Abstract
Multiple strategies involving the parasite-host-vector triad are necessary to control leishmaniasis. One option is to prevent or reduce transmission of the pathogen by the phlebotomine sand fly vectors. In this sense, it is essential to explore compounds that may influence the vectorial capacity of the insect and reduce its longevity. We investigated the effect of anti-Leishmania drugs administered via the sugar meal on longevity, blood feeding, oviposition, and parasite load on the third day of infection of the sand flies, to identify the most promising candidates for vector infection tests. We identified compounds that affected the longevity of sand flies (three pterocarpanquinones – LQB-475, LQB-181, and LQ-03; one hydroxyethylpiperazine, PMIC-4, and Pentamidine), reduced oviposition of females after blood feeding (LQB-181 and PMIC-4), but did not decrease infection rates or parasite loads. The results provide the effect of antiparasitic drugs from the perspective of the insect vector.
Introduction
Leishmaniasis is a group of diseases induced by protists of the genus Leishmania, transmitted via the bite of female phlebotomine sand flies [1–3]. It is listed as one of the top ten neglected tropical diseases, with more than 12 million people currently infected (0.9 to 1.6 million new cases yearly) and between 20,000 and 30,000 deaths per year [1,4]. This group of diseases is widely distributed in tropical and subtropical areas of the world [5].
Leishmania species are transmitted through the bite of female phlebotomines of the genera Phlebotomus in the Old World and Lutzomyia in the New World [4,6–8]. Lu. longipalpis is one of the main species of medical importance in Brazil for being the primary vector in VL and for its adaptive capacity in varied anthropic environments and permissiveness for different species of Leishmania, besides its typical, Le. infantum [8–12].
The Leishmania parasite has a digenetic life cycle, existing in two distinct forms: the amastigote form, which is rounded and intracellular, multiplying inside mammalian cells, particularly macrophages, and the promastigote, an extracellular, flagellated form found in the gut of sandflies [3,6,13,14].
Adult sand flies primarily feed on plant-derived sugars or aphid secretions, which serve as their primary sources of carbohydrates to meet their energetic requirements [8,10,15,16]. Females are also hematophagous and require blood to acquire the nutrients necessary for the development and maturation of their eggs. If the blood they ingest is infected, the parasites can be transmitted to another host during a subsequent blood meal [6,7,17,18]. The development of the parasite within the insect gut is crucial for transmission, as it undergoes developmental changes until reaching the infective metacyclic promastigote form. During this stage, the parasite remains in direct contact with the blood and sugar meals ingested by the insect.
Given the importance of the carbohydrate diet for sand flies, some groups have been investigating disease control strategies by using attractive sugar baits containing insecticidal components for controlling the insect vector [17,19–26]. The use of sugar-based toxic baits can be a valuable tool for delivering insecticidal compounds or antiparasitic agents inhibiting the parasite’s development within the insect vector’s midgut.
Recently, our group identified that secondary plant metabolites, added to the sugar solution, could reduce L. mexicana development in Lu. longipalpis [27]. The investigation of natural compounds impacting the development of Leishmania is a concept developed by Schlein and Jacobson [17,28,29]. By employing this innovative approach of delivering anti-Leishmania compounds through toxic sugar baits, this research has the potential to offer a more targeted strategy than conventional methods that use spraying of chemical insecticides, contributing to the ongoing efforts in combating this disease.
Overall, this research aims to identify compounds that could effectively control the spread of leishmaniasis by targeting the insect vector and disrupting parasite development inside the insect by using a new strategy: delivering the compounds through toxic sugar baits. For this, we selected molecules that had an effect on promastigote forms of Leishmania sp. in vitro: two pterocarpanquinones (LQB-475, LQB-181), one furanonapthoquinone (LQ-03); one hydroxyethylpiperazine (PMIC-4), Pentamidine (Pent), and Amphotericin B (Amp). These compounds were used to evaluate the following aspects: the effects on adult longevity, influence on blood feeding (oviposition and quantity ingested), and the effect on the infection of Lu. longipalpis with Le. amazonensis. We designed this study as a proof-of-concept work for this series of compounds, choosing to target initially Le. amazonensis because it was used in previous studies and develops well in Lu. longipalpis, being a model already explored for the study of parasite-sand fly interactions [30]. Besides that, this parasite-sand fly association was already observed in the field, making Lu. longipalpis as a candidate for the transmission of cutaneous Leishmaniasis in Brazil [31].
Antimonials have been the main drugs used in the treatment of patients with leishmaniasis for over a hundred years. The second line option is pentamidine, amphotericin B, miltefosine, or azithromycin [2,3,7,12]. However, these drugs have limitations such as toxicity or adverse effects. Another concern is the emergence of treatment-resistant strains of Leishmania [2,3,7,12,32,33].
In this context, many groups have been studying natural or synthetic compounds of analogous formulation to expand the arsenal of drugs available for leishmaniasis treatment. For example, chalcones, flavonoids, quinones, alkaloids, and terpenoids, which are phytoconstituents with anti-Leishmania activity and with the advantage of being environmentally acceptable [33–35]. Some compounds are also evaluated on the promastigote form of the parasite. Pterocarpanoquinones can be highlighted as synthetic compounds analogous to natural ones, which have shown promising results in Leishmania promastigotes and amastigotes [36–42].
The development of toxic sugar baits provides an opportunity to test the antiparasitic effect of these compounds within the insect vector. In this study, we evaluated the influence of different synthetic compounds with anti-Leishmania activity, including positive controls, amphotericin B and pentamidine, in different aspects of Lu. longipalpis physiology when added to the sugar diet. In previous publications, we found that these drugs affect the behavior of the vector insect when added to the sugar solution [27].
Materials and methods
Anti-Leishmania compounds
The drugs tested in this study were mostly modeled and synthesized at the Laboratory of Bioorganic Chemistry of the Nucleus for Research on Natural Products at the Federal University of Rio de Janeiro, Brazil [42]. They are synthetic compounds similar to those of natural origin with anti-promastigote action, mainly produced from naphthoquinones and pterocarpanes [37,41,42]. Pentamidine and amphotericin B, generally used in the treatment of leishmaniasis, were used as reference compounds. These compounds were received in solution in a stock concentration of 10 mM using dimethyl sulfoxide (DMSO) as solvent. Table 1 provides information on the compounds evaluated.
Table 1. Properties and experimental conditions of the anti-Leishmania compounds used in this work. The IC50 values represent half of the maximum inhibitory concentration for promastigotes of Le. infantum or Le. amazonensis. The concentration used in the experiments with LQB-475 and Pent was 10 times the value of the higher IC50observed for both Leishmania species. For LQB-181, PMIC-4, and Amp the Le. amazonensis IC50 was used, and for LQ-3 the Le. infantum IC50 was used. N.D.: Not determined. Structures of compounds are presented in Supplementary Table 1.
| Compound | Class | IC50 Le. infantum/Le. amazonensis (μ mol L − 1) | Concentration (μ mol L −1) | Reference |
|---|---|---|---|---|
| LQB-475 | Pterocarpan-quinone | 1.4/ 0.4 | 14.0 | [41] |
| LQB-181 | Pterocarpan-quinone | N.D./ 1.98 | 20.0 | [43] |
| LQ-03 | Pterocarpan-quinone | 1.7/ N.D. | 17.0 | [44]; this work |
| PMIC-4 | Hydroxyethyl-piperazine | N.D./ 1.8 | 18.0 | [45,46] |
| Pentamidine (Pent) | Aromatic-diamidine | 5.7/ 4.8 | 57.0 | [41] |
| Amphotericin B (Amp) | Polyene | N.D./ 2.2 | 22.0 | This work |
| DMSO | Solvent | N.D./ N.D. | 0.57% | – |
For anti-Leishmania activity, promastigotes of Leishmania amazonensis were adjusted to a concentration of 1 × 106 cells/mL in Schneider medium supplemented with 10% of fetal bovine serum and incubated at 26 °C for 72 h with Amphotericin B or LQ-03 (0–10 µM). The antileishmanial activity was evaluated by adding in each well 22 μL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] at 5 mg/mL (Sigma®). After 2 h, 80 μL of DMSO was added into each well to solubilize the formazan crystals. The optical density was determined at a wavelength of 570 nm in microplate reader (µQuant Bio-Tek Instruments®, Winooski, state, USA). Logarithm regression analysis was performed using GraphPad Prism 5.0 (San Diego, CA, USA) to obtain the values of IC50.
Preparation of sugar solution containing compounds
The sugar solution used in all experiments was prepared with commercial sucrose (Sigma) in a concentration of 70% (w/v), sterilized in an autoclave, and refrigerated at 4ºC. Compounds LQB-475, LQB-181, LQ-03, PMIC-4, Pentamidine (Pent), Amphotericin B (Amp) (10 mM stock solutions in DMSO) were added to the sucrose 70% (w/v) solution to achieve a final concentration corresponding to 10x the value of the selected IC50 for Leishmania sp. (see Table 1 for details). For the experiments, two control groups were used: Control 1 was a sucrose 70% (w/v) solution, and Control 2 (referred to as DMSO) was a solution of DMSO 5.7% (v/v) in the sucrose 70% (w/v) solution. In all experiments, the sucrose solution was offered to the insects in 20 drops of 5 µL each, on top of a petri dish covered with parafilm [47]. See Supplementary Table 1 for details.
Effect of compounds on the longevity of sand flies
Insects used in all experiments were from the colony of Lu. longipalpis of the Laboratory of Insect Biochemistry and Physiology of the Oswaldo Cruz Institute, established from insects collected in Jacobina (Bahia state, Brazil). The colony was kept according to Refs 16 and 27.
Experiments were performed with 20 newly emerged adults per cage (males or females, separated). For each cage, either the sucrose solution containing the compounds, or only sucrose 70% (w/v) (control 1), or 5.7% (v/v) DMSO in sucrose solution (control 2) was offered to the insects as described above [47]. The sugar solution was changed every two days, and the stocks were kept at −20 ºC. The mortality was observed daily. Insects were kept in an incubator under controlled conditions (26 ºC and 70% humidity), according to previously described studies [27,48]. In accordance with our previous reports [47], all the sand flies fed on the sugar solutions in these conditions. The experiments were carried out in triplicate.
Effects of compounds on blood feeding and oviposition
Blood feeding.
Cages (n = 7) containing about 30 females and 30 males, 1–2 days after emergence, were used in this experiment. As described above, the sucrose solution containing compounds, or the control (sucrose only or DMSO) was offered to the insects in each cage. The solutions were changed every two days, and the stocks were kept in a freezer (−20 ºC). After seven days, the females were offered an anesthetized hamster for 30 minutes (License CEUA/IOC – L-029/2016). After the feeding, the engorged females were counted, and these insects were separated and dissected on the same day to observe the gut and quantify the ingested blood. The quantification was performed through protein quantification using the bicinchoninic acid method (PierceTM et al. - Thermo Fisher Scientific) [49], following the instructions provided by the manufacturer for microplate procedures, using bovine serum albumin as a standard [50]. The amount of protein originated from insect tissues was considered to be a minor component of the sample, and total protein measurements were assumed to reflect the nutritional intake of each insect.
Fecundity and Fertility.
The female mortality was evaluated before blood feeding (7 days of contact with compounds) and three days after post-blood feeding. Around two hours after feeding, blood-fed females were separated into small plastic containers containing plaster in the bottom to maintain humidity. These containers create a favorable environment for the females to lay eggs. Aspects related to oviposition were evaluated, including the number of eggs, fecundity (eggs/female), and fertility (whether the eggs hatched or not), which were monitored up to 14 days after blood feeding. The number of eggs laid was quantified by visually counting the eggs in a photo taken in a stereoscope (Carl Zeiss – Stemi DV4, Werk Gottingen, Germany). Editing tools such as Photoscape (v3.7), the ImageJ program (1.53e), and image enlargement (zoom) were utilized. In ImageJ, the same image underwent an editing process involving 8-bit conversion, with threshold adjustment, black and white scale, selection of the area of interest, and particle analysis, as recommended by the program. The visual counting and ImageJ quantification data were used to calculate an average with the standard deviation of the average (SEM). No eggs were observed on the sides or in the top of the oviposition pots.
For compound LBQ-181, it was necessary to increase the initial number of females per replicate from 30 (replicate 1) to an average of 50 (replicates 2–5). Controls (sucrose and DMSO) and PMIC-4 had an average of 30 females (5 replicates). For the other compounds, 3 replicates with 30 insects each were performed.
Effect of compounds on Lu. longipalpis infection with Leishmania
Animal handling.
Mice aged 12–16 weeks were infected with Le. amazonensis (JOSEFA strain) by direct injection containing 2 × 106 parasites mL-1. The infection of the insects was done 70 days after mouse infection when the paws contained a high parasite load. The infection process with Le. amazonensis simulates a natural infection, where female sand flies fed directly from the infected foot of Balb/c mice. To facilitate blood feeding, Balb/c mice were anesthetized with xylazine (10 mg/kg) plus ketamine (100 mg/kg) before exposure to the sand flies (Ethic Committee License Numbers L-029/2016 CEUA-IOC, and 080−18 UFRJ).
Sand fly infections.
In this experiment, only the most promising compounds, with significant results in the previous test, were used to evaluate the effect on Lu. longipalpis infection with Le. amazonensis. The sucrose solution containing compounds LQB-475, LQ-03, Pent, and DMSO were used. Another control group was included, with insects feeding only on sucrose 70% (w/v) before and after blood feeding.
For infections, about 200 females 1–2 days after emergence were separated into fabric cages. Insects were fed for two days on a control diet (sucrose 70% (w/v)) or a test diet (sucrose plus compound or DMSO) before the infective feeding. Mice were placed outside the top part of the cage, standing over a plastic cover to prevent insects from biting other portions of the mice. The insects accessed the infected foot through a hole in the top of the cage. Blood feeding was conducted for 75 min, and two mice were used for each cage. After feeding, the mice were removed from the top of the cage and euthanized. This experiment was repeated two to three times.
Leishmania countings.
After blood feeding, only the engorged females were kept in the cage and maintained on the same sugar diet as previously established (control – sucrose or test – sucrose plus compound or DMSO), according to described above. For analyzing the infection, 10–15 samples were collected on the 3rd, 5th, and 7th days after blood feeding in each repetition, corresponding to the different stages of evolution of the Le. amazonensis infection in Lu. longipalpis. Each gut was dissected and gently homogenized in 10 μL of PBS, and quantification was performed with the aid of a hemocytometer.
Statistical analysis
Data was analyzed using GraphPad software Prism (version 6) for Windows. The D’Agostino and Pearson test was used to verify the normal distribution of the data. Data with normal distribution were submitted to the unpaired t-test or Analysis of Variance (One-way ANOVA) with Tukey’s multiple comparison post-test. Non-normally distributed data were analyzed trough the nonparametric Mann-Whitney or Kruskal-Wallis test with Dunn’s multiple comparison post-test. For survival, results were analyzed using the Kaplan-Meier survival curve, and the median survival time was determined in each condition. The log-rank Mantel-Cox test was used to compare survival curves, and Dunn’s multiple comparison post-test was used to compare tree survival replicates’ mean and median values. All comparisons with p-value < 0.05 were considered statistically significant.
Results
Effect of compounds on the longevity of sand flies
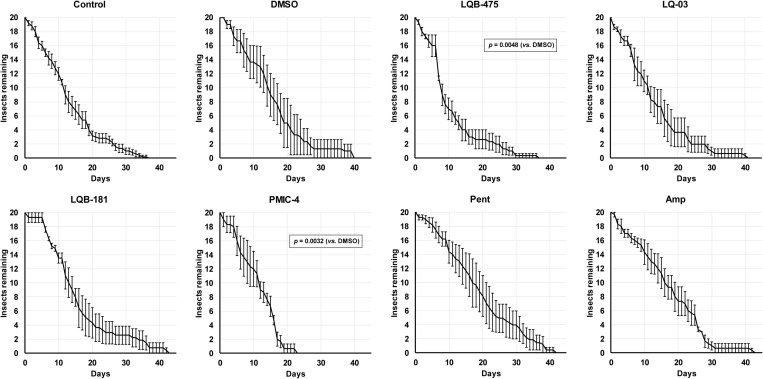
We analyzed the impact of the anti-Leishmania compounds on the longevity of both male and female sand flies using sucrose diets. Because the stock solutions of the compounds were prepared in DMSO, we included a control group fed on sucrose with DMSO, and all the statistical comparisons were performed against this group. However, because we were also concerned about the physiological impact of DMSO, we also performed a comparison of insects fed with DMSO against insects fed with sucrose only. Importantly, addition of DMSO has a significant impact in the longevity of males when compared to insects fed only with sucrose (Fig. 1, p = 0.034, Log-rank test). The longevity of insects fed with the LQB-181 compound was significantly shorter than the longevity of insects fed with sucrose plus DMSO (Log-rank test, p < 0.0001). Compounds LQ-03 (Log-rank test, p < 0.001), LQB-475 (Log-rank test, p < 0.05), and Pent (Log-rank test, p < 0.05; Fig 1) also resulted in longevities significantly different to the longevity of insects fed with sucrose plus DMSO. The LQB-181 compound shortened the average lifespan (ALS) to 10 ± 1 days, while DMSO led to an ALS of 15 ± 1 days. An opposite effect was observed in ALS of males fed on Pent, LQB-475, and LQ-03, which increased the ALS respectively to 17 ± 1, 18 ± 1, and 19 ± 2 days (Table 2).
Fig 1. Effect of anti-Leishmania drugs added to a sugar diet on the longevity of Lu. longipalpis males.
Insects were kept under controlled conditions (26 ºC and 70% humidity), and mortality was checked daily. In the control group, insects were fed with a solution containing only sucrose 70% (w/v). All results are the mean ± SEM. Comparison test between log-rank survival curves (Mantel-Cox). Experiments were performed at least three times independently, with n = 20 each. See Material and Methods and Table 2 for details.
Table 2. Median and average lifespans (ALS) of Lu. longipalpis feeding on sugar baits containing anti-Leishmania compounds. Male and female controls were fed only the sucrose diet (Ctrl). These values were obtained by survival analyses and p values were calculated by Log-rank survival curves (Mantel-Cox) comparative test for males: Ctrl/DMSO – p = 0.034, LQB-475/DMSO = 0.0133, LQ-03/DMSO = 0.0007, LQB-181/DMSO < 0.0001, and Pent/DMSO = 0.0499, and females: LQB-475/DMSO = 0.0048, and PMIC-4/DMSO = 0.0032. ALS was calculated considering the lifespan of all individuals.
| males | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ctrl | DMSO | LQB-475 | LQ-03 | LQB-181 | PMIC-4 | Pent | Amp | |
| median | 17 | 15 | 19 | 20 | 10 | 13 | 18 | 15 |
| ALS ± SEM | 17 ± 1 | 15 ± 1* | 18 ± 1* | 19 ± 2*** | 10 ± 1**** | 13 ± 1 | 17 ± 1* | 15 ± 1 |
| n | 120 | 80 | 60 | 60 | 60 | 60 | 60 | 60 |
| FEMALES | ||||||||
| Ctrl | DMSO | LQB-475 | LQ-03 | LQB-181 | PMIC-4 | Pent | Amp | |
| median | 12 | 15 | 8 | 12 | 13 | 12 | 19 | 17 |
| ALS ± SEM | 14 ± 1 | 16 ± 1 | 11 ± 1** | 13 ± 1 | 16 ± 1 | 11 ± 1** | 19 ± 1 | 17 ± 1 |
| n | 100 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
Interestingly, adding DMSO to the sugar did not affect significantly the longevity of females (Fig 2, p > 0.05, Log-rank test). The longevities of females fed on LQB-475 (Log-rank, p < 0.01), and PMIC-4 (Log-rank, p < 0.01) (Fig 2), were significantly lower to the longevity of females feeding on sucrose containing DMSO. We detected a reduction in ALS when comparing LQB-475 and PMIC-4 groups vs. the DMSO group, from 16 ± 1 days to 11 ± 1 (Table 2).
Fig 2. Effect of anti-Leishmania drugs added to a sugar diet on the longevity of Lu. longipalpis females.
Insects were kept under controlled conditions (26 ºC and 70% humidity), and mortality was checked daily. In the control group insects were fed with sucrose 70% (w/v). All results are the mean ± SEM. Comparison test between log-rank survival curves (Mantel-Cox). Experiments were performed at least three times independently, with n = 20 each. See Material and Methods and Table 2 for details.
Effects of anti-Leishmania compounds on blood feeding and oviposition
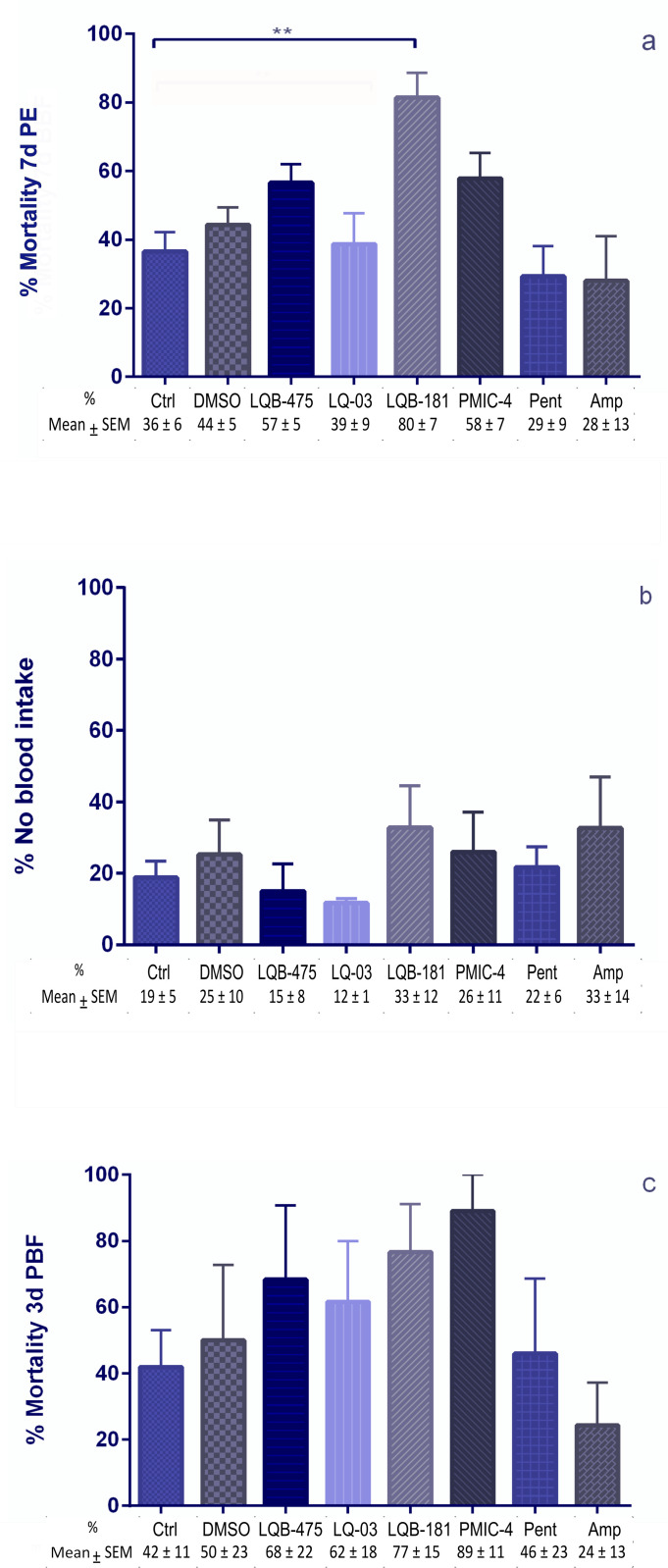
Besides evaluating the effect of compounds on the longevity of insects, we also analyzed how these compounds affect female blood feeding and their impact on other aspects like fecundity and fertility. Evaluating the effects of the drugs added to a sugar diet before a blood meal, we did not see significant differences in mortalities when comparing the group fed with sugar plus DMSO to the insects fed with sugar only. LQB-181 caused higher mortality in females and reduced the number of insects available for blood feeding when comparing to sugar-fed controls. However, there was no significant difference when comparing the insects treated with LBQ-181 to the insects exposed to sugar containing DMSO (Fig 3a). No statistical difference was detected in the number of engorged females (Fig 3b) or survival of females three days after blood feeding (Fig 3c) between any of the groups considered. Although there is a reduction tendency caused by LQB-181 and PMIC-4 in the female fecundity, no statistical differences were noticed between all groups tested (Fig 4a).
Fig 3. Effect of anti-Leishmania compounds added to a sugar diet on females of Lu. longipalpis pre and post-blood feeding.
(a) Mortality of females before blood feeding. These females were offered the anti-Leishmania compound in a sugar diet for seven days before blood feeding. 7d PE: 7 days post-emergence. (b) Percentage of females that are not engorged after blood feeding. Females were exposed to the source of blood for 30 min. (c) Mortality of females three days after blood feeding. Females continued to be offered anti-Leishmania compounds in the sugar diet after blood feeding. 3d PBF: 3 days post-blood feeding. All percentage results are the mean ± SEM. For LQB-181, 5 replicates were performed, 1 with 30 insects and 4 with 50 insects each. For Controls and PMIC-4, 5 replicates with 30 insects each, and for the remaining compounds, 3 replicates with 30 insects each were performed. Dunn’s multiple comparison test, ** p = 0.007.
Fig 4. Effect of anti-Leishmania compounds on fecundity of females and egg fertility.
The compounds were added to a sugar diet and offered to females seven days before blood feeding and three days after when females were separated for oviposition. (a) Fecundity (eggs laid per female). The number of eggs was accessed seven days after oviposition. (b) Fertility (whether the eggs hatched or not). The hatching was evaluated 14 days after oviposition. The percentage results consider the total number of eggs hatching or not in each replicate (three to four repetitions). Different letters beside the bars indicate groups that are statistically different from each other, according to Fisher’s exact test (p < 0.05). For DMSO and PMIC-4, 4 replicates with 30 insects were performed. For LQB-181, 4 replicates with 50 insects were performed. For the other compounds, 3 replicates with 30 insects each were performed.
We assessed the viability of the eggs, by observing the hatching and if these larvae were alive and active. For this aspect, we checked the hatching rate of the eggs in each group tested. The group fed with sugar plus DMSO had a hatching rate significantly lower than the group fed with sucrose only (Fisher’s exact test, p = 0.037). There was no significant difference between the treatments with LQB-181 and DMSO (Fisher’s exact test, p = 0.567), but the groups treated with PMIC-4 and DMSO showed significant differences to each other (Fisher’s exact test, p < 0.0001). Fig 4b illustrates the hatching percentages for each compound.
Five replicates were performed to test the effect of the LQB-181 compound. As previously demonstrated, the compound caused a high mortality of females after seven days of feeding (sucrose + compound) before blood feeding. In these experimental replicas, one did not have enough females for oviposition (these samples were directed to dissection and protein quantification), and the females laid no egg in the other two. Females laid eggs in the other two replicates, but only in one of the two experimental replicas did the eggs hatch days later. That means that 36% of the total number of females (4/11) separated for oviposition did not lay eggs.
For tests with compound PMIC-4, of the four experimental replicates performed in which we had females for oviposition, females did not lay eggs in one of the replicas, corresponding to 26% of total number of females (4/15). In the other three replicas, even with posture, the eggs hatched only in one of the replicas.
We investigated whether adding these compounds to the sugar diet before blood feeding could inhibit the amount of ingested blood. For this, we quantified the proteins in the gut content of engorged females. Adding DMSO to the sugar did not affect the amount of blood ingested when compared to insects previously fed with sugar only. None of the compounds resulted in significantly different amounts of blood ingested when compared to the insects fed with sugar plus DMSO. The average amount of protein ingested by the females was between 2.2 ± 0.2 and 4.0 ± 0.4 µg/µL. Fig 5 shows the result of these quantifications.
Fig 5. Quantification of ingested proteins by females after 30 minutes of blood feeding.
Females were previously fed with a sugar solution containing anti-Leishmania compounds for seven days. Proteins were quantified from the blood present in the intestinal contents of females immediately after blood feeding, using the BCA method. The percentage results are the mean ± SEM of three to five (LQB-181 and PMIC-4) biological replicates. Dunn’s multiple comparisons test, **p < 0.01. Each symbol (square, triangle, diamond or circle) shows the measurement from one individual insect.
Effect of compounds on Lu. longipalpis infection with Le. amazonensis
To determine the effect of compounds added to the sugar diet on the infection of Lu. longipalpis with Le. amazonensis, the compounds LQB-475, LQ-03, and Pent were selected. This was based on the in vitro effect of the compounds over promastigote forms and on the impact on insect physiology described in this study and the previous study published by our group [47]. Compounds LQB-181 and PMIC-4 were not used for this further evaluation. Compound LQB-181 affected the longevity of females before blood feeding (Fig 3a), which limits the number of females available for infective blood feed and the assessment of the course of infection. Compound PMIC-4 affected the longevity of males and females (Table 2; Figs 1 and 2) and has more pronounced effects related to oviposition.
Of the infections carried out with Le. amazonensis, we observed that insects fed with sugar plus DMSO had significantly lower parasite counts than the controls fed with sugar only, in the third-day post infection (PI) (p = 0.0108, Mann-Whitney test) (Fig 6). Interestingly, on this day insects fed with Pent showed higher parasite counts than the insects treated with DMSO (p = 0.0048, Mann-Whitney test, Fig. 6). However, the progression of infection was not affected by any of the compounds tested, since any differences between all groups were not observed on the fifth- or seventh-day PI (Fig 6).
Fig 6. Influence of anti-Leishmania compounds added to the sugar diet on the infection of Lu. longipalpis with Le. amazonensis.
Parasite counts were measured on the 3rd, 5th, and 7th day after infection. Significant differences were investigated by Dunn’s multiple comparisons test and Mann-Whitney test.
In terms of the percentage of infected (positive) females compared with the total number of insects dissected in the three days analyzed, we observed a trend similar to what was seen on parasite counts. On the third day PI, insects fed with sugar plus DMSO showed a significant lower prevalence of infection (46%) when compared to controls fed with sugar only (75%) (p = 0.0192, Fisher’s exact test, Table 3). Besides that, on the same day the insects treated with Pent showed a prevalence of infection (81%) significantly higher than the insects fed with sugar plus DMSO (46%) (p = 0.01, Fisher’s exact test, Table 3). On the 5th and 7th after infection, no significant differences were observed between any of the groups (Table 3). Table 4 summarizes all results obtained for the compounds described in this work.
Table 3. Effect of compounds added to the sugar diet on the prevalence of infection of Lu. Longipalpis with Le. amazonensis. Numbers and percentages of females infected with Le. amazonensis over the post-infection days.
| Ctrl (Suc.) | DMSO | LQB-475 | LQ-03 | Pent | |
|---|---|---|---|---|---|
| Ni/ Nt | 36/ 48 | 11/ 24 | 10/ 22 | 13/ 26 | 22/ 27 |
| 3 DPI | 75 | 46 | 48 | 50 | 81 |
| Ni/ Nt | 33/ 49 | 9/ 17 | 15/ 21 | 13/ 17 | 19/ 23 |
| 5 DPI | 67 | 53 | 71 | 76 | 83 ± 1% |
| Ni/ Nt | 32/ 51 | 7/ 8 | 10/ 20 | 23/ 32 | 23/ 33 |
| 7 DPI | 63 | 88* | 50 | 72 | 770 |
Abbreviations: Ni = number of infected females; Nt = total number of females analyzed; DPI = days post infection.
Table 4. Summary of the effects of antiparasitic drugs on the physiology of Lu. longipalpis. Summary of the data on longevity (males and females), mortality of females before blood feeding, egg hatching, blood intake, and infection with Le. amazonensis.
| DMSO | LQB-475 | LQB-181 | LQ-03 | PMIC-4 | Pent | Amp | |
|---|---|---|---|---|---|---|---|
| Longevity | ↓♂ | ↑♂ | ↓↓↓♂ | ↑♂ | ↑♂ | — | |
| — | ↓↓↓♀ | — | — | ↓↓↓♀ | — | ||
| Mortality before blood-feeding | — | — | — | — | — | — | — |
| Hatching of eggs | 81% | 100% | 72% | 100% | 41% | 100% | 100% |
| Blood intake | — | — | — | — | — | — | — |
| Parasitic load Leishmania amazonensis | ↓ (3 DPI) | — | n.d. | — | n.d. | ↑ (3DPI) | n.d. |
| % Infection | ↓ (3 DPI) | — | n.d. | — | n.d. | ↑ (3DPI) | n.d. |
Abbreviations — = no significant change compared to controls with a diet containing only sucrose 70% (w/v); n.d. = not determined; ↓ = reduction or ↑ = increase of the analyzed parameter; DPI = days post infection.
Discussion
In this study, we added compounds to the sugar diets to examine the effects of antiparasitic drugs on some aspects of the biology of sand flies by adding these substances to the sugar diet. Sugar baits are a well stablished strategy to reduce insect populations and can be a new approach to reduce their vectorial capacity. In this context, we must consider a new perspective as treating insects with antiparasitic drugs, using sugar baits to prevent infection. In this respect, it is strategic to select for compounds with no anti-feeding effect. We evaluated the effect of selected compounds on the longevity of both female and male sand flies, the effect on blood feeding and oviposition, and lastly, the infection of Lu. longipalpis with Le. amazonensis. The compounds tested were chosen based on their known activity against Leishmania, and in the absence of repellent and anti-feeding activity on sugar meals [47]. The characterization of the effects of the ingestion of these compounds in sugar meals can help in the development of a new generation of transmission-blocking baits, aiming at the parasite, vectorial capacity, and other aspects of vector fitness, like blood feeding or reproduction.
The first aspect evaluated was the effect of the compounds on the longevity of insects. Of the compounds tested, only the Amphotericin B (Amp) did not affect longevity in males or females. Compounds LQB-181 and the solvent DMSO caused a reduction in ALS in males, and compounds LQB-475, LQ-03, and Pent unexpectedly increased lifespan. For females, LQB-475 and PMIC-4 caused a decrease in ALS. Three of the compounds tested are classified as pterocarpanoquinones (LQB-475, LQB-181, and LQ-03), and compound PMIC-4, which exhibited a longevity-reducing effect in females, is a hydroxyethylpiperazine derivative. These results are promising since we are not using high concentrations of these substances (between 14 and 57 μmol. L-1) to evaluate the possibility of using them in baits for insect control. The sugar bait containing Amphotericin B (Amp) (924.09 g·mol-1), for example, had only 0.002% of the antiparasitic compound (22 µmol. L-1), a concentration at least twenty times lower than the 0.05 to 0.25% concentration of active compound in insect control commercial products [51].
Some studies have demonstrated the effect of quinones on the mortality and feeding of insects from different orders as Diptera, Isoptera and Coleoptera [52–58].. The possible mechanism of these compounds involves damage the midgut after digestion, and inhibition of the mitochondrial complex III. Quinones, known as maesanin and juglone, possibly affect the energy generation mechanism in insects by inhibiting the mitochondrial electron transport chain [59]. These effects may explain why the reduction in longevity in sand flies is not immediate but rather a long-term effect.
Furthermore, this fact agrees with another capacity of quinones, such as naphthoquinones, that are redox active and can generate oxidative stress mainly in oxygen-rich environments [60,61]. These substances can also generate anti-feed or toxic effects due to their ability to alkylate essential nutrients and cellular components [60]. Inhibitory feeding effects were also observed in pterocarpanes extracted from Pterocarpus macrocarpus in tests carried out with Spodoptera litura [62].
The unexpected increase in ALS caused by LQ-03 and LQB-475 in males could be related to a structural detail in this pterocarpanquinone, an ortho-quinone. Molecules with this configuration are involved in sclerotization and cuticle darkening. This process occurs by reaction of a protein or an amino acid with a secreted polyphenolic derivative in the presence of an oxidase. Tissue hardness and stiffness involve the oxidation of these compounds to ortho-quinones primarily, followed by condensation with the NH2 (or still SH) groups of proteins [63,64]. The sclerotization can also promote the darkening of the cuticular color (brown/black color), which favors the absorption of solar radiation, keeping the insects warm [64,65]. However, further studies would need to be conducted to assess whether LQ-03 and LQB-475 would benefit the cuticular structure of adult sand flies.
Of those compounds, LQB-181 generated the most significant effects in reducing male longevity. Structurally different from other quinone derivatives, this compound has an amide group linking oxygen, suggesting that this nitrogenous portion might be responsible for enhancing the effect in males. Previous results demonstrated an anti-feeding effect of compound LQB-181. When offered a choice, males prefer to feed on a diet containing solely sucrose (54% vs 33%). Besides that, males fed on the sugar bait containing LQB-181 compound ingested the same volume as males fed on a sucrose diet [45]. The results demonstrate that even though LBQ-181 presented a toxic effect, males fed on a diet containing this drug due to a lack of another source of sugar.
PMIC-4, a hydroxyethyl piperazine derivative, caused a reduction in ALS in females and showed promise for use as an insecticidal compound for sand flies. Previously, PMIC-4 was ignored by males when there was a choice between diets [47], and even the volume of solution ingested was reduced compared to the control diet. Similarly to LQB-181, males ingested a smaller amount of PMIC-4, but since they had no other diet option it increased their mortality. Literature data show that piperazine-derived synthetic compounds exhibit larvicidal or growth inhibitory effects on caterpillars and mosquito larvae [66–68].
Compounds LQ-03 and Pent (pentamidine) caused an increase in the average lifespan of males. When looking at preference and ingestion results [47], we did not detect preference or higher ingestion in solutions containing these compounds. Pent seems to be palatable for sand flies since, in the experiments of ingestion of the sugar diet, the ingested volume of the pentamidine solution (68 ± 10 nL) was one of the highest compared to the other diets. These results suggest that Pent was beneficial for the insect’s fitness.
These data highlight the potential of these drugs to influence the epidemiology of the disease, especially those related to pentamidine, which is already one of the reference drugs used to treat leishmaniasis [2,3]. In this work, we verified the influence of this compound via the sugar diet; nonetheless, it would be relevant to assess whether an increase in longevity would also occur in a female that obtained a blood meal from a vertebrate host, e.g., a dog, being treated with this drug. In this scenario, we would have a potentially infected female with an increased lifespan and higher chances of making new blood feeds and spreading the pathogen. Our results infecting sand flies with Le. amazonensis also demonstrated that the insect’s ingestion of pentamidine does not inhibit the parasite’s development. All this reinforces the non-recommendation of treating dogs with the same drugs used to treat humans. Besides that, medications such as amphotericin B and pentamidine isothionate seem to have low efficacy in treating dogs and increase the risk of selecting parasites resistant to these drugs [2].
It is also worth mentioning that although compounds LQB-181 and PMIC-4 did not cause a statistically significant reduction in the number of eggs/females (Fig 4a), they seem to influence the laying and hatching of eggs (Fig 4b). These results reinforce that LQB-181 and PMIC-4 were the most deleterious compounds for the physiology of sand flies, suggesting that digestion or alterations in digestive products could affect egg production. These results can be incorporated into future studies to assess whether these effects would persist at different concentrations or experimental designs.
It is important to highlight, in the context of future applications of the compounds above for sand fly control, that LQB-475 has low toxicity against mammalian cells, with selective indexes of 23.76, 5.53, and >40 for Le. amazonensis, Le. brasiliensis, and Le. infantum, respectively [39]. Besides that, LQB-181 was shown to be selective for cancer cells and Le. amazonensis, with selective indexes of 13.7 and 2.8, respectively [43]. PMIC-4 was shown to be safe in a murine model, as after 5-days treatment no alterations in serological markers of toxicity were observed [46]. However, there is no information about LQ-03 safety, or about the stability of any of these compounds in environmental conditions. These are important aspects to consider for the future design of sugar baits against sand flies or other insects.
The investigation of natural compounds impacting the development of Leishmania is a concept developed by Schlein and Jacobson for nearly three decades [17,28,29]. Over the years, this line of research has continued and expanded to other vectors, such as mosquitoes [20,24–26,69,70].
None of the drugs reduced the parasite load in sand flies infected with Le. amazonensis. This may be interpreted as low effectiveness, as we added the compounds to the sugar diet at a concentration ten times greater than the IC50 calculated for Le. infantum or amazonensis, according to Table 1. For LQB-475, we used the IC50 value from Table 1 for Le. infantum, as it was the previously established concentration. However, possibly LQB-475 was used at a concentration 35 times higher than the IC50 since this value for Le. amazonensis reported in the literature is 0.40 ± 0.06 µM [39]. Moreover, our group has made significant progress in this area; we identified that secondary plant metabolites, added to the sugar solution, were able to reduce the percentage of infected females and the parasite load in infected Lu. longipalpis with L. mexicana [27], so we expected a similar effect.
Sand flies store sugar in their crop, and the sugar passage to the digestion site (thoracic midgut) takes place slowly, according to the insect’s physiological requirement for sugar consumption [10,71–75]. Sugar feeding is more frequent in gravid females or before the next blood feed [76]. Thus, after blood digestion, females may need to replenish their energy levels with more sugar intake. At this moment, the nectomonad forms would escape from the matrix and migrate to the anterior region of the gut, a region with a higher concentration of sugar mixed with the tested compounds.
A pivotal consideration pertains to the volume ingested by females when sugar solution is mixed with different compounds. It is important to highlight that the compounds LQB-475, LQB-181, PMIC-4 and Pent had no significant effect in the volumes of sugar solution ingested by females when compared to controls, with a low effect of LQ-03 in this parameter. However, LQ-03 did not affected the preference of females for control or experimental sugar meals [47]. So, the volume of solution containing LQB-475 ingested by females was probably higher than that of solution with LQ-03. As a result, the parasites may have been exposed to different drug concentrations, unlike the observed for pentamidine; when the females possibly ingested volumes close to 60 nL.
We expected that the in vivo effects of these pterocarpanquinones would be closer to those observed in vitro since they present promastigote growth inhibition effects, alteration in mitochondrial membrane potential (ΔΨm), DNA fragmentation at dose-dependent concentrations (≤ 10 µM) and increased production of ROS, with LQB-475 having the lowest IC50 values for the promastigote forms of Le. amazonensis, Le. braziliensis, and Le. infantum compared to the other drugs tested, including LQB-474, LQB-182 and LQB-118 [41,42,77]. Quinone derivatives also could cause oxidative stress or even interact with DNA topoisomerases, leading Leishmania to death by apoptosis [78].
We also did not observe statistically significant changes in the percentage of infected females. It is important to consider that for LQ-03, the IC50 used was the one determined previously for Le. infantum and that a very different sensibility to the drug in the two parasite species can’t be discarded, as the IC50 for Le. amazonensis is still unknown.
The absence and loss of anti-Leishmania activity in females of Lu. longipalpis treated with any of the tested compounds may be related to the metabolism of these compounds by the insect. The metabolism of MSPs of several classes, including quinones, in the gut of lepidopterans has been documented [79], and mechanisms of anthraquinone degradation have been proposed in termites [80]. In this sense, it would be essential to evaluate the possible excretion of these compounds in feces and urine or even their recruitment in catabolic pathways or detoxification by the insect. Another perspective is to assess the permanence of these compounds in the female diverticulum or other organs.
Another possible mechanism of inactivation of anti-Leishmania activity after insect ingestion may involve intestinal microbiota’s metabolism or modification of these compounds. The action of the intestinal microbiota on MSP and its inactivation has been well documented [81], and it is possible that other microorganisms indirectly benefit the parasites. Given the potential antimicrobial properties of these compounds, continuous insect consumption might have favored the selection of drug-resistant bacteria with a high capacity to metabolize them. Thus, future studies should also consider the influence of the intestinal microbiota. In this context, it is important to consider that this study was performed with sand flies from a laboratory colony that was established in the 70’s. It is possible that these sand flies have a microbiota different from field insects’, being associated with particular microorganisms that can interfere with the relationship with the compounds tested in this work.
Another perspective is the selection of parasites resistant to the compounds during continuous exposure. The insect phase of the parasite life cycle facilitates genetic material exchange between strains, with possible diversification and selection of resistant parasites [82]. In general terms, the observed results indicate that the exposure of Leishmania to antiparasitic compounds in the intestine of sand flies should consider metabolic interactions between parasite, vector, and microbiota. The complexity of this biological system and the emergence of resistance phenotypes to the target compounds are limiting factors of the proposed transmission block strategy and should be the subject of future studies.
Conclusions
We concluded that compounds LQB-181 and PMIC-4 reduced the longevity of adult sand flies and influenced the hatching of eggs, being promising candidates as lead compounds for the development of new insecticides due to their toxic effects. Compounds LQ-03 and Pent benefited insects and increased the lifespan of males and females, respectively. Compound LQB-475 reduced the longevity of Lu. longipalpis females, but did not decrease infection rates or parasite loads.
Our study offers some novel insights into the multifaceted impacts of compounds on insect biology, encompassing longevity, blood feeding, fecundity, fertility, and protein intake. These findings shed light on the intricate interplay between compounds and various physiological aspects, contributing to a deeper understanding of their effects on insects. The study suggests that assessing Leishmania exposure to antiparasitic compounds within the sand fly intestine through sugar baits necessitates the knowledge of the physiology of sugar digestion and acknowledging the potential metabolic interplay between parasite, vector, and microbiota.
Supporting information
Volumes of stock solutions (10 mM in DMSO) of each compound added to 1 mL of final mixture to achieve working concentrations as described in Table 1, and properties of working sugar solutions added to the sugar baits. DMSO corresponds to the group “Control 2”, and for this group the stock solution in the table below corresponds to the pure DMSO solvent.
(DOCX)
Raw experimental data used for statistical analysis and to make Figures 1–6.
(XLSX)
Acknowledgments
The authors are thankful to Bianca dos Santos Carvalho (IOC-Fiocruz) and Dr. Samara Graciane da Costa Latgé (IOC-Fiocruz) for maintaining the sand fly colony.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Funding. This work was funded by the Brazilian Research agencies FAPERJ (Grant No. E-26/200.454/2023), CNPq (Grant No. 312305/2022-2), and Fiocruz (Grant No. IOC-008-FIO-22-2-21). TNF was a Ph.D. student of the Post-graduate Program in Parasite Biology of the Oswaldo Cruz Institute (CAPES). SGCL, TDR, EFCJ, PRRC, CDN, ECTS, MAM, HLMG, and FAG are staff members of their respective departments.
References
- 1.World Health Organization. Leishmaniasis. In: WHO/Home/Newsroom/Fact sheets/Detail/Leishmaniasis. 2023. Jan 12 [cited May 19]. Geneva: WHO 2024; [about 8 screens]. Available from: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis [Google Scholar]
- 2.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Brasil. Manual de Vigilância e Controle da Leishmaniose Visceral. 1st edition. Brasília: Ministério da Saúde; 2014. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral_1edicao.pdf [Google Scholar]
- 3.Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, Departamento de Vigilância das Doenças Transmissíveis, Brasil. Manual de vigilância da leishmaniose tegumentar. 2nd edition. Brasília: Ministério da Saúde, 2017. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf [Google Scholar]
- 4.Pan American Health Organization. Leishmaniasis. In: PAHO/Home/Topics/Leishmaniasis. 2022. [cited September 19]. Washington: PAHO 2024. [about 5 screens]. Available from: https://www.paho.org/en/topics/leishmaniasis [Google Scholar]
- 5.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organización Panamericana de la Salud. PAS. Manual de procedimientos para vigilancia y control de las leishmaniasis en las Américas. 1st edition. Washignton: OPS, 2019. Available from http://iris.paho.org/xmlui/bitstream/handle/123456789/50524/9789275320631_spa.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 7.Mansueto P, Seidita A, Vitale G, Cascio A. Leishmaniasis in travelers: a literature review. Travel Med Infect Dis. 2014;12(6 Pt A):563–81. doi: 10.1016/j.tmaid.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 8.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–50. doi: 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 9.Brazil RP, Brazil BG. Binomia. In: Rangel EF, Lainson R, editors. Flebotomíneos do Brasil. Rio de Janeiro: Editora Fiocruz; 2003. p. 257–74. [Google Scholar]
- 10.Brazil RP, Brazil BG. Bionomy: Biology of Neotropical Phlebotomine Sand Flies. In: Rangel FP, Shaw JJ, editors. Brazilian Sand Flies. Cham: Springer; 2018. p. 299–318. [Google Scholar]
- 11.Salomón OD, Feliciangeli MD, Quintana MG, Afonso MM dos S, Rangel EF. Lutzomyia longipalpis urbanisation and control. Mem Inst Oswaldo Cruz. 2015;110(7):831–46. doi: 10.1590/0074-02760150207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rêgo FD, Soares RP. Lutzomyia longipalpis: an update on this sand fly vector. An Acad Bras Cienc. 2021;93(3):e20200254. [DOI] [PubMed] [Google Scholar]
- 13.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl Trop Dis. 2016;10(3):e0004349. doi: 10.1371/journal.pntd.0004349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27(2):123–47. doi: 10.1111/j.1365-2915.2012.01034.x [DOI] [PubMed] [Google Scholar]
- 15.Añez N, Lugo A, Loaiza A, Nieves E, Orozco J. Sugars in the alimentary canal of Lutzomyia youngi (Diptera: phlebotominae). Med Vet Entomol. 1994;8(1):38–42. doi: 10.1111/j.1365-2915.1994.tb00381.x [DOI] [PubMed] [Google Scholar]
- 16.Moraes CS, Aguiar-Martins K, Costa SG, Bates PA, Dillon RJ, Genta FA. Second Blood Meal by Female Lutzomyia longipalpis: Enhancement by Oviposition and Its Effects on Digestion, Longevity, and Leishmania Infection. Biomed Res Int. 2018;2018:2472508. doi: 10.1155/2018/2472508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlein Y. Leishmania and Sandflies: interactions in the life cycle and transmission. Parasitol Today. 1993;9(7):255–8. doi: 10.1016/0169-4758(93)90070-v [DOI] [PubMed] [Google Scholar]
- 18.Steverding D. The history of leishmaniasis. Parasit Vectors. 2017;10(1):82. doi: 10.1186/s13071-017-2028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diarra RA, Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, et al. Testing configurations of attractive toxic sugar bait (ATSB) stations in Mali, West Africa, for improving the control of malaria parasite transmission by vector mosquitoes and minimizing their effect on non-target insects. Malar J. 2021;20(1):184. doi: 10.1186/s12936-021-03704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiorenzano JM, Koehler PG, Xue R-D. Attractive Toxic Sugar Bait (ATSB) For Control of Mosquitoes and Its Impact on Non-Target Organisms: A Review. Int J Environ Res Public Health. 2017;14(4):398. doi: 10.3390/ijerph14040398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraser KJ, Mwandigha L, Traore SF, Traore MM, Doumbia S, Junnila A, et al. Estimating the potential impact of Attractive Targeted Sugar Baits (ATSBs) as a new vector control tool for Plasmodium falciparum malaria. Malar J. 2021;20(1):151. doi: 10.1186/s12936-021-03684-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junnila A, Müller GC, Schlein Y. Attraction of Phlebotomus papatasi to common fruit in the field. J Vector Ecol. 2011;36 Suppl 1:S206-11. doi: 10.1111/j.1948-7134.2011.00132.x [DOI] [PubMed] [Google Scholar]
- 23.Maia-Elkhoury ANS, Albuquerque R, Salomón OD. Leishmaniasis-Vector Surveillance and Control in Brazil: A Challenge to Control Programs. In: Rangel FP, Shaw JJ, editors. Brazilian Sand Flies. Cham: Springer; 2018. p. 467–494. doi: 10.1007/978-3-319-75544-1 [DOI] [Google Scholar]
- 24.Müller GC, Schlein Y. Different methods of using attractive sugar baits (ATSB) for the control of Phlebotomus papatasi. J Vector Ecol. 2011;36 Suppl 1:S64-70. doi: 10.1111/j.1948-7134.2011.00113.x [DOI] [PubMed] [Google Scholar]
- 25.Qualls WA, Müller GC, Khallaayoune K, Revay EE, Zhioua E, Kravchenko VD, et al. Control of sand flies with attractive toxic sugar baits (ATSB) and potential impact on non-target organisms in Morocco. Parasit Vectors. 2015;8:87. doi: 10.1186/s13071-015-0671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saghafipour A, Vatandoost H, Zahraei-Ramazani AR, Yaghoobi-Ershadi MR, Rassi Y, Karami Jooshin M, et al. Control of zoonotic cutaneous leishmaniasis vector, Phlebotomus papatasi, using attractive toxic sugar baits (ATSB). PLoS One. 2017;12(4):e0173558. doi: 10.1371/journal.pone.0173558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira TN, Pita-Pereira D, Costa SG, Brazil RP, Moraes CS, Díaz-Albiter HM, et al. Transmission blocking sugar baits for the control of Leishmania development inside sand flies using environmentally friendly beta-glycosides and their aglycones. Parasit Vectors. 2018;11(1):614. doi: 10.1186/s13071-018-3122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlein Y, Jacobson RL, Messer G. Leishmania infections damage the feeding mechanism of the sandfly vector and implement parasite transmission by bite. Proc Natl Acad Sci U S A. 1992;89(20):9944–8. doi: 10.1073/pnas.89.20.9944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlein Y, Jacobson RL. Mortality of Leishmania major in Phlebotomus papatasi caused by plant feeding of the sand flies. Am J Trop Med Hyg. 1994;50(1):20–7. [PubMed] [Google Scholar]
- 30.da Rocha Silva FB, Miguel DC, Minori K, Grazzia N, Machado VE, de Oliveira CM, et al. Attractiveness of Golden Hamster infected with Leishmania amazonensis (Kinetoplastida: Trypanosomatidae) to laboratory-reared Lutzomyia longipalpis (Diptera: Psychodidae). Acta Trop. 2024;255:107238. doi: 10.1016/j.actatropica.2024.107238 [DOI] [PubMed] [Google Scholar]
- 31.Carvalho-Silva R, Ribeiro-da-Silva RC, Cruz LNPD, Oliveira M da S de, Amoedo PM, Rebêlo JMM, et al. Predominance of Leishmania (Leishmania) amazonensis DNA in Lutzomyia longipalpis sand flies (Diptera: Psychodidae) from an endemic area for leishmaniasis in Northeastern Brazil. Rev Inst Med Trop Sao Paulo. 2022;64:e32. doi: 10.1590/S1678-9946202264032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BPD, Nakamura CV. Recent advances in leishmaniasis treatment. Int J Infect Dis. 2011;15(8):e525-32. doi: 10.1016/j.ijid.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 33.Tiwari N, Gedda MR, Tiwari VK, Singh SP, Singh RK. Limitations of Current Therapeutic Options, Possible Drug Targets and Scope of Natural Products in Control of Leishmaniasis. Mini Rev Med Chem. 2018;18(1):26–41. doi: 10.2174/1389557517666170425105129 [DOI] [PubMed] [Google Scholar]
- 34.Gervazoni LFO, Barcellos GB, Ferreira-Paes T, Almeida-Amaral EE. Use of Natural Products in Leishmaniasis Chemotherapy: An Overview. Front Chem. 2020;8:579891. doi: 10.3389/fchem.2020.579891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sen R, Chatterjee M. Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine. 2011;18(12):1056–69. doi: 10.1016/j.phymed.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 36.Andrade-Neto VV, Cunha-Junior EF, Dos Santos Faioes V, Pereira TM, Silva RL, Leon LL, et al. Leishmaniasis treatment: update of possibilities for drug repurposing. Front Biosci (Landmark Ed). 2018;23(5):967–96. doi: 10.2741/4629 [DOI] [PubMed] [Google Scholar]
- 37.Buarque CD, Militão GCG, Lima DJB, Costa-Lotufo LV, Pessoa C, de Moraes MO, et al. Pterocarpanquinones, aza-pterocarpanquinone and derivatives: synthesis, antineoplasic activity on human malignant cell lines and antileishmanial activity on Leishmania amazonensis. Bioorg Med Chem. 2011;19(22):6885–91. doi: 10.1016/j.bmc.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 38.Costa L, Pinheiro RO, Dutra PML, Santos RF, Cunha-Júnior EF, Torres-Santos EC, et al. Pterocarpanquinone LQB-118 induces apoptosis in Leishmania (Viannia) braziliensis and controls lesions in infected hamsters. PLoS One. 2014;9(10):e109672. doi: 10.1371/journal.pone.0109672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunha-Júnior EF, Martins TM, Canto-Cavalheiro MM, Marques PR, Portari EA, Coelho MGP, et al. Preclinical Studies Evaluating Subacute Toxicity and Therapeutic Efficacy of LQB-118 in Experimental Visceral Leishmaniasis. Antimicrob Agents Chemother. 2016;60(6):3794–801. doi: 10.1128/AAC.01787-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.da Cunha-Júnior EF, Pacienza-Lima W, Ribeiro GA, Netto CD, do Canto-Cavalheiro MM, da Silva AJM, et al. Effectiveness of the local or oral delivery of the novel naphthopterocarpanquinone LQB-118 against cutaneous leishmaniasis. J Antimicrob Chemother. 2011;66(7):1555–9. doi: 10.1093/jac/dkr158 [DOI] [PubMed] [Google Scholar]
- 41.Faiões VDS, da Frota LCRM, Cunha-Junior EF, Barcellos JCF, Da Silva T, Netto CD, et al. Second-generation pterocarpanquinones: synthesis and antileishmanial activity. J Venom Anim Toxins Incl Trop Dis. 2018;24:35. doi: 10.1186/s40409-018-0174-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro GA, Cunha-Júnior EF, Pinheiro RO, da-Silva SAG, Canto-Cavalheiro MM, da Silva AJM, et al. LQB-118, an orally active pterocarpanquinone, induces selective oxidative stress and apoptosis in Leishmania amazonensis. J Antimicrob Chemother. 2013;68(4):789–99. doi: 10.1093/jac/dks498 [DOI] [PubMed] [Google Scholar]
- 43.Gomes S, Militão G, Costa A, Pessoa C, Costa-Lotufo L, Cunha-Junior E, et al. Suzuki-Miyaura Coupling between 3-Iodolawsone and Arylboronic Acids. Synthesis of Lapachol Analogues with Antineoplastic and Antileishmanial Activities. J Braz Chem Soc. 2016. doi: 10.21577/0103-5053.20160326 [DOI] [Google Scholar]
- 44.Demidoff F, de Souza F, Netto C. Synthesis of Stilbene-Quinone Hybrids through Heck Reactions in PEG-400. Synthesis. 2017;49(23):5217–23. doi: 10.1055/s-0036-1589095 [DOI] [Google Scholar]
- 45.Cunico W, Gomes CRB, Moreth M, Manhanini DP, Figueiredo IH, Penido C, et al. Synthesis and antimalarial activity of hydroxyethylpiperazine derivatives. Eur J Med Chem. 2009;44(3):1363–8. doi: 10.1016/j.ejmech.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 46.de Vasconcelos MF, da Cunha-Júnior EF, de Andrade-Neto VV, Siqueira LM, d’Avila-Levy CM, Moreth M, et al. Oral effectiveness of PMIC4, a novel hydroxyethylpiperazine analogue, in Leishmania amazonensis. Int J Parasitol Drugs Drug Resist. 2014;4(3):210–3. doi: 10.1016/j.ijpddr.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira TN, Brazil RP, McDowell MA, Cunha-Júnior EF, Costa PRR, Netto CD, et al. Effects of anti-Leishmania compounds in the behavior of the sand fly vector Lutzomyia longipalpis. Pest Manag Sci. 2022;78(7):2792–805. doi: 10.1002/ps.6900 [DOI] [PubMed] [Google Scholar]
- 48.Díaz-Albiter HM, Ferreira TN, Costa SG, Rivas GB, Gumiel M, Cavalcante DR, et al. Everybody loves sugar: first report of plant feeding in triatomines. Parasit Vectors. 2016;9:114. doi: 10.1186/s13071-016-1401-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7 [DOI] [PubMed] [Google Scholar]
- 50.da Costa SG, Moraes C da S, Bates P, Dillon R, Genta FA. Development of Leishmania mexicana in Lutzomyia longipalpis in the absence of sugar feeding. Mem Inst Oswaldo Cruz. 2019;114:e180482. doi: 10.1590/0074-02760180482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakagawa LE, Costa AR, Polatto R, do Nascimento CM, Papini S. Pyrethroid concentrations and persistence following indoor application. Environ Toxicol Chem. 2017;36(11):2895–8. doi: 10.1002/etc.3860 [DOI] [PubMed] [Google Scholar]
- 52.Silva RL, Demarque DP, Dusi RG, Sousa JPB, Albernaz LC, Espindola LS. Residual Larvicidal Activity of Quinones against Aedes aegypti. Molecules. 2020;25(17):3978. doi: 10.3390/molecules25173978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaelakis A, Strongilos AT, Bouzas EA, Koliopoulos G, Couladouros EA. Larvicidal activity of naturally occurring naphthoquinones and derivatives against the West Nile virus vector Culex pipiens. Parasitol Res. 2009;104(3):657–62. doi: 10.1007/s00436-008-1242-7 [DOI] [PubMed] [Google Scholar]
- 54.Mbatchou VC, Tchouassi DP, Dickson RA, Annan K, Mensah AY, Amponsah IK, et al. Mosquito larvicidal activity of Cassia tora seed extract and its key anthraquinones aurantio-obtusin and obtusin. Parasit Vectors. 2017;10(1):562. doi: 10.1186/s13071-017-2512-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castillo L, Rossini C. Bignoniaceae metabolites as semiochemicals. Molecules. 2010;15(10):7090–105. doi: 10.3390/molecules15107090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mozaina K, Cantrell CL, Mims AB, Lax AR, Tellez MR, Osbrink WLA. Activity of 1,4-benzoquinones against formosan subterranean termites (Coptotermes formosanus). J Agric Food Chem. 2008;56(11):4021–6. doi: 10.1021/jf800331r [DOI] [PubMed] [Google Scholar]
- 57.Akhtar Y, Isman MB, Niehaus LA, Lee C-H, Lee H-S. Antifeedant and toxic effects of naturally occurring and synthetic quinones to the cabbage looper, Trichoplusia ni. Crop Protection. 2012;31(1):8–14. doi: 10.1016/j.cropro.2011.09.009 [DOI] [Google Scholar]
- 58.Sreelatha T, Hymavathi A, Babu KS, Murthy JM, Pathipati UR, Rao JM. Synthesis and insect antifeedant activity of plumbagin derivatives with the amino acid moiety. J Agric Food Chem. 2009;57(14):6090–4. doi: 10.1021/jf901760h [DOI] [PubMed] [Google Scholar]
- 59.Magiri EN, Konji VN, Makawiti DW, Midiwo J. Effect of Plant Quinones on Insect Flight Muscle Mitochondria. Int J Trop Insect Sci. 1995;16(2):183–9. doi: 10.1017/s1742758400017094 [DOI] [Google Scholar]
- 60.Barbehenn RV, Peter Constabel C. Tannins in plant-herbivore interactions. Phytochemistry. 2011;72(13):1551–65. doi: 10.1016/j.phytochem.2011.01.040 [DOI] [PubMed] [Google Scholar]
- 61.Rahman-Soad A, Dávila-Lara A, Paetz C, Mithöfer A. Plumbagin, a Potent Naphthoquinone from Nepenthes Plants with Growth Inhibiting and Larvicidal Activities. Molecules. 2021;26(4):825. doi: 10.3390/molecules26040825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morimoto M, Fukumoto H, Hiratani M, Chavasiri W, Komai K. Insect antifeedants, pterocarpans and pterocarpol, in heartwood of Pterocarpus macrocarpus Kruz. Biosci Biotechnol Biochem. 2006;70(8):1864–8. doi: 10.1271/bbb.60017 [DOI] [PubMed] [Google Scholar]
- 63.Bittner S. When quinones meet amino acids: chemical, physical and biological consequences. Amino Acids. 2006;30(3):205–24. doi: 10.1007/s00726-005-0298-2 [DOI] [PubMed] [Google Scholar]
- 64.Sugumaran M. Chemistry of Cuticular Sclerotization. Advances in Insect Physiology. Elsevier. 2010. p. 151–209. doi: 10.1016/b978-0-12-381387-9.00005-1 [DOI] [Google Scholar]
- 65.Klowden MJ. Integumentary Systems. In: Klowden MJ, editor. Physiological Systems in Insects. 2nd edition. Cambridge: Academic Press; 2008. p. 75–135. doi: 10.1016/B978-012369493-5.50003-1 [DOI] [Google Scholar]
- 66.Cai M, Li Z, Fan F, Huang Q, Shao X, Song G. Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl)ethyl]-4-[3-(trifluoromethyl)phenyl]piperazine (PAPP). J Agric Food Chem. 2010;58(5):2624–9. doi: 10.1021/jf902640u [DOI] [PubMed] [Google Scholar]
- 67.Xu G, Yang X, Jiang B, Lei P, Liu X, Wang Q, et al. Synthesis and bioactivities of novel piperazine-containing 1,5-Diphenyl-2-penten-1-one analogues from natural product lead. Bioorg Med Chem Lett. 2016;26(7):1849–53. doi: 10.1016/j.bmcl.2016.01.088 [DOI] [PubMed] [Google Scholar]
- 68.Aretz CD, Kharade SV, Chronister K, Rusconi Trigueros R, Martinez Rodriguez EJ, Piermarini PM, et al. Further SAR on the (Phenylsulfonyl)piperazine Scaffold as Inhibitors of the Aedes aegypti Kir1 (AeKir) Channel and Larvicides. ChemMedChem. 2021;16(2):319–27. doi: 10.1002/cmdc.202000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lehane MJ. The Biology of Blood-Sucking in Insects. 1st edition. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 70.Maia MF, Tenywa FC, Nelson H, Kambagha A, Ashura A, Bakari I, et al. Attractive toxic sugar baits for controlling mosquitoes: a qualitative study in Bagamoyo, Tanzania. Malar J. 2018;17(1):22. doi: 10.1186/s12936-018-2171-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bates PA. Leishmania sand fly interaction: progress and challenges. Curr Opin Microbiol. 2008;11(4):340–4. doi: 10.1016/j.mib.2008.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bates PA. Revising Leishmania’s life cycle. Nat Microbiol. 2018;3(5):529–30. doi: 10.1038/s41564-018-0154-2 [DOI] [PubMed] [Google Scholar]
- 73.Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes?. Trends Parasitol. 2006;22(9):439–45. doi: 10.1016/j.pt.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 74.Pimenta PFP, de Freitas VC, Monteiro CC, Pires ACMA, Secundino NFC. Biology of the Leishmania−Sand Fly Interaction. In: Rangel E, Shaw J, editors. Brazilian Sand Flies. Cham: Springer; 2018. pp. 319–39. doi: 10.1007/978-3-319-75544-1_6 [DOI] [Google Scholar]
- 75.Brazil RP, Carneiro VL, Andrade Filho JD, Alves JCM, Falcão AL. Biology of Lutzomyia lenti (Mangabeira) (Diptera: Psychodidae). An Soc Entomol Bras. 1997;26(1):191–3. doi: 10.1590/s0301-80591997000100026 [DOI] [Google Scholar]
- 76.Takken W, Koenraadt CJM. Ecology of parasite-vector interactions. 1st edition. Cham: Springer; 2013. [Google Scholar]
- 77.da Silva AJM, Netto CD, Pacienza-Lima W, Torres-Santos EC, Rossi-Bergmann B, Maurel S, et al. Antitumoral, antileishmanial and antimalarial activity of pentacyclic 1,4-naphthoquinone derivatives. J Braz Chem Soc. 2009;20(1):176–82. doi: 10.1590/s0103-50532009000100026 [DOI] [Google Scholar]
- 78.Pérez-Pertejo Y, Escudero-Martínez JM, Reguera RM, Balaña-Fouce R, García PA, Jambrina PG, et al. Antileishmanial activity of terpenylquinones on Leishmania infantum and their effects on Leishmania topoisomerase IB. Int J Parasitol Drugs Drug Resist. 2019;11:70–9. doi: 10.1016/j.ijpddr.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salminen J-P, Lahtinen M, Lempa K, Kapari L, Haukioja E, Pihlaja K. Metabolic modifications of birch leaf phenolics by an herbivorous insect: detoxification of flavonoid aglycones via glycosylation. Z Naturforsch C J Biosci. 2004;59(5–6):437–44. doi: 10.1515/znc-2004-5-627 [DOI] [PubMed] [Google Scholar]
- 80.Osbrink WLA, Tellez MR, Kobaisy M, Lax AR. Assessment of natural products for control of Formosan subterranean termites. In: Petroski RJ, Tellez MR, Behle RW, editors. Semiochemicals in pest and weed control. 1st edition. Washington: American Chemical Society; 2005. p. 73–87. [Google Scholar]
- 81.Genta FA, Dillon RJ, Terra WR, Ferreira C. Potential role for gut microbiota in cell wall digestion and glucoside detoxification in Tenebrio molitor larvae. J Insect Physiol. 2006;52(6):593–601. doi: 10.1016/j.jinsphys.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 82.Van Bockstal L, Hendrickx S, Maes L, Caljon G. Sand Fly Studies Predict Transmission Potential of Drug-resistant Leishmania. Trends Parasitol. 2020;36(9):785–95. doi: 10.1016/j.pt.2020.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Volumes of stock solutions (10 mM in DMSO) of each compound added to 1 mL of final mixture to achieve working concentrations as described in Table 1, and properties of working sugar solutions added to the sugar baits. DMSO corresponds to the group “Control 2”, and for this group the stock solution in the table below corresponds to the pure DMSO solvent.
(DOCX)
Raw experimental data used for statistical analysis and to make Figures 1–6.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.