Abstract
Background/objectives
Diets enriched with live microbes offer multiple health benefits. This study aimed to investigate the relationship between dietary intake of live microbes and accelerated biological age, as well as to examine the mediating role of insulin resistance.
Methods
The study included 4,909 participants from the National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2018. Dietary live microbes intake was assessed using a self-report questionnaire. Insulin resistance was measured using the HOMA-IR and plasma insulin levels. Biological age and accelerated aging were assessed using PhenoAge and PhenoAgeAccel. We performed weighted multiple linear regression, logistic regression, restricted cubic spline (RCS) analysis, mediation analyses, and interaction analyses to explore the relationships between dietary live microbe intake, insulin resistance, and accelerated aging.
Results
Higher intake of live dietary microbes, compared to lower intake, was associated with a slower rate of accelerated aging after full adjustment for confounders (β = 0.15; 95% CI: − 0.26 to − 0.04; P = 0.008). RCS curves indicated an L-shaped dose–response relationship (P-nonlinear = 0.0003). Insulin resistance-related metrics (HOMA-IR and insulin) partially mediated these effects, with mediation proportions ranging from 24.65% to 30.03%. Interaction analyses revealed no significant interaction between dietary live microbe intake and other stratification factors regarding phenotypic age.
Conclusions
The intake of dietary live microbes is nonlinearly associated with accelerated biological age. IR partially mediated the relationship.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-025-03104-6.
Keywords: Dietary live microbes, Aging, Phenoage, NHANES
Introduction
Aging is an inevitable process that has become a significant socio-economic issue. According to the World Health Organization, by 2030, approximately one-fifth of the global population will be 65 years of age or older. The number of individuals aged 80 and above is projected to double between 2020 and 2050, reaching approximately 426 million [1]. These figures underscore the need for a more comprehensive understanding of the aging process. A prerequisite for an in-depth knowledge of the aging process is the accurate measurement of aging [2]. As a consequence of the general increase in life expectancy resulting from social progress and medical technology, chronological age alone is insufficient for assessing the degree and risk of aging [3]. Individuals of the same chronological age may exhibit markedly different susceptibility to age-related diseases and mortality. This is because there are differences in their underlying biological aging processes, which cannot be fully characterized by actual age. To address this issue, researchers have developed algorithms based on DNA methylation data [4–6], as well as those based on blood chemistry and other clinical data [7, 8]. Compared to DNA methylation-based algorithms, those relying on clinically observable data demonstrate stronger predictive performance and greater utility [9]. Phenotypic age is a calculation based on clinically observable data proposed by Levine in 2018 that captures differences in mortality risk and physiological status among individuals of the same chronological age [10].
The topic of delaying aging remains a longstanding issue. Various interventions have been identified to reduce the risk of accelerated aging, and dietary modifications are one of those interventions. Foods containing live dietary microbes, such as unpeeled fruits, vegetables, and fermented products, have been demonstrated to benefit human health in numerous studies over the past decades [11]. Increased consumption of fermented foods may offer cardiovascular protection, enhance gut flora, modulate the immune system, and improve cognitive function [12–15]. This may be achieved through mechanisms such as reducing inflammatory responses, reducing oxidative stress, and lowering insulin resistance [16–18]. Existing studies have focused on the intake of fermented foods. However, the range of live microorganisms that can be ingested in the daily diet may be broader. For example, raw, unpeeled fruits and vegetables, as well as milk or cooked foods that are not commercially asepticized or pasteurized all have some amount of live microbes [19]. A recent study found that non-fermented pasteurized milk shortened lifespan and increased all-cause mortality by enhancing mTORC1 signaling associated with aging [20]. This effect may result from pasteurization and refrigeration inhibiting the growth and activity of milk-fermenting bacteria, suggesting potential benefits of including certain live microbes in the diet. Nonetheless, evidence on whether a broader dietary intake of live microbes can mitigate accelerated aging remains limited.
Insulin resistance is a common pathogenetic mechanism for several chronic diseases and is also implicated in the pathology of aging. Recent research has demonstrated that hyperinsulinemia and associated insulin resistance (IR) accelerate the aging of human adipocytes, hepatocytes, and neurons [21]. Clinical studies have corroborated these findings. In the Baltimore Longitudinal Study of Aging, men with lower plasma insulin levels had a higher survival rate than men with higher insulin levels [22]. In an Italian study, healthy centenarians have glucose tolerance and insulin action better than individuals aged > 75 years [23]. These studies are further illustrative in suggesting that lower insulin levels and high insulin receptor sensitivity may contribute to lower accelerated aging. Dietary live microbes are strongly associated with insulin resistance, which is supported by clinical studies and experiment [24–27]. A single-blinded and parallel 10-week clinical trial study indicated that fermented food benefits the subjects on improving plasma glycolipids and insulin sensitivity [28]. Similarly, fermented functional foods for three months alleviated insulin resistance in SD rats, as evidenced by the return of fasting insulin levels and the HOMA-IR index to control levels [29]. However, whether insulin resistance mediates the association between dietary live microorganisms and accelerated aging is unclear. Therefore, we aimed to conduct a well-controlled, population-based study to provide a deeper understanding of the relationship between dietary live microbes and phenotypic age acceleration. In addition, we explored whether the relationship is mediated by insulin resistance.
Materials and methods
Subsection
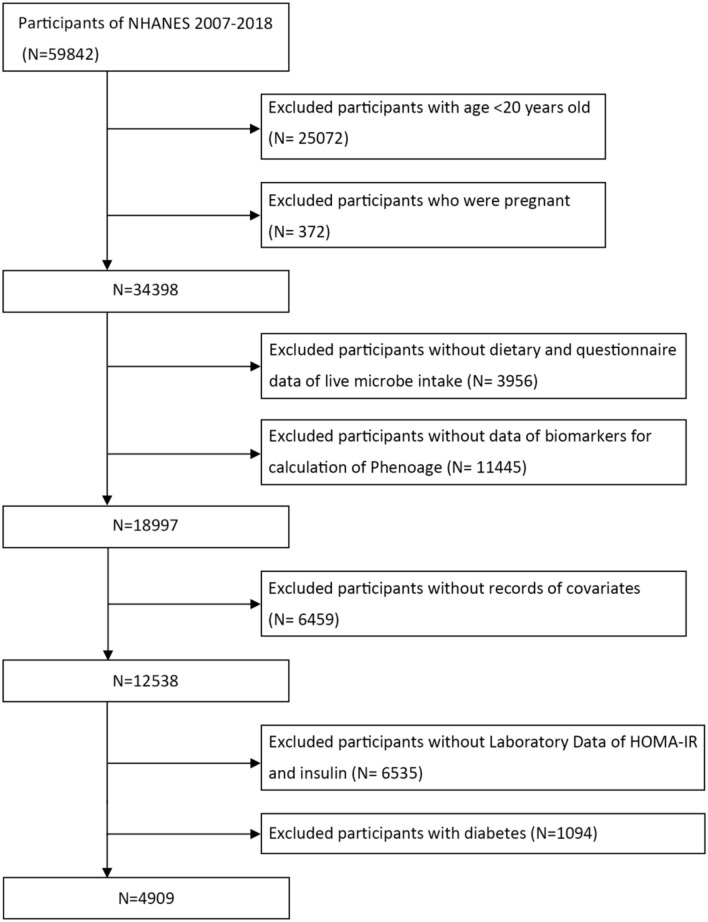
In this study, data were obtained from the publicly available National Health and Nutrition Examination Survey (NHANES) from 2007–2018. The NHANES is conducted biennially by the Centers for Disease Control and Prevention (CDC) to assess the health and nutritional status of the U.S. population. It employs a complex, stratified, and multistage sampling design to select a representative sample from noninstitutionalized U.S. population. These individuals were interviewed at their residences and subsequently underwent a health screening at a mobile examination centers (MECs). The CDC National Center for Health Statistics Ethics Review Board approved the NHANES protocol, and all participants provided written informed consent(https://www.cdc.gov/nchs/nhanes/irba98.htm). This study utilized data from six cycles of NHANES (2007–2018) with a total of 59,842 participants. Inclusion criteria included participants who completed a dietary information interview within 24 h and had clinical data on their biological age. Exclusion criteria applied to participants younger than 20 years of age, those who were pregnant, missing data on demographics, physical examinations, dietary habits, laboratory tests, or questionnaire responses. Additionally, individuals with diabetes were identified by fasting glucose, HbA1c, and self-report questionnaire. They were excluded due to varying degrees of insulin resistance. Figure 1 presents a comprehensive flowchart illustrating the participant selection process.
Fig. 1.
Flowchart of the study design and participants
Dietary live microbe intake
Dietary live microbe intake was assessed as previously described. Briefly, NHANES 24-h dietary recall data were used in this study. The categorization of live microbe levels in foods was derived from a study by Sanders [19]. The live microbe content of 9388 individual food codes across 48 subgroups, expressed as colony-forming units per gram (CFU/g), was estimated from the literature by four experts in the field (MLM, MES, RH, and CH). Foods were categorized into 3 groups based on estimated levels of live microorganisms (bacteria and fungi): low (Lo; < 104 CFU/g), medium (Med; 104–107 CFU/g), and high (Hi; > 107 CFU/g). Processed foods that ordinarily are heat-treated (milk, prepared meat, pork, poultry, and seafood dishes; sauces and gravies) were classified as "Lo" due to their minimal levels of microorganisms. The foods assigned to "Med" primarily consisted of fresh vegetables and fruits, along with certain fermented foods such as miso and sauerkraut. Fermented dairy products constituted the majority of foods assigned to "Hi". For each participant, we calculated the amount of medium and high-level food consumed (in 100 g) and defined this as MedHi. MedHi was also transformed into categorical variables for the analysis: G1, individuals who did not consume any MedHi food; G2, individuals who consumed MedHi food but less than the median MedHi intake; and G3, individuals who consumed more MedHi food than the median MedHi intake. These analytical methods have been employed in several studies [30–32].
PhenoAge and PhenoAgeAccel
PhenoAge was calculated from 10 aging-related biomarkers: actual age, albumin (liver), creatinine (kidney), glucose (metabolism), C-reactive protein (inflammation), percentage of lymphocytes (immunization), mean cell volume (immunization), erythrocyte distribution width (immunization), alkaline phosphatase (liver), and white blood cell count (immunization). These biomarkers were selected using the Cox proportional risk elastic net mortality model validated through tenfold cross-validation [9, 10]. Details of the specific calculations have been described in detail in previous literature. PhenoAge Acceleration (PhenoAgeAccel) was determined as the residual obtained from the linear regression model, reflecting the deviation of the PhenoAge from the chronological age. Positive PhenoAgeAccel values denote accelerated aging, while negative values signify a younger-than-expected phenotype. PhenoAge calculations were performed using the “BioAge” package [33].
Insulin resistance
Insulin resistance was assessed using both the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and plasma insulin levels. Previous studies have found good agreement between HOMA-IR and the normoglycemic high insulin clamp technique for subject categorization, and HOMA-IR can be used in large-scale or epidemiologic studies. Higher values of HOMA-IR indicate decreased sensitivity to insulin, suggesting the presence of IR. Since the distribution is non-normal, the HOMA-IR is used in the regression model and mediation effects log and log insulin to minimize the effect of outliers and to improve the interpretation of relevant results. The formula for HOMA-IR is as follows: HOMA-IR = (FPI × FPG)/22.5 [34]. FPI is fasting plasma insulin concentration (mU/l) and FPG is fasting blood glucose (mmol/l).
Covariates
To control for confounding factors, we adjusted for known covariates. These factors included age, gender(Male, Female), race(Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, Other race), poverty income ratio(PIR < 1.3, 1.3–3.5, > 3.5), education(Less Than 9th Grade, 9–11th Grade, High School Graduate/GED, Some College or AA Degree, College Graduate or above), body mass index(BMI)(Normal, Overweight, Obese), smoking status(Current, Former, Never), drinking status(Current, Former, Never), physical activity, CVD, hypertension. Smoking status was categorized into three groups: nonsmokers (never smoked 100 or more cigarettes in their lifetime), former smokers (smoked 100 or more cigarettes in the past but do not currently smoke), and current smokers (smoked 100 or more cigarettes in the past and currently smoke). Drinking status was categorized into three groups: nondrinkers (those who had not had 12 or more drinks in the past year or in their lifetime), former drinkers (participants who had had at least 12 drinks in their lifetime but not in the past year), and current drinkers (those who had had at least 12 drinks in the past year and who reported a frequency of drinking that was not 0 in the past 12 months). Physical activity was categorized according to the Physical Activity Guidelines for Americans [35]. Exercise ≥ 600 MET-min/week (equivalent to 150 min/week of moderate-intensity or 75 min/week of vigorous-intensity recreational activity) was classified as active, and less than this threshold classified as inactive. Diabetes mellitus was confirmed based on the following questions: glycated hemoglobin (HbA1c) ≥ 6.5%, fasting blood glucose ≥ 7 mmol/L, the questionnaire “Doctor told you have diabetes” and “Taking insulin now”. Hypertension was confirmed based on the following questions: systolic blood pressure (SBP) ≥ 140 mmHg, or diastolic blood pressure (DBP) ≥ 90 mmHg, and the questionnaire “Ever told you had high blood pressure”. Cardiovascular disease was determined on the questionnaire “Ever told you had a stroke/coronary heart disease/angina/heart attack”.
Statistical analysis
To comply with NHANES guidelines and account for the complex survey design, we weighted the data to ensure the representativeness of the entire U.S. population. The basic characteristics of participants were summarized and expressed as mean ± SD for continuous variables and as unweighted frequencies and weighted percentages for categorical variables. Differences were assessed using the Wilcoxon rank sum test for continuous variables and the Rao-Scott chi-square test for categorical variables. Weighted multiple linear and logistic regression was used to assess the correlation between dietary intake of live microbe and PhenoAgeAccel. Model 1 did not include any adjustments. Model 2 included adjustments for age, sex, race, PIR, and education. Model 3 included full adjustment for all covariates with the addition of BMI, smoking status, drinking status, physical activity, hypertension, and CVD. To assess the nonlinear relationship between dietary intake of live microbes and the risk of accelerated biological aging, a restricted cubic spline (RCS) analysis was performed.
In models adjusted for all covariates, we used mediation analyses to verify the presence of mediating effects and the strength of the effects. For the analysis of mediated effects, continuous PhenoAge served as the outcome, dietary intake of live microbes as the exposure, and indices related to insulin resistance (HOMA-IR, Insulin) as mediators. The significance of mediated effects was tested using the R “Mediation” software package. Subgroup analyses were conducted based on age (≤ 50 and > 50 years), sex (male and female), body mass index (overweight, obese and normal weight), and physical activity (inactive and active). All statistical analyses were performed using R software and statistical significance was determined by a two-tailed test with a P value of less than 0.05.
Results
Participant characteristics
In this study, we analyzed data from 4,909 participants with a mean age of 46.72 years, of whom 53% were female (Table 1). Participants consumed an average of 136.67 g of Medhi food per day. Among the participants, the distribution of participants among the G1, G2, and G3 groups was 32%, 32%, and 36%, respectively. On average, PhenoAge progression decreased by 2.94 years relative to their chronological age, indicating that the biological age of the participants was younger than their actual age. Those with higher Medhi food intake exhibited distinct characteristics. These included older age, Non-Hispanic White, High School Grad/GED, lower BMI, better economic status, more physical activity, and lower insulin resistance. In addition, G3 participants had a smaller PhenoAgeAccel compared to those in G1 and G2.
Table 1.
Baseline characteristics of included participants based on dietary live microbes intake group
| Dietary live microbe group | |||||
|---|---|---|---|---|---|
| Characteristic | Overall, N = 4909 (100%)1 | G1, N = 1766 (32%)1 | G2, N = 1560 (32%)1 | G3, N = 1583 (36%)1 | P Value2 |
| Gender | 0.001 | ||||
| Female | 2,520 (53%) | 827 (47%) | 839 (55%) | 854 (56%) | |
| Male | 2,389 (47%) | 939 (53%) | 721 (45%) | 729 (44%) | |
| Age | 46.72(16.52) | 44.83(16.45) | 46.60(16.57) | 48.49(16.35) | 0.002 |
| Age (subgroup) | 0.024 | ||||
| < 40 | 1,810 (36%) | 686 (41%) | 601 (37%) | 523 (32%) | |
| 40–59 | 1,665 (40%) | 585 (38%) | 542 (42%) | 538 (41%) | |
| ≥ 60 | 1,434 (23%) | 495 (21%) | 417 (22%) | 522 (27%) | |
| Race | < 0.001 | ||||
| Non-Hispanic White | 2,379 (72%) | 750 (66%) | 787 (72%) | 842 (78%) | |
| Non-Hispanic Black | 832 (8.9%) | 435 (14%) | 223 (8.4%) | 174 (5.2%) | |
| Mexican American | 817 (7.4%) | 263 (7.3%) | 283 (8.2%) | 271 (6.8%) | |
| other Hispanic | 526 (4.8%) | 170 (5.0%) | 170 (5.0%) | 186 (4.5%) | |
| Other race | 355 (6.5%) | 148 (8.3%) | 97 (6.2%) | 110 (5.2%) | |
| PIR | < 0.001 | ||||
| < 1.3 | 1,490 (20%) | 681 (29%) | 432 (18%) | 377 (14%) | |
| 1.3–3.5 | 1,880 (34%) | 691 (38%) | 614 (35%) | 575 (31%) | |
| > 3.5 | 1,539 (46%) | 394 (33%) | 514 (47%) | 631 (55%) | |
| Education | < 0.001 | ||||
| Less Than 9th Grade | 817 (7.4%) | 263 (7.3%) | 283 (8.2%) | 271 (6.8%) | |
| 9-11th Grade | 526 (4.8%) | 170 (5.0%) | 170 (5.0%) | 186 (4.5%) | |
| High School Grad/GED | 2,379 (72%) | 750 (66%) | 787 (72%) | 842 (78%) | |
| Some College or AA degree | 832 (8.9%) | 435 (14%) | 223 (8.4%) | 174 (5.2%) | |
| College Graduate or above | 355 (6.5%) | 148 (8.3%) | 97 (6.2%) | 110 (5.2%) | |
| BMI | 0.003 | ||||
| Normal(< 25) | 1,540 (32%) | 528 (28%) | 492 (32%) | 520 (36%) | |
| Overweight(25 to < 30) | 1,739 (35%) | 592 (35%) | 572 (36%) | 575 (34%) | |
| Obese(≥ 30) | 1,630 (33%) | 646 (38%) | 496 (32%) | 488 (30%) | |
| Smoking status | < 0.001 | ||||
| Current | 1,056 (20%) | 493 (28%) | 329 (20%) | 234 (13%) | |
| Former | 1,178 (26%) | 385 (23%) | 360 (24%) | 433 (31%) | |
| Never | 2,675 (54%) | 888 (49%) | 871 (56%) | 916 (56%) | |
| Drinking status | 0.016 | ||||
| Current | 3,147 (70%) | 1,043 (66%) | 1,051 (73%) | 1,053 (71%) | |
| Former | 1,132 (20%) | 466 (23%) | 326 (18%) | 340 (19%) | |
| Never | 630 (10%) | 257 (11%) | 183 (8.6%) | 190 (10%) | |
| Physical activity | < 0.001 | ||||
| No | 3,227 (62%) | 1,262 (71%) | 1,013 (60%) | 952 (55%) | |
| Yes | 1,682 (38%) | 504 (29%) | 547 (40%) | 631 (45%) | |
| CVD | 0.066 | ||||
| No | 4,529 (94%) | 1,606 (93%) | 1,440 (93%) | 1,483 (95%) | |
| Yes | 380 (6.2%) | 160 (6.8%) | 120 (7.1%) | 100 (5.0%) | |
| Hypertension | 0.8 | ||||
| No | 3,158 (68%) | 1,105 (67%) | 1,024 (69%) | 1,029 (68%) | |
| Yes | 1,751 (32%) | 661 (33%) | 536 (31%) | 554 (32%) | |
| Dietary livemicrobe in grams | 136.67(189.69) | 0.00(0.00) | 60.51(37.87) | 324.58(203.34) | < 0.001 |
| Phenoage | 43.77(17.23) | 43.04(17.42) | 43.54(17.23) | 44.62(17.03) | 0.2 |
| PhenoAgeAccel | -2.94(4.95) | -1.79(4.95) | -3.05(4.82) | -3.86(4.85) | < 0.001 |
| HOMA-IR | 17.82(16.60) | 19.64(19.65) | 18.26(15.99) | 15.84(13.75) | < 0.001 |
| Insulin | 70.80(61.52) | 77.48(71.88) | 72.69(59.34) | 63.23(51.98) | < 0.001 |
1Mean(SD) for continuous; n (%) for categorical
2Chi-squared test with Rao & Scott's second-order correction; Wilcoxon rank-sum test for complex survey samples
Associations of dietary live microbes Intake and PhenoAgeAccel
We investigated the association between dietary live microbes and PhenoAgeAccel using weighted linear regression models. In three models, when Medhi consumption was considered a continuous variable, each additional 100 g of Medhi food consumed reduced PhenoAgeAccel by 0.35 (95% CI: − 0.47, − 0.23; P < 0.001), 0.23 (95% CI: − 0.34, − 0.12; P < 0.001), and 0.15 (95% CI: − 0.26, − 0.04; P = 0.008) years. When Medhi consumption was considered as a categorical variable, groups G2 and G3 were associated with lower PhenoAgeAccel compared to group G1. In particular, in the fully adjusted model, G3 showed a greater decrease in PhenoAgeAccel than G2 (β = − 1.1; 95% CI: − 1.6, − 0.56; P < 0.001) (Table 2). This suggests that individuals consuming more dietary live microbes experienced a slower aging process compared to those consuming less or no Medhi foods.
Table 2.
Linear regression analyses of associations between microbe intake and acceleration of aging
| Model1 | Model2 | Model3 | ||||
|---|---|---|---|---|---|---|
| β(95%CI) | P-value | β(95%CI) | P-value | β(95%CI) | P-value | |
| Medhi(grams) | − 0.35(− 0.47, − 0.23) | < 0.001 | − 0.23(− 0.34, − 0.12) | < 0.001 | − 0.15(− 0.26, − 0.04) | 0.008 |
| G1 | Reference | Reference | Reference | |||
| G2 | − 1.3(− 1.8, − 0.76) | < 0.001 | − 0.83(− 1.3, − 0.33) | 0.002 | − 0.64(− 1.2, − 0.12) | 0.018 |
| G3 | − 2.1(− 2.6, − 1.6) | < 0.001 | − 1.4(− 2.0, − 0.90) | < 0.001 | − 1.1(− 1.6, − 0.56) | < 0.001 |
Model 1: non-adjusted model; Model 2: adjusted for age + gender + race + education level + PIR; Model 3: adjusted for age + gender + race + education level + PIR + smoking status + drinking status + Physical activity + BMI + hypertension + CVD
The population was categorized into accelerated and delayed aging groups by comparing their biological age progression with a zero value. We constructed multivariate logistic regression. In the initial model, higher Medhi intake was associated with a reduced risk of accelerated aging. However, in Models 2 and 3, G2 did not show a statistically significant association with reduced accelerated aging compared to G1, while G3 continued to be significantly associated with a lower incidence of accelerated aging (Supplement Table 1). In the fully adjusted model, the risk of accelerated aging was reduced by 36% in the G3 group compared to the G1 group (P = 0.007). This indicates that a higher Medhi intake, rather than a relatively lower intake, decreases the risk of accelerated aging.
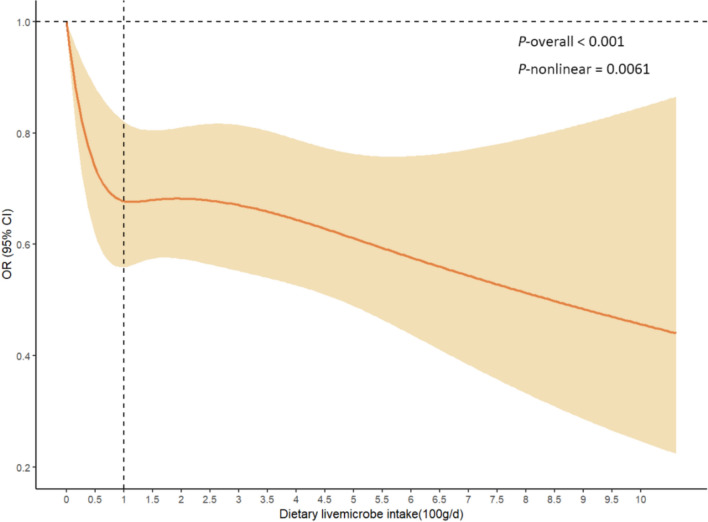
Further, a nonlinear relationship between dietary live microbes and biomarkers of accelerated aging was revealed by RCS curves. The results showed that the risk of accelerated aging was significantly reduced with increasing Medhi intake, and there was a nonlinear relationship (P for nonlinear = 0.0061). The inflection point of the curve was near an intake of 100 g/d (Fig. 2).
Fig. 2.
Nonlinear associations between dietary live microbes intake and the PhenoAgeAccel
Associations of IR and PhenoAge
We investigated the connection between dietary live microbes and insulin resistance, as well as the association between insulin resistance and PhenoAgeAccel. Insulin resistance was assessed using two indicators: HOMA-IR and insulin levels.
Supplement Table 2 presents the results of the linear regression analysis of the relationship between dietary live microbes and HOMA-IR. Specifically, in the fully adjusted model, each additional 100 g of Medhi food intake was associated with a 0.02 unit reduction in HOMA-IR and a similar 0.02 unit reduction in Insulin. The effects of G2 on HOMA-IR and Insulin were not statistically significant compared to G1. Conversely, G3 was associated with lower HOMA-IR and insulin levels. In conclusion, a greater intake of dietary live microbes was associated with reduced insulin resistance. Further, we explored the relationship between IR and PhenoAgeAccel, with linear regression analyses indicating that higher insulin resistance was associated with accelerated aging (Supplement Table 3).
Mediating role of IR indicators
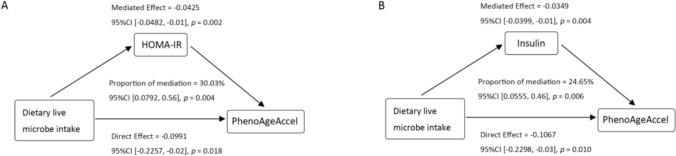
Subsequently, we performed mediation effect analysis based on model 3 (fully adjusted model) to investigate the potential mediating role of insulin resistance. The results showed that insulin resistance significantly mediated the relationship between dietary live microbes and PhenoAgeAccel. The mediation proportions of HOMA-IR and Insulin were 30.03% and 24.65% (P < 0.01), respectively (Fig. 3).
Fig. 3.
The mediation effect of IR on the association between Dietary live microbes intake and PhenoAgeAccel. HOMA-IR and insulin were both log-transformed. Adjusted for age + gender + race + education level + PIR + smoking status + drinking status + Physical activity + BMI + hypertension + CVD
Subgroup analysis
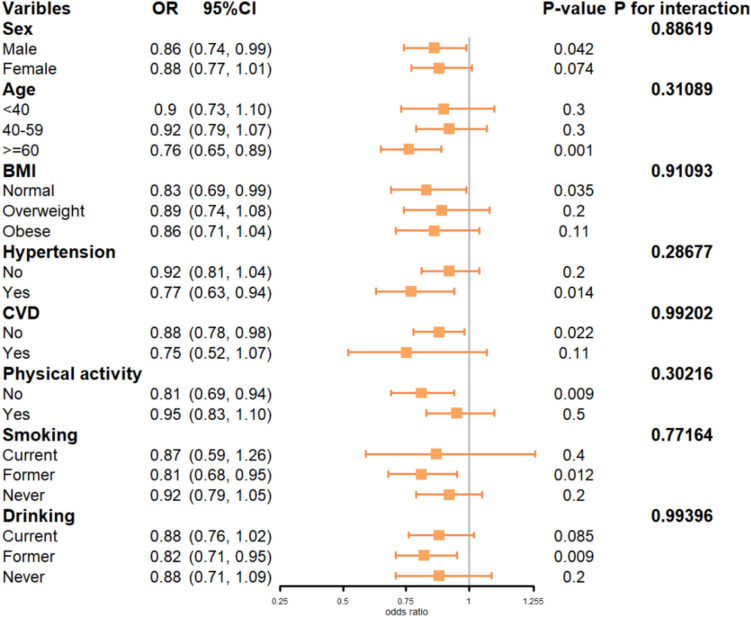
To further explore whether other variables influenced the relationship between dietary live microbes and PhenoAge Accel, we examined interactions and conducted subgroup analyses. As shown in Fig. 4, in stratified populations—men, individuals aged ≥ 60 years, those with normal BMI, individuals with hypertension, those without CVD, those without physical activity, past smokers, and past alcohol consumers—each 100-g increase in Medhi food intake was associated with a reduced risk of accelerated aging (P < 0.05). No significant interaction was observed between Medhi food intake and stratification variables in terms of influencing PhenoAge Accel (P > 0.05).
Fig. 4.
Forest plot of stratified analyses of the associations between Dietary live microbes intake and PhenoAgeAccel. Adjusted for age + gender + race + education level + PIR + smoking status + drinking status + physical activity + BMI + hypertension + CVD
Discussion
This cross-sectional analysis aimed to assess the relationship between dietary live microbes intake and PhenoAge. We found a nonlinear negative correlation between higher dietary live microbes intake, not moderate intake, and biological aging. This correlation remained significant even after adjusting for potential confounders including demographic, socioeconomic, and lifestyle-related factors. HOMA-IR and insulin partially mediated these effects, with mediation proportions of 30.03% and 24.65%, respectively.
Medhi foods include raw, unprocessed fruits and vegetables, unpasteurized fermented foods, and probiotic supplements that contain moderate to high levels of live microbes [19]. Recent studies suggest an association between live microbes intake and various health benefits. For example, foods rich in live microbes constitute up to 40% of both the Mediterranean diet and the DASH diet, which have been shown to provide clear cardiovascular benefits [36]. In traditional societies, such as Hadza hunter gatherers, there are less prone to many of the characteristics of “diseases of aging” than industrialized societies because of their higher levels of microbial richness and biodiversity [18]. Consumption of fermented foods has been linked to improved cognitive function, reduced insulin resistance, and lower fasting glucose, total cholesterol, and body fat percentage [37, 38]. In summary, current evidence supports the hypothesis that daily intake of live, safe microbes from the diet may enhance the ability of human symbionts to reduce susceptibility to chronic diseases [39]. Further, several routes for health promotion by fermented foods are proposed, including nutritive alteration of raw ingredients and the biosynthesis of bioactive compounds, modification of the human gut microbiota, and development and modification of the immune system. Microorganisms, their residual extracellular polysaccharides, microbial building blocks, proteins, or metabolites may directly or indirectly interact with the resident gut microbiome or intestinal epithelial cells and the immune system, thereby affecting human health [40]. Several studies based on in vitro or in vivo experiments have explored the association between live microbes, especially those present in fermented foods, and aging. These studies suggest that fermented food intake may reduce age-related oxidative damage, attenuate inflammatory responses, and improve mitochondrial function, potentially contributing to a longer healthy lifespan. However, there is a deficiency of clinical trials, especially those with large sample sizes, to determine whether foods enriched with live microbes can decelerate aging and to what extent they may reverse the aging process in humans [16]. Our study provides valuable insights into the relationship between dietary live microbes intake and biological age.
We observed that insulin resistance was previously reported in the literature as an important mechanism of biological aging. However, dietary live microbes intake may attenuate insulin resistance. As early as the 1990s, researchers proposed that enhancing insulin sensitivity could decelerate the aging process [41]. Insulin resistance and its secondary hyperinsulinemia can accelerate aging by enhancing oxidative stress by promoting protein oxidation and stimulating free radical generators and antioxidant enzymes [42, 43]. In vitro studies have demonstrated that oral administration of Lactobacillus casei CCFM419 [44], Bifidobacterium animalis subsp. lactis 420 [45], Bacteroides thetaiotaomicron [46], Bacillus amyloliquefaciens [47] attenuated insulin resistance in a type 2 diabetes and obesity model by modulating gut microbiota. A few clinical studies have reached similar conclusions [48]. Hence, we hypothesized that insulin resistance may be an intermediate link between live microbes and PhenoAge acceleration, which was confirmed by mediation analysis. Mediation analysis showed that HOMA-IR and high insulin levels partially mediated the relation between dietary live microbes and PhenoAgeAccel.
Although there is a burgeoning amount of literature supporting the role of foods enriched with live microbes in providing health factors (more often fermented foods), the decision on how much live microbes food to consume remains unresolved. The current study found no statistically significant effect of consuming fewer Medhi foods on the progression of biological aging compared to not consuming any Medhi foods. A recent review similarly noted that live microbes, when ingested in sufficient amounts, provide health benefits to the host [40]. This suggests that there is a threshold for ingesting live microbes for health benefits, but unfortunately, the review did not define a specific value for “adequate amounts. Determining the recommended intake of live microbes remains a priority for future research [49–51]. Our study offers some insights into this issue. The non-linear relationship between Medhi intake and the risk of accelerated aging was revealed by the RCS curve. The curve nodes were located near 100 g/d where the marginal benefit begins to level off. Derrien concluded that consumption of 1010 cells would be necessary to induce an effect on the microbiota and host health, which was achieved by consuming 100 g of fermented food containing 108 cells/g [52]. This is similar to our findings. Given that similar trends and nodes have been observed in other studies using the same samples and live microbes counting methods [31, 32, 53], we hypothesize that this could also be related to the definitional grouping of Medhi foods. Furthermore, as our analysis utilized a single dataset, the derived threshold may be influenced by misclassification or recall bias from the 24-h recall. In conclusion, the recommended intake of Medhi foods should not be simply considered to be around 100 g, and more studies are needed to support this observation.
Subgroup analyses also suggested some interesting findings. It seems that PhenoAge acceleration cannot be delayed by increasing dietary live microbes intake in individuals under 60 years of age, those with a BMI outside the normal range, or those with existing cardiovascular disease (CVD). This may be due to the low baseline risk of PhenoAge acceleration in relatively younger individuals. In contrast, increasing dietary live microbes intake may be an option to reduce PhenoAge acceleration in individuals over 60 years old, those with hypertension, or those who are less physically active. Previous studies have found that the composition of the gut microbiota undergoes significant changes with aging and associated disease [54]. Individuals over 60 experience a reduction in gut microbiota diversity, with a decrease in glycolytic bacteria and an increase in protein-hydrolyzing bacteria [55]. A more fragile gut environment may be one of the reasons why they gain a more pronounced degree of delayed aging through the intake of live microbes. These hypotheses require further empirical investigation.
In conclusion, our study found a nonlinear negative association between the dietary live microbes intake and biological age, suggesting that a higher intake of dietary live microbes may delay the biological age progression. Further, insulin resistance was found to partially mediate this relationship. Utilizing the NHANES study design, known for its robust survey methodology and stringent quality control, our research offers new insights into the effects of dietary live microbes on PhenoAge. However, several limitations must be noted. Firstly this is a cross-sectional study and cannot demonstrate a direct causal or temporal relationship between the three. Secondly, we excluded missing data rather than interpolated, which may have reduced the representativeness of the sample. Third, dietary live microbes intake data were derived from 24-h dietary recalls, which may contain potential recall bias versus underreporting bias and reflect only the most recent level of dietary intake. Fourth, our study did not further classify the amount of alcohol consumed and did not take into account heavy drinkers, whose life expectancy may differ from that of moderate drinkers. Finally, although we adjusted for several potential confounders, it remains unknown whether the relationship between dietary live microbes and PhenoAge was influenced by other confounding effects. For instance, long-term exposure to air pollutants such as PM2.5 and PM10 exhibited a potential correlation with accelerated biological aging by increasing systemic inflammation [56, 57]. Future well-designed randomized controlled trials and prospective cohort studies are needed to validate these results, and a wider sample population and more accurate methods for live microbes intake are needed to better understand the relationship between dietary live microbes and PhenoAge.
Conclusions
The intake of live dietary microbes is nonlinearly associated with reduced accelerated biological age. Higher intake of live dietary microbes may be necessary to produce a notable effect. IR partially mediated the relationship.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
YYH, SZY, and LJG conceived and designed the study. TT, SXN, and ZXX extracted and analyzed data. YYH wrote the manuscript. YKW, WQQ, and LC contributed to the manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Central High-Level Traditional Chinese Medicine Hospital Project of eye Hospital China Academy of Chinese medical science(GSP2-02 and GSP2-18).
Data Availability
The dataset(s) supporting the conclusions of this article is(are) available in the NHANES, https://www.cdc.gov/nchs/nhanes/. No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participant
The program was approved by the National Center for Health Statistics Ethics Review Board. All of the participants provided written informed consent. No additional ethical review board approval was required to analyze the anonymized NHANES data.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO). Ageing and health [Internet]. [cited 2024 Sep 13]. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
- 2.Spinelli R, Baboota RK, Gogg S, Beguinot F, Blüher M, Nerstedt A, et al. Increased cell senescence in human metabolic disorders. J Clin Invest [Internet]. 2023 Jun 15 [cited 2024 Sep 2];133(12). Available from: https://www.jci.org/articles/view/169922 [DOI] [PMC free article] [PubMed]
- 3.Burch JB, Augustine AD, Frieden LA et al (2014) Advances in geroscience: impact on healthspan and chronic disease. J Gerontol: Ser A 69:S1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:R115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu AT, Quach A, Wilson JG et al (2019) DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY) 11:303–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shireby GL, Davies JP, Francis PT et al (2020) Recalibrating the epigenetic clock: implications for assessing biological age in the human cortex. Brain 143:3763–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murabito JM, Zhao Q, Larson MG et al (2018) Measures of biologic age in a community sample predict mortality and age-related disease: the Framingham offspring study. J Gerontol A Biol Sci Med Sci 73:757–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belsky DW, Moffitt TE, Cohen AA et al (2018) Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol 187:1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Kuo PL, Horvath S et al (2018) A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med 15:e1002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine ME, Lu AT, Quach A et al (2018) An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 10:573–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez-Ozaeta I, Astiazaran OJ (2022) Fermented foods: an update on evidence-based health benefits and future perspectives. Food Res Int 156:111133 [DOI] [PubMed] [Google Scholar]
- 12.Ton AMM, Campagnaro BP, Alves GA et al (2020) Oxidative stress and dementia in Alzheimer’s patients: effects of synbiotic supplementation. Oxid Med Cell Longev 13:2638703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hütt P, Songisepp E, Rätsep M et al (2015) Impact of probiotic Lactobacillus plantarum TENSIA in different dairy products on anthropometric and blood biochemical indices of healthy adults. Benef Microbes 6:233–243 [DOI] [PubMed] [Google Scholar]
- 14.Fathi Y, Ghodrati N, Zibaeenezhad MJ et al (2017) Kefir drink causes a significant yet similar improvement in serum lipid profile, compared with low-fat milk, in a dairy-rich diet in overweight or obese premenopausal women: a randomized controlled trial. J Clin Lipidol 11:136–146 [DOI] [PubMed] [Google Scholar]
- 15.Zhou F, Li YL, Zhang X et al (2021) Polyphenols from Fu brick tea reduce obesity via modulation of gut microbiota and gut microbiota-related intestinal oxidative stress and barrier function. J Agric Food Chem 69:14530–14543 [DOI] [PubMed] [Google Scholar]
- 16.Das G, Paramithiotis S, Sundaram Sivamaruthi B et al (2020) Traditional fermented foods with anti-aging effect: a concentric review. Food Res Int 134:109269 [DOI] [PubMed] [Google Scholar]
- 17.Şanlier N, Gökcen BB, Sezgin AC (2019) Health benefits of fermented foods. Crit Rev Food Sci Nutr 59:506–527 [DOI] [PubMed] [Google Scholar]
- 18.Mafra D, Borges NA, Alvarenga L et al (2023) Fermented food: should patients with cardiometabolic diseases go back to an early neolithic diet? Crit Rev Food Sci Nutr 63:10173–10196 [DOI] [PubMed] [Google Scholar]
- 19.Marco ML, Hutkins R, Hill C et al (2022) A classification system for defining and estimating dietary intake of live microbes in US adults and children. J Nutr 152:1729–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnik BC, Schmitz G (2021) Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev 67:101270 [DOI] [PubMed] [Google Scholar]
- 21.Kolb H, Kempf K, Martin S (2023) Insulin and aging - a disappointing relationship. Front Endocrinol (Lausanne) 14:1261298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth GS, Lane MA, Ingram DK et al (2002) Biomarkers of caloric restriction may predict longevity in humans. Science 297:811 [DOI] [PubMed] [Google Scholar]
- 23.Paolisso G, Gambardella A, Ammendola S et al (1996) Glucose tolerance and insulin action in healthy centenarians. Am J Physiol 270:E890-894 [DOI] [PubMed] [Google Scholar]
- 24.Zhang XF, Qi Y, Zhang YP et al (2024) Fermented foods and metabolic outcomes in diabetes and prediabetes: a systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 64:9514–9531 [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Zhang B, Li L et al (2022) Fermented noni (Morinda citrifolia L.) fruit juice improved oxidative stress and insulin resistance under the synergistic effect of Nrf2/ARE pathway and gut flora in db/db mice and HepG2 cells. Food Funct 13:8254–8273 [DOI] [PubMed] [Google Scholar]
- 26.Daniel N, Nachbar RT, Tran TTT et al (2022) Gut microbiota and fermentation-derived branched chain hydroxy acids mediate health benefits of yogurt consumption in obese mice. Nat Commun 13:1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang C, Zhao H, Kong L et al (2023) Probiotic yogurt alleviates high-fat diet-induced lipid accumulation and insulin resistance in mice via the adiponectin pathway. J Agric Food Chem 71:1464–1476 [DOI] [PubMed] [Google Scholar]
- 28.Pan R, Xu T, Bai J et al (2020) Effect of Lactobacillus plantarum fermented barley on plasma glycolipids and insulin sensitivity in subjects with metabolic syndrome. J Food Biochem 44:e13471 [DOI] [PubMed] [Google Scholar]
- 29.Dhuique-Mayer C, Boada S, Meile JC et al (2025) A fermented functional food enriched in phytosterol and carotenoids improves lipid profile and insulin resistance and restores vitamin A status in high-fat diet-induced metabolic syndrome rats. Food Funct 16:2881–2892 [DOI] [PubMed] [Google Scholar]
- 30.Han L, Wang Q (2022) Association of dietary live microbe intake with cardiovascular disease in US adults: a cross-sectional study of NHANES 2007–2018. Nutrients 14:4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huo X, Jia S, Sun L et al (2024) Association of dietary live microbe intake with frailty in US adults: evidence from NHANES. J Nutr Health Aging 28:100171 [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Wang H, Yu Q et al (2024) High dietary live microbe intake is correlated with reduced risk of depressive symptoms: a cross-sectional study of NHANES 2007–2016. J Affect Disord 1:198–206 [DOI] [PubMed] [Google Scholar]
- 33.Kwon D, Belsky DW (2021) A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. Geroscience 43:2795–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 35.The U.S. Department of Health and Human Services (HHS). 2008 Physical Activity Guidelines for Americans [Internet]. [cited 2024 Sep 13]. Available from: https://health.gov/our-work/nutrition-physical-activity/physical-activity-guidelines/previous-guidelines/2008-physical-activity-guidelines
- 36.Estruch R, Lamuela-Raventós RM (2023) Cardiovascular benefits of fermented foods and beverages: still up for debate. Nat Rev Cardiol 20:789–790 [DOI] [PubMed] [Google Scholar]
- 37.Kim EK, An SY, Lee MS et al (2011) Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr Res 31:436–443 [DOI] [PubMed] [Google Scholar]
- 38.Kwon DY, Daily JW, Kim HJ et al (2010) Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr Res 30:1–13 [DOI] [PubMed] [Google Scholar]
- 39.Marco ML, Sanders ME, Gänzle M et al (2021) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol 18:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeuwendaal NK, Stanton C, O’Toole PW et al (2022) Fermented foods, health and the gut microbiome. Nutrients 14:1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paolisso G, Tagliamonte MR, Rizzo MR et al (1999) Advancing age and insulin resistance: new facts about an ancient history. Eur J Clin Invest 29:758–769 [DOI] [PubMed] [Google Scholar]
- 42.Parr T (1996) Insulin exposure controls the rate of mammalian aging. Mech Ageing Dev 88:75–82 [DOI] [PubMed] [Google Scholar]
- 43.Janssen JAMJL (2021) Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci 22:7797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Wang E, Yin B et al (2017) Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef Microbes 8:421–432 [DOI] [PubMed] [Google Scholar]
- 45.Uusitupa HM, Rasinkangas P, Lehtinen MJ et al (2020) Bifidobacterium animalis subsp. lactis 420 for Metabolic Health: Review of the Research. Nutrients 12:892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H, Wang XK, Tang M et al (2024) Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 16:2304159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeong DY, Daily JW, Lee GH et al (2020) Short-term fermented soybeans with bacillus amyloliquefaciens potentiated insulin secretion capacity and improved gut microbiome diversity and intestinal integrity to alleviate asian type 2 diabetic symptoms. J Agric Food Chem 68:13168–13178 [DOI] [PubMed] [Google Scholar]
- 48.Hulston CJ, Churnside AA, Venables MC (2015) Probiotic supplementation prevents high-fat, overfeeding-induced, insulin resistance in humans. Br J Nutr 113:596–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaine PC, Balentine DA, Erdman JW et al (2013) Are dietary bioactives ready for recommended intakes?12. Adv Nutr 4:539–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marco ML, Hill C, Hutkins R et al (2020) Should there be a recommended daily intake of microbes? J Nutr 150:3061–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hill C (2018) RDA for microbes—are you getting your daily dose? Biochemist 40:22–25 [Google Scholar]
- 52.Derrien M, van Hylckama Vlieg JET (2015) Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol 23:354–366 [DOI] [PubMed] [Google Scholar]
- 53.Wang L, Wang S, Wang Y et al (2024) Association between dietary live microbe intake and Life’s Essential 8 in US adults: a cross-sectional study of NHANES 2005–2018. Front Nutr 11:1340028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lakshminarayanan B, Stanton C, O’Toole PW et al (2014) Compositional dynamics of the human intestinal microbiota with aging: Implications for health. J Nutr Health Aging 18:773–786 [DOI] [PubMed] [Google Scholar]
- 55.Vaiserman AM, Koliada AK, Marotta F (2017) Gut microbiota: a player in aging and a target for anti-aging intervention. Ageing Res Rev 1:36–45 [DOI] [PubMed] [Google Scholar]
- 56.Zhao Y, Yang X, He D, Wei W, Cheng B, Zhang F. Associations between environmental air pollution, greenspace and apparent biological aging: a cross-sectional study. Geroscience. 2025; [DOI] [PMC free article] [PubMed]
- 57.Dolcini J, Landi R, Ponzio E et al (2024) Association between TNF-α, cortisol levels, and exposure to PM10 and PM2.5: a pilot study. Environ Sci Europe. 36:141 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset(s) supporting the conclusions of this article is(are) available in the NHANES, https://www.cdc.gov/nchs/nhanes/. No datasets were generated or analysed during the current study.