Abstract
Background
There are limited epidemiological data on metabolic syndrome (MetS) during gestation in many developing nations. Available information on MetS prevalence in pregnancy is of clinical importance as it could aid in identifying pregnant women at risk of adverse perinatal outcomes. This study was designed to determine the prevalence of MetS, and its associated risk factors in pregnant women in Auchi, Edo Stage, Nigeria.
Methods
This hospital-based cross-sectional study involved 223 pregnant women aged 17-45 years recruited from selected hospitals in Auchi and its environs. Information on socio-demography and nutritional habits were obtained using a self-administered questionnaire. Anthropometric indices were determined using appropriate methods for height, weight and BMI. Blood glucose, triglyceride, high-density lipoprotein, low-density lipoprotein and total cholesterol were determined from the serum of the study participants using enzymatic assay. The prevalence of MetS was determined using the HNLBI/AHA and NECP ATP III definitions, modified for pregnant women.
Results
The findings revealed the following prevalences: MetS 10.8%, low HDL 35.7%, obesity 14.4%, high blood pressure 4.5%, hyperglycaemia 21.6% and hypertriglyceridemia 11.8%. There was no significant association between social-demographic and life style parameters with MetS.
Conclusion
The findings emphasize the importance of vigilant monitoring of pregnant women to reduce the risk of metabolic syndrome.
Keywords: Metabolic syndrome, Pregnancy, High-density lipoprotein, Triglycerides, Total cholesterol, Components of metabolic syndrome
Introduction
Metabolic syndrome (MetS) is a multifarious cluster of cardiovascular disease and type II diabetes mellitus risk factors that frequently occur together.[1] Obesity, hyperglycemia and hyperlipidemia, are all disorders associated with MetS that are brought on by changes in insulin sensitivity and persistent low-grade inflammation.[2]
Currently, it is a cause of great concern in both developed and developing nations because it affects a considerable proportion of the world population.[3] About 24% to 34% of Americans and up to 36% of Europeans aged 40 to 55 are affected by this epidemic, which is linked to the increased prevalence of obesity in both industrialized and developing nations.[4] In the US, almost one in three adults has MetS,[2] and Its prevalence varies between 24% and 33% in India and between 20.4% and 39.68% in China.[5, 6] Similar trends are seen in Africa, where the prevalence of MetS varies between nations and is dependent on the diagnostic criteria used. The prevalence was reported as being 34.6% in Kenya, 34.8% in Ethiopia, 42.6% in South Africa and 19.1% in Uganda,[7, 8] and 34.3% in Nigeria.[9]
In Nigeria, MetS prevalence has been reported in some cities and states in the country using different diagnostic criteria. This includes Sokoto, 20.5% (WHO), Enugu, 15.9% (IDF), Lagos, 34.3% (NCEP-ATP), Osogbo, 17.9% (IDF). Lagos, 86% (IDF), Jos 63.6% (IDF), Uyo 62.5% (NCEP-ATP III) and Ibadan 16.3% (IDF).[10,29] In Africa, where MetS is relatively common, women appear to be more impacted by MetS and its components than men regardless of age and other risk factors.[11]
Pregnancy-related MetS poses a serious risk to the mother's health as well as the health of the unborn child.[12] Few studies have demonstrated a link between MetS in the first trimester of conception and unfavorable maternal and neonatal outcomes during pregnancy.
Furthermore, perinatal consequences like diabetes mellitus during pregnancy, premature delivery, malformations of the neural tube, and a high risk for the newborn to develop obesity, MetS, or type 2 diabetes mellitus later in life have also been reported.[13 – 15] In Nigeria, information is sparse on the association between MetS with pregnancy outcomes. The purpose of this study was to ascertain the prevalence of MetS and its components among expectant mothers in Auchi, Etsako West, Edo State, Nigeria.
Method
Study Design and Setting
This cross-sectional study was conducted in hospitals in Auchi metropolis, in the Etsako west region of Edo State, Nigeria. The study involved 223 pregnant women receiving antenatal care, of these, 200 participants were recruited from Favour Hospital, 10 from Auchi Central hospital, and 13 from Blossom Hospital. The study was carried out from November 2022 to March 2023.
Sample Size and Sampling Method
The sample size for this study was determined using this formula n = (z)2 p (1 – p) / d2, with a prevalence of MetS of 12%,[16] a 5% acceptable margin of error, with a 95% confidence level. A minimum of 162 participants made up the sample size. For a final minimum sample size of 223 individuals, a 10% imponderable proportion was included to the model. All pregnant women who met the selection criteria were recruited using simple random sampling during their antenatal session.
Eligibility Criteria
Apparently, healthy pregnant women who were between the ages of 17 and 45 years old who came to the above hospitals in Auchi for antenatal appointments were recruited into this study. This study excluded pregnant women who had diabetes mellitus, cardiovascular disease, pre-pregnancy hypertension and hyperuricemia.
Determination of Anthropometric Parameters
The height of each study participant was measured using a stadiometer. The participant was asked to put on light clothing and take off their shoes and headgear. The body weight of each participant was measured using a weighing balance. BMI was determined using the formula: BMI = Weight (kg)/Height (m2) and BMI ≥ 30.[5, 14, 22]
Determination of Biochemical Parameters
Preparation sample: Following an overnight fast of at least 12 hours, a 5 ml blood sample was taken from each participant via venipuncture and 3ml of blood was put into a lithium heparin bottle for lipid profile test and 2ml was put into a fluoride oxalate bottle for blood glucose test. Within one hour of blood collection, the blood samples were centrifuged for 5 minutes at a speed of 300 revolutions per minute (rpm) and the plasma was separated into a plain sample bottle labelled properly and kept in the freezer at -20°C until analyzed.
(b) Determination of lipid profile: The enzyme assay spectrophotometric method was used to determine the level of total cholesterol, HDL, LDL and TG concentrations (Precision Kit, India).[5, 17]
(c) Blood glucose determination: Fasting plasma glucose level was determined using the glucose oxidase method following the manufacturer's instruction (Precision Kit, India).[5]
Measurement of Blood Pressure
At a comfortable sitting position, an automatic digital blood pressure monitor was used to measure the diastolic (DBP) and systolic (SBP) blood pressure. Two measurements were obtained at 5-minute intervals after the study participants were given at least 25 minutes to relax. The average of the two readings that were obtained from the subjects was used as the final blood pressure in mmHg.
Diagnosis of Metabolic Syndrome
Identification of MetS used the National Heart, Lung, and Blood Institute/American Heart Association (HNLBI/AHA), Adult Treatment Panel III (ATP III) definitions, modified for pregnant women.[14] A participant is said to have MetS if she possesses at least three of the following criteria: BMI >30 kg/m2; TG ≥150 mg/dl HDL <50mg/dl, SBP ≥130/DBP ≥85 mm/Hg.[5]
Statistical Analyses
The IBM SPSS for Microsoft Windows version 21 (IBM Corp, Armonk, NY, USA), was used for data analysis. The comparison of continuous variables presented as mean and standard deviation (SD) was done using ANOVA. Chi square test was used for the comparison of categorical variables and to assess the prevalence of MetS among the study participants. Binary logistic regression was used to predict and identify the risk factors of MetS. The statistical significance of P<0.05 was used as the threshold.
Ethical Considerations
The Ethical Committee of the Faculty of Basic Medical Sciences at Edo State University Uzairue issued the ethical approval (EDSUREC 22/0079). Prior to conducting the research, the study participants were asked to provide written consent. Detailed information regarding the nature of study and purpose was provided, and the pregnant women voluntarily enrolled in the project. They were assured of their safety and the confidentiality of their responses throughout the study.
Results
The socio-demographics of study participants, are shown in Table 1, whereby the prevalence of MetS was 0.9% among participants between the ages of 17-22 years old, 4.0% among participants between the age of 23–28 years old, 2.2% among participants between the age of 29–34 years old, 3.1% among participants between the age of 35-40 years old and 0.4% among participants between the ages of 41-45 (p 0.021).
Table 1.
Socio-demographic status and ages of study participants with MetS and Non-MetS (N=223)
| Parameters | Response | Metabolic syndrome n (%) | ||
|---|---|---|---|---|
|
|
||||
| MetS n (%) |
Non-MetS n (%) |
P value | ||
| Educational level | Primary | 3(1.3) | 9(4.0) | 0.223 |
| Secondary | 14(6.3) | 114(51.1) | ||
| Tertiary | 7(3.2) | 76(34.1) | ||
| Marital status | Married | 20(9.0) | 150(67.2) | 0.520 |
| Single | 4(1.8) | 41(18.4) | ||
| Divorced | 0(0) | 8(3.6) | ||
| Occupation | Employed | 2(0.9) | 19(8.5) | 0.219 |
| Self-employed | 21(9.5) | 145(65.0) | ||
| Unemployed | 1(0.4) | 35(15.7) | ||
| Religion | Christian | 15(6.7) | 128(57.4) | 0.860 |
| Muslim | 9(4.1) | 71(31.8) | ||
| Age group | 17-22 | 2(0.9) | 35(15.7) | 0.021 |
| 23-28 | 9(4) | 90(40.4) | ||
| 29-34 | 5(2.2) | 41(18.4) | ||
| 35-40 | 7(3.1) | 33(14.4) | ||
| 41-45 | 1(0.4) | 0(0.0) | ||
MetS=Metabolic syndrome; Non-MetS= Non Metabolic Syndrome.
The prevalence of MetS was higher amongst married participant (9.0%) compared to single and divorced though it was not significant (p = 0.520). The prevalence of MetS was found to be insignificantly higher in participant with secondary school education (6.3%) compared to tertiary (3.1%), (p = 0.223).
MetS prevalence was higher in participants who are self-employed (9.4%) compared to the employed (0.9%) and unemployed (0.4%). MetS prevalence was higher amongst participants who are Christians (6.7%) compared to Muslim (4.1%) (p = 0.860).
Family history of chronic diseases, lifestyle and level of physical activities of study participants are shown on Table 2. There was no significant difference in the consumption of alcohol pre- and during pregnancy, family history of chronic diseases and physical activities in the study participants.
Table 2.
Family History of Chronic Diseases, Level of Physical Activities and Lifestyle Parameters of study participants with MetS and Non-MetS
| Parameters | MetS n (%) |
Non MetS n (%) |
P value | |
|---|---|---|---|---|
| Life style and level of physical activities | ||||
| Alcohol before pregnancy | No | 17(7.6) | 164(73.5) | 0.171 |
| Yes | 7(3.2) | 35(15.7) | ||
| Alcohol during pregnancy | No | 23(10.4) | 185(83.0) | 0.596 |
| Yes | 1(0.4) | 14(6.2) | ||
| Physical activity levels | Inactive | 63(28.2) | 0.362 | |
| Active | 14(6.3) | 86(38.6) | ||
| Very active | 4(1.8) | 50(22.4) | ||
| Farm work | No | 22(9.9) | 184(82.5) | 0.890 |
| Yes | 2(0.9) | 15(6.7) | ||
| Stress | No | 13(5.9) | 132(59.2) | 0.238 |
| Yes | 11(4.9) | 67(30.0) | ||
| Family History of Chronic Diseases | ||||
| Type 2 Diabetes | No | 21(9.4) | 178(78.9) | 0.771 |
| Yes | 0(0.0) | 2(0.9) | ||
| Cardiovascular diseases | No | 24(10.8) | 197(88.3) | 0.662 |
| Yes | 0(0.0) | 2(0.9) | ||
| Hypertension | No | 23(10.4) | 178(79.8) | 0.322 |
| Yes | 1(0.4) | 21(9.4) | ||
| Gestational diabetes | No | 24(10.8) | 199(89.2) | |
Note: Data are presented as relative frequencies (in %) and absolute frequencies (n), MetS=Metabolic syndrome; Non-MetS= Non Metabolic Syndrome.
Table 3 shows the dietary patterns of study participants. There was no difference in eating close to bedtime, skipping meals, number of meals per day, or consumption of fruits, vegetables, fish, meat, tea and dairy products.
Table 3.
Dietary Pattern of Study Participants with MetS and Non-MetS
| Parameters | Response | MetS (%) | Non-MetS (%) | P values |
|---|---|---|---|---|
| Eaten close to bed time | Yes | 16(7.2) | 121(54.2) | 0.577 |
| No | 8(3.6) | 78(35.0) | ||
| Weekly skipping of meals | Nil | 17(7.6) | 119(53.4) | 0.753 |
| Once a week | 1(0.4) | 17(7.6) | ||
| 2 times a week | 2(0.9) | 24(10.8) | ||
| 3 times a week | 3(1.3) | 14(6.3) | ||
| 4 times a week | 1(0.4) | 7(3.1) | ||
| 5 times a week | 0(0.0) | 3(1.3) | ||
| 7 times a week | 0(0.0) | 11(4.9) | ||
| 14 times a week | 0(0.0) | 4(1.8) | ||
| Weekly consumption of fresh fruits | Yes | 24(10.8) | 192(86.1) | 0.351 |
| No | 0(0.0) | 7(3.1) | ||
| Number of meals per day | 2 times a day | 1(0.4) | 26(11.7) | 0.514 |
| 3 times a day | 16(7.2) | 113(50.7) | ||
| 4 times a day | 7(3.1) | 49(22.0) | ||
| 5 times a day | 0(0.0) | 6(2.7) | ||
| 6 times a day | 0(0.0) | 5(2.2) | ||
| Weekly consumption of raw vegetables | Yes | 14(6.3) | 98(43.9) | 0.400 |
| No | 10(4.5) | 101(45.3) | ||
| Weekly consumption of cooked vegetables | Yes | 24(10.8) | 189(84.8) | 0.261 |
| No | 0(0.0) | 10(4.5) | ||
| Weekly consumption of eggs | Yes | 18(8.1) | 159(71.3) | 0.575 |
| No | 6(2.7) | 40(17.9) | ||
| Consumption of meat | Yes | 24(10.8) | 192(86.1) | 0.351 |
| No | 0(0.0) | 7(3.1) | ||
| Consumption of fish | Yes | 24(10.8) | 183(82.1) | 0.149 |
| No | 0(0.0) | 16(7.2) | ||
| Consumption of milk | Yes | 17(7.6) | 152(68.2) | 0.549 |
| No | 7(3.1) | 47(21.1) | ||
| Weekly consumption of tea or coffee | Yes | 15(6.7) | 129(57.8) | 0.822 |
| No | 9(4.0) | 70(31.4) |
MetS=Metabolic syndrome; Non-MetS= Non Metabolic Syndrome
Table 4 shows the prevalence of MetS and its determinants among participants recruited in this study. The most occurring metabolic component of MetS was low HDL, with a prevalence of 35.7%, followed by hyperglycemia with a prevalence of 21.6%. Hypertension was the least prevalent (4.5%) among the various components of MetS.
Table 4.
Prevalence of metabolic syndrome and its individual components
| Components of MetS | Prevalence (%) |
Components | OR | 95%CI | P value |
|---|---|---|---|---|---|
| Low HDL-cholesterol | 35.7 | Low HDL | 14.16 | 3.24-61.86 | <0.001 |
| High fasting blood glucose | 21.6 | High FBG | 0.14 | 0.05-0.34 | <0.001 |
| Body mass index (BMI) | 14.4 | High BMI | 0.08 | 0.03-0.21 | <0.001 |
| Hypertriglyceridemia | 11.8 | High TG | 0.03 | 0.01-0.09 | <0.001 |
| High Systolic blood pressure (SBP) | 5.2 | High SBP | 0.22 | 0.07-0.70 | 0.006 |
| High Diastolic blood pressure (DBP) | 9.5 | High DBP | 0.24 | 0.09-0.62 | 0.002 |
| Elevated Blood pressure | 4.5 | ||||
| Metabolic syndrome | 10.8 |
MetS=Metabolic syndrome
Figure 1 shows that the mean value of BMI, SBP, DBP, plasma glucose level and TG levels were significantly higher in participants with MetS compared to those without MetS, while the mean value of HDL-cholesterol level, TC and LDL were higher in pregnant women without MetS compared to participants with MetS.
Figure 1.
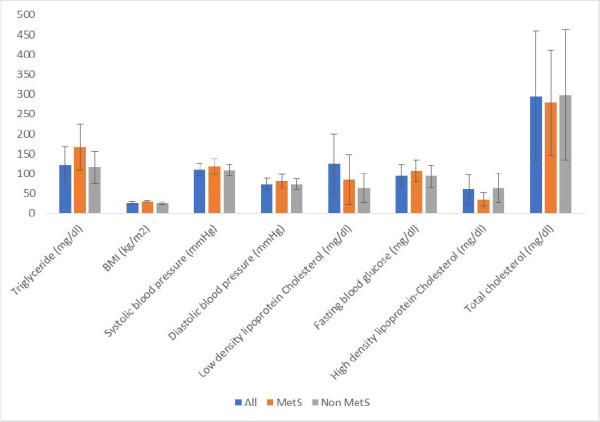
Metabolic profile of study participants, showing mean ± standard deviation (SD) of various components
Discussion
In this study, there was no significant difference between educational levels in study participants with MetS and Non-MetS. This is in contrast with the research on prevalence of metabolic syndrome and relationships with socio-demographic characteristics and physical activity in urban population of Iranian adults,[18] which indicated a higher prevalence of MetS among participants with lower educational status. Participants between the ages of 23 and 28 had a greater prevalence of MetS than other age groups. This result is consistent with that reported in the West region of Cameroon.[5] This could be because most study participants fall within this age range, and age is recognized to be a risk factor for many chronic diseases, such as obesity and MetS.[2]
This study found no significant comparison between the level of physical activity and MetS, which is in contrast to a previous report,[19] which showed that maintaining an active lifestyle can prevent the development of the MetS. The prevalence of MetS in this study was 10.8% and this was similar to a previous report.[20,5] Low HDL and hyperglycemia observed could be the reason for the high prevalence of MetS reported in this study, because low HDL and hyperglycemia were the most occurring metabolic components among the study participants. Among the risk factors of MetS, low HDL (35.7%; 95% CI: 3.24.16–61.85) and hyperglycemia (21.6%; 95% CI: 0.05–0.34) were the most recurrent in the study population. This report is in accordance with the study carried out amongst pregnant women in Dschang District Hospital in the West Region of Cameroon.[5] The reduction of HDL cholesterol has been linked to a variety of genetic, dietary, and environment-related factors.[21] In contrast, more than 90% of cases of low HDL cholesterol levels are caused by lifestyle factors such as smoking, inactivity, obesity, diet and advanced alcohol-related liver disease.[21,22]
Studies have shown that there is a significant correlation between low HDL cholesterol levels and the physiological manifestations of MetS during pregnancy. Observation from the study conducted amongst pregnant women has shown that hypertriglyceridemia was identified as the most prevalent MetS component.[20] This prevalence of hypertriglyceridemia and low HDL cholesterol may be the result of alteration in lipid metabolism caused by MetS-associated insulin resistance.[23]
The prevalence of obesity in this present study (14.4%) is lower than the value reported amongst pregnant women in the West Region of Cameroon.[5] This could be because the study was carried out in the region of Cameroon with the highest occurrence of obesity in the country and the people had an ideology that being overweight is considered a sign of good living. [24] A sedentary lifestyle and overfeeding are the major causes of obesity. However, this prevalence is similar to the 11.1 % prevalence reported in a review of metabolic syndrome and pregnancy.[13]
The abdominal circumference cannot be used to determine obesity during pregnancy as it may not reflect a true state of abdominal adiposity, particularly in women with polyhydramnios and foetal macrosomia.[25, 26] The prevalence of hypertension in this study (4.5%) was lower than the prevalence of hypertension in a report of pregnant women followed up at the Dschang District Hospital of West Region Cameroon (11.9%). [5] The lower prevalence of hypertension in this study could be attributed to the healthy lifestyle and dietary practices of the study participants. In comparison to previous studies,[5,13] hyperglycaemia prevalence of 21.6% in study was higher. This can be a result of the study participant's eating habits and type of diet.[27] On the other hand, in this study, there was a lower prevalence of hypertriglyceridemia (11.8%) than in previous reports.[5,28] Studies have shown that severe hypertriglyceridemia typically develops in the presence of a hereditary TG metabolism defect.[19]
Strengths and Limitations of Study
This study focused on pregnant women from Auchi and its environs that have received comparatively little research in terms of women's reproductive health.
In the course of this study, below are some limitations of the study:
This study was conducted in Auchi and its environs, it is challenging to extrapolate its findings to all pregnant women in the whole of Nigeria.
Measurement errors caused by self-reporting of socio-demographic characteristics and economic position may result in misclassification and lack of differentiation, which could push a relationship closer to zero or have less significance.
Conclusion
It could be concluded from this study that the prevalence of MetS was 10.8% among pregnant women in Auchi, and low HDL and hyperglycemia were the major components. There is the need for regular monitoring of pregnant women in order to reduce the risk of metabolic syndrome.
Acknowledgement
The chief medical officers of Auchi Central Hospital (Now Edo State University Teaching Hospital), Favour, and Blossom Hospitals, as well as all the pregnant women who participated in the study, are gratefully acknowledged. The laboratory and antenatal consulting staff at Edo State University Teaching Hospital are also appreciated.
Contributions of Authors
AMU and OOA designed the study. KIU did patients' recruitment and the laboratory analysis. AMU and OOA supervised the study. KIU wrote the first draft which was critiqued by other authors. All authors approved the final version of the manuscript.
Conflicting Interest
The authors have no conflicting interest to report.
Funding
This research work was self-funded.
References
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood Institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. doi: 10.1161/circulationaha.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):1–8. doi: 10.1007/s11906-018-0812-z. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajian-Tilaki K, Heidari B, Firouzjahi A, Bagherzadeh M, Hajian-Tilaki A, Halalkhor S. Prevalence of metabolic syndrome and the association with socio-demographic characteristics and physical activity in urban population of Iranian adults: a population-based study. Diabetes Metab Syndr: Clinical Research & Reviews. 2014;8(3):170–176. doi: 10.1016/j.dsx.2014.04.012. doi: 10.1016/j.dsx.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Balkau B, Charles MA, Drivsholm T, Borch-Johnsen K, Wareham N, Yudkin JS, Morris R, Zavaroni I, van Dam R, Feskins E, Gabriel R. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes Metab. 2002;28(5):364–376. https://pubmed.ncbi.nlm.nih.gov/12461473. [PubMed] [Google Scholar]
- 5.Dabou S, Ongbayokolak NS, Fonkeng Sama L, Matene Foking E, Kamdom NM, Telefo PB. Metabolic Syndrome During Pregnancy: Prevalence and Determinants Among Pregnant Women Followed-Up at the Dschang District Hospital, West Region of Cameroon. Diabetes Metab Syndr Obes: Targets and Therapy. 2022;1:743–753. doi: 10.2147/DMSO.S348040. doi: 10.2147/dmetso.S348040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harikrishnan S, Sarma S, Sanjay G, Jeemon P, Krishnan MN, Venugopal K, Mohanan PP, Jeyaseelan L, Thankappan KR, Zachariah G. Prevalence of metabolic syndrome and its risk factors in Kerala, South India: Analysis of a community based cross-sectional study. PloS one. 2018;13(3):e0192372. doi: 10.1371/journal.pone.0192372. doi: 10.1371/journal.pone.0192372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaduka LU, Kombe Y, Kenya E, Kuria E, Bore JK, Bukania ZN, Mwangi M. Prevalence of metabolic syndrome among an urban population in Kenya. Diabetes care. 2012;35(4):887–893. doi: 10.2337/dc11-0537. doi: 10.2337/dc11-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruger MJ, Nell TA. The prevalence of the metabolic syndrome in a farm worker community in the Boland district, South Africa. BMC Public Health. 2017. pp. 1–10. doi:10.1186/s12889-016-3973-1. [DOI] [PMC free article] [PubMed]
- 9.Akintunde AA, Ayodele OE, Akinwusi PO, Opadijo GO. Metabolic syndrome: comparison of occurrence using three definitions in hypertensive patients. Clin. Med. Res. 2011;9(1):26–31. doi: 10.3121/cmr.2010.902. doi:10.3121/cmr.2010.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okafor CI. The metabolic syndrome in Africa: Current trends. Indian J Endocrinol Metab. 2012;16(1):56–66. doi: 10.4103/2230-8210.91191. doi: 10.4103/2230-8210.91191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambachew S, Endalamaw A, Worede A, Tegegne Y, Melku M, Biadgo B. The prevalence of metabolic syndrome in ethiopian population: a systematic review and meta-analysis. J. Obes. 2020. pp. 1–14. DOI: 10.1155/2020/2701309. [DOI] [PMC free article] [PubMed]
- 12.Vryonidou A, Paschou SA, Muscogiuri G, Orio F, Goulis DG. Mechanisms in endocrinology: metabolic syndrome through the female life cycle. Eur. J. Endocrinol. 2015;173(5):153–163. doi: 10.1530/EJE-15-0275. doi: 10.1530/EJE-15-0275. [DOI] [PubMed] [Google Scholar]
- 13.Dos Prazeres Tavares H, Arantes MA, Tavares SB, Abbade JF, Calderon ID, Rudge MV. Metabolic syndrome and pregnancy, its prevalence, obstetrical and newborns complications. Open J. Obstet Gynecol. 2015;5(11):618. Doi: 10.4236/ojog.2015.511087. [Google Scholar]
- 14.Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, Kafatos A, Koutis A, Kogevinas M. Metabolic syndrome in early pregnancy and risk of preterm birth. Am. J. Epidemiol. 2009;170(7):829–836. doi: 10.1093/aje/kwp211. doi: 10.1093/aje/kwp211. [DOI] [PubMed] [Google Scholar]
- 15.Ray JG, Thompson MD, Vermeulen MJ, Meier C, Wyatt PR, Wong PY, Summers AM, Farrell SA, Cole DE. Metabolic syndrome features and risk of neural tube defects. BMC Pregnancy Childbirth. 2007;7(1):1–5. doi: 10.1186/1471-2393-7-21. doi: 10.1186/1471-2393-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adeloye D, Basquill C, Aderemi AV, Thompson JY, Obi FA. An estimate of the prevalence of hypertension in Nigeria: a systematic review and meta-analysis. J. Hyperten. 2015;33(2):230–242. doi: 10.1097/HJH.0000000000000413. doi: 10.1097/HJH.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 17.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969;6(1):24–27. doi:10.1177/000456326900600108. [Google Scholar]
- 18.Hajian-Tilaki K, Heidari B, Firouzjahi A, Bagherzadeh M, Hajian-Tilaki A, Halalkhor S. Prevalence of metabolic syndrome and the association with socio-demographic characteristics and physical activity in urban population of Iranian adults: a population-based study. Diabetes Metab. Syndr. Clin. Res. Rev. 2014;8(3):170–176. doi: 10.1016/j.dsx.2014.04.012. doi: 10.1016/j.dsx.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg AS, Hegele RA. Severe hypertriglyceridemia in pregnancy. J. Clin. Endocrinol. Metab. 2012;97(8):2589–2596. doi: 10.1210/jc.2012-1250. doi: 10.1210/jc.2012-1250. [DOI] [PubMed] [Google Scholar]
- 20.Grieger JA, Bianco-Miotto T, Grzeskowiak LE, Leemaqz SY, Poston L, McCowan LM, Kenny LC, Myers JE, Walker JJ, Dekker GA, Roberts CT. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Medicine. 2018;15(12):e1002710. doi: 10.1371/journal.pmed.1002710. doi:10.1371/journal.pmed.1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashid S, Genest J. Effect of obesity on high-density lipoprotein metabolism. Obesity. 2007;15(12):2875–2888. doi: 10.1038/oby.2007.342. doi: 10.1038/oby.2007.342. [DOI] [PubMed] [Google Scholar]
- 22.Von Eckardstein A, Assmann G. Prevention of coronary heart disease by raising high-density lipoprotein cholesterol? Curr. Opin. Lipidol. 2000;11(6):627–637. doi: 10.1097/00041433-200012000-00010. doi: 10.1097/00041433-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Wani K, Sabico S, Alnaami AM, Al-Musharaf S, Fouda MA, Turkestani IZ, Al-Ajlan A, Alshingetti NM, Alokail MS, Al-Daghri NM. Early-pregnancy metabolic syndrome and subsequent incidence in gestational diabetes mellitus in Arab women. Front. Endocrinology. 2020;11:98. doi: 10.3389/fendo.2020.00098. doi:10.3389/fendo.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marbou WJ, Kuete V. Prevalence of metabolic syndrome and its components in Bamboutos Division's adults, west region of Cameroon. BioMed Res. Int. 2019. doi: 10.1155/2019/9676984. [DOI] [PMC free article] [PubMed]
- 25.Desai M, Beall M, Ross MG. Developmental origins of obesity: programmed adipogenesis. Curr. Diabetes Rep. 2013;13:27–33. doi: 10.1007/s11892-012-0344-x. doi: 10.1007/s11892-012-0344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schack-Nielsen L, Michaelsen KF, Gamborg M, Mortensen EL, Sørensen TI. Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int. J. Obes. 2010;34(1):67–74. doi: 10.1038/ijo.2009.206. doi: 10.1038/ijo.2009.206. [DOI] [PubMed] [Google Scholar]
- 27.Panagiotakos DB, Tzima N, Pitsavos C, Chrysohoou C, Papakonstantinou E, Zampelas A, Stefanadis C. The relationship between dietary habits, blood glucose and insulin levels among people without cardiovascular disease and type 2 diabetes; the ATTICA study. Rev. Diabet Stud. 2005;2(4):208. doi: 10.1900/RDS.2005.2.208. doi: 10.1900/RDS.2005.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatzi L, Plana E, Pappas A, Alegkakis D, Karakosta P, Daraki V, Vassilaki M, Tsatsanis C, Kafatos A, Koutis A, Kogevinas M. The metabolic syndrome in early pregnancy and risk of gestational diabetes mellitus. Diabetes Metab. 2009;35(6):490–494. doi: 10.1016/j.diabet.2009.07.003. doi: 10.1016/j.diabet.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Ladapo OO, Salako L, Sodiq O, Shoyinka K, Adedapo K, Falase AO. A prevalence of cardiometabolic risk factors among a rural Yoruba south-western Nigerian population: a population-based survey. Cardiovasc. J. Africa. 2010;21(1):26–31. [PMC free article] [PubMed] [Google Scholar]


