Abstract
Background
Emerging evidence suggests that senescent microglia play a role in β-amyloid (Aβ) pathology and neuroinflammation in Alzheimer’s disease (AD). Targeting senescent cells with naturally derived compounds exhibiting minimal cytotoxicity represents a promising therapeutic strategy.
Objectives
This study aimed to investigate whether delphinidin, a naturally occurring anthocyanin, can alleviate AD-related pathologies by mitigating microglial senescence and to elucidate the underlying molecular mechanisms.
Methods
We employed APP/PS1 mice, naturally aged mice, and an in vitro model using Aβ42-induced senescent BV2 microglia. Delphinidin’s effects were evaluated through assessments of cognitive function, synaptic integrity (synapse loss), Aβ plaque burden, senescent microglia gene signatures, and cellular senescence markers (including senescence-associated β-galactosidase activity, SASP factor expression, oxidative stress, and cyclin p21/p16 levels). Mechanistic studies involved analyzing the AMPK/SIRT1 signaling pathway, testing direct delphinidin-SIRT1 interaction, and using the AMPK inhibitor Compound C.
Results
Delphinidin treatment significantly alleviated cognitive deficits, synapse loss, Aβ peptides plaques of APP/PS1 mice via downregulated senescent microglia gene signature, prevented cell senescence, including senescence-associated β-galactosidase activity, senescence-associated secretory phenotype (SASP), oxidative stress, cyclin p21 and p16. And delphinidin treatment also prevented microglial senescence in naturally aged mice. In vitro, delphinidin treatment attenuated cell senescence induced by Aβ42 in BV2 microglia cells. Further research indicated that delphinidin treatment enhanced the AMPK/SIRT1 signaling pathway. Additionally, delphinidin was found to directly interact with SIRT1. It’s noteworthy that AMPK inhibitor Compound C inversed the protective effect of delphinidin against microglial senescence.
Conclusion
Our study reveals for the first time that delphinidin effectively improved cognitive deficits, alleviated synapse loss and Aβ pathology in APP/PS1 mice by mitigating microglial senescence. These findings highlight delphinidin as a promising natural anti-aging agent against the development of aging and age-related diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-025-01783-x.
Keywords: Alzheimer's disease, Microglial senescence, Aging, Neuroinflammation, AMPK
Introduction
Alzheimer's disease (AD) is an age-related neurodegenerative disorder, with extensive accumulation of amyloid β-peptide (Aβ) plaques and tau neurofibrillary tangles in vulnerable brain regions [1]. Emerging evidence indicates that cellular senescence plays a crucial role in AD pathophysiology [2–4]. Recent research revealed that Aβ triggered cell senescence, including increased the senescence-associated secretory phenotype (SASP), reactive oxygen species (ROS) production, cell cycle arrest and activated DNA damage response [5–8]. Senolytic treatment can slow down aging processes and ameliorate aging-related pathophysiological changes in various disease models, removal of senescent cells alleviates Aβ load and improves memory in AD model mice [8, 9].
As individuals age, the immune system becomes less efficient at recognizing and eliminating senescent cells, which are damaged or dysfunctional cells that no longer divide but remain metabolically active. Normally, senescent cells are removed by immune cells like macrophages and microglia. However, with aging, the efficiency of this clearance process declines, leading to an accumulation of senescent cells within tissues, including the brain [10, 11]. As the primary resident immune cells of the central nervous system, microglia may be particularly susceptible to the effects of aging. Microglia exhibit inflammatory characteristics during the early stages of AD, but transition to a senescent state in later stages [12]. Senescent microglia exhibit structural changes associated with aging, characterized by a decreased ability to clear Aβ and an increased release of inflammatory cytokines [13, 14]. Given this, it is reasonable to investigate whether the removal of senescent microglia can restore their phagocytic capacity and limit amyloid accumulation.
Delphinidin, a prevalent anthocyanidin found in grapes, berries, and a variety of colorful vegetables, has demonstrated robust antioxidant and anti-inflammatory properties in various disease models [15–18]. Previous research has shown that delphinidin ameliorates calcium influx and tau hyperphosphorylation in vitro, and improves memory cognition in Meynert lesioned Rats [19, 20]. Emerging evidence has highlighted the potential anti-aging properties of anthocyanidins [21–23]. While the anti-aging effects of delphinidin in the brain remain unclear, a recent study has demonstrated the promising anti-aging effects of delphinidin against oxidative stress-induced senescence in human nucleus pulposus cells [24]. However, the mechanisms underlying the neuroprotective effects of delphinidin in AD remain elusive.
Delphinidin exerts protective effects by activating the AMPK and SIRT1 signaling pathways in various diseases [25–28]. AMP-activated protein kinase (AMPK) is a pivotal regulator of biological energy metabolism. Notably, AMPK activation exerts multiple neuroprotective effects in AD, including reducing Aβ accumulation, restoring cellular antioxidant capacity and improving cognitive function [29–31]. Accumulating evidence suggests that AMPK plays a critical role in modulating cellular senescence across various disease models [32–34]. As a well-known longevity gene, SIRT1 is intimately associated with the aging process and the prevention of age-related diseases. Furthermore, SIRT1 regulates cell cycle arrest by downregulating the expression of p53 and p21 during cellular senescence [35, 36]. The activation of SIRT1 promotes the clearance of Aβ, regulates synaptic plasticity, and inhibits oxidative stress, neuroinflammation and apoptosis in AD brains [37]. Latest research suggested that AMPK regulating metabolism and cellular senescence through activates SIRT1 [38].
This study aims to explore the therapeutic potential of delphinidin in AD mice models (APP/PS1) by mitigating microglial senescence. To investigate whether Delphinidin exerts neuroprotective effects by reversing senescent microglia, naturally aged mice were further applied. Additionally, we explored the molecular mechanisms of Delphinidin in mitigating microglial senescence through the activation of the AMPK/SIRT1 signaling pathway.
Materials and methods
Chemicals and reagents
Delphinidin (purity ≥97%) was purchased from Cayman (Michigan, USA). The human Aβ1–42 peptide was obtained from GL Biochemical Ltd (Shanghai, China). Superoxide dismutase (SOD) assay kit, malondialdehyde (MDA) assay kit, reactive oxygen species (ROS) assay kit and Pierce BCA protein assay kit were obtained from Beyotime Biotechnology (Shanghai, China). Compound C (AMPK inhibitor) was purchased from MedChemExpress (Monmouth Junction, NJ, USA).
Experimental mice and pharmacological treatment
Seven-month-old male APPswe/PSEN1dE9 (APP/PS1) double transgenic model mice and male C57BL/6 J mice were obtained from Jiangsu Aniphe Biolaboratory Inc (Licence No. SYXK [Jiangsu] 2023–0066). All mice were housed in a controlled environment with 22–25 °C temperature at 55–60% humidity with a 12 h light/dark cycle. After acclimatization, APP/PS1 mice were randomly divided into two groups (n = 8): APP/PS1 mice orally administered equal amounts of PBS (APP/PS1), and APP/PS1 mice orally administered 15 mg/kg/day delphinidin in PBS (APP/PS1 + Dp) for 8 weeks. C57BL/6 J mice (7-month-old) were administered equal amounts of PBS (WT) for 8 weeks as previously described [39, 40]. Body weight was measured once a week. The experimental design is shown in Fig. 1A. Furthermore, delphinidin has no significant impact on the body weight or liver function of the mice (Supplementary Fig. 1).
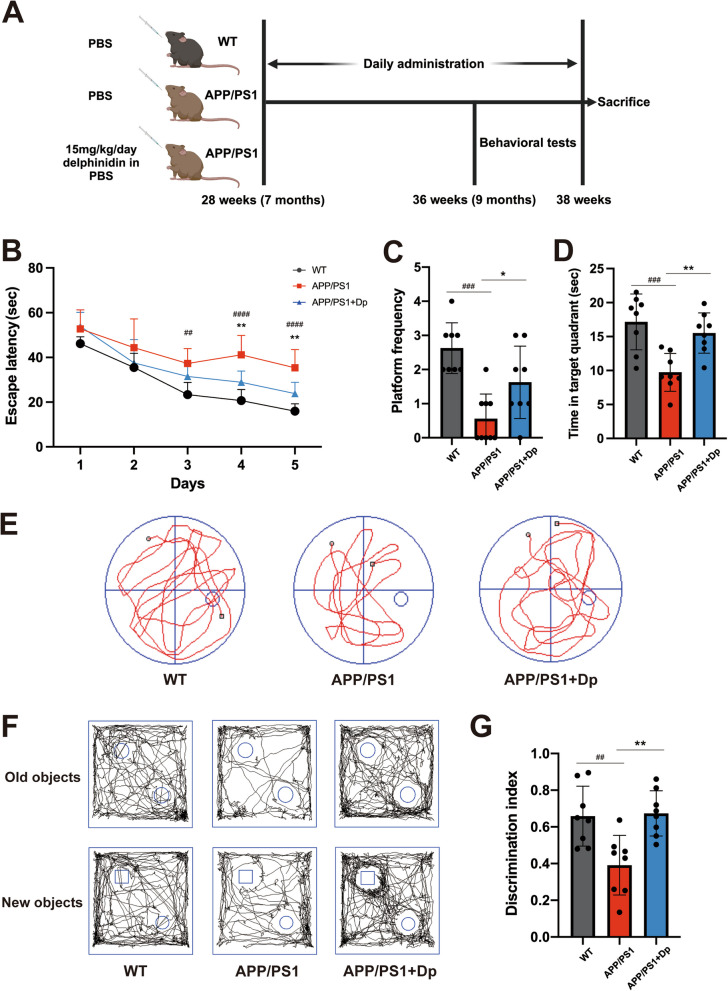
Fig. 1.
Delphinidin ameliorates learning and memory deficits in APP/PS1 mice. A This schematic diagram outlines the experimental design used to investigate the effects of delphinidin treatment on APP/PS1 mice. B-E Evaluating the effects of delphinidin on learning and memory through the Morris Water Maze (MWM) test. B The escape latency during spatial acquisition training. C, D Number of platform crossings and time spent in the target quadrant in the spatial probe test. E Representative motion track during the spatial probe test. After the MWM test was completed, the mice were subjected to the object recognition test. F Representative motion track and (G) the discrimination index of the object recognition test. n = 8 mice. Data were presented as mean ± SD. ## p < 0.005, ### p < 0.0005 versus vehicle-treated WT mice, * p < 0.05, ** p < 0.005 versus vehicle-treated APP/PS1 mice. Dp: delphinidin; WT: wildtype
In order to further explore the effect of delphinidin on aging mice: young wild type mice (2-month-old) were categorized into two groups(n = 4) by treatment: delphinidin (Young + Dp) and vehicle (Young + Veh). Aged wild type mice (18-month-old) were categorized into two groups(n = 4): delphinidin (Aged + Dp) and vehicle (Aged + Veh).
Morris water maze test
The Morris Water Maze (MWM) test was performed to detect spatial learning and memory in mice. The water maze consisted of a 120-cm circular pool filled with opaque water (22–24 °C). A 10-cm circular platform was submerged 1.0 cm below the water surface. During the training phase, mice were given 60 s to freely swim and locate the submerged platform. A trial concluded when the mouse found the platform or after 60 s had passed. Mice underwent four training trials daily for five consecutive days. Twenty-four hours after the final training trial, memory retention was assessed in a probe trial without the platform. The performance of mice was automatically measured with a DigBehv Animal Behavior Analysis System.
Novel objection recognition (NOR) test
The NOR test for investigating recognition and memory ability of the mice was conducted as previously described. In the habituation phase, mice were allowed to explore the environment (50 cm × 50 cm × 50 cm plastic box, empty) for 5 min before testing. For training day, two similar objects in two corners in opposite directions and mice were allowed to explore the objects for 10 min. After a 24-h retention period, in the testing day, the upper left object was replaced by a novel object, and the mice were put back in the box and allowed to freely explore for 10 min. Animal Behavior Analysis System was used to record the time mice spent exploring each object. The results are presented as a discrimination index, calculated using the formula: Discrimination index (%) = (time with novel object − time with familiar object)/(time with novel object + time with familiar object).
Preparation of tissue samples
Following the behavioral tests, the mice were anesthetized, and blood was collected via cardiac puncture. The brains were then removed after cardiac perfusion with PBS and bisected along the sagittal plane. The right hemispheres of four brains were fixed in 4% paraformaldehyde overnight, embedded in paraffin wax, and stored at room temperature. In contrast, the right brain samples from other animals were embedded in OCT and stored at −80℃ for subsequent immunofluorescence (IF) analysis and Senescence-Associated β-Galactosidase (SA‑β‑gal) staining. Meanwhile, the left brain samples from eight animals were placed in 1.5 ml tubes and preserved at −80 ℃ for future analysis.
Aβ oligomer preparation
The Aβ oligomers were prepared as previously described [41]. Human Aβ1–42 peptide (GL Biochem, Shanghai) was initially dissolved in 1,1,1,3,3,3- hexafluoroisopropanol (HFIP, Sigma-Aldrich) and sonicated for 30 min to yield a clear solution. Next, HFIP was removed via lyophilization, and the resulting Aβ1–42 monomer powder was dissolved in sterile dimethyl sulfoxide (DMSO) at a concentration of 2 mM. After this, the DMSO was evaporated using a SpeedVac (Thermo Scientific), leaving behind a clear peptide film. This film can be stored at − 80 °C for up to six months. Before use, the peptide film was re-dissolved in anhydrous DMSO and sonicated for 10 min to ensure complete resuspension. The solution was then diluted to 100 μM with culture medium and incubated overnight at 4 °C prior to use. Next, the sample was subjected to 0.45 μm membrane filtration for aggregate removal. We validated oligomer stability via DLS post-incubation (Z-Average diameter = 23.07 nm, PDI = 0.265).
Cell culture and treatments
The mouse BV2 microglia cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Logan, UT, USA) with 10% fetal bovine serum and 1% penicillin/streptomycin at 37 °C with 5% CO2. BV2 cells were pretreated with or without delphinidin (30um) for 1 h, and then treated with Aβ42 oligomers for 24 h. The concentration of Compound C (MedChemExpress) used to treat BV2 cells was 5 μM.
Thioflavin‑S staining
Thioflavin-S staining was performed to detect the amyloid peptides in the brain tissue. Paraffin sections were deparaffinized in 3 times of xylene, with 10 min for each, and then dehydrated in 3 times of pure ethanol, with 5 min for each, and washed in distilled water. Thioflavin-S staining working solution was prepared with 50% alcohol with a concentration of 0.3% filtered Thioflavine-S (Yuanye Biotech, Shanghai, China). Then sections were incubated with thioflavin-S staining working solution at room temperature for 8 min. Sections were washed with 80% alcohol for 10 s and washed with distilled water for 10 s. Then sections were incubated with DAPI dye solution (Servicebio, Wuhan, China) in the dark at room temperature for 10 min. The sections were washed with PBS 3 times and washed for 5 min each time, then sealed with anti-fluorescence quenching agent and imaged with confocal microscopy (Leica, Tokyo, Japan). The results were analyzed using ImageJ (Maryland, USA) to evaluate the fluorescence intensity of Th-S.
Immunofluorescence staining
Brain sections were fixed in 4% paraformaldehyde for 30 min at room temperature. All washing steps were performed with PBS 3 times and washed for 5 min each time. Sections were washed and subsequently permeabilized with 0.3% Triton X-100. Next, the sections were washed and then blocked in 10% donkey serum for 1 h at room temperature. Then sections were incubated with primary antibodies overnight at 4 °C. The following day, sections were washed and incubated in appropriate secondary antibodies (Invitrogen, California, USA) for 1 h at room temperature. Sections were washed and incubated with DAPI for 5 min at room temperature. Finally, the samples were sealed with an anti-fluorescence quencher and imaged with confocal microscopy (Leica, Tokyo, Japan). The antibodies used for immunofluorescence staining are listed in Supplementary Table 1.
Immunohistochemical staining
Immunohistochemistry was performed as previously described. The sections were incubated with primary antibodies at 4 °C overnight. The following day, sections were washed in PBS and incubated with appropriate secondary antibodies (Boster, Wuhan, China) for 30 min at 37 °C. Then sections were incubated with a 3,3′-diaminobenzidine (DAB) kit (Boster, Wuhan, China) and inspected under a scanning microscope (Leica, Tokyo, Japan). Quantitative analysis of immuno-positive cells present in the sections was assessed in each section (three sections per animal) using ImageJ (Maryland, USA). For the quantification of positive cells or Aβ staining area in cortex, the data was presented as the average of three random fields of view in each section.
Senescence‑associated beta‑galactosidase (SA‑β‑gal) staining
SA-β-gal activity was performed by an SA-β-Gal Staining Kit (Solarbio, Beijing, China), as in previous studies. Brain sections and cells were washed with PBS once for 3 min and fixed with β-Gal Fixative solution at room temperature for 15 min. Brain sections and cells were washed with PBS 3 times for 3 min each time. Brain sections and cells were treated with β-galactosidase staining working solution at 37℃ and sealed with sealing film. After overnight incubation, removed the β-galactosidase staining working solution and added 2 ml PBS. Then cells were imaged by an inverted microscope (Leica, Tokyo, Japan).
Western blotting
The protein concentration in each sample was measured using a pierce BCA protein assay kit (Beyotime, Shanghai, China), following the manufacturer's instructions. The protein samples were separated by SDS-PAGE and transferred onto polyvinylidene membranes (Millipore, Massachusetts, USA). Then the membranes were incubated with 5% non-fat powdered milk (Beyotime, Shanghai, China) for 1 h at room temperature. After that, the membranes were incubated with corresponding primary antibodies (summarized in Supplementary Table 1) overnight at 4 °C. Afterward, membranes were washed for 3 times and incubated with corresponding secondary antibodies (Beyotime, Shanghai, China) for 1 h at room temperature. The membranes were washed for 3 times and protein blots were observed by the enhanced chemiluminescence (Tanon, Shanghai, China). The density of each band was quantified using ImageJ software.
RNA extraction and RT-qPCR
Total RNA was extracted using the EZ-press RNA Purification Kit (EZBioscience, Suzhou, China) and reverse transcribed into cDNA by the 4X EZscript Reverse Transcription Mix II (EZBioscience, Suzhou, China). Real-time PCR analysis was performed with 2X Color SYBR qPCR Master Mix (EZBioscience, Suzhou, China). The primer sequences were listed in Supplementary Table 2. RT‐PCR was performed using the following conditions: initial denaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Relative quantification of qPCR signals was conducted using GAPDH as the reference gene.
Detection of SOD and MDA
Total SOD activity was measured using the WST-8 method. Based on the reaction of WST-8 and superoxide anion catalyzed by xanthine oxidase, producing water-soluble formazan dye and absorbance was measured at 450 nm. As in previous studies, the final result was calculated by the unit of SOD enzyme activity/mg protein of each sample. Based on a reaction of MDA and thiobarbituric acid (TBA), producing a red product and absorbance was measured at 532 nm. According to the standard curve, the measured absorbance was converted to MDA content. The final result was presented as the level of MDA content/mg protein of each sample.
Detection of reactive oxygen species production
To determine the level of intracellular reactive oxygen species (ROS), different groups of BV2 cells were loaded with a final concentration of 10 μM DCFH-DA dye in the dark at 37 °C. After 20 min of incubation, the cells were washed using serum-free culture medium. Then samples were assessed by flow cytometry (FACS, BD, UK) or microscope (Leica, Tokyo, Japan).
Transcriptome sequencing analysis
Mice hippocampus RNA (4 mice per group) was rapidly extracted and sent to Novogene (Beijing, China) for transcriptome analysis, comprising cDNA library construction, and RNA sequencing (RNA-seq). Corrected P < 0.05 and [log2Fold Change (FC)] > 1 was set as the thresholds for significant differential expressed genes (DEGs). Pathway enrichment analysis was performed using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
Molecule docking
Before molecular docking, the structure of delphinidin was obtained from the PubChem database and converted from SDF files to PDB format using Open Babel version 2.3.2. The structure of SIRT1 was retrieved from the Protein Data Bank (PDB) database. During molecular docking, the receptor protein was initially processed with PYMOL 2.3.4 to remove water molecules and bound ligands. Further preparation involved adding hydrogen atoms and assigning charges to the receptor using AutoDockTools. Both the SIRT1 and the delphinidin were converted to the PDBQT format. Molecular docking simulations were then carried out using AutoDock Vina 1.1.2, and the resulting docking interactions were analyzed using PLIP. The predicted docking poses were visualized using PyMOL.
Molecular dynamics simulation
To further investigate the binding interactions between the SIRT1 and delphinidin, a 100 ns molecular dynamics (MD) simulation was performed on the docked conformation. The MD simulation was carried out using the Gromacs 2019.6 program, with the amber99sb-ildn force field for protein and the GAFF force field for small molecules, and the TIP3P water model. The simulation was conducted under constant temperature and pressure conditions, with periodic boundary conditions. During the MD simulation of small molecules, all hydrogen bonds involved were constrained using the LINCS algorithm, with an integration time step of 2 fs. Electrostatic interactions were calculated using the Particle-Mesh Ewald (PME) method. The cutoff value for non-bonded interactions was set to 12 Å, with updates every 20 steps. The V-rescale method was employed for temperature coupling, maintaining the simulation temperature at 300 K, while pressure was controlled using the Parrinello-Rahman method at 1 bar. Initially, the steepest descent method was applied for energy minimization of the system to eliminate close atomic contacts. Subsequently, all atoms in the system were heated to 300 K over a duration of 100 ps. Finally, a 100 ns MD simulation was conducted, saving conformational snapshots every 20 ps. Visualization of the simulation results was accomplished using Gromacs embedded tools and PyMOL.
Statistical analysis
All values are presented as mean ± standard error of the mean (SEM) and were analyzed using GraphPad Prism 10. For the analysis of escape latency during the Morris water maze test, a two-way repeated measures analysis of variance (ANOVA) with Tukey’s post-hoc test was employed to compare multiple groups. One-way ANOVA followed by Tukey's post-hoc test was used to analyze all other experiments. A significance level of P < 0.05 was adopted.
Results
Delphinidin ameliorates learning and memory deficits in APP/PS1 mice
Following the experimental design in Fig. 1A, APP/PS1 mice were randomly divided into two groups (n = 8): APP/PS1 mice orally administered equal amounts of PBS (APP/PS1), and APP/PS1 mice orally administered 15 mg/kg/day delphinidin in PBS (APP/PS1 + Dp) for 8 weeks. C57BL/6 J mice (7-month-old) were administered equal amounts of PBS (WT) for 8 weeks as previously described [39]. Body weight was measured once a week. The experimental design is shown in Fig. 1A. Subsequently, the MWM and Novel Object Recognition NOR tests were conducted to assess spatial learning and memory, evaluating delphinidin’s effect on spatial learning and memory ability. During the spatial acquisition training period of MWM, the APP/PS1 mice presented a higher escape latency compared to the WT mice. The escape latency of the delphinidin-treated APP/PS1 mice was significantly decreased compared to the vehicle-treated APP/PS1 mice (Fig. 1B). During the probe trial, vehicle-treated with APP/PS1 mice exhibited reduced platform crossing frequency and spent significantly less time in the target quadrant compared to WT mice. However, delphinidin-treated APP/PS1 mice demonstrated an increased frequency of platform crossings and longer duration in the target quadrant compared to vehicle-treated APP/PS1 mice (Fig. 1C-E).
In the novel object recognition test, wild-type mice exhibited a preference for the novel object, whereas vehicle-treated APP/PS1 mice showed no significant preference between the familiar and novel objects. Following treatment with delphinidin, APP/PS1 mice spent significantly more time exploring the new objects (Fig. 1F-G). These results suggest that delphinidin treatment may ameliorate cognitive deficits and enhance learning and memory abilities in APP/PS1 mice.
Transcriptome sequencing analysis reveals that delphinidin exhibits an anti-aging signature in the hippocampus of APP/PS1 mice
To further investigate how delphinidin ameliorates cognitive deficits in APP/PS1 mice, we performed bulk RNA sequencing on the brain of mice from three different groups. The Venn diagram was created to identify differentially expressed genes (DEGs) shared between WT mice versus APP/PS1 mice and APP/PS1 mice versus delphinidin-treated APP/PS1 mice (Supplementary Fig. 2). Our analysis revealed the gene expression profiles of APP/PS1 mice differed significantly from those of WT mice. The heatmap illustrates that the DEGs profile of delphinidin-treated APP/PS1 mice closely resembles that of WT mice, indicating that delphinidin treatment may effectively restore gene expression patterns disrupted by the pathological change in APP/PS1 mice (Fig. 2A). As depicted in the volcano plot, the transcriptomic analysis revealed 870 DEGs in the brains of APP/PS1 versus WT mice, with 581 genes showing elevated expression and 289 genes displaying reduced expression (Fig. 2B).
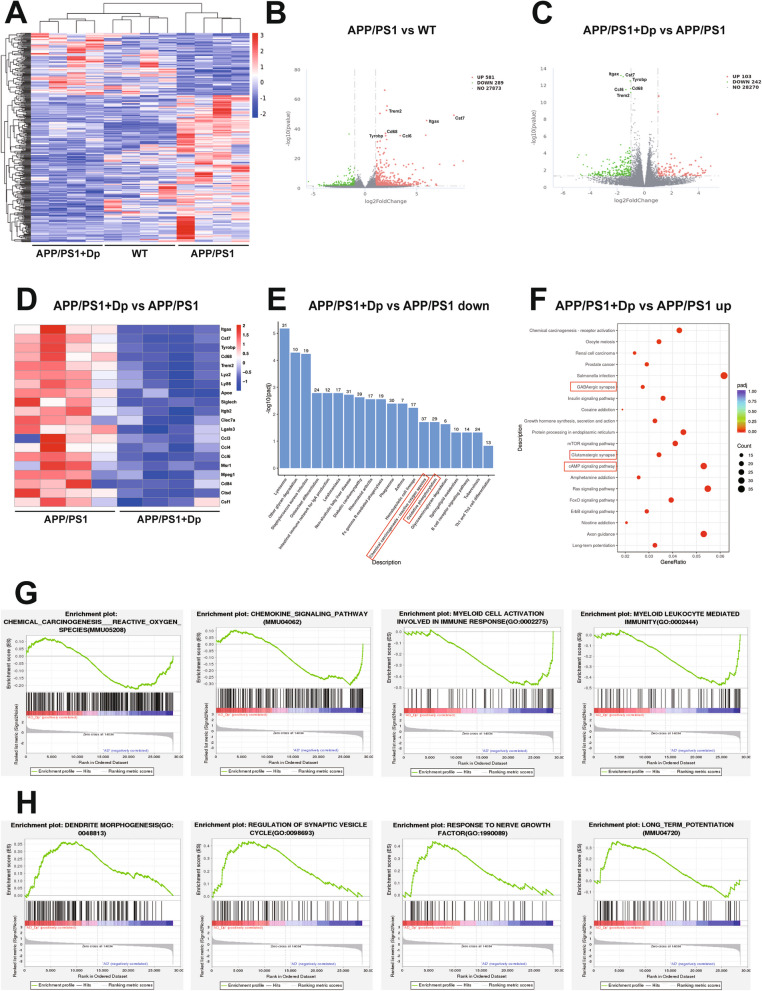
Fig. 2.
Effects of delphinidin on the gene expression profiles in APP/PS1 mice. A A heatmap illustrating hierarchical clustering of co-regulated differentially expressed genes (DEGs) across three distinct experimental groups. B, C Volcano plotting displaying DEGs between WT versus APP/PS1 mice and APP/PS1 versus delphinidin-treated APP/PS1 mice. D The heatmap showing DEGs associated with cell senescence. E, F The diagram showed the top 20 significantly down-regulated (E) and up-regulated (F) KEGG pathways of DEGs between APP/PS1 and delphinidin-treated APP/PS1 mice. G, H KEGG and GO term of gene set enrichment analysis between APP/PS1 and delphinidin-treated APP/PS1 mice. n = 4 mice. Dp: delphinidin; WT: wildtype
Our findings revealed that the transcriptomic comparison between delphinidin-treated APP/PS1 mice and vehicle-treated APP/PS1 mice identified 103 upregulated genes and 242 downregulated genes (Fig. 2C). Notably, among these genes, we discovered a principal cluster of genes that was downregulated in delphinidin-treated APP/PS1 mice compared to vehicle-treated APP/PS1 mice (Fig. 2D). This suggests that delphinidin treatment induces changes in the differential gene expression profile of APP/PS1 mice, which appear to exhibit an anti-aging signature [42, 43]. Further analysis showed that delphinidin treatment significantly downregulated genes associated with senescent microglia in APP/PS1 mice, including TREM2, ApoE, Cst7, Itgax, Tyrobp, Ccl4, Lyz2 and Cd68. These results suggest that delphinidin has the potential to resist aging-related molecular changes in AD mice, likely by reversing the cellular senescence of microglia.
Next, DEGs between delphinidin-treated APP/PS1 mice and vehicle-treated APP/PS1 mice submitted to KEGG pathway enrichment analysis. In the top 20 significantly down-regulated KEGG pathways, delphinidin treatment significantly reduced the pathways associated with redox reaction in APP/PS1 mice (Fig. 2E). Additionally, gene set enrichment analysis (GSEA) revealed that most DEGs related to reactive oxygen species, myeloid cell activation involved in the immune response, and myeloid leukocyte mediated immunity were downregulated in delphinidin-treated APP/PS1 mice compared to vehicle-treated APP/PS1 mice (Fig. 2G). The KEGG pathway analysis revealed an enrichment of synapse-related transcriptional upregulation in delphinidin-treated APP/PS1 mice compared to vehicle-treated APP/PS1 mice (Fig. 2E). Additionally, GSEA indicated that DEGs associated with dendrite morphogenesis, regulation of synaptic vesicle cycle, nerve growth factor and long-term potentiation were upregulated in the delphinidin-treated group (Fig. 2H). The SAUL_SEN_MAYO gene set, a newly identified signature of aging-related genes, is primarily composed of SASP factors [44]. Our research initially demonstrated an upregulation of the SAUL_SEN_MAYO gene set in APP/PS1 mice. Treatment with delphinidin downregulated the genes within the SAUL_SEN_MAYO gene set. These findings suggest that delphinidin may play a potential role in mitigating aging-related SASP changes in APP/PS1 mice. Among the top 20 significantly enriched KEGG pathways, our analysis highlighted the cAMP signaling pathway as particularly prominent in delphinidin-treated APP/PS1 mice compared to vehicle-treated APP/PS1 mice, suggesting its potential role in the observed biological effects. Considering that cAMP is a crucial precursor in AMP biosynthesis, which subsequently influences the activation energy of the AMPK signaling pathway. To summarize, the functional analysis of DEGs indicated that delphinidin may alleviate cognitive impairment by reducing oxidative stress, modulating the immune response, inhibiting cell senescence, and upregulating synapse-related process, while also enhancing AMPK signaling pathway.
Delphinidin prevents synapse loss and reduces Aβ plaques in APP/PS1 mice
It is well-established that the loss of synaptic function is associated with cognitive impairment. Our RNA sequencing enrichment analysis results suggest that delphinidin treatment promotes synaptogenesis and long-term potentiation in APP/PS1 mice (Fig. 2H). To further investigate the effects on synaptic plasticity, we assessed hippocampal synaptic integrity by analyzing postsynaptic PSD-95 and presynaptic Synaptophysin via immunofluorescence and western blot. Consistent with our quantitative data, visual inspection of these new confocal images reveals a sparser structure and reduced density of both PSD-95 and Synaptophysin puncta in the hippocampal CA1 region of vehicle-treated APP/PS1 mice compared to WT mice. Importantly, the images from the delphinidin-treated APP/PS1 mice clearly show increased density and a more robust appearance of both PSD-95 and Synaptophysin puncta in the CA1 region, indicating improved synaptic structures (Fig. 3A-B). Supporting this, Western blots confirmed delphinidin mitigated the overall hippocampal loss of PSD-95 and Synaptophysin protein in APP/PS1 mice (Fig. 3C-E). Additionally, Immunofluorescence staining for the dendritic marker Map2 revealed structural deficits in APP/PS1 mice that were significantly improved by delphinidin treatment (Supplementary Fig. 3A-B). These findings indicate an enhancement of synaptic plasticity in APP/PS1 mice following delphinidin treatment.
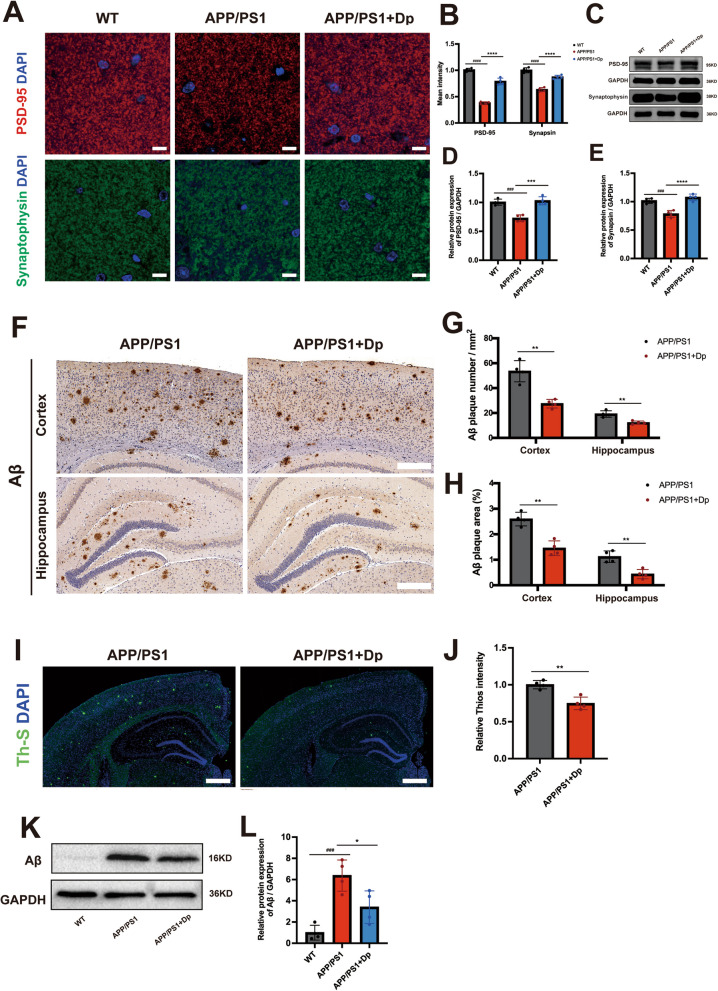
Fig. 3.
Delphinidin prevents synaptic loss and reduces Aβ plaques in APP/PS1 mice. A Representative PSD-95 and Synaptophysin immunostaining in the CA1. Scale bar = 50 μm. B Quantitative intensity analysis of PSD-95 and Synaptophysin immunostaining in the hippocampus. C Western blot analysis of PSD-95 and Synaptophysin in the hippocampus of mice. D, E Quantification of PSD-95/GAPDH (D) and Synaptophysin/GAPDH (E) in C. F Representative immunohistochemistry (IHC) staining of Aβ plaques in the brain. Scale bar = 200 μm. G, H Quantitative analysis of Aβ IHC staining number (G) and area (H) in the cortex and hippocampus. I Representative Th-S positive Aβ plaques in the brain. Scale bar = 500 μm. J Quantitative intensity analysis of Th-S positive staining in the brain. K Western blot analysis of Aβ and GAPDH in the hippocampus of mice. L Quantification of Aβ/GAPDH in I. n = 4 mice. Data were presented as mean ± SD. ###p < 0.0005, #### p < 0.0001 versus WT mice treated with vehicle, * p < 0.05, ** p < 0.005 versus APP/PS1 mice treated with vehicle. Dp: delphinidin; WT: wildtype
Extracellular accumulation of Aβ is a well-established pathological marker in APP/PS1 mice. To investigate whether delphinidin treatment can reduce Aβ plaque formation in the brain of APP/PS1 mice, we conducted immunohistochemistry and thioflavin S (Th-S) staining assays. Our findings revealed the presence of senile plaques in both the cortex and hippocampus of APP/PS1 mice. Notably, delphinidin treatment significantly decreased both the area and number of Aβ plaques in the cortex and hippocampus of APP/PS1 mice (Fig. 3F-H). Correspondingly, delphinidin treatment effectively decreased the intensity of Th-S staining in APP/PS1 mice (Fig. 3I-J). The hippocampal Aβ protein level was decreased in delphinidin-treated APP/PS1 mice compared with that in vehicle-treated APP/PS1 mice (Fig. 3K-L).
Investigating a potential mechanism for delphinidin’s effects on Aβ, we assessed microglial phagocytosis in vitro. Primary microglia treated with delphinidin showed significantly increased uptake of Cy3-labeled Aβ₄₂, measured by flow cytometry and immunofluorescence (Supplementary Fig. 4A-D). This suggests delphinidin promotes Aβ clearance by enhancing microglial phagocytic activity, explaining the reduced in vivo Aβ plaque load. These results suggest that delphinidin treatment significantly alleviates Aβ pathological alterations. Collectively, our findings indicate that delphinidin may delay or prevent neurodegenerative changes in APP/PS1 mice.
Delphinidin suppresses microglia activation, neuroinflammation and oxidative stress while upregulating the AMPK/SIRT1 pathway in APP/PS1 mice
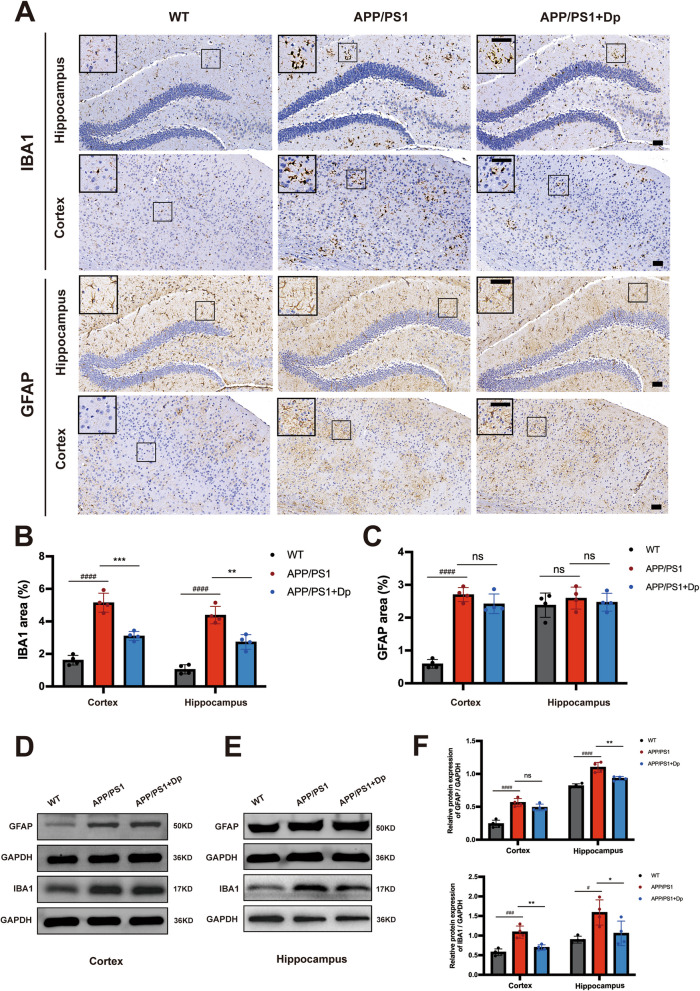
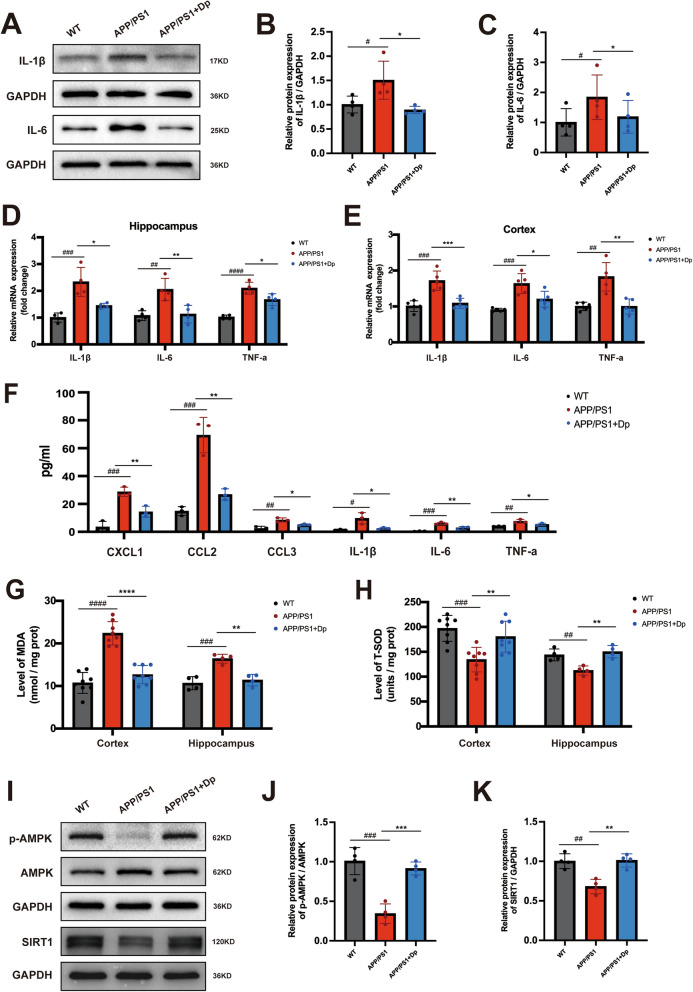
Neuroinflammation and oxidative stress play key roles in the pathogenesis of Aβ pathology. Given the important role of glial cells in neuroinflammation, we assessed reactive astrocytes and microglia by GFAP and Iba-1 immunostaining, respectively. Delphinidin treatment significantly decreased reactive microglia in both the cortex and hippocampus of the APP/PS1 mice (Fig. 4A-B). Immunohistochemistry results showed prominent GFAP activation in the cortex of APP/PS1 mice but no significant effect of delphinidin treatment when quantifying astrocyte area (Fig. 4C). To further investigate delphinidin’s impact on astrocytes and microglia, we performed Western Blot analyses on protein lysates from both hippocampal and cortical tissues. Consistent with reactive astrogliosis, we confirmed that GFAP protein levels were significantly increased in both the cortex and hippocampus of APP/PS1 mice compared to WT mice. Interestingly, our results revealed that delphinidin treatment significantly reduced the elevated GFAP protein levels specifically in the hippocampus and cortex of APP/PS1 mice. In the cortex, delphinidin showed a slight decreasing trend in GFAP protein levels, but this did not reach statistical significance (Fig. 4D-F). Western blot analysis showed the increased levels of IBA1 in the hippocampus and cortex of APP/PS1 mice compared to those of the WT mice. Delphinidin treatment reduced protein level of IBA1 in the hippocampus and cortex of APP/PS1 mice (Fig. 4D-F).
Fig. 4.
Delphinidin suppresses microglia activation in APP/PS1 mice. A Representative IHC staining of IBA1 and GFAP in the brains of APP/PS1 mice treated with delphinidin or vehicle and their WT littermates treated with vehicle. Scale bar = 50 μm. B Quantitative analysis of IBA1 immunostaining area in A. C Quantitative analysis of GFAP immunostaining area in A. D Western blot analysis of GFAP, IBA1 and GAPDH in the cortex of mice. E Western blot analysis of GFAP, IBA1 and GAPDH in the hippocampus of mice. F Quantification of GFAP/GAPDH and IBA1/GAPDH in D and E. n = 4 mice. Data were presented as mean ± SD. # p < 0.05, #### p < 0.0001 versus vehicle-treated WT mice, * p < 0.05, ** p < 0.005, *** p < 0.0005 versus vehicle-treated APP/PS1 mice, ns, not significant. Dp: delphinidin; WT: wildtype
To further explore the levels of inflammatory cytokines, we found that protein levels of IL-1β and IL-6 in the hippocampus of the APP/PS1 mice were higher than those of WT mice, whereas delphinidin markedly decreased the levels of these proinflammatory cytokines in the APP/PS1 mice (Fig. 5A-C). In addition, delphinidin administration reduced the mRNA levels of inflammatory mediators IL-1β, IL-6 and TNF-α in both the cortex and hippocampus of APP/PS1 mice (Fig. 5D-E). Similar to the brain, the levels of proinflammatory cytokines and chemokines CXCL1, CCL2, CCL3, IL-1β, IL-6, and TNF-α were significantly increased in the serum of APP/PS1 mice. These levels decreased following treatment with delphinidin (Fig. 5F).
Fig. 5.
Delphinidin reduces pro-inflammatory cytokine production and oxidative stress while upregulating the AMPK/SIRT1 pathway in APP/PS1 mice. A Western blot analysis of IL-1β, IL-6 and GAPDH in the hippocampus of mice. (n = 4 mice). B, C Quantification of IL-1β/GAPDH (B) and IL-6/GAPDH (C) in A. D The mRNA expression of IL-1β, IL-6 and TNF-α in the hippocampus were detected by qRT-PCR. (n = 4 mice). E The mRNA expression of IL-1β, IL-6, and TNF-α in the cortex were detected by qRT-PCR. (n = 5 mice). F Proinflammatory cytokines or chemokines CXCL1, CCL2, CCL3, IL-1β, IL6 and TNF-α levels were detected by cytokine array in the serum of APP/PS1 mice treated with delphinidin or vehicle and their WT littermates treated with vehicle. (n = 3 mice). G The level of MDA in the cortex and hippocampus of mice. (n = 8 mice for cortex, n = 4 mice for hippocampus). H The level of T-SOD activity in the cortex and hippocampus of mice. (n = 8 mice for cortex, n = 4 mice for hippocampus). I Western blot analysis of p-AMPK, AMPK, SIRT1 and GAPDH in the hippocampus of mice. (n = 4 mice). J, K Quantification of p-AMPK/AMPK (J) and SIRT1/GAPDH (K) in I. Data were presented as mean ± SD. # p < 0.05, ## p < 0.005, ### p < 0.0005, #### p < 0.0001 versus vehicle-treated WT mice, * p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001 versus vehicle-treated APP/PS1 mice. Dp: delphinidin; WT: wildtype
Increased levels of MDA and decreased activity of SOD are associated with Aβ plaque aggregation and the progression of AD. To investigate the potential impact of delphinidin on MDA levels and T-SOD activity, we measured MDA levels and T-SOD activity in both the cortex and hippocampus of the mice. The MDA content was significantly higher in the cortex and hippocampus of APP/PS1 mice compared to that in WT mice, as shown in Fig. 5G, indicating that APP/PS1 mice exhibit elevated levels of free radicals and cellular damage. Delphinidin treatment significantly reduced the MDA levels in the cortex and hippocampus of APP/PS1 mice. Furthermore, T-SOD activity was lower in the cortex and hippocampus of APP/PS1 mice compared to WT mice, while delphinidin treatment significantly increased T-SOD activity in APP/PS1 mice (Fig. 5H).
Since our RNA sequencing data indicated enrichment of the cAMP signaling pathway, and given that cAMP is a crucial precursor in AMP biosynthesis, which in turn influences the activation energy of the AMPK signaling pathway, we employed the western blotting analysis to determine whether delphinidin affects the AMPK signaling pathway. A significant decrease in the p-AMPK/AMPK ratio was observed in the hippocampus of APP/PS1 mice compared to WT mice (Fig. 5I-J). However, treatment with delphinidin markedly increased the p-AMPK/AMPK ratio in the APP/PS1 mice. Given the close relationship between SIRT1 and AMPK, we further investigated the expression levels of SIRT1. The protein expression of SIRT1 was decreased in the hippocampus of APP/PS1 mice compared to WT mice, while delphinidin treatment significantly enhanced the levels of this protein in APP/PS1 mice (Fig. 5I and K). To determine the cellular localization, we performed immunofluorescence co-staining. Significant co-localization of SIRT1 with the microglial marker IBA1 was observed in both primary microglia and BV2 microglial cells. This provides direct cellular evidence that SIRT1 activation by delphinidin takes place in microglia (Supplementary Fig. 6 A).
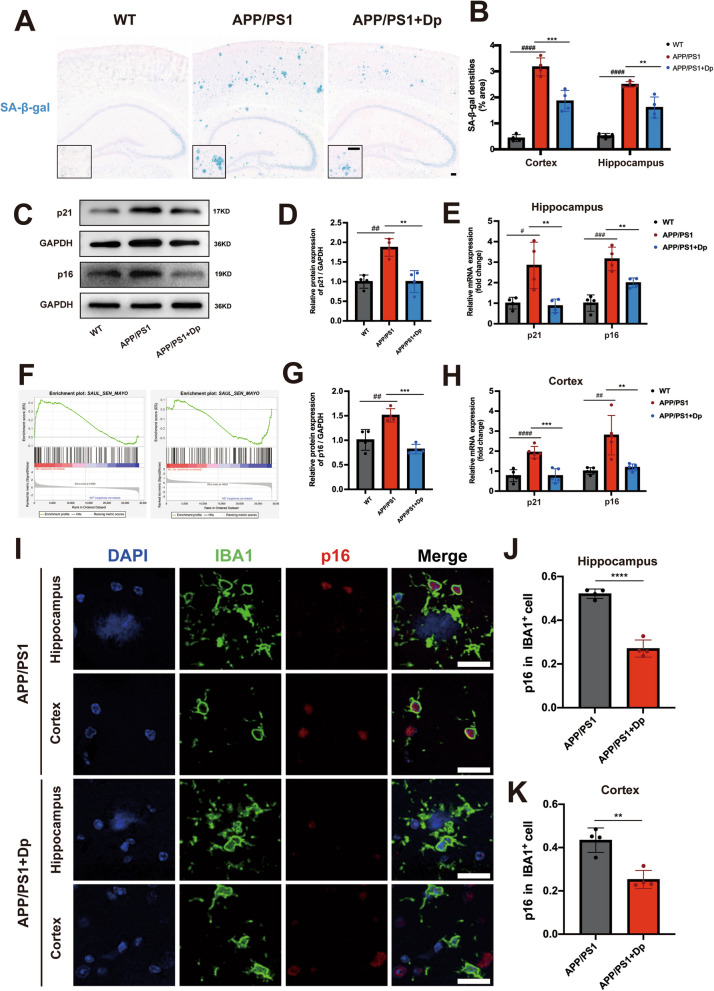
Delphinidin prevents microglial senescence in APP/PS1 mice
Previous studies have strongly indicated that inflammatory responses are a hallmark of cellular senescence, and recent findings suggest that cell senescence occurs in AD. To further investigate whether delphinidin influences cellular senescence, we examined senescence phenotypes using SA-β-gal staining. There was more SA-β-gal staining in both the hippocampus and cortex of APP/PS1 mice compared to WT mice. Notably, delphinidin treatment reduced SA-β-gal staining in the hippocampus and cortex of APP/PS1 mice (Fig. 6A-B). Western blot experiments showed that the protein level of senescence markers p16INK4a and p21cip1 in the hippocampus of APP/PS1 mice were significantly higher than those in WT mice. However, this increase was reversed by treatment delphinidin (Fig. 6C-D). Furthermore, the mRNA levels of p16INK4a and p21cip1 were also elevated in the hippocampus of APP/PS1 mice, but decreased following treatment with delphinidin (Fig. 6E and H).
Fig. 6.
Delphinidin prevents microglial senescence in APP/PS1 mice. A Representative SA-β-gal staining in the brains of APP/PS1 mice treated with delphinidin or vehicle and their WT littermates treated with vehicle. Scale bar = 100 μm. B Quantification of SA-β-gal staining densities in A. (n = 4 mice). C Western blot analysis of p16INK4a, p21cip1 and GAPDH in the hippocampus of mice. (n = 4 mice). D Quantification of p21cip1/GAPDH in C. (n = 4 mice). E The mRNA expression of p16INK4a and p21cip1 in the hippocampus were detected by qRT-PCR. (n = 4 mice). F Gene set enrichment analysis of SenMayo gene set between WT versus APP/PS1 mice and APP/PS1 versus delphinidin-treated APP/PS1 mice. G Quantification of p16INK4a/GAPDH in C. (n = 4 mice). H The mRNA expression of p16INK4a and p21cip1 in the cortex were detected by qRT-PCR. (n = 5 mice). I Immunofluorescence of IBA1 (green) with p16INK4a (red) in the hippocampus and cortex of APP/PS1 mice treated with delphinidin or vehicle. Scale bar = 20 μm. J, K Quantification the proportion of p16INK4a-positive cells among IBA+ cells in the hippocampus (J) and cortex (K) of APP/PS1 mice treated with delphinidin or vehicle. (n = 4 mice). Data were presented as mean ± SD. # p < 0.05, ## p < 0.005, ### p < 0.0005, #### p < 0.0001 versus vehicle-treated WT mice, ** p < 0.005, *** p < 0.0005, **** p < 0.0001 versus vehicle-treated APP/PS1 mice, ns, not significant. Dp: delphinidin; WT: wildtype
As mentioned earlier, the SASP as a marker of cellular senescence and involves the production of pro-inflammatory cytokines. The levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α were significantly reduced following delphinidin treatment (Fig. 5D and E). Following treatment delphinidin, the levels of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α were significantly reduced in APP/PS1 mice. The SAUL_SEN_MAYO gene set, a newly identified list of senescence-associated genes, is primarily composed of SASP factors [44]. Our research initially demonstrated that delphinidin treatment downregulated SAUL_SEN_MAYO genes in APP/PS1 mice (Fig. 6F). These results further confirm that delphinidin play a potential role in mitigating cellular senescence in AD mice.
Senescent microglia-mediated neuroinflammatory responses are believed to exacerbate neuronal damage, thereby driving the progression of age-related neurodegeneration in AD. Based on the RNA sequencing data revealed that delphinidin treatment down-regulated several genes associated with senescent microglia, including Apoe, Cst7, Tyrobp, Cd68, Itgax and Trem2 (Fig. 2C-D). To explore whether delphinidin inhibit microglia senescence in the brains of APP/PS1 mice, we co-stained for the cellular senescence marker p16INK4a alongside the microglia marker IBA1. Following delphinidin intervention, we observed a statistically significant reduction in the proportion of p16INK4a-positive cells among IBA+ cells in both the hippocampal and cortical regions of APP/PS1 mice, indicative of a mitigated senescent signature in brain (Fig. 6I-K).
To evaluate neuronal contribution and delphinidin treatment specificity, p16INK4a+/NeuN+ co-localization was performed. A minor increase in p16INK4a+ neurons was observed in APP/PS1 mice relative to WT, but delphinidin treatment showed only a minor trend towards reducing these p16INK4a-positive neurons (Supplementary Fig. 3C-D). Overall, our transcriptomic and immunofluorescence results indicate that delphinidin primarily alleviates microglial senescence, with minimal impact on neuronal senescence. Our data indicate for the first time that delphinidin exhibits anti-aging properties and alleviates cellular senescence in the brain of AD mice.
Delphinidin decreases microglial senescence in BV2 microglia cells
To investigate the effect of delphinidin on microglial senescence in vitro, we treated BV2 microglia cells with varying concentrations of delphinidin. Initially, we assessed the impact of different concentrations of delphinidin on BV2 microglia cells viability to determine a suitable concentration with minimal effects on cell viability (Supplementary Fig. 5).
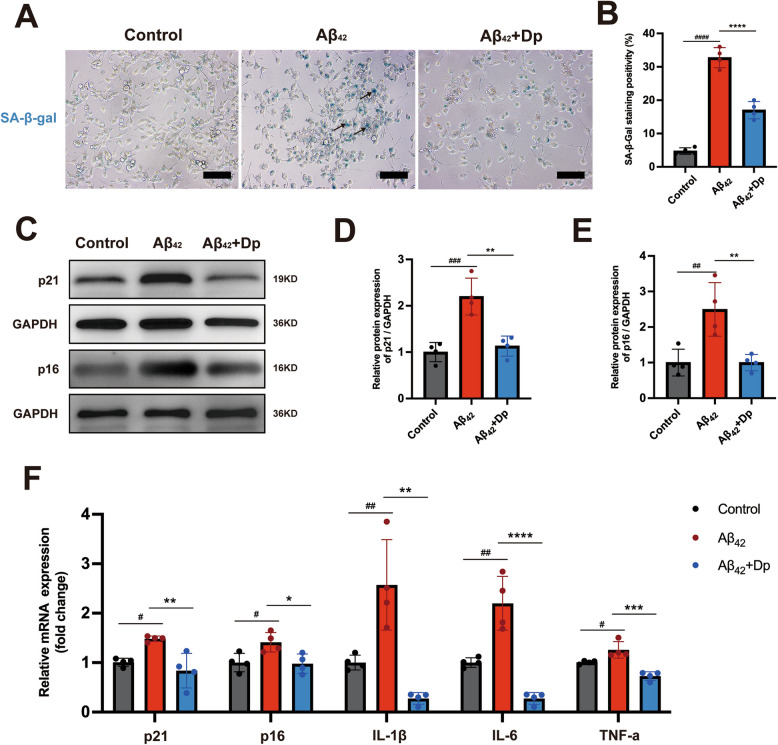
Since Aβ induces microglial activation, triggering an inflammatory response and accelerating microglial senescence [45]. We next evaluated the effect of delphinidin on Aβ₄₂-induced microglial senescence in vitro, following the experimental setup detailed schematically in Supplementary Fig. 7. Our results demonstrated a significant increase in SA-β-Gal positive cells following Aβ42 treatment, indicating the induction of cellular senescence (Fig. 7A-B). Conversely, delphinidin treatment attenuated the Aβ42-induced increase in SA-β-Gal positive cells. Furthermore, delphinidin treatment significantly reduced the protein and mRNA levels of p16INK4a and p21Cip1 induced by Aβ42 (Fig. 7C-F). Our data indicate that delphinidin eliminates senescent cells induced by Aβ42. We further investigated whether delphinidin treatment could reduce the inflammatory response in BV2 microglia cells induced by Aβ42. The results indicated that Aβ42 increased the mRNA levels of IL-1β, IL-6, and TNF-α inflammatory cytokines in BV2 microglia cells, while delphinidin treatment significantly reduced the levels of these cytokines (Fig. 7F). We further investigated delphinidin’s effect on senescence induced by different amyloid-beta oligomers, specifically using Aβ₂₅₋₃₅ in BV2 microglia. Delphinidin treatment effectively counteracted Aβ₂₅₋₃₅-induced senescence, significantly reduced SA-β-Gal positive cells, attenuating p16 and p21 mRNA expression (Supplementary Fig. 8A-C). This demonstrates that delphinidin’s protective anti-senescence effects extend beyond Aβ₄₂ oligomers.
Fig. 7.
Delphinidin ameliorates microglial senescence induced by the Aβ42 oligomers in BV2 microglia cells. A Representative SA-β-gal staining in BV2 microglia cells. Black arrows point to representative SA-β-gal staining positive cells. Scale bar = 20 μm. B Quantification of SA-β-gal staining positive cells in A. C Western blot analysis of p21cip1, p16INK4a and GAPDH in BV2 microglia cells. D, E Quantification of p21cip1/GAPDH (D) and p16INK4a/GAPDH (E) in C. F The mRNA expression of p16INK4a, p21cip1, IL-1β, IL-6, and TNF-α in BV2 microglia cells were detected by qRT-PCR. n = 4. Data are presented as mean ± SD. # p < 0.05, ## p < 0.005, ### p < 0.0005, #### p < 0.0001 versus vehicle-treated cells, * p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001 versus Aβ42 oligomers-treated cells. Dp: delphinidin
Delphinidin decreases excessive ROS production while upregulating the AMPK/SIRT1 pathway in BV2 microglia cells
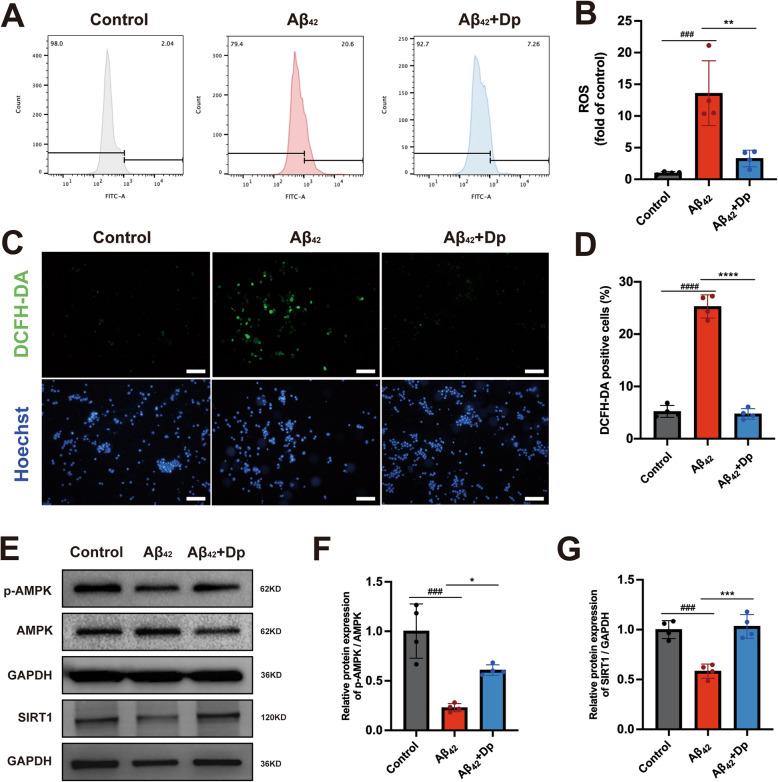
Excessive ROS production, as senescence marker, was significantly upregulated in BV2 cells treated with Aβ42, as assessed by flow cytometry and microscopy. Notably, delphinidin treatment attenuated the production of ROS induced by Aβ42 (Fig. 8A-D). Similarly, delphinidin treatment markedly reduced the intracellular levels of ROS induced by Aβ₂₅₋₃₅ (Supplementary Fig. 8D-G). Given delphinidin's potent antioxidant properties, it may alleviate cellular senescence by decreasing ROS production.
Fig. 8.
Delphinidin decreases excessive ROS production while enhancing the AMPK/SIRT1 pathway induced by the Aβ42 oligomers in BV2 microglia cells. A, B Assessment of ROS production in BV2 microglia cells via flow cytometry following loading with the ROS indicator DCFH-DA. C Representative image of BV2 microglia cells loaded with DCFH-DA (green) and Hoechst (blue). Scale bar = 50 μm. D Quantification the proportion of DCFH-DA positive cells in C. E Western blot analysis of p-AMPK, AMPK, SIRT1 and GAPDH in BV2 microglia cells. F, G Quantification of p-AMPK/AMPK (F) and SIRT1/GAPDH (G) in E. n = 4. Data are presented as mean ± SD. ### p < 0.0005, #### p < 0.0001 versus vehicle-treated cells, * p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001 versus Aβ42 oligomers-treated cells. ROS, reactive oxygen species; Dp: delphinidin
We further evaluated the effect of delphinidin on the AMPK and SIRT1 in BV2 cells induced by Aβ42. A significant decrease in the p-AMPK/AMPK ratio and SIRT1 expression was observed in BV2 cells treated with Aβ42. However, treatment with delphinidin markedly increased the p-AMPK/AMPK ratio and SIRT1 expression in the Aβ42 induced BV2 cells (Fig. 8E-G).
Delphinidin inhibits microglial senescence induced by Aβ42 through the AMPK/SIRT1 pathway
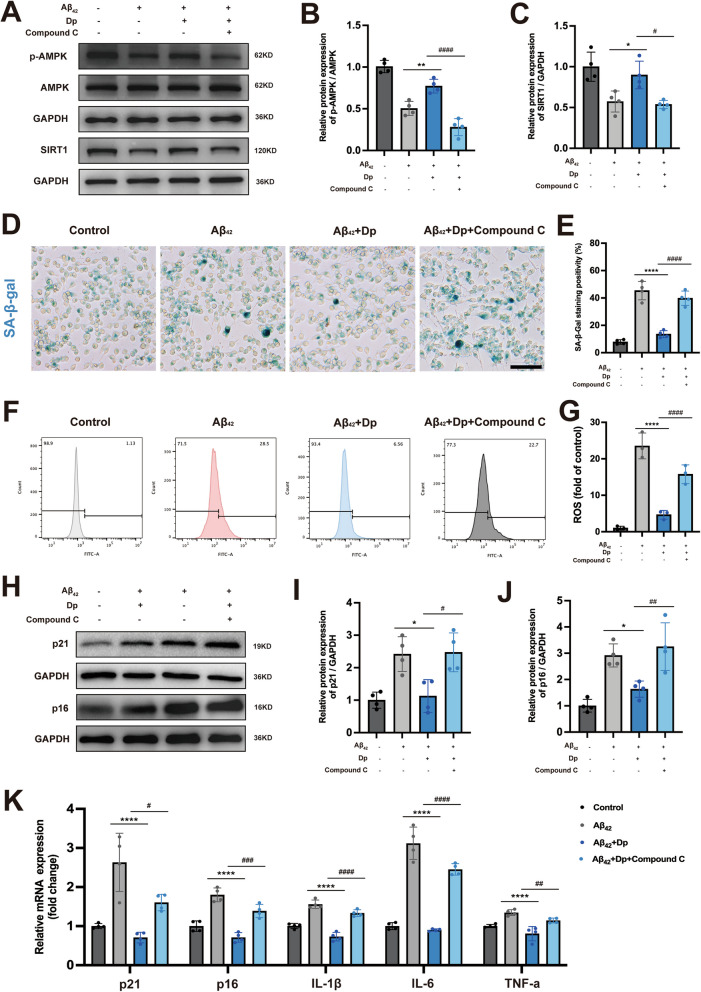
To investigate whether delphinidin protects against Aβ42-induced microglial senescence by modulating the AMPK pathway, we conducted in vitro experiments co-administering delphinidin with the AMPK inhibitor Compound C. As anticipated, the results showed that Compound C effectively inhibited AMPK activation and suppressed SIRT1 expression (Fig. 9A-C).
Fig. 9.
Delphinidin inhibits microglial senescence induced by Aβ42 through the AMPK/SIRT1 pathway. A Western blot analysis of p-AMPK, AMPK, SIRT1 and GAPDH in BV2 microglia cells. B, C Quantification of p-AMPK/AMPK (B) and SIRT1/GAPDH (C) in A. D Representative SA-β-gal staining in BV2 microglia cells. Scale bar = 20 μm. E Quantification of SA-β-gal staining positive cells in D. F, G Assessment of ROS production in BV2 microglia cells via flow cytometry following loading with the ROS indicator DCFH-DA. H Western blot analysis of p21cip1, p16INK4a and GAPDH in BV2 microglia cells. (I-J) Quantification of p21cip1/GAPDH (I) and p16INK4a/GAPDH (J) in C. K The mRNA expression of p16INK4a, p21cip1, IL-1β, IL-6, and TNF-α in BV2 microglia cells were detected by qRT-PCR. n = 4. Data are presented as mean ± SD. # p < 0.05, ## p < 0.005, ### p < 0.0005, #### p < 0.0001 versus Aβ42 oligomers and delphinidin treated cells, * p < 0.05, ** p < 0.005, **** p < 0.0001 versus Aβ42 oligomers-treated cells. ROS, reactive oxygen species; Dp: delphinidin
SA-β-gal staining revealed that Compound C inhibited the ability of delphinidin to reverse the reduction in the proportion of SA-β-gal-positive cells induced by Aβ42 (Fig. 9D-E). Flow cytometry analysis demonstrated that, in the presence of Compound C, delphinidin was unable to mitigate the ROS production induced by Aβ42 (Fig. 9F-G). Similarly, Compound C inhibited the ability of delphinidin to reverse the protein expression and mRNA level of P21CIP1 and P16INK4a triggered by Aβ42 (Fig. 9H-K). Similarly, Compound C inhibited the ability of delphinidin to reverse the mRNA levels of IL-1β, IL-6, and TNF-α inflammatory cytokines induced by Aβ42 (Fig. 9K).
In conclusion, these findings suggest that delphinidin exerts its protective effects against Aβ42-induced microglial senescence through targeted regulation of the AMPK/SIRT1 pathway.
Delphinidin directly binds to SIRT1
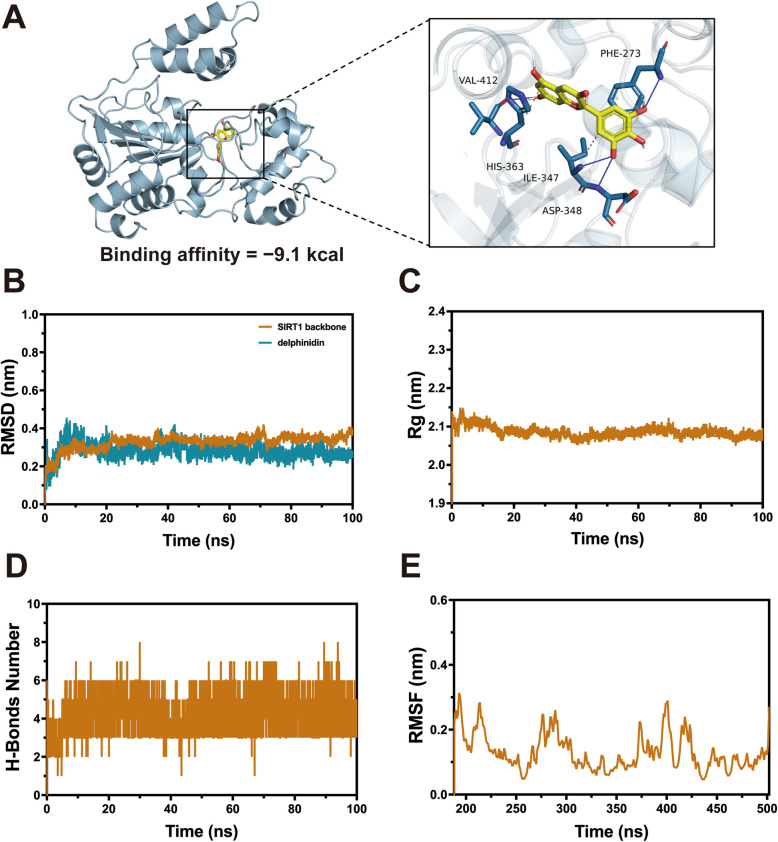
Recent research suggested that SIRT1 regulating metabolism and cellular senescence through activates AMPK [38]. Molecular docking simulation was performed to investigate the binding mode of delphinidin within the active site of the SIRT1 protein using AutoDock Vina 1.1.2 and PLIP. The molecular docking results indicated that hydrogen bonds were formed at the PHE-273, ILE-347, ASP-348, and VAL-412 residues between delphinidin and SIRT1 (Fig. 10A). The theoretical binding affinity of A to SIRT1 was calculated to be − 9.1 kcal/mol (mean value). This indicates a strong binding between delphinidin and SIRT1, which may potentially influence the structural function and biological activity of SIRT1.
Fig. 10.
Direct interaction between SIRT1 and delphinidin. A The docking mode of delphinidin with SIRT1 protein. B Root Mean Square Deviation (RMSD) analysis showing the binding of delphinidin with SIRT1 protein. C Gyration radius (Rg) analysis showing the binding of delphinidin with SIRT1 protein. D The number of hydrogen bonds formed between delphinidin and SIRT1 protein. E Root Mean Square Fluctuation (RMSF) analysis showing the binding of delphinidin with SIRT1 protein
To further validate the binding affinity and stability between delphinidin and SIRT1, we performed molecular dynamics simulations on the docking results. The Root Mean Square Deviation (RMSD) values of the backbone atoms stabilized at approximately 0.30–0.42 nm after 20 ns, indicating that the system had reached a well-equilibrated state. The RMSD of the delphinidin stabilized at around 0.25–0.45 nm, suggesting that it could stably bind to the SIRT1's hydrophobic pocket (Fig. 10B). Furthermore, the radius of gyration (Rg) of the SIRT1 protein was analyzed during the simulation, and it was found to remain stable at around 2.0–2.2 nm, indicating that the protein-small molecule complex was relatively stable throughout the simulation (Fig. 10C). The number of hydrogen bonds formed directly between SIRT1 protein and delphinidin during the simulation was also analyzed. It was found that 3–6 stable hydrogen bonds were formed between SIRT1 protein and delphinidin, which played a crucial role in their stable binding (Fig. 10D). Additionally, the root-mean-square fluctuation (RMSF) of the SIRT1 protein backbone was analyzed, revealing that the regions spanning residues 184–196, 207–219, 273–305, 372–375, 395–405, and 414–425 exhibited higher flexibility, while the remaining regions showed relatively rigid structures (Fig. 10E).
These findings suggest that the complex formed between delphinidin and SIRT1 is remarkably stable and exhibits strong binding affinity. The results further demonstrate that delphinidin treatment significantly enhances the thermal stability of SIRT1. Overall, it is likely that delphinidin exerts its protective effects by specifically targeting and binding to SIRT1, thereby enhancing its stability and function.
Delphinidin also mitigates microglial senescence in naturally aged mice
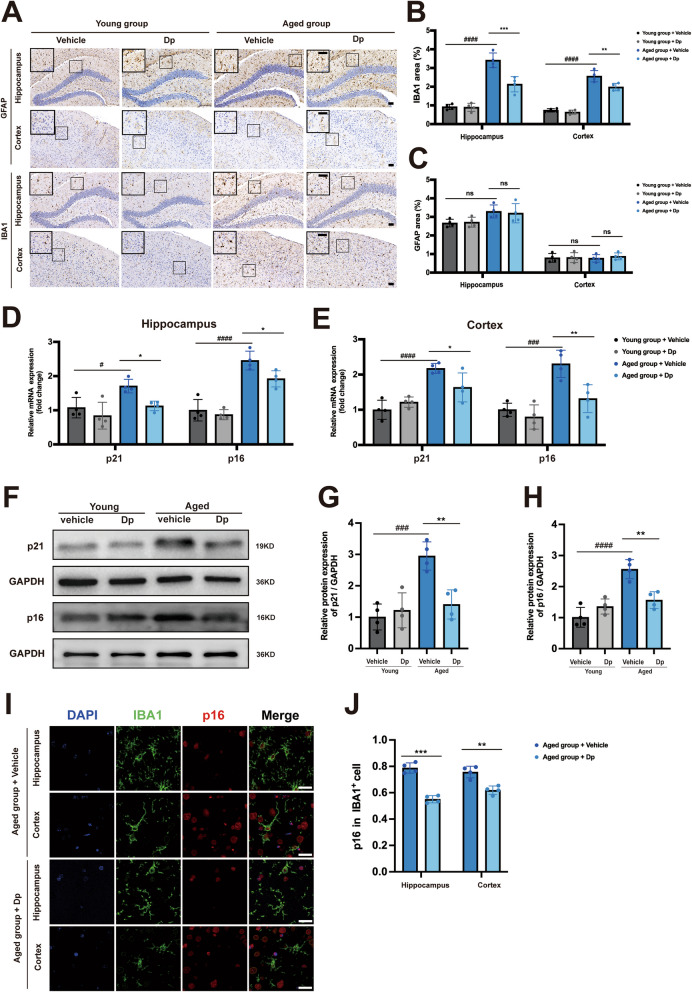
Glial activation, characterized by astrocyte and microglial activation, is a hallmark of brain aging. Immunohistochemical staining revealed a significant increase in IBA1 area in both the hippocampus and cortex of aged mice compared to young mice, but delphinidin treatment significantly reduced IBA1 area in both the hippocampus and cortex of aged mice (Fig. 11 A-B). Delphinidin treatment had no discernible effect on the GFAP area of young mice and aged mice (Fig. 11C).
Fig. 11.
Delphinidin also reduces microglial senescence in aged mice. A Representative IHC staining of GFAP and IBA1 in the brains of young mice treated with delphinidin or vehicle and aged mice treated with delphinidin or vehicle. Scale bar = 50 μm. B Quantitative analysis of GFAP immunostaining area in A. C Quantitative analysis of IBA1 immunostaining area in A. D, E The mRNA expression of p16INK4a and p21cip1 in the hippocampus (D) and cortex (E) were detected by qRT-PCR. F Western blot analysis of p16INK4a, p21cip1 and GAPDH in the hippocampus of mice. (n = 4 mice). G, H Quantification of p21cip1/GAPDH (G) and p16INK4a/GAPDH (H) in F. I Immunofluorescence of IBA1 (green) with p16INK4a(red) in the hippocampus and cortex of aged mice treated with delphinidin or vehicle. Scale bar = 20 μm. J Quantification the proportion of p16INK4a-positive cells among IBA+ cells in the hippocampus and cortex of aged mice treated with delphinidin or vehicle. n = 4 mice. Data are presented as mean ± SD. # p < 0.05, ### p < 0.0005, #### p < 0.0001 versus vehicle-treated young mice, * p < 0.05, ** p < 0.005, *** p < 0.0005 versus vehicle-treated aged mice, ns, not significant. Dp: delphinidin
To further investigate cellular senescence, we examined the expression of senescence markers p16INK4a and p21Cip1 in young and aged mice. Notably, our findings revealed that the mRNA levels of p16INK4a and p21Cip1 were significantly upregulated in both the hippocampus and cortex of aged mice, but were notably reduced following treatment with delphinidin (Fig. 11D-E). Similarly, delphinidin treatment also decreased the protein levels of p16INK4a and p21Cip1 in the hippocampus of aged mice (Fig. 11F-H). We evaluated microglial senescence by co-staining p16INK4a with the microglia marker IBA1. Following treatment with delphinidin, a statistically significant reduction in the proportion of p16INK4a-positive cells among IBA+ cells was observed in both the hippocampal and cortical regions of aged mice (Fig. 11I-J). These findings indicate for the first time that delphinidin mitigates microglia senescence in the brain of aged mice.
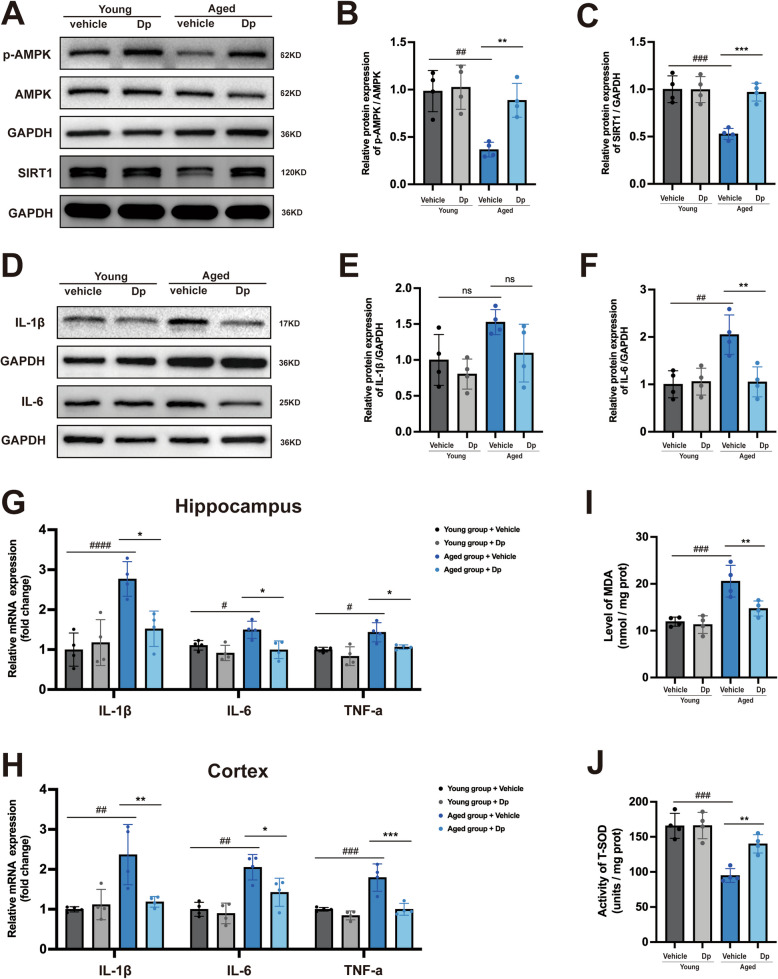
Delphinidin suppresses neuroinflammation, oxidative stress while upregulating the AMPK/SIRT1 pathway in naturally aged mice
Furthermore, we investigated the effect of delphinidin on AMPK/SIRT1 signaling pathway in young and aged mice. A significant decrease in the p-AMPK/AMPK ratio and SIRT1 expression was observed in the brains of aged mice compared to those in young mice. However, treatment with delphinidin notably restored both the p-AMPK/AMPK ratio and SIRT1 expression in the brains of aged mice (Fig. 12A-C). The protein levels of IL-1β and IL-6, as well as the mRNA levels of IL-1β, IL-6, and TNF-α inflammatory cytokines, were significantly increased in the brains of aged mice compared to those of young mice. Delphinidin treatment significantly decreased the protein levels of IL-1β and IL-6 inflammatory cytokines in the brains of aged mice (Fig. 12D-F). Similarly, delphinidin treatment reduced the mRNA levels of IL-1β, IL-6, and TNF-α in aged mice (Fig. 12G-H).
Fig. 12.
Delphinidin suppresses pro-inflammatory cytokine production, oxidative stress while upregulates the AMPK/SIRT1 pathway in aged mice. A Western blot analysis of p-AMPK, AMPK, SIRT1 and GAPDH in the hippocampus of mice. B, C Quantification of p-AMPK/AMPK (B) and SIRT1/GAPDH (C) in A. D Western blot analysis of IL-1β, IL-6 and GAPDH in the hippocampus of mice. E, F Quantification of IL-1β/GAPDH (E) and IL-6/GAPDH (F) in D. G, H The mRNA expression of IL-1β, IL-6, and TNF-α in the hippocampus (G) and cortex (H) were detected by qRT-PCR. I The level of MDA in the hippocampus of mice. J The level of T-SOD activity in the hippocampus of mice. n = 4 mice. Data are presented as mean ± SD. # p < 0.05, ## p < 0.005, ### p < 0.0005, #### p < 0.0001 versus vehicle-treated young mice, * p < 0.05, ** p < 0.005, *** p < 0.0005 versus vehicle-treated aged mice, ns, not significant. Dp: delphinidin
Oxidative stress plays a crucial role in aged-related alternations. We further investigated the potential effect of delphinidin on oxidative stress in both the cortex and hippocampus of aged mice. The results showed that the MDA content was significantly higher in the cortex of aged mice compared to that in young mice. Delphinidin treatment significantly reduced the MDA levels in the cortex of aged mice (Fig. 12I). Furthermore, T-SOD activity was lower in the cortex of aged mice compared to that in young mice, while delphinidin treatment significantly increased T-SOD activity in aged mice (Fig. 12J).
Discussion
As a progressive and age-related neurodegenerative disease, AD is characterized by cognitive decline and is the primary cause of dementia. Unfortunately, there are currently no effective therapeutic agents available to slow or halt the progression of AD. Abnormal deposition of Aβ is believed to play a crucial role in AD, with excessive aggregation of Aβ into plaques being a hallmark of early-stage AD brain pathology. Disaggregating Aβ fibrils represents a promising therapeutic strategy for AD. Previous studies have demonstrated that delphinidin has the ability to disaggregate amyloid β fibrils, as evidenced by total-internal-reflection fluorescence microscopy and quartz-crystal microbalance techniques. However, the potential of delphinidin to regulate key pathological processes in APP/PS1 mice and naturally aged mice—such as Aβ plaques accumulation, synaptic loss, cell senescence, neuroinflammation and oxidative stress—has yet to be explored.
Delphinidin, one of the most valuable anthocyanidins, can be extracted from a variety of colorful vegetables and fruits. Anthocyanins have emerged as promising therapeutic agents for the treatment of AD, owing to their diverse array of bioactive properties, including potent antioxidant and anti-inflammatory effects. Previous studies have shown that flavonoid compounds can enhance cognitive function in AD mice [41, 46]. Research has shown that anthocyanins, including delphinidin, are efficiently absorbed and distributed, with evidence of their presence in the brain following oral administration [47, 48]. This demonstrates that delphinidin can effectively cross the blood–brain barrier. Our current study provides the first evidence that delphinidin treatment effectively ameliorates cognitive deficits, reduces Aβ plaque accumulation, mitigates microglial senescence, decreases neuroinflammation, alleviates oxidative stress, and enhances synaptic plasticity in the brains of APP/PS1 mice. Consistent with previous findings that delphinidin alleviates cognitive deficits Meynert-lesioned rats, our results further suggest that delphinidin has the potential to enhance cognitive function in AD mouse models.
Notably, delphinidin treatment also modulates the gene expression profile associated with senescent microglia, potentially restoring the disrupted gene expression patterns caused by pathological changes in APP/PS1 mice. Recent studies show that the disassembly of Aβ fibrils by anthocyanins depends on the number of hydroxyl groups in ring B. Delphinidin-3-galactoside, with three hydroxyl groups, exhibits high disassembly activity, destabilizing Aβ protofibrils [49]. In our study, delphinidin treatment reduced Aβ deposits in the cortex and hippocampus of APP/PS1 mice, suggesting that delphinidin effectively inhibited Aβ pathology of APP/PS1 mice. Pathological studies have revealed that Aβ plaque deposition triggers a cascade of detrimental effects, including mitochondrial dysfunction, oxidative stress, and neuroinflammation, ultimately leading to synaptic loss and neuronal degeneration [50]. The progressive loss of synapses in the hippocampus is a key contributor to cognitive decline in both AD mouse models and human patients. The postsynaptic protein PSD-95 and the presynaptic protein Synaptophysin are crucial for synaptic plasticity. Our study found that delphinidin significantly increased the protein expression and immunofluorescence intensity of both PSD-95 and Synaptophysin in APP/PS1 mice. This coincided with enhanced transcriptional activity related to synaptic function. Furthermore, delphinidin treatment also increased staining intensity for the dendritic marker Map2, suggesting improved dendritic integrity. Furthermore, GSEA indicated that delphinidin treatment upregulated DEGs associated with dendrite morphogenesis, GABAergic synapses, nerve growth factor and long-term potentiation in APP/PS1 mice. These findings suggest that delphinidin promotes synaptic plasticity in AD mouse model.
The accumulation of senescent cells releases SASP factors that promote inflammation, oxidative stress, synaptic dysfunction and neuron loss [51, 52]. Previous studies have indicated that excessive oxidative stress in APP/PS1 mice triggers senescence characteristics, including heightened SA-β-gal activity and cell cycle arrest, highlighting the pathogenic link between oxidative stress and senescence in AD [53]. Therefore, reducing excessive production of ROS is an effective strategy for mitigating cellular senescence and enhancing longevity. Growing evidence suggests that anthocyanins can delay aging and alleviate age-related diseases [22, 42, 43, 54]. We demonstrated for the first time the antioxidant properties of delphinidin in AD mice in this study. Delphinidin treatment significantly reduced the pathways associated with redox reaction in APP/PS1 mice. Furthermore, delphinidin treatment significantly reduced the MDA levels and increased T-SOD activity in APP/PS1 mice.
Recently, the flavonoid procyanidin C1 has been shown to effectively eliminate senescent cells, exerting significant biological effects in naturally aged mice [21]. Furthermore, a previous study has shown that luteolin, another abundant flavonoid, can delay the onset of senescence and extend the lifespan of naturally aged mice, potentially by disrupting the interaction between p16 and CDK6 [42]. Delphinidin has been previously identified to promote antioxidant and anti-inflammatory effects in various models [16, 18, 55]. A recent study has revealed the promising anti-aging effects of delphinidin against oxidative stress-induced senescence in human nucleus pulposus cells [24]. However, it remains unclear whether delphinidin possesses anti-aging properties in the brain. Our results showed delphinidin treatment significantly decreased SA-β-gal activity and the level of p16INK4a and p21Cip1 in APP/PS1 mice. The SAUL_SEN_MAYO gene set has been identified as a newly recognized subcluster of senescence-associated genes [44]. In this study, we found that delphinidin significantly downregulated the genes within SAUL_SEN_MAYO gene set. In another study, a subcluster of genes was identified that is upregulated in aging muscle [42]. Consistent with this finding, our study also observed similar upregulation of aging-related genes in APP/PS1 mice, and delphinidin treatment significantly decreased the expression of these aging-related genes. Thus, our results demonstrated that delphinidin exhibits anti-aging properties and alleviates cellular senescence in the brain of AD mice.
Senescent microglia release pro-inflammatory cytokines and other mediators, ultimately contributing to neuronal damage in the AD brain. The previous study revealed that dietary supplementation with delphinidin-rich extract effectively inhibits obesity-induced hippocampal inflammation in mice [56]. To our knowledge, few studies have investigated the effects of delphinidin on microglia in AD. In this study, we found that delphinidin treatment reduced microglia activation and inhibited pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in both AD mice and aged mice. The anti-inflammatory effects of delphinidin are also supported by other studies [16, 17, 55, 57, 58]. The senolytic treatment reduced senescent microglia, resulting in improved cognition and decreased neuroinflammation in 5 × FAD mice [59]. Senolytic treatment effectively eliminates autophagy-deficient senescent microglia and AD-related pathology in 5 × FAD mice [60]. These findings suggest that targeting senescent microglia may represent a promising therapeutic strategy for AD. Recent research identified senescent microglia expressing genes such as TREM2, ApoE, Lpl, Cst7, Cd63, Itgax, Cd83, Cd9, Tyrobp, Ccl4, Lyz2 and Cd68 [59]. These genes were also observed to be upregulated in aged retina [43]. Although the transcriptional profile of senescent microglia is similar to that of disease-associated microglia (DAM), but they display distinct protein profiles that set them apart from DAM. Interestingly, treatment with a Bcl2 family inhibitor, which acts as a senolytic agent, significantly reduced the level of senescent microglia without impacting the DAM population, and was associated with enhanced cognitive performance in an AD mouse model. These previous studies suggested that progressive senescent microglia play a more significant role in the pathology of AD than activate microglia. Our RNA-seq analysis of APP/PS1 mice revealed a dominant transcriptional signature characterized by the upregulation of microglial activation and senescence-associated genes, such as Trem2, Cst7, and Clec7a. This finding aligns with published studies [61] and reflects the strong neuroinflammatory response to Aβ pathology inherent to this model. While neuronal pathway-related genes, including App and Apbb1ip, also showed significant upregulation in APP/PS1 mice compared to wild-type controls, the magnitude and statistical significance of these changes were markedly less pronounced than those observed for the genes associated with microglial activation and senescence. This disparity likely reflects the robust and coordinated microglial response, whose strong transcriptional signal can statistically overshadow more subtle neuronal alterations in bulk RNA-seq analyses. Therefore, given the prominent nature of these microglial changes, our study prioritized the investigation of microglial senescence. Consistent with this focus, our data show that delphinidin treatment significantly downregulated genes associated with senescent microglia in APP/PS1 mice, including TREM2, ApoE, Cst7, Itgax, Tyrobp, Ccl4 and Lyz2. Further experiment showed that delphinidin treatment reduced the proportion of p16INK4a-positive cells among IBA1+ microglia in APP/PS1 mice. Consequently, these findings provide evidence that delphinidin may mitigate microglial senescence and neuroinflammatory responses in APP/PS1 mice. Given our primary focus on microglia, supported by transcriptomics, we did not further investigate astrocyte-specific mechanisms. Whether delphinidin inhibits or modulates astrocytes requires future study. Accumulating evidence suggests that Aβ induces microglial senescence, oligodendrocyte progenitor cells senescence and accelerates neuronal senescence [5–8].
Our findings indicate that delphinidin significantly reduced the proportion of SA-β-Gal positive cells and downregulated the levels of senescence markers p21Cip1 and p16INK4a induced by Aβ in BV2 cells. Furthermore, delphinidin eliminated Aβ-induced excessive ROS production and pro-inflammatory cytokines in BV2 cells. In addition to its role in regulating oxidative stress and inflammation, delphinidin may also mitigate microglial senescence through other mechanisms. Previous research has reported that procyanidin C1 can eliminate senescent cells by inducing apoptosis in these cells [21].
Furthermore, delphinidin treatment reduced the accumulation of senescent microglia, neuroinflammation, and oxidative stress in naturally aged mice. To elucidate the underlying mechanisms, we employed specific inhibitor of AMPK in microglia, confirming that delphinidin decreases Aβ-induced microglial senescence in an AMPK/SIRT1-dependent manner.
AMPK has been extensively investigated in the context of various aging-related diseases. It exerts neuroprotective effects by enhancing autophagy, reducing oxidative stress, inhibiting inflammatory response, and improving insulin sensitivity and mitochondrial biogenesis [62]. Furthermore, AMPK is implicated in the pathogenesis of AD through its regulation of amyloid precursor protein (APP) processing by inhibiting BACE1 expression, accelerating Aβ degradation, and modulating tau protein phosphorylation [63, 64]. Recent research indicates that the deletion of SIRT1 accelerates the translocation of APP to the endosome, thereby facilitating the amyloidogenic processing of APP in AD mice [65]. Previous studies have shown that AMPK activation and SIRT1 expression are significantly down-regulated in the AD models [66–69]. Moreover, recent research indicates that activating the AMPK/SIRT1 pathway reduces neuroinflammation and oxidative stress, thereby mitigating cognitive impairment in AD models [70]. Notably, a growing body of evidence suggests that delphinidin exerts protective effects by inducing autophagy, enhancing lipid metabolism, and promoting mitochondrial biogenesis through the regulation of AMPK and SIRT1 signaling pathways in various diseases [25–28]. Consistent with previous reports, we observed significant suppression of AMPK activation and SIRT1 expression in the hippocampus of APP/PS1 mice and in microglia in vitro. However, treatment with delphinidin enhanced AMPK activation and stimulated SIRT1 expression in APP/PS1 mice and microglia in vitro.
Additionally, activated AMPK effectively prolonging cell survival during energy deficiency [71]. SIRT1 plays a crucial role in anti-aging by deacetylating p53, inhibiting cell senescence, and promoting cell proliferation [38]. Notably, recent clinical trials have underscored the significant impact of AMPK and SIRT1 on the progression of senescence [72–76]. Our results revealed that treatment with delphinidin notably enhanced AMPK activation and stimulated SIRT-1 expression in the brains of aged mice. Naturally derived compounds targeting cellular senescence have shown promise in enhancing lifespan across various models [77]. Consequently, botanical extracts present valuable developing therapeutics aimed at promoting healthy aging and alleviating the economic burdens with increasing aging population [42]. Our study revealed that delphinidin alleviates microglial senescence, neuroinflammation, and oxidative stress in the brains of aged mice, suggesting its potential as a therapeutic strategy for promoting healthy aging. Understanding delphinidin’s effects on behavior (cognition, anxiety) during normal aging, alongside its potential impact on lifespan and systemic aging across organs, is crucial for future research to fully uncover its therapeutic potential.
The precise mechanisms by which AMPK and SIRT1 coordinate to regulate cellular senescence remain unclear. However, it is established that a complex interplay exists between AMPK and SIRT1. Previous studies have shown that AMPK can increase cellular levels of Nicotinamide adenine dinucleotide (NAD +), thereby inducing the activation of SIRT1 [33]. Conversely, SIRT1 has been found to activate AMPK through the deacetylation of LKB1, a key upstream kinase that phosphorylates and activates AMPK [78]. In the present study, we sought to further investigate the potential role of the AMPK/SIRT1 pathway in mediating the effects of delphinidin on microglial senescence. Our results indicate that delphinidin protects against microglial senescence through an AMPK/SIRT1 pathway by using Compound C, an AMPK inhibitor. Additionally, delphinidin was found to directly interact with SIRT1. Therefore, delphinidin may exert neuroprotective effects against microglial senescence by enhancing SIRT1 through the regulation of AMPK activation, or it may directly bind to SIRT1. In conclusion, delphinidin appears to influence both AMPK and SIRT1 through multiple mechanisms, including upstream signaling modulation, direct binding interactions, and enhancement of NAD⁺ availability. While molecular docking suggests a potential direct interaction with SIRT1, the functional consequences of this binding, particularly in terms of SIRT1 activation and downstream metabolic effects, should be further explored. The integration of these molecular pathways could provide a deeper understanding of how delphinidin mediates its beneficial effects on metabolic health.
Conclusion
In summary, our study revealed for the first time that delphinidin effectively improved cognitive deficits, alleviated synapse loss and Aβ pathology in APP/PS1 mice. This study is the first to demonstrate that delphinidin exhibits anti-aging properties in the brain of AD mice and naturally aged mice. Moreover, the AMPK/SIRT1 signaling pathway is involved in the protective effects of delphinidin against microglial senescence. Given its antioxidant and anti-inflammatory, delphinidin has the potential to function as a senomorphic drug. Its ability to modulate senescence-related pathways, particularly through the activation of the AMPK/SIRT1 signaling axis, positions it as a promising candidate for further investigation in the context of aging and age-related diseases. These findings support delphinidin’s potential as a novel natural senolytic treatment and the prospects of natural anti-aging agents for the development of AD therapy. Furthermore, our results highlight delphinidin as a promising natural anti-aging agent against the development of aging and age-related diseases.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- Aβ
β-Amyloid
- AD
Alzheimer’s disease
- AMPK
AMP-activated protein kinase
- ANOVA
Analysis of variance
- APP/PS1
APPswe/PSEN1dE9
- DAB
3,3′-Diaminobenzidine
- DCFH-DA
Dichlorodihydrofluorescein diacetate
- DEGs
Differentially expressed genes
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- GO
Gene Ontology
- GSEA
Gene set enrichment analysis
- HFIP
1,1,1,3,3,3-Hexafluoroisopropanol
- IF
Immunofluorescence
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- MD
Molecular dynamics
- MDA
Malondialdehyde
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- MWM
Morris water maze
- NOR
Novel Object Recognition
- PBS
Phosphate buffered saline
- PDB
Protein Data Bank
- PME
Particle-Mesh Ewald
- RT-qPCR
Real time quantitative Polymerase Chain Reaction
- RIPA
Radio-Immunoprecipitation Assay
- ROS
Reactive oxygen species
- RNA-seq
RNA sequencing
- SA‑β‑gal
Senescence-Associated β-Galactosidase
- SASP
Senescence-associated secretory phenotype
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM
Standard error of mean
- SOD
Superoxide dismutase
- TBA
Thiobarbituric acid
- Th-S
Thioflavin S
- WT
Wild-type
Authors’ contributions
Wenshi Wei and Aijuan Yan contributed equally to the study design. Ying Liu drafted the manuscript. Ying Liu and Ting Hong contribute to the manuscript modification. Ying Liu, Ting Hong, Mingxuan Lv, Xiaoyu Guo and Panpan Zhang carried out the experiments. All authors reviewed and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Number 82201565 and 82302417), the Natural Science Foundation of Shanghai (Grant Number 24ZR1420800), the Investigator Initiated Research Projects of Huadong Hospital (Grant Number: HDLC2022003), the Qingmiao Talent Program of Huadong Hospital (Grant Number: QMRC2203), the Shanghai Hospital Development Center Foundation (Grant Number: SHDC22022304), the Shanghai Sailing Program (Grant Number: 21YF1411600).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Approval of the study protocol was obtained from the Experimental Animal Ethics Committee of Fudan University (License No. 2022110017S). All animal experimental were conducted in compliance with the guidelines established by the Animal Research: Reporting of In Vivo Experiments (ARRIVE).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Liu and Ting Hong contributed equally to this work.
Contributor Information
Aijuan Yan, Email: yanaijuan1928@163.com.
Wenshi Wei, Email: wenshiwei1994@163.com.
References
- 1.van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21(1):21–35. [DOI] [PubMed] [Google Scholar]
- 2.Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128(4):1208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garwood CJ, Simpson JE, Al Mashhadi S, Axe C, Wilson S, Heath PR, et al. DNA damage response and senescence in endothelial cells of human cerebral cortex and relation to Alzheimer’s neuropathology progression: a population-based study in the Medical Research Council Cognitive Function and Ageing Study (MRC-CFAS) cohort. Neuropathol Appl Neurobiol. 2014;40(7):802–14. [DOI] [PubMed] [Google Scholar]
- 4.Hu Y, Fryatt GL, Ghorbani M, Obst J, Menassa DA, Martin-Estebane M, et al. Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Rep. 2021;35(10): 109228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li R, Li Y, Zuo H, Pei G, Huang S, Hou Y. Alzheimer's Amyloid-beta Accelerates Cell Senescence and Suppresses SIRT1 in Human Neural Stem Cells. Biomolecules. 2024;14(2):189. [DOI] [PMC free article] [PubMed]
- 6.He N, Jin WL, Lok KH, Wang Y, Yin M, Wang ZJ. Amyloid-beta(1–42) oligomer accelerates senescence in adult hippocampal neural stem/progenitor cells via formylpeptide receptor 2. Cell Death Dis. 2013;4(11): e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei Z, Chen XC, Song Y, Pan XD, Dai XM, Zhang J, et al. Amyloid beta Protein Aggravates Neuronal Senescence and Cognitive Deficits in 5XFAD Mouse Model of Alzheimer’s Disease. Chin Med J (Engl). 2016;129(15):1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci. 2019;22(5):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562(7728):578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9(1):5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin O, Agrawal A, Porat Z, Krizhanovsky V, Alon U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat Commun. 2019;10(1):5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rim C, You MJ, Nahm M, Kwon MS. Emerging role of senescent microglia in brain aging-related neurodegenerative diseases. Transl Neurodegener. 2024;13(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017;169(7):1276–90 e17. [DOI] [PubMed]
- 14.Shahidehpour RK, Higdon RE, Crawford NG, Neltner JH, Ighodaro ET, Patel E, et al. Dystrophic microglia are associated with neurodegenerative disease and not healthy aging in the human brain. Neurobiol Aging. 2021;99:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie X, Zhao R, Shen GX. Influence of delphinidin-3-glucoside on oxidized low-density lipoprotein-induced oxidative stress and apoptosis in cultured endothelial cells. J Agric Food Chem. 2012;60(7):1850–6. [DOI] [PubMed] [Google Scholar]
- 16.Haseeb A, Chen D, Haqqi TM. Delphinidin inhibits IL-1beta-induced activation of NF-kappaB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology (Oxford). 2013;52(6):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carey AN, Fisher DR, Rimando AM, Gomes SM, Bielinski DF, Shukitt-Hale B. Stilbenes and anthocyanins reduce stress signaling in BV-2 mouse microglia. J Agric Food Chem. 2013;61(25):5979–86. [DOI] [PubMed] [Google Scholar]
- 18.Song SE, Jo HJ, Kim YW, Cho YJ, Kim JR, Park SY. Delphinidin prevents high glucose-induced cell proliferation and collagen synthesis by inhibition of NOX-1 and mitochondrial superoxide in mesangial cells. J Pharmacol Sci. 2016;130(4):235–43. [DOI] [PubMed] [Google Scholar]
- 19.Kim HS, Sul D, Lim JY, Lee D, Joo SS, Hwang KW, et al. Delphinidin ameliorates beta-amyloid-induced neurotoxicity by inhibiting calcium influx and tau hyperphosphorylation. Biosci Biotechnol Biochem. 2009;73(7):1685–9. [DOI] [PubMed] [Google Scholar]
- 20.Heysieattalab S, Sadeghi L. Effects of Delphinidin on Pathophysiological Signs of Nucleus Basalis of Meynert Lesioned Rats as Animal Model of Alzheimer Disease. Neurochem Res. 2020;45(7):1636–46. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. 2021;3(12):1706–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Y, Wu X, Han L, Bian J, He C, El-Omar E, et al. The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases. Nutrients. 2022;14(14):2836. [DOI] [PMC free article] [PubMed]
- 23.Jung YH, Chae CW, Choi GE, Shin HC, Lim JR, Chang HS, et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J Biomed Sci. 2022;29(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahar ME, Hwang JS, Lai TH, Byun JH, Kim DH, Kim DR. The Survival of Human Intervertebral Disc Nucleus Pulposus Cells under Oxidative Stress Relies on the Autophagy Triggered by Delphinidin. Antioxidants (Basel). 2024;13(7):759. [DOI] [PMC free article] [PubMed]
- 25.Jin X, Chen M, Yi L, Chang H, Zhang T, Wang L, et al. Delphinidin-3-glucoside protects human umbilical vein endothelial cells against oxidized low-density lipoprotein-induced injury by autophagy upregulation via the AMPK/SIRT1 signaling pathway. Mol Nutr Food Res. 2014;58(10):1941–51. [DOI] [PubMed] [Google Scholar]
- 26.Lai D, Huang M, Zhao L, Tian Y, Li Y, Liu D, et al. Delphinidin-induced autophagy protects pancreatic beta cells against apoptosis resulting from high-glucose stress via AMPK signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2019;51(12):1242–9. [DOI] [PubMed] [Google Scholar]
- 27.Park M, Sharma A, Lee HJ. Anti-Adipogenic Effects of Delphinidin-3-O-beta-Glucoside in 3T3-L1 Preadipocytes and Primary White Adipocytes. Molecules. 2019;24(10):1848. [DOI] [PMC free article] [PubMed]
- 28.Cremonini E, Da Silva LME, Lanzi CR, Marino M, Iglesias DE, Oteiza PI. Anthocyanins and their metabolites promote white adipose tissue beiging by regulating mitochondria thermogenesis and dynamics. Biochem Pharmacol. 2024;222: 116069. [DOI] [PubMed] [Google Scholar]
- 29.Jia J, Yin J, Zhang Y, Xu G, Wang M, Jiang H, et al. Thioredoxin-1 Promotes Mitochondrial Biogenesis Through Regulating AMPK/Sirt1/PGC1alpha Pathway in Alzheimer’s Disease. ASN Neuro. 2023;15:17590914231159226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khandelwal M, Manglani K, Upadhyay P, Azad M, Gupta S. AdipoRon induces AMPK activation and ameliorates Alzheimer’s like pathologies and associated cognitive impairment in APP/PS1 mice. Neurobiol Dis. 2022;174: 105876. [DOI] [PubMed] [Google Scholar]
- 31.She L, Xiong L, Li L, Zhang J, Sun J, Wu H, et al. Ginsenoside Rk3 ameliorates Abeta-induced neurotoxicity in APP/PS1 model mice via AMPK signaling pathway. Biomed Pharmacother. 2023;158: 114192. [DOI] [PubMed] [Google Scholar]
- 32.Liang J, Yao F, Fang D, Chen L, Zou Z, Feng L, et al. Hyperoside alleviates photoreceptor degeneration by preventing cell senescence through AMPK-ULK1 signaling. FASEB J. 2023;37(11): e23250. [DOI] [PubMed] [Google Scholar]
- 33.Rakshe PS, Dutta BJ, Chib S, Maurya N, Singh S. Unveiling the interplay of AMPK/SIRT1/PGC-1alpha axis in brain health: Promising targets against aging and NDDs. Ageing Res Rev. 2024;96: 102255. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez DF, Mojica L, Cervantes EL, Gonzalez de Mejia E. Common bean polyphenolic enriched extracts decrease reactive oxygen species induced by heavy metals and polycyclic aromatic hydrocarbons in Hs27 and Hs68 human fibroblasts. Food Chem. 2024;459:140371. [DOI] [PubMed]
- 35.Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187: 111215. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Lu J, Hou Y, Huang S, Pei G. Alzheimer’s Amyloid-beta Accelerates Human Neuronal Cell Senescence Which Could Be Rescued by Sirtuin-1 and Aspirin. Front Cell Neurosci. 2022;16: 906270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Tang Z. Therapeutic potential of natural molecules against Alzheimer’s disease via SIRT1 modulation. Biomed Pharmacother. 2023;161: 114474. [DOI] [PubMed] [Google Scholar]
- 38.Han Y, Liu Y, Zhang Y, Wang W, Lv T, Huang J, et al. The Role and Application of the AMPK-Sirtuins Network in Cellular Senescence. Front Biosci (Landmark Ed). 2023;28(10):250. [DOI] [PubMed] [Google Scholar]
- 39.Parra-Vargas M, Sandoval-Rodriguez A, Rodriguez-Echevarria R, Dominguez-Rosales JA, Santos-Garcia A, Armendariz-Borunda J. Delphinidin Ameliorates Hepatic Triglyceride Accumulation in Human HepG2 Cells, but Not in Diet-Induced Obese Mice. Nutrients. 2018;10(8):1060. [DOI] [PMC free article] [PubMed]
- 40.Chen Y, Ge Z, Huang S, et al. Delphinidin attenuates pathological cardiac hypertrophy via the AMPK/NOX/MAPK signaling pathway. Aging (Albany NY). 2020;12(6):5362–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Z, Li X, Wang Z, Cao Y, Han S, Li N, et al. Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor gamma-dependent mechanism in Alzheimer’s disease. Redox Biol. 2023;66: 102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zumerle S, Sarill M, Saponaro M, Colucci M, Contu L, Lazzarini E, et al. Targeting senescence induced by age or chemotherapy with a polyphenol-rich natural extract improves longevity and healthspan in mice. Nat Aging. 2024;4(9):1231-48. [DOI] [PMC free article] [PubMed]
- 43.Liu Y, Liu X, Chen X, Yang Z, Chen J, Zhu W, et al. Senolytic and senomorphic agent procyanidin C1 alleviates structural and functional decline in the aged retina. Proc Natl Acad Sci U S A. 2024;121(18): e2311028121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saul D, Kosinsky RL, Atkinson EJ, Doolittle ML, Zhang X, LeBrasseur NK, et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat Commun. 2022;13(1):4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu Y, Huang Y, Xing S, Chen C, Shen D, Chen J. Aβ promotes CD38 expression in senescent microglia in Alzheimer’s disease. Biol Res. 2022;55(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baek H, Sanjay, Park M, Lee HJ. Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1DeltaE9 transgenic mice model. J Neuroinflammation. 2023;20(1):268. [DOI] [PMC free article] [PubMed]
- 47.Ozdemir-Kaynak E, Qutub AA, Yesil-Celiktas O. Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front Physiol. 2018;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8(2):111–20. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Zhan C, Li X, Pan T, Yao Y, Tan Y, et al. Five similar anthocyanidin molecules display distinct disruptive effects and mechanisms of action on Abeta(1–42) protofibril: A molecular dynamic simulation study. Int J Biol Macromol. 2024;256(Pt 2): 128467. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Du L, Zhang T, Chu Y, Wang Y, Wang Y, et al. Edaravone Dexborneol ameliorates the cognitive deficits of APP/PS1 mice by inhibiting TLR4/MAPK signaling pathway via upregulating TREM2. Neuropharmacology. 2024;255: 110006. [DOI] [PubMed] [Google Scholar]
- 51.Gaikwad S, Senapati S, Haque MA, Kayed R. Senescence, brain inflammation, and oligomeric tau drive cognitive decline in Alzheimer’s disease: Evidence from clinical and preclinical studies. Alzheimers Dement. 2024;20(1):709–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ngoi NY, Liew AQ, Chong SJF, Davids MS, Clement MV, Pervaiz S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021;45: 102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suelves N, Saleki S, Ibrahim T, Palomares D, Moonen S, Koper MJ, et al. Senescence-related impairment of autophagy induces toxic intraneuronal amyloid-beta accumulation in a mouse model of amyloid pathology. Acta Neuropathol Commun. 2023;11(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X, Yang Y, Tang S, Chen Q, Zhang M, Ma J, et al. Anti-Aging Effects of Anthocyanin Extracts of Sambucus canadensis Caused by Targeting Mitochondrial-Induced Oxidative Stress. Int J Mol Sci. 2023;24(2):1528. [DOI] [PMC free article] [PubMed]
- 55.Daveri E, Cremonini E, Mastaloudis A, Hester SN, Wood SM, Waterhouse AL, et al. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018;18:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muhammad I, Cremonini E, Mathieu P, Adamo AM, Oteiza PI. Dietary Anthocyanins Mitigate High-Fat Diet-Induced Hippocampal Inflammation in Mice. J Nutr. 2024;154(9):2752–62. [DOI] [PubMed] [Google Scholar]
- 57.Sogo T, Terahara N, Hisanaga A, Kumamoto T, Yamashiro T, Wu S, et al. Anti-inflammatory activity and molecular mechanism of delphinidin 3-sambubioside, a Hibiscus anthocyanin. BioFactors. 2015;41(1):58–65. [DOI] [PubMed] [Google Scholar]
- 58.Nam OH, Ro ST, Lee HW, Jeong J, Chae YK, Lee KE, et al. Evaluation of delphinidin as a storage medium for avulsed teeth. BMC Oral Health. 2023;23(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rachmian N, Medina S, Cherqui U, Akiva H, Deitch D, Edilbi D, et al. Identification of senescent, TREM2-expressing microglia in aging and Alzheimer’s disease model mouse brain. Nat Neurosci. 2024;27(6):1116–24. [DOI] [PubMed] [Google Scholar]
- 60.Choi I, Wang M, Yoo S, Xu P, Seegobin SP, Li X, et al. Autophagy enables microglia to engage amyloid plaques and prevents microglial senescence. Nat Cell Biol. 2023;25(7):963–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, et al. NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer's disease via cGAS-STING. Proc Natl Acad Sci USA. 2021;118(37):e2011226118. [DOI] [PMC free article] [PubMed]
- 62.Zhao Z, Yan J, Huang L, Yang X. Phytochemicals targeting Alzheimer’s disease via the AMP-activated protein kinase pathway, effects, and mechanisms of action. Biomed Pharmacother. 2024;173: 116373. [DOI] [PubMed] [Google Scholar]
- 63.Won JS, Im YB, Kim J, Singh AK, Singh I. Involvement of AMP-activated-protein-kinase (AMPK) in neuronal amyloidogenesis. Biochem Biophys Res Commun. 2010;399(4):487–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koppel J, Jimenez H, Adrien L, Greenwald BS, Marambaud P, Cinamon E, et al. Haloperidol inactivates AMPK and reduces tau phosphorylation in a tau mouse model of Alzheimer’s disease. Alzheimers Dement (N Y). 2016;2(2):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N, Bai N, Zhao X, Cheng R, Wu X, Jiang B, et al. Cooperative effects of SIRT1 and SIRT2 on APP acetylation. Aging Cell. 2023;22(10): e13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Shi FX, Li N, Cao Y, Lei Y, Wang JZ, et al. AMPK Ameliorates Tau Acetylation and Memory Impairment Through Sirt1. Mol Neurobiol. 2020;57(12):5011–25. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Wang X, Xiao A, Han J, Wang Z, Wen M. Ketogenic diet prevents chronic sleep deprivation-induced Alzheimer’s disease by inhibiting iron dyshomeostasis and promoting repair via Sirt1/Nrf2 pathway. Front Aging Neurosci. 2022;14: 998292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu QQ, Su ZR, Hu Z, Yang W, Xian YF, Lin ZX. Patchouli alcohol ameliorates the learning and memory impairments in an animal model of Alzheimer’s disease via modulating SIRT1. Phytomedicine. 2022;106: 154441. [DOI] [PubMed] [Google Scholar]
- 69.Xiong W, Li D, Feng Y, Jia C, Zhang X, Liu Z. CircLPAR1 Promotes Neuroinflammation and Oxidative Stress in APP/PS1 Mice by Inhibiting SIRT1/Nrf-2/HO-1 Axis Through Destabilizing GDF-15 mRNA. Mol Neurobiol. 2023;60(4):2236–51. [DOI] [PubMed] [Google Scholar]
- 70.Khamies SM, El-Yamany MF, Ibrahim SM. Canagliflozin Mitigated Cognitive Impairment in Streptozotocin-Induced Sporadic Alzheimer’s Disease in Mice: Role of AMPK/SIRT-1 Signaling Pathway in Modulating Neuroinflammation. J Neuroimmune Pharmacol. 2024;19(1):39. [DOI] [PubMed] [Google Scholar]
- 71.Qu Q, Chen Y, Wang Y, Long S, Wang W, Yang HY, et al. Lithocholic acid phenocopies anti-ageing effects of calorie restriction. Nature. 2025;638(8050):E6. [DOI] [PMC free article] [PubMed]
- 72.de Kreutzenberg SV, Ceolotto G, Cattelan A, Pagnin E, Mazzucato M, Garagnani P, et al. Metformin improves putative longevity effectors in peripheral mononuclear cells from subjects with prediabetes. A randomized controlled trial. Nutr Metab Cardiovasc Dis. 2015;25(7):686–93. [DOI] [PubMed]
- 73.Davari M, Hashemi R, Mirmiran P, Hedayati M, Sahranavard S, Bahreini S, et al. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and Sirtuin-1 (SIRT1) in type 2 diabetes: a randomized, double blind, and controlled clinical trial. Nutr J. 2020;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Martinez BI, Ruiz-Ramos M, Pedraza-Chaverri J, Santiago-Osorio E, Mendoza-Nunez VM. Effect of Resveratrol on Markers of Oxidative Stress and Sirtuin 1 in Elderly Adults with Type 2 Diabetes. Int J Mol Sci. 2023;24(8):7422. [DOI] [PMC free article] [PubMed]
- 75.Nayyar D, Yan X, Xu G, Shi M, Garnham AP, Mathai ML, et al. Gynostemma Pentaphyllum Increases Exercise Performance and Alters Mitochondrial Respiration and AMPK in Healthy Males. Nutrients. 2023;15(22):4721. [DOI] [PMC free article] [PubMed]
- 76.Hengist A, Davies RG, Walhin JP, Buniam J, Merrell LH, Rogers L, et al. Ketogenic diet but not free-sugar restriction alters glucose tolerance, lipid metabolism, peripheral tissue phenotype, and gut microbiome: RCT. Cell Rep Med. 2024;5(8): 101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fan X, Fan Z, Yang Z, Huang T, Tong Y, Yang D, et al. Flavonoids-Natural Gifts to Promote Health and Longevity. Int J Mol Sci. 2022;23(4):2176. [DOI] [PMC free article] [PubMed]
- 78.Ji Z, Liu GH, Qu J. Mitochondrial sirtuins, metabolism, and aging. J Genet Genomics. 2022;49(4):287–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.