Abstract
Background
Knowledge of sand fly–Leishmania attachment determinants is pivotal for providing evidence on vector status. Considering the Amazonian transmission context of Trichophoromyia spp.–L. (Viannia) lainsoni, the present study aimed to assess in vitro interactions and detect gut glycoconjugates associated with this vector–parasite association.
Methods
Field-caught Trichophoromyia brachipyga and Trichophoromyia ubiquitalis were tested. Lutzomyia longipalpis reared in the laboratory was used as a control. The intestines were obtained by dissection, and the species were confirmed by morphology. Interactions for each sand fly–Leishmania association were individually performed via an in vitro incubation system. N-acetyl-d-glucosamine (GlcNAc), galactose-(β 1,3)-GalNAc (Gal/GalNAc), and N-acetyl-d-galactosamine (GalNAc) glycoconjugates of Trichophoromyia spp. were analyzed by Western blotting using corresponding peroxidase-conjugated lectins.
Results
No difference was found between Th. ubiquitalis and Lu. longipalpis attachment with L. (V.) lainsoni, and Lu. longipalpis with Leishmania (Leishmania) infantum (control). However, Th. brachipyga-L. (V.) lainsoni attachment was weaker than that of the control. Trichophoromyia spp. were negative for residues of GlcNAc terminally exposed. Trichophoromyia ubiquitalis was positive for GalNAc and Gal/GalNAc, whereas Th. brachipyga presented only residues of GalNac terminally exposed.
Conclusions
The present study suggests that Trichophoromyia spp. sand flies, particularly Th. brachipyga, are susceptible to L. (V.) lainsoni, based on the observed vector–parasite attachment profiles and detection of GalNAc in their midguts. This supports early field data suggesting the vector status of these sand fly species.
Graphical Abstract
Keywords: Sand fly, Attachment, Glycoconjugates, Vector, Leishmania
Background
Cutaneous leishmaniasis (CL) is a neglected tropical disease with a wide geographic distribution and can cause severe clinical manifestations [1]. In the Amazon region, a myriad of parasite–vector–host arrangements reflect a complex network of interactions [2]. Despite their medical and epidemiological relevance, some transmission cycles remain understudied, which limits the effectiveness of specific surveillance strategies.
Leishmania (Viannia) lainsoni was first described in the 1980s in the state of Pará, Brazil, as a causative agent of cutaneous leishmaniasis in humans [3]. Since then, it has been identified as a causative agent of the disease in other Brazilian states and neighboring countries [4–10]. Trichophoromyia species have been associated with the transmission of L. (V.) lainsoni [11]. Whereas Th. ubiquitalis has long been regarded as a vector of this parasite [12, 13], Th. brachipyga has recently been included in the suspicion list on the basis of a natural infection diagnosis [11, 14].
Attachment to the sand fly midgut is a crucial step for Leishmania survival in the vector and is included in the determinant set of vector competence [15, 16]. Carbohydrate epitopes, primarily N-acetyl-d-galactosamine, present on the midgut microvillar surface, bind to Leishmania. [17, 18]. Furthermore, under laboratory conditions, the attachment capacity of a given sand fly to one or more Leishmania species can be classified as specific or permissive, respectively [19]. In vitro attachment experiments have been performed to allow rapid analyses of the interaction between insect midguts and promastigote forms of Leishmania [18, 20–28], providing alternative evidence of that in vivo, where unproductive offspring and unsuccessful artificial blood-feeding of wild-caught species may limit sample size [29, 30].
Given the epidemiological relevance of CL agents in the Amazon region and the limited knowledge of sand fly–Leishmania interactions, studies addressing these gaps are essential. This work aimed to evaluate the in vitro interaction between Trichophoromyia spp. and the associated parasite L. (V.) lainsoni, as well as to detect glycoconjugates in the sand fly midgut.
Methods
Sand flies and Leishmania spp.
The field-caught sand flies used in this study were obtained from an urban park located in the Belém Metropolitan Region, Pará State, Brazil (1° 25′ 48.2′′ S, 48° 27′ 24.9′′ W), where the ecology of these insects has been previously studied; in that environment, Th. ubiquitalis and Th. brachipyga are abundant [14]. Entomological capture was performed using Centers for Disease Control and Prevention (CDC) light traps (John W. Hock Company, Gainesville, USA) installed between 6:00 pm and 6:00 am, close to the ground (~1.5 m). The sampling effort was not established, as it was based on the availability of specimens, the success of the experiments, and, consequently, the statistical support for the findings.
An old, closed colony of Lutzomyia longipalpis from the 1990s (Abaetetuba, Brazil, F245) was used as a control, as this species is recognized as a permissive vector [17, 19]. The insects were reared and maintained under a 14:10 h light/dark regime at a temperature of 25 °C, a relative humidity of 80%, and a diet of 10% sucrose. Previous evidence has demonstrated that this species is experimentally susceptible to L. (V.) lainsoni [3].
Promastigotes of L. (V.) lainsoni (MHOM/BR/1981/M6426) and L. (L.) infantum (MCER/BR/1981/M6445) were obtained from the Leishmania cryobank of the Ralph Lainson leishmaniases lab (Instituto Evandro Chagas, Ananindeua, Brazil) and maintained in Roswell Park Memorial Institute (RPMI) culture medium supplemented with inactivated 10% fetal bovine serum (FBS) at 25 °C to estimate the growth curve.
Dissection of midguts
Field-caught samples were immediately transported to the laboratory in a nylon cage as described elsewhere [29]. Insects were placed in a container, immobilized by cooling (4 °C), and then successively washed with phosphate-buffered saline (PBS) [31]. The sand flies were morphologically screened to triage the target species. Females were dissected under sterile conditions in Grace’s insect medium (Merck, Darmstadt, DE) within concave wells of glass slides with the aid of entomological forceps following decapitation and removal of the last abdominal segments to obtain the spermatheca and digestive system [28]. The spermatheca was placed on a slide and covered with a coverslip to confirm the species under an optical microscope, following the taxonomic identification keys of Galati [32]. After taxonomic determination, the midgut and Malpighian tubules were removed, and the midgut was retained. These procedures were continuously performed to ensure sufficient material for the experiments described in the following sections.
In vitro interactions
The following sand fly–Leishmania associations were considered: Th. ubiquitalis–L. (V.) lainsoni and Th. brachipyga–L. (V.) lainsoni as tests and Lu. longipalpis–L. (L.) infantum and Lu. longipalpis–L. (V.) lainsoni as controls.
Late logarithmic phase promastigotes were centrifuged at 1800 × g for 10 min and resuspended in 1 mL of Grace’s insect medium (GIM) [28]. A dilution for counting was subsequently performed in the Neubauer chamber to estimate and subsequently adjust the inoculum to 2 × 107 cells/mL [25].
The midgut was dissected, and the remaining structures were removed as previously described. The concave wells of glass slides containing 5 μL of GIM were longitudinally opened and incubated for 45 min with 50 μL of promastigotes (2 × 107 cells/mL) in a dark and humid chamber. After incubation, the midgut was washed three times in PBS using two pairs of needles and syringes (one pair to remove PBS from the slide and one pair to add sterile PBS again) to remove promastigotes that did not adhere to the intestinal epithelium [25, 26]. After washing, the well of the slide containing the midgut was covered with a coverslip and observed under 400× magnification with an AxioScope optical microscope (Zeiss, Oberkochen, DE). The observed adhesion profile was classified as weak (fewer than 100 promastigotes), moderate (100–1000 promastigotes), or heavy (more than 1000 promastigotes), adapting the criteria of Myskova et al. [17] for in vitro interaction assays. Each combination of sand fly–Leishmania was evaluated in 40 dissected midguts.
Detection of glycoconjugates
Previously standardized samples of seven pooled midguts were stored in microtubes containing 25 μL of cell lysis buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM Na2-Ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% Triton) with protease inhibitor cocktail (Complete, Mini EDTA-free) (Roche, Basileia, CH) at 4 °C. The midguts were then macerated, heated to 100 °C for 5 min, and then frozen in liquid nitrogen until the experiments. Subsequently, samples were thawed and 15 μL mixed with 5 μL 4 × SDS sample buffer (final concentration: 250 mM Tris–HCl pH 6.8, 40% glycerol; 8% SDS; 20% 2-mercaptoethanol; 0.2% bromophenol blue) and heated at 100 °C for 3 min using a Thermo-Shaker (KASVI, Pinhais, BR). After that, the samples and molecular weight marker were applied to a 10% polyacrylamide gel for the separation of glycoconjugates by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 120 V for 1 h) using a Mini-Protean Tetra chamber (Bio-Rad, Hercules, USA) [17, 18].
Then, the transfer to a nitrocellulose membrane was performed using the ENDURO™ Semi-Dry Blotter (Labnet, Edison, USA) at 15 V for 30 min. To block nonspecific sites, the membrane was incubated overnight with skim milk/tris-buffered saline-Tween (TBST) (0.05 g/mL) at pH 7.6. After blocking, the nitrocellulose membrane was incubated for 1 h with the lectins from wheat germ agglutinin (WGA) (Sigma-Aldrich, St. Louis, USA), peanut agglutinin (PNA) (Sigma-Aldrich, St. Louis, USA), and Helix pomatia agglutinin (HPA) (Sigma-Aldrich, St. Louis, USA), conjugated with peroxidase in TBST (1:100) to determine GlcNAc, Gal/GalNAc, and GalNAc, respectively. The membrane was washed three times with TBST followed by incubation with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, USA) for a few seconds until the bands could be visualized. Carbohydrate labeling was analyzed using ImageJ 1.5a software (National Institutes of Health, Bethesda, USA) [17, 18].
Data analysis
To analyze statistically significant differences between in vitro interactions, the G test was performed for two independent samples with categorical variables. The confidence interval was set at 95%. Tests were performed via Bioestat 5.0 software (Instituto Mamirauá, Tefé, Brazil) [33], and graphical elements were created via Prism 10.3.1 (GraphPad Software, Boston, USA).
Results and discussion
Sand fly–Leishmania in vitro interactions
Each of the four sand fly–Leishmania associations was assessed using 40 midguts; thus, 160 in vitro interactions were performed.
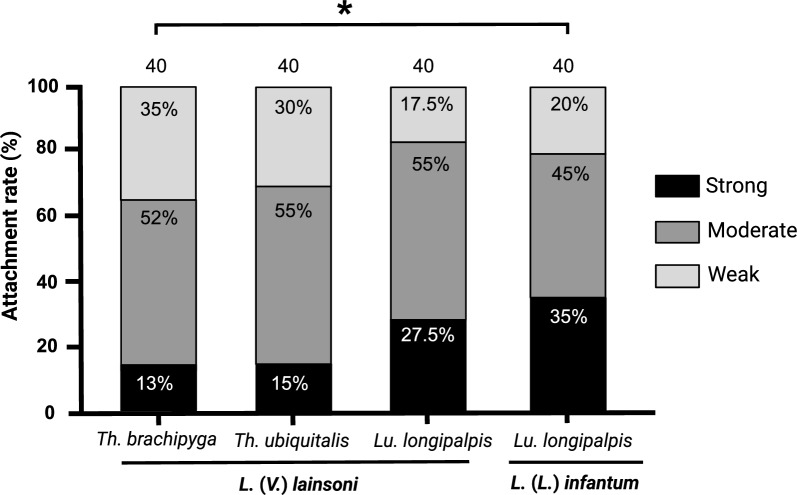
Attachment profiles of sand flies with L. (V.) lainsoni and Lu. longipalpis with L. (L.) infantum with the respective intensity percentages (weak, moderate, and heavy) are presented in Fig. 1. There was no difference between the attachment profiles in the following comparisons: Th. ubiquitalis–L. (V.) lainsoni versus Lu. longipalpis–L. (V.) lainsoni–L. (L.) infantum (Table 1). Despite the limitations of in vitro infection assays, the similar adhesion profile to that of Lu. longipalpis, a species known to be permissive [19], provides initial experimental evidence for investigating the potential role of Th. ubiquitalis in the transmission cycle of cutaneous leishmaniasis caused by L. (V.) lainsoni, widely described in the literature [11]. However, additional in vivo susceptibility assays with different Leishmania spp. are essential.
Fig. 1.
In vitro interactions between Trichophoromyia brachipyga–Leishmania (Viannia) lainsoni, Th. ubiquitalis–L. (V.) lainsoni, and Lutzomyia longipalpis–L. (V.) lainsoni. The susceptible association Lu. longipalpis–L. (L.) infantum was used as a control. The attachment profiles were classified into three categories: weak (less than 100 parasites per gut), moderate (100–1000 parasites per gut), and heavy (more than 1000 parasites per gut); values inside the bars indicate percentages of each category; values on the bars indicate the number of guts assessed. *P < 0.05
Table 1.
Summary statistics for the comparison of in vitro binding in different parasite–vector combinations
| Sand fly species | Leishmania spp. | N | Statistics |
|---|---|---|---|
| Th. ubiquitalis | L. (V.) lainsoni | 40 | G test = 2.8240; df = 2; P = 0.2437 |
| Lu. longipalpis | 40 | ||
| Th. ubiquitalis | L. (V.) lainsoni | 40 | G test = 4.4974; df = 2; P = 0.1055 |
| Lu. longipalpis | L. (L.) infantum | 40 | |
| Th. brachipyga | L. (V.) lainsoni | 40 | G test = 4.7078; df = 2; P = 0.0950 |
| Lu. longipalpis | 40 | ||
| Th. brachipyga | L. (V.) lainsoni | 40 | G test = 6.3272; df = 2; P = 0.0423 |
| Lu. longipalpis | L. (L.) infantum | 40 |
Significant differences are highlighted in bold
Interestingly, the adhesion profile of Th. brachipyga–L. (V.) lainsoni was similar to that of Lu. longipalpis–L. (V.) lainsoni but weaker than that of the Lu. longipalpis–L. (L.) infantum control (Fig. 1; Table 1), a classical parasite–vector binomial association [19, 22]. Considering that the success of an infection depends, in the first instance, on the establishment of the parasite’s attachment to the sand fly midgut [16], attachment differences herein observed may converge with the hypothesis that the natural infection of Th. brachipyga appears to be more occasional or still emerging, without ruling out a possible role as an alternative or secondary vector [11, 14].
There was no difference between the adhesion profiles of Lu. longipalpis with L. (V.) lainsoni and L. (L.) infantum. However, when Sánchez-Uzcátegui et al. [30] compared the susceptibility of Lu. longipalpis with these Leishmania spp. on the eighth day post-blood meal (pbm), they observed greater parasitosis with L. (L.) infantum. This suggests that, although the initial adhesion profiles are proportionally similar, the maintenance during late-stage infections is better established when considering the natural association between sand flies and Leishmania spp.
Furthermore, the development of infection in the vector may also be related to the growth characteristics of the species involved in the infection [34]. Aspects of the molecular challenges imposed by the vector’s digestive environment and the adaptations developed by Leishmania parasites have already been reviewed [35]. Still, in this regard, the present authors are aware of limitations. As stated by Wilson et al. [28], in several vector–parasite pairs, the specificity of in vitro binding alone is insufficient to explain overall vector specificity since other significant barriers to development must exist in certain refractory Leishmania–sand fly vector combinations.
Glycoconjugate detection
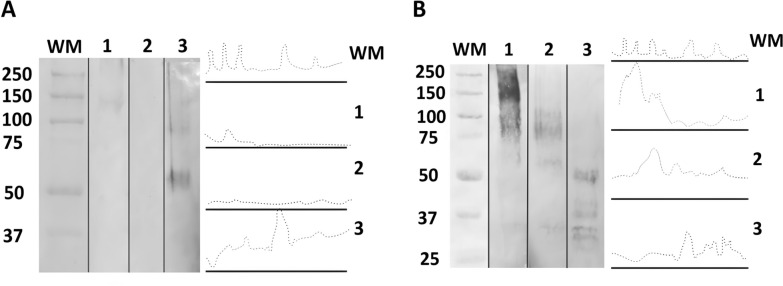
To evaluate the presence of possible ligands for Leishmania in the sandfly species tested, each carbohydrate detection (GlcNAc, Gal/GalNAc, and GalNAc) was performed in triplicate with seven pooled midgut samples of three sand fly species; thus, 189 midgut samples were used. Regarding intestinal glycoconjugates, the WGA lectin showed no reactivity in any of the species tested, while PNA lectin was reactive only in Th. ubiquitalis and Lu. longipalpis. In Th. ubiquitalis, the reactivity was weaker, presenting bands of ~150–250 kDa, whereas the Lu. longipalpis presented the highest labeling reactivity, with bands ranging from ~50 to 250 kDa (Fig. 2). The lack of PNA reactivities in Th. brachipyga and WGA in all species suggest that Gal/GalNAc and GlcNAc residues are not terminally exposed in the midgut glycoconjugates of the sand fly species tested, making them inaccessible to lectins. The absence of terminal GlcNAc residues apparently did not affect promastigote adhesion, as the parasite interactions with the permissive vector Lu. longipalpis predominantly exhibited strong and moderate adhesion, and only comparison with Th. brachipyga showed significant differences. Similarly, the absence of terminal Gal/GalNAc residues in Th. brachipyga alone does not appear to be sufficient to reduce adhesion, as observed in comparison with other vector–parasite systems that possess this terminal carbohydrate. Therefore, it is unlikely that these sugar residues act as binding sites for the tested Leishmania species, even if ligands for these carbohydrates are possibly present in Leishmania spp. [36].
Fig. 2.
Western blot and densitometry analyses of peroxidase-conjugated lectin reaction profiles for the detection of sand fly gut glycoconjugates. A Peanut agglutinin (PNA), indicating galactose/GalNAc (Gal/GalNAc); B Helix pomatia agglutinin (HPA), indicating N-acetyl-d-galactosamine (GalNac). 1 indicates Trichophoromyia ubiquitalis; 2 indicates Th. brachipyga; 3 indicates Lutzomyia longipalpis. WM, molecular weight marker (values in kilodaltons)
N-Acetyl-d-galactosamine (GalNAc) is particularly important, as it was identified as a component of glycoconjugates present exclusively in the intestines of permissive sand flies and later demonstrated as part of the midgut mucins capable of binding to Leishmania, possibly by a lectin-like molecule present on the surface of the promastigote [17, 37]. Our data demonstrated HPA lectin reactivity in all tested sand fly species. Th. ubiquitalis presented greater reactivity, with bands ranging from ~70 to 250 kDa. In Th. brachipyga, the reactivity was slightly lower and presented bands ranging from ~60 to 110 kDa (Fig. 2). In Lu. longipalpis, the intensity was lower than that of Th. ubiquitalis but close to Th. brachipyga with bands of ~30–50 kDa. The results, visually observed in the run, were confirmed by densitometry, suggesting preliminary evidence of possible permissiveness for Th. ubiquitalis and Th. brachipyga. However, the limitations of these findings should be overcome with supporting in vivo experimental data and further characterization of the Leishmania molecule possibly mediating this binomial interaction.
Conclusions
The attachment profiles and glycoconjugates presented herein provide a better understanding of the interactions between the sand fly and medically important Leishmania spp. in the Amazon region. Accumulating, sometimes controversial data should be carefully considered when unraveling these complex and specific relationships.
Acknowledgements
The authors would like to thank Fábio Márcio Medeiros da Silva Freire, Edna de Freitas Leão, Luciene Aranha da Silva Santos, Maria Sueli Barros Pinheiro, Raimundo Nonato Barbosa Pires, and Lucivaldo João Conceição Ferreira (Evandro Chagas Institute, Ministry of Health, Brazil) for their technical support with the field and laboratory work; Jerson Marques de Araujo Costa and Isabella Luiza Dinis da Silva (Laboratory of Structural Biology, Federal University of Pará, Brazil) for their support with the characterization of glycoconjugates; Alexandre Mesquita, Valéria Batista Libonati, Sarah Santos Carneiro, and Carlos Alberto Silva Sousa (Department of Management of Special Areas, Municipal Secretariat of Environment, Belém, Brazil); and the Bosque Rodrigues Alves staff for their logistical facilities to access the park.
Author contributions
Study design: T.G.M., E.O.S., and T.V.S. Data acquisition: T.G.M., Y.V.S.U., C.B.C.S., and R.R.F. Resources: F.T.S., E.O.S., and T.V.S. Data analysis: T.G.M., Y.V.S.U., C.B.C.S., R.R.F., F.T.S., E.O.S., and T.V.S. Manuscript, original draft: T.G.M., E.O.S., and T.V.S. Manuscript, final version: T.G.M., Y.V.S.U., C.B.C.S., R.R.F., F.T.S., E.O.S., and T.V.S. All the authors read and approved the final version of the manuscript.
Funding
This research was financially supported by the Instituto Evandro Chagas (IEC)/Ministry of Health, Brazil. T.G.M. and Y.V.S.U. received, respectively, Master’s and Ph.D. scholarships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES; Financial code 001). R.R.F. received an international mobility scholarship supported by the Programa de Doutorado-Sanduíche no Exterior (PDSE) from CAPES (44/2022; 01/2023). E.O.S. received a research productivity scholarship from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grant no. 314890/2021–1).
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
The capture and processing of invertebrate fauna (Diptera: Psychodidae) were authorized by the Sistema de Autorização e Informação em Biodiversidade (SISBio) under protocol no. 89906–1.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edilene Oliveira da Silva and Thiago Vasconcelos dos Santos have contributed equally to this work.
References
- 1.World Health Organization. Leishmaniasis. 2024. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/leishmaniasis. Accessed 20 Jun 2024.
- 2.de Souza AAA, da Rocha Barata I, das Graças Soares Silva M, Lima JAN, Jennings YLL, Ishikawa EAY, et al. Natural Leishmania (Viannia) infections of phlebotomines (Diptera: Psychodidae) indicate classical and alternative transmission cycles of American cutaneous leishmaniasis in the Guiana Shield, Brazil. Parasite. 2017;24:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silveira FT, Shaw JJ, Braga RR, Ishikawa E. Dermal leishmaniasis in the Amazon region of Brazil: Leishmania (Viannia) lainsoni sp.n., a new parasite from the State of Pará. Mem Inst Oswaldo Cruz. 1987;82:289–91. [DOI] [PubMed] [Google Scholar]
- 4.Corrêa JR, Brazil RP, Soares MJ. Leishmania (Viannia) lainsoni (Kinetoplastida: Trypanosomatidae), a divergent Leishmania of the Viannia subgenus—a mini review. Mem Inst Oswaldo Cruz. 2005;100:587–92. [DOI] [PubMed] [Google Scholar]
- 5.Lainson R. The Neotropical Leishmania species: a brief historical review of their discovery, ecology, and taxonomy. Rev Pan-Amaz Saude. 2010;1:13–32. [Google Scholar]
- 6.Benítez IR, Andrade-Zampieri R, Silveira-Elkhoury AN, Floeter-Winter LM, Lauletta-Lindoso JA, Pereira J, et al. Detección molecular de Leishmania (Viannia) lainsoni Silveira, Shaw, Braga & Ishikawa, 1987 (Kinetoplastida: Trypanosomatidae) en humanos de Paraguay. Rev Inst Med Trop. 2019;14:21–8. [Google Scholar]
- 7.Ducharme O, Simon S, Ginouves M, Prévot G, Couppie P, Demar M, et al. Leishmania naiffi and lainsoni in French Guiana: clinical features and phylogenetic variability. PLoS Negl Trop Dis. 2020;14:e0008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonçalves LP, Santos TVD, Campos MB, Lima LVD, Ishikawa EAY, Silveira FT, et al. Further insights into the eco-epidemiology of American cutaneous leishmaniasis in the Belem metropolitan region, Pará State, Brazil. Rev Soc Bras Med Trop. 2020;53:e20200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brilhante AF, Zampieri RA, Souza EA, Carneiro ACG, Barroso EP, Ávila MM, et al. Preliminary observations of the urbanization and domiciliation of the American cutaneous leishmaniasis in Rio Branco, Acre, Western Amazon. Rev Soc Bras Med Trop. 2022;55:e0359-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezemer JM, Freire-Paspuel BP, Schallig HDFH, de Vries HJC, Calvopiña M. Leishmania species and clinical characteristics of Pacific and Amazon cutaneous leishmaniasis in Ecuador and determinants of health-seeking delay: a cross-sectional study. BMC Infect Dis. 2023;23:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasconcelos dos Santos T, Silveira FT. Increasing putative vector importance of Trichophoromyia phlebotomines (Diptera: Psychodidae). Mem Inst Oswaldo Cruz. 2020;115:e190284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silveira FT, Souza AA, Lainson R, Shaw JJ, Braga RR, Ishikawa EE. Cutaneous leishmaniasis in the Amazon region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará State, Brazil. Mem Inst Oswaldo Cruz. 1991;86:127–30. [DOI] [PubMed] [Google Scholar]
- 13.Lainson R, Shaw JJ, Souza AA, Silveira FT, Falqueto A. Further observations on Lutzomyia ubiquitalis (Psychodidae: Phlebotominae), the sandfly vector of Leishmania (Viannia) lainsoni. Mem Inst Oswaldo Cruz. 1992;87:437–9. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez Uzcátegui YDV, Vasconcelos dos Santos T, Silveira FT, Ramos PKS, dos Santos EJM, Póvoa MM. Phlebotomines (Diptera: Psychodidae) from an urban park of Belém, Pará State, Northern Brazil and potential implications in the transmission of American cutaneous leishmaniasis. J Med Entomol. 2020;57:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–89. [DOI] [PubMed] [Google Scholar]
- 16.Serafim TD, Coutinho-Abreu IV, Dey R, Kissinger R, Valenzuela JG, Oliveira F, et al. Leishmaniasis: the act of transmission. Trends Parasitol. 2021;37:976–87. [DOI] [PubMed] [Google Scholar]
- 17.Myskova J, Svobodova M, Beverley SM, Volf P. A lipophosphoglycan-independent development of Leishmania in permissive sand flies. Microb Infect. 2007;9:317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira DM, da Silva BJ, de Sena CB, Lima JA, Vasconcelos dos Santos T, Silveira FT, et al. Comparative analysis of carbohydrate residues in the midgut of phlebotomines (Diptera: Psychodidae) from colony and field populations from Amazon. Brazil Exp Parasitol. 2016;168:31–8. [DOI] [PubMed] [Google Scholar]
- 19.Volf P, Myskova J. Sand flies and Leishmania: specific versus permissive vectors. Trends Parasitol. 2007;23:91–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pimenta PF, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, Sacks DL. Stage-specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256:1812–5. [DOI] [PubMed] [Google Scholar]
- 21.Pimenta PF, Saraiva EM, Rowton E, Modi GB, Garraway LA, Beverley SM, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. PNAS. 1994;91:9155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soares RP, Macedo ME, Ropert C, Gontijo NF, Almeida IC, Gazzinelli RT, et al. Leishmania chagasi: lipophosphoglycan characterization and binding to the midgut of the sand fly vector Lutzomyia longipalpis. Mol Biochem Parasitol. 2002;121:213–24. [DOI] [PubMed] [Google Scholar]
- 23.Soares RPP, Margonari C, Secundino NFC, Macêdo ME, Costa SM, Rangel EF, et al. Differential midgut attachment of Leishmania (Viannia) braziliensis in the sand flies Lutzomyia (Nyssomyia) whitmani and Lutzomyia (Nyssomyia) intermedia. J Biomed Biotechnol. 2010;2010:439174. 10.1155/2010/439174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares RP, Altoé ECF, Ennes-Vidal V, da Costa SM, Rangel EF, de Souza NA, et al. In vitro inhibition of Leishmania attachment to sandfly midguts and LL-5 cells by divalent metal chelators, anti-GP63 and phosphoglycans. Protist. 2017;168:326–34. [DOI] [PubMed] [Google Scholar]
- 25.Soares RP, Nogueira PM, Secundino NF, Marialva EF, Ríos-Velásquez CM, Pessoa FAC. Lutzomyia umbratilis from an area south of the Negro River is refractory to in vitro interaction with Leishmania guyanensis. Mem Inst Oswaldo Cruz. 2018;113:202–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coutinho-Abreu IV, Oristian J, de Castro W, Wilson TR, Meneses C, Soares RP, et al. Binding of Leishmania infantum lipophosphoglycan to the midgut is not sufficient to define vector competence in Lutzomyia longipalpis sand flies. mSphere. 2020;5:e00594-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamhawi S, Modi GB, Pimenta PF, Rowton E, Sacks DL. The vectorial competence of Phlebotomus sergenti is specific for Leishmania tropica and is controlled by species specific-specific, lipophosphoglycan-mediated midgut attachment. Parasitol. 2000;121:25–33. [DOI] [PubMed] [Google Scholar]
- 28.Wilson R, Bates MD, Dostalova A, Jecna L, Dillon RJ, Volf P, et al. Stage-specific adhesion of Leishmania promastigotes to sand fly midguts assessed using an improved comparative binding assay. PLoS Negl Trop Dis. 2010;4:e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sánchez Uzcátegui YDV, Dos Santos EJM, Matos ER, Silveira FT, Vasconcelos dos Santos T, Póvoa MM. Artificial blood-feeding of phlebotomines (Diptera: Psychodidae: Phlebotominae): is it time to repurpose biological membranes in light of ethical concerns? Parasit Vectors. 2022;15:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez Uzcátegui YDV, Silveira FT, de Morais TG, Furtado RR, Vasconcelos dos Santos T, Póvoa MM. Experimental susceptibility of Nyssomyiaantunesi and Lutzomyialongipalpis (Psychodidae: Phlebotominae) to Leishmania (Viannia) lainsoni and L. (V.) lindenbergi (Trypanosomatidae: Leishmaniinae). Microorganisms. 2024;12:809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan L, Lainson R, Shaw JJ. Leishmaniasis in Brazil. XXIV Natural flagellate infections of sandflies (Diptera: Psychodidae) in Pará State, with particular reference to the rôle of Psychodopyguswellcomei as the vector of Leishmaniabraziliensisbraziliensis in the Serra dos Carajás. Trans R Soc Trop Med Hyg. 1987;81:353–9. [DOI] [PubMed] [Google Scholar]
- 32.Galati EA. Phlebotominae (Diptera, Psychodidae): classification, morphology and terminology of adults and identification of American taxa. In: Rangel EF, Shaw JJ, editors. Brazilian sand flies: biology, taxonomy, medical importance and control. Cham: Springer; 2018. p. 9–212. [Google Scholar]
- 33.Ayres M, Junior AM. BioEstat 20: aplicações estatísticas nas áreas das ciências biológicas e médicas. Belém: Civil Society Mamirauá; 2000. p. xii–259. [Google Scholar]
- 34.Alexandre J, Sadlova J, Lestinova T, Vojtkova B, Jancarova M, Podesvova L, et al. Experimental infections and co-infections with Leishmania braziliensis and Leishmania infantum in two sand fly species, Lutzomyia migonei and Lutzomyia longipalpis. Sci Rep. 2020;10:3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dostálová A, Volf P. Leishmania development in sand flies: parasite-vector interactions overview. Parasit Vectors. 2012;5:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kock NP, Gabius HJ, Schmitz J, Schottelius J. Receptors for carbohydrate ligands including heparin on the cell surface of Leishmania and other trypanosomatids. Trop Med Int Health. 1997;2:863–74. [DOI] [PubMed] [Google Scholar]
- 37.Myskova J, Dostálová A, Penicková L, et al. Characterization of a midgut mucin-like glycoconjugate of Lutzomyia longipalpis with a potential role in Leishmania attachment. Parasit Vectors. 2016;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.