Abstract
Plant molecular farming is a promising platform for biopharmaceutical production, however, downstream processing remains a challenge due to cost and complexity. In this study, we present natural peptidoglycan nanoparticles (NPNs) derived from Gram-positive lactic acid bacteria as a novel tool for plant-based vaccine purification and delivery. Sequential treatment with trichloroacetic acid and trypsin effectively reduced NPN size, removing residual host subcellular constructs and proteins while preserving protein-binding capacity. Optimizing trimeric protein anchors and trimerization elements for plant-based expression enabled protein binding at low temperatures, minimizing proteolytic degradation. NPNs conjugated with plant-derived hemagglutinin elicited strong humoral immune responses in mice. Additionally, NPNs enhanced the retention of GFP at the injection site and supported efficient polyclonal antibody generation. These findings establish NPNs as a versatile platform for plant-based recombinant vaccine purification and delivery.
Key words: plant molecular pharming, Nicotiana benthamiana, bacterium-like particle, vaccine, adjuvant, pep, tidoglycan, protein purification
This study reports the development of natural peptidoglycan nanoparticles (NPNs), derived from bacterium-like particles of lactic acid bacteria, as a novel tool for plant-based vaccine purification and delivery. NPNs streamline the purification of recombinant proteins from plant lysates while simultaneously serving as a biodegradable vaccine carrier. This dual functionality both reduces production costs and significantly enhances the resulting immune responses.
Introduction
Plant molecular farming for biopharmaceuticals—including vaccines (Ward et al., 2021; Jung et al., 2022), antibodies (Ma et al., 1995), and enzymes (Shaaltiel et al., 2007; Tekoah et al., 2015; Ruderfer et al., 2018)—has been extensively studied over the past 30 years. Its advantages over traditional bioreactors are well documented. Plants are naturally free of human pathogens and endotoxins, significantly reducing the risk of contamination during processing. Additionally, they require only basic greenhouse conditions—light, water, and nutrients—thereby eliminating the need for costly sterile environments and culture media (Chung et al., 2021; Kole et al., 2024; Song et al., 2024). Importantly, plant-based platforms can produce millions of doses within weeks, making them ideal for emergency pandemic response or large-scale applications (D’Aoust et al., 2008). Despite these advantages in upstream production, downstream processing remains a significant challenge. Plants produce abundant endogenous proteins and metabolites, which complicate purification and account for up to 80% of total production costs (Fischer and Buyel, 2020). To overcome this limitation and enable broader application, an affordable downstream purification system tailored to plant-based production is urgently needed.
Over the past few decades, vaccines have provided effective protection against numerous pathogens by eliciting immune responses. Traditional vaccines, such as Havrix for hepatitis A and the measles, mumps, and rubella vaccine, use inactivated or attenuated pathogens to stimulate immunity (Andey et al., 2024). In contrast, subunit vaccines—which consist of antigenic protein components produced in bioreactors like Escherichia coli (E. coli) (Huang et al., 2017), yeast (Kulagina et al., 2021), mammalian cells (Xu et al., 2022), insect cells (Tian et al., 2021), or plants (Song et al., 2022)—have gained prominence due to advances in DNA technology. These vaccines are safer, easier to produce, and associated with fewer adverse reactions. However, their lower immunogenicity, due to the absence of pathogen-associated molecular patterns essential for innate immune recognition, necessitates the use of adjuvants such as alum, AS04, AS03, AS01, and CpG ODN 1018 to elicit a stronger immune response (Zhao et al., 2023). Consequently, researchers are exploring innovative delivery systems to improve vaccine efficacy against both existing and emerging infectious diseases.
Gram-positive lactic acid bacteria (LAB) have emerged as promising candidates for mucosal vaccine delivery owing to their nonpathogenic nature and lack of lipopolysaccharides in their cell walls (Pontes, 2011; Bermúdez-Humarán et al., 2013; Wang et al., 2016). Genetically engineered LAB have demonstrated potential as oral and intranasal vaccine platforms targeting influenza virus (Lei, 2015a, 2015b; Lei et al., 2015a, 2015b; Mohseni et al., 2020). To address concerns regarding genetically modified organisms, researchers have developed bacterium-like particles (BLPs) by acid-treating LAB to remove lipoteichoic acid and host proteins, leaving behind a peptidoglycan (PGN) matrix that serves as a scaffold for recombinant antigens (Zadravec et al., 2015; Mao et al., 2016; Zhou et al., 2023). These freestanding BLPs act as effective adjuvants by enhancing systemic and mucosal immune responses (De Haan et al., 2012), shifting the T helper 1/T helper 2 balance (Saluja et al., 2010a, 2010b), reducing required antigen doses (Saluja et al., 2010c), and providing robust protection against various infections across multiple administration routes, as demonstrated in both preclinical and clinical studies. In addition to their adjuvant properties, BLP-based vaccines have shown promising clinical efficacy in enhancing systemic, mucosal, and cellular immune responses against diverse pathogens, including HIV-1 (Bi et al., 2020), respiratory syncytial virus (RSV) (Van Braeckel-Budimir et al., 2013), Middle East respiratory syndrome coronavirus (Li et al., 2019), and Rift Valley fever virus (Zhang et al., 2022). A recent clinical trial found that intranasal administration of the BLP-based seasonal influenza vaccine FluGEM was safe and well-tolerated in healthy adults aged 18–49 and 65 years and older, with the highest immunogenicity observed at the 1.25- and 2.5-mg doses, suggesting a potential non-linear dose-response relationship (van der Plas et al., 2024).
As a major structural component of LAB cell walls, PGN is recognized by the innate immune system as a key pathogen-associated molecular pattern during bacterial infection in vivo. This recognition involves pattern-recognition receptors such as Toll-like receptor 2 (TLR2), peptidoglycan recognition proteins (PGLYRPs), and cytosolic NOD-like receptors (NOD1 and NOD2), which detect PGN fragments like muramyl dipeptide and initiate inflammatory signaling (Wolf and Underhill, 2018). PGN is degraded by lysosomal enzymes, releasing immunostimulatory fragments. This process bridges innate and adaptive immunity by shaping cytokine responses, T cell polarization, immunoglobulin A (IgA) secretion, and memory T cell development, enabling tailored immune defense (Wolf and Underhill, 2018). These properties highlight the potential of PGN-based adjuvants for enhancing vaccine efficacy, particularly for intramuscular delivery. Recently, intramuscularly administered BLP-presented antigens, especially recombinant hemagglutinin (HA) derived from Nicotiana benthamiana (N. benthamiana),demonstrated potent antibody induction without the need for additional adjuvants (Song et al., 2021). However, challenges remain, including limited antigen display capacity and residual host antigenic components in BLPs, which hinder their broader application.
In this study, we developed natural peptidoglycan nanoparticles (NPNs) based on BLPs. The NPNs are significantly smaller than BLPs, with undetectable subcellular structures and host proteins, while retaining full protein binding capacity. To improve protein expression, we optimized plant-adapted trimeric domains fused with efficient NPN binding domains, which enhanced protein folding efficiency and allowed for efficient large-scale NPN binding at low temperatures. NPN-mediated antigen delivery resulted in slow, sustained release in muscle tissue, inducing robust humoral immune responses. These responses were comparable to those triggered by BLPs and significantly stronger than those generated by soluble antigens alone. These results provide a strong foundation for advancing NPN-based platforms in vaccine development, polyclonal antibody production, and plant-based recombinant protein purification.
Results
Screening of domains with higher levels of plant expression and BLP binding efficiency
The binding capacity of HAs–mCor1–LysM to the surface of BLPs is largely dependent on its trimeric structure, as seen in our previous study. In this construct, the HA is from the avian influenza virus, and mCor1 denotes a coiled-coil domain derived from mouse coronin 1, which promotes trimerization of the LysM domain, thereby enhancing its binding affinity to BLPs. In this study, we evaluated the foldon domain (FD), a widely used trimerization domain in mammalian systems for proteins such as influenza virus HA (McMillan et al., 2021), RSV fusion protein (Gilman et al., 2019), and SARS-CoV-2 spike protein (Juraszek et al., 2021), due to its structural advantages. Structural alignment revealed that, unlike mCor1, the N-terminus of FD contains a flexible linker rather than a consecutive β-helix (Supplemental Figure 1), potentially reducing its impact on flanking proteins. To compare the effectiveness of these trimerization domains in a plant-based expression system, we generated GFP–mCor1–LLysM and GFP–FD–LLysM constructs using the pTEX1L vector (Figure 1A). These constructs were transiently expressed in N. benthamiana, and both GFP fluorescence imaging (Figure 1B) and western blot analysis (Supplemental Figure 3A) revealed higher expression levels for GFP–FD–LLysM. To further validate the functions of mCor1 and FD, we substituted the LLysM domain with an alternative LysM domain derived from Enterococcus faecalis (ELysM; Supplemental Figure 2), creating the GFP–mCor1–ELysM and GFP–FD–ELysM constructs. GFP fluorescence imaging (Figure 1C) and western blot analysis (Supplemental Figure 3B) again confirmed higher expression levels for GFP–FD–ELysM. Taken together, these results demonstrate superior compatibility of the FD domain with plant-based expression systems.
Figure 1.
Screening of plant-preferred trimeric domains and BLP-binding domains
(A and F) Schematic representation of recombinant GFP constructs in the pTEX1L binary vector. FD, foldon domain; GFP, green fluorescent protein; HDEL, ER retention signal; mCor1, the trimerization motif of mouse Coronin 1A; ProMacT, promoter of AtMacT; RD29B T, terminator of AtRD29B; SP, the signal peptide of AtBIP1.
(B, C, and G) GFP fluorescence images of indicated constructs expressed in N. benthamiana leaves via agroinfiltration, captured 3 DPI. Scale bar, 1 cm.
(D) Schematic diagram of BLP incubated with a gradient amount of total soluble proteins (TSPs) extracted from recombinant GFP construct infiltrated leaf tissues.
(E and H) SDS-PAGE and CBB analyses of BLP-bound recombinant GFP proteins from gradient amounts of TSP extracted from infiltrated leaf tissues. Bovine serum albumin (BSA) standards were loaded on the same gel for generating the standard curve. M, molecular weight.
(I) Quantitative results of protein binding shown as mean ± SD (n = 3).
(J–N) Binding assay of GFP–FD–LLysM and GFP–FD–SCpl7 at different temperatures. TSP extracted from 0.1 g of leaf tissue was incubated with BLPs prepared from L. sakei cells (1 mL, OD600 = 1) at 37 °C or 4 °C for 15 min. The pellet was collected for SDS-PAGE analysis. Red asterisks indicate the main bands of GFP–FD–LLysM and GFP–FD–SCpl7. Band densities were measured and are shown in (K) and (M), respectively. The blue arrowhead indicates the aggregated GFP–FD–LLysM and GFP–FD–SCpl7. Band densities were measured and are shown in (L) and (N). The black arrowhead indicates a non-specific protein that binds to BLP at 37 °C. Data represent mean ± SD (n = 3). Statistical significance was determined by the Student’s t test using GraphPad Prism.
Using the optimized FD domain, we conducted a comprehensive screening of FD-assembled trimeric protein anchors (PAs), composed of LysMs, S-layers, and Cpl7s (Figure 1F), to identify candidates that are compatible with plant expression systems and capable of efficient BLP binding. Among the candidates tested, the cell wall binding domains of the Cpl-7 endolysin from Streptococcus phage CP-7 and from Lactococcus lactis subsp. lacti (Supplemental Figure 2) exhibited high compatibility with plant-based expression systems (Figure 1G and Supplemental Figure 3C). Binding efficiency tests using gradient concentrations of total soluble protein (TSP; Figure 1D) showed that trimeric PAs, including FD–LLysM, FD–LSCpl7, and FD–SCpl7, exhibited strong binding to BLPs (Figure 1E, 1H, and 1I).
Previous studies have explored various PAs derived from Lactobacillus and Lactococcus, including numerous LysMs from AcmA, for their ability to bind BLPs in vaccine delivery and related applications. Additional PAs, including Muro (Bosma et al., 2006), LcsB (Hu et al., 2011), Lyb5 (Hu et al., 2010), MltD (Bosma et al., 2006), and AcmD (Bosma et al., 2006), have also been evaluated for BLP binding. Notably, the BLP binding process typically requires incubation at 37 °C or room temperature—conditions that are suboptimal for plant-based systems due to the activation of endogenous plant proteases, which degrade recombinant proteins. In contrast, the trimeric PAs developed in this study demonstrated higher binding capacity and reduced aggregation at 4 °C compared to 37 °C, as evidenced by fewer non-specific bands (Figure 1J–1N). This low-temperature binding ability effectively reduces proteolytic degradation during protein purification, offering a significant advantage for plant-based systems in vaccine purification.
Preparation and representation of natural peptidoglycan nanoparticles
The cell wall of Gram-positive LAB consists mainly of a thick PGN layer, which functions as a scaffold for the attachment of cell wall components such as lipoteichoic acids, polysaccharides, and proteins (Pasquina-Lemonche et al., 2020). These surface components broadly block the interaction between PGN and PAs. Therefore, live LAB cells are typically pretreated with 10% trichloroacetic acid (TCA) at 100 °C for 10 min to disrupt lipoteichoic acids and protein complexes, thereby exposing the entire PGN surface for PA binding and generating BLPs (Zhou et al., 2023). Although BLPs are inactive and lose most of their surface components during TCA treatment, residual host proteins on both the exterior and interior limit their application as vaccine or drug carriers in vivo.
To further clean BLPs, we first screened various LAB strains for the lowest host protein content. Lactobacillus sakei was identified as a superior strain for BLP preparation due to its low residual host protein levels following 10% TCA treatment (Supplemental Figure 4). Next, we conducted protein digestion experiments using robust proteases, including trypsin, pepsin, and proteinase K (Figure 2A). Both trypsin and proteinase K effectively removed residual host proteins according to the Bradford assay (Figure 2B). This result was confirmed by SDS-PAGE and Coomassie Brilliant Blue (CBB) staining, which showed undetectable protein levels for BLPs treated with either protease (Figure 2C). However, proteinase K almost completely degraded the BLP, as evidenced by the minimal pellet remaining after incubation. As expected, proteinase K-treated BLPs lost their protein binding capability, whereas trypsin-treated BLPs retained this functionality (Figure 2D). Based on these findings, we selected trypsin for BLP host protein removal. Further optimization demonstrated that incubation of BLPs at 37 °C for 2 h with 2 μg/mL trypsin was sufficient for the effective removal of residual host proteins (Supplemental Figure 5). The resulting trypsin-treated BLPs were designated NPNs.
Figure 2.
Preparation and representation of natural peptidoglycan nanoparticles (NPNs)
(A) Schematic representation of BLP and NPN preparation. Live cells were heated in 10% TCA for 10 min for BLP preparation. BLPs were digested by proteases at 37 °C for 2 h for NPN preparation.
(B) Total protein content of BLP after treatment with trypsin, pepsin, and proteinase K. BLPs (1 mL reaction volume) were treated with various proteases, centrifuged, and the pellet was crushed by vortexing with glass beads. Protein levels were quantified by Bradford assay. Data represent mean ± SD (n = 3).
(C) SDS-PAGE and CBB staining analysis of total proteins from live cells (1), BLPs (2), trypsin-treated BLPs (3), pepsin-treated BLPs (4), and proteinase K-treated BLPs (5). Equal amounts of live L. sakei cells (from 1 mL culture, OD600 = 1) were processed as described in Methods. Pellets were crushed with glass beads, followed by boiling in SDS sample buffer for SDS-PAGE analysis. Whole lane densities were quantified using ImageJ. Data represent mean ± SD (n = 3).
(D) Binding capacity of GFP–FD–SCpl7 to BLPs before and after trypsin, pepsin, or proteinase K treatment. The black arrowhead indicates the main band of GFP–FD–SCpl7.
(E) Suspension and precipitation states of the Live cells, BLPs and NPNs. Equal amounts of L. sakei cells (4 mL, OD600 = 1) were treated as live cells, BLPs, and NPNs, respectively. Resuspension status of each (100 μL) was observed in PCR tubes.
(F) The OD600 value of live cells, BLPs, and NPNs. Equal amounts of L. sakei cells (1 mL, OD600 = 1) were treated as BLPs and NPNs for measurement. Data are mean ± SD (n = 3).
(G) Particle size measurements of live cells, BLPs, and NPNs determined from TEM images using ImageJ. Over 30 particles were analyzed per group. Data are mean ± SD (n > 30).
(H) Overview and morphology of live cells, BLPs, and clean NPNs observed by optical microscopy and transmission electron microscopy. Statistical significance was determined using the Student’s t test in GraphPad Prism.
While optimizing digestion conditions, we observed a significant reduction in pellet volume following trypsin digestion (Figure 2E), accompanied by a notably lower optical density at 600 nm (OD600) upon resuspension (Figure 2F). This reduction was unlikely due to a decrease in particle number, as the protein binding capacity remained unaffected. Instead, it suggested a loss of protein components both outside and inside the BLPs, which could also lead to a decrease in particle size. To investigate this hypothesis, we prepared live LAB cells, BLPs, and NPNs for optical microscopy observation (Figure 2H). Live cells and BLPs showed no notable morphological differences, whereas NPNs appeared smaller. For more detailed characterization, the samples were fixed, dehydrated, embedded in resin, and subjected to transmission electron microscopy (TEM) (Figure 2H). TEM analysis revealed that live cells exhibited full, spherical structures with dense internal contents, whereas BLPs retained a similar morphology but contained fewer internal components. Consistent with our hypothesis, NPNs exhibited a significantly smaller size (∼100 × 500 nm) (Figure 2G) and a nearly hollow structure, characterized by a shrunken PGN coat. To enable potential applications of NPNs for in vivo protein drug delivery in animals, it is essential to ensure purity and eliminate contamination by live pathogens. Autoclave testing demonstrated that NPNs retained comparable protein binding capacity even after 3 months of storage at room temperature, indicating excellent long-term stability (Supplemental Figure 6). Furthermore, NPNs maintained high binding capacity for recombinant proteins at 4 °C, further confirming their suitability for pharmaceutical applications (Supplemental Figure 7).
Preparation and functional evaluation of NPN-sHA1 for vaccination
Influenza A viruses are a major cause of acute respiratory infections in swine, resulting in significant economic losses in the global pork industry. Three primary subtypes—H1N1, H3N2, and H1N2—are currently co-circulating worldwide (Mancera Gracia et al., 2020), necessitating the development of efficient, cost-effective vaccines to overcome this challenge.
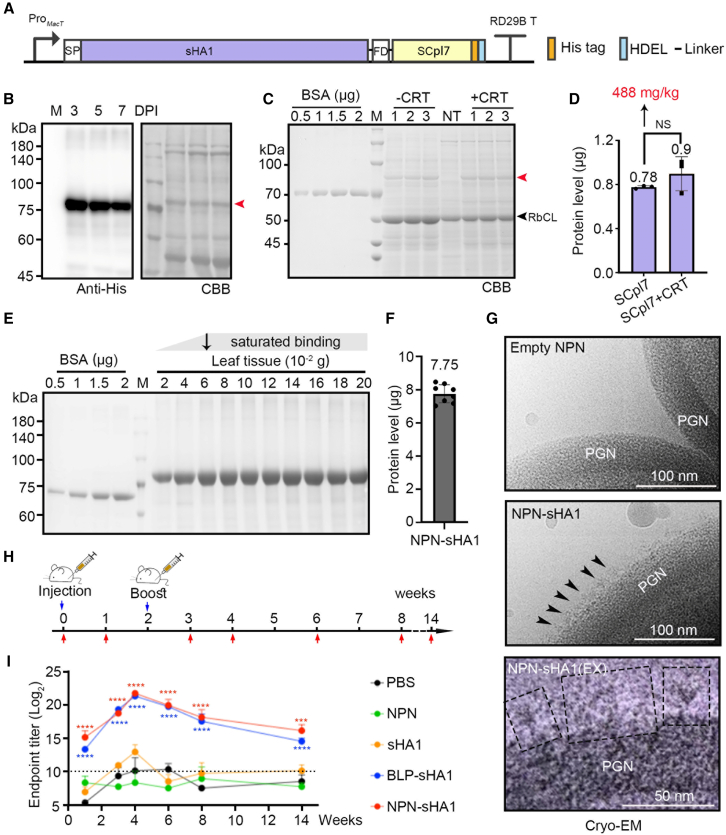
To evaluate the potential of NPNs for in vivo antigen delivery, we selected the HA gene from A/swine/Korea/CY0423-33/2013(H1N2) (amino acids [aa] 16–530, designated sHA1) for further study. We generated the sHA1–FD–SCpl7 construct and infiltrated the associated Agrobacterium into N. benthamiana leaves, with or without co-infiltration of CRT (Calreticulin), for expression efficiency testing (Figure 3A). Co-expression with CRT did not enhance the expression of sHA1–FD–SCpl7. A total of 0.1 g infiltrated leaf tissue was homogenized in 1 mL of extraction buffer, followed by high-speed centrifugation to obtain the supernatant. For SDS-PAGE and CBB staining, 16 μL of supernatant was mixed with 4 μL of 5× SDS loading buffer and boiled prior to electrophoresis. sHA1–FD–SCpl7 alone achieved a high expression yield of approximately 488 mg/kg in N. benthamiana (Figure 3B–3D). Remarkably, only 0.06 g of infiltrated fresh plant tissue was sufficient to saturate NPNs prepared from 1 mL of live cells (OD600 = 1), achieving a binding capacity of up to 7.75 μg, as determined by the main band density (Figure 3E and 3F). This is four times higher than the binding capacity observed with BLP-tHA (trimeric hemagglutinin) in our previous studies (Song et al., 2021). Structural analysis of NPN-conjugated sHA1 (NPN–sHA1) using cryo-electron microscopy (cryo-EM) revealed a uniform trimeric HA structure on the NPN surface (Figure 3G), closely resembling the trimeric HA configuration observed in our earlier research.
Figure 3.
Preparation and functional test of NPN-sHA1.
(A) Schematic representation of recombinant sHA1 constructs in the pTEX1L binary vector. HDEL, ER retention signal; sHA1, HA gene from swine H1N1; SP, the signal peptide of AtBIP1.
(B) SDS-PAGE and western blot analysis of sHA1–FD–SCpl7 expression in Nicotiana benthamiana. Target proteins were transiently expressed and harvested at 3, 5, and 7 DPI. Red arrowhead indicates the target protein band.
(C and D) SDS-PAGE and CBB staining analysis of sHA1–FD–SCpl7. A BSA gradient (0.5, 1, 1.5, and 2 μg) was loaded for standard curve generation. Expression with or without co-expression of the chaperone CRT was compared. Results are shown in (D). Data are mean ± SD (n = 3). NT, non-treated plants. The red arrowhead indicates the target proteins. Statistical significance was determined using the Student’s t test in GraphPad Prism.
(E) SDS-PAGE and CBB staining of NPN-conjugated sHA1–FD–SCpl7. Total protein extracts from increasing amounts of infiltrated leaf tissue (0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.18, and 0.2 g) were incubated with equal amounts of NPNs. The pellets after incubation were separated by SDS-PAGE and subjected to CBB analysis. Varying amounts of BSA (0.5, 1, 1.5, and 2 μg) were loaded on the same gel for standard curve generation. The black arrowhead indicates that sHA1–FD–SCpl7 protein extracted from 0.06 g of fresh leaf tissue can saturably bind NPNs prepared from 1 mL of L. sakei culture (OD600 = 1).
(F) The level of NPN-conjugated sHA1-FD-SCpl7 protein extracted from 0.06 g to 0.2 g of fresh leaf tissue is saturated, as shown in Figure 3E. The eight bands were quantified using ImageJ, and the mean values were calculated.
(G) Cryo-EM of NPN-sHA1. PGN, peptidoglycan. The black arrowheads indicate sHA1–FD–SCpl7 trimers presented on the surface of NPNs. Representative sHA1–FD–SCpl7 trimers are shown in black dashed boxes. EX, expanded.
(H and I) (H) Schematic of the immunization protocol. Five B5 mice per group were injected intramuscularly with 0.5 μg of soluble sHA1, BLP-sHA1, NPN-sHA1, or control treatments (PBS, BLPs, or NPNs), followed by a booster at 2-weeks. Mice sera were collected before and after immunization to determine humoral immune responses. Endpoint IgG antibody titers were measured by ELISA using plates coated with soluble sHA1–FD–SCpl7 protein (I). Dotted lines indicate the positive cutoff value, calculated as the mean endpoint titer of the PBS group. Statistical differences were determined by two-way ANOVA followed by Tukey’s multiple comparisons tests. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. Error bars represent SEM.
To evaluate the immunogenicity of NPN–sHA1, we intramuscularly immunized C57BL/6 (B6) mice twice at a 2-week interval with 0.5 μg of soluble sHA1 and equivalent doses of BLP–sHA1 or NPN–sHA1 (based on sHA1 protein content). Control groups received PBS and NPNs alone. Sera were collected at various time points to measure sHA1-specific IgG levels before and after immunization (Figure 3H). End-point ELISA revealed that both BLP-sHA1 and NPN-sHA1 elicited a significantly stronger immune response compared to soluble sHA1 (Supplemental Figure 8). Notably, antibodies remained in circulation for a significantly longer period, indicating that NPN–sHA1 produced a robust and sustained immune response in vivo (Figure 3I). These results highlight the superior immunogenic and functional performance of NPNs for vaccine delivery in vivo.
NPN–rGFP persists longer in muscle tissue and induces robust GFP-specific polyclonal antibodies in both mice and rabbit
To further validate the enhanced immune response induced by NPNs, we selected plant-derived recombinant GFP–FD–LLysM (rGFP) as a second target protein for antibody production testing in BALB/c mice (Figure 4A). Six-week-old BALB/c mice were intramuscularly administered 1 μg soluble rGFP, BLP-rGFP, or NPN-rGFP. Control groups received PBS, BLP, or NPN alone in equivalent volumes. A booster dose was administered 2 weeks later. Sera were collected 2 weeks post-boost and analyzed by western blot (1:1000 dilution) using a commercial anti-His antibody as a positive control (Figure 4B). The results showed that both BLP–rGFP and NPN–rGFP, but not soluble rGFP, elicited GFP-specific antibodies capable of detecting rGFP by western blot analysis (Figure 4C–4H). Consistent with these findings, endpoint ELISA confirmed that both BLP–rGFP and NPN–rGFP, but not soluble rGFP, elicited GFP-specific antibodies (Supplemental Figure 9). To investigate the mechanism underlying the higher immunogenicity of NPN–rGFP, we examined the retention of rGFP delivered by NPN. We monitored the GFP signal after intramuscular injection of 5 μg soluble rGFP or NPN–rGFP into the shaved legs of mice under a fluorescent stereomicroscope (Figure 4I and 4J). Soluble rGFP rapidly disappeared within 1 h, rendering the GFP signal undetectable. In contrast, rGFP delivered by NPN remained concentrated near the injection site, with the signal persisting for up to 10 h (Figure 4K). These findings underscore the potential of NPNs as a delivery platform for enhancing antigen stability and retention, laying the groundwork for further exploration of its immunological benefits.
Figure 4.
NPN-rGFP remains in muscular tissue longer and induces GFP-specific polyclonal antibodies
(A) Schematic overview of the immunization protocol and GFP-specific antibody analysis. BALB/c mice (n = 3 per group) were intramuscularly immunized with 1 μg of soluble rGFP, BLP-rGFP, or NPN-rGFP or an equivalent volume of control (PBS, BLP, or NPN), followed by a booster at 2 weeks. Sera were collected after immunization to determine GFP polyclonal antibody production via western blot. Samples loaded on the SDS-PAGE included total protein from live cells (1), BLP s(2), NPNs (3), NT (4), and GFP-FD-LLysM (GFP-FD-LLysM infiltrated plants) (5).
(B–H) Western blot analysis of samples using a commercial anti-His antibody (B) and sera collected from mice immunized with PBS (C), BLPs (D), NPNs (E), soluble rGFP (F), BLP-rGFP (G), and NPN-rGFP (H). Red asterisks indicate the bands of GFP–FD–LLysM.
(Iand J) Injection and imaging procedure. Equal doses (5 μg) of soluble rGFP and NPN-rGFP were injected into the muscular tissue of the mouse leg (I). Intramuscular GFP signals were captured using a Leica fluorescence microscope (Z16 APO A) equipped with a mercury lamp as the excitation source and a monochromatic camera (DMC6200). The shank-feathering-removed mouse was anesthetized with isoflurane during observation, and its hairless leg was fixed using a scaffold for imaging(J).
(K) Temporal tracking of GFP signal post-injection captured at 0, 1, 3, 6, 10, and 24 h.
Scale bars, 2 mm.
The low-cost and robust plant-derived, NPN-mediated vaccine purification and delivery system prompted us to evaluate its applicability in polyclonal antibody production. Rabbits are commonly used for generating polyclonal antibodies due to their robust immune responses and the ability to generate high-affinity antibodies. Their relatively large size also allows for sufficient blood collection, making them ideal for both research and commercial antibody production. To assess the potential of BLP–rGFP for polyclonal antibody production, we immunized 6-week-old rabbits with 5, 50, 100, or 200 μg of BLP-rGFP, administering three doses at 2-week intervals (Supplemental Figure 10A). Sera collected 2 weeks after the final boost were diluted 1:10 000 and analyzed by western blot for GFP-specific polyclonal antibodies, using a commercial rabbit anti-GFP polyclonal antibody (Abcam, ab6556) at the same dilution as a positive control. Notably, 50 μg of BLP–rGFP induced an antibody response comparable to the commercial product in terms of GFP detection (Supplemental Figure 10B). This GFP polyclonal antibody has since been used by several laboratories for research purposes. Typically, generating polyclonal antibodies in rabbits using a soluble antigen requires more than 100 μg of protein, even with the aid of a strong adjuvant. However, BLP-mediated antigen delivery significantly reduces the required antigen dose compared to soluble antigen formulations.
Discussion
In this study, we systematically screened domain elements and optimized conditions for developing a plant-based vaccine purification and delivery system using NPNs.
We identified plant-compatible trimeric domains and BLP binding motifs that enhanced expression in N. benthamiana and enabled high-affinity binding to BLPs at low temperatures. We also selected an appropriate LAB strain to minimize residual host proteins after TCA treatment, followed by screening proteases and refinement of the NPN preparation protocol. The resulting NPNs exhibited a shrunken morphology and lacked detectable host proteins, enabling safer delivery of recombinant proteins. NPNs enabled controlled antigen release at the injection site in muscle tissue, inducing a robust humoral immune response. These features highlight the dual functionality of NPNs as both an efficient purification agent and an effective delivery vehicle. Ultimately, we summarized an overall workflow for the plant-based production and NPN-mediated purification system (Figure 5), underscoring the versatility and promise of this innovative approach in plant molecular farming.
Figure 5.
NPN production and its conjugation process to plant-derived antigens.
(A) NPNs were prepared from live lactic acid bacteria (LAB) through sequential treatment with 10% trichloroacetic acid and trypsin.
(B) Codon-optimized foreign genes were efficiently expressed in N. benthamiana via Agrobacterium-mediated transient transformation. Harvested leaves were homogenized in extraction buffer, filtered through Miracloth, and centrifuged three times to obtain TSPs. GOI, gene(s) of interest encoding antigens; PA, protein anchors.
(C) Purified NPNs were incubated with TSP at 4°C for 15 min. The mixture was then filtered to collect antigen-displayed NPNs and washed three times to generate NPN-conjugated vaccine complexes.
Efficient production of recombinant proteins is a major focus in plant molecular farming. One common challenge is the disruption of proper protein folding by structural elements from different sources, which reduces overall yield. To address this, researchers often insert linker sequences between domains to minimize interference and improve folding efficiency. In this study, we compared two trimerization domains used to stabilize recombinant proteins. We found that inserting the FD domain between the target protein and PA significantly increased expression levels compared to the commonly used mCor1 domain. This improvement may be attributed to the greater flexibility of the N-terminal sequence of FD, which likely reduces its impact on the folding of the adjacent target protein and supports more efficient overall folding. Previously, our HA–mCor1–LysM construct yielded 150–250 mg/kg of fresh leaf tissue. In contrast, the optimized HA–FD–SCpl7 construct developed in this study reached ∼480 mg/kg, representing a two- to three-fold increase. These results highlight the critical role of domain design in enhancing recombinant protein expression in plants.
Downstream processing in plant molecular farming is particularly challenging due to the presence of insoluble particles, host cell proteins, and metabolites in plant extracts, which contribute to over 80% of total production costs. To further enhance antigen conjugation efficiency, we evaluated several PA domains. Among these, SCpl7 showed a significant improvement in binding efficiency. Our earlier construct, HA–mCor1–LysM, bound approximately 1.8 μg of protein on BLPs derived from 1 mL of LAB culture (OD600 = 1). In comparison, the HA–FD–SCpl7 construct developed in this study achieved a binding efficiency of 7.5 μg, representing a roughly four-fold increase. Notably, the system also achieved high purity, with no detectable non-specific binding of endogenous plant proteins to NPNs. A key breakthrough in this study was the ability of the trimeric PA system to achieve higher NPN binding at low temperatures. This feature is particularly advantageous for potential one-step purification directly from plant TSP. However, the mechanism by which temperature affects protein binding to NPNs remains unclear.
NPNs facilitated the sustained release of GFP at the injection site, providing prolonged stimulation of the immune system and resulting in significantly higher antibody titers compared to soluble GFP. Notably, the antibody titer induced by NPNs has no significant difference from that of BLPs for the sHA1 antigen, suggesting that immune-relevant components remain intact—or are even better exposed—despite trypsin treatment. The primary component of NPNs, PGN, is widely recognized for its adjuvant properties, including TLR2 binding, cytokine induction, and the regulation of cellular immunity via activation of multiple signaling pathways. However, this study did not directly investigate the mechanisms by which NPNs activate innate immunity or modulate adaptive immune responses. While our preliminary data suggest that NPNs function as effective immunostimulators, a deeper mechanistic understanding is needed. Future studies should aim to elucidate the molecular pathways involved in NPN-mediated immune activation, including potential engagement of pattern recognition receptors, downstream signaling cascades, and effects on antigen-presenting cells and lymphocyte responses.
In addition to mechanistic insights, the homogeneity and safety profile of NPNs must be rigorously evaluated in preclinical models. We isolated NPNs from LAB through sequential treatment with TCA and trypsin. Electron microscopy revealed that these particles formed intact PGN shells without signs of fragmentation, demonstrating uniform structural integrity. We also found no residual subcellular debris or endogenous proteins, further confirming the high purity and consistency of the NPN preparations. Despite these promising results, the current characterization is insufficient to support the use of NPNs as vaccine adjuvants or delivery vehicles in animals or humans. Additional studies are required to comprehensively assess their safety and functional potential.
Furthermore, although no mortality was observed after NPN injection in this study, comprehensive toxicological and biodistribution assessments are necessary to determine the biocompatibility and potential immunotoxicity of NPNs when used as a vaccine delivery platform. These evaluations should be conducted in vertebrate models and, ultimately, in non-human primates to better approximate human immune responses. Understanding both the immunological functions and safety characteristics of NPNs will be critical for their advancement as novel adjuvants or delivery vehicles in next-generation vaccine formulations. Thorough preclinical validation will not only ensure safety but also support regulatory approval for future clinical trials, paving the way for their broader application in both human and veterinary vaccines.
Methods
Construction of recombinant genes
DNA fragments encoding GFP, together with the trimerization motifs of mouse Coronin 1A (mCor1; VSRLEEDVRNLNAIVQKLQERLDRLEETVQAK) or FD (GYIPEAPRDGQAYVRKDGEWVLLSTFL), were fused at the C-terminus with various LAB binding domains (LysMs, S-Layers, or Cpl7s), followed by a His-tag and the endoplasmic reticulum (ER) retention signal HDEL. The ER leader peptide of Arabidopsis BiP1 was fused at the N-terminus of all constructs to enable ER targeting. The genes encoding various LAB binding domains were codon optimized and synthesized by Gene Universal (Newark, DE), and separately subcloned into the GFP reporter construct using the restriction enzymes XmaI and XhoI. The HA gene from the swine influenza virus (A/swine/Korea/CY0423-33/2013(H1N2); sHA1, aa 16–530) was also codon optimized and synthesized by Gene Universal (Newark, United States), and subcloned into the GFP reporter construct using the restriction enzymes BamHI and SpeI to replace the GFP coding sequence. All sequences used in this study are listed in Supplemental Table 1.
Production of transient transgenic plants
Expression vectors were introduced into Agrobacterium tumefaciens strain GV3101 by electroporation. A single colony harboring expression vectors was inoculated with LB broth (LPS Solution, catalog no. LBL-05) and cultured overnight at 28 °C. Four-week-old N. benthamiana plants, grown in a greenhouse at 25 °C under a 16-h light/8-h dark cycle, were used for agroinfiltration by syringe. Infiltrated leaves were harvested at 3, 5, and 7 days post-infiltration (DPI) for expression analysis. A vacuum chamber was used for the large-scale agroinfiltration.
GFP fluorescence imaging in N. benthamiana
Infiltrated leaves expressing various GFP fusion proteins were harvested at 3, 5, and 7 DPI. GFP fluorescence in leaves was visualized using the LAS3000 imaging system (Fujifilm, Tokyo, Japan).
SDS-PAGE and western blot analysis
Infiltrated leaves were ground and homogenized in protein extraction buffer (Tris-buffered saline [TBS] containing 1 mΜ EDTA, 0.1% [v/v] Tween 20, and 1 mM PMSF). Live cells, BLPs, and NPNs were washed and resuspended in TBS buffer (Tris 50 mM, pH 7.5, NaCl 150 mM). Approximately 10–50 mg of glass beads (425–600 μm, Sigma, St. Louis, MO) were added to the tubes, and the samples were vortexed for 7 min. Total protein extracts or purified proteins were separated on 7.5%–15% SDS-PAGE. Western blot analysis was performed using the mouse anti-His antibody (1:1000 dilution, Novus, AD1.1.10) or sera collected 28 days post-immunization from vaccinated mice. The secondary antibody was a horseradish peroxidase (HRP)-conjugated sheep anti-mouse IgG (HRP; 1:5000 dilution; Bethyl Laboratories, Boston, MA). Immunoblots were developed with an enhanced chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ), and images were captured using the LAS3000 system (Fujifilm) or the Amersham Imager 680 (GE Healthcare, Chicago, IL).
BLP and NPN preparation
L. sakei cells were cultured overnight at 37 °C under anaerobic conditions in MRS broth (Millipore [Burlington, MA], catalog no. 69 966-500G) supplemented with 0.1% Tween 80 (Sigma-Aldrich, catalog no. P1754), without shaking. Cells were collected by centrifugation and treated with 10% TCA at 100 °C for 10 min. The resulting pellets were washed three times with TBS to remove residual TCA and resuspended for further use as BLPs. To prepare NPNs, BLPs were resuspended in Tris buffer (50 mM, pH 7.8) containing 2 μg/mL trypsin (Sigma) and incubated at 37 °C for 2 h. Samples were then centrifuged and washed three times with double-distilled H2O (ddH2O) to remove residual trypsin.
BLP and NPN binding assay
Plant tissues were thoroughly ground in liquid nitrogen and dissolved in extraction buffer (50 mM Tris, pH 7.5; 150 mM NaCl, 1 mΜ EDTA, 0.1% Tween 20, and 1 mM PMSF) at a tissue-to-buffer ratio of 10:1 (v:v). Homogenates were centrifuged two to four times to remove debris as much as possible, and the resulting supernatants were filtered through 0.22-μm sterile filters. Filtered extracts were incubated with BLPs or NPNs at 4 °C, 25 °C, or 37 °C for 15 min. All tools used for sample preparation, including tips and tubes, were sterilized.
Optical microscopy and TEM imaging
Live L. sakei, BLPs, and NPNs were resuspended and initially observed using an optical microscope (Axioplan 2 imaging, Zeiss, Oberkochen, Germany) and then by EM. Samples were fixed in 2% paraformaldehyde and 2% glutaraldehyde in 0.05 M sodium cacodylate buffer (pH 7.2) for 4 h at 4 °C, followed by a second fixation in 1% osmium tetroxide in the same buffer for 2 h at 4°C. Fixed samples were dehydrated through a graded ethanol series up to 100%, transitioned into 100% propylene oxide via infiltration, and embedded for ultramicrotomy. Ultrathin sections (∼80 nm) were cut with an ultramicrotome, mounted on Cu 200-mesh grids (Quantifoil), and stained with 2.5% uranyl acetate for 7 min and 2.5% lead citrate for 3 min. Dry samples were observed by a JEOL JEM-1011 TEM (JEOL, Peabody, MA) at 80 kV.
Cryo-EM sample preparation and imaging
Cryo-EM grids were prepared using a Vitrobot Mark IV (Thermo Fisher Scientific, Hillsboro, OR) at 8 °C and 100% humidity. A 4-μL aliquot of each sample (0.2 mg/mL) was applied to glow-discharged holey carbon grids (Quantifoil R1.2/1.3). After 5 s, the grids were blotted for 1–3 s and plunged into liquid ethane for quick freezing. Grids were screened using a Tecnai Arctica microscope (Thermo Fisher Scientific) operated at 200 kV with a Falcon II direct electron detector (Thermo Fisher Scientific).
Qualified grids were transferred into a Titan Krios microscope (Thermo Fisher Scientific) operated at 300 kV and equipped with an energy filter (slit width 20 eV; GIF Quantum LS, Gatan, Pleasanton, CA). Images were recorded using a K3 Summit direct electron detector (Gatan) in super-resolution mode at a nominal magnification of 105, 000×, corresponding to a calibrated pixel size of 0.42165 Å. Data acquisition was automated using AutoEMation2.0 in movie mode. Each stack was recorded with a frame exposure time of 0.08 s and a total exposure time of 2.56 s, generating 32 frames per movie. The total electron dose per stack was approximately 50 e− Å−2. All 32 frames in each stack were aligned and summed using the whole-image motion correction program MotionCor2 and binned to a pixel size of 0.8433 Å.
Immunization of C57BL/6 mice and ELISA for sHA1-specific IgG detection
Eight-week-old male C57BL/6 mice (n = 5) were intramuscularly injected in the thigh with 0.5 μg of soluble sHA1, or equivalent doses of BLP–sHA1 and NPN–sHA1 based on sHA1 protein content. Control groups received either PBS or NPN alone at doses matching the NPN content in the NPN–sHA1 formulation. Two weeks later, a booster injection was administered following the same protocol. Serum samples were collected before and after immunization to assess humoral immune responses. All mouse experiments were performed in accordance with the guidelines and authorization of the Pohang University of Science and Technology (POSTECH) Institutional Animal Care and Use Committee (project no. POSTECH-2021-0121).
To measure serum HA1-specific IgG levels in immunized mice, 96-well plates were coated with 50 ng of sHA1–FD–SCpl7 dissolved in a coating buffer (15 mM Na2CO3, 35 mM NaHCO3, and 3 mM NaN3) and incubated overnight at 4°C. Plates were then washed three times with washing buffer (0.1% Tween-20 in PBS) and blocked for 2 h at room temperature with blocking buffer (3% skim milk and 0.1% Tween-20 in PBS). Serial two-fold dilutions of serum samples in blocking buffer were added to the wells and incubated for 2 h at room temperature. After three washes, plates were incubated with HRP-conjugated goat anti-mouse IgG (Southern Biotech, Birmingham, AL) for 2 h at room temperature. Plates were washed again three times, and 100 μL of TMB chromogen solution (Invitrogen, Carlsbad, CA) was added to each well and incubated at room temperature in the dark. The reaction was terminated by adding 100 μL of 0.18 M H2SO4, and absorbance was measured at 450 nm using a Tecan Infinite 200Pro microplate reader. Endpoint titers were defined as the reciprocal of the highest serum dilution yielding an OD value above the cutoff, calculated as the mean OD of the negative control (without serum) plus 3 standard deviations (SDs).
Immunization of BALB/c mice and GFP fluorescence acquisition
Specific pathogen-free six-week-old female BALB/c mice were used for polyclonal antibody production and in vivo GFP retention studies. For polyclonal antibody production, mice (n = 3 per group) were immunized intramuscularly with 1 μg per dose of soluble rGFP, BLP-rGFP, NPN-rGFP, or control formulas (TBS, BLP, and NPN) without any adjuvant. A booster was administered 14 days after initial immunization. Sera were collected at 0 and 28 days post-immunization to detect GFP-specific IgG polyclonal antibodies via western blot. For in vivo GFP retention study, the mice (n = 3 per group) were anesthetized with isoflurane, and their hairless legs were immobilized using a scaffold for imaging. A dose of 5 μg soluble rGFP or NPN–rGFP was injected into the hairless leg, and GFP fluorescence was visualized using a Leica fluorescence microscope (catalog no. Z16 APO A) equipped with a mercury lamp excitation source and a monochrome camera (catalog no. DMC6200). These animal studies were conducted under the approval of the POSTECH Institutional Animal Care and Use Committee (project nos. POSTECH-2022-0005 and POSTECH-2022-0018).
Immunization of rabbit
Six-week-old rabbits were administered three doses of 5, 50, 100, or 200 μg BLP-rGFP at 2-week intervals. Sera collected 2 weeks after the final boost were diluted 1:10 000 for further analysis. All rabbit experiments were conducted in accordance with the guidelines and authorization of the Gyeongsang National University Institutional Animal Care and Use Committee (project no. GNU-240108-B0008).
Statistical analysis
Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparisons test or the unpaired two-tailed Student’s t test using GraphPad Prism software.
Funding
This research was supported by the Science and Technology Foundation of Beijing Life Science Academy (grant no. 2024200CB0110), the Natural Science Foundation of Shandong Province (grant no. ZR2024QC068), and the National Research Foundation of Korea grant funded by the Korean government (grant no. MSIT-2022R1A5A1031361) to W.-Y.K.
Acknowledgments
No conflict of interest declared.
Author contributions
I.H. and S.-J.S. conceived the study and wrote the manuscript. S.-J.S. and H.-P.D. constructed the expression vectors and conducted most experiments with assistance from H.-X.M. and Y.-F.G. S.-S.Z. and C.L. performed cryo-EM under the guidance of M.-J.Y. S.K. conducted the C57BL/6 mouse immunization and ELISA, supervised by K.S.K. S.-J.S., S.K., and J.Y. acquired GFP fluorescence images of BALB/c mice, guided by K.H.K. H.M. and W.-Y.K. performed the rabbit immunization experiment.
Published: June 16, 2025
Footnotes
Supplemental information is available at Plant Communications Online.
Contributor Information
Shi-Jian Song, Email: songshijian@caas.cn.
Inhwan Hwang, Email: ihhwang@postech.ac.kr.
Supplemental information
References
- Andey T., Soni S., Modi S. In: Advanced Vaccination Technologies for Infectious and Chronic Diseases. Chavda V.P., Vora L.K., Apostolopoulos V., editors. Academic Press; 2024. Chapter 3 - Conventional vaccination methods: Inactivated and live attenuated vaccines; pp. 37–50. [Google Scholar]
- Bermúdez-Humarán L.G., Aubry C., Motta J.-P., Deraison C., Steidler L., Vergnolle N., Chatel J.-M., Langella P. Engineering lactococci and lactobacilli for human health. Curr. Opin. Microbiol. 2013;16:278–283. doi: 10.1016/j.mib.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Bi J., Li F., Zhang M., Wang H., Lu J., Zhang Y., Ling H., Wang J., Gao F., Kong W., et al. An HIV-1 vaccine based on bacterium-like particles elicits Env-specific mucosal immune responses. Immunol. Lett. 2020;222:29–39. doi: 10.1016/j.imlet.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Bosma T., Kanninga R., Neef J., Audouy S.A.L., Van Roosmalen M.L., Steen A., Buist G., Kok J., Kuipers O.P., Robillard G., Leenhouts K. Novel Surface Display System for Proteins on Non-Genetically Modified Gram-Positive Bacteria. Appl. Environ. Microbiol. 2006;72:880–889. doi: 10.1128/AEM.72.1.880-889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y.H., Church D., Koellhoffer E.C., Osota E., Shukla S., Rybicki E.P., Pokorski J.K., Steinmetz N.F. Integrating plant molecular farming and materials research for next-generation vaccines. Nat. Rev. Mater. 2022;7:372–388. doi: 10.1038/s41578-021-00399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aoust M., Lavoie P.O., Couture M.M.-J., Trépanier S., Guay J.M., Dargis M., Mongrand S., Landry N., Ward B.J., Vézina L.P. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008;6:930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- De Haan A., Haijema B.J., Voorn P., Meijerhof T., Van Roosmalen M.L., Leenhouts K. Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration. Vaccine. 2012;30:4884–4891. doi: 10.1016/j.vaccine.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Fischer R., Buyel J.F. Molecular farming – The slope of enlightenment. Biotechnol. Adv. 2020;40 doi: 10.1016/j.biotechadv.2020.107519. [DOI] [PubMed] [Google Scholar]
- Gilman M.S.A., Furmanova-Hollenstein P., Pascual G., B van 't Wout A., Langedijk J.P.M., McLellan J.S., McLellan J.S. Transient opening of trimeric prefusion RSV F proteins. Nat. Commun. 2019;10:2105. doi: 10.1038/s41467-019-09807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Kong J., Kong W., Guo T., Ji M. Characterization of a Novel LysM Domain from Lactobacillus fermentum Bacteriophage Endolysin and Its Use as an Anchor To Display Heterologous Proteins on the Surfaces of Lactic Acid Bacteria. Appl. Environ. Microbiol. 2010;76:2410–2418. doi: 10.1128/AEM.01752-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Kong J., Sun Z., Han L., Kong W., Yang P. Heterologous protein display on the cell surface of lactic acid bacteria mediated by the s-layer protein. Microb. Cell Fact. 2011;10:86. doi: 10.1186/1475-2859-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Wang X., Zhang J., Xia N., Zhao Q. Escherichia coli-derived virus-like particles in vaccine development. NPJ Vaccines. 2017;2:3. doi: 10.1038/s41541-017-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J.W., Zahmanova G., Minkov I., Lomonossoff G.P. Plant-based expression and characterization of SARS-CoV-2 virus-like particles presenting a native spike protein. Plant Biotechnol. J. 2022;20:1363–1372. doi: 10.1111/pbi.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraszek J., Rutten L., Blokland S., Bouchier P., Voorzaat R., Ritschel T., Bakkers M.J.G., Renault L.L.R., Langedijk J.P.M. Stabilizing the closed SARS-CoV-2 spike trimer. Nat. Commun. 2021;12:244. doi: 10.1038/s41467-020-20321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole C., Chaurasia A., Hefferon K.L., Panigrahi J., editors. Applications of Plant Molecular Farming. Springer Nature Singapore; 2024. [Google Scholar]
- Kulagina N., Besseau S., Godon C., Goldman G.H., Papon N., Courdavault V. Yeasts as Biopharmaceutical Production Platforms. Front. Fungal Biol. 2021;2 doi: 10.3389/ffunb.2021.733492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Peng X., Jiao H., Zhao D., Ouyang J. Broadly protective immunity against divergent influenza viruses by oral co-administration of Lactococcus lactis expressing nucleoprotein adjuvanted with cholera toxin B subunit in mice. Microb Cell Fact. 2015;14:111. doi: 10.1186/s12934-015-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Peng X., Zhao D., Ouyang J., Jiao H., Shu H., Ge X. Lactococcus lactis displayed neuraminidase confers cross protective immunity against influenza A viruses in mice. Virology. 2015;476:189–195. doi: 10.1016/j.virol.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Lei H., Peng X., Ouyang J., Zhao D., Jiao H., Shu H., Ge X. Intranasal immunization of recombinant Lactococcus lactis induces protection against H5N1 virus in ferrets. Virus Res. 2015;196:56–59. doi: 10.1016/j.virusres.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Lei H., Peng X., Shu H., Zhao D. Intranasal immunization with live recombinant Lactococcus lactis combined with heat-labile toxin B subunit protects chickens from highly pathogenic avian influenza H5N1 virus. J. Med. Virol. 2015;87:39–44. doi: 10.1002/jmv.23983. [DOI] [PubMed] [Google Scholar]
- Li E., Chi H., Huang P., Yan F., Zhang Y., Liu C., Wang Z., Li G., Zhang S., Mo R., et al. A Novel Bacterium-Like Particle Vaccine Displaying the MERS-CoV Receptor-Binding Domain Induces Specific Mucosal and Systemic Immune Responses in Mice. Viruses. 2019;11:799. doi: 10.3390/v11090799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.K., Hiatt A., Hein M., Vine N.D., Wang F., Stabila P., Van Dolleweerd C., Mostov K., Lehner T. Generation and Assembly of Secretory Antibodies in Plants. Science. 1995;268:716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- Mancera Gracia J.C., Pearce D.S., Masic A., Balasch M. Influenza A Virus in Swine: Epidemiology, Challenges and Vaccination Strategies. Front. Vet. Sci. 2020;7:647. doi: 10.3389/fvets.2020.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao R., Wu D., Wang Y. Surface display on lactic acid bacteria without genetic modification: strategies and applications. Appl. Microbiol. Biotechnol. 2016;100:9407–9421. doi: 10.1007/s00253-016-7842-8. [DOI] [PubMed] [Google Scholar]
- McMillan C.L.D., Cheung S.T.M., Modhiran N., Barnes J., Amarilla A.A., Bielefeldt-Ohmann H., Lee L.Y.Y., Guilfoyle K., Van Amerongen G., Stittelaar K., et al. Development of molecular clamp stabilized hemagglutinin vaccines for Influenza A viruses. NPJ Vaccines. 2021;6:135. doi: 10.1038/s41541-021-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni A.H., Taghinezhad S.S., Keyvani H. The First Clinical Use of a Recombinant Lactococcus lactis Expressing Human Papillomavirus Type 16 E7 Oncogene Oral Vaccine: A Phase I Safety and Immunogenicity Trial in Healthy Women Volunteers. Mol. Cancer Therapeut. 2020;19:717–727. doi: 10.1158/1535-7163.MCT-19-0375. [DOI] [PubMed] [Google Scholar]
- Pasquina-Lemonche L., Burns J., Turner R.D., Kumar S., Tank R., Mullin N., Wilson J.S., Chakrabarti B., Bullough P.A., Foster S.J., Hobbs J.K. The architecture of the Gram-positive bacterial cell wall. Nature. 2020;582:294–297. doi: 10.1038/s41586-020-2236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes, D. S. (2011). Lactococcus lactis as a live vector: Heterologous protein production and DNA delivery systems. Protein Expression and Purification Advance Access published 2011. [DOI] [PubMed]
- Ruderfer I., Shulman A., Kizhner T., Azulay Y., Nataf Y., Tekoah Y., Shaaltiel Y. Development and Analytical Characterization of Pegunigalsidase Alfa, a Chemically Cross-Linked Plant Recombinant Human α-Galactosidase-A for Treatment of Fabry Disease. Bioconjug. Chem. 2018;29:1630–1639. doi: 10.1021/acs.bioconjchem.8b00133. [DOI] [PubMed] [Google Scholar]
- Saluja V., Amorij J.P., Van Roosmalen M.L., Leenhouts K., Huckriede A., Hinrichs W.L.J., Frijlink H.W. Intranasal Delivery of Influenza Subunit Vaccine Formulated with GEM Particles as an Adjuvant. AAPS J. 2010;12:109–116. doi: 10.1208/s12248-009-9168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saluja V., Visser M.R., Van Roosmalen M.L., Leenhouts K., Huckriede A., Hinrichs W.L.J., Frijlink H.W. Gastro-intestinal delivery of influenza subunit vaccine formulation adjuvanted with Gram-positive enhancer matrix (GEM) particles. Eur. J. Pharm. Biopharm. 2010;76:470–474. doi: 10.1016/j.ejpb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Saluja V., Visser M.R., Ter Veer W., Van Roosmalen M.L., Leenhouts K., Hinrichs W.L.J., Huckriede A., Frijlink H.W. Influenza antigen-sparing by immune stimulation with Gram-positive enhancer matrix (GEM) particles. Vaccine. 2010;28:7963–7969. doi: 10.1016/j.vaccine.2010.09.066. [DOI] [PubMed] [Google Scholar]
- Shaaltiel Y., Bartfeld D., Hashmueli S., Baum G., Brill-Almon E., Galili G., Dym O., Boldin-Adamsky S.A., Silman I., Sussman J.L., et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol. J. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Song S.J., Shin G.I., Noh J., Lee J., Kim D.H., Ryu G., Ahn G., Jeon H., Diao H.P., Park Y., et al. Plant-based, adjuvant-free, potent multivalent vaccines for avian influenza virus via Lactococcus surface display. JIPB. 2021;63:1505–1520. doi: 10.1111/jipb.13141. [DOI] [PubMed] [Google Scholar]
- Song S.J., Kim H., Jang E.Y., Jeon H., Diao H.P., Khan M.R.I., Lee M.S., Lee Y.J., Nam J.H., Kim S.R., et al. SARS-CoV -2 spike trimer vaccine expressed in Nicotiana benthamiana adjuvanted with Alum elicits protective immune responses in mice. Plant Biotechnol. J. 2022;20:2298–2312. doi: 10.1111/pbi.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.-J., Diao H.-P., Guo Y.-F., Hwang I. Advances in Subcellular Accumulation Design for Recombinant Protein Production in Tobacco. Biodes. Res. 2024;6 doi: 10.34133/bdr.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekoah Y., Shulman A., Kizhner T., Ruderfer I., Fux L., Nataf Y., Bartfeld D., Ariel T., Gingis–Velitski S., Hanania U., Shaaltiel Y. Large-scale production of pharmaceutical proteins in plant cell culture—the protalix experience. Plant Biotechnol. J. 2015;13:1199–1208. doi: 10.1111/pbi.12428. [DOI] [PubMed] [Google Scholar]
- Tian J.-H., Patel N., Haupt R., Zhou H., Weston S., Hammond H., Logue J., Portnoff A.D., Norton J., Guebre-Xabier M. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021;12:372. doi: 10.1038/s41467-020-20653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Braeckel-Budimir N., Haijema B.J., Leenhouts K. Bacterium-Like Particles for Efficient Immune Stimulation of Existing Vaccines and New Subunit Vaccines in Mucosal Applications. Front. Immunol. 2013;4:282. doi: 10.3389/fimmu.2013.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas J.L., Haijema B.J., Leenhouts K., Paul Zoeteweij J., Burggraaf J., Kamerling I.M.C. afety, reactogenicity and immunogenicity of an intranasal seasonal influenza vaccine adjuvanted with gram-positive matrix (GEM) particles (FluGEM): A randomized, double-blind, controlled, ascending dose study in healthy adults and elderly. Vaccine. 2024;42:125836. doi: 10.1016/j.vaccine.2024.03.063. [DOI] [PubMed] [Google Scholar]
- Wang M., Gao Z., Zhang Y., Pan L. Lactic acid bacteria as mucosal delivery vehicles: a realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016;100:5691–5701. doi: 10.1007/s00253-016-7557-x. [DOI] [PubMed] [Google Scholar]
- Ward B.J., Gobeil P., Séguin A., Atkins J., Boulay I., Charbonneau P.-Y., Couture M., D’Aoust M.-A., Dhaliwall J., Finkle C., et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021;27:1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A.J., Underhill D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018;18:243–254. doi: 10.1038/nri.2017.136. [DOI] [PubMed] [Google Scholar]
- Xu K., Gao P., Liu S., Lu S., Lei W., Zheng T., Liu X., Xie Y., Zhao Z., Guo S., et al. Protective prototype-Beta and Delta-Omicron chimeric RBD-dimer vaccines against SARS-CoV-2. Cell. 2022;185:2265–2278.e14. doi: 10.1016/j.cell.2022.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadravec P., Štrukelj B., Berlec A. Heterologous surface display on lactic acid bacteria: non-GMO alternative? Bioengineered. 2015;6:179–183. doi: 10.1080/21655979.2015.1040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Yan F., Liu D., Li E., Feng N., Xu S., Wang H., Gao Y., Yang S., Zhao Y., Xia X. Bacterium-Like Particles Displaying the Rift Valley Fever Virus Gn Head Protein Induces Efficacious Immune Responses in Immunized Mice. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.799942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T., Cai Y., Jiang Y., He X., Wei Y., Yu Y., Tian X. Vaccine adjuvants: mechanisms and platforms. Sig Transduct Target Ther. 2023;8:283. doi: 10.1038/s41392-023-01557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Gao M., De X., Sun T., Bai Z., Luo J., Wang F., Ge J. Bacterium-like particles derived from probiotics: progress, challenges and prospects. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1263586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.