Abstract
The gut virome, an essential component of the intestinal microbiome, constitutes ∼0.1% of the total microbial biomass but contains a far greater number of particles than bacteria, with phages making up 90%–95% of this virome. This review systematically examines the developmental patterns of the gut virome, focusing on factors influencing its composition, including diet, environment, host genetics, and immunity. Additionally, it explores the gut virome's associations with various diseases, its interactions with gut bacteria and the immune system, and its emerging clinical applications.
Keywords: gut virome, inflammatory bowel disease, fecal microbiota transplantation, colorectal cancer, probiotics, dietary intervention
Graphical Abstract
Graphical Abstract.
Clinical applications of the gut virome in disease therapeutics.
Introduction
The gut microbiome, often referred to as the body's “second genome”, consists of bacteria, archaea, and fungi, coexisting with the gut virome, a distinct entity overwhelmingly dominated by bacteriophages, with eukaryotic viruses representing the remainder (Fig. 1) [1]. This complex and dynamic ecosystem is essential for host metabolism, immune regulation, and disease resistance. Among its microbial inhabitants, the gut virome plays a crucial regulatory role. Though comprising only 0.1% of the gut microbial population by relative abundance, viral particles can outnumber bacteria by a factor of 1 to 10, with bacteriophages accounting for 90%–95% of this virome [2]. This vast abundance suggests a potential regulatory role in shaping microbial communities and maintaining gut homeostasis, as bacteriophages influence bacterial dynamics through predation and horizontal gene transfer [3].
Figure 1.
Composition of the human gut virome.
The gut virome is marked by its remarkable plasticity, often displaying greater sensitivity to environmental and host-derived factors than bacterial communities. Host-specific characteristics such as age and genetic background provide the fundamental framework for viral diversity, while environmental factors (e.g. geographic location and urbanization) drive regional variations. Lifestyle choices, including diet and hygiene practices, contribute to daily fluctuations in the viral population, and medical interventions (e.g. antibiotic use and fecal transplants) can cause rapid shifts in the virome composition. These factors, interacting through a complex network of host–microbe relationships, collectively shape the structure and function of the gut virome.
In addition to commensal phages, the gut virome has historically included pathogenic enteric viruses with major implications for human health [4]. Before the advent of widespread vaccination, viruses such as poliovirus, coxsackievirus, and rotavirus were leading causes of severe childhood gastrointestinal diseases, some of which remain endemic today [5, 6]. In recent years, the gut virome's critical role in maintaining intestinal homeostasis and influencing disease outcomes has become an increasingly prominent area of research. Dysregulation of the gut virome has been implicated in several intestinal disorders, including inflammatory bowel disease (IBD) and Clostridium difficile infection (CDI), where certain phage populations often become significantly enriched, potentially exacerbating inflammation by activating specific immune pathways, such as the Toll-like receptor 9 (TLR9) and interferon-gamma (IFN-γ) signaling pathway [7]. Furthermore, therapeutic approaches like fecal microbiota transplantation (FMT) have shown promise in modulating phage populations and improving disease outcomes [8]. This paper systematically reviews the developmental patterns of the gut virome, delves into the factors influencing its composition—including diet, environment, host genetics, and immunity—and explores its association with disease, interactions with bacteria and the host immune system, as well as potential clinical applications.
Impact of the gut virome on human health
Developmental trajectories of the gut virome
The human gut virome undergoes significant changes throughout an individual's lifespan. In infancy, the gut is predominantly colonized by phages, with relatively fewer bacteria and even fewer eukaryotic viruses [9]. Early-life factors, such as mode of delivery, diet (e.g. breastfeeding vs. formula feeding), and antibiotic exposure, have a profound impact on the initial establishment and diversity of the gut virome. Recent studies have shown that the infant virome is not only highly diverse but also uniquely individualized. A 2023 study expanded the catalog of viral species in healthy infants, revealing a vast unexplored diversity in early-life viral communities [10]. Building on this, a 2024 metagenome-assembled genome study tracked the longitudinal development of the infant gut virome and bacteriome, demonstrating rapid turnover and dynamic shifts during the first few years of life [11]. These findings highlight the complexity and individualized trajectories of the early-life virome, which may have long-term implications for microbial ecosystem development and immune system education. During adolescence, shifts in diet, hormone levels, and immune system maturation further refine the gut virome. As individuals mature, the gut microbiota stabilizes, with phage and bacterial populations reaching a dynamic equilibrium, while eukaryotic viruses remain a minority. In adulthood, the gut microbiome maintains a cooperative balance that supports intestinal homeostasis [12, 13]. However, with aging, there is a notable increase in lysogenic phages, particularly those associated with Akkermansia and Ruminococci [14]. It is therefore hypothesized that the enrichment of specific phages (e.g. lysogenic phages) in the elderly gut microbiome may play a role in age-related changes by modulating the host microbiota.
Major factors influencing the composition of the gut virome
Dietary structure and living environment
Dietary habits significantly influence the composition and function of the gut virome. A high-fiber diet fosters a more favorable environment for lysogenic phages by promoting fermentation within the intestinal microbiota, which, in turn, enhances their proliferation capacity [15]. In contrast, a diet high in fat and sugar, typical of Western diets, may facilitate the survival and proliferation of certain pathogenic phages in the gut [16]. Dietary components modulate the composition of the gut virome not only directly but also indirectly by altering bacterial communities, which serve as hosts for bacteriophages. For instance, high-fiber diets promote the growth of beneficial bacteria such as Bacteroides and Firmicutes, which in turn influence the abundance and diversity of lysogenic phages [17].
Urbanization also plays a crucial role in shaping the composition and diversity of the gut virome. Environmental changes associated with urbanization, such as overcrowding and improved sanitation, can impact the origin and diversity of the gut virome. Studies have shown that urbanization reduces exposure to natural microbial reservoirs, which may lead to a decline in environmentally derived viruses that contribute to the gut virome, consequently reducing gut virome diversity [18]. For example, the diversity of the gut virome in healthy Chinese adults is significantly influenced by geographic location and dietary habits. Rural residents, who consume more fiber-rich foods, exhibit distinct gut virome profiles compared to their urban counterparts [19]. Geographic variations in gut virome composition have been observed globally, with individuals from non-Western, rural environments exhibiting higher viral diversity, often linked to traditional diets rich in fiber and lower antibiotic exposure.
Host genetics and immunity
Host genetics and immune mechanisms are essential in shaping the gut virome. Certain genetic variants can influence the expression or function of pattern recognition receptors, thereby modulating antiviral immune responses [20]. Such variations may also affect the recognition and clearance of enteric viruses, influencing the composition of the gut virome.
Under normal conditions, innate and mucosal immunity work together to maintain gut virome homeostasis. Innate immune mechanisms, such as type I and III IFN responses, limit viral replication and spread, while IgA secretion reduces viral interaction with intestinal epithelial cells. Additionally, antigen-presenting cells can recognize enteric viruses, activating T-cell responses and cytokine production [e.g. interleukin (IL)-22, IL-15, IFNs], further influencing the gut virome and host immunity [21].
However, immune dysfunction, such as in human immunodeficiency virus (HIV)-induced immunodeficiency, can lead to the uncontrolled expansion of certain viral populations [22]. The depletion of CD4+ T cells in gut-associated lymphoid tissue impairs viral replication control, allowing opportunistic viruses like adenovirus and cytomegalovirus (CMV) to proliferate. This dysregulation not only alters the composition of the gut virome but also exacerbates gastrointestinal complications in acquired immunodeficiency syndrome patients, highlighting the intricate interplay between the immune system and enteric virome stability [23].
Multidimensional interactions of the gut virome with the bacteriome and the mammalian host
Mechanisms of interaction between the gut virome and bacteriome
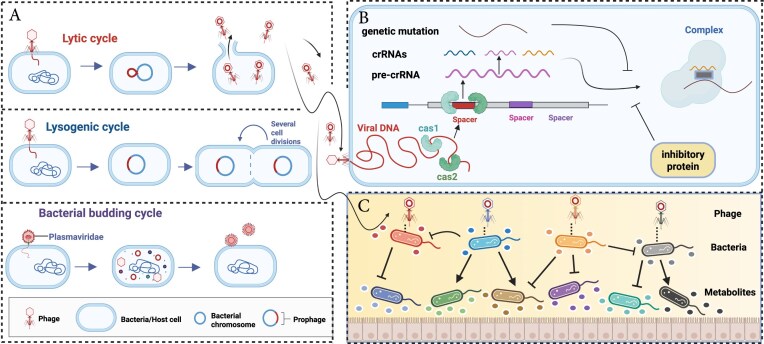
In the intestinal microcosm, viruses that directly interact with bacteria are predominantly phages. These phages regulate the structure and function of bacterial communities through dynamic life-cycle transitions, including lysis, lysogeny, and budding (Fig. 2A). Under physiological homeostasis, lysogenic phages integrate into the host genome as prophages, forming a symbiotic relationship that enhances the ecological competitiveness of the host bacterium. This symbiosis can confer advantages such as antibiotic resistance, toxin production, and metabolic stress adaptation [24]. The lysogenic system possesses multi-level environmental sensing capabilities, allowing it to detect stress signals—including antibiotics, ultraviolet radiation, and pH fluctuations—and respond by excising prophages to initiate the lytic cycle [25–29]. This transition plays a key role in microbial community stability, as controlled prophage induction can help to maintain homeostasis by regulating bacterial population densities. In contrast, budding phages continuously release viral particles through the host cell membrane without lysing the host, maintaining a long-term symbiotic relationship. This mechanism supports the persistence of specific bacterial species in the gut environment and may contribute to biofilm stability.
Figure 2.
Mechanisms of interaction between the gut virome and bacteriome. Created in BioRender. Zuo, T. (2025) https://BioRender.com/jasye5j. (A) Bacteriophage life cycles. Phages replicate through three cycles: lytic (host lysis), lysogenic (genome integration), and budding (non-lethal release). (B) Bacterial defense vs. phage countermeasures. Bacteria combat phage infection using systems like CRISPR-Cas, which recognizes and cleaves phage DNA. In response, phages promote the generation of inhibitory proteins and genetic mutations to evade detection, driving a constant evolutionary arms race. (C) Effects of phages on bacterial functions. Phages regulate metabolism, biofilm formation, and virulence. Some phages transfer genes that enhance adherence and invasion to shape bacterial behavior and host interactions.
However, when the intestinal microenvironment becomes imbalanced, the phage–bacteria interaction network is restructured. In such cases, lysogenic phages become activated, selectively targeting and reducing pathogenic bacterial populations by 40%–60%. Yet, excessive lysis can disrupt the microbial equilibrium, potentially leading to secondary dysbiosis and increased inflammation [30].
This interplay between phages and bacteria extends beyond simple defense mechanisms; it represents a complex evolutionary arms race. Bacteria have evolved sophisticated anti-phage defense strategies, such as the clustered regularly interspaced palindromic repeats [CRISPR)/CRISPR-associated (Cas)] systems, which enables them to recognize and cleave phage genetic material, establishing an adaptive immune response (Fig. 2B) [31]. In response, phages evade host recognition through inhibitory proteins or genetic mutations [32]. This constant "offensive and defensive" exchange not only influences bacterial community composition but also significantly regulates their metabolism [31]. Beyond classical genetic immunity, both bacterial quorum sensing (QS) signals and virus-encoded communication systems have been shown to influence the lysis–lysogeny decision in temperate phages. These ecological regulatory layers—such as QS-mediated host sensing [33] and the arbitrium phage communication system [34]—collectively shape and complicate the landscape of phage–bacterium interactions. The role of phages extends beyond defense and immune modulation; they are central regulators of host bacterial metabolism and pathogenicity (Fig. 2C). For example, Escherichia coli carrying the Φ24B prophage exhibits enhanced survival in acidic environments due to phage-encoded acid-tolerant regulatory elements [35]. Furthermore, filamentous phages can remodel biofilm structures and regulate the synthesis and release of virulence factors, such as cholera toxin, by altering host gene expression networks [36]. Genomic studies have revealed that phages can directly encode pathogenic factors, such as cholera toxin genes carried by the CTXφ phage in Vibrio cholerae [37] and adenosine diphosphate ribosyltransferases [38, 39], which significantly enhance the adherence and invasive capabilities of pathogenic bacteria. This underscores the dual role of phages as both regulators of microbial balance and potential drivers of bacterial pathogenicity.
From an evolutionary perspective, phages act as mobile genetic elements that drive phenotypic innovation in host bacteria, thereby shaping the functional diversity of bacterial populations through continuous horizontal gene transfer [40]. In the gut microbiome, phages play a crucial role in facilitating horizontal gene transfer, which not only promotes genetic diversity among gut bacteria but also enables them to rapidly adapt to environmental changes. This process occurs through the transfer of non-viral DNA into bacterial communities via transduction, a mechanism that is vital for bacterial evolution. Research has demonstrated that phage-mediated horizontal gene transfer, particularly phage transduction, profoundly influences the function and stability of gut bacterial communities [41]. This gene flow accelerates the spread of antibiotic resistance, fosters metabolic adaptations, and reshapes bacterial pathogenic potential by integrating virulence islands. Consequently, phages are key drivers of microbial co-evolution, serving as essential mediators of both symbiosis and conflict within the gut ecosystem.
Mechanisms of interaction between the gut virome and mammalian host immunity
The virus–bacteria–mammalian host triad forms a supersystem that achieves bidirectional regulation of physiological homeostasis and pathological processes through dynamic plasticity (Fig. 3). Under physiological conditions, this system maintains balance through multiple regulatory levels. First, phages colonize the intestinal mucosa and establish a basal defense layer. For example, T4-like phages can anchor mucin glycans through the IgG-like structural domains of Hoc proteins, forming a physical antimicrobial barrier [42]. Second, phages modulate T/B cell activity and macrophage function in a dose-dependent manner, shaping adaptive immune responses [43, 44]. This immunomodulatory role is crucial in maintaining tolerance to commensal bacteria while preventing pathogenic invasion. Finally, eukaryotic viruses contribute to immune homeostasis by modulating responses via the TLR and retinoic acid-inducible gene I (RIG-I) pathway. For instance, in mouse models, norovirus resists pathogen invasion by activating specific immunity [45]. Additionally, murine astrovirus plays a crucial role in maintaining intestinal defenses during immunodeficiency through an IFN-γ compensatory mechanism, likely by enhancing alternative immune pathways [46]. Together, these mechanisms form a ternary interplay of defense networks.
Figure 3.
Mechanisms of interaction between the gut virome and mammalian host immunity. Phages within the intestinal mucosa act as a frontline defense, forming a physical barrier while modulating T/B cell activity and macrophage function in a dose-dependent manner. Meanwhile, eukaryotic viruses contribute to immune homeostasis through TLR and RIG-I signaling pathways. However, external disturbances such as infections or antibiotic exposure can disrupt these pathways, reprogramming the immune response from protective to pathological. This shift triggers TLR9-mediated overactivation of Th17 cell responses, resulting in excessive IL-17 production and subsequent mucosal damage. Additionally, phage-induced microbial dysbiosis exacerbates immune imbalance, generating a self-perpetuating cycle that contributes to inflammatory diseases such as IBD. Created in BioRender. Zuo, T. (2025) https://BioRender.com/kexdnt4.
However, when this supersystem is subjected to external disturbances (e.g. infection, antibiotic abuse, or immunodeficiency), the homeostatic defense network can undergo maladaptive reprogramming, shifting from a protective to a pathological architecture. Under pathological conditions, phage-derived nucleic acids can induce aberrant activation of the TLR pathway, such as TLR9-mediated T helper 17 (Th17) cell overactivation. This, in turn, triggers an IL-17 cytokine storm and remodels pro-inflammatory factor networks, exacerbating mucosal injury [47, 48]. Disruptions in phage-mediated microbial control, such as the loss of commensal bacterial ecological niches or an increase in antibiotic resistance gene reservoirs, can create a vicious cycle of immune intolerance, ultimately driving inflammatory diseases like IBD [49]. Beyond local inflammation, recent studies suggest that phages may also influence systemic immune responses, particularly in the setting of cancer immunotherapy. Notably, MHC class I-restricted epitopes encoded by the tail length tape measure protein of certain prophages have been shown to suppress the activation of commensal-specific memory T cells [50]. This immune dampening effect may reduce the efficacy of immune checkpoint blockade in tumor-bearing hosts, revealing a previously unrecognized axis between the gut virome and anti-tumor immunity [50]. These findings call for careful consideration of virome composition in personalized cancer treatment strategies, particularly in patients undergoing immune checkpoint blockade. This shift from a “defense barrier” to a “disease-promoting engine” not only reveals the adaptive properties of the supersystem under microenvironmental stress but also provides a theoretical basis for targeted regulation of the intestinal microecology–immunity axis. By intervening at key nodes (e.g. the phage–mammalian host interface or TLR signaling hubs), it may be possible to regulate the intestinal microecology–immunity axis, reversing pathological processes and restoring system homeostasis. Phage therapy and engineered bacteriophages could be leveraged to selectively eliminate pathogenic bacterial populations while preserving commensal microbiota, offering a precision-medicine approach for treating gut inflammation and dysbiosis.
Gut virome and diseases
Imbalances in the gut virome play a crucial role in the pathogenesis of IBD, CDI, colorectal cancer (CRC), and other conditions (Table 1). In patients with IBD, the gut virome is characterized by a marked expansion of Caudovirales [51] and a concurrent reduction in Microviridae abundance [52] compared to healthy individuals. The gut virome can exacerbate disease progression through two interrelated pathways: directly modulating the host immune response and disrupting microbial equilibrium. Caudovirales phages, for instance, amplify the inflammatory cascade by activating CD4+ T cells and promoting IFN-γ secretion through a TLR9-dependent pathway [21]. In addition to bacteriophages, pathogenic eukaryotic enteric viruses, such as norovirus and rotavirus, have also been implicated in IBD. Noroviruses can alter host gene expression and trigger intestinal inflammation [53]. Animal studies further revealed that norovirus infection accelerates intestinal pathology in genetically susceptible mice, such as those deficient in IL-10, a key immune regulator, or autophagy-related 16-like 1, which is essential for autophagy and gut homeostasis. This underscores the pathogenic potential of viral elements in individuals with underlying genetic predispositions [54, 55]. Rotavirus infection contributes to mucosal damage by increasing intestinal permeability, impairing epithelial cell turnover [56], and eliciting strong immune responses via non-structural protein 4 (NSP4)-mediated signaling and dendritic cell activation [57], contributing to mucosal damage in IBD-prone hosts. Beyond these well-known viruses, recent studies have also implicated Epstein–Barr virus (EBV) and CMV in IBD exacerbations, particularly in immunocompromised patients, suggesting a broader role for eukaryotic viruses in intestinal inflammation [58, 59]. Collectively, these findings highlight the pathogenic potential of gut virome elements—both phages and eukaryotic viruses—particularly in individuals with underlying genetic susceptibility.
Table 1.
Gut virome features in diseases.
| Disease | Human observational studies | Supporting references | Mechanistic studies | Supporting references |
|---|---|---|---|---|
| IBD |
Caudovirales↑ Microviridae↓ EnterovirusB↑ Picornaviridae↑ Faecalibacterium prausnitzii phages↑ |
Liang et al. [52] Norman et al. [60] Cornuault et al. [61] Zuo et al. [51] Massimino et al. [62] Wagner et al. [63] Liang et al. [64] Adiliaghdam et al. [65] |
Induction of IFN-γ production via TLR9 signaling. Likely attributed to increased fluid flow or the shedding of mucosal layers that fail to retain phages. Increased temperate phage-induced mortality of F. prausnitzii. |
Liang et al. [52] Norman et al. [60] Cornuault et al. [61] Zuo et al. [51] Massimino et al. [62] Wagner et al. [63] Liang et al. [64] |
| IBS |
Microviridae↑; Myoviridae↑; Podoviridae↑ Lactobacillus virus LBR48↑ |
Mihindukulasuriya et al. [66] Li et al. [67] |
Diminished beneficial activity of Lactobacillus brevis. | Li et al. [67] |
| CDI | Caudovirales; Anelloviridae↑; Microviridae↓ | Zuo et al. [68] | None. | None. |
| CRC |
Fusobacterium nucleatum phage↑ Parvimonas micra phage↑ Peptacetobacter hiranonis phage↑ Enterobacteria phage↑ Autographiviridae↑; Gratiaviridae↑ Siphoviridae↑; Drexlerviridae↑ Inoviridae↑; Podoviridae↑; Myoviridae↑ |
Shen et al. [69] Chen et al. [70] Zuo et al. [71] Broecker et al. [72] |
FadA promotes cancer cell proliferation through Wnt signaling, highlighting its potential as a CRC biomarker; impact on bacterial dysbiosis. |
Shen et al. [69] Zuo et al. [71] Broecker et al. [72] |
In irritable bowel syndrome (IBS), the virome of IBS patients is distinct, characterized by an increased abundance of several viral families, such as Microviridae, Myoviridae, and Podoviridae, alongside elevated levels of Lactobacillus phages, including Lactobacillus bacteriophage LBR48, which specifically target Lactobacillus species [66, 67]. These alterations may inhibit the activity of beneficial bacteria like Lactobacillus brevis, disrupting intestinal homeostasis and exacerbating IBS symptoms [67]. Additionally, norovirus and rotavirus have been linked to the onset of post-infectious IBS, where acute viral gastroenteritis precedes the development of chronic symptoms, suggesting that viral triggers may initiate or sustain gut–brain axis dysregulation [73].
In CDI, viral imbalances manifest as an overrepresentation of Caudovirales and Anelloviridae, alongside a reduction in Microviridae [68]. This dysbiosis may enhance the pathogenicity of C. difficile by modulating bacterial competition, influencing colonization dynamics, and regulating toxin expression. Recent data also suggest that viral co-infections, such as CMV or norovirus, may exacerbate CDI severity, especially in hospitalized or immunocompromised individuals, by further disrupting mucosal integrity and immune defenses [74, 75].
In CRC, the gut virome exhibits significantly greater diversity, dominated by phage families such as Siphoviridae, Myoviridae, Drexlerviridae, and Podoviridae [71]. Furthermore, CRC patients demonstrate an increased abundance of phages associated with Fusobacterium nucleatum, Parvimonas micra, and Peptostreptobacter hiranonis [69]. These viral alterations may contribute to oncogenesis through multiple mechanisms, including phage-mediated horizontal gene transfer, which facilitates the spread of oncogenic bacteria and drug-resistance genes. Fusobacterium nucleatum, for instance, promotes tumorigenesis by activating the Wnt signaling pathway via the FadA (fusobacterium adhesin A) adhesin protein, and CRC-associated phages may further modulate this process [69]. Clinical subgroup analyses have identified >20 viral genera that distinguish CRC patients from healthy individuals, with viral community composition correlating with cancer stage and prognosis, suggesting that the gut virome may serve as a potential biomarker for CRC [76]. Recent findings suggest that certain phages can influence bacterial biofilm formation, a key factor in microbial persistence and pathogenicity. For instance, phages associated with F. nucleatum may contribute to biofilm stabilization, promoting CRC-associated dysbiosis and inflammation [77].
Although bacteriophages, interacting with their bacterial hosts, are frequently associated with the development of CRC, pathogenic eukaryotic enteric viruses also play a significant role in CRC progression. Notably, eukaryotic viruses such as JC virus, human papillomavirus (HPV), and EBV have been detected in colorectal tumors. JC virus' T-antigen may promote carcinogenesis by activating β-catenin signaling, while HPV infection has been associated with epigenetic dysregulation in colorectal tissues. Although the mechanistic evidence remains limited, these findings raise the possibility that certain eukaryotic viruses may directly contribute to colorectal oncogenesis [78, 79]. Viral infections, including EBV and HPV, account for ∼1%–6% of the global cancer burden [80], yet their direct mechanistic involvement in CRC is still unclear.
It is worth noting that phage abundance in the gut often reflects the dynamics of their bacterial hosts, suggesting that many observed virome shifts in diseases may result from bacterial changes rather than directly causing pathology. Similar to studies of the bacterial microbiome, the causal relationship between viral dysbiosis and diseases remains to be definitively established. However, emerging evidence suggests the virome may independently influence disease progression. For instance, expanded Escherichia and Bacterioides phages have been shown to exacerbate colitis via TLR9 and IFN-γ, independent of detectable endogenous inhabitant bacterial hosts in a mouse colitis model [7] Additionally, the infant gut virome is associated with disease risk independently of the bacteriome [81]. Furthermore, fecal virome transplantation has demonstrated greater efficacy than bacteriome transplantation in alleviating intestinal inflammation in certain diet-associated contexts [82]. The partial congruence between virome and bacteriome in diseases may be due to the disruption of the typical phage–host dynamic under the disease-related inflammation. These findings highlight the complex tripartite relationship among the gut virome, bacteriome, and disease, underscoring the need for further experimental studies to elucidate their causal connections.
Despite the growing recognition that the gut virome functions not only as a disease marker but also as an active participant in disease pathology by modulating microbial homeostasis and the host immune response, discrepancies in study findings remain a challenge. Some studies, for instance, have reported no significant differences in viral abundance between IBD patients and healthy individuals [83], highlighting the potential impact of factors such as sample source, sequencing technology (e.g. viral shotgun next-generation sequencing), and analytical methodologies on study outcomes [60]. Future research integrating multi-omics approaches with functional validation will be essential for elucidating the precise contributions of the gut virome to disease processes and for advancing our understanding of its potential as a therapeutic target.
Clinical applications of the gut virome
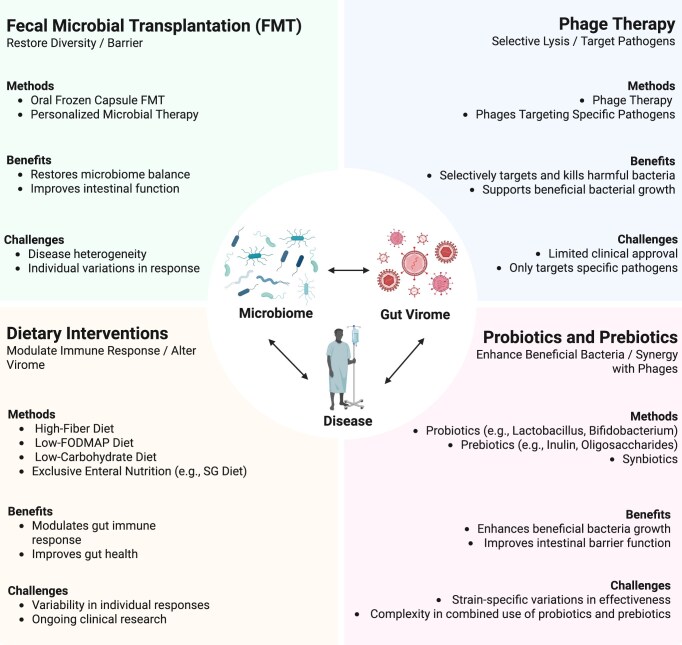
The gut virome plays a pivotal role in modulating the effectiveness of therapies such as FMT, phage therapy, dietary interventions, and probiotics. By modulating gut health and immune responses, and by shaping microbial interactions, the gut virome directly impacts the success of these treatments in managing gastrointestinal disorders, including IBD, IBS, CRC, and CDI (Fig. 4). Recent studies suggest that bacteriophages regulate bacterial populations through lytic and lysogenic cycles, which may enhance or hinder therapeutic efficacy. Additionally, eukaryotic viruses can directly interact with the host immune system, influencing inflammation and disease progression. However, challenges remain in standardizing gut virome analyses and understanding the complex interplay between virome, microbiome, and host health. This review highlights the gut virome's involvement in improving therapeutic outcomes and its potential to redefine disease management strategies.
Figure 4.
Clinical applications of the gut virome in disease therapeutics. Created in BioRender. Zuo, T. (2025) https://BioRender.com/c0hlynx.
FMT
FMT has emerged as a promising strategy for restoring gut homeostasis and treating various diseases, including metabolic disorders and recurrent infections, demonstrating efficacy, particularly in CDI, and showing potential in IBD and CRC. However, its precise mechanisms are not yet fully understood. While most research has focused on the restoration of bacterial eubiosis, growing evidence suggests that the gut virome—particularly bacteriophages and eukaryotic viruses—plays a crucial role in mediating FMT outcomes. In IBD, FMT has shown the potential to reduce inflammation, enhance microbial diversity, and support intestinal barrier repair [84]. However, therapeutic success varies due to disease heterogeneity, patient selection, and administration routes. A meta-analysis reported clinical remission rates of 35.0% in ulcerative colitis (UC) and 47.6% in Crohn's disease (CD) [85]. Interestingly, oral frozen capsules have demonstrated superior efficiency compared to traditional delivery methods [86]. Beyond bacterial shifts, the virome composition, particularly the balance between bacteriophages and eukaryotic viruses, appears to influence treatment outcomes. In UC patients, lower baseline eukaryotic viral loads correlate with better FMT responses [87], suggesting that modulating the viral component may enhance therapeutic efficacy.
FMT has revolutionized CDI treatment by modulating bile acid metabolism—raising secondary bile acids while reducing primary bile acids—to suppress C. difficile overgrowth and restore microbial balance [88]. Clinical studies report cure rates of up to 93% with multiple FMT procedures, far surpassing conventional antibiotic therapy [89]. While bacterial restoration plays a central role, phages may act as additional regulators by selectively targeting pathogenic strains and stabilizing the gut ecosystem [90]. However, the effectiveness of FMT in cases with severe CDI remains inconsistent, and concerns have been raised over potential adverse effects from frozen fecal transplants, particularly due to the risk of transmitting pathogenic viruses from donor samples, thus, underscoring the need for refined protocols [91].
In CRC, FMT is being explored for its ability to modulate dysbiosis and potentially slow tumor progression. Preclinical models suggest that a healthy microbiome transfer can suppress tumor growth possibly through microbial-mediated modulation of inflammatory pathways [92]. However, clinical applications are still in the early stages. The role of the virome in CRC therapy remains largely unexplored, but phage-mediated bacterial regulation may influence tumor-associated microbial communities, offering a novel therapeutic avenue. Further research is needed to elucidate virome–microbiota interactions in CRC and identify potential intervention targets.
Beyond bacterial restoration, the gut virome is emerging as a key determinant of FMT efficacy. While bacterial composition takes months to stabilize, phage populations rapidly align with donor profiles post-FMT, suggesting that viruses—especially bacteriophages—could serve as early modulators of gut homeostasis [90]. The expansion of donor-derived phages in recipients suggests that these phages contribute to shaping microbial dynamics, for example by suppressing pathogenic bacteria and facilitating the growth of beneficial microbial taxa [93–96].
Eukaryotic viruses also warrant attention, particularly in IBD. Elevated viral abundance has been observed in UC patients [87], with FMT responders exhibiting lower viral loads before and after transplantation compared to non-responders. This suggests that modulating the eukaryotic virome may be crucial for sustaining remission. However, the mechanisms by which eukaryotic viruses interact with gut microbiota and host immunity remain poorly understood, and their potential role in FMT efficacy requires further investigation.
Despite its promise, integrating virome-targeted approaches into FMT regimens faces multiple challenges. Inter-individual virome variability complicates standardization, and current donor screening protocols largely overlook viral components. Future research should focus on establishing virome-based donor biomarkers, leveraging phage therapy to enhance FMT, and optimizing delivery methods to preserve viral stability, ultimately refining FMT protocols for more precise and durable therapeutic outcomes.
Phage therapy
FMT effectively restores gut homeostasis but carries inherent risks, including unpredictable microbial shifts and potential pathogen transmission. This has led to the development of more precise alternatives, such as phage therapy, which selectively eliminates pathogenic bacteria while preserving beneficial microbial populations. Studies suggest that phage therapy during the remission phase of IBD can foster the growth of beneficial microorganisms [97]. By reshaping the gut microbiota composition, phage therapy enhances immune-metabolic functions, suppresses pathogenic bacterial overgrowth, and restores intestinal microecological balance.
Preclinical studies have demonstrated the therapeutic potential of phage therapy in intestinal disorders. A triple-phage cocktail targeting E. coli strain LF82, implicated in CD, significantly reduced bacterial colonization and alleviated dextran sulfate sodium-induced colitis in a carcinoembryonic antigen-10 transgenic mouse model [98]. Similarly, a pentaphage regimen, composed of phages MCoc5c, 8M-7, 1.2–3s, KP2-5-1, and PKP-55, has successfully targeted and inhibited Klebsiella pneumoniae, a pathogen associated with human IBD, effectively treating intestinal inflammation [99]. Beyond IBD, phage therapy is also being explored in CRC. Targeting Clostridium scindens, a bacterium linked to CRC, shows promise in animal models, offering a strategy to reduce tumor burden by eliminating deoxycholic acid-producing bacteria [100]. Additional studies suggest that bacteriophage-mediated modulation of the gut microbiota can remodel the tumor–immune microenvironment and inhibit tumor progression [101]. To further enhance therapeutic precision, engineered bacteriophages have been developed to improve host specificity, expand target range, and deliver functional cargos such as CRISPR/Cas systems or immune-modulating genes [102]. These synthetic phages have shown potential to reshape the tumor microenvironment [103], suppress bacteria-driven tumorigenesis, enhance responses to immune checkpoint blockade [104], and augment chemotherapy efficacy in CRC models [105]. Although still largely preclinical, this strategy highlights the virome's untapped therapeutic potential beyond traditional antimicrobial applications [106].
Despite its promise, phage therapy faces several challenges before it can be widely applied in clinical settings. While phage-based formulations are already approved as prebiotics in some Western countries [107], their therapeutic applications have yet to receive formal regulatory approval. The high specificity of phages, though advantageous for precision targeting, limits their broader use, necessitating the development of broad-host-range phages or multi-targeted phage formulations. Moreover, most research remains confined to animal models, highlighting the urgent need for large-scale randomized controlled trials to validate clinical efficacy and safety.
As research into phage-host interactions advances, personalized phage therapies hold great promise for the precise treatment of IBD and other gastrointestinal diseases. Phage therapy represents a novel approach to microbial modulation, offering a refined, more targeted alternative to traditional FMT strategies.
Dietary interventions
Dietary intervention plays a crucial role in regulating the gut virome. Nutrient composition influences the balance between lytic and lysogenic phages, with fiber-rich diets altering the gut environment through their metabolites, promoting the proliferation of lysogenic phages, which in turn regulate bacterial populations. These dietary interventions have shown considerable potential in the treatment of IBD, IBS, CDI, and CRC.
In patients with IBD, dietary interventions help to modulate the immune system, reduce inflammation, and improve quality of life. Through dietary guidance and lifestyle changes provided by dieticians, IBD patients experience significant improvements in diet quality and reductions in disease-related fatigue and daily life disruptions [108]. Additionally, a high-animal-fat diet results in lower levels of short-chain fatty acid (SCFA)-producing bacteria, such as Faecalibacterium prausnitzii, which are essential for maintaining intestinal immune homeostasis [109]. SCFAs, particularly butyrate, have been shown to influence phage–bacteria interactions, potentially modulating lysogenic conversion and altering the stability of the bacterial community.
Beyond SCFA-mediated effects, emerging evidence suggests that diet serves as a key regulator of the gut phageome–bacteriome network, shifting the paradigm from a bacteriome-centered perspective to one that encompasses trans-kingdom interactions within the gut microbiota. For instance, dietary whey protein has been shown to attenuate intestinal inflammation by modulating cross-kingdom interactions between gut phages and commensal bacteria, thereby promoting gut health. These findings highlight whey protein's potential as a dietary supplement in IBD management, while phages could be leveraged to selectively target pathobionts, aiding in symptom control and disease prevention in CD. Integrating dietary strategies with targeted modulation of the gut phageome and bacteriome may thus provide novel therapeutic avenues for CD [82].
Enteral nutrition (EEN) represents another cornerstone of IBD management, providing both essential nutrients and potential immunomodulatory effects through regulation of the microbiome [110]. However, research on the impact of EEN on the gut virome is limited. Given the virome's role in immune modulation, future studies should explore potential effects of EEN on the virome to enhance IBD treatment strategies.
Dietary modification is a key therapeutic approach for IBS patients. Both low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) and low-carbohydrate diets have been shown to significantly improve IBS symptoms, with these dietary interventions proving more effective than traditional medications [111]. Furthermore, digital health tools such as app-based low-FODMAP diet interventions have demonstrated promising results in primary care settings, offering high patient compliance and no serious adverse effects [112].
In the treatment of CRC, the serine/glycine-free (SG) diet, an emerging dietary intervention strategy, has been shown to inhibit tumor cell proliferation and migration, enhance the antitumor activity of immune cells, and synergize with immunotherapy [113]. This multifaceted efficacy raises an important question: could the gut virome, an often overlooked immunomodulator, play a key role in optimizing the effectiveness of the SG diet? While current microbiome research predominantly focuses on bacterial communities, recent evidence suggests that phages actively regulate microbial ecology and mucosal immunity [114]. A comprehensive investigation of the triad comprising host metabolism, gut virome, and immune system may uncover new strategies to enhance CRC immunotherapy
For CDI, dietary intervention can reduce recurrence rates by regulating the intestinal microbiome and reducing the colonization of harmful bacteria. A growing body of evidence suggests that fiber-rich diets, particularly those rich in fermentable fibers such as inulin and arabinoxylan, promote the expansion of beneficial Bacteroides and Lactobacillus species, which compete with C. difficile for niche space and nutrients [115].
Overall, dietary interventions play a vital role in treating a range of intestinal disorders, including IBD, IBS, CDI, and CRC, by modulating the intestinal microecological and metabolic environments. These interventions have shown positive effects in improving symptoms and enhancing patients' quality of life. Future research should explore the potential of diet-based virome modulation as a complementary strategy to current microbiome-targeted therapies, paving the way for personalized nutrition approaches in gastrointestinal disease management.
Probiotics and prebiotics
Probiotics help to maintain or restore gut homeostasis by introducing beneficial live bacteria, such as Lactobacillus and E. coli Nissle 1917. Notably, E. coli Nissle 1917 can induce remission in UC by upregulating anti-inflammatory cytokine IL-10 while suppressing pro-inflammatory mediators IL-2 and tumor necrosis factor alpha [116, 117]. Prebiotics, in contrast, are indigestible compounds that selectively stimulate beneficial bacteria. They have been shown to alleviate intestinal inflammation and reduce mucosal damage in IBD models [118]. For instance, psyllium supplementation significantly improved clinical outcomes in inactive UC patients compared to placebo (69% vs. 24%) [119].
Symbiotics, the combination of probiotics and prebiotics, enhance probiotic benefits by providing a competitive advantage in the gut. They suppress synthesis of pro-inflammatory cytokines like IL-6 and IL-8 in colitis models [120] and restore gut permeability disrupted by a Westernized diet [121]. In IBS, probiotics—especially Lactobacillus and Bifidobacterium strains—have significantly reduced symptoms like abdominal pain and bloating [112,122].
As research on gut microecology advances, growing evidence highlights the role of the virome in microbial balance. Traditionally, gut modulation has focused on probiotics and prebiotics, but recent studies identify bacteriophages as a key component. By selectively lysing pathogenic bacteria, phages reduce harmful bacterial loads while fostering a favorable environment for beneficial microbes [123].
Although phage therapy dates back to the early 20th century, its impact on gut ecology remains an evolving field. A 2019 clinical study (ClinicalTrials.gov NCT03269617) demonstrated that the PreforPro® E. coli phage cocktail significantly reduced fecal E. coli without disrupting the microbial balance. Notably, it increased butyrate-producing bacteria while reducing Clostridium perfringens and inflammatory markers such as aspartate aminotransferase and alkaline phosphatase [124]. These findings suggest phages hold promise as dietary supplements and therapeutic tools for gut microbiota modulation.
While probiotics act gradually and non-specifically, phages offer targeted, rapid effects, making them a powerful complement in microbiota regulation. A probiotic–phage synergy could enhance gut stability and therapeutic outcomes [125]. A 2020 study (ClinicalTrials.gov NCT04511221) found that a 4-week regimen of PreforPro® combined with Bifidobacterium bifidum BL04 significantly increased Lactobacillus and B. bifidum abundance, suggesting phages may function similarly to prebiotics in enhancing probiotic efficacy [126].
Several commercial products, such as InnovixLabs® Multi-Strain Probiotics, BioSchwartz® Probiotics, and Natrol® Immune-Biotic, now incorporate PreforPro® to enhance probiotic efficacy. By selectively lysing certain bacteria and releasing their cellular components, PreforPro® promotes phage–prebiotic–probiotic interactions, generating an integrated microbiota-modulating system. This synergy advances precision therapeutics, with targeted phage cocktails offering next-generation solutions for gut health. Further research is crucial to unlocking their full therapeutic potential, particularly in defining the optimal phage–probiotic pairings for specific clinical conditions.
Virome-based biomarkers for disease diagnosis and prognosis
The gut virome is emerging as a stable and disease-specific biomarker for gastrointestinal disorders such as IBD and CRC. Unlike bacterial markers, viral signatures remain less affected by transient environmental changes, making them valuable for early detection and prognosis.
In IBD, a decreased diversity of Caudovirales bacteriophages and an increased abundance of Microviridae phages correlate with disease severity, while a higher ratio of temperate to lytic phages is linked to chronic inflammation [51]. In CRC, distinct viral signatures include Fusobacterium-infecting phages, reflecting the pathogenic role of Fusobacterium nucleatum, as well as elevated levels of HPV and polyomaviruses, suggesting a potential link to oncogenesis [76].
Advances in shotgun metagenomic sequencing allow for non-invasive disease prediction and monitoring. Machine learning models trained on virome data can identify preclinical IBD and CRC cases [127, 128], while virome profiling helps to assess treatment responses, such as the success of FMT in IBD and CDI [129]. Additionally, shifts in virome composition post-chemotherapy correlate with immune modulation and treatment efficacy in CRC [130].
Future research should focus on developing standardized virome reference databases and integrating virome biomarkers with microbiome and metabolome data for comprehensive diagnostic models. Targeted virome-based therapeutics, such as phage therapy, could further enhance precision medicine in gastroenterology. With continued advancements in sequencing and computational analysis, virome-guided diagnostics has the potential to revolutionize disease detection and treatment strategies.
Conclusion
With advancements in metagenomic sequencing and bioinformatics, the once-hidden complexity of the gut virome is now being uncovered. As an integral component of the intestinal ecosystem, the virome is shaped by multiple factors, including diet, environment, host genetics, and immunity. Understanding these influences is crucial for deciphering the virome's interactions with gut microbiota and the immune system, highlighting its growing clinical relevance. The gut virome plays a critical role in enhancing the efficacy of microbiome-targeted therapies such as FMT, phage therapy, dietary interventions, and probiotics. By modulating gut health and immune responses, the virome contributes to improved treatment outcomes for gastrointestinal disorders, including IBD, CRC, and CDI. These findings underscore its potential to refine therapeutic strategies and optimize clinical outcomes.
Despite these promising prospects, several challenges still hinder the clinical application of virome-based interventions. Limitations in virome analysis, including sequencing biases and incomplete viral genome databases, complicate the identification of precise therapeutic targets. Furthermore, the spatial variability of the virome across different intestinal regions and fecal samples adds another layer of complexity, making the standardization of diagnostic and therapeutic approaches difficult. A key challenge in virome research is distinguishing causality from correlation. Many phage alterations observed in disease contexts may reflect downstream effects of bacterial shifts, owing to their host dependence. However, this pattern is also shaped by technical limitations in phage profiling, isolation, and culturing. Unlike bacteria, the absence of universal marker genes and reliance on shotgun metagenomics constrain exploration of the virome's role in intestinal disease. The gut virome remains a largely uncharted component of the microbiome, often referred to as its “dark matter”. Furthermore, the difficulty of culturing and isolating phages limits understanding of their causal roles in disease mechanisms and therapy development. Although advances in shotgun metagenomic sequencing have improved viral identification and biomarker discovery, these methods lack the simplicity of bacterial 16S rRNA-based sequencing for clinical diagnostics and phage profiling.
To overcome these challenges, future research should focus on refining viral genome databases, improving bioinformatic tools for viral identification, and developing targeted viral interventions. Furthermore, optimizing delivery methods for phage therapy and virome-modulating interventions will be essential for translating these approaches into clinical practice.
In conclusion, addressing these challenges will pave the way for precision medicine, where gut virome-based interventions could transform the treatment of microbiome-associated diseases. By integrating virome-based diagnostics and therapeutics, we can move toward more effective, personalized strategies that significantly enhance clinical outcomes.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants No. 82172323, 32100134, 823B2010), Guangdong Provincial Natural Science Foundation (Grant No. 2024A1515010533), Guangzhou Key R&D program (Grant No. 202206010014), and a seed fund from the Sixth Affiliated Hospital of Sun Yat-sen University and Sun Yat-sen University (Grant No. 2022JBGS03).
Contributor Information
Zhiyang Feng, Guangdong Institute of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510655, China; Key Laboratory of Human Microbiome and Chronic Diseases (Sun Yat-sen University), Ministry of Education, Guangzhou 510655, China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510655, China.
Elke Burgermeister, Department of Medicine II, Medical Faculty Mannheim, University of Heidelberg, Mannheim 69120, Germany.
Anna Philips, Institute of Bioorganic Chemistry, Polish Academy of Sciences, Poznan 61704, Poland.
Tao Zuo, Guangdong Institute of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510655, China; Key Laboratory of Human Microbiome and Chronic Diseases (Sun Yat-sen University), Ministry of Education, Guangzhou 510655, China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510655, China.
Weijie Wen, Guangdong Institute of Gastroenterology, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510655, China; Key Laboratory of Human Microbiome and Chronic Diseases (Sun Yat-sen University), Ministry of Education, Guangzhou 510655, China; Biomedical Innovation Center, The Sixth Affiliated Hospital, Sun Yat-sen University, Guangzhou 510655, China.
Author contributions
Zhiyang Feng (Writing—original draft), Elke Burgermeister (Writing—review & editing), Anna Philips (Writing—review & editing), Tao Zuo (Conceptualization, Supervision, Writing—review & editing) and Weijie Wen (Conceptualization, Supervision, Writing—review & editing).
Conflict of interest
None declared. In addition, as an Editorial Board Member of Precision Clinical Medicine, the corresponding author Tao Zuo was blinded from reviewing and making decision on this manuscript.
References
- 1. Gregory AC, Zablocki O, Zayed AA et al. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe. 2020;28:724–40. 10.1016/j.chom.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shkoporov AN, Clooney AG, Sutton TDS et al. The human gut virome is highly diverse, stable, and individual specific. Cell Host Microbe. 2019,26:527–41. 10.1016/j.chom.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 3. Cao Z, Sugimura N, Burgermeister E et al. The gut virome: A new microbiome component in health and disease. EBioMedicine. 2022;81:104113. 10.1016/j.ebiom.2022.104113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Virgin HW The virome in mammalian physiology and disease. Cell. 2014;157:142–50. 10.1016/j.cell.2014.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Troeger C, Blacker BF, Khalil IA et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1211–28. 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tate JE, Burton AH, Boschi-Pinto C et al. Global, regional, and national estimates of rotavirus mortality in children< 5 years of age, 2000–2013. Clin Infect Dis. 2016;62: S96–S105. 10.1093/cid/civ1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gogokhia L, Buhrke K, Bell R et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25:285–99. 10.1016/j.chom.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ooijevaar RE, Terveer EM, Verspaget HW et al. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. 2019;70:335–51. 10.1146/annurev-med-111717-122956 [DOI] [PubMed] [Google Scholar]
- 9. Lim ES, Zhou Y, Zhao G et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015;21:1228–34. 10.1038/nm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shah SA, Deng L, Thorsen J et al. Expanding known viral diversity in the healthy infant gut. Nat Microbiol. 2023;8:986–98. 10.1038/s41564-023-01345-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tisza MJ, Lloyd RE, Hoffman K et al. Longitudinal phage–bacteria dynamics in the early life gut microbiome. Nat Microbiol. 2025;10:420–30. 10.1038/s41564-024-01906-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Minot S, Bryson A, Chehoud C et al. Rapid evolution of the human gut virome. Proc Natl Acad Sci USA. 2013;110:12450–5. 10.1073/pnas.1300833110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martino C, Dilmore AH, Burcham ZM et al. Microbiota succession throughout life from the cradle to the grave. Nat Rev Micro. 2022;20:707–20. 10.1038/s41579-022-00768-z [DOI] [PubMed] [Google Scholar]
- 14. Johansen J, Atarashi K, Arai Y et al. Centenarians have a diverse gut virome with the potential to modulate metabolism and promote healthy lifespan. Nat Microbiol. 2023;8:1064–78. 10.1038/s41564-023-01370-6 [DOI] [PubMed] [Google Scholar]
- 15. Avellaneda-Franco L, Xie L, Nakai M et al. Dietary fiber intake impacts gut bacterial and viral populations in a hypertensive mouse model. Gut Microbes. 2024;16:2407047. 10.1080/19490976.2024.2407047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu TC, Kern JT, Jain U et al. Western diet induces Paneth cell defects through microbiome alterations and farnesoid X receptor and type I interferon activation. Cell Host Microbe. 2021;29:988–1001. 10.1016/j.chom.2021.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Mantrana I, Selma-Royo M, Alcantara C et al. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front Microbiol. 2018;9:890. 10.3389/fmicb.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Z, Li Y, Park H et al. Unveiling and harnessing the human gut microbiome in the rising burden of non-communicable diseases during urbanization. Gut Microbes. 2023;15:2237645. 10.1080/19490976.2023.2237645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuo T, Sun Y, Wan Y et al. Human-gut-DNA virome variations across geography, ethnicity, and urbanization. Cell Host Microbe. 2020;28:741–51. 10.1016/j.chom.2020.08.005 [DOI] [PubMed] [Google Scholar]
- 20. Delgado-Wicke P, Fernández de Córdoba-Oñate S, Roy-Vallejo E et al. Genetic variants regulating the immune response improve the prediction of COVID-19 severity provided by clinical variables. Sci Rep. 2024;14:20728. 10.1038/s41598-024-71476-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zuo T Gut bacteriophages ignite mammalian immunity. Nat Rev Micro. 2023;21:634. 10.1038/s41579-023-00911-4 [DOI] [PubMed] [Google Scholar]
- 22. Lozupone CA, Li M, Campbell TB et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329–39. 10.1016/j.chom.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monaco CL, Gootenberg DB, Zhao G et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe. 2016;19:311–22. 10.1016/j.chom.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chevallereau A, Pons BJ, van Houte S et al. Interactions between bacterial and phage communities in natural environments. Nat Rev Micro. 2022;20:49–62. 10.1038/s41579-021-00602-y [DOI] [PubMed] [Google Scholar]
- 25. Federici S, Nobs SP, Elinav E Phages and their potential to modulate the microbiome and immunity. Cell Mol Immunol. 2021;18:889–904. 10.1038/s41423-020-00532-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yue WF, Du M, Zhu MJ High temperature in combination with UV irradiation enhances horizontal transfer of stx 2 gene from E. coli O157: H7 to non-pathogenic E. coli. PLoS One. 2012;7:e31308. 10.1371/journal.pone.0031308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X, Lin S, Liu T et al. Xenogeneic silencing relies on temperature-dependent phosphorylation of the host H-NS protein in Shewanella. Nucleic Acids Res. 2021;49:3427–40. 10.1093/nar/gkab137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boling L, Cuevas DA, Grasis JA et al. Dietary prophage inducers and antimicrobials: toward landscaping the human gut microbiome. Gut Microbes. 2020;11:721–34. 10.1080/19490976.2019.1701353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Korkina L, Ozben T, Saso L Modulation of oxidative stress: pharmaceutical and pharmacological aspects. Oxid Med Cell Long. 2016;2016:6023417. 10.1155/2016/6023417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shkoporov AN, Turkington CJ, Hill C Mutualistic interplay between bacteriophages and bacteria in the human gut. Nat Rev Micro. 2022;20:737–49. 10.1038/s41579-022-00755-4 [DOI] [PubMed] [Google Scholar]
- 31. Camara-Wilpert S, Mayo-Muñoz D, Russel J et al. Bacteriophages suppress CRISPR–Cas immunity using RNA-based anti-CRISPRs. Nature. 2023;623:601–7. 10.1038/s41586-023-06612-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan X, Huang Z, Zhu Z et al. Recent advances in phage defense systems and potential overcoming strategies. Biotechnol Adv. 2023;65:108152. 10.1016/j.biotechadv.2023.108152 [DOI] [PubMed] [Google Scholar]
- 33. Silpe JE, Bassler BL A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell. 2019;176:268–80. 10.1016/j.cell.2018.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erez Z, Steinberger-Levy I, Shamir M et al. Communication between viruses guides lysis–lysogeny decisions. Nature. 2017;541:488–93. 10.1038/nature21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bloch S, Nejman-Faleńczyk B, Licznerska K et al. Complex effects of the exo-xis region of the Shiga toxin-converting bacteriophage Φ24B genome on the phage development and the Escherichia coli host physiology. J Appl Genetics. 2024;65:191–211. 10.1007/s13353-023-00799-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veses-Garcia M, Liu X, Rigden DJ et al. Transcriptomic analysis of Shiga-toxigenic bacteriophage carriage reveals a profound regulatory effect on acid resistance in Escherichia coli. Appl Environ Microb. 2015;81:8118–25. 10.1128/AEM.02034-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mai-Prochnow A, Hui JGK, Kjelleberg S et al. Big things in small packages: the genetics of filamentous phage and effects on fitness of their host. FEMS Microbiol Rev. 2015;39:465–87. 10.1093/femsre/fuu007 [DOI] [PubMed] [Google Scholar]
- 38. Jahn MT, Arkhipova K, Markert SM et al. A phage protein aids bacterial symbionts in eukaryote immune evasion. Cell Host Microbe. 2019;26:542–50. 10.1016/j.chom.2019.08.019 [DOI] [PubMed] [Google Scholar]
- 39. Aktories K, Schwan C, Jank T Clostridium difficile toxin biology. Annu Rev Microbiol. 2017;71:281–307. 10.1146/annurev-micro-090816-093458 [DOI] [PubMed] [Google Scholar]
- 40. Brown EM, Arellano-Santoyo H, Temple ER et al. Gut microbiome ADP-ribosyltransferases are widespread phage-encoded fitness factors. Cell Host Microbe. 2021;29:1351–65. 10.1016/j.chom.2021.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borodovich T, Shkoporov AN, Ross RP et al. Phage-mediated horizontal gene transfer and its implications for the human gut microbiome. Gastroenterol Rep. 2022;10:goac012. 10.1093/gastro/goac012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barr JJ, Auro R, Furlan M et al. Bacteriophage adhering to mucus provide a non–host-derived immunity. Proc Natl Acad Sci USA. 2013;110:10771–6. 10.1073/pnas.1305923110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bichet MC, Chin WH, Richards W et al. Bacteriophage uptake by mammalian cell layers represents a potential sink that may impact phage therapy. iScience. 2021;24:102287. 10.1016/j.isci.2021.102287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dąbrowska K Phage therapy: What factors shape phage pharmacokinetics and bioavailability? Systematic and critical review. Med Res Rev. 2019;39:2000–25. 10.1002/med.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Neil JA, Matsuzawa-Ishimoto Y, Kernbauer-Hölzl E et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat Microbiol. 2019;4:1737–49. 10.1038/s41564-019-0470-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ingle H, Lee S, Ai T et al. Viral complementation of immunodeficiency confers protection against enteric pathogens via interferon-λ. Nat Microbiol. 2019;4:1120–8. 10.1038/s41564-019-0416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sweere JM, Van Belleghem JD, Ishak H et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science. 2019;363:eaat9691. 10.1126/science.aat9691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ivanov II, Littman DR Modulation of immune homeostasis by commensal bacteria. Curr Opin Microbiol. 2011;14:106–14. 10.1016/j.mib.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Q, Wang M, Yang Q et al. The role of bacteriophages in facilitating the horizontal transfer of antibiotic resistance genes in municipal wastewater treatment plants. Water Res. 2025;268:122776. 10.1016/j.watres.2024.122776 [DOI] [PubMed] [Google Scholar]
- 50. Fluckiger A, Daillère R, Sassi M et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science. 2020;369:936–42. 10.1126/science.aax0701 [DOI] [PubMed] [Google Scholar]
- 51. Zuo T, Lu XJ, Zhang Y et al. Gut mucosal virome alterations in ulcerative colitis. Gut. 2019;68:1169–79. 10.1136/gutjnl-2018-318131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liang G, Cobián-Güemes AG, Albenberg L et al. The gut virome in inflammatory bowel diseases. Curr Opin Virol. 2021;51:190–8. 10.1016/j.coviro.2021.10.005 [DOI] [PubMed] [Google Scholar]
- 53. Yang Y, An R, Lyu C et al. Interactions between human norovirus and intestinal microbiota/microbes: A scoping review. Food Microbiol. 2024;119:104456. 10.1016/j.fm.2023.104456 [DOI] [PubMed] [Google Scholar]
- 54. Basic M, Keubler LM, Buettner M et al. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm Bowel Dis. 2014;20:431–43. 10.1097/01.MIB.0000441346.86827.ed [DOI] [PubMed] [Google Scholar]
- 55. Cadwell K, Patel KK, Maloney NS et al. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–45. 10.1016/j.cell.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Amimo JO, Raev SA, Chepngeno J et al. Rotavirus interactions with host intestinal epithelial cells. Front Immunol. 2021;12:793841. 10.3389/fimmu.2021.793841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ball JM, Mitchell DM, Gibbons TF et al. Rotavirus NSP4: a multifunctional viral enterotoxin. Viral Immunol. 2005;18:27–40. 10.1089/vim.2005.18.27 [DOI] [PubMed] [Google Scholar]
- 58. Nissen LHC, Nagtegaal ID, de Jong DJ et al. Epstein–Barr virus in inflammatory bowel disease: the spectrum of intestinal lymphoproliferative disorders. Journal of Crohn's and Colitis. 2015;9:398–403. 10.1093/ecco-jcc/jjv040 [DOI] [PubMed] [Google Scholar]
- 59. Vega R, Bertran X, Menacho M et al. Cytomegalovirus infection in patients with inflammatory bowel disease. Korean J Gastroenterol. 2022;80:60–5. 10.4166/kjg.2022.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Norman JM, Handley SA, Baldridge MT et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–60. 10.1016/j.cell.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cornuault JK, Petit MA, Mariadassou M et al. Phages infecting Faecalibacterium prausnitzii belong to novel viral genera that help to decipher intestinal viromes. Microbiome. 2018;6:65. 10.1186/s40168-018-0452-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Massimino L, Lovisa S, Lamparelli LA et al. Gut eukaryotic virome in colorectal carcinogenesis: Is that a trigger?. Comput Struct Biotechnol J. 2021;19:16–28. 10.1016/j.csbj.2020.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wagner J, Maksimovic J, Farries G et al. Bacteriophages in gut samples from pediatric Crohn's disease patients: metagenomic analysis using 454 pyrosequencing. Inflamm Bowel Dis. 2013;19:1598–608. 10.1097/MIB.0b013e318292477c [DOI] [PubMed] [Google Scholar]
- 64. Liang G, Conrad MA, Kelsen JR et al. Dynamics of the stool virome in very early-onset inflammatory bowel disease. Journal of Crohn's and Colitis. 2020;14:1600–10. 10.1093/ecco-jcc/jjaa094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Adiliaghdam F, Amatullah H, Digumarthi S et al. Human enteric viruses autonomously shape inflammatory bowel disease phenotype through divergent innate immunomodulation. Sci Immunol. 2022;7:eabn6660. 10.1126/sciimmunol.abn6660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mihindukulasuriya KA, Mars RAT, Johnson AJ et al. Multi-omics analyses show disease, diet, and transcriptome interactions with the virome. Gastroenterology. 2021;161:1194–207. 10.1053/j.gastro.2021.06.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li M, Wang C, Guo Q et al. More positive or more negative? Metagenomic analysis reveals roles of virome in human disease-related gut microbiome. Front Cell Infect Microbiol. 2022;12:846063. 10.3389/fcimb.2022.846063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zuo T, Wong SH, Lam K et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2018;67:634–43. 10.1136/gutjnl-2017-313952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shen S, Huo D, Ma C et al. Expanding the colorectal cancer biomarkers based on the human gut phageome. Microbiol Spectr. 2021;9:e00090–21. 10.1128/Spectrum.00090-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen F, Li S, Guo R et al. Meta-analysis of fecal viromes demonstrates high diagnostic potential of the gut viral signatures for colorectal cancer and adenoma risk assessment. J Adv Res. 2023;49:103–14. 10.1016/j.jare.2022.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zuo W, Michail S, Sun F Metagenomic analyses of multiple gut datasets revealed the association of phage signatures in colorectal cancer. Front Cell Infect Microbiol. 2022;12:918010. 10.3389/fcimb.2022.918010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Broecker F, Moelling K The roles of the virome in cancer. Microorganisms. 2021;9:2538. 10.3390/microorganisms9122538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thabane M, Marshall JK Post-infectious irritable bowel syndrome. World J Gastroenterol. 2009;15:3591. 10.3748/wjg.15.3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rodemann JF, Dubberke ER, Reske KA et al. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5:339–44. 10.1016/j.cgh.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 75. Koo HL, Ajami NJ, Jiang ZD et al. A nosocomial outbreak of norovirus infection masquerading as Clostridium difficile infection. Clin Infect Dis. 2009;48:e75–7. 10.1086/597299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nakatsu G, Zhou H, Wu WKK et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology. 2018;155:529–41. 10.1053/j.gastro.2018.04.018 [DOI] [PubMed] [Google Scholar]
- 77. Ou S, Wang H, Tao Y et al. Fusobacterium nucleatum and colorectal cancer: From phenomenon to mechanism. Front Cell Infect Microbiol. 2022;12:1020583. 10.3389/fcimb.2022.1020583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Laghi L, Randolph AE, Chauhan DP et al. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc Natl Acad Sci USA. 1999;96:7484–9. 10.1073/pnas.96.13.7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baandrup L, Thomsen LT, Olesen TB et al. The prevalence of human papillomavirus in colorectal adenomas and adenocarcinomas: a systematic review and meta-analysis. Eur J Cancer. 2014;50:1446–61. 10.1016/j.ejca.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 80. de Martel C, Georges D, Bray F et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180–90. 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 81. Leal Rodríguez C, Shah SA, Rasmussen MA et al. The infant gut virome is associated with preschool asthma risk independently of bacteria. Nat Med. 2024;30:138–48. 10.1038/s41591-023-02685-x [DOI] [PubMed] [Google Scholar]
- 82. Su RP, Wen WJ, Jin YF et al. Dietary Whey Protein protects against Crohn's disease by orchestrating cross-kingdom interaction between the gut phageome and bacteriome. Gut. 2025;; gutjnl-2024-334516. 10.1136/gutjnl-2024-334516. [DOI] [PubMed] [Google Scholar]
- 83. Clooney AG, Sutton TDS, Shkoporov AN et al. Whole-virome analysis sheds light on viral dark matter in inflammatory bowel disease. Cell Host Microbe. 2019;26:764–78. 10.1016/j.chom.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 84. Wagner PL, Waldor MK Bacteriophage control of bacterial virulence. Infect Immun. 2002;70:3985–93. 10.1128/IAI.70.8.3985-3993.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Caldeira LDF, Borba HH, Tonin FS et al. Fecal microbiota transplantation in inflammatory bowel disease patients: a systematic review and meta-analysis. PLoS One. 2020;15:e0238910. 10.1371/journal.pone.0238910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Haifer C, Paramsothy S, Kaakoush NO et al. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol hepatol. 2022;7:141–51. 10.1016/S2468-1253(21)00400-3 [DOI] [PubMed] [Google Scholar]
- 87. Conceição-Neto N, Deboutte W, Dierckx T et al. Low eukaryotic viral richness is associated with faecal microbiota transplantation success in patients with UC. Gut. 2018;67:1558–9. 10.1136/gutjnl-2017-315281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Weingarden AR, Chen C, Bobr A et al. Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiology Gastrointestinal Liver Physiol. 2014;306:G310–9. 10.1152/ajpgi.00282.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kelly CR, Ihunnah C, Fischer M et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–71. 10.1038/ajg.2014.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Broecker F, Russo G, Klumpp J et al. Stable core virome despite variable microbiome after fecal transfer. Gut Microbes. 2017;8:214–20. 10.1080/19490976.2016.1265196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McGill SK Fecal microbiota transplant for severe Clostridioides difficile infection: Let's halt the raging fire. Clin Infect Dis. 2021;73:720–1. 10.1093/cid/ciab047 [DOI] [PubMed] [Google Scholar]
- 92. Yu H, Li XX, Han X et al. Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses. Front Microbiol. 2023;14:1126808. 10.3389/fmicb.2023.1126808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park H, Laffin MR, Jovel J et al. The success of fecal microbial transplantation in Clostridium difficile infection correlates with bacteriophage relative abundance in the donor: a retrospective cohort study. Gut Microbes. 2019;10:676–87. 10.1080/19490976.2019.1586037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fujimoto K, Kimura Y, Allegretti JR et al. Functional restoration of bacteriomes and viromes by fecal microbiota transplantation. Gastroenterology. 2021;160:2089–102. 10.1053/j.gastro.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Broecker F, Klumpp J, Moelling K Long-term microbiota and virome in a Zürich patient after fecal transplantation against Clostridium difficile infection. Ann NY Acad Sci. 2016;1372:29–41. 10.1111/nyas.13100 [DOI] [PubMed] [Google Scholar]
- 96. Draper LA, Ryan FJ, Smith MK et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6:220. 10.1186/s40168-018-0598-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li N, Li Y, Huang Z et al. Faecal phageome transplantation alleviates intermittent intestinal inflammation in IBD and the timing of transplantation matters: a preclinical proof-of-concept study in mice. Gut. 2025;74:868–70. 10.1136/gutjnl-2024-333598 [DOI] [PubMed] [Google Scholar]
- 98. Galtier M, Sordi LD, Sivignon A et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn's disease. J Crohns Colitis. 2017;11:840–7. 10.1093/ecco-jcc/jjw224 [DOI] [PubMed] [Google Scholar]
- 99. Federici S, Kredo-Russo S, Valdés-Mas R et al. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell. 2022;185:2879–98. 10.1016/j.cell.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 100. Cong J, Liu P, Han Z et al. Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8+ T cell effector functions. Immunity. 2024;57:876–89. 10.1016/j.immuni.2024.02.014 [DOI] [PubMed] [Google Scholar]
- 101. Dong X, Pan P, Zheng DW et al. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6:eaba1590. 10.1126/sciadv.aba1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Khambhati K, Bhattacharjee G, Gohil N et al. Phage engineering and phage-assisted CRISPR-Cas delivery to combat multidrug-resistant pathogens. Bioeng Transl Med. 2023;8:e10381. 10.1002/btm2.10381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ragothaman M, Yoo SY Engineered phage-based cancer vaccines: Current advances and future directions. Vaccines. 2023;11:919. 10.3390/vaccines11050919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li HR, Zhou Y, Ye BC Tumor-targeted delivery of PD-1-displaying bacteriophages by Escherichia coli for adjuvant treatment of colorectal cancer. ACS Synth Biol. 2025;14:407–19. 10.1021/acssynbio.4c00570 [DOI] [PubMed] [Google Scholar]
- 105. Zheng DW, Dong X, Pan P et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3:717–28. 10.1038/s41551-019-0423-2 [DOI] [PubMed] [Google Scholar]
- 106. Islam MS, Fan J, Pan F The power of phages: revolutionizing cancer treatment. Front Oncol. 2023;13:1290296. 10.3389/fonc.2023.1290296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sabino J, Hirten RP, Colombel JF bacteriophages in gastroenterology—from biology to clinical applications. Aliment Pharmacol Ther. 2020;51:53–63. 10.1111/apt.15557 [DOI] [PubMed] [Google Scholar]
- 108. Lamers CR, De Roos NM, Heerink HH et al. Lower impact of disease on daily life and less fatigue in patients with inflammatory bowel disease following a lifestyle intervention. Inflamm Bowel Dis. 2022;28:1791–9. 10.1093/ibd/izac027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang S, Martins R, Sullivan MC et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome. 2019;7:126. 10.1186/s40168-019-0740-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zeng W, Wu J, Xie H et al. Enteral nutrition promotes the remission of colitis by gut bacteria-mediated histidine biosynthesis. EBioMedicine. 2024;100:104959. 10.1016/j.ebiom.2023.104959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nybacka S, Törnblom H, Josefsson A et al. A low FODMAP diet plus traditional dietary advice versus a low-carbohydrate diet versus pharmacological treatment in irritable bowel syndrome (CARIBS): a single-centre, single-blind, randomised controlled trial[J]. Lancet Gastroenterol Hepatol. 2024;9:507–20. 10.1016/S2468-1253(24)00045-1 [DOI] [PubMed] [Google Scholar]
- 112. Ringel-Kulka T, McRorie J, Ringel Y Multi-center, double-blind, randomized, placebo-controlled, parallel-group study to evaluate the benefit of the probiotic Bifidobacterium infantis 35624 in non-patients with symptoms of abdominal discomfort and bloating. American J Gastroenterol. 2017;112:145–51. 10.1038/ajg.2016.511 [DOI] [PubMed] [Google Scholar]
- 113. Tong H, Jiang Z, Song L et al. Dual impacts of serine/glycine-free diet in enhancing antitumor immunity and promoting evasion via PD-L1 lactylation. Cell Metab. 2024;36:2493–510. 10.1016/j.cmet.2024.10.019 [DOI] [PubMed] [Google Scholar]
- 114. Cao Z, Fan D, Sun Y et al. The gut ileal mucosal virome is disturbed in patients with Crohn's disease and exacerbates intestinal inflammation in mice. Nat Commun. 2024;15:1638. 10.1038/s41467-024-45794-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thomson C, Garcia AL, Edwards CA Interactions between dietary fibre and the gut microbiota. Proc Nutr Soc. 2021;80:398–408. 10.1017/S0029665121002834 [DOI] [PubMed] [Google Scholar]
- 116. Huang M, Chen Z, Lang C et al. Efficacy of mesalazine in combination with bifid triple viable capsules on ulcerative colitis and the resultant effect on the inflammatory factors. Cell Host Microbe. 2018;24:500. 10.1016/j.chom.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 117. Scaldaferri F, Gerardi V, Mangiola F et al. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: an update. World J Gastroenterol. 2016;22:5505. 10.3748/wjg.v22.i24.5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rufino MN, Aleixo GFP, Trombine-Batista IE et al. Systematic review and meta-analysis of preclinical trials demonstrate robust beneficial effects of prebiotics in induced inflammatory bowel disease. J Nutr Biochem. 2018;62:1–8. 10.1016/j.jnutbio.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 119. Hallert C, Kaldma M, Petersson BG Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand J Gastroenterol. 1991;26:747–50. 10.3109/00365529108998594 [DOI] [PubMed] [Google Scholar]
- 120. Hijová E, Šoltésová A, Salaj R et al. Preventive use of Lactobacillus plantarum LS/07 and inulin to relieve symptoms of acute colitis. Acta Biochim Pol. 2015;62:553–7. 10.18388/abp.2015_1008 [DOI] [PubMed] [Google Scholar]
- 121. Schroeder BO, Birchenough GMH, Ståhlman M et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe. 2018;23:27–40. 10.1016/j.chom.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wu Y, Li Y, Zheng Q et al. The efficacy of probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in irritable bowel syndrome: a systematic review and network meta-analysis. Nutrients. 2024;16:2114. 10.3390/nu16132114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gindin M, Febvre HP, Rao S et al. Bacteriophage for gastrointestinal health (PHAGE) study: evaluating the safety and tolerability of supplemental bacteriophage consumption. J Am Coll Nutr. 2019;38:68–75. 10.1080/07315724.2018.1483783 [DOI] [PubMed] [Google Scholar]
- 124. Febvre HP, Rao S, Gindin M et al. PHAGE study: effects of supplemental bacteriophage intake on inflammation and gut microbiota in healthy adults. Nutrients. 2019;11:666. 10.3390/nu11030666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. D'Accolti M, Soffritti I, Mazzacane S et al. Bacteriophages as a potential 360-degree pathogen control strategy. Microorganisms. 2021;9:261. 10.3390/microorganisms9020261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Grubb DS, Wrigley SD, Freedman KE et al. PHAGE-2 study: Supplemental bacteriophages extend Bifidobacterium animalis subsp. lactis BL04 benefits on gut health and microbiota in healthy adults. Nutrients. 2020;12:2474. 10.3390/nu12082474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tian X, Li S, Wang C et al. Gut virome-wide association analysis identifies cross-population viral signatures for inflammatory bowel disease. Microbiome. 2024;12:130. 10.1186/s40168-024-01832-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Murovec B, Deutsch L, Stres B Predictive modeling of colorectal cancer using exhaustive analysis of microbiome information layers available from public metagenomic data. Front Microbiol. 2024;15:1426407. 10.3389/fmicb.2024.1426407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fanizzi F, D'Amico F, Bombassaro IZ et al. The role of fecal microbiota transplantation in IBD.Microorganisms. 2024;12:1755. 10.3390/microorganisms12091755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Huang H, Yang Y, Wang X et al. Gut virome dysbiosis impairs antitumor immunity and reduces 5-fluorouracil treatment efficacy for colorectal cancer. Front Oncol. 2024;14:1501981. 10.3389/fonc.2024.1501981 [DOI] [PMC free article] [PubMed] [Google Scholar]