Abstract
Objective: To identify risk factors for pulmonary arterial hypertension (PAH) in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) and develop a nomogram model to facilitate early clinical identification of high-risk patients and guide personalized treatment plans. Methods: This retrospective study included 602 AECOPD patients treated at Zhoushan Women and Children’s Hospital from June 2018 to May 2023. Patients were divided into two groups based on the presence or absence of PAH. Univariate and multivariate logistic regression analyses were performed to identify independent risk factors for AECOPD with PAH. A nomogram model was then established based on these factors. The Bootstrap self-sampling method was used to evaluate the predictive performance of the model. Indicators such as the area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and consistency index (C-index) were calculated to evaluate the discrimination and calibration of the model. Results: Among 602 AECOPD patients, 8.31% developed PAH. Compared with the non-PAH group, the PAH group exhibited a higher proportion of Chronic Obstructive Lung Disease (GOLD) grade IV, hypertension, and elevated neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and N-terminal pro-brain natriuretic peptide (NT-proBNP) levels. Multivariate logistic regression analysis identified GOLD grade, hypertension, NLR, PLR, and NT-proBNP as independent risk factors for AECOPD-associated PAH. A nomogram prediction model was developed based on these variables. The model’s AUC, sensitivity, and specificity in the training set were 0.906 (95% confidence interval (CI): 0.847-0.966), 0.850, and 0.862, respectively, and those in the validation set were 0.861 (95% CI: 0.715-0.932), 0.700, and 0.948, respectively. The C-index for the calibration curves of the model in both the training and validation sets was high (0.906 and 0.861, respectively). Decision curve analysis indicated a positive net benefit within a certain risk threshold. Conclusion: PAH in AECOPD patients was associated with GOLD grade, hypertension, NLR, PLR, and NT-proBNP. The developed nomogram demonstrated strong predictive performance and clinical utility.
Keywords: Acute exacerbation of chronic obstructive pulmonary disease, exacerbation period, pulmonary arterial hypertension, risk factors, nomogram, prediction
Introduction
Chronic obstructive pulmonary disease (COPD) is a prevalent and globally significant chronic respiratory disorder. According to the latest epidemiological data, COPD is among the leading causes of morbidity and mortality worldwide, imposing a substantial burden on healthcare systems and society [1]. It is primarily characterized by persistent airflow limitation that progressively worsens over time, closely linked to an enhanced chronic inflammatory response in the airways and lung tissues due to exposure to harmful gases and particles, such as cigarette smoke, biomass fuel smoke, and industrial pollutants.
Patients with COPD typically manifest a range of symptoms, including cough, expectoration, shortness of breath, and dyspnea. These symptoms substantially impair daily activities, reduce work capacity, and limit social participation. In severe cases, COPD can be life-threatening, with a high risk of respiratory failure and other serious complications [2].
The acute exacerbation of COPD (AECOPD) represents a critical stage in the disease’s progression, characterized by sudden symptom worsening, increased airway inflammation, mucus hypersecretion, and further lung function deterioration. The development of pulmonary arterial hypertension (PAH) as a complication during AECOPD further exacerbates disease severity and significantly elevates the mortality risk of patients [3].
In recent years, the global prevalence of COPD has risen, heightening awareness of its significant public health impact. Consequently, there has been growing interest in understanding the complex pathophysiology of AECOPD and its associated complications. The coexistence of PAH in AECOPD patients has become a major clinical concern, as it exacerbates respiratory and cardiovascular symptoms while complicating diagnosis, treatment, and prognosis [4]. Despite advances in medical technology and expanding research on COPD, the specific mechanisms underlying PAH development in AECOPD patients remain incompletely understood, necessitating further in-depth investigation.
Some potential risk factors for PAH in AECOPD patients have been identified, including age, impaired lung function, hypoxemia, and the inflammatory response [5]. Aging is associated with structural and functional changes in the pulmonary vasculature, making older patients more susceptible to PAH. Impaired lung function, commonly measured by forced expiratory volume in one second (FEV1), is closely related to COPD severity and linked to an increased risk of PAH. Hypoxemia, frequently observed in AECOPD patients, induces pulmonary vasoconstriction and vascular remodeling, contributing to PAH development. The inflammatory response, characterized by elevated levels of cytokines and chemokines, also plays a role in PAH pathogenesis in AECOPD. However, the precise contributions and interactions of these factors, as well as other potential risk factors, require further exploration.
The development of an accurate and reliable nomogram model has become an essential approach in clinical medicine. A nomogram is a graphical tool that integrates multiple risk factors to provide a comprehensive assessment of disease risk or prognosis [6]. In the context of AECOPD complicated by PAH, a well-developed nomogram model can assist clinicians in rapidly and accurately evaluating the risk of PAH in patients, enabling more informed treatment decisions. This approach has the potential to improve treatment outcomes and prognosis in AECOPD patients with PAH.
This study is motivated by the urgent need to address the knowledge gaps regarding the risk factors for PAH in AECOPD and the lack of a practical, accurate risk assessment tool. By conducting a comprehensive analysis of a large clinical dataset, we aim not only to identify key risk factors but also to establish a clinically applicable nomogram model. We hope that this study will contribute to a better understanding of disease mechanisms and provide valuable insights for clinicians, ultimately improving diagnostic accuracy, treatment strategies, and patient outcomes.
Materials and methods
Research subjects
A total of 602 AECOPD patients treated at Zhoushan Women and Children’s Hospital between June 1, 2018, and May 31, 2023, were retrospectively included in this study.
Inclusion criteria: (1) Patients diagnosed with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [7]; (2) Patients aged 18 years or older; (3) Patients without mental disorders; (4) Patients with complete clinical data.
Exclusion criteria: (1) Incomplete laboratory test results; (2) Severe comorbidities, including liver and kidney failure; (3) History of allergic diseases; (4) Recent (within two weeks) severe lung infection or immunosuppressant use; (5) Presence of other respiratory diseases that could interfere with COPD management; (6) Malignant tumors or severe hematological disorders.
This study was approved by the Ethics Committee of Zhoushan Women and Children’s Hospital.
Data collection
Clinical data were collected for all patients, including sex, age, body mass index (BMI), smoking history, COPD duration, GOLD grade, respiratory failure status, underlying diseases/comorbidities (pneumonia, hypertension, atrial fibrillation, fever), and pulmonary function parameters (forced expiratory volume in one second [FEV1], forced vital capacity [FVC], FEV1/FVC ratio, residual volume [RV], and total lung capacity [TLC]). Additionally, arterial blood gas parameters (PaO2, PaCO2), hematological markers (neutrophil-to-lymphocyte ratio [NLR], platelet-to-lymphocyte ratio [PLR]), N-terminal pro-brain natriuretic peptide (NT-proBNP), and other relevant indicators were recorded.
The GOLD classification [8] was determined based on the percentage of predicted FEV1 (FEV1% pred) after bronchodilator administration. Grade I: FEV1% pred ≥80% (mild airflow limitation); Grade II: 50%≤FEV1% pred <80% (moderate airflow limitation); Grade III: 30%≤FEV1% pred <50% (severe airflow limitation); Grade IV: FEV1% pred <30% (extremely severe airflow limitation).
A 6 mL fasting blood sample was collected in the morning using sodium citrate for anticoagulation. Samples were centrifuged at 2000 r/min for 15 minutes to separate the serum. Hematological parameters (neutrophils, lymphocytes, and platelets) were measured using a Mindray BC-7500 CS fully automated hematology analyzer. NLR and PLR were calculated as follows: NLR = platelets/lymphocytes; PLR = Platelets/Lymphocytes. The Beckman Coulter DX1800 fully automatic chemiluminescence immunoassay analyzer was used to determine NT-proBNP levels.
Diagnostic methods and criteria for PAH
PAH was diagnosed based on the 2018 Chinese Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension [9]. Clinical manifestations include dyspnea, fatigue, chest pain, which may be accompanied by an accentuated second heart sound over the pulmonary valve area. At sea level and under resting conditions, a mean pulmonary artery pressure (mPAP) of ≥25 mmHg, measured by right heart catheterization, was used as the diagnostic threshold.
Risk factor analysis
AECOPD patients without PAH were assigned into the non-PAH group, and those with PAH-complicated AECOPD were assigned into the PAH group. Potential risk factors were initially assessed using univariate analysis. Variables showing statistical significance were further analyzed using multivariate logistic regression to identify independent risk factors associated with PAH in AECOPD patients.
Establishment and evaluation of nomogram model
According to the results of multivariate analysis, independent risk factors were used to develop a nomogram model.
(1) Construction method and principle of nomogram model: First, the independent risk factors associated with PAH in AECOPD patients were identified. Each risk factor was assigned a score according to its regression coefficient. The scales for each factor and their corresponding scores were plotted on a coordinate axis. By summing the scores based on the patient’s specific risk factor values, a total score was obtained, which was then mapped to the probability of concurrent PAH. The principle of the nomogram is to comprehensively quantify multiple risk factors, visually demonstrating the contribution of each factor to the outcome and providing an overall prediction result.
(2) Model evaluation method: We used R software to divide the dataset into a training set and a validation set at a ratio of 7:3. The training set was used to develop the nomogram model, while the validation set was used to evaluate the performance of the model.
In the training set, internal verification was performed using the Bootstrap self-sampling method with 500 repetitions. The consistency index (C-index) was calculated to assess the discrimination and accuracy of the model. This approach helps evaluate the model’s ability to distinguish between patients with and without PAH in the training data. It also provides an estimate of the model’s predictive accuracy within the dataset used for its construction.
For the validation set, additional evaluation metrics such as calibration plots and the area under the receiver operating characteristic curve (AUC) were used to further assess the model’s performance in an independent dataset. These methods further assess the model’s performance on an independent dataset, ensuring its generalizability to new patients and different clinical settings.
By dividing the dataset into a training set and a validation set and employing robust internal and external verification methods, the performance and reliability of the nomogram model for predicting PAH in AECOPD patients were comprehensively evaluated.
Statistical processing
The data were statistically analyzed using SPSS 23.0. In univariate analysis, for continuous variables, independent sample t-test (for data conforming to a normal distribution, mean±standard deviation) or Mann-Whitney U test (data not conforming to normal distribution) was used to compare the differences between the PAH group and non-PAH group. For categorical variables, chi-square test was used to analyze the differences between the two groups.
In multivariate analysis, multivariate logistic regression was used to identify independent risk factors for PAH in AECOPD patients. Variables showing statistical significance in univariate analysis were included in the multivariate logistic regression model, whether concurrent PAH was taken as the dependent variable (0 = no complication, 1 = complication), and the significant variables screened out by univariate analysis were taken as independent variables. The independent risk factors were finally screened out by stepwise regression method, with regression coefficient, odds ratio (OR) and its 95% confidence interval (CI) of each risk factor being determined. OR>1 indicated an increased risk of concurrent PAH, while OR<1 indicated a protective effect against PAH. The predictive ability of each independent influencing factor for AECOPD complicated with PAH was analyzed using receiver operating characteristic curve (ROC). Pearson correlation analysis was used to assess relationships between normally distributed continuous variables, and Spearmen rank correlation analysis was applied for associations between ordinal and continuous variables. A P-value <0.05 was considered statistically significant.
Results
Comparison of demographic characteristics between the PAH and non-PAH groups
Among the 602 AECOPD patients, 50 cases were diagnosed with concurrent PAH, resulting in an incidence rate of 8.31%. The proportion of GOLD grade IV and hypertension, as well as the levels of NLR, PLR, and NT-proBNP in the PAH group were significantly higher than those in the AECOPD group (all P<0.05) (Table 1).
Table 1.
Comparison of baseline information between the PAH and non-PAH groups
| Group | PAH group (n = 50) | Non-PAH group (n = 552) | χ2/t | P |
|---|---|---|---|---|
| Sex (Male/Female, n) | 37/13 | 335/217 | 3.441 | 0.064 |
| Age (year, x̅±s) | 68.23±6.38 | 66.68±7.29 | 1.454 | 0.147 |
| Body mass index (kg/m2, x̅±s) | 22.39±4.37 | 22.62±3.52 | 0.433 | 0.665 |
| Smoking history (yes/no, n) | 26/24 | 274/278 | 0.102 | 0.749 |
| Course of COPD (year, x̅±s) | 9.18±2.64 | 9.46±2.88 | 0.663 | 0.508 |
| GOLD grade [n (%)] | 14.455 | 0.002 | ||
| I | 2 (4.00) | 132 (23.91) | ||
| II | 17 (34.00) | 134 (24.28) | ||
| III | 8 (16.00) | 121 (21.92) | ||
| IV | 23 (46.00) | 165 (29.89) | ||
| Respiratory failure [n (%)] | 0.013 | 0.910 | ||
| yes | 8 (16.00) | 85 (15.40) | ||
| no | 42 (84.00) | 467 (84.60) | ||
| Underlying diseases/comorbidities (yes/no, n) | ||||
| pneumonia | 19/31 | 268/284 | 2.046 | 0.153 |
| Hypertension | 40/10 | 302/250 | 11.951 | 0.001 |
| Atrial fibrillation | 4/46 | 54/498 | 0.167 | 0.682 |
| Fever | 5/45 | 56/496 | 0.001 | 0.974 |
| FEV1 (%, x̅±s) | 47.15±4.28 | 46.72±5.13 | 0.575 | 0.566 |
| FVC (%, x̅±s) | 62.71±11.39 | 63.18±11.22 | 0.283 | 0.777 |
| FEV1/FVC (x̅±s) | 0.75±0.17 | 0.73±0.16 | 0.842 | 0.400 |
| RV (%,x̅±s) | 148.23±30.28 | 147.69±32.16 | 0.114 | 0.909 |
| TLC (%,x̅±s) | 125.61±23.24 | 124.39±25.82 | 0.322 | 0.747 |
| PaO2 (mmHg,x̅±s) | 68.33±3.39 | 68.52±3.52 | 0.367 | 0.714 |
| PaCO2 (mmHg,x̅±s) | 50.28±4.67 | 49.24±5.59 | 1.276 | 0.203 |
| NLR (x±s) | 3.91±1.03 | 2.82±0.65 | 6.188 | <0.001 |
| PLR (x±s) | 248.33±62.15 | 209.42±56.19 | 3.228 | 0.002 |
| NT-proBNP (pg/mL,x̅±s) | 138.60±42.39 | 91.58±28.23 | 6.388 | <0.001 |
PAH: pulmonary arterial hypertension; AECOPD: acute exacerbation of chronic obstructive pulmonary disease; COPD: chronic obstructive pulmonary disease; GOLD: the Global Initiative for Chronic Obstructive Lung Disease; FEV1: Forced expiratory volume in one second; FVC: Forced vital capacity; RV: residual volume; TLC: Total lung capacity; PaO2: Partial pressure of oxygen; PaCO2: Partial pressure of carbon dioxide; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Identification of risk factors for PAH in AECOPD patients
Multivariate logistic regression analysis was conducted on the potential risk factors identified as significant in univariate analysis. The results showed that GOLD grade, hypertension, NLR, PLR, and NT-proBNP were independent influencing factors for PAH in AECOPD patients (all P<0.05) (Table 2).
Table 2.
Risk factor analysis
| B | SE | Wals | P | OR (95% CI) | |
|---|---|---|---|---|---|
| GOLD grade | 6.614 | 0.045 | |||
| I | -1.566 | 0.796 | 3.874 | 0.049 | 0.209 (0.044-0.993) |
| II | -0.691 | 0.517 | 1.782 | 0.182 | 0.501 (0.182-1.382) |
| III | -1.108 | 0.597 | 3.447 | 0.063 | 0.330 (0.103-1.064) |
| Hypertension | 0.929 | 0.496 | 3.508 | 0.041 | 2.531 (0.958-6.688) |
| NLR | 1.737 | 0.299 | 33.745 | <0.001 | 5.680 (3.161-10.206) |
| PLR | 0.011 | 0.004 | 9.467 | 0.002 | 1.011 (1.004-1.018) |
| NT-proBNP | 0.044 | 0.007 | 38.160 | <0.001 | 1.045 (1.031-1.060) |
GOLD: the Global Initiative for Chronic Obstructive Lung Disease; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide; SE: standard error; OR: odds ratio; CI: confidence interval.
Predictive performance of independent risk factors for PAH in AECOPD
ROC curves were drawn to evaluate the predictive ability of each independent risk factor for PAH in AECOPD (Figure 1). The area under the receiver operating characteristic curve (AUC) for GOLD grade, hypertension, ET-1, NLR, PLR, and NT-proBNP for predicting PAH in AECOPD all exceeded 0.6, indicating moderate predictive value. Among these, NT-proBNP demonstrated the highest predictive accuracy (AUC = 0.811), followed by NLR (AUC = 0.789) (Table 3).
Figure 1.
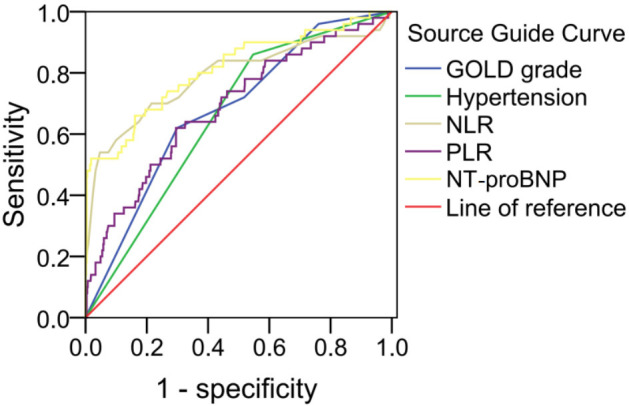
Receiver operating characteristic (ROC) curve analysis for each independent risk factor in predicting PAH in AECOPD. AECOPD: acute exacerbation of chronic obstructive pulmonary disease; PAH: pulmonary arterial hypertension; GOLD: the Global Initiative for Chronic Obstructive Lung Disease; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Table 3.
The predictive performance of each independent risk factor for PAH in AECOPD patients
| Variables | AUC (95% CI) | SE | P | Sensitivity | Specificity | Optimum cutoff value |
|---|---|---|---|---|---|---|
| GOLD grade | 0.678 (0.605-0.751) | 0.037 | <0.001 | 62.00% | 70.10% | - |
| Hypertension | 0.656 (0.587-0.726) | 0.035 | <0.001 | 86.00% | 45.30% | - |
| NLR | 0.789 (0.706-0.872) | 0.042 | <0.001 | 54.00% | 95.30% | 3.85 |
| PLR | 0.688 (0.607-0.768) | 0.041 | <0.001 | 62.00% | 70.50% | 242.35 |
| NT-proBNP | 0.811 (0.738-0.884) | 0.037 | <0.001 | 52.00% | 98.20% | 146.55 |
AECOPD: acute exacerbation of chronic obstructive pulmonary disease; PAH: pulmonary arterial hypertension; GOLD: the Global Initiative for Chronic Obstructive Lung Disease; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide; AUC: the area under the receiver operating characteristic curve; CI: confidence interval; SE: standard error.
Correlation analysis
Correlation analysis showed that there was no linear relationship among GOLD grade, hypertension, ET-1, NLR, PLR, and NT-proBNP (P>0.05) (Table 4).
Table 4.
Correlation analysis among independent risk factors
| Variable | GOLD grade | Hypertension | NLR | PLR | NT-proBNP | |
|---|---|---|---|---|---|---|
| GOLD grade | r | 1.000 | 0.058 | 0.071 | -0.010 | 0.098 |
| P | 0.159 | 0.081 | 0.815 | 0.016 | ||
| Hypertension | r | 1.000 | 0.113 | 0.066 | 0.046 | |
| P | 0.006 | 0.107 | 0.257 | |||
| NLR | r | 1.000 | 0.098 | 0.068 | ||
| P | 0.016 | 0.097 | ||||
| PLR | r | 1.000 | 0.008 | |||
| P | 0.843 | |||||
| NT-proBNP | r | 1.000 | ||||
| P |
GOLD: the Global Initiative for Chronic Obstructive Lung Disease; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Establishment of a nomogram prediction model
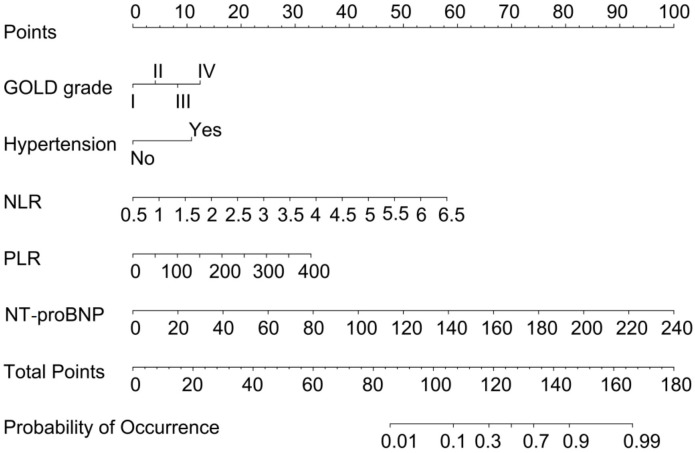
The influencing indicators of the training set and the validation set are shown in Table 5. A nomogram prediction model was developed based on the identified independent risk factors (GOLD grade, hypertension, NLR, PLR, NT-proBNP), providing an intuitive assessment of the contribution of each factor to PAH risk in AECOPD patients (Figure 2). Each risk factor was assigned a score proportional to its impact on PAH risk. The total score was calculated by summing the individual scores of all risk factors, with higher total scores indicating a greater likelihood of PAH. Risk factors with stronger predictive power were assigned higher scores.
Table 5.
The influencing indicators of the training set and the validation set
| Group | Training set (n = 418) | Validation set (n = 184) | χ2/t | P |
|---|---|---|---|---|
| GOLD grade [n (%)] | 5.731 | 0.125 | ||
| I | 95 (22.73) | 39 (21.20) | ||
| II | 105 (25.12) | 43 (23.37) | ||
| III | 95 (22.73) | 31 (16.85) | ||
| IV | 123 (29.43) | 71 (38.59) | ||
| Hypertension | 235/203 | 110/74 | 1.971 | 0.160 |
| NLR (x̅±s) | 2.92±0.78 | 2.90±0.69 | 0.300 | 0.764 |
| PLR (x̅±s) | 216.08±57.97 | 208.89±56.01 | 1.416 | 0.157 |
| NT-proBNP (pg/mL, x̅±s) | 95.46±30.66 | 95.52±31.49 | 0.022 | 0.983 |
GOLD: the Global Initiative for Chronic Obstructive Lung Disease; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
Figure 2.
The nomogram prediction model based on risk factors. GOLD: the Global Initiative for Chronic Obstructive Lung Disease; NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; NT-proBNP: N-terminal pro-brain natriuretic peptide.
ROC analysis
A total of 184 patients were included in the validation set, among which 10 patients had PAH and 174 patients had non-PAH. The distribution of independent risk factors in the validation set was as follows: In terms of the GOLD classification, there were 39 patients at grade I, 43 patients at grade II, 31 patients at grade III, and 71 patients at grade IV. Regarding hypertension, there were 92 patients with hypertension and 92 patients without hypertension. The NLR was 2.90±0.69. The PLR was 204.89±56.01. The level of NT-proBNP was (95.52±31.49) pg/mL.
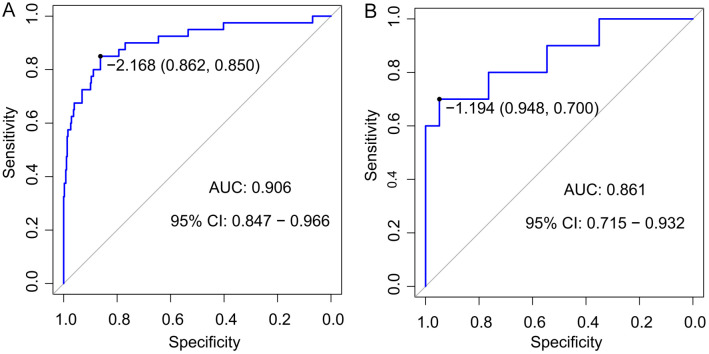
The nomogram model demonstrated strong predictive performance in both the training set and validation set based on ROC results. In the training set, the model achieved a high AUC (0.906, 95% CI: 0.847-0.966), indicating excellent discrimination between positive and negative cases. Additionally, the high sensitivity of 0.850 indicated that the model can effectively identify true positive samples; and the specificity of 0.862 indicated the model’s ability in reducing the likelihood of false positives (Figure 3A).
Figure 3.
Receiver operating characteristic (ROC) curve analysis for the model’s predictive performance in training (A) and validation (B) sets. AUC: the area under the receiver operating characteristic curve; CI: confidence interval.
In the validation set, the model also performs well, with an AUC of 0.861, (95% CI: 0.715-0.932), confirming its stability and generalization ability. The sensitivity (0.700) and specificity (0.948) remained within a reasonable limits, further validating its reliability across different datasets (Figure 3B).
Calibration curve analysis
In the training set, the discrimination index shows good performance. The C-index (0.906) indicated excellent discrimination ability, distinguishing samples of different risk levels effectively. The calibration curve closely aligned with the ideal curve, demonstrating that the model’s predictions were highly consistent with actual outcomes (Figure 4A).
Figure 4.
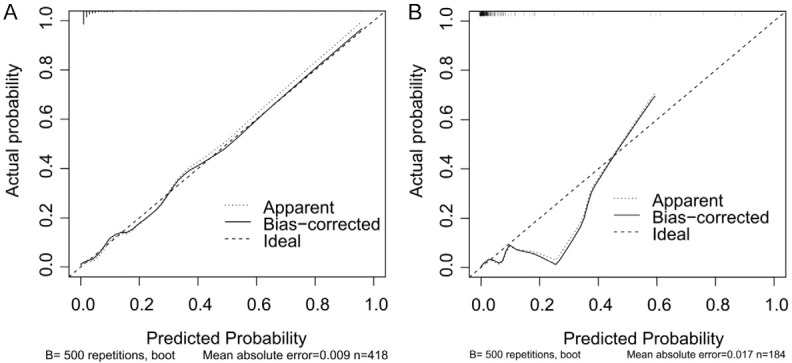
Calibration curve for the predictive model. A. Training set; B. Validation set.
In the validation set, the model also showed good discrimination and calibration with a C-index of 0.861, verifying its robust generalization ability. The calibration curve in the validation set similarly showed good agreement between predicted and actual outcomes, further reinforcing the model’s reliability and accuracy (Figure 4B).
Decision curve analysis
In the training set, the decision curve demonstrated a positive net benefit across a defined risk threshold range (Figure 5A), suggesting that using the nomogram for clinical decision-making provides practical value. In the validation set, this nomogram model also showed a positive net benefit within an appropriate risk threshold range (Figure 5B), further corroborating its clinical applicability and generalization ability across datasets.
Figure 5.
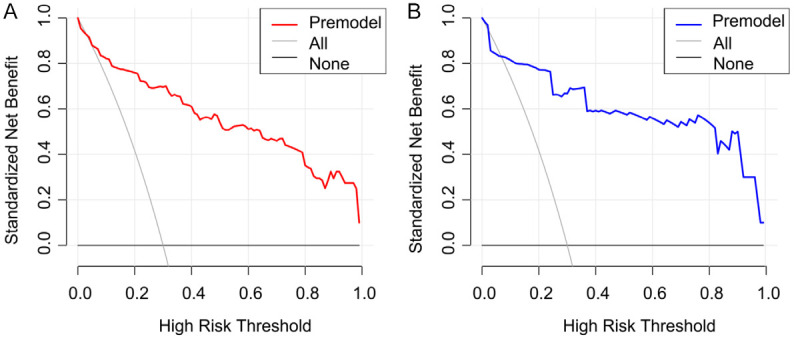
Decision curve analysis for the predictive model. A. Training set; B. Validation set.
Discussion
In AECOPD, the presence of PAH significantly worsens disease severity and increases mortality risk. Through an extensive analysis of clinical cases, we identified several potential risk factors, including impaired lung function and inflammatory response, both of which contribute to PAH development. Impaired lung function directly affects pulmonary gas exchange, while inflammatory response may damage pulmonary vascular structure and function [10]. The nomogram model developed in this study integrates multiple risk factors, providing clinicians with an efficient and practical risk assessment tool. This facilitates early intervention and personalized treatment strategies, ultimately improving patient prognosis and quality of life [11]. Given the severe impact of PAH on AECOPD patients and the potential clinical benefits of this model, our findings offer important insights for risk assessment and disease management.
This study analyzed 602 AECOPD patients, revealing a PAH incidence of 8.31%, which is lower than the 21% reported by Nakayama et al. [12]. Compared to the non-PAH group, patients in the PAH group exhibited significantly higher proportions of GOLD grade IV, hypertension, NLR, PLR, and NT-proBNP levels, suggesting that these factors may be associated with PAH development in AECOPD patients. Multivariate logistic regression analysis identified GOLD grade, hypertension, NLR, PLR, and NT-proBNP as independent risk factors for PAH in AECOPD. This indicates that these factors play a crucial role in predicting PAH occurrence and are not confounded by other variables. The nomogram prediction model, which was developed on these independent risk factors, demonstrated strong predictive performance in both the training set and the validation set. In the training set, the nomogram demonstrated an AUC of 0.906 (95% CI: 0.847-0.966), with sensitivity and specificity of 0.850 and 0.862, respectively. The validation set showed an AUC of 0.861 (95% CI: 0.715-0.932), and sensitivity and specificity of 0.700 and 0.948, respectively. In addition, the C-index values for the calibration curves were 0.906 (training set) and 0.861 (validation set), indicating strong model discrimination. The decision curve analysis further demonstrated a positive net benefit within a defined risk threshold, reinforcing the model’s clinical applicability and reliability.
GOLD grade IV is widely recognized as an independent risk factor for PAH in AECOPD patients, with multiple studies providing strong supporting evidence. For example, Gupta et al. [13] found a significant association between GOLD grade and PAH risk in COPD patients. Their study showed that as GOLD grade increases, lung function gradually deteriorates, leading to pulmonary vascular remodeling and increased pulmonary vascular resistance, thereby heightening PAH risk. Similarly, Song et al. [14] conducted a comprehensive analysis of lung function tests, blood gas analysis, and cardiac ultrasound in AECOPD patients and confirmed a strong correlation between GOLD grade IV and PAH development. Their findings also highlighted that patients with GOLD grade IV often experience more severe hypoxemia and hypercapnia, which further contribute to PAH progression. In addition, higher GOLD grades in AECOPD patients have been linked to elevated inflammatory marker levels [15]. These inflammatory factors may promote pulmonary vascular remodeling and PAH development by damaging pulmonary vascular endothelial cells and stimulating pulmonary vascular smooth muscle cell proliferation. These results underscore the close association between GOLD grade and PAH in AECOPD patients. Clinicians should closely monitor AECOPD patients with higher GOLD grades, conduct early screening for PAH, and implement timely interventions to improve patient prognosis.
Patients with hypertension already exhibit vascular endothelial dysfunction and vascular remodeling, which may compromise pulmonary vascular structure and function [16]. In AECOPD patients, hypertension may further aggravate pulmonary pulmonary vascular damage, promoting the occurrence and development of PAH. NLR and PLR serve as inflammatory markers, and in AECOPD patients, the inflammatory response is often more pronounced. Elevated NLR and PLR may indicate inflammation-mediated pulmonary vascular damage [17]. Cytokines and reactive oxygen species released by inflammatory cells can damage pulmonary vascular endothelial cells, induce smooth muscle cell proliferation and fibrosis, facilitating PAH development. NT-proBNP, a marker of cardiac function, is frequently elevated in AECOPD patients, reflecting increased cardiac load [18]. Cardiac dysfunction may lead to pulmonary circulation congestion and elevated pulmonary vascular pressure, further contributing to PAH progression. According to Lewis et al. [19], NT-proBNP plays a crucial role in the diagnosis and risk stratification of PAH and correlates with pulmonary hemodynamics. In conclusion, hypertension, NLR, PLR, and NT-proBNP are key risk factors for PAH in AECOPD patients, contributing to disease progression through vascular, inflammatory, and cardiac-related mechanisms.
The nomogram model accurately predicts PAH risk in AECOPD patients based on GOLD grade, hypertension, NLR, PLR, and NT-proBNP, enabling early identification of high-risk patients. This allows clinicians to implement preventive and therapeutic measures to reduce PAH incidence. For AECOPD patients with concurrent PAH, the nomogram model facilitates personalized treatment planning [20]. Based on individual risk factors and prediction results, doctors can adjust treatment strategies, such as optimizing oxygen therapy, managing blood pressure, and administering vasodilators, to improve treatment outcomes. The nomogram model can serve as a disease monitoring tool. By regularly assessing key clinical indicators, clinicians can use the model for risk assessment, allowing for timely adjustments to treatment plans and early intervention to prevent PAH progression. Researchers can use this model to further investigate pathogenesis, risk factors, and treatment methods for PAH, providing stronger evidence base for clinical practice. The results of our study highlight the multiple applications and benefits of the nomogram model in clinical management and research of AECOPD complicated with PAH, including risk prediction, personalized treatment, disease monitoring, and the facilitation of further research.
Our study still has several limitations. First, the study population was limited to a specific number and source of patients, which may not fully represent all AECOPD patients, potentially introducing selection bias. Second, the identification of risk factors was constrained by current detection methods and research parameters, meaning potential risk factors may have been overlooked. In addition, the nomogram model was developed based on a specific dataset, and its applicability across different populations and geographic regions requires further validation. Finally, the study did not include long-term follow-up, limiting its ability to assess the long-term prognosis of AECOPD patients with PAH. In general, acknowledging these limitations, sample selection bias, restricted identification of risk factors, model generalizability concerns, and the lack of long-term follow-up, is crucial. Future studies should focus on addressing these limitations to enhance the study’s robustness and clinical applicability.
In conclusion, PAH in AECOPD patients is significantly associated with GOLD grade, hypertension, NLR, PLR, and NT-proBNP. The nomogram model developed in this study demonstrated high predictive performance and a positive net benefit in both the training and validation sets, highlighting its clinical utility. Future research should focus on further optimizing the model, expanding the sample size, and conducting multicenter studies to improve accuracy and reliability. Additionally, in-depth investigations into the pathogenesis of independent risk factors and PAH will provide a stronger theoretical foundation for the prevention and treatment of PAH in AECOPD patients.
Disclosure of conflict of interest
None.
References
- 1.Watanabe N, Fujita Y, Nakayama J, Mori Y, Kadota T, Hayashi Y, Shimomura I, Ohtsuka T, Okamoto K, Araya J, Kuwano K, Yamamoto Y. Anomalous epithelial variations and ectopic inflammatory response in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2022;67:708–719. doi: 10.1165/rcmb.2021-0555OC. [DOI] [PubMed] [Google Scholar]
- 2.Liu W, Liu Y, Li X. Impact of exercise capacity upon respiratory functions, perception of dyspnea, and quality of life in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1529–1534. doi: 10.2147/COPD.S311221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu CT, Li GH, Huang CT, Cheng YC, Chen CH, Chien JY, Kuo PH, Kuo LC, Lai F. Acute exacerbation of a chronic obstructive pulmonary disease prediction system using wearable device data, machine learning, and deep learning: development and cohort study. JMIR Mhealth Uhealth. 2021;9:e22591. doi: 10.2196/22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roca M, Verduri A, Corbetta L, Clini E, Fabbri LM, Beghé B. Mechanisms of acute exacerbation of respiratory symptoms in chronic obstructive pulmonary disease. Eur J Clin Invest. 2013;43:510–21. doi: 10.1111/eci.12064. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Cheng Z, Zha B, Chen X, Gong Z, Ji L, Wei L. Risk factors of pulmonary arterial hypertension in patients with systemic lupus erythematosus: a meta-analysis. Lupus. 2023;32:1310–1319. doi: 10.1177/09612033231202398. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Huan C, Hu Y, Xiao S, Xu T, Guo M, Wang X, Liu A, Sun J, Wang C, Wang J, Zhu H, Pan D. Development and validation of a nomogram for predicting all-cause mortality in patients with hemodialysis having pulmonary hypertension. Cardiorenal Med. 2023;13:282–291. doi: 10.1159/000533674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, Bourbeau J, Han MK, Martinez FJ, Montes de Oca M, Mortimer K, Papi A, Pavord I, Roche N, Salvi S, Sin DD, Singh D, Stockley R, López Varela MV, Wedzicha JA, Vogelmeier CF. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Arch Bronconeumol. 2023;59:232–248. doi: 10.1016/j.arbres.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Agusti A, Böhm M, Celli B, Criner GJ, Garcia-Alvarez A, Martinez F, Sin DD, Vogelmeier CF. GOLD COPD DOCUMENT 2023: a brief update for practicing cardiologists. Clin Res Cardiol. 2024;113:195–204. doi: 10.1007/s00392-023-02217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oishi K, Asami-Noyama M, Yamamoto T, Matsumori K, Yonezawa K, Watanabe M, Hisamoto Y, Fukatsu A, Matsuda K, Hamada K, Suetake R, Ohata S, Murata Y, Yamaji Y, Sakamoto K, Ito K, Osoreda H, Edakuni N, Kakugawa T, Hirano T, Matsunaga K. Detection of impaired gas exchange using the 1-minute sit-to-stand test in patients with interstitial lung disease. Respir Investig. 2023;61:186–189. doi: 10.1016/j.resinv.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Z, Ren J, Liu Q, Li S, Xu J, Wu X, Xiao Y, Zhang Z, Jia W, Bai H, Zhang J. A nomogram for predicting the risk of cancer-related cognitive impairment in breast cancer patients based on a scientific symptom model. Sci Rep. 2024;14:14566. doi: 10.1038/s41598-024-65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Working Group on Pulmonary Vascular Diseases of Chinese Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of pulmonary hypertension 2018. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46:933–964. doi: 10.3760/cma.j.issn.0253-3758.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama S, Chubachi S, Sakurai K, Irie H, Tsutsumi A, Hashiguchi M, Itabashi Y, Murata M, Nakamura H, Asano K, Fukunaga K. Characteristics of chronic obstructive pulmonary disease patients with pulmonary hypertension assessed by echocardiography in a three-year observational cohort study. Int J Chron Obstruct Pulmon Dis. 2020;15:487–499. doi: 10.2147/COPD.S230952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta KK, Roy B, Chaudhary SC, Mishra A, Patel ML, Singh J, Kumar V. Prevalence of pulmonary artery hypertension in patients of chronic obstructive pulmonary disease and its correlation with stages of chronic obstructive pulmonary disease, exercising capacity, and quality of life. J Family Med Prim Care. 2018;7:53–57. doi: 10.4103/jfmpc.jfmpc_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y, Ma LN, Yang YH. The role of pulmonary arterial pressure in chronic obstructive pulmonary disease phenotypes based on cluster analysis and its prognostic value. Zhonghua Yi Xue Za Zhi. 2020;100:97–103. doi: 10.3760/cma.j.issn.0376-2491.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Ge J, Geng S, Jiang H. Long noncoding RNAs antisense noncoding RNA in the INK4 locus (ANRIL) correlates with lower acute exacerbation risk, decreased inflammatory cytokines, and mild GOLD stage in patients with chronic obstructive pulmonary disease. J Clin Lab Anal. 2019;33:e22678. doi: 10.1002/jcla.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balistrieri A, Makino A, Yuan JX. Pathophysiology and pathogenic mechanisms of pulmonary hypertension: role of membrane receptors, ion channels, and Ca2+ signaling. Physiol Rev. 2023;103:1827–1897. doi: 10.1152/physrev.00030.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poto R, Loffredo S, Palestra F, Marone G, Patella V, Varricchi G. Angiogenesis, lymphangiogenesis, and inflammation in chronic obstructive pulmonary disease (COPD): few certainties and many outstanding questions. Cells. 2022;11:1720. doi: 10.3390/cells11101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assadi H, Matthews G, Chambers B, Grafton-Clarke C, Shabi M, Plein S, Swoboda PP, Garg P. Cardiac magnetic resonance left ventricular filling pressure is associated with NT-proBNP in patients with new onset heart failure. Medicina (Kaunas) 2023;59:1924. doi: 10.3390/medicina59111924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis RA, Durrington C, Condliffe R, Kiely DG. BNP/NT-proBNP in pulmonary arterial hypertension: time for point-of-care testing? Eur Respir Rev. 2020;29:200009. doi: 10.1183/16000617.0009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen E, Yap TA, Lee EK, Højgaard M, Mettu NB, Lheureux S, Carneiro BA, Plummer R, Fretland AJ, Ulanet D, Xu Y, McDougall R, Koehler M, Fontana E. Development of a practical nomogram for personalized anemia management in patients treated with ataxia telangiectasia and Rad3-related inhibitor camonsertib. Clin Cancer Res. 2024;30:687–694. doi: 10.1158/1078-0432.CCR-23-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]