Abstract
Objectives
This research investigates how the SERPINE1-associated tumor microenvironment influences anti-PD-1 treatment response in gastric carcinoma (GC).
Methods
Bioinformatics analysis, cellular experiments, and animal models were employed to quantify the levels of NFATC2, SERPINE1, JAK3, STAT3, and to explore their associations with various biological behaviors of GC cells, encompassing proliferation, migration, invasiveness, EMT, and immune cell infiltration. Additionally, by constructing a GC tumor-bearing model, we assessed the efficacy of knocking down SERPINE1 in combination with anti-PD-1 therapy.
Results
Elevated SERPINE1 expression in GC correlated with enhanced tumor aggressiveness, lymphatic dissemination, and adverse prognostic indicators. NFATC2, a potential transcription factor of SERPINE1, showed high expression that correlated with poor prognosis in GC patients. NFATC2 orchestrates JAK3/STAT3 pathway activation via SERPINE1 induction, culminating in STAT3 upregulation. Concurrently, STAT3 regulates the upregulation of NFATC2, which in turn further enhances SERPINE1 levels, establishing a positive feedback loop. This loop facilitates the proliferation, clonogenic growth, migration, invasion, and EMT processes of GC cells, thereby accelerating the progression of GC. Additionally, the NFATC2/SERPINE1 axis may facilitate immune evasion in GC by increasing the presence of PD-L1+ M2 macrophages. Importantly, silencing SERPINE1 enhanced the sensitivity of GC xenografts to anti-PD-1 therapy.
Conclusion
Our study reveals the critical function of the NFATC2/SERPINE1/JAK3/STAT3 positive feedback loop in gastric carcinogenesis while identifying its plausible contribution to anti-PD-1 therapy resistance mechanisms.
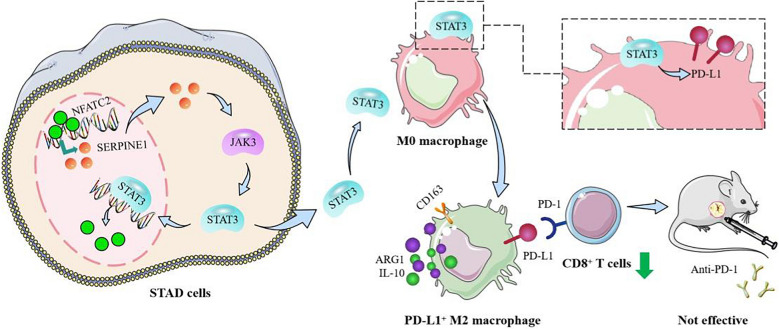
Graphical Abstract
Schematic diagram of NFATC2 in stomach adenocarcinoma. The positive feedback loop of NFATC2/SERPINE1/JAK3/STAT3 reshapes the immune microenvironment to mediate resistance to anti-PD-1 therapy in stomach adenocarcinoma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10565-025-10050-6.
Keywords: NFATC2, SERPINE1, JAK3/STAT3, Gastric cancer, Immune microenvironment, Anti-PD-1 therapy, Drug resistance
Introduction
Gastric carcinoma (GC) remains a predominant global malignancy characterized by elevated incidence and mortality. WHO reports indicate nearly 1 million annual GC diagnoses, with 70% presenting at advanced stages. While conventional therapies (surgery, chemotherapy, radiotherapy) enhance survival outcomes, clinical prognoses remain suboptimal (Siegel et al. 2024). Immune checkpoint inhibitors targeting PD-1/PD-L1 signaling have revolutionized therapeutic strategies by augmenting antitumor immunity through pathway blockade. However, many patients develop resistance to these therapies (Abaza et al. 2023). Research indicates that multiple factors within the tumor microenvironment (TME) may contribute to resistance mechanisms (Zhang et al. 2022). In GC, the infiltration and activation of immunosuppressive cells are key to immune evasion (Wei et al. 2024). M2-type macrophages, for instance, promote tumor growth by secreting factors that inhibit T-cell activity. Therefore, understanding the regulatory mechanisms of immunosuppressive cells in the TME is crucial for overcoming resistance.
SERPINE1 (plasminogen activator inhibitor-1), a serpin superfamily member, is genomically mapped to chromosome 7q21.3-q22 (Haynes et al. 2022). This protein is primarily secreted into the extracellular matrix and bloodstream by endothelial cells, among others, regulating fibrinolysis (Batiha et al. 2022). In cancer, SERPINE1 expression is often upregulated and associated with tumor malignancy, invasiveness, and poor prognosis (Chen et al. 2022a). Elevated SERPINE1 levels in GC clinically correlate with diminished overall and disease-free survival rates (Lv et al. 2023). Pan-cancer analyses demonstrate elevated SERPINE1 expression correlates with adverse prognoses across 21 types of cancer, and its expression levels are related to immune regulatory factors and immune cell infiltration (Zhai et al. 2023). Additionally, high SERPINE1 expression has been validated in clear cell renal cell carcinoma (Li et al. 2023). Therefore, SERPINE1 serves as both an important tumor marker and a potential therapeutic target.
NFATC2 (Nuclear factor of activated T-cells cytoplasmic 2), a calcineurin-regulated transcription factor modulated by calcium signaling, which influences various biological processes, including immune responses and cell proliferation (Maksoud et al. 2021). The role of NFATC2 has been studied in several cancers; for example, dysregulation of NFAT family members promotes malignant growth and cancer development in lung and colorectal cancers (Liu et al. 2019). Additionally, the function of NFATC2 in non-immune cells has been confirmed, such as in colorectal cancer cells, where it regulates the expression of genes including cyclooxygenase-2 (COX-2), thereby affecting the phenotype of tumor cells (Kim et al. 2022). Furthermore, studies demonstrate NFATC2 orchestrates T cell activation and functional programming, thereby modulating differentiation-proliferation dynamics (Zhu et al. 2022). NFATC2 mechanistically drives inflammation-driven colorectal carcinogenesis via IL-6 transcriptional orchestration (Gerlach et al. 2012). In pancreatic cancer, NFATC2 induces heterochromatin formation and silencing by targeting the p15 (INK4b) promoter, thus promoting tumor growth (Baumgart et al. 2012). These findings imply that NFATC2 facilitates tumor progression through modulation of particular gene expression.
The JAK3/STAT3 axis serves as a critical regulator of oncogenic processes, orchestrating fundamental cellular functions including proliferative activity, differentiation programs, and immunomodulation, and has a substantial impact on tumorigenesis, cancer progression, and treatment resistance (Ahmad et al. 2024). Although the roles of NFATC2, SERPINE1, JAK3, and STAT3 in GC have been studied to some extent, it is not fully understood whether there exists a positive feedback loop among them and how this loop specifically contributes to the reshaping of the immune microenvironment and the mediation of resistance to anti-PD-1 therapy. This study examines how the NFATC2/SERPINE1/JAK3/STAT3 autoregulatory circuit modulates immune microenvironment reprogramming and drives anti-PD-1 therapy resistance in GC. This feedback mechanism is proposed to exert essential functions in driving gastric cancer advancement and immune escape, providing a foundation for novel GC treatment approaches.
Methods
Cell culture
GC cell lines (MKN45, HGC27, AGS, MKN1, SNU-1) were acquired from ATCC, while THP1 monocytic leukemia cells were sourced from the Chinese Academy of Sciences Cell Bank. Cells were maintained in RPMI1640/DMEM (Gibco) supplemented with 10% FBS and 1% antibiotic cocktail at 37 °C with 5% CO₂ under humidification.
Human monocytic THP1 cells were maintained in complete growth medium for differentiation protocols, followed by macrophage induction through 100 nM phorbol 12-myristate 13-acetate (PMA) treatment. These cells were cultured in PMA-supplemented medium for 48 h to facilitate macrophage maturation, while GC cells underwent parallel transfection and 48-h incubation. The supernatant from these transfected GC cells was collected. The differentiated THP1-derived macrophages were then co-cultured with this GC cell supernatant. The co-cultured cells were subsequently prepared for analysis, such as flow cytometry and qRT-PCR, to assess relevant biological parameters.
Plasmid construction and viral packaging
Overexpression and knockdown plasmids were constructed using pLVXIRESZsGreen1 (Clontech, USA) and pLVXshRNA1 (Clontech, USA) vectors, respectively. The cDNA sequences of the target genes were synthesized and cloned into the corresponding plasmids. shRNA sequences targeting NFATC2 and SERPINE1 were designed using online tools, with the following sequences:
shRNA sequences for NFATC2
(shNFATC2-1)5'-CCGGAAGCTTCTTGGAGGACCTTTCAAACTCGAGTTTGAAAGGTCC TCCAAGAAGCTTTTTTG-3'.
(shNFATC2-2)5'-CCGGGCTTGTCCAGCTTCTTGGTCTTCAAGAGAGACC AAGAAGCTGGACAAGCTTTTTTG-3'.
(shNFATC2-3)5'-CCGGGAGGACCTTTCAAACTTCTTCAAGAGAGAAGAAGTTTGAAAGGT CTCCTTTTTTG-3'.
shRNA sequences for SERPINE1 (human)
(shSERPINE1-1)5'-CCGGTGGCTGCTGTTTCTGTTTCTTCAAGAGAGAAACAGAAACAGCAGCCA TTTTTTG-3'.
(shSERPINE1-2)5'-CCGGAGCTTGCTTCTGCTTCTTCTTCAAGAGAGAAGAAGCAGAAGCAAGCT TTTTTTG-3'.
shRNA sequences for Serpine1 (mice)
(shSerpine1-1)5′-CCGGTGCTGAGATGTGGAGTTTCTCGAGAACTCCACATCTCAGCACCTTTTTG-3′
(shSerpine1-2)5′-CCGGGAGCTGTCAAGTTCATGTCTCGAGACATGAACTTGACAGCTCTTTTG-3′
Custom-designed shRNA sequences were generated and ligated into the pLVXshRNA1 vector. Recombinant plasmids were co-introduced with packaging vectors (pMD2.G/psPAX2, Addgene) into HEK293 T cells (ATCC) via Lipofectamine 3000-mediated transfection (Invitrogen). Viral supernatants were collected following a 48-h culture period, aseptically filtered, and subsequently employed for target cell transduction.
Cell transfection
Stable cell lines were generated through Lipofectamine 3000-mediated plasmid delivery (Invitrogen), with 48 h culture and 2 μg/mL puromycin selection (Sigma-Aldrich). Selection Conditions: After 2 weeks of selection, overexpressing cell lines were confirmed by observing ZsGreen1 expression under a fluorescence microscope. Knockdown cell lines underwent target gene expression verification via Western blot and qRT-PCR analysis.
qRT-PCR
RNA isolation was performed with TRIzol (Invitrogen), followed by PrimeScript-mediated cDNA synthesis (Takara) and SYBR Premix Ex Taq II-based qRT-PCR amplification on an ABI 7500 platform (Applied Biosystems). Primer sequences are detailed below. NFATC2, F: GCTTCTTGGAGGACCTTTCA; R: CTTGTCCAGCTTCTTGGTCT. SERPINE1, F: TGGCTGCTGTTTCTGTTTCT; R: AGCTTGCTTCTGCTTCTTCT. JAK3, F: CAGGAGGAGGAGGAGGAGGA; R: GCTGCTGCTGCTGCTGCTGC. STAT3, F: TGGAGGAGGAGGAGGAGGAG; R: GCTGCTGCTGCTGCTGCTGC. GAPDH, F: ACCCAGAAGACTGTGGATGG; R: CCTGGAAGATGGTGATGGG.
Western blot
Proteins were extracted using RIPA lysis buffer (Beyotime) with protease inhibitors (Roche). Protein concentrations were quantified via BCA assay (Thermo Fisher), separated on 10% SDS-PAGE gels, and transferred to PVDF membranes (Millipore). Membranes were blocked with 5% non-fat milk in TBST for 1 h before overnight primary antibody incubation at 4 °C. GAPDH (1:5000, Santa Cruz); JAK3/STAT3 (1:1000, CST); NFATC2/SERPINE1/PD-L1 (1:1000, Abcam). Post three TBST washes (10 min each), membranes were probed with HRP-conjugated anti-rabbit IgG (1:5000, Santa Cruz) for 1 h at RT. Chemiluminescent detection utilized an ECL kit (Thermo Fisher) with exposure imaging.
Immunohistochemistry
Tumor specimens underwent 4% paraformaldehyde fixation, paraffin embedding, and 4 μm-thick sectioning. Antigen retrieval was conducted using microwave-mediated citrate buffer heating (10 min). Sections underwent 30-min blocking with 5% BSA prior to overnight 4 °C incubation with primary antibodies: NFATC2/SERPINE1/CD163 (1:100, Abcam). Following three PBS washes (10 min each), sections were exposed to HRP-conjugated anti-rabbit IgG (1:200, Santa Cruz) for 1 h at RT. Tissue sections were developed with a DAB staining system (Beyotime, China), underwent hematoxylin counterstaining, and finally mounted in neutral resin.
Cell viability assay (CCK-8)
Cells were plated in 96-well culture dishes at 5 × 103 cells/well, with experimental groups prepared in triplicate. Post-attachment, cells were exposed to experimental regimens involving pharmacological agents or plasmid constructs. Following 48-h exposure, 10 μL CCK-8 solution was administered per well, with subsequent 2-h maintenance. OD450 measurements were conducted using a Thermo Fisher microplate reader.
Colony formation assay
Cells were cultured in 6-well plates at 1 × 103/well density, with experimental groups prepared in triplicate. Post-adhesion, cells were subjected to dose-escalated drug/plasmid treatments. Cellular colonies were maintained for 10–14 days until macroscopic emergence. Colonies were processed with 4% PFA (15 min), crystal violet-stained (0.1%, 30 min), then analyzed by imaging/quantification.
Transwell assays
Invasion assays were performed via plating 5 × 104 cells in serum-deprived medium (200μL) into Matrigel-precoated Transwell inserts (Corning), using 10% FBS chemoattractant (600μL) in lower chambers. Following 24-h incubation, non-invasive cells were mechanically cleared from chamber surfaces, while migrated cells underwent 15-min 4% paraformaldehyde fixation and 30-min 0.1% crystal violet staining prior to imaging and quantification, following identical migration assay protocols.
Flow cytometry
Cells were seeded in 6-well plates at 1 × 10⁶ per well and subjected to 48-h treatment. Following PBS washing, primary antibodies (CD163/PD-L1: 1:100, Abcam; CD8: 1:100, BD Biosciences) were incubated at 4 °C for 30 min. Following 3 × 5 min PBS rinses, Alexa Fluor 488-goat anti-rabbit IgG (1:500; #Invitrogen) was probed for 30 min at RT under darkness. Flow cytometry analysis was conducted on a BD FACSCalibur system, with data processed through FlowJo software.
ELISA
Culture supernatants were harvested and clarified by centrifugation to pellet cellular debris. Cytokine quantification (TGF-β/IL-10) was performed with ELISA kits (R&D Systems) following manufacturer protocols. Optical density measurements were assessed spectrophotometrically (Thermo Fisher Scientific).
Animal experiments
Male BALB/c mice (6–8-week-old, 18–20 g) were procured from Shanghai SLAC Laboratory Animal Co.; SAHFMMU Ethics Committee-approved protocols were implemented; AGS cells (1 × 10⁶ cells/100μL PBS) were subcutaneously inoculated into right axillary regions, followed by tumor harvesting and weight quantification. BALB/c nude mice were used to establish xenografts with human GC cells, as their immunodeficient status minimizes immune rejection of human tumor cells.
Male C57BL/6 J mice (6-week-old) were housed under SPF conditions with unbiased allocation to four experimental cohorts. Six days prior to treatment, 1 × 106 YTN16 cells stably expressing sh-NC or sh-Serpine1 were implanted subcutaneously on the right flank of the mice, allowed to grow, with five mice per group (n = 5). C57BL/6 J mice were used for anti-PD-1 therapy experiments due to their intact immune system, allowing evaluation of immunotherapeutic effects on tumor-immune interactions. Mice were administered anti-PD-1 antibody (10 mg/kg, Bio X Cell, USA) or isotype control IgG antibody (10 mg/kg, Sigma-Aldrich, USA) twice weekly for a total of five injections. Tumor volume was determined using the conventional ellipsoid equation: (L × W2)/2. Upon study completion, animals were humanely sacrificed for tumor tissue collection, with subsequent weighing and processing for downstream analyses.
Bioinformatics analysis
Bioinformatics analyses utilized the following resources: 1) TIMER (https://cistrome.shinyapps.io/timer/) for SERPINE1 expression profiling and immune infiltration correlations in GC. 2) CIBERSORT algorithm (default parameters) to quantify 22 immune cell subtypes from TCGA data. 3) Spearman's correlation analysis (p < 0.05 significance threshold) between SERPINE1 and immune infiltration levels. 4) UALCAN (http://ualcan.path.uab.edu/) validating SERPINE1 upregulation in GC. 5) GEPIA (http://gepia.cancer-pku.cn/) assessing NFATC2-SERPINE1 and NFATC2-STAT3 co-expression patterns. 6) JASPAR (http://jaspar.genereg.net/) identifying NFATC2 DNA-binding motifs. 7) Protein Atlas (https://www.proteinatlas.org/) comparing SERPINE1 expression in GC vs normal tissues. Data processing and visualization were performed using R language and Bioconductor packages. The main analytical steps included data normalization, differential expression analysis, correlation analysis, and survival analysis.
Luciferase reporter assay
Pre-transfection preparation involved plating AGS or MKN1 cells (2 × 105 cells/well) in 6-well plates. Transfection complexes were formulated by combining individual plasmid DNA (2 μg) with Renilla luciferase reporter plasmid (Promega, 0.3 μg) and Lipofectamine 3000 transfection reagent (Germany, 10 μL). After 20 min equilibration under ambient conditions (25 °C), PBS-rinsed cellular monolayers received the DNA-lipid complexes, followed by 6 h incubation under standard culture parameters (5% CO2, 37 °C). Post-transfection media exchange was implemented using serum-supplemented growth medium. In combinatorial transcriptional factor studies, parallel transfections with pGL3 null vector (Promega) served as baseline controls. Throughout experimental manipulations, equivalent DNA dosages (2 μg/well) were maintained across treatment groups. Transgene-expressing cells received triple PBS rinses prior to 48 h extended culture. Cytolytic harvest was performed using passive lysis buffer (Promega), followed by quantification of bioluminescent signals via Dual-Luciferase Reporter technology (Promega) using spectrophotometric detection. All enzymatic measurements were executed in biological duplicates with technical triplicates, ensuring ≥ 2 independent experimental replicates.
Statistical analysis
All experiments were performed in triplicate with data presented as mean ± SD. Statistical analyses employed GraphPad Prism 9.5, where intergroup comparisons utilized Student's t-tests and multigroup analyses implemented ANOVA with Tukey's post hoc tests for multigroup analyses, with significance defined as p < 0.05.
Results
High expression of SERPINE1 in gastric cancer
Bioinformatic analysis via TIMER revealed elevated the expression of SERPINE1 in GC versus normal tissues (Fig. 1A). UALCAN validation confirmed its upregulation in GC specimens (Fig. 1B). Stage-dependent escalation analysis demonstrated progressive SERPINE1 elevation, particularly showing marked increases from Stage III to IV (Fig. 1C). This suggests that SERPINE1 may be associated with tumor progression or malignancy. With increasing tumor grade, the expression of SERPINE1 showed a gradual upward trend, indicating that SERPINE1 may be related to the malignancy of gastric cancer, i.e., SERPINE1 expression escalates proportionally with tumor grade progression (Fig. 1D). Figure 1E revealed SERPINE1's progressive upregulation in GC lymph node metastasis (normal/N0: low; N1-N3: high; peak in N3). Survival analyses demonstrated significant clinical correlations: elevated SERPINE1 predicted reduced overall survival (OS, Fig. 1F) and shortened disease-free survival (DFS, Fig. 1G) compared to low-expression cohorts (p < 0.05). Differential expression analysis identified upregulated SERPINE1 mRNA in GC versus normal tissues (p < 0.0001, Fig. 1H). Protein-level validation through western blot (Fig. 1I) and immunohistochemistry (Fig. 1J) confirmed concordant overexpression patterns. These results implicate SERPINE1 in gastric carcinogenesis progression, demonstrating clinical correlations with advanced tumor staging, unfavorable survival outcomes, and therapeutic target potential.
Fig. 1.
Expression of SERPINE1 in Gastric Cancer and Its Correlation with Clinical Features. (A-B) Bioinformatics via TIMER (A) and UALCAN (B) databases for SERPINE1 mRNA in GC vs. normal tissues. (C-E) Correlation of SERPINE1 with cancer stage (C), tumor grade (D), and lymph node metastasis (E) via one-way ANOVA. (F-G) Kaplan–Meier survival analysis (log-rank test) for SERPINE1 vs. overall survival (F) and DFS (G). (H) qRT-PCR of SERPINE1 mRNA in GC and normal tissues (Student’s t-test). (I) Western blot analysis of SERPINE1 protein expression in GC and normal tissues. (J) IHC staining for SERPINE1 in GC and normal tissues. **p < 0.01
NFATC2 as a potential transcription factor of SERPINE1
To investigate SERPINE1's regulatory mechanisms in gastric carcinogenesis, we used the PROMO database to predict its potential transcription factors, and NFATC2 was identified as a potential transcription factor of SERPINE1. Using the JASPAR database, we obtained the DNA sequence motif bound by NFATC2 (Fig. 2A). The Bioinformatic analyses (GEPIA/TIMER) revealed NFATC2 overexpression in GC with positive SERPINE1 co-expression (Fig. 2B-C). Western blot analysis confirmed elevated NFATC2 protein levels across GC cell lines (AGS/SNU-1/HGC27/MKN45/MKN1), showing maximal expression in AGS and MKN1 (Fig. 2D), which were selected for subsequent experiments. NFATC2 silencing significantly reduced SERPINE1 expression at transcriptional (p < 0.01, Fig. 2E) and translational levels (p < 0.01, Fig. 2F). However, knockdown of SERPINE1 did not result in significant changes in NFATC2 at either the mRNA (Fig. 2G) or protein level (Fig. 2H), indicating that NFATC2 is upstream of SERPINE1. Dual-luciferase reporter assays showed that NFATC2 exerted its biological effects by reducing the activity of the luciferase reporter gene, and this effect was abolished or weakened in the mutant construct (Fig. 2I). ChIP-qPCR experiments further confirmed that NFATC2 protein was significantly enriched in the promoter region of SERPINE1 in AGS and MKN1 cell lines, with higher enrichment in MKN1 cells (Fig. 2J).
Fig. 2.
NFATC2 as a Potential Transcription Factor of SERPINE1. (A) Prediction of NFATC2-binding DNA motif in the SERPINE1 promoter via JASPAR database. (B-C) Correlation analysis of NFATC2 and SERPINE1 expression in GC using GEPIA (B) and TIMER (C) databases. (D) Western blot detection of NFATC2 protein levels in human GC cell lines (AGS, SNU-1, HGC27, MKN45, MKN1). (E–F) qRT-PCR (E) and Western blot (F) analysis of SERPINE1 expression after NFATC2 knockdown in AGS cells (shNFATC2 vs. shNC), performed via Student’s t-test. (G-H) qRT-PCR (G) and Western blot (H) analysis of NFATC2 expression after SERPINE1 knockdown in AGS cells (shSERPINE1 vs. shNC), via Student’s t-test. (I) Dual-luciferase reporter assay to validate NFATC2 binding to the SERPINE1 promoter in AGS cells (wild-type vs. mutant constructs). (J) ChIP-qPCR analysis of NFATC2 enrichment in the SERPINE1 promoter region in AGS and MKN1 cells. **p < 0.01, ***p < 0.001
These findings establish NFATC2 as a direct transcriptional regulator of SERPINE1, critically involved in gastric carcinogenesis and clinical progression. Mechanistically, NFATC2 overexpression drives oncogenic advancement and adverse clinical outcomes through SERPINE1 upregulation.
Regulation of gastric cancer malignant phenotype by SERPINE1-mediated NFATC2
Given that NFATC2 is a potential transcription factor of SERPINE1 and SERPINE1 potentially contributes to gastric carcinogenesis, while the role of NFATC2 in GC remains to be explored, we conducted functional rescue experiments to investigate whether SERPINE1 is involved in the regulation of malignant biological behaviors of GC cells by NFATC2. Lentiviral transfection was used to achieve overexpression of SERPINE1 in AGS and MKN1 cells after knocking down NFATC2. Figure 3A demonstrates NFATC2 silencing suppressed SERPINE1 expression, whereas SERPINE1 ectopic expression rescued this regulatory effect. CCK-8 assays revealed NFATC2 knockdown impaired GC cell proliferative capacity, which was counteracted by SERPINE1 overexpression. Additionally, overexpression of SERPINE1 partially restored the decrease in cell viability caused by NFATC2 knockdown, suggesting that SERPINE1 serves as a key effector in NFATC2-mediated regulation of cellular proliferative capacity (Fig. 3B). Clonogenic analysis demonstrated NFATC2 silencing impaired GC cell colony formation, while overexpression of SERPINE1 increased the colony-forming ability. Furthermore, overexpression of SERPINE1 partially restored the decrease in colony-forming ability caused by NFATC2 knockdown, further indicating that SERPINE1 plays a role in the regulation of cell proliferation mediated by NFATC2 (Fig. 3C). Transwell assays revealed NFATC2 knockdown suppressed gastric cancer cell migration/invasion, while SERPINE1 overexpression potentiated these malignant phenotypes. Moreover, SERPINE1 overexpression partially rescued the reduction in migration and invasion abilities induced by NFATC2 silencing, indicating that SERPINE1 is involved in regulating cell migration and invasion mediated by NFATC2 (Fig. 3D). Immunoblotting of EMT markers demonstrated NFATC2 ablation attenuated EMT progression in gastric cancer cells, manifested through E-cadherin elevation with concomitant N-cadherin/Vimentin reduction. Overexpression of SERPINE1 partially promoted this EMT process (Fig. 3E). Overall, our results show that SERPINE1 overexpression enhances GC cell survival, proliferation, migration, and invasion, and promotes the EMT process, while NFATC2 knockdown has opposite effects. Significantly, SERPINE1 overexpression can partially reverse the impacts of NFATC2 knockdown on these biological processes of GC cells.
Fig. 3.
Regulation of Gastric Cancer Malignant Phenotype by SERPINE1-mediated NFATC2. (A) Lentiviral transfection for NFATC2 knockdown (shNFATC2) and SERPINE1 overexpression (OE-SERPINE1) in AGS cells, followed by Western blot to detect SERPINE1 protein levels. (B) CCK-8 assay to evaluate cell viability after NFATC2 knockdown and/or SERPINE1 overexpression in AGS cells. (C) Colony formation assay to assess clonogenic potential under NFATC2 knockdown and/or SERPINE1 overexpression conditions in AGS cells. (D) Transwell migration and invasion assays to measure motility of AGS cells with NFATC2 knockdown and/or SERPINE1 overexpression. (E) Western blot analysis of EMT-related proteins (E-cadherin, N-cadherin, Vimentin) in AGS cells after NFATC2 knockdown and/or SERPINE1 overexpression. *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. sh-NFATC2#1
SERPINE1 is associated with tumor microenvironment immune infiltration
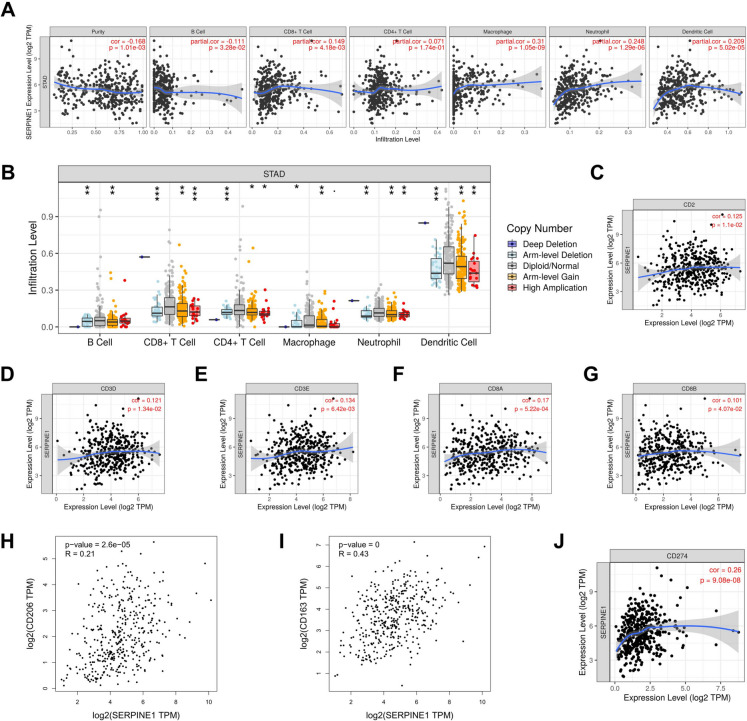
Following the examination of SERPINE1’s role in mediating NFATC2-regulated malignant biological behaviors of GC cells, we additionally explored SERPINE1’s role in tumor microenvironment immune infiltration. Figure 4A shows the association between SERPINE1 gene expression levels and infiltration levels of different immune cell types in GC. Findings reveal that SERPINE1 expression is significantly linked to infiltration levels of certain immune cells, particularly showing a positive correlation with macrophage, neutrophil, and dendritic cell infiltration. Figure 4B demonstrates that copy number variations (CNVs) of the SERPINE1 gene are closely associated with the infiltration levels of multiple immune cells in GC. Elevated SERPINE1 copy number alterations demonstrated reduced CD8+ T cell and dendritic cell infiltration; contrastingly, neutrophils exhibited mild elevation. This suggests that SERPINE1 amplification may correlate with immune evasion via suppressing cytotoxic T cell recruitment. This suggests that CNVs of the SERPINE1 gene may influence the immune response in the GC microenvironment, thereby affecting disease progression and development. Figure 4C-G reveal associations between SERPINE1 gene expression levels and expression levels of CD2, CD3D, CD3E, CD8 A, and CD8B in GC. Results indicate SERPINE1 expression is positively correlated with these markers, with the strongest correlation noted for CD8 A. This suggests SERPINE1 expression in GC may be linked to T cell activity or presence, particularly CD8+ T cells, with potential implications for the immune microenvironment and disease progression in GC. Figure 4H-I demonstrate the associations between SERPINE1 gene expression and CD206/CD163 expression levels in GC. Results reveal that SERPINE1 expression is positively correlated with both CD206 and CD163, with a stronger association observed for CD163. Both markers are indicative of M2-type macrophages, suggesting that the expression of SERPINE1 in GC may be related to the presence or activity of tumor-associated macrophages (TAMs). Figure 4J illustrates the association between SERPINE1 and CD274 (PD-L1) in GC, showing a modest positive correlation between SERPINE1 and PD-L1 expression levels. This may imply a regulatory link between SERPINE1 and PD-L1 expression in the GC immune microenvironment. Given that PD-L1 is a target for immune checkpoint inhibitors and SERPINE1 is a protein involved in immune regulation and tumor progression, this positive correlation may have important implications for immunotherapy strategies in GC.
Fig. 4.
Relationship Between SERPINE1 and Immune Infiltration in the Tumor Microenvironment. A. Correlation between SERPINE1 gene expression levels and infiltration levels of different immune cells. B. Relationship between SERPINE1 copy number variations (CNVs) and infiltration levels of various immune cells. C-G. Correlation between SERPINE1 gene expression levels and CD2, CD3D, CD3E, CD8 A, and CD8B expression levels. H-I. Correlation between SERPINE1 gene expression levels and CD206 and CD163 expression levels. J. Relationship between SERPINE1 and CD274 (PD-L1) in GC, showing a slight positive correlation between their expression levels
NFATC2 induces macrophage polarization via SERPINE1
To further validate the roles of NFATC2 and SERPINE1 on macrophage polarization, complementary experimental validation was performed through in vitro and in vivo mechanistic approaches. GC cells were co-cultured with macrophages (THP1/M0) as depicted in Fig. 5A. Subsequently, flow cytometric profiling and quantitative RT-PCR analysis were implemented to quantify expression dynamics of macrophage surface markers, including CD163, ARG1, and IL10. Quantitative RT-PCR analysis demonstrated NFATC2 silencing downregulated the expression of CD163, ARG1, and IL10, while overexpression of SERPINE1 increased the expression of these genes. Additionally, overexpression of SERPINE1 partially restored the decrease in the expression of these genes caused by NFATC2 knockdown, indicating that SERPINE1 plays a role in NFATC2-mediated M2 polarization of macrophages (Fig. 5B). Flow cytometry analysis revealed that NFATC2 silencing decreased the proportion of CD206+ macrophages (typically associated with M2-type macrophages) induced by GC cells, while overexpression of SERPINE1 increased this percentage. Similarly, SERPINE1 overexpression partially restored the decreased percentage of CD206-positive macrophages caused by NFATC2 knockdown. This indicates that SERPINE1 is involved in NFATC2-mediated regulation of M2 macrophage polarization (Fig. 5C).
Fig. 5.
NFATC2 Induces Macrophage Polarization via SERPINE1. (A) Schematic of co-culture system between GC cells (AGS/MKN1) and THP1-derived M0 macrophages. (B) qRT-PCR analysis of M2 macrophage markers (CD163, ARG1, IL10) in THP1 cells co-cultured with AGS cells transfected with shNFATC2 and/or OE-SERPINE1. (C) Flow cytometry to quantify CD206 + M2 macrophages after NFATC2 knockdown and/or SERPINE1 overexpression in GC cells. (D-E) Nude mouse xenograft model: tumor images (D) and weights (E) after subcutaneous injection of AGS cells with shNC, shNFATC2, OE-SERPINE1, or shNFATC2 + OE-SERPINE1 (n = 5/group). (F) Immunohistochemistry staining for CD163 in xenograft tumors to assess M2 macrophage infiltration. (G) ELISA detection of TGF-β and IL-10 secretion in co-culture supernatants or tumor lysates. △p < 0.05, △△△p < 0.001 vs. Blank; *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. sh-NFATC2#1
In vivo gastric cancer xenograft studies utilized athymic murine models (n = 5), stratified into four experimental cohorts: NC, shNFATC2#1, OE-SERPINE1, and shNFATC2#1 + OE-SERPINE1. The xenograft tumor images (Fig. 5D) and the changes in tumor weight under different treatment conditions (Fig. 5E) showed that knockdown of NFATC2 significantly inhibited tumor growth, while overexpression of SERPINE1 significantly promoted tumor growth. When both NFATC2 knockdown and SERPINE1 overexpression were performed, the tumor weight increased but did not reach the level seen with SERPINE1 overexpression alone, suggesting that the absence of NFATC2 may partially counteract the promoting effect of SERPINE1 overexpression on tumor growth. Further immunohistochemistry results (Fig. 5F) confirmed these findings. Macrophage polarization is regulated by various cytokines, with TGF-β and IL-10 being two important pro-inflammatory factors that play key roles in M2-type macrophage polarization (Yeh et al. 2018; Li et al. 2022). ELISA results for TGF-β and IL-10 (Fig. 5G) showed that knockdown of NFATC2 reduced the secretion of these cytokines, while overexpression of SERPINE1 increased their secretion. Even under conditions of NFATC2 knockdown, overexpression of SERPINE1 still promoted the secretion of TGF-β and IL-10, although the effect was somewhat diminished. These results provide crucial mechanistic insights into NFATC2/SERPINE1-mediated gastric carcinogenesis and their immunomodulatory functions, identifying novel therapeutic targets for GC intervention. Collectively, our data establish NFATC2/SERPINE1 as critical regulators of gastric oncogenesis via M2 macrophage polarization to influence tumor growth and microenvironment, potentially offering novel therapeutic targets for gastric cancer.
NFATC2/SERPINE1/JAK3/STAT3 signaling forms a positive feedback loop
To explore the mechanisms by which NFATC2 and SERPINE1 regulate GC, we conducted multiple experiments. Western blot results showed that silencing the NFATC2 gene could inhibit the activation of the JAK3/STAT3 pathway, while overexpression of the SERPINE1 gene promoted the activation of this pathway (Fig. 6A). Further analysis using the TIMER and GEPIA databases revealed that STAT3 and NFATC2 expression levels were positively correlated in GC (Fig. 6B-C). STAT3 overexpression induced NFATC2 mRNA upregulation (Fig. 6D). Conversely, when the STAT3 gene was specifically silenced using siRNA interference technology, the expression level of NFATC2 mRNA significantly decreased (Fig. 6E). Our analysis using the JASPAR database identified 93 potential STAT3 binding sites within the NFATC2 promoter (score = 10.21011), with the conserved binding motifs depicted in Fig. 6F. Dual-luciferase reporter systems revealed STAT3 overexpression markedly enhanced WT promoter-driven transcriptional activity (Fig. 6G). This effect was abrogated when mutations were introduced into the promoter to disrupt STAT3 binding sites (Fig. 6G). Chromatin immunoprecipitation (ChIP)-qPCR assays validated the direct interaction between STAT3 and the NFATC2 promoter. Specifically, the anti-STAT3 antibody showed enrichment of promoter DNA fragments relative to control IgG, and this enrichment was further enhanced under conditions of STAT3 overexpression (Fig. 6H). Collectively, these findings confirm that STAT3 functions as a transcription factor for NFATC2, directly activating its promoter to drive gene expression. Our findings further demonstrate SERPINE1 enhances NFATC2 expression via JAK3/STAT3 activation, forming a self-reinforcing positive regulatory circuit that drives gastric carcinogenesis.
Fig. 6.

SERPINE1 Promotes NFATC2 Expression via Activation of the JAK3/STAT3 Signaling Pathway, Forming a Positive Feedback Loop. (A) Western blot analysis of JAK3/STAT3 signaling pathway activation (p-JAK3, p-STAT3) in AGS cells with NFATC2 knockdown (shNFATC2) or SERPINE1 overexpression (OE-SERPINE1). (B-C) GEPIA/TIMER database correlation analysis of STAT3 and NFATC2 expression in GC. (D-E) qRT-PCR detection of NFATC2 mRNA levels after STAT3 overexpression (D) or knockdown (E) in AGS cells, via Student’s t-test. (F) JASPAR database prediction of STAT3-binding motifs in the NFATC2 promoter. (G) Dual-luciferase reporter assay of wild-type (WT) and mutant (Mut) NFATC2 promoter constructs in the presence of STAT3 overexpression. (H) ChIP-qPCR assay of STAT3 binding to the NFATC2 promoter in AGS cells. (I) Western blot for p-JAK3/p-STAT3 in AGS cells with OE-NFATC2 and/or sh-JAK3. (J-L) CCK-8 (J), colony formation (K), and Transwell (L) assays to evaluate cell viability, proliferation, and migration/invasion under NFATC2 overexpression and JAK3 inhibition. (M) Western blot analysis of EMT markers (E-cadherin, N-cadherin, Vimentin) in AGS cells with OE-NFATC2 and/or sh-JAK3. *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. OE-NFATC2
To further validate the relationship between NFATC2 and the JAK3/STAT3 signaling pathway, AGS cells were divided into four groups: NC, OE-NFATC2, sh-JAK3, and OE-NFATC2 + sh-JAK3. Western blot analysis (Fig. 6I) demonstrated NFATC2 overexpression upregulated JAK3/STAT3 phosphorylation, whereas JAK3 silencing abrogated this signaling activation. These findings suggest NFATC2 contributes to gastric carcinogenesis via JAK3/STAT3 pathway activation, as evidenced by CCK-8 assays (Fig. 6J) results showed that knocking down JAK3 could reverse the enhancement of GC cell viability caused by overexpression of NFATC2. Colony formation assays (Fig. 6K) indicated that NFATC2 overexpression enhanced cell proliferation and clonogenic ability, while JAK3 knockdown reduced these capabilities. Transwell migration and invasion assays (Fig. 6L) showed that NFATC2 overexpression promoted GC cell migration and invasion, whereas JAK3 depletion attenuated this promoting effect. Western blot analysis of EMT-related proteins (Fig. 6M) revealed that NFATC2 overexpression induced EMT, characterized by E-cadherin suppression with concomitant N-cadherin/Vimentin induction, while JAK3 knockdown reversed this process with opposite protein changes. Collectively, these data demonstrate NFATC2-mediated JAK3/STAT3 activation through SERPINE1, resulting in STAT3 elevation. Simultaneously, STAT3 regulates the upregulation of NFATC2, which further upregulates SERPINE1, forming a positive feedback loop. This oncogenic circuit drives malignant phenotypes (proliferation/clonogenicity), metastatic potential, and EMT progression in gastric cancer cells, fueling disease advancement.
SERPINE1/JAK3/STAT3 increasesPD-L1+ M2 macrophages
After exploring the mechanism by which SERPINE1 promotes GC progression by upregulating NFATC2 through the activation of the JAK3/STAT3 signaling pathway, we further investigated the role of this signaling pathway in the tumor immune microenvironment, particularly its effects on PD-L1+ M2 macrophages. GEPIA database interrogation revealed significant STAT3-PD-L1 co-expression in gastric cancer (Fig. 7A). To further explore this relationship, we designed co-culture experiments, as shown in Fig. 7B, AGS GC cells with STAT3 silencing were cocultured with THP1 macrophages. Immunoblotting demonstrated STAT3 knockdown suppressed PD-L1 expression (Fig. 7C). In further co-culture experiments, we examined the proportion of PD-L1+ M2 macrophages after co-culturing GC cells with macrophages. Flow cytometry analysis revealed that NFATC2 overexpression significantly enhances PD-L1 expression in M2 macrophages, while JAK3 knockdownexerted an opposing effect. Notably, JAK3 depletion partially reversed the NFATC2 overexpression-driven upregulation of PD-L1 (Fig. 7D). These findings suggest STAT3 potentially facilitates GC immune evasion through PD-L1 induction, while NFATC2 promotes macrophage PD-L1 expression and M2 polarization via JAK3 to remodel the tumor immune landscape.
Fig. 7.
The SERPINE1/JAK3/STAT3 Pathway Increases PD-L1+ M2 Macrophages and Inhibits CD8+ T Cell Activation via the PD-1/PD-L1 Interaction. (A) Pearson correlation analysis via GEPIA database to assess STAT3 and PD-L1 (CD274) expression correlation in GC. (B) Schematic of THP1 macrophage-AGS cell co-culture system for immune phenotype analysis. (C) Western blot detection of PD-L1 protein levels in THP1 cells after STAT3 knockdown (si-STAT3 vs. si-NC), analyzed by Student’s t-test. **p < 0.01, ***p < 0.001 vs. si-NC. (D) Flow cytometry to quantify PD-L1+ CD206+ M2 macrophages and CD8+ T cell activation markers in co-cultures with AGS cells overexpressing NFATC2 and/or knocking down JAK3, performed via one-way ANOVA. △△p < 0.01, △△△p < 0.001 vs. Blank; *p < 0.05, **p < 0.01, ***p < 0.001 vs. NC; #p < 0.05, ##p < 0.01 vs. OE-NFATC2
Knockdown of serpine1 sensitizes gastric cancer xenografts to anti-PD-1 therapy
After gaining a deeper understanding of the regulatory role of the SERPINE1/JAK3/STAT3 signaling pathway in the immune microenvironment of GC, we further explored the impact of Serpine1 knockdown on the sensitivity of GC xenografts to anti-PD-1 therapy. Utilizing a lentiviral-mediated gene silencing approach, we downregulated Serpine1 expression in YTN16 cells, followed by assessing protein levels via Western blot analysis (Fig. 8A). C57BL/6 J mice received subcutaneous injections of YTN16 cells with stable shRNA constructs (sh-NC controls or sh-Serpine1) in the right flank. The mice were then treated with anti-PD-1 antibodies or isotype-matched control IgG as per the experimental protocol (Fig. 8B). Figure 8C displays the size and shape of tumors in different treatment groups, including sh-NC (negative control), sh-Serpine1 (Serpine1 gene knockdown), anti-PD-1 (anti-PD-1 treatment), and sh-Serpine1 + anti-PD-1 (Serpine1 gene knockdown plus anti-PD-1 treatment). The changes in tumor volume over time, showed that compared to the sh-NC group, the sh-Serpine1 and anti-PD-1 groups had slower tumor volume growth, and the sh-Serpine1 + anti-PD-1 group had the slowest tumor volume growth, further confirming the synergistic effect of the combined treatment (Fig. 8D). The tumor weights indicated that Serpine1 knockdown inhibited tumor growth, and anti-PD-1 treatment also showed some inhibitory effect, with the lowest tumor weight in the sh-Serpine1 + anti-PD-1 group, suggesting a synergistic effect in inhibiting tumor growth (Fig. 8E). Serpine1 knockdown combined with PD-1 blockade synergistically enhanced tumor-infiltrating CD4+/CD8+ T cell populations, with the most significant effect observed in the combination treatment (Fig. 8F). Collectively, these findings demonstrate dual targeting synergistically suppresses gastric cancer progression by augmenting antitumor immunity.
Fig. 8.
Knockdown of SERPINE1 Sensitizes Gastric Cancer Xenografts to Anti-PD-1 Therapy. (A) Western blot validation of SERPINE1 knockdown in YTN16 cells via lentiviral shRNA (sh-SERPINE1 vs. sh-NC). (B) Experimental design: C57BL/6 J mice inoculated with sh-NC or sh-SERPINE1 YTN16 cells, treated with anti-PD-1 antibody (10 mg/kg) or IgG control (n = 5/group). (C) Representative tumor images from each treatment group (sh-NC, sh-SERPINE1, anti-PD-1, sh-SERPINE1 + anti-PD-1). Tumor weights (D) and growth curves (E) measured over 28 days, analyzed by one-way ANOVA. (F) Flow cytometry analysis of CD8+ T cell infiltration in tumors from each group. **p < 0.01, ***p < 0.001
Discussion
Clinical evidence links SERPINE1 overexpression in gastric cancer to advanced-stage progression (lymph node metastasis; unfavorable prognosis). Chen et al. further established SERPINE1 overexpression as an independent predictor of reduced overall and disease-free survival in GC cohorts (Chen et al. 2022a). Integrative bioinformatics analysis of transcriptomic data (microarray/TCGA) revealed significant SERPINE1 upregulation in gastric cancer versus normal counterparts. Kaplan–Meier survival curves correlated elevated SERPINE1 with reduced overall/disease-free survival, with parallel observations in colorectal cancer progression and prognosis (Wang et al. 2021). Emerging evidence demonstrates SERPINE1 dysregulation across pan-cancer malignancies, modulating tumor immunity and driving oncogenic progression (Li et al. 2023). Collectively, these data indicate SERPINE1 serves as a pan-oncogenic regulator, highlighting its actionable therapeutic target value in gastric cancer.
Our findings indicated that NFATC2 acted as a critical transcription factor for SERPINE1, significantly impacting the progression and prognosis of GC. Our study demonstrated that elevated expression of NFATC2 may facilitate the progression of GC by upregulating SERPINE1 expression, which is associated with poor patient prognosis. This study elucidates NFATC2's novel mechanistic role in gastric carcinogenesis and pinpoints SERPINE1 as a therapeutic target, expanding upon established calcineurin-regulated nuclear shuttling mechanisms that drive oncogenic transcription (Bourajjaj et al. 2008). Our results are consistent with these findings, further emphasizing the multifunctionality of NFATC2 in tumor biology. Lang et al. demonstrated NFATC2 upregulation in colorectal cancer correlates with tumor progression and sustains stemness in CRC stem cells (Lang et al. 2018). NFATC2 sustains colorectal cancer stemness through AJUBA elevation and YAP dephosphorylation-mediated transcriptional activation, while in lung cancer it mediates tumor-initiating cell preservation and chemoresistance (Yang et al. 2022). Specifically, NFATC2 enhances drug resistance and tumor-initiating ability in lung cancer cells by binding to the SOX2 enhancer and upregulating ALDH expression. These findings suggest that NFATC2 may function through different mechanisms in various cancer types. Our study further expands the functional scope of NFATC2, particularly in GC. We found that NFATC2 may directly regulate the expression of This study identifies NFATC2-mediated SERPINE1 regulation as a novel mechanistic axis in gastric cancer. SERPINE1, an oncogenic factor upregulated in GC, correlates with aggressive disease progression and poor prognosis, establishing NFATC2 as a pivotal regulator of gastric carcinogenesis through SERPINE1 modulation.
Our follow-up studies further confirmed that NFATC2 drives the proliferation, migration, invasion, and EMT process of GC cells by upregulating SERPINE1 expression. Knockdown of either NFATC2 or SERPINE1 markedly suppressed the malignant cellular behaviors of GC cells. This finding deepens our understanding of the molecular mechanisms underlying GC and provides a critical theoretical foundation for developing novel therapeutic strategies. Related studies (Yang et al. 2019; Kuai et al. 2023) have shown that SERPINE1 is critical in GC cell invasion and metastasis by modulating the expression of EMT-related genes such as FN1, TIMP1, MMP2, and SPARC. Significantly, these genes exhibit positive correlation with SERPINE1, and their overexpression in GC patients is strongly linked to poor prognosis (Xu et al. 2019). Our findings confirm SERPINE1's immunomodulatory role in gastric cancer through tumor microenvironment remodeling, promoting M2 macrophage polarization, suppressing CD8+ T cell responses, and facilitating immune evasion. Related research indicates that SERPINE1 shows abnormal expression in many cancers and is strongly correlated with tumor mutational burden (TMB), immune cell infiltration, microsatellite instability (MSI), immune checkpoint genes, and the ESTIMATE score, which evaluates stromal and immune cell content in tumor tissues using expression data (Lv et al. 2023; Li et al. 2023; Gao et al. 2023; Ren et al. 2023). These findings propose SERPINE1 modulates tumor immune evasion through orchestrating immune cell infiltration dynamics. Ye et al. elucidated gastric cancer-derived SERPINE1 functions as a central oncogenic effector, mediating exosomal let-7 g-5p trafficking via autocrine JAK2/STAT3 axis activation to drive both tumor progression and TAM M2 polarization (Ye et al. 2025). Our findings corroborate SERPINE1's capacity to drive M2 macrophage polarization. Melanoma analyses further demonstrate SERPINE1 overexpression correlates with enhanced immune infiltration and upregulated immune checkpoints (PD-1/CTLA-4) (Samarkina et al. 2023; Zila et al. 2024). These findings validate SERPINE1 as a key modulator of tumor microenvironment immune infiltration dynamics.
Further exploration was conducted into the mechanism by which NFATC2 facilitates the malignant progression of GC cells. NFATC2 was shown to promote gastric cancer cell proliferation, survival, invasion, and migration through JAK3/STAT3 pathway activation. Additionally, it established a positive feedback mechanism, thereby intensifying these malignant traits. The JAK/STAT signaling axis critically regulates cellular signal transduction, with its dysregulation established as a key driver in gastric carcinogenesis (Buzzelli et al. 2019; Khanna et al. 2015; Huang et al. 2022; Wang et al. 2024). The JAK3/STAT3 signaling axis critically regulates gastric carcinogenesis through modulating oncogenic processes (proliferation/survival/metastasis) (Yoon et al. 2015). The sustained activation of STAT3 directly fuels tumorigenesis through cell proliferation enhancement and apoptosis prevention, and it is linked to tumor immune escape mechanisms (Hashimoto et al. 2022; Xiao et al. 2023). For instance, STAT3 activation can suppress CD8+ T cell activation by upregulating PD-L1 expression, thus enabling tumor cells to avoid immune recognition and elimination (Zerdes et al. 2019; Peng et al. 2022; Chen et al. 2022b; Hu et al. 2024). Our results are consistent with these findings, indicating that NFATC2, through the activation of the JAK3/STAT3 signaling pathway, enhances the expression of NFATC2 and SERPINE1 and promotes the upregulation of PD-L1, leading to tumor immune escape. Specifically, NFATC2 forms a positive feedback loop by directly regulating the expression of SERPINE1 and activating the JAK3/STAT3 signaling pathway. This signaling circuit drives reciprocal amplification of NFATC2/SERPINE1 with concomitant PD-L1 upregulation, establishing an immunosuppressive niche through CD8+ T cell suppression. Ju et al. also discovered a similar positive feedback regulation mechanism when studying the lactyl transferase activity of AARS1 and its role in GC. AARS1 functions as a lactate sensor, nuclear-translocating to engage YAP-TEAD complex activation. This axis establishes a self-reinforcing circuit through YAP-TEAD-mediated AARS1 transcriptional upregulation, mechanistically driving gastric cancer proliferation (Ju et al. 2024). Our integrative analysis establishes the JAK3/STAT3 axis as a critical regulator of gastric carcinogenesis. Through this pathway, NFATC2 facilitates the malignant progression of GC cells and simultaneously influences tumor immune escape mechanisms by modulating crucial immune microenvironment molecules, including PD-L1. Our findings establish a critical foundation for designing JAK3/STAT3-targeted therapeutic interventions.
Despite the significant achievements of our study, there are some limitations. First, our current findings are confined to preclinical validation (in vitro models/animal studies), pending verification in clinical cohorts. Second, the interaction mechanisms between NFATC2 and SERPINE1 in GC may be more complex, involving additional signaling pathways and molecules, warranting mechanistic exploration in translational studies.
In summary, our study revealed the mechanism by which SERPINE1 promotes GC progression by upregulating NFATC2 through the activation of the JAK3/STAT3 signaling pathway, forming a positive feedback loop, and explored the pathway by which SERPINE1/JAK3/STAT3 increases PD-L1+ M2 macrophages and inhibits CD8+ T cell activation via PD-1/PD-L1 (Fig. 9). Furthermore, we found that knockdown of SERPINE1 sensitizes GC xenografts to anti-PD-1 therapy.
Fig. 9.
The positive feedback loop of NFATC2/SERPINE1/JAK3/STAT3 reshapes the immune microenvironment to mediate resistance to anti-PD-1 therapy in stomach adenocarcinoma
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Zhenyu Yang and Dingwen Zhong conducted the experiments and drafted the manuscript. Xi’e Hu collected and analyzed the data. Wenhui Chen prepared figures, contributed the methodology and edited the manuscript. Yonghui Liao and Xianli He designed and supervised the study. All authors reviewed the manuscript.
Funding
This study was funded by Recruitment Assistance Scheme of Tangdu Hospital (No.2023BTDQNB013) and Natural Science Foundation of Jiangxi Province (No. 20212BAB206083).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethical approval
All experiments were conducted in accordance with the guidelines approved by the Ethics Committee of the Second Affiliated Hospital of Air Force Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhenyu Yang, Dingwen Zhong, and Xi’e Hu have contributed equally to this work.
Contributor Information
Yonghui Liao, Email: liaohepato@163.com.
Xianli He, Email: wanghe@fmmu.edu.cn.
References
- Abaza A, Sid Idris F, Anis Shaikh H, Vahora I, Moparthi KP, Al Rushaidi MT, et al. Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) Immunotherapy: A Promising Breakthrough in Cancer Therapeutics. Cureus. 2023;15(9): e44582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Chen L, Yuan Z, Ma X, Yang X, Wang Y, et al. Pyrimidine compounds BY4003 and BY4008 inhibit glioblastoma cells growth via modulating JAK3/STAT3 signaling pathway. Neurotherapeutics. 2024;21(5): e00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha GE, Al-Kuraishy HM, Al-Maiahy TJ, Al-Buhadily AK, Saad HM, Al-Gareeb AI, et al. Plasminogen activator inhibitor 1 and gestational diabetes: the causal relationship. Diabetol Metab Syndr. 2022;14(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart S, Glesel E, Singh G, Chen NM, Reutlinger K, Zhang J et al. Restricted heterochromatin formation links NFATc2 repressor activity with growth promotion in pancreatic cancer. Gastroenterology. 2012;142(2):388–98.e1–7. [DOI] [PMC free article] [PubMed]
- Bourajjaj M, Armand AS, da Costa Martins PA, Weijts B, van der Nagel R, Heeneman S, et al. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem. 2008;283(32):22295–303. [DOI] [PubMed] [Google Scholar]
- Buzzelli JN, O'Connor L, Scurr M, Chung Nien Chin S, Catubig A, Ng GZ et al. Overexpression of IL-11 promotes premalignant gastric epithelial hyperplasia in isolation from germline gp130-JAK-STAT driver mutations. Am J Physiol Gastrointest Liver Physiol. 2019;316(2):G251-g62. [DOI] [PubMed]
- Chen S, Li Y, Zhu Y, Fei J, Song L, Sun G, et al. SERPINE1 Overexpression Promotes Malignant Progression and Poor Prognosis of Gastric Cancer. J Oncol. 2022a;2022:2647825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bai B, Ying K, Pan H, Xie B. Anti-PD-1 combined with targeted therapy: Theory and practice in gastric and colorectal cancer. Biochim Biophys Acta Rev Cancer. 2022b;1877(5): 188775. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhao Z, Zhang H, Huang S, Xu M, Pan H. Transcriptomic characterization and construction of M2 macrophage-related prognostic and immunotherapeutic signature in ovarian metastasis of gastric cancer. Cancer Immunol Immunother. 2023;72(5):1121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach K, Daniel C, Lehr HA, Nikolaev A, Gerlach T, Atreya R, et al. Transcription factor NFATc2 controls the emergence of colon cancer associated with IL-6-dependent colitis. Cancer Res. 2012;72(17):4340–50. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Hashimoto A, Muromoto R, Kitai Y, Oritani K, Matsuda T. Central Roles of STAT3-Mediated Signals in Onset and Development of Cancers: Tumorigenesis and Immunosurveillance. Cells. 2022;11(16):2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes LM, Huttinger ZM, Yee A, Kretz CA, Siemieniak DR, Lawrence DA, et al. Deep mutational scanning and massively parallel kinetics of plasminogen activator inhibitor-1 functional stability to probe its latency transition. J Biol Chem. 2022;298(12): 102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Chen S, Deng R, Deng H, Peng M, Wang X, et al. Exosomal PDL1 Suppresses the Anticancer Activity of CD8(+) T Cells in Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2024;2024:1608582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang IH, Chung WH, Wu PC, Chen CB. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: An updated review. Front Immunol. 2022;13:1068260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J, Zhang H, Lin M, Yan Z, An L, Cao Z, et al. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J Clin Invest. 2024;134(10): e174587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Chua PJ, Bay BH, Baeg GH. The JAK/STAT signaling cascade in gastric carcinoma (Review). Int J Oncol. 2015;47(5):1617–26. [DOI] [PubMed] [Google Scholar]
- Kim DK, Synn CB, Yang SM, Kang S, Baek S, Oh SW, et al. YH29407 with anti-PD-1 ameliorates anti-tumor effects via increased T cell functionality and antigen presenting machinery in the tumor microenvironment. Front Chem. 2022;10: 998013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuai X, Lv J, Zhang J, Xu M, Ji J. Serpin Family A Member 1 Is Prognostic and Involved in Immunological Regulation in Human Cancers. Int J Mol Sci. 2023;24(14):11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Ding X, Kong L, Zhou X, Zhang Z, Ju H, et al. NFATC2 is a novel therapeutic target for colorectal cancer stem cells. Onco Targets Ther. 2018;11:6911–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Rong Z, Zhang R, Niu S, Di X, Ni L et al. Vascular restenosis reduction with platelet membrane coated nanoparticle directed M2 macrophage polarization. iScience. 2022;25(10):105147. [DOI] [PMC free article] [PubMed]
- Li L, Li F, Xu Z, Li L, Hu H, Li Y, et al. Identification and validation of SERPINE1 as a prognostic and immunological biomarker in pan-cancer and in ccRCC. Front Pharmacol. 2023;14:1213891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Pan CG, Luo ZQ. High expression of NFAT2 contributes to carboplatin resistance in lung cancer. Exp Mol Pathol. 2019;110: 104290. [DOI] [PubMed] [Google Scholar]
- Lv J, Yu C, Tian H, Li T, Yu C. Expression of Serpin Family E Member 1 (SERPINE1) Is Associated with Poor Prognosis of Gastric Adenocarcinoma. Biomedicines. 2023;11(12):3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud MJE, Tellios V, Lu WY. Nitric oxide attenuates microglia proliferation by sequentially facilitating calcium influx through TRPV2 channels, activating NFATC2, and increasing p21 transcription. Cell Cycle. 2021;20(4):417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Huang H, Huan Q, Liao C, Guo Z, Hu D, et al. Adiponectin Deficiency Enhances Anti-Tumor Immunity of CD8(+) T Cells in Rhabdomyosarcoma Through Inhibiting STAT3 Activation. Front Oncol. 2022;12: 847088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Pan X, Ning L, Gong D, Huang J, Deng K, et al. Construction of a Combined Hypoxia-related Genes Model for Hepatocellular Carcinoma Prognosis. Curr Comput Aided Drug des. 2023;19(2):150–61. [DOI] [PubMed] [Google Scholar]
- Samarkina A, Youssef MK, Ostano P, Ghosh S, Ma M, Tassone B, et al. Androgen receptor is a determinant of melanoma targeted drug resistance. Nat Commun. 2023;14(1):6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. [DOI] [PubMed] [Google Scholar]
- Wang S, Pang L, Liu Z, Meng X. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: a novel therapeutic target. BMC Cancer. 2021;21(1):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Z, Li S, Ma J, Dai X, Lu J. Deciphering JAK/STAT signaling pathway: A multifaceted approach to tumorigenesis, progression and therapeutic interventions. Int Immunopharmacol. 2024;131: 111846. [DOI] [PubMed] [Google Scholar]
- Wei R, Song J, Liu X, Huo S, Liu C, Liu X. Immunosuppressive MFAP2(+) cancer associated fibroblasts conferred unfavorable prognosis and therapeutic resistance in gastric cancer. Cell Oncol (Dordr). 2024;47(1):55–68. [DOI] [PubMed] [Google Scholar]
- Xiao D, Zeng T, Zhu W, Yu ZZ, Huang W, Yi H, et al. ANXA1 Promotes Tumor Immune Evasion by Binding PARP1 and Upregulating Stat3-Induced Expression of PD-L1 in Multiple Cancers. Cancer Immunol Res. 2023;11(10):1367–83. [DOI] [PubMed] [Google Scholar]
- Xu B, Bai Z, Yin J, Zhang Z. Global transcriptomic analysis identifies SERPINE1 as a prognostic biomarker associated with epithelial-to-mesenchymal transition in gastric cancer. PeerJ. 2019;7: e7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JD, Ma L, Zhu Z. SERPINE1 as a cancer-promoting gene in gastric adenocarcinoma: facilitates tumour cell proliferation, migration, and invasion by regulating EMT. J Chemother. 2019;31(7–8):408–18. [DOI] [PubMed] [Google Scholar]
- Yang MH, Wang YS, Shi XQ, Zhao XW, Li B. Arsenic Trioxide Restrains Lung Cancer Growth and Metastasis by Blocking the Calcineurin-NFAT Pathway by Upregulating DSCR1. Curr Cancer Drug Targets. 2022;22(10):854–64. [DOI] [PubMed] [Google Scholar]
- Ye Z, Yi J, Jiang X, Shi W, Xu H, Cao H, et al. Gastric cancer-derived exosomal let-7 g-5p mediated by SERPINE1 promotes macrophage M2 polarization and gastric cancer progression. J Exp Clin Cancer Res. 2025;44(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HW, Chiang CF, Chen PH, Su CC, Wu YC, Chou L, et al. Axl Involved in Mineral Trioxide Aggregate Induces Macrophage Polarization. J Endod. 2018;44(10):1542–8. [DOI] [PubMed] [Google Scholar]
- Yoon J, Ko YS, Cho SJ, Park J, Choi YS, Choi Y, et al. Signal transducers and activators of transcription 3-induced metastatic potential in gastric cancer cells is enhanced by glycogen synthase kinase-3β. APMIS. 2015;123(5):373–82. [DOI] [PubMed] [Google Scholar]
- Zerdes I, Wallerius M, Sifakis EG, Wallmann T, Betts S, Bartish M, et al. STAT3 Activity Promotes Programmed-Death Ligand 1 Expression and Suppresses Immune Responses in Breast Cancer. Cancers (Basel). 2019;11(10):1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Liu X, Huang Z, Zhang J, Stalin A, Tan Y, et al. Data mining combines bioinformatics discover immunoinfiltration-related gene SERPINE1 as a biomarker for diagnosis and prognosis of stomach adenocarcinoma. Sci Rep. 2023;13(1):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Miao K, Sun H, Deng CX. Tumor heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci. 2022;18(7):3019–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Zhou X, Gu M, Kim J, Li Y, Ko CJ, et al. Dapl1 controls NFATc2 activation to regulate CD8(+) T cell exhaustion and responses in chronic infection and cancer. Nat Cell Biol. 2022;24(7):1165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zila N, Eichhoff OM, Steiner I, Mohr T, Bileck A, Cheng PF, et al. Proteomic Profiling of Advanced Melanoma Patients to Predict Therapeutic Response to Anti-PD-1 Therapy. Clin Cancer Res. 2024;30(1):159–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.