Abstract
Circulating tumor DNA (ctDNA) testing has transformed precision oncology by enabling the non-invasive detection of actionable mutations. To facilitate broader clinical adoption and improve testing accuracy, standardized quality criteria must be clearly defined and universally implemented. The International Society of Liquid Biopsy (ISLB) established the Quality Control and Accreditation Committee to develop consensus-based minimal standards for ctDNA analysis in oncology. Ensuring reliable and reproducible ctDNA testing necessitates standardization across the pre-analytical, analytical, and post-analytical phases. Key considerations include appropriate blood collection, efficient cfDNA isolation and purification, thorough assay validation, and precise data interpretation. The ISLB is committed to leading collaborative efforts among laboratories, regulatory bodies, and professional organizations to advance standardization and ensure high-quality ctDNA testing worldwide. Through initiatives led by the Quality Control and Accreditation Committee, educational programs, and multidisciplinary stakeholder workshops, ISLB provides a structured framework to promote standardization and foster innovation. By addressing current challenges and advocating for robust quality standards, ctDNA testing can reach its full potential in advancing personalized cancer care, enabling more precise and timely interventions for patients. This manuscript provides the first global initiative for quality control in liquid biopsy, presenting the ISLB perspective on minimal requirements for ctDNA testing in solid tumors.
Keywords: Circulating tumor DNA (ctDNA), Precision oncology, Standardization, Quality assurance, Liquid biopsy
1. Introduction
Circulating tumor DNA (ctDNA) testing has emerged as a transformative tool in precision oncology, providing unprecedented opportunities to advance cancer diagnosis, monitoring, and treatment [1]. Unlike solid tissue biopsies, liquid-biopsy-based ctDNA testing facilitates the minimal-invasive detection and analysis of tumor-specific alterations, offering real-time insights into the dynamic progression of cancer [[2], [3], [4]]. This technology has garnered significant attention for its potential to detect minimal residual disease (MRD), monitor therapeutic responses, and identify actionable mutations that are critical in the era of personalized cancer care [5]. Despite remarkable progress in research and clinical implementation, conflicting data and variable sensitivity and specificity have been reported, which reflects the lack of widely recognized minimal quality requirements for institutes and laboratories conducting molecular testing using ctDNA. This underscores an urgent need for standardization [6,7].
To address this challenge, the International Society of Liquid Biopsy (ISLB) has convened an international panel of experts to form the Quality Control and Accreditation Committee. This committee is dedicated to establishing consensus on minimal quality requirements for institutes, centers and laboratories performing molecular testing in oncology using ctDNA. The overarching objective is to ensure that ctDNA testing can be seamlessly and reliably integrated into routine clinical practice, thereby enabling more precise, personalized, and timely interventions for cancer patients worldwide.
2. Pre-Analytical considerations
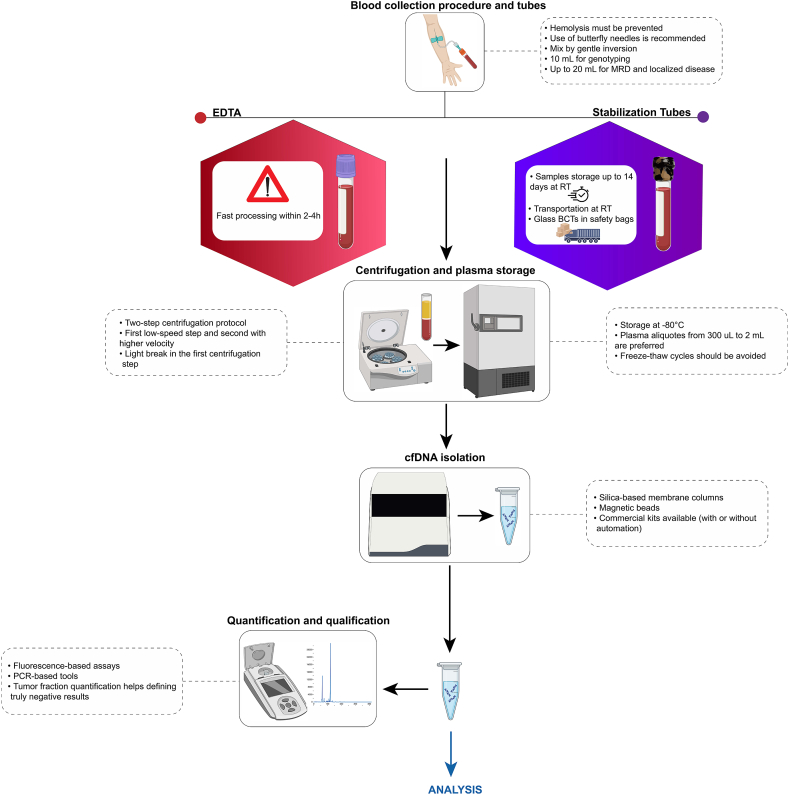
The pre-analytical phase is fundamental to the success of circulating free DNA (cfDNA) testing, as it directly impacts the quality, integrity, and quantity of cfDNA, which are crucial for reliable and reproducible results. This phase includes critical steps such as blood collection, handling, processing, and storage, all of which demand rigorous standardization and documentation to ensure the high quality of cfDNA isolation and subsequently influence test sensitivity and specificity [8]. While various circulating nucleic acid analytes, such as RNA and methylated cfDNA, have emerging clinical applications, this section focuses exclusively on ctDNA. The considerations outlined below regard specifically the pre-analytical handling of cfDNA for ctDNA-based assays, without addressing RNA-based or methylation-specific platforms. Strict adherence to these protocols ensures the production of high-quality plasma suitable for cfDNA extraction (Fig. 1).
Fig. 1.
Workflow of liquid biopsy pre-analytical steps and recommendations for circulating tumor DNA (ctDNA) analysis. MRD, Minimal residual disease; RT, Room temperature; cfDNA, Circulating-free DNA.
2.1. Blood collection methods and best practices
Blood collection represents the initial and arguably most important step in the ctDNA testing workflow. Proper techniques are essential to minimize contamination with high molecular weight DNA and hemolysis, both of which can dilute or degrade cfDNA. The use of butterfly needles is recommended to reduce shear stress on blood cells during venipuncture [9]. To prevent clot formation and maintain cfDNA integrity, blood should be gently mixed by inversion immediately after collection [10]. Comprehensive training and education for nursing staff and phlebotomists on these techniques are imperative to uphold sample quality. Plasma is the preferred sample type for cfDNA analysis, as it reduces contamination from genomic DNA released by lysed blood cells [11]. However, in some retrospective studies and biobanks, serum samples have been stored and may still be considered for analysis [12]. It is important to note that cfDNA concentrations in serum tend to be higher due to leukocyte lysis during clotting, which can introduce additional background DNA and impact assay sensitivity and specificity [13]. Therefore, whenever possible, plasma should be used to ensure more reliable and reproducible results. Timing of blood collection must align with the clinical objective, as samples drawn during specific treatment phases or disease states yield the most relevant clinical insights. Depending on the type of tube and absence/presence of DNA preservatives, the time from collection to processing should be considered.
2.2. Selection of blood collection tubes
The choice of blood collection tubes (BCTs) is critical for preserving cfDNA and reducing white blood cell lysis and must align with laboratory workflows. Two primary types are commonly used, including EDTA tubes and BCT including preservatives. EDTA tubes are cost-effective but necessitate processing within two to 4 h to prevent the release of genomic DNA from lysed cells [14]. In contrast, specialized cfDNA stabilizing tubes (e.g., Streck, Cell-Free DNA BCT or PAXgene Blood ccfDNA Tube) can preserve cfDNA for up to 14 days, making them an ideal choice for workflows requiring sample transport or delayed processing [7,15]. When conducting multi-modal liquid biopsy studies, it is important to note that not all stabilizing tubes are effective for preserving various analytes such as circulating tumor cells (CTCs) and extracellular vesicles (EVs), since many stabilizing reagents and BCT are specifically designed to preserve cfDNA profiles in whole blood. The choice of BCTs must weigh the trade-offs between cost, logistical requirements, and the demands of downstream analyses.
2.3. Sample volume and centrifugation protocols
Adequate sample volume is essential for high sensitivity cfDNA assays. A minimum of 10 mL of blood is recommended to yield sufficient plasma for reliable analysis [14,16]. When possible, higher volumes should be collected in case of test failure or result confirmation. Following collection, plasma separation should adhere to a two-step centrifugation protocol: an initial low-speed spin to separate plasma from cellular components, followed by a high-speed centrifugation to remove residual debris [17].
2.4. Plasma storage and handling
Proper storage and handling of plasma are critical to preserving cfDNA stability. Plasma should be aliquoted in low binding tubes and stored at −80 °C immediately after centrifugation to prevent nucleic acid degradation [18]. Plasma aliquots from 300 μL to 2 ML are preferred. During handling, particularly during thawing, plasma must be kept on ice to minimize degradation and contamination [19].
2.5. cfDNA purification
The extraction of cfDNA from plasma is a highly sensitive process that requires careful optimization. The optimal plasma volume for cfDNA extraction depends on the intended application. While 1–2 mL plasma may be sufficient for basic research and low sensitivity assays (e.g. shallow whole genome sequencing), at least 4 mL is recommended for routine cfDNA applications. When disease is still at localized setting and for minimal residual disease (MRD) detection higher volumes (8–20 mL) are required as tumor-derived ctDNA is often present at extremely low fractions. Laboratories may choose between manual and automated extraction methods, with automated systems providing scalability and improved reproducibility [20]. The choice of extraction chemistry, whether silica-based membranes, magnetic beads, or alternative technologies, should be guided by the laboratory's throughput needs and analytical requirements. Several studies have compared the efficiency of commercial cfDNA extraction kits in terms of yield and quality [21,22]. Terp et al. found that the QIAamp Circulating Nucleic Acid Kit (manual and semi-automated) outperformed the QIAamp MinElute ccfDNA Kit (QIAcube) and QIAsymphony DSP Circulating DNA Kit (QIAsymphony), offering higher recovery rates and cfDNA quantities, measured by droplet digital PCR and TapeStation [20]. In a multicenter study, Lampignano et al. evaluated six extraction kits, including QIAGEN's QIAamp Circulating Nucleic Acid Kit, QIAsymphony, MinElute ccfDNA Kit, and kits from Maxwell (AX1115, AS1480) and Chemagic. They observed that within a single laboratory, extraction results were more consistent, with the QIAamp CNA kit showing the broadest yield range, while the Maxwell AX1115 had lower variation but yielded less cfDNA. Notably, Qubit quantification revealed higher recovery of cfDNA from Streck plasma with the Maxwell AX1115. The Maxwell AS1480 and Chemagic kit, tested at fewer sites, yielded similar results, but the Chemagic kit showed the lowest cfDNA recovery [23]. Overall, careful selection and validation of cfDNA extraction methods are crucial for ensuring consistent, high-quality results that meet the needs of downstream applications.
2.6. Quantification and quality control of input ctDNA
The quality and quantity of input ctDNA are critical determinants of successful analysis. Accurate quantification using fluorometric or qPCR-based methods ensures that sufficient DNA is available for downstream applications [23,24]. Evaluating fragment size distribution and purity is equally important to optimize the performance of molecular techniques [25]. Poor-quality input DNA, and/or genomic DNA contamination, can result in low library yields and conversion rates incomplete library preparation, sequencing artifacts, or reduced assay sensitivity, ultimately compromising clinical interpretation. In this regard, the assessment of tumor fraction (TF) is gaining interest, particularly when reporting negative results [26]. Determining TF helps evaluate whether a negative result truly reflects the absence of detectable alterations or whether it may result from insufficient tumor-derived ctDNA in the sample. This distinction is essential to decide whether a new sample is needed to rule out a false-negative result or if the original finding is a true negative, thereby guiding further clinical decision-making. TF can be estimated through various approaches, including the allele frequency of somatic mutations, copy number variations (CNVs), or genome-wide methylation and fragmentation patterns [27]. In targeted sequencing assays, TF is often inferred by analyzing the variant allele frequency (VAF) of known somatic mutations, adjusted for tumor ploidy and clonality. When genome-wide data are available, bioinformatic tools can also estimate TF based on the amplitude and distribution of copy number changes across the genome [28]. Laboratories must establish strict criteria for input DNA quality to mitigate these risks. Regular participation in an external quality assessment (EQA)/proficiency testing (PT) scheme for ctDNA extraction provides external validation of this process, ensuring that a laboratory's quality control (QC) processes are accurate, robust, and reproducible. Given the fundamental importance of ctDNA extraction to the downstream analytical pipeline, reinforcing quality assurance at this stage is essential to maintaining the integrity and reliability of subsequent analyses.
3. ANALYTICAL workflow
The analytical phase of cf/ctDNA testing involves the precise processing of DNA samples using the appropriate molecular technique to detect and quantify tumor-specific alterations. This phase plays a pivotal role in determining the accuracy and reliability of liquid biopsy results, requiring careful method selection, rigorous assay validation, and robust quality controls. Below, we outline key considerations and best practices for achieving reliable analytical outcomes [29].
3.1. Selection of analytical methods
The choice of cfDNA assay depends on multiple factors, including the intended clinical or research application, detection sensitivity, multiplexing capability, cost, and turnaround time [30]. Each cfDNA-based application requires distinct detection technologies. For MRD detection and monitoring, ultra-sensitive techniques are necessary due to the extremely low abundance of tumor-derived cfDNA [31]. In this context, ultra-sensitive detection through tumor-informed approaches using NGS or dPCR are most commonly used. Tumor-agnostic approaches, such as methylation-based sequencing and fragmentation analysis, have also demonstrated promise in MRD detection as well as for early cancer detection [32]. These methods do not require prior knowledge of tumor mutations, making them applicable to a wide range of cancer types. However, their clinical validation is still evolving, and they may require higher volumes of cfDNA for effective analysis. Other liquid biopsy applications, including detection of mutations for targeted therapy selection and to monitor evidence of resistance, benefit from targeted NGS approaches using hotspot panel or comprehensive genomic profiling for simultaneous biomarker detection [33,34]. Modern deep sequencing methods and error suppression techniques, including unique molecular identifiers (UMIs) and duplex sequencing, have significantly improved the resolution of these assays [35]. Their ability to identify a wide range of genetic alterations, including single nucleotide variants (SNVs), CNVs, and structural rearrangements, makes it an essential tool for tumor-agnostic testing and monitoring genomic evolution over time [29]. For targeted mutation detection, dPCR is a very precise and cost-effective alternative. However, its narrow genomic coverage may limit its usefulness in scenarios that demand a broader analysis of genetic analyses [[36], [37], [38]]. dPCR is particularly valuable in clinical scenarios where the target is well-defined, such as detecting hotspot mutations or specific viral sequences [39]. For instance, dPCR has been successfully applied to detect circulating viral ctDNA in virus-associated cancers, including HPV-related head and neck or cervical cancers, offering a sensitive approach for disease monitoring and early detection [34].
3.2. Assay design and validation
The design and validation of ctDNA assays must align with well-defined clinical objectives, ensuring optimal sensitivity, specificity, and reproducibility [40]. Recent advancements in assay development emphasize a tailored approach, where different clinical applications dictate distinct validation requirements [41]. Current best practices also account for potential sources of variability, including sample input quality, pre-analytical processing, library preparation, sequencing efficiency, and bioinformatics pipelines [42]. Furthermore, the validation process must incorporate state-of-the-art reference standards, including well-characterized reference materials and diverse clinical samples representing various tumor types and patient demographics [43]. Key analytical performance metrics such as limit of detection (LOD), limit of quantification (LOQ), sensitivity, specificity, accuracy, and precision, must be rigorously evaluated under both analytical and clinical validation frameworks [44]. To ensure assay robustness and clinical reliability, rigorous internal and EQAs are mandatory throughout the analytical workflow. This includes proficiency testing, batch-to-batch consistency checks, and real-time QC monitoring, reinforcing the reproducibility and clinical applicability of ctDNA assays. For hotspot mutation assays (e.g., PIK3CA, EGFR), achieving a balance between sensitivity and specificity is critical to minimize false positives and false negatives [45,46]. These assays must undergo rigorous analytical validation to confirm their ability to detect low-frequency variants with high confidence. Broader applications, such as whole-genome sequencing (WGS) or whole-exome sequencing (WES), demand a focus on coverage depth, read uniformity, and bioinformatic accuracy to ensure comprehensive variant detection [29]. Assays designed for monitoring therapeutic response, resistance mutations, or longitudinal disease progression typically operate at moderate sensitivity thresholds but require stringent reproducibility across time points. Conversely, assays developed for MRD detection must reliably identify ultra-low frequency variants, often below 0.01 % of VAF, may require different validation setups [47]. By integrating these state-of-the-art validation strategies, laboratories can establish ctDNA assays as highly reliable tools for precision oncology, enabling improved cancer detection, disease monitoring, and personalized therapeutic decision-making.
3.3. In-house vs. centralized testing
The decision of whether to conduct ctDNA analysis in-house or outsource it to external providers involves multiple complexities and requires careful evaluation not only by laboratories but also by entire institutions, individual investigators, and treating physicians, with consideration of the healthcare reimbursement model which differs by country. In-house testing offers complete control over raw data, allowing investigation of sequencing metrics and optimization of analyses [48]. It also enables skill development among personnel and fosters innovation in assay design. However, in-house workflows may require substantial infrastructure and expertise and is often less cost-effective for smaller institutions [49]. Testing centralization provides logistical convenience, especially for institutes with a low number of requests, but may introduce challenges such as reduced data availability, limited capacity for customization, and longer turnaround times. Only summarized reports are typically provided by external labs, which may limit clinical flexibility. Healthcare professionals and health institutions must balance considerations of cost, speed, and control when determining the most appropriate strategy for their analytical needs. Laboratories electing to use the out-sourcing route should ensure that this is clear on any clinical report associated with a patient test.
3.4. POST-ANALYTICAL phase
The post-analytical phase is critical for ensuring that the results of ctDNA testing are effectively translated into actionable clinical insights. This phase encompasses data interpretation, report generation, and communication of findings to the relevant healthcare providers. Proper execution of this phase ensures that the full value of the ctDNA testing process is realized.
3.5. Data interpretation and validation
Interpreting ctDNA results requires an understanding of the biological and technical contexts of the detected variants. Analytical findings need to be coupled with clinical parameters, such as tumor type, stage, and prior treatments, to derive meaningful conclusions [50]. The interpretation must also consider the assay's limitations, such as detection thresholds and potential for false positives or negatives [51]. To enhance reliability, internal validation of results is critical. This includes cross-referencing variant calls against established databases and using orthogonal methods, such as sanger sequencing or digital PCR, for confirmation of key findings. Implementation of standard operating procedures (SOPs) for variant prioritization, ensuring clinical relevance and reproducibility, are necessary.
Moreover, robust criteria for calling positive versus negative results should be established, including the use of replicates to increase confidence in variant detection and minimize false positives. Quantifying ctDNA also warrants careful consideration, as expressing ctDNA as a fraction of cfDNA can be misleading due to interindividual variability in baseline cfDNA levels. Instead, quantification approaches should focus on mutant ctDNA as a ratio to wild-type or as copies per milliliter of plasma, which is generally preferred for consistent reporting and clinical interpretation.
3.6. Scientific reporting and clinical communication
The format and content of ctDNA test reports are pivotal in facilitating clinical decision-making. Reports should provide a concise summary of the findings, including the nature of detected alterations, their clinical significance, and potential therapeutic implications [52]. Reports which comply with guidelines for detailing all aspects needed for clinical interpretation are essential [53]. Key elements of the report should include a description of the assay used, its performance metrics, and the analytical context of the results [4]. These aspects are outlined both in European and American guidelines [41,54,55]. Clinical annotations, such as known drug-target relationships or evidence from clinical trials, should be integrated to support treatment recommendations. In certain occasions, treatment decision-making may also be performed by a local molecular tumor board (MTB) [56,57]. Effective communication with clinicians is fundamental to the success of the post-analytical phase. Results must be contextualized within the broader scope of patient management, considering tumor evolution, treatment history, and emerging clinical guidelines. Multidisciplinary discussions, including tumor boards, can help interpret complex cases and foster a collaborative approach to treatment planning [57,58].
4. Regulatory and quality considerations
The regulatory framework for ctDNA testing is complex and rapidly evolving. In the European Union, the In Vitro Diagnostic Regulation (IVDR) governs laboratory practices, emphasizing validation and traceability, particularly for laboratory-developed tests (LDTs) [59]. In the US, the Food and Drug Administration (FDA) and Centers for Medicare and Medicaid Services (CMS) have consistently supported each other in overseeing the analytical and clinical validity of LDTs. While LDTs are crucial in healthcare, poorly performing tests or those lacking scientific rationale pose risks to patients. In this regard, the FDA has recently finalized a rule to enhance the oversight of LDTs to ensure their safety and effectiveness. This decision follows years of growing concern over the variability and potential risks associated with unregulated LDTs, including false results that may compromise patient care. The final rule, published on April 29, 2024, establishes that IVD tests are classified as medical devices under the Federal Food, Drug, and Cosmetic Act, even when manufactured by clinical laboratories [60]. The FDA's oversight will be implemented through a phased four-year plan consisting of five stages. Laboratories must demonstrate compliance with analytical performance standards, including biological reference intervals and clinical decision limits, ensuring consistent and clinically meaningful results. Accreditation under ISO 15189:2022 adds another layer of quality assurance, focusing on laboratory competence [61]. However, the absence of specific standards for liquid biopsy assays under this framework poses challenges. Laboratories can address this gap by implementing rigorous internal quality controls, participating in EQA programs, and benchmarking performance against international standards [62]. Workflow optimization and stringent quality control are key to meeting regulatory demands. Dedicated spaces to prevent contamination, comprehensive SOPs, and regular staff training ensure transparency and reproducibility. Internal controls should monitor assay sensitivity, specificity, and reproducibility, while EQA programs provide independent performance evaluation, especially for detecting low-frequency variants or managing degraded samples. Collaboration with professional societies like European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO), European Society of Pathology (ESP), and EQA networks fosters standardization, providing guidelines and consensus recommendations. These initiatives harmonize protocols and promote best practices, supporting laboratories in delivering reliable results across diverse settings. Despite progress, gaps remain in regulatory alignment and standardization. Dedicated accreditation pathways and updated frameworks are needed to accommodate emerging technologies, such as multi-omic analyses, while prioritizing patient safety and clinical utility. By adhering to rigorous quality standards throughout the various stages of a clinical test, and engaging in collaborative networks, laboratories can ensure ctDNA testing remains reliable, actionable, and aligned with evolving clinical needs.
5. Conclusions
ctDNA testing success relies on rigorous standardization across pre-analytical, analytical, and post-analytical phases. Key considerations include proper sample handling to preserve ctDNA integrity, the use of validated technologies tailored to clinical needs, and precise result interpretation to inform decision-making. A major barrier to broader adoption is the lack of harmonized protocols and quality assurance frameworks. Establishing universal standards for assay development, laboratory practices, and reporting is essential to ensure reliable and reproducible outcomes. Collaboration among regulatory bodies, laboratories, and professional organizations will further enhance the utility and integration of ctDNA testing into routine clinical practice. By addressing these challenges, ctDNA testing can achieve its full potential, driving advancements in precision oncology and improving patient outcomes.
Ethical Approval - patient consent
Not applicable.
Declaration of interest statement
Nicola Fusco: Has received honoraria for consulting, advisory roles, speakers' bureau participation, travel, and/or research grants from Merck Sharp & Dohme (MSD), Merck, Novartis, AstraZeneca, Roche, Menarini Group, Daiichi Sankyo, GlaxoSmithKline (GSK), Gilead, Sysmex, Veracyte Inc., Sakura, Leica Biosystems, Thermo-Fisher Scientific, Lilly, Pfizer, Abbvie.
Konstantinos Venetis: Has received honoraria for speaker bureau from Merck Sharp & Dohme (MSD), Roche, AstraZeneca, and Johnson & Johnson.
Francesco Pepe: Has received personal fees (as speaker bureau or advisor) from Menarini and Roche.
Simon Heeke: Has received speaker fees from Guardant health and AstraZeneca and reports intellectual property on the classification of lung cancer.
Simon Patton: has received financial support from AstraZeneca, MSD, and Johnson & Johnson for EMQN CIC to deliver external quality assessment activities to laboratories worldwide, has received honoraria from AstraZeneca for delivering webinar series, and has received travel costs from AstraZeneca to support delivery of a lecture at a major European conference.
Ellen Heitzer: Has received unrelated funding from Illumina, Roche, Servier, Freenome, and PreAnalytiX, and received honoraria from Roche and Astra Zeneca for advisory boards unrelated to our study.
Paul Hoffman: has received honoraria for travel support and consulting/advisory roles for AstraZeneca, Roche, Bristol-Myers Squibb, Biocartis, Bayer, Ed Lilly, Diaceutics, Novartis, Pfizer, MSD, Qiagen, Thermo-Fisher Scientist, Janssen, Amgen, Abbvie, Biocartis, Pierre Fabre, and Sanofi, outside the submitted work.
Massimo Cristofanilli: reports personal fees from Lilly, Sermonix, Data Genomics, Foundation Medicine, Guardant Health, Celcuity, Iylon, and Ellipses and grants and personal fees from Pfizer, AZ and Menarini, all outside the submitted work.
David R. Gandara: Honoraria: Merck Consulting or Advisory Role: AstraZeneca (Inst), Guardant Health (Inst), OncoCyte (Inst), IO Biotech (Inst), Roche/Genentech (Inst), Adagene (Inst), Guardant Health (Inst), OncoHost (Inst) Research Funding: Merck (Inst), Amgen (Inst), Genentech (Inst), AstraZeneca (Inst), Astex Pharmaceuticals (Inst).
Christian Rolfo: speaker honoraria: AstraZeneca, Roche and MSD; advisory board honoraria: Inivata, Archer, Boston Pharmaceuticals, EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA, Novartis, Bayer, Invitae, Regeneron, Janssen, Bostongene, Novocure; scientific advisory board member: Imagene; institutional research funding: LCRF- Pfizer and NCRF; non-renumerated research support: Guardant Health and Foundation Medicine; non-renumerated leadership roles: the International Society of Liquid Biopsy (ISLB), the International Association for Study of Lung Cancer (IASLC), and the European School of Oncology (ESO).
Umberto Malapelle: Has received personal fees (as consultant and/or speaker bureau) from Boehringer Ingelheim, Roche, MSD, Amgen, Thermo Fisher Scientific, Eli Lilly, Diaceutics, GSK, Merck and AstraZeneca, Janssen, Diatech, Novartis and Hedera, all unrelated to the current work.
No other potential conflicts of interest are reported.
Contributor Information
Nicola Fusco, Email: nicola.fusco@ieo.it.
Konstantinos Venetis, Email: konstantinos.venetis@ieo.it.
Christian Rolfo, Email: Christian.Rolfo@osumc.edu.
Umberto Malapelle, Email: umbertomalapelle@gmail.com.
References
- 1.Rolfo C., Mack P., Scagliotti G.V., Aggarwal C., Arcila M.E., Barlesi F., et al. Liquid biopsy for advanced nsclc: a consensus statement from the international association for the study of lung cancer. J Thorac Oncol. 2021;16(10):1647–1662. doi: 10.1016/j.jtho.2021.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Boukovala M., Westphalen C.B., Probst V. Liquid biopsy into the clinics: current evidence and future perspectives. J Liq Biopsy. 2024;4 doi: 10.1016/j.jlb.2024.100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusco N., Jantus-Lewintre E., Serrano M.J., Gandara D., Malapelle U., Rolfo C. Role of the International Society of Liquid Biopsy (ISLB) in establishing quality control frameworks for clinical integration. Crit Rev Oncol-Hematol. 2025 doi: 10.1016/j.critrevonc.2025.104619. [DOI] [PubMed] [Google Scholar]
- 4.Guerini-Rocco E., Venetis K., Cursano G., Mane E., Frascarelli C., Pepe F., et al. Standardized molecular pathology workflow for ctDNA-based ESR1 testing in HR+/HER2- metastatic breast cancer. Crit Rev Oncol Hematol. 2024;201 doi: 10.1016/j.critrevonc.2024.104427. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S.A., Liu M.C., Aleshin A. Practical recommendations for using ctDNA in clinical decision making. Nature. 2023;619(7969):259–268. doi: 10.1038/s41586-023-06225-y. [DOI] [PubMed] [Google Scholar]
- 6.Bronkhorst A.J., Holdenrieder S. The changing face of circulating tumor DNA (ctDNA) profiling: factors that shape the landscape of methodologies, technologies, and commercialization. Med Genet. 2023;35(4):201–235. doi: 10.1515/medgen-2023-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Leest P., Schuuring E. Critical factors in the analytical work flow of circulating tumor DNA-based molecular profiling. Clin Chem. 2024;70(1):220–233. doi: 10.1093/clinchem/hvad194. [DOI] [PubMed] [Google Scholar]
- 8.Greytak S.R., Engel K.B., Parpart-Li S., Murtaza M., Bronkhorst A.J., Pertile M.D., et al. Harmonizing cell-free DNA collection and processing practices through evidence-based guidance. Clin Cancer Res. 2020;26(13):3104–3109. doi: 10.1158/1078-0432.CCR-19-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnaby D.P., Wollowitz A., White D., Pearlman S., Davitt M., Holihan L., et al. Generalizability and effectiveness of butterfly phlebotomy in reducing hemolysis. Acad Emerg Med. 2016;23(2):204–207. doi: 10.1111/acem.12858. [DOI] [PubMed] [Google Scholar]
- 10.Simundic A.M., Bölenius K., Cadamuro J., Church S., Cornes M.P., van Dongen-Lases E.C., et al. Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015–2038. doi: 10.1515/cclm-2018-0602. [DOI] [PubMed] [Google Scholar]
- 11.Pittella-Silva F., Chin Y.M., Chan H.T., Nagayama S., Miyauchi E., Low S.K., et al. Plasma or serum: which is preferable for mutation detection in liquid biopsy? Clin Chem. 2020;66(7):946–957. doi: 10.1093/clinchem/hvaa103. [DOI] [PubMed] [Google Scholar]
- 12.Lommen K., Odeh S., Theije C.C., Smits K.M. Biobanking in molecular biomarker research for the early detection of cancer. Cancers (Basel) 2020;12(4) doi: 10.3390/cancers12040776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cervena K., Vodicka P., Vymetalkova V. Diagnostic and prognostic impact of cell-free DNA in human cancers: systematic review. Mutat Res Rev Mutat Res. 2019;781:100–129. doi: 10.1016/j.mrrev.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Diaz I.M., Nocon A., Held S.A.E., Kobilay M., Skowasch D., Bronkhorst A.J., et al. Pre-analytical evaluation of Streck cell-free DNA blood collection tubes for liquid profiling in oncology. Diagnostics. 2023;13(7) doi: 10.3390/diagnostics13071288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorber L., Zwaenepoel K., Jacobs J., De Winne K., Van Casteren K., Augustus E., et al. Specialized blood collection tubes for liquid biopsy: improving the pre-analytical conditions. Mol Diagn Ther. 2020;24(1):113–124. doi: 10.1007/s40291-019-00442-w. [DOI] [PubMed] [Google Scholar]
- 16.Heitzer E., van den Broek D., Denis M.G., Hofman P., Hubank M., Mouliere F., et al. Recommendations for a practical implementation of circulating tumor DNA mutation testing in metastatic non-small-cell lung cancer. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungerer V., Bronkhorst A.J., Holdenrieder S. Preanalytical variables that affect the outcome of cell-free DNA measurements. Crit Rev Clin Lab Sci. 2020;57(7):484–507. doi: 10.1080/10408363.2020.1750558. [DOI] [PubMed] [Google Scholar]
- 18.Malapelle U., Pisapia P., Addeo A., Arrieta O., Bellosillo B., Cardona A.F., et al. Liquid biopsy from research to clinical practice: focus on non-small cell lung cancer. Expert Rev Mol Diagn. 2021;21(11):1165–1178. doi: 10.1080/14737159.2021.1985468. [DOI] [PubMed] [Google Scholar]
- 19.van Ginkel J.H., van den Broek D.A., van Kuik J., Linders D., de Weger R., Willems S.M., et al. Preanalytical blood sample workup for cell-free DNA analysis using Droplet Digital PCR for future molecular cancer diagnostics. Cancer Med. 2017;6(10):2297–2307. doi: 10.1002/cam4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terp S.K., Pedersen I.S., Stoico M.P. Extraction of cell-free DNA: evaluation of efficiency, quantity, and quality. J Mol Diagn. 2024;26(4):310–319. doi: 10.1016/j.jmoldx.2024.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Diefenbach R.J., Lee J.H., Kefford R.F., Rizos H. Evaluation of commercial kits for purification of circulating free DNA. Cancer Genet. 2018;228–229:21–27. doi: 10.1016/j.cancergen.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Kresse S.H., Brandt-Winge S., Pharo H., Flatin B.T.B., Jeanmougin M., Vedeld H.M., et al. Evaluation of commercial kits for isolation and bisulfite conversion of circulating cell-free tumor DNA from blood. Clin Epigenet. 2023;15(1):151. doi: 10.1186/s13148-023-01563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampignano R., Neumann M.H.D., Weber S., Kloten V., Herdean A., Voss T., et al. Multicenter evaluation of circulating cell-free DNA extraction and downstream analyses for the development of standardized (Pre)analytical work flows. Clin Chem. 2020;66(1):149–160. doi: 10.1373/clinchem.2019.306837. [DOI] [PubMed] [Google Scholar]
- 24.Pallisgaard N., Spindler K.L., Andersen R.F., Brandslund I., Jakobsen A. Controls to validate plasma samples for cell free DNA quantification. Clin Chim Acta. 2015;446:141–146. doi: 10.1016/j.cca.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Martignano F. Cell-free DNA: an overview of sample types and isolation procedures. Methods Mol Biol. 2019;1909:13–27. doi: 10.1007/978-1-4939-8973-7_2. [DOI] [PubMed] [Google Scholar]
- 26.Rolfo C.D., Madison R.W., Pasquina L.W., Brown D.W., Huang Y., Hughes J.D., et al. Measurement of ctDNA tumor fraction identifies informative negative liquid biopsy results and informs value of tissue confirmation. Clin Cancer Res. 2024;30(11):2452–2460. doi: 10.1158/1078-0432.CCR-23-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husain H., Pavlick D.C., Fendler B.J., Madison R.W., Decker B., Gjoerup O., et al. Tumor fraction correlates with detection of actionable variants across > 23,000 circulating tumor DNA samples. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.22.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galant N., Nicoś M., Kuźnar-Kamińska B., Krawczyk P. Variant allele frequency analysis of circulating tumor DNA as a promising tool in assessing the effectiveness of treatment in non-small cell lung carcinoma patients. Cancers (Basel) 2024;16(4) doi: 10.3390/cancers16040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranghiero A., Frascarelli C., Cursano G., Pescia C., Ivanova M., Vacirca D., et al. Circulating tumour DNA testing in metastatic breast cancer: integration with tissue testing. Cytopathology (Oxf) 2023;34:519–529. doi: 10.1111/cyt.13295. [DOI] [PubMed] [Google Scholar]
- 30.Santonja A., Cooper W.N., Eldridge M.D., Edwards P.A.W., Morris J.A., Edwards A.R., et al. Comparison of tumor-informed and tumor-naïve sequencing assays for ctDNA detection in breast cancer. EMBO Mol Med. 2023;15(6) doi: 10.15252/emmm.202216505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu L., Xu R., Yang L., Shi W., Zhang Y., Liu J., et al. Minimal residual disease (MRD) detection in solid tumors using circulating tumor DNA: a systematic review. Front Genet. 2023;14 doi: 10.3389/fgene.2023.1172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G., Huang S.H., Ailles L., Rey-McIntyre K., Melton C.A., Shen S.Y., et al. Clinical validation of a tissue-agnostic genome-wide methylome enrichment molecular residual disease assay for head and neck malignancies. Ann Oncol. 2025;36(1):108–117. doi: 10.1016/j.annonc.2024.08.2348. [DOI] [PubMed] [Google Scholar]
- 33.Ma L., Guo H., Zhao Y., Liu Z., Wang C., Bu J., et al. Liquid biopsy in cancer current: status, challenges and future prospects. Signal Transduct Targeted Ther. 2024;9(1):336. doi: 10.1038/s41392-024-02021-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartolomucci A., Nobrega M., Ferrier T., Dickinson K., Kaorey N., Nadeau A., et al. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. npj Precis Oncol. 2025;9(1):84. doi: 10.1038/s41698-025-00876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heidrich I., Ačkar L., Mossahebi Mohammadi P., Pantel K. Liquid biopsies: potential and challenges. Int J Cancer. 2021;148(3):528–545. doi: 10.1002/ijc.33217. [DOI] [PubMed] [Google Scholar]
- 36.Gezer U., Bronkhorst A.J., Holdenrieder S. The clinical utility of droplet digital PCR for profiling circulating tumor DNA in breast cancer patients. Diagnostics. 2022;12(12) doi: 10.3390/diagnostics12123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venetis K., Pepe F., Pescia C., Cursano G., Criscitiello C., Frascarelli C., et al. ESR1 mutations in HR+/HER2-metastatic breast cancer: enhancing the accuracy of ctDNA testing. Cancer Treat Rev. 2023;121 doi: 10.1016/j.ctrv.2023.102642. [DOI] [PubMed] [Google Scholar]
- 38.Venetis K., Pepe F., Munzone E., Sajjadi E., Russo G., Pisapia P., et al. Analytical performance of next-generation sequencing and RT-PCR on formalin-fixed paraffin-embedded tumor tissues for PIK3CA testing in HR+/HER2− breast cancer. Cells. 2022;11(22):3545. doi: 10.3390/cells11223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venetis K., Cursano G., Pescia C., D'Ercole M., Porta F.M., Blanco M.C., et al. Liquid biopsy: cell-free DNA based analysis in breast cancer. J Liq Biopsy. 2023 doi: 10.1016/j.jlb.2023.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lockwood C.M., Borsu L., Cankovic M., Earle J.S.L., Gocke C.D., Hameed M., et al. Recommendations for cell-free DNA assay validations: a joint consensus recommendation of the association for molecular pathology and college of American pathologists. J Mol Diagn. 2023;25(12):876–897. doi: 10.1016/j.jmoldx.2023.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Pascual J., Attard G., Bidard F.C., Curigliano G., De Mattos-Arruda L., Diehn M., et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2022;33(8):750–768. doi: 10.1016/j.annonc.2022.05.520. [DOI] [PubMed] [Google Scholar]
- 42.Larkey N.E., Paulson V.A. Joint consensus recommendations for validating cell-free DNA assays. Clin Chem. 2024;70(7):1000–1001. doi: 10.1093/clinchem/hvae028. [DOI] [PubMed] [Google Scholar]
- 43.Geeurickx E., Hendrix A. Targets, pitfalls and reference materials for liquid biopsy tests in cancer diagnostics. Mol Aspect Med. 2020;72 doi: 10.1016/j.mam.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Armbruster D.A., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29(Suppl 1):S49–S52. Suppl 1. [PMC free article] [PubMed] [Google Scholar]
- 45.McCall C.M., Mosier S., Thiess M., Debeljak M., Pallavajjala A., Beierl K., et al. False positives in multiplex PCR-based next-generation sequencing have unique signatures. J Mol Diagn. 2014;16(5):541–549. doi: 10.1016/j.jmoldx.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blais J., Lavoie S.B., Giroux S., Bussières J., Lindsay C., Dionne J., et al. Risk of misdiagnosis due to allele dropout and false-positive PCR artifacts in molecular diagnostics: analysis of 30,769 genotypes. J Mol Diagn. 2015;17(5):505–514. doi: 10.1016/j.jmoldx.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Widman A.J., Shah M., Frydendahl A., Halmos D., Khamnei C.C., Øgaard N., et al. Ultrasensitive plasma-based monitoring of tumor burden using machine-learning-guided signal enrichment. Nat Med. 2024;30(6):1655–1666. doi: 10.1038/s41591-024-03040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofman P. Implementation of the clinical practice of liquid biopsies for thoracic oncology the experience of the RespirERA university hospital institute (Nice, France) J Liq Biopsy. 2023;1 doi: 10.1016/j.jlb.2023.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholas C., Beharry A., Bendzsak A.M., Bisson K.R., Dadson K., Dudani S., et al. Point of care liquid biopsy for cancer treatment-early experience from a community center. Cancers (Basel) 2024;16(14) doi: 10.3390/cancers16142505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malapelle U., Donne A.D., Pagni F., Fraggetta F., Rocco E.G., Pasello G., et al. Standardized and simplified reporting of next-generation sequencing results in advanced non-small-cell lung cancer: practical indications from an Italian multidisciplinary group. Crit Rev Oncol Hematol. 2024;193 doi: 10.1016/j.critrevonc.2023.104217. [DOI] [PubMed] [Google Scholar]
- 51.Underhill H.R. Leveraging the fragment length of circulating tumour DNA to improve molecular profiling of solid tumour malignancies with next-generation sequencing: a pathway to advanced non-invasive diagnostics in precision oncology? Mol Diagn Ther. 2021;25(4):389–408. doi: 10.1007/s40291-021-00534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Jager V.D., Giacomini P., Fairley J.A., Toledo R.A., Patton S.J., Joosse S.A., et al. Reporting of molecular test results from cell-free DNA analyses: expert consensus recommendations from the 2023 European Liquid Biopsy Society ctDNA Workshop. EBioMedicine. 2025;114 doi: 10.1016/j.ebiom.2025.105636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malapelle U., Leighl N., Addeo A., Hershkovitz D., Hochmair M.J., Khorshid O., et al. Recommendations for reporting tissue and circulating tumour (ct)DNA next-generation sequencing results in non-small cell lung cancer. Br J Cancer. 2024;131(2):212–219. doi: 10.1038/s41416-024-02709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakravarty D., Johnson A., Sklar J., Lindeman N.I., Moore K., Ganesan S., et al. Somatic genomic testing in patients with metastatic or advanced cancer: ASCO provisional clinical opinion. J Clin Oncol. 2022;40(11):1231–1258. doi: 10.1200/JCO.21.02767. [DOI] [PubMed] [Google Scholar]
- 55.Febbo P.G., Allo M., Alme E.B., Cuyun Carter G., Dumanois R., Essig A., et al. Recommendations for the equitable and widespread implementation of liquid biopsy for cancer care. JCO Precis Oncol. 2024;8 doi: 10.1200/PO.23.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tack V., Spans L., Schuuring E., Keppens C., Zwaenepoel K., Pauwels P., et al. Describing the reportable range is important for reliable treatment decisions: a multiple laboratory study for molecular tumor profiling using next-generation sequencing. J Mol Diagn. 2018;20(6):743–753. doi: 10.1016/j.jmoldx.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Repetto M., Crimini E., Boscolo Bielo L., Guerini-Rocco E., Ascione L., Bonfanti A., et al. Molecular tumour board at European Institute of Oncology: report of the first three year activity of an Italian precision oncology experience. Eur J Cancer. 2023;183:79–89. doi: 10.1016/j.ejca.2023.01.019. [DOI] [PubMed] [Google Scholar]
- 58.El Helali A., Lam T.C., Ko E.Y., Shih D.J.H., Chan C.K., Wong C.H.L., et al. The impact of the multi-disciplinary molecular tumour board and integrative next generation sequencing on clinical outcomes in advanced solid tumours. Lancet Reg Health West Pac. 2023;36 doi: 10.1016/j.lanwpc.2023.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahles A., Goldschmid H., Volckmar A.L., Ploeger C., Kazdal D., Penzel R., et al. Structure and content of the EU-IVDR : current status and implications for pathology. Pathologie (Heidelb). 2023;44(Suppl 2):73–85. doi: 10.1007/s00292-022-01176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aaron D.G., Adashi E.Y., Cohen I.G. The US FDA's new rule for regulating laboratory-developed tests. JAMA Health Forum. 2024;5(10) doi: 10.1001/jamahealthforum.2024.2917. [DOI] [PubMed] [Google Scholar]
- 61.Ahmad-Nejad P., Ashavaid T., Vacaflores Salinas A., Huggett J., Harris K., Linder M.W., et al. Current and future challenges in quality assurance in molecular diagnostics. Clin Chim Acta. 2021;519:239–246. doi: 10.1016/j.cca.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Theodorsson E., Meijer P., Badrick T. External quality assurance in the era of standardization. Clin Chim Acta. 2024;557 doi: 10.1016/j.cca.2024.117876. [DOI] [PubMed] [Google Scholar]