Highlights
-
•
The establishment of a novel fluorescence-based assay tailored for 2-E channel inhibitor screening.
-
•
The structural and functional characterization of a 2-E channel blocker.
-
•
The validation of 2-E channel as a promising antiviral target.
Keywords: SARS-CoV-2 envelope protein, Viroporin, Fluorescence release, Antivirus, Drug target
Abstract
The SARS-CoV-2-encoded 2-E channel is critical in the viral life cycle and pathogenesis. By facilitating viral replication, it promotes the dysregulation of inflammatory pathways, leading to cytokine storm, and triggers DNA damage response (DDR), thus exacerbating disease progression. The 2-E channel, a viroporin, is a promising antiviral target. However, the lack of specific inhibitors and effective screening methods has hindered therapeutic exploitation of the 2-E channel. To address this gap, we report on a fluorescence-based screening assay that targets the 2-E channel activity, resulting in the identification of potential inhibitory molecules. After performing both electrophysiological studies and surface plasmon resonance (SPR) analyses, we identified the top-ranked candidate, TPN10518, as a pore-blocking inhibitor of the 2-E channel. TPN10518 binds to a hydrophobic pocket in the C-terminal vestibule of the 2-E channel, thereby inhibiting its activity. Functional evaluation showed that TPN10518 exhibits significant antiviral efficacy in vitro, while, at the same time, effectively protecting against 2-E channel-mediated host damage and suppressing cytokine storm caused by dysregulated homeostasis of inflammatory pathways in vivo. Therefore, our work introduces a screening method for targeting 2-E channels, establishes the 2-E channel as a viable therapeutic target against SARS-CoV-2, and identifies TPN10518 as a promising antiviral candidate.
Graphical abstract
1. Introduction
The SARS-CoV-2 envelope (2-E) protein is the smallest of four structural proteins (Cao et al., 2021). It forms homopentameric channels within the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) membrane of infected cells (Mandala et al., 2020; Medeiros-Silva et al., 2023; Surya et al., 2023; Wilson et al., 2004), thus becoming the gateway wherein 2-E can exert multiple functions during the viral life cycle and contribute substantially to host tissue damage (Zhou et al., 2023).
During the viral life cycle, 2-E channels facilitate ion flux across membranes, which is essential for viral assembly and budding, as well as for maintaining the ionic environment necessary for efficient virion production. Within the ERGIC, 2-E channel activity regulates viral particle formation, and through functional coordination with the spike (S) protein, it supports the viral life cycle (Boson et al., 2021). Additionally, the amphipathic C-terminal domain of the 2-E protein induces membrane curvature, further enhancing virion release efficiency (Kuzmin et al., 2022). At the host level, the 2-E channel impacts host ion homeostasis, signaling pathways, and immune responses. By disrupting transmembrane calcium and proton gradients, the 2-E channel induces electrolyte imbalances, leading to intracellular and extracellular homeostasis dysregulation (Lippi et al., 2020; Poggio et al., 2023).
These imbalances not only impair host cell functions but also facilitate viral replication by restructuring inter-organelle contact sites (Poggio et al., 2023; Xu et al., 2024). Furthermore, the 2-E protein activates Toll-like receptor (TLR) signaling pathways, triggering the release of pro-inflammatory cytokines and contributing to airway inflammation and acute respiratory distress syndrome (ARDS) (Xia et al., 2021; Xu et al., 2024; Zheng et al., 2021). Through modulation of the c-Jun N-terminal kinase (JNK) signaling pathway, the 2-E protein enhances inflammatory responses, while simultaneously disrupting tight junction protein regulation, thereby compromising host barrier functions (Xu et al., 2024).
Notably, the evolutionary conservation of the 2-E channel makes it a potential antiviral target. Compared to the SARS-CoV-E protein, the SARS-CoV-2 E protein shows high sequence conservation with no changes identified in the ion channel-forming region across SARS-CoV-2 isolates (Hassan et al., 2020). This structural conservation supports functional stability and reduces the risk of resistance mutations, presenting opportunities for combination therapy (Hassan et al., 2020). These mechanisms and multifaceted functions of the E protein allow it to address challenges associated with single therapeutic strategies, making it an ideal antiviral target.
Intervention strategies targeting the 2-E protein include antibodies, vaccines, and small-molecule inhibitors. Although some inhibitors targeting the 2-E channel have been reported, such as hexamethylene amiloride (HMA) (Somberg et al., 2023; Wilson et al., 2006), amantadine (AMT) (Toft-Bertelsen et al., 2021; Torres et al., 2007), sinapic acid (SA) (Orfali et al., 2021), proanthocyanidins (BE-33) (Wang et al., 2022), gliclazide and memantine (Singh Tomar et al., 2020), these compounds do not meet clinical needs. High-throughput screening methods targeting the 2-E protein, including pharmacophore-based virtual screenings (Mukherjee et al., 2021), computational multistage structure-driven screenings (Jalily et al., 2022), and cell-based high-throughput screenings (Wang et al., 2022), have been developed. However, the lack of specific screening methods targeting functional aspects of the 2-E channel remains a key limitation in the development of 2-E channel-targeted therapies.
In this study, we developed a screening assay based on 2-E channel-induced Tl+ fluorescence release and identified six promising compounds. Among them, TPN10518, a novel artemisinin derivative (Xie et al., 2023), exhibited potent inhibitory effects on 2-E channel activity and antiviral capability. Furthermore, TPN10518 showed its ability to effectively alleviate 2-E channel-induced pathological effects in vivo. These findings provide evidence supporting the feasibility of targeting 2-E protein as an antiviral strategy and establish a basis for subsequent drug development.
2. Materials and methods
2.1. Plasmids and compounds
4-Aminopyridine (4-AP, Sigma, USA, #275,875) was prepared as a 500 mM stock solution with ddH2O. Wild-type SARS-CoV-2-E sequences (NCBI reference sequence YP_009724392.1, residues 1–75) were constructed in the eukaryotic (pcDNA3.1) and prokaryotic (pGEX-6P-1) expression vectors. Point mutations were generated using site-directed mutagenesis and confirmed by sequencing. All compounds applied for screening are in-house compounds. TPN10518 was provided by Xiangrui Jiang (Shanghai Institute of Materia Medica, Chinese Academy of Sciences).
2.2. Cell culture
The A549 human lung carcinoma cell line and Vero E6 cells were purchased from the American Type Culture Collection (ATCC, USA). A549 cells and Vero E6 cells were cultured in RPMI-1640 (Gibco, USA, #11,875,500) and DMEM (Gibco, USA, #11,054,001), respectively. Both RPMI-1640 and DMEM were supplemented with 10 % fetal bovine serum (FBS, Gibco, USA, #10,100) and 100 units/mL penicillin/streptomycin (Gibco, USA, #15,070,063). All cells were grown in a 37 °C humidified incubator with 5 % CO₂ and passaged when the density reached 80–90 %.
2.3. Cytotoxicity assay
A549 cells were seeded at 8000 cells per well in a 96-well plate overnight and incubated with the addition of compounds for 24 h. Cell viability was measured using the CCK-8 kit (Yeasen, China, #40203ES60) following the manufacturer's instructions. Absorbance was measured at 450 nm using a microplate reader manufactured by Thermo Fisher Scientific (Waltham, MA, USA).
2.4. FDSS Tl Ion fluorescence release assay
A549 cells were seeded in wells of a 96-well plate at 8000 cells per well, and 250 ng of 2-E plasmid per well was transfected after 24 h using Lipofectamine 3000 transfection reagent (Thermo Fisher, USA, #L3000015) according to the manufacturer’s protocol. The culture medium was replaced 6 h after compound addition. 18 h post-replacement, the medium was aspirated and detection was initiated. We used the FluxOR™ Potassium Ion Channel Assay kit from Thermo Fisher (USA, #F10016) following the manufacturer’s instructions with modifications, including the addition of 80 μL loading buffer per well and incubation for 1 h at 18–24 °C in the dark. This was followed by aspirating the loading buffer and adding 100 μL assay buffer containing 1 mM 4-AP to each well. The final incubation lasted 40 min at 18–24 °C in the dark.
The assay was then performed using the FDSS/μCell screening system (Hamamatsu, Japan). The protocol required recording the fluorescence intensity of each well every second for 10 s (excitation wavelength of 460–490 nm, emission wavelength of 520–540 nm), followed by the addition of 20 μL stimulus buffer to each well. Afterward, the recording of fluorescence intensity lasted 10 min. Data analysis defined the control group as A549 cells transfected with pcDNA3.1 vector plasmid alone, the model group as A549 cells transfected with 2-E plasmid alone, and the drug group as A549 cells transfected with 2-E plasmid followed by the addition of compounds. Compounds with Adrug/Amodel < 1 were selected for rescreening. Inhibition (%) = [1 - (Adrug - Acontrol) / (Amodel - Acontrol)] × 100.
2.5. Planar lipid bilayer (BLM)
2-E protein was expressed in E.coli BL21/DE3 pLysS (TransGen Biotech, China, #CD901) and purified using a Ni-NTA column, and detected by LC-MS/MS as described in our previous work (Xia et al., 2021). Lipid membranes were formed from phosphatidylcholine (PC, #850356P): phosphatidylserine (PS, #850408P) = 3:2. All lipids were purchased from Avanti Polar Lipids (USA). We defined the cup side as the cis side and the chamber side as the trans side. The cis side was 1 mL of 500 mM KCl and 5 mM HEPES, and the trans side was 1 mL of 50 mM KCl and 5 mM HEPES, both at pH 6.8. The currents of purified 2-E proteins were recorded in a voltage clamp mode using a Warner BC-535 bilayer clamp amplifier (Warner Instruments, USA) filtered at 1–2 kHz. The currents were digitized using pClamp 10.2 software (Molecular Devices, USA). The open time was determined by fitting a single-/double-exponential function. Open times <0.5–1.5 ms were ignored. The open probability (Po) of single-channel currents was determined from the amplitude histogram by calculating the ratio of the area corresponding to open channels to the total area. We defined Po, control, and Po, drug as the probability of 2-E channel opening before and after adding compounds. Channel inhibition (%) = [(1 - Po, drug/Po, control)] × 100.
2.6. Size exclusion chromatography (SEC)
The Superdex 75 Increase 10/300 column (GE Healthcare, USA, #29,148,721) was washed with 50 mL of ddH2O. Then, the column was equilibrated with TBS buffer (150 mM NaCl, 20 mM Tris-base, 0.06 % DDM, pH 8.0) until a stable baseline was obtained at a flow rate of 0.5 mL/min. Subsequently, 500 μL of concentrated 2-E protein solution was injected into the sample loop of the AKTA purifier using a syringe, with care taken to avoid bubble formation, and the loading was performed at a flow rate of 0.5 mL/min. Elution was monitored continuously by UV absorption at 280 nm. Fractions corresponding to the 2-E protein elution peak were collected based on the chromatographic profile.
2.7. In vivo pharmacodynamic evaluation
Eight-week-old male C57BL/6 mice were provided by Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All animal handling was performed under the National Institutes of Health Guide for the Care and Use of Laboratory Animals, strictly following protocols approved by the Institutional Animal Care and Use Committee (IACUC).
We constructed a SARS-CoV-2-E-induced mouse model by delivering 2-E protein (2.5 mg/kg body weight) via intratracheal nebulization to mice. TBS and GST groups were used as negative controls. The 2-E protein group containing ion channel activity-impaired mutations (2-ET9I/T11A) was used as a functional control. 5 mg/kg or 10 mg/kg TPN10518 was administrated by intraperitoneal (IP) injections to mice 1 h before tracheal nebulization. Mice receiving an equal volume of DMSO were used as controls. Mice were sacrificed 24 h later, and the spleens and lungs were harvested for pathological characterization and assayed for mRNA levels of inflammatory cytokines and chemokines.
2.8. Histology/Immunofluorescence
Tissues from mice were fixed in 4 % PFA for 48 h, paraffin-embedded, and then cut into 3 μm sections following the standard procedure. The sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. The pathological score was assessed based on the degree of lung tissue lesions, including alveolar septal thickening, hemorrhage, inflammatory cell infiltration, and consolidation. The semi-quantitative assessment was performed by scoring 0 when no alveolar septal thickening was observed, 1 when alveolar septal thickening was very mild and the area of alveolar septal thickening, hemorrhage, and inflammatory cells infiltration was <10 %, 2 when alveolar septal thickening was mild and the area of alveolar septal thickening, hemorrhage, and inflammatory cells infiltration was 10–25 %, 3 when alveolar septal thickening was moderate and the area of alveolar septal thickening, hemorrhage, and inflammatory cells infiltration was 25–50 %, 4 when alveolar septal thickening was marked and the area of alveolar septal thickening, hemorrhage, inflammatory cells infiltration, and consolidation was 50–75 %, and 5 when alveolar septal thickening was very marked and the area of alveolar septal thickening, hemorrhage, inflammatory cells infiltration, and consolidation was greater than 75 %. The images were taken by a NanoZoomer®S360 scanner.
2.9. Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from lung tissues with Total RNA Extraction Reagent (Yeasen, China, #10606ES60). The obtained RNA was reverse-transcribed to cDNA through ABScript III RT Master Mix (Abclonal, China, #RK20429). Each experimental result represents three independent replicates using SYBR Green Master Mix (Yeasen, China, #11184ES03) to quantify the mean values of ΔCt and SD (Standard Deviation). The primers used for quantification are listed in Supplementary Information, Table S1.
2.10. Surface Plasmon Resonance (SPR)
The binding affinity of compounds to 2-E protein was assessed using the Biacore T200 Surface Plasmon Resonance (SPR) System (GE Healthcare, USA). 2-E protein was immobilized onto the surface of a CM5 chip by amine coupling in 10 mM sodium acetate buffer (pH 4.5) at a flow rate of 10 μL per minute. A mixture of 50 mM N-hydroxysuccinimide (NHS) and 200 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide was injected for 7 min to activate the sensor surface. Subsequently, 50 μg/mL of protein was injected to a target level of approximately 1400 RU, and the surface was blocked with 1 M ethanolamine (pH 8.5). Compounds were injected into the flow system at serial concentrations (12.5, 25, 50, 100, and 200 μM) with an analysis time of 90 s and a separation time of 120 s. The compounds were analyzed and separated in the flow system. All binding analyses were performed in phosphate-buffered saline (pH 7.4, 25 °C) containing 0.05 % (v/v) Tween 20 and 1 % DMSO. Overall refractive index variations, injection noise, and data drift were eliminated by double reference subtraction before analysis. Binding affinity was determined by fitting a Langmuir 1:1 binding model in Biacore evaluation software (GE Healthcare, USA).
2.11. Binding site detection and molecular docking
The solid-state nuclear magnetic resonance (ssNMR) structure of the transmembrane domain (TM) of 2-E protein (PDB code: 7K3G) (Mandala et al., 2020) was employed to perform molecular docking. To detect the ligand-binding site, we used DiffDock (Corso et al., 2022) to perform blind-docking of TPN10518 to the TM of the protein. Pocket binding to TPN10518 with a low confidence score (<2.0) was considered to be suggestive of a potential binding site. Based on that reference point, TPN10518 was then docked to possible binding sites using AutoDock Vina (Santos-Martins et al., 2021). The TPN10518-binding model with the lowest docking score was selected for analysis. A Schrödinger MM-GBSA module was used to characterize protein-ligand interactions.
2.12. Antiviral activity assay
SARS-CoV-2 strain BA.2.2 was stored at Fudan University It was propagated and titrated in Vero E6 cells, and its associated operations were performed in a biosafety level 3 (BSL-3) facility. Vero E6 cells were seeded in a 96-well plate with 1 × 104 cells per well and incubated at 37 °C in a cell culture incubator for 12 h. After mixing equal volumes of the compound serially diluted in DMEM medium and SARS-CoV-2 (MOI=0.01) thoroughly, 100 μL of the mixture was added to the 96-well plate with pre-removed supernatant. The viral copy number was measured in the cell supernatant collected 48 h later. Viral RNA was extracted from the cell supernatant using the Easy Pure Viral DNA/RNA Kit (TransGen Biotech, China, #ER201-02) as the instruction described. Reverse transcription was then performed using the One Step PrimeScript RT-PCR Kit (TaKaRa, Japan, #RR064A), followed by quantitative PCR to measure the SARS-CoV-2 RNA copy number. The primer sequences targeting the SARS-CoV-2 N gene are listed in Supplementary Information, Table S1.
2.13. Statistical analysis
Analyses were performed in GraphPad Prism. All statistical significance tests were conducted using ANOVA or unpaired Student’s t-test. The data are presented as the mean ± SD. The dose-effect curve was fitted using the Hill equation.
3. Results
3.1. Development of a fluorescence release-based screening method targeting the 2-E channel
Our previous studies demonstrated that the 2-E protein forms a potassium-permeable ion channel and mediates intracellular potassium flux (Xia et al., 2021). To advance the development of functional therapeutics targeting the 2-E channel, we aimed to establish a specific screening method based on the unique biophysical properties of the 2-E channel. This approach was designed to reduce nonspecific responses and false positives often encountered in cell death-based screening systems wherein multiple proteins are activated downstream of 2-E channel activation (Liu et al., 2024; Wang et al., 2022). We employed the FluxOR™ assay, a fluorescence-based technique that evaluates potassium channel activity via thallium ion (Tl⁺) permeability (Weaver et al., 2004). While FluxOR™ has been widely used for cellular potassium channel studies, its application to viral-encoded ion channels remains unexplored. In our experiments, cells expressing the 2-E channel were incubated with a thallium-sensitive fluorescent dye. To minimize background activity from endogenous potassium channels, we included 1 mM 4-AP, a classical potassium channel inhibitor (Fig. 1A). Control groups included cells transfected with empty vectors as a negative control and the 2-E channel-specific inhibitor BE-33 as a positive control. Upon extracellular addition of thallium ions, cells expressing the 2-E channel exhibited significantly higher fluorescence intensity compared to the controls. Conversely, treatment with BE-33 or HMA markedly suppressed the thallium-induced fluorescence signal (Fig. 1B, Supplementary Figure S2A). These results suggested the feasibility and specificity of a 2-E channel screening assay based on a thallium-sensitive fluorescent dye. To further validate the utility of this screening system, we defined compounds with the fluorescence intensity ratio Adrug/Amodel < 1 as effective inhibitors of 2-E channel activity. Using this criterion, we screened a library of 38 structurally diverse compounds synthesized in-house. Compound concentrations were carefully controlled to avoid cytotoxic effects (Fig. 1C, Supplementary Figure S1). Following two rounds of screening, we identified six candidate molecules that significantly suppressed 2-E-induced potassium flux fluorescence signals (Fig. 1D).
Fig. 1.
Fluorescence-based preliminary screening of potential 2-E inhibitors using the Tl+ release assay. (A) Schematic diagram of the FDSS Tl+ fluorescence assay. (B) Relative fluorescence intensity of Tl+ in A549 cells transfected with 2-E plasmid or vector, either with or without 1 μM BE-33 added in the 2-E expression group. Left: Fluorescence curves for each group; Right: Relative fluorescence units of each group at the detection time of 10 min. (C) Overview of results from the first round of screening. All compounds and their corresponding activities are represented by circles at non-cytotoxic concentrations. The fluorescence intensity ratio of Adrug/Amodel is displayed along the vertical axis. The compounds with a ratio of <1 were considered as effective inhibitors. Data represent the mean of relative fluorescence intensity results. (D) Relative fluorescence intensity of the six candidates obtained from the second round of screening. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; unpaired Student’s t-test. All error bars are SD (n = 3–6).
3.2. Evaluation of the inhibitory effects of candidate compounds on 2-E channels and antiviral activities
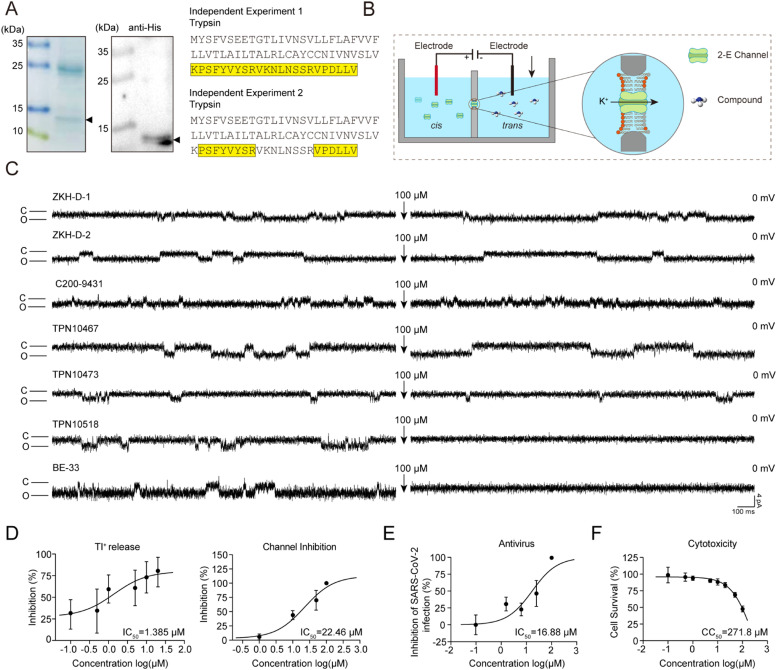
To further validate the inhibitory effects of the six candidate molecules at the channel level, we employed a planar lipid bilayer system for in vitro functional assessment (Fig. 2A, B). Purified 2-E protein was incorporated into an artificial planar lipid bilayer (Supplementary Figure S3), and we defined the added protein side as the cis side and the contralateral side as the trans side. Upon detection of channel currents formed by 2-E proteins, we added 100 μM compounds to the trans side, while stirring to disperse the compounds uniformly in the solution to promote their binding to 2-E channels. Here, BE-33 and HMA were used as positive controls. Notably, TPN10518 significantly inhibited 2-E channel-mediated current, whereas the other five candidate molecules did not (Fig. 2C, Supplementary Figure S2B). To further exclude non-specific interactions, we also tested the inhibitory effect of TPN10518 on another channel, TMEM41B, a characterized ion channel previously reported in our laboratory (Ma et al., 2025). The results showed that TPN10518 did not inhibit TMEM41B channel activity (Supplementary Figure S2C). These data suggested that TPN10518 is a potential inhibitor of the 2-E channel.
Fig. 2.
Validation of candidate compounds with both antiviral activity and 2-E channel inhibitory ability. (A) Purification of 2-E protein by Ni-NTA affinity chromatography. Left: 12 % SDS-PAGE gel stained with Coomassie blue. Triangles indicate 2-E protein; Middle: Western blot probed with anti-his-tag antibody; Right: Peptides of 2-E (yellow) were detected by LC-MS/MS. The data were derived from two independent experiments. (B) Schematic representation of the planar lipid bilayer system for screening 2-E channel inhibitors. (C) Representative single-channel traces after 2-E exposure to the indicated compounds at 100 μM. Once ion channel conductance was detected, the compounds were added to the trans side, while stirring to promote binding of the compounds to the channel. Black arrows indicate the application of compounds. Each compound was tested in duplicate (n = 3). "C" indicates channel closed; "O" indicates channel open. (D) IC50 of TPN10518 on Tl⁺ flux inhibition (n = 5–6) and 2-E channel inhibition (n = 3–4). cis: trans = 500:50 mM KCl (E) IC50 of TPN10518 on anti-SARS-CoV-2 (n = 3). (F) CC50 of TPN10518 on cell cytotoxicity (n = 3–6). All error bars are SD.
Having demonstrated the inhibitory activity of TPN10518 at both cellular and channel levels, we next examined its dose-dependent inhibition of the 2-E channel. To more accurately assess the impact of TPN10518 on the 2-E channel, the dose-dependence study was conducted using the two aforementioned assay systems. At the cellular level, TPN10518 inhibited Tl+ permeability with an IC50 of 1.385 μM, while its IC50 at the channel level was determined to be 22.46 μM (Fig. 2D). Subsequently, we evaluated the antiviral capability of TPN10518 in vitro. We evaluated the inhibitory activity of TPN10518 against SARS-CoV-2 in a BSL-3 laboratory. Our results demonstrated that TPN10518 inhibited the replication of SARS-CoV-2 in a dose-dependent manner with an antiviral IC50 of 16.88 μM (Fig. 2E). Importantly, no significant cytotoxicity was observed within the tested concentration range, with a CC50 exceeding 200 μM (Fig. 2F). These results identified TPN10518 as a candidate compound with potential anti-SARS-CoV-2 activity. Of note, TPN10518 is a derivative of artemisinin, a compound with established antimalarial therapeutic potential, suggesting its favorable developmental prospects.
3.3. Validation of TPN10518 as an antiviral candidate targeting 2-E channel
To verify whether TPN10518 exerts its inhibitory effects by directly binding to the 2-E channel, we systematically evaluated its binding characteristics using SPR (Fig. 3A, Supplementary Figure S4A). Binding affinity of the compound for the 2-E protein was demonstrated by the rapid binding rate and slow dissociation rate. Response units at reaction equilibrium were plotted according to the concentration of TPN10518, and binding affinity (KD) was calculated by nonlinear regression analysis, which showed that TPN10518 bound to 2-E with a KD value of 110 μM.
Fig. 3.
Interaction model of TPN10518 with the 2-E channel. (A) Binding ability of TPN10518 to 2-E channel via SPR. (B) Representative docking pose of TPN10518. The TM of 2-E is shown in cartoon representation. The compound and key residues are shown as sticks. (C) The amino acid sequence of 2-EL28R and 2-EA32Y proteins. (D) Representative single-channel trace of 2-E mutant after exposure to 100 μM TPN10518 at 0 mV. Once ion channel conductance was detected, compounds were added to the trans side, while stirring to promote compound binding to the channel. Black arrows indicate compound application. "C" indicates channel closed; "O" indicates channel open. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001; unpaired Student’s t-test. All error bars are SD (n = 3).
To further explore the interaction mechanism between TPN10518 and the TM of the 2-E channel, we performed molecular docking studies based on the ssNMR structure of the TM of the 2-E protein. The analyzed docking score of TPN10518 to the TM of 2-E was −8.4 kcal/mol. The docking model showed that TPN10518 binds to the channel area of 2-E protein, suggesting that TPN10518 inhibits ion conduction by blocking the channel area (Fig. 3B). In the C-terminal vestibule of the TM of 2-E, 6 different residues (L28, V29, A32, I33, T35, A36) from 5 subunits constitute a binding pocket for the TPN10518. Residues L28, V29, A32, I33, T35, and A36 hydrophobically interact with TPN10518. The 3-dimethylbutanoic acid group of L28 at the entry of the channel area binds to TPN10518, while A32, located at the middle region of the pocket, contributes to stabilizing the binding of TPN10518.
To validate the binding site predicted by the molecular docking model, we designed single-point mutations targeting two residues (L28R and A32Y) (Fig. 3C, Supplementary Figure S3). Functional assays revealed that these mutations significantly attenuated the inhibitory effects of TPN10518 on 2-E protein (Fig. 3D, Supplementary Figure S4B), consistent with predictions from the docking studies. The above results further support a mechanism whereby TPN10518 directly binds to the 2-E channel to inhibit its function.
3.4. TPN10518 alleviated lung damage induced by the 2-E channel
As a pathogenic factor of SARS-CoV-2, 2-E protein induces ARDS-like lung injury and triggers cytokine storm in vivo (Xia et al., 2021). To evaluate the protective effects of TPN10518 against 2-E-induced lung injury, we employed an intratracheal nebulization model to simulate infection (Fig. 4A, Supplementary Figure S6A). Compared to traditional intratracheal instillation, aerosol delivery reduced surgical trauma and significantly improved mouse survival rates (Wang et al., 2023). Mice were injected intraperitoneally with 5 mg/kg TPN10518, 10 mg/kg TPN10518, or an equal volume of DMSO 1 h before administration of 2-E protein (2.5 mg/kg) (Supplementary Figure S5), 2-E protein containing ion channel-impaired mutations (2-ET9I/T11A, 2.5 mg/kg), TBS or GST (2.5 mg/kg). All mice survived and were sacrificed to harvest the lungs and spleens 24 h after administration of 2-E protein. In contrast to mice administered with TBS, GST, or 2-ET9I/T11A, gross examination showed that those treated with 2-E displayed pulmonary edema and hemorrhage. Conversely, the administration of TPN10518 was associated with mitigation of these pathological manifestations (Fig. 4B, Supplementary Figure S6B). Histopathological analysis showed significant inflammatory cell infiltration, edema, interstitial congestion, hemorrhage, and alveolar atrophy in the lungs of mice given 2-E protein. In contrast, only minimal hemorrhage and slight damage were observed in the lungs of mice administered with TPN10518 (Fig. 4C, D, Supplementary Figure S6C, D). To further assess the regulatory effects of TPN10518 on 2-E-induced inflammatory responses, we measured the mRNA levels of inflammatory cytokines and chemokines in the left lung by qRT-PCR (Fig. 4E, Supplementary Figure S6E). The mRNA levels of TNF-α, IL-1β, IL-6, CXCL9, CXCL11, CCL5, and CCL3 were significantly lower in mice treated with TPN10518 than those in mice injected with DMSO. In summary, these in vitro and in vivo findings demonstrated that TPN10518 is a promising anti-SARS-CoV-2 candidate compound targeting 2-E protein.
Fig. 4.
TPN10518 attenuates lung inflammation caused by 2-E protein in vivo. (A) Flow schematic of the pharmacodynamics experiment. (B) Pictures of lungs obtained from sacrificed mice. Scale bar, 5 mm. (C) Histopathology of lungs from each group. Scale bar, 10 μm. (D) Histopathological scores of mice lung sections after 24 h of tracheal nebulization with 2-E protein. Both the TBS and GST groups consisted of 3 mice. The 2-ET9I/T11A, 2-EWT, and 2-EWT + TPN10518 groups each consisted of 4 mice. (E) mRNA levels of cytokines and chemokines in the left lung by qRT-PCR. Each sample was tested in duplicate (n = 3). ####P < 0.0001; * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001, ns: not significant. The TBS, GST, 2-ET9I/T11A, and 2-EWT groups were analyzed using ANOVA. The 2-EWT and 2-EWT with 10 mg/kg TPN10518 groups were analyzed using unpaired Student’s t-test. All error bars are SD.
4. Discussion
Through the development of a fluorescence-based screening system, we demonstrated the functional role of 2-E protein as a promising antiviral target and successfully identified a potent small-molecule inhibitor, TPN10518. Our findings not only support the direct interaction of TPN10518 with 2-E channels and characterize its molecular mechanism, providing a strategy for antiviral drug discovery.
Using a high-sensitivity fluorescence-based Tl⁺ flux assay, we identified 6 candidate compounds, among which TPN10518 exhibited specific inhibitory activity against 2-E channels. In comparison to conventional cell death-based approaches and single-channel methods, our system demonstrates improved performance in both throughput and sensitivity. The high detection sensitivity, broad dynamic range, and robust model performance enable precise screening for channel inhibitors. Unlike prior strategies that assessed 2-E function indirectly via cell death induction, our system targets direct inhibition of channel activity, boosting specificity and applicability. Furthermore, this approach facilitates the optimization of combination therapies, supporting the development of multidrug strategies.
Currently, antiviral targets for SARS-CoV-2 include S protein, Mpro, and RNA-dependent RNA polymerase (RdRp), leading to the development of such drugs as arbidol (Vankadari, 2020), lopinavir/ritonavir (Cao et al., 2020; Nutho et al., 2020), and remdesivir (Arba et al., 2021). In contrast, the 2-E protein, which is known for its highly conserved structure and critical roles in viral replication, ion homeostasis, and inflammatory activity, has gained attention as an attractive therapeutic target. Its conservation across variants underscores its potential to address resistance issues, making it an ideal target for novel antiviral therapeutics.
TPN10518 was derived from artemisinin, which was initially identified in studies of experimental autoimmune encephalomyelitis (EAE) through AP1 downregulation that suppresses Th1 and Th17 cell differentiation and reduces CNS inflammation (Xie et al., 2023). Here, we identified TPN10518 as a potential 2-E channel inhibitor and elucidated its mechanism of interaction. Molecular docking studies predicted the binding of TPN10518 to the C-terminal vestibule of 2-E, blocking ion channel activity. Site-directed mutagenesis of predicted residues further validated this interaction, providing a molecular basis for its inhibitory effects.
Some limitations in this study should be noted. We could not use human angiotensin-converting enzyme 2 (hACE2) transgenic mouse models to directly test the effect of TPN10518 on SARS-CoV-2 infection in vivo (DeDiego et al., 2008; Lutz et al., 2020). However, our ARDS-like animal model, supported by previous studies, offered reliable results (Xia et al., 2021). 2-E is predominantly incorporated into mature virions and rarely present on the cell surface. However, its involvement in physiological and pathological processes beyond viral assembly underscores the complexity of E protein dynamics during infection (Venkatagopalan et al., 2015). Regarding the nebulized 2-E protein mouse model, we acknowledge its limitations but emphasize that it is designed to simulate the acute effects of E protein overexpression. Given that E protein induces host cell stress and pathological changes during infection, this model, while not fully replicating physiological conditions, provides a controlled system to assess its direct impact on respiratory tissues. Similar approaches using viral protein overexpression have induced acute pathological changes in vivo (Tang et al., 2023; Xia et al., 2021; Xu et al., 2024), supporting the rationale of our approach. Additionally, the reduced efficiency of protein delivery via aerosolization compared to intravenous injection, particularly for large molecular weight proteins, combined with potential degradation of unmodified proteins upon organismal entry, necessitates increased aerosolized protein concentration (Patton et al., 2007). Therefore, our model requires future refinement to enhance protein aerosolization efficiency. Moreover, future work should improve the screening system, broaden its use, and include more models to increase accuracy and consistency. Despite these limitations, our screening platform enabled identification for finding 2-E protein inhibitors. These data suggested that targeting 2-E is a promising antiviral approach and supporting further exploration for drug development. Future studies aimed at optimizing the screening method and confirming the in vivo efficacy of TPN10518 are expected to advance therapeutic strategies against emerging coronavirus variants.
Author contributions
B. X., X. J., X. W., Z. G., and S. J. conceived the project, designed the experiments, and supervised the study; H. Z., S. L., P. L., and Z. L. carried out the cell-based assays; H. Z. and S. L. performed the electrophysiological recordings; H. Z., S. S., and P. L. purified the proteins; X. W. and L. S. carried out the virus inhibition assays in vitro; X. C. and J. H. analyzed the sequence; J. S., X. J., and Z. Y. synthesized and purified the compounds. All authors analyzed and discussed the data. B. X., X. J., X. W., Z. G., and S. J. wrote the manuscript. All authors read and approved the manuscript.
CRediT authorship contribution statement
Han Zhang: Investigation, Writing – original draft, Visualization, Validation. Shuxin Shi: Investigation, Writing – original draft, Validation. Lujia Sun: Investigation, Visualization. Shuangqu Li: Investigation. Yan Zhang: Investigation. Ziyue Li: Investigation. Jingjing Hou: Formal analysis. Pingan Li: Investigation. Jingshan Shen: Methodology, Supervision. Xi Cheng: Formal analysis, Methodology. Shibo Jiang: Conceptualization, Methodology, Supervision. Zhaobing Gao: Conceptualization, Methodology, Supervision. Xinling Wang: Conceptualization, Methodology, Supervision, Writing – review & editing. Xiangrui Jiang: Conceptualization, Methodology, Supervision, Writing – review & editing. Bingqing Xia: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We are grateful to the National Natural Science Foundation of China (92169202,82341058, 82202491, 82394463), the Scientific Instrument Developing Project of the Chinese Academy of Sciences (PTYQ2024YZ0008), Fund of Shanghai Science and Technology (20ZR1474200, 22QA1411000), the Science and Technology Commission of Shanghai Municipality (23JC1404300), and the National Key R&D Program of China (2023YFC2307800).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2025.100409.
Contributor Information
Zhaobing Gao, Email: zbgao@simm.ac.cn.
Xinling Wang, Email: xinlingwang@fudan.edu.cn.
Xiangrui Jiang, Email: jiangxiangrui@simm.ac.cn.
Bingqing Xia, Email: xiabingqing@simm.ac.cn.
Appendix. Supplementary materials
Data availability
No data was used for the research described in the article.
References
- Arba M., Wahyudi S.T., Brunt D.J., et al. Mechanistic insight on the remdesivir binding to RNA-dependent RNA polymerase (RdRp) of SARS-cov-2[J/OL] Comput. Biol. Med. 2021;129 doi: 10.1016/j.compbiomed.2020.104156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boson B., Legros V., Zhou B., et al. The SARS-CoV-2 envelope and membrane proteins modulate the maturation and retention of the spike protein, allowing assembly of virus-like particles[J/OL] J. Biol. Chem. 2021;296 doi: 10.1074/jbc.RA120.016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., et al. A trial of Lopinavir-Ritonavir in adults hospitalized with severe covid-19[J/OL] N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Yang R., Lee I., et al. Characterization of the SARS-CoV-2 E protein: sequence, structure, viroporin, and inhibitors[J/OL] Protein Sci. 2021;30(6):1114–1130. doi: 10.1002/pro.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso G., Stärk H., Jing B., et al. DiffDock: diffusion steps, twists, and turns for molecular docking[A/OL] arXiv[2024-12-06] 2022 doi: 10.48550/arXiv.2210.01776. http://arxiv.org/abs/2210.01776 [DOI] [Google Scholar]
- DeDiego M.L., Pewe L., Alvarez E., et al. Pathogenicity of severe acute respiratory coronavirus deletion mutants in hACE-2 transgenic mice[J/OL] Virology. 2008;376(2):379–389. doi: 10.1016/j.virol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Sk S, Choudhury P.P., Roy B. Molecular phylogeny and missense mutations at envelope proteins across coronaviruses[J/OL] Genomics. 2020;112(6):4993–5004. doi: 10.1016/j.ygeno.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalily P.H., Jalily Hasani H., Fedida D. Silico evaluation of hexamethylene amiloride derivatives as potential luminal inhibitors of SARS-CoV-2 E protein[J/OL] Int. J. Mol. Sci. 2022;23(18) doi: 10.3390/ijms231810647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A., Orekhov P., Astashkin R., et al. Structure and dynamics of the SARS-CoV-2 envelope protein monomer[J/OL] Proteins: Struct. Funct. Bioinform. 2022;90(5):1102–1114. doi: 10.1002/prot.26317. [DOI] [PubMed] [Google Scholar]
- Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19)[J/OL] Ann. Clin. Biochem. 2020;57(3):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zhang L., Hao X., et al. Coronavirus envelope protein activates TMED10-mediated unconventional secretion of inflammatory factors[J/OL] Nat. Commun. 2024;15(1):8708. doi: 10.1038/s41467-024-52818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C., Maher L., Lee C., et al. COVID-19 preclinical models: human angiotensin-converting enzyme 2 transgenic mice[J/OL] Hum, Genom. 2020;14(1):20. doi: 10.1186/s40246-020-00272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang Y., Zhao X., et al. TMEM41B is an endoplasmic reticulum Ca2+ release channel maintaining naive T cell quiescence and responsiveness[J/OL] Cell Discov. 2025;11(1):18. doi: 10.1038/s41421-024-00766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala V.S., McKay M.J., Shcherbakov A.A., et al. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers[J/OL] Nat., Struct., Mol., Biol. 2020;27(12):1202–1208. doi: 10.1038/s41594-020-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros-Silva J., Dregni A.J., Somberg N.H., et al. Atomic structure of the open SARS-CoV-2 E viroporin[J/OL] Sci. Adv. 2023;9(41):eadi9007. doi: 10.1126/sciadv.adi9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Harikishore A., Bhunia A. Targeting C-terminal helical bundle of NCOVID19 envelope (E) protein[J/OL] Int, J. Biol, Macromol. 2021;175:131–139. doi: 10.1016/j.ijbiomac.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutho B., Mahalapbutr P., Hengphasatporn K., et al. Why are Lopinavir and Ritonavir effective against the newly emerged Coronavirus 2019? Atomistic insights into the inhibitory mechanisms[J/OL] Biochemistry. 2020;59(18):1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- Orfali R., Rateb M.E., Hassan H.M., et al. Sinapic acid suppresses SARS CoV-2 replication by targeting its envelope protein[J/OL] Antibiotics. 2021;10(4):420. doi: 10.3390/antibiotics10040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J.S., Byron P.R. Inhaling medicines: delivering drugs to the body through the lungs[J/OL] Nat. Rev. Drug Discov. 2007;6(1):67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- Poggio E., Vallese F., Hartel A.J.W., et al. Perturbation of the host cell Ca<SUP>2+</SUP>homeostasis and ER-mitochondria contact sites by the SARS-CoV-2 structural proteins E and M[J/OL] Cell Death Dis. 2023;14(4):297. doi: 10.1038/s41419-023-05817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Martins D., Solis-Vasquez L., Tillack A.F., et al. Accelerating AutoDock4 with GPUs and gradient-based local search[J/OL] J. Chem. Theory Comput. 2021;17(2):1060–1073. doi: 10.1021/acs.jctc.0c01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh Tomar P.P., Arkin I.T. SARS-CoV-2 E protein is a potential ion channel that can be inhibited by Gliclazide and Memantine[J/OL] Biochem, Biophys., Res., Commun. 2020;530(1):10–14. doi: 10.1016/j.bbrc.2020.05.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somberg N.H., Medeiros-Silva J., Jo H., et al. Hexamethylene amiloride binds the SARS-CoV-2 envelope protein at the protein-lipid interface[J/OL] Protein Sci. 2023;32(10):e4755. doi: 10.1002/pro.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surya W., Tavares-Neto E., Sanchis A., et al. The complex proteolipidic behavior of the SARS-CoV-2 envelope protein channel: weak selectivity and heterogeneous oligomerization[J/OL] Int. J. Mol. Sci. 2023;24(15) doi: 10.3390/ijms241512454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Xu Y., Tan Y., et al. CD36 mediates SARS-CoV-2-envelope-protein-induced platelet activation and thrombosis[J/OL] Nat. Commun. 2023;14(1):5077. doi: 10.1038/s41467-023-40824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft-Bertelsen T.L., Jeppesen M.G., Tzortzini E., et al. Amantadine has potential for the treatment of COVID-19 because it inhibits known and novel ion channels encoded by SARS-CoV-2[J/OL] Commun. Biol. 2021;4(1):1347. doi: 10.1038/s42003-021-02866-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J., Maheswari U., Parthasarathy K., et al. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein[J/OL] Protein Sci. 2007;16(9):2065–2071. doi: 10.1110/ps.062730007. DOI:2023092718165100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein[J/OL] Int. J. Antimicrob. Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatagopalan P., Daskalova S.M., Lopez L.A., et al. Coronavirus envelope (E) protein remains at the site of assembly[J/OL] Virology. 2015;478:75–85. doi: 10.1016/j.virol.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Fang S., Wu Y., et al. Discovery of SARS-CoV-2-E channel inhibitors as antiviral candidates[J/OL] Acta. Pharmacol., Sin. 2022;43(4):781–787. doi: 10.1038/s41401-021-00732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Pan X., Ji H., et al. Impact of SARS-CoV-2 envelope protein mutations on the pathogenicity of Omicron XBB[J/OL] Cell Discov. 2023;9(1):80. doi: 10.1038/s41421-023-00575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver C.D., Harden D., Dworetzky S.I., et al. A thallium-sensitive, fluorescence-based assay for detecting and characterizing potassium channel modulators in mammalian cells[J/OL] SLAS Discov. 2004;9(8):671–677. doi: 10.1177/1087057104268749. [DOI] [PubMed] [Google Scholar]
- Wilson L., Gage P., Ewart G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication[J/OL] Virology. 2006;353(2):294–306. doi: 10.1016/j.virol.2006.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Mckinlay C., Gage P., et al. SARS coronavirus E protein forms cation-selective ion channels[J/OL] Virology. 2004;330(1):322–331. doi: 10.1016/j.virol.2004.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B., Shen X., He Y., et al. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target[J/OL] Cell Res. 2021;31(8):847–860. doi: 10.1038/s41422-021-00519-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Lv J., Saimaier K., et al. The novel small molecule TPN10518 alleviates EAE pathogenesis by inhibiting AP1 to depress Th1/Th17 cell differentiation[J/OL] Int., Immunopharmacol. 2023;123 doi: 10.1016/j.intimp.2023.110787. [DOI] [PubMed] [Google Scholar]
- Xu J.B., Guan W.J., Zhang Y.L., et al. SARS-CoV-2 envelope protein impairs airway epithelial barrier function and exacerbates airway inflammation via increased intracellular Cl<SUP>-</SUP>concentration[J/OL] Signal Transduct. Target. Ther. 2024;9(1):74. doi: 10.1038/s41392-024-01753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Karki R., Williams E.P., et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines[J/OL] Nat., Immunol. 2021;22(7):829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Lv P., Li M., et al. SARS-CoV-2 E protein: pathogenesis and potential therapeutic development[J/OL] Biomed. Pharmacother. 2023;159 doi: 10.1016/j.biopha.2023.114242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.