Abstract
Introduction
Multiple sclerosis (MS) is a heterogeneous disease that disproportionately impacts Black people with MS (PwMS), who experience more severe disease and higher relapse rates compared with non-Black populations. Despite widespread use of fumarates and sphingosine-1-phosphate (S1P) receptor modulators as oral disease-modifying therapies (DMTs) for relapsing MS, their comparative effectiveness in Black PwMS has not been studied. This study aims to help address this gap using real-world claims data.
Methods
This retrospective analysis using the Komodo Health Claims Database included Black PwMS. Patients were aged 18–64 years with ≥ 1 claim for MS diagnosis (International Classification of Diseases, Tenth Revision, Clinical Modification code G35) and ≥ 1 prescription claim for fumarates (dimethyl fumarate or diroximel fumarate) or an S1P receptor modulator (fingolimod, siponimod, ozanimod, or ponesimod) between January 2017 and April 2023. Outcomes included annualized relapse rate (ARR) and time to first relapse. Propensity score matching (2:1) and inverse probability weighting were used to balance baseline characteristics. Relapse events were identified using a claims-based algorithm.
Results
The analysis included 1664 Black PwMS (1231 and 433 in fumarate and S1P treatment arms, respectively). Post-index ARRs were comparable between groups (rate ratio [RR] 1.18, p = 0.423). Kaplan–Meier analyses showed similar relapse-free proportions at 24 months (72.6% and 74.7% in fumerate and S1P populations, respectively; p = 0.152). These findings were consistent in both the propensity score-matched and inverse probability weighted populations.
Conclusions
This real-world, claims-based analysis demonstrates that fumarates and S1P receptor modulators have similar effectiveness in reducing relapses among Black PwMS, with > 72% of patients in both treatment groups remaining relapse-free at 24 months. Given the underrepresentation of Black patients in MS clinical trials, these results provide valuable real-world evidence to guide treatment decisions for this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-025-00774-2.
Keywords: African American populations, Dimethyl fumarate, Diroximel fumarate, Effectiveness, Multiple sclerosis, Sphingosine-1-phosphate
Key Summary Points
| Why carry out this study? |
| Black people with multiple sclerosis (PwMS) experience more frequent relapses, faster disability progression, and a more severe disease course compared with non-Black populations |
| Black PwMS are underrepresented in MS clinical trials, limiting understanding of how fumarates and sphingosine-1-phosphate (S1P) receptor modulators perform in this population |
| What was learned from this study? |
| Fumarates and S1Ps demonstrated comparable effectiveness in reducing relapse rates and maintaining relapse-free status over 24 months among Black PwMS |
| Over 72% of Black PwMS in both treatment groups remained relapse-free at 24 months, with no significant differences in annualized relapse rates or time to first relapse |
| This study is the first real-world comparative effectiveness analysis of these therapies in Black PwMS, contributing valuable insights to address the treatment data gap for this underrepresented population |
Introduction
Multiple sclerosis (MS) is a complex and heterogeneous disease affecting nearly 1 million people in the USA and approximately another 2 million people outside of the USA [1]. In the USA, people with Black or African American race origins account for 13.7% of the US population [2]. People from minority groups are underrepresented in MS clinical trials, with the majority of trials not reporting race or ethnicity enrollment data [3]. This underrepresentation limits the understanding of how MS therapies perform in this population, particularly given the observed differences in disease characteristics among Black patients with MS (PwMS), who tend to experience more frequent relapses and accelerated disability progression compared with the non-Black population [4–6].
In the USA, fumarates, including dimethyl fumarate (DMF) and diroximel fumarate (DRF), and sphingosine-1-phosphate (S1P) receptor modulators, such as fingolimod, siponimod, ozanimod, and ponesimod, are widely used oral disease-modifying therapies (DMTs) approved for treating relapsing forms of MS [7–12]. Comparative studies, including registry data and indirect analyses of clinical trial results, suggest that both fumarates and S1Ps demonstrate similar efficacy in reducing relapse rates, slowing disability progression, and improving magnetic resonance imaging (MRI) outcomes [13–17]. Despite these findings, the comparative effectiveness of these treatments in Black PwMS remains entirely unexplored. Black individuals are consistently underrepresented in clinical trials in MS, and few studies provide real-world data on the effectiveness of these DMTs in this population [18].
Claims analyses are increasingly being used as an avenue to fill these knowledge gaps by leveraging real-world data to observe treatment utilization patterns and outcomes [19, 20]. These analyses are especially valuable in underrepresented populations, where traditional clinical trial data are often scarce. The study reported here helps to address this gap by focusing on the comparative effectiveness of fumarates compared to S1Ps in Black PwMS. We hypothesize that both treatments will demonstrate comparable effectiveness across key MS outcomes. This research builds on prior studies [13–15], while also addressing a critical unmet need for more inclusive data on MS treatments in minority populations.
Methods
Study Design
This retrospective observational analysis used data from the Komodo Health Claims Database to estimate the annualized relapse rate (ARR) and time to first relapse in Black PwMS who initiated treatment with fumarates versus those who initiated treatment with S1P receptor modulators. The Komodo Health database comprises de-identified medical and pharmacy claims from a large, commercially and government-insured population across the USA. Patient-level information on diagnoses, procedures, prescription medications, and enrollment status are captured. The study period spanned from 01 January 2016 to 15 April 2023.
Patients
Eligible patients were aged 18–64 years at the index date and had ≥ 1 medical claims with a diagnosis of MS (International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] code G35) during the study period. The index date was defined as the first prescription claim for either a fumarate (DMF or DRF) or an S1P receptor modulator (fingolimod, siponimod, ozanimod, or ponesimod) between 01 January 2017 and 15 April 2023. Data on eligible patients also showed continuous medical and pharmacy enrollment for ≥ 12 months prior to the index date and ≥ 3 months after the index date, allowing for coverage gaps of ≤ 45 days.
Patients were excluded if they had multiple DMTs prescribed on the index date or if they had any claim for a monoclonal antibody DMT (e.g., natalizumab, alemtuzumab, ocrelizumab, ofatumumab) at any time prior to the index date.
Race and ethnicity were self-reported. To ensure consistency, the Komodo Health Claims Database consolidates race and ethnicity data into one field. The designation for Black people included those who reported their race as Black or African American [21].
Follow-Up and Outcomes
Patients were followed from the index date until the end of the study (15 April 2023), loss of insurance eligibility, a gap in index DMT coverage > 45 days, or a switch from index DMT to another DMT.
The primary outcomes were ARR and the time to first relapse post-index DMT among Black PwMS. The ARR was calculated as the total number of relapses divided by the total patient-years of follow-up. Time to first relapse was defined as the duration from the index date to the first identified relapse event. Relapses were identified using a previously published claims-based algorithm that has been validated against relapses identified based on medical chart review [22–25]. Relapses were defined as an MS-related inpatient claim with a primary diagnosis of MS (ICD-10 code G35) or an outpatient MS-related diagnosis plus a prescription claim for an intravenous (IV) steroid (not including IV steroids that were administered on the same day as an IV DMT, such as natalizumab, alemtuzumab, ocrelizumab, or rituximab), adrenocorticotropic hormone, total plasma exchange, or a high-dose oral corticosteroid (≥ 500 mg/day prednisone or equivalent) ≤ 7 days after the outpatient visit.
Compliance with Ethics Guidelines
This study was a retrospective analysis of de-identified data from the Komodo Health Sentinel database and did not involve any new studies with human or animal subjects. As such, ethical approval or informed consent was not required.
Statistical Analyses
Baseline characteristics were summarized using descriptive statistics, with continuous variables presented as means with standard deviations (SDs) and categorical variables presented as frequencies and percentages. To adjust for potential baseline confounders, a 2:1 nearest-neighbor propensity score (PS)-matching was performed which included age, gender, MS Severity Score, Charlson Comorbidity Index, insurance type, ARR, any pre-index DMT, and index year. A sensitivity analysis implementing stabilized inverse probability weighting (IPW) was also conducted to assess consistency in results with the alternative confounder adjustment method. ARRs and rate ratios (RRs) were estimated using Poisson generalized linear models that included an offset for patient-specific follow-up time, accommodating varying durations of observation among patients. Kaplan–Meier survival analyses and weighted Cox proportional hazards models were utilized to estimate the survival functions for time to first relapse.
Results
Patient Population
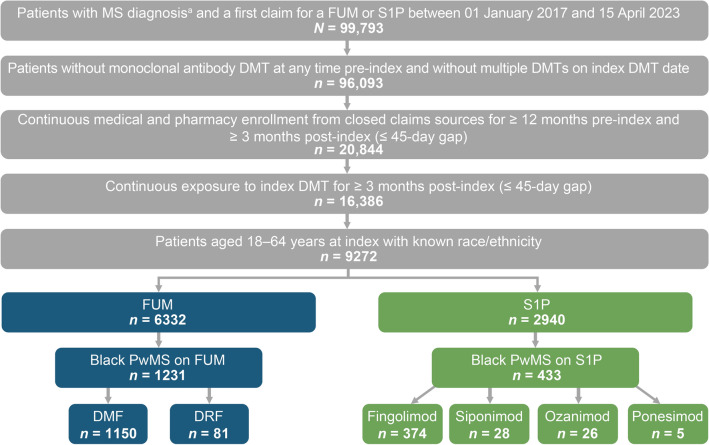
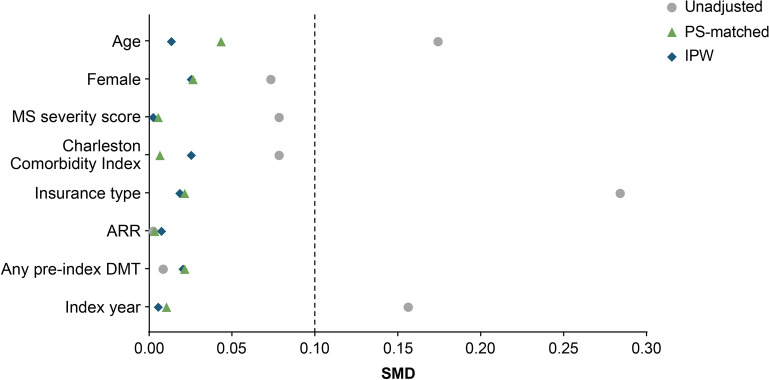
Overall, there were 99,793 people with a diagnosis for MS and ≥ 1 claim for a fumarate or S1P between 01 January 2017 and 15 April 2023 (Fig. 1). After applying exclusion criteria, the analysis included a total of 1664 Black PwMS, comprising 1231 patients in the fumarates group and 433 patients in the S1P group. Before any adjustment or weighting, baseline characteristics differed between the fumarates and S1P populations (Table 1), with standardized mean differences (SMDs) ≥ 0.1 for variables including age, insurance type, and index year (Fig. 2). Specifically, the unadjusted population had mean (SD) ages of 42.2 (11.0) years for the fumarates group and 40.3 (11.4) years for the S1P group, and differing proportions of insurance types between the groups.
Fig. 1.
Study sample. a ≥1 claim with a diagnosis for MS (ICD-10 code G35). DMF Dimethyl fumarate, DMT disease-modifying therapy, DRF diroximel fumarate, ICD-10 International Classification of Diseases, Tenth Revision, FUM fumarate, MS multiple sclerosis, PwMS patients with MS, S1P sphingosine-1-phosphate receptor modulator
Table 1.
Patient demographics and characteristics
| Demographics/characteristics | Unadjusted population | PS-matched population | IPW population | |||
|---|---|---|---|---|---|---|
| FUM group (n = 1231) | S1P group (n = 433) | FUM group (n = 866) | S1P group (n = 433) | FUM group (n = 1231.6) | S1P group (n = 431.3) | |
| Femalea, n (%) | 1004 (81.6) | 365 (84.3) | 738 (85.2) | 365 (84.3) | 1014.1 (82.3) | 359.2 (83.3) |
| Age,a years, mean (SD) | 42.2 (11.0) | 40.3 (11.4) | 40.7 (10.9) | 40.3 (11.4) | 41.7 (11.0) | 41.5 (11.6) |
| Age group, years, n (%) | ||||||
| 18–29 | 183 (14.9) | 85 (19.6) | 159 (18.4) | 85 (19.6) | 198.0 (16.1) | 72.6 (16.8) |
| 30–39 | 363 (29.5) | 128 (29.6) | 269 (31.1) | 128 (29.6) | 372.8 (30.3) | 120.8 (28.0) |
| 40–49 | 335 (27.2) | 128 (29.6) | 231 (26.7) | 128 (29.6) | 329.5 (26.8) | 129.6 (30.0) |
| 50–59 | 285 (23.2) | 65 (15.0) | 176 (20.3) | 65 (15.0) | 271.3 (22.0) | 73.4 (17.0) |
| 60–64 | 65 (5.3) | 27 (6.2) | 31 (3.6) | 27 (6.2) | 60.1 (4.9) | 35.0 (8.1) |
| Region, n (%) | ||||||
| Midwest | 296 (24.0) | 93 (21.5) | 202 (23.3) | 93 (21.5) | 293.4 (23.8) | 92.4 (21.4) |
| Northeast | 237 (19.3) | 74 (17.1) | 158 (18.2) | 74 (17.1) | 234.0 (19.0) | 76.8 (17.8) |
| South | 606 (49.2) | 239 (55.2) | 447 (51.6) | 239 (55.2) | 614.8 (49.9) | 232.7 (53.9) |
| West | 92 (7.5) | 27 (6.2) | 59 (6.8) | 27 (6.2) | 89.3 (7.3) | 29.4 (6.8) |
| Insurance typea, n (%) | ||||||
| Commercial | 479 (38.9) | 229 (52.9) | 450 (52.0) | 229 (52.9) | 525.2 (42.6) | 186.6 (43.3) |
| Medicaid | 607 (49.3) | 166 (38.3) | 341 (39.4) | 166 (38.3) | 571.4 (46.4) | 199.5 (46.2) |
| Medicare | 145 (11.8) | 38 (8.8) | 75 (8.7) | 38 (8.8) | 135.0 (11.0) | 45.2 (10.5) |
| Reside in state with high level of povertyd, n (%) | 454 (36.9) | 172 (39.7) | 316 (36.5) | 172 (39.7) | 456.0 (37.0) | 168.9 (39.2) |
| ARRa,b,c, mean (SD) | 0.34 (0.81) | 0.35 (0.84) | 0.32 (0.73) | 0.35 (0.84) | 0.35 (0.81) | 0.35 (0.88) |
| Patients with relapses by count, n (%) | ||||||
| 0 | 914 (74.2) | 323 (74.6) | 650 (75.1) | 323 (74.6) | 913.8 (74.2) | 321.0 (74.4) |
| 1 | 261 (21.2) | 89 (20.6) | 178 (20.6) | 89 (20.6) | 260.7 (21.2) | 88.9 (20.6) |
| 2 | 37 (3.0) | 14 (3.2) | 25 (2.9) | 14 (3.2) | 37.6 (3.1) | 15.2 (3.5) |
| 3 | 7 (0.6) | 4 (0.9) | 6 (0.7) | 4 (0.9) | 7.4 (0.6) | 3.3 (0.8) |
| 4+ | 12 (1.0) | 3 (0.7) | 7 (0.8) | 3 (0.7) | 12.0 (1.0) | 2.9 (0.7) |
| MS severity scorea, mean (SD) | 7.14 (4.23) | 6.82 (4.12) | 6.80 (3.90) | 6.82 (4.12) | 7.06 (4.20) | 7.06 (4.25) |
| MS severity, n (%) | ||||||
| Low | 370 (30.4) | 144 (33.6) | 281 (32.8) | 144 (33.6) | 381.2 (31.3) | 131.1 (30.6) |
| Moderate | 629 (51.6) | 222 (51.7) | 442 (51.6) | 222 (51.7) | 625.0 (51.3) | 225.5 (52.7) |
| High | 220 (18.0) | 63 (14.7) | 133 (15.5) | 63 (14.7) | 212.9 (17.5) | 71.5 (16.7) |
| Charlson Comorbidity Index,a mean (SD) | 0.67 (1.23) | 0.58 (1.12) | 0.59 (1.14) | 0.58 (1.12) | 0.65 (1.21) | 0.62 (1.13) |
| Index yeara, mean (SD) | 2018.98 (1.71) | 2018.71 (1.71) | 2018.73 (1.64) | 2018.71 (1.71) | 2018.91 (1.70) | 2018.92 (1.77) |
| Any pre-index DMTa, n (%) | 323 (26.2) | 112 (25.9) | 232 (26.8) | 112 (25.9) | 323.0 (26.2) | 116.9 (27.1) |
| Pre-index DMT, n (%) | ||||||
| Glatiramer acetate | 186 (15.1) | 57 (13.2) | 136 (15.7) | 57 (13.2) | 186.0 (15.1) | 60.5 (14.0) |
| Interferon beta-1a | 63 (5.1) | 25 (5.8) | 46 (5.3) | 25 (5.8) | 62.0 (5.0) | 25.8 (6.0) |
| Interferon beta-1a/albumin human | 42 (3.4) | 20 (4.6) | 27 (3.1) | 20 (4.6) | 43.1 (3.5) | 20.3 (4.7) |
| Interferon beta-1b | 17 (1.4) | 8 (1.8) | 10 (1.2) | 8 (1.8) | 16.7 (1.4) | 8.5 (2.0) |
| Peginterferon beta-1a | 15 (1.2) | 2 (0.5) | 13 (1.5) | 2 (0.5) | 15.3 (1.2) | 1.8 (0.4) |
| None | 908 (73.8) | 321 (74.1) | 634 (73.2) | 321 (74.1) | 908.5 (73.8) | 314.4 (72.9) |
| Index DMT, n (%) | ||||||
| DMF | 1150 (93.4) | 0 (0.0) | 820 (94.7) | 0 (0.0) | 1154.2 (93.7) | 0.0 (0.0) |
| DRF | 81 (6.6) | 0 (0.0) | 46 (5.3) | 0 (0.0) | 77.4 (6.3) | 0.0 (0.0) |
| Fingolimod HCL | 0 (0.0) | 374 (86.4) | 0 (0.0) | 374 (86.4) | 0.0 (0.0) | 360.4 (83.6) |
| Ozanimod HCL | 0 (0.0) | 26 (6.0) | 0 (0.0) | 26 (6.0) | 0.0 (0.0) | 32.3 (7.5) |
| Ponesimod | 0 (0.0) | 5 (1.2) | 0 (0.0) | 5 (1.2) | 0.0 (0.0) | 6.0 (1.4) |
| Siponimod | 0 (0.0) | 28 (6.5) | 0 (0.0) | 28 (6.5) | 0.0 (0.0) | 32.7 (7.6) |
ARR Annualized relapse rate, DMF dimethyl fumarate, DMT disease-modifying therapy, DRF diroximel fumarate, FUM fumarates, IPW inverse probability of treatment weighting, MS multiple sclerosis, PS propensity score, S1P sphingosine-1-phosphate receptor modulators, SD standard deviation, SMD standardized mean difference
aVariable was included in the PS and IPW models
bARR calculated as the total number of relapses observed divided by the total number of patient-years observed. Estimated via Poisson model adjusting for repeated measures correlation
cRelapses were defined as an MS-related inpatient claim with a primary diagnosis of MS or an outpatient MS-related diagnosis and prescription claim for an intravenous steroid, adrenocorticotropic hormone, total plasma exchange, or a high-dose oral corticosteroid ≤ 7 days after the outpatient visit
dState ranks in the top quartile for the proportion of residents who live in poverty based on 2021 US Census Bureau of Statistics (LA, MS, NM, DC, WV, AL, KY, OK, SC, GA, TX, NV, NY)
Fig. 2.
The SMDs of baseline characteristics in the unadjusted population, the PS-matched population, and the IPW population. ARR Annualized relapse rate, DMT disease-modifying therapy, IPW inverse probability of treatment weights, MS multiple sclerosis, PS propensity score, SMD absolute standardized mean difference. Threshold ≤0.10 was considered to be well balanced [26]
After applying PS-matching and IPW adjustments, all baseline variables demonstrated SMDs < 0.1 (Fig. 2), indicating adequate balance between the treatment groups [26]. In the 2:1 PS-matched population, a total of 1299 Black PwMS were included, with 866 patients in the fumarates group and 433 in the S1P group (Table 1). The mean (SD) age was 40.7 (10.9) years for patients receiving fumarates and 40.3 (11.4) years for those receiving S1P. The majority of patients in both groups were female, with 85.2% in the fumarates group and 84.3% in the S1P group.
The average post-index duration of treatment exposure (SD) was 441 days (392) for the fumarates group and 506 days (423) for the S1P group (Electronic Supplementary Material Table S1). Baseline characteristics such as the MS Severity Score and Charlson Comorbidity Index were similar between the groups after matching, with mean (SD) MS Severity Scores of 6.80 (3.90) for the fumarates group and 6.82 (4.12) for the S1P group, and Charlson Comorbidity Index scores of 0.59 (1.14) and 0.58 (1.12), respectively. Prior to index treatment, 26.8% of patients in the fumarates group and 25.9% in the S1P group had used any DMT, with glatiramer acetate and interferon beta-1a being the most common. Additionally, a similar proportion of patients resided in states with high levels of poverty, accounting for 36.5% of the fumarates group and 39.7% of the S1P group in the matched population.
Effectiveness Outcomes
In the 2:1 PS-matched population, there were no significant differences in post-index ARRs between the fumarates and S1P groups. In the PS-matched population, the ARR (95% confidence interval [CI]) was 0.251 (0.205–0.307) for the fumarates group and 0.212 (0.150–0.300) for the S1P group. The RR was 1.18 with a p value of 0.423, indicating that patients in the fumarates group had a similar risk of relapse compared with those in the S1P group (Fig. 3). In the IPW population, the ARR (95% CI) was 0.237 (0.203–0.278) for the fumarates group and 0.234 (0.156–0.350) for the S1P group. The RR was 1.02 with a p value of 0.944, further demonstrating no significant difference in ARRs between the two treatment groups.
Fig. 3.
The ARR on FUM versus S1P. Relapses were defined as an MS-related inpatient claim with a primary diagnosis of multiple sclerosis (MS) or an outpatient MS-related diagnosis and prescription claim for an intravenous steroid, adrenocorticotropic hormone, total plasma exchange, or a high-dose oral corticosteroid ≤7 days after the outpatient visit. ARR was calculated as the total number of relapses observed divided by the total number of patient-years observed. The 95% CI was based on Poisson distribution. ARR Annualized relapse rate, CI confidence interval, FUM fumarates, IPW inverse probability of treatment weights, PS propensity score, S1P sphingosine-1-phosphate receptor modulators
There was no significant difference in the time to first relapse between the treatment groups (2:1 PS-matched: hazard ratio [HR] 1.21, 95% CI 0.91–1.60, p = 0.19; IPW: HR 1.16, 95% CI 0.88–1.52, p = 0.30). There was no separation between the Kaplan–Meier curves (Fig. 4), and the expected medians were not reached in either treatment group. Kaplan–Meier survival analyses showed comparable proportions of relapse-free patients at 24 months between the fumarates and S1P groups. In the 2:1 PS-matched population, 72.6% of patients in the fumarates group (95% CI 68.3–77.1%) remained relapse-free at month 24, compared to 74.7% in the S1P group (95% CI 68.9–80.9%), with a p value of 0.152 (Fig. 4). Similarly, in the IPW population, the estimated relapse-free proportions at month 24 were 73.3% (95% CI 69.4–76.8%) for the fumarates group and 74.9% (95% CI 68.3–80.3%) for the S1P group, with a p value of 0.229. These findings indicate that there were no statistically significant differences in the effectiveness of fumarates and S1P treatments in reducing relapse rates and time to first relapse among Black PwMS over the 24-month follow-up period.
Fig. 4.
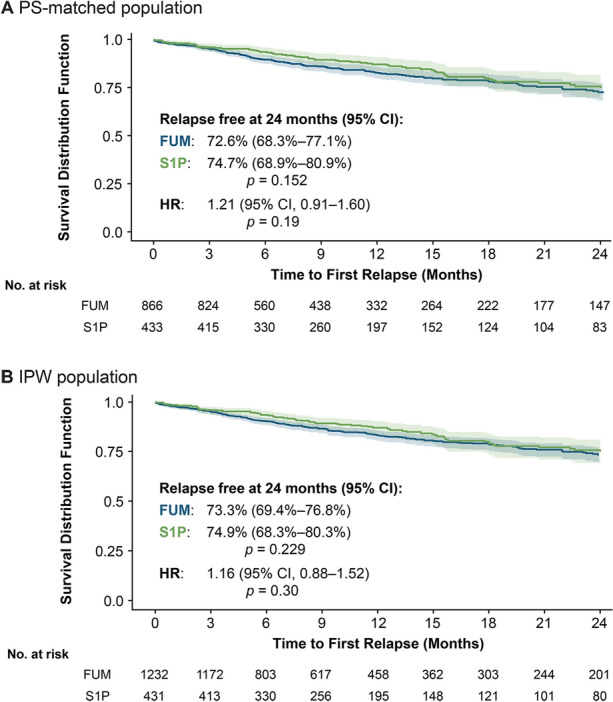
Time to first post-index relapse in the PS-matched population (a) and in the IPW population (b). Kaplan–Meier models were used to estimate the time to first relapse survival functions. CI Confidence interval, FUM fumarates, HR hazard ratio, IPW inverse probability of treatment weights, PS propensity score, S1P sphingosine-1-phosphate receptor modulators
Discussion
This retrospective observational analysis utilized real-world data from the US Komodo Health Claims Database to compare the effectiveness of fumarates and S1P receptor modulators in Black PwMS. Our findings indicate that both treatments demonstrate comparable effectiveness in this population, with no significant differences observed in relapse outcomes between the two groups. Specifically, in both the PS-matched and IPW analyses, the ARRs and time to first relapse were similar for patients initiating fumarates and those initiating S1P receptor modulators. At 24 months post-initiation, the proportion of patients remaining relapse-free was > 70% in both treatment groups across both analytical methods. The observed 24-month relapse-free rate (72–74%) is generally consistent with reported outcomes in white populations treated with S1P modulators and fumarates. For fumarates, a real-world analysis in the USA found a 24-month relapse-free rate of 80.5% (95% CI 70.0–82.1%) in white PwMS, which overlaps with the observed range, suggesting similar effectiveness [27, 28]. However, differences in study design, population characteristics, and real-world factors may contribute to variations. For S1P modulators, the FREEDOMS trial (which did not report racial breakdown) found relapse-free rates of 74.7% (95% CI 70.4–78.9%) for fingolimod 1.25 mg and 70.4% (95% CI 66.0–74.8%) for fingolimod 0.5 mg, which closely aligns with the observed data [29].
These comparisons indicate that the observed efficacy is broadly in line with prior findings, although further analysis may be warranted to account for potential population-specific differences. Additionally, re-baselining to accommodate for the time from initiation of treatment until onset of DMT effectiveness was not done. Therefore, effectiveness outcomes may be confounded by initial disease activity that occurs before the full effect of a DMT has been achieved; however, this represents a real-world treatment scenario (or something similar).
Previous studies have also demonstrated comparable effectiveness between fumarates and S1P receptor modulators (fingolimod, siponimod, ozanimod, and ponesimod), as well as improved effectiveness compared to lower efficacy DMTs such as interferon beta-1a, glatiramer acetate, and teriflunomide [13–15]. Prior studies demonstrating comparable effectiveness between fumarates and S1P receptor modulators consisted of predominantly white PwMS. The present study is the first known comparative effectiveness analysis specifically focusing on these oral DMTs in Black PwMS. Given that Black individuals are often underrepresented in MS clinical trials and may experience a more aggressive disease course with more frequent relapses and accelerated disability progression [4–6], our findings provide valuable insights into the management of MS within this population.
The comparable effectiveness observed between fumarates and S1P receptor modulators suggests that both classes of oral DMTs are viable treatment options for Black PwMS. This is particularly important considering potential disparities in healthcare access, social determinants of health, and differences in MS presentation that may influence treatment outcomes in this group [30]. By leveraging real-world data, our study contributes to a more inclusive understanding of MS treatment effectiveness and supports clinicians in making informed decisions tailored to the needs of Black PwMS.
Limitations
First, as an observational study based on administrative claims data, there is the potential for residual confounding due to unmeasured variables such as disease duration, disability status, or socioeconomic factors not captured in the database. Second, the reliance on claims data to identify relapses may result in misclassification or underestimation of relapse events, particularly mild relapses that do not prompt medical intervention or are not captured within the specified timeframe for relapse definition. Third, the lack of clinical measures such as MRI findings or Expanded Disability Status Scale scores limits our ability to assess other aspects of disease activity and progression beyond relapse events. Additionally, the claims data do not allow us to reliably capture treatment-related complications (e.g., hospitalizations related to side effects, lymphopenia, infections, or other comorbidities). Although these limitations exist, analyzing claims data remains a valuable method that has been previously used to evaluate real-world efficacy and outcomes of DMT use in PwMS [31, 32].
Furthermore, while our study focuses exclusively on Black PwMS, the findings may not be generalizable to other racial or ethnic groups or to populations outside the USA. Future research should aim to include diverse populations and incorporate additional clinical and patient-reported outcomes to provide a more comprehensive understanding of treatment effectiveness across different subgroups of PwMS.
Conclusions
This real-world, claims-based analysis is the first known study to specifically compare the effectiveness of fumarates and S1Ps in Black PwMS. The study findings showed that fumarates and S1Ps are equally effective in reducing relapse rates among Black PwMS. Over 72% of patients in both treatment groups remained relapse-free at 24 months.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Biogen (Cambridge, MA, USA) sponsored this study and provided funding for medical writing in the development of this paper. The authors had full editorial control of the paper and provided their final approval of all content.
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Samantha Pluta, PharmD, on behalf of Excel Medical Affairs, and the manuscript was edited by Cara Farrell. Support for this assistance was provided by Biogen.
Author Contributions
Nicholas Belviso, Sai L. Shankar, Jason P. Mendoza, Boyang Bian, James B. Lewin, and Kinyee Fong contributed to the study conception and design. Nicholas Belviso, Sai L. Shankar, and Kinyee Fong played a major role in data acquisition. All authors (Sophia Woodson, Edward J. Gettings, Chu-Yueh Guo, Sylvia Klineova, Jong-Mi Lee, Rebecca S. Romero, Aljoeson Walker, Nicholas Belviso, Sai L. Shankar, Jason P. Mendoza, Boyang Bian, James B. Lewin, and Kinyee Fong) contributed to manuscript drafting and revision. All authors read and approved the final manuscript.
Funding
This study was funded by Biogen, which also provided funding for the journal’s Rapid Service Fees.
Data Availability
The Komodo Health database is publicly available through licensing. Biogen obtained a license for the data used in this analysis.
Declarations
Conflicts of Interest
Sophia Woodson served on scientific advisory or data safety monitoring boards for MSSA and Sanofi, and served on speaker bureaus for Biogen, Bristol Myers Squibb, and Genentech. Edward J Gettings, Jong-Mi Lee, and Aljoeson Walker declare that they have no competing interests. Chu-Yueh Guo has received consulting fees from Genentech and Horizon. Sylvia Klineova has received consulting fees from Amgen and TG Therapeutics and for contracted research for Biogen, and served on speakers bureaus for Alexion and Biogen. Rebecca S Romero has received consulting fees from Alexion, EMD Serono, Genentech, Horizon, and TG Therapeutics. Nicholas Belviso, Sai L Shankar, Jason P Mendoza, Boyang Bian, James B Lewin, and Kinyee Fong are all employees of Biogen and may hold stock in the company.
Ethical Approval
This study was a retrospective analysis of de-identified data from the Komodo Health Sentinel database and did not involve any new studies with human or animal subjects. As such, ethical approval or informed consent was not required.
References
- 1.MS International Federation. Atlas of MS. 2023. https://www.atlasofms.org/map/united-states-of-america/epidemiology/number-of-people-with-ms#about. Accessed 25 Sept 2024.
- 2.US Census Bureau. QuickFacts. 2023. https://www.census.gov/quickfacts/fact/table/US/RHI225222. Accessed 25 Sept 2024.
- 3.Turner BE, Steinberg JR, Weeks BT, Rodriguez F, Cullen MR. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health Am. 2022;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19(11):87. [DOI] [PubMed] [Google Scholar]
- 5.Khan O, Williams MJ, Amezcua L, et al. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract. 2015;5(2):132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology. 2010;75(3):217–23. [DOI] [PubMed] [Google Scholar]
- 7.Tecfidera [prescribing information]. Biogen Inc., Cambridge, MA, 2024. https://www.tecfidera.com/content/dam/commercial/multiple-sclerosis/tecfidera/pat/en_us/pdf/full-prescribing-info.pdf. Accessed 18 Dec 2024.
- 8.Vumerity [prescribing information]. Biogen Inc., Cambridge MA, 2024. https://www.vumerityhcp.com/content/dam/commercial/vumerity/hcp/en_us/pdf/vumerity-prescribing-information.pdf. Accessed 18 Dec 2024.
- 9.Novartis. Gilenya prescribing information. 2024. https://www.novartis.com/us-en/sites/novartis_us/files/gilenya.pdf. Accessed 18 Dec 2024.
- 10.Novartis. Mayzent prescribing information. 2024. https://www.novartis.com/us-en/sites/novartis_us/files/mayzent.pdf. Accessed 18 Dec 2024.
- 11.Janssen Pharmaceuticals. Ponvory prescribing information. 2023. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/PONVORY-pi.pdf. Accessed 18 Dec 2024.
- 12.Bristol Myers Squibb. Zeposia prescribing information. 2024. https://packageinserts.bms.com/pi/pi_zeposia.pdf. Accessed 18 Dec 2024.
- 13.Braune S, Grimm S, van Hovell P, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus interferon, glatiramer acetate, teriflunomide, or fingolimod: results from the German NeuroTransData registry. J Neurol. 2018;265(12):2980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang T, Ziemssen T, Wray S, et al. Matching-adjusted indirect comparisons of diroximel fumarate, ponesimod, and teriflunomide for relapsing multiple sclerosis. CNS Drugs. 2023;37(5):441–52. [DOI] [PubMed] [Google Scholar]
- 15.Jiang T, Shanmugasundaram M, Bozin I, et al. Comparative efficacy of diroximel fumarate, ozanimod and interferon beta-1a for relapsing multiple sclerosis using matching-adjusted indirect comparisons. J Comp Eff Res. 2024;13(10): e230161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ontaneda D, Nicholas J, Carraro M, et al. Comparative effectiveness of dimethyl fumarate versus fingolimod and teriflunomide among MS patients switching from first-generation platform therapies in the US. Mult Scler Relat Disord. 2019;27:101–11. [DOI] [PubMed] [Google Scholar]
- 17.Vollmer B, Nair KV, Sillau SH, et al. Comparison of fingolimod and dimethyl fumarate in the treatment of multiple sclerosis: two-year experience. Mult Scler J Exp Transl Clin. 2017;3(3):2055217317725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perumal J, Balabanov R, Balcer L, et al. Long-term effectiveness and safety of natalizumab in african american and hispanic/latino patients with early relapsing-remitting multiple sclerosis: STRIVE data analysis. Neurol Ther. 2023;12(3):833–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhard A, Toliver J, Rascati K. Association between multiple sclerosis disease severity and adherence to disease-modifying therapies. J Manag Care Spec Pharm. 2021;27(7):915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W, Tang X, Heyman RA, et al. Patterns of utilization and expenditure across multiple sclerosis disease-modifying therapies: a retrospective cohort study using claims data from a commercially insured population in the United States, 2010–2019. Neurol Ther. 2022;11(3):1147–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komodo Health Inc. Komodo race & ethnicity data: user guide version V23.8. Komodo Health Inc., San Francisco; 2024.
- 22.Nicholas J, Ontaneda D, Carraro M, et al. Development of an algorithm to identify multiple sclerosis (MS) disease severity based on healthcare costs in a US administrative claims database (P2.052). Neurology. 2017;88(16):2. [Google Scholar]
- 23.Toliver J, Barner JC, Lawson K, Sonawane K, Rascati K. Replication of a claims-based algorithm to estimate multiple sclerosis disease severity in a commercially insured population. Mult Scler Relat Disord. 2020;46:102539. [DOI] [PubMed] [Google Scholar]
- 24.Toliver JC, Barner JC, Lawson KA, Rascati KL. Use of a claims-based algorithm to estimate disease severity in the multiple sclerosis Medicare population. Mult Scler Relat Disord. 2021;49:102741. [DOI] [PubMed] [Google Scholar]
- 25.Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–25. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, Acharya KP. Propensity score matching for causal inference and reducing the confounding effects: statistical standard and guideline of Life Cycle Committee. Life Cycle. 2022;2:e18. [Google Scholar]
- 27.Woodson S, Gettings E, Guo C-Y, et al. Real-world treatment outcomes in Black, Hispanic, Asian, and White people with multiple sclerosis treated with fumarates (P4–6.005). Neurology. 2024;102(7):6308. [DOI] [PMC free article] [PubMed]
- 28.Woodson S, Gettings EJ, Guo C-Y, et al. Real world treatment outcomes in Black, Hispanic, Asian, and White people with multiple sclerosis treated with fumarates. In: ACTRIMS Forum, 29 February-2 March 2024, West Palm Beach; P479.
- 29.Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401. [DOI] [PubMed] [Google Scholar]
- 30.Dobson R, Rice DR, D’Hooghe M, et al. Social determinants of health in multiple sclerosis. Nat Rev Neurol. 2022;18(12):723–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergvall N, Makin C, Lahoz R, et al. Comparative effectiveness of fingolimod versus interferons or glatiramer acetate for relapse rates in multiple sclerosis: a retrospective US claims database analysis. Curr Med Res Opin. 2013;29(12):1647–56. [DOI] [PubMed] [Google Scholar]
- 32.Freeman L, Kee A, Tian M, Mehta R. Retrospective claims analysis of treatment patterns, relapse, utilization, and cost among patients with multiple sclerosis initiating second-line disease-modifying therapy. Drugs Real World Outcomes. 2021;8(4):497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Komodo Health database is publicly available through licensing. Biogen obtained a license for the data used in this analysis.