Abstract
Nectin cell adhesion molecule 4 (Nectin-4) is specifically overexpressed in most cancers of epithelial origin but downregulated in normal tissue, representing an ideal target for positron emission tomography imaging. The development of positron emission tomography imaging probes targeting Nectin-4 has gained significant attention in recent years, especially after the approval in December 2019 by the US Food and Drug Administration of enfortumab vedotin—an antibody drug conjugate targeting Nectin-4—in patients with locally advanced or metastatic bladder cancer. This article aims to comprehensively review original research articles discussing preclinical development or early translational clinical applications of radiolabeled probes targeting Nectin-4. The main radioactive compounds investigated belong to two classes, antibody-based radiopharmaceuticals and peptide-drug conjugates, in particular novel bicyclic peptides. While monoclonal antibody-based probes have demonstrated theranostic potential in preclinical studies, their clinical application has been hindered by their slow pharmacokinetic properties. However, peptide-based positron emission tomography/computed tomography tracers offer several advantages, such as ease of handling in synthesis, a more favorable biodistribution, and lower immunogenicity and have been tested in preliminary clinical experiences.
Key Points
| Nectin cell adhesion molecule 4 (Nectin-4) is a cell adhesion molecule overexpressed in various cancers, particularly urothelial carcinoma. Its high tumor-specific expression makes it a promising target for both therapeutic agents, such as antibody-drug conjugates, and molecular imaging tracers used in positron emission tomography/single photon emission computed tomography imaging for cancer diagnosis and treatment monitoring. |
| Small peptide-based radiopharmaceuticals can serve as valuable tools for precision imaging, enhancing the diagnosis and treatment planning for patients with Nectin-4-overexpressing tumors. |
| Radiotracers targeting Nectin-4 could have a potential clinical role in several tumors, including bladder, triple-negative breast, pancreatic, and serous ovarian cancer. For pharmacokinetic issues, peptide-based probes appear more suitable for diagnostic purposes, meanwhile monoclonal antibody-based probes may have a role in theranostic models. |
Introduction
Nectin cell adhesion molecule 4 (Nectin-4) is a calcium-independent immunoglobulin-like protein involved in cell adhesion [1]. Although its expression progressively decreases from embryonic life to the adult age, Nectin-4 is overexpressed in several solid neoplasms (i.e., bladder cancer, triple-negative breast cancer, head and neck carcinoma, and melanoma) [2–5]. The selective overexpression of Nectin-4 in cancer cells makes it an ideal candidate for precision oncology (Fig. 1). Moreover, Nectin-4 is a type I transmembrane polypeptide with an extracellular domain that is targeted by the antibody-drug conjugate enfortumab-vedotin (EV). Since 2020, EV has been approved by the US Food and Drug Administration and European Medicines Agency for the treatment of locally advanced, or metastatic bladder cancer [1, 3]. Further clinical applications are expected in the next few years.
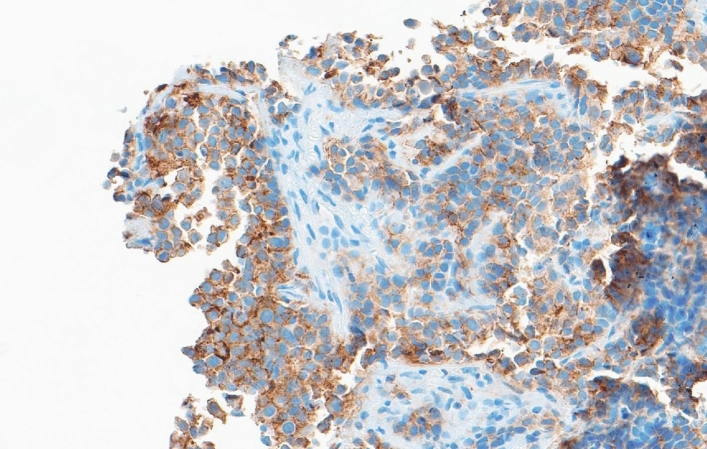
Fig. 1.
Diffuse expression of Nectin cell adhesion molecule 4 in a urothelial carcinoma of the bladder
With the availability of EV, several open questions have arisen. Indeed, EV is an effective treatment leading to improved overall survival versus chemotherapy [6]. However, the use of EV is impaired by high costs and the emergence of drug-resistant lesions. Thus, the availability of a selective imaging probe able to depict the whole inter-lesion pattern of Nectin-4 expression would be very significant to better select patients as candidates for EV, or—in the case of emergence of drug resistance—to discontinue or initiate focal therapies.
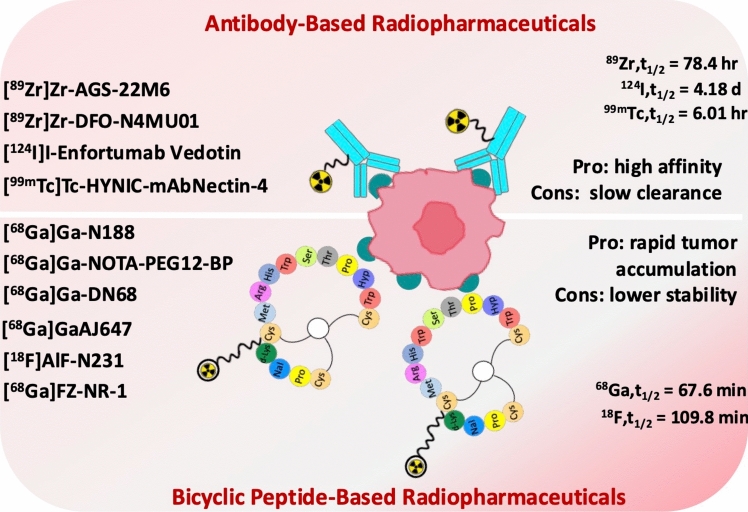
The presence of an extra-cellular domain allows the design of targeted radio-diagnostic probes. Recently, various radiopharmaceuticals have been studied to target Nectin-4, including antibody-based radiopharmaceuticals and peptide-drug conjugates, in particular novel bicyclic peptides (Fig. 2) with optimized pharmacokinetics (Table 1). This article outlines the main characteristics of the positron emission tomography (PET) and single photon emission computed tomography (SPECT) radiopharmaceuticals developed to target Nectin-4, highlighting their imaging potential and results obtained in preclinical or clinical studies.
Fig. 2.
Radiopharmaceuticals targeting Nectin-4 cell adhesion molecule. d day, hr hour, min minutes
Table 1.
Studies on PET imaging of Nectin-4 expression
| Study | Year | Location | Pre-clinical and clinical | Tumor model | Tracer | Device | Comments |
|---|---|---|---|---|---|---|---|
| Ren et al. [8] | 2024 | China | Pre-clinical | Bladder cancer with Nectin-4-positive cells (SW780 cells) | 124/125I Enfortumab Vedotin | Small-animal PET-CT | The tracer was successfully prepared with high specificity and binding affinity of Nectin-4. It simulates the internal circulation of ADC drugs |
| Zhang et al. [17] | 2024 | China | Clinical | 16 types of cancer | [68Ga]Ga-N188 | PET-CT | PET with [68Ga]Ga-N188 could be an effective tool for selecting patients who may benefit from enfortumab vedotin treatment in a variety of tumors |
| Ge et al. [13] | 2024 | China | Pre-clinical | MC38-Nectin-4 and MDA-MB-468 tumors | [68Ga]Ga-DN68 | PET-CT | In vitro experiments showed that [68Ga]Ga-DN68 could effectively target Nectin-4 in tumor cells. Biodistribution and PET imaging studies showed that [68Ga]Ga-DN68 was specifically accumulated in MC38-Nectin-4 and MDA-MB-468 tumors, which was significantly higher than that of MC38 |
| Duan [9] | 2023 | China | Pre-clinical and clinical | UC cell lines and xenograft mouse models, healthy volunteers and a cohort of patients with advanced UC | [68Ga]Ga-N188 | PET-CT | Preclinical investigation and first-in-human study in 14 patients demonstrated excellent specificity and sensitivity of [68Ga]Ga-N188 in detecting metastases |
| Campbell [6] | 2016 | USA/Japan | Pre-clinical | MDA-MB-231-Nectin-4 tumors | [89Zr]Zr-AGS-22M6 and [18F]FAGS-22M6 | Micro-PET/CT | A positive correlation was demonstrated between tumor Nectin-4 expression and [89Zr]Zr-AGS-22M6 uptake. [18F]FAGS-22M6 showed limited uptake in cynomolgus monkey tissues |
| Wan [12] | 2024 | China | Pre-clinical | SW780/5637 cells | [68Ga]Ga-NOTA-PEG12-BP | Micro-PET/CT | The tracer demonstrated optimal pharmacokinetics. Compared with [18F]FDG, PET imaging, in vivo blocking assays of [68Ga]Ga-NOTA-PEG12-BP and histological staining confirmed that specific tumor uptake was mediated by Nectin-4 receptors |
| Duan [15] | 2024 | China | Pre-clinical | Nectin-4+SW780 tumor group | [18F]AlF−N230; [18F]F-AlF −N231 | Micro-PET/CT | [18F]AlF−N231 has promising capability for non-invasive Nectin-4 detection in vivo |
| Mishra [14] | 2024 | USA | Pre-clinical | UC xenografts | [68Ga]GaAJ647 | Micro-PET/CT | A strong correlation was demonstrated between PET-derived measures of target engagement and therapeutic outcomes |
| Babeker [10] | 2025 | Canada | Pre-clinical | TNBC xenograft and syngeneic models | N4MU01 was radiolabeled with 89Zr and 225Ac for imaging and radiotherapy, respectively | [89Zr]Zr-DFO-N4MU01 showed high tumor uptake. [225Ac]Ac-Macropa-N4MU01 was effectively internalized in MDA-MB-468 and was cytotoxic to the cells with a 50% inhibition concentration of 1.2 kBq/mL | |
| Sun [16] | 2025 | China | Pre-clinical and clinical | TNBC. MDA-MB-468 (Nectin-4–positive) and MDA-MB-231 (Nectin-4–negative) cell lines | [68Ga]Ga -FZ-NR-1, [68Ga]Ga -DOTA-polyethylene glycol 5-Nectin-4 ([68Ga]Ga -FZ-NR-2), and [68Ga]Ga-DOTA-polyethylene glycol 10- Nectin-4 ([68Ga]Ga-FZ-NR-3) |
Micro-PET/CT and PET/CT |
[68Ga]Ga -FZ-NR-1 PET/CT effectively identified tumors in 9 patients with TNBC, which was confirmed by [18F]FDG PET/CT. Biopsy samples of the tumor lesions revealed that the positive lesions identified by [68Ga]Ga-FZ-NR-1 PET/CT corresponded to areas of high Nectin-4 expression |
| Shao [7] | 2022 | China | Pre-clinical | MDA-MB-468 TNBC cells | [99mTc]Tc-HYNIC-mAbNectin-4 for SPECT imaging mAbNectin-4-ICG for photothermal therapy | MicroSPECT/CT | [99mTc]Tc-HYNIC-mAbNectin-4 has a strong tumor retention and good imaging performance for TNBC diagnosis and classification |
[18F]FDG [18F]fluorodeoxyglucose, ADC antibody-drug conjugate, CT computed tomography, Nectin-4 Nectin cell adhesion molecule 4, PET positron emission tomography, SPECT single-photon emission computed tomography, TNBC triple-negative breast cancer, UC urothelial carcinoma
Preclinical Studies
Antibody-Based Radiopharmaceuticals
[89Zr]Zr-AGS-22M6
Campbell et al. [7] developed an immune-PET probe targeting Nectin-4 for preclinical tumor imaging in mice. The probe, [89Zr]Zr-AGS-22M6, was derived from the full-length monoclonal antibody AGS-22M6, conjugated to desferrioxamine (DFO), and radiolabeled with zirconium-89 at 94% radiochemical purity. Immunoreactivity exceeded 90%, confirming high target specificity. In MDA-MB-231-Nectin-4 tumors, uptake peaked at 45.3 ± 2.4 %ID/g on day 3, significantly higher than in control tumors (18.2 ± 2.8 %ID/g). Tumor uptake was influenced by architecture, vascularization, and probe permeability. The tracer effectively identified tumors, providing a quantitative measure of Nectin-4 expression, demonstrating its potential for tumor characterization and therapy monitoring. Although the liver exhibits a strong signal, as it is the primary site for the elimination of full-length antibodies from the body, the drug maintains a signal-to-background ratio in the organ that facilitates the detection of Nectin-4-positive lesions. Furthermore, studies evaluating the tracer’s ability to identify metastatic bone lesions yielded promising results with high intensity uptake in Nectin-4-positive lesions (66.56 ± 23.3 vs 11.94 ± 3.52 %ID/g in MDA-MB-231 and the control, respectively).
[99mTc]Tc-HYNIC-mAbNectin-4
In 2022, Shao et al. [8] developed diagnostic and therapeutic agents targeting Nectin-4, including [99mTc]Tc-HYNIC-mAbNectin-4 for SPECT imaging and mAbNectin-4-ICG for photothermal therapy. [99mTc]Tc-HYNIC-mAbNectin-4 was synthesized with a 73.03% radiolabeling yield and 95.03% radiochemical purity, demonstrating strong tumor-targeting properties in triple-negative breast cancer (TNBC). MicroSPECT/computed tomography (CT) imaging in tumor-bearing mice showed a high tumor-to-background contrast, with tumor uptake increasing over time (peaking at 24–36 h post-injection). Early radioactivity was observed in the heart and liver, but it decreased over time, whereas tumor uptake remained high. Conversely, control and blocked groups (after co-administration of cold EV) had significantly lower tumor uptake while liver retention persisted. Biodistribution studies further confirmed specific binding to Nectin-4-positive tumors. MDA-MB-468 xenografts exhibited significantly higher uptake (15.32 ± 1.04% ID/g) compared with MCF-7 tumors (3.02 ± 0.20% ID/g, p < 0.001) and blocked MDA-MB-468 tumors (4.33 ± 0.48% ID/g, p < 0.001). The tumor-to-muscle and tumor-to-blood ratios were also superior in the MDA-MB-468 model. These findings highlight [99mTc]Tc-HYNIC-mAbNectin-4 as a highly specific imaging agent for Nectin-4-positive tumors, offering strong tumor retention and good imaging performance for TNBC diagnosis and classification.
[124I]I-EV
In 2024, Ren et al. [9] reported the development of a iodine-124-labeled PET probe, [124I]I-EV, designed to target Nectin-4 for tumor-specific imaging. The radiolabeled [124I]I-EV was successfully synthesized without requiring bifunctional chelators, preserving its structural integrity. Thus, the tracer mimics the pharmacokinetics of EV, including its tumor uptake and retention, making it appealing for clinical translation. Biodistribution and PET imaging showed predominant accumulation in the blood and liver, with gradual clearance via hepatic metabolism. [124I]I-EV enables a urothelial carcinoma diagnosis and Nectin-4 visualization, aiding in treatment classification. The tracer effectively distinguished between Nectin-4-positive and Nectin-4-negative tumors, showing high uptake in SW780 tumors. Compared with [68Ga]Ga-N188, a bicyclic peptide, [124I]I-EV exhibited a lower KD (23.7 nM vs 13.8 nM) and achieved a higher maximum standardized uptake value in SW780 tumors (1.50 ± 0.01 at 24 h p.i. vs < 1 at 1 h p.i. for [68Ga]Ga-N188) [10], indicating excellent tumor-targeting capabilities. Notably, preliminary imaging and biodistribution studies in animal models revealed that the highest tumor uptake occurred at 24 h p.i., with overall organ uptake decreasing over time and without showing significant radiotoxicity.
[89Zr]Zr-DFO-N4MU0
In 2025, Babeker et al. [11] developed anti-Nectin-4 theranostics based on N4MU01. The diagnostic agent, N4MU01, was prepared by conjugating the antibody with deferoxamine (using p-SCN-Bn-DFO) and radiolabeling it with zirconium-89 to obtain a PET imaging agent. The radiotherapeutic version of N4MU01 was produced by conjugating the antibody with an 18-membered ring macrocyclic chelator, Macropa (using p-SCN-Macropa), followed by radiolabeling with actinium-225 for alpha-particle therapy. [89Zr]Zr-DFO-N4MU01 exhibited a high radiochemical yield (>90%) and strong tumor uptake (13.2 ± 1.12 %IA/g) at 120 h in MDA-MB-468 xenograft models. Blocking experiments confirmed the superior specificity compared with [89Zr]Zr-DFO-AGS-22M6. The radiotherapeutic agent, [225Ac]Ac-Macropa-N4MU01, demonstrated significant cytotoxic effects (IC50 = 1.2 kBq/mL) and led to dose-dependent tumor growth inhibition in mice. Treatment with [225Ac]Ac-Macropa-N4MU01 (13 kBq) achieved complete tumor remission in 83.3% of mice in a syngeneic 4T1-Nectin-4 model. A safety assay exhibited high radiation doses in the liver, lungs, and spleen. However, a very favorable dosimetry in all these healthy organs was observed because of the excellent clearance rates of the [225Ac]Ac-Macropa-N4MU01 from Nectin-4-negative healthy tissues. These findings highlight the potential of N4MU01 for both imaging and targeted radiotherapy in Nectin-4-expressing tumors, making it a promising candidate for future clinical applications.
Bicyclic Peptide-Based Radiopharmaceuticals
Small peptide-based radiopharmaceuticals, particularly bicyclic peptides, have emerged as promising alternatives to antibody-based agents for Nectin-4 imaging. Bicyclic peptides are typically composed of 10–20 amino acids (15 in the specific case of Nectin-4 imaging). Three of these amino acids are specifically modified to create three stable bonds, resulting in a distinctive double-ring structure. Unlike linear or monocyclic peptides, bicyclic peptides possess constrained conformations, which enhance their affinity and selectivity. Additionally, they address several challenges, such as susceptibility to degradation by terminal enzymes and structural instability [12]. Their compact structure enables rapid clearance from non-target tissues and improved tumor penetration, while maintaining high radiochemical purity and strong affinity for the target.
[68Ga]Ga-N188
In 2023, Duan et al. [10] developed [68Ga]Ga-N188, a PET probe based on a bicyclic N188 peptide (derived from BT8009). The peptide was synthesized in the solid phase, purified by high-performance liquid chromatography with a purity exceeding 95% and functionalized at the N-terminal end with DOTA to allow radiolabeling. The peptide showed high affinity for the target protein (Kd = 23.7 nM) and high stability in saline and serum, retaining > 95% intact after 30 min. After the success of in vitro tests, the probe was tested in a xenograft mouse model with tumors either positive or negative for the target protein (SW780 and 5637 respectively); in this context, PET/CT acquired 1 h post-injection of 7.4 MBq of the tracer validated its greater uptake in SW780 mice (2.94 ± 0.36 %ID/g) compared with the 5637 control (2.02 ± 0.22 %ID/g) and blocking group (1.44 0.17% ID/g). Finally, [68Ga]Ga-N188 showed favorable pharmacokinetic properties being rapidly cleared through the kidneys, resulting in a suitable tumor-to-muscle contrast for SW780, with values of 3.98 ± 2.48 after 1 h. Overall, the results of the preclinical imaging study suggest that the use of [68Ga]Ga-N188 for tumor-specific Nectin-4 imaging is viable and has a tolerable safety profile.
[68Ga]Ga-NOTA-PEG12-BP
To further enhance the pharmacokinetic properties of Nectin-4-targeting PET tracers, recent efforts have focused on optimizing peptide-based probes through PEGylation. In this context, Wan et al. [13] introduced a series of PEGylated molecular probes [68Ga]Ga-NOTA-PEG2-24-BP, among which [68Ga]Ga-NOTA-PEG12-BP emerged as the most promising candidate. By strategically incorporating a PEG12 linker between the bicyclic peptide and the chelator, [68Ga]Ga-NOTA-PEG12-BP successfully balanced hydrophilicity, tumor targeting, and metabolic clearance. Preclinical studies demonstrated that it achieved a superior tumor uptake compared with its predecessor [68Ga]Ga-N188 while maintaining rapid background clearance, leading to an improved tumor-to-background ratio. Notably, [68Ga]Ga-NOTA-PEG12-BP exhibited a three-fold higher tumor-to-background contrast in the blood, liver, and muscle at 60 min post-injection, addressing a major limitation of earlier probes.
While [68Ga]Ga-NOTA-PEG12-BP demonstrates strong potential for clinical translation, one limitation is its urinary excretion, which may pose challenges for in situ bladder cancer detection because of high background activity in the urinary tract. However, the main potential of this diagnostic probe is to target metastatic sites of both bladder cancer and other Nectin-4-expressing tumors, such as TNBC. Future efforts will likely focus on modifying its structure to alter the excretion pathway and further enhance its affinity and specificity.
[68Ga]Ga-DN68
Building on these advancements, Ge et al. [14] further optimized Nectin-4-targeting bicyclic peptides by developing [68Ga]Ga-DN68, a novel PET tracer designed to enhance imaging specificity and pharmacokinetics. Unlike its predecessor [68Ga]Ga-N188, DN68 incorporates PEG4 and metal-chelating groups via d-Lys conjugation, which significantly improves its stability and reduces non-specific background uptake. This modification resulted in a higher binding affinity to Nectin-4 (Kd = 0.64 nM) and a superior target-to-non-target ratio (>20 1-h post-injection), ensuring clearer tumor delineation in PET imaging. Preclinical studies demonstrated that [68Ga]Ga-DN68 achieves rapid tumor visualization within 30 min, outperforming earlier probes in terms of both contrast and retention. A biodistribution analysis revealed a predominantly renal excretion pathway, reducing hepatic accumulation and enhancing imaging accuracy. Moreover, competitive binding assays confirmed the tracer’s high specificity for Nectin-4-positive tumors, reinforcing its potential for non-invasive detection and therapeutic monitoring.
[68Ga]GaAJ647
In a preprint, Mishra et al. [15] explored [68Ga]Ga-AJ647 as a PET imaging agent for real-time monitoring of Nectin-4 engagement in EV therapy in metastatic urothelial carcinoma. AJ647, derived from AJ632, was optimized with a PEG linker and a NOTA chelator for effective gallium-68 labeling. The radiolabeling process achieved high purity and molar specific activity, demonstrating a PET-based target engagement analysis as a reliable predictor of therapeutic efficacy.
Positron emission tomography imaging revealed dose-dependent variations in Nectin-4 engagement, with lower EV doses leading to incomplete engagement and reduced tumor response. A receiver operating characteristic analysis established a threshold for therapeutic response prediction, emphasizing PET imaging’s role in optimizing treatment strategies and addressing tumor heterogeneity. Despite its advantages, [68Ga]Ga-AJ647 PET imaging is not without limitations. While it accurately quantifies target engagement, it does not fully account for the intracellular processing and payload delivery of EV, which also influence therapeutic efficacy. Additionally, longitudinal studies are needed to validate its predictive power in clinical settings and assess whether integrating PET-based biomarkers with genomic or transcriptomic profiling could further refine patient stratification strategies.
[18F]AlF-N231
To overcome the limitations of [68Ga]Ga-N188, previously developed by the same authors, owing to the relatively short half-life and low positron emission rate of 68Ga, Duan et al. have recently developed the radiopharmaceutical [18F]AlF-N231 [16]. This probe consists of a bicyclic N231 peptide, specific for Nectin-4, labeled with 18F. Specifically, the peptide was synthesized in the solid phase, purified by high-performance liquid chromatography (demonstrating > 95% purity) and finally conjugated to 18F by NOTA. In vitro studies showed increased uptake of [18F]AlF-N231 in Nectin-4-positive SW780 tumor cells compared with Nectin-4-negative 5637 cells. In Kunming mice injected with 18.5 MBq of the tracer, micro-PET/CT imaging confirmed a higher tumor uptake in SW780 xenografts (1.91 ± 0.50 %ID/g) than in 5637 tumors (1.32 ± 0.43 %ID/g) and block groups (0.51 ± 0.05 %ID/g). High-dose toxicity tests (925 MBq/kg) showed no adverse effects. Given its promising properties, [18F]AlF-N231 could serve as a PET probe for Nectin-4 imaging, pending further studies.
[68Ga]Ga-FZ-NR-1
This new probe, recently developed by Sun et al. [17], consists of a bicyclic peptide that binds specifically to the target protein, labeled with gallium-68. The synthesis of the peptide was performed by a similar approach to that used for other previously reported PET probes, while the DOTA chelator was employed for radiolabeling. This probe is characterized by strong hydrophilicity (log D7.4 = −3.55 ± 0.10) and high specificity for Nectin-4 (Kd = 2.81 nM). In early in vitro studies, the probe demonstrated significantly higher accumulation in Nectin-4-positive tumor cells (MDA-MB-468) than in negative tumor cells (MDA-MB-231). Moreover, even in preclinical studies, tumor uptake in mice xenografted with MDA-MB-468 was significantly higher (3.53 ± 0.33 %ID/g) than in the Nectin-4 negative tumors (2.4 ± 0.4 %ID/g) and the blocking group (2.4 ± 0.18 %ID/g). Finally, toxicity tests performed on mice showed no relevant side effects even at high doses, as confirmed by the maintenance of a stable body weight, normal blood parameters, and the absence of alterations in major organs. Taken together, these advancements reinforce the idea that small peptide-based radiopharmaceuticals can be valuable tools for precision imaging, ultimately improving the diagnosis and treatment planning for patients with Nectin-4-overexpressing tumors.
Preliminary Clinical Experiences
The research of PET radiotracers targeting Nectin-4 is very recent and to date only three papers have completed the translation from preclinical to clinical feasibility [10, 17, 18]. Duan et al. [10] tested [68Ga]Ga-N188 in 16 humans (clinical study NCT05321316, two healthy volunteers and 14 patients affected by metastatic bladder cancer). The authors, using a long-axial field-of-view PET/CT scanner (uEXPLORER; United Imaging Healthcare, Shanghai, China) [19, 20], performed a dynamic 40-minute scan in the two healthy volunteers and in four patients, confirming the favorable biodistribution of the tracer already demonstrated in their preclinical study. The radiotracer was mainly excreted by the kidneys, with very minimal background activity detectable in most of the tissues except the spleen and liver, esophagus, and prostate gland. Indeed, the medium uptake in the liver could represent a drawback of the radiotracer, limiting the exportability of the eventual hepatic metastatic spread. Late scans (40 min and 120 min post-injection) were undertaken, with 40-minute images showing the higher tracer uptake. Furthermore, all patients underwent [18F]fluorodeoxyglucose ([18F]FDG) PET/CT, which represents a pivotal imaging modality in metastatic bladder cancer, within a week from [68Ga]Ga-N188 PET/CT. Of note, [68Ga]Ga-N188 PET/CT demonstrated a superior tumor-to-background ratio than conventional [18F]FDG PET/CT. Finally, a correlation between the uptake intensity at [68Ga]Ga-N188 PET/CT and the Nectin-4 expression in tumor samples was demonstrated (p < 0.001). Taken altogether, the preliminary results by Duan and colleagues [10] paved the way for further clinical trials with this radiotracer, which have been recently published by Zhang et al. [18]. In this article, the authors used [68Ga]Ga-N188 PET/CT to identify the neoplasms more suitable for Nectin-4 imaging in 62 patients affected by 16 different cancers. Bladder cancer, intra-hepatic cholangiocarcinoma, cutaneous squamous cell carcinoma, cervical cancer, and pancreatic cancer seem to be the tumors with higher Nectin-4 expression. Of note, the high intensity of the tracer uptake (expressed as a high maximum standardized uptake value) significantly correlated with the membranous Nectin-4 expression, while it was not correlated with the cytoplasmatic expression. The patients enrolled were imaged with both [68Ga]Ga-N188 and [18F]FDG PET/CT within 1 week. The comparison of the two radiotracers in the whole group demonstrated that [18F]FDG has a higher sensitivity, accuracy, and specificity to identify primary tumors and lymph node metastases. However, [68Ga]Ga-N188 PET/CT retained a higher overall specificity (63.6% vs 36.4%) and a comparable overall detection rate (95% vs 93.3%) with respect to [18F]FDG PET/CT. In particular, [68Ga]Ga-N188 PET/CT outperformed [18F]FDG PET/CT in the detection of recurrent pancreatic cancer (100% vs 66.7%) and peritoneal metastatic involvement from high-grade serous ovarian cancer.
Another clinical trial of Nectin-4 targeting PET radiotracers was performed by Sun et al. [17], focusing on TNBC. Nine patients with metastatic TNBC were imaged with both [68Ga]Ga-FZ-NR-1 and [18F]FDG PET/CT. [68Ga]Ga-FZ-NR-1 PET/CT detected all the tumor lesions identified by [18F]FDG PET/CT but with a higher target-to-background ratio. Of note, no adverse events were identified in patients enrolled in both the clinical trials with Nectin-4 PET radiotracers.
Discussion
Nectin-4 is a very promising target for future theranostics. In a modern concept of PET imaging, visualizing the expression of a target throughout the body means guiding effective personalized treatments, enabling precision oncology [21]. Historically, the rise of PET imaging has been sustained by [18F]FDG. [18F]Fluorodeoxyglucose is a metabolic radiotracer with low specificity, suitable for imaging several oncological and inflammatory diseases [22–24]. However, because of its nature, [18F]FDG lacks the potential for a theranostic application. As a consequence, cutting-edge research is focusing on radiotracers targeting specific tumor markers, similarly to the work that has been done with prostate-specific membrane antigen and somatostatin receptors in prostate cancer and neuroendocrine tumors, respectively [22, 25, 26]. With this approach, the above-mentioned radio-theranostic probes targeting Nectin-4 could represent a new frontier to detect and treat several metastatic neoplasms. To date, only three articles report a clinical translation of Nectin-4-targeting radiotracers in cancer, mainly bladder cancer and TNBC [10, 17, 18]. The pilot study by Zhang et al. [18] suggests that PET radiotracers targeting Nectin-4 also deserve attention in pancreatic and high-grade serous ovarian cancer, in addition to bladder cancer and TNBC. Indeed, more experiments and trials need to be performed to see if these radiotracers can become part of daily clinical practice. However, their use could allow a precise selection of patients with high Nectin-4 expression that could benefit from EV. This is particularly relevant as poorly differentiated bladder cancer (i.e., sarcomatoid or rhabdoid urothelial carcinoma) does not overexpress Nectin-4 and would not be suitable for treatment with EV as it is unlikely to be effective [27]. Moreover, one of the limits of EV is the emergence of resistance to the treatment, with the reduction in Nectin-4 expression described in some metastases of patients with urothelial cancer [28, 29]. Nectin-4-targeting PET probes could enable the timely visualization of Nectin-4 expression loss and lead to discontinuation of EV in favor of other treatments, improving patients’ response rates and reducing the costs for the healthcare system. Moreover, a theranostic model targeting Nectin-4 could be a useful therapeutic weapon to damage cells overexpressing Nectin-4 and to hinder the establishment of the anti-Nectin-4 drug resistance mechanism mediated by the expression of the ABCB1 gene, which encodes the multidrug-resistant protein/P-glycoprotein that is typical of EV [30, 31] (Fig. 3).
Fig. 3.
Theranostic model with radiolabeled Nectin cell adhesion molecule 4 (Nectin-4) probes in bladder cancer, breast cancer, and melanoma. Created with Biorender.com
The identification of the most suitable radiotracer for targeting Nectin-4 remains inconclusive until further data become available in the literature. To date, most researchers focused on PET radiotracers, probably because of the best spatial resolution of PET imaging, and only one radiotracer for SPECT imaging has been proposed. However, SPECT is still the most available nuclear medicine imaging worldwide and further investigations on [99mTc]Tc-HYNIC-mAbNectin-4 are warranted [8].
Antibody-based radiotracers have been tested only in preclinical settings so far. These radiotracers move slowly through the body, taking a long time to reach their target and be cleared. As a result, it takes longer for them to accumulate in sufficient amounts in the target tissues to provide good imaging contrast. This necessitates the use of radioisotopes with long half-lives to ensure that the signal remains detectable throughout the tracer’s distribution. Consequently, image acquisition occurs after several days, limiting their appeal for clinical use in busy congested PET centers. A Nectin-4 targeting radioligand with suitable pharmacokinetics fitting the half-life of commonly available fluorine-18 and gallium-68 would facilitate clinical translation. Another limit of antibody-based radiotracers targeting Nectin-4 is their physiological distribution. The high liver retention may limit the diagnostic identification of the metastatic spread, which is non-negligible in tumors such as breast cancer or melanoma [32, 33]. Moreover, it represents a limit for therapeutic agents, although Babeker et al. [11] reported that [225Ac]Ac-Macropa-N4MU01 seems to show rapid hepatic and lung clearance, thus limiting radiation exposure.
Bicyclic peptides represent a promising alternative owing to their stability, high affinity, and selective targeting. In bicycle peptides designed to bind Nectin-4, such as those used in the radiopharmaceuticals [68Ga]Ga-N188, [68Ga]Ga-NOTA-PEG12-BP, and [68Ga]Ga-DN68, interaction with Nectin-4 occurs through specific amino acid residues within the peptide sequence. Studies have shown that Met4, Trp7, Ser8, Pro10, and Trp12 are essential for high-affinity and specific binding to Nectin-4. Substitution of these residues with alanine led to a significant reduction in binding affinity, confirming their critical role in the interaction with Nectin-4 [34].
To further improve binding affinity and pharmacokinetic properties, several modifications can be introduced into the peptide sequence, such as the substitution of phenylalanine with larger aromatic analogs, such as 1-naphthylalanine or 2-naphthylalanine, to increase binding affinity. Introduction of non-natural amino acids, such as azetidine-2-carboxylic acid and pipecolic acid, to explore ring expansion or contraction in the peptide. These modifications have led to peptides with nanomolar-range binding affinities and improved solubility and stability [34].
Considering the bicyclic peptide-based radiopharmaceuticals that have been developed, [68Ga]Ga-DN68 seems to outperform [68Ga]Ga-N188 in terms of the tumor-to-non-target ratio (20 vs 3.98 1-h post-injection) and demonstrated a lower physiological tracer uptake in the spleen, lungs, and intestine [14]. Furthermore, a PET probe using fluorine-18 has been recently proposed, [18F]AlF-N231 [16]. Indeed, fluorine-18 is the most versatile radionuclide for PET imaging, combining favorable spatial resolution and allowing central production and shipment to satellite centers. Nonetheless, several challenges persist, including the need to increase the radiochemical yield and improve tumor uptake to achieve greater sensitivity. Finally, all of the three experiments reported in humans have been performed with bicyclic peptides. This might be considered a hint that these types of radiotracers have a greater possibility of clinical transition than antibody-based radiotracers. Although diagnostic bicyclic peptide-based radiopharmaceuticals have made significant research progress, current challenges focus on developing compounds better suited for the detection of primary urothelial lesions. Structure–activity relationship studies are therefore essential to optimize the lipophilicity and polarity of the radiotracers, enabling these compounds to be excreted through the pancreas or the hepatobiliary system. These studies would also facilitate the development of therapeutic radiopharmaceutical analogs. The radioligand therapy based on bicyclic peptide targeting Nectin-4 represents a promising strategy, given their high binding affinity, metabolic stability, and favorable pharmacokinetic profiles; however, further preclinical and clinical studies are required to fully elucidate their efficacy and translational potential.
Conclusions
Radiolabeled agents targeting Nectin-4 deserve further investigations in an attempt to identify new theranostic agents with potential for clinical translation. These radiolabeled compounds could enable effective detection of Nectin-4 expression status in metastases and may become therapeutic agents if radiolabeled with high-energy radionuclides. Further research efforts are needed to pursue the clinical translation of these promising radiotracers in several oncologic diseases.
Declarations
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Conflicts of Interest/Competing Interests
Giorgia Speltri, Ilham Badrane, Rebecca Napolitano, Alessandra Boschi, Licia Uccelli, Luca Filippi, Massimo Guidoboni, Matteo Brunelli, Federica Lancia, Petra Martini, Antonella Iudicello, Corrado Cittanti, Mirco Bartolomei, and Luca Urso have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Ethics approval was waived because no patient data were analyzed.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: LU, AB; writing first draft: GS, IB, RN, LU, AB; editing and revision: LU, LF, MG, MB, FL, PM, AI, CC, MB. All authors read and approved the final version of the manuscript.
Footnotes
Giorgia Speltri, Ilham Badrane are Co-first authors.
References
- 1.Li K, Zhou Y, Zang M, Jin X, Li X. Therapeutic prospects of nectin-4 in cancer: applications and value. Front Oncol. 2024;14:1354543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee S, Sinha S, Kundu CN. Nectin cell adhesion molecule-4 (NECTIN-4): a potential target for cancer therapy. Eur J Pharmacol. 2021;911: 174516. [DOI] [PubMed] [Google Scholar]
- 3.Filippi L, Schillaci O. NECTIN-4 targeted theranostics for urothelial cancer: getting ready for primetime? Expert Rev Anticancer Ther. 2024;24:1–4. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto H, Tanaka Y, Murata M, Ito T. Nectin-4: a novel therapeutic target for skin cancers. Curr Treat Options Oncol. 2022;23:578–93. [DOI] [PubMed] [Google Scholar]
- 5.Swiecicki PL, Yilmaz E, Rosenberg AJ, Fujisawa T, Bruce JY, Meng C, et al. Phase II trial of enfortumab vedotin in patients with previously treated advanced head and neck cancer. J Clin Oncol. 2025;43:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg JE, Powles T, Sonpavde GP, Loriot Y, Duran I, Lee J-L, et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann Oncol. 2023;34:1047–54. [DOI] [PubMed] [Google Scholar]
- 7.Campbell DO, Noda A, Verlinsky A, Snyder J, Fujita Y, Murakami Y, et al. Preclinical evaluation of an anti-Nectin-4 immunoPET reagent in tumor-bearing mice and biodistribution studies in cynomolgus monkeys. Mol Imaging Biol. 2016;18:768–75. [DOI] [PubMed] [Google Scholar]
- 8.Shao F, Pan Z, Long Y, Zhu Z, Wang K, Ji H, et al. Nectin-4-targeted immunoSPECT/CT imaging and photothermal therapy of triple-negative breast cancer. J Nanobiotechnol. 2022;20:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren Y, Liu T, Li S, Ma X, Xia L, Wang P, et al. An iodine-labelled antibody-drug conjugate PET probe for noninvasive monitoring of Nectin-4 expression in urothelial carcinoma. Int J Pharmaceutics. 2024;651: 123756. [DOI] [PubMed] [Google Scholar]
- 10.Duan X, Xia L, Zhang Z, Ren Y, Pomper MG, Rowe SP, et al. First-in-human study of the radioligand 68Ga-N188 targeting Nectin-4 for PET/CT imaging of advanced urothelial carcinoma. Clin Cancer Res. 2023;29:3395–407. [DOI] [PubMed] [Google Scholar]
- 11.Babeker H, Njotu FN, Pougoue Ketchemen J, Monzer A, Tikum AF, Doroudi A, et al. 225 Ac/89 Zr-labeled N4MU01 radioimmunoconjugates as theranostics against Nectin-4-positive triple-negative breast cancer. J Nucl Med. 2025;66(4):592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes CA, Pei D. Bicyclic peptides as next-generation therapeutics. Chemistry. 2017;23:12690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan Q, Yuan H, Cai P, Liu Y, Yan T, Wang L, et al. Effects of PEGylation on imaging contrast of68 Ga-labeled bicyclic peptide PET probes targeting Nectin-4. Mol Pharmaceutics. 2024;21:4430–40. [DOI] [PubMed] [Google Scholar]
- 14.Ge S, Jia T, Shi J, Cao J, Sang S, Li J, et al. A cutting-edge 68Ga-labeled bicyclic peptide PET molecular probe for noninvasive assessment of Nectin4 expression. Bioorganic Chem. 2024;152: 107745. [DOI] [PubMed] [Google Scholar]
- 15.Mishra A, Sharma AK, Gupta K, Banka DR, Johnson BA, Hoffman-Censits J, et al. Nectin-4 PET for optimizing enfortumab vedotin dose-response in urothelial carcinoma. Available from: 10.1101/2024.12.25.630315. Accessed 10 Mar 2025.
- 16.Duan X, Zhang Z, Xu H, Zhang J, Yan Y, Yang X. Preclinical evaluation of an Al18 F-radiolabeled bicyclic peptide targeting Nectin-4. Mol Pharmaceutics. 2025;22:221–8. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Sun Y, Zuo K, Fan L, Wang X, Zhang J, et al. Pilot study of Nectin-4-targeted PET imaging agent68 Ga-FZ-NR-1 in triple-negative breast cancer from bench to first-in-human. J Nucl Med. 2025;66:473–9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Duan X, Chen X, Zhang Z, Sun H, Shou J, et al. Translational PET imaging of Nectin-4 expression in multiple different cancers with68 Ga-N188. J Nucl Med. 2024;65:12S-18S. [DOI] [PubMed] [Google Scholar]
- 19.Urso L, Frantellizzi V, De Vincentis G, Schillaci O, Filippi L, Evangelista L. Clinical applications of long axial field-of-view PET/CT scanners in oncology. Clin Transl Imaging. 2023;11:365–80. [Google Scholar]
- 20.Jiang D, Lan X, Cai W. PET Imaging of Nectin-4: a promising tool for personalized/precision oncology. Clin Cancer Res. 2023;29:3259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodei L, Herrmann K, Schöder H, Scott AM, Lewis JS. Radiotheranostics in oncology: current challenges and emerging opportunities. Nat Rev Clin Oncol. 2022;19:534–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urso L, Panareo S, Castello A, Ambrosio MR, Zatelli MC, Caracciolo M, et al. Glucose metabolism modification induced by radioligand therapy with [177Lu]Lu/[90Y]Y-DOTATOC in advanced neuroendocrine neoplasms: a prospective pilot study within FENET-2016 Trial. Pharmaceutics. 2022;14:2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urso L, Quartuccio N, Caracciolo M, Evangelista L, Schirone A, Frassoldati A, et al. Impact on the long-term prognosis of FDG PET/CT in luminal-A and luminal-B breast cancer. Nucl Med Commun. 2022;43:212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casali M, Lauri C, Altini C, Bertagna F, Cassarino G, Cistaro A, et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: a guide for image acquisition and interpretation. Clin Transl Imaging. 2021;9:299–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. [DOI] [PubMed] [Google Scholar]
- 26.Hofman MS, Lau WFE, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–16. [DOI] [PubMed] [Google Scholar]
- 27.Brunelli M, Gobbo S, Malpeli G, Sirgiovanni G, Caserta C, Munari E, et al. TROP-2, NECTIN-4 and predictive biomarkers in sarcomatoid and rhabdoid bladder urothelial carcinoma. Pathologica. 2024;116:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klümper N, Ralser DJ, Ellinger J, Roghmann F, Albrecht J, Below E, et al. Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res. 2023;29:1496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chou J, Trepka K, Sjöström M, Egusa EA, Chu CE, Zhu J, et al. TROP2 expression across molecular subtypes of urothelial carcinoma and enfortumab vedotin-resistant cells. Eur Urol Oncol. 2022;5:714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urso L, Uccelli L, Boschi A, Schillaci O, Filippi L. Molecular imaging in cancer chemoresistance: what’s brewing? J Nucl Med. 2025;66:344–8. [DOI] [PubMed] [Google Scholar]
- 31.Cabaud O, Berger L, Crompot E, Adélaide J, Finetti P, Garnier S, et al. Overcoming resistance to anti-Nectin-4 antibody-drug conjugate. Mol Cancer Ther. 2022;21:1227–35. [DOI] [PubMed] [Google Scholar]
- 32.Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–7. [DOI] [PubMed] [Google Scholar]
- 33.Wohlfeil SA, Häfele V, Dietsch B, Weller C, Sticht C, Jauch AS, et al. Angiogenic and molecular diversity determine hepatic melanoma metastasis and response to anti-angiogenic treatment. J Transl Med. 2022;20:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudd GE, Scott H, Chen L, Van Rietschoten K, Ivanova-Berndt G, Dzionek K, et al. Discovery of BT8009: a Nectin-4 targeting bicycle toxin conjugate for the treatment of cancer. J Med Chem. 2022;65:14337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]