Abstract
Overcoming challenges in drug targeting and modulating the immunosuppressive microenvironment are critical for treating chronic bacterial infections, which are often characterized by intracellular bacteria and biofilms. To overcome these barriers, we report a multifunctional nanomedicine (CpE@BMV). The prodrug conjugate (CpE), composed of two phenylboronic acid-modified ciprofloxacin (Cip-pba) molecules and ellagic acid (Ea), self-assembles due to its hydrophobic nature and π–π stacking. Bacterial membrane vesicles (BMVs) derived from Escherichia coli aid in CpE assembly and structural stabilization. Upon administration, pathogen-associated molecular patterns on CpE@BMV engage toll-like receptors on macrophages, activating these cells and enhancing their phagocytic response. Once internalized, CpE responds to elevated intracellular H₂O₂ levels, releasing Cip to eliminate intracellular bacteria. Additionally, Ea scavenges excess reactive oxygen species in inflamed macrophages and modulates the expression of inflammatory factors, preventing an exaggerated inflammatory response. The CpE@BMV formulation also penetrates biofilms, eliminating bacteria and releasing antigens. These antigens are transported to draining lymph nodes, where they induce dendritic cell maturation and trigger a robust T and B cell-mediated immune response, helping restore immune balance and combat pathogens effectively in female mouse models. Therefore, our CpE@BMV provide an efficient strategy combining chemical and immunological therapies for chronic bacterial infection management.
Subject terms: Drug delivery, Bacterial infection, Biomedical materials
Effective delivery of therapeutic agents to the infection site and the modulation of the immunosuppressive microenvironment at the site of infection are vital for the treatment of chronic bacterial infections but remain elusive. Here, the authors address these issues by developing a nanomedicine that combines chemical and immunological therapies for chronic bacterial infection management.
Introduction
Chronic bacterial infections pose a substantial challenge in healthcare, primarily due to the persistence of intracellular bacteria and the formation of resilient bacterial biofilms1. Intracellular bacteria, capable of residing within host cells, evade immune surveillance and antimicrobial treatments, leading to persistent infections that are difficult to eradicate2,3. These intracellular pathogens exploit host cells as a niche for replication, avoiding direct exposure to the immune system’s defenses and some antimicrobial treatments4. In addition, bacterial biofilms further exacerbate the complexity of chronic infections by providing a protective environment for bacteria to thrive5–7. Biofilms are structured communities encased in a self-produced extracellular matrix, shielding bacteria from host immune responses and rendering them highly resistant to antibiotics8. The formation of biofilms not only enhances bacterial survival but also contributes to the chronicity and recurrent nature of infections9. Therefore, in the treatment of chronic bacterial infections, two major challenges arise: the first being the delivery of therapeutic agents to overcome barriers and reach the site of infection, and the second involving the modulation of the immunosuppressive microenvironment at the site of infection.
Overcoming delivery barriers entails leveraging strategies such as employing nanocarriers, liposomes, or nanoparticles to bolster drug penetration and retention at the infection site, surmount biofilm obstacles, and enhance intracellular drug delivery for targeting persistent bacteria10,11. Addressing the immunosuppressive microenvironment necessitates the development of immunomodulatory therapies12, including immune checkpoint inhibitors, cytokine treatments, or immunostimulatory agents, to revive immune function, amplify immune responses, and facilitate bacterial clearance. While immunomodulatory therapies have become a cornerstone of cancer treatment, their application to chronic bacterial infections presents unique challenges, particularly when modulating adaptive immunity13. One of the key hurdles is the limited immunogenicity of bacterial antigens within the biofilm matrix, which impairs antigen presentation and subsequent T cell activation14. Additionally, the immunosuppressive milieu is characterized by diminished antigen-presenting cell efficacy, impaired T cell function, reduced B cell somatic hypermutation, and attenuated antibody responses15,16. Chronic infections also lead to the aberrant activation of immune-suppressive cells, such as regulatory T cells (Tregs), which further complicates the immune response against biofilm-associated bacteria17. Moreover, the disrupted balance of co-inhibitory and co-stimulatory signals within adaptive immune pathways undermines the effectiveness of antibody-based therapies targeting the bacteria. Given these challenges, we propose that dual strategies—aiming to optimize drug delivery and enhance immune responses—hold promise for effectively combating chronic bacterial infections18.
Bacteria-derived membrane vesicles (BMVs) hold significant promise for cargo delivery and immunomodulatory therapy. These naturally occurring MVs exhibit a remarkable ability to transport virulence factors, toxins, and pathogen-associated molecular patterns (PAMPs) from the parent bacterium to host cells, thereby triggering a cascade of inflammatory and immunomodulatory responses19. The notable features of MVs include their stability, non-replicative nature, and the diverse array of PAMPs inherent to the parent bacterium, thus positioning them as compelling candidates for vaccine development20. MVs have demonstrated remarkable efficacy in delivering genetically engineered peptides or proteins derived from the parent bacterium, exemplified by the presence of autolysin murein hydrolase in Pseudomonas vesicles21 or specific antigens in Escherichia coli (E. coli) outer membrane vesicles22. Despite their advantageous properties, the robust structure of bacteria-derived MVs presents a challenge in effectively delivering chemotherapeutic agents, impeding the optimal penetration and diffusion of pharmaceutical compounds.
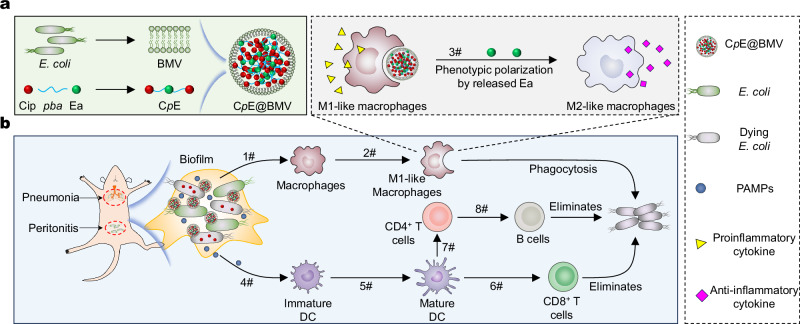
In this study, we have developed a prodrug conjugation strategy for antibiotics that can autonomously assemble in the presence of bacterial membranes, resulting in the creation of a multifunctional nanomedicine (CpE@BMV) designed for the treatment of chronic bacterial infections. We hypothesize that the prodrug conjugation (CpE), formed by combining two phenylboronic acid (pba)-modified ciprofloxacin (Cip) (Cip-pba) molecules with one ellagic acid (Ea), can self-assemble owing to its hydrophobic properties (Fig. 1a). Bacterial membranes sourced from E. coli were utilized to facilitate the assembly of CpE and stabilize the resultant structures. Upon administration, the PAMPs on the surface of BMVs can engage with toll-like receptors on macrophages, activating these immune cells and augmenting their phagocytic activity against the infecting bacteria (Fig. 1b). Furthermore, once internalized by macrophages, the encapsulated CpE can respond to elevated intracellular H2O2 levels, leading to the release of Cip to eradicate intracellular bacteria. Additionally, Ea can scavenge excess reactive oxygen species (ROS) within inflamed macrophages and downregulate the pro-inflammatory factors, thereby preventing an exaggerated inflammatory response, such as an inflammatory storm, that could harm normal tissues. Moreover, our CpE@BMV formulation exhibits the ability to penetrate bacterial biofilms and eliminate the bacteria residing within, releasing bacterial antigens in the process. Subsequently, these generated antigens are transported to draining lymph nodes. Within lymph nodes, the maturation of dendritic cells (DCs) is induced by antigens, enabling them to efficiently present to T cells and initiating T and B cells -mediated immune response. This coordinated immune response serves to maintain immune homeostasis and effectively combat external pathogens.
Fig. 1. Schematic diagram illustrating the preparation of CpE@BMV and the proposed mechanism of action to enhance antimicrobial immune response.
a Preparation of CpE@BMV via BMV cloaked prodrug CpE. BMV was extracted from E. coli. Prodrug CpE was synthesized using Cip and Ea with a pba linker. b CpE@BMV facilitated bacterial killing and immune regulator. 1#: E. coli was killed by CpE@BMV, resulting in the release of PAMPs that combined with BMV to induce macrophage polarization; 2#: M0 macrophage polarized into M1-like macrophage for efficient phagocytosis of E. coli; 3#: uptakes of CpE@BMV nanoparticles led to Ea release, further reprogramming M1-like to M2-like macrophage; 4#: generated PAMPs was delivered into immature DC; 5#: promoted DC maturation; 6#: naïve T cells were activated and differentiated into specific CD8+ T cells for eliminated E.coli; 7#: activated naïve T cells differentiated into CD4+ T cells; 8#: B cells was activated by CD4+ T cells and contributed to the elimination of E. coli.
Results
BMV stabilizes CpE
The prodrug molecule, CpE, was synthesized by utilizing dynamic boronate bonds between ellagic acid (Ea) and boronic acid-modified ciprofloxacin (Cip-pba) in dimethyl sulfoxide at a molar ratio of EA: Cip-pba of 1:2. Cip-pba was prepared following our established procedure through the reaction of Cip with 4-bromophenylboronic acid (Supplementary Fig. 1)23,24. After the reaction, the chemical shift at 28.44 ppm of Cip-pba disappeared in their corresponding 11B NMR spectra. Conversely, the appearance of a new peak at 8.78 ppm indicated the formation of a boronate ester (Supplementary Fig. 2a), consistent with our previous observations25. Furthermore, mass spectrometry showed characteristic peaks at 1161.43 and 1183.42, confirming the formation of CpE (Supplementary Fig. 2b). Further, 1H–1H COSY and NOSY spectra illustrated that the protons on Ea and Cip are coupled, suggesting that Ea and Cip are adjacent (Supplementary Fig. 2c, d). In the infrared spectrum, a sharp absorption peak near 3478 cm–1 (O–H stretching of Ea) disappeared post-reaction, while CpE retained the B-OC absorption peak around 1015 cm–1 (Supplementary Fig. 3a). The UV-vis spectrum of CpE showed an absorption peak at 273 nm, indicating the presence of Cip-pba (Supplementary Fig. 3b). These results collectively suggest the successful preparation of the CpE molecule.
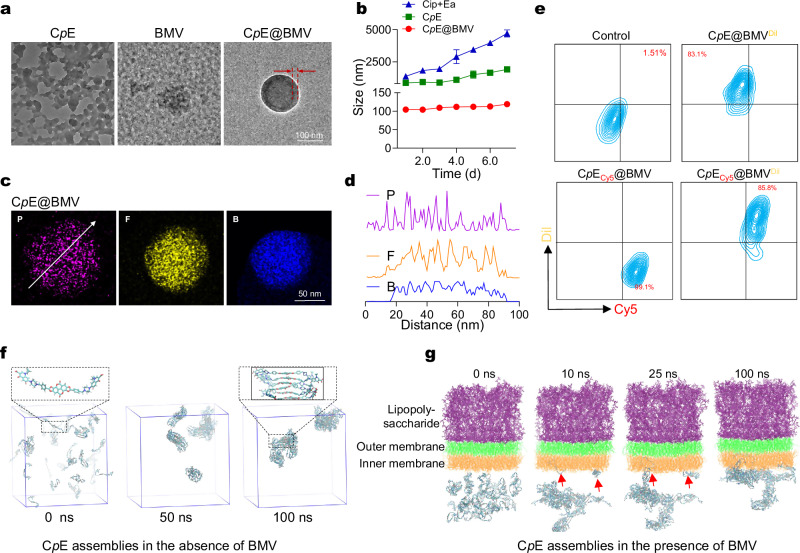
Concurrently, BMV was extracted from E. coli DH5α, one of the most common laboratory strains, using a chemical lysis method with a yield of 29%. The isolated BMV exhibited an amorphous structure (Fig. 2a). Notably, when dispersing Cip+Ea (physical mixture) and CpE in phosphate buffer (PB, pH 7.4, 10 mM), rapid precipitation occurred, resulting in aggregates with sizes exceeding 1000 nm (Supplementary Fig. 3c). This phenomenon is likely attributed to the high hydrophobicity of CpE, with a calculated LogP (partition coefficient) (ClogP) of approximately 3.7 (Supplementary Fig. 1b). A higher cLogP value indicates increased hydrophobicity of a substance. Interestingly, when CpE was dispersed in the BMV suspension and subjected to extrusion, a stable new suspension was produced, with a diameter of around 100 nm with narrow distribution and negatively charged surfaces (Supplementary Fig. 3c, d). This observation aligns with our previous publication, which demonstrated that the cell membrane can stabilize the prodrug conjugate26. Transmission electron microscopy (TEM) imaging revealed the formation of a core-shell structure in the BMV-stabilized CpE (referred to as CpE@BMV thereafter). The thickness of the shell was determined to be around 10 nm, consistent with the BMV thickness27, indicating that BMV contributes to the formation and stability of CpE in an aqueous environment. As a proof-of-concept, the size of CpE@BMV exhibited minimal variations during a 7-day observation period at room temperature, while the size of CpE aggregates gradually increased to up to 2000 nm under the same storage conditions (Fig. 2b). Further elemental mapping images of CpE@BMV (Fig. 2c, d) indicated the distribution of phosphorus (P) elements on the surface of BMV, while boron (B) and fluorine (F) elements are solely distributed within the interior. Furthermore, Dil-labeled BMV was used to encapsulate Cy5-labeled CpE, followed by analysis using flow cytometry. The percentage of Dil+/Cy5+ assemblies reached 85.8% (Fig. 2e), indicating co-localization of BMV and CpE in the CpE@BMV.
Fig. 2. Characterization of CpE@BMV.
a Representative TEM images of CpE, BMV, and CpE@BMV. Scale bar: 100 nm. For CpE@BMV, the thickness of the BMV film in CpE@BMV was around 20 nm. b Colloidal diameter of Cip+Ea, CpE, and CpE@BMV in phosphate buffer (PB, pH 7.4, 10 mM) over a 7-day period was measured using DLS. The CpE and CpE@BMV were diluted to a final concentration of 0.1 mg/mL for size measurement. Data are presented as mean ± s.d. (n = 3 biologically independent samples). c Elemental mapping image of CpE@BMV showing the distribution of P, F, and B elements. d Gray value distribution along the arrow in (c) for P, F, and B elements in CpE@BMV. e Representative nano-flow cytometry analysis of CpE@BMV. BMV and prodrug CpE core were labeled with Dil (yellow) and Cy5 (red), respectively. f Snapshots from the all-atom simulations illustrate the assembly process of CpE without BMV at different time points, with an enlargement showing the planar structure of Ea and the stacking of Ea driving aggregate formation. g Snapshots from the all-atom simulations depict the assembly process of CpE in the presence of BMV at different time points. The insertion of CpE into the bacterial membrane is indicated by the red arrows. The upper leaflet, middle leaflet, and lower leaflet of the bacterial outer membrane vesicles are represented in purple, green, and orange, respectively.
To further explore the self-assembly behavior of CpE in the presence or absence of BMV, all-atom simulations were conducted. In the absence of BMV, the planar structure of Ea facilitates the parallel stacking of CpE molecules through π-π interactions (Fig. 2f, Supplementary Movie 1), resulting in the formation of numerous small aggregates within 50 ns and a larger irregular structure at 100 ns. These larger aggregates demonstrate a lack of long-range order, assembling randomly to create disordered aggregates, consistent with our experimental observations.
Conversely, in the presence of BMV, within the initial 10 ns, most CpE molecules coalesce similarly to their behavior in the absence of BMV, owing to their hydrophobic properties. Subsequently, after 10 ns, some small CpE aggregates began to associate with BMV. By 25 ns, as the CpE aggregate diffused and came into contact with the outer membrane surface, it was attracted to the exposed regions of the CpE molecules embedded in the membrane, thereby becoming anchored to the membrane surface. Over time, the entire aggregate gradually adhered to the membrane surface, culminating in the formation of a stable binding configuration by the conclusion of the 100 ns simulation (Fig. 2g, Supplementary Movie 2). These in-silico findings once again underscore the significant role of BMV in the self-assembly process and stability.
Analysis of the protein composition in CpE@BMV via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) revealed that most membrane proteins from BMV were retained in CpE@BMV (Supplementary Fig. 3e). At physiological pH (pH 7.4), the release of Cip from CpE@BMV was relatively slow, with only 15% release efficiency achieved after 48 h. However, exposure to a microenvironment with pH 6.5 and the presence of hydrogen peroxide significantly accelerated Cip release from CpE@BMV, achieving 70% release efficiency (Supplementary Fig. 4a). Further size measurements confirmed the stability of CpE@BMV at pH 7.4. In contrast, their size increased to approximately 2 μm when exposed to pH 6.5 and hydrogen peroxide conditions (Supplementary Fig. 4b), possibly due to the triggering of the cleavage of pba and release of free Cip when CpE@BMV are exposed to hydrogen peroxide.
To determine if antimicrobial agent binding and BMV encapsulation affect the antimicrobial efficacy of the composed antimicrobial agents, Staphylococcus aureus WHGFP (S. aureus WHGFP), E. coli Xen14, Salmonella Typhimurium 15,649 (S. Typhimurium 15,649) were treated with Cip, CpE, and CpE@BMV in solution to determine their minimum inhibitory concentration and bactericidal concentration (MIC and MBC) against these two multidrug-resistant bacteria. Cip and CpE demonstrated the same concentration MIC and MBC values, while CpE@BMV demonstrated a 4-fold lower MIC and a 2-fold lower MBC (Supplementary Table 1). Similar results were observed with live/dead staining, where CpE@BMV demonstrated significantly higher bactericidal efficacy compared to other treatments (Supplementary Fig. 5a). Therefore, it can be conservatively concluded that either conjugation or membrane encapsulation does not have a negative impact on antimicrobial efficacy.
Immune-stimulatory CpE@BMV boosts the immune responses of RAW264.7 macrophages
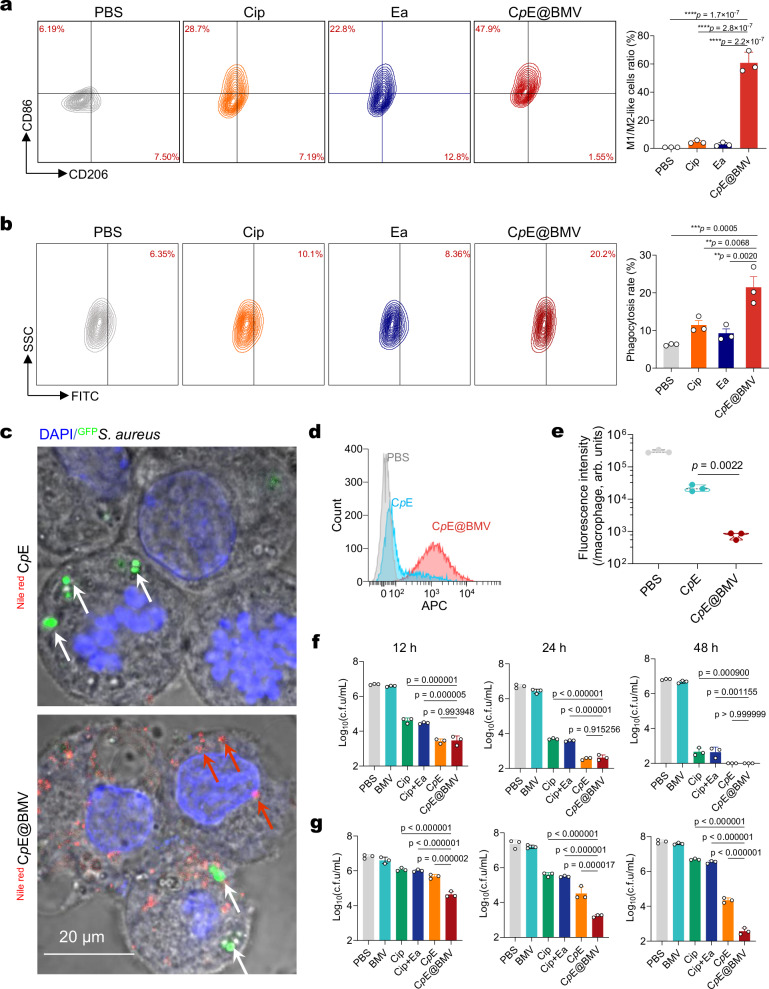
Macrophages are essential for effective innate and adaptive immune responses against bacterial infections. To assess whether the changes in immune suppression and co-stimulatory molecules induced by CpE@BMV stimulate subsequent immune responses, the polarization state and phagocytic ability of RAW264.7 macrophages were analyzed. Flow cytometry analysis showed a significant increase in the percentage of M1-phenotype macrophages in the CpE@BMV group, indicating successful activation of M0 macrophages into M1 pro-inflammatory macrophages (Fig. 3a). Further studies demonstrated that RAW264.7 macrophages treated with CpE@BMV exhibited a higher bacterial phagocytosis rate compared to other treatments (Fig. 3b). Additionally, to further confirm whether CpE@BMV was successfully taken up by infected macrophages, infected RAW264.7 macrophages were co-incubated with CpE and CpE@BMV for 2 h. Compared to bare CpE, CpE@BMV is more readily internalized by activated macrophages. This is because the presence of BMV facilitates its uptake by macrophages, which in turn internalize the encapsulated CpE. Confocal laser scanning microscopy (CLSM) (Fig. 3c) and Flow cytometry (Fig. 3d) confirmed the successful internalization of CpE@BMV by infected macrophages. Importantly, CpE@BMV taken up by infected macrophages released Cip under acidic and hydrogen peroxide conditions, aiding in the clearance of bacteria within macrophages. This was validated by a significant decrease in the green fluorescence intensity of S. aureus WHGFP in the CLSM images of the macrophages receiving the treatment of CpE@BMV (Supplementary Fig. 5b). Quantitative analysis of the green fluorescence intensity of S. aureus WHGFP within macrophages in each group supported this conclusion (Fig. 3e). Next, to demonstrate the bactericidal effect of CpE@BMV on extracellular and intracellular S. aureus WHGFP and E. coli Xen14, bacteria were mixed with CpE@BMV at a Cip concentration of 0.44 μg/mL. At the same concentration, the marginal inhibitory difference in extracellular bacteria was observed after the treatments of Cip-pba, CpE, and CpE@BMV (Fig. 3f). However, CpE@BMV was significantly more efficient in eradicating intercellular bacteria, especially in a shorter period of exposure time (Fig. 3f, g, Supplementary Fig. 6a). In addition, similar results were observed with S. Typhimurium 15,649, a clinical isolate and typical intracellular pathogen, where our CpE@BMVs were significantly more effective in eradicating intracellular bacteria compared to all other treatments (Supplementary Fig. 6b). These findings highlight the exceptional bactericidal efficacy of CpE@BMVs in eliminating intracellular bacteria.
Fig. 3. CpE@BMV infiltrated macrophages and stimulated them for intracellular bacterial eradication.
a Flow cytometry analysis illustrating the changes in CD86 (M1 marker) and CD206 (M2 marker) expression in RAW264.7 macrophages following different treatments and statistical analysis of the ratio of M1/M2 macrophages, equivalent to CD86-positive/CD206-positive cells. b Representative flow cytometry image and quantitative analysis of the phagocytosis of S. aureus WHGFP by RAW264.7 macrophages treated as specified. c Representative CLSM image depicting RAW264.7 cells with intracellular S. aureus WHGFP (white arrow) post-treatment with Nile Red-labeled CpE and CpE@BMV (red arrow). Each experiment was repeated three times independently with similar results. d Flow cytometry analysis of fluorescence intensity in the infected RAW264.7 macrophages after co-incubation with Nile Red-labeled CpE and CpE@BMV for 2 h. e Quantitative analysis of green fluorescence intensity of S. aureus WHGFP (live bacteria) within macrophages following treatment with PBS, CpE, and CpE@BMV. f, g Statistical analysis of the c.f.u. of extracellular (f) and intracellular (g) surviving S. aureus WHGFP in cocultures of bacteria and RAW264.7 macrophages after exposure to different treatments for 12, 24, and 48 h. Data are mean ± s.d. of n = 3 biologically independent samples. Statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons test, p > 0.05, no significance (ns), *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. Source data are provided as a Source Data file.
ROS scavenging and anti-inflammatory activity of CpE@BMV
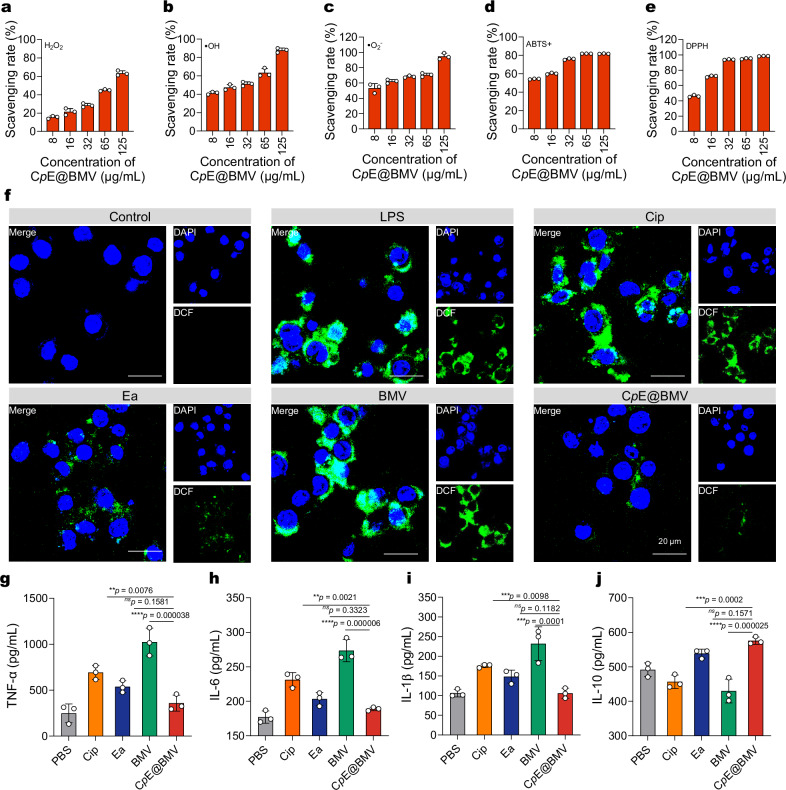
To study the ROS scavenging ability of CpE@BMV, representative ROS and reactive nitrogen species, namely H2O2 (hydrogen peroxide), •OH (hydroxyl radical), and •O2− (superoxide anion radical), ABTS+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), and DPPH (2,2-diphenyl-1-picrylhydrazyl) were used. They are generated by enzymatic catalysis reactions, Fenton reactions, xanthine and xanthine oxidase reactions, where •OH and •O2− are reactive oxygen species that can cause DNA damage, lipid peroxidation, and protein breakage. Over 60% of H2O2 (Fig. 4a), over 85% of •OH (Fig. 4b), and over 90% of •O2− (Fig. 4c) were eliminated by CpE@BMV with a concentration over 125 µg/mL. Furthermore, the excellent ROS scavenging ability of CpE@BMV was further confirmed through classical ABTS+ and DPPH radical scavenging assays (Fig. 4d, e). The multiple antioxidant properties of CpE are likely attributed to the presence of pba and Ea in CpE25. It is important to note that the concentrations used here were approximately 10-fold higher than those typically found in vivo, to meet the sensitivity requirements of the assay kit. Despite this, even the lowest nanoparticle concentration (3.9 μg/mL of Cip) effectively scavenged 20%–60% of the high levels of ROS, demonstrating the strong ROS scavenging capacity of CpE@BMVs.
Fig. 4. CpE@BMV scavenges the excessive ROS of LPS-induced macrophages.
a–e ROS scavenging efficiency of CpE@BMV toward H2O2 (a), •OH (b), •O2– (c), ABTS+ (d), and DPPH (e) at different nanoparticles concentrations (n = 3 independent samples). f Fluorescence images of intracellular ROS in LPS-treated RAW264.7 cells after various treatments. RAW264.7 cells were stained with a DCFH-DA fluorescence probe. Scale bar: 20 μm. g–j The levels of TNF-α (g), IL-6 (h), IL-1β (i), and IL-10 (j) in the supernatant of RAW264.7 macrophages after different treatments for 24 h were examined using ELISAs. Data are presented as means ± s.d. (n = 3 biologically independent samples). Statistical significance was determined using one-way ANOVA with Tukey’s post hoc test, p > 0.05, no significance (ns), *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
Further investigation was conducted to determine if CpE@BMV could eliminate intracellular ROS and protect against ROS-mediated damage. Moreover, to simulate an environment with high ROS expression in vitro, macrophages (RAW264.7) were pretreated with lipopolysaccharide (LPS) to induce inflammation and excessive ROS production. As shown in Fig. 4f, under LPS stimulation, cells produced an excess of ROS under oxidative stress conditions, evidenced by a significant increase in strong green fluorescence signal via dichlorofluorescein (DCF). However, treatment with CpE@BMV significantly reduced the green fluorescence signal, and the cell viability was significantly increased (Supplementary Fig. 7), validating the cell-protective ability of CpE@BMV against ROS-mediated damage. In addition to mitigating intracellular ROS overexpression, CpE@BMV effectively downregulated pro-inflammatory cytokine production. The anti-inflammatory potential of CpE@BMV was further evaluated, showing significant reductions in pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β), compared to other treatments. Conversely, the anti-inflammatory cytokine IL-10 was upregulated following CpE@BMV treatment (Fig. 4g–j). As anticipated, CpE@BMV reduced all major pro-inflammatory cytokines, highlighting its broad anti-inflammatory activity. These findings suggest that CpE@BMV possesses strong ROS scavenging capabilities and notable anti-inflammatory properties.
CpE@BMV eliminates E. coli biofilms
Next, biofilm elimination was evaluated in the presence of Cip, CpE, and CpE@BMV. The results showed that only a minimal amount of E. coli biofilm was dispersed following treatment with free Cip or CpE, indicating a lack of significant biofilm dispersal capability. This finding was further corroborated by the corresponding CLSM images, which revealed minimal disruption of the biofilm structure in both treatments (Supplementary Fig. 8a). In contrast, under the same conditions, CpE@BMV effectively eradicated the mature E. coli biofilms. Subsequent quantitative data showed that the green fluorescence intensity of biofilms treated with CpE@BMV was significantly lower than those treated with free Cip or CpE (Supplementary Fig. 8b). Detection of biomass, thickness, and roughness of biofilms treated with various agents revealed that the biomass and thickness of biofilms after treatment with CpE@BMV were significantly lower than those treated with free Cip or CpE (Supplementary Fig. 8c, d), with higher roughness compared to biofilms treated with free Cip or CpE (Supplementary Fig. 8e). Similar results were observed in crystal violet-stained biofilms, where the total biofilm volume of biofilms treated with CpE@BMV was significantly lower than those treated with free Cip or CpE (Supplementary Fig. 7f). Additionally, colony-forming unit (c.f.u.) enumeration of bacteria embedded in biofilms after various treatments was counted. Biofilms treated with CpE@BMV showed significantly fewer viable bacteria compared to those treated with Cip or CpE (Supplementary Fig. 8g). These results collectively demonstrate that our CpE@BMV can effectively eliminate E. coli biofilm formation. Of note, BMVs induce biofilm formation by delivering signaling molecules and adhesion factors that promote bacterial aggregation and surface attachment, while also facilitating the production of extracellular matrix components28. In our study, the effect of BMVs on biofilm formation was minimal, likely due to the short 4-h incubation, which did not allow sufficient time for the BMVs to exert their effects.
CpE@BMV eliminates bacteria and inflammation in a pneumonia model
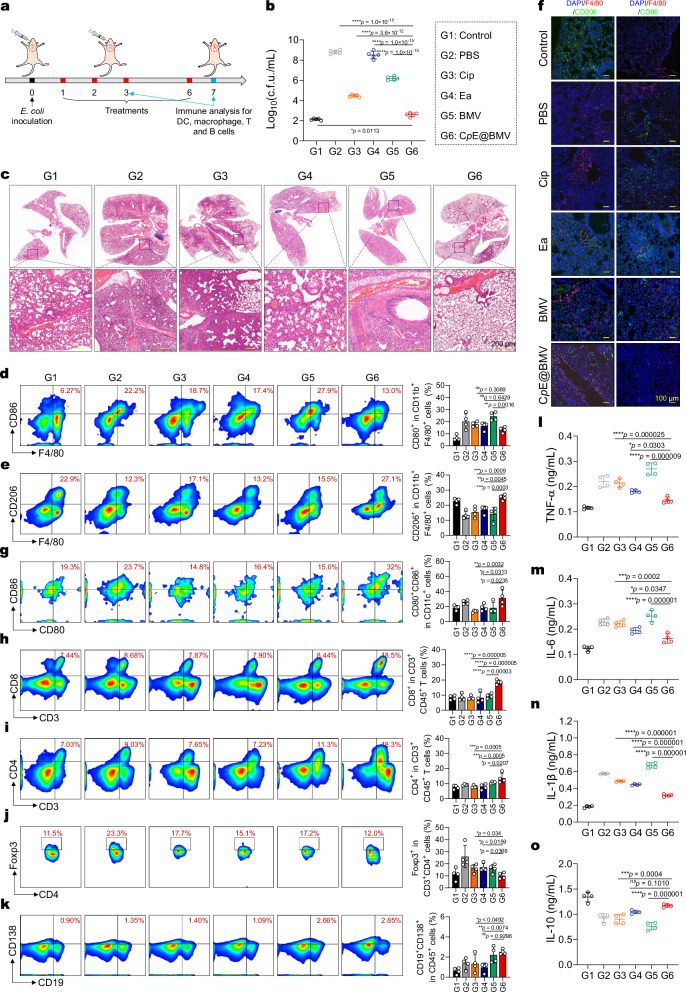
Furthermore, an animal model of mouse pneumonia was established using E. coli Xen14 to further evaluate the antimicrobial action of CpE@BMV. The pneumonia model was constructed using the reported method29, and infected mice were treated with various therapies via tracheal intubation on days 1, 2, 3, and 6 (Fig. 5a). On the 7th day, all infected mice were sacrificed, and lung tissues were homogenized for c.f.u. enumeration. Compared to the PBS-treated group, treatment with CpE@BMV resulted in a reduction of c.f.u. by over 4 log units (Fig. 5b). Compared to free Cip, CpE@BMV induced a more pronounced reduction in c.f.u. (p < 0.0001). Additionally, histological assessment using Hematoxylin and Eosin (H&E) staining of lung tissue sections was conducted to evaluate the extent of tissue necrosis. As shown in Fig. 5c, lung alveoli in healthy, uninfected lungs were intact and hollow. In contrast, lung alveoli fusion and incomplete inflation were observed in the PBS group, indicating severe bacterial infection. In comparison, the CpE@BMV-treated group maintained relatively healthy lung tissue, suggesting successful eradication of the bacterial infection. Furthermore, an immune profiling analysis of the infected draining lymph nodes (IDLN) and lung tissues demonstrated that CpE@BMV activated various immune cells in the pneumonia model. Treatment with CpE@BMV significantly reduced the proportion of pro-inflammatory M1-like macrophages (F4/80+CD86+) and increased the proportion of anti-inflammatory M2-like macrophages (F4/80+CD206+) in the lungs on days 3 and 7 post-treatment (Fig. 5d, e, Supplementary Fig. 9). Immunofluorescence staining was used to determine the phenotypes of macrophages after various treatments. Immunofluorescence imaging (Fig. 5f) revealed a significant presence of F4/80+CD86+ macrophages in the lung tissues of the PBS-treated mice, indicating macrophage activation and transition to the pro-inflammatory M1 phenotype. In contrast, CpE@BMV reduced the levels of F4/80+CD86+ macrophages and increased the levels of F4/80+CD206+ macrophages. Further flow cytometry indicated that the CpE@BMV treatment notably increases the expression of mature dendritic cells (CD11c+CD80+CD86+), facilitates CD8+ T cells (CD3+CD8+), CD4+ T cells (CD3+CD4+), infiltrated T cells (CD4+Foxp3+) activation and proliferation, increases the numbers of plasma cells (CD138+CD19+) (Fig. 5g–k, Supplementary Fig. 9). Importantly, mice treated with CpE@BMV exhibited a significant reduction in pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and a significant increase in the anti-inflammatory cytokine interleukin-10 (IL-10) in the infected tissue and serum (Fig. 5l–o, Supplementary Figs. 10–12). These results further demonstrate that the ability of CpE@BMV to enhance immune cell infiltration in lung tissues, eliciting a robust antibacterial immune response in the case of bacterial infection. Therefore, our CpE@BMV can prevent inflammation caused by bacterial infections and effectively eradicate bacteria, while exhibiting minimal cytotoxicity towards model cells and normal tissues (Supplementary Figs. 13–15). In the context of multidrug-resistant bacterial infections, antibody responses are considered a key protective factor for neutralizing bacteria. Furthermore, we observed an increase in pro-inflammatory M1-like macrophage expression after BMV treatment, reflecting the activation of immune cells in vivo. This data confirms that BMVs, rather than CpE, play a crucial role in triggering antibacterial immune responses.
Fig. 5. CpE@BMV effectively suppresses E. coli pulmonary infection in vivo.
a Experimental scheme of the E. coli-induced pulmonary infection model in female BALB/c mice. Different formulations administered via tracheal needle on days 1, 2, 3, and 6 using equivalent doses of Cip, Ea, and BMV were 1.76 mg/kg, 0.76 mg/kg, and 1.12 mg/kg, respectively (n = 4 biologically independent mice). b Quantified c.f.u. of E. coli in excised lung tissues from treatment endpoints was performed (n = 4 biologically independent mice). c Representative H&E staining images of the collected lung tissues from different groups after various treatments on day 7. Each experiment was repeated three times independently with similar results. d, e Representative scatter plots of flow cytometry data and quantitative results demonstrated the percentage of M1-phenotype macrophages (d) and M2-phenotype macrophages (e) within the CD11b+F4/80+ cell population in the lung tissues on day 7 after various treatments (n = 4 biologically independent mice). f Representative immunofluorescent images were captured for lung tissues. Each experiment was repeated three times independently with similar results. g–k Representative scatter plots of flow cytometry data and quantitative results showing the number of mature DCs (CD80+CD86+) (g), CD8+ T cells (CD3+CD8+) (h), CD4+ T cells (CD3+CD4+) (i), regulatory T cells (CD3+CD4+Foxp3+) (j), plasmablasts (CD138+CD19+) and plasma cells (CD138+CD19−) (k) (n = 4 biologically independent mice). l–o Cytokine concentrations of TNF-α (l), IL-6 (m), IL-1β (n), and IL-10 (o) in the infected lung tissue from the mice that received various treatments as measured by ELISA (n = 4 biologically independent mice). Data are presented as means ± s.d. Statistical significance was determined using one-way ANOVA with Tukey’s post hoc test, p > 0.05, no significance (ns), *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
Mechanisms of CpE@BMV in the treatment of bacterial pneumonia in vivo
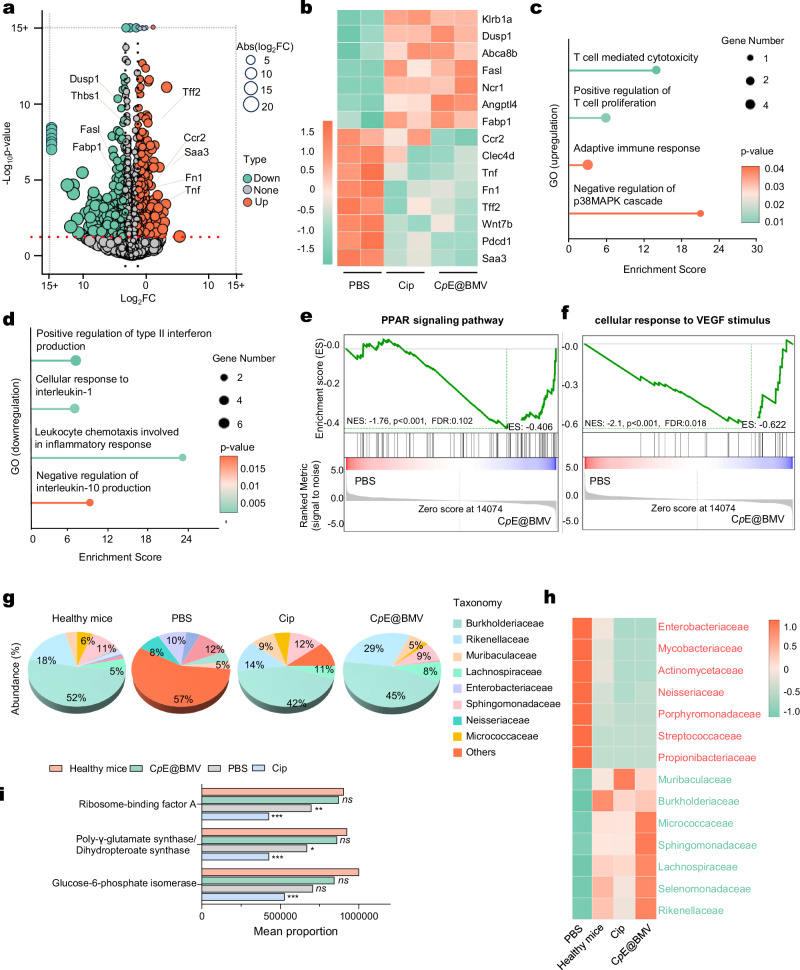
To analyze the underlying mechanisms of CpE@BMV in the treatment of bacterial pneumonia, an RNA-seq of lung tissue was conducted. Lung tissue samples from BALB/c mice after 3 days and 7 days of treatment with PBS, Cip, or CpE@BMV were collected and analyzed. A total of 392 differentially expressed genes (p < 0.05 & |log2FC | > 1.0) were identified between the PBS-treated and CpE@BMV-treated mice, after 3 days and 7 days of treatment, respectively (Fig. 6a, Supplementary Fig. 16a). A cluster heatmap was generated to visualize the expression of some differentially expressed genes in the two groups (Fig. 6b, Supplementary Fig. 16b). In the CpE@BMV-treated mice, some genes related to anti-inflammation and tissue repair were expressed higher compared to the PBS-treated group both after 3 days and 7 days of treatment, such as Dusp1, which helps maintain mitochondrial function and structural integrity after tissue injury30, and Fasl, which is related to immune privilege to protect the tissue site31 (Fig. 6b, Supplementary Fig. 16b). Conversely, genes related to tissue damage and inflammatory response were overexpressed in PBS-treated mice, including Wnt7b, which is related to abnormally activated Wnt signaling in lung tissue and severe pulmonary epithelial injury32, Tnf, which is strongly related to cell death and chronic inflammation33, and Saa3, which stimulates pro-inflammatory cytokine expression34 (Fig. 6b). Gene ontology (GO) network analysis revealed that, compared to the PBS-treated group, pathways involved in adaptive immune response and anti-inflammatory pathways were upregulated in the CpE@BMV-treated group, both after 3 days and 7 days of treatment. This included the enhanced T cell proliferation and T cell-mediated cytotoxicity, an enhanced adaptive immune response, and the negative regulation of the inflammatory signaling pathway p38MAPK, which produces interleukin-1 and tumor-necrosis factor35 (Fig. 6c), as well as T cell-mediated immunity and B cell activation after 3 days treatment (Supplementary Fig. 16c). Meanwhile, some GO terms related to pro-inflammation were downregulated in the CpE@BMV-treated group both after 3 days and 7 days of treatment, including the positive regulation of type II interferon production, cellular response to interleukin-1, and negative regulation of interleukin-10 production36 (Fig. 6d), as well as tumor necrosis factor binding after 3 days treatment (Supplementary Fig. 16d). Gene set enrichment analysis (GSEA) revealed that integration of energy metabolism had higher activity in CpE@BMV-treated group compared to the CpE@BMV-treated group after 3 days treatment (Supplementary Fig. 16e). The PPAR signaling pathway and cellular response to VEGF in the PBS-treated group showed lower activity compared to the CpE@BMV-treated group (Fig. 6e, f). The PPAR signaling pathway controls cellular metabolism and exerts anti-inflammatory effects on immune cells37,38. The cellular response to VEGF is a key signaling pathway mediating physiological angiogenesis, crucial for tissue repair39. The lower expression of PPAR and the cellular response to VEGF in the PBS-treated group indicates that the lung tissue failed to transition from the inflammatory phase to the lung repair phase, whereas the CpE@BMV-treated group showed greater success in anti-inflammation and infected lung tissue repair (Fig. 6e, f).
Fig. 6. Transcriptome sequencing and microbiota analysis in the pulmonary model on day 7 post various treatments.
a Volcano plot displayed the distributions of upregulated and downregulated genes (≥2-fold difference, p-value < 0.05) in the CpE@BMV group compared to the PBS group. None, non-differentially expressed genes (DEGs). b Heatmaps of different groups of upregulated and downregulated DEGs related to immune response pathways. c, d Gene ontology (GO) enrichment analysis of DEGs performed for both upregulated (c) and downregulated (d) GO terms between the CpE@BMV-treated mice and PBS-treated mice. e, f Gene set enrichment analysis (GSEA) of DEGs enriched in PPAR signaling pathway (e) and cellular response to VEGF stimulus (f). g Relative abundance of the top 7 family-level taxa in the lung microbiome across healthy mice, PBS, Cip, and CpE@BMV treatments. h Relative abundance of various taxa in the healthy mice, PBS, Cip, and CpE@BMV treatments. Red, pathogenic strains in lung infection. Green, non-pathogenic strains. i Predicted functional compositions of the microbial communities in the healthy mice, PBS, Cip, and CpE@BMV treatments. Data are presented as means ± s.d. Statistical significance was determined using one-way ANOVA with Tukey’s post hoc test, p > 0.05, no significance (ns), *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
CpE@BMV reshapes the lung microbiome after infection in vivo
In pneumonia, a large number of bacteria invade the lung tissue, resulting in dysbiosis of the mouse lung microbiome. To reveal changes in the lung microbiome, a 2bRAD sequencing for microbiome (2bRAD-M) was conducted. Compared to ribosomal 16s RNA sequencing, 2bRAD-M offers higher accuracy and sensitivity, generating species-level taxonomic profiles and enabling qualitative and relative quantitative analysis of microbes, providing information on most bacterial species. Lung tissue samples from BALB/c mice after 3 days and 7 days of treatment with PBS, Cip, or CpE@BMV were collected and analyzed. The relative abundance of different taxa at the family-level changed significantly after infection, especially with an increase in the abundance of Enterobacteriaceae and a decrease in Burkholderiaceae (Supplementary Fig. 16f). Meanwhile, the CpE@BMV-treated mice showed a lower abundance of Enterobacteriaceae and a higher abundance of Burkholderiaceae after 3 days of treatment, compared to PBS and Cip-treated mice, indicating a faster recover of microbiome (Supplementary Fig. 16f). After 7 days treatment, the community abundance in the PBS-treated mice is still quite different from that in healthy mice, Cip-treated and CpE@BMV-treated mice. The top three abundant families in PBS-treated mice are Neisseriaceae (12%), Enterobacteriaceae (10%), and Porphyromonadaceae (8%), indicating that pathogenic E. coli remains the major bacterium after PBS treatment. The top three families in healthy mice, Cip-treated mice, and CpE@BMV-treated mice are the same: Burkholderiaceae (52% in healthy mice, 42% in Cip-treated mice, and 45% in CpE@BMV-treated mice), Rikenellaceae (18% in healthy mice, 14% in Cip-treated mice, and 29% in CpE@BMV-treated mice), and Sphingomonadaceae (11% in healthy mice, 12% in Cip-treated mice, and 8% in CpE@BMV-treated mice), none of these families belong to pathogenic bacteria. (Fig. 6g). Subsequently, the relative abundance of species between different groups was displayed using a heatmap (Fig. 6h, Supplementary Fig. 16g). The species abundance in the PBS group differs significantly from the healthy group, while Cip and CpE@BMV effectively restore the abundance of some pathogenic strains to normal levels, including the reduced considerably abundance of Enterobacteriaceae, Neisseriaceaeand Porphyromonadaceae, and maintained non-pathogenic Burkholderiaceae, Sphingomonadaceae, and Lachnospiracesain at a higher level (Fig. 6h, Supplementary Figs. 16g and 17a). There are also significant differences in microbial metabolism and bacterial functional changes after CpE@BMV treatment. We used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) to predict the functional composition of the microbial communities in different groups (Fig. 6i). The results show that the mean proportion of ribosome-binding factor A, folypolyglutamates synthase/dihydropteroate synthase, and glucose-6-phosphate isomerase in the PBS-treated and Cip-treated mice were significantly lower than those in the CpE@BMV-treated and healthy mice, indicating that mitochondrial ribosome biogenesis, protein synthesis, and glycolysis in the lung microbiome are less active (Fig. 6i, Supplementary Fig. 17b). This situation was significantly improved in the CpE@BMV-treated mice, as evidenced by the similar proportions between healthy mice and CpE@BMV-treated mice (Fig. 6i). These results indicate that the CpE@BMV treatment can reshape the lung microbiome after infection and help it recover to a healthy level.
CpE@BMV responds to recurrent peritonitis by enhancing long-term immunity
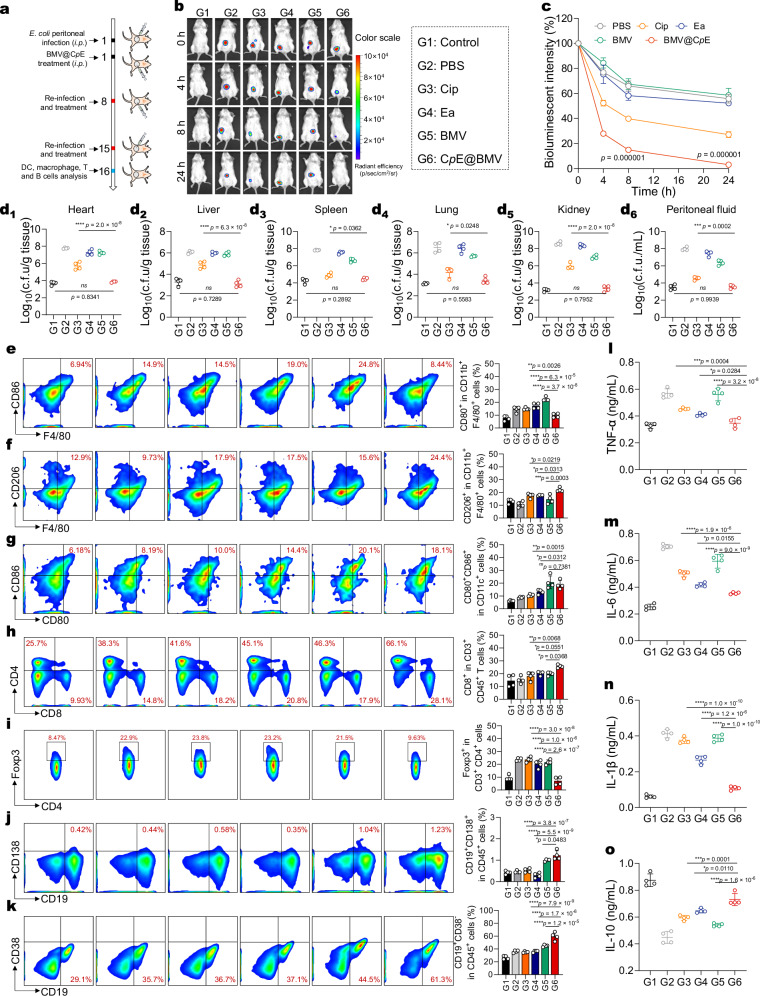
To study the efficacy of CpE@BMV to treat the widespread peritonitis in vivo, we used E. coli Xen14 to establish a peritonitis animal model. Two hours post-infection, mice were treated with various formulations, and all measurements were taken during a 24-h observation period (Fig. 7a). During the 24-h observation period, a decreasing trend in bioluminescence intensity at the infection site was observed in mice treated with free Cip, but notably, CpE@BMV treatment resulted in a significant and rapid decrease in bioluminescence intensity at the infection site (Fig. 7b). Quantitative analysis of bioluminescence intensity showed that over 85% of the bioluminescence intensity decreased within the first 8 h after treatment with CpE@BMV, indicating rapid bacterial killing and prevention of further bacterial infection development (Fig. 7c). The bacterial counts of E. coli per gram of homogenized organ tissues from five organs were quantified, revealing that the CpE@BMV treatment exhibited a significant reduction (p < 0.05) in c.f.u. compared to the Cip treatment, particularly showing significant bactericidal effects in the heart, liver, and kidneys (Fig. 7d1–d5). For bacteria in ascites, both free Cip and CpE@BMV demonstrated considerable clearance efficiency. While free Cip exhibited notable extracellular bactericidal performance, its intracellular inhibition of E. coli was quite low. In contrast, CpE@BMV effectively killed intracellular E. coli and demonstrated optimal therapeutic efficacy (Fig. 7d6).
Fig. 7. CpE@BMV exhibits bacterial eradication activity in the peritonitis model and established immunological memory in vivo.
a Experimental scheme of the E. coli peritonitis model in female BALB/c mice. Different formulations administered via i.p. on day 1 using equivalent doses of Cip, Ea, and BMV were 1.76 mg/kg, 0.76 mg/kg, and 1.12 mg/kg, respectively (n = 4 biologically independent mice). b Bioluminescent images of the mice with peritonitis model after various treatments. c Quantified bioluminescence intensity from the images in (b) (n = 4 biologically independent mice). d1–d6 Quantitative analysis of survival E. coli in d1 heart, d2 liver, d3 spleen, d4 lung, d5 kidney, and d6 ascites fluid of the mice receiving various treatments on day 2 (n = 4 biologically independent mice). e–k Representative scatter plots of flow cytometry data and quantitative results showing the number of M1-phenotype macrophages (F4/80+CD80+) (e), M2-phenotype macrophages (F4/80+CD206+) (f), mature DCs (CD80+CD86+) (g), CD8+ and CD4+ T cells (h), regulatory T cells (CD3+CD4+Foxp3+) (i), plasmablasts (CD138+CD19+) (j), and memory B cells (CD19+CD38−) (k) (n = 4 biologically independent mice). l–o Cytokine concentrations of TNF-α (l), IL-6 (m), IL-1β (n), and IL-10 (o) in the peritoneal fluid from the mice that received various treatments as measured by ELISA (n = 4 biologically independent mice). Data are presented as means ± s.d. Statistical significance was determined using one-way ANOVA with Tukey’s post hoc test, p > 0.05, no significance (ns), *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
Encouraged by the high efficacy of CpE@BMV to inhibit bacterial proliferation, we further evaluated the ability of CpE@BMV for long-term immune memory in vivo, providing enduring protective immunity against reinfection with Gram-negative bacteria. In addition to the initial peritoneal infection and CpE@BMV treatment, the immune mice were infected and treated post-infection on days 7 and 14, followed by immune cell analysis of macrophages, DC cells, B cells, and T cells in the spleen after sacrificing the mice on days 3 and 15. Similar to the immune response in the pneumonia infection model, a decrease in the expression levels of M1-like macrophages (F4/80+CD86+) and an increase in the expression levels of M2-like macrophages (F4/80+CD206+) were observed in the CpE@BMV treatment group, along with higher levels of mature DCs (CD11c+CD80+CD86+), CD8+ T cells (CD3+CD8+), CD4+ helper T cells (CD3+CD4+), and plasma cells (CD19+CD138+) infiltration (Fig. 7e–j, Supplementary Figs. 18 and 19). To evaluate the level of pathogen-specific immune memory stimulation, memory B cells (CD45+CD19+CD38–) significantly increased in mice compared to untreated mice (Fig. 7k). To study the inflammatory response in mice receiving various treatments, pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, and anti-inflammatory cytokine IL-10 in ascites fluid and serum were measured. In ascites fluid, treatment with CpE@BMV significantly neutralized pro-inflammatory cytokines, outperforming other treatments. Furthermore, treatment with CpE@BMV significantly promoted the expression of IL-10 in ascites fluid (Fig. 7l–o) and the serum (Supplementary Fig. 20). These results collectively demonstrate that CpE@BMV can effectively eradicate bacteria in the peritonitis model, alleviate inflammation, and help prevent reinfection while enhancing the body’s resistance by activating memory B cells.
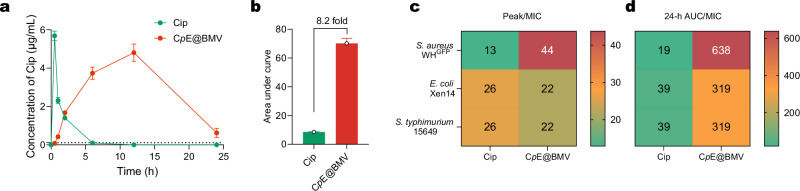
Moreover, the pharmacokinetic/pharmacodynamic (PK/PD) parameters were analyzed to evaluate the efficacy of CpE@BMV in comparison to free Cip. PK/PD parameters describe the relationship between drug exposure and bacterial killing, aiding in the optimization of antibiotic dosing strategies to achieve maximal therapeutic effects while minimizing the risk of resistance development40. Based on the blood Cip concentration, we observed that CpE@BMV significantly prolonged the bioavailability of Cip, with its 24-h AUC being 8.2 times higher than that of free Cip (Fig. 8a, b, and Supplementary Fig. 21). This substantial increase suggests that the nanoparticle-based delivery system enhances systemic circulation time and drug retention, potentially leading to improved clinical outcomes.
Fig. 8. CpE@BMV enhances the pharmacokinetic profile of Cip.
a Cip concentration in plasma following peritoneal infection with either Cip or CpE@BMV at different time intervals. Data are presented as mean ± S.D. (n = 3 biological replicates). b Area under the curve calculated from (a). c Heatmap showing the Peak/MIC ratio of Cip and CpE@BMV against the various pathogens. d Heatmap showing the 24-h AUC/MIC ratio of Cip and CpE@BMV against the various pathogens.
Peak/MIC (Cmax/MIC) represents the ratio of the maximum drug concentration to the MIC, which is crucial for concentration-dependent antibiotics like Cip, where bacterial killing efficacy increases with higher peak concentrations. The recommended target for Peak/MIC is ≥8–10 to ensure optimal antimicrobial activity41. In our study, the Peak/MIC ratio of free Cip ranged from 13 to 26, already exceeding the minimum threshold. However, CpE@BMV further elevated the Peak/MIC ratio to a range of 22–44 (Fig. 8c), indicating a greater potential for rapid bacterial eradication. The significantly higher Peak/MIC of CpE@BMV suggests improved drug delivery efficiency and greater potential for achieving therapeutic success, particularly in severe infections requiring high peak concentrations for bacterial eradication.
24-h AUC/MIC (AUC24/MIC) reflects total drug exposure over 24 h relative to MIC and is particularly important for time-dependent or exposure-dependent antibiotics like Cip. Recommended AUC24/MIC targets for fluoroquinolones are ≥125 for Gram-negative bacteria and ≥30–50 for Gram-positive bacteria, ensuring sufficient drug exposure to suppress bacterial growth and prevent resistance selection. In our study, the AUC24/MIC of free Cip ranged from 19 to 39, which is below the required threshold, particularly for Gram-negative pathogens. This suboptimal exposure may contribute to inadequate bacterial clearance and a higher likelihood of resistance development over time. In contrast, CpE@BMV exhibited an AUC24/MIC range of 319–638 (Fig. 8d), far exceeding the necessary levels. This remarkable improvement underscores the enhanced drug retention and sustained release profile of CpE@BMV, which may lead to prolonged bacterial suppression and reduced dosing frequency, thereby improving patient compliance and treatment efficacy.
These findings highlight the superior pharmacokinetic performance of CpE@BMV compared to free Cip, emphasizing its potential to improve treatment outcomes in bacterial infections. The significant enhancements in both Peak/MIC and AUC24/MIC suggest that CpE@BMV could offer more effective bacterial eradication, prolonged therapeutic coverage, and a reduced risk of resistance development42. Further investigations, including in vivo efficacy studies and clinical trials, are warranted to confirm these advantages and assess the long-term benefits of this drug delivery system.
Discussion
Prodrug assemblies amalgamate the benefits of prodrugs and nanomedicines43–45. Typically, prodrug assemblies exhibit high drug loading capacities and mitigate premature drug release and potential drug-related side effects. In this study, we employed Ea bearing two catechol domains to engage in a reaction with Cip-pba, resulting in the formation of the CpE prodrug through dynamic covalent boronate bonding. A notable advantage of this dynamic covalent bonding process is the generation of two water molecules without the involvement of harmful organic solvents, thus averting the potential side effects stemming from residual organic solvent remnants46–48. Moreover, the CpE prodrug showcases a significantly high drug loading content (>95%), surpassing the drug loading contents typically observed in conventional drug delivery systems, which usually fall below 10%. This underscores the paramount significance of our CpE prodrug, which is facilitated by dynamic covalent bonding.
Another notable advantage of our CpE prodrug, which incorporates Cip and Ea, lies in its potential to surmount bacterial drug resistance and inflammation by leveraging a combination of antibiotics and their adjuvants. Cip typically functions by inhibiting bacterial DNA gyrase and topoisomerase IV enzymes, crucial for bacterial DNA replication, transcription, repair, and recombination processes. Conversely, polyphenols often target bacterial cell membranes. By concurrently targeting distinct bacterial elements, the likelihood of bacteria developing drug resistance is diminished, and in certain cases, existing drug resistance may even be reversed49,50. While the amalgamation of drugs holds significant promise in combating bacterial drug resistance, the dissimilar pharmacological characteristics of individual drugs can impede their efficacy in reaching their intended targets spontaneously. Our CpE prodrug, which combines two distinct drugs, presents an efficacious strategy for the spontaneous delivery of drugs, thereby enhancing therapeutic outcomes.
Moreover, the presence of Ea in the CpE formulation enhances its self-assembly, facilitated by the planar structure of Ea and the strong π–π stacking interactions. It is worth noting that the irregular structure of Cip at the terminals of CpE leads to the self-assembled structures of CpE exhibiting a disordered arrangement, prone to forming amorphous structures and aggregates. To enhance the stability of CpE self-assemblies, we introduced the BMV. Our all-atom simulation findings indicate that BMV effectively mitigates the excessive aggregation of CpE, with the Cip domain of CpE potentially embedding into the membrane and acting as an anchoring point. Consequently, the spherical configuration of BMV acts as a confinement for CpE assemblies, thereby stabilizing the resulting suspensions. Remarkably, we have observed that our CpE aggregates adhere to the inner surface of the BMV, which is distinguished by a reduced presence of lipopolysaccharides. This phenomenon may be attributed to the enhanced interactions between CpE and lipids, surpassing those with lipopolysaccharides. Moreover, the nanoparticulate nature of CpE@BMV also facilitates the penetration of drugs into bacterial biofilms, as has been extensively observed in numerous previous studies11,51, ultimately enhancing the efficacy of bacterial eradication in their biofilm growth mode.
An alternative effective strategy in addressing antimicrobial resistance involves the utilization of vaccines. Bacteria-derived membrane vesicles containing a plethora of PAMPs have the ability to trigger innate immune signaling pathways and have been harnessed as vaccine adjuvants and carriers in cancer therapy52. Gram-negative bacteria utilize two primary mechanisms for vesicle formation: outer membrane blebbing and explosive cell lysis. In contrast, Gram-positive bacteria undergo a process called “bubbling cell death,” triggered by endolysin, which leads to the formation of cytoplasmic membrane vesicles53. Since BMVs derived from Gram-negative bacteria are easier to prepare and more commonly used, we employed BMVs from E. coli in our study as a model system to encapsulate the prodrug conjugate and stimulate the immune response. These BMV exhibit remarkable stability, are non-pathogenic, and can be genetically engineered nanoparticles housing crucial immunogenic proteins from the originating bacterium22,54. They have the capacity to induce immune responses from both the innate and adaptive immune systems, making them well-suited for roles as vaccine candidates and immune modulators55. The chemical lysis method and bacterial cell wall disruption were used instead of traditional OMV isolation methods because they provide a higher yield and are more efficient in breaking open bacterial cells. This approach is faster, cost-effective, and allows for better control over the release of cellular contents. Traditional OMV isolation methods, like ultracentrifugation, can be more time-consuming and may not always yield consistent or pure results53. The higher yield of our BMV (29%) compared with the commonly used outer membrane vesicles56 for vaccine (<0.01%)57 is a big advantage for broader application. Antigen-presenting cells (APCs) play a crucial role in bacterial infection, as the occurrence of infection significantly affects the activity and function of APCs, impacting immune responses and regulation58. The transformation of APCs suggests that CpE@BMV promotes the processing and presentation of bacterial antigens. The significant increase in the expression of mature dendritic cells (CD11c+CD80+CD86+) facilitates T cell activation and proliferation. The increase in CD8+ T cells (CD3+CD8+) infiltration indicates the essential role of cytotoxic T cells in immune regulation, involved in clearing infected and abnormal cells, participating in immune responses, maintaining immune homeostasis, and protecting the body from pathogens and abnormal cells. Additionally, an increase in CD4+ T cells (CD3+CD4+) infiltration further suggests that CpE@BMV enhances humoral immune responses, regulates inflammatory responses, and maintains the normal function of the immune system59. Furthermore, a sharp decrease in infiltrated T cells (CD4+Foxp3+) indicates that CpE@BMV restricts the migration of T cells to the immune microenvironment, leading to a reduction in immune suppression and increased immune response activity. B cells, as specialized APCs, differentiate into plasma cells and memory B cells upon infection, participating in humoral immune responses by producing antibodies to neutralize and eliminate invading bacteria, thereby protecting the body from infection60. An increase in plasma cells (CD138+CD19+) in the immune microenvironment following treatment with CpE@BMV suggests that CpE@BMV induces antigen-specific humoral immune responses, enhancing humoral immune efficacy and enabling mice to produce a large quantity of antibodies more rapidly and effectively upon encountering the same bacteria again, leading to a faster and stronger immune response61.
Bacterial pneumonia arises when host defenses are compromised and/or when the host is exposed to highly virulent or a significant number of microorganisms. Additionally, prior infections and chronic lung conditions can contribute to the development of pneumonia62. The process of the host immune system clearing pathogen infections and further repairing lung tissue typically involves two sequential phases: the pro-inflammatory phase and the anti-inflammatory phase. During the pro-inflammatory phase, pathogen eradication is promoted through enhanced phagocytic activity and ROS generation, while the subsequent anti-inflammatory phase aids in tissue repair and remodeling63. Transcriptomic data analysis of lung tissue collected after 7 days of treatment demonstrated that the CpE@BMV-treated mice exhibited downregulated pro-inflammatory genes and signaling pathways (Fig. 6b–e), consistent with measurements of inflammatory cytokines (Fig. 5l–o). Concurrently, the pathogen quantity significantly decreased in the CpE@BMV-treated group (Fig. 5b). These findings indicate that CpE@BMV treatment effectively clears the pathogen and facilitates the transition of mice from the pro-inflammatory phase to the anti-inflammatory phase. Additionally, GO network analysis revealed the CpE@BMV-treated group had a higher response in adaptive immune compared to the PBS-treated group (Fig. 6c, d), aligning with the results from flow cytometry (Fig. 5d–k), further confirming enhanced adaptive immunity in the CpE@BMV-treated group.
Of note, it has been clear that the healthy lungs have a unique microbiota which includes around 100 different taxa. Disruption of this microbial balance, known as dysbiosis, occurs during bacterial infections, as evidenced in the 2bRAD-M results (Fig. 6g–i). The CpE@BMV treatment successfully reconfigured the lung microbiota from an infected state, similar to the PBS-treated group, to a healthy mouse status. This transformation is supported by the changes in microbiota composition and abundance (Fig. 6g–i), along with the quantification of pneumonia-causing pathogens in lung tissue (Fig. 5b). Lung microbiota not only influences microbial diversity and taxonomy abundance but also plays a crucial role in immune responses. Consequently, the reshaping of the microbiota further contributed to the recovery from pneumonia.
Methods
Materials and reagents
Ciprofloxacin (Cip, 97%) was purchased from Bidepharm (Shanghai, China). Ellagic acid (EA) was purchased from J&K Scientific (Beijing, China). H2O2, •OH, •O2– kits were purchased from Jiancheng Bioengineering Institute (Nanjing, China). Nile red (95%), ammonium persulfate (97%), 2,2-Diphenyl-1-picrylhydrazyl (DPPH, 95%), N, N, N′, N′-Tetramethylethylenediamine (TEMED, 95%) were purchased from Macklin (Shanghai, China). SDS-PAGE, Hoechst 33,342, and 4′,6-diamidino-2-phenylindole (DAPI, 95%) were purchased from Thermo Fisher (Shanghai, China). Dil (95%), bicinchoninic acid (BCA) kits, 30% Acr-Bis (29:1), sodium 1-dodecanesulfonate (SDS), RIPA lysate, coomassie blue, and cell counting kit-8 (CCK-8) were purchased from Beyotime (Shanghai, China). Cy5 (Sulfo-Cyanine5, 95%) was purchased from MedChemExpress (New Jersey, USA). Anti-mouse CD16/32 antibody (Clone: 93), anti-mouse CD3-FITC (Clone: 17A2), anti-mouse CD4-BV421 (Clone: RM4-4), anti-mouse CD8a-APC (Clone: 53-6.7), anti-mouse Foxp3-PE (Clone: MF-14), anti-mouse CD19-APC (Clone: 6D5), anti-mouse CD138-APC (Clone: 281-2), anti-mouse CD86-BV421 (Clone: GL-1), anti-mouse CD80-APC (Clone: 16-10A1), anti-mouse CD11c-FITC (Clone: N418), anti-mouse CD206-FITC (Clone: C068C2), anti-mouse CD86-BV421 (Clone: GL-1) and anti-mouse F4/80-APC (Clone: BM8) were purchased from BioLegend (California, USA). Dulbecco’s Modified Eagle Medium (DMEM), penicillin-streptomycin, phosphate-buffered saline (PBS), and fetal bovine serum (FBS) were purchased from Gibco Life Technologies, Inc. (Grand Island, USA). Mouse TNF-α, IL-1β, IL-6, IL-10 ELISA kits, and macrophage colony-stimulating factor 1 (M-CSF) were purchased from iCell Bioscience Inc. (Shanghai, China). Bovine serum albumin (BSA) and Triton-X-100 were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China).
Bacterial strains
S. aureus WHGFP, E. coli Xen14 (PerkinElmer Inc., Waltham, MA, USA), E. coli DH5α, and S. typhimurium 15,649 were employed in this study. S. typhimurium 15,649 was kindly provided by Zhou Lab at the First Affiliated Hospital of Wenzhou Medical University. For experiments, one colony of E. coli DH5α on lysogeny broth (LB, OXOID, Basingstoke, UK) plates was inoculated into 10 mL of LB at 37 °C for 24 h in ambient air. Preculture was diluted 1:20 in 200 mL LB and grown statically (37 °C, 16 h) for the main culture to harvest bacteria. S. aureus WHGFP with green fluorescence and E. coli Xen14 with bioluminescence are multidrug-resistant strains cultured at 37 °C under ambient atmosphere in tryptone soy broth (TSB, OXOID, Basingstoke, UK) or LB medium, respectively. Bacteria were cultured using the same protocol described above, with plate growth and preculture performed in medium containing strain-specific antibiotics: 10 μg/mL tetracycline for S. aureus WHGFP or 30 μg/mL kanamycin for E. coli Xen14. S. typhimurium 15,649 was incubated for 24 h at 37 °C, followed by main culture using the same protocol described above. Finally, bacteria were harvested by centrifugation at 5000 g for 5 min. The collected bacteria were washed twice in PBS (10 mL, 5 mM K2HPO4, 5 mM KH2PO4, and 150 mM NaCl, pH 7.4), and bacterial concentration was determined in a Bürker-Türk counting chamber.
Cell lines
Murine macrophage cell line (RAW264.7) and mouse fibroblast (L929) cells were involved in this study and were purchased from the American Type Culture Collection (ATCC). RAW264.7 and L929 cells were cultured in DMEM containing 10% FBS and 1% penicillin−streptomycin solution.
Animals
Female BALB/c mice (6–8 weeks) were provided by Vital River Laboratory Animal Technology Co., Ltd (Beijing, China). The mice were housed under controlled conditions (20 ± 2 °C, 50% humidity, and 12/12 light cycle). Experiments followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. All animal studies were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at Wenzhou Institute, University of Chinese Academy of Sciences (No. WIUCAS22081605, WIUCAS25010207).
Preparation of bacterial membrane
The collected and lyophilized E. coli DH5α (2 g) was dispersed in Tris-HCl buffer (40 mL, 20 mM, pH 8.0) containing lysozyme (15 mg/mL). The samples were incubated at 37 °C under constant shaking for 3 h. Subsequently, the bacterial solution was incubated with 400 mg SDS and disrupted using a probe sonicator while cooling in an ice/water bath before being lyophilized. The resulting dried bacteria lysate was suspended in a mixture of chloroform-methanol-water (5 mL, 30:15:1, v/v/v), placed on a shaker at 37 °C for 1 h, and then filtered by Millipore HVLP Durapore® membrane (0.45 μm). The residue was extracted by chloroform-methanol-water three more times, and then the filtrate was combined and dried under vacuum to yield BMV (0.58 g, yield: 29%). The yield (%) was determined by dividing the weight of BMV by the total bacterial weight. Finally, the protein concentration of the bacterial membrane was determined using the BCA assay.
Synthesis of Cip-pba-EA prodrug
The phenylboronic acid-functionalized ciprofloxacin (Cip-pba) was synthesized according to our previous report24. Briefly, ciprofloxacin (Cip, 252 mg, 0.73 mmol) and K2CO3 (300 mg, 2.17 mmol) were dissolved in DMF/H2O (4.0 mL, v/v = 2/1), followed by the addition of 4-(bromomethyl)-phenylboronic acid (163 mg, 1.35 mmol). The reaction mixture was allowed to react overnight. The pH of the solution was adjusted to 7.4 using an HCl solution. Subsequently, the precipitation was filtered and dried under a vacuum to obtain the white powder. 1H NMR (400 MHz, DMSO-d6) of Cip-pba: 1H NMR (400 MHz, DMSO) δ 15.18 (s, 1H), 8.67 (s, 1H), 8.01 (s, 2H), 7.91 (d, J = 13.3 Hz, 1H), 7.79 (d, J = 7.8 Hz, 2H), 7.58 (d, J = 7.4 Hz, 1H), 7.33 (d, J = 7.7 Hz, 2H), 3.83 (s, 1H), 3.59 (s, 2H), 2.62 (s, 4H), 1.33 (d, J = 6.4 Hz, 2H), 1.19 (s, 2H).
The conjugated prodrug, Cip-pba-Ea was synthesized by a dynamic covalent bond. Briefly, Cip-pba (5.0 mg, 10.0 μmol) and Ea (1.5 mg, 5.0 μmol) were reacted in DMSO (500 μL) under stirring at 25 °C for 12 h. The resulting product is denoted as CpE. The chemical structure of CpE was verified by 1H–1H COSY and 1H–1H NOSY spectra using NMR spectroscopy (AVANCE III 400 MHz, Bruker, Germany) in methanol-d4, mass spectrum (Agilent 6520Q, California, USA), Fourier infrared spectrometer (Bruker Tensor II, Karlsruhe, Germany) and UV-vis spectrum (UV-1900i, Shimadzu, Japan).
Preparation of CpE@BMV
Cip-pba (5.0 mg, 10.0 μmol) and Ea (1.5 mg, 5.0 μmol) were reacted in DMSO (500 μL) under stirring at 25 °C for 12 h. The resulting product CpE was coated with BMV through extrusion. In brief, CpE (100 μL, 13 mg/mL) and BMV (900 μL, 500 μg/mL in ultrapure water) were mixed, and a noticeable Tyndall effect was observed immediately. Then, the mixture of BMV and CpE was extruded 20 times successively through a 200 nm polycarbonate porous membrane with an Avanti mini extruder to obtain the CpE@BMV, and the CpE@BMV was kept at 4 °C for further use.
In addition, the Dil-labeled CpE@BMV (CpE@BMVDil) was prepared by adding Dil (3%, w/w of BMV) to the BMV, followed by mixing with CpE according to a similar protocol as described above. The free Dil was removed by centrifugation. For the preparation of Cy5- or Nile Red-labeled CpE@BMV (CpECy5@BMV or Nile redCpE@BMV), Cy5 (3%, w/w of Cip-pba) or Nile Red (3%, w/w of Cip-pba) was added during CpE formation, and a similar protocol was used to prepare CpECy5@BMV or Nile RedCpE@BMV. Free Cy5 or Nile Red was removed by centrifugation. The CpECy5@BMVDil was prepared by adding Dil (3%, w/w of BMV) to the BMV, followed by mixing with Cy5-labeled CpE (3%, w/w) using a similar protocol. Free Cy5 and Dil were removed by centrifugation.
The loading content and encapsulation efficiency of Cip and Ea were determined by HPLC.
| 1 |
| 2 |
Characterization of CpE@BMV
The size distribution, colloidal stability, and zeta potential were measured using Malvern Nano ZS Zen3600 (Malvern, UK). For size and Zeta potential measurement, CpE and CpE@BMV were diluted with phosphate buffer (PB, pH 7.4, 10 mM) to a final concentration of approximately 0.1 mg/mL. The sample morphology was observed by transmission electron microscope (TEM, FEI Talos-F200S, Hillsboro, USA) with an accelerating voltage of 120 kV. EDS mapping was used to analyze the spatial distribution of various elements.
To determine the BMV coating, the membrane proteins of CpE@BMV were identified through sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In brief, the obtained CpE@BMV was incubated with RIPA lysate (1:100, v/v) for 30 min on ice and subsequently centrifuged (15,000 g, 4 °C) for 20 min to isolate the membrane protein. Total protein concentration was determined using the BCA protein assay kit. Equivalent amounts of total proteins from BMV and CpE@BMV were denatured by boiling in an SDS loading buffer at 99 °C for 15 min, and CpE was treated similarly as a control. All samples were separated by electrophoresis on a 10% SDS-PAGE gel, further stained with Coomassie blue for 2 h, and decolorized overnight before being photographed using a mobile phone camera. In addition, CpECy5@BMV, CpE@BMVDil, or CpECy5@BMVDil were analyzed by flow cytometry (Beckman CytoFlex, California, USA) to prove the BMV coating.
Molecular dynamics simulation
In order to understand the experimental results from a microscopic perspective, all-atom simulations were carried out to investigate the self-assembly of CpE molecules and their interactions with the outer membrane of Gram-negative bacteria. Similar to our previous work64, we used the ATB tool65 to build an all-atom model of the CpE molecule, employed the CHARMM-GUI tool66 to build a model of the outer membrane of Gram-negative bacteria67, and then adopted the combination rule to construct the hybrid system of these two parts.
To prepare the initial configurations for self-assembly simulations, the Gromacs insert-molecules tool was employed. Fifty CpE molecules were randomly positioned within a periodic cubic box with a side length of 15 nm. The system was then solvated with TIP3P water molecules to mimic a realistic biological environment. To maintain the electrical neutrality of the system, calcium and chloride ions were strategically added by replacing water molecules at random until a concentration of 0.1 M was achieved. Finally, to promote the formation of the most stable self-assembled structure, an annealing simulation was conducted for 100 ns. During this process, the temperature of the system was gradually decreased from 450 K to 298.15 K. Nose-Hoover thermostat and isotropic Parrinello–Rahman barostat were utilized throughout the simulation to maintain the desired temperature and pressure conditions.
To study how CpE molecules interact with the outer membrane of Gram-negative bacteria, they were initially positioned under the outer membrane. Then the system evolves freely for 100 ns under constant temperature and pressure conditions. The conformational changes were examined and analyzed after the simulation. The simulations were run with Gromacs version 2021.4 and analyzed with VMD software68,69.
Evaluation of ROS scavenging
Hydrogen peroxide (H2O2) scavenging assay: the H2O2 scavenging capacity of CpE@BMV was evaluated using a hydrogen peroxide assay kit. Briefly, Amplite IR peroxidase substrate and peroxidase were mixed to prepare the working solution according to the protocol. Then, CpE@BMV (50 μL, at different concentrations of 16, 31, 62, 125, and 250 μg/mL) was added to the working solution (50 μL) at room temperature. After 30 min, the solution was measured using a microplate reader with the fluorescence mode (Ex/Em: 640 nm/680 nm). The inhibition rate was calculated based on an H2O2 standard curve.
Hydroxyl radical (•OH) scavenging test: a hydroxyl free radical assay kit was used to study the •OH scavenging capacity of CpE@BMV. Briefly, CpE@BMV (200 μL, at different concentrations of 16, 31, 62, 125, and 250 μg/mL) was added into a fluorescence solution (200 μL) and incubated for 1 min at 37 °C. Then, hydroxyl radical initiator and Fenton reagent were added and incubated for 20 min at 25 °C. The absorbance of samples at 550 nm was recorded with a microplate reader. The inhibition rate was calculated based on a standard curve.
Superoxide anion (•O2–) scavenging assay: the •O2– scavenging capacity of CpE@BMV was evaluated using a superoxide anion assay kit. Briefly, CpE@BMV (20 μL, at different concentrations of 16, 31, 62, 125, and 250 μg/mL) was mixed with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium sodium salt (WST-1) working solution (200 μL). Subsequently, xanthine oxidase solution (20 μL) was added to each tube and incubated for 10 min at 37 °C. The absorbance of samples at 550 nm was recorded with a microplate reader. The inhibition rate was calculated based on a standard curve.
ABTS•·radical scavenging activity: ABTS• was added to potassium persulfate to produce an ABTS radical cation (ABTS + •). Then, CpE@BMV nanoparticles (100 μL, at different concentrations of 16, 31, 62, 125, and 250 μg/mL) were added to the ABTS + • solution. The absorbance of both ABTS + • solution (AB) and the mixture solution containing ABTS + • and CpE@BMV nanoparticles (AE) was measured at 734 nm. The percentage scavenging of ABTS + • was calculated as follows:
| 3 |
DPPH• scavenging assay: CpE@BMV nanoparticles (100 μL, at different concentrations of 16, 31, 62, 125, and 250 μg/mL) were incubated with DPPH ethanol solution (0.1 mM, 100 μL) for 30 min. The absorbance of DPPH• solution (Ac), CpE@BMV nanoparticles (Ad), and the mixture solution containing DPPH• and CpE@BMV nanoparticles (As) was measured at 517 nm. The percentage scavenging of DPPH• was calculated as follows:
| 4 |
ROS scavenging capability of CpE@BMV in vitro
RAW264.7 cells (1 × 10⁵ cells/well) were plated in 24-well plates and cultured for 24 h (37 °C, 5% CO₂). After PBS washing, the cells were cultured with LPS (200 ng/mL, 24 h). The cells were treated with Cip (4.4 μg/mL), CpE (8.2 μg/mL), BMV (2.8 μg/mL), and CpE@BMV (with Cip concentration of 4.4 μg/mL) for 24 h, and further incubated with DCFH-DA (10 μM) for 30 min. Meanwhile, the cell nuclei were stained with Hoechest33324 and imaged on a confocal laser scanning microscope (CLSM, Nikon A1, Japan), with the excitation of 405/488 nm, collecting fluorescence at 420–460 nm (DAPI) and 500–535 nm (DCFH-DA). In parallel experiments, culture supernatants were analyzed for inflammatory cytokines of IL-6, IL-1β, TNF-α, and IL-10 using ELISA kits according to the protocols.
Cell-protective ability of CpE@BMV against LPS and H2O2 damage
RAW264.7 cells were plated in 96-well plates (1 × 104 cells/well) and cultured overnight. The LPS (2 μg/mL) or H2O2 (1 mM) was added to cells for 2 h. The cells were then treated with Cip (4.4 μg/mL), Ea (1.9 μg/mL), BMV (2.8 μg/mL), and CpE@BMV (with Cip concentration of 4.4 μg/mL) for 24 h. The treated RAW264.7 cells of all groups were harvested, and the cell viability was assessed using CCK-8 kits according to the protocols.
Red blood cell hemolysis analysis
Mouse whole blood (1 mL) was diluted in PBS (1:10), centrifuged (4000 × g, 15 min), and washed with PBS (10 mL × 5) to collect red blood cells (RBCs). The RBCs were resuspended to 4% (v/v) in PBS. Serial dilutions of CpE@BMV (4–400 μg/mL) were incubated with 4% RBC suspensions (1:1 v/v) for 3 h at 37 °C. After centrifugation (3000 × g, 15 min), supernatant absorbance (ODtreated, 545 nm) was measured to calculate hemolysis. The PBS was used as a negative control (OD negative control) and 0.1% Triton-X as a positive control (OD positive control). The percentage of hemolysis was determined using the formula:
| 5 |
Cip and Ea release from CpE@BMV
The release profile of Cip and Ea from CpE@BMV was evaluated by dialysis and measured by a High-performance liquid chromatography system (HPLC, Agilent, US). A solution of CpE@BMV (1 mL, [Cip] = 350 μg/mL, [Ea] = 150 μg/mL) was transferred into a dialysis bag (MWCO 3000 Da), and suspended in different buffers at 37 °C in an incubator: PBS (20 mL, 10 mM) at pH 7.4, PBS (20 mL, 10 mM) at pH 6.5, PBS (20 mL, 10 mM) at pH 7.4 containing H2O2 (100 μM), PBS (20 mL, 10 mM) at pH 6.5 containing H2O2 (100 μM). The elevated concentration of H₂O₂ at the infection site, which can reach up to 1 mM, is a result of the oxidative burst generated by immune cells to combat invading pathogens. This serves as a critical component of the host’s defense mechanism64,70. At predetermined intervals, samples (3 mL) were withdrawn from the buffer solution and replaced with fresh isopycnic phosphate buffer. All collected solutions were lyophilized and resolved in DMSO to measure the content of Cip and Ea using the HPLC. HPLC analysis was conducted using an Agilent 1260 Infinity II HPLC system (Agilent, US) with an Alltima C18 column (4.6 × 100 mm, 2.7 μm). The UV detection was set at 280 nm, with a mobile phase of 2% acetic acid in water/ACN (84:16, v/v). The flow rate was 0.7 mL/min, and the injection volume was 5 μL.
MIC and MBC determination
We tested minimum inhibitory concentration (MIC) as well as bactericidal concentration (MBC) values of the tested formulations according to our published protocal71,72. Briefly, Cip, Ea, CpE, and CpE@BMV (100 μL) with an equivalent amount of Cip ranging from 0 to 7.0 μg/mL and an equivalent amount of Ea ranging from 0 to 3.0 μg/mL were applied to suspensions of S. aureus WHGFP (in TSB), E. coli Xen14, and S. typhimurium 15,649 (in LB) (100 μL, 2 × 105 bacteria/mL). The MIC values represented the lowest antibiotic concentrations preventing visible growth. The MBC values were tested by plating aliquots (10 μL) from wells showing no visible growth on agar plates after incubation for 24 h at 37 °C, and the lowest concentration at which colony formation remained absent was considered as the MBC value.
Macrophage phenotypic regulation of CpE@BMV in vitro
RAW264.7 cells were plated in 24-well plates (5 × 104 cells/well) and cultured overnight. Treatments included PBS, Cip (4.4 μg/mL), Ea (1.9 μg/mL), and CpE@BMV (with the concentration of Cip 4.4 μg/mL and Ea 1.9 μg/mL) for 12 h. The treated RAW264.7 cells of all groups were harvested and blocked with anti-mouse CD16/32 antibody (10 μL, 0.5 mg/mL) for Fc receptors. Subsequently, the cell surface was stained with anti-mouse F4/80-APC (10 μL, 0.5 mg/mL) and anti-mouse CD86-BV421 (10 μL, 0.5 mg/mL), while the intracellular staining was performed using anti-mouse CD206-FITC (10 μL, 0.5 mg/mL) for flow cytometry (Beckman CytoFlex, California, USA). In addition, cytokine levels of IL-6, IL-1β, TNF-α, and IL-10 in the cell supernatant were measured using ELISA kits according to the protocols.
Cellular uptake of CpE@BMV by S. aureus WHGFP infected RAW264.7 cells in vitro
The cell uptake of CpE, CpE@BMV by S. aureus WHGFP infected RAW264.7 cells was studied. Briefly, RAW264.7 cells were plated in six-well plates and grown for 12 h at 37 °C (4 × 105 cells/mL). The cell was mixed with S. aureus WHGFP (8 × 106 bacteria/well) for 4 h. Gentamicin (100 μg/mL) was then added to inhibit extracellular growth during 24 h of coculture. Following coculture, wells were gently washed once with PBS to remove extracellular S. aureus WHGFP. The freshly prepared Nile red-loaded CpE or CpE@BMV (with the concentration of Cip 0.44 μg/mL) were cocultured with cells for another 2 h. Cells were washed with PBS (1 mL/well), fixed with 4% paraformaldehyde (15 min, room temperature), and stained with 4′,6-diamidino-2-phenylindole (10 μM, 30 min, in the dark). RAW264.7 cells containing internalized red-fluorescent CpE or CpE@BMV were visualized using confocal laser scanning microscopy (A1, Nikon, Japan), with the excitation of 405/525 nm, collecting fluorescence at 420–460 nm (DAPI) and 560–625 nm (Nile red). Furthermore, the red fluorescence intensity in infected macrophages was detected using flow cytometry (Beckman CytoFlex, California, USA).
Live/dead staining of bacteria in vitro
E. coli Xen14 was seeded onto a confocal dish and incubated with Cip, CpE, and CpE@BMV (with the concentration of Cip 0.44 μg/mL) for 6 h. Subsequently, the bacteria were stained with SYTO 9 (green, for live bacteria) and PI (red, for dead bacteria) for 30 min before being observed using confocal laser scanning microscopy (A1, Nikon, Japan), with the excitation of 488/525 nm, collecting fluorescence at 420–460 nm (SYTO 9) and 560–625 nm (PI). Meanwhile, the bacteria were treated with the treatment of PBS as the control group.
Antibacterial activity against extracellular and intracellular bacteria in RAW264.7 cells in vitro
For extracellular bacteria eradication in vitro: RAW264.7 cells were seeded at a 1.5 × 105 cells/well density in 24-well plates and grown for 24 h at 37 °C. The cells were infected by S. aureus WHGFP or E. coli Xen14 (3 × 106 bacteria/well) for 2 h. After washing the cells with PBS (1 mL × 3), the infected macrophage cells were treated with Cip, Cip-pba, CpE, and CpE@BMV (with the concentration of Cip 0.44 μg/mL) for 12 h, 24 h, and 48 h, respectively. The supernatant was collected, and the extracellular S. aureus WHGFP or E. coli was measured by serial dilution and plated on TSB or LB agar for c.f.u. enumeration.
For intracellular bacteria eradication in vitro: RAW264.7 cells were seeded at a 1.5 × 105 cells/well density in 24-well plates and grown for 24 h at 37 °C. RAW264.7 cells were infected with S. aureus WHGFP or E. coli Xen14 (3 × 106 bacteria/well) for 2 h. The cells were washed with PBS (1 mL × 3) to remove the extracellular bacteria. The infected macrophage cells were treated with Cip, Cip-pba, CpE, and CpE@BMV (with the concentration of Cip 0.44 μg/mL) for 12 h, 24 h, and 48 h, respectively. Cells were collected through centrifugation (1000 × g, 3 min) and lysed in PBS supplemented with 0.1% bovine serum albumin and 0.1% Triton-X. The harvested lysate was diluted serially and plated on TSB or LB agar for c.f.u. enumeration. The detection limit for c.f.u. was 200, using 100 µL of the undiluted suspension.
Phagocytosis assay induced by CpE@BMV in vitro
RAW264.7 cells were seeded in 24-well plates (1.5 × 105 cells/well) and cultured for 24 h. The cells were pretreated with Cip, Ea, and CpE@BMV ([Cip] = 7.0 μg/mL, [Ea] = 3.0 μg/mL, [CpE@BMV] = 17.5 μg/mL in which the concentration of Cip is 7.0 μg/mL) for 24 h. Subsequently, the cells were cocultured with SYTO 9-labeled S. aureus WHGFP (1.5 × 106 bacteria/well) for 60 min, followed by washing with PBS and staining with Dil (10 μM). Red fluorescence of RAW264.7 and green fluorescence of S. aureus WHGFP were detected using flow cytometry (Beckman CytoFlex, California, USA).
Cytotoxicity of CpE@BMV in vitro
L929 cells and RAW264.7 cells were cultured in a 96-well plate (1 × 104 cells/well) overnight. The cells were incubated with CpE@BMV at different concentrations (0.1–400 μg/mL) for 24 h. The cell cytotoxicity was assessed using a CCK-8 kit according to the protocol.
Antibacterial efficacy of CpE@BMV in a pneumonia model
BALB/c mice were intratracheally administered with E. coli Xen14 (1 × 109 bacteria/mL, 50 μL per mouse) to establish a pneumonia model. The mice were randomly allocated into six groups (n = 7): Control group (healthy mice), the E. coli Xen14 infected mice with different formulations using equivalent doses of Cip, Ea, and BMV were 1.76 mg/kg, 0.76 mg/kg, and 1.12 mg/kg, respectively. Different formulations were treated via intratracheal instillation. Body weights were monitored every 48 h during treatment. At day 3 and day 7 post-treatment, the blood was collected from the eyes (n = 5) to measure the major blood parameters using Mindray, Shenzhen, China, and the serum was further collected and applied for cytokine quantification (TNF-α, IL-6, IL-β, IL-10) with ELISA Kits. The mice were sacrificed on day 7. The heart, liver, spleen, lung, and kidney were harvested (n = 3) for H&E staining according to the protocol. The frozen slices of lung tissue were prepared, and the immunofluorescence staining was performed to analyze macrophage phenotypes labeled with CD86 and CD206. The lung tissue with PBS-treated and CpE@BMV-treated groups was also collected for RNA sequencing (n = 3) and 2bRAD sequencing for microbiome (n = 3). Additionally, the lung tissue (n = 4) was homogenized in PBS and centrifuged to collect supernatants, 10 μL supernatants were diluted serially and plated on LB agar for c.f.u. enumeration, and the other 10 μL supernatants were applied for cytokine quantification (TNF-α, IL-6, IL-β, IL-10) with ELISA Kits.
Antibacterial efficacy of CpE@BMV in a peritonitis model
BALB/c mice were randomly allocated into six groups (n = 5) and intraperitoneally injected with E. coli Xen14 solution (100 μL, 1 × 108 bacteria/mL) on day 0, 7, and 14. After 4 h of infection, the mice were intraperitoneally administered with different formulations using equivalent doses of Cip, Ea, and BMV were 1.76 mg/kg, 0.76 mg/kg, and 1.12 mg/kg, respectively, on days 0, 7, and 14. Bioluminescence of bacteria in the mouse peritoneal cavity was recorded at 0, 4, 8, and 24 h after the first treatment, using a bio-optical imaging system (IVIS®, 45 s exposure time, medium binning, 1F/Stop, Open Emission Filter).
On day 15, the blood of mice was collected from eyes (n = 5) to measure the major blood parameters using (Mindray, Shenzhen, China), and the serum was further collected and applied for cytokine quantification (TNF-α, IL-6, IL-β, IL-10) with ELISA Kits. The mice were sacrificed on day 15. The peritoneal fluid of mice was collected and split for c.f.u. counting (n = 4), and cytokine quantification (TNF-α, IL-6, IL-β, IL-10) with ELISA Kits, the spleen tissues were harvested and homogenized in PBS to obtain the supernatant for cytokine quantification with ELISA Kits. The major organs of mice (n = 4) were homogenized in PBS and centrifuged to collect supernatants, 10 μL supernatants were diluted serially and plated on LB agar for c.f.u. enumeration.
Infiltrating immune cells analysis in vivo
Immune cells in lung and spleen tissues at different time points (pneumonia model: days 3 and 7; peritonitis model: day 15) were analyzed by multiparameter flow cytometry following various treatments. The excised lung/spleen tissue was mechanically disrupted using the tip of a syringe, then 4 mL of digestion solution (Sigma, Germany) was added, and the mixture was put on a shaker (150 rpm) for 1 h to ensure complete digestion. Finally, the suspension was filtered through a 200-mesh sieve and centrifuged at 450 × g for 5 min to obtain a single-cell suspension. The single-cell suspension was subsequently stained with antibodies and analyzed using flow cytometry. Firstly, a single-cell suspension was incubated with anti-mouse CD16/32 (10 μL, 0.5 mg/mL) at 4 °C for 15 min to block Fc receptors. For macrophages, cells were stained with anti-mouse CD45-BV605 (10 μL, 0.2 mg/mL), anti-mouse CD11b-FITC (10 μL, 0.2 mg/mL), anti-mouse F4/80-BV421 (10 μL, 0.2 mg/mL), anti-mouse CD86-APC (10 μL, 0.2 mg/mL), anti-mouse CD206-PE (10 μL, 0.2 mg/mL) for 30 min; For T cells, cells were stained with anti-mouse CD45-BV605 (10 μL, 0.2 mg/mL), anti-mouse CD3-FITC (10 μL, 0.5 mg/mL), anti-mouse CD4-BV421 (10 μL, 0.2 mg/mL), anti-mouse CD8-APC (10 μL, 0.2 mg/mL), anti-mouse Foxp3-PE (10 μL, 0.2 mg/mL) for 30 min; For B cells, cells were stained with anti-mouse CD45-BV605 (10 μL, 0.2 mg/mL), anti-mouse CD19-BV421 (10 μL, 0.2 mg/mL), anti-mouse CD138-APC (10 μL, 0.2 mg/mL) for 30 min. Flow cytometry was performed by Beckman Coulter (CytoFlex, California, USA), and the result was analyzed using FlowJo software. Besides, the infection-draining lymph nodes (IDLN) were separated to collect immune cells, and the cells were stained with anti-mouse CD16/32 antibody (10 μL, 0.5 mg/mL), anti-mouse CD45-BV605 (10 μL, 0.2 mg/mL), anti-mouse CD11c-BV421 (10 μL, 0.2 mg/mL), anti-mouse CD86-APC (10 μL, 0.2 mg/mL), and anti-mouse CD80-FITC (10 μL, 0.2 mg/mL) for 30 min, and analyzed by Beckman Coulter.
RNA sequencing
Total RNA in lung tissue was extracted using TRIzol according to the user guide. Next, the purity and quantification of the collected RNA were measured by a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA), and RNA integrity was checked by an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). The transcriptome library was built using a VAHTS Universal V5 RNA-seq Library Prep Kit. The sequencing was conducted using an Illumina Novaseq 6000 sequencing platform. DESeq2 software was employed to analyze differentially expressed genes (DEG). The DEG was further investigated by GO and KEGG Pathway enrichment based on the hypergeometric distribution algorithm to identify the enriched GO and KEGG terms. RNA sequencing was conducted at the OE Biotech Co., Ltd. (Qingdao, China).
2bRAD-M sequencing for microbiome
The 2bRAD-M library construction was based on the method reported by Mikhail V Matz et al.73. Genomic DNA of lung tissue was extracted using a MagPure Soil DNA KF Kit (MGBio, China), and the collected DNA (1 pg–200 ng) was further treated with the enzyme BcgI (4 U, NEB) for three hours. After ligating adapters to the DNA fragments by mixing 10 μL digested DNA with a master mix (10 μL, with 0.2 μL adapters, and 800 U T4 DNA ligase) at 4 °C overnight, the mixture was amplified using an 8% polyacrylamide gel, in which the bands around 100 bp were excised and DNA was collected. Sample-specific barcodes were incorporated via PCR using platform-compatible indexing primers, which were further purified by a QIAquick PCR purification kit. The 2BRAD-M sequencing was performed by the Illumina Nova PE150 platform.
The databases of GTDB and Ensembl were applied to identify the species-level of 2bRAD-M markers. The set of 2bRAD tags derived from each genome was assigned under the corresponding GCF number, along with the taxonomic information associated with the whole genome. At the end, all single-copy 2bRAD tags (occurring once per genome) from each GCF were compared across all genomes to develop species-specific 2bRAD markers. The sequenced 2bRAD tags were mapped against the database to calculate relative abundance. The relative abundance of a specific species was determined by the proportion of microbial individuals in the species relative to the total number of detectable species in a sample.
Statistical analysis
All result data were presented as mean ± standard deviation (s.d.). Unless otherwise stated, all experiments used biological replication. Statistical analyses were analyzed using an unpaired Student’s t-test (two-tailed) for two groups, and one-way ANOVA with Tukey’s multiple comparisons was used for three or more groups. Significance levels were defined as follows: n.s., no significant difference, p > 0.05; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001; p-values were calculated by GraphPad Prism v8.3.0 (GraphPad Software) and marked on the figures.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 52293383, 52422307, 22475156, 52303181, 52203184, 22275043); the National Key Research and Development Program (2023YFB3809902); Zhejiang Provincial Natural Science Foundation Outstanding Youth Project (LR24H180001); Wenzhou Municipal Science and Technology Major Project (ZY2023031) and Scientific Research Project (Y20240113); and the Infectious Diseases X Discipline Cluster, the First Affiliated Hospital of Wenzhou Medical University.
Author contributions
Y. Li and Y. Liu conceived the project and strategies. L.S. and Y. Liu supervised the work and revised the manuscript. W.H., R.H., D.L. and Y. Li designed and conducted the experiments, analyzed the data and drafted the initial manuscript. Y.P. and Y.W. cultured the bacteria and conducted the biofilm-related experiments. M.P. carried out the TEM observations and H.L. performed the all-atom simulations. All authors reviewed and approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Jeremy Brown, Man-Wah Tan, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated in this study are provided in the paper, Supplementary Information, and Source Data file. The full image dataset is available from the corresponding author upon request. The data for 16s RNA sequencing and 2bRAD-M analysis used in this study are available in the Figshare database under accession DOI code 10.6084/m9.figshare.28792304.v1. Source data are provided in this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuanfeng Li, Wei He, Yinzi Piao.
Contributor Information
Rongdang Hu, Email: hurongdang@hotmail.com.
Dongdong Li, Email: ldd@ucas.ac.cn.
Yong Liu, Email: y.liu@nankai.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-60570-2.
References
- 1.Young, D., Hussell, T. & Dougan, G. Chronic bacterial infections: living with unwanted guests. Nat. Immunol.3, 1026–1032 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Hou, X. et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol.15, 41–46 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao, X. et al. Guiding antibiotics towards their target using bacteriophage proteins. Nat. Commun.15, 5287 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu, J. et al. Multimodal nanoimmunotherapy engages neutrophils to eliminate Staphylococcus aureus infections. Nat. Nanotechnol. 19, 1032–1043 (2024). [DOI] [PMC free article] [PubMed]
- 5.Wolcott, R. D. & Ehrlich, G. D. Biofilms and chronic infections. JAMA299, 2682–2684 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Flemming, H.-C. et al. Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol.14, 563–575 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Sauer, K. et al. The biofilm life cycle: expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol.20, 608 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov.2, 114–122 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Fisher, M. C. et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol.20, 557–571 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makabenta, J. M. V. et al. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol.19, 23–36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, Y. et al. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev.48, 428–446 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Amin Yavari, S., Castenmiller, S. M., van Strijp, J. A. G. & Croes, M. Combating implant infections: shifting focus from bacteria to host. Adv. Mater.32, 2002962 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Perciani, C. T., Liu, L. Y., Wood, L. & MacParland, S. A. Enhancing immunity with nanomedicine: employing nanoparticles to harness the immune system. ACS Nano15, 7–20 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Zhang, S. et al. Immunomodulatory biomaterials against bacterial infections: progress, challenges, and future perspectives. Innovation4, 100503 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arciola, C. R., Campoccia, D. & Montanaro, L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol.16, 397–409 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Heim, C. E. et al. Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat. Microbiol.5, 1271–1284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belkaid, Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol.7, 875–888 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Tyers, M. & Wright, G. D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol.17, 141–155 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Nürnberger, T. & Kemmerling, B. Pathogen-associated molecular patterns (PAMP) and PAMP-triggered immunity. In Annual Plant Reviews Volume 34: Molecular Aspects of Plant Disease Resistance 16–47 (2008).
- 20.Kaparakis-Liaskos, M. & Ferrero, R. L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol.15, 375–387 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Gan, Y. et al. Bacterial membrane vesicles: physiological roles, infection immunology, and applications. Adv. Sci.10, 2301357 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng, K. et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat. Commun.12, 2041 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding, Y. et al. Lipid prodrug nanoassemblies via dynamic covalent boronates. ACS Nano17, 6601–6614 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Zhan, Y. et al. Antimicrobial hybrid amphiphile via dynamic covalent bonds enables bacterial biofilm dispersal and bacteria eradication. Adv. Funct. Mater.33, 2214299 (2023). [Google Scholar]
- 25.Li, Y. et al. Dynamic covalent nano-networks comprising antibiotics and polyphenols orchestrate bacterial drug resistance reversal and inflammation alleviation. Bioact. Mater.27, 288–302 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Y. et al. Coating of a novel antimicrobial nanoparticle with a macrophage membrane for the selective entry into infected macrophages and killing of intracellular staphylococci. Adv. Funct. Mater.30, 2004942 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun, J., Rutherford, S. T., Silhavy, T. J. & Huang, K. C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol.20, 236–248 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen, N. et al. Bacterial extracellular vesicle: a non-negligible component in biofilm life cycle and challenges in biofilm treatments. Biofilm8, 100216 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao, Y.-Z. et al. GOx-encapsulated iron-phenolic networks power catalytic cascade to eradicate bacterial biofilms. J. Control. Release352, 1–14 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Shi, L. et al. DUSP1 protects against ischemic acute kidney injury through stabilizing mtDNA via interaction with JNK. Cell Death Dis.14, 724 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green, D. R. & Ferguson, T. A. The role of Fas ligand in immune privilege. Nat. Rev. Mol. Cell Biol.2, 917–924 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther.7, 3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol.23, 289–303 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, R. et al. Serum amyloid protein A in inflammatory bowel disease: from bench to bedside. Cell Death Discov.9, 154 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar, S., Boehm, J. & Lee, J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov.2, 717–726 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Ivashkiv, L. B. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol.18, 545–558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy, R. C., Rehan, V. K., Roman, J. & Sime, P. J. PPARs: regulators and translational targets in the lung. PPAR Res.2012, 1–2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montaigne, D., Butruille, L. & Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol.18, 809–823 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Pérez-Gutiérrez, L. & Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol.24, 816–834 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Zelenitsky, S. A., Ariano, R. E., Iacovides, H., Sun, S. & Harding, G. K. M. AUC0–t/MIC is a continuous index of fluoroquinolone exposure and predictive of antibacterial response for Streptococcus pneumoniae in an in vitro infection model. J. Antimicrob. Chemother.51, 905–911 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Pickerill, K. E., Paladino, J. A. & Schentag, J. J. Comparison of the fluoroquinolones based on pharmacokinetic and pharmacodynamic parameters. Pharmacother. J. Hum. Pharmacol. Drug Ther.20, 417–428 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Craig, W. A. Does the dose matter?. Clin. Infect. Dis.33, S233–S237 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Hu, X. et al. Polyprodrug amphiphiles: hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc.135, 17617–17629 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Couvreur, P., Lepetre-Mouelhi, S., Garbayo, E. & Blanco-Prieto, M. J. Self-assembled lipid–prodrug nanoparticles. Nat. Rev. Bioeng.1, 749–768 (2023). [Google Scholar]
- 45.Hu, X. et al. Reaction-induced self-assembly of polymyxin mitigates cytotoxicity and reverses drug resistance. Adv. Mater.36, 2406156 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Li, Y. et al. Microenvironment-adaptive metallo-polymeric nanodecoys via subcomponent coordination for bacterial biofilm eradication and immunomodulation. Adv. Funct. Mater.34, 2405487 (2024). [Google Scholar]
- 47.Hu, X. et al. Two-tailed dynamic covalent amphiphile combats bacterial biofilms. Adv. Mater.35, 2301623 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Wu, H. et al. Dynamic covalent prodrug nanonetworks via reaction-induced self-assembly for periodontitis treatment. ACS Nano18, 34884–34901 (2024). [DOI] [PubMed] [Google Scholar]
- 49.Baym, M., Stone, L. K. & Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science351, aad3292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brooks, B. D. & Brooks, A. E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev.78, 14–27 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Chen, Y., Gao, Y., Huang, Y., Jin, Q. & Ji, J. Inhibiting quorum sensing by active targeted pH-sensitive nanoparticles for enhanced antibiotic therapy of biofilm-associated bacterial infections. ACS Nano17, 10019–10032 (2023). [DOI] [PubMed] [Google Scholar]
- 52.Toyofuku, M., Schild, S., Kaparakis-Liaskos, M. & Eberl, L. Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol.21, 415–430 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol.17, 13–24 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Liu, G. et al. Bacteria-derived nanovesicles enhance tumour vaccination by trained immunity. Nat. Nanotechnol.19, 387–398 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Yang, C. et al. Inorganic nanosheets facilitate humoral immunity against medical implant infections by modulating immune co-stimulatory pathways. Nat. Commun.13, 4866 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weyant, K. B. et al. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens. Nat. Commun.14, 464 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi, R. et al. High-yield, magnetic harvesting of extracellular outer-membrane vesicles from Escherichia coli. Small18, 2204350 (2022). [DOI] [PubMed] [Google Scholar]
- 58.Fritz, J. H., Le Bourhis, L., Magalhaes, J. G. & Philpott, D. J. Innate immune recognition at the epithelial barrier drives adaptive immunity: APCs take the back seat. Trends Immunol.29, 41–49 (2008). [DOI] [PubMed] [Google Scholar]
- 59.Parkin, J. & Cohen, B. An overview of the immune system. Lancet357, 1777–1789 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Batista, F. D. & Harwood, N. E. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol.9, 15–27 (2009). [DOI] [PubMed] [Google Scholar]
- 61.Strugnell, R. A. & Wijburg, O. L. C. The role of secretory antibodies in infection immunity. Nat. Rev. Microbiol.8, 656–667 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Torres, A. et al. Pneumonia. Nat. Rev. Dis. Prim.7, 25 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Wu, F. et al. Single-atom Cu anchored on carbon nitride as a bifunctional glucose oxidase and peroxidase nanozyme for antibacterial therapy. ACS Nano19, 10816–10828 (2025). [DOI] [PMC free article] [PubMed]
- 64.Piao, Y.-Z. et al. One-component lipidic bicontinuous nanospheres as a smart drug loading platform to eradicate candida biofilms in oral and vaginal infection. Nano Today54, 102123 (2024). [Google Scholar]
- 65.Malde, A. K. et al. An automated force field topology builder (ATB) and repository: version 1.0. J. Chem. Theory Comput.7, 4026–4037 (2011). [DOI] [PubMed] [Google Scholar]
- 66.Wu, E. L. et al. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem.35, 1997–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiao, Y. et al. Microwave assisted antibacterial action of Garcinia nanoparticles on Gram-negative bacteria. Nat. Commun.13, 2461 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraham, M. J. et al. Gromacs: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX1, 19–25 (2015). [Google Scholar]
- 69.Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph.14, 33–38 (1996). [DOI] [PubMed] [Google Scholar]
- 70.Dahlgren, C. & Karlsson, A. Respiratory burst in human neutrophils. J. Immunol. Methods232, 3–14 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Liu, Y. et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano10, 4779–4789 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Liu, Y. et al. Eradication of multidrug-resistant staphylococcal infections by light-activatable micellar nanocarriers in a murine model. Adv. Funct. Mater.27, 1701974 (2017). [Google Scholar]
- 73.Wang, S., Meyer, E., McKay, J. K. & Matz, M. V. 2b-RAD: a simple and flexible method for genome-wide genotyping. Nat. Methods9, 808–810 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated in this study are provided in the paper, Supplementary Information, and Source Data file. The full image dataset is available from the corresponding author upon request. The data for 16s RNA sequencing and 2bRAD-M analysis used in this study are available in the Figshare database under accession DOI code 10.6084/m9.figshare.28792304.v1. Source data are provided in this paper. Source data are provided with this paper.