Abstract
Animals make decisions based on the value of potential outcomes. This perceived value is not fixed; it changes depending on internal needs, such as hunger or thirst, and past experiences. The basolateral amygdala (BLA) is known to be crucial for updating predicted reward values. However, it has been unclear how the BLA represents the specific value of different rewards. Two-photon calcium imaging in male mice showed that population response magnitude scaled with subjective value, and different rewards recruited distinct neuronal subpopulations. Value representations quickly re-scaled when a novel, higher-value reward appeared, and internal state shaped them: thirst selectively boosted responses to water, whereas aversive experience dampened sucrose responses. Thus, BLA circuits carry flexible, stimulus-specific value signals that integrate relative value and current affective or homeostatic conditions, providing a neural basis for adaptive decision making and learning. Our findings reveal that the BLA maintains adaptable, reward-specific value signals, essential for guiding choices according to current needs and changing circumstances.
Subject terms: Neural circuits, Reward
How basolateral amygdala represents the specific value of rewards remains unclear. Here the authors find that basolateral amygdala neurons assign stimulus-specific values to different rewards and rapidly re-scale these signals with changing reward context, thirst or stress, illuminating how the brain guides flexible, state-dependent choices.
Introduction
A major challenge for animals is to rapidly adjust their behavior to accommodate variable environmental conditions and need states. One of the main mechanisms to structure this interaction is associative learning in which actions or neutral sensory cues become predictive of appetitive or aversive outcomes. During associative learning, the value of the expected outcome is defined by its valence, which can be appetitive or aversive, and by its subjective importance or value.
Value signals have been reported throughout the brain1,2, with the basolateral amygdala (BLA) identified to be a key brain area for the acquisition, retrieval, and updating of associations of cues or actions with the current value of specific outcomes3,4. Impairing BLA function prevents the formation and retrieval of both appetitive and aversive stimulus-specific associations5–7. Moreover, the BLA was found to be necessary to adapt behavioral responses whenever the perceived value of the expected outcome changed8–11. For instance, associating the outcome (a specific food) with malaise, through conditioned taste aversion (CTA), leads to a BLA-dependent reduction in instrumental actions evoked by an outcome-associated cue12. However, the BLA is not implicated in general motivation13 and contrary to other brain regions, optogenetic activation does not cause excessive food consumption or strong place avoidance14–16. These findings suggest that behavioral adaptations are driven by dynamic changes in the representation of specific outcomes in the BLA.
Previous neurophysiological investigations of outcome representations in the BLA have suggested sensory-invariant value representations17–19, which would not allow for flexibly updating value associations in a stimulus-specific manner and can not account for behavioral evidence of sensory-specific value updating and learning. Previous work studying associative learning have proposed stimulus-specific responses20–22, however their stimulus sets were either not sufficiently orthogonalized or focused on cue representation, which makes it difficult to draw conclusions.
As stimulus specificity and dynamic value updating of outcome representations can only be characterized by exposing animals to a diverse set of rewarding outcomes, we performed two-photon calcium imaging in head-fixed mice consuming a range of appetitive gustatory stimuli. By measuring lick bout size, an established read-out for perceived value in experimental psychology23,24 and recently shown to be bidirectionally modulated by BLA activity25, we were able to quantify the relative values of these stimuli as a function of the rewards available and an animal’s internal state.
Our study reconciles previous findings at the behavioral and the neurophysiological level by demonstrating that the BLA represents the value of distinct appetitive outcomes in largely separate neuronal populations. Furthermore, we extend the concept of stimulus-specific reward value by showing that these representations are dynamically updated according to reward context, homeostatic and affective states, while population-level responses could be integrated to achieve a stimulus-invariant representation.
Results
BLA reward responses are locked to consumption onset
To study BLA activity during value assignment to innately valuable stimuli, we developed a head-fixed task in which food-restricted mice were presented with sets of unsignaled liquid rewards, delivered in a random sequence at random intervals, while recording general motor activity (running wheel motion), arousal (pupil dilation, whisker pad motion), and licking behavior (Fig. 1a). To minimize predictability and potential interactions between successive stimuli, we chose long inter-trial intervals of an average of 20 s (Supplementary Fig. S1a). Quantification of licking behavior upon presentation with water or 20% sucrose demonstrated that animals continued to lick the reward spout long after the reward had been consumed, which takes about 3 licks (Fig. 1b-e and Supplementary Fig. S1b). Sucrose rewards resulted in significantly more licks per reward compared to water (Fig. 1d, e). The number of licks per reward was defined as the number of uninterrupted licks (inter-lick interval <500 ms) after reward delivery, also referred to as lick bout size. The difference in lick bout size was predominantly driven by a change in lick duration rather than frequency (Fig. 1d), as described previously26. To assess whether reward history influenced subsequent reward consumptions, we compared pupil dilation in trials with no change in reward and in trials in which there was a shift from water to 20% sucrose. We found no significant difference within the range of mean reward size fluctuations and therefore considered reward responses in subsequent trials to be independent from each other (Supplementary Fig. S1c–e).
Fig. 1. BLA activity is locked to reward consumption onset.
a Schema of recorded variables during head-fixed behavioral paradigm with random reward deliveries. b Example licking from three animals consuming water and 20% sucrose rewards. c Lick pattern surrounding the first consummatory lick (n = 10 (animals) - sessions = 4). d Mean lick probability following consumption onset. e Mean number of uninterrupted licks (inter-lick interval <500 ms) following consumption onset for the different rewards (paired t-test, p = 0.0044, n = 10 (animals) − sessions = 4). f Example tongue tip trajectories during unrewarded licks as well as consummatory lick bouts whilst consuming water and sucrose 20%. Single interpolated lick trajectories with average trajectory overlaid. g Left: matrix comparing the procrustes distance of different lick categories (reward type and lick number within lick bout). Right: Quantification of interpolated lick trajectory shape differences using Procrustes distance within a lick category (same reward and lick within lick bout) compared to across the two reward types (repeated measures ANOVA, followed by Tukey test, p = 0.0014; Within water to within sucrose: p = 0.33, within water to across: p = 0.0011, within sucrose to across: p = 0.071; n = 16 (sessions) - animals = 4). h PCA1 of the combined behavioral read-outs of whisker pad motion, pupil dilation and running speed (left) and the delta between the combined read-outs between water and sucrose 20% (right). Dashed line indicates a 3 standard deviation increase from baseline. i Schema of the viral and surgical strategy for two-photon recordings in the BLA with an example recording plane shown in (j). Red outlines indicate the spatial footprints of the extracted neurons. k Example traces of six simultaneously recorded neurons. l Single (opaque) and mean (solid) reward-triggered activity of the neurons in (k). m Calcium transient probability in response to reward consumption (n = 1846 (neurons) - animals = 10, sessions = 4). Dashed lines indicate a 3 standard deviation increase from baseline transient probability.
To address whether licking behavior is reward-specific, we analyzed tongue movements at higher temporal resolution using video tracking at 194 Hz (Supplementary Movie 1). Tongue motion was stereotypical, with probing tongue movements to check for reward availability and a transition to a lapping motion after reward detection (Fig. 1f). Comparing lick trajectories revealed that licks in response to water were more similar to each other than licks in response to 20% sucrose (Fig. 1g), while 20% sucrose licks were as similar to each other as to water. To test whether such lick pattern differences could systematically bias our neuronal recordings, we quantified if differences in lick pattern scale with sucrose concentration differences and found that they did not (Supplementary Fig. S1f–h). To further assess at which point consumption behavior started to differ, we compared the first principal component of the combined behavioral metrics (whisking pad motion, running speed, and pupil dilation) for water and sucrose consumption lick bouts (Fig. 1h—left).
We found that the behavior started to significantly deviate 1.28 s after consumption bout onset (Fig. 1h—right), indicating that during this time window neural activity is likely related to the sensory content or value of the stimulus, rather than to differences in motor patterns27.
To characterize the neuronal representation of reward value, we expressed the calcium indicator GCaMP6f in principal neurons in the BLA (Fig. 1i and Supplementary Fig. S2) and imaged neurons through a gradient refractive index (GRIN) lens during the task using a two-photon microscope. Aligning the neuronal responses to the onset of reward consumption indicated that BLA principal neurons showed calcium responses time-locked to consumption onset (Fig. 1i–l). Rather than spanning the entire time of a given lick bout, reward responses were primarily onset responses. The probability of calcium transient onset was elevated above baseline during the first 1.5 s after consumption onset (Fig. 1m). This timing makes it unlikely that the motor pattern differences described above contribute to the measured differences in neuronal activity. To further control for a possible contribution of lick-related motor signals, we sub-selected trials with similar lick bout sizes for both rewards and found that reward response differences were maintained (Supplementary Fig. S3a–c).
BLA population activity reflects perceived reward value
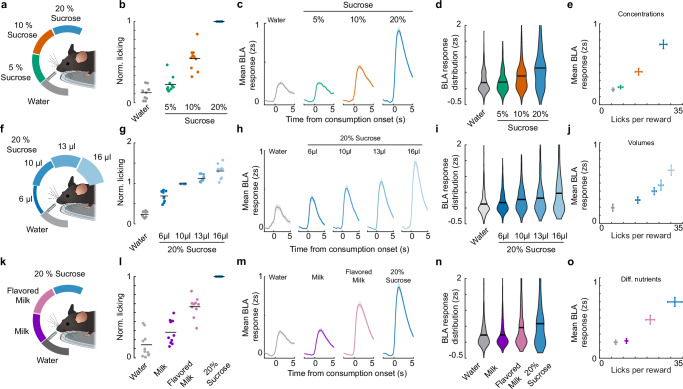
To address how the BLA represents the value of rewards across a range of gustatory stimuli, we exposed mice to three different reward sets (Fig. 2a, f, k). Lick bout sizes were normalized to the average lick bout size in response to 10 µl 20% sucrose reward (normalized licking), which was present in all reward sets. This analysis showed that normalized licking scaled with the animals’ preference for higher sucrose concentrations (Fig. 2b) and larger volumes at a given concentration (Fig. 2g). We also observed differential licking for distinct rewards in a set composed of rewards containing different nutrients (milk, flavored milk, and sucrose; Fig. 2l), where value assignment was unclear a priori. As previous work used lick bout size as a proxy for relative value assessment28, we conclude that licking behavior can also be used to measure the perceived value of unpredicted gustatory rewards in our head-fixed task.
Fig. 2. The BLA represents the value of gustatory rewards at the population level.
a–e Characterization of reward concentration effects. a Schema of the randomly provided sucrose concentrations during the behavioral session (10 µl per reward). b Number of licks normalized to the 10 µl, 20% sucrose response in this session (repeated measures ANOVA, p = 9.29*10^(−15), n = 10 (animals), sessions = 4). Time-resolved mean population response of active BLA neurons (c) with corresponding quantification (Two-way RM ANOVA: rewards × animals, p = 1.81*10^(−27), n = 427 (neurons); animals = 10, sessions = 4) (d). e Average mean BLA response plotted against the mean licking for the Concentration session (n = 10 (animals), sessions = 4). f–j Characterization of reward volume effects. f Schema of the randomly provided volumes of 20% sucrose during the behavioral session. g Number of licks normalized to the 10 µl, 20% sucrose response in this session (repeated measures ANOVA, p = 1.25*10^(−21), n = 10 (animals) - sessions = 2). Time-resolved mean population response of active BLA neurons (h) with corresponding quantification (Two-way RM ANOVA: rewards × animals, p = 0.013, n = 185 (neurons) − animals = 10, sessions = 2) (i). j Average mean BLA response plotted against the mean licking for the Volume session (n = 10 (animals) − sessions = 2). k–o Characterization of reward type effects. k Schema of the randomly provided reward types during the behavioral session (10 µl per reward). l Number of licks normalized to the 10 µl, 20% sucrose response in this session (repeated measures ANOVA, p = 6.86*10^(−10), n = 10 (animals) - sessions = 3). Time-resolved mean population response of active BLA neurons (m) with corresponding quantification (Two-way RM ANOVA: rewards × animals, p = 5.62*10^(−19), n = 510 (neurons) − animals = 10, sessions = 4) (n). o Average mean BLA response plotted against the mean licking for the Diff. nutrient session (n = 10 (animals) - sessions = 3). Error bars depict SEM.
For each reward set, we observed an increase in mean BLA activity across session averaged single neuron responses for preferred rewards (Fig. 2c–e, h–j, m–o). Due to the biophysical properties of the calcium sensor, full differentiation of the sources for this increased activity is not possible29. It is however likely that it reflects a combination of the recruitment of more neurons, an increase in single-neuron response amplitude and/or response probability (Supplementary Fig. S3e–g). A positive correlation of the mean neuronal response magnitude with normalized licking was also observed when subdividing responses based on lick bout size alone (Supplementary Fig. S3h) and when subdividing for individual rewards and lick bout size (Supplementary Fig. S3i). Thus, consistent with previous studies concluding that BLA reward representations are stimulus-invariant18,19, our results demonstrate that mean BLA activity correlates with lick bout size independent of the currently consumed reward, and that perceived reward values are represented in a stimulus-invariant manner at the population level.
Neuronal subpopulations encode stimulus-specific reward value
The monotonically increasing relationship of perceived value and population response could be a result of stimulus-invariant value neurons that each have a monotonically increasing relationship with reward value or arise through the combination of subpopulations of neurons specifically responding to distinct rewards.
To distinguish between these two possibilities, we performed a regressor analysis to test whether neurons had stimulus-invariant responses by correlating mean responses with a regressor containing all stimuli and comparing it to the best correlating individual stimulus regressor (see “Methods”, Fig. 3a). In the sessions in which mice were exposed to sucrose solutions of different volumes we found that a similar number of neurons were better correlated with a single stimulus than all stimuli regressor. In the sucrose concentration sessions, in which stimulus features were still very similar, correlations for the best maximum individual regressor were slightly better than the all stimuli’ regressor. This difference was increased during the session in which mice were exposed to distinct stimuli containing different nutrients, where we found a strong bias towards neurons correlating better with the maximum individual stimulus regressor (Fig. 3b, c). This finding indicates that BLA value coding is not stimulus-invariant. To test this further, we compared the number of neurons that were significantly responding to only one reward and found that most neurons were responding exclusively to a single reward, with higher overlap during sessions with higher reward similarity (Fig. 3d–f). To understand whether the observed findings were only true for session averages or also observable at the individual trial level, we calculated population vector (PV) correlations (Fig. 3g). Comparing the across vs. within PV-stimulus correlations revealed that even at the single trial level the population response was significantly different for sessions in which different rewards were consumed (Fig. 3h).
Fig. 3. Distinct BLA sub-populations track stimulus-specific value.
a Schema of linear regression analysis performed to characterize single neuron tuning. b Correlation of individual neuron responses with lick bout size and the respective stimuli in the Concentration (left), Volume (middle) and Diff. nutrient (right) sessions with corresponding quantification in (c) (KS test, Bonferroni-corrected, Conc: p = 4.4*10^(−30), Vol: p > 0.5, Diff. nutrients: p = 9.43*10^(−74); Conc.-Vol. p = 1.7*10^(−6), Conc. - Diff. nutrients p = 4.63*10^(−13), Diff. nutrients - Vol. p = 2.84*10^(−21); - Concentrations: n = 427 (neurons) - animals = 10, sessions = 4; Volume: n = 185 (neurons); animals = 10, sessions = 2; Diff. nutrients: n = 510 (neurons) - animals = 10, sessions = 4). d Time-resolved activity of all significantly modulated neurons across the different behavioral sessions. e Venn diagrams displaying the reward specificity of individual neurons across different reward sessions with corresponding quantification of neurons active to only one reward shown in (f) (Chi² test, Bonferroni-corrected, Conc. - Vol. p = 0.0018, Conc. - Diff. nutrients p = 0.0209, Diff. nutrients - Vol. p = 3.83*10^(−8), Conc: n = 427 (neurons) — animals = 10, sessions = 4; Vol: n = 185 (neurons) - animals = 10, sessions = 2; Diff nutrients: n = 510 (neurons) - animals = 10, sessions = 4). g, h Trial-to-Trial correlation of the population vector (PV) for three example sessions of the types: Concentration, Volume, and Diff. nutrient with corresponding quantification of within reward vs. across reward PV-correlation for the individual sessions in (h) (repeated measures ANOVA, followed by Tukey test, Conc. - Vol. p = 0.97, Conc. – Diff. nutrients. p = 0.02, Diff. nutrients - Vol. p = 0.011, Conc.: n = 10 (animals) - sessions = 4; Vol.: n = 10 (animals) - sessions = 2; Diff. nutrients: n = 10 (animals) - sessions = 4).
To investigate the stability of representations, we focused on sessions with different appetitive rewards, which, given the lower overlap in responses, should have the largest differences across days. We find that many neurons maintain very similar response profiles (Supplementary Fig. S4a) and that a larger number of neurons than expected by chance maintain appetitive responsiveness (Supplementary Fig. S4b). When comparing the change of significant responses towards specific rewards across days, we find that most responsive neurons maintained their response profile (51.49%) or became unresponsive (38.06%). A much smaller fraction lost or gained a significant response for one reward but maintained at least one previous significant response (8.2%). Only a very small fraction of neurons (2.2%) changed their responses completely (Supplementary Fig. S4c). To avoid potential biases due to thresholding, we also compare PV correlations and find that the average PV response across rewards of aligned neurons, split within and across session, maintains high correlation. This further indicates that stimulus responses are stable across days (Supplementary Fig. S4d).
Taken together the presented data demonstrate that the BLA maintains stimulus-specific value representations at the single neuron level, while a stimulus-invariant value representation could be computed via the integration over the positive outcome responsive BLA population.
BLA neurons dynamically encode value
To address how BLA reward value representations change upon exposure to a preferred reward and to expand on previous work focusing on individual neurons30, we first performed a cross-session comparison of the neural response to the same stimulus (10 µl of 20% sucrose) in the presence and absence of a better, preferred reward. This comparison (Fig. 4a) revealed that animals licked less for the same reward when they experienced a larger 16 µl reward of the same concentration in the same session, while responses for water were not affected (session II; Fig. 4b). Similarly, we found that BLA responses were reduced for the 10 µl reward (session II) and that the 16 µl reward, at the time the most valuable available reward (session II), evoked a similar response as the 10 µl reward in sessions where mice were presented with 10 µl as the highest reward (session I; Fig. 4c, d). To overcome potential shortcomings of cross-session comparisons and to test whether reward response re-scaling also occurs within the same experimental session, we performed an additional experiment where we alternated two different reward scales between blocks (Fig. 4e and Supplementary Fig. S2c), an experimental design known as simultaneous contrast31. Consistent with the previous findings, we observed larger lick bout sizes (Supplementary Fig. S5e) and a larger BLA population response to the same 20% sucrose reward within a reward block that did not contain 40% sucrose compared to blocks with 40% sucrose rewards available (Fig. 4f). Analyzing block transitions showed that BLA population responses adapted within a single session. 20% sucrose responses rapidly adapted within the blocks (5–10 min.) and recovered to baseline levels after termination of the 40% block (Fig. 4g, h). This effect was mediated by a group of neurons (Supplementary Fig. S5a) with stimulus-specific responses, consistent with our previous observations (Supplementary Fig. S5b). Within-session adaptation suggests an integration of local reward history, which we did not observe in previous experiments (Supplementary Fig. S1c–e). This discrepancy is likely explained by the higher mean reward fluctuations in the simultaneous contrast session (Supplementary Fig. S5c, d), an interpretation supported by control experiments in which we introduced an equal number of 20% sucrose trials instead of 40% sucrose (Supplementary Fig. S5f), where we did not observe an adaptation between the different blocks (Supplementary Fig. S5g–j).
Fig. 4. BLA maintains coherence of value representation by re-scaling upon exposure to a new reward environment.
a Schema of the sessions, as presented in Fig. 2. b Number of licks in response in the concentration session (Session I) on a per animal basis (repeated measures ANOVA, followed by Tukey test, Conc. 10 µl - Vol. 10 µl: p = 0.0011; Vol. 16 µl - Vol. 10 µl: p = 0.0019; Conc. 10 µl - Vol. 16 µl: p > 0.5; Conc. water - Vol. water: p > 0.5; n = 10 (animals) - session = 4/2). c Time-resolved mean BLA response to rewards in the volume (dashed line) and concentration session (solid line) (d) with corresponding quantification of the relative BLA response of significantly responding neurons in the volume session (session II) subtracted from the mean response of significantly active neurons in the concentration session (session I) of the corresponding rewards depicted in (c) (repeated measures ANOVA, followed by Tukey test, water - 10 µl: p = 9.72*10^(−10); 10–16 µl: p = 2.33*10^(−6); water – 16 µl: p = 0.013; n = 185 (neurons) - animals = 10, sessions = 4/2). e Schema of the experimental design for simultaneous contrast characterization. Animals were given alternating blocks of rewards including (yellow line) or excluding (blue line) 40% sucrose. f Split violin plots without (left) and with (right) 40% sucrose present in the current block (paired t-test, p = 0.029, n = 104 (neurons) - animals = 4, sessions = 3). g Time-resolved mean BLA response to 20% in blocks without (blocks 1, 3, 5, 7) and the blocks with 40% sucrose (blocks 2, 4, 6). h Quantification of the BLA response change between 20% blocks (blocks 1–3, 3–5, 5–7, left), transitions from 20% blocks to 40% blocks (1–2, 3–4, 5–6, middle) and transitions from 40% blocks to 20% blocks (2–3, 4–5, 6–7, right) (one-sample t-tests, Bonferroni-corrected, p > 0.5; p = 0.01; p = 0.005; n = 218/174/176 (ΔNeuron activity) - animals = 4, sessions = 3, blocks = 3, neurons = 104). Error bars and shaded regions depict SEM.
Value representations reflect homeostatic and affective states
To examine whether the BLA response scaling does not only take the distribution of all available rewards into account but also incorporates changes in an animal’s internal state, we first measured reward responses in acutely water-restricted animals. At the behavioral level, we observed a strong and specific increase in the lick response to water, indicating a shift in the relative value of water in comparison to sucrose (Fig. 5a–c). Accordingly, the BLA population response to water was specifically increased in water-restricted animals, resulting in an equal BLA population response magnitude to water and sucrose rewards (Fig. 5d–f). This increased water response was predominantly driven by an increase in the number of neurons showing a water-specific response that we did not observe in non-water-restricted animals (Fig. 5e, f).
Fig. 5. Value representations in BLA are internal state dependent.
a Schema of water deprivation session design. b Comparison of the licking response to water during the water deprivation session with the concentration sessions (see Fig. 2b; paired t-test, p = 0.00022, n = 10 (animals) - sessions = 4/1). c Time-resolved mean BLA response to different rewards of significantly active neurons sorted by mean activity to water for water restriction (left) and control condition (right). d Quantification of the difference of all significantly active neurons for water responses in the control condition (concentration session) and water restriction session (two-sample t-test, Bonferroni-corrected, FR - WR: p = 2.4*10^(−10); FR: p = 8.48*10^(−36); WR: p = 0.27; FR: n = 427 (neurons); animals = 10, sessions = 4, WR: n = 104 (neurons); animals = 10, sessions = 1). e Time resolved activity of all BLA neurons during water restriction, and control session (see Fig. 2b). f Visualization of the overlap of significantly water and 20% sucrose responsive neurons in control session (upper panel) and water restricted session (lower panel). Normalized to the fraction of significantly 20% sucrose active neurons in the control session. g Schema of aversive session design. h Licking response comparing the licking during control session with this session (paired t-test, p = 4.14 *10^(−36); n = 10 (animals) - sessions = 3). i, j Time-resolved mean BLA response to 20% sucrose reward during the aversive session (solid line) and the food restricted session (dashed line; see Fig. 2b) with corresponding quantifications (two-sample t-test, p = 2.33*10^(−31), n = 427/258 (neurons) - animals = 10, sessions = 4/3) (j). k Less than 1% overlap of significantly responding neurons responding to aversive events and 20% sucrose. Shaded regions depict SEM.
Finally, we examined how a negative affective state would influence reward value representations by exposing mice to unpredicted aversive stimuli randomly interspersed throughout the session (random ITI 45–60 s, Fig. 5h). We further modified the paradigm to trigger reward delivery upon spout licking, (closed-loop reward delivery), allowing us to dissociate motivational drive from assigned value32, which could both be influenced by repeated aversive exposures. Aversive experiences led to a marked decrease in the lick bout size after consumption but did not result in a strong reduction of reward seeking behavior, as quantified by lick-triggered reward deliveries (Fig. 5h and Supplementary Fig. S6a–d). While reward-seeking behavior was only partially affected and the BLA strongly responded to aversive stimuli (Supplementary Fig. S6e, f), we observed a strong attenuation of neuronal sucrose responses despite continued consumption (Fig. 5i, j). Reward-responsive neurons were not overlapping with aversive-responsive neurons in this experiment, suggesting that the investigated appetitive value representation is likely valence specific (Fig. 5k).
To characterize the effect of aversive on appetitive experiences further, we performed open-loop appetitive/aversive experiments in three conditions: (1) appetitive only, (2) aversive onset and (3) aversive offset (Supplementary Fig. S7a and Supplementary Fig. S2d). We find that behavioral engagement is stable across the session for water and sucrose in the appetitive only and aversive offset conditions but drops to about 50% in animals that experience aversive stimuli starting only after several appetitive trials (aversive onset – Supplementary Fig. S7b). Licking to rewards was stable in the appetitive only condition and did not recover after aversive offset but were reduced in the aversive onset conditions (Supplementary Fig. S7c). Similar to the closed-loop experiment (Fig. 5k, l), we observed a stable response across session for the appetitive only session (Supplementary Fig. S7d) and a strong reduction in water and sucrose responses after aversive stimulus onset (Supplementary Fig. S7e). When aversive stimuli ended, both the licking behavior and neuronal reward responses remained suppressed (Supplementary Fig. S7f), showing no recovery to baseline levels. This stands in contrast to what was observed in the simultaneous contrast experiments (Fig. 4e–h), where recovery did occur. This persistent suppression suggests that exposure to aversive stimuli creates a lasting change in how subjects evaluate and respond to rewards, rather than just a temporary suppression of reward value.
Discussion
Here we show that the BLA represents the perceived value of stimuli and that this effect is not a mere reflection of general arousal or systematic differences in licking microstructure. Furthermore, we find that this stimulus-invariant value response emerges from largely separate neuronal subpopulations specifically activated by distinct gustatory stimuli. Exposing mice to larger rewards within the same stimulus set resulted in re-scaling of reward representations, demonstrating that value representations are relative and maintained through rapid adaptation of neural response magnitudes. Finally, we show that changes in the internal state can bi-directionally modulate the magnitude of value responses in the BLA. These data thus support a pivotal role for the BLA at the interface between stimulus-specific sensory processing and stimulus-invariant value computation.
Our finding of consumption onset locked responses is in agreement with previous reports of BLA activity in response to reward exposure33. However, we did not observe a reduction of consumption-onset locked responses across time17, potentially due to the lack of predictability of reward availability in our paradigm. Interestingly and contrary to reports in other brain areas, we find that single neurons in the BLA show stimulus-specific responses and are not dominated by orofacial movement (Supplementary Fig. S3).
There have been indirect lines of evidence suggesting that at least a fraction of BLA neurons display stimulus-specific responses22. For instance, 10% of task-related BLA neurons showed an outcome specific response in a visually cued choice task20. On the other hand, the same stimulus leads to the activation of different sets of neurons before and after CTA21, showing that BLA activity is not merely reflecting sensory properties of the stimulus. From these studies we can deduce that BLA has neither a sensory-invariant value representation nor a value independent stimulus-specific representation. However, given the mixing with appetitive, aversive, and learning aspect, we cannot deduce an integrated view of what aspects of the stimuli are represented and how they are updated to allow for dynamic cue to outcome-value associations.
Dedicated studies, investigating value representation in BLA in contrast, have reported the existence of a stimulus-invariant value code in BLA18,19,33 which we find only at the population level. We extend these findings by demonstrating that even though the total population does indeed encode stimulus-invariant reward value, this representation arises from stimulus-specific value representations that vary in number of neurons, magnitude and response probability according to the perceived value of a given specific stimulus. This stands in contrast to regions like the orbitofrontal cortex34, globus pallidus35, ventral pallidum36, and nucleus accumbens37, where individual neurons have been shown to encode value in a more stimulus-invariant manner, suggesting a hierarchical transformation of value representation across the reward circuit. The high stimulus specificity even for very similar rewards like different sucrose concentrations cannot be sustained with increasing stimulus set size and would lead to suboptimal coding38. Due to the lower overlap of neurons coding for sugar and fat-based rewards (Fig. 3) it is likely that, at least in the gustatory domain, the BLA projects stimulus features onto relevant axes, such as carbohydrate, protein, fat, and mineral content.
Our discovery of different sets of neurons with high stimulus-specific response properties in the BLA allows to resolve a longstanding disconnect between behavioral studies, showing the necessity of BLA function when the value of specific rewards needs to be updated, for example during outcome devaluation, revaluation, reinstatement, conditioned taste aversion or sensory specific associations8–11,13 and neurophysiological reports of stimulus-invariant valence responses in the BLA18,19,33. This allows for the modulation of specific reward channels, like water in our water deprivation experiment (Fig. 5a–f), while preserving others. Our findings are well aligned with recent work from our lab, describing the transfer of specific stimulus value to instrumental actions and the necessity for this representation for actions execution39 and are supported by holographic stimulation of BLA neurons responsive to sucrose, leading to an increase in the perceived value of sucrose25.
Our findings are also complementary to the characterization of learned responses to predicting neutral stimuli (such as tones) that are paired with appetitive or aversive stimuli40 and suggest a potential mechanism for the rapid update of sensory-specific associations5–7. The adaptation of single neurons to the best currently available stimulus in the BLA have been reported in primates30,41 using different reward volumes. Here we show that this adaptation is more general and applies to volumes as well as concentrations. Moreover, we demonstrate that neurons adapt within minutes and recover the original representation once the previous reward set is presented. Interestingly, while previous characterizations of value representation in the orbital-frontal cortex (OFC)42 found an adjustment of single neuron firing rates according to divisive normalization34, we find that the implementation in the BLA is different. While the BLA population response shows adaptation in agreement with a divisive normalization model (Fig. 4), this is the result of largely non-overlapping sets of BLA neurons representing 20% sucrose when it is the best available rewards vs. neurons representing 20% sucrose when it is the second-best available reward and neurons representing 40% sucrose (Supplementary Fig. S5a). Downstream integration over many BLA neurons representing appetitive stimuli could lead to a single cell value representation as described in the OFC. Given the known impact of BLA on OFC function43, our findings further suggest a direction of information transfer. However, a comparative study with simultaneous recordings in multiple brain regions such as the OFC, striatum, and gustatory cortex would be needed to determine the unique computations of each brain area conclusively.
Thirst modulated neuronal activity has been reported throughout the brain1 and it is yet unclear how the subfornical organ drives changes in value assignment. Given our results that show that water responses in the BLA are strongly increased upon water deprivation, and in the light of previous reports on the specific role of the BLA in updating stimulus-specific action and Pavlovian associations10,13, we speculate that the BLA is a key site in implementing this homeostatic-state dependent update.
The impact of an aversive state on food consumption has been observed at the level of the hypothalamus44, directly impacting the activity of hormonal driven hunger signals of AgRP neurons. Due to the unchanged high motivation of animals exposed to aversive stimuli to ingest food (Supplementary Fig. S6), it is unlikely that the decrease in activity in BLA following reward consumption is a consequence of the altered hunger signal broadcasted to the brain though the above-mentioned hypothalamic system, and strongly indicates that BLA reward representations are strongly modulated by an animal’s affective state.
Our results complement findings in lateral hypothalamus (LHA), a major target of BLA projections, that demonstrated that stimulus unspecific appetitive behaviors can be driven with optogenetic stimulation45, thus suggesting that the BLA is situated at the interface between stimulus-specific sensory processing and stimulus-invariant motivation. These findings also align well with observations in humans, where the amygdala has been found to be important for the integration of value, while its dysregulation has been linked to affective disorders accompanied by anhedonia46–48, suggesting an important role for the BLA in mediating value assignment under physiological conditions.
Methods
Animals
All animal procedures were performed in accordance with institutional guidelines and with current European Union guidelines and were approved by the Veterinary Department of the Canton of Basel-Stadt, Switzerland. Male C57BL6/J (Envigo, in house breeding) were housed on a 12 h light/dark cycle and had ad libitum access to food and water in their home cage. Environmental enrichment was provided in the form of a running wheel, bedding material, a cardboard tunnel and a shelter. Animals were between 12 and 20 weeks at the time of experiments at which time they were separated for the duration of the experiment. All experiments were performed during the light cycle. No statistical methods were used to pre-determine sample size and due to the automatic scoring and extraction of behavior the experimenters were not blinded to the experimental conditions.
Surgical procedures, viral vector injections and GRIN lens implantation
One day prior to the surgery and 3 days after the animals received drinking water supplemented with the nonsteroidal anti-inflammatory drug Carprofen (Rimadyl, 67 μg/ml, 10 mg/kg, Pfizer) to provide pain relief without the necessity of injections. Before anesthesia, buprenorphine (Temgesic, Indivior UK Limited; 0.1 mg/kg body weight (BW)) was injected subcutaneously 30 min before the surgery. Anesthesia of mice was induced with 5% and maintained with 1–2% isoflurane (Attane, Provet) in oxygen enriched air (Oxymat 3, Weinmann), animals’ heads shaved and placed in a stereotactic frame (Model 1900, Kopf Instruments). Mice were kept on a heating pad controlled by a feedback-based DC temperature control system (FHC) and received an ocular gel to prevent drying of the eye’s surface (Viscotears, Novartis). Before the first incision was made the skin above the skull was injected subcutaneously with a local anesthetic (1:1 mixture of Lidocaine: 10 mg/kg, Bichsel and Ropivacaine: 3 mg/kg; Naropin, AstraZeneca). A hole of 1.3 mm diameter was drilled using a surgical drill (Kopf Instruments) at the following coordinates: AP−1.6 mm (from bregma), ML−3.35/+3.35 mm (from bregma) and an adeno-associated virus (AAV2/5.CaMK2.GCaMP6f - 500 nl, University of Pennsylvania Vector Core, UPenn) unilaterally injected into the BLA using a precision micropositioner (Model 2650, Kopf Instruments) and pulled volume-calibrated glass capillaries (Drummond Scientific, Cat.-No. 2-000-001, tip diameter about 30 μm) connected to a Picospritzer III microinjection system (Parker Hannifin Corporation) at the following depth: DV -4.2 mm (from pia). Following the retraction of the pulled glass capillary, a hollow injection needle with 1.1 mm diameter was lowered to −4.3 mm (from pia) and immediately retracted before we lowered a 1 mm GRIN lens into the skull (Inscopix, part ID: 1050-002177) using the micropositioner and fixed at −4.2 mm (from pia) using a UV-curing glue (Henkel, Loctite 4305). The surface of the exposed skull was scratched, covered with Vetbond (3 M) and a custom-built titanium head-bar fixed using dental acrylic (Paladur, Heraeus). To protect the surface of the GRIN lens before the experiment it was covered with a rapid-curing silicone elastomer (KauPo).
Immunohistochemistry
Following the completion of behavioral paradigms, the brains of the mice were post-processed for histological analyses. Mice were anesthetized with an intraperitoneal injection of an anesthetic mixture (250 mg/kg ketamine and 2.5 mg/kg medetomidine) to achieve deep anesthesia. The mice were then perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) for 2 min followed by 4% paraformaldehyde in PBS for 40 min. Then, the brains were removed from the skull and cut into 80 or 100 μm coronal slices using a vibratome (VT1000S). Perfusion-fixed sections containing the amygdala were subsequently washed in 0.1 M phosphate buffer (PB, pH 7.4) and Tris-buffered saline (TBS, pH 7.4) and blocked in a solution of 1% human serum albumin (Sigma-Aldrich) and 0.1% Triton-X (Sigma-Aldrich) in TBS and then incubated in a mixture of primary antibodies containing 0.1% Triton-X for 48–72 h. This was followed by extensive washes in TBS, and incubation in the mixture of appropriate secondary antibodies overnight. Following this, sections were subsequently washed in TBS and PB, dried on slides and covered with Aquamount (PolySciences). Sections were scanned with a Zeiss Axio Scan Z1 Slide scanner equipped with a Zeiss objective (Fluor 5×/0.25) to confirm the expression of the different viruses and GRIN lens implantation sites. GRIN lens placements were matched against a mouse brain atlas (Allen Brain Atlas)49.
Behavioral protocols and recordings
Five days prior to the start of the experiment animals were food restricted and maintained at 85% of their ad libitum food body weight throughout the experiment. The feeding schedule was maintained throughout the experimental process and happened immediately after the daily experimental session was completed. Acute water restriction was performed on non-food restricted animals and constituted a 24 h period of water removal before the experiment was conducted. After the experiment finished animals were again given ad libitum access to water.
Head-fixed paradigms
Two to six weeks after the surgical procedure, animals were placed under a two-photon microscope (Ultima Investigator, Bruker) to check for expression of the calcium indicator GCaMP6f. If animals had sufficient expression, they were put under food restriction (see Behavioral protocols and recordings) and the experiment commenced 5–7 days afterwards.
First, mice were habituated to head-fixation and reward delivery in two brief 15 min sessions. Rewards were delivered using up to five NE-1000 syringe pumps (NewEra Pumpsystems Inc, Milan) controlled by an analog signal output from the two-photon microscope (Ultima Investigator, Bruker). The output pattern was generated using a Matlab 2017 (Mathworks) script that randomized deliveries and inter-trial intervals (ITI’s) and generated individual.txt files used by the system to control the voltage supplied to pumps that control the deliveries through a RS232 to BNC cable. Following habituation, mice underwent several sessions of each type (up to 4, separated by 24 h, Figs. 1– 4a–f, 5) in which the rewards were changed (order: concentrations, volumes, different nutrients, aversive, water restriction). All sucrose rewards were mixed according to the specified concentration of sucrose (Sigma-Aldrich) and delivered with different volumes. Milk (whole-milk, COOP Switzerland) and flavored milk (Emmy Energy Milk Vanilla, COOP Switzerland). Experiments pertaining to Fig. 4e–f were performed on an additional set of mice that followed a slightly altered protocol. In each session rewards were delivered through a single custom-built spout that had five independent openings, one connected to each pump. Spout licks were detected when the mouse closed a circuit between its metal head-bar and the spout (P. Buchmann, P. Argast, J. Hinz—custom built). Conductance from the headbar to the mouse was facilitated by an ultrasound gel bridge to the neck skin. Additionally we monitored the running speed of the animals on the saucer wheel using a rotary encoder (Yumo rotary encoder E6B2-CWZ3E: 1024 pulses per rotation) and the pupil dilation with a single monochrome camera at 60 Hz (The Imaging Source, part ID: DMK 22BUC03) through ICcapture (The Imaging Source) or lick trajectories and pupil dilation with multiple monochrome cameras (FLIR, part ID: BFS-U3-16S2M-CS) at 194.1 Hz through Bonsai (version 2.3.1)50 and Python (version 3.8.8, based on https://github.com/ThomasAkam/Point_Grey_Bonsai_multi_camera_acquisition) for Fig. 1f–h. To allow for a good dynamic range in pupil size, we installed a custom build 415 nm UV back light (ILS ILH-XC01-S410-SC211WIR200 filtered through a 445/45 nm bandpass) and adjusted the light power to result in a medium sized pupil at the beginning of each experimental session.
During the appetitive open-loop sessions, rewards were delivered at random intervals (ITI 15–25 s, equal probability) and no structure imposed on the distribution of rewards. During experiments pertaining to Fig. 4e–h and Supplementary Fig. S5, rewards were organized in blocks of 25/35 (either including or excluding additional reward), where each block contained the same number of rewards, randomized within the block, and delivered at random ITIs (15–25 s, equal probability).
During the aversive sessions, reward delivery was contingent on the animals licking and therefore licking was additionally processed by a RP2.1 real-time processor (Tucker Davis). Rewards were delivered when the animal had licked 3 times and didn’t incur a reward time-out (8 s after the last reward was delivered).
In experiments pertaining to Supplementary Fig. S7, appetitive rewards and aversive stimuli were delivered independent of mouse behavior (open-loop), as for all experiments other than Fig. 5g–k. Rewards were structured in Blocks of 70 trials with the 10-20-20-20 trials for water, milk, flavored milk and sucrose, respectively. Within the block, rewards were delivered in randomized order. In aversive onset sessions animals received one block of appetitive trials before four blocks of appetitive trials interspaced with aversive stimuli. In the aversive offset session this pattern was reversed, with only the last block not containing aversive stimuli. In the appetitive only session, the same protocol was used as in Figs. 2 and 3.
Behavioral analysis
Data extraction
Behavioral data was synchronized with the two-photon imaging frames using the two-photon system’s GPIO channel voltage recording (Ultima Investigator, Bruker) at 1 kHz. The running speed was determined from the rotary encoder signal by normalizing to the running saucer wheel circumference at the mouse position. Video camera frames were timestamped by recording the frame trigger signal. Pupil dilation, whisking power, and lick trajectories were extracted from the video camera recordings via DeepLabCut (DLC)51, using a network trained on manually labeled sample frames (8 points around the pupil, 4 points around the whisker pad, 3 points on the tongue, 3 points on the lick spout).
Data analysis
To ensure that we only included actual licks and not spout contacts by other body parts, like hand digits, were detected, we used the length of contact to exclude other events. Tongue contacts were the most common spout interaction and had a stereotypic duration. We therefore fitted a normal distribution to the duration of all detected spout contacts and excluded events that were longer or shorter than 3*std of the mean contact duration. Given the delivery speed of the syringe pumps, we defined the first rewarded lick to be the first lick 100 ms after pump onset. This definition comes with some level of uncertainty so that, given the average lick frequency of 6.8 Hz, the first rewarded lick can in some cases be 150 ms earlier or later than the one we defined. To calculate the lick bout size, we counted the number of consecutive licks that were not interrupted for more than 500 ms. We normalized these lick bout sizes to the average within animal, 10 µl, 20% sucrose lick bout size. To exclude an impact of reward prediction error on the observed neuronal signals we checked whether pupil dilation was impacted by positive or negative reward difference. To this end we fit the distribution of pupil dilations and checked whether there was a significant deviation from the mean of the corresponding distributions for positive and negative reward shifts (see Supplementary Fig. S1c–e). To investigate the motor patterns during consumption we analyzed running speed and pupil dilation, as well as whisker pad motion and lick trajectories. Whisker pad motion was defined as the average pixel value change within the whisker pad area. To compensate for potential lick speed differences, lick trajectories were interpolated to the same number of coordinates per lick using a modified Akima cubic Hermite interpolation (Matlab, interp1, option makima, upscale factor 10) from the DLC tracked tongue tip trajectory around the electrically detected licks times. Lick trajectory similarity was defined as the mean Procrustes distance (Matlab, procrustes, no reflection) between all compared lick trajectories.
Two-photon imaging
Imaging was conducted using a two-photon microscope (Ultima Investigator, Bruker) during all behavioral sessions on three planes separated by 70–80 μm using a 400 μm mechanical piezo (Bruker) with bi-directional scanning at a volume rate of 8.5 Hz and a spatial resolution of 256 by 256 pixels through a water immersion objective (16×, 0.8 numerical aperture (NA), Nikon). Ultrasound gel (G008, FIAB spA) was used to interface the objective and the GRIN lens. Excitation light was provided with a mode-locked laser system operating at 920 nm, 80-MHz pulse rate, 120 fs pulse width (Insight X3, Spectra Physics). We used standard Bruker PMT (photomultipliers, Hamamatsu Photonics) for light detection. For the same mice, imaging parameters were kept the same across repeated behavioral sessions and recorded using Prairie View (versions 5.2–5.6, Bruker).
Data extraction
We extracted the images using custom software written in Matlab 2017 (Mathworks Inc.) available on GitHub39. Briefly, after loading individual.tif and converting them to.mat files they were mapped to RAM and rigid motion correction performed using the Matlab implementation of NoRMCorre52. Animals that displayed non-rigid motion were excluded in a manual intervention step and ROIs identified using the Matlab implementation of CNMF53. The resulting ROIs were initially manually sorted and these manually sorted components used to train an SVM classifier that was subsequently used to re-sort all previous components and all future datasets. After the automatic sorting procedure, we excluded components that had high temporal correlation within and across planes when they were in close spatial proximity. We then aligned the remaining components across sessions by first performing rigid field-of-view alignment using landmark registration and then aligning the components using the cross-day alignment code of the package CIAtah54. Next, we combined the data for the multiplane acquisition that needed separate analysis and took the timestamp of the middle image as the timestamp for the three planes (±4 ms).
Data analysis
All analysis of calcium data was performed on deconvolved traces (variable C_dec) extracted through CNMF and performed using Matlab 2017b (Mathworks, code available on Github).
The first step was to align the first rewarded lick, we obtained by aligning the licking to the reward delivery (see behavioral analysis), to the imaging. Therefore, we used the timestamp of acquisition for each image and found the closest image recorded to the first rewarded lick and extracted a window of −3 s to +5 s around this timestamp for further processing. As pointed out previously there are some uncertainties about the first rewarded lick and given the volume imaging rate of the two-photon system of 8.5 Hz and the associated confounds we estimate that the actual onset of neuronal activity associated with the reward consumption is in the range of a max./min. of 250 ms of what we state as the time of consumption. This means that there is a jitter around zero of about ±2 frames most likely explaining imperfect alignment of the activity to time point 0 s.
To present peri-stimulus time histograms across the paper we computed local z-scores by normalizing each individual trial. To this end we subtracted the mean activity of each baseline before consummatory onset from the whole extracted period and divided it by the variance of the whole neuronal trace. Through this process we avoided the issue of division by zero, which often happens when using deconvolved traces and corrected for the overall activity level of the neuron. The mean activity for neurons displayed in the paper is the mean of the normalized individual presentations.
To calculate the p(Ca2+-transients) we used the estimated spike variable, binarized it and calculated the mean and SEM around the time of consumption of the different rewards for each neuron. Displayed is the mean across neurons and the threshold derived by calculating the mean and 3 standard deviations of the baseline preceding the consumption onset.
To quantify matched lick bout trials, we identified sessions that had at least three repetitions for each of the stimuli within the normalized lick range of 0.25–0.65 of the mean 20% sucrose response. These trials were then pooled across animals and used for quantification and visual display.
We calculated the population vector correlation (PV-correlation) by extracting the mean response, for individual trials across all recorded neurons resulting in a 1d vector, which we used to calculate the Pearson correlation between each single reward presentation. We subsequently sorted these correlations by reward identity. To calculate the correlation of single neurons with all stimuli or the individual stimulus regressors, we correlated the average activity of significantly active neurons during each trial (pre- and post-stimulus periods) with either a regressor containing all ones for the post-stimulus periods or ones for the post-stimulus period of one stimulus and zeros for the rest.
To quantify the mean activity for specific lick bouts, we calculated the mean response of active neurons in the specific lick bout, either split according to stimulus identity (Supplementary Fig. S3i) or summed over all stimuli (Supplementary Fig. S3h).
During simultaneous contrast experiments we calculated the change of the 10 µl 20% sucrose response between the different blocks by subtracting the mean response of neurons with at least three measured events and calculating whether these differences were deviating from 0. Similarly we also calculated the relative BLA response in Fig. 5d (between different rewards) and Fig. 4d, where we subtracted the mean BLA response from the indicated rewards (e.g., 10 µl Sucrose response during Session II mean BLA population response of 10 µl Sucrose during Session I).
Antibodies
We used DAPI (Thermo Fisher Scientific, Cat# D1306), Goat-anti-Calretinin (Dilution 1:5000, Swant, CG1), Chicken-anti-GFP (Dilution 1:2000, Lifetech, A10262), Rabbit-anti-vAchT (Dilution 1:3000, Synaptic Systems, 139103) and Mouse-anti-FoxP2 (Dilution 1:3000, Millipore, MABE415) as primary Antibodies in the identification of lens positions. We visualized them with the following secondary antibodies: Donkey-anti-Chicken, Cy2 conjugated (Dilution 1:1000, Jackson Immunoresearch Inc., 703-225−155), Donkey-anti-Mouse, 488 conjugated (Dilution 1:500, Molecular Probes, A21202), Goat-anti-Chicken, 488 conjugated (Dilution 1:1000, Molecular Probes, A11039), Donkey-anti-Rabbit, Cy3 conjugated (Dilution 1:1000, Sigma, AP182C), Donkey-anti-Goat, 568 conjugated (Dilution 1:500, Molecular Probes, A11057) and Donkey-anti-Mouse, 647 conjugated (Dilution 1:500–1:1000, Molecular Probes, A31571). The specificities of the primary antibodies were extensively tested, using knock-out mice if possible. Secondary antibodies were extensively tested for possible cross-reactivity with the other antibodies used, and possible tissue labeling without primary antibodies was also tested to exclude auto-fluorescence or specific background labeling. No specific-like staining was observed under these control conditions.
Statistical analyses and data presentation
Statistical analysis was performed with Matlab 2017b and Matlab 2022a (Mathworks). No methods were used to predetermine sample size. The sample sizes used here match those usual for the field. Experimenters were not blind to conditions, but all sorting and data extraction performed automatically, and sorting algorithms found on GitHub. We excluded animals if at least one of the following conditions were met: (i) GRIN lens placement outside of the BLA, (ii) non-rigid motion artifacts in the recordings, (iii) insufficient GCaMP6f expression or (iiii) insufficient interaction with the spout after habituation. All data are expressed as mean ± SEM unless stated otherwise. All data is always included in the visualization, no data was excluded from the analysis. Datasets were acquired from mice stemming from multiple litters and were reproducible. The statistical significance threshold was set at p < 0.05 and significance indicated by * and the exact p-value reported in the text and/or figure legend. For figure display, traces were normalized to represent the local z-score as described under Data analysis. We display multiple comparison corrected p-values using the Bonferroni correction. We used repeated measures one-way analysis of variance (ANOVA) for more than two groups and a Tukey-Kramer test if individual differences needed to be compared. When less than three groups were compared, paired or unpaired student’s t-tests were used. A Fisher’s exact test was conducted to compare proportions between two groups.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors thank Yue Zhang, Martin Zeller, Nikolaos Karalis, Julien Courtin and all members of the Lüthi laboratory for discussion about project design and feedback on earlier versions of the Manuscript. We thank P. Argast and P. Buchmann for technical assistance, G. Ferrand, B. Heller-Stilb and the animal caretakers for help with animal husbandry and Charlotte Sonseson (Bioinformatics, FMI) for help with the statistical analysis. This work was supported by the Novartis Research Foundation, the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (Grant Agreements 669582 to A.L. and under the Marie Skłodowska-Curie grant agreement 844492 to M.M.), the Swiss National Science Foundation (310030B_170268; TMAG-3_209270; all to A.L. and Ambizione grant agreement No PZ00P3_209032 to M.M.), and the European Molecular Biology Organization (EMBO-ALTF-233-2020 to A.S.).
Author contributions
Conceptualization: J.H., M.M., A.L. Methodology: J.H., M.M., A.S. Investigation: J.H., M.M., S.M., A.S., T.E., A.L. Visualization: J.H., M.M. Funding acquisition: M.M., A.S., A.L. Project administration: J.H., A.L. Supervision: A.L. Writing—original draft: J.H., M.M., A.L. Writing—review & editing: J.H., M.M., A.L. All authors have read and agreed to the published version of the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available at: 10.5281/zenodo.15298470.
Code availability
Custom codes can be found in the following GitHub repository: https://github.com/fmi-basel/2Photon_Analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Julian Hinz, Mathias Mahn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-60414-z.
References
- 1.Allen, W. E. et al. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science364, eaav3932 (2019). [DOI] [PMC free article] [PubMed]
- 2.Vickery, T. J., Chun, M. M. & Lee, D. Ubiquity and specificity of reinforcement signals throughout the human brain. Neuron72, 166–177 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci.16, 317–331 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Baxter, M. G. & Murray, E. A. The amygdala and reward. Nat. Rev. Neurosci.3, 563–573 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Dębiec, J., Díaz-Mataix, L., Bush, D. E. A., Doyère, V. & LeDoux, J. E. The amygdala encodes specific sensory features of an aversive reinforcer. Nat. Neurosci.13, 536–537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Mataix, L., Debiec, J., LeDoux, J. E. & Doyere, V. Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J. Neurosci.31, 9538–9543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sias, A. C. et al. Dopamine projections to the basolateral amygdala drive the encoding of identity-specific reward memories. Nat. Neurosci.27, 728–736 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balleine, B. W., Killcross, A. S. & Dickinson, A. The effect of lesions of the basolateral amygdala on instrumental conditioning. J. Neurosci.23, 666–675 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassum, K. M., Cely, I. C., Balleine, B. W. & Maidment, N. T. μ-Opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J. Neurosci.31, 1591–1599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin, R. J. & Floresco, S. B. The role of different subregions of the basolateral amygdala in cue-induced reinstatement and extinction of food-seeking behavior. Neuroscience146, 1484–1494 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Corbit, L. H., Leung, B. K. & Balleine, B. W. The role of the amygdala-striatal pathway in the acquisition and performance of goal-directed instrumental actions. J. Neurosci.33, 17682–17690 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland, P. C. & Rescorla, R. A. The effect of two ways of devaluing the unconditioned stimulus after first- and second-order appetitive conditioning. J. Exp. Psychol. Anim. Behav. Process.1, 355–363 (1975). [DOI] [PubMed] [Google Scholar]
- 13.Corbit, L. H. & Balleine, B. W. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J. Neurosci.25, 962–970 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuber, G. D. & Wise, R. A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci.19, 198–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betley, J. N. et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature521, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim, H. et al. Genetically- and spatially-defined basolateral amygdala neurons control food consumption and social interaction. Nat. Commun.15, 1–22 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyeler, A. et al. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron90, 348–361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namburi, P. et al. A circuit mechanism for differentiating positive and negative associations. Nature520, 675–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci.19, 1636–1646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jezzini, A. & Padoa-Schioppa, C. Neuronal activity in the primate amygdala during economic choice. J. Neurosci.40, 1286–1301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman, S. E., Fontanini, A., Wieskopf, J. S. & Katz, D. B. Learning-related plasticity of temporal coding in simultaneously recorded amygdala–cortical ensembles. J. Neurosci.28, 2864–2873 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontanini, A., Grossman, S. E., Figueroa, J. A. & Katz, D. B. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J. Neurosci.29, 2486–2495 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge, K. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev.24, 173–198 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Berridge, K. C. Affective valence in the brain: modules or modes? Nat. Rev. Neurosci.20, 225–234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piantadosi, S. C. et al. Holographic stimulation of opposing amygdala ensembles bidirectionally modulates valence-specific behavior via mutual inhibition. Neuron112, 593–610.e5 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dwyer, D. M. Licking and liking: the assessment of hedonic responses in rodents. Q. J. Exp. Psychol.65, 371–394 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Stringer, C. et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science. 364, 255 (2019). [DOI] [PMC free article] [PubMed]
- 28.Johnson, A. W. Characterizing ingestive behavior through licking microstructure: Underlying neurobiology and its use in the study of obesity in animal models. Int. J. Dev. Neurosci.64, 38–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledochowitsch, P. et al. On the correspondence of electrical and optical physiology in in vivo population-scale two-photon calcium imaging. bioRxiv 800102 10.1101/800102 (2019).
- 30.Bermudez, M. A. & Schultz, W. Reward magnitude coding in primate amygdala neurons. J. Neurophysiol.104, 3424–3432 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dwyer, D. M., Lydall, E. S. & Hayward, A. J. Simultaneous contrast: evidence from licking microstructure and cross-solution comparisons. J. Exp. Psychol. Anim. Behav. Process.37, 200–210 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Berridge, K. C. & Robinson, T. E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol.71, 670–679 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, X. & Li, B. Population coding of valence in the basolateral amygdala. Nat. Commun.9, 1–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louie, K., Grattan, L. E. & Glimcher, P. W. Reward value-based gain control: divisive normalization in parietal cortex. J. Neurosci.31, 10627–10639 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephenson-Jones, M. et al. A basal ganglia circuit for evaluating action outcomes. Nat539, 289–293 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, K. S., Tindell, A. J., Aldridge, J. W. & Berridge, K. C. Ventral pallidum roles in reward and motivation. Behav. Brain Res.196, 155–167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taha, S. A. & Fields, H. L. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J. Neurosci.25, 1193–1202 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manookin, M. B. & Rieke, F. Two sides of the same coin: efficient and predictive neural coding. Annu. Rev. Vis. Sci.9, 293–311 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Courtin, J. et al. A neuronal mechanism for motivational control of behavior. Science375, eabg7277 (2022). [DOI] [PubMed]
- 40.Paton, J. J., Belova, M. A., Morrison, S. E. & Salzman, C. D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature439, 865–870 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bermudez, M. A. & Schultz, W. Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity). J. Neurophysiol.103, 1158–1170 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi, S., De Carvalho, O. P. & Schultz, W. Adaptation of reward sensitivity in orbitofrontal neurons. J. Neurosci.30, 534–544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoenbaum, G., Chiba, A. A. & Gallagher, M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat. Neurosci.1, 155–159 (1998). [DOI] [PubMed] [Google Scholar]
- 44.de Araujo Salgado, I. et al. Toggling between food-seeking and self-preservation behaviors via hypothalamic response networks. Neuron111, 2899–2917.e6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sander, D. & Nummenmaa, L. Reward and emotion: an affective neuroscience approach. Curr. Opin. Behav. Sci.39, 161–167 (2021). [Google Scholar]
- 47.Der-Avakian, A. & Markou, A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci.35, 68–77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Husain, M. & Roiser, J. P. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat. Rev. Neurosci.19, 470–484 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Sunkin, S. M. et al. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res.41, D996–D1008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopes, G. et al. Bonsai: an event-based framework for processing and controlling data streams. Front. Neuroinform. 9, 7 (2015). [DOI] [PMC free article] [PubMed]
- 51.Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci.21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Pnevmatikakis, E. A. & Giovannucci, A. NoRMCorre: an online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods291, 83–94 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Pnevmatikakis, E. A. et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron89, 285–299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corder, G. et al. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science363, 276–281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data that support the findings of this study are available at: 10.5281/zenodo.15298470.
Custom codes can be found in the following GitHub repository: https://github.com/fmi-basel/2Photon_Analysis.