Abstract
Introduction
Sotorasib and adagrasib are the only treatments approved in the USA and Europe for advanced/metastatic KRAS G12C-mutated non-small cell lung cancer (NSCLC). In the absence of head-to-head trials, a matching-adjusted indirect comparison (MAIC) was conducted to assess the relative efficacy and safety of sotorasib versus adagrasib using phase 3 trials.
Methods
Patient-level data from CodeBreaK 200 were reweighted to match the baseline characteristics reported in KRYSTAL-12. The analysis evaluated progression free-survival (PFS), objective response rate (ORR), and treatment-related adverse events (TRAE). Age, sex, region, prior treatment, brain metastases, and liver metastases were selected for adjustment in the primary analysis per clinical guidance, using an unanchored approach (no common comparator). We conducted sensitivity analyses including additional covariates or anchoring the analysis via common comparator (docetaxel). Additional subgroup analysis was performed in patients with baseline brain metastases, assessing systemic PFS.
Results
Following adjustment, the reweighted patient characteristics from CodeBreaK 200 and KRYSTAL-12 were well balanced. In the primary analysis, sotorasib and adagrasib showed similar efficacy: PFS (HR [hazard ratio] 0.93; 95% confidence interval [CI] 0.70–1.22; p = 0.589) and ORR (odds ratio 0.86; 95% CI 0.53–1.38; p = 0.524). Among patients with brain metastases, sotorasib demonstrated a 39% reduced risk of progression compared with adagrasib (HR 0.61; 95% CI 0.38–0.98; p = 0.040). Sotorasib also demonstrated a more favorable safety profile than adagrasib, with lower odds of TRAEs, TRAEs leading to dose reduction or dose interruption, and all eight individual TRAEs evaluated. Sensitivity analyses supported the robustness of base-case results.
Conclusion
In this MAIC, sotorasib and adagrasib showed comparable efficacy in previously treated advanced KRAS G12C-mutated NSCLC. Among patients with baseline brain metastases, PFS point estimates favored sotorasib. Sotorasib also demonstrated a favorable overall safety profile. These findings may help inform payer decisions and clinical practice in the treatment of KRAS G12C-mutated NSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-025-03259-8.
Keywords: KRAS G12C mutation, Sotorasib, Adagrasib, Matching-adjusted indirect comparison, Non-small cell lung cancer, Objective response rate, Progression-free survival, Treatment-related adverse event
Plain Language Summary
Two drugs, sotorasib and adagrasib, are approved in the USA and Europe for patients with advanced or metastatic non-small cell lung cancer who have a KRAS G12C gene mutation. However, there are no clinical trials that directly compare these two treatments. To address this, a method called matching-adjusted indirect comparison can help compare results from different clinical trials by reducing differences in patient characteristics between the trials and minimizing bias. This study conducted a matching-adjusted indirect comparison to compare how well sotorasib and adagrasib perform and how safe they are, based on data from pivotal clinical trials. The findings show that both drugs were similarly effective at slowing down cancer progression and shrinking tumors. Additionally, sotorasib had fewer side effects compared with adagrasib. These findings provide important insights to help patients, doctors, and healthcare decision-makers choose the most suitable treatment option for non-small cell lung cancer with a KRAS G12C mutation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-025-03259-8.
Key Summary Points
| Why carry out this study? |
| Sotorasib and adagrasib are the only KRAS G12C inhibitors approved by both the US Food and Drug Administration and the European Medicines Agency for the treatment of adults with KRAS G12C‑mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) who have received at least one prior systemic therapy. |
| No head-to-head clinical trials between sotorasib and adagrasib exist; however, the availability of data from the pivotal phase 3 trials allowed for matching-adjusted indirect comparison to provide relative efficacy and safety comparison of the two treatments. |
| These findings can help patients and healthcare providers make more informed decisions for the treatment of KRAS G12C-mutated advanced NSCLC. |
| What was learned from this study? |
| Sotorasib and adagrasib demonstrated similar efficacy in terms of progression-free survival (PFS) and objective response rate. |
| Sotorasib showed favorable PFS point estimates in a subgroup analysis of patients with brain metastases. |
| Sotorasib also showed a favorable safety profile compared with adagrasib. |
Introduction
Lung cancer is the leading cause of cancer morbidity and mortality worldwide, accounting for almost 2.5 million new cases (12.4%) and 18.7% of total cancer deaths in 2022, with a global incidence rate of 32 per 100,000 men and 16 per 100,000 women [1]. Incidence rates vary widely by country, mainly attributable to differences in smoking patterns [2], and are projected to increase by approximately 86% by 2050 [3].
Non-small cell lung cancer (NSCLC) comprises 80% to 85% of all lung cancers [4]. It is a molecularly heterogeneous disease with poor prognosis [5]. The 5-year relative survival rate for NSCLC for all stages combined is 28% [6]. Kirsten rat sarcoma viral oncogene homolog (KRAS) represents the most prevalent isoform mutation in lung cancers, with more than 30% of patients harboring this mutation [7]. Among these, the KRAS G12C variant is the most dominant, comprising around 40% of all KRAS mutations and present in 10% to 13% of advanced non-squamous NSCLC cases [8].
Sotorasib and adagrasib are the only small-molecule KRAS G12C inhibitors approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of adults with KRAS G12C‑mutated locally advanced or metastatic NSCLC who have received at least one prior systemic therapy [9–12]. Sotorasib, which specifically and irreversibly inhibits the KRAS G12C mutation, demonstrated durable clinical benefit with no new safety signals in CodeBreaK 100 (NCT03600883), a phase 2, single-arm, open-label trial [13]. Adagrasib demonstrated similar results in its respective phase 2, single-arm, open-label trial KRYSTAL-1 (NCT03785249) [14]. A matching-adjusted indirect treatment comparison (MAIC) based on the corresponding phase 2 data was conducted to assess the relative efficacy of sotorasib versus adagrasib [15]. No significant differences were found between the two treatments in terms of progression-free survival (PFS), overall survival (OS), and objective response rate (ORR).
In the phase 3 trials of sotorasib (CodeBreaK 200 [NCT04303780] [16]) and adagrasib (KRYSTAL-12 [NCT04685135] [17]), both drugs demonstrated improved PFS over docetaxel in the population of interest. In the absence of a head-to-head clinical trial between sotorasib and adagrasib, the objective of this study was to conduct an MAIC using the two phase 3 randomized controlled trials (RCT): individual patient data (IPD) for CodeBreaK 200 and aggregate data for KRYSTAL-12 for adagrasib.
MAIC is a pairwise indirect comparison technique designed to enhance the accuracy of trial comparisons and reduce potential biases by accounting for between-trial variations in patient characteristics [18]. This approach involves reweighting patient-level data from one trial to align more closely with the population of a second trial. Endpoint data are then recalculated based on the weighted study population, enabling a more balanced comparison than a simple side-by-side evaluation of trial outcomes [18]. MAIC is a well-established methodology, frequently employed for comparing therapies in NSCLC [19–21].
The current MAIC is the first study to evaluate the relative efficacy and safety of sotorasib versus adagrasib in patients with previously treated advanced NSCLC with KRAS G12C mutation using data from phase 3 trials.
Methods
Data Sources
A systematic literature review (SLR) of RCTs was initially conducted in January 2021 and consequently updated in September 2024 to synthesize evidence for the efficacy and safety of systemic therapies approved in the USA or European Union for second or subsequent line of treatment in adults with advanced or metastatic NSCLC. CodeBreaK 200 and KRYSTAL-12 corresponding to sotorasib and adagrasib, respectively, were identified as eligible phase 3 trials for the current study.
CodeBreaK 200 [16] and KRYSTAL-12 [17] recruited patients from multiple countries in Asia, Australia, Europe, and North America and were similar regarding other inclusion and exclusion criteria. Baseline patient characteristics from the two trials were summarized using percentages for categorical variables and mean or median for continuous variables.
Both studies were open-label, phase 3 RCTs that evaluated the efficacy and safety of sotorasib or adagrasib versus docetaxel among adults with previously treated NSCLC with KRAS G12C mutation. CodeBreaK 200 [16] and KRYSTAL-12 [17] reported PFS and ORR as efficacy outcomes, and reported safety outcomes with a focus on treatment-related adverse events (TRAE). The median follow-up for efficacy outcomes in CodeBreaK 200 [16] was 17.7 months compared with 7.2 months in KRYSTAL-12 [17].
In this MAIC analysis, IPD were available for CodeBreaK 200 (data cutoff date August 2, 2022) [16] and aggregate data were sourced from the KRYSTAL-12 publication (data cutoff date December 31, 2023) [17].
Covariates
Covariates that may have a prognostic or effect-modifying impact for NSCLC were identified based on extensive, documented discussions with six NSCLC physicians in Europe and North America as well as from a literature review of other MAIC studies in the disease. Covariates relevant to safety outcomes were also selected based on discussion with clinical experts.
Age, sex, race (region was used in the current analysis: Asia–Pacific [APAC] versus non-APAC), prior chemotherapy + immunotherapy, brain metastases per independent neuroradiologist review, and liver metastases per blinded independent central review (BICR) were considered key variables for adjustment in the base case.
Eastern Cooperative Oncology Group (ECOG) performance status (PS) was unanimously deemed the most important prognostic factor of patients with advanced NSCLC. However clinical expert opinion and literature [22, 23] suggested that the prognostic impact is most pronounced between ECOG ≥ 2 versus 0 to 1. Additionally, CodeBreaK 200 and KRYSTAL-12 only included patients with an ECOG PS score of 0 or 1, and ECOG PS distribution appeared comparable between the overall populations of the two trials; therefore, this variable was only adjusted for in sensitivity analyses.
Per physician interviews, programmed death ligand 1 (PD-L1) expression was also determined to be a strong predictor for NSCLC, but only if immunotherapy is involved; none of the treatments evaluated (i.e., sotorasib, adagrasib, and docetaxel) are immunotherapies. Therefore, adjustment for PD-L1 expression (i.e., < 1%, 1% to 49%, or ≥ 50%) was restricted to sensitivity analysis. Other variables included in sensitivity analysis, in addition to ECOG PS and PD-L1 expression, were disease stage (metastatic versus locally advanced), bone metastases per BICR, and smoking history (i.e., current, former, or never).
Outcomes
The outcomes of interest used to assess the comparative efficacy of sotorasib versus adagrasib were PFS by BICR and ORR by BICR. OS was not included as an outcome since these data were not reported for KRYSTAL-12 at the time of study conduct (December 2024) [17]. Systemic PFS by BICR was also evaluated for the subgroup of patients with brain metastases [24, 25].
The outcomes used to assess the safety of sotorasib versus adagrasib were any TRAEs, grade ≥ 3 TRAEs, TRAEs leading to discontinuation, TRAEs leading to dose reduction, TRAEs leading to dose interruption, and individual TRAEs of any grade. Eight individual TRAEs were selected based on the top 10 TRAEs reported in both the trials (i.e., diarrhea, nausea, decreased appetite, alanine aminotransferase [ALT] increased, aspartate aminotransferase [AST] increased, fatigue, vomiting, and asthenia). The analysis of grade ≥ 3 individual TRAEs was not conducted because of the low event rates for most of the individual TRAEs in both trials that could lead to unstable estimates.
Statistical Methods
A MAIC was conducted as described by Phillippo et al. [18].
The Kaplan–Meier PFS curves reported in the KRYSTAL-12 trial were digitized via Engauge Digitizer [26] and were used to simulate pseudo patient-level data by applying the Guyot algorithm [27]. Reconstructed Kaplan–Meier curves were compared with published literature to ensure the similarity of the curves and the corresponding hazard ratios (HR).
Weights were estimated by method of moments, as per National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) Technical Support Document (TSD) 18 [28]. The odds were calculated as , where is the vector of baseline variables included for matching and is the intercept. Effective sample size (ESS) was calculated as . A small ESS usually indicates little overlap between baseline characteristics of the two trials and an irregular distribution of weights across patients, which can lead to unstable or invalid estimates.
The time-to-event data from CodeBreaK 200 with MAIC weights and the digitized pseudo IPD from KRYSTAL-12 were used to estimate the relative treatment effect for PFS between sotorasib and adagrasib. For ORR and TRAEs, the outcome of each individual patient from CodeBreaK 200 with MAIC weights and the number of patients with events in KRYSTAL-12 were used.
Weighted Cox regression/weighted logistic regression with a robust variance estimator was used to generate HRs/odds ratios (OR) with 95% confidence intervals (CI) that reflect the expected relative effect between sotorasib and adagrasib on PFS (HR), ORR (OR), and TRAEs (OR). The reference in all analyses was adagrasib. Naïve comparison (unweighted) was also conducted without any reweighting or variable adjustment.
A patient was excluded from analysis if information on one or more matching covariates was missing, or if a patient had missing information on the outcome analyzed. No imputation was performed for missing values.
Base Case, Sensitivity Analyses, and Subgroup Analysis
In both trials, the control arm involved administering docetaxel 75 mg/m2 intravenously every 3 weeks. Both trials also allowed crossover from the control arm to the intervention arm after disease progression. However, there were key differences between the trials that could introduce substantial bias in an anchored MAIC. In CodeBreaK 200, out-of-protocol crossover led to participants receiving sotorasib while still formally in the control arm, potentially affecting PFS assessments. Additionally, informative censoring occurred when patients progressed according to investigator assessment but had not yet met the criteria for progression as confirmed by BICR. Furthermore, the early dropout rate in the control arm of CodeBreaK 200 was 65% higher than the corresponding rate in KRYSTAL-12 [16, 17].
These differences resulted in notable discrepancies in outcomes in the control arm between the two trials. For example, in the docetaxel control arm, median PFS (4.5 months versus 3.8 months) and ORR (13% versus 9%) were higher in CodeBreaK 200 [16] compared with KRYSTAL-12 [17], respectively. Importantly, these differences cannot be explained by population characteristics at baseline, further highlighting the variability between the control arms. Such variations could introduce substantial bias in an anchored MAIC by confounding the comparison of intervention arms through differences in the control arms.
However, these biases are not relevant for the intervention arms in the two trials, which makes an unanchored MAIC a more suitable approach. For this reason, the unanchored MAIC was chosen as the base-case analysis. An anchored MAIC, using the control arm as the anchor, was conducted as a sensitivity analysis to assess the robustness of the findings.
In the base case using an unanchored MAIC, patient-level data from CodeBreaK 200 were reweighted for comparison with aggregate data of KRYSTAL-12, adjusting for age, sex, region, prior chemotherapy + immunotherapy, brain metastases, and liver metastases.
Two sensitivity analyses were conducted. The first was an unanchored comparison which included ECOG PS, disease stage, bone metastases, PD-L1 expression, and smoking history in addition to the variables included in the base case. The second was an anchored MAIC that used the docetaxel arm as the anchor, adjusting for the same variables as in the base case, despite major limitations described above.
Approximately 40% of patients with KRAS G12C-mutated NSCLC develop brain metastases and there is a high unmet need for effective therapies in this population [29]. Systemic efficacy, PFS per BICR in accordance with Response Evaluation Criteria in Solid Tumours v1.1, was reported in both CodeBreaK 200 and KRYSTAL-12 for patients with baseline treated and stable brain metastases identified by independent neuroradiologist review [24, 25]. Therefore, subgroup analysis was conducted for patients with brain metastases in terms of systemic PFS, using both unanchored and anchored MAIC with age, sex, prior chemotherapy + immunotherapy, and liver metastases as matching variables. Sensitivity analysis using additional covariates was not conducted for the subgroup of patients with brain metastases since they were not reported in KRYSTAL-12.
The statistical analysis plan was pre-specified and executed without protocol amendments or deviations. All planned analyses are reported in the manuscript. The analysis was conducted independently in accordance with the pre-defined protocol.
Statistical Software
Data analyses were conducted using R version 4.3.1 within the R Studio environment [30]. The codes for MAIC weighting followed the example provided in Appendix D of NICE DSU TSD 18 [28].
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The CodeBreak 200 trial used in the study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The protocol and amendments were approved by the institutional review board at each participating site and regulatory authorities of participating countries. The protocol for the KRYSTAL-12 trial states that the study will be conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Patients (Council for International Organizations of Medical Sciences 2002), Guidelines for Good Clinical Practice (GCP) (International Council for Harmonisation [ICH] 1996), ICH E6 (R2) and concepts that have their origin in the Declaration of Helsinki (World Medical Association 1996, 2008 & 2013); the study will be conducted under a protocol reviewed and approved by an institutional review board/ethics committee.
Results
Study and Patient Characteristics
Detailed information from CodeBreaK 200 and KRYSTAL-12 can be found in the Supplementary Materials: study characteristics (Supplementary Table 1), patient characteristics (Supplementary Table 2), efficacy outcomes reported (Supplementary Table 3), and safety outcomes reported (Supplementary Table 4).
CodeBreaK 200 and KRYSTAL-12 had similar distributions of age, sex, ECOG PS, smoking status, disease stage, histology, liver and bone metastases. KRYSTAL-12 had a higher proportion of patients from the APAC region (26% versus 13%) and higher proportion of patients with concurrent prior chemotherapy + immunotherapy (73% versus 48%) than CodeBreaK 200. Compared with CodeBreaK 200, more patients in KRYSTAL-12 had PD-L1 expression from 1% to 50% (50% versus 35%), but fewer patients in KRYSTAL-12 had PD-L1 expression ≥ 50% (26% versus 31%).
MAIC Matching
The intention-to-treat (ITT) populations from CodeBreaK 200 and KRYSTAL-12 were used for the efficacy analysis. Out of 171 patients in the sotorasib ITT population in CodeBreaK 200, 12 patients were excluded from the base-case analysis and 20 were excluded in sensitivity analysis 1 (additional covariates) because of the missing information on matching covariates. In sensitivity analysis 2 (anchored MAIC), out of 345 patients in the ITT population (171 sotorasib, 174 docetaxel), 57 were excluded (12 sotorasib, 45 docetaxel) because of missing information on matching covariates.
After reweighting, the patient characteristics from CodeBreaK 200 and KRYSTAL-12 were well balanced across all analyses. The ESS was 70.7% of the original size from CodeBreaK 200 in the base case, 56.3% in sensitivity analysis 1 (additional covariates), 72.9% in sensitivity analysis 2 (anchored MAIC), 79.4% in the unanchored subgroup analysis (brain metastases), and 73.3% in the anchored subgroup analysis (brain metastases). The complete MAIC matching results for PFS and ORR are provided in Table 1.
Table 1.
MAIC matching results for PFS and ORR: base case, sensitivity analysis 1 and 2, and subgroup analysis
| Trial | Intervention | Comparison | ESS | Mean age |
Sex male |
Region APAC |
Chemo + IO conc | Brain mets | Liver mets | ECOG PS 1 |
Stage metastatic | Bone mets | Smokingb | PD-L1b | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Former | Current | ≥ 1% to < 50% | ≥ 50% | |||||||||||||
| Base case | ||||||||||||||||
| CodeBreaK 200 | Sotorasib | Unweighted | 159 (100.0%)a | 63.7 | 64.8% | 14.5% | 45.3% | 25.2% | 15.1% | |||||||
| CodeBreaK 200 | Sotorasib | Weighted | 112.4 (70.7%) | 64.0 | 64.0% | 26.0% | 73.0% | 25.9% | 15.0% | |||||||
| KRYSTAL-12 | Adagrasib | Reference | 301 | 64.0 | 64.0% | 26.0% | 73.0% | 25.9% | 15.0% | |||||||
| Sensitivity analysis 1 (additional covariates) | ||||||||||||||||
| CodeBreaK 200 | Sotorasib | Unweighted | 151 (100.0%)a | 63.4 | 65.6% | 14.6% | 46.4% | 24.5% | 15.9% | 66.2% | 94.7% | 29.8% | 78.8% | 18.5% | 27.8% | 37.7% |
| CodeBreaK 200 | Sotorasib | Weighted | 85.0 (56.3%) | 64.0 | 64.0% | 26.0% | 73.0% | 25.9% | 15.0% | 68.0% | 94.0% | 23.0% | 76.0% | 19.0% | 48.8% | 27.9% |
| KRYSTAL-12 | Adagrasib | Reference | 301 | 64.0 | 64.0% | 26.0% | 73.0% | 25.9% | 15.0% | 68.0% | 94.0% | 23.0% | 76.0% | 19.0% | 48.8% | 27.9% |
| Sensitivity analysis 2 (anchored MAIC) | ||||||||||||||||
| CodeBreaK 200 | Sotorasib | Unweighted | 288 (100.0%)a | 63.5 | 62.8% | 13.9% | 47.9% | 24.0% | 15.3% | |||||||
| CodeBreaK 200 | Docetaxel | 63.6 | 54.6% | 13.2% | 49.4% | 16.7% | 14.9% | |||||||||
| CodeBreaK 200 | Sotorasib | Weighted | 210.0 (72.9%) | 64.3 | 66.7% | 26.0% | 73.0% | 25.2% | 14.0% | |||||||
| CodeBreaK 200 | Docetaxel | 65.0 | 72.0% | 26.0% | 73.0% | 24.0% | 14.0% | |||||||||
| KRYSTAL-12 | Adagrasib | Reference | 453 | 64.3 | 66.7% | 26.0% | 73.0% | 25.2% | 14.0% | |||||||
| KRYSTAL-12 | Docetaxel | 65.0 | 72.0% | 26.0% | 73.0% | 24.0% | 14.0% | |||||||||
| Subgroup analysis (brain metastases, unanchored) | ||||||||||||||||
| CodeBreaK 200 | Sotorasib | Unweighted | 40 (100.0%) | 61.5 | 62.5% | 55.0% | 10.0% | |||||||||
| CodeBreaK 200 | Sotorasib | Weighted | 31.8 (79.4%) | 64.0 | 60.0% | 67.0% | 19.0% | |||||||||
| KRYSTAL-12 | Adagrasib | Reference | 78 | 64.0 | 60.0% | 67.0% | 19.0% | |||||||||
| Subgroup analysis (brain metastases, anchored) | ||||||||||||||||
| CodeBreaK 200 | Sotorasib | Unweighted | 69 (100.0%) | 60.4 | 62.3% | 53.6% | 11.6% | |||||||||
| CodeBreaK 200 | Sotorasib | Weighted | 50.6 (73.3%) | 63.5 | 61.3% | 72.1% | 19.0% | |||||||||
| KRYSTAL-12 | Adagrasib | Reference | 114 | 63.5 | 61.3% | 72.1% | 19.0% | |||||||||
APAC Asia–Pacific, chemo chemotherapy, conc concurrent, ECOG Eastern Cooperative Oncology Group, ESS effective sample size, IO immunotherapy, ITT intention to treat, MAIC matching-adjusted indirect comparison, mets metastases, ORR objective response rate, PD-L1 programmed death ligand 1, PFS progression-free survival, PS performance status
aFor variables with two categories, one of the two categories was matched on; for variables with three categories, two of the three categories were matched on
bIn CodeBreaK 200, patients were excluded from analysis if information on one or more matching covariates required in the model was missing. In the base case, out of 171 patients in the sotorasib ITT population, 12 were excluded: four did not have both prior chemo and IO, and eight did not have liver metastases information. In sensitivity analysis 1, out of 171 patients in sotorasib ITT population, 20 were excluded: four did not have both prior chemo and IO, eight were without liver and bone metastases information, and eight did not have PD-L1 information. In sensitivity analysis 2, out of 345 patients in the ITT population (171 sotorasib, 174 docetaxel), 57 were excluded (12 sotorasib, 45 docetaxel): eight did not have both prior chemo and IO, and 49 were without liver metastases information
After matching on key baseline characteristics, notable differences in docetaxel outcomes remained between trials (median PFS, months—CodeBreaK 200 unadjusted: 4.47 [95% CI 3.02–5.84]; CodeBreaK reweighted: 5.42 [95% CI 4.20–6.99]; KRYSTAL-12: 3.80 [95% CI 2.70–4.70]; median PFS, months [central nervous system (CNS) subgroup]—CodeBreaK unadjusted: 4.50 [95% CI 2.07–7.0]; CodeBreaK reweighted: 3.97 [95% CI 2.07–6.73]; KRYSTAL-12: 2.90 [95% CI 2.0–6.2]).
For the evaluation of safety outcomes, safety populations were used instead of ITT populations. MAIC reweighting results were similar to those from the efficacy analysis, with ESS of 70.7%, 56.3%, and 73.2% of the original size for base case, sensitivity analysis 1 (additional covariates), and sensitivity analysis 2 (anchored MAIC), respectively.
Efficacy Analyses
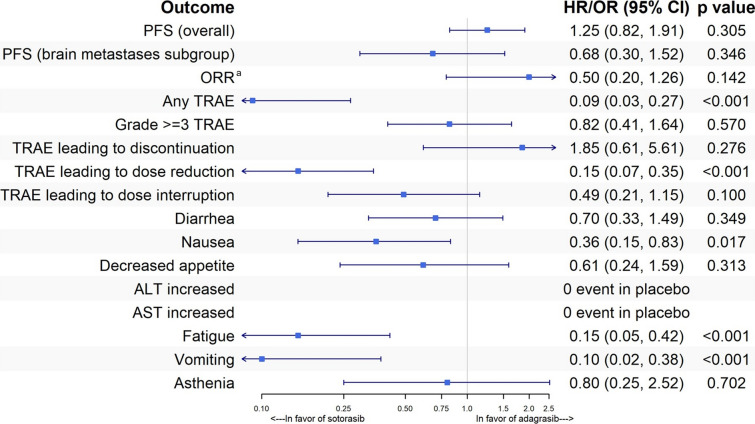
In the base-case analysis, sotorasib and adagrasib showed comparable efficacy for PFS (HR 0.93, 95% CI 0.70–1.22; p = 0.589) and ORR (OR 0.86, 95% CI 0.53–1.38; p = 0.524). The base-case MAIC results across all outcomes are provided in Fig. 1.
Fig. 1.
MAIC results—base case. ALT alanine aminotransferase, AST aspartate aminotransferase, HR hazard ratio, MAIC matching-adjusted indirect comparison, OR odds ratio, ORR objective response rate, PFS progression-free survival, TRAE treatment-related adverse event. aThe odds ratio for ORR was inverted to align with the directional convention used in the forest plots. The base-case model adjusted for age, sex, region, prior treatment (chemotherapy + immunotherapy), brain metastases, and liver metastases
The results of sensitivity analysis 1 (additional covariates; Fig. 2) and sensitivity analysis 2 (anchored MAIC; Fig. 3) were consistent with those of the base case, with no statistically significant difference in efficacy between the two treatments. Kaplan–Meier curves for the base case and sensitivity analysis 1 are shown in Figs. 4 and 5, respectively.
Fig. 2.
MAIC results—sensitivity analysis 1 (additional covariates). ALT alanine aminotransferase, AST aspartate aminotransferase, HR hazard ratio, MAIC matching-adjusted indirect comparison, OR odds ratio, ORR objective response rate, PFS progression-free survival, TRAE treatment-related adverse event. aThe odds ratio for ORR was inverted to align with the directional convention used in the forest plots. The sensitivity analysis 1 model adjusted for age, sex, region, prior treatment (chemotherapy + immunotherapy), brain metastases, liver metastases, Eastern Cooperative Oncology Group performance status, programmed death ligand 1 expression, disease stage (metastatic versus locally advanced), bone metastases, and smoking history
Fig. 3.
MAIC results—sensitivity analysis 2 (anchored MAIC). ALT alanine aminotransferase, AST aspartate aminotransferase, HR hazard ratio, MAIC matching-adjusted indirect comparison, OR odds ratio, ORR objective response rate, PFS progression-free survival, TRAE treatment-related adverse event. aThe odds ratio for ORR was inverted to align with the directional convention used in the forest plots. The sensitivity analysis 2 model adjusted for age, sex, region, prior treatment (chemotherapy + immunotherapy), brain metastases, and liver metastases
Fig. 4.
PFS—base case—Kaplan–Meier curves. PFS progression-free survival. The base-case model adjusted for age, sex, region, prior treatment (chemotherapy + immunotherapy), brain metastases, and liver metastases
Fig. 5.
PFS—sensitivity analysis 1 (additional covariates)—Kaplan–Meier curves. PFS progression-free survival. The sensitivity analysis 1 model adjusted for age, sex, region, prior treatment (chemotherapy + immunotherapy), brain metastases, liver metastases, Eastern Cooperative Oncology Group performance status, programmed death ligand 1 expression, disease stage (metastatic versus locally advanced), bone metastases, and smoking history
More detailed results, including naïve comparison, are provided in Supplementary Table 5 for PFS and Supplementary Table 6 for ORR.
Subgroup Efficacy Analyses (Brain Metastases)
In the base-case MAIC analysis for patients with baseline brain metastases, sotorasib was associated with a 39% lower risk of progression than adagrasib, with an HR of 0.61 (95% CI 0.38–0.98; p = 0.040) (Fig. 1). Kaplan–Meier curves for the unanchored subgroup analysis depict early and sustained separation of curves between sotorasib and adagrasib (Fig. 6).
Fig. 6.
PFS—subgroup analysis (brain metastases)—Kaplan–Meier curves. PFS progression-free survival. The subgroup analysis adjusted for age, sex, prior treatment (chemotherapy + immunotherapy), and liver metastases
Using anchored MAIC, the estimated HR for sotorasib versus adagrasib for patients with baseline brain metastases was 0.68 (95% CI 0.30–1.52; p = 0.346) (Fig. 3).
Safety Analyses
The safety analyses favored sotorasib over adagrasib. In the base-case MAIC analysis (Fig. 1), the odds of experiencing any TRAE were significantly lower for patients treated with sotorasib compared with adagrasib (OR 0.20, 95% CI 0.10–0.37; p < 0.001). Similarly, sotorasib was associated with a markedly reduced likelihood of TRAEs leading to dose reduction (OR 0.25, 95% CI 0.15–0.44; p < 0.001) and dose interruption (OR 0.44, 95% CI 0.28–0.70; p < 0.001).
Notably, sotorasib demonstrated consistent safety benefits across all eight individual TRAEs (any grade) evaluated, including diarrhea, nausea, decreased appetite, elevated AST, elevated ALT, fatigue, vomiting, and asthenia. In each case, sotorasib was associated with lower odds of occurrence (ORs < 1, p values < 0.05).
The robustness of these findings was further confirmed in sensitivity analyses (Figs. 2 and 3), which supported the reliability of the base-case results. A detailed summary of all safety analysis outcomes is provided in Supplementary Table 7.
Discussion
In the absence of head-to-head clinical trials, this study represents the first MAIC analysis using phase 3 trials to compare sotorasib and adagrasib, the only KRAS G12C inhibitors approved by the US FDA and EMA for the treatment of adults with locally advanced or metastatic NSCLC harboring a KRAS G12C mutation who have received at least one prior systemic therapy. While previous studies have reported naïve comparisons of sotorasib and adagrasib [31, 32], the current analysis is distinguished by its adjustment for differences in key baseline variables between the trial populations, ensuring a more robust and meaningful comparison. Both CodeBreaK 200 and KRYSTAL-12 exhibited substantial biases in the control (docetaxel) arm, stemming from early dropout, crossover to the intervention arm, and protocol deviations. These biases resulted in discrepancies in outcomes between the control arms of the two trials. Despite matching on key baseline characteristics, notable differences in docetaxel outcomes remained between trials as suggested in the results section which could not be explained by baseline characteristics. Given these limitations, an anchored MAIC using the control arm as the anchor would have been prone to confounding, making an unanchored MAIC the most appropriate choice for this analysis.
The unanchored MAIC approach, while more susceptible to residual confounding if effect modifiers or prognostic factors are not fully adjusted for, allowed for a more reliable comparison by focusing on the intervention arms. This method has been widely validated for pairwise indirect treatment comparisons and has been successfully applied in numerous studies in NSCLC [20, 21, 33].
After matching on key covariates in CodeBreaK 200 to those of KRYSTAL-12, sotorasib and adagrasib demonstrated similar efficacy for PFS and ORR, with no statistically significant findings in the base case or sensitivity analyses. In the subgroup analysis for patients with brain metastases, sotorasib was associated with a favorable PFS point estimate compared with adagrasib.
The base-case results aligned with findings from a previous MAIC study which assessed the relative efficacy of sotorasib versus adagrasib based on phase 2 trial data [15]. Two other studies (an MAIC and a multilevel meta-regression) [34, 35] reported favorable results for adagrasib over sotorasib in terms of ORR. However, both studies compared phase 2 data (from KRYSTAL-1 [14]) for adagrasib with phase 3 results from CodeBreaK 200. In addition, adjustments for key covariates such as metastases of the brain, liver, or bone were not performed in either study.
Regarding safety outcomes, sotorasib exhibited a favorable safety profile compared with adagrasib. Sotorasib was found to be associated with lower odds of events compared with adagrasib for 11 of the 13 safety outcomes analyzed in the base case and sensitivity analysis 1 (additional covariates). This aligns with a previous MAIC based on KRYSTAL-1 and CodeBreaK 100/200 that demonstrated higher risk of grade ≥ 3 TRAEs for adagrasib [34], as well as a recent real-world study using the FDA Adverse Event Reporting System which showed that adagrasib was associated with higher risk of serious adverse events compared with sotorasib [36].
In the primary MAIC, the ESS was more than 70% of the original sotorasib cohort, reflecting adequate population overlap. Although ESS was lower in sensitivity analyses since it involved additional covariates, the significance of results remained unaltered, supporting the validity and robustness of the findings.
Limitations
The results of this MAIC should be interpreted in the context of the following limitations.
In the base case, unanchored MAIC was deemed the most suitable approach due to different magnitude of bias introduced by early dropout, crossover, and protocol deviations in the control arms in CodeBreaK 200 and KRYSTAL-12, and a large number of patients with missing information on adjusted variables in the docetaxel arm of CodeBreaK 200. Unanchored comparisons are more susceptible to bias and systematic error from improper model specification when there is residual confounding, as it assumes that all effect modifiers and prognostic factors are accounted for, an assumption that is strong and unlikely to be met in practice. Additionally, the MAIC approach can only account for differences in patient-level characteristics that affect outcomes, while other differences at the study level remain unaccounted for. However, given the similar study design, minimal differences in patient populations, and the majority of the key confounding factors being adjusted in either the base case or sensitivity analysis 1 (additional covariates), the residual bias was expected to be low. Anchored MAIC was also conducted despite major limitations with the control arm as anchor, and the findings were consistent with the base case.
Certain important variables, such as presence of other gene alterations and number of prior lines of therapy, were not available in KRYSTAL-12 and therefore could not be adjusted for. However, adjusting for prior chemotherapy + immunotherapy and PD-L1 expression is expected to potentially account for the confounding which might be attributed to number of prior lines of therapy as an unmeasured confounder.
Both CodeBreaK 200 and KRYSTAL-12 were methodologically aligned across evaluation of efficacy, safety, and CNS imaging. Minor procedural differences such as slightly more frequent post-week 49 imaging (CodeBreaK 200: 8 to 10 weeks; KRYSTAL-12: 12 weeks) and a slightly longer adverse event assessment window (CodeBreaK 200: 30 days; KRYSTAL-12: 28 days) post-treatment are unlikely to substantially affect outcomes. The minimal bias, if any, would be against sotorasib. Thus, the observed treatment effects in our study are unlikely to be in favor of sotorasib due to the methodological differences noted above.
For the subgroup of patients with brain metastases, the sample size was relatively small, which limited the power of the analysis and should be interpreted with caution. Furthermore, there were limitations with the covariate availability and the definition of brain metastases, making a comparison challenging. Both naïve and adjusted analyses were conducted in the context of these limitations, given the importance of identifying efficacy differences in this important subgroup according to clinical expert opinion.
The trials had different follow-up periods: KRYSTAL-12 had a median follow-up of 7.2 months compared with 17.7 months of CodeBreaK 200. In terms of safety outcomes, this translated to a bias in favor of adagrasib. Additionally, OS data were not available for KRYSTAL-12 at the time of the analysis.
The digitization of Kaplan–Meier curves using statistical methods can “recreate” numerical values and provide a reasonable estimate of time-to-event data in KRYSTAL-12, but it may not perfectly replicate true patient-level data. However, because the Kaplan–Meier curves were closely replicated, the bias is expected to be minimal.
MAICs are not randomized comparisons and cannot be interpreted as such. The generalizability of this analysis was limited to the patients included in the CodeBreaK 200 and KRYSTAL-12 trials and may not reflect real-world populations.
This MAIC does not report results separately for subgroups stratified by sex because of data availability. Specifically, for KRYSTAL-12, to date no sex-specific results have been reported. Even if such data were available, conducting a subgroup MAIC would additionally require covariate distributions stratified by sex. This represents a limitation in terms of the generalizability of the findings across subgroups stratified by sex. However, evidence from CodeBreaK 200 suggests consistency across sex subgroups [16]. In that study, the HR for PFS was 0.56 (0.39, 0.80) for men and 0.69 (0.45, 1.08) for women. Given the absence of a clear sex-based difference in CodeBreaK 200, it is likely that the findings from this MAIC are generalizable to both men and women.
Conclusions
In this first MAIC analysis of sotorasib and adagrasib based on phase 3 data, efficacy estimates for the two treatments were similar in patients with previously treated advanced NSCLC with KRAS G12C mutation. In a subgroup of patients with brain metastases, PFS point estimates favored sotorasib. Sotorasib also demonstrated a favorable safety profile compared with adagrasib.
These findings can support decision-making among healthcare providers and payers, particularly given the lack of direct comparative effectiveness data for novel treatments such as sotorasib and adagrasib. Additional research is needed to evaluate the generalizability of this analysis to real-world practice and population.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Thanking Patients
We thank the individuals who participated in the CodeBreaK 200 and KRYSTAL-12 trials.
Medical Writing/Editorial Assistance
Writing and editorial assistance in the preparation of this article was provided by Colleen Dumont, BS, of Cytel, Inc. Support for this assistance was funded by Amgen, Inc.
Author Contributions
Divyan Chopra, Zhiyi Lan, David M. Waterhouse, Jürgen Wolf, Enriqueta Felip, Hoora Moradian, Nadia Karim, Cynthia Obiozor and Björn Stollenwerk contributed to the study conception and design. Material preparation and data collection were performed by Divyan Chopra. Material preparation and data analysis were performed by Zhiyi Lan. The first draft of the manuscript was written by Zhiyi Lan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Amgen, Inc. The journal’s Rapid Service Fee was funded by Amgen Inc.
Data Availability
All aggregate data generated or analyzed during this study are included in this published article/as supplementary information files.
Declarations
Conflicts of Interest
Divyan Chopra, Nadia Karim, Cynthia Obiozor, and Björn Stollenwerk are employees of and stockholders in Amgen. Hoora Moradian is an employee of Cytel that received consultancy fees from Amgen. Zhiyi Lan was employed by Cytel, Inc., at the time of study conduct. Jürgen Wolf received advisory board and lecture fees from AbbVie, Amgen, AstraZeneca, BeiGene, Blueprint, Bristol Myers Squibb (BMS), Boehringer Ingelheim, Chugai, Daiichi Sankyo, Ellipses Pharma, Genmab, Janssen, Lilly, Loxo, Merck, Murati, Merck Sharp & Dohme (MSD), Novartis, Novalent, Pfizer, Pierre-Fabre, Regeneron, Roche, Seattle Genetics, Takeda, and Turning Point as well as research support from AstraZeneca, BMS, Janssen Pharmaceutica, Novartis, and Pfizer. David M. Waterhouse has served in a consulting or advisory role for BMS, AZTherapies, AbbVie, Amgen, McGivenny Global, Janssen Oncology, Seattle Genetics, Exelixis, Eisai, EMD Serono, Merck, Pfizer, Mirati Therapeutics, Regeneron/Sanofi, Fresenius KBI, Lilly, Sanofi, Astellas Pharma, Gilead Sciences, Takeda, Daiichi Sankyo, Novartis, Bayer, AVEO; has participated in speakers’ bureaus for BMS, Janssen Oncology, Merck, AstraZeneca, Amgen, and EMD Serono; has received travel, accommodations, expenses from BMS. Enriqueta Felip has received consulting fees from AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, F. Hoffmann-La Roche, Gilead, GlaxoSmithKline, Iteos Therapeutics, Janssen, Johnson & Johnson, MSD, Novartis, Pierre-Fabre, Pfizer, Regeneron, and Turning Point; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, AstraZeneca, BMS, Daiichi Sankyo, Eli Lilly, F. Hoffmann-La Roche, Genentech, Gilead, Janssen, Johnson & Johnson, Medical Trends, Medscape, Merck Serono, MSD, Novartis, Peervoice, Pfizer, and Regeneron; has received support for attending meetings and/or travel from AstraZeneca, Janssen, and Roche; is an independent member of the board of Grifols.
Disclosure
Zhiyi Lan was employed by Cytel, Inc. at the time of study conduct. Zhiyi’s current affiliation is IQVIA Inc., CA, USA.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
The CodeBreak 200 trial used in the study was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and the principles of the Declaration of Helsinki. The protocol and amendments were approved by the institutional review board at each participating site and regulatory authorities of participating countries. The protocol for the KRYSTAL-12 trial states that the study will be conducted in accordance with International Ethical Guidelines for Biomedical Research Involving Human Patients (Council for International Organizations of Medical Sciences 2002), Guidelines for Good Clinical Practice (GCP) (International Council for Harmonisation [ICH] 1996), ICH E6 (R2) and concepts that have their origin in the Declaration of Helsinki (World Medical Association 1996, 2008 & 2013), the study will be conducted under a protocol reviewed and approved by an institutional review board/ethics committee.
Footnotes
Prior Presentation: Some of the findings from this study were presented at European Lung Cancer Conference (ELCC) in Paris, France, 26–29 March 2025.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 2.Wéber A, Morgan E, Vignat J, et al. Lung cancer mortality in the wake of the changing smoking epidemic: a descriptive study of the global burden in 2020 and 2040. BMJ Open. 2023;13(5):e065303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou J, Xu Y, Liu J, Feng L, Yu J, Chen D. Global burden of lung cancer in 2022 and projections to 2050: incidence and mortality estimates from GLOBOCAN. Cancer Epidemiol. 2024;93:102693. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. What is lung cancer? 2019. https://www.cancer.org/cancer/lung-cancer/about/what-is.html. Accessed Feb 11, 2021.
- 5.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Lung cancer survival rates. https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed Jan 10, 2025.
- 7.Prior IA, Hood FE, Hartley JL. The frequency of Ras mutations in cancer. Cancer Res. 2020;80(14):2969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim TKH, Skoulidis F, Kerr KM, et al. KRAS G12C in advanced NSCLC: prevalence, co-mutations, and testing. Lung Cancer. 2023;184:107293. [DOI] [PubMed] [Google Scholar]
- 9.United States Food and Drug Administration. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc. Accessed Jan 9, 2025.
- 10.United States Food and Drug Administration. FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-adagrasib-kras-g12c-mutated-nsclc. Accessed Jan 9, 2025.
- 11.European Medicines Agency. Lumykras (sotorasib). 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/lumykras. Accessed Jan 10, 2025.
- 12.European Medicines Agency. Krazati (adagrasib). 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/krazati. Accessed Jan 10, 2025.
- 13.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C) mutation. N Engl J Med. 2022;387(2):120–31. [DOI] [PubMed] [Google Scholar]
- 15.Chopra D, Waterhouse DM, Sylvester B, Jamotte A, Stollenwerk B. EP.12C.01 sotorasib vs adagrasib: matching-adjusted indirect comparison in prior treated KRAS G12C advanced non-small cell lung cancer. J Thorac Oncol. 2024;19(10):S647–8. [Google Scholar]
- 16.de Langen AJ, Johnson ML, Mazieres J, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet. 2023;401(10378):733–46. [DOI] [PubMed] [Google Scholar]
- 17.Mok TSK, Yao W, Duruisseaux M, et al. KRYSTAL-12: Phase 3 study of adagrasib versus docetaxel in patients with previously treated advanced/metastatic non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. J Clin Oncol. 2024;42(17):8509. [Google Scholar]
- 18.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018;38(2):200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halmos B, Burke T, Kalyvas C, et al. A matching-adjusted indirect comparison of pembrolizumab + chemotherapy vs nivolumab + ipilimumab as first-line therapies in patients with PD-L1 TPS ≥1% metastatic NSCLC. Cancers. 2020;12(12):3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reckamp K, Lin HM, Huang J, et al. Comparative efficacy of brigatinib versus ceritinib and alectinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small cell lung cancer. Curr Med Res Opin. 2019;35(4):569–76. [DOI] [PubMed] [Google Scholar]
- 21.Chu P, Antoniou M, Bhutani MK, Aziez A, Daigl M. Matching-adjusted indirect comparison: entrectinib versus crizotinib in ROS1 fusion-positive non-small cell lung cancer. J Comp Eff Res. 2020;9(12): 861–76. [DOI] [PubMed] [Google Scholar]
- 22.Dall'Olio FG, Maggio I, Massucci M, Mollica V, Fragomeno B, Ardizzoni A. ECOG performance status ≥2 as a prognostic factor in patients with advanced non small cell lung cancer treated with immune checkpoint inhibitors—a systematic review and meta-analysis of real world data. Lung Cancer. 2020;145:95–104. [DOI] [PubMed] [Google Scholar]
- 23.Yang F, Markovic SN, Molina JR et al. Association of sex, age, and Eastern Cooperative Oncology Group performance status with survival benefit of cancer immunotherapy in randomized clinical trials: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(8):e2012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dingemans AMC, Syrigos K, Livi L et al. Intracranial efficacy of sotorasib versus docetaxel in pretreated KRAS G12C-mutated advanced non-small cell lung cancer (NSCLC): Practice-informing data from a global, phase 3, randomized, controlled trial (RCT). J Clin Oncol. 2023;41(17):9016. [Google Scholar]
- 25.Barlesi F, Yao W, Duruisseaux M et al. LBA57 Adagrasib (ADA) vs docetaxel (DOCE) in patients (pts) with KRASG12C-mutated advanced NSCLC and baseline brain metastases (BM): results from KRYSTAL-12. Ann Oncol. 2024;35:S1247–8. [Google Scholar]
- 26.Mitchell M, Muftakhidinov B, Winchen T. Engauge Digitizer Software. 2023. http://markummitchell.github.io/engauge-digitizer. Accessed 9 Jan 2025.
- 27.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. 2016.
- 29.Lamberti G, Aizer A, Ricciuti B et al. Incidence of brain metastases and preliminary evidence of intracranial activity with sotorasib in patients with KRAS(G12C)-mutant non-small-cell lung cancer. JCO Precis Oncol. 2023;7:e2200621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Development Core Team. R: a language and environment for statistical computing. Vienna. Austria. Austria: R Foundation for Statistical Computing. 2010.
- 31.Oya Y, Mitsudomi T. Is adagrasib just another sotorasib?—or, should we differentiate their usage according to patients’ clinical presentation? Transl Lung Cancer Res. 2023;12(5):940–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mausey N, Halford Z. Targeted therapies for previously “undruggable” KRAS-mutated non-small cell lung cancer: a review of sotorasib and adagrasib. Ann Pharmacother. 2024;58(6):622–35. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Albuquerque de Almeida A, Ines M, Iadeluca L, Cooper M. Matching-adjusted indirect comparisons of lorlatinib versus chemotherapy for patients with second-line or later anaplastic lymphoma kinase-positive non-small cell lung cancer. Value Health. 2023;26(1):64–70. [DOI] [PubMed] [Google Scholar]
- 34.Bouwmeester W, Laurie M, Korytowsky B, et al. CO95 matching-adjusted indirect comparison (MAIC) in previously treated KRAS G12C-mutated advanced/metastatic non-small cell lung cancer (a/mNSCLC): adagrasib versus sotorasib. Value Health. 2023;26(12):S31. [Google Scholar]
- 35.Maciel D, Bouwmeester W, Korytowsky B, et al. CO82 Bayesian pan-tumor multilevel meta-regression: an unanchored indirect treatment comparison (ITC) of adagrasib versus sotorasib in previously treated KRASG12C-mutated advanced/metastatic solid tumors. Value Health. 2024;27(6):S32. [Google Scholar]
- 36.Chen M, Huang Y, Jiang S, Ke C. Safety assessment of KRAS (G12C) inhibitors based on the FDA Adverse event reporting system (FAERS) database: a real-world pharmacovigilance study. Lung Cancer. 2024;196:107966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All aggregate data generated or analyzed during this study are included in this published article/as supplementary information files.