Abstract
Introduction
Atherosclerosis cardiovascular disease (ASCVD), especially coronary artery disease (CAD), remains the leading cause of death worldwide, with several well-identified risk factors. This case report presents a premenopausal female with low calculated ASCVD risk, hypertension, elevated lipoprotein(a) [Lp(a)], and clinically significant CAD.
Case report
A 44-year-old premenopausal White female with controlled stage 2 hypertension, and overall low calculated 10-year ASCVD risk, was found to have severe CAD. She presented to the clinic with worsening chest discomfort during exertion and was diagnosed with a heavily calcified proximal left anterior descending artery stenosis, necessitating percutaneous coronary intervention.
Discussion
The global prevalence of elevated Lp(a) >50 mg/dL is around 1.43 billion. Elevated lipoprotein(a) is now recognized, based on the preponderance of the evidence, by several international scientific statements as an independent risk factor for ASCVD, including CAD. Nevertheless, the current 2018 American College of Cardiology (ACC) and American Heart Association (AHA) multi-society guideline on the Management of Blood Cholesterol only classifies Lp(a) as a risk enhancer. This recommendation, along with the lack of approved pharmacotherapy has contributed to limited testing in current United States clinical practice (<1 % for the general population). Furthermore, the inadequate assessment of Lp(a) may lead to an underestimation of ASCVD risk.
Conclusion
This case highlights the shortcomings of inadequate assessment of Lp(a) leading to the underestimation of cardiovascular risk. Accordingly, with multiple recent international scientific statements, clinicians should universally screen for elevated Lp(a). In the future, investigational therapies for lowering Lp(a) may be crucial for improving patient outcomes.
Highlights
-
•
Elevated lipoprotein(a) is an independent risk factor for atherosclerotic cardiovascular disease.
-
•
Premenopausal women, as well as men, may be at increased cardiovascular risk from elevated lipoprotein(a).
-
•
Risk calculators may underestimate disease in patients with elevated lipoprotein(a).
-
•
Clinicians should screen for elevated lipoprotein(a) for early identification of coronary artery disease.
1. Case report
A 44-year-old premenopausal White female was referred to the cardiology clinic for hypertension management and cardiovascular risk assessment. She had a past medical history of isolated episodes of supraventricular tachycardia, treated with intermittent beta-blocker pharmacotherapy, Raynaud's phenomenon, situational anxiety, and mild positional sleep apnea (apnea-hypopnea index 5.6, normal <5 events/h). The patient had four pregnancies, including one miscarriage and three full-term deliveries via Cesarean section. Each pregnancy was complicated by hypertension, without eclampsia, and 6–8 weeks of diuretic therapy after delivery. She had no history of gestational diabetes and reported intermittent use of progesterone and estrogen oral contraceptives, as well as etonogestrel/ethinyl estradiol vaginal ring for over 10 years. Her body mass index (BMI) was 20 kg/m2 and Hemoglobin A1C (HbA1C) was 5.5 %. Her family history included maternal premature coronary artery disease (CAD) with severe hypercholesterolemia and coronary artery bypass grafting at age 62. Her father's history included hypertension. The patient consumed a glass of wine nightly, but had no tobacco or illicit drug use. Her physical activity routine comprised of running approximately 30 min two to four times per week.
At the initial visit, the patient's medications included metoprolol tartrate 25 mg as needed for palpitations and citalopram 10 mg daily for anxiety. To better define the extent and severity of her blood pressure burden, an ambulatory blood pressure monitor was completed and confirmed a diagnosis of hypertension stage 2. A total of 42 measurements were obtained. The overall average was 148/104 mm Hg (98 % measurement success). Daytime average was 148/105 (97 % measurement success). The nighttime average was 147/102 mm Hg (100 % measurement success). The patient started amlodipine 5 mg daily and subsequently achieved blood pressure control with a mean office blood pressure of 120/79 mm Hg.
To examine the extent of target organ damage, transthoracic echocardiography was completed. The study demonstrated an ejection fraction of 60 %–65 %, no regional wall motion abnormalities, no diastolic dysfunction, or valvular stenosis or insufficiency. Laboratory results prior to lipid-modifying medication revealed a total cholesterol of 180 mg/dL (<200 mg/dL), triglycerides 54 mg/dL (<150 mg/dL), HDL-C 71 mg/dL (>50 mg/dL), and LDL-C of 108 mg/dL (<100 mg/dL). Additional findings included TSH 1.99 uIU/mL (0.358–3.74 uIU/mL), and glomerular filtration rate (GFR) 96 mL/min (>60 mL/min). The patient's 10-year atherosclerosis cardiovascular disease (ASCVD) risk was calculated by two methods: the Pooled Cohort Risk Assessment Equations (PCE) and the Framingham Risk Score (FRS). This patient's pooled cohort 10-year ASCVD risk was 0.5 % prior to starting blood pressure medication. The patient's 10-year ASCVD FRS was 0.3 %. Therefore, the patient was encouraged to pursue lifestyle modifications including a low saturated fat and low cholesterol diet in consideration of her mildly elevated LDL-C.
Considering the patient's family history of premature CAD and the emerging evidence of the independent ASCVD risk associated with lipoprotein(a) [Lp(a)], additional lipid testing was ordered. The patient's Lp(a) was 219 nmol/L, indicating a high risk for ASCVD, as Lp(a) level >125 nmol/L (50 mg/dL) is considered high risk. In association, Lp(a) concentration is considered intermediate risk if between 75 and 125 nmol/L (30–50 mg/dL), and low risk if less than <75 nmol/L (30 mg/dL) [1]. Rosuvastatin 10 mg was initiated subsequently based on the patient's history and Lp(a) results.
Considering that high Lp(a) levels are >90 % genetically determined [2], a comprehensive genetic analysis was completed. The analysis revealed heterozygosity for the rs3798220 (p.Ile1891Met) variant in the LPA gene. An additional finding in her genetic profile was heterozygosity for the p.Cys1359Arg (rs77542162) variant in the ABCA6 gene, which is associated with hypercholesterolemia, higher total LDL-C, and apoB-100. As part of shared decision-making, the patient was informed of the benefits of intense preventive ASCVD strategies and Lp(a) screening in relatives.
Four months after the initial evaluation, the patient presented to the clinic with complaints of intermittent postprandial epigastric pain ongoing for three weeks. The resting electrocardiogram (ECG) showed normal sinus rhythm with T wave inversion in V1, V2, and V3. Coronary computed tomographic angiography reported a total calcium score of 318 in the left descending artery (LAD) 187, zero in the left circumflex artery (LCx), and 131 in the right coronary artery (RCA). Stenosis of the proximal LAD and the RCA were estimated to be 60 % and 50 % respectively. After a discussion with the patient, it was concluded no invasive coronary angiography was needed at that time. To more intensively lower the patient's LDL-C, rosuvastatin was increased to 20 mg, resulting in an LDL-C of 62 mg/dL. The patient could not continue a trial of ezetimibe 10 mg due to gastrointestinal side effects.
Seven months later while running, the patient experienced a sudden feeling of “being punched in the chest” that radiated to the left shoulder and scapula. The pain was rated a 3/10 and resolved after 1 min of rest. She subsequently noted prolonged episodes of fatigue with minimal activity. Two days later she presented to a local emergency department with sharp, shooting, intermittent chest pain, rated 3/10. Despite having no new ECG changes and no elevated troponin, coronary angiography was performed to assess for significant CAD given the results of the CT angiogram and concern for unstable angina. For this, the right radial artery was used for access, and a Judkins Right 4.0 and a Judkins Left 3.5 catheter were used to perform right and left coronary artery angiograms, respectively. This revealed a focal 95 % stenosis of the proximal LAD. A percutaneous coronary intervention of the proximal LAD was attempted using an extra-backup (EBU) 3.5 guide catheter. A Balanced Middleweight (BMW) guidewire was utilized to cross the LAD stenosis. The PCI was guided by intravascular ultrasound (IVUS) imaging to assess the lesion and guide stenting. The IVUS revealed plaque containing both soft and calcified areas. Initial pre-dilation of the stenosis via balloon angioplasty was suboptimal.
To modify the plaque and obtain as much luminal area as possible for this relatively young female patient, a staged procedure was performed, via right femoral artery access using an EBU 3.75 guide catheter. This PCI was assisted by atherectomy using a 1.5 mm burr (Boston Scientific ROTAPRO Rotational Atherectomy System), followed by further pre-dilation with balloon angioplasty. Finally, a drug-eluting stent was placed with minimal residual stenosis and Thrombolysis in Myocardial Infarction (TIMI) 3 flow post-PCI.
In consideration of the patient's relatively young age, positive family history, elevated Lp(a), rapid progression of CAD, no Lp(a) lowering benefit with rosuvastatin [3], and ezetimibe intolerance, the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab 140 mg every 2 weeks was started. Despite her relatively young age, the calcified plaque burden was complex and likely present for an extended period. Her CAD may have been impacted by elevated Lp(a) combined with hypertension and a strong family history of premature coronary atherosclerosis.
2. Overview of Lp(a)
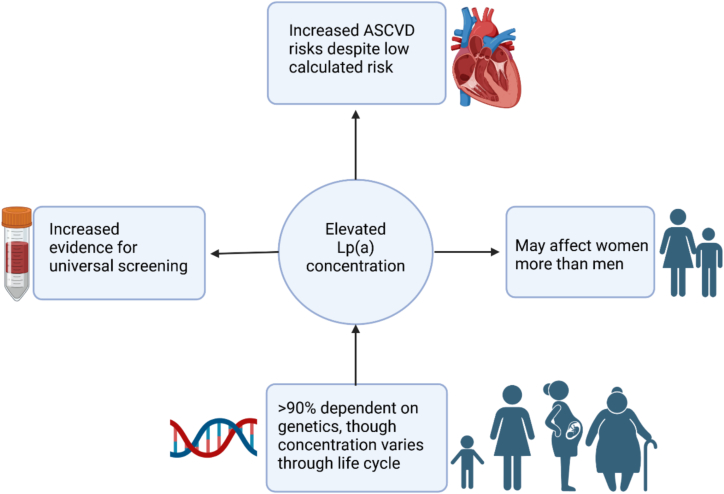
Cardiovascular disease (CVD) is the leading cause of mortality worldwide, accounting for 19.91 million deaths in 2021 [4]. Accordingly, comprehensive assessment must be performed early in the disease process to identify high-risk individuals and enable access to timely treatments. Optimal management includes lifestyle modifications, evidence-based pharmacotherapy, and device-based interventions. Traditional ASCVD risk factors include advanced age, tobacco use, hypertension, obesity, diabetes, dyslipidemia, and historically, race [5]. Nevertheless, elevated Lp(a) is now identified as an independent and causal risk factor for ASCVD, including CVD [6,7]. Notably, Lp(a) is linked to increased CVD risk in premenopausal women which may modify future risk stratification [8]. This case report exemplifies the potential benefit of universal Lp(a) screening as a means of identifying individuals at risk to facilitate earlier, more intense interventions for CVD prevention.
Structurally, Lp(a) is composed of apolipoprotein(a) [apo(a)] bound to a single molecule of apolipoprotein B-100 (apoB-100) through non-covalent interactions and a disulfide bridge [3]. Lp(a) is structurally similar to plasminogen, having evolved from the plasminogen gene and sharing 94 % of its amino acids [9]. Hence, Lp(a) may interfere with tissue plasminogen activator, potentially reducing fibrinolytic activity and increasing thrombotic risk [10]. The ability of Lp(a) to inhibit the fibrinolytic activity of plasmin, contributing to thrombotic risk, has not been proven to be of clinical significance [2]. Although the Lp(a) synthesis pathway is incompletely understood, it is known to be independent of LDL-C synthesis, and not a product of the catabolism of other lipoproteins [11]. Moreover, standard total cholesterol screening does not account for Lp(a), a distinct subtype of LDL inadequately reflected in conventional LDL-C estimates [12]. Additionally, apo(a) is synthesized in hepatocytes, and the binding apoB-100 appears on the hepatocyte surface. Similarly, the catabolism of Lp(a) is not well established, although renal activity may potentially play a role [13].
In some published reports, Lp(a) is expressed in mass units (mg/dL), reflecting the total mass of the entire particle, including apo(a), apoB-100, cholesterol, cholesteryl esters, phospholipids, triglycerides, and carbohydrates [14]. Conversely, Lp(a) measured by immunoassays quantifies only the protein component, excluding lipid and carbohydrate content. In this case review, the units are presented as originally published, without conversion between mg/dL and nmol/L concentrations, which is often discouraged. Further standardization may improve reliability and repeatability, with nmol/L as the optimal unit of measurement and using apo(a) isoforms-insensitive assays traceable to International Federation of Clinical Chemistry reference material [15]. The 2022 European Atherosclerosis Society (EAS) consensus offers valuable guidelines on the superiority of molar measurements, apo(a) isoform-insensitive lab methods, mass-to-molar conversion, and the pathogenic threshold of Lp(a) in ASCVD [2].
It is well-documented that Lp(a) is highly genetically determined, with studies showing >90 % of Lp(a) variance controlled by the LPA gene [2]. Plasma Lp(a) levels arise from the codominant expression of two LPA alleles, resulting in considerable variation among individuals and across population groups. People of African and South Asian descent have higher Lp(a) levels compared to those of European and East/Southeast Asian origin [16,17]. The United Kingdom (UK) biobank analysis by Patel et al., the largest study to date on Lp(a) and ASCVD risk (N = 460,506), reported median Lp(a) levels of 75, 31, 19, and 16 nmol/L for Black, South Asian, White, and Chinese populations respectively [6]. Although the median Lp(a) levels were approximately three times higher in Black individuals compared to White and Asian individuals, the association of Lp(a) levels with increased cardiovascular risk appears similar across ethnicities [18,19]. Based on self-identification, ancestral genetic testing, and family history, this patient was classified as non-Hispanic white. Some individuals exhibit markedly elevated Lp(a) levels despite lacking known LPA single-nucleotide polymorphisms, indicating the presence of small apo(a) isoforms [20,21]. While the clinical utility of genetic testing continues to evolve, Lp(a) genetic insights are valuable in understanding the causal link between Lp(a) and CVD. Since Lp(a) levels remain relatively stable throughout life, a one-time measurement may be cost-effective for CVD risk assessment [22,23]. Since the cost of genetic testing may pose a barrier for some patients [15], measuring plasma Lp(a) levels alone may be sufficient for cardiovascular risk assessment [20].
Additionally, certain diseases affect Lp(a) levels. Kidney disease has a pronounced impact on Lp(a) concentrations [24]. As the glomerular filtration rate (GFR) declines and kidney function becomes progressively impaired, Lp(a) concentrations rise in patients with large apo(a) isoforms. In cases of nephrotic syndrome, plasma Lp(a) concentrations become 3–5 times those seen in control groups, independent of apo(a) isoform size [2]. Interestingly, even in those without primary renal disease, there is an inverse correlation between renal function and Lp(a) levels of large apo(a) isoform carriers. Similarly, dialysis treatment is associated with elevated Lp(a) levels in patients with large isoforms [2]. In contrast, kidney transplantation has been shown to normalize Lp(a) levels associated with kidney disease. Furthermore, hyperthyroidism and hypothyroidism are associated with decreased and increased levels of Lp(a), respectively [25]. Treatment for overt hyperthyroidism and hypothyroidism is shown to return Lp(a) to baseline.
Although lifestyle interventions have not been shown to significantly lower Lp(a) levels [2,26], a recent study suggests a low-carbohydrate diet may offer some benefit [27]. Additionally, findings from the Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) support evidence for a reduction in major adverse cardiovascular events across various Lp(a) levels [28].
3. Lp(a) levels and cardiovascular risk
Understanding the pathophysiology of how Lp(a) contributes to CVD is crucial for developing effective treatments and interventions. Nevertheless, much progress remains to be made. For instance, Lp(a) contains not only the proatherogenic property of LDL but also apo(a), with many hypothesizing that Lp(a) serves as a link between atherosclerosis and atherothrombosis [29]. One possible mechanism is attributed to Lp(a)’s pro-inflammatory effects. Lp(a) particles carry oxidized phospholipids (oxPLs), which trigger monocyte activation, upregulate endothelial cell adhesion, and promote cytokine release [7,29,30]. Additionally, Schnitzler et al. demonstrated endothelial cells exposed to Lp(a) exhibited higher glycolytic activity through the 6-phosphofructo-2-kinase/fructose-2,6 biphosphatase 3 (PFKFB3) pathway. Inhibition of PFKFB3 reverses the inflammatory response [30].
A high level of Lp(a) is associated with an increased risk of CVD, including CAD, ischemic stroke, aortic stenosis (AS), peripheral arterial disease, heart failure, and overall decreased longevity [2,10,31,32]. Despite notable differences in Lp(a) concentration distribution among racial groups, the UK Biobank analysis estimated comparable hazard ratios per every 50 nmol/L increase: 1.11 for White, 1.10 for South Asian, and 1.07 for the Black population [6]. At Lp(a) level exceeding 70 mg/dL, the hazard ratio reaches 1.5 denoting a 50 % increased risk of an ASCVD event. Interestingly, high Lp(a) poses a higher relative risk for individuals without pre-existing ASCVD compared to those with it (HR 1.1 for those without ASCVD and 1.04 for those with ASCVD) [6], suggesting a strong case for Lp(a) screening in those without prior ASCVD events. Participants of the Multi-Ethnic Study of Atherosclerosis (MESA) with elevated Lp(a) ≥ 50 mg/dL were observed to have an increased risk of CAD, both independent of and in conjunction with increased LDL-C [33]. A recently published meta-analysis of six randomized controlled statin trials, with 27,658 total participants, demonstrated a log-linearly association with ASCVD risk across all LDL-C levels [34]. Furthermore, Lp(a) >50 mg/dL posed an independent increase in ASCVD risk regardless of LDL-C levels or reductions. In a further analysis with 49,699 participants from the Copenhagen General Population Study conducted by Langsted et al., each 50 mg/dL increase in Lp(a) corresponded to an age and sex-adjusted hazard ratio of 1.2 for ischemic stroke [35].
Moreover, elevated Lp(a) has been shown to promote the development of aortic valve calcification [36,37], leading to calcific aortic valve stenosis independent of CAD [38]. The prospective Rotterdam Study found that Lp(a) is strongly associated with both baseline and new-onset of aortic valve calcium but not with progression in patients with pre-existing aortic valve calcium [39].
4. Impact of Lp(a) on women's cardiovascular health
There are unique aspects of the predictive power of Lp(a) as an independent ASCVD risk factor in women (Fig. 1). For example, a recent study including 1858 patients by Yurtseven et al. demonstrated a need for a lower Lp(a) threshold in women compared to men [8]. Specifically, an Lp(a) level ≥ 50 mg/dL increases CAD risk in both sexes, whereas an Lp(a) level ≥ 30 mg/dL is only predictive in women. Furthermore, Lp(a) concentration is slightly higher in women aged 50 years and older than in men of the same age group [6,8,40], further underscoring the impact of Lp(a). Interestingly, in a study with 28,024 participants, Lp(a) was observed to be significantly elevated in women with premature coronary heart disease who were younger than 65 but not in those 65 and older [41]. A recent paper indicated that Lp(a) concentrations are low at birth and reach a stable level within the first 2 years of life, and may increase during pregnancy and menopause [42].
Fig. 1.
Impact of elevated Lp(a) on cardiovascular disease risk in Women.
Despite a suggestion that there may be a greater impact of elevated Lp(a) in women and even sex-specific thresholds for interpretation of Lp(a) levels, a recently published meta-analysis (N = 27,658) from 6 placebo-controlled statin trials found no significant interaction between Lp(a) and sex for ASCVD risk (P = 0.310), with similar risk associated outcomes in men and women [34]. Moreover, in consideration of the patient's relatively young age of 44, the relationship was similar to Lp(a) and LDL-C among those younger than or equal to the median age of 63 years (P = 0.710). This particular case focuses on a premenopausal female, similar to what most adult cardiologists may see in clinic practice.
Menopausal status also uniquely impacts the effect of Lp(a) on women's CVD risk. For instance, a meta-analysis of 17 studies involving 4686 premenopausal and 8274 postmenopausal women, reveals Lp(a) concentrations were higher in postmenopausal compared to premenopausal women [43]. On the other hand, hormone replacement therapy with estrogen is shown to decrease Lp(a) level, even at low doses [44]. However, aging cannot be ruled out as the main contributor to the increase [43]. Further research needs to be done to determine the underlying mechanism of Lp(a) increase in postmenopausal women.
5. Current recommendations and treatments
Currently, there is no definitive cut-off for Lp(a). In the National Lipid Association (NLA)’s consensus statement, high risk is considered at ≥ 125 nmol/L (≥ 50 mg/dL) [1]. The National Heart, Lung, and Blood Institute (NHLBI) Working Group considers the traditional threshold for elevated Lp(a) to be > 30 mg/dL, which is the 75th percentile of the White population [45]. Despite the global prevalence of elevated Lp(a) > 50 mg/dL being around 1.43 billion, Lp(a) testing rate in current clinical practice is low even in those with diagnosed or family history of CVD [46]. Among the 5,553,654 adults evaluated by the University of California health system, the rate of Lp(a) testing was <1 % [47].
Considering the increasing awareness of Lp(a) as an independent ASCVD risk factor and recommendations including scientific statements from Europe, Canada, and the US National Lipid Association, the use of Lp(a) testing may expand in the near future. However, a 2025 published analysis of 5.3 million veterans revealed that < 1 % had undergone Lp(a) testing [48]. The 2018 multi-society guideline published by the American College of Cardiology (ACC) and American Heart Association (AHA) designated Lp(a) ≥ 50 mg/dL or ≥ 125 nmol/L as a risk-enhancing factor [5]. However, more recent guidelines and consensus statements provide a more updated view of current evidence. According to the consensus statement published by EAS, measuring Lp(a) is recommended in individuals with a high risk for CVD or those with a significant family history of premature CVD [2]. The EAS considers Lp(a) level below 30 mg/dL normal and a level exceeding 50 mg/dL (80th percentile) high risk for CVD. They also suggest the interim level between 30 and 50 mg/dL should be considered in conjunction with other traditional risk factors. Additionally, the Canadian Cardiovascular Society Guidelines recommend once-in-a-lifetime Lp(a) screening to assess CVD risk [22]. Regarding Lp(a) testing in children, two longitudinal studies demonstrated that youths (9–24 years) exposed to Lp(a) levels ≥ 30 mg/dL were twice at greater risk of developing ASCVD in adulthood [49].
Importantly, this case report showcases the gap between traditional risk calculators and actual CVD risk. Both risk calculators do not account for Lp(a) levels and would not assist our care team with predicting this patient's risk. A useful approach to risk calculation may include Lp(a) and imaging modality. Reyes et al. suggest a modified 10-year risk estimate accounting for elevated Lp(a) level using the following formula: Modified 10-year risk = Traditionally Predicted 10-year risk×[] [7]. Additionally, a recent multimodality writing group of major cardiovascular organizations suggests coronary artery calcium (CAC) scoring would be an appropriate imaging modality within certain clinical scenarios where risk-enhancing factors, including high Lp(a), are present [50]. In a European population with relatively low cardiovascular risk, imaging modalities such as CAC scoring and computed tomography (CT) angiography appear to enhance diagnostic accuracy and can reclassify the 10-year risk of first-onset CVD [51].
The current best approach for reducing CVD risk in patients with high Lp(a) is to modify traditional risk factors as well as target LDL-C/apoB. When considering antiplatelet therapies like aspirin following the result of CAC scoring and CT, it is essential to balance the potential benefits with associated risks. Data from MESA suggest aspirin use may reduce the risk of CAD events by 46 % in individuals with Lp(a) > 50 mg/dL [52]. However, it was also associated with major bleeding complications.
Currently, there is no Food and Drug Administration (FDA) approved medication for Lp(a) elevation specifically. The only current treatment available is lipoprotein apheresis, which is FDA-approved in the United States for individuals with familial hypercholesterolemia who have LDL-C ≥ 100 mg/dL, Lp(a) levels ≥ 60 mg/dL, and coronary or other artery disease [53]. After treatment, a 50–80 % reduction in Lp(a) is observed. More data are needed on the effectiveness of Lp(a) apheresis in reducing the incidence of CVD. Another potential approach is LDL-C reduction with PCSK9 inhibitors (evolocumab and alirocumab), which induce a 20–25 % reduction in Lp(a) [53,54]. Additionally, studies on the effect of statin therapy on Lp(a) levels have indicated mixed results, with older trials suggesting minor decreases and recent meta-analyses indicating slight increases or no clinically significant changes [54].
6. Future directions
To address the CVD risk posed by elevated Lp(a), research on developing and evaluating strategies for lowering Lp(a) levels is being conducted. Several promising therapies are being explored, including pelacarsen, a single-stranded antisense oligonucleotide that inhibits mRNA production from the Lp(a) gene [17]. Additionally, small interfering RNAs (siRNA) such as olpasiran, zerlasiran, and lepodisiran inhibit mRNA production, while muvalaplin acts to prevent Lp(a) formation. Pelacarsen, olpasiran, zerlasiran, and lepodisiran are administered by subcutaneous injection while muvalaplin is taken orally.
Recent phase 2 trials had demonstrated the efficacy of these therapies. Pelacarsen had shown up to an 80 % reduction in Lp(a) levels [55]. In a phase 2 trial involving 281 patients with ASCVD and elevated Lp(a) (above 150 nmol/L), olpasiran placebo-adjusted Lp(a) levels were significantly reduced by 101.1 % and 100.5 % at a 225 mg dose administered every 12 and 24 weeks, respectively (P < 0.0001 for both comparisons with baseline) [56]. Similarly, in another phase 2 trial, lepodisiran use resulted in a 94 % reduction in Lp(a) levels at the highest dosage [57].
Muvalaplin also showed promising data in a new report from a global phase 2, placebo-controlled, randomized, double-blind trial (N = 233), with Lp(a) concentrations ≥ 175 nmol/L with ASCVD, diabetes, or familial hypercholesterolemia [58]. In this cohort, muvalaplin resulted in placebo-adjusted reductions in lipoprotein(a) as much as 85.8 % (95 % CI, 83.1 %–88.0 %) with the highest dosage. Most recently, a phase 2 trial evaluating zerlasiran in 178 patients with stable ASCVD and Lp(a) concentrations ≥ 125 nmol/L demonstrated zerlasiran was well-tolerated and achieved reduced time-averaged Lp(a) levels by > 80 % over 36 weeks [59].
Based on these findings, several phase 3 trials are in progress. Pelacarsen is being evaluated in the HORIZON trial (NCT04023552), which includes 8323 participants and is expected to be completed in 2026 [17]. Additional phase 3 trials include olpasiran in OCEAN(a) (NCT05581303), and lepodisiran (NCT06292013) in ACCLAIM-Lp(a) [17].
7. Conclusion
This case report demonstrates the potentially profound impact of Lp(a) on premature and often severe CAD, despite conventionally low calculated ASCVD risk. Although a potent cardiovascular risk factor, Lp(a) is being tested in < 1 % of the general population. Clinicians should embrace universal screening for Lp(a), particularly in premenopausal women, whose ASCVD risk burden is often underestimated by conventional risk calculators. While there is currently no approved treatment specifically targeting Lp(a), identifying high-risk individuals leads to early interventions that can reduce their risk of complications associated with CAD through conventional means. This approach ensures proper diagnoses, timely treatments and potentially saves lives. In addition, this case supports further studies to understand the cardiovascular risk posed by Lp(a) and the inclusion of women and diverse populations in clinical trials.
CRediT authorship contribution statement
Nguyen Yen Nhi Ngo: Writing – review & editing, Writing – original draft, Visualization. Chloé Davidson Villavaso: Writing – review & editing, Writing – original draft. Chisom Joan Orakwue: Writing – review & editing, Writing – original draft. William Zachary Rowalt: Writing – review & editing, Writing – original draft. Madhur Roberts: Writing – review & editing, Writing – original draft. Keith C. Ferdinand: Writing – review & editing, Conceptualization.
Ethics statement
We confirm that the following are true:
-
•
The work described has not been published previously except in the form of a preprint, an abstract, a published lecture, academic thesis or registered report.
-
•
The article is not under consideration for publication elsewhere.
-
•
The article's publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out.
-
•
If accepted, the article will not be published elsewhere in the same form, in English or in any other language, including electronically, without the written consent of the copyright-holder.
Declaration of competing interest
Dr. Ferdinand is the steering committee chair for A Randomized Double-blind, Placebo-controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability of Pelacarsen (TQJ230) in US Black/African American & Hispanic Patient Populations With Elevated Lp(a) and Established Atherosclerotic Cardiovascular Disease NCT06267560
Dr. Ferdinand is an investigator for Lp(a) HORIZON is a pivotal, global multicenter, double-blind, placebo-controlled pivotal Phase 3 study conducted by Novartis NCT04023552
Chloé Davidson Villavaso is an investigator for Novartis.
References
- 1.Koschinsky M.L., et al. A focused update to the 2019 NLA scientific statement on use of lipoprotein(a) in clinical practice. J. Clin. Lipidol. 2024;18(3):e308–e319. doi: 10.1016/j.jacl.2024.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F., et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur. Heart J. 2022;43(39):3925–3946. doi: 10.1093/eurheartj/ehac361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lian P., et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and proprotein convertase subtilisin/kexin-type 9 inhibitors. Clin. Chim. Acta. 2025;565 doi: 10.1016/j.cca.2024.119982. [DOI] [PubMed] [Google Scholar]
- 4.Martin S.S., et al. 2024 Heart Disease and Stroke Statistics: a report of US and global data from the American Heart Association. Circulation. 2024;149(8):e347–e913. doi: 10.1161/CIR.0000000000001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy S.M., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel A.P., et al. Lipoprotein(a) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large national biobank. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):465–474. doi: 10.1161/ATVBAHA.120.315291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reyes-Soffer G., et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2022;42(1):e48–e60. doi: 10.1161/ATV.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yurtseven E., et al. Is there a need for sex-tailored lipoprotein(a) cut-off values for coronary artery disease risk stratification? Clin. Cardiol. 2024;47(9) doi: 10.1002/clc.70012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J. Am. Coll. Cardiol. 2017;69(6):692–711. doi: 10.1016/j.jacc.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Tasdighi E., Adhikari R., Almaadawy O., Leucker T.M., Blaha M.J. LP(a): structure, genetics, associated cardiovascular risk, and emerging therapeutics. Annu. Rev. Pharmacol. Toxicol. 2024;64(1):135–157. doi: 10.1146/annurev-pharmtox-031023-100609. [DOI] [PubMed] [Google Scholar]
- 11.Krempler F., Kostner G., Bolzano K., Sandhofer F. Lipoprotein (a) is not a metabolic product of other lipoproteins containing apolipoprotein B. Biochim. Biophys. Acta. 1979;575(1):63–70. doi: 10.1016/0005-2760(79)90131-0. [DOI] [PubMed] [Google Scholar]
- 12.Feingold K.R. In: Endotext. Feingold K.R., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., de Herder W.W., Dhatariya K., Dungan K., Hofland J., Kalra S., Kaltsas G., Kapoor N., Koch C., Kopp P., Korbonits M., Kovacs C.S., Kuohung W., Laferrère B., Levy M., McGee E.A., McLachlan R., New M., Purnell J., Sahay R., Shah A.S., Singer F., Sperling M.A., Stratakis C.A., Trence D.L., Wilson D.P., editors. MDText.com, Inc.; South Dartmouth (MA): 2000. Utility of advanced lipoprotein testing in clinical practice.http://www.ncbi.nlm.nih.gov/books/NBK355893/ Accessed: Mar. 03, 2025. [Online]. Available: [Google Scholar]
- 13.Hopewell J.C., Haynes R., Baigent C. The role of lipoprotein (a) in chronic kidney disease. J. Lipid Res. 2018;59(4):577–585. doi: 10.1194/jlr.R083626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cegla J., France M., Marcovina S.M., Neely R.D.G. Lp(a): when and how to measure it. Ann. Clin. Biochem. 2021;58(1):16–21. doi: 10.1177/0004563220968473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes-Soffer G., Yeang C., Michos E.D., Boatwright W., Ballantyne C.M. High lipoprotein(a): actionable strategies for risk assessment and mitigation. Am. J. Prevent. Cardiol. 2024;18 doi: 10.1016/j.ajpc.2024.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enkhmaa B., Anuurad E., Berglund L. Lipoprotein (a): impact by ethnicity and environmental and medical conditions. J. Lipid Res. 2016;57(7):1111–1125. doi: 10.1194/jlr.R051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordestgaard B.G., Langsted A. Lipoprotein(a) and cardiovascular disease. Lancet. 2024;404(10459):1255–1264. doi: 10.1016/S0140-6736(24)01308-4. [DOI] [PubMed] [Google Scholar]
- 18.Reyes-Soffer G. The impact of race and ethnicity on lipoprotein (a) levels and cardiovascular risk. Curr. Opin. Lipidol. 2021;32(3):163–166. doi: 10.1097/MOL.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong N.D., et al. Lipoprotein(a) and long-term cardiovascular risk in a multi-ethnic pooled prospective cohort. J. Am. Coll. Cardiol. 2024;83(16):1511–1525. doi: 10.1016/j.jacc.2024.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Langsted A., Nordestgaard B.G. Value of genetic testing for lipoprotein(a) variants. Circulation: Genom. Precision Med. 2022;15(2) doi: 10.1161/CIRCGEN.122.003737. [DOI] [PubMed] [Google Scholar]
- 21.Epstein E.S. Genetic testing in patients with high lipoprotein(a): experience from the UCSD lipoprotein(a) specialty clinic†. J. Clin. Lipidol. 2022;16(1):e12–e13. doi: 10.1016/j.jacl.2021.09.018. [DOI] [Google Scholar]
- 22.Pearson G.J., et al. 2021 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can. J. Cardiol. 2021;37(8):1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Enas E.A., Varkey B., Dharmarajan T.S., Pare G., Bahl V.K. Lipoprotein(a): an independent, genetic, and causal factor for cardiovascular disease and acute myocardial infarction. Indian Heart J. 2019;71(2):99–112. doi: 10.1016/j.ihj.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronenberg F. Causes and consequences of lipoprotein(a) abnormalities in kidney disease. Clin. Exp. Nephrol. 2014;18(2):234–237. doi: 10.1007/s10157-013-0875-8. [DOI] [PubMed] [Google Scholar]
- 25.Kotwal A., et al. Treatment of thyroid dysfunction and serum lipids: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2020;105(12):3683–3694. doi: 10.1210/clinem/dgaa672. [DOI] [PubMed] [Google Scholar]
- 26.Enkhmaa B., Petersen K.S., Kris-Etherton P.M., Berglund L. Diet and Lp(a): does dietary change modify residual cardiovascular risk conferred by Lp(a)? Nutrients. 2020;12(7):2024. doi: 10.3390/nu12072024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebbeling C.B., et al. Effects of a low-carbohydrate diet on insulin-resistant dyslipoproteinemia—a randomized controlled feeding trial. Am. J. Clin. Nutr. 2021;115(1):154–162. doi: 10.1093/ajcn/nqab287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szarek M., et al. Lipoprotein(a) blood levels and cardiovascular risk reduction with icosapent ethyl. J. Am. Coll. Cardiol. 2024;83(16):1529–1539. doi: 10.1016/j.jacc.2024.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Vuorio A., Watts G.F., Schneider W.J., Tsimikas S., Kovanen P.T. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J. Intern. Med. 2020;287(1):2–18. doi: 10.1111/joim.12981. [DOI] [PubMed] [Google Scholar]
- 30.Schnitzler J.G., et al. Atherogenic lipoprotein(a) increases vascular glycolysis, thereby facilitating inflammation and leukocyte extravasation. Circ. Res. 2020;126(10):1346–1359. doi: 10.1161/CIRCRESAHA.119.316206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudbjartsson D.F., et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J. Am. Coll. Cardiol. 2019;74(24):2982–2994. doi: 10.1016/j.jacc.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Langsted A., Kamstrup P.R., Nordestgaard B.G. High lipoprotein(a) and high risk of mortality. Eur. Heart J. 2019;40(33):2760–2770. doi: 10.1093/eurheartj/ehy902. [DOI] [PubMed] [Google Scholar]
- 33.Rikhi R., et al. Relationship of low-density lipoprotein-cholesterol and lipoprotein(a) to cardiovascular risk: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2022;363:102–108. doi: 10.1016/j.atherosclerosis.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatia H.S., et al. Independence of lipoprotein(a) and low-density lipoprotein cholesterol-mediated cardiovascular risk: a participant-level meta-analysis. Circulation. 2024 doi: 10.1161/CIRCULATIONAHA.124.069556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langsted A., Nordestgaard B.G., Kamstrup P.R. Elevated lipoprotein(a) and risk of ischemic stroke. J. Am. Coll. Cardiol. 2019;74(1):54–66. doi: 10.1016/j.jacc.2019.03.524. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser Y., et al. Lipoprotein(a) is robustly associated with aortic valve calcium. Heart. 2021;107(17):1422. doi: 10.1136/heartjnl-2021-319044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thanassoulis G., et al. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 2013;368(6):503. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perrot N., et al. Genetic variation in LPA, calcific aortic valve stenosis in patients undergoing cardiac surgery, and familial risk of aortic valve microcalcification. JAMA Cardiol. 2019;4(7):620. doi: 10.1001/jamacardio.2019.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaiser Y., et al. Lipoprotein(a) is associated with the onset but not the progression of aortic valve calcification. Eur. Heart J. 2022;43(39):3960–3967. doi: 10.1093/eurheartj/ehac377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Afshar M., Kamstrup P.R., Williams K., Sniderman A.D., Nordestgaard B.G., Thanassoulis G. Estimating the population impact of Lp(a) lowering on the incidence of myocardial infarction and aortic stenosis—brief report. Arterioscler. Thromb. Vasc. Biol. 2016;36(12):2421–2423. doi: 10.1161/ATVBAHA.116.308271. [DOI] [PubMed] [Google Scholar]
- 41.Dugani S.B., et al. Plasma biomarker profiles for premature and nonpremature coronary heart disease in women. Clin. Chem. 2024;70(5):768–779. doi: 10.1093/clinchem/hvae007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pablo C., et al. Lipoprotein(a) throughout life in women. Am. J. Prev. Cardiol. 2024:100885. doi: 10.1016/j.ajpc.2024.100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anagnostis P., Antza C., Trakatelli C., Lambrinoudaki I., Goulis D.G., Kotsis V. The effect of menopause on lipoprotein (a) concentrations: a systematic review and meta-analysis. Maturitas. 2023;167:39–45. doi: 10.1016/j.maturitas.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Anagnostis P., et al. The effect of hormone replacement therapy and tibolone on lipoprotein (a) concentrations in postmenopausal women: a systematic review and meta-analysis. Maturitas. 2017;99:27–36. doi: 10.1016/j.maturitas.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Tsimikas S., et al. NHLBI Working Group Recommendations to Reduce Lipoprotein(a)-Mediated Risk of Cardiovascular Disease and Aortic Stenosis. J. Am. Coll. Cardiol. 2018;71(2):177–192. doi: 10.1016/j.jacc.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rendler, J., Murphy, M., and Yeang, C. Lipoprotein(a) is a prevalent yet vastly underrecognized risk factor for cardiovascular disease. Health Care Curr. Rev., 12, 2, p. 397, 2024. Accessed: Nov. 25, 2024. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10959503/. [PMC free article] [PubMed]
- 47.Bhatia H.S., Hurst S., Desai P., Zhu W., Yeang C. Lipoprotein(a) testing trends in a large academic health system in the United States. J. Am. Heart Assoc. 2023;12(18) doi: 10.1161/JAHA.123.031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T., et al. Lipoprotein a testing patterns in the veterans health administration. JAMA Netw. Open. 2025;8(1):e2453300. doi: 10.1001/jamanetworkopen.2024.53300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stürzebecher P.E., et al. Lipoprotein(a) serum concentrations in children in relation to body mass index, age and sex. Pediatr. Res. 2024;96(1):177–183. doi: 10.1038/s41390-024-03108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.M. W. G. for C. C. D. Multimodality Writing Group for Chronic Coronary Disease, et al. ACC/AHA/ASE/ASNC/ASPC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2023 multimodality appropriate use criteria for the detection and risk assessment of chronic coronary disease. J. Am. Coll. Cardiol. 2023;81(25):2445–2467. doi: 10.1016/j.jacc.2023.03.410. [DOI] [PubMed] [Google Scholar]
- 51.Tokgozoglu L., Torp-Pedersen C. Redefining cardiovascular risk prediction: is the crystal ball clearer now? Eur. Heart J. 2021;42(25):2468–2471. doi: 10.1093/eurheartj/ehab310. [DOI] [PubMed] [Google Scholar]
- 52.Bhatia H.S., et al. Aspirin and cardiovascular risk in individuals with elevated lipoprotein(a): the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2024;13(3) doi: 10.1161/JAHA.123.033562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.CDC, About Lipoprotein (a), Heart Dis. Fam. Health Hist. Fam. Hypercholesterolemia. Accessed: Mar. 03, 2025. [Online]. Available: https://www.cdc.gov/heart-disease-family-history/about/about-lipoprotein-a.html.
- 54.Kaur G., et al. Lipoprotein(a): emerging insights and therapeutics. Am. J. Prevent. Cardiol. 2024;18 doi: 10.1016/j.ajpc.2024.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsimikas S., et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 2020;382(3):244–255. doi: 10.1056/NEJMoa1905239. [DOI] [PubMed] [Google Scholar]
- 56.O’Donoghue M.L., et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N. Engl. J. Med. 2022;387(20):1855–1864. doi: 10.1056/NEJMoa2211023. [DOI] [PubMed] [Google Scholar]
- 57.Nissen S.E., et al. Lepodisiran, an extended-duration short interfering RNA targeting lipoprotein(a): a randomized dose-ascending clinical trial. JAMA. 2023;330(21):2075. doi: 10.1001/jama.2023.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicholls S.J., et al. Oral muvalaplin for lowering of lipoprotein(a): a randomized clinical trial. JAMA. 2024 doi: 10.1001/jama.2024.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nissen S.E., et al. Zerlasiran—a small-interfering RNA targeting lipoprotein(a): a phase 2 randomized clinical trial. JAMA. 2024 doi: 10.1001/jama.2024.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]