Abstract
Plateau environment represents a common terrestrial characterized by multistress conditions including hypobaric hypoxia, low temperature, and intense radiation, yet sustain over 100 million permanent or transient inhabitants. While this extreme environment exerts profound impacts on cerebral architecture and gut microbiota homeostasis, precipitating cognitive deficits and microbiome-derived intestinal pathologies, the mechanistic interplay between plateau environment adaptation and microbial dynamics remains contentious. Here, we employ a microbiota-gut-brain axis framework to investigate whether probiotic intervention can ameliorate hippocampal impairments induced by simulated plateau environment exposure (3500–4000 m) in mice. Through simulated plateau environment exposure experiments, we revealed that extreme high-altitude conditions induced hippocampal memory dysfunction in mice, exacerbated oxidative stress damage in hippocampal tissues, and altered synaptic plasticity-related biomarkers including CREB transcription factor, BDNF protein levels, and electrophysiological power spectra. Administration of HL79 alleviated these burdens, including memory dysfunction and tissue damage, though complete reversal was not achieved. Combined hippocampal transcriptomic analyses suggested that HL79’s beneficial effects primarily involved modulation of lipid-related gene expression in the hippocampus, consistent with prior reports of plateau environmental impacts on gene expression. Serum metabolomic results further reinforced this inference that differential metabolites regulated by HL79 are mainly enriched in bile secretion, taurine and hypotaurine metabolism, linoleic acid metabolism, and PPAR signaling pathways, though the precise regulatory mechanisms require further elucidation. This research provides a novel microbiota-gut-brain axis-based regulatory strategy for adaptation to extreme plateau environments and offers new evidence for understanding the relationship between gut microbiota and plateau environment adaptation at high elevations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-025-01898-2.
Keywords: Plateau environment, Microbiota-gut-brain axis, Probiotics, Spatial memory dysfunction
Introduction
Plateaus, defined in geographical terms as elevated landforms exceeding 500 m in altitude formed through crustal tectonic processes, constitute approximately 45% of Earth’s terrestrial surface (Danielson and Gesch 2011). Despite multistressor plateau environments (hypothermia, hypobaric hypoxia, arid conditions, intense UV radiation), ~ 4% of the global population permanently inhabits plateaus, with 14.5 million residents concentrated on the Qinghai-Tibet Plateau, the planet’s largest plateau ecosystem (Favre et al. 2015; Tian et al. 2022). Annually, millions experience acute plateau environmental exposure through occupational, military, or recreational engagements requiring rapid-altitude transitions. The extreme environment of plateau has been proven to have a direct and important impact on human beings. Hypobaric hypoxia in plateau environments may induce neurophysiological impairments, manifesting as acute mountain sickness (headache, cerebral edema) and multiorgan dysfunction (gastrointestinal distress, sleep disruption, dizziness) in non-acclimatized individuals, which has aroused widespread medical attention (Benedetti et al. 2015; Honigman et al. 1993; Lawley et al. 2014; Roach et al. 2018; Song et al. 2016). The brain's oxygen dependency (consuming 20% systemic O2) renders hippocampal, cerebellar, and striatal regions particularly vulnerable to plateaus hypoxic insult (Clarke 1999). Mechanistically, plateaus hypobaric hypoxia triggers cortical/hippocampal neurodegeneration via oxidative stress-mediated mitochondrial dysfunction, metabolic dysregulation, and blood–brain barrier compromise, collectively impairing affective, behavioral, and cognitive domains (Clarke 1999; Ji et al. 2021; Li et al. 2013; Liu et al. 2022; Ma et al. 2020; McMorris et al. 2017). Studies have shown that cognitive deficits in non-acclimatized populations at > 3,048 m altitudes—notably attenuated visual/auditory processing, attention deficits, and impaired executive function—exhibit partial recovery following 14-day acclimatization (Guo et al. 2015; Ma et al. 2019; Pun et al. 2018; Yang et al. 2005).
At present, the symptoms and cognitive impairment caused by plateau environment hypobaric hypoxia can be prevented and protected by means of adaptive training, increasing oxygen intake, slowing down entry time, drug, and nutrient intervention (Liu et al. 2021). Notably, emerging evidence delineates the microbiota-gut-brain axis as a critical pathway through which neurological functions may be modulated via targeted gut microbiota interventions. Our prior plateau exposure studies demonstrated that antibiotic-induced gut microbiota depletion significantly exacerbated hippocampal memory impairments in murine models, suggest the microbiota's regulatory role in plateau hypoxia-induced neurological dysfunction (Zhang et al. 2023). However, the therapeutic potential of probiotic regimens against plateau environment induced neurocognitive deficits remains underexplored.
Probiotics, defined as live microorganisms conferring quantified health benefits, enhance intestinal barrier integrity, modulate innate and adaptive immunity, and produce systemic-acting metabolites through gut microbiota regulation (Hill et al. 2014). Their clinical utility in dietary and therapeutic formulations persists despite interindividual variability in host responsiveness. Growing evidence documents the efficacy of mono- and poly-strain formulations in ameliorating cognitive deficits, including learning and spatial memory impairments associated with neurological disorders (e.g., Alzheimer’s disease, autism spectrum disorders, and depression-anxiety comorbidities), conditions frequently linked to gut microbial dysregulation (Baião et al. 2023; Chang et al. 2023; Wang et al. 2022; Zha et al. 2025). In particular, Lactobacillus and Bifidobacterium can participate in the regulation of microbiota-gut-brain axis through interaction with the host gut microbiota, affect the development of neurons and the content of brain chemicals and improve the cognitive and behavioral ability of the host, which has mental health effects (Pasam et al. 2024; Tian et al. 2021; Wang et al. 2019). Recent studies have revealed that the diversity, species composition, and functional characteristics of the human gut microbiota exhibit unique altitudinal patterns, generally showing a decrease in diversity and species richness with increasing altitude (Ma et al. 2025; Peng et al. 2025). A 108-day longitudinal study found that Blautia A species play a more significant role in the rapid response of gut microbiota to plateau (Su et al. 2024a, b). Acute exposure to plateau results in substantial changes in the gut microbiota, while prolonged exposure leads to structural and functional remodelling (Ma et al. 2025). These findings suggest that gut microbes may help lowlanders adapt to plateau, although extensive research on gut microbiota related to altitude adaptation remains limited (Liu et al. 2023; Pennisi 2024; Su et al. 2024a, b). Similarly, recent studies have provided new evidence that microbial dysbiosis can aggravate cognitive dysfunction caused by plateau environmental exposure. The structure of the gut microbiota of people who migrate to plateau areas has changed significantly. The classification of bacteria, such as Bifidobacterium and Lactobacillus, which are usually defined as beneficial bacteria, has decreased, while the conditional pathogenic bacteria, such as Proteobacteria and Escherichia coli, have increased. Intestinal barrier function is impaired and diarrheal symptoms are high (Bhushan et al. 2021; Karl et al. 2018; Li et al. 2016; Zhao et al. 2023). Acutely exposed to simulated physiological plateau environment (4500 m), the gut microbiota affected the spatial memory of mice by affecting the intestinal barrier and inducing immunosuppression (Bai et al. 2022; Suzuki et al. 2019; Wan et al. 2022; Zhang et al. 2023).
The established link between plateau-associated cognitive decline and microbial dysregulation provides a rationale for probiotic interventions. We propose that targeted probiotic supplementation may mitigate plateau hypoxia-induced cognitive deficits and related pathophysiological consequences. Therefore, in this study, a strain of Lactobacillus johnsonii HL79 isolated from human feces at plateau environment has the potential to regulate cognitive impairment caused by high altitude environment (using a hypobaric oxygen chamber to simulate a height of 3500–4000 m). Its possible mechanism were evaluated by simulating the equivalent plateau environment to provide new ideas and probiotic solutions for the study of the negative effects caused by plateau environment.
Materials and methods
Bacterial strains and culture
Lactobacillus johnsonii HL79 (CCTCC M 2025192) was preserved and provided by the Plateau Brain Science Research Center, Tibet University, Lhasa, Tibet, China. A single colony was selected and purified, then inoculated into de Man, Rogosa and Sharpe (MRS) medium and cultured at 37 °C for 24 h. HL79 was collected by centrifugation at 4,000 g for 15 min, resuspended in physiological saline, and centrifuged again for washing three times. From the purified bacterial-saline suspension, 1 mL was transferred into a centrifuge tube containing 9 mL of saline and mixed thoroughly, resulting in a 10−1 dilution. Then, 1 mL was taken from the 10−1 bacterial suspension and added into a new centrifuge tube containing 9 mL of saline, yielding a 10−2 dilution. This serial dilution process was continued accordingly, generating dilutions of 10−3, 10−4, 10−5, 10−6, 10−7, 10−8, and 10⁻9.
From each dilution, 50 μL of the bacterial suspension was spread onto MRS solid agar plates. The plates were incubated at 37 °C for 24 h. After incubation, the number of HL79 colonies on each plate corresponding to the different dilutions was counted. An appropriate dilution was selected to calculate the original concentration of HL79 in the purified bacterial-saline suspension. The purified bacterial suspension was diluted to a final concentration of 2 × 109 CFU/mL based on the calculations, and this concentration was used for subsequent animal experiments.
Animal and experiment design
Seventy-two 8-week-old specific pathogen-free C57BL/6 mice were purchased from Spfbiotech (Beijing) Biotechnology Co., Ltd. Mice were randomly divided into 3 groups: control group (CON), high altitude group (HA), high altitude probiotic group (HAP). All mice were given a week to adapt to their new environment after receiving.
During the experiment, the HA and HAP groups were fed in a hypoxic oxygen chamber simulator to simulate an equivalent physiological altitude of 3500–4000 m, and the pressure was set to 60–65 kPa. Group CON was fed in an ordinary environment outside the hypobaric oxygen chamber at a pressure of 94.5 kPa, and the other conditions were the same. The HAP group was intragastrically administered with 0.2 mL HL79 solution (1 × 109 CFU/mL) once a day, and the CON and HA groups were intragastrically administered with 0.2 mL normal saline.
The rest of the environmental conditions such as temperature, humidity, consistent, 12 h of light–dark cycle, free eating and drinking water, six mice per cage. The hypobaric oxygen chamber simulator was opened for 1 h every day for food supply and gavage treatment.
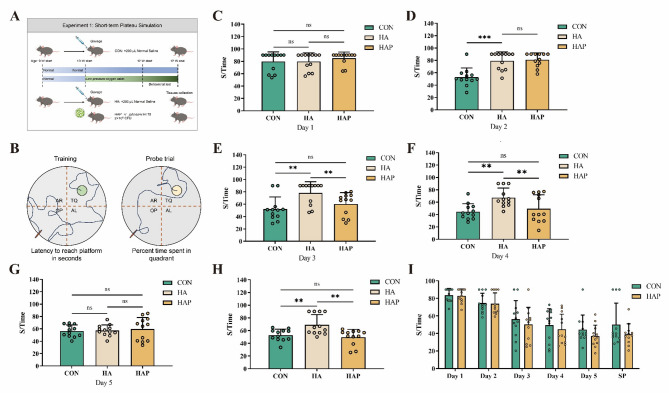
In Experiment 1 (as shown in Fig. 1A), a total of 36 mice (n = 12/group) were used with an experimental period of 21 days. Morris Water Maze (MWM) behavioral testing was conducted on days 16–20 of the experiment. On day 21, all animals were humanely euthanized via cervical dislocation under deep anesthesia. Brain tissues were rapidly dissected, with hippocampal regions isolated and immediately cryopreserved in liquid nitrogen at − 80 °C for subsequent mRNA quantification and immunoblot analysis, or immersion-fixed in 4% paraformaldehyde (PFA) solution (pH 7.4, 24 h at 4 °C) prior to immunofluorescence.
Fig. 1.
HL79 rescues plateau environment exposure-induced spatial memory deficits. A schematic of HL79 intervention in plateau-environment-exposed experimental design. B Schematic representation of the Morris water maze. C–I Escape latency across training days in different experimental groups. CON: Normoxic control. HA: Hypobaric hypoxia (simulated 3,500–4,000 m plateau). HAP: Hypoxia cohort supplemented with 1 × 101⁰ CFU/day HL79 via gastric gavage. Abbreviations maintained throughout subsequent analyses. Data are presented as mean ± standard deviation. One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 were considered significant, and ns means no significant difference
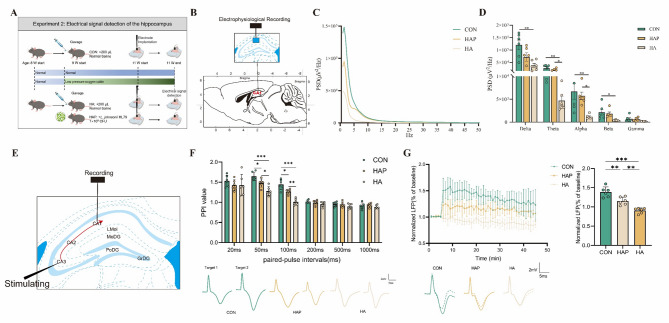
In Experiment 2 (as shown in Fig. 4A), a total of 36 mice (n = 12/group) underwent Michigan electrode implantation surgery on day 15 of the experiment. On day 21, six mice were randomly selected from the surviving mice in each group for electroencephalogram (EEG) signal experiments.
Fig. 4.
Electrophysiological signal recording and analysis in the hippocampal CA1 subregion. A Position of the recording site in the left CA1 region. B, C The effects of high-altitude environment and HL79 on the power spectral density of LFP. D Schematic diagram of recording in the Schaffer collateral pathway-CA1 synapses of the left hippocampus, a stimulating electrode was positioned in the CA3 region and a recording electrode was located in the CA1 region. E Changes in paired-pulse index at different time intervals (top) and representation examples of evoked LFP responses of paired-pulse stimulation (down). F Representation examples of pre- and post-TBS traces (down), changes in LFP amplitude over recording (top left), and percentage change of LFP amplitude (top right). Data are presented as mean ± standard deviation. n = 6, one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 were considered significant, and ns means no significant difference
Transcriptome sequencing and analysis
Total RNA was isolated using the Trizol Reagent (No: 15596018CN, Invitrogen Life Technologies) and E.Z.N.A.® Total RNA Kit (No: R6834-02, OMEGA Bio-Tek), after which the concentration, quality and integrity were determined using a NanoDrop spectrophotometer (NanoDrop 2000C, Thermo Scientific). Three micrograms of RNA were used as input material for the RNA sample preparations. The libraries were constructed using the NEBNext Ultra II RNA Library Prep Kit for Illumina (No: E7770S). Sequencing libraries were generated according to the following steps. Firstly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Fragmentation was carried out using divalent cations under elevated temperature in an Illumina proprietary fragmentation buffer. First strand cDNA was synthesized using random oligonucleotides and Super Script II. Second strand cDNA synthesis was subsequently performed using DNA Polymerase I and RNase H. Remaining overhangs were converted into blunt ends via exonuclease/polymerase activities and the enzymes were removed. After adenylation of the 3’ ends of the DNA fragments, Illumina PE adapter oligonucleotides were ligated to prepare for hybridization. To select cDNA fragments of the preferred 400–500 bp in length, the library fragments were purified using the AMPure XP system (Beckman Coulter,Beverly, CA, USA). DNA fragments with ligated adaptor molecules on both ends were selectively enriched using Illumina PCR Primer Cocktail in a 15 cycle PCR reaction. Products were purified (AMPure XP system) and quantified using the Agilent high sensitivity DNA assay on a Bioanalyzer 2100 system (Agilent). The sequencing library was then sequenced on NovaSeq 6000 platform (Illumina) Shanghai Personal Biotechnology Cp. Ltd. There were 7 samples in each group, totaling 21 samples that were sequenced.
We use fastp (0.22.0) software to filter the sequencing data to get high quality sequence (Clean Data) for further analysis. The filtered reads were mapping to the reference genome using HISAT2 (v2.1.0). Then used FPKM (Fragments Per Kilo bases per Million fragments) to standardize the expression. Then difference expression of genes was analyzed by DESeq2 (v1.38.3) with screened conditions as follows: expression difference multiple|log2FoldChange|> 1, significant P-value < 0.05. Using ClusterProfiler (v4.6.0) to perform GO enrichment analysis on the differential genes, calculate P-value by hypergeometric distribution method (the standard of significant enrichment is P-value < 0.05), and find the GO term with significantly enriched differential genes to determine the main biological functions performed by differential genes. ClusterProfiler (v4.6.0) software was used to carry out the enrichment analysis of the KEGG pathway of differential genes, focusing on the significant enrichment pathway with P-value < 0.05.
Metabolite detection from mouse serum samples
Corresponding to the transcriptome sequencing samples, there were 7 samples per group, totaling 21 serum samples that underwent untargeted metabolite profiling. Of methanol, 800 µl was added to 200 µl of serum in 1.7-ml Eppendorf tubes. The tubes were sonicated for 1 min followed by another 10 min of vigorous stirring. Extracts were pelleted at 5,000 g for 5 min, and supernatants were transferred to 2-ml HPLC vials. Remaining pellets were further extracted with another 10 min of vigorous stirring in 500 µl ethanol. Extracts were pelleted at 5,000 g for 5 min, and the combined supernatants were dried in a SpeedVac (Thermo Fisher Scientific) vacuum concentrator. Samples were resuspended in 100 μl of methanol and pelleted at 5,000 g for 5 min. Clarified extracts were transferred to fresh HPLC vials and stored at − 20 °C until analysis. An ACQUITY UPLC HSS T3 column (100 Å, 1.8 µm, 2.1 mm × 100 mm) was used with a flow rate of 0.4 mL/min, a column temperature of 40 °C, an autosampler temperature of 8 °C, and an injection volume of 2 μL. Thermo Orbitrap Exploris 120 mass spectrometer was used to collect DDA mass spectrometric data in positive and negative ion modes under the control of Xcalibur software (version: 4.7, Thermo). HESI source, spray voltage 3.5 kV/− 3.0 kV, sheath gas 40 arb, auxiliary gas 15 arb, capillary temperature 325℃, auxiliary gas temperature 300℃, primary resolution 60,000, scan range 100–1000 m/z, AGC Target Standard, Max IT 100 ms, the top 4 ions were screened for secondary fragmentation, dynamic exclusion time was 8 s, secondary resolution 15,000, HCD collision energy 30%, AGC Target Standard, Max IT Auto. The raw format data was imported into the commercial software Compound Discoverer™ 3.3 (version 3.3.2.31, Thermo, Waltham, USA). Based on the new peak detection and peak quality scoring algorithm of the software, peak extraction, alignment, correction and other operations were performed. The unique peak quality rating calculation and filter greatly reduced the interference of background peaks and low-quality peaks. The peaks that were not detected in more than 50% of the QC samples were filtered, and the missing13 values of the undetected peaks were filled based on the software Fill Gaps algorithm, and the Sum total peak area was normalized. The identification of metabolites was based on self-built library PSNGM Database, mzCloud online library (https://www.mzcloud.org/), LIPID MAPS (https://www.lipidmaps.org/), HMDB (https://hmdb.ca/), MoNA (https://mona.fiehnlab.ucdavis.edu/) and NIST_2020_MSMS spectral library. The MS1 mass tolerance was set to 15 ppm and the MS2 Match Factor Threshold was set to 50.
Electrical signal detection of the hippocampus
Refer to the method of Xin et al. (2023). In short, under anesthesia, Michigan recording electrodes (16-channels, 8 channels per shank to confirm the electrode at the appropriate position, 50 µm diameter, 100 µm distance, KedouBC) were stereotaxically (Stereotaxic apparatus, KW-DWY-S, Nanjing Calvin Biotechnology Co., Ltd.) implanted into the CA1 region (A/P =– 2.2 mm, L/M = + 1.5 mm, D/V =– 1.2 mm relative to bregma), while stimulating electrodes were positioned in CA3 (A/P =– 2.2 mm, L/M = + 2.8 mm, D/V =– 1.8 mm relative to bregma) using mouse brain atlas coordinates (Paxinos and Franklin 2019). The signal of PSD analysis is the spontaneous activity signal recorded by a single brain region without external stimulation. A cerebellar ground electrode served as the common reference for all recordings. Local field potentials (LFPs) were acquired from the left hippocampal CA1 subfield in response to Schaffer collateral-CA1 pathway stimulation.
Synaptic responses in the CA1 region of the hippocampus evoked by stimulation with the CA3 Schaffer were recorded. Short-term synaptic plasticity (STP) was measured using paired-pulse stimulation. Stimulations (300 μA, 50 μs duration, square pulse, 10-s interval) were delivered through a pulse stimulator (Master-9, A.M.P.I). Paired-pulse stimuli were delivered at interstimulus intervals (ISIs) of 20, 50, 100, 200, 500, and 1000 ms, with 20 trials at 10-s intervals to assess short-term plasticity (STP) dynamics. All electrophysiological recordings were conducted using the Plexon Data Acquisition System (Plexon, USA). All electrical data were analyzed off-line using built-in and custom-written MATLAB codes.
Morris water maze test
The Morris Water Maze (MWM) test was carried out in a circular plastic pool with a diameter of 120 cm and a depth of 50 cm. The Morris water maze video analysis system (WMT-100S Morris, Chengdu TECHMAN Software Co., Ltd.) divides the maze into four equal quadrants, and cards with different shapes and colors was fixed on the wall of the pool in different quadrants to distinguish. The video recording camera model is 1/3" SONY CCD. The center of quadrant I in the pool contains a transparent plastic escape platform 6.5 cm, hidden 1 cm below the water surface, and the three times distance of the radius around the escape platform is defined as the effective area. During the test, the reference marker and position of the escape platform remained unchanged, and potato starch was used to adjust the water to opaque white. The test is divided into two parts: (1) Place navigation test: facing the wall of quadrant III, release the mice and let them explore freely. Each mouse had 90 s to find the platform. If mice did not find the platform within 90 s, they were guided to the platform and stay for 15 s. The escape latency and movement trajectory of all mice search platform were recorded. Place navigation test was conducted once a day and spanned over five consecutive days. (2) Space exploration test: After the plate navigation test was completed on the 5th day, the escape platform was withdrawn from the pool. Each mouse rested for 30 min after the place navigation test for space exploration. The mice, with their heads facing the wall, were released in water, and the trajectory within 90 s was recorded. The escape delay, the time spent in the effective area and the number of platform crossings were calculated and analyzed.
Real-time quantitative polymerase chain reaction (qPCR)
Six samples were selected from each group, totaling 18 samples that underwent RNA extraction and reverse transcription. Isolation of hippocampal RNA was performed according to a previously described method (Xin et al. 2021). Briefly, total RNA was isolated from 10 mg of hippocampus using the E.Z.N.A.® Total RNA Kit (No: R6834-02, OMEGA Bio-Tek) and reverse-transcribed to first-strand complementary DNA using the PrimeScript RT reagent kit with gDNA Eraser (No: RR047B, Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Gene expression of related mRNAs was performed using SYBR green (No: 1725121, Bio-Rad, Hercules, CA, USA) and primers using a LightCycler 96 (No: 05815916001, Roche, UK). β-actin was used as a reference gene to normalize the relative mRNA expression levels of the target genes, with values presented as 2−ΔΔCt. The primer sequences and optimum annealing temperatures are listed in Supplementary Table S1. Total DNA was isolated from 10 mg of hippocampus using an E.Z.N.A.® Tissue DNA Kit (No: R6834-02, OMEGA Bio-Tek), according to the manufacturer’s instructions. Copy numbers of mitochondrial DNA relative to diploid chromosomal DNA content were analyzed by RT-qPCR in a LightCycler 96 using SYBR green and primers of mRNA and β-actin (Table.S1).
Immunofluorescence
After fixation, the mouse brains were dehydrated through a gradient of 15%, 20%, and 30%, and then embedded in optimal cutting temperature (OCT) compound (No. 4583, Sakura Finetek). Sections of 40 μm were cut using a Leica cryostat (Leica CM1950, Leica Biosystems). Five brain sections per group were stained, with each section representing an individual mouse. The sections were placed in a repair cassette filled with citric acid (pH 6.0) antigen repair solution in a microwave oven for antigen repair. The samples were then subjected to a medium fire for 8 min to reach a boil, the fire was turned off for 8 min, and then the fire was turned to low and medium for 7 min. After natural cooling, the glass slides were placed in PBS (pH 7.4) and washed three times with shaking on a decolorization shaker for 5 min. The sections were shaken slightly, circled around the tissue using a histochemical pen, dried in phosphate buffered saline (PBS), and closed for 30 min with a drop of BSA. Next, the blocking solution was gently shaken off and rabbit polyclonal anti-BDNF (1:200) (ab108319), rabbit polyclonal anti-SYP (1:100) (GB11533), rabbit anti-CREB pAb (1:400) (GB11539-100) were added dropwise to the sections, which were placed flat in the sections and incubated overnight at 4 °C in a wet box. Thereafter, the slides were placed in PBS (pH 7.4) and washed three times for 5 min each by shaking on a decolorization shaker. The sections were then removed and washed with PBS. Goat anti-rabbit IgG (1:300) was added dropwise, and the slides were incubated for 50 min at room temperature before being placed in PBS (pH 7.4) and washed three times for 5 min each by shaking on a decolorization shaker. The slides were shaken and washed three times for 5 min each in PBS (pH 7.4) on a decolorization shaker. The autofluorescence quencher was added to the circles for 5 min and then rinsed under running water for 10 min. The slides were shaken, sealed with an antifluorescence quenching sealer, and imaged using an OLYMPUS microscope. All above-mentioned reagents were purchased from Servicebio Technology Co., Ltd. (Wuhan, China), except for BDNF, which was obtained from Abcam. All sections were imaged using a Nikon confocal microscope (Nikon A1/C2, NIS-Elements 5.30.07 Build 1569, Nikon).
Biochemical analysis
Antioxidant indices in the hippocampus were measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China), including total antioxidant capacity (T-AOC) (A015-3), glutathione peroxidase (GSH-Px) (A006-1-1), superoxide dismutase, and malondialdehyde (MDA) (A003-1-1). All reagents were purchased from Servicebio Technology Co., Ltd. (Wuhan, China).
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Normal distributions were assessed using the Shapiro–Wilk normality test. If the data were not normally distributed, normality tests were repeated after log-transformation. Data that did not reach a normal distribution were tested using the Kruskal–Wallis test at P < 0.05, followed by the Wilcoxon rank-sum test. If the data were normally distributed, one-way analysis of variance (ANOVA) was performed. Statistical data analysis was performed using the IBM SPSS Statistics 25 (IBM Corporation) software. *P < 0.05 and **P < 0.01 indicate a significant difference, while ***P < 0.001 indicates an extremely significant difference. The mapping software used was GraphPad Software 7.0.
Results
HL79 rescued memory dysfunction caused by simulated plateau environment exposure
Morris’s water maze was used to evaluate the possible role of HL79 in the intervention of brain cognitive function caused by plateau environment (Fig. 1A, B). As described in the previous animal handling procedures, a total of three groups were established. The group housed in the hypobaric chamber and treated with saline was designated as the HA group, the group housed in the hypobaric chamber and administered HL79 via gavage was designated as the HAP group, and the group maintained in a normal, non-hypobaric environment and treated with saline was designated as the CON group. As shown in Fig. 1C, D, there was no significant difference between the HA group and the HAP group in the first two days of the navigation test. However, compared with HA, the escape latency of the control group was significantly (P < 0.001) shortened on the second day, and this trend continued until the fifth day, until the escape latency of the HA group was shortened (Fig. 1E–G). This suggests that the plateau environment causes brain spatial memory impairment in mice. It is worth noting that from the third day, there was no statistical difference in the escape latency time between the HAP group fed with HL79 and the control group, but it was still significantly different from the HA group (P < 0.01), indicating that HL79 alleviated the memory impairment caused by high altitude low pressure (Fig. 1E). In the space exploration experiment on the fifth day, there was no significant difference between the HAP group and the control group, but the escape latency of these two groups was significantly (P < 0.01) lower than that of the HA group (Fig. 1H). During the whole experimental period, there was no significant difference in the escape latency between the HAP group and the control group (Fig. 1I). This shows that the plateau environment can cause spatial memory damage in mice, and the application of HL79 may shorten the recovery time of the damage.
HL79 rescues hippocampal damage caused by simulated plateau environment exposure.
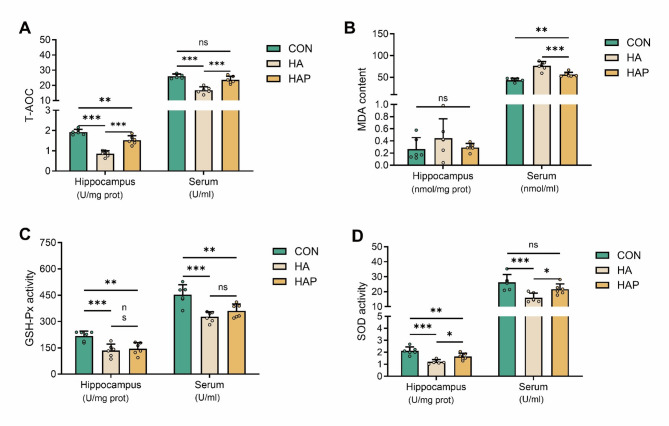
We continued to evaluate the levels of oxidative stress in mouse serum and memory-related hippocampal regions. Compared with the plain control group, the total antioxidant capacity, glutathione peroxidase and superoxide dismutase activities in the hippocampus of the HA group were significantly (P < 0.001) reduced in the plateau environment (Fig. 2A–D). Although the application of HL79 probiotics was able to reverse this trend (total antioxidant capacity and superoxide dismutase), it was still significantly (P < 0.01) lower than the control group. In addition, the decrease of glutathione peroxidase activity was not reversed. At the same time, we also noticed that no matter the plateau environment or the application of HL79, there was no significant difference in the content of malondialdehyde products in the hippocampus of mice (Fig. 2B). For the serum antioxidant performance of each group, the difference trend between the HA group and the control group was consistent with the trend of hippocampus. The total antioxidant capacity, glutathione peroxidase and superoxide dismutase activity of serum were significantly (P < 0.001) decreased in plateau environment (Fig. 2A, C, D). However, it is noteworthy that the application of HL79 almost completely reversed the total antioxidant capacity and superoxide dismutase enzyme activity in serum, which was significantly (P < 0.05) different from the HA group and not statistically different from the control group (Fig. 2A, D). At the same time, the activity of glutathione peroxidase in serum was also increased, but not significantly compared with the HA group (Fig. 2C). In addition, different from the hippocampus, the application of HL79 could significantly (P < 0.001) inhibit the increase of malondialdehyde content in serum of mice caused by plateaus environment, but it was still significantly (P < 0.01) lower than that of the control group (Fig. 2B). These results suggest that plateau environment can cause oxidative stress damage in hippocampus and serum of mice, and the application of HL79 can partially alleviate these negative effects.
Fig. 2.
Assessment of key oxidative stress biomarkers in hippocampus and serum. A Total antioxidant capacity (T-AOC). B Lipid peroxidation product malondialdehyde (MDA). C Glutathione peroxidase activity (GSH-Px). D Superoxide dismutase activity (SOD). Data are presented as mean ± standard deviation. One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 were considered significant, and ns means no significant difference
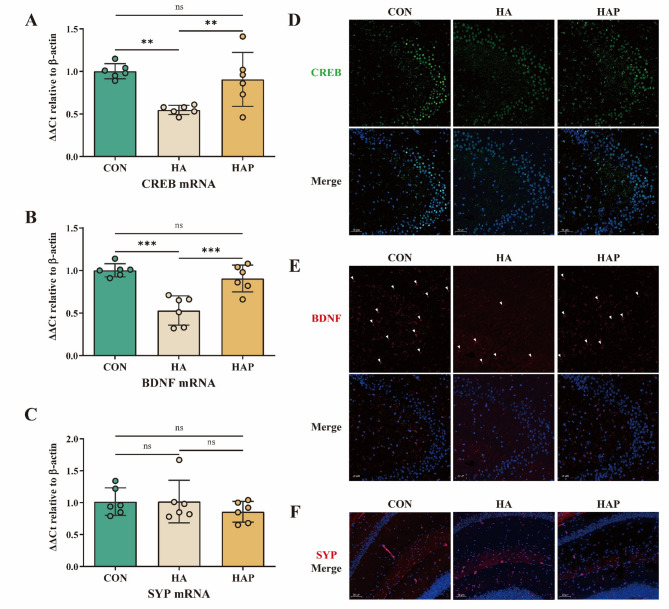
Changes in brain memory function are often related to biological processes such as synaptic plasticity and synaptic regeneration. In addition to the spatial memory and oxidative damage of hippocampus in mice caused by plateau environment, it may also change the expression of some important proteins in hippocampus. Compared with the control group, the mRNA transcription level of cAMP-response element binding protein in the HA group was significantly decreased (P < 0.01), and the transcription level of brain-derived neurotrophic factor BDNF, a downstream protein of CREB, was significantly decreased (P < 0.001), but the transcription level of synaptic protein was not significantly changed (Fig. 3A, B). Contrary to this trend, the transcription levels of CREB and BNDF proteins in HAP group treated with HL79 were significantly (P < 0.01) higher than those in HA group, but still lower than those in control group. In addition, there was no significant change in the transcription of SYP protein (Fig. 3C). Based on immunofluorescence, the actual expression of the above proteins in the tissues was further evaluated. As shown in Fig. 3D, E, compared with the control group, the CREB and BDNF proteins in the hippocampus of the HA group were significantly reduced, while the HAP group reversed this phenomenon, similar to the control group. Similarly, in the immunofluorescence results of hippocampus, the SYP protein fluorescence signals between the three groups were basically the same, and there was no significant difference (Fig. 3F). The trend of the results was consistent with the results of mRNA transcription level. In general, the results of mRNA transcription and immunofluorescence suggested that the spatial memory impairment caused by plateau environment may be related to the decrease of CREB transcription factor protein expression and the decrease of downstream protein BDNF expression, while HL79 can inhibit this negative effect and prevent the decrease of related protein expression caused by plateau environment.
Fig. 3.
Assessment of hippocampal synaptic plasticity-associated markers. A–C mRNA relative quantification of CREB, BDNF, and SYP genes in hippocampal tissue, normalized to CON group (set as 1). D–F Representative immunofluorescence images of CREB, BDNF, and SYP proteins in the hippocampus (scale bar: 50 μm). D Green represents CREB staining, blue represents DAPI staining. E Red represents BDNF staining, with DAPI. F Red represents SYP staining, and blue represents DAPI. Data are presented as mean ± standard deviation. One-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 were considered significant, and ns means no significant difference
Simulated plateau environment exposure and application of probiotics HL79 altered the neuronal firing of hippocampal
Behavioural studies have shown that the mouse brain has undergone changes. We first detected changes in neural oscillations and neuronal firing activity in the hippocampus. Extracellular recording of neurons in the hippocampal CA1 region of mice was performed by implanting electrodes (Fig. 4B). The power spectral density (PSD) of brain signals quantifies the energy distribution of three groups in the frequency domain (Fig. 4C), thereby enabling further analysis of brain rhythm patterns. Exposure to simulated plateau environment resulted in a significant decrease (P < 0.05) in the power of local field potential (LFP) bands (except Gamma) (Fig. 4D). Compared with the control group, the power of theta and alpha activities in HA and HAP groups was significantly reduced (P < 0.05). It is worth noting that after the application of HL79, HAP significantly (P < 0.05) improved the average power of theta and alpha compared with HA group, but still lower than CON group (P > 0.05). In addition, Delta, Beta and Gamma have improved, but not significantly.
Then we used different paired-pulse stimulation intervals to detect short-term plasticity in the hippocampal CA1 pathway in mice. STP is considered to be the dynamic synaptic effect of transient transmission processing (from milliseconds to seconds) during regulation and plays an important role in the biological sensory system. We measured the induced LFP amplitude and the pair-pulse index (PPI) of each interval (Fig. 4E, F). HA group and HAP group had lower PPI values than CON group at 20 ms, 50 ms, 100 ms, 200 ms, 500 ms and 1000 ms. However, we only found that weak PPF with significantly decreased PPI values occurred at 50 ms and 100 ms, in the comparison between HA and CON (P < 0.001). The CON group showed clear and early paired-pulse facilitation (PPF) at 50 ms and 100 ms. However, the HA group significantly reduced PPI values at 50 ms and 100 ms (P < 0.001), and had no paired-pulse depression (PPD) ability at 100 ms. Although HAP significantly increased the PPI value (P < 0.05) compared with HA, and failed to fully restore the decrease of PPI value caused by simulated plateau environment, it still had strong PPD ability. In general, HA and HAP were almost unaffected at 20 ms and longer intervals (200 ms, 500 ms and 1000 ms), but significantly decreased at 50 ms and 100 ms intervals, with the greatest impact in the HA group. Theta-burst stimulation consisting of 5 bursts of 5 stimuli at 100 Hz with an interval of 200 ms and 10 repetitions was used to induce stable LTP in CA1 of CON group and HAP group. The results showed that the LTP of HAP group was significantly lower (P < 0.01) than that of CON group, but still significantly (P < 0.01) higher than that of HA group. We noted that the HA group induced LTP E2 / E1 ratio < 100%, indicating that TBS failed to successfully induce LTP of CA1 in the HA group, and the HA group had a stronger LTP inhibition. This suggests that the ability to induce LTP after exposure to simulated plateau environment is impaired, and the application of HL79 probiotics partially alleviates the impaired LTP response ability (Fig. 4G).
The glucose and lipid metabolism genes in the hippocampus after simulated plateau environment exposure were affected by HL79
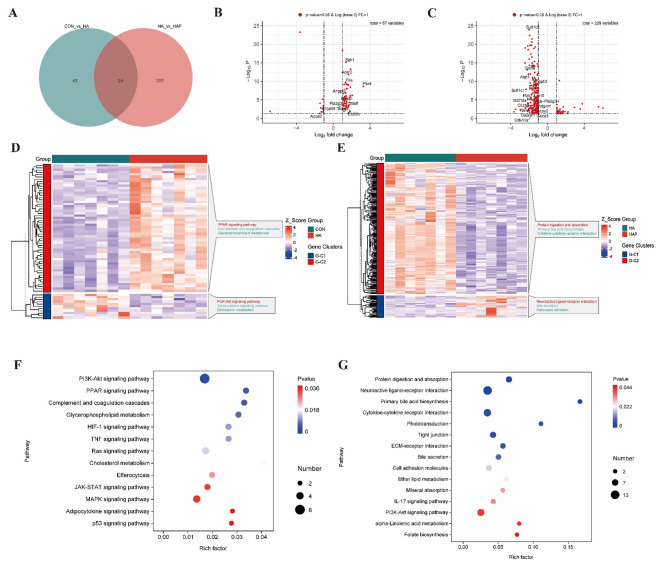
We further explored what changes in the mouse hippocampal transcriptome caused by simulated plateau environment and the application of HL79 strain. Transcriptome sequencing was performed on the hippocampus of three groups of mice (Fig.S1A-C). As shown in Fig. 5A, 67 (56 up-regulated, 11 down-regulated) and 229 (33 up-regulated, 196 down-regulated) mRNA levels were significantly (P < 0.05) changed between CON and HA, HA and HAP groups (Fig.S1D, Extended Data Table 1–2), respectively. In the comparison of CON and HA, these differentially expressed genes were mainly enriched in the KEGG Complement and coagulation cascades, Glycerophospholipid metabolism, Cholesterol metabolism, Efferocytosis pathway, among which Cholesterol metabolism had the highest rich factor (Fig. 5F). Similarly, in the comparison of HA and HAP, these differential genes were mainly enriched in KEGG's Protein digestion and absorption, Primary bile acid biosynthesis, Bile secretion, Ether lipid metabolism, alpha-Linolenic acid metabolism, Folate biosynthesis (Fig. 5G). These pathways have been reported to be involved in fat metabolism or related to the host body. This suggests that the lipid metabolism of our host body may change significantly under simulated high-altitude conditions or after the application of HL79. We further specially annotated mRNAs with significant changes in the comparison of CON and HA, HA and HAP and reported to be related to processes such as fat synthesis and metabolism in the volcano plot (Fig. 5B, C). Among the differentially expressed genes identified between CON and HA groups, 12 associated mRNAs were detected, with 11 demonstrating significant upregulation in the HA group (Fig. 5B). A total of 18 associated mRNAs were identified among differentially expressed genes in HA versus HAP comparison, with all transcripts exhibiting significant downregulation in the HAP group (Fig. 5C). Therefore, we speculate that the plateau simulated environment has an important impact on the biological process of host fat synthesis or metabolism, and the application of HL79 can improve this process.
Fig. 5.
Effects of plateau environment exposure and HL79 gavage colonization on hippocampal gene transcription profiles. A Venn diagram illustrating differentially expressed genes (DEGs) among experimental groups, with the comparison between HA and CON groups designated as CON vs HA. Subsequent comparisons follow this nomenclature. B, C Volcano plots displaying DEGs for CON vs HA and HA vs HAP comparisons, respectively. Genes meeting the threshold criteria (P < 0.05 and|Log2FoldChange|> 1) are shown, with lipid metabolism-associated genes explicitly labeled. D, F Hierarchical clustering heatmap of DEG expression patterns and corresponding KEGG pathway enrichment analysis for the CON vs HA comparison. E, G Parallel analyses (gene expression clustering and pathway annotation) for the HA vs HAP comparison are presented correspondingly
HL79 mainly changed glucose and lipid metabolism in serum.
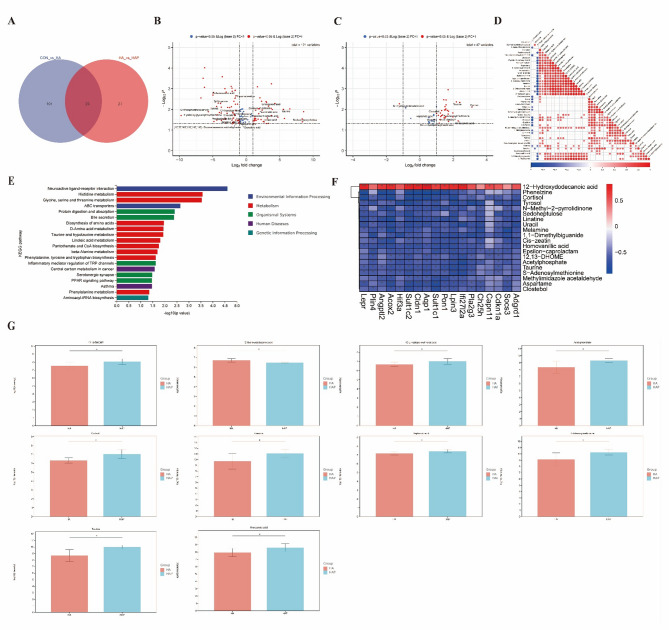
Based on the above transcriptome results, it is suggested that the host fat synthesis metabolic pathway is significantly affected, and whether it can be reacted on metabolites. Therefore, we further detected the serum metabolites of each group of mice. In the comparison between CON and HA group, a total of 121 significantly (P < 0.05) changed metabolites were found, of which 45 were up-regulated and 76 were down-regulated in HA group (Fig. S1E, Extended Data Table 3). In the comparison between HA and HAP groups, a total of 47 metabolites with significant (P < 0.05) changes were found, of which 44 were up-regulated and 3 were down-regulated in HAP group (Fig.S1E, Extended Data Table 4). Among the significantly (P < 0.05) changed metabolites, we found that there were 23 potential substances related to lipid metabolism in CON VS HA, and 10 potential substances related to lipid metabolism in HA VS HAP (Fig. 6B, C). Pearson correlation was calculated for the abundance of these differential metabolites (Fig. 6D, Fig.S1F-G). Enrichment pathway display of differential metabolites based on KEGG data (Fig. 6E). The metabolites of HA vs HAP were mainly involved in Neuroactive ligand-receptor interaction, Bile secretion, Taurine and hypotaurine metabolism, Linoleic acid metabolism, PPAR signaling pathway, Pantothenate and CoA biosynthesis, Protein digestion and absorption, Glycine. These pathways have been reported to affect lipid transport, lipid digestion and absorption, lipid oxidation, and sphingomyelin synthesis (Fig. 6E). Among the metabolites of CON vs HA, Sphingolipid signaling pathway, Linoleic acid metabolism, Cholesterol metabolism and Sphingolipid metabolism were also found to be directly involved in the process of lipid metabolism (Fig.S2A). The changes of these serum metabolites are consistent with the changes of the above transcriptome, which shows that the genes or substances related to the lipid metabolism process of the host are mainly affected. We performed Pearson correlation analysis on 18 mRNAs related to lipid metabolism found in HA vs HAP and metabolites with significant (P < 0.05) changes, and showed the top 20 metabolites with the most significant (P < 0.05) correlation (Fig. 6F). Among them, 12-Hydroxydodecanoic acid, 12,13-DHOME, Sedoheptulose, 1,1-Dimethylbiguanide and Cortisol have been reported to regulate and participate in glucose metabolism and lipid metabolism. Phenelzine and Homovanillic acid have been reported to be related to neurotransmitter metabolism, while Tyrosol and 12,13-DHOME have been reported to be related to antioxidant and inflammatory processes. Glucose 6-phosphate, L-arabinose and dodecanedioic acid are directly involved in glucose and lipid metabolism in CON vs HA (Fig.S1H). There is evidence that Serotonin and Benzylamine are involved in neurotransmitter metabolism, and Fisetin and Ascorbate are involved in host antioxidant and inflammatory regulation (Fig.S1H). Based on the results of the analysis, we show the abundance of some representative metabolites separately (Fig. 6G, Fig.S2B).
Fig. 6.
Impacts of plateau environment exposure and HL79 gavage colonization on serum metabolomic profiles. A Venn diagram of differential metabolites identified through pairwise comparisons across experimental groups. B, C Volcano plots visualizing differential metabolites in CON vs HA and HA vs HAP comparisons, respectively. Red dots denote metabolites meeting significance thresholds (P < 0.05 and|Log2FoldChange|> 1), with lipid-related metabolites text-labeled. D Pearson correlation matrix of differentially abundant metabolites in the HA vs HAP comparison. E KEGG pathway enrichment analysis of HA vs HAP differential metabolites. F Heatmap depicting Pearson correlations between lipid-associated hippocampal DEGs and the top 20 most significantly correlated differential metabolites from HA vs HAP serum profiles. G Abundance profiles of lipid metabolism-related metabolites among HA vs HAP differential serum metabolites. Data are presented as mean ± standard deviation. n = 6, Student's t test. *P < 0.05, **P < 0.01, ***P < 0.001 were considered significant, and ns means no significant difference
Discussion
The harsh environment of the plateau is the biggest challenge for staying or permanent residence. Its low pressure, low oxygen, low temperature and other environmental conditions have a lasting impact on the physiology and psychology of the adaptor. In the exposure stage of ≤ 1 month, the body often reacts violently to the external environment. However, due to the inability to adapt to the process quickly, individuals often show different degrees of executive function, attention, psychological processing speed, language ability and memory. At present, the main way to deal with these negative reactions is to strengthen adaptive training before entering the plateau environment, while in the plateau environment, reducing activity, reasonable oxygen inhalation, plateau oxygen cabin or taking drugs such as acetazolamide and dexamethasone. In addition, it is worth mentioning that there is evidence indicates that gut microbiota significantly impacts an organism's ability to adapt to plateau environments by adjusting its composition, and hence impacting its function and ability to release microbial metabolites. However, based on the perspective of gut microbiota, there is still a lack of research on how the body regulates and adapts to the process of plateau environment, as well as external intervention and improvement of its negative effects.
Plateau environment can cause gut microbiota dysbiosis, intestinal barrier function damage, and induce intestinal inflammation. In our previous study, it was found that the exposure to plateau environment made the Lactobacillus in the gut microbiota of mice almost completely disappear, and the opportunistic pathogen Staphylococcus replaced its main position (Wan et al. 2022). In fact, the response of individual bacterial genera to Plateau environment exposure is not the same. Studies have shown that Plateau environment exposure will reduce the abundance of Bacteroides, Parabacteroides, Lactobacillus, and Fecalibacterium, while Prevotella, Alistipes, and Desulfovibrio are significantly increased (Han et al. 2022; Pan et al. 2022; Qi et al. 2023). These reduced microbiotas are mainly reported to be involved in the production of short-chain fatty acids in gut. When these microorganisms change, the whole community structure is unbalanced, the original flora structure is broken and then after a long time of recovery, and then tends to be similar to the microbial community with plateau environmental structure (Jia et al. 2020; Su et al. 2024a, b). In addition, plateau environment exposure regulates the expression of inflammatory factors such as IL-1β, TNF-α and TGF-β in intestinal tissue, which may lead to intestinal inflammation in mice, increase intestinal mucosal permeability and destroy intestinal barrier (Wan et al. 2022). Although the effects of plateau environment exposure on the intestine are mentioned above, the impact on the brain is more important, which is directly reflected in the structural and functional changes of the brain. Studies have shown that the gray matter volume of the left inferior temporal gyrus of the exposed population in the plateau environment increased, and the anisotropy index of the white matter structure of the corpus callosum, corona radiata and superior and inferior longitudinal fasciculus increased (Bao et al. 2022; Chen et al. 2019). The increase of gray matter structure is related to the proliferation of nerve cells and angiogenesis, while the increase of white matter FA index indicates that the microstructure of white matter is damaged, and this change may be the basis of cognitive function change. It has been confirmed that exposure to plateau environment has a moderate negative impact on brain cognitive ability (Bao et al. 2023; Su et al. 2024a, b). Gut microbiota dysbiosis or clearance also exacerbates cognitive dysfunction in mice exposed to high altitude conditions, such as working and spatial memory dysfunction (Wan et al. 2022; Zhang et al. 2023). Based on the microbiota-gut-brain axis hypothesis, the brain functions such as cognitive impairment, learning and spatial memory can be improved by reconstructing gut microbiota or regulating microbiota homeostasis. Therefore, we propose whether the negative effects of brain function caused by plateau environment exposure can also be alleviated by probiotic intervention.
In this study, we gave HL79 strain intragastric intervention during simulated plateau environment exposure. In the simulated plateau environment exposure test (Fig. 1), 1 × 109 CFU intervention accelerated the recovery of spatial memory and learning ability in mice. Consistent with previous studies, the untreated plateau environment exposure group (HA) showed learning and memory deficits and continued until the 5th day of the test, and the escape latency was significantly increased compared with the control group (Bao et al. 2023; Zhang et al. 2023; Zhao et al. 2022). Synaptic plasticity is one of the key mechanisms to explain this change in learning and memory deficits. It refers to the ability of synaptic connections to form, strengthen, weaken, and change. Brain-derived neurotrophic factor is a key mediator of synaptic plasticity in the brain. It can promote the growth of neurons, promote the formation and stability of synapses in brain nerve cells, and promote long-term potentiation (Castrén and Monteggia 2021). It is worth noting that the level of BDNF mRNA is precisely regulated by the CREB family. The CREB family and co-transcription factors mediate Ca2+ dependent transcriptional regulation by binding to the CRE element in the BDNF gene, thereby affecting synaptic function (Tao et al. 1998). Synaptophysin (SYP) is a vesicle-adsorbing protein closely related to synaptic structure and function. It is involved in Ca2+ dependent neurotransmitter release and synaptic vesicle cycle, and is considered to be an important marker of synaptic initiation and synaptic remodelling (Wang et al. 2024). In this study, we found that plateau environment exposure significantly reduced the mRNA transcription and protein expression of CREB and BDNF in the hippocampus of mice, which further emphasized its effect on the memory function of the hippocampus of mice. Zhang et al. reported that exposure to the plateau environment significantly reduced the antioxidant capacity of the hippocampus, which was further aggravated after the use of antibiotics to remove the intestinal flora (Zhang et al. 2023). Similarly, we examined common indicators of oxidative stress damage, such as T-AOC, GSH-PX, MDA, and SOD. Obviously, the oxidative damage of the hippocampus was aggravated in the plateau environment. However, the use of HL79 alleviated some of its oxidative damage, although its mechanism of action is not yet clear.
Current electrophysiological techniques can enable the amplification of weak bioelectrical signals from different tissues for easy observation. When the brain is working, the neurons in the corresponding brain area are activated synchronously and the postsynaptic potential is formed by the sum of the brain waves, which record the changes of the brain activity (Reinhart and Nguyen 2019). It is the overall reflection of the electrophysiological activity of the brain nerve cells in the cerebral cortex or the surface of the scalp. In this study, we used optical fiber electrodes to record the electrical signals generated by the neuronal population in the CA1 region under plateau environment exposure conditions. The power spectral density of the brain waves of the species was significantly lower than that of the control group, which explained the memory dysfunction of the HA group to some extent. After intervention with HL79, the power spectra of Beta and Gamma were significantly improved, and these two waves have been reported to reflect cognitive reasoning, learning, memory and processing ability. The Beta and Gamma power intensities of individuals with mental disorders and learning disabilities are often lower than the average. The θ burst stimulation from CA3 to CA1 suggests that exposure to the plateau environment also causes the projected neurons to lose the potential to amplify the neuronal signals they receive (Cole et al. 2024), indicating that the hippocampus cannot recall information, and the application of HL79 can only save part of it and cannot be completely reversed. This is consistent with the results of the water maze. Plateau environment exposure affects the special area of the brain, resulting in oxidative damage to the hippocampus and memory impairment.
The plateau environment induces profound alterations in host organismal gene expression and clinical blood parameters (Liu et al. 2023). Large-scale genomic analyses of Tibetan populations and comparative genomics data have revealed that the genetic diversity of EPAS1/EGLN1 loci underlies the molecular basis for the hypoxia-insensitive response observed in Tibetans at the transcriptomic level (Lorenzo et al. 2014; Xin et al. 2020). Under hypoxic conditions, EGLN protein activity is suppressed, resulting in negative regulation of HIF protein stability. Experimental translocation of sheep from low to high altitudes revealed that organs and tissues integral to rapid hypoxia adaptation-including the kidneys, colon, adipose tissue, and cerebellum, which mediate energy metabolism, endocrine regulation, and neurological functions-are preferentially affected. Notably, the cerebellum exhibited tissue-specific differentially expressed genes (DEGs) associated with synaptic signaling (e.g., NTNG1, TNF, and GABBR2) and cellular stress response modulation (e.g., HIF1A, ATF4, and PARG) (Yan et al. 2024). In this study, plateau environment exposure induced differential expression of 67 genes, predominantly enriched in KEGG pathways including the PPAR signaling pathway, Complement and coagulation cascades, Glycerophospholipid metabolism, Cholesterol metabolism, and Efferocytosis pathway (Fig. 5B, D, F). Consistent with prior findings, these pathways directly or indirectly regulate systemic lipid metabolism. For example: Serum/glucocorticoid-regulated kinase 1 (SGK1) (Fig. 5B) activates mTORC1/SREBP to stimulate de novo lipogenesis (Vaidyanathan et al. 2022). Fatty acid synthase (FAS) (Fig. 5B) catalyzes the conversion of acetyl-CoA and malonyl-CoA into long-chain saturated fatty acids in the presence of NADPH. PLIN4 (Fig. 5B), a key regulator of lipid droplet mobilization and signaling in the brain, modulates neuronal mitophagy in mice (Han et al. 2018). APOA2 (Fig. 5B) encodes apolipoprotein A-II (apoA-II), whose elevated plasma levels correlate with hypertriglyceridemia and reduced HDL cholesterol (Florea et al. 2022). Intriguingly, administration of HL79 maintained significant enrichment of lipid metabolism-related pathways in the hippocampus of plateau environments-exposed mice, such as Protein digestion and absorption, Primary bile acid biosynthesis, Bile secretion, Ether lipid metabolism, α-linolenic acid metabolism, and Folate biosynthesis. Notably, LPIN3 (Fig. 5C) functions not only as a phosphatidic acid phosphatase (PAP) essential for triglyceride synthesis but also as a transcriptional coactivator regulating lipid synthesis genes (Reue and Zhang 2007; Zhou et al. 2022). AQP1 (Fig. 5C) knockout in mice revealed its role as a modulator of brown adipose tissue quiescence (Cheng et al. 2024). ANGPTL2 (Fig. 5C) deficiency ameliorates adipose tissue inflammation and systemic insulin resistance in diet-induced obese mice (Tabata et al. 2009).These results collectively suggest that HL79 mitigates plateau-environment-induced hippocampal dysfunction (e.g., memory impairment, oxidative stress, and metabolic disruption) by modulating lipid-associated genes and pathways. Subsequent serum metabolomics corroborated this hypothesis. Comparative analyses identified 23 and 10 lipid metabolism-related metabolites differentially abundant between CON vs HA groups and HA vs HAP groups, respectively. KEGG enrichment of these metabolites highlighted pathways including Neuroactive ligand-receptor interaction, Bile secretion, Taurine/hypotaurine metabolism, Linoleic acid metabolism, PPAR signaling, Pantothenate/CoA biosynthesis, Protein digestion/absorption, and Glycine metabolism (Fig. 6E). Correlation analysis revealed 12-hydroxydodecanoic acid as the sole metabolite positively associated with lipid metabolism-related DEGs (Fig. 6F). As a fatty acid, it directly participates in lipid storage and energy mobilization, while other metabolites are implicated in glucolipid metabolism, neurotransmitter regulation, antioxidant defense, and inflammatory modulation (Macêdo et al. 2022; Muriana et al. 2017; Simpson et al. 2012; Stanford et al. 2018; Zhao et al. 2024). These findings collectively support the hypothesis that Lactobacillus johnsonii HL79 intervention rescues hippocampal memory deficits and oxidative stress in plateau-environment-exposed mice, likely through reprogramming hippocampal gene expression and modulating circulating metabolites in peripheral blood.
Conclusion
Prolonged exposure at plateau environment imposes multifaceted adverse effects on physiological systems. In mice subjected to a 21-day plateau environments regimen, spatial cognitive memory deficits in the hippocampus were accompanied by reduced expression of CREB and BDNF-critical proteins regulating synaptic plasticity—alongside exacerbated oxidative damage in both hippocampal tissue and serum. Guided by the microbiota-gut-brain axis hypothesis, we propose that Lactobacillus johnsonii HL79 partially rescues high-altitude-induced hippocampal memory impairment and oxidative stress, likely through modulating lipid metabolism-associated gene expression in the hippocampus and altering peripheral blood concentrations of lipids, neurotransmitters, and oxidative stress markers. However, the precise molecular mechanisms underlying these regulatory effects remain to be elucidated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Additionally, as the author of this article, Baoxing Gan would like to express his gratitude to his wife, Zhijun Liu, and to forever commemorate the day of January 30, 2023.
Author contributions
Baoxing Gan: Formal analysis, Data curation, Writing–original draft. Xufei Zhang: Data curation, Formal analysis. Jinge Xin: Resources, Writing–original draft. Lixiao Duan: Validation. Ning Sun: Validation. Yu Chen: Validation. Junqi Zeng and Yueying Lian: Validation. Hao Li: Project administration. Hesong Wang: Funding acquisition, Supervision. Xueqin Ni: Funding acquisition, Methodology, Project administration. Hailin Ma: Writing–review and editing. All authors reviewed the manuscript.
Funding
This study was supported by Joint Funds of the National Natural Science Foundation of China (No. U23A20476), the Key Science and Technology Project of Lhasa, Tibet (No. LSKJ202309, No. XZ202303ZY0013G), the President Foundation of The People's Hospital of Baiyun District Guangzhou (BYYZ24010), and the Key Research and Development Project of the Tibet Autonomous Region (2023ZYJM001).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The experimental protocol was approved by the Research Ethics Committee, College of Veterinary Medicine, Sichuan Agricultural University, China (approval number SYXKchuan2024-0187).
Consent for publication
All authors consent to the publication of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baoxing Gan, Xufei Zhang, Jinge Xin and Jingjie Chen contributed to the work equally and should be regarded as co-first authors.
Contributor Information
Hesong Wang, Email: sunasa1030@foxmail.com.
Xueqin Ni, Email: xueqinni@foxmail.com.
Hailin Ma, Email: David_ma79@163.com.
References
- Bai X, Liu G, Yang J, Zhu J, Wang Q, Zhou Y, Gu W, La L, Li X (2022) Changes in the gut microbiota of rats in high-altitude hypoxic environments. Microbiol Spectrum 10(6):e01626–e11622. 10.1128/spectrum.01626-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baião R, Capitão LP, Higgins C, Browning M, Harmer CJ, Burnet PWJ (2023) Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: a randomised, double-blind, placebo-controlled study. Psychol Med 53(8):3437–3447. 10.1017/S003329172100550X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, He X, Wang F, Kang D (2022) Study of brain structure and function in chronic mountain sickness based on fMRI. Front Neurol 12:763835. 10.3389/fneur.2021.763835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Zhang D, Li X, Liu M, Ma H (2023) Long-term high-altitude exposure influences task-related representations in visual working memory. Front Neurol 14:1–12. 10.3389/fneur.2023.1149623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Durando J, Giudetti L, Pampallona A, Vighetti S (2015) High-altitude headache: the effects of real vs sham oxygen administration. Pain 156(11):2326–2336. 10.1097/j.pain.0000000000000288 [DOI] [PubMed] [Google Scholar]
- Bhushan B, Eslavath MR, Yadav AP, Srivastava AK, Reddy MPK, Norboo T, Kumar B, Singh SB, Ganju L (2021) Metagenomic sequencing reveals altered gut microbiota of sojourners at high altitude: a longitudinal study. J Proteins Proteomics 12:271–288. 10.1007/s42485-021-00077-8 [Google Scholar]
- Castrén E, Monteggia LM (2021) Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiat 90(2):128–136. 10.1016/j.biopsych.2021.05.008 [DOI] [PubMed] [Google Scholar]
- Chang C-H, Shih J-H, Tsai Y-C, Hsieh H-M (2023) Study the potential of PS23 probiotics in Alzheimer’s disease model mice. Alzheimers Dement 19:e062054. 10.1002/alz.062054 [Google Scholar]
- Chen X, Li H, Zhang Q, Wang J, Zhang W, Liu J, Li B, Xin Z, Liu J, Yin H, Chen J, Kong Y, Luo W (2019) Combined fractional anisotropy and subcortical volumetric abnormalities in healthy immigrants to high altitude: a longitudinal study. Hum Brain Mapp 40(14):4202–4212. 10.1002/hbm.24696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CM, Blay CJ, Tsai P-Y, Li M, Edwards K, Qu Y, Liu Y, Buettner N, Walter C, Snyder M, Costa IPD, Devuyst O, Barrow JJ (2024) AQP1—a regulatory factor associated with brown adipose tissue-silencing. bioRxiv. 10.1101/2024.09.23.61459939803522 [Google Scholar]
- Clarke DD (1999) Circulation and energy metabolism of the brain. Basic neurochemistry: molecular, cellular, medical aspects, pp 638–667
- Cole E, O’Sullivan SJ, Tik M, Williams NR (2024) Accelerated Theta burst stimulation: safety, efficacy, and future advancements. Biol Psychiat 95(6):523–535. 10.1016/j.biopsych.2023.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson JJ, Gesch DB (2011) Global multi-resolution terrain elevation data 2010 (GMTED2010) (2011–1073). Retrieved from https://pubs.usgs.gov/publication/ofr20111073
- Favre A, Päckert M, Pauls SU, Jähnig SC, Uhl D, Michalak I, Muellner-Riehl AN (2015) The role of the uplift of the Qinghai-Tibetan Plateau for the evolution of Tibetan biotas. Biol Rev 90(1):236–253. 10.1111/brv.12107 [DOI] [PubMed] [Google Scholar]
- Florea G, Tudorache IF, Fuior EV, Ionita R, Dumitrescu M, Fenyo IM, Bivol VG, Gafencu AV (2022) Apolipoprotein A-II, a player in multiple processes and diseases. Biomedicines 10(7):1578. 10.3390/biomedicines10071578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LI, Zhang J, Jin J, Gao X-B, Yu J, Geng Q, Li H, Huang L (2015) Genetic variants of endothelial PAS domain protein 1 are associated with susceptibility to acute mountain sickness in individuals unaccustomed to high altitude: a nested case-control study. Exp Ther Med 10(3):907–914. 10.3892/etm.2015.2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Zhu J, Zhang X, Song Q, Ding J, Lu M, Sun S, Hu G (2018) Plin4-dependent lipid droplets hamper neuronal mitophagy in the MPTP/p-induced mouse model of Parkinson’s disease. Front Neurosci 12:397. 10.3389/fnins.2018.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Xu J, Yan Y, Zhao X (2022) Dynamics of the gut microbiota in rats after hypobaric hypoxia exposure. PeerJ 10:e14090. 10.7717/peerj.14090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S (2014) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- Honigman B, Theis MK, Koziol-McLain J, Roach R, Yip R, Houston C, Moore LG (1993) Acute mountain sickness in a general tourist population at moderate altitudes. Ann Intern Med 118(8):587–592. 10.7326/0003-4819-118-8-199304150-00003 [DOI] [PubMed] [Google Scholar]
- Ji W, Zhang Y, Luo J, Wan Y, Liu J, Ge R-L (2021) Memantine ameliorates cognitive impairment induced by exposure to chronic hypoxia environment at high altitude by inhibiting excitotoxicity. Life Sci 270:119012. 10.1016/j.lfs.2020.119012 [DOI] [PubMed] [Google Scholar]
- Jia Z, Zhao X, Liu X, Zhao L, Jia Q, Shi J, Xu X, Hao L, Xu Z, Zhong Q, Yu K, Cui S, Chen H, Guo J, Li X, Han Y, Song X, Zhao C, Bo X, Tian Y, Wang W, Xie G, Feng Q, He K (2020) Impacts of the plateau environment on the gut microbiota and blood clinical indexes in han and tibetan individuals. mSystems 5(1):e00660-e619. 10.1128/mSystems.00660-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl JP, Berryman CE, Young AJ, Radcliffe PN, Branck TA, Pantoja-Feliciano IG, Rood JC, Pasiakos SM (2018) Associations between the gut microbiota and host responses to high altitude. Am J Physiol Gastrointest Liver Physiol 315(6):1003–1015. 10.1152/ajpgi.00253.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley JS, Alperin N, Bagci AM, Lee SH, Mullins PG, Oliver SJ, Macdonald JH (2014) Normobaric hypoxia and symptoms of acute mountain sickness: elevated brain volume and intracranial hypertension. Ann Neurol 75(6):890–898. 10.1002/ana.24171 [DOI] [PubMed] [Google Scholar]
- Li J, Qi Y, Liu H, Cui Y, Zhang Li, Gong H, Li Y, Li L, Zhang Y (2013) Acute high-altitude hypoxic brain injury: identification of ten differential proteins. Neural Regen Res 8(31):2932. 10.3969/j.issn.1673-5374.2013.31.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Dan Z, Gesang L, Wang H, Zhou Y, Du Y, Ren Yi, Shi Y, Nie Y (2016) Comparative analysis of gut microbiota of native Tibetan and Han populations living at different altitudes. PLoS ONE 11(5):e0155863. 10.1371/journal.pone.0155863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gao X, Huang X, Fan Y, Wang Y-E, Zhang Y, Chen X, Wen J, He H, Hong Y, Liang Y, Zhang Y, Liu Z, Chen S, Li X (2023) Moderate altitude exposure impacts host fasting blood glucose and serum metabolome by regulation of the intestinal flora. Sci Total Environ 905:167016. 10.1016/j.scitotenv.2023.167016 [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang L, Song C, Wang D, Suolang B, Duan G (2021) Effect of hypoxia on human cognitive ability and indoor oxygen environment demand for sojourners at high altitude. Build Environ 194:107678. 10.1016/j.buildenv.2021.107678 [Google Scholar]
- Liu Y, Xue C, Lu H, Zhou Y, Guan R, Wang J, Zhang Q, Ke T, Aschner M, Zhang W (2022) Hypoxia causes mitochondrial dysfunction and brain memory disorder in a manner mediated by the reduction of Cirbp. Sci Total Environ 806:151228. 10.1016/j.scitotenv.2021.151228 [DOI] [PubMed] [Google Scholar]
- Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li Ge, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorde LB, Kaelin WG, Koivunen P, Prchal JT (2014) A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46(9):951–956. 10.1038/ng.3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhang D, Li X, Ma H, Wang N, Wang Y (2019) Long-term exposure to high altitude attenuates verbal and spatial working memory: evidence from an event-elated potential study. Brain Behavior 9(4):e01256. 10.1002/brb3.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang C, Sun Y, Pang L, Zhu S, Liu Y, Zhu L, Zhang S, Wang L, Du L (2020) Comparative study of oral and intranasal puerarin for prevention of brain injury induced by acute high-altitude hypoxia. Int J Pharm 591:120002. 10.1016/j.ijpharm.2020.120002 [DOI] [PubMed] [Google Scholar]
- Ma X, Duan C, Wang X, Tao Y, Yang L, Teng Y, Pan Y, Zhang M, Xu J, Sheng J, Wang X, Jin P (2025) Human gut microbiota adaptation to high-altitude exposure: longitudinal analysis over acute and prolonged periods. Microbiol Spectrum. 10.1128/spectrum.02916-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macêdo AP, Azevêdo M, Rosetto V, Cintra DE, Pauli JR (2022) 12,13-diHOME as a new therapeutic target for metabolic diseases. Life Sci 290:120229. 10.1016/j.lfs.2021.120229 [DOI] [PubMed] [Google Scholar]
- McMorris T, Hale BJ, Barwood M, Costello J, Corbett Jo (2017) Effect of acute hypoxia on cognition: a systematic review and meta-regression analysis. Neurosci Biobehav Rev 74:225–232. 10.1016/j.neubiorev.2017.01.019 [DOI] [PubMed] [Google Scholar]
- Muriana FJG, la Paz M-d, Sergio L, Ricardo B, Beatriz J, Sara M, Juan C, Abia R, Lopez S (2017) Tyrosol and its metabolites as antioxidative and anti-inflammatory molecules in human endothelial cells. Food Funct 8(8):2905–2914. 10.1039/c7fo00641a [DOI] [PubMed] [Google Scholar]
- Pan Z, Hu Y, Huang Z, Han Ni, Li Y, Zhuang X, Yin J, Peng H, Gao Q, Zhang W, Huang Y, Cui Y, Bi Y, Xu ZZ, Yang R (2022) Alterations in gut microbiota and metabolites associated with altitude-induced cardiac hypertrophy in rats during hypobaric hypoxia challenge. Sci China Life Sci 65(10):2093–2113. 10.1007/s11427-021-2056-1 [DOI] [PubMed] [Google Scholar]
- Pasam T, Padhy HP, Dandekar MP (2024) Lactobacillus helveticus improves controlled cortical impact injury-generated neurological aberrations by remodeling of gut-brain axis mediators. Neurochem Res 50(1):3. 10.1007/s11064-024-04251-4 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2019) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. Academic Press, Cambridge [Google Scholar]
- Peng L-L, Qi F-L, Tan K, Xiao W (2025) The altitudinal patterns of global human gut microbial diversity. BMC Microbiol 25(1):267. 10.1186/s12866-025-03974-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi E (2024) Gut microbe may ward off altitude sickness. Science 385(6716):1404–1404. 10.1126/science.adt3871 [DOI] [PubMed] [Google Scholar]
- Pun M, Guadagni V, Bettauer KM, Drogos LL, Aitken J, Hartmann SE, Furian M, Muralt L, Lichtblau M, Bader PR (2018) Effects on cognitive functioning of acute, subacute and repeated exposures to high altitude. Front Physiol 9:1131. 10.3389/fphys.2018.01131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi P, Lv J, Bai L-H, Yan X-D, Zhang L (2023) Effects of hypoxemia by acute high-altitude exposure on human intestinal flora and metabolism. Microorganisms 11(9):2284. 10.3390/microorganisms11092284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG, Nguyen JA (2019) Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci 22(5):820–827. 10.1038/s41593-019-0371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K, Zhang P (2007) The lipin protein family: dual roles in lipid biosynthesis and gene expression. FEBS Lett 582(1):90–96. 10.1016/j.febslet.2007.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach, Robert C, Hackett, Peter H, Oelz, Oswald, Bärtsch, Peter, Luks, Andrew M, MacInnis, Martin J, Baillie, J Kenneth, & Committee, Lake Louise AMS Score Consensus (2018) The 2018 Lake Louise acute mountain sickness score. High Alt Med Biol 19(1):4–6. 10.1089/ham.2017.0164 [DOI] [PMC free article] [PubMed]
- Simpson SM, Hickey AJ, Baker GB, Reynolds JN, Beninger RJ (2012) The antidepressant phenelzine enhances memory in the double Y-maze and increases GABA levels in the hippocampus and frontal cortex of rats. Pharmacol Biochem Behav 102(1):109–117. 10.1016/j.pbb.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Song T-T, Bi Y-H, Gao Y-Q, Huang R, Hao Ke, Xu G, Tang J-W, Ma Z-Q, Kong F-P, Coote JH (2016) Systemic pro-inflammatory response facilitates the development of cerebral edema during short hypoxia. J Neuroinflammation 13:1–14. 10.1186/s12974-016-0528-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, Chen EY, Gao F, Narain NR, Distefano G, Shettigar VK, Hirshman MF, Ziolo MT, Kiebish MA, Tseng Y-H, Coen PM, Goodyear LJ (2018) 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27(5):1111-1120.e1113. 10.1016/j.cmet.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Zhuang D-H, Li Y-C, Chen Yu, Wang X-Y, Ge M-X, Xue T-Y, Zhang Q-Y, Liu X-Y, Yin F-Q, Han Y-M, Gao Z-L, Zhao L, Li Y-X, Lv M-J, Yang L-Q, Xia T-R, Luo Y-J, Zhang Z, Kong Q-P (2024a) Gut microbiota contributes to high-altitude hypoxia acclimatization of human populations. Genome Biol 25(1):232. 10.1186/s13059-024-03373-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R, Jia S, Zhang N, Wang Y, Li H, Zhang D, Ma H, Su Y (2024b) The effects of long-term high-altitude exposure on cognition: a meta-analysis. Neurosci Biobehav Rev 161:105682. 10.1016/j.neubiorev.2024.105682 [DOI] [PubMed] [Google Scholar]
- Suzuki TA, Martins FM, Nachman MW (2019) Altitudinal variation of the gut microbiota in wild house mice. Mol Ecol 28(9):2378–2390. 10.1111/mec.14905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y (2009) Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab 10(3):178–188. 10.1016/j.cmet.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Tao Xu, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20(4):709–726. 10.1016/s0896-6273(00)81010-7 [DOI] [PubMed] [Google Scholar]
- Tian P, Chen Y, Qian X, Zou R, Zhu H, Zhao J, Zhang H, Wang G, Chen W (2021) Pediococcus acidilactici CCFM6432 mitigates chronic stress-induced anxiety and gut microbial abnormalities. Food Funct 12(22):11241–11249. 10.1039/d1fo01608c [DOI] [PubMed] [Google Scholar]
- Tian Y, Tian M, Liu J, Wei J (2022) Spatial distribution pattern and evolution characteristics of population in Qinghai-Tibet Plateau. Sci Technol Dev 18(5):620–628 [Google Scholar]
- Vaidyanathan S, Salmi TM, Sathiqu RM, McConville MJ, Cox AG, Brown KK (2022) YAP regulates an SGK1/mTORC1/SREBP-dependent lipogenic program to support proliferation and tissue growth. Dev Cell 57(6):719-731.e718. 10.1016/j.devcel.2022.02.004 [DOI] [PubMed] [Google Scholar]
- Wan Z, Zhang X, Jia X, Qin Y, Sun N, Xin J, Zeng Y, Jing Bo, Fang J, Pan K, Zeng D, Bai Y, Wang H, Ma H, Ni X (2022) Lactobacillus johnsonii YH1136 plays a protective role against endogenous pathogenic bacteria induced intestinal dysfunction by reconstructing gut microbiota in mice exposed at high altitude. Front Immunol 13:1007737. 10.3389/fimmu.2022.1007737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jiang W, Leitz J, Yang K, Esquivies L, Wang X, Shen X, Held RG, Adams DJ, Basta T, Hampton L, Jian R, Jiang L, Stowell MHB, Baumeister W, Guo Q, Brunger AT (2024) Structure and topography of the synaptic V-ATPase–synaptophysin complex. Nature 631(8022):899–904. 10.1038/s41586-024-07610-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Braun C, Murphy EF, Enck P (2019) Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. Am J Gastroenterol 114(7):1152. 10.14309/ajg.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Prajapati SK, Mishra SP, Jain S, Yadav H (2022) Protection of cognitive decline and Alzheimer’s disease progression by a human origin-probiotic biotherapy. Alzheimers Dement 18:e066137. 10.1002/alz.066137 [Google Scholar]
- Xin J, Wang H, Sun N, Bughio S, Zeng D, Li L, Wang Y, Khalique A, Zeng Y, Pan K, Jing Bo, Ma H, Bai Y, Ni X (2021) Probiotic alleviate fluoride-induced memory impairment by reconstructing gut microbiota in mice. Ecotoxicol Environ Saf 215:112108. 10.1016/j.ecoenv.2021.112108 [DOI] [PubMed] [Google Scholar]
- Xin J, Zhu B, Wang H, Zhang Y, Sun N, Cao Xi, Zheng L, Zhou Y, Fang J, Jing Bo, Pan K, Zeng Y, Zeng D, Li F, Xia Y, Xu P, Ni X (2023) Prolonged fluoride exposure induces spatial-memory deficit and hippocampal dysfunction by inhibiting small heat shock protein 22 in mice. J Hazard Mater 456:131595. 10.1016/j.jhazmat.2023.131595 [DOI] [PubMed] [Google Scholar]
- Xin J, Zhang H, He Y, Duren Z, Bai C, Chen L, Luo X, Yan D-S, Zhang C, Zhu X, Yuan Q, Feng Z, Cui C, Qi X, Ouzhuluobu W, Hung W, Wang Y, Su B (2020) Chromatin accessibility landscape and regulatory network of high-altitude hypoxia adaptation. Nat Commun 11(1):4928. 10.1038/s41467-020-18638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Yang J, Wei W-T, Zhou M-L, Mo D-X, Wan X, Ma R, Wu M-M, Huang J-H, Liu Y-J, Lv F-H, Li M-H (2024) A time-resolved multi-omics atlas of transcriptional regulation in response to high-altitude hypoxia across whole-body tissues. Nat Commun 15(1):3970. 10.1038/s41467-024-48261-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Feng Z, Qin A, Liu Y, Wang T, Liao Y, Wang J, Liu J (2005) A follow-up study on cognitive function of soldiers during altitude training. J Fourth Mil Med Univ 26(3):272–275 [Google Scholar]
- Zha X, Liu X, Wei M, Huang H, Cao J, Liu S, Bian X, Zhang Y, Xiao F, Xie Y, Wang W, Zhang C (2025) Microbiota-derived lysophosphatidylcholine alleviates Alzheimer’s disease pathology via suppressing ferroptosis. Cell Metab 37(1):169-186.e169. 10.1016/j.cmet.2024.10.006 [DOI] [PubMed] [Google Scholar]
- Zhang X, Jia X, Wang S, Xin J, Sun N, Wang Y, Zhang X, Wan Z, Fan J, Li H (2023) Disrupted gut microbiota aggravates spatial memory dysfunction induced by high altitude exposure: a link between plateau environment and microbiome–gut–brain axis. Ecotoxicol Environ Saf 259:115035. 10.1016/j.ecoenv.2023.115035 [DOI] [PubMed] [Google Scholar]
- Zhao J, Yao Y, Dong M, Xiao H, Xiong Y, Yang S, Li D, Xie M, Ni Q, Zhang M (2023) Diet and high altitude strongly drive convergent adaptation of gut microbiota in wild macaques, humans, and dogs to high altitude environments. Front Microbiol 14:1067240. 10.3389/fmicb.2023.1067240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Ren Z, Zhao A, Tang Y, Kuang J, Li M, Chen T, Wang S, Wang J, Zhang H, Wang J, Zhang T, Zeng J, Liu X, Xie G, Liu P, Sun N, Bao T, Nie T, Lin J, Liu P, Zheng Y, Zheng X, Liu T, Jia W (2024) Gut bacteria-driven homovanillic acid alleviates depression by modulating synaptic integrity. Cell Metab 36(5):1000-1012.e1006. 10.1016/j.cmet.2024.03.010 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Cui D, Wu G, Ren H, Zhu X, Xie W, Zhang Y, Yang L, Peng W, Lai C, Huang Y, Li H (2022) Disrupted gut microbiota aggravates working memory dysfunction induced by high-altitude exposure in mice. Front Microbiol 13:1054504. 10.3389/fmicb.2022.1054504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Fan X, Miao Y (2022) LPIN1 promotes triglycerides synthesis and is transcriptionally regulated by PPARG in buffalo mammary epithelial cells. Sci Rep 12(1):2390. 10.1038/s41598-022-06114-w [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.