Abstract
OBJECTIVES:
The aims of this scoping review were to: 1) explore factors driving surgical ICU (SICU) admission decisions, 2) provide an environmental scan of SICU admission practices, and 3) identify underexamined domains relevant for SICU triage, admission, and discharge inquiries.
DATA SOURCES:
Embase, PubMed, and Medline were queried from inception to April 18, 2024, for English-language peer-reviewed studies related to adult SICU admission criteria and decision-making; neonatal ICU, PICU, veterinary ICU, and military ICU data and gray literature were excluded. Studies were not limited by design.
STUDY SELECTION:
Following duplicate removal, 363 of the initial 625 abstracts remained. After content screening, 54 abstracts remained topic aligned. Full-text review identified 44 articles appropriate for analysis.
DATA EXTRACTION:
Abstracted data addressed SICU structure, function, findings, and potential future directions.
DATA SYNTHESIS:
Most included studies (n = 23, 52%) focused on identifying risk factors for SICU admission or risk factors for the need for SICU admission, including demographics, comorbidities, and procedural specifics. Admission protocol evaluation studies were less common (n = 5, 11%), but offered promise in reducing unnecessary admissions using preoperative or postoperative interventions. Future inquiry domains included admission and discharge protocol development (n = 17, 39%), risk factors for ICU admission or the need for ICU admission (n = 16, 36%), multicenter studies (n = 16, 36%), additional or specific patient populations (n = 15, 34%), prospective studies (n = 14, 32%), costs (n = 6, 14%), and implementation of embedded clinical decision-support aids to inform SICU triage decision-making (n = 2, 5%). No included studies presented results regarding SICU discharge decision-making or ICU stress adaptations relevant during surge episodes.
CONCLUSIONS:
Research on SICU triage decision-making primarily focuses at admission risk factor discovery, with less emphasis on protocol evaluation and implementation practices. Future research should focus on refining existing SICU triage approaches that include discharge and surge-based decision-making coupled with deployable clinical decision-support aids.
Keywords: decision support, intensive care, postoperative, surgery, triage
KEY POINTS
Question: What drives surgical ICU (SICU) triage decisions?
Findings: A scoping review of 44 studies found that inquiry primarily focuses on specific risk factors for SICU admission rather than protocol evaluation or discharge decision-making. Risk factors involved age, comorbidities, features of the disease necessitating surgery, specifics of surgery, postoperative complications, and scoring systems.
Meaning: Future research should evaluate and refine existing SICU triage protocols, discharge decision-making during periods of ICU stress, and deployable clinical decision-support aids to reduce unnecessary SICU admission and care.
Hundreds of millions of operations are performed worldwide (1). Following each of these procedures, surgeons determine a postoperative level of care for patients requiring acute care facility admission. A subset of these patients is admitted to a surgical ICU (SICU)—a process that may be governed by physiology, monitoring needs, resources, surgeon discretion, or uncertainty regarding postoperative trajectory. Appropriate ICU bed allocation impacts outcomes by ensuring bed availability for those in need and beneficially influences resource utilization and healthcare cost. Low-acuity patients (a.k.a. “low-risk monitor”) who are postoperatively overtriaged to an ICU constitute approximately 5% of SICU admissions (2). This unique group enjoys comparable outcomes at the expense of higher costs and therefore lower care value compared with similar patients admitted to an acute care unit. Relatedly, those undertriaged to an acute care unit constitute approximately 12% of such admissions (2). Similarly, they receive lower care value, endure a longer hospital length of stay, and higher rates of mortality, cardiac arrest, and acute kidney injury rates compared with similar patients admitted to ICUs (2). It is unclear if both over and undertriage events have occurred in the context of a specific set of SICU admission criteria or simply reflect clinician caprice or facility acute care unit care limitations.
To mitigate ICU over and undertriage, the Society of Critical Care Medicine (SCCM) has articulated general recommendations regarding admission, discharge, and triage (ADT) that may guide decision-making around ICU bed utilization. Specifically, ICU triage decisions should reflect needs for life-supporting therapies or specific clinical expertise that is informed by the patient’s physiology, diagnosis, bed availability, and the potential to benefit from interventions coupled with prognosis (3). While comprehensive, these recommendations are not specific to SICUs and demonstrate limited granularity due to the absence of high-quality evidence. Furthermore, it is unclear how surgeons—with and without fellowship training in critical care medicine—approach ICU triage decision-making. Additionally, since only half of all U.S. ICUs are staffed by an intensivist, ICU ADT practices across institutions may be quite divergent (4). A more recent evaluation of intensivist staffing led to a much higher estimate, but was skewed (69.8% had training programs in place) and clearly underrepresented community, rural, and critical-access facilities (5). Therefore, it is relevant to assess the evidence base addressing SICU triage decision-making. We aim to describe factors that drive SICU triage decisions and provide an environmental scan of current practice. This scoping review intends to provide a platform from which to identify high-value domains for future investigations of SICU ADT processes to align the right care with the right patient at the right time.
METHODS
Embase, PubMed, and Medline were queried from inception to April 18, 2024, for English-language peer-reviewed articles having titles or abstracts related to surgery, postoperative triage, and care intensity. Articles addressing neonatal ICU, PICU, veterinary ICU, or military ICU were excluded (Supplemental Fig. 1, https://links.lww.com/CCX/B520).
Two reviewers (P.H., P.P.P.) used Covidence (Melbourne, VIC, Australia) (6) to screen titles and abstracts describing evidence informing SICU admission decision-making. Two reviewers (K.L.A., P.H.) similarly used Covidence to screen selected full-text articles. Search terms and exclusions following screening and full-text review are presented in Supplemental Figure 1 (https://links.lww.com/CCX/B520). Reviewers resolved disagreements regarding article inclusion via discussion and consensus. Interrater reliability was assessed using Cohen’s κ, calculated by Covidence.
For each remaining article, one reviewer (K.L.A.) extracted information relevant to ICU structure and function, findings, and potential future inquiries. Result reporting conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews guideline (Supplemental Table 1, https://links.lww.com/CCX/B520). Data are reported as number with percentage or median with interquartile range. As a scoping review, this study did not require ethical oversight from an Institutional Review Board.
RESULTS
The initial search yielded 625 abstracts with 363 remaining after duplicate removal. Following screening, 54 abstracts remained. Cohen’s κ for interrater reliability was 0.39 for title and abstract screening and 0.76 for full-text review. After full-text review, 44 articles provided study data.
Summary of Included Studies
Abstracted data are shown in Supplemental Tables 2 and 3 (https://links.lww.com/CCX/B520). All 44 included studies were cohort studies. The median number of patients was 391 (231–1634). Approximately, one-third of studies (36%) included patients spanning multiple surgical specialties. Slightly more than half of the studies (52%) came from the United States. Other settings included Italy (9%), The Netherlands (7%), Denmark (7%), and dozens of other countries, with one multinational study (7).
Studies were heterogeneous regarding SICU structure. Some SICUs (n = 2, 5%) reported also admitting medical patients (8, 9), and some (n = 4, 9%) reported only admitting subspecialty patients (e.g., only neurosurgery patients) (10–13). Most studies (n = 38, 86%) were nonspecific regarding possible inclusion of both medical and surgical patients. Beds, when reported (n = 6, 14%), ranged from eight to 26 (8, 14–17), with a median of 11 across institutions in the multinational study (7).
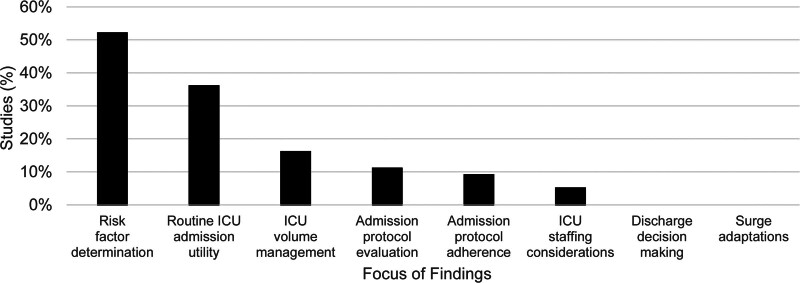
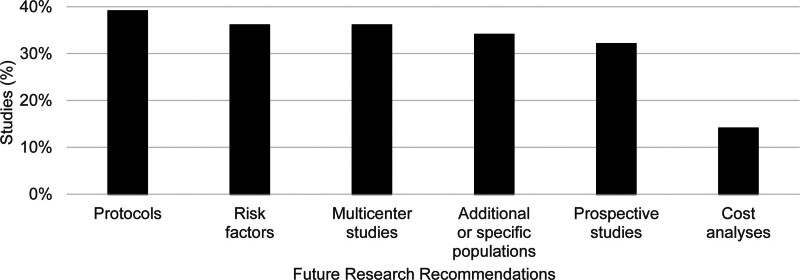
Study findings predominantly related to risk factor determination (52%), routine ICU admission utility (36%), ICU volume management (16%), admission protocol evaluation (11%), admission protocol adherence (9%), or ICU staffing considerations (5%) (Fig. 1). Future research recommendations generally involved protocols (39%), risk factors (36%), multicenter studies (36%), additional or specific patient populations (34%), prospective studies (32%), and cost analyses (14%) (Fig. 2). No studies specifically presented results regarding ICU discharge decision-making or ICU stress adaptations during surge episodes.
Figure 1.
Primary study findings.
Figure 2.
Study recommendations for future research.
Comorbidities and Risk Scores
Studies examined a variety of SICU admission predictors. Risk factors associated with SICU admission are presented in (Table 1). Commonly deployed objective risk assessment scores were also associated with SICU admission, including: Acute Physiology and Chronic Health Evaluation II greater than or equal to 11 (27), Charlson Comorbidity Index greater than 8 (25), Karnofsky Performance Score (KPS) less than 70 (7), multiple variants of medically necessary time sensitive (28), Simplified Acute Physiology Score (SAPS) II score greater than 36 (7), and Surgical Procedure Assessment score greater than or equal to 2B (29). Conversely, factors suggesting that SICU admission was less likely included advanced age (7), previous triages for ICU admission during the same hospital stay (7), ability of the triaging physician to examine the patient, ICU physician seniority, advanced age, certain underlying diseases, self-sufficiency, and low bed availability (8). No studies presented results regarding patient-to-staff ratios or geographic coverage approaches for surgical patients cared for in another ICU.
TABLE 1.
Risk Factors for Either ICU Admission or the Need for ICU Admission (Distinct From Directly Predicting ICU Admission Because Not All ICU Admissions Are Necessary, Due to Over-Triage [2])
| Risk Factors for ICU Admission | Risk Factors for Need for ICU Admission |
|---|---|
| Advanced age (18) | Advanced age (19, 20) |
| History of hypertension (21) | History of hypertension (22) |
| History of smoking for ≥ 50 pack years (23) | History of myocardial infarction (19) |
| History of vascular or liver disease (7) | History of chronic obstructive pulmonary disease (22) |
| History of hematologic malignancy (7) | Low physical status (22) |
| No comorbidities (7) | Body mass index > 35 (19) |
| Hypotension (18) | Decreased creatinine clearance (19) |
| Subarachnoid hemorrhage or ruptured aneurysm (21) | General endotracheal anesthesia (11) |
| Ratio of red blood cell distribution width to serum calcium (24) | Extensive resection (22) |
| Serum albumin ≤ 3.5 (25) | Prolonged operative time (20, 22, 26) |
| Blood pH < 7.3 (18) | Large estimated blood loss (22) |
| Trauma (7) | Elevated lactate (22) |
| Surgery (7, 16) | Early postoperative complications (11) |
| Invasive mechanical ventilation (7) | |
| Neurologic deficits (21) |
Risk Factors for Requiring SICU Admission
Studies also examined a variety of predictors of requiring unplanned postoperative SICU admission or later SICU admission for complication management. These types of admissions are distinct from those for whom SICU care is already planned based upon procedure, monitor requirements, or unique patient characteristics. Risk factors for requiring SICU admission are presented in Table 1.
Objective risk assessment scores were similarly found to predict requiring SICU admission, including: electronic Cardiac Arrest Risk Triage greater than or equal to 2.1% (30), Emergency Surgery Score greater than or equal to 7 (31), KPS less than 70 (11), Predictive Optimal Trees in Emergency Surgery Risk-ICU (POTTER-ICU) probability (32), Surgical Outcome Risk Tool risk of greater than or equal to 5% (33), SAPS III score greater than 40 (26), and machine-learning models, such as a decision tree that outperformed clinical judgment for ICU admission (34), and a deep learning model for prolonged length of stay (2). The POTTER-ICU study included the largest sample size with 464,861 patients, and achieved an area under the receiver operating characteristic curve of 0.88 for predicting SICU admission. Conversely, factors suggesting that SICU admission was not needed included the absence of anticoagulation (10), age younger than 55 (10), Glasgow Coma Scale score equal to 15 (10), and limited risk for neurologic decline (10).
Routine SICU Admission
Studies of routine SICU admission following specific procedures, or with airway-based comorbidities, advanced that this practice was unnecessary for a wide variety of patients provided that sufficient staffing and monitoring was present in non-SICU care locations (Table 2).
TABLE 2.
Procedures for Which Routine Surgical ICU Admission Was Examined and Found to Be Unnecessary
| Procedures Not Routinely Requiring Surgical ICU Admission |
|---|
| Orthopedic surgery for patients with sleep apnea (35) |
| Sleep apnea surgery (36) |
| Carotid endarterectomy (37, 38) |
| Lower extremity bypass (39) |
| Robotic pancreaticoduodenectomy (40) |
| Liver transplantation (41) |
| Resection of epithelial ovarian, peritoneal, or fallopian tube carcinoma (42) |
| Decompression for blunt traumatic brain injury (10) |
| Elective supratentorial craniotomy (12, 17, 43) |
| Eventless microvascular decompression for neuralgias (13) |
| Elective neuroradiology procedures (21) |
SICU Admission Protocols
Several studies noted the presence of—or specifically described—protocols for SICU admission, including one that used the SCCM guideline (16). Several other studies identified a locally generated protocol (42), reserving beds for acute respiratory failure or ventilator-dependent patients (22, 44), patients with large-volume blood loss (44), hemodynamic instability (22, 44), continuous vasopressor infusion (22), intensive monitoring for myocardial infarction evolution, or evaluation for progression of other specific intraoperative events (i.e., open body cavity management for intra-abdominal hypertension) (22, 44). One study described admission as a function of bed availability in a temporally driven fashion with one bed reserved for emergency cases (14).
Studies evaluating SICU admission protocols found that these safely reduced admission both preoperatively (45, 46) and postoperatively (14, 36, 44). Two protocols addressed sleep apnea, with one study noting reduced SICU admissions (9.1–2.0%), length of stay (2.3–1.3 d), and cost (50%), with no adverse impact on postoperative complications (46). Another study found that a postoperative protocol safely triaged a small proportion (5/104, 4.8%) of patients to non-ICU care, although one such patient later required SICU management (36). A postcardiac surgery fast-track protocol led to 84% of patients transferring from the postanesthesia care unit to acute care unit, with a small proportion (1.2%) later requiring SICU care (44). Relatedly, a total hip arthroplasty preoperative triage protocol increased SICU admissions (from an unspecified percent to 11.4%) but reduced unplanned admissions (7.1–2.2%), major complications (12.5–2.0%), and mortality (observed-to-expected mortality from 4.77 to 1.62) (45). One study suggested that SICU admission for high-risk patients may be unnecessary beyond 48 hours—the time when postoperative mortality appeared highest (14).
Future Research Potentials
Inquiries emphasized the need for further exploration of preoperative, intraoperative, and postoperative risk factors that drive the requirement for SICU admission and their related surgical outcomes (2, 8, 12, 13, 17, 22, 25, 26, 29, 31, 32, 34, 42, 44, 47, 48) (n = 14, 36%), protocol generation based on the identification of such risk factors (7, 9, 11–15, 19–22, 28, 36, 37, 44, 45, 47) (n = 17, 39%), and the construction of embedded clinical decision-support tools (2, 48) (n = 2, 5%). Nearly one-third advanced the need for prospective (2, 7, 10–12, 16, 18, 19, 21, 24, 27, 34, 38, 48) (n = 14, 32%), multicenter (2, 8, 10–12, 28, 30, 33, 38–41, 43, 44, 48, 49) (n = 16, 36%) inquiries, including patient populations undergoing previously unaddressed procedures, as well as specific populations based on unique characteristics (2, 8, 21, 24, 26, 30, 32–35, 39–41, 47, 49) (n = 15, 34%). A smaller set of studies proposed expanding cost—as opposed to charge—data capture (26, 28, 40, 41, 45, 47) (n = 6, 14%). No study suggested inquiries into SICU triage decision-making based upon the following delimiters: intensivist-staffed vs. unstaffed, training program vs. no trainees, PharmD-staffed vs. unstaffed, nor physician trainee vs. advanced practice provider (APP)-staffed SICU settings.
DISCUSSION
Our scoping review findings are unsurprising as they represent what is conveniently assessable and readily examinable, reflecting current practice. Nonetheless, the benefit of this scoping review lies in what was not assessed or addressed. These absences should inform the extent and focus of subsequent inquiries. We noted the conspicuous absence of a uniform SICU admission and outcome database and ontology or controlled vocabulary to facilitate opportunities at the national and medical professional organization levels. For example, the National Surgical Quality Improvement Program (50), the Veterans Affairs Surgical Quality Improvement Program (51), and the Trauma Quality Improvement Program (52) are surgically focused databases that could meet those goals with the inclusion of specific fields that address SICU admission. Relatedly, the Extracorporeal Life Support Organization (53) is a key exemplar of an international database that drives care delivery improvement by reducing variation in care. Recently, the United Network for Organ Sharing has been asked to craft a similar database for normothermic regional perfusion assessment in the United States.
Other key domains that were not addressed among included studies include ICU discharge criteria; decision-making during patient surge episodes; the impact of ICU stress on triage decision-making; the evolution of geographic coverage by provider teams across ICUs, ICU staffing with trainees, PharmDs, or APPs; and the role of critical care organizations in driving interfacility transfer, although the article search criteria were not engineered to focus specifically on those domains. In particular, interfacility transfer shapes admission between facilities of disparate complexities (54–56). Relatedly, the role of within-system transfers across a critical care organization will also drive discharge that is tied to originating facility repatriation—another element that is infrequently assessed. Each of these are fruitful areas of investigation that would be aided by a national database with a consistent data dictionary.
Currently, postoperative SICU admission appears to be driven by some patient-specific factors as well as many other systems-based factors that often vary by institution and resources (57). Accordingly, protocols are only one part of that calculus (4). This kind of admission is distinct from preoperative admission for resuscitation ahead of surgical management of emergency conditions other than mesenteric ischemia, necrotizing soft-tissue infection, and hemorrhage; each of those conditions benefit from concomitant resuscitation and operation. Admission may be also driven by intensivist staffing—or lack thereof—as well as gatekeeping approaches that relate to SICU function. For example, SICUs tend to be collaboratively structured (intensivist as consultant) as opposed to medical ICUs (MICUs) that tend to be closed (intensivist as the attending of record) (58, 59). When the SICU is staffed with an intensivist-led team, approval for unplanned admissions more commonly requires discourse with, and approval by, the attending intensivist. SICUs that do not benefit from intensivist direction are more likely to be open, devoid of gatekeeping, and have admission driven by surgeon preference regardless of physiology or monitoring requirement. Furthermore, admission practices may reflect the influence of training programs, although articles included in our analysis did not present results regarding ICU staffing with trainees. Community facilities are more likely to house a single ICU that addresses medical and surgical patients as opposed to training facilities that have distinct ICUs that align with parent disciplines (i.e., medical, surgical, cardiac, and neuro ICUs) (60). Importantly, studies included in this review did not focus on closed, open, and collaborative ICU approaches as an influencer of postoperative SICU admission, but shed light on other metrics that may guide admission decision-making.
Objective ICU admission risk scores may help identify patients at higher risk for intervention or decompensation. However, they are often forced into binary variables and static thresholds and fail to consider interactions between comorbidities and other risk factors. When static thresholds are applied, there is a failure to weight the intensity of the comorbidity along a continuum (e.g., atrial fibrillation with rate control may have a different implication from atrial fibrillation with rapid ventricular response, and classifying heart rate values as ≥ vs. < 100 beats/min treats values of 99 and 100 differently, while treating values of 100 and 150 as being the same) (61). These issues may be addressed by applying machine-learning approaches to raw values. If they are difficult to implement or do not integrate with existing digital workflows, they will be poorly used in practice, driving the need for embedded and readily implementable clinical decision-making aids. Recent examples of pragmatic decision-support include those that help guide clinicians to evaluate the prescribed tidal volume for acute respiratory distress syndrome patients or select particular antimicrobial agents for those with septic shock ahead of and following culture data that will tailor the selection (62). SICU admission and discharge can be similarly guided, especially given the wealth of information encoded in the electronic health record (EHR).
ICU discharge—equally important to admission—is underevaluated in studies and is a key element within evidence-informed recommendations. Resources and acute care unit staffing play a large role in such decisions and will influence data that are published and used for performance improvement projects within a single institution or healthcare system. Furthermore, the inability to discharge individuals with homelessness, complex wound care, or those who are transferred from remote locations to a tertiary or quaternary care center impedes SICU discharge (63). In addition, SICU discharge hinges on the comfort and capability of the operative service or attending in managing their patient outside of the SICU. More generally oriented surgical services may have greater comfort so doing while there remain issues related to recovering organ failure, nutrition, and infection management; some subspecialty services may be uncomfortable with such issues. The latter barrier to SICU discharge may be offset by the rise of hospitalist services. Indeed, some hospitalists care for patients in the ICU in community or critical-access facilities that are not staffed or not reliably staffed by intensivists (64). Discharge reticence is also shaped by the notion that there is a service caring for the patient while they remain in the SICU and the admitting service would need to provide that care if the patient were outside the ICU. Furthermore, some outpatient-oriented surgical services have limited capacity for inpatient management, further damping their desire for independent management of medical issues. This key resource influencer was not explored among included studies. Even when there are guideline-based recommendations, the extent to which individuals and services embrace the recommendations is notoriously less than desired (3).
Failure to implement guideline-based recommendations is aptly termed the “knowledge-to-practice” gap. Compliance with guideline measures—including the SCCM ICU ADT guideline—are reported as limited unless there is an associated financial or regulatory requirement (i.e., Surviving Sepsis Campaign guideline-based resuscitation and Centers for Medicare and Medicaid Services Severe Sepsis and Septic Shock Early Management Bundle) (3). Compliance is impeded by local resources, practice inertia, lack of awareness, and low frequency events, establishing a lag time that may exceed a decade after guideline publication for practice adoption. Therefore, new guidelines should also be accompanied by implementation strategies that are readily deployable by clinicians across diverse settings. Of course, certain recommendations are tethered to resource-replete spaces forcing guideline recommendation adaptation to resource-limited realities. Inclusion of stakeholders from such spaces can inform guideline recommendations but may also serve as a platform for focused guideline articulation. Currently, SICU admission and discharge guidelines are unaccompanied by implementation strategies, nor adaptations for resource-limited spaces, such as low- or middle-income countries and underresourced healthcare systems within high-income countries. Gamification may help with guideline deployment and compliance by linking EHR-based decision-support to enact guideline-based recommendations with visual reinforcements of success. Regardless, SICU physician admission and discharge decision-making must also consider related resources.
Staffing ratios and clinician cognitive and technical burden also influence triage decisions as repeatedly highlighted during the recent severe acute respiratory syndrome coronavirus 2 pandemic (65, 66). In certain centers, the key driver might be nursing availability, whereas in others, it might be the number or acuity of patients cared for by a clinical team. MICU services are more permeated by patient allocation limits (i.e., “capping”), precluding additional patients being added to their care roster. The influence of “capping” may also drive overflow into other units that will impact SICU metrics and decision-making (67). MICU capping leads to emergency department (ED) overcrowding and is a well-described phenomenon. During periods of overcrowding, an available SICU bed presents a reasonable patient care destination to unencumber an ED bed. This practice is termed “geographic coverage” and leverages the commonality of critical care medicine imperatives across disciplines (68). Of necessity, the SICU team encounters clinical conditions that often exist as comorbidities in surgical patients (e.g., chronic obstructive pulmonary disease [COPD]) but which do not generally drive the need for ICU admission (e.g., COPD exacerbation). Depending on the penetrance of this practice across ICUs (which is not assessed among included studies), there may be important implications for surgical critical care training to broaden exposure and develop specific competences. One mechanism to do so may be an increase in MICU rotations during surgical critical care fellowship; other approaches may be equally or more efficacious.
SICU triage may be also influenced by geographically driven aspects of training and practice. For instance, in the United States, especially at quaternary care centers, SICU intensivists are commonly general surgeons with critical care fellowship training. Increasingly, emergency medicine and neurocritical care clinicians are represented within the SICU faculty roster (69). In the community, surgical intensivist penetrance is more limited and is instead characterized by anesthesia, medical, or pulmonary intensivists (70). Furthermore, in the community, ICUs are often combined MICUs/SICUs that may have different triage criteria based upon disease process and required interventions. Outside of the United States, most intensive care specialists are parent-specialty trained in anesthesiology (71). It remains to be investigated how the parent training discipline—as well as financial imperatives, such as a relative value unit-driven compensation system—influence triage decision-making.
As a scoping review, there are relevant limitations that influence how our results are interpreted and used. First, the scope of included perspectives was limited by our exclusion of neonatal ICU, PICU, and military ICU data and gray literature. Second, Cohen’s κ for interrater reliability for title and abstract screening was only fair, suggesting potential for selection bias. Third, most included studies were retrospective observational and cohort, which are susceptible to selection bias. Fourth, while this review is international in scope, the generalizability of specific findings may be limited by the narrow focus or small sample size of the included studies. Nearly half of the studies came from the United States, but ICU admission practices vary across healthcare systems and international settings. For example, in some countries, intensivists may have greater autonomy for admission decisions, with reduced influence from surgical teams, leading to differing patterns of constraints related to bed availability or other facets of ICU management. Fifth, all studies are peer-reviewed, which introduces a risk of publication bias, including the absence of negative studies. Relatedly, only sampling peer-reviewed studies does not provide a granular evaluation of currently embraced practices—it only offers insight into reported practices. Sixth, only English-language studies were accessed, potentially excluding relevant studies. Seventh, as we limited our evaluation to surgical patients, the data may not represent practices that influence admission to other ICUs, including specialty ones, such as neurologic ICUs. Eighth, whereas we assessed SICU triage, it is worth noting that ICUs that care for surgical patients are not homogenous, also potentially limiting applicability to specialty SICUs, such as those addressing cardiothoracic patient care.
CONCLUSIONS
This scoping review highlights existing areas of inquiry as well as gaps in the current understanding of SICU triage decision-making. Despite general guidance, specific criteria for SICU admission remain underexplored and principally focus on identification of admission risk factors and assessment scores. Comparatively fewer investigations assess implementation and evaluation of SICU triage protocols or approaches parsed by setting and patient population. The broad range of knowledge gaps uncovered in this scoping review offer multiple avenues for local, medical professional organization, as well as national and international knowledge discovery collaboration.
Supplementary Material
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Veterans Health Administration, the Department of Defense, or the federal government of the United States.
Dr. Abbott was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number T32 GM008721. Dr. Kaplan is the coprincipal investigator of a Veterans Affairs Cooperative Studies Program investigation under award number CSP 2040, funded by the Biomedical Advanced Research and Development Agency (solely provides project funding without salary support); he is a past president of the Society of Critical Care Medicine (2020–2021); he serves as an associate editor for Critical Care Explorations; and he sits on the editorial boards of Critical Care Medicine, Injury, Orthoplastic Surgery, and Surgical Infections. Dr. Loftus was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers K23 GM140268 and R01 GM149657. Drs. Loftus and Efron sit on the editorial board of Critical Care Explorations. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (https://journals.lww.com/ccejournal).
Contributor Information
Philip Hong, Email: philip.hong@surgery.ufl.edu.
Matthew M. Ruppert, Email: matthew.ruppert@surgery.ufl.edu.
Purvi P. Patel, Email: purvi.p.patel@gmail.com.
Philip A. Efron, Email: philip.efron@surgery.ufl.edu.
Natasha Keric, Email: Natasha.Keric@bannerhealth.com.
Lewis J. Kaplan, Email: Lewis.Kaplan@pennmedicine.upenn.edu.
Niels D. Martin, Email: Niels.Martin@pennmedicine.upenn.edu.
Tyler J. Loftus, Email: tyler.loftus@surgery.ufl.edu.
REFERENCES
- 1.Weiser TG, Haynes AB, Molina G, et al. : Estimate of the global volume of surgery in 2012: An assessment supporting improved health outcomes. Lancet 2015; 385:S11. [DOI] [PubMed] [Google Scholar]
- 2.Loftus TJ, Ruppert MM, Shickel B, et al. : Overtriage, undertriage, and value of care after major surgery: An automated, explainable deep learning-enabled classification system. J Am Coll Surg 2023; 236:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nates JL, Nunnally M, Kleinpell R, et al. : ICU admission, discharge, and triage guidelines: A framework to enhance clinical operations, development of institutional policies, and further research. Crit Care Med 2016; 44:1553–1602 [DOI] [PubMed] [Google Scholar]
- 4.Halpern NA, Tan KS, DeWitt M, et al. : Intensivists in U.S. acute care hospitals. Crit Care Med 2019; 47:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershengorn HB, Garland A, Costa DK, et al. : ICU staffing in the United States. Chest 2024; 166:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covidence Systematic Review Software: Veritas Health Innovation. 2024. Available at: www.covidence.org. Accessed April 19, 2024 [Google Scholar]
- 7.Iapichino G, Corbella D, Minelli C, et al. : Reasons for refusal of admission to intensive care and impact on mortality. Intensive Care Med 2010; 36:1772–1779 [DOI] [PubMed] [Google Scholar]
- 8.Garrouste-Orgeas M, Montuclard L, Timsit JF, et al. : Triaging patients to the ICU: A pilot study of factors influencing admission decisions and patient outcomes. Intensive Care Med 2003; 29:774–781 [DOI] [PubMed] [Google Scholar]
- 9.Hampers MJ, Surgenor SD, Spanjian K, et al. : ICU care for patients with gastrointestinal bleeding: Impact on cost and outcome. Clin Intensive Care 2002; 13:109–113 [Google Scholar]
- 10.Bardes JM, Turner J, Bonasso P, et al. : Delineation of criteria for admission to step down in the mild traumatic brain injury patient. Am Surg 2016; 82:36–40 [PMC free article] [PubMed] [Google Scholar]
- 11.Franko LR, Hollon T, Linzey J, et al. : Clinical factors associated with ICU-specific care following supratentoral brain tumor resection and validation of a risk prediction score. Crit Care Med 2018; 46:1302–1308 [DOI] [PubMed] [Google Scholar]
- 12.Sun MZ, Babayan D, Chen JS, et al. : Postoperative admission of adult craniotomy patients to the neuroscience ward reduces length of stay and cost. Neurosurgery 2021; 89:85–93 [DOI] [PubMed] [Google Scholar]
- 13.Hatipoglu Majernik G, Wolff Fernandes F, Al-Afif S, et al. : Routine postoperative admission to the neurocritical intensive care unit after microvascular decompression: Necessary or can it be abandoned? Neurosurg Rev 2023; 46:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shanker J, Ghorpode A, Upasani CB: Triage for surgical ICU: Anesthesiologist and intensivist as gatekeepers to ICU. Indian J Crit Care Med 2006; 10:167–170 [Google Scholar]
- 15.Cavaliere F, Conti G, Costa R, et al. : Intensive care after elective surgery: A survey on 30-day postoperative mortality and morbidity. Minerva Anestesiol 2008; 74:459–468 [PubMed] [Google Scholar]
- 16.Shum HP, Chan KC, Lau CW, et al. : Triage decisions and outcomes for patients with triage priority 3 on the Society of Critical Care Medicine scale. Crit Care Resusc 2010; 12:42–49 [PubMed] [Google Scholar]
- 17.Bui JQH, Mendis RL, Van Gelder JM, et al. : Is postoperative intensive care unit admission a prerequisite for elective craniotomy? J Neurosurg 2011; 115:1236–1241 [DOI] [PubMed] [Google Scholar]
- 18.Bjørn M, Simonsen JW, Mogensen CB: A combination of clinical parameters and blood-gas analysis identifies patients at risk of transfer to intensive care upon arrival to the emergency department. Eur J Emerg Med 2016; 23:305–310 [DOI] [PubMed] [Google Scholar]
- 19.Kamath AF, McAuliffe CL, Baldwin KD, et al. : Unplanned admission to the intensive care unit after total hip arthroplasty. J Arthroplasty 2012; 27:1027–1032 [DOI] [PubMed] [Google Scholar]
- 20.Lee CC, Gandotra S, Lahey ET, et al. : Is intensive care unit monitoring necessary after maxillomandibular advancement for management of obstructive sleep apnea? J Oral Maxillofac Surg 2022; 80:456–464 [DOI] [PubMed] [Google Scholar]
- 21.Shamim F, Asghar A, Karam K: Frequency of intensive care unit admission after elective interventional neuroradiological procedures under general anesthesia in a tertiary care hospital. Saudi J Anaesth 2015; 9:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pecorelli N, Turi S, Salvioni MT, et al. : Development of a predictive model for unplanned intensive care unit admission after pancreatic resection within an enhanced recovery pathway. Surg Endosc 2023; 37:2932–2942 [DOI] [PubMed] [Google Scholar]
- 23.Møller AM, Pedersen T, Villebro N, et al. : A study of the impact of long-term tobacco smoking on postoperative intensive care admission. Anaesthesia 2003; 58:55–59 [DOI] [PubMed] [Google Scholar]
- 24.Han TY, Cheng T, Liu BF, et al. : Evaluation of the prognostic value of red cell distribution width to total serum calcium ratio in patients with acute pancreatitis. Gastroenterol Res Pract 2021; 2021:6699421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruskin R, Urban RR, Sherman AE, et al. : Predictors of intensive care unit utilization in gynecologic oncology surgery. Int J Gynecol Cancer 2011; 21:1336–1342 [DOI] [PubMed] [Google Scholar]
- 26.Silva JM, Rocha HMC, Katayama HT, et al. : SAPS 3 score as a predictive factor for postoperative referral to intensive care unit. Ann Intensive Care 2016; 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprung CL, Geber D, Eidelman LA, et al. : Evaluation of triage decisions for intensive care admission. Crit Care Med 1999; 27:1073–1079 [DOI] [PubMed] [Google Scholar]
- 28.Koltka AK, Dinçer MB, Güzel M, et al. : Integration of functional capacity to medically necessary, time-sensitive scoring system: A prospective observational study. Saudi Med J 2023; 44:921–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagener G, Minhaz M, Wang S, et al. : The Surgical Procedure Assessment (SPA) score predicts intensive care unit length of stay after cardiac surgery. J Thorac Cardiovasc Surg 2011; 142:443–450 [DOI] [PubMed] [Google Scholar]
- 30.Bartkowiak B, Snyder AM, Benjamin A, et al. : Validating the electronic Cardiac Arrest Risk Triage (eCART) score for risk stratification of surgical inpatients in the postoperative setting: Retrospective cohort study. Ann Surg 2019; 269:1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kongkaewpaisan N, Lee JM, Eid AI, et al. : Can the emergency surgery score (ESS) be used as a triage tool predicting the postoperative need for an ICU admission? Am J Surg 2019; 217:24–28 [DOI] [PubMed] [Google Scholar]
- 32.Gebran A, Vapsi A, Maurer LR, et al. : POTTER-ICU: An artificial intelligence smartphone-accessible tool to predict the need for intensive care after emergency surgery. Surgery 2022; 172:470–475 [DOI] [PubMed] [Google Scholar]
- 33.Jeon A, Nagappan L: Postoperative complications and disposition for vascular surgery. Anaesth Intensive Care 2023; 51:193–198 [DOI] [PubMed] [Google Scholar]
- 34.Wang D, Carrano FM, Fisichella PM, et al. : A quest for optimization of postoperative triage after major surgery. J Laparoendosc Adv Surg Tech A 2019; 29:203–205 [DOI] [PubMed] [Google Scholar]
- 35.Schuster ST, Bondarsky E, Hardwick CJ, et al. : Safety of a novel obstructive sleep apnea triage tool for postoperative orthopedic surgery patients. J Perianesth Nurs 2022; 37:174–183 [DOI] [PubMed] [Google Scholar]
- 36.Rocke D, Sharp S, Wiener D, et al. : Effectiveness of a postoperative disposition protocol for sleep apnea surgery. Am J Otolaryngol 2013; 34:273–277 [DOI] [PubMed] [Google Scholar]
- 37.Melissano G, Castellano R, Mazzitelli S, et al. : Safe and cost-effective approach to carotid surgery. Eur J Vasc Endovasc Surg 1997; 14:164–169 [DOI] [PubMed] [Google Scholar]
- 38.Sakawi Y, Groudine S, Roberts K, et al. : Carotid endarterectomy surgery and ICU admissions: A regional anesthesia perspective. J Neurosurg Anesthesiol 1998; 10:211–217 [DOI] [PubMed] [Google Scholar]
- 39.Alshaikh HN, Hicks CW, DiBrito SR, et al. : Elective infrainguinal lower extremity bypass for claudication is associated with high postoperative intensive care utilization. J Vasc Surg 2019; 69:1863–1873.e1 [DOI] [PubMed] [Google Scholar]
- 40.Cunningham KE, Zenati MS, Petrie JR, et al. : A policy of omitting an intensive care unit stay after robotic pancreaticoduodenectomy is safe and cost-effective. J Surg Res 2016; 204:8–14 [DOI] [PubMed] [Google Scholar]
- 41.Mandell MS, Lezotte D, Kam I, et al. : Reduced use of intensive care after liver transplantation: Influence of early extubation. Liver Transpl 2002; 8:676–681 [DOI] [PubMed] [Google Scholar]
- 42.van Stein RM, Aronson SL, Sikorska K, et al. : Is routine admission to a critical care setting following hyperthermic intraperitoneal chemotherapy for ovarian cancer necessary? Eur J Surg Oncol 2023; 49:107084. [DOI] [PubMed] [Google Scholar]
- 43.Florman JE, Cushing D, Keller LA, et al. : A protocol for postoperative admission of elective craniotomy patients to a non-ICU or step-down setting. J Neurosurg 2017; 127:1392–1397 [DOI] [PubMed] [Google Scholar]
- 44.Haanschoten MC, Van Straten AHM, Ter Woorst JF, et al. : Fast-track practice in cardiac surgery: Results and predictors of outcome. Interact Cardiovasc Thorac Surg 2012; 15:989–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamath AF, Gutsche JT, Kornfield ZN, et al. : Prospective study of unplanned admission to the intensive care unit after total hip arthroplasty. J Arthroplasty 2013; 28:1345–1348 [DOI] [PubMed] [Google Scholar]
- 46.Alexis SL, Draper PN, Harris D, et al. : Improving bed utilization in a cohort of bariatric surgical patients using a perioperative obstructive sleep apnea treatment and bed triage protocol. Obes Surg 2022; 32:1926–1934 [DOI] [PubMed] [Google Scholar]
- 47.Thevathasan T, Copeland CC, Long DR, et al. : The impact of postoperative intensive care unit admission on postoperative hospital length of stay and costs: A prespecified propensity-matched cohort study. Anesth Analg 2019; 129:753–761 [DOI] [PubMed] [Google Scholar]
- 48.Loftus TJ, Ruppert MM, Ozrazgat-Baslanti T, et al. : Postoperative overtriage to an intensive care unit is associated with low value of care. Ann Surg 2023; 277:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon K, Allorto N, Wise R: Analysis of referrals and triage patterns in a South African metropolitan adult intensive care service. S Afr Med J 2015; 105:491–495 [DOI] [PubMed] [Google Scholar]
- 50.Cohen ME, Liu Y, Ko CY, et al. : Improved surgical outcomes for ACS NSQIP hospitals over time: Evaluation of hospital cohorts with up to 8 years of participation. Ann Surg 2016; 263:267–273 [DOI] [PubMed] [Google Scholar]
- 51.Khuri SF, Daley J, Henderson W, et al. : The Department of Veterans Affairs’ NSQIP: The first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998; 228:491–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shafi S, Nathens AB, Cryer HG, et al. : The Trauma Quality Improvement Program of the American College of Surgeons Committee on Trauma. J Am Coll Surg 2009; 209:521–530.e1 [DOI] [PubMed] [Google Scholar]
- 53.Brogan TV, Lequier L, Lorusso R, et al. : Extracorporeal Life Support: The ELSO Red Book. Fifth Edition. Ann Arbor, MI, Extracorporeal Life Support Organization, 2017 [Google Scholar]
- 54.Droogh JM, Smit M, Absalom AR, et al. : Transferring the critically ill patient: Are we there yet? Crit Care 2015; 19:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loftus TJ, Wu Q, Wang Z, et al. : Delayed interhospital transfer of critically ill patients with surgical sepsis. J Trauma Acute Care Surg 2020; 88:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilcox SR, Wax RS, Meyer MT, et al. : Interfacility transport of critically ill patients. Crit Care Med 2022; 50:1461–1476 [DOI] [PubMed] [Google Scholar]
- 57.Sprung CL, Danis M, Iapichino G, et al. : Triage of intensive care patients: Identifying agreement and controversy. Intensive Care Med 2013; 39:1916–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Q, Du JL, Shao F: Mortality rate and other clinical features observed in open vs closed format intensive care units: A systematic review and meta-analysis. Medicine (Baltimore) 2019; 98:e16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Multz AS, Chalfin DB, Samson IM, et al. : A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU. Am J Respir Crit Care Med 1998; 157(5 Pt 1):1468–1473 [DOI] [PubMed] [Google Scholar]
- 60.Nelson JB, Jr: The role of an intensive care unit in a community hospital. A ten-year review with observations on utilization past, present, and future. Arch Surg 1985; 120:1233–1236 [DOI] [PubMed] [Google Scholar]
- 61.Singh PP, Zeng IS, Srinivasa S, et al. : Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 2014; 101:339–346 [DOI] [PubMed] [Google Scholar]
- 62.Patel B, Mumby S, Johnson N, et al. ; DeVENT Study Group: Decision support system to evaluate ventilation in the acute respiratory distress syndrome (DeVENT study)-trial protocol. Trials 2022; 23:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin FF, Peet J, Murray L, et al. : Who gets the bed: Factors influencing the intensive care exit block: A qualitative study. Int J Nurs Stud 2025; 161:104949. [DOI] [PubMed] [Google Scholar]
- 64.Hamel MB, Drazen JM, Epstein AM: The growth of hospitalists and the changing face of primary care. N Engl J Med 2009; 360:1141–1143 [DOI] [PubMed] [Google Scholar]
- 65.McKenzie MS, Auriemma CL, Olenik J, et al. : An observational study of decision making by medical intensivists. Crit Care Med 2015; 43:1660–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiest KM, Krewulak KD, Plotnikoff KM, et al. : Allocation of intensive care resources during an infectious disease outbreak: A rapid review to inform practice. BMC Med 2020; 18:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar MV, Cheater L, Raw D, et al. : Overflow critical care facility in a teaching hospital: How often do we use it? Crit Care 2008; 12(Suppl 2):P529 [Google Scholar]
- 68.Kapoor R, Gupta N, Roberts SD, et al. : Impact of geographical cohorting in the ICU: An academic tertiary care center experience. Crit Care Explor 2020; 2:e0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiu WC, Marcolini EG, Simmons DE, et al. : Training dedicated emergency physicians in surgical critical care: Knowledge acquisition and workforce collaboration for the care of critically ill trauma/surgical patients. J Trauma 2011; 71:43–48 [DOI] [PubMed] [Google Scholar]
- 70.Pastores SM, Kostelecky N, Halpern NA: Graduates of a multidisciplinary critical care training program from 2000 to 2020: Looking at their first job. ATS Sch 2023; 4:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bion J, Rothen HU: Models for intensive care training. A European perspective. Am J Respir Crit Care Med 2014; 189:256–262 [DOI] [PubMed] [Google Scholar]