4s (Murat et al. 2014), ZIKV rG4s (Zou et al. 2021), and transmembrane serine protease 2 (TMPRSS2) rG4s, which are involved in regulation of SARS-CoV-2 infection (Liu et al. 2022). Elsewhere, the binding of PDS to G4s in the pre-mRNA of EWSR1, involved in translocation of Ewing sarcomas, regulated splicing events by blocking hnRNPH1, and thus decreased EWS-FLI1 transcriptional activity (Neckles et al. 2019). However, like TMPyP4, PDS also demonstrates binding to and regulation of non-G4 forming sequences in important regions, inhibiting telomeric C-strand synthesis (Johnson et al. 2024).
In 2012, Di Antonio et al. developed a PDS derivative carboxypyridostatin (cPDS), which selectively binds and stabilizes rG4s but not dG4s (Fig. 2A; Di Antonio et al. 2012). Using in situ click chemistry, they synthesized a series of PDS derivatives. They functionalized PDS with an alkyne group and reacted it with azide substituents bearing diverse moieties, including positively/negatively charged groups, aromatic substrates, and neutral sugars, aimed to probe specific interaction modes critical for G4 recognition, such as π-stacking, electrostatic and hydrogen bonding. Notably, the reactions were catalyzed by either hTELO dG4 or TERRA rG4. Among them, the negatively charged carboxylate adduct (cPDS) was the most prevalent product in TERRA rG4-catalyzed reactions, while other adducts with an aromatic moiety formed less efficiently. cPDS synthesis could also be achieved by NRAS rG4 catalyst. FRET melting assays confirmed cPDS as a potent TERRA rG4 stabilizer, exhibiting a ΔTm value of 20.7 ± 0.1 K at 1 μM compound. This stabilization remained robust even in the presence of a 100-fold molar excess of DNA competitor, highlighting its specificity for rG4s. Through molecular dynamics and docking simulations, Rocca et al. revealed that the preferential binding of cPDS to rG4s is attributed to its conformational properties and enhanced solvation effects, as well as the inherently higher stability of rG4s compared to dG4s (Rocca et al. 2017). The introduction of the carboxyl group into PDS benefits its selective rG4s targeting with reduced negative electrostatic repulsion caused by the formation of hydrogen bonds between the ribonucleic hydroxyl groups and the phosphate groups (Rocca et al. 2017).
In addition to the above two structures most commonly studied for targeting rG4s, other distinct scaffolds and strategies were also explored to target rG4s. Derived from porphyrins, Kawauchi et al. developed an anionic phthalocyanine, ZnAPC, which selectively bound to the 5′-UTR NRAS mRNA G4 with low micromolar affinity (Kd = 3.1 ± 0.3 μM). This binding affinity remained unchanged even in the presence of a 50-fold excess of dsRNA, leading to downregulation of NRAS expression, along with selective cleavage of the targeted rG4 upon photo-irradiation (Kawauchi et al. 2018). Natural alkaloids with intriguing aromaticity and potential medicinal activity have also been used in rG4-targeting small molecules (Che et al. 2018). Tan et al. used two derivatives of jatrorrhizine to bind and induce the formation of a dimeric miRNA-1587 rG4 (Tan et al. 2018). Additionally, a polyaromatic compound, RGB-1 (Fig. 2A), showed specific binding and stabilization of rG4s with enhanced ΔTm over 5°C, but not dG4s with different topologies, or other non-rG4 structures (Katsuda et al. 2016). It also intensified G4-mediated inhibition of NRAS expression in breast cancer cells. Through biomimetics of G-quartets, Monchaud designed a series of template-assembled synthetic G-quartets (TASQs) for investigation of dG4 and rG4 biology (Monchaud 2023). Among them, a DOTA-templated synthetic G-quartet assembled with four peptide nucleic acid (PNA) monomers of guanine (PNADOTASQ) exhibited good binding affinity for telomeric transcript TERRA rG4s, TRF2 rG4s, and VEGF rG4s with ΔTm > 10°C, albeit with slight competition from highly stable dG4s (Haudecoeur et al. 2013). In addition, they applied biotinylated TASQs to pull down G4s from cell lysates, with preferential pull down of rG4s over dG4s (Yang et al. 2018; Renard et al. 2019). Combining rG4-specific precipitation with sequencing, they achieved transcriptome-wide identification of transient rG4 formation in human cells. Drug repurposing is a cost- and time-efficient strategy in pharmaceutical research. Recently, Chen et al. developed a new cell-based G-quadruplex-triggered fluorescence hybridization (GTFH) probe displacement method and used it for large-scale screening of NRAS rG4-specific ligands from a clinical compound library (Chen et al. 2023). Octenidine, a clinical antiseptic agent, was identified as a potent and selective NRAS rG4 binder and NRAS repressor with strong downregulation of NRAS expression and antiproliferation against NRAS-mutant melanoma cells in a mouse xenograft model. The superior effect of octenidine compared with clinical antimelanoma agents highlights the potential of rG4-targeting therapy.
Peptides that target RNA G-quadruplex structure
G4-specific peptides are alternative tools for rG4 targeting (Table 1; Banerjee et al. 2022; Basak et al. 2023). Several endogenous proteins, highly involved in DNA and RNA remodeling and metabolism, also exhibit effective binding and unwinding abilities for rG4 structures through their RNA-binding domains (e.g., RGG motifs, zinc knuckles), such as nucleolin, ZNF9/CNBP, DHX36, DDX21, DHX9, and eIF4a (von Hacht et al. 2014; Caterino and Paeschke 2022). Notably, RNA helicases like DHX36 and DHX9 enable preferential recognition and resolution of rG4 motifs in functional contexts including translation or RNA granule dynamics (Chakraborty and Grosse 2011; Sauer et al. 2019; Vester et al. 2019; Pipier et al. 2021). Designing peptides by truncating the rG4-binding domains of RNA helicases has proven to be an effective strategy for developing rG4-targeting tools (Basak et al. 2023). Their structures generally possess relatively few α-helixes or β-sheets, which preserves their parent proteins’ binding affinity and preference while allowing for easy modification and engineering for desired properties (Nguyen and Dang 2023).
DHX36, also referred to as RNA helicase associated with AU-rich element (RHAU), is an ATP-dependent RNA helicase belonging to the DEAH (Asp-Glu-Ala-His) box family, exhibiting specific binding and resolvase activity to parallel dG4s and rG4s with picomolar affinity (Meier et al. 2013; Chen et al. 2018a; Caterino and Paeschke 2022; Nguyen and Dang 2023). Biochemical and mutagenesis analysis has revealed that the essential and minimal functional domain of RHAU responsible for recognition and resolution of rG4s is a 13-amino acid terminal region (residues 54–66), termed RHAU-specific motif (RSM) (Chalupnikova et al. 2008; Lattmann et al. 2010). To further investigate RHAU–rG4 interactions, truncated RHAU variants containing RSM flanked by additional residues were engineered, such as RHAU53, RHAU29, RHAU23, and RHAU18 (Meier et al. 2013; Ariyo et al. 2015; Heddi et al. 2015, 2020). These constructs demonstrated potent and selective binding to parallel G4, displaying affinities in the nanomolar to submicromolar range.
Elucidating the molecular recognition of RHAU for rG4s may yield critical insights for applying these tools. Notably, Heddi et al. provided solution structure of RHAU18 with different parallel G4 including dG4s and rG4s via NMR spectroscopy (Heddi et al. 2015). They found that, like most small-molecule G4 binders, RHAU18 occupies the terminal G-tetrad of the G4 structure, while three cationic residues in RHAU interact electrostatically with the anionic phosphate groups in G4, providing a tight three-point anchoring for RHAU18 to clamp G4. Two peptides bind to the 5′-end/3′-end of one G4 motif simultaneously. In 2020, Mou and Kwok investigated the effect of rG4 sequence context and stereochemistry on rG4–RHAU53 interaction. G-quartet number, thermostability, overhanging nucleotides, and RNA base chirality were all found to be important influences on rG4–RHAU53 interaction (Mou and Kwok 2020). Exploiting the strong interaction between RHAU and rG4, Truong et al. fused two RHAU peptides with either a fluorescent protein pair CFP/YFP or two inactive monomers of apoptotic casp9, to detect rG4-mediated dimerization by FRET, and activate enzymatic activity of apoptotic casp9 monomers (Truong et al. 2020).
The arginine-glycine-rich motif RGG is widespread as an important motif for binding high order DNA and RNA structures (Chong et al. 2018). The RGG-box of hnRNPA1 specifically binds to loop-containing intramolecular TERRA rG4s, as demonstrated by NMR spectroscopy, but exhibits no interaction with single-stranded RNA (ssRNA) (Ghosh and Singh 2020). This motif also displays ∼31-fold stronger affinity for TERRA rG4s compared to telomere dG4s. Furthermore, the RGG-box cooperatively enhances the efficiency of UP1 domain of hnRNPA1 to destabilize TERRA rG4s, underscoring its role in promoting the unfolding of rG4s (Ghosh and Singh 2020). The three RGG boxes in fused in sarcoma (FUS), also known as translocated in liposarcoma (TLS), have also been proven to be the critical binding domain for TLS's interaction with TERRA rG4, and G4s from the 3′ UTR of two neuronal mRNAs (postsynaptic density 95 and Shank1a mRNAs) (Table 1; Takahama et al. 2013; Yagi et al. 2018; Imperatore et al. 2020). By substituting tyrosine for phenylalanine in the RGG domain, Takahama and Oyoshi developed an engineered RGG domain of TLS (TLSRGG3Y), which presented significantly higher binding specificity toward loop-containing TERRA rG4 with a reduction in binding affinity for telomere dG4 by ∼100 orders of magnitude (Takahama and Oyoshi 2013). This binding preference of TLSRGG3Y is attributable to the recognition of the 2′-OH of the ribose of loops within TERRA rG4. Additionally, Yoshida et al. that showed methyltransferase METTL3/METTL14 heterodimers, which contain RGG repeats at the C terminus (MTD3/MTD14-RGG), selectively bound rG4 with a six- to 10-fold stronger binding affinity compared to non-G4 RNA sequences, and methylated adenosines near rG4 sequences, elucidating the regulatory role of rG4s in epitranscriptomics (Yoshida et al. 2022).
In addition to obtaining rG4-targeting peptides through truncation of identified rG4-BPs, synthetic peptides have also started to emerge (Basak et al. 2023). Pur-alpha (Pura), a Pur family DNA/RNA-BP, was found to bind to the RNA hexanucleotide repeat expansion (HRE; GGGGCC) of C9orf72 (C9), from which a G4-forming sequence, consisting of three GGGGCC-repeats and adjacent GGGGC nucleotides, termed the minimal C9orf72 G-quadruplex repeat unit (C9Gru), was identified (Fratta et al. 2012; Daigle et al. 2016). To target this, Wortman et al. developed a synthetic polypeptide, TZIP, derived from conserved Pur domain consensus sequences and modified with a transporter motif (Wortman et al. 2020). TZIP bound specifically to both the G4 and linear forms of (GGGGCC)4 RNA repeats, showing negligible affinity for nontarget sequences. Notably, TZIP was shown to unwind rG4s to form higher-order RNA structures observed by EMSA, revealing a potential application for modulating G4 conformation in C9 repeats.
In 2023, Mou and Kwok developed a new platform, G4-mRNA display-seq, for recognition of short peptides specifically targeting G4 structures (Fig. 2B; Mou and Kwok 2023). Using this platform, a de novo peptide, pep11, was identified. Pep11 shows high binding preference to human telomerase RNA (hTERC) G4, with a dissociation constant (Kd) of 1.37 ± 0.22 μM, compared to other tested dG4s and rG4s. Furthermore, the tandem and cyclic version of pep11 demonstrated a two- to threefold increase in binding affinity and ∼30% downregulation of rG4-mediated gene expression, as shown by dual luciferase reporter gene assay.
Aptamers that target RNA G-quadruplex structure
L-RNA is the “mirror image” enantiomer of natural D-RNA, and does not hybridize with D-RNA/D-DNA (Young et al. 2019). L-RNA aptamers show strong stability under biological conditions owing to their resistance to nuclease degradation. Systematic evolution of ligands by exponential enrichment (SELEX) is a popular method to select aptamers with the potential for target binding (Tuerk and Gold 1990). Based on this, Umar et al. developed rG4-SELEX for selecting rG4-targeting L-RNA aptamers (Umar et al. 2022). In 2020, Chan and Kwok reported the first L-RNA aptamer, Ap3-7, that specifically targets and binds D-RNA G4 with a Kd of 99.0 ± 20.3 nM, showing no binding to other dG4s, rG4s, and RNA hairpin tested (Table 1; Chan and Kwok 2020). Ap3-7 can also effectively interfere with TERRA rG4–RHAU53 binding with an IC50 of 975.3 ± 1.1 nM. In that year, Umar and Kwok developed the shortest L-RNA aptamer yet reported (Umar and Kwok 2020). L-Apt.4-1c has only 25 nt and preferentially binds rG4s rather than dG4s or non-G4s demonstrated by EMSA. Using hTERC rG4 as an example, L-Apt.4-1c suppressed hTERC rG4–nucleolin interactions with comparable effectiveness to G4-specific ligands such as cPDS and QUMA-1 (Fig. 2C). L-Apt.4-1c can also suppress the interaction between rG4s in MALAT1 lncRNAs and NONO protein (Mou et al. 2022). The determined IC50 value of 618.1 ± 110.7 nM was comparable to that observed with PDS and RHAU53. Furthermore, in 2022, a new L-RNA aptamer, L-Apt.8f, was developed to target rG4s at the 3′-UTR mRNA of amyloid precursor protein (APP), which is closely linked to Alzheimer's disease (Zhao et al. 2022). L-Apt.8f downregulated the expression of APP reporter gene and native transcript translation in cells (Fig. 2C). Recently, an L-RNA aptamer targeting pUG fold, a noncanonical rG4 structure, was developed as well. L-apt3.1 binds to pUG fold strongly (Kd = 13.5 ± 2.8 nM), both in vitro and in vivo, inhibiting gene silencing in Caenorhabditis elegans (Liew et al. 2025).
In 2023, the same group further refined the in vitro selection platform, termed G4-SELEX-seq, by integrating SELEX with next generation sequencing (Ji et al. 2023). They applied parallel dG4, c-kit 1 dG4, to capture aptamer candidates in G4-SELEX and identified a L-RNA aptamer L-Apt12-6, which selectively targets parallel dG4 and rG4 structures rather than antiparallel or hybrid dG4s demonstrated by EMSA (Ji et al. 2023). L-Apt12-6 strongly bound to c-kit 1 dG4 (Kd = 42.3 ± 1.9 nM), thus downregulating the gene expression thereof. Recently, using a low GC content template library in G4-SELEX-seq, Lau et al. developed the first non-G4-containing L-RNA aptamer, L-Apt.1-1, exhibiting nanomolar binding affinity to APP rG4. By binding to APP rG4 within HeLa cells, L-Apt.1-1 downregulated endogenous APP protein expression by 38% (Lau et al. 2024). All reported that L-RNA aptamers targeting rG4s adopt a stem–loop (SL) structure, in which the loops primarily mediate G4 targeting, whereas the stem regions provide structural reinforcement. Thus, the SL structure is hypothesized to be favored by G4 binding. Motivated by this, Ji et al. applied a predefined library with SL structures during the SELEX process to reduce selection failures and enhance selection efficiency (Ji et al. 2024). Furthermore, a negative selection step with another rG4 target was incorporated, aiming at improving aptamer specificity. They developed an innovative and effective selection platform for rG4-targeting L-RNA aptamers, termed G4-SLSELEX-Seq. A G-triplex-containing the L-RNA aptamer L-Apt1-12 was identified in only three rounds of selection, demonstrating greater effectiveness of G4-SLSELEX-Seq in L-RNA aptamer selections. L-Apt1-12 shows strong binding to EBNA1 rG4s (Kd = 107.4 ± 10.4 nM) but considerably lower affinity to the negative rG4 target with Kd above 1000 nM. L-Apt1-12 could effectively downregulate the expression of endogenous EBNA1 in EBV-positive cancer cells. Additionally, they further demonstrated that G4-SLSELEX-Seq is a potent and generic platform in aptamer selection for APP rG4 and HCV-1a rG4.
Various modifications and optimizations on L-RNA aptamer have also been carried out. To further improve the binding properties of L-RNA aptamers, Ji et al. used cyclization strategy on L-RNA aptamers in 2021. They cyclized the linear L-Apt.4-1c via click chemistry, developing the first circular L-RNA aptamer, cycL-Apt.4-1c, which exhibited a 10-fold improvement in binding affinity with a Kd from 74.9 ± 7.5 to 7.76 ± 0.95 nM, and greater selectivity toward hTERC rG4 over dG4 (Ji et al. 2021). To improve cell penetration of L-RNA aptamers, Zhang et al. reported a robust strategy of conjugating L-RNA aptamers with cell-penetrating peptides (CPPs) in 2024 (Zhang et al. 2024). They developed the first L-RNA aptamer–CPP conjugate, specifically L-Apt.4-1c and polyarginine residues (R8), by click chemistry. The L-Apt.4-1c–R8 conjugate performed cell uptake and gene regulation of various mRNAs forming G4s in cells without transfection reagents, with comparable effectiveness to that of L-Apt.4-1c by transfection. In addition, to improve selectivity of L-RNA aptamers to a specific rG4 target, Feng and Kwok developed an L-RNA aptamer, L-Apt.T8, for targeting the HIV-1 U3-III rG4, and further conjugated it with 10 nt DNA antisense oligonucleotides (ASOs) complementary to the extension sequence downstream HIV-1 U3-III rG4 (Feng and Kwok 2024). L-Apt.T8-10D presented enhanced binding affinity and selectivity for the target with a Kd from 58.1 ± 4.9 to 12.5 ± 5.17 nM, and disruption to the target's interaction with HIV-1 U3-tRNA segments and nucleocapsids, while suppressing in vitro HIV-1 minus strand transfer. Similarly, another L-aptamer–ASO conjugate comprising L-Apt.4-1c and a 15 nt ASO was designed for specific recognition and stabilization of APP rG4 (Zhao et al. 2024). L-Apt.4-1c–ASO15nt(APP) inhibited APP expression through helicase dissociation from rG4 and RNase H-mediated mRNA knockdown.
Antibodies that target RNA G-quadruplex structure
Fernando et al. pioneered the development of G4-specific antibodies using in vitro phage display methodologies (Fernando et al. 2008; Biffi et al. 2013). Through screening a library of 2.3 × 1010 single-chain antibody clones, BG4 was identified as the optimal selective binder of dG4s, enabling visualization of these structures in human cells (Biffi et al. 2013). Enzyme-linked immunosorbent assays revealed that BG4 binds to both intramolecular and intermolecular dG4s with high affinity (Kd ≤ 2.0 nM), exhibiting no detectable binding to non-G4 nucleic acids or conformational preference toward specific G4 topologies. Subsequently, they further investigated BG4's interaction with rG4 structures, revealing its low nanomolar binding affinity to rG4s, which is comparable to that of dG4s (Biffi et al. 2014). Through using a secondary antibody that recognizes a FLAG tag on BG4, followed by a tertiary fluorescent antibody for amplification of the signal, they observed the BG4 nuclear foci and then cytoplasmic foci for longer exposure, demonstrating the visualization of endogenous rG4 structures within the cytoplasm of human cells by BG4 (Fig. 2D; Biffi et al. 2014).
Using BG4-based quantification, they also observed the cPDS-mediated selective targeting and trapping of cytoplasmic rG4 structures in human cells, resulting in approximately a 2.3-fold increase in BG4 cytoplasmic foci but not in nuclear foci (Biffi et al. 2014). Although BG4 is not an rG4-specific tool, it has been used as a significant auxiliary tool in a series of rG4-related studies, such as validation of new-found G4s in RNA, including human ribosomal RNA (rRNA) G4s, HCV rG4s, SARS-CoV-2 rG4s (Wang et al. 2016a; Mestre-Fos et al. 2019; Zhao et al. 2021; Qin et al. 2022), the imaging verification of rG4-targeting probes/agents by colocalization (Chen et al. 2018b; Luo et al. 2019; Yu et al. 2020; Zou et al. 2021), and studies of rG4-BPs as well as its interaction with rG4s (Neckles et al. 2019; Herviou et al. 2020; Asamitsu et al. 2023).
Other tools that target RNA G-quadruplex structure
Sequence-selective recognition of several specific rG4s has been achieved using ASOs to hybridize to the quadruplex-forming sequence (Table 1; Cadoni et al. 2021). In 2014, Murat et al. reported the use of a 21-mer RNA ASO and its DNA analog for identification and destabilization of rG4s forming in EBNA1 mRNA (Murat et al. 2014). ASO dAS2 effectively “opened” the rG4 of EBNA1 and thus promoted mRNA translation. In 2015, Rouleau et al. applied the small-ASO-based strategy to block or promote rG4 formation in the 5′ UTR of the H2AFY gene by hybridizing the long loop of rG4s or the sequences neighboring G tracts, thus achieving translational switches of specific mRNAs (Rouleau et al. 2015). This strategy is promising for modulation of H2AFY, a clinically relevant cancer biomarker. Similarly, Marin and Armitage attempted to use two PNAs, P7C and P7H, to invade rG4s forming in an RNA aptamer (RDQ) that selectively binds FMRP (Marin and Armitage 2005). They observed that invasion and hybridization by P7C, the PNA complementary to the rG4-forming sequence, resulted in formation of PNA–RNA duplexes, while invasion by P7H, the PNA homologous to rG4, resulted in stable hybrid quadruplex structures. In 2016, Oyaghire et al. developed several new complementary γPNA oligomers exhibiting strong binding affinities to complementary nucleic acid targets (Kd = 1.9–4.2 nM) (Oyaghire et al. 2016). γPNA oligomers effectively inhibited mRNA translation in luciferase reporter gene assay by invading a stable G4 structure of NRAS mRNA at nanomolar concentrations. Compared with the homologous PNA and 2′-OMe oligomers, γPNA oligomers demonstrate superior selectivity and potency in target binding compared to control mRNA and translation inhibition. In 2021, Sarkar and Armitage used complementary γPNA oligomers to invade a newly identified rG4 in the NS5 protein-coding region of the West Nile virus genome (Fig. 2E; Sarkar and Armitage 2021). The γPNAs exhibited strong binding to the rG4 target with femtomolar affinity.
Affinity and selectivity must be traded off in many current ligands, which hinders the development and improvement of rG4-targeting tools (Banerjee et al. 2022). Dual binding is a proven strategy for improving the efficiency, selectivity, or both in rG4 targeting (Table 1). Yangyuoru et al. investigated the dual binding of the rG4-specfic ligand cPDS and the antibody BG4 to TERRA rG4, to verify whether a small molecule and an antibody could form a ternary complex with rG4s and generate improved stabilization cooperatively (Fig. 2F; Yangyuoru et al. 2015). They found that the rupture forces of TERRA rG4 with a mixture of cPDS and BG4 increased from 23/36 to 30/50 pN, and the free energy of unfolding (ΔGunfold) increased from 17 to 25 kcal/mol compared with mono-binding. They further proposed a two-stage binding mechanism in the TERRA rG4–cPDS–BG4 complex. In the first stage, cPDS outcompetes BG4 for initial binding to TERRA rG4 due to its significantly greater acceleration on TERRA rG4 folding kinetics and pronounced enhancement of rG4 folding rates. In the second stage, BG4 binds to the prefolded cPDS–TERRA rG4 complex, followed by a slow conformational rearrangement that finalized the formation of the ternary complex. This simultaneous binding of small molecule cPDS and antibody BG4 significantly increased the mechanical and thermodynamic stability of TERRA rG4. In addition, by linking a guanine moiety to the G4-binding peptide RHAU23, He et al. developed a bifunctional guanine-RHAU23 peptide conjugate (GRPC) to selectively target the “imperfect” G-vacancy-bearing G-quadruplexes (GVBQs) (Fig. 2G; He et al. 2020). The guanine groups occupied the G-vacancies in GVBQs, directing RHAU23 specifically toward binding to these structures with enhanced stability and low nanomolar affinity. In addition to interacting with DNA GVBQs, the GRPC could also stabilize two RNA equivalents, namely MYOG-3332 and ABTB2-3233, increasing their temperature of thermal melting to nearly 30°C, much higher than when using guanosine and RHAU23 individually or together. The GRPC also inhibited both RNA reverse transcription and RNA translation in vitro.
RNA G-QUADRUPLEX STRUCTURE IMAGING AND BIOSENSING
Visualizing G4 structures in cells facilitates the understanding of their biological role in various diseases (Suseela et al. 2018). In 2013, Biffi et al. developed the antibody BG4, and achieved dG4 imaging using BG4, BG4-specific secondary antibody, and tertiary fluorochrome-labeled antibody (Biffi et al. 2013). In the following years, the BG4 antibody was applied to rG4 imaging (Chen et al. 2018b; Luo et al. 2019; Zhang et al. 2021; Robinson et al. 2024). Subsequently, various dyes have been developed to target either dG4s or rG4s (Suseela et al. 2018). In this section, we introduce the different classes and mechanisms of rG4-imaging tools and their applications in rG4 imaging and disease research. The current imaging tools for rG4s are either small molecule–based or aptamer-based, and can be divided into three classes: fluorescent probes that directly bind and light up rG4s, nonfluorescent probes with conjugated fluorescent molecules, and fluorogenic bifunctional aptamers (FLAPs) (Fig. 3; Table 2).
FIGURE 3.
Principle of rG4 imaging techniques. (A) Fluorescent small molecules that light up upon binding to rG4 structures. (B) Immunofluorescent staining of rG4 structure using immuno-tag G4 binding ligand. (C) Selective rG4 imaging using an antisense conjugated rG4 fluorescent ligand, or proximity-induced rolling circle amplification (RCA) triggered by click reaction between antisense and rG4-binding ligand of close proximity. (D) Fluorogenic bifunctional aptamer (FLAP) by connecting rG4 targeting aptamer and fluorogenic aptamer to image rG4 structure in the presence of its corresponding fluorescent ligand.
TABLE 2.
Summary of current rG4 structure imaging studies
| Name | Target rG4 | Ex. | Em. | Biophysical approaches | Biological system | Applications | References |
|---|---|---|---|---|---|---|---|
| Small molecules | |||||||
| QUMA-1 | rG4s in cells | 555 | 660 | Fluorescence spectroscopy, competition assay, CD, NMR, HPLC, MS | Cell line: HeLa | General rG4 dynamic | Chen et al. 2018b |
| ThT-NE | HCV RNA CG2a | 461 | 495 | Fluorescence spectroscopy: 1693-fold in vitro, CD, UV-Vis spectroscopy, gel electrophoresis, NMR | Cell line: Huh7, FCA1, GG2, GG2-G4Mut and HLCZ01 | Hepatitis C virus (HCV) infection | Luo et al. 2019 |
| NIR-2 | HCV RNA CG2a | 591 | 689 | Fluorescence spectroscopy, CD, NMR, HRMS | Cell line: Huh7, FCA1, GG2-G4-Mut and GG2 Organ: Huh7 or GG2 cell xenograft mice 3.4-fold ex vivo |
HCV infection | Zhang et al. 2021 |
| ThT-NA | rG4s in cells | 510 | 610 | Fluorescence spectroscopy, CD, NMR | Cell line: HepG2 and HeLa Tissue: HeLa cell xenograft mice |
Cancer | Lu et al. 2022 |
| TCB-1 | Abnormal NOP56 RNA | 490 | 526 | Fluorescence spectroscopy: 150 to 438-fold with dG4/rG4 in vitro, UV-Vis spectroscopy, NMR, MS | Cell line: HeLa and MCF-7, 8-Fold | Autosomal dominant spinocerebellar ataxia 36 (SCA36) | Sun et al. 2023 |
| TOR-G4 | rG4s in cells | 467 | 540 | Fluorescence spectroscopy, UV-Vis spectroscopy, NMR, MS | Cell line: U2OS | Fluorescent lifetime-based imaging | Robinson et al. 2024 |
| NIRG-2 | dG4s and rG4s | 850 | 940 | Fluorescence spectroscopy, CD, NMR | Cell line: 4T1, HEK293, A549, MCF-7, LX-2, and L929 Tissue: chicken breast Tumor: 4T1 subcutaneous xenograft mice: 47-fold in vivo |
Cancer lymphatic metastasis | Wang et al. 2024 |
| SCY-5 | Parallel dG4s and rG4s | 575 | / | Fluorescence spectroscopy, UV-vis spectroscopy, native gel electrophoresis, NMR | Cell line: HUVECs, MCF-10A, MCF-7, HeLa, A549, and SMMC7721, >3.5-fold in tumor cells, twofold in normal cells Blood: greater than twofold for cancer patient, twofold for normal patient |
Cancer | Sun et al. 2024 |
| 4S-2TO-4a | dG4s and rG4s | 498 | 680 | Fluorescence spectroscopy, CD, UV-Vis spectroscopy, native gel electrophoresis, NMR, HRMS | Cell line: HeLa | General G4 imaging | Parveen et al. 2024 |
| ThT | dG4s and rG4s | 404 | 490 | Fluorescence spectroscopy, TCSPC time-resolved decays, UV-Vis spectroscopy, | Cell line: U2OS | General G4 imaging | Bradford et al. 2024 |
| TzN-TASQ | dG4s and rG4s | 286 | 414 | Fluorescence spectroscopy, UV-Vis spectroscopy, NMR, molecular dynamics simulations | General G4 detection | Ledvinka et al. 2024 | |
| Small molecule + antibody | |||||||
| 5-BrdU PDC + antibody | rG4s in cells | / | 450 | G4-FID, click reaction | Cell line: A549 and A2780 | General G4 imaging | Masson et al. 2021 |
| Small molecule + antisense | |||||||
| ISCH-app | APP rG4 | 570 | 650 | Fluorescence spectroscopy, CD, UV-Vis spectroscopy, RTS assay, SHALiPE assays | Cell line: HEK293T and HeLa, 28–50-fold in cells | Alzheimer's Disease (AD) | Lyu et al. 2019 |
| MAMPA | BCL2 rG4 | 488 | 520 | Fluorescence spectroscopy, MS | Cell line: HeLa, MCF 10A, MDA-kb2, MCF-7, BT-474, SK-BR3, MDA-MB-231, mESC, and 293FT | Proto-oncogene | Dai et al. 2022 |
| Aptamer | |||||||
| L-Apt.1-1_Pepper | APP rG4 | 485 | 530 | Fluorescence spectroscopy: 44.5 ± 1.4-fold in vitro, CD, native gel electrophoresis | Cell line: HEK293T and HeLa | AD | Lau et al. 2024 |
In the following paragraph, we will introduce recently developed fluorescent small-molecule probes for rG4 imaging in cells. Currently, most fluorescent small-molecule probes are derived from cyanine, coumarin-hemicyanine, thiazole orange (TO), benzothiazole, and other dyes. Their planar structure allows them to intercalate between or stack at the end of the G-tetrads of rG4s, a binding interaction that stabilizes the fluorescent small molecules and activates their fluorescence. Using fluorescent small molecules to image rG4 structures is advantageous due to their small size, allowing them to penetrate cells, and their broad spectral range.
In recent years, fluorescent probes have been applied to study the formation and dynamics of rG4 structures in cells (Table 2). In 2018, Chen et al. developed a red-colored coumarin-hemicyanine fluorophore, QUMA-1, by condensing a coumarin aldehyde and an N-methylated quinoline moiety. QUMA-1 can light up various rG4s but has no response to double- or single-stranded RNA in vitro, the Kd of QUMA-1 against TERRA rG4 was 0.57 µM. QUMA-1 was applied to track the dynamics of rG4s; significant decreases or increase of fluorescence in cells were observed in responses to the activation or inhibition of the RNA helicase DHX36, respectively. This study used QUMA-1 to prove the potential involvement of RNA BPs in rG4 cellular dynamics, and it can be a potential ligand for researchers to study the nature of rG4 folding in cells (Chen et al. 2018b).
Later, several TO fluorescent probes were developed for rG4 structures. Sun et al. developed the green-fluorescent probe TCB-1, derived from TO, to monitor the abnormal NOP56 gene rG4 of autosomal dominant spinocerebellar ataxia (Sun et al. 2023). To improve its selectivity to G4s, TO was linked to a methylene bridge and ethyl group to sterically hinder its interaction with double-stranded DNA. Fluorescent measurement showed TCB-1 responds to both dG4s and rG4s, but not to non-G4s. Using SCA36 model cells overexpressing repeated amplifications of GGGCCT units, an eightfold fluorescent increase was observed in the cytoplasm (Fig. 3A; Sun et al. 2023).
In the same year, Robinson developed the first fluorescence-lifetime-based probe, TOR-G4, to image rG4s in cells. TOR-G4 is a green dye also derived from TO. To improve its G4 selectivity, a benzyl-styryl unit was added to the original TO motif to facilitate its π-stacking with G-quartets. By performing time-resolved fluorescence trace of TOR-G4 in solution, it was proven that TOR-G4 can impart long fluorescence lifetimes to both dG4s and rG4s, but shorter lifetimes to non-G4 RNAs, overcoming the limitations of intensity-based switch-on probes. Moreover, TOR-G4 preferentially binds and lights up rG4s instead of dG4s in cells, which may be due to the blockage of its intercalation to dG4s in chromatin, compared with small molecules that can bind the groove of dG4s in chromatin (Fig. 3A; Robinson et al. 2024).
In 2024, Parveen et al. developed a near-infrared (NIR) fluorescent dye, 4S-2TO-4a, derived from TO. 4S-2TO-4a showed significant fluorescence enhancement in the present of antiparallel dG4s and parallel rG4s, and it has a lower cellular toxicity compared with TO, which was then applied to stain both rG4 and dG4 structures in cells (Fig. 3A; Parveen et al. 2024). In the same year, Sun et al. developed a new anionic cyanine dye, SCY-5, which has an absorbance peak at 575 nm in the presence of parallel dG4s and rG4s, but not in that of hybrid or antiparallel G4s. SCY-5's selectivity to parallel G4s arises from the stronger steric hindrance of lateral and diagonal loops in hybrid or antiparallel G4s. In cellular imaging, SCY-5 was able to selectively image rG4s instead of dG4s due to its preference to bind parallel G4 structures. This was proven by fixed cells stained with SCY-5 DNase I or RNase T1 treatment, whereas fluorescence decrease was only observed in the RNase treated group. SCY-5 was then applied to detect rG4s in total cellular RNA from clinical blood samples, with fluorescence intensity ratio (F/F0) values of above 2 for cancer patients, but ∼2 for the control group. This study demonstrated the possibility of cancer diagnosis via the detection of rG4s as biomarkers (Fig. 3A; Sun et al. 2024).
The rG4 structures have also been proven to form in viral RNA. However, many viral RNA live-cell imaging techniques require chemical labeling or modification of the viral genome, hindering the study of native virus infection. Responding to the need for new tools to image unmodified viral genomes, in 2019 Luo et al. developed ThT-NE, derived from thioflavin T (ThT), to light up the unmodified HCV viral genome. ThT-NE contains methylbenzothiazole and N,N-diethylaniline moieties, and can light up the HCV subtype 2a genome by binding to the CG2a rG4 with Kd = 1.77 ± 0.19 μM. ThT-NE's application to monitor HCV infection progress in host cells will benefit diagnosis and drug development (Fig. 3A; Luo et al. 2019). In 2022, Lu et al. from the same group developed ThT-NA to light up rG4s in deep tissue. ThT-NA was synthesized by replacing the methyl-benzothiazole moiety in ThT with a stronger electron donor to generate a large stokes shift with red emission, reducing interference of cellular autofluorescence and improving tissue penetration. ThT-NA has high specificity for parallel and hybrid G4s, and was applied to image endogenous G4s in living cells and deep tissue using two-photon fluorescence (Fig. 3A; Lu et al. 2022). The in vivo imaging of rG4 structures in animals requires fluorescent probes in the NIR region. In 2021, Zhang et al. developed the first NIR-emissive fluorescent probe, NIR-2, for in vivo imaging in live animals, classed as an infrared G-quadruplex mimic of fluorescent probes. NIR-2 binds CG2a rG4s with a Kd of 1.58 ± 0.18 μM and detection limit of 0.015 μM. NIR-2 was applied to image HCV RNA in living mice and achieved a 3.4-fold fluorescent change compared with non-HCV RNA controls (Fig. 3A; Zhang et al. 2021). In 2024, Wang et al. developed another NIR-emissive fluorescent probe, NIRG-2, for in vivo G4 detection. NIRG-2 was synthesized by linking a trimethine chain to anthocyanin and introducing a sulfonic group to the anthocyanin scaffold to facilitate its binding to G4 structures. NIRG-2 exhibited a high turn-on selectivity for parallel dG4s and rG4s, and was applied to image the oncogene Myc in living mice (Fig. 3A; Wang et al. 2024).
The second class of rG4-imaging tools—nonfluorescent probes with various conjugations—are produced by conjugating G4-binding small molecules with immuno-tag or antisense strand (Table 2). In 2021, Masson et al. developed a new approach to visualizing rG4 structures in cells, called G4-ligand-guided immunofluorescence staining. 5-Bromo-2′-deoxyuridine (5-BrdU) was synthesized by linking the 5-BrdU immuno-tag to the selective G4 ligand pyridodicarboxamide (PDC) by click reaction. Immunofluorescence labeling of rG4 structures in cells was performed by using anti-5-BrdU antibodies, HRP-conjugated secondary antibodies (AbII), and an enzymatic substrate that allows chromogenic detection of AbII. Fluorescent signal was observed in the nucleoli and cytoplasm, and was greatly reduced after RNase treatment, which indicated the light-up was from rG4s. This approach overcomes the weak ligand accumulation that impairs ligand visibility when using fluorescent probes, thus achieving high sensitivity for rG4 detection (Fig. 3B; Masson et al. 2021). Apart from sensitivity, selectivity in the imaging of individual rG4s is also important for understanding rG4 biology. In 2019, Lyu imaged the APP rG4 via GTFH using “ISCH-app,” which consists of a fluorescent light-up moiety that binds G4 structures and an antisense DNA molecule that hybridizes to the APP 3′-UTR sequence, thus selectively targeting individual rG4s. The ISCH signal overlaps well with wild-type (wt) APP rG4 and has a signal 28-fold stronger than mutant APP rG4 in HEK293T cells and 50-fold stronger in HeLa cells (Fig. 3C; Lyu et al. 2019). In 2022, Dai et al. developed the module-assembled multifunctional probe assay (MAMPA) to image endogenous proto-oncogene BCL2 in cells. MAMPA was designed by linking the G4 ligand cPDS and antisense strand via click reaction in the presence of BCL2, and the assembly of the two probes triggered rolling circle amplification to amplify the fluorescent signal. MAMPA was applied to monitor the G4 ratio of a BCL2 mRNA with or without the presence of its G4 regulator DHX9 and should be also applicable to other G4-BPs (Fig. 3C; Dai et al. 2022).
The final class of rG4-imaging tools is FLAPs (Table 2). By conjugating an rG4-targeting aptamer and a fluorogenic aptamer, a bifunctional aptamer that binds both rG4s and fluorescent ligands was developed for rG4 imaging. In 2024, Lau et al. developed L-Apt.1-1_Pepper by conjugating the rG4-binding aptamer L-Apt.1-1 and the fluorogenic Pepper L-aptamer for the detection and imaging of the APP rG4 with the HBC530 fluorescent ligand. This system generated a fluorescent signal improvement of 44.5 ± 1.4-fold upon the addition of APP rG4 in vitro, and achieved an ∼0.7 colocalization ratio in cells with transfected wt APP rG4 but not with mutant APP rG4. Their fluorogenic L-aptamer system introduces a new approach for cellular imaging of rG4 structures using L-aptamers (Fig. 3D; Lau et al. 2024).
CURRENT CHALLENGES AND FUTURE PERSPECTIVES
In recent decades, the development of small molecules with diverse structures targeting G4s has become a primary approach in this field (Banerjee et al. 2022). These small molecules hold significant promise for advancing therapeutic agents aimed at G4-mediated treatments (Yan et al. 2023). Several commonly used G4-targeting agents, such as TMPyP4 and PDS, have been widely applied to verify and regulate newly identified rG4s (Table 1), while their application in rG4-mediated treatments remains limited (Sanchez-Martin et al. 2021). More importantly, their less effective interaction with rG4s than with dG4s, as well as their poor selectivity for rG4s over dG4s and non-G4 structures, are likely to exacerbate the difficulty of application (Collie et al. 2009; Asamitsu et al. 2019). For instance, TMPyP4 and PDS have been shown to bind a telomeric DNA sequence without consecutive G tracts, which are structurally unfavorable for G4 formation, and thus inhibiting telomeric C-strand DNA synthesis (Johnson et al. 2024).This highlights potential off-target effects in a few of these developed G4-targeted ligands. Unfortunately, there has been less focus on developing new small molecules specifically for targeting rG4s compared to dG4s, despite the abundance of rG4s in different regions of coding mRNAs and different classes of ncRNAs (Lyu et al. 2021; Sanchez-Martin et al. 2021).
The distinct presence of 2′-OH groups in rG4s versus dG4s serves as a critical determinant for ligand selectivity due to their alteration on G4 structure properties such as groove depth and loop geometry (Collie et al. 2009). Functionalizing existing scaffolds with substituents optimized for electrostatic and solvation interactions (e.g., carboxylated ligands like cPDS) represents a viable strategy (Di Antonio et al. 2012). However, careful optimization of ligand side chains is essential due to the potential for restricted spatial accessibility caused by the 2′-OH groups in rG4s. An inappropriate side chain may lead to unfavorable electrostatic repulsion, resulting in reduced affinity for rG4s (Collie et al. 2009). More attention also needs to be paid to developing new scaffolds and structures for specific binding to rG4s (Duarte et al. 2018). Besides traditional experimental approaches to screen and identify potential rG4-targeting small molecules, computational tools offer a rapid and cost-effective way (Neidle 2016; Haider 2018), using techniques such as automated molecular docking and virtual screening (De Magalhães et al. 2004; Monsen and Trent 2018). Furthermore, small-molecule microarrays have also demonstrated effectiveness in high-throughput screening for rG4-targeting compounds (Prestwood et al. 2024). Additionally, fragment-based drug discovery, when used to develop high-affinity and selective RNA binders, presents a promising strategy for creating rG4-specific small molecules (Zeller et al. 2022; Suresh et al. 2023).
Besides small molecules, new rG4-targeting L-RNA aptamers and peptides have continually emerged in recent years (Chan and Kwok 2020; Banerjee et al. 2022). These burgeoning tools, possessing diverse and intricate composition and structures, present great potential in targeting complex biomolecules with high affinity and specificity (Basak et al. 2023; Lam et al. 2024). Also, they tend to be more stable and biocompatible in biological environments, exhibiting lower cytotoxicity and lower immunogenicity, which makes them suitable for therapeutic applications. However, the therapeutic use of these tools is still relatively underreported. Thus, further verification and application of L-RNA aptamers and peptides in cells and in vivo will make them even more promising as potent agents for rG4 targeting. In addition, selectivity to a specific rG4 remains a challenge for these new rG4-targeting tools. This limitation could be explained and addressed by understanding and characterizing the molecular basis of the rG4–peptide/aptamer complexes, which is also a key challenge and unsolved task for most rG4-targeting aptamers and peptides. To address this gap, advanced structural studies using techniques such as NMR spectroscopy, X-ray crystallography, or cryo-EM, are urgently needed to resolve the molecular architecture of these complexes (Santos et al. 2021; Troisi and Sica 2024). Detailed insights into binding interfaces, conformational dynamics, and interaction networks could reveal the determinants of specificity, enabling rational sequence/structure modifications or design strategies to improve their selectivity. For the future development of new rG4-specific L-RNA aptamers and peptides, artificial intelligence and machine learning could also be integrated to expedite the refinement and discovery process (Attique et al. 2020; Bashir et al. 2021; Perez Tobia et al. 2023; Wan et al. 2024).
Antibody is also a highly effective tool for binding rG4 structures. In addition to the widely used BG4, several antibodies capable of binding to dG4s have been reported, including hf2 (Fernando et al. 2008), HF1 (Fernando et al. 2009), 1H6 (Henderson et al. 2014), D1 (Liu et al. 2016a), and nanobody SG4 (Galli et al. 2022). While their binding to rG4s requires further investigation, and the development of rG4-specific antibodies remains underexplored, studies on existing dG4 targeting antibodies may provide important insights and ideas for the development of rG4-specific antibodies. Using rG4s as targets in antibody phage display and hybridoma technology could be a promising strategy for generating antibodies specific for rG4s (Fernando et al. 2008; Henderson et al. 2014; Liu et al. 2016a). We believe that advancing antibodies may significantly enhance the tools available for rG4 targeted applications.
Meanwhile, various designs and strategies are emerging to enhance and functionalize rG4-targeting tools. Approaches such as tandem design, cyclization, and combined/conjugated methods show potential for future optimization and application. For example, conjugating with G4 flanking sequence–complementary oligonucleotides has been proven to enhance the binding affinity and selectivity of small molecules and L-RNA aptamers for rG4 targets (Chen et al. 2016; Dai et al. 2022; Feng and Kwok 2024; Zhao et al. 2024). To improve cell penetration, linking with CPPs and incorporation into nanoparticles present feasible and effective strategies for delivery of rG4-targeting tools (Liu et al. 2016b; Yang et al. 2023; Zhang et al. 2024). Moreover, an aptamer stapling strategy for stabilizing the binding conformation of aptamers can significantly improve their serum stability, binding affinity, and therapeutic efficacy, which could be applied in rG4-targeting oligonucleotides and peptides (Amu et al. 2024). Additionally, PROTACs/RIBOTACs could be developed using rG4-binding tools as targeting ligands for selective degradation of rG4-BPs or related RNAs (Zhang et al. 2022; Fang et al. 2024). Recently, the use of G4-stabilizing proteins and ligands combined with CRISPR technology has allowed for the selective promotion of single or multiple targeted dG4 formations, thereby enabling G4-mediated gene regulation (Qin et al. 2024). Thus, the conjunction of rG4-targeting tools with CRISPR may underpin a more precise exploration of the biological functions of de novo rG4s.
In addition, a significant challenge in this field is the lack of standardized assessment for rG4-trageting tools across studies, especially for their selectivity. Variability in experimental approaches makes cross-comparison of reported metrics difficult for researchers (Table 1). Studies that claim rG4 targeting often neglect critical validation to rule out off-target binding to competing nucleic acid structures, or apply these tools without systematically addressing off-target effects in their analyses (Johnson et al. 2024). Several actionable strategies could be considered by researchers in their own works: (1) perform experimental validation like competition assays with rigorous negative controls, using binding affinity ratios to quantify selectivity (Ren and Chaires 1999); (2) complement orthogonal validation with structural techniques (e.g., NMR or cryo-EM) to confirm specific binding, and with computational tools (e.g., machine learning–based prediction, transcriptome-wide sequencing or proteomics) to assess off-target effect (Chen et al. 2021b; Santos et al. 2021; Revikumar et al. 2022); and (3) provide transparent reporting including off-targets, limitations, and negative results to define the tool's validated specificity scope.
The critical regulatory roles of rG4s in various cellular processes make them significant targets in a range of diseases, including malignant tumors, viral infections, and neurological disorders (Goering et al. 2020; Goldberg et al. 2020). The emerging rG4-targeting tools thus have evident therapeutic potential in diverse diseases. However, this strongly depends on their interaction with rG4s, which necessitates both stabilization and destabilization strategies. Most reported rG4-targeting tools stabilize rG4 structures by forming strong interactions with guanine bases and disrupting the binding of helicase to rG4s, leading to inhibition of disease-related gene/protein expression, or modification of their function for treatment of diseases (Lejault et al. 2021). Meanwhile, many neurological disorders and genetic and age-related diseases stem from an accumulation of G4s, highlighting the potential applications of G4-destabilizing tools to reduce G4 formation (Moruno-Manchon et al. 2020; Vijay Kumar et al. 2023). In recent decades, several rG4 unwinders have exhibited destabilizing effects on rG4s, including small molecules, peptides, and ASOs, and may have desirable uses in gene regulation and therapy (Rouleau et al. 2015; Lejault et al. 2021; Basak et al. 2023; Fracchioni et al. 2024). Moreover, several small molecules exhibit opposing effects on G4s depending on conditions such as the ligand concentration ratio and temperature, for example, TMPyP4, TAP1, and stiff stilbenes (Waller et al. 2009; O'Hagan et al. 2019; Haldar et al. 2022). Mimicking the key domain of rG4 helicase is a feasible and efficient approach to developing peptides that unwind rG4s (Wortman et al. 2020). Meanwhile, invasion followed by interaction with the Gs in G4s through the formation of cytosine–guanine hydrogen bonds is a major mechanism for disrupting rG4s adopted by many ASOs and small molecules, including a phenylpyrrolocytosine-based G-clamp analog (PhpC) (Fig. 2A; Mitteaux et al. 2024) and a dual naphthyridine carbamate (DANC) (Liu et al. 2024). Recently, a 2′-F cytidine trimer (2′-F C3) was identified as a new rG4 unfolder that binds to G runs. 2′-F C3 upregulates G4-forming mRNA without causing DNA damage or genomic instability, unlike TMPyP4 and most ASOs (Teng et al. 2024).
Visualizing rG4s in cells is important for understanding G4 biology and G4-regulated diseases. Many fluorescent probes have been developed to image the existence of rG4 structures and dynamics in cells. In recent years, researchers have focused on improving the specificity of these probes for rG4 structures. Several modified G4-specific small-molecule fluorescent probes have been chemically engineered to recognize and bind rG4s with improved specificity (Chen et al. 2018b; Luo et al. 2019; Zhang et al. 2021; Lu et al. 2022; Sun et al. 2023, 2024; Parveen et al. 2024; Robinson et al. 2024; Wang et al. 2024). However, imaging individual rG4s from a pool of structurally similar rG4 motifs using these small-molecule fluorescent probes is still difficult. To solve this problem, techniques such as conjugation of antisense strand to G4-targeting fluorescent probes have been applied (Lyu et al. 2019; Masson et al. 2021; Dai et al. 2022). This strategy combines the sequence specificity of antisense oligonucleotides with the G4-binding ability of small molecules to selectively target and visualize specific rG4 structures. Besides, aptamers have been applied to specific rG4 imaging. By using FLAPs and their corresponding fluorogenic ligands, rG4 structures can be visualized in cells, which provides a new direction in rG4 imaging (Lau et al. 2024). In the future, other cellular imaging approaches can be applied for specific rG4 structure imaging, for example, CRISPR technology (Huang et al. 2023; Van Tricht et al. 2023). By modifying catalytically inactive Cas proteins (dCas) with fluorescent tags, and a guide RNA that is designed to bind to the target of interest, visualization of a specific target without being cleaved can be achieved, including the genome (Chen et al. 2013; Wang et al. 2019a) and transcripts (Yang et al. 2019; Huang et al. 2023; Van Tricht et al. 2023). CRISPR imaging allows flexible design, engineering, and a combination of targeting probe and signaling molecule, which can be further explored and applied in rG4 imaging.
While advancements have been made in selectively visualizing rG4 structures, quantitative imaging of rG4s has not been developed. Quantifying rG4 structures, abundance and distribution in cells is important for understanding their biological roles in cell metabolism (Varshney et al. 2020; Kosiol et al. 2021). One of the most representative rG4 structures in cells is the TERRA rG4, which has proven to be involved in telomere maintenance (Azzalin 2007; Schoeftner 2008; Xu 2010; Biffi 2012). An L-RNA aptamer, L-Ap3-7, was developed to bind TERRA rG4 (Chan and Kwok 2020), which is a potential ligand for the imaging of TERRA rG4 with high specificity for understanding the localization and abundance of TERRA rG4 to telomere, as well as the studies on genome stability and cancer research. In neurological diseases, abnormal levels of G4-forming RNAs play a crucial role in disease progression and pathogenesis by altering translation and stress granule formation, for example, in repeat expansion disorders such as the (CGG)n repeats found in FMR1 causing fragile X-associated disorders (FXds) (Broniarek et al. 2024), and the (GGGGCC)n repeats found in C9orf72 causing ALS and FTD (DeJesus-Hernandez et al. 2011; Reddy et al. 2013; Zamiri et al. 2014; Wortman et al. 2020). It was suggested that only short C9orf72 repeats fold into G4 structures while longer repeats form predominantly hairpins with bulged Gs (Wang et al. 2019b). It is important to cautiously consider and further understand the regulatory contributions of G4-hairpin structure switch in C9orf72 to disease mechanisms for therapy development. Recently, live cell imaging on the elongation and frameshifting dynamics of C9orf72 repeats was performed to understand their production of toxic dipeptide repeat (Latallo et al. 2023). It is believed that live cell imaging of their rG4 structures is also possible if ligands that specifically target these rG4s can be developed. Besides, rG4 structures have also been found in the viral RNAs such as SARS-CoV-2 (Tong et al. 2023), HIV (Amrane et al. 2022), HCV (Wang et al. 2016a), etc., and they contribute to the viral replication by affecting the host metabolisms. Currently, small molecule–based probes were developed to image the HCV viral rG4 (Luo et al. 2019; Zhang et al. 2021); further exploration and the imaging of other viral rG4s will be beneficial to understanding viral biology and the identification of potential diagnostic and therapeutic targets.
Another challenge in rG4 imaging is tracking the dynamic nature of rG4 structures, which are tightly linked to their biological functions (Dumas et al. 2021). Several proteins have been identified to interact with rG4 structures, such as hnRNP H/F (Herviou et al. 2020), DHX36 (Murat et al. 2018; Chen et al. 2021a; Lyu et al. 2022), nucleolin (Ghosh et al. 2023), etc. These proteins were reported to regulate the translation of G4-containing mRNA by stabilizing or unwinding rG4 structures (Murat et al. 2018; Chen et al. 2021a; Lyu et al. 2022). Some of them are also involved in G4-related RNA processing, such as miRNA biogenesis (Liu et al. 2020). Therefore, monitoring rG4 dynamics and stability in live cells is important to understand rG4–protein biology as well as their relation with cell metabolisms. The SiR-PyPDS was developed to monitor dG4 dynamics during cell cycles (Di Antonio et al. 2020); their relationship with G4-BPs can be further explored in the future. Besides, the QUMA-1 fluorescent probe was developed and applied to monitor DHX36-responsive rG4 folding in cells (Chen et al. 2018b). It is believed that the development of other highly sensitive and responsive probes to monitor real-time rG4 dynamics against different rG4-BPs is possible and significant.
Lastly, super-resolution microscopy such as PALM, STORM, and SIM have been applied to visualize biological structures including RNAs or proteins (Schermelleh et al. 2019; Eng et al. 2019; Klevanski et al. 2020; Sunbul et al. 2021; He et al. 2022), and with some adaptations in the molecular design and strategy, rG4 imaging should be feasible. With the help of super-resolution imaging, rG4 dynamics at the single molecule level can be studied with high spatial resolution, and the detailed rG4 formation, dissociation, and localization in cells can be revealed, especially for disease-related rG4s. Additionally, dual-color or multicolor imaging of different rG4 structures or other cellular components, including G4-BPs, may be a new direction. Currently, the studies on these G4–protein interactions are mainly based on chromatography, mass spectrometry and pull-down assays (Shu et al. 2022). However, these assays cannot reveal the real G4–protein interaction in complex cellular environments. By performing super-resolution imaging on rG4–protein complexes, rG4–protein interactions in biological systems and their related biological pathways can be better understood, which can enhance our understanding on disease pathogenesis and the corresponding therapy monitoring or development.
SUMMARY
The emerging interest in and exciting findings regarding rG4 biology and function have greatly stimulated the development of new targeting and imaging tools for these functional structures in cells. As described, recent advances in the targeting and imaging of rG4s have unveiled their importance in studying rG4 biology in cells, as well as their potential as therapeutic targets and biomarkers for diseases such as cancer and neurodegenerative disorders. We are cautiously positive that optimization of the described methods and the development of innovative tools will enable us to solve the challenges presented in the above section. With the extensive networks of international scientists working in related research areas, we look forward to more scientific breakthroughs in rG4 targeting and imaging in the near future.
ACKNOWLEDGMENTS
We apologize to colleagues whose works are not cited due to space limitations. This work was supported by the National Natural Science Foundation of China (NSFC) Projects (32471343, 32222089); Research Grants Council (RGC) of the Hong Kong Special Administrative Region (RFS2425-1S02, CityU 11100123, CityU 11100222, CityU 11100421); Croucher Foundation Project (9509003); State Key Laboratory of Marine Pollution Seed Collaborative Research Fund (SCRF/0037, SCRF/0040, SCRF0070); and City University of Hong Kong projects (7030001, 9678302).
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.080587.125.
Freely available online through the RNA Open Access option.
REFERENCES
- Amrane S, Jaubert C, Bedrat A, Rundstadler T, Recordon-Pinson P, Aknin C, Guedin A, De Rache A, Bartolucci L, Diene I, et al. 2022. Deciphering RNA G-quadruplex function during the early steps of HIV-1 infection. Nucleic Acids Res 50: 12328–12343. 10.1093/nar/gkac1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amu G, Zhang G, Jing N, Ma Y. 2024. Developing stapled aptamers with a constrained conformation for osteogenesis imperfect therapeutics. J Med Chem 67: 18883–18894. 10.1021/acs.jmedchem.4c01293 [DOI] [PubMed] [Google Scholar]
- Andreeva DV, Tikhomirov AS, Shchekotikhin AE. 2021. Ligands of G-quadruplex nucleic acids. Russ Chem Rev 90: 1. 10.1070/rcr4968 [DOI] [Google Scholar]
- Antariksa NF, Di Antonio M. 2024. The emerging roles of multimolecular G-quadruplexes in transcriptional regulation and chromatin organization. Acc Chem Res 57: 3397–3406. 10.1021/acs.accounts.4c00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyo EO, Booy EP, Patel TR, Dzananovic E, McRae EK, Meier M, McEleney K, Stetefeld J, McKenna SA. 2015. Biophysical characterization of G-quadruplex recognition in the PITX1 mRNA by the specificity domain of the helicase RHAU. PLoS One 10: e0144510. 10.1371/journal.pone.0144510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamitsu S, Bando T, Sugiyama H. 2019. Ligand design to acquire specificity to intended G-quadruplex structures. Chemistry 25: 417–430. 10.1002/chem.201802691 [DOI] [PubMed] [Google Scholar]
- Asamitsu S, Yabuki Y, Matsuo K, Kawasaki M, Hirose Y, Kashiwazaki G, Chandran A, Bando T, Wang DO, Sugiyama H, et al. 2023. RNA G-quadruplex organizes stress granule assembly through DNAPTP6 in neurons. Sci Adv 9: eade2035. 10.1126/sciadv.ade2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Chou HL, Bevilacqua PC. 2023. Rock, scissors, paper: how RNA structure informs function. Plant Cell 35: 1671–1707. 10.1093/plcell/koad026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attique M, Farooq MS, Khelifi A, Abid A. 2020. Prediction of therapeutic peptides using machine learning: computational models, datasets, and feature encodings. IEEE Access 8: 148570–148594. 10.1109/access.2020.3015792 [DOI] [Google Scholar]
- Azzalin C, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. 2007. Telomeric repeat–containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 318: 798–801. 10.1126/science.1147182 [DOI] [PubMed] [Google Scholar]
- Bai LP, Liu J, Han L, Ho HM, Wang R, Jiang ZH. 2014. Mass spectrometric studies on effects of counter ions of TMPyP4 on binding to human telomeric DNA and RNA G-quadruplexes. Anal Bioanal Chem 406: 5455–5463. 10.1007/s00216-014-7943-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N, Panda S, Chatterjee S. 2022. Frontiers in G-quadruplex therapeutics in cancer: selection of small molecules, peptides and aptamers. Chem Biol Drug Des 99: 1–31. 10.1111/cbdd.13910 [DOI] [PubMed] [Google Scholar]
- Basak S, Biswas S, Naskar J. 2023. Peptides and peptide derivatives as Gquadruplex targeting ligands: a brief review. ChemistrySelect 8: e202302177. 10.1002/slct.202302177 [DOI] [Google Scholar]
- Bashir A, Yang Q, Wang J, Hoyer S, Chou W, McLean C, Davis G, Gong Q, Armstrong Z, Jang J, et al. 2021. Machine learning guided aptamer refinement and discovery. Nat Commun 12: 2366. 10.1038/s41467-021-22555-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat V, Disney MD. 2015. RNA structures as mediators of neurological diseases and as drug targets. Neuron 87: 28–46. 10.1016/j.neuron.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G, Tannahill D, Balasubramanian S. 2012. An intramolecular G-quadruplex structure is required for binding of telomeric repeat-containing RNA to the telomeric protein TRF2. J Am Chem Soc 134: 11974–11976. 10.1021/ja305734x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G, Tannahill D, McCafferty J, Balasubramanian S. 2013. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat Chem 5: 182–186. 10.1038/nchem.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffi G, Di Antonio M, Tannahill D, Balasubramanian S. 2014. Visualization and selective chemical targeting of RNA G-quadruplex structures in the cytoplasm of human cells. Nat Chem 6: 75–80. 10.1038/nchem.1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdon S, Herviou P, Dumas L, Destefanis E, Zen A, Cammas A, Millevoi S, Dassi E. 2023. QUADRatlas: the RNA G-quadruplex and RG4-binding proteins database. Nucleic Acids Res 51: D240–D247. 10.1093/nar/gkac782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford T, Summers PA, Majid A, Sherin PS, Lam JYL, Aggarwal S, Vannier JB, Vilar R, Kuimova MK. 2024. Imaging G-quadruplex nucleic acids in live cells using thioflavin T and fluorescence lifetime imaging microscopy. Anal Chem 96: 20223–20229. 10.1021/acs.analchem.4c04207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broniarek I, Niewiadomska D, Sobczak K. 2024. Contribution of DNA/RNA structures formed by expanded CGG/CCG repeats within the FMR1 locus in the pathogenesis of fragile X-associated disorders. Wiley Interdiscip Rev RNA 15: e1874. 10.1002/wrna.1874 [DOI] [PubMed] [Google Scholar]
- Bugaut A, Balasubramanian S. 2012. 5′-UTR RNA G-quadruplexes: translation regulation and targeting. Nucleic Acids Res 40: 4727–4741. 10.1093/nar/gks068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugaut A, Rodriguez R, Kumari S, Hsu ST, Balasubramanian S. 2010. Small molecule-mediated inhibition of translation by targeting a native RNA G-quadruplex. Org Biomol Chem 8: 2771–2776. 10.1039/c002418j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovskaya E, Solda P, Scalabrin M, Nadai M, Richter SN. 2019. HIV-1 nucleocapsid protein unfolds stable RNA G-quadruplexes in the viral genome and is inhibited by G-quadruplex ligands. ACS Infect Dis 5: 2127–2135. 10.1021/acsinfecdis.9b00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni E, De Paepe L, Manicardi A, Madder A. 2021. Beyond small molecules: targeting G-quadruplex structures with oligonucleotides and their analogues. Nucleic Acids Res 49: 6638–6659. 10.1093/nar/gkab334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammas A, Millevoi S. 2017. RNA G-quadruplexes: emerging mechanisms in disease. Nucleic Acids Res 45: 1584–1595. 10.1093/nar/gkw1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Zhang Y, Ding Y, Wan Y. 2024. Identification of RNA structures and their roles in RNA functions. Nat Rev Mol Cell Biol 25: 784–801. 10.1038/s41580-024-00748-6 [DOI] [PubMed] [Google Scholar]
- Carvalho J, Santos T, Carrilho R, Sousa F, Salgado GF, Queiroz JA, Cruz C. 2020. Ligand screening to pre-miRNA 149 G-quadruplex investigated by molecular dynamics. J Biomol Struct Dyn 38: 2276–2286. 10.1080/07391102.2019.1632743 [DOI] [PubMed] [Google Scholar]
- Caterino M, Paeschke K. 2022. Action and function of helicases on RNA G-quadruplexes. Methods 204: 110–125. 10.1016/j.ymeth.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR, Steitz JA, Atkins JF. 2018. RNA worlds: new tools for deep exploration. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Chakraborty P, Grosse F. 2011. Human DHX9 helicase preferentially unwinds RNA-containing displacement loops (R-loops) and G-quadruplexes. DNA Repair (Amst) 10: 654–665. 10.1016/j.dnarep.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Chalupnikova K, Lattmann S, Selak N, Iwamoto F, Fujiki Y, Nagamine Y. 2008. Recruitment of the RNA helicase RHAU to stress granules via a unique RNA-binding domain. J Biol Chem 283: 35186–35198. 10.1074/jbc.M804857200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Kwok CK. 2020. Specific binding of a d-RNA G-quadruplex structure with an l-RNA aptamer. Angew Chem Int Ed Engl 59: 5293–5297. 10.1002/anie.201914955 [DOI] [PubMed] [Google Scholar]
- Che T, Wang YQ, Huang ZL, Tan JH, Huang ZS, Chen SB. 2018. Natural alkaloids and heterocycles as G-quadruplex ligands and potential anticancer agents. Molecules 23: 493. 10.3390/molecules23020493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Gilbert LA, Cimini BA, Schnitzbauer J, Zhang W, Li GW, Park J, Blackburn EH, Weissman JS, Qi LS, et al. 2013. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 155: 1479–1491. 10.1016/j.cell.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SB, Hu MH, Liu GC, Wang J, Ou TM, Gu LQ, Huang ZS, Tan JH. 2016. Visualization of NRAS RNA G-quadruplex structures in cells with an engineered fluorogenic hybridization probe. J Am Chem Soc 138: 10382–10385. 10.1021/jacs.6b04799 [DOI] [PubMed] [Google Scholar]
- Chen MC, Tippana R, Demeshkina NA, Murat P, Balasubramanian S, Myong S, Ferre-D'Amare AR. 2018a. Structural basis of G-quadruplex unfolding by the DEAH/RHA helicase DHX36. Nature 558: 465–469. 10.1038/s41586-018-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Chen SB, Dai J, Yuan JH, Ou TM, Huang ZS, Tan JH. 2018b. Tracking the dynamic folding and unfolding of RNA G-quadruplexes in live cells. Angew Chem Int Ed Engl 57: 4702–4706. 10.1002/anie.201801999 [DOI] [PubMed] [Google Scholar]
- Chen X, Yuan J, Xue G, Campanario S, Wang D, Wang W, Mou X, Liew SW, Umar MI, Isern J, et al. 2021a. Translational control by DHX36 binding to 5′UTR G-quadruplex is essential for muscle stem-cell regenerative functions. Nat Commun 12: 5043. 10.1038/s41467-021-25170-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hu L, Zhang BT, Lu A, Wang Y, Yu Y, Zhang G. 2021b. Artificial intelligence in aptamer-target binding prediction. Int J Mol Sci 22: 3605. 10.3390/ijms22073605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Tang GX, Dai J, Dai LT, Wu TY, Li WW, Ou TM, Huang ZS, Tan JH, Chen SB. 2023. Discovery of clinically used octenidine as NRAS repressor that effectively inhibits NRAS-mutant melanoma. J Med Chem 66: 5171–5184. 10.1021/acs.jmedchem.3c00094 [DOI] [PubMed] [Google Scholar]
- Chong PA, Vernon RM, Forman-Kay JD. 2018. RGG/RG motif regions in RNA binding and phase separation. J Mol Biol 430: 4650–4665. 10.1016/j.jmb.2018.06.014 [DOI] [PubMed] [Google Scholar]
- Collie G, Reszka AP, Haider SM, Gabelica V, Parkinson GN, Neidle S. 2009. Selectivity in small molecule binding to human telomeric RNA and DNA quadruplexes. Chem Commun 48: 7482–7484. 10.1039/b901889a [DOI] [PubMed] [Google Scholar]
- Cusanelli E, Chartrand P. 2015. Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity. Front Genet 6: 143. 10.3389/fgene.2015.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabral P, Babu J, Zareie A, Verma SC. 2020. LANA and hnRNP A1 regulate the translation of LANA mRNA through G-quadruplexes. J Virol 94: e01508-19. 10.1128/JVI.01508-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Teng X, Li J. 2022. Single-cell visualization of monogenic RNA G-quadruplex and occupied G-quadruplex ratio through a module-assembled multifunctional probes assay (MAMPA). Angew Chem Int Ed Engl 61: e202111132. 10.1002/anie.202111132 [DOI] [PubMed] [Google Scholar]
- Dai Y, Teng X, Zhang Q, Hou H, Li J. 2023. Advances and challenges in identifying and characterizing G-quadruplex-protein interactions. Trends Biochem Sci 48: 894–909. 10.1016/j.tibs.2023.06.007 [DOI] [PubMed] [Google Scholar]
- Daigle JG, Krishnamurthy K, Ramesh N, Casci I, Monaghan J, McAvoy K, Godfrey EW, Daniel DC, Johnson EM, Monahan Z, et al. 2016. Pur-alpha regulates cytoplasmic stress granule dynamics and ameliorates FUS toxicity. Acta Neuropathol 131: 605–620. 10.1007/s00401-015-1530-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cian A, Guittat L, Shin-ya K, Riou J-F, Mergny J-L. 2005. Affinity and selectivity of G4 ligands measured by FRET. Nucleic Acids Symp Ser 49: 235–236. 10.1093/nass/49.1.235 [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, Nicholson AM, Finch NA, Flynn H, Adamson J, et al. 2011. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72: 245–256. 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magalhães CS, Barbosa HJ, Dardenne LE. 2004. A genetic algorithm for the ligand-protein docking problem. Genet Mol Biol 27: 605–610. 10.1002/prot.21423 [DOI] [Google Scholar]
- Di Antonio M, Biffi G, Mariani A, Raiber EA, Rodriguez R, Balasubramanian S. 2012. Selective RNA versus DNA G-quadruplex targeting by in situ click chemistry. Angew Chem Int Ed Engl 51: 11073–11078. 10.1002/anie.201206281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Antonio M, Ponjavic A, Radzevičius A, Ranasinghe RT, Catalano M, Zhang X, Shen J, Needham L-M, Lee SF, Klenerman D, et al. 2020. Single-molecule visualization of DNA G-quadruplex formation in live cells. Nat Chem 12: 832–837. 10.1038/s41557-020-0506-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AR, Cadoni E, Ressurreicao AS, Moreira R, Paulo A. 2018. Design of modular G-quadruplex ligands. ChemMedChem 13: 869–893. 10.1002/cmdc.201700747 [DOI] [PubMed] [Google Scholar]
- Dumas L, Herviou P, Dassi E, Cammas A, Millevoi S. 2021. G-quadruplexes in RNA biology: recent advances and future directions. Trends Biochem Sci 46: 270–283. 10.1016/j.tibs.2020.11.001 [DOI] [PubMed] [Google Scholar]
- Elliott D, Ladomery M. 2016. Molecular biology of RNA. Oxford University Press, Oxford. [Google Scholar]
- Eng CL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, Yun J, Cronin C, Karp C, Yuan GC, et al. 2019. Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature 568: 235–239. 10.1038/s41586-019-1049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Wu Q, Wang F, Liu Y, Zhang H, Yang C, Zhu Z. 2024. AptamerRIBOTAC strategy enabling tumorspecific targeted degradation of microRNA for precise cancer therapy. Small Methods 25: e2400349. 10.1002/smtd.202400349 [DOI] [PubMed] [Google Scholar]
- Faudale M, Cogoi S, Xodo LE. 2012. Photoactivated cationic alkyl-substituted porphyrin binding to g4-RNA in the 5′-UTR of KRAS oncogene represses translation. Chem Commun 48: 874–876. 10.1039/c1cc15850c [DOI] [PubMed] [Google Scholar]
- Fay MM, Lyons SM, Ivanov P. 2017. RNA G-quadruplexes in biology: principles and molecular mechanisms. J Mol Biol 429: 2127–2147. 10.1016/j.jmb.2017.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Kwok CK. 2024. A selective HIV-1 RNA G-quadruplex-targeting L-aptamer-D-antisense conjugate inhibits HIV-1 minus strand transfer. Chem Commun 60: 13558–13561. 10.1039/d4cc03448a [DOI] [PubMed] [Google Scholar]
- Ferino A, Nicoletto G, D'Este F, Zorzet S, Lago S, Richter SN, Tikhomirov A, Shchekotikhin A, Xodo LE. 2020. Photodynamic therapy for ras-driven cancers: targeting G-quadruplex RNA structures with bifunctional alkyl-modified porphyrins. J Med Chem 63: 1245–1260. 10.1021/acs.jmedchem.9b01577 [DOI] [PubMed] [Google Scholar]
- Fernando H, Rodriguez R, Balasubramanian S. 2008. Selective recognition of a DNA G-quadruplex by an engineered antibody. Biochemistry 47: 9365–9371. 10.1021/bi800983u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando H, Sewitz S, Darot J, Tavare S, Huppert JL, Balasubramanian S. 2009. Genome-wide analysis of a G-quadruplex-specific single-chain antibody that regulates gene expression. Nucleic Acids Res 37: 6716–6722. 10.1093/nar/gkp740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fracchioni G, Vailati S, Grazioli M, Pirota V. 2024. Structural unfolding of G-quadruplexes: from small molecules to antisense strategies. Molecules 29: 3488. 10.3390/molecules29153488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasson I, Pirota V, Richter SN, Doria F. 2022. Multimeric G-quadruplexes: a review on their biological roles and targeting. Int J Biol Macromol 204: 89–102. 10.1016/j.ijbiomac.2022.01.197 [DOI] [PubMed] [Google Scholar]
- Fratta P, Mizielinska S, Nicoll AJ, Zloh M, Fisher EM, Parkinson G, Isaacs AM. 2012. C9orf72 hexanucleotide repeat associated with amyotrophic lateral sclerosis and frontotemporal dementia forms RNA G-quadruplexes. Sci Rep 2: 1016. 10.1038/srep01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli S, Melidis L, Flynn SM, Varshney D, Simeone A, Spiegel J, Madden SK, Tannahill D, Balasubramanian S. 2022. DNA G-quadruplex recognition in vitro and in live cells by a structure-specific nanobody. J Am Chem Soc 144: 23096–23103. 10.1021/jacs.2c10656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Singh M. 2020. Structure specific recognition of telomeric repeats containing RNA by the RGG-box of hnRNPA1. Nucleic Acids Res 48: 4492–4506. 10.1093/nar/gkaa134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Ekka MK, Tawani A, Kumar A, Chakraborty D, Maiti S. 2019. Restoration of miRNA-149 expression by TmPyP4 induced unfolding of quadruplex within its precursor. Biochemistry 58: 514–525. 10.1021/acs.biochem.8b00880 [DOI] [PubMed] [Google Scholar]
- Ghosh A, Pandey SP, Joshi DC, Rana P, Ansari AH, Sundar JS, Singh P, Khan Y, Ekka MK, Chakraborty D, et al. 2023. Identification of G-quadruplex structures in MALAT1 lncRNA that interact with nucleolin and nucleophosmin. Nucleic Acids Res 51: 9415–9431. 10.1093/nar/gkad639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R, Hudish LI, Guzman BB, Raj N, Bassell GJ, Russ HA, Dominguez D, Taliaferro JM. 2020. FMRP promotes RNA localization to neuronal projections through interactions between its RGG domain and G-quadruplex RNA sequences. eLife 9: e52621. 10.7554/eLife.52621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg DC, Fones L, Vivinetto AL, Caufield JT, Ratan RR, Cave JW. 2020. Manipulating adult neural stem and progenitor cells with G-quadruplex ligands. ACS Chem Neurosci 11: 1504–1518. 10.1021/acschemneuro.0c00194 [DOI] [PubMed] [Google Scholar]
- Gomez D, Lemarteleur T, Lacroix L, Mailliet P, Mergny JL, Riou JF. 2004. Telomerase downregulation induced by the G-quadruplex ligand 12459 in A549 cells is mediated by hTERT RNA alternative splicing. Nucleic Acids Res 32: 371–379. 10.1093/nar/gkh181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez D, Guedin A, Mergny JL, Salles B, Riou JF, Teulade-Fichou MP, Calsou P. 2010. A G-quadruplex structure within the 5′-UTR of TRF2 mRNA represses translation in human cells. Nucleic Acids Res 38: 7187–7198. 10.1093/nar/gkq563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JU, Bartel DP. 2016. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science 353: aaf5371. 10.1126/science.aaf5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Lu H. 2017. Conjunction of potential G-quadruplex and adjacent cis-elements in the 5′ UTR of hepatocyte nuclear factor 4-alpha strongly inhibit protein expression. Sci Rep 7: 17444. 10.1038/s41598-017-17629-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S. 2018. Computational methods to study G-quadruplex–ligand complexes. J Indian Inst Sci 98: 325–339. 10.1007/s41745-018-0083-3 [DOI] [Google Scholar]
- Haldar S, Zhang Y, Xia Y, Islam B, Liu S, Gervasio FL, Mulholland AJ, Waller ZAE, Wei D, Haider S. 2022. Mechanistic insights into the ligand-induced unfolding of an RNA G-quadruplex. J Am Chem Soc 144: 935–950. 10.1021/jacs.1c11248 [DOI] [PubMed] [Google Scholar]
- Haudecoeur R, Stefan L, Monchaud D. 2013. Multitasking water-soluble synthetic G-quartets: from preferential RNA-quadruplex interaction to biocatalytic activity. Chemistry 19: 12739–12747. 10.1002/chem.201300791 [DOI] [PubMed] [Google Scholar]
- He YD, Zheng KW, Wen CJ, Li XM, Gong JY, Hao YH, Zhao Y, Tan Z. 2020. Selective targeting of guanine-vacancy-bearing G-quadruplexes by G-quartet complementation and stabilization with a guanine-peptide conjugate. J Am Chem Soc 142: 11394–11403. 10.1021/jacs.0c00774 [DOI] [PubMed] [Google Scholar]
- He S, Bhatt R, Brown C, Brown EA, Buhr DL, Chantranuvatana K, Danaher P, Dunaway D, Garrison RG, Geiss G, et al. 2022. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat Biotechnol 40: 1794–1806. 10.1038/s41587-022-01483-z [DOI] [PubMed] [Google Scholar]
- Heddi B, Cheong VV, Martadinata H, Phan AT. 2015. Insights into G-quadruplex specific recognition by the DEAH-box helicase RHAU: solution structure of a peptide-quadruplex complex. Proc Natl Acad Sci 112: 9608–9613. 10.1073/pnas.1422605112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddi B, Cheong VV, Schmitt E, Mechulam Y, Phan AT. 2020. Recognition of different base tetrads by RHAU (DHX36): X-ray crystal structure of the G4 recognition motif bound to the 3′-end tetrad of a DNA G-quadruplex. J Struct Biol 209: 107399. 10.1016/j.jsb.2019.10.001 [DOI] [PubMed] [Google Scholar]
- Henderson A, Wu Y, Huang YC, Chavez EA, Platt J, Johnson FB, Brosh RM Jr, Sen D, Lansdorp PM. 2014. Detection of G-quadruplex DNA in mammalian cells. Nucleic Acids Res 42: 860–869. 10.1093/nar/gkt957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herviou P, Le Bras M, Dumas L, Hieblot C, Gilhodes J, Cioci G, Hugnot JP, Ameadan A, Guillonneau F, Dassi E, et al. 2020. hnRNP H/F drive RNA G-quadruplex-mediated translation linked to genomic instability and therapy resistance in glioblastoma. Nat Commun 11: 2661. 10.1038/s41467-020-16168-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Dai R, Zhang Z, Zhang H, Zhang M, Li Z, Zhao K, Xiong W, Cheng S, Wang B, et al. 2023. CRISPR/Cas-based techniques for live-cell imaging and bioanalysis. Int J Mol Sci 24: 13447. 10.3390/ijms241713447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatore JA, McAninch DS, Valdez-Sinon AN, Bassell GJ, Mihailescu MR. 2020. FUS recognizes G quadruplex structures within neuronal mRNAs. Front Mol Biosci 7: 6. 10.3389/fmolb.2020.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert C, Bedrat A, Bartolucci L, Di Primo C, Ventura M, Mergny JL, Amrane S, Andreola ML. 2018. RNA synthesis is modulated by G-quadruplex formation in Hepatitis C virus negative RNA strand. Sci Rep 8: 8120. 10.1038/s41598-018-26582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lyu K, Zhao H, Kwok CK. 2021. Circular L-RNA aptamer promotes target recognition and controls gene activity. Nucleic Acids Res 49: 7280–7291. 10.1093/nar/gkab593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Yuan JH, Chen SB, Tan JH, Kwok CK. 2023. Selective targeting of parallel G-quadruplex structure using L-RNA aptamer. Nucleic Acids Res 51: 11439–11452. 10.1093/nar/gkad900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wang B, Lo KW, Tsang CM, Kwok CK. 2024. Pre-defined stem-loop structure library for the discovery of L-RNA aptamers that target RNA G-quadruplexes. Angew Chem Int Ed Engl 64: e202417247. 10.1002/anie.202417247 [DOI] [PubMed] [Google Scholar]
- Johnson K, Seidel JM, Cech TR. 2024. Small molecule telomerase inhibitors are also potent inhibitors of telomeric C-strand synthesis. RNA 30: 1213–1226. 10.1261/rna.080043.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda Y, Sato S, Asano L, Morimura Y, Furuta T, Sugiyama H, Hagihara M, Uesugi M. 2016. A small molecule that represses translation of G-quadruplex-containing mRNA. J Am Chem Soc 138: 9037–9040. 10.1021/jacs.6b04506 [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Sugimoto W, Yasui T, Murata K, Itoh K, Takagi K, Tsuruoka T, Akamatsu K, Tateishi-Karimata H, Sugimoto N, et al. 2018. An anionic phthalocyanine decreases NRAS expression by breaking down its RNA G-quadruplex. Nat Commun 9: 2271. 10.1038/s41467-018-04771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi K, Urano R, Kinoshita N, Kuwamoto S, Torii T, Hashimoto Y, Taniguchi S, Tsuruta M, Miyoshi D. 2020. Photosensitizers based on G-quadruplex ligand for cancer photodynamic therapy. Genes (Basel) 11: 1340. 10.3390/genes11111340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharel P, Balaratnam S, Beals N, Basu S. 2020. The role of RNA G-quadruplexes in human diseases and therapeutic strategies. Wiley Interdiscip Rev RNA 11: e1568. 10.1002/wrna.1568 [DOI] [PubMed] [Google Scholar]
- Kharel P, Fay M, Manasova EV, Anderson PJ, Kurkin AV, Guo JU, Ivanov P. 2023. Stress promotes RNA G-quadruplex folding in human cells. Nat Commun 14: 205. 10.1038/s41467-023-35811-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevanski M, Herrmannsdoerfer F, Sass S, Venkataramani V, Heilemann M, Kuner T. 2020. Automated highly multiplexed super-resolution imaging of protein nano-architecture in cells and tissues. Nat Commun 11: 1552. 10.1038/s41467-020-15362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiol N, Juranek S, Brossart P, Heine A, Paeschke K. 2021. G-quadruplexes: a promising target for cancer therapy. Mol Cancer 20: 40. 10.1186/s12943-021-01328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok CK, Marsico G, Sahakyan AB, Chambers VS, Balasubramanian S. 2016. rG4-seq reveals widespread formation of G-quadruplex structures in the human transcriptome. Nat Methods 13: 841–844. 10.1038/nmeth.3965 [DOI] [PubMed] [Google Scholar]
- Kwok CK, Marsico G, Balasubramanian S. 2018. Detecting RNA G-quadruplexes (rG4s) in the transcriptome. Cold Spring Harb Perspect Biol 10: a032284. 10.1101/cshperspect.a032284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SY, Umar MI, Zhao H, Zhao J, Kwok CK. 2024. Capture of RNA G-quadruplex structures using an l-RNA aptamer. RSC Chem Biol 5: 1045–1051. 10.1039/d4cb00161c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latallo MJ, Wang S, Dong D, Nelson B, Livingston NM, Wu R, Zhao N, Stasevich TJ, Bassik MC, Sun S, et al. 2023. Single-molecule imaging reveals distinct elongation and frameshifting dynamics between frames of expanded RNA repeats in C9ORF72-ALS/FTD. Nat Commun 14: 5581. 10.1038/s41467-023-41339-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattmann S, Giri B, Vaughn JP, Akman SA, Nagamine Y. 2010. Role of the amino terminal RHAU-specific motif in the recognition and resolution of guanine quadruplex-RNA by the DEAH-box RNA helicase RHAU. Nucleic Acids Res 38: 6219–6233. 10.1093/nar/gkq372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HL, Zhao H, Feng H, Kwok CK. 2024. Specific targeting and imaging of RNA G-quadruplex (rG4) structure using non-G4-containing l-RNA aptamer and fluorogenic L-aptamer. Small Methods 9: e2401097. 10.1002/smtd.202401097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledvinka J, Rota Sperti F, Paragi G, Marc P, Cheron N, Valverde I, Menova P, Monchaud D. 2024. Fluorescence detection of DNA/RNA G-quadruplexes (G4s) by twice-as-smart ligands. ChemMedChem 20: e202400829. 10.1002/cmdc.202400829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Weissbein U, Blum R, Lee JT. 2024. G-quadruplex folding in Xist RNA antagonizes PRC2 activity for stepwise regulation of X chromosome inactivation. Mol Cell 84: 1870–1885.e1879. 10.1016/j.molcel.2024.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejault P, Mitteaux J, Sperti FR, Monchaud D. 2021. How to untie G-quadruplex knots and why? Cell Chem Biol 28: 436–455. 10.1016/j.chembiol.2021.01.015 [DOI] [PubMed] [Google Scholar]
- Li F, Tan W, Chen H, Zhou J, Xu M, Yuan G. 2019. Up- and downregulation of mature miR-1587 function by modulating its G-quadruplex structure and using small molecules. Int J Biol Macromol 121: 127–134. 10.1016/j.ijbiomac.2018.10.017 [DOI] [PubMed] [Google Scholar]
- Li F, Guo D, Xie T, Zhang S, Wang A, Li Y, Zhou J. 2023. G-quadruplex from precursor miR-1587 modulated its maturation and function. Int J Biol Macromol 231: 123279. 10.1016/j.ijbiomac.2023.123279 [DOI] [PubMed] [Google Scholar]
- Liano D, Chowdhury S, Di Antonio M. 2021. Cockayne Syndrome B protein selectively resolves and interact with intermolecular DNA G-quadruplex structures. J Am Chem Soc 143: 20988–21002. 10.1021/jacs.1c10745 [DOI] [PubMed] [Google Scholar]
- Liew SW, Cao D, Petersen RJ, Butcher SE, Kennedy SG, Kwok CK. 2025. A novel L-RNA aptamer to regulate the pUG fold RNA-induced gene expression in vivo. Nucleic Acids Res 53: gkaf137. 10.1093/nar/gkaf137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lista MJ, Martins RP, Billant O, Contesse MA, Findakly S, Pochard P, Daskalogianni C, Beauvineau C, Guetta C, Jamin C, et al. 2017. Nucleolin directly mediates Epstein-Barr virus immune evasion through binding to G-quadruplexes of EBNA1 mRNA. Nat Commun 8: 16043. 10.1038/ncomms16043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Zhao Q, Zhang TP, Wu Y, Xiong YX, Wang SK, Ge YL, He JH, Lv P, Ou TM, et al. 2016a. Conformation selective antibody enables genome profiling and leads to discovery of parallel G-quadruplex in human telomeres. Cell Chem Biol 23: 1261–1270. 10.1016/j.chembiol.2016.08.013 [DOI] [PubMed] [Google Scholar]
- Liu J, Wei T, Zhao J, Huang Y, Deng H, Kumar A, Wang C, Liang Z, Ma X, Liang XJ. 2016b. Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance. Biomaterials 91: 44–56. 10.1016/j.biomaterials.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Liu G, Du W, Xu H, Sun Q, Tang D, Zou S, Zhang Y, Ma M, Zhang G, Du X, et al. 2020. RNA G-quadruplex regulates microRNA-26a biogenesis and function. J Hepatol 73: 371–382. 10.1016/j.jhep.2020.02.032 [DOI] [PubMed] [Google Scholar]
- Liu G, Du W, Sang X, Tong Q, Wang Y, Chen G, Yuan Y, Jiang L, Cheng W, Liu D, et al. 2022. RNA G-quadruplex in TMPRSS2 reduces SARS-CoV-2 infection. Nat Commun 13: 1444. 10.1038/s41467-022-29135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qi Q, Xiong W, Shen W, Zhang K, Fan R, Zhang Y, Zhao Y, Xu X, Li M, et al. 2024. Unveiling a potent small molecule disruptor for RNA G-quadruplexes tougher than DNA G-quadruplex disruption. ACS Chem Biol 19: 2032–2040. 10.1021/acschembio.4c00357 [DOI] [PubMed] [Google Scholar]
- Lu X, Wu X, Kuang S, Lei C, Nie Z. 2022. Visualization of deep tissue G-quadruplexes with a novel large stokes-shifted red fluorescent benzothiazole derivative. Anal Chem 94: 10283–10290. 10.1021/acs.analchem.2c02049 [DOI] [PubMed] [Google Scholar]
- Luo X, Xue B, Feng G, Zhang J, Lin B, Zeng P, Li H, Yi H, Zhang XL, Zhu H, et al. 2019. Lighting up the native viral RNA genome with a fluorogenic probe for the live-cell visualization of virus infection. J Am Chem Soc 141: 5182–5191. 10.1021/jacs.8b10265 [DOI] [PubMed] [Google Scholar]
- Lyu K, Chen SB, Chan CY, Tan JH, Kwok CK. 2019. Structural analysis and cellular visualization of APP RNA G-quadruplex. Chem Sci 10: 11095–11102. 10.1039/c9sc02768h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu K, Chow EY, Mou X, Chan TF, Kwok CK. 2021. RNA G-quadruplexes (rG4s): genomics and biological functions. Nucleic Acids Res 49: 5426–5450. 10.1093/nar/gkab187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu K, Chen SB, Chow EY, Zhao H, Yuan JH, Cai M, Shi J, Chan TF, Tan JH, Kwok CK. 2022. An RNA G-quadruplex structure within the ADAR 5′UTR interacts with DHX36 helicase to regulate translation. Angew Chem Int Ed Engl 61: e202203553. 10.1002/anie.202203553 [DOI] [PubMed] [Google Scholar]
- Majee P, Pattnaik A, Sahoo BR, Shankar U, Pattnaik AK, Kumar A, Nayak D. 2021. Inhibition of Zika virus replication by G-quadruplex-binding ligands. Mol Ther Nucleic Acids 23: 691–701. 10.1016/j.omtn.2020.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin VL, Armitage BA. 2005. RNA guanine quadruplex invasion by complementary and homologous PNA probes. J Am Chem Soc 127: 8032–8033. 10.1021/ja051102y [DOI] [PubMed] [Google Scholar]
- Masson T, Landras Guetta C, Laigre E, Cucchiarini A, Duchambon P, Teulade-Fichou MP, Verga D. 2021. BrdU immuno-tagged G-quadruplex ligands: a new ligand-guided immunofluorescence approach for tracking G-quadruplexes in cells. Nucleic Acids Res 49: 12644–12660. 10.1093/nar/gkab1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M, Patel TR, Booy EP, Marushchak O, Okun N, Deo S, Howard R, McEleney K, Harding SE, Stetefeld J, et al. 2013. Binding of G-quadruplexes to the N-terminal recognition domain of the RNA helicase associated with AU-rich element (RHAU). J Biol Chem 288: 35014–35027. 10.1074/jbc.M113.512970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestre-Fos S, Penev PI, Suttapitugsakul S, Hu M, Ito C, Petrov AS, Wartell RM, Wu R, Williams LD. 2019. G-quadruplexes in human ribosomal RNA. J Mol Biol 431: 1940–1955. 10.1016/j.jmb.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitteaux J, Raevens S, Wang Z, Pirrotta M, Valverde IE, Hudson RHE, Monchaud D. 2024. PhpC modulates G-quadruplex-RNA landscapes in human cells. Chem Commun 60: 424–427. 10.1039/d3cc05155b [DOI] [PubMed] [Google Scholar]
- Monchaud D. 2023. Template-assembled synthetic G-quartets (TASQs): multiTASQing molecular tools for investigating DNA and RNA G-quadruplex biology. Acc Chem Res 56: 350–362. 10.1021/acs.accounts.2c00757 [DOI] [PubMed] [Google Scholar]
- Monsen RC, Trent JO. 2018. G-quadruplex virtual drug screening: a review. Biochimie 152: 134–148. 10.1016/j.biochi.2018.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MJ, Wingate KL, Silwal J, Leeper TC, Basu S. 2012. The porphyrin TmPyP4 unfolds the extremely stable G-quadruplex in MT3-MMP mRNA and alleviates its repressive effect to enhance translation in eukaryotic cells. Nucleic Acids Res 40: 4137–4145. 10.1093/nar/gkr1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moruno-Manchon JF, Lejault P, Wang Y, McCauley B, Honarpisheh P, Morales Scheihing DA, Singh S, Dang W, Kim N, Urayama A, et al. 2020. Small-molecule G-quadruplex stabilizers reveal a novel pathway of autophagy regulation in neurons. eLife 9: e52283. 10.7554/eLife.52283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou X, Kwok CK. 2020. Effect of RNA sequence context and stereochemistry on G-quadruplex-RHAU53 interaction. Biochem Biophys Res Commun 533: 1135–1141. 10.1016/j.bbrc.2020.09.045 [DOI] [PubMed] [Google Scholar]
- Mou X, Kwok CK. 2023. Peptides selected by G4-mRNA display-seq enable RNA G-quadruplex recognition and gene regulation. J Am Chem Soc 145: 18693–18697. 10.1021/jacs.3c04534 [DOI] [PubMed] [Google Scholar]
- Mou X, Liew SW, Kwok CK. 2022. Identification and targeting of G-quadruplex structures in MALAT1 long non-coding RNA. Nucleic Acids Res 50: 397–410. 10.1093/nar/gkab1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye AL, Porter KC, Cohen SB, Phan T, Zyner KG, Sasaki N, Lovrecz GO, Beck JL, Bryan TM. 2015. Telomeric G-quadruplexes are a substrate and site of localization for human telomerase. Nat Commun 6: 7643. 10.1038/ncomms8643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland K, Sullivan H-J, Garner J, Cai J, Chen B, Wu C. 2019. Three-dimensional structure of RNA monomeric G-quadruplex containing ALS and FTD related G4C2 repeat and its binding with TMPyP4 probed by homology modeling based on experimental constraints and molecular dynamics simulations. ACS Chem Neurosci 11: 57–75. 10.1021/acschemneuro.9b00572 [DOI] [PubMed] [Google Scholar]
- Murat P, Zhong J, Lekieffre L, Cowieson NP, Clancy JL, Preiss T, Balasubramanian S, Khanna R, Tellam J. 2014. G-quadruplexes regulate Epstein-Barr virus-encoded nuclear antigen 1 mRNA translation. Nat Chem Biol 10: 358–364. 10.1038/nchembio.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murat P, Marsico G, Herdy B, Ghanbarian AT, Portella G, Balasubramanian S. 2018. RNA G-quadruplexes at upstream open reading frames cause DHX36- and DHX9-dependent translation of human mRNAs. Genome Biol 19: 229. 10.1186/s13059-018-1602-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckles C, Boer RE, Aboreden N, Cross AM, Walker RL, Kim BH, Kim S, Schneekloth JS Jr, Caplen NJ. 2019. HNRNPH1-dependent splicing of a fusion oncogene reveals a targetable RNA G-quadruplex interaction. RNA 25: 1731–1750. 10.1261/rna.072454.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle S. 2016. Quadruplex nucleic acids as novel therapeutic targets. J Med Chem 59: 5987–6011. 10.1021/acs.jmedchem.5b01835 [DOI] [PubMed] [Google Scholar]
- Nguyen LTA, Dang DT. 2023. RHAU peptides specific for parallel G-quadruplexes: potential applications in chemical biology. Mol Biotechnol 65: 291–299. 10.1007/s12033-022-00552-7 [DOI] [PubMed] [Google Scholar]
- Norseen J, Johnson FB, Lieberman PM. 2009. Role for G-quadruplex RNA binding by Epstein-Barr virus nuclear antigen 1 in DNA replication and metaphase chromosome attachment. J Virol 83: 10336–10346. 10.1128/JVI.00747-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofer N, Weisman-Shomer P, Shklover J, Fry M. 2009. The quadruplex r(CGG)n destabilizing cationic porphyrin TMPyP4 cooperates with hnRNPs to increase the translation efficiency of fragile X premutation mRNA. Nucleic Acids Res 37: 2712–2722. 10.1093/nar/gkp130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan MP, Haldar S, Duchi M, Oliver TAA, Mulholland AJ, Morales JC, Galan MC. 2019. A photoresponsive stiff-stilbene ligand fuels the reversible unfolding of G-quadruplex DNA. Angew Chem Int Ed Engl 58: 4334–4338. 10.1002/anie.201900740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaghire SN, Cherubim CJ, Telmer CA, Martinez JA, Bruchez MP, Armitage BA. 2016. RNA G-quadruplex invasion and translation inhibition by antisense gamma-peptide nucleic acid oligomers. Biochemistry 55: 1977–1988. 10.1021/acs.biochem.6b00055 [DOI] [PubMed] [Google Scholar]
- Parveen S, Chaurasia N, Gupta S, Vidyarthi S, Gupta N, Pandey P, Pant B, Srivastava KR, Kumar N, Goel A. 2024. Rationally designed G-quadruplex selective “turn-on” NIR fluorescent probe with large stokes shift for nucleic acid research-based applications. ACS Appl Bio Mater 7: 7233–7243. 10.1021/acsabm.4c00940 [DOI] [PubMed] [Google Scholar]
- Pasternack RF, Gibbs EJ. 1996. Porphyrin and metalloporphyrin interactions with nucleic acids. Met Ions Biol Syst 33: 367–397. 10.1021/bk-1989-0402.ch004 [DOI] [PubMed] [Google Scholar]
- Pasternack RF, Gibbs EJ, Villafranca JJ. 1983. Interactions of porphyrins with nucleic acids. Biochemistry 22: 5409–5417. 10.1021/bi00292a024 [DOI] [PubMed] [Google Scholar]
- Perez Tobia J, Huang PJ, Ding Y, Saran Narayan R, Narayan A, Liu J. 2023. Machine learning directed aptamer search from conserved primary sequences and secondary structures. ACS Synth Biol 12: 186–195. 10.1021/acssynbio.2c00462 [DOI] [PubMed] [Google Scholar]
- Perrone R, Butovskaya E, Daelemans D, Palu G, Pannecouque C, Richter SN. 2014. Anti-HIV-1 activity of the G-quadruplex ligand BRACO-19. J Antimicrob Chemother 69: 3248–3258. 10.1093/jac/dku280 [DOI] [PubMed] [Google Scholar]
- Phan AT, Kuryavyi V, Darnell JC, Serganov A, Majumdar A, Ilin S, Raslin T, Polonskaia A, Chen C, Clain D, et al. 2011. Structure-function studies of FMRP RGG peptide recognition of an RNA duplex-quadruplex junction. Nat Struct Mol Biol 18: 796–804. 10.1038/nsmb.2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipier A, Devaux A, Lavergne T, Adrait A, Coute Y, Britton S, Calsou P, Riou JF, Defrancq E, Gomez D. 2021. Constrained G4 structures unveil topology specificity of known and new G4 binding proteins. Sci Rep 11: 13469. 10.1038/s41598-021-92806-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood PR, Yang M, Lewis GV, Balaratnam S, Yazdani K, Schneekloth JS Jr. 2024. Competitive microarray screening reveals functional ligands for the DHX15 RNA G-quadruplex. ACS Med Chem Lett 15: 814–821. 10.1021/acsmedchemlett.3c00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Yang C, Xia Y, Guo S, Song D, Su H. 2019. Preferential binding of π-ligand porphyrin targeting 5′-5′ stacking interface of human telomeric RNA G-quadruplex dimer. J Phys Chem Lett 10: 2143–2150. 10.1021/acs.jpclett.9b00637 [DOI] [PubMed] [Google Scholar]
- Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. 2019. Revealing lncRNA structures and interactions by sequencing-based approaches. Trends Biochem Sci 44: 33–52. 10.1016/j.tibs.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Qin G, Zhao C, Liu Y, Zhang C, Yang G, Yang J, Wang Z, Wang C, Tu C, Guo Z, et al. 2022. RNA G-quadruplex formed in SARS-CoV-2 used for COVID-19 treatment in animal models. Cell Discov 8: 86. 10.1038/s41421-022-00450-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G, Liu Z, Yang J, Liao X, Zhao C, Ren J, Qu X. 2024. Targeting specific DNA G-quadruplexes with CRISPR-guided G-quadruplex-binding proteins and ligands. Nat Cell Biol 26: 1212–1224. 10.1038/s41556-024-01448-1 [DOI] [PubMed] [Google Scholar]
- Ramos CIV, Monteiro AR, Moura NMM, Faustino MAF, Trindade T, Neves M. 2021. The interactions of H(2)TMPyP, analogues and its metal complexes with DNA G-quadruplexes—an overview. Biomolecules 11: 1404. 10.3390/biom11101404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K, Zamiri B, Stanley SYR, Macgregor RB Jr, Pearson CE. 2013. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem 288: 9860–9866. doi: 10.1074/jbc.C113.452532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Chaires JB. 1999. Sequence and structural selectivity of nucleic acid binding ligands. Biochemistry 38: 16067–16075. 10.1021/bi992070s [DOI] [PubMed] [Google Scholar]
- Renard I, Grandmougin M, Roux A, Yang SY, Lejault P, Pirrotta M, Wong JMY, Monchaud D. 2019. Small-molecule affinity capture of DNA/RNA quadruplexes and their identification in vitro and in vivo through the G4RP protocol. Nucleic Acids Res 47: 5502–5510. 10.1093/nar/gkz215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revikumar A, Kashyap V, Palollathil A, Aravind A, Raguraman R, Kumar KMK, Vijayakumar M, Prasad TSK, Raju R. 2022. Multiple G-quadruplex binding ligand induced transcriptomic map of cancer cell lines. J Cell Commun Signal 16: 129–135. 10.1007/s12079-021-00637-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznichenko O, Quillevere A, Martins RP, Loaec N, Kang H, Lista MJ, Beauvineau C, Gonzalez-Garcia J, Guillot R, Voisset C, et al. 2019. Novel cationic bis(acylhydrazones) as modulators of Epstein-Barr virus immune evasion acting through disruption of interaction between nucleolin and G-quadruplexes of EBNA1 mRNA. Eur J Med Chem 178: 13–29. 10.1016/j.ejmech.2019.05.042 [DOI] [PubMed] [Google Scholar]
- Robinson J, Stenspil SG, Maleckaite K, Bartlett M, Di Antonio M, Vilar R, Kuimova MK. 2024. Cellular visualization of G-quadruplex RNA via fluorescence-lifetime imaging microscopy. J Am Chem Soc 146: 1009–1018. 10.1021/jacs.3c11908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca R, Talarico C, Moraca F, Costa G, Romeo I, Ortuso F, Alcaro S, Artese A. 2017. Molecular recognition of a carboxy pyridostatin toward Gquadruplex structures: Why does it prefer RNA? Chem Biol Drug Des 90: 919–925. 10.1111/cbdd.13015 [DOI] [PubMed] [Google Scholar]
- Roschdi S, Yan J, Nomura Y, Escobar CA, Petersen RJ, Bingman CA, Tonelli M, Vivek R, Montemayor EJ, Wickens M, et al. 2022. An atypical RNA quadruplex marks RNAs as vectors for gene silencing. Nat Struct Mol Biol 29: 1113–1121. 10.1038/s41594-022-00854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau SG, Beaudoin JD, Bisaillon M, Perreault JP. 2015. Small antisense oligonucleotides against G-quadruplexes: specific mRNA translational switches. Nucleic Acids Res 43: 595–606. 10.1093/nar/gku1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahayasheela VJ, Sugiyama H. 2024. RNA G-quadruplex in functional regulation of noncoding RNA: challenges and emerging opportunities. Cell Chem Biol 31: 53–70. 10.1016/j.chembiol.2023.08.010 [DOI] [PubMed] [Google Scholar]
- Sanchez-Martin V, Soriano M, Garcia-Salcedo JA. 2021. Quadruplex ligands in cancer therapy. Cancers (Basel) 13: 3156. 10.3390/cancers13133156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos T, Salgado GF, Cabrita EJ, Cruz C. 2021. G-quadruplexes and their ligands: biophysical methods to unravel G-quadruplex/ligand interactions. Pharmaceuticals (Basel) 14: 769. 10.3390/ph14080769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Armitage BA. 2021. Targeting a potential G-quadruplex forming sequence found in the West Nile virus genome by complementary gamma-peptide nucleic acid oligomers. ACS Infect Dis 7: 1445–1456. 10.1021/acsinfecdis.0c00793 [DOI] [PubMed] [Google Scholar]
- Sauer M, Juranek SA, Marks J, De Magis A, Kazemier HG, Hilbig D, Benhalevy D, Wang X, Hafner M, Paeschke K. 2019. DHX36 prevents the accumulation of translationally inactive mRNAs with G4-structures in untranslated regions. Nat Commun 10: 2421. 10.1038/s41467-019-10432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen GPC. 2019. Super-resolution microscopy demystified. Nat Cell Biol 21: 72–84. 10.1038/s41556-018-0251-8 [DOI] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA. 2008. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol 10: 228–236. 10.1038/ncb1685 [DOI] [PubMed] [Google Scholar]
- Schubert C, Schalk-Hihi C, Struble GT, Ma HC, Petrounia IP, Brandt B, Deckman IC, Patch RJ, Player MR, Spurlino JC, et al. 2007. Crystal structure of the tyrosine kinase domain of colony-stimulating factor-1 receptor (cFMS) in complex with two inhibitors. J Biol Chem 282: 4094–4101. 10.1074/jbc.M608183200 [DOI] [PubMed] [Google Scholar]
- Sharp PA. 2009. The centrality of RNA. Cell 136: 577–580. 10.1016/j.cell.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Shu H, Zhang R, Xiao K, Yang J, Sun X. 2022. G-quadruplex-binding proteins: promising targets for drug design. Biomolecules 12: 648. 10.3390/biom12050648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla C, Datta B. 2024. G-quadruplexes in long non-coding RNAs and their interactions with proteins. Int J Biol Macromol 278: 134946. 10.1016/j.ijbiomac.2024.134946 [DOI] [PubMed] [Google Scholar]
- Spitale RC, Incarnato D. 2023. Probing the dynamic RNA structurome and its functions. Nat Rev Genet 24: 178–196. 10.1038/s41576-022-00546-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Guo X, Yang D, Cai X, Li Q, Yao L, Sun H, Tang Y. 2023. RNA G-quadruplex in live cells lighted-up by a thiazole orange analogue for SCA36 identification. Int J Biol Macromol 229: 724–731. 10.1016/j.ijbiomac.2022.12.231 [DOI] [PubMed] [Google Scholar]
- Sun H, Sun R, Yang D, Li Q, Jiang W, Zhou T, Bai R, Zhong F, Zhang B, Xiang J, et al. 2024. A cyanine dye for highly specific recognition of parallel G-quadruplex topology and its application in clinical RNA detection for cancer diagnosis. J Am Chem Soc 146: 22736–22746. 10.1021/jacs.4c07698 [DOI] [PubMed] [Google Scholar]
- Sunbul M, Lackner J, Martin A, Englert D, Hacene B, Grun F, Nienhaus K, Nienhaus GU, Jaschke A. 2021. Super-resolution RNA imaging using a rhodamine-binding aptamer with fast exchange kinetics. Nat Biotechnol 39: 686–690. 10.1038/s41587-020-00794-3 [DOI] [PubMed] [Google Scholar]
- Suresh BM, Taghavi A, Childs-Disney JL, Disney MD. 2023. Fragment-based approaches to identify RNA binders. J Med Chem 66: 6523–6541. 10.1021/acs.jmedchem.3c00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suseela YV, Narayanaswamy N, Pratihar S, Govindaraju T. 2018. Far-red fluorescent probes for canonical and non-canonical nucleic acid structures: current progress and future implications. Chem Soc Rev 47: 1098–1131. 10.1039/c7cs00774d [DOI] [PubMed] [Google Scholar]
- Takahama K, Oyoshi T. 2013. Specific binding of modified RGG domain in TLS/FUS to G-quadruplex RNA: tyrosines in RGG domain recognize 2'-OH of the riboses of loops in G-quadruplex. J Am Chem Soc 135: 18016–18019. 10.1021/ja4086929 [DOI] [PubMed] [Google Scholar]
- Takahama K, Takada A, Tada S, Shimizu M, Sayama K, Kurokawa R, Oyoshi T. 2013. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem Biol 20: 341–350. 10.1016/j.chembiol.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Tan W, Yi L, Zhu Z, Zhang L, Zhou J, Yuan G. 2018. Hsa-miR-1587 G-quadruplex formation and dimerization induced by NH4+, molecular crowding environment and jatrorrhizine derivatives. Talanta 179: 337–343. 10.1016/j.talanta.2017.11.041 [DOI] [PubMed] [Google Scholar]
- Teng X, Hu D, Dai Y, Jing H, Hu W, Zhang Q, Zhang N, Li J. 2024. Discovery of a G-quadruplex unwinder that unleashes the translation of G-quadruplex-containing mRNA without inducing DNA damage. Angew Chem Int Ed Engl 63: e202407353. 10.1002/anie.202407353 [DOI] [PubMed] [Google Scholar]
- Tomaszewska M, Szabat M, Zielinska K, Kierzek R. 2021. Identification and structural aspects of G-quadruplex-forming sequences from the influenza A virus genome. Int J Mol Sci 22: 6031. 10.3390/ijms22116031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Liu G, Sang X, Zhu X, Fu X, Dou C, Jian Y, Zhang J, Zou S, Zhang G, et al. 2023. Targeting RNA G-quadruplex with repurposed drugs blocks SARS-CoV-2 entry. PLoS Pathog 19: e1011131. 10.1371/journal.ppat.1011131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Sica F. 2024. Structural overview of DNA and RNA G-quadruplexes in their interaction with proteins. Curr Opin Struct Biol 87: 102846. 10.1016/j.sbi.2024.102846 [DOI] [PubMed] [Google Scholar]
- Truong TTT, Cao C, Dang DT. 2020. Parallel G-quadruplex-mediated protein dimerization and activation. RSC Adv 10: 29957–29960. 10.1039/d0ra06173e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C, Gold L. 1990. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249: 505–510. 10.1126/science.2200121 [DOI] [PubMed] [Google Scholar]
- Umar MI, Kwok CK. 2020. Specific suppression of D-RNA G-quadruplex-protein interaction with an L-RNA aptamer. Nucleic Acids Res 48: 10125–10141. 10.1093/nar/gkaa759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar MI, Chan CY, Kwok CK. 2022. Development of RNA G-quadruplex (rG4)-targeting L-RNA aptamers by rG4-SELEX. Nat Protoc 17: 1385–1414. 10.1038/s41596-022-00679-6 [DOI] [PubMed] [Google Scholar]
- Van Tricht C, Voet T, Lammertyn J, Spasic D. 2023. Imaging the unimaginable: leveraging signal generation of CRISPR-Cas for sensitive genome imaging. Trends Biotechnol 41: 769–784. 10.1016/j.tibtech.2022.10.003 [DOI] [PubMed] [Google Scholar]
- Varshney D, Spiegel J, Zyner K, Tannahill D, Balasubramanian S. 2020. The regulation and functions of DNA and RNA G-quadruplexes. Nat Rev Mol Cell Biol 21: 459–474. 10.1038/s41580-020-0236-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester K, Eravci M, Serikawa T, Schutze T, Weise C, Kurreck J. 2019. RNAi-mediated knockdown of the Rhau helicase preferentially depletes proteins with a Guanine-quadruplex motif in the 5′-UTR of their mRNA. Biochem Biophys Res Commun 508: 756–761. 10.1016/j.bbrc.2018.11.186 [DOI] [PubMed] [Google Scholar]
- Vijay Kumar MJ, Morales R, Tsvetkov AS. 2023. G-quadruplexes and associated proteins in aging and Alzheimer's disease. Front Aging 4: 1164057. 10.3389/fragi.2023.1164057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hacht A, Seifert O, Menger M, Schutze T, Arora A, Konthur Z, Neubauer P, Wagner A, Weise C, Kurreck J. 2014. Identification and characterization of RNA guanine-quadruplex binding proteins. Nucleic Acids Res 42: 6630–6644. 10.1093/nar/gku290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller ZAE, Sewitz SA, Hsu S-TD, Balasubramanian S. 2009. A small molecule that disrupts G-quadruplex DNA structure and enhances gene expression. J Am Chem Soc 131: 12628–12633. 10.1021/ja901892u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F, Wong F, Collins JJ, de la Fuente-Nunez C. 2024. Machine learning for antimicrobial peptide identification and design. Nat Rev Bioeng 2: 392–407. 10.1038/s44222-024-00152-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-R, Min Y-Q, Wang J-Q, Liu C-X, Fu B-S, Wu F, Wu L-Y, Qiao Z-X, Song Y-Y, Xu G-H, et al. 2016a. A highly conserved G-rich consensus sequence in hepatitis C virus core gene represents a new anti–hepatitis C target. Sci Adv 2: e1501535. 10.1126/sciadv.1501535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SR, Zhang QY, Wang JQ, Ge XY, Song YY, Wang YF, Li XD, Fu BS, Xu GH, Shu B, et al. 2016b. Chemical targeting of a G-quadruplex RNA in the ebola virus L gene. Cell Chem Biol 23: 1113–1122. 10.1016/j.chembiol.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Wang H, Nakamura M, Abbott TR, Zhao D, Luo K, Yu C, Nguyen CM, Lo A, Daley TP, Russa ML et al. 2019a. CRISPR-mediated live imaging of genome editing and transcription. Science 365: 1301–1305. 10.1126/science.aax7852 [DOI] [PubMed] [Google Scholar]
- Wang X, Goodrich KJ, Conlon EG, Gao J, Erbse AH, Manley JL, Cech TR. 2019b. C9orf72 and triplet repeat disorder RNAs: G-quadruplex formation, binding to PRC2 and implications for disease mechanisms. RNA 25: 935–947. 10.1261/rna.071191.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Thombre R, Shah Y, Latanich R, Wang J. 2021a. G-quadruplexes as pathogenic drivers in neurodegenerative disorders. Nucleic Acids Res 49: 4816–4830. 10.1093/nar/gkab164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Liu CX, Chen LL, Zhang QC. 2021b. RNA structure probing uncovers RNA structure-dependent biological functions. Nat Chem Biol 17: 755–766. 10.1038/s41589-021-00805-7 [DOI] [PubMed] [Google Scholar]
- Wang RX, Ou Y, Chen Y, Ren TB, Yuan L, Zhang XB. 2024. Rational design of NIR-II G-quadruplex fluorescent probes for accurate in vivo tumor metastasis imaging. J Am Chem Soc 146: 11669–11678. 10.1021/jacs.3c13851 [DOI] [PubMed] [Google Scholar]
- Wheelhouse RT, Sun D, Han H, Han FX, Hurley LH. 1998. Cationic porphyrins as telomerase inhibitors: the interaction of tetra-(N-methyl-4-pyridyl)porphine with quadruplex DNA. J Am Chem Soc 120: 3261–3262. 10.1021/ja973792e [DOI] [Google Scholar]
- Wortman MJ, Dagdanova AV, Clark AM, Godfrey EW, Pascal SM, Johnson EM, Daniel DC. 2020. A synthetic Pur-based peptide binds and alters G-quadruplex secondary structure present in the expanded RNA repeat of C9orf72 ALS/FTD. Biochim Biophys Acta Mol Cell Res 1867: 118674. 10.1016/j.bbamcr.2020.118674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong YX, Huang ZS, Tan JH. 2015. Targeting G-quadruplex nucleic acids with heterocyclic alkaloids and their derivatives. Eur J Med Chem 97: 538–551. 10.1016/j.ejmech.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Xu Y, Suzuki Y, Ito K, Komiyama M. 2010. Telomeric repeat-containing RNA structure in living cells. Proc Natl Acad Sci 107: 14579–14584. 10.1073/pnas.1001177107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Huang H, Zhou X. 2021. G-quadruplexes in neurobiology and virology: functional roles and potential therapeutic approaches. JACS Au 1: 2146–2161. 10.1021/jacsau.1c00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi R, Miyazaki T, Oyoshi T. 2018. G-quadruplex binding ability of TLS/FUS depends on the beta-spiral structure of the RGG domain. Nucleic Acids Res 46: 5894–5901. 10.1093/nar/gky391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan MP, Wee CE, Yen KP, Stevens A, Wai LK. 2023. G-quadruplex ligands as therapeutic agents against cancer, neurological disorders and viral infections. Future Med Chem 15: 1987–2009. 10.4155/fmc-2023-0202 [DOI] [PubMed] [Google Scholar]
- Yang SY, Lejault P, Chevrier S, Boidot R, Robertson AG, Wong JMY, Monchaud D. 2018. Transcriptome-wide identification of transient RNA G-quadruplexes in human cells. Nat Commun 9: 4730. 10.1038/s41467-018-07224-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LZ, Wang Y, Li SQ, Yao RW, Luan PF, Wu H, Carmichael GG, Chen LL. 2019. Dynamic imaging of RNA in living cells by CRISPR-Cas13 systems. Mol Cell 76: 981–997.e7. 10.1016/j.molcel.2019.10.024 [DOI] [PubMed] [Google Scholar]
- Yang X, Cheema J, Zhang Y, Deng H, Duncan S, Umar MI, Zhao J, Liu Q, Cao X, Kwok CK, et al. 2020. RNA G-quadruplex structures exist and function in vivo in plants. Genome Biol 21: 226. 10.1186/s13059-020-02142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yu H, Duncan S, Zhang Y, Cheema J, Liu H, Benjamin Miller J, Zhang J, Kwok CK, Zhang H, et al. 2022. RNA G-quadruplex structure contributes to cold adaptation in plants. Nat Commun 13: 6224. 10.1038/s41467-022-34040-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li Z, Binzel DW, Guo P, Williams TM. 2023. Targeting oncogenic KRAS in non-small cell lung cancer with EGFR aptamer-conjugated multifunctional RNA nanoparticles. Mol Ther Nucleic Acids 33: 559–571. 10.1016/j.omtn.2023.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yangyuoru PM, Di Antonio M, Ghimire C, Biffi G, Balasubramanian S, Mao H. 2015. Dual binding of an antibody and a small molecule increases the stability of TERRA G-quadruplex. Angew Chem Int Ed Engl 54: 910–913. 10.1002/anie.201408113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Oyoshi T, Suda A, Futaki S, Imanishi M. 2022. Recognition of G-quadruplex RNA by a crucial RNA methyltransferase component, METTL14. Nucleic Acids Res 50: 449–457. 10.1093/nar/gkab1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BE, Kundu N, Sczepanski JT. 2019. Mirror-image oligonucleotides: history and emerging applications. Chemistry 25: 7981–7990. 10.1002/chem.201900149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z-Y, Luo W-H, Chen X-C, Chen S-B, Huang Z-S, Tan J-H. 2020. Efficient and rational development of a new fluorescent probe specific for RNA G-quadruplex imaging in cells. Sens Actuators B Chem 324: 128770. 10.1016/j.snb.2020.128770 [DOI] [Google Scholar]
- Zamiri B, Reddy K, Macgregor RB, Pearson CE. 2014. TMPyP4 porphyrin distorts RNA G-quadruplex structures of the disease-associated r(GGGGCC)n repeat of the C9orf72 gene and blocks interaction of RNA-binding proteins. J Biol Chem 289: 4653–4659. 10.1074/jbc.C113.502336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller MJ, Favorov O, Li K, Nuthanakanti A, Hussein D, Michaud A, Lafontaine DA, Busan S, Serganov A, Aube J, et al. 2022. SHAPE-enabled fragment-based ligand discovery for RNA. Proc Natl Acad Sci 119: e2122660119. 10.1073/pnas.2122660119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, El Omari K, Duman R, Liu S, Haider S, Wagner A, Parkinson GN, Wei D. 2020a. Native de novo structural determinations of non-canonical nucleic acid motifs by X-ray crystallography at long wavelengths. Nucleic Acids Res 48: 9886–9898. 10.1093/nar/gkaa439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Jiang H, Deng H, Dong C, Shen W, Chen H, Gao C, Xiao S, Liu ZF, et al. 2020b. G(2)-quadruplex in the 3′UTR of IE180 regulates Pseudorabies virus replication by enhancing gene expression. RNA Biol 17: 816–827. 10.1080/15476286.2020.1731664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li H, Lin B, Luo X, Yin P, Yi T, Xue B, Zhang XL, Zhu H, Nie Z. 2021. Development of near-infrared nucleic acid mimics of fluorescent proteins for in vivo imaging of viral RNA with turn-on fluorescence. J Am Chem Soc 143: 19317–19329. 10.1021/jacs.1c04577 [DOI] [PubMed] [Google Scholar]
- Zhang L, Li L, Wang X, Liu H, Zhang Y, Xie T, Zhang H, Li X, Peng T, Sun X, et al. 2022. Development of a novel PROTAC using the nucleic acid aptamer as a targeting ligand for tumor selective degradation of nucleolin. Mol Ther Nucleic Acids 30: 66–79. 10.1016/j.omtn.2022.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Nie Q, Chi-Kong Lau T, Kit Kwok C. 2024. Rational design of L-RNA aptamer-peptide conjugate for efficient cell uptake and G-quadruplex-mediated gene control. Angew Chem Int Ed Engl 63: e202310798. 10.1002/anie.202310798 [DOI] [PubMed] [Google Scholar]
- Zhao C, Qin G, Niu J, Wang Z, Wang C, Ren J, Qu X. 2021. Targeting RNA G-quadruplex in SARS-CoV-2: a promising therapeutic target for COVID-19? Angew Chem Int Ed Engl 60: 432–438. 10.1002/anie.202011419 [DOI] [PubMed] [Google Scholar]
- Zhao H, Wong HY, Ji D, Lyu K, Kwok CK. 2022. Novel L-RNA aptamer controls APP gene expression in cells by targeting RNA G-quadruplex structure. ACS Appl Mater Interfaces 14: 30582–30594. 10.1021/acsami.2c06390 [DOI] [PubMed] [Google Scholar]
- Zhao H, Lau HL, Zhang K, Kwok CK. 2024. Selective recognition of RNA G-quadruplex in vitro and in cells by L-aptamer-D-oligonucleotide conjugate. Nucleic Acids Res 52: 13544–13560. 10.1093/nar/gkae1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Li JY, Zhang MJ, Li JH, Huang JT, You PD, Liu SW, Zhou CQ. 2021. G-quadruplex binder pyridostatin as an effective multi-target ZIKV inhibitor. Int J Biol Macromol 190: 178–188. 10.1016/j.ijbiomac.2021.08.121 [DOI] [PubMed] [Google Scholar]