Abstract
Background
Fibrinogen-to-albumin ratio (FAR) has been widely examined for its prognostic value in esophageal cancer (EC), although findings across studies have been inconsistent. This meta-analysis aimed to assess the predictive role of FAR in EC.
Methods
A comprehensive search was conducted across Web of Science, Embase, PubMed, and Cochrane Library. The prognostic value of FAR in EC was assessed by pooling hazard ratios (HRs) and 95% confidence intervals (CIs). Additionally, the correlation between FAR and clinicopathological features of EC was evaluated using pooled odds ratios (ORs) and 95%CIs.
Results
A total of six studies involving 2,616 patients were included. The analysis revealed that a high FAR was significantly associated with poor overall survival (OS) in EC (HR = 1.98, 95%CI = 1.48–2.65, p < 0.001). Furthermore, elevated FAR correlated significantly with male sex (OR = 1.38, 95%CI = 1.09–1.74, p = 0.007), T3–T4 stages (OR = 2.36, 95%CI = 1.93–2.87, p < 0.001), N1–N3 stages (OR = 1.58, 95%CI = 1.32–1.91, p < 0.001), TNM III–IV stages (OR = 2.68, 95%CI = 1.52–4.73, p = 0.001), and tumor length > 3 cm (OR = 2.36, 95%CI = 1.15–4.87, p = 0.020). However, FAR showed no significant association with age (OR = 0.87, 95%CI = 0.48–1.60, p = 0.660), tumor location (OR = 0.98, 95%CI = 0.77–1.25, p = 0.886), or tumor differentiation (OR = 1.09, 95%CI = 0.76–1.56, p = 0.634).
Conclusion
This meta-analysis highlights that an elevated FAR is a strong prognostic indicator of poor OS in patients with EC. Moreover, high FAR is significantly associated with clinical features indicative of tumor progression and metastasis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12957-025-03886-z.
Keywords: Fibrinogen-to-albumin ratio, Esophageal cancer, Evidence-based medicine, Meta-analysis, Biomarker
Introduction
Esophageal cancer (EC), a malignant tumor, represents a serious threat to human health, ranking as the 11th most frequently diagnosed cancer and the 7th leading cause of cancer-related deaths globally [1, 2]. According to the latest GLOBOCAN data, 510,716 new cases of EC and 445,129 deaths due to this disease were reported in 2022 worldwide [1]. EC is primarily classified into esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC) histopathological subtypes, with ESCC accounting for approximately 90% of cases annually [3]. Currently, surgical resection remains the primary treatment modality for EC, often combined with immunotherapy, targeted therapy, or adjuvant chemoradiotherapy [4, 5]. Despite advancements in treatment strategies in recent years, the prognosis for EC remains poor, with the 5-year survival rate persistently below 30% [6]. Prognostic markers play a crucial role in guiding clinical decision-making and improving survival outcomes [7]. Therefore, identifying novel and reliable prognostic biomarkers for EC is critical.
Serum markers and their impact on cancer survival have recently attracted considerable interest in cancer epidemiology, likely due to their affordability and accessibility. Several hematological markers, including lymphocyte-to-monocyte ratio [8], systemic immune-inflammation index [9], prognostic nutritional index [10], C-reactive protein to albumin ratio [11], and the controlling nutritional status score [12], have been previously suggested to possess significant predictive value across various cancers. The fibrinogen-to-albumin ratio (FAR), which combines fibrinogen and albumin levels, reflects the immune and nutritional status of individuals. Numerous studies have highlighted the prognostic relevance of FAR in various malignancies, including hepatocellular carcinoma [13], diffuse large B-cell lymphoma [14], non-small cell lung cancer [15], laryngeal cancer [16], and colorectal cancer [17]. FAR has also been explored for its predictive value in EC; however, findings across studies remain inconsistent [18–23]. Therefore, we conducted a comprehensive literature search and performed this meta-analysis to investigate the precise prognostic value of FAR in EC. Furthermore, we examined the correlations between FAR and various clinicopathological characteristics of EC.
Materials and methods
Study guideline
The meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplemental file 1) [24]. This meta-analysis has been registered on INPLASY under the registration number INPLASY202560005. The link to this protocol is https://inplasy.com/inplasy-2025-6-0005/.
Search strategy
A comprehensive literature search was conducted across Web of Science, Embase, PubMed, and the Cochrane Library up to March 4, 2025. The search strategy used was: (albumin-to-fibrinogen OR fibrinogen/albumin OR fibrinogen-to-albumin) AND (Esophageal Cancers OR Esophageal Neoplasms OR Esophagus Cancers OR Esophageal Cancer OR Cancer of Esophagus OR Esophagus Cancer). Only articles published in English were included. The detailed search strategies for each database were provided in Supplemental file 2. Furthermore, the reference lists of the selected studies were carefully reviewed to identify additional relevant publications.
Eligibility criteria
Studies were included based on the following criteria: (1) EC diagnosis confirmed by pathological examination; (2) investigation of the relationship between FAR and EC survival outcomes; (3) reporting of hazard ratios (HRs) and 95% confidence intervals (CIs); (4) availability of a FAR threshold; and (5) publications in English. Studies were excluded if they met any of the following criteria: (1) case reports, reviews, comments, meeting abstracts, or letters; (2) studies with duplicate data; and (3) animal studies.
Data extraction and quality evaluation
Data extraction from eligible studies was independently performed by two reviewers (Y.L. and X.L.), with any disagreements resolved through discussion with a third reviewer (X.G.). The following information was collected: first author, publication year, country, sample size, age, sex, study design, study period, study center, cancer type, TNM stage, treatment details, FAR threshold, threshold determination method, survival endpoints, follow-up duration, survival analysis types, HRs, and corresponding 95%CIs. The primary survival endpoint was overall survival (OS). Study quality was evaluated using the Newcastle-Ottawa Scale (NOS) [25], which examines three key domains: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). NOS scores range between 0 and 9, with studies scoring ≥ 6 considered to be of high quality.
Statistical analysis
The prognostic value of FAR in EC was evaluated by calculating pooled HRs and 95%CIs. Between-study heterogeneities were assessed using Cochran’s Q test and I2 statistic, with I2 ≥ 50% and p < 0.10 indicating significant heterogeneity. A fixed-effects model was applied when heterogeneity was low, while a random-effects model was used in cases of substantial heterogeneity. Threshold values for FAR were determined using different methods across studies. Three articles [19, 21, 22] employed receiver operating characteristic (ROC) curve analysis, while the other three [18, 20, 23] used X-tile software. The optimal cutoff value was determined according to the ROC curve in three studies [19, 21, 22]. The X-tile software was utilized to find the best cutoff values for FAR in predicting OS using the minimal P-value method [18, 20, 23]. Subgroup analyses were performed to further investigate the prognostic value of FAR across various patient subgroups. The relationships between FAR and clinicopathological features of EC were assessed by pooling odds ratios (ORs) and 95%CIs. The stability of the findings was assessed through sensitivity analysis by sequentially excluding each study. Potential publication bias was examined using both Begg’s and Egger’s tests. All statistical analyses were performed using Stata software version 12.0 (Stata Corp., College Station, Texas, USA), and a p < 0.05 was considered statistically significant.
Results
Process of literature retrieval
The initial search yielded 48 studies, of which 35 were retained after removing duplicates (Fig. 1). Following a review of titles and abstracts, 23 studies were excluded for being irrelevant or animal studies. The full texts of the remaining 12 studies were then assessed, resulting in the exclusion of six studies for the following reasons: irrelevance to FAR (n = 4), no survival data (n = 1), and irrelevance to EC (n = 1). Ultimately, six studies involving 2,616 patients [18–23] were included in the meta-analysis (Fig. 1).
Fig. 1.
PRISMA flowchart for study selection in this meta-analysis
Enrolled study features
The included articles [18–23] were all published in English between 2017 and 2025 (Table 1). Five of the studies were conducted in China [18–21, 23], while one in Japan [22], with sample sizes of 88–1,135 (median: 314.5). All studies were of retrospective design. Five articles focused on ESCC [18, 20–23], whereas one study examined esophageal small-cell carcinoma [19]. Regarding cancer staging, five studies included patients with stage I–III disease [18, 20–23], while one study included patients with stage I–IV disease [19]. The FAR threshold range was 0.065–0.111 (median: 0.081). Therefore, a cut-off value of 0.08 was used for stratification in the subgroup analysis. Threshold determination methods varied: three studies [19, 21, 22] used ROC curve analysis, whereas the other three [18, 20, 23] employed X-tile software. All six studies [18–23] evaluated the association between FAR and OS. Three studies derived HRs and 95%CIs using univariate regression analysis [20, 22, 23], whereas the other three used multivariate regression analysis [18, 19, 21]. The quality of the included studies was high, with NOS scores ranging from 7 to 9.
Table 1.
Baseline characteristics of included studies in this meta-analysis
| Study | Year | Country | Sample size | Gender (M/F) | Age (year) Median(range) |
Study period | Cancer type | TNM stage | Treatment | Cut-off value | Cut-off determination | Survival endpoints | Follow-up (month) Median(range) |
Survival analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tan, Z. | 2017 | China | 1135 | 888/247 | 58(28–88) | 2008–2010 | ESCC | I-III | Surgery | 0.08 | X-tile | OS | 1–80 | Multivariate | 9 |
| Wang, Y. | 2020 | China | 88 | 66/22 | 61(42–83) | 2010–2019 | Small cell carcinoma | I-IV | Mixed | 0.081 | ROC curve | OS | 6(1–91) | Multivariate | 7 |
| Zhang, H. | 2021 | China | 641 | 525/116 | 61(33–88) | 2005–2013 | ESCC | I-III | Surgery | 0.065 | X-tile | OS | 36(3-144) | Univariate | 8 |
| Zheng, Z. | 2021 | China | 215 | 154/61 | 60(34–80) | 2013–2015 | ESCC | I-III | Surgery | 0.083 | ROC curve | OS | 44.7(1.6–73) | Multivariate | 8 |
| Aoyama, T. | 2024 | Japan | 123 | 100/23 |
< 70y: 67 ≥ 70y: 56 |
2005–2020 | ESCC | I-III | Surgery | 0.081 | ROC curve | OS | 1–60 | Univariate | 8 |
| Zhang, X. S. | 2025 | China | 414 | 392/22 |
< 60y: 184 ≥ 60y: 230 |
2014–2022 | ESCC | I-III | Surgery | 0.111 | X-tile | OS | 1-120 | Univariate | 8 |
ESCC, esophageal squamous cell carcinoma; ROC, receiver operating characteristic; OS, overall survival; NOS, Newcastle-Ottawa Scale
FAR and OS
All six studies, comprising 2,626 patients [18–23], reported the predictive value of FAR for OS in EC. Given the evident heterogeneity (I2 = 70.2%, p = 0.005), a random-effects model was used. As demonstrated, elevated FAR was significantly associated with poorer OS in EC (HR = 1.98, 95%CI = 1.48–2.65, p < 0.001; Table 2; Fig. 2). Subgroup analyses confirmed that the significant predictive value of FAR for OS remained consistent regardless of country, sample size, cancer type, threshold, threshold determination, or survival analysis (all p < 0.05; Table 2).
Table 2.
Subgroup analysis of prognostic value of FAR for OS in patients with EC
| Subgroups | No. of studies | No. of patients | Effects model | HR (95%CI) | p | Heterogeneity I2(%) Ph |
|
|---|---|---|---|---|---|---|---|
| Total | 6 | 2616 | Random | 1.98(1.48–2.65) | < 0.001 | 70.2 | 0.005 |
| Country | |||||||
| China | 5 | 2493 | Random | 1.97(1.41–2.75) | < 0.001 | 74.5 | 0.003 |
| Japan | 1 | 123 | - | 2.14(1.26–3.63) | 0.005 | - | - |
| Sample size | |||||||
| < 300 | 3 | 426 | Fixed | 2.24(1.55–3.24) | < 0.001 | 0 | 0.697 |
| ≥ 300 | 3 | 2190 | Random | 1.85(1.29–2.78) | 0.003 | 86.1 | 0.001 |
| Cancer type | |||||||
| ESCC | 5 | 2528 | Random | 1.92(1.42–2.58) | < 0.001 | 73.8 | 0.004 |
| Small cell carcinoma | 1 | 88 | - | 3.49(1.18–10.31) | 0.024 | - | - |
| Cut-off value | |||||||
| ≤ 0.08 | 2 | 1776 | Random | 1.53(1.14–2.06) | 0.005 | 66.3 | 0.085 |
| > 0.08 | 4 | 840 | Fixed | 2.53(2.00-3.20) | < 0.001 | 0 | 0.704 |
| Cut-off determination | |||||||
| X-tile | 3 | 2190 | Random | 1.85(1.73–2.78) | 0.003 | 86.1 | 0.001 |
| ROC curve | 3 | 426 | Fixed | 2.24(1.55–3.24) | < 0.001 | 0 | 0.697 |
| Survival analysis | |||||||
| Univariate | 3 | 1178 | Random | 2.18(1.63–2.92) | < 0.001 | 52.6 | 0.121 |
| Multivariate | 3 | 1438 | Random | 1.77(1.09–2.89) | 0.021 | 57.0 | 0.098 |
FAR, fibrinogen-to-albumin ratio; OS, overall survival; ROC, receiver operating characteristic; ESCC, esophageal squamous cell carcinoma; EC, esophageal cancer
Fig. 2.
Forest plots for the prognostic value of FAR for OS in patients with EC. Increased FAR exhibited close relation to poor OS in EC (HR = 1.98, 95%CI = 1.48–2.65, p < 0.001)
Associations of FAR with clinicopathological features
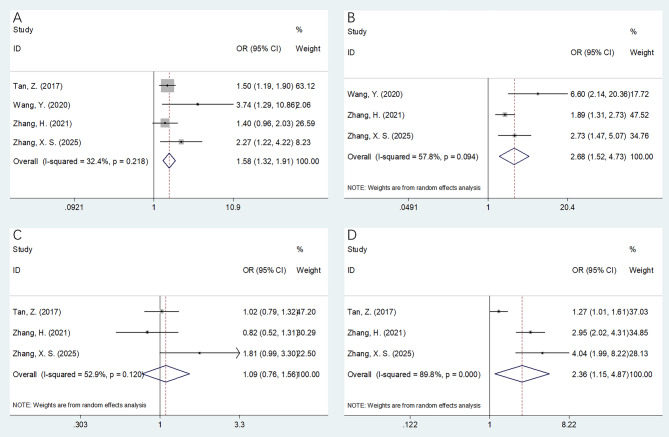
Four studies involving 2,278 patients [18–20, 23] provided data on the correlations between FAR and clinicopathological features of EC. High FAR was significantly associated with the following factors: male sex (OR = 1.38, 95%CI = 1.09–1.74, p = 0.007) (Fig. 3A), T3–T4 stages (OR = 2.36, 95%CI = 1.93–2.87, p < 0.001) (Fig. 3C), N1–N3 stages (OR = 1.58, 95%CI = 1.32–1.91, p < 0.001) (Fig. 4A), TNM III–IV stages (OR = 2.68, 95%CI = 1.52–4.73, p = 0.001) (Fig. 4B), and tumor length > 3 cm (OR = 2.36, 95%CI = 1.15–4.87, p = 0.020) (Fig. 4D) (Table 3). However, FAR was not significantly associated with age (OR = 0.87, 95%CI = 0.48–1.60, p = 0.660) (Fig. 3B), tumor location (OR = 0.98, 95%CI = 0.77–1.25, p = 0.886) (Fig. 3D), or tumor differentiation (OR = 1.09, 95%CI = 0.76–1.56, p = 0.634) (Fig. 4C) (Table 3).
Fig. 3.
Forest plots presenting the association between FAR and clinicopathological features of EC. (A) Gender (male vs. female); (B) Age (years) (≥ 60 vs. < 60); (C) T stage (T3-T4 vs. T1-T2); and (D) Tumor location (middle/lower vs. upper)
Fig. 4.
Forest plots presenting the association between FAR and clinicopathological features of EC. (A) N stage (N1-N3 vs. N0); (B) TNM stage (III-IV vs. I-II); (C) Tumor differentiation (poor vs. well/moderate); and (D) Tumor length (> 3 cm vs. ≤ 3 cm)
Table 3.
The association between FAR and clinicopathological factors in patients with EC
| Variables | No. of studies | No. of patients | Effects model | OR (95%CI) | p | Heterogeneity I2(%) Ph |
|
|---|---|---|---|---|---|---|---|
| Gender (male vs. female) | 4 | 2278 | Fixed | 1.38(1.09–1.74) | 0.007 | 0 | 0.765 |
| Age (years) (≥ 60 vs. < 60) | 4 | 2278 | Random | 0.87(0.48–1.60) | 0.660 | 83.3 | < 0.001 |
| T stage (T3-T4 vs. T1-T2) | 4 | 2278 | Fixed | 2.36(1.93–2.87) | < 0.001 | 37.5 | 0.187 |
| Tumor location (middle/lower vs. upper) | 4 | 2278 | Fixed | 0.98(0.77–1.25) | 0.886 | 0 | 0.686 |
| N stage (N1-N3 vs. N0) | 4 | 2278 | Fixed | 1.58(1.32–1.91) | < 0.001 | 32.4 | 0.218 |
| TNM stage (III-IV vs. I-II) | 3 | 1143 | Random | 2.68(1.52–4.73) | 0.001 | 57.8 | 0.094 |
| Tumor differentiation (poor vs. well/moderate) | 3 | 2190 | Random | 1.09(0.76–1.56) | 0.634 | 52.9 | 0.120 |
| Tumor length (> 3 cm vs. ≤ 3 cm) | 3 | 2190 | Random | 2.36(1.15–4.87) | 0.020 | 89.8 | < 0.001 |
FAR, fibrinogen-to-albumin ratio; EC, esophageal cancer
Sensitivity analysis
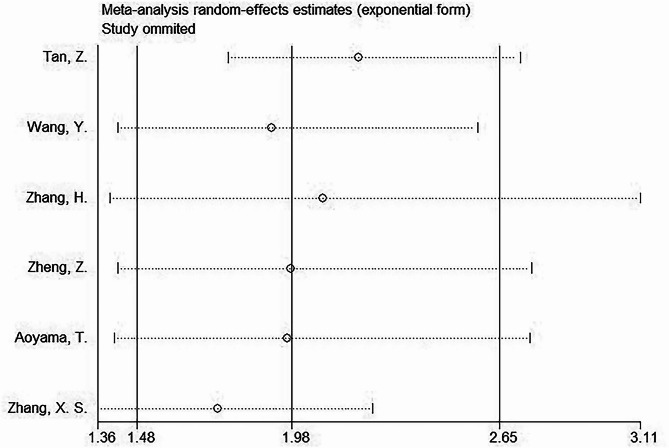
In the sensitivity analysis, individual studies were removed sequentially. The HRs derived from the remaining studies in each analysis remained within the expected range (Fig. 5). These results indicate the robustness and reliability of the overall findings.
Fig. 5.
Sensitivity analysis. Individual studies were removed separately in the sensitivity analysis. The HRs derived from the aggregated results of the remaining studies in each analysis stayed within the anticipated range. Sensitivity analysis revealed reliable study results
Publication bias
This study assessed potential publication bias using Begg’s and Egger’s tests. The funnel plots appeared symmetrical (Fig. 6), and the results revealed no significant publication bias (p = 0.260 for Begg’s test; p = 0.239 for Egger’s tests) (Fig. 6).
Fig. 6.
Publication bias. (A) Begg’s test for OS, p = 0.260; and (B) Egger’s test for OS, p = 0.239. This study analyzed possible publication bias through Begg’s and Egger’s tests. Funnel plots were symmetrical. The results indicated no significant publication bias
Discussion
The predictive value of FAR in EC remains inconsistent across studies. This meta-analysis combined data from six studies involving 2,616 patients to investigate these inconsistencies. Our findings demonstrated that elevated FAR significantly predicted poorer OS in EC. Moreover, higher FAR levels were significantly associated with advanced TNM stage, advanced T stage, lymph node metastasis, and larger tumor length in EC. The reliability of these results was verified through sensitivity and publication bias analyses. Overall, FAR appears to have a significant prognostic value in EC. To our knowledge, this study is the first meta-analysis to comprehensively analyze the predictive value of FAR in EC.
FAR is calculated as a ratio of fibrinogen to albumin (FAR = fibrinogen/albumin). Therefore, an elevated FAR may result from increased fibrinogen levels and/or decreased albumin levels. While the exact mechanisms underlying the predictive value of FAR in EC remain unclear, several interpretations have been proposed. On the one hand, fibrinogen levels are known to rise under various pathophysiological conditions such as tumors, surgeries, infections, inflammations, or trauma [26]. Additionally, fibrinogen has been shown to promote tumor cell invasion and migration while stimulating angiogenesis [27]. It also contributes to chronic low-grade inflammation and facilitates cancer metastasis by influencing leukocyte and platelet movement while also serving as a scaffold for tumor growth, invasion, and metastasis [28, 29]. On the other hand, serum albumin serves as a marker of nutritional status. Low albumin levels are linked to malnutrition, immune system dysfunction, and systemic inflammation [30]. Inflammatory factors can suppress albumin synthesis, and oxidative stress may cause its denaturation, leading to a rapid decline in serum albumin levels among inflammatory conditions [31]. Malnutrition adversely impacts organ and cellular function, worsens clinical outcomes, and is closely linked to reduced survival and quality of life among patients with cancer [32]. Therefore, FAR serves as a rational prognostic marker, reflecting the combined impact of fibrinogen and albumin levels.
This meta-analysis shows that FAR is a significant prognostic marker for EC with several clinical implications. First, patients with EC who present with a high pretreatment FAR (> 0.08) may have a poorer prognosis and should be considered for multimodality therapy. Second, FAR could be monitored during the follow-up to help assess disease progression. Third, current prognostic models for EC should incorporate FAR as a potential predictive factor.
Notably, significant heterogeneity was observed in the meta-analysis for overall OS. This heterogeneity may be attributed to several factors. First, all included studies had a retrospective design, which introduces potential selection bias. Second, the cut-off values for FAR varied across the included studies. Third, the treatment methods were not consistent among the studies. These variations could lead to heterogeneity among studies. The reported FAR thresholds ranged from 0.065 to 0.111, with a median of 0.081. Therefore, we selected 0.08 as the cut-off value for subgroup analysis, which indicated that FAR remained a significant prognostic marker for EC, regardless of cut-off values (Table 2). Currently, a standard FAR cut-off value is lacking for EC. Based on the findings of our meta-analysis, we suggest 0.08 as a potential standard FAR cut-off value for EC.
Although the funnel plot appeared symmetrical and Begg’s/Egger’s tests showed no statistically significant results, potential publication bias may still exist due to the following reasons. First, this meta-analysis included only published studies, excluding unpublished literature, which may introduce bias. Second, only English-language articles were considered, potentially introducing language bias. Third, we used pooled HRs and 95%CIs rather than individual patient data, which may affect the precision and reliability of the findings.
Recently, many studies have highlighted the significant prognostic value of FAR across various tumor types through meta-analyses [33–35]. Wang et al. conducted a meta-analysis involving 4,094 patients and found that elevated FAR was significantly linked to poorer OS and disease-free survival in breast cancer [33]. Similarly, Li et al. performed a meta-analysis involving 5,926 patients, showing a strong correlation between elevated FAR and unfavorable clinical outcomes in malignant tumors [34]. Another recent meta-analysis comprising 19 studies also reported that increased FAR was closely linked to higher rates of tumor recurrence and mortality [35]. The results of the current meta-analysis were in line with the findings on other cancer types.
This study has some limitations. First, the sample size was relatively small, as only six articles involving 2,616 patients were included despite a comprehensive search of recent literature. Second, all eligible studies were retrospective. Consequently, selection bias may be present due to the inherent limitations of retrospective designs. Each study also used different inclusion criteria for patient selection, which may have further contributed to this bias. Third, the FAR threshold varied among the included studies. A standard FAR cut-off value is currently lacking for EC. Based on our findings, we suggest 0.08 as a potential standard FAR cut-off value for EC. Fourth, this meta-analysis included only publications in English, excluding non-English studies. While English-language articles are often considered to be of relatively high quality and are widely accessible, the exclusion of non-English studies may introduce language bias. Therefore, large, prospective, multi-regional trials with standardized FAR thresholds are required to validate our findings.
Conclusion
This meta-analysis demonstrated that elevated FAR is significantly associated with poor OS in EC. Moreover, high FAR is closely linked to factors indicative of tumor progression and metastasis in EC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations
- FAR
Fibrinogen-to-albumin ratio
- EC
Esophageal cancer
- HR
Hazard ratio
- CI
Confidence interval
- OR
Odds ratio
- ESCC
Esophageal squamous cell carcinoma
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NOS
Newcastle-Ottawa Scale
- LN
Lymph node
- ROC
Receiver operating characteristic
- OS
Overall survival
Author contributions
YL, XL, and XG collected data and wrote the manuscript. XG and XJ prepared the figures and tables. XJ provided suggestions for revisions. YL provided guidance. All authors reviewed the manuscript.
Funding
This research received no external funding.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that there is no conflict of interest.
Supplemental files
Supplemental file 1 The PRISMA checklist.
Supplemental file 2 The detailed search strategies for each database.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2024;74(3):229–63. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Zhang B, Xu J, Xue L, Wang L. Current status and perspectives of esophageal cancer: a comprehensive review. Cancer Commun (London England) 2024. [DOI] [PMC free article] [PubMed]
- 3.Liu CQ, Ma YL, Qin Q, Wang PH, Luo Y, Xu PF, Cui Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer. 2023;14(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakanaka K. Treatment strategy for early-stage esophageal cancer. Jpn J Radiol. 2024;42(7):677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moughnyeh MM, Green M, Katuwal B, Hammoud ZT. Current landscape of immunotherapy in esophageal cancer: a literature review. J Thorac Dis. 2024;16(12):8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leowattana W, Leowattana P, Leowattana T. Systemic treatments for resectable carcinoma of the esophagus. World J Gastroenterol. 2023;29(30):4628–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong X, Jin M, Wang L, Zhang D, Yin Y, Shen Q. Prognostic biomarkers for immunotherapy in esophageal cancer. Front Immunol. 2024;15:1420399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zapała Ł, Kunc M, Sharma S, Biernat W, Radziszewski P. Low Lymphocyte-to-Monocyte ratio is the potential Indicator of worse overall survival in patients with renal cell carcinoma and venous tumor Thrombus. Diagnostics (Basel Switzerland) 2021, 11(11). [DOI] [PMC free article] [PubMed]
- 9.Liu P, Chen S, Gao X, Liang H, Sun D, Shi B, Zhang Q, Guo H. Preoperative sarcopenia and systemic immune-inflammation index can predict response to intravesical Bacillus Calmette-Guerin instillation in patients with non-muscle invasive bladder cancer. Front Immunol. 2022;13:1032907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Özcan P, Çarkman MS. The relationship between the prognostic nutritional index and lymphovascular and perineural invasion of the tumor in patients diagnosed with gastric cancer, and its effect on overall survival. Med (Baltim). 2024;103(42):e40087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki T, Tateishi K, Sonehara K, Hirota S, Komatsu M, Yamamoto M, Kanda S, Kuraishi H, Hanaoka M, Koizumi T. Clinical utility of the C-reactive protein:albumin ratio in non-small cell lung cancer patients treated with nivolumab. Thorac Cancer. 2021;12(5):603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu J, Xu X, Tian M, Wang H, Jin X. The controlling nutritional status score as a new prognostic predictor in patients with cervical cancer receiving radiotherapy: a propensity score matching analysis. BMC Cancer. 2024;24(1):1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H, Ma J, Lu J, Yao ZH, Ran HL, Zhou H, Yuan ZQ, Huang YC, Xiao YY. Fibrinogen-to-albumin ratio predicts overall survival of hepatocellular carcinoma. World J Gastrointest Oncol. 2023;15(9):1662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi X, Wu C, Deng W, Wu J. Prognostic value of lactate dehydrogenase to absolute lymphocyte count ratio and albumin to fibrinogen ratio in diffuse large B-cell lymphoma. Med (Baltim). 2024;103(30):e39097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma S, Wang L. Fibrinogen-to-albumin ratio (FAR) is the best biomarker for the overall survival of patients with non-small-cell lung cancer. Front Oncol. 2024;14:1396843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Meng Z, Lu M, Ruan S, Zhou J, Zhang M, Huang Y, Chen K, Luo X, Xie CK, et al. Study of the significance of the combination of the fibrinogen-albumin ratio and sarcopenia in predicting the prognosis of laryngeal cancer patients undergoing radical surgery. BMC Cancer. 2024;24(1):1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K, Yan J, Zhang H, Lu C, Wang W, Guo M, Zhang X, Zhang Z. Prognostic value of preoperative white blood cell to hemoglobin ratio and fibrinogen to albumin ratio in patients with colorectal cancer. Med (Baltim). 2024;103(3):e37031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan Z, Zhang M, Han Q, Wen J, Luo K, Lin P, Zhang L, Yang H, Fu J. A novel blood tool of cancer prognosis in esophageal squamous cell carcinoma: the fibrinogen/albumin ratio. J Cancer. 2017;8(6):1025–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Li J, Chang S, Zhou K, Che G. Low albumin to fibrinogen ratio predicts poor overall survival in esophageal small cell carcinoma patients: A retrospective study. Cancer Manag Res. 2020;12:2675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Ren P, Ma M, Zhu X, Zhu K, Xiao W, Gong L, Tang P, Yu Z. Prognostic significance of the preoperative albumin/fibrinogen ratio in patients with esophageal squamous cell carcinoma after surgical resection. J Cancer. 2021;12(16):5025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z, Lin D, Chen Q, Zheng B, Liang M, Chen C, Zheng W. Prognostic value of combined detection of preoperative Albumin-to-Fibrinogen ratio and Neutrophil-to-Lymphocyte ratio in operable esophageal squamous cell carcinoma patients without neoadjuvant therapy. Cancer Manag Res. 2021;13:2359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoyama T, Maezawa Y, Hashimoto I, Hara K, Kazama K, Komori K, Kato A, Otani K, Tamagawa A, Cho H, et al. The clinical impact of the pretreatment albumin to fibrinogen ratio in esophageal Cancer patients who receive curative treatment. Vivo. 2024;38(3):1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XS, Li XY, Xing L, Ren P. Combining the Albumin-to-fibrinogen ratio and pathologic factors predicts survival in surgically treated patients with esophageal squamous cell carcinoma. Oncologie. 2025;27(1):137–47. [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 26.Pieters M, Wolberg AS. Fibrinogen and fibrin: an illustrated review. Res Pract Thromb Haemost. 2019;3(2):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li R, Song S, He X, Shi X, Sun Z, Li Z, Song J. Relationship between fibrinogen to albumin ratio and prognosis of Gastrointestinal stromal tumors: A retrospective cohort study. Cancer Manag Res. 2020;12:8643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin WZ, Li QL, Chen WF, Xu MD, Zhang YQ, Zhong YS, Ma LL, Hu JW, Cai MY, He MJ, et al. Overexpression of fibrinogen-like protein 2 induces epithelial-to-mesenchymal transition and promotes tumor progression in colorectal carcinoma. Med Oncol (Northwood Lond Engl). 2014;31(9):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang F, Wang Y, Sun P, Wang ZQ, Wang DS, Zhang DS, Wang FH, Fu JH, Xu RH, Li YH. Fibrinogen promotes malignant biological tumor behavior involving epithelial-mesenchymal transition via the p-AKT/p-mTOR pathway in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(12):2413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moujaess E, Fakhoury M, Assi T, Elias H, El Karak F, Ghosn M, Kattan J. The therapeutic use of human albumin in cancer patients’ management. Crit Rev Oncol/Hematol. 2017;120:203–9. [DOI] [PubMed] [Google Scholar]
- 31.Bito R, Hino S, Baba A, Tanaka M, Watabe H, Kawabata H. Degradation of oxidative stress-induced denatured albumin in rat liver endothelial cells. Am J Physiol Cell Physiol. 2005;289(3):C531–542. [DOI] [PubMed] [Google Scholar]
- 32.Yan X, Zhang S, Jia J, Yang J, Song Y, Duan H. Exploring the malnutrition status and impact of total parenteral nutrition on the outcome of patients with advanced stage ovarian cancer. BMC Cancer. 2021;21(1):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Shen X. Prognostic and clinicopathological significance of fibrinogen-to-albumin ratio (FAR) in patients with breast cancer: a meta-analysis. World J Surg Oncol. 2024;22(1):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li B, Deng H, Lei B, Chen L, Zhang X, Sha D. The prognostic value of fibrinogen to albumin ratio in malignant tumor patients: A meta-analysis. Front Oncol. 2022;12:985377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun DW, An L, Lv GY. Albumin-fibrinogen ratio and fibrinogen-prealbumin ratio as promising prognostic markers for cancers: an updated meta-analysis. World J Surg Oncol. 2020;18(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.