Abstract
Objectives:
Adolescents represent a significant demographic in hospital outpatient visits, and it is important to give them special consideration and conduct a thorough examination for any adnexal pathology. The research aimed to study adnexal masses in adolescent females aged 10–19 years, including their clinical, biochemical, and radiological evaluations. The goal was to develop a treatment plan and analyze its relationship with histopathological findings.
Materials and Methods:
The study was carried out at a single center and included 124 participants in a descriptive research design.
Results:
During the study, 6.2% of adolescents had adnexal masses, with abdominal discomfort being the most common symptom (66.12%). Ultrasound scans showed cystic, solid, or complex masses in 41.6%, 23.4%, and 35% of cases, respectively. In 53.4% of the patients, tumors exceeding 10 cm in size were identified, while 66.67% exhibited elevated levels of tumor markers. Surgical procedures were necessary for 48.5% of adolescents, with laparoscopic cystectomy being carried out in 48.34% and staging laparotomy in 46.66%.
Conclusion:
Small asymptomatic cysts need to be monitored, but larger complex or solid tumors must all be thoroughly examined to rule out malignancy. Early diagnosis and preservation of the ovary should be the aim of treatment. Although guidelines exist for the treatment of adnexal masses in adults, there is limited information available in the literature regarding adolescents. Therefore, it is crucial to establish an appropriate surgical plan that preserves fertility in this vulnerable age group.
Keywords: Adnexal mass, adolescent, cystectomy, ovarian cyst, teenage, tumor
INTRODUCTION
An adnexal mass or tumor refers to any abnormal growth that originates from the ovary, fallopian tube, or nearby connective tissue. These masses are an important aspect of adolescent gynecology. The estimated occurrence of adnexal tumors in adolescents is 2.6 per 106 of the adolescent population.[1] Ovarian tumors make up 1%–1.5% of all childhood malignancies and 8% of all abdominal tumors in this age group. The majority (95%) of gynecologic tumors at this age are of ovarian origin, and among these, 10%–30% are malignant.[1,2,3,4]
Adolescents may discover ovarian tumors incidentally during routine scans or examinations, experiencing symptoms such as abdominal pain, lumps, or menstrual irregularities.[5] The occurrence of ovarian cysts increases with age during puberty, ranging from 3.8% to 31.3% throughout adolescence, with the highest incidence around age 15 years.[6] Ultrasonography is the primary imaging technique for detecting adnexal cysts, but further characterization and staging may require magnetic resonance imaging (MRI) or computed tomography (CT) scans if there are indeterminate cysts or suspicion of ovarian cancer.
Limited access to health care and hesitancy among adolescents often leads to delays in seeking help. This can result in time-consuming diagnosis and treatment processes at primary healthcare facilities, ultimately worsening conditions by the time they are referred to tertiary care centers.[7] The main goal of surgical treatment is to save the ovary when dealing with adnexal tumors, usually benign. However, treating ovarian cancer in adolescents requires caution due to the lack of specific guidelines for this age group, leading to varying opinions among gynecologists on the best methods for preserving the ovary. Ovarian cancer is often diagnosed at an advanced stage due to the lack of early symptoms and reliable screening methods.[8]
Surgery is fundamental in treating large or symptomatic ovarian tumors. Laparoscopic techniques have emerged as a practical choice for managing sizable ovarian masses in women.[9,10,11] However, the potential for a higher-than-expected malignancy rate underscores the importance of careful patient selection and comprehensive preoperative counseling. Intraoperative frozen section analysis of ovarian tumors is commonly employed to differentiate between benign and malignant cases, guiding surgical decisions. The frozen section results for benign tumors show impressive statistics: 95% sensitivity, 100% specificity, 100% positive predictive value, and 88% negative predictive value.[12]
Unfortunately, there is a lack of comprehensive studies focused on adolescent adnexal pathology. Our main goal in this research is to thoroughly examine the various types of adnexal pathology, explaining their clinical signs and ultrasound (USG) characteristics to aid in making informed decisions, while also establishing connections with histopathological findings.
MATERIALS AND METHODS
Study design
This was a single-center, descriptive study conducted after approval by the institute ethics committee. The study was conducted in accordance with the Declaration of Helsinki and was approved by AIIMS ethics committe with (approval numbe: IEC/AIIMS/ PAT /110/2016). Informed consent was obtained from all participant.
Settings
This study aimed to evaluate the presentation and management of adnexal masses in female adolescents aged 10–19 years in a tertiary care center with a total population of 104.2 million. This study was conducted over 7 years from January 2016 to December 2022. All adolescent females who visited the department of obstetrics and gynecology during the mentioned period and had adnexal masses were included in the study, whereas females with nongynecological pathologies were excluded from the study.
Participants
All adolescent females who had either radiological or clinical findings of adnexal masses during the study period were included in this study.
Data collection
After explaining the nature of the study and obtaining written informed consent, privacy and confidentiality were maintained, and a detailed medical history and any family history of malignancy were obtained. In all adnexal mass with solid components or complex structure or with a size of >7 cm on transabdominal USG, a panel of tumor markers was performed as part of the treatment protocol. In all cases where the USG examination revealed a solid component or equivocal findings, a CT scan or MRI scan was performed to plan further treatment. In all surgical cases, the histopathologic diagnosis was done and compared with the preoperative diagnosis.
Statistical methods
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA: IBM Corp. Results on continuous measurement are presented as mean ± standard deviation and categorical as frequency and percentage.
RESULTS
Over 7 years, 49,700 new female patients were recruited for a gynecology clinic, with 4.02% being teenagers. Among the teenagers, 6.2% were diagnosed with adnexal masses. Masses were found through clinical palpation in 39.5% of cases and during USGs in 60.5% of cases. The average age of these patients was 16.6 years, with an average BMI of 22.3 and an average age at menarche of 11.5 years. 12% were overweight, 4.9% were obese, and 8.8% were married. The majority were in the 17–19 age group and lived in rural areas, making up 62.9% of the total cohort [Table 1].
Table 1.
Demographics
| Characteristics | Frequency (%) |
|---|---|
| Total patients | 124 (100) |
| Age (years) | |
| 10–13 | 16 (21) |
| 14–16 | 33 (27) |
| 17–19 | 75 (61) |
| Mean age (years) | 16.6 |
| Premenarche | 3 |
| Postmenarche | 121 |
| The mean age of menarche (years) | 11.5 |
| Residence | |
| Rural | 78 (62.9) |
| Urban | 46 (37.1) |
| BMI | |
| Underweight (<18.5) | 11 (8.9) |
| Normal (18.5–24.9) | 92 (74.2) |
| Overweight (25–29.9) | 15 (12) |
| Obese (>30) | 6 (4.9) |
| Mean BMI | 22.3 |
| Married | 11 (8.8) |
| Unmarried | 113 (91.2) |
BMI: Body mass index
A total of 64 patients were conservatively managed, with 30.6% (38 of 64) being asymptomatic and incidentally diagnosed with simple cysts measuring <7 cm on ultrasonography. These patients were closely observed and monitored with USG examinations every 3 months, while 21% (26 of 64) required medical intervention like nonsteroidal anti-inflammatory drugs or combined oral contraceptives. The highest incidence of age-related ovarian cysts was observed in late adolescence, accounting for 53.2% of cases. The majority of cysts were under 3 cm in length (51.5%), followed by those between 3 cm and 5 cm (38.2%) and 5–7 cm (15.7%) [Supplementary Table 1].
Supplementary Table 1.
Characteristics of ovarian cysts managed conservatively (n=64/124; 51.6%)
| Age | Laterality | Total, n (%) | Size (cm) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Right, n (%) | Left, n (%) | <3, n (%) | 3–5, n (%) | 5–7, n (%) | ||
| 10–13 | 5 | 1 | 6 (9.3) | 2 | 3 | 1 |
| 13–16 | 13 | 11 | 24 (37.5) | 12 | 9 | 3 |
| 17–19 | 24 | 10 | 34 (53.2) | 19 | 9 | 6 |
| Total | 42 (65.6) | 22 (34.4) | 64 (100) | 33 (51.5) | 21 (32.8) | 10 (15.7) |
Among the patients, abdominal pain was the most common complaint, reported by 66.12% (n = 82) of individuals, either on its own or in combination with other symptoms such as abdominal mass sensation, menstrual irregularity, or pressure symptoms. Acute abdominal pain was experienced by 4% (n = 5) of cases that required emergency surgery, with torsion being identified intraoperatively in all instances. Menstrual irregularities were the next most common presentation, affecting 30.6% (n = 38) of patients, followed by the presence of a lump in the abdomen, reported by 17.7% (n = 22) of individuals [Figure 1].
Figure 1.
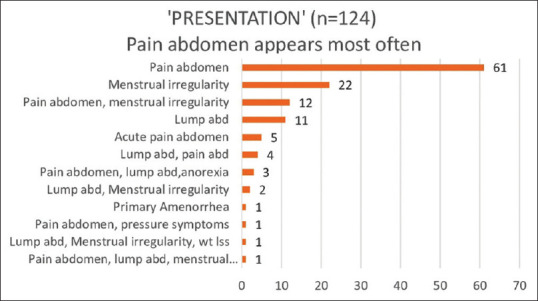
Diverse presenting symptoms
In various surgical interventions, it was observed that laparoscopic cystectomy was carried out in 18.6% (23 of 124) of cases. In addition, salpingo-oophorectomy was performed in 23.4% (29 of 124) of cases. In a singular case, salpingectomy was necessary due to tubal rupture. Interestingly, in 5.6% (7 of 124) of scenarios, the presence of endometriosis-related thick adhesions required adhesiolysis, before cystectomy. Noteworthy findings also revealed the distribution of cysts in the adnexal region. It was found that 62% (72 of 124) of cysts were located in the right adnexal region, while 34% (42 of 124) were found in the left adnexal region. Only a mere 4% (5 of 124) of cases had cysts present on both sides. This indicates a clear predominance of cysts on the right side, as depicted in Table 2.
Table 2.
Management of ovarian masses
| Management | n=124, n (%) |
|---|---|
| Observation and follow-up | 38 (30.6) |
| Medical management | 26 (21) |
| Nonsteroidal anti-inflammatory | 11 (8.9) |
| Oral contraceptive pills | 15 (12) |
| Surgical management | 60 (48.4) |
| Cystectomy | 23 (18.6) |
| Unilateral salpingo-oophorectomy | 29 (23.4) |
| Adhesiolysis and cystectomy | 7 (5.6) |
| Salpingectomy | 1 (0.8) |
| Total | 124 (100) |
| Side | |
| Right adnexal | 77 (62) |
| Left adnexal | 42 (34) |
| Bilateral | 5 (4) |
| Total | 124 (100) |
USG results showed cystic adnexal masses in 41.6% of cases, solid masses in 23.4%, and complex masses in 35%. About 58.34% of patients had CT scans for precise treatment planning, with 46.6% having tumors smaller than 10 cm and 53.4% having tumors larger than 10 cm. About 23.34% of cases had large masses palpable exceeding 28 weeks, adding an interesting aspect to the study. Elevated tumor markers were found in 66.67% of cases, leading to diagnoses of nonneoplastic tumors in 40%, benign tumors in another 40%, and malignant tumors in 20 (12%) cases. About 48.4% of adolescents underwent surgical treatment based on a comprehensive evaluation of clinical, radiological, and tumor marker data [Table 3]. Twenty-nine patients (48.34%) underwent laparoscopic surgery, 28 patients (46.66%) had laparotomy, and 3 patients (5%) required laparoscopic conversion due to dense adhesions [Table 4].
Table 3.
Correlation of radiological, clinical, and tumor markers with different adnexal masses
| Surgical management (n=60) | Total, n (%) | |||
|---|---|---|---|---|
|
| ||||
| Nonneoplastic (n=24; 40%) | Benign (n=24; 40%) | Malignant (n=12; 20%) | ||
| USG | 24 | 24 | 12 | 60 |
| Cystic | 18 | 7 | 0 | 25 (41.6) |
| Solid | 0 | 6 | 8 | 14 (23.4) |
| Complex | 6 | 11 | 4 | 21 (35) |
| CT scan | 6 | 17 | 12 | 35 (58.34) |
| Benign | 6 | 15 | 1 | 22 (62.8) |
| Malignant | 0 | 2 | 11 | 13 (37.2) |
| Size on USG (cm) | ||||
| <10 | 19 | 9 | 0 | 28 (46.6) |
| >10 | 5 | 15 | 12 | 32 (53.4) |
| Clinical size | ||||
| Nonpalpable | 5 | 5 | 0 | 10 (16.66) |
| Up to 16 weeks | 14 | 6 | 0 | 20 (33.34) |
| Lump of 16–20 weeks | 2 | 3 | 0 | 5 (8.3) |
| Lump of 20–24 weeks | 2 | 2 | 2 | 6 (10) |
| Lump of 24–28 weeks | 1 | 3 | 1 | 5 (8.3) |
| Lump of 28–32 weeks | 0 | 4 | 5 | 9 (15) |
| >32 weeks | 0 | 1 | 4 | 5 (8.3) |
| Tumor markers raised | n=9; 22.5% | n=6; 15%) | n=25; 62.5% | n=40; 66.67% |
| AFP | 0 | 1 | 10 | 11 |
| CA-125 | 6 | 2 | 6 | 14 |
| CA 19-9 | 1 | 0 | 1 | 2 |
| LDH | 2 | 3 | 8 | 13 |
USG: Ultrasonography, CT: Computer tomography, AFP: Alpha fetoprotein, CA: Cancer antigen, LDH: Lactate dehydrogenase
Table 4.
Route of surgery and final diagnosis
| Final histopathological diagnosis | n (%) | Laparoscopy (n=29), n (%) | Laparotomy (n=28), n (%) | Laparoscopy converted to laparotomy (n=3), n (%) |
|---|---|---|---|---|
| Nonneoplastic (n=14; 23.3%) | ||||
| Endometrioma | 10 (16.7) | 5 | 3 | 2 |
| Simple cyst | 6 (10) | 6 | - | - |
| Hemorrhagic cyst | 3 (5) | 2 | 1 | |
| Para tubal cyst | 2 (3.2) | 2 | ||
| Tubercular | 1 (1.6) | 1 | - | - |
| Ectopic pregnancy | 1 (1.6) | 1 | - | - |
| Benign (n=33; 55%) | ||||
| Dermoid | 14 (24) | 11 | 3 | 0 |
| Serous cystadenoma | 8 (13.2) | 1 | 6 | 1 |
| Mucinous cystadenoma | 2 (3.3) | - | 2 | - |
| Malignant (n=13; 21.7%) | ||||
| Dysgerminoma | 5 (8.3) | - | 5 | - |
| Immature teratoma | 3 (5) | - | 3 | - |
| Mixed GCT | 3 (5) | - | 3 | - |
| Mucinous carcinoma | 1 (1.6) | - | 1 | - |
| Sex cord-stromal tumor | 1 (1.6) | - | 1 | - |
| Total | 60 (100) | 29 (48.34) | 28 (46.66) | 3 (5) |
GCT: Germ cell tumor
The histopathology examination findings showed that 23.3% of cases had nonneoplastic pathology, 55% had benign pathology, and 21.7% had malignant pathology. Dermoid was the most common diagnosis, accounting for 24% of cases. Dysgerminoma was the most prevalent malignant pathology at 8.3%, followed by mixed germ cell tumor and immature teratoma at 5% each [Figure 2]. Adnexal masses were overall less common in early adolescents aged 10–13 years, benign tumors were more prevalent in middle adolescence, while malignant tumors were more common in late adolescence [Supplementary Figure 1 (84.9KB, tif) ].
Figure 2.
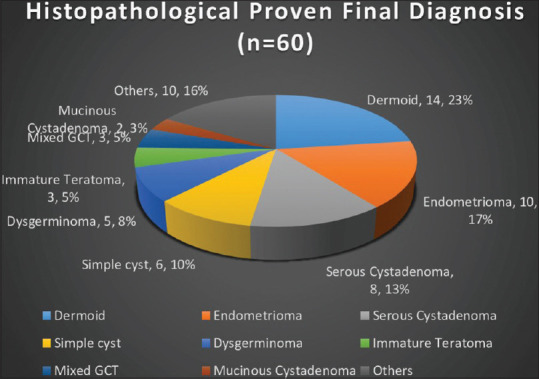
Histopathological Proven Final Diagnosis
DISCUSSION
Adnexal masses are usually not neoplastic, but their diagnosis is always a concern for both parents and clinicians. The main concern of the parents is the future fertility, the nature of the mass, and its prognosis. Similarly, clinicians face the challenge of finding out the low percentage of malignant tumors among them and their optimal treatment plan. Establishing an appropriate surgical plan that preserves fertility is very important in this vulnerable age group.[13,14,15] Although there is a clear guideline for the treatment of adnexal masses in adults, little is found in the literature about the adolescent population. Here, we prospectively studied 124 patients with adnexal masses and aimed to study the different spectra of presentation, their clinical, biochemical, and radiological evaluations before formulating a conservative or surgical treatment plan and finally their histopathology correlation.
Our research has revealed a fascinating statistic, indicating that a staggering 80% of adnexal masses were discovered in the age group of 14–19 years. Furthermore, a separate study has shown that approximately 61.7% of these masses were found in patients aged between 15 and 19 years. These findings shed light on the prevalence of such masses in young individuals, highlighting the importance of early detection and intervention.[9]
The research found that the mean age of participants was 16.6 years, with an average age at menarche of 11.5 years. These statistics provide insights into developmental phases and factors influencing adnexal mass development.[16,17] Three girls had not yet reached menarche, showing adnexal masses can develop before menstruation. These girls had rudimentary horns with endometrioma, highlighting the need for thorough evaluation and management.[18,19]
In terms of the distribution of the adnexal masses, the majority of our patients (96%) displayed unilateral disease, with a notable predominance on the right side (62%). These findings align with previous reports, suggesting a consistent pattern in the localization of these masses.[6,20]
Our research has revealed that abdominal pain (66.12%) is the prevailing symptom among the patients we studied. Following closely behind, menstrual irregularity was reported by 30.6% of patients, while lumps in the abdomen were reported by 17.7% of individuals. Various studies also confirmed that abdominal pain and the presence of a palpable abdominal mass were the most commonly observed symptoms.[7,21,22,23,24]
In addition to the prevalence of abdominal pain, our study also uncovered a significant correlation between tumor size and malignancy. Remarkably, tumors measuring over 10 cm on USG were found to be malignant on histopathology in a staggering 40% of cases.[25,26,27,28] This finding emphasizes the importance of closely monitoring tumor size as it can serve as a valuable predictor of malignancy. The larger the tumor, the higher the likelihood of it being malignant.
USG is a highly regarded imaging technique used to assess ovarian tumors. With a sensitivity of 90.7% and specificity of 91.4%, it is invaluable in distinguishing between benign and malignant masses.[29] In the realm of clinical practice, USG reigns supreme as the preferred method for the initial evaluation of ovarian pathology, serving as the primary screening tool to discern between malignant and benign diseases.[30] In fact, in every single patient, USG was the initial imaging examination, with a supplementary CT scan being performed in 35.4% of cases (44 of 124). While the necessity for additional imaging beyond USG remains uncertain for benign ovarian diseases, its significance has been firmly established in the context of malignant diseases.[31] It is worth noting that CT and MRI, while valuable in their own right, are considered supplementary and are not routinely recommended.[32,33]
In addition to the radiologic findings, the utilization of tumor marker tests proves to be immensely valuable in the assessment of preoperative risks. A comprehensive study, spanning over an impressive 15½ years, and a meticulous retrospective review of surgical ovarian cases have unequivocally established a strong association between elevated levels of beta-human chorionic gonadotropin, alpha-fetoprotein, and cancer antigen 125 (CA-125) with malignancy, with a remarkable statistical significance of P < 0.02.[34] The remarkable CA-125 exhibits an impressive sensitivity of 81% and a specificity of 75% when it comes to detecting ovarian malignancy. In our meticulous examination of all 60 cases that underwent surgical treatment, we discovered that both AFP and CA-125 were significantly elevated in cases where malignancy was histologically proven, further solidifying their importance as reliable tumor markers.
In nearly half of the cases, specifically 48.3%, we successfully conducted fertility-sparing laparoscopic cystectomy, a minimally invasive procedure that preserves the ability to conceive. However, for the remaining 46.66% of cases, we had to resort to laparotomy followed by unilateral salpingo-oophorectomy. This higher rate of unilateral salpingo-oophorectomy was primarily due to the referral of patients with larger tumors to a specialized tertiary center, where more extensive surgical intervention was deemed necessary.
During the initial two decades of life, germ cells and stromal tumors are commonly seen, with germ cell tumors in adolescents ranging from 67% to 85%. Conversely, epithelial tumors are uncommon during this period. Mature cystic teratoma is the most prevalent benign tumor (27.6%), while dysgerminomas are the primary malignant ovarian neoplasms.[35,36,37] Remarkably, our own findings align with these patterns, as 24% of the tumors we examined were identified as dermoid or mature cystic teratomas, while dysgerminomas constituted the majority of malignant tumors at an impressive rate of 38.5% (5 of 13 cases).
In a recent retrospective study of 1102 cases of ovarian tumors, our findings were further supported by the results, showing that 65.2% of the tumors were benign and 34.8% were malignant. This aligns with our own research, which revealed that 55% of the tumors we analyzed were benign, 23.3% were nonneoplastic, and 21.7% were malignant ovarian tumors.[21] It is worth noting that another study conducted at a referral center reported a higher frequency of malignant ovarian tumors, accounting for 59.49% of cases. Conversely, a separate study observed a much lower incidence of malignancy at only 4%.[38] These variations in findings can be attributed to the specific study settings and patient populations involved.
The study revealed an alarmingly high rate of malignancy, with 21.7% of the total cases (13 out of 60) being affected. This significant occurrence can be attributed to the study’s location in a prestigious tertiary referral center, known for its expertise in treating complex and advanced malignancies.
Several studies have identified potential risk factors associated with the formation of ovarian cysts in teenagers. Hormonal imbalances that result in irregular menstrual cycles are the predominant factor contributing to the formation of cysts within this age demographic. In addition, pelvic infections, early menarche, and conditions such as endometriosis and polycystic ovary disease can further predispose individuals to the formation of ovarian cysts. A family history of ovarian cysts, ovarian cancer, or breast cancer also raises one’s risk, emphasizing the need to discuss family medical history with healthcare professionals. Furthermore, certain lifestyle choices, including tobacco use and obesity, can contribute to the development of ovarian cysts, making it vital to embrace healthier habits for overall health and well-being.[39,40]
Strengths and limitations
The current study stands out due to its cross-sectional design and long duration, which are considered its main strengths. However, it is crucial to recognize a slight drawback of the study, as it did not monitor the follow-up of cancerous tumors or the requirement for postoperative chemoradiotherapy. Nevertheless, the study’s overall discoveries and perspectives continue to hold importance and make a substantial contribution to the established knowledge in the field.
CONCLUSION
The research emphasizes the diverse display and attributes of adnexal masses in adolescents. However, detecting them early in teenagers is challenging due to the absence of dependable screening methods. It is of utmost importance to promptly assess any adnexal pathology in young patients. When surgery becomes necessary, the primary objective should be to preserve the ovary, ensuring the most favorable results for young individuals who may be confronted with potential ovarian malignancies.
Author contributions
Mukta Agarwal: Conceptualisation, supervision, and final editing. Smita Singh: Data collection, methodology, and manuscript writing. Sudwita Sinha: Data curation and editing. All authors have read and agreed to the final version of the manuscript.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY MATERIAL
Diagram showing the frequency of histopathologically proven nonneoplastic, benign, and malignant adnexal lump in different age groups
Acknowledgments
The authors acknowledge the Government of India for the Rashtriya Bal Swasthya Karyakram (RBSK) Yojna, under which many adolescents with Mullerian anomaly were operated on free of charge, and for the Pradhan Mantri Jan Arogya Yojana (PM-JAY), under which many poor adolescents were operated on free of charge.
Funding Statement
Nil.
REFERENCES
- 1.Zolton JR, Maseelall PB. Evaluation of ovarian cysts in adolescents. Open J Obstet Gynecol. 2013;3:12–16. [doi: 10.4236/ojog.2013.37A1004] [Google Scholar]
- 2.Bhattacharyya NK, De A, Bera P, Sristidhar M, Chakraborty S, Bandopadhyay R. Ovarian tumors in pediatric age group – A clinicopathologic study of 10 years'cases in West Bengal, India. Indian J Med Paediatr Oncol. 2010;31:54–7. doi: 10.4103/0971-5851.71656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quddusi H, Alvi AN, Masood E. Ovarian tumors in adolescent females, an experience at Nishtar Hospital Multan. Pak J Med Health Sci. 2013;7:93–4. [Google Scholar]
- 4.Baloch S, Khaskheli M, Malik AM, Sheeba A, Khushk IA. Clinical spectrum and management of ovarian tumours in young girls up to 20 years of age. J Ayub Med Coll Abbottabad. 2008;20:14–7. [PubMed] [Google Scholar]
- 5.Zhang M, Jiang W, Li G, Xu C. Ovarian masses in children and adolescents – An analysis of 521 clinical cases. J Pediatr Adolesc Gynecol. 2014;27:e73–7. doi: 10.1016/j.jpag.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 6.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins-Gynecology. Practice bulletin no. 174: Evaluation and management of adnexal masses. Obstet Gynecol. 2016;128:e210–26. doi: 10.1097/AOG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 7.Emeksiz HC, Derinöz O, Akkoyun EB, GüçlüPınarlı F, Bideci A. Age-specific frequencies and characteristics of ovarian cysts in children and adolescents. J Clin Res Pediatr Endocrinol. 2017;9:58–62. doi: 10.4274/jcrpe.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Łuczak J, Bagłaj M. Selecting treatment method for ovarian masses in children – 24 years of experience. J Ovarian Res. 2017;10:59. doi: 10.1186/s13048-017-0353-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinay T, Kizilkaya Y, Altinbas SK, Tapisiz OL, Ustun YE. Feasibility and safety of laparoscopic surgery in large ovarian masses. Gynecol Minim Invasive Ther. 2022;11:215–20. doi: 10.4103/gmit.gmit_122_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham TH, Nguyen PN, Ho QN. Timely laparoscopic intervention for ovarian tumor-related autoimmune encephalitis: A challenging pathology at Tu Du Hospital in Vietnam and literature review. Gynecol Minim Invasive Ther. 2023;12:185–8. doi: 10.4103/gmit.gmit_36_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vatsa R, Kulshrestha V, Bharti J, Singhal S, Malhotra N. Synchronous bilateral torsion of nonpathological ovaries in an adolescent girl with unilateral recurrence. Gynecol Minim Invasive Ther. 2023;12:246–8. doi: 10.4103/gmit.gmit_32_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palakkan S, Augestine T, Valsan MK, Vahab KP, Nair LK. Role of frozen section in surgical management of ovarian neoplasm. Gynecol Minim Invasive Ther. 2020;9:13–7. doi: 10.4103/GMIT.GMIT_2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brookfield KF, Cheung MC, Koniaris LG, Sola JE, Fischer AC. A population-based analysis of 1037 malignant ovarian tumors in the pediatric population. J Surg Res. 2009;156:45–9. doi: 10.1016/j.jss.2009.03.069. [DOI] [PubMed] [Google Scholar]
- 14.Renaud EJ, Sømme S, Islam S, Cameron DB, Gates RL, Williams RF, et al. Ovarian masses in the child and adolescent: An American Pediatric Surgical Association outcomes and evidence-based practice committee systematic review. J Pediatr Surg. 2019;54:369–77. doi: 10.1016/j.jpedsurg.2018.08.058. [DOI] [PubMed] [Google Scholar]
- 15.Northridge JL. Adnexal masses in adolescents. Pediatr Ann. 2020;49:e183–7. doi: 10.3928/19382359-20200227-01. [DOI] [PubMed] [Google Scholar]
- 16.Northridge JL. Adnexal masses in adolescents. Pediatr Ann. 2020;49:e183–7. doi: 10.3928/19382359-20200227-01. [DOI] [PubMed] [Google Scholar]
- 17.Kelleher CM, Goldstein AM. Adnexal masses in children and adolescents. Clin Obstet Gynecol. 2015;58:76–92. doi: 10.1097/GRF.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 18.Scheibner K, Kuhn A, Raio L, Brühwiler H, Müller MD. The non-communicating rudimentary horn: Diagnostic and therapeutic challenges. Gynecol Surg. 2007;4:207–11. [doi: 10.1007/s10397-006-0269-y] [Google Scholar]
- 19.Jalil RA, Alsada AI. Unicornuate uterus with a functional non-communicating horn in adolescent. BMJ Case Rep. 2021;14:e242874. doi: 10.1136/bcr-2021-242874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.User İR, Karakuş SC, Özokutan BH, Akçaer V, Burulday B, Ceylana H. Can preoperative findings help to interpret neoplastic and non-neoplastic lesions of ovary and affect surgical decisions in children and adolescents? Arch Argent Pediatr. 2019;117:294–400. doi: 10.5546/aap.2019.eng.294. [DOI] [PubMed] [Google Scholar]
- 21.Rathore R, Sharma S, Arora D. Spectrum of childhood and adolescent ovarian tumors in India: 25 years experience at a single institution. Open Access Maced J Med Sci. 2016;4:551–5. doi: 10.3889/oamjms.2016.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta B, Guleria K, Suneja A, Vaid NB, Rajaram S, Wadhwa N. Adolescent ovarian masses: A retrospective analysis. J Obstet Gynaecol. 2016;36:515–7. doi: 10.3109/01443615.2015.1103721. [DOI] [PubMed] [Google Scholar]
- 23.AlDakhil L, Aljuhaimi A, AlKhattabi M, Alobaid S, Mattar RE, Alobaid A. Ovarian neoplasia in adolescence: A retrospective chart review of girls with neoplastic ovarian tumors in Saudi Arabia. J Ovarian Res. 2022;15:105. doi: 10.1186/s13048-022-01033-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilli GS, Suneeta KP, Dhaded AV, Yenni VV. Ovarian tumours: A study of 282 cases. J Indian Med Assoc. 2002;100:420. 423-4, 447. [PubMed] [Google Scholar]
- 25.Péroux E, Franchi-Abella S, Sainte-Croix D, Canale S, Gauthier F, Martelli H, et al. Ovarian tumors in children and adolescents: A series of 41 cases. Diagn Interv Imaging. 2015;96:273–82. doi: 10.1016/j.diii.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Yu D, Wang F. Clinical and computed tomographic features of ovarian lesions in infants, children, and adolescents: A series of 222 cases. J Pediatr Adolesc Gynecol. 2021;34:387–93. doi: 10.1016/j.jpag.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Ryoo U, Lee DY, Bae DS, Yoon BK, Choi D. Clinical characteristics of adnexal masses in Korean children and adolescents: Retrospective analysis of 409 cases. J Minim Invasive Gynecol. 2010;17:209–13. doi: 10.1016/j.jmig.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Kang GG, So KA, Hwang JY, Kim NR, Yang EJ, Shim SH, et al. Ultrasonographic diagnosis and surgical outcomes of adnexal masses in children and adolescents. Sci Rep. 2022;12:3949. doi: 10.1038/s41598-022-08015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hafeez S, Sufian S, Beg M, Hadi Q, Jamil Y, Masroor I. Role of ultrasound in characterization of ovarian masses. Asian Pac J Cancer Prev. 2013;14:603–6. doi: 10.7314/apjcp.2013.14.1.603. [DOI] [PubMed] [Google Scholar]
- 30.Prasad S. Role of ultrasonography to evaluate ovarian masses and its correlation with histopathological findings. J Med Sci Clin Res. 2019;7:266–72. [doi: 10.18535/jmscr/v7i4.47] [Google Scholar]
- 31.Łuczak J, Bagłaj M, Dryjański P, Kalcowska A, Banaszyk-Pucała N, Boczar M, et al. What should be the topics of a prospective study on ovarian masses in children? Results of a multicenter retrospective study and a scoping literature review. Curr Oncol. 2022;29:1488–500. doi: 10.3390/curroncol29030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor EC, Irshaid L, Mathur M. Multimodality imaging approach to ovarian neoplasms with pathologic correlation. Radiographics. 2021;41:289–315. doi: 10.1148/rg.2021200086. [DOI] [PubMed] [Google Scholar]
- 33.Amendola MA. The role of CT in the evaluation of ovarian malignancy. Crit Rev Diagn Imaging. 1985;24:329–68. [PubMed] [Google Scholar]
- 34.Oltmann SC, Garcia N, Barber R, Huang R, Hicks B, Fischer A. Can we preoperatively risk stratify ovarian masses for malignancy? J Pediatr Surg. 2010;45:130–4. doi: 10.1016/j.jpedsurg.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Amatya A, Rana A, Gurung G. Ovarian tumors in childhood and adolescents – Our eight years experiences. Nepal J Obstet Gynaecol. 1970;3:39–42. [Google Scholar]
- 36.Khatoon F, Begum SA, Choudhury N, Yeasmin S, Begum M. Clinical spectrum and management of ovarian masses in children and adolescent up to 20 years of age. Bangladesh Med J. 2021;50:1–9. [Google Scholar]
- 37.Grapsa D, Kairi-Vassilatou E, Kleanthis C, Dastamani C, Fillipidou A, Kondi-Pafiti A. Epithelial ovarian tumors in adolescents: A retrospective pathologic study and a critical review of the literature. J Pediatr Adolesc Gynecol. 2011;24:386–8. doi: 10.1016/j.jpag.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Spinelli C, Pucci V, Buti I, Liserre J, Messineo A, Bianco F, et al. The role of tumor markers in the surgical approach of ovarian masses in pediatric age: A 10-year study and a literature review. Ann Surg Oncol. 2012;19:1766–73. doi: 10.1245/s10434-012-2249-y. [DOI] [PubMed] [Google Scholar]
- 39.Parazzini F, Moroni S, Negri E, La Vecchia C, Dal Pino D, Ricci E. Risk factors for functional ovarian cysts. Epidemiology. 1996;7:547–9. [PubMed] [Google Scholar]
- 40.Parazzini F, La Vecchia C, Franceschi S, Negri E, Cecchetti G. Risk factors for endometrioid, mucinous and serous benign ovarian cysts. Int J Epidemiol. 1989;18:108–12. doi: 10.1093/ije/18.1.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagram showing the frequency of histopathologically proven nonneoplastic, benign, and malignant adnexal lump in different age groups
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.


