Abstract
The uncontrolled distribution of genetically modified organisms (GMO)-based food and feed is an increasing global concern, primarily due to limited information about their potential harmful effects. The growing diversity and complexity of GMOs present significant challenges for their detection, traceability, and safety monitoring. Traditionally, GMOs are detected using molecular methods, among which PCR methods are the most explored and are considered the gold standard. However, isothermal nucleic acid amplification methods, though less explored, hold great potential, especially when integrated with biosensor platforms, enabling the development of highly efficient and versatile biosensing systems. This paper provides a comprehensive overview of the recent advances in biosensors utilizing methods of isothermal nucleic acid amplification, highlighting their current progress and future perspectives. We discuss molecular methods for GMO detection, focusing on reaction conditions, amplification efficiency, and compatibility with various detection modalities. Additionally, we investigate the integration of various nanomaterials into transducers, such as electrochemical platforms, together with the electrochemical techniques and detection mechanisms, aiming to outline their synergistic effects with molecular techniques to improve detection sensitivity and enable real-time monitoring. Furthermore, we discuss the applications of GMO biosensors across diverse fields, including food safety and environmental monitoring, while addressing existing challenges and potential strategies for improving the performance, robustness, and practicality of biosensing platforms. Overall, this review highlights the significant progress achieved in GMO biosensors and underscores their promising role in advancing diagnostic and monitoring capabilities.
Graphical abstract

Keywords: GMO, Nucleic acid amplification, Transducers, Electrochemical biosensors, Field-effect transistor biosensors, Food safety, Environmental monitoring, Point-of-need
Introduction
Genetically modified organisms (GMOs) are living organisms with artificially modified genomes. These modifications are made using advanced biotechnology [1], i.e., advanced breeding technologies, also called precision breeding techniques [2]. The process by which GMOs are formed is known as genetic transformation and includes transferring a DNA fragment originating from foreign organisms into a host organism or cell, creating a permanent difference in the hosts’ genome [3]. However, genetic transformation is not the only method for creating genetically engineered organisms. Gene editing, or genome editing, using species-specific nucleases can be used to insert, knock out, or modify specific genes [4]. The most popular technology for producing transgene-free genetic modifications is based on the utility of the CRISPR/Cas 9 system, which enables the precise modification of an organism’s genome using natural or artificial genes to introduce desirable traits [5].
The new transgene-free genome editing techniques are slowly replacing traditional GMO production technologies, offering a safer and cheaper way to improve crop yield and overcome uncertainties related to the transfer of foreign genetic material into genetic surroundings of unrelated species [5]. However, despite the high costs associated with developing new GMOs—due to advanced technologies, strict regulations, and time-intensive procedures—existing GMOs remain widely used and are already present in the environment [3, 5].
The introduction of genetically modified (GM) crops enhanced agricultural productivity and reduced environmental pollution through decreased chemical usage (indirectly improving food safety, environmental restoration, soil health, etc.) [3]. However, production of GMOs is often considered “imperfect technology” due to potential health implications (i.e., toxicity, allergenicity, and the possibility of unforeseen genetic hazards, possibly caused by potential pleiotropic effects of inserted genes) and environmental effects (e.g., invasive species, evolution of superbugs and superweeds, cross hybridization with non-GM plants) [3, 6]. Globally, approximately 11.5% of total agricultural land dedicated to crop production is used for GM crops, mostly for GM soybean and GM maize [7]. In contrast, within the European Union, agricultural cropland covers approximately 175 million hectares, of which only 0.05% is allocated to GM crop production, primarily for GM maize (90%) [7]. The most abundant genetic modifications in EU crops are the ones for glyphosate tolerance (5-enolpyruvylshikimate-3-phosphate synthase (EPSPS)), Cry genes for insect defense (e.g., Cry1, Cry2), glufosinate resistance (phosphinothricin-N acetyltransferase (PAT)), MON810 (coding insecticidal protein), herbicide resistance (bialaphos resistance gene (Bar)), and β-glucuronidase (GUS) [8–11]. The EU enforces strict GMO regulation through the European Food Safety Authority (EFSA)—leading health and environmental risk assessment [12]. Additionally, at the EU level, GMO labeling is required for food products that contain or are derived from GMOs. The threshold below which the product is considered GMO-free is 0.9%. While some EU countries completely forbid the consumption and production of GMOs by their national laws, consumer concerns about GMOs in the EU have decreased over the years. Nevertheless, the majority of consumers continue to support labeling, preferring to be directly informed about their food choices [13].
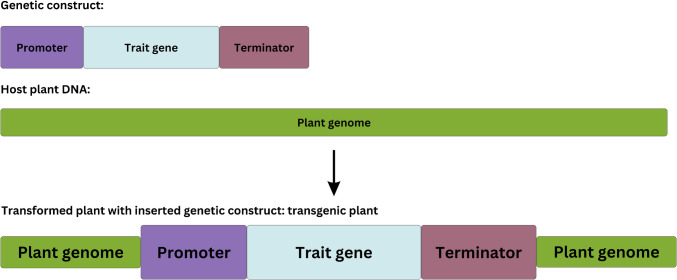
The presence of GMO can be easily confirmed using molecular methods, with the most commonly utilized techniques being polymerase chain reaction (PCR)-based detection methods, isothermal amplification methods, and high-throughput sequencing. These methods can detect common genetic elements (promoter or terminator regions), transgenes, or a whole transgenic construct (Fig. 1). Current GMO legal regulations have directed molecular GMO screening methods to fit into point-of-need (PON) standards for on-site applicability in GMO monitoring. To achieve this demand, molecular techniques are coupled with different sensor technologies and electrode materials in specially designed DNA sensor devices with advanced properties. Biosensors based on DNA detection have the advantages of high sensitivity as well as specificity, which is achieved through highly specific DNA amplification, followed by hybridization with a complementary oligonucleotide probe integrated into the DNA sensor. While conventional amplification methods, such as PCR, rely on thermal cycling and require specialized equipment (thermal cycler), isothermal amplification methods have been developed as efficient alternatives. Isothermal amplification techniques enable fast, highly sensitive, specific amplification, carried out at constant temperature, which makes them suitable for PON applications and integration into biosensor devices [14–16].
Fig. 1.
Integration of a genetic construct into a plant genome for the expression of desired traits
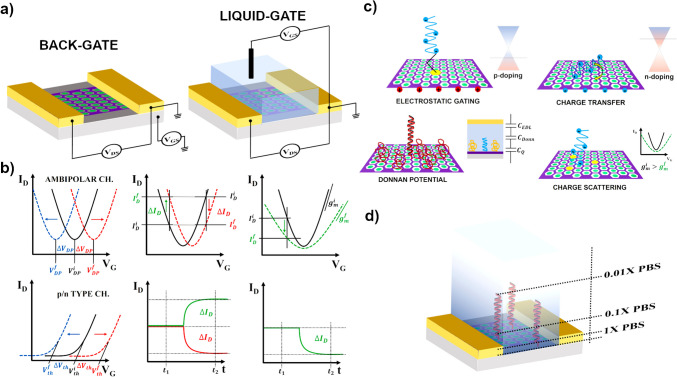
The detection of GM organisms using electrochemical DNA biosensors primarily involves converting DNA hybridization signals into measurable electrochemical signals. In this process, single-strand DNA (ssDNA) probes are first immobilized on the surface of signal transducers, such as glassy carbon electrodes, gold electrodes (GCE), or indium tin oxide. DNA-based electrochemical biosensors utilize a variety of chemistries, all of which capitalize on nanoscale interactions between the target in solution, the recognition layer, and the solid electrode surface. Various approaches to electrochemical detection have been developed, such as direct electrochemistry of DNA, electrochemistry on polymer-modified electrodes, the use of DNA-specific redox reporters, electrochemical amplification with nanoparticles, and electrochemical devices that rely on DNA-mediated charge transport chemistry [17]. Furthermore, a special type of electrochemical-based biosensors is referred to as field-effect transistors (FETs), which operate in a so-called liquid-gate configuration, and they utilize the electrical modulation of the channel material for signal development, unlike classical electrochemical systems. With the advancement of 2D nanomaterials, a new class of FETs, denoted as 2D-FETs, has emerged and they have been extensively developed for DNA biosensing. Figure 2 showcases the workflow of this review paper, covering all important steps toward GMO screening using innovative electrochemical solutions for nucleic acid hybridization detection, and future perspectives of the PON GMO detection.
Fig. 2.
Frame diagram representing a workflow of the review paper
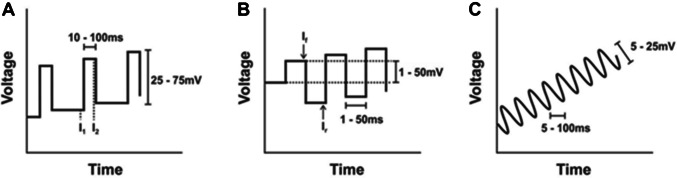
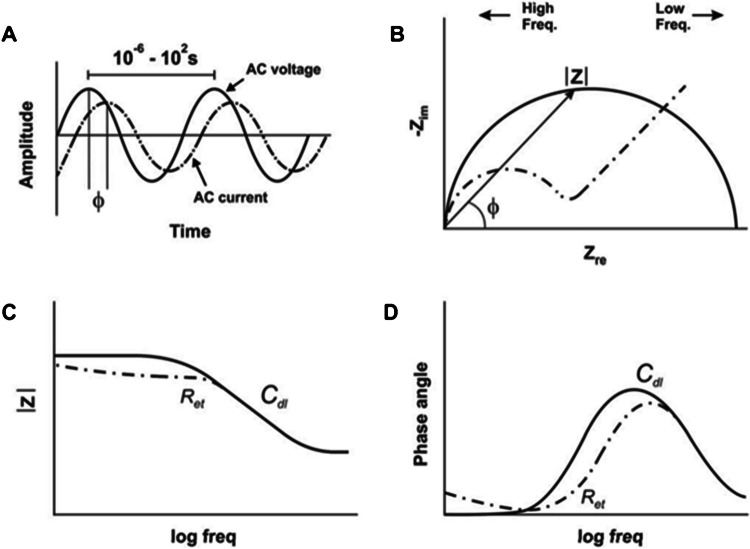
Not many review publications on this topic discuss the electrochemical techniques used in electrochemical DNA sensors. Even in scientific experimental publications, authors only mention certain electrochemical techniques used in that particular work, but without their comparison or the explanation why one is better than another. The electrochemical technique drives the biosensor, gives the input signal to the sensing element at the working electrode, and reads out specific parameters, such as potential and current with time, resistance, conductance, or capacitance. Some of the mostly used are linear sweep voltammetry (LSV), cyclic voltammetry (CV), alternating current voltammetry (ACV), chronoamperometry (CA), chronopotentiometry (CP), chronocoulometry (CC), square wave voltammetry (SWV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS). Depending on the system and the detection mechanism used at the surface of the working electrode, some techniques will give a better electrochemical response than others. The goal is to obtain the possible relative signal difference between the baseline (usually empty buffer solution) and the target DNA molecules. Baseline of the detection (usually a buffer of a certain pH without DNA target) is of crucial importance for any electrochemical technique.
Considering the importance of GMO detection in a cost-effective and time-efficient manner and the possibilities of electrochemical biosensors, this review highlights as follows: (a) challenges in GMO detection and available molecular methods; (b) the most relevant aspects of the current advances in development and manufacturing electrochemical biosensors; (c) application, challenges and opportunities of electrochemical biosensors in GMO monitoring using DNA detection strategy; and (d) research and development toward improvements in reliability and lowering the cost of electrochemical biosensors.
Materials and methods
A literature review was conducted by identifying and analyzing research and reviewing scientific articles as well as technical reports and guidelines provided by international organizations related to GMO detection using molecular approaches and electrochemical-based DNA biosensors. Schematic figures are prepared in Biorender and MS Office PowerPoint software. Images from published articles are adapted and arranged using GIMP (GNU Image Manipulation Software), an open access software. All images reproduced from published articles and book chapters are reprinted with permission, which is stated in the figures’ captions.
Molecular methods to detect GMOs
Polymerase chain reaction-based methods of GMO detection
As one of the foundational tools in molecular biology, a PCR has an everlasting importance in diagnostic screenings. Considering the extensive database of available primers, alongside the specificity and capabilities for quantification, this method integrates fundamental aspects of a molecular biology tool. Therefore, numerous recent studies are focused on the optimization of PCR methodologies. However, in terms of on-site testing, a strong downside of PCR is the need for sophisticated equipment, namely thermal cyclers as the essential equipment for the reaction. The reaction mechanism is reliant on changes in temperatures between each step, to allow strand separation (92–95℃), primer annealing (around 50–60℃), and elongation (72℃). This and the fact that around 30 cycles are needed to produce reliable results cause the reaction time to go up to two and a half hours. The following text summarizes the latest research on applications of PCR for GM testing in agriculture.
In 2019, Bak and Emerson [18] developed a multiplex qPCR for the detection of GM in plants and the identification of false-positive GM plants that were infected by cauliflower mosaic virus (CaMV). They designed TaqMan qPCR probes with different fluorescence wavelengths, enabling simultaneous detection of different target regions. The developed assay targeted GM-related regions P-35S and T-NOS, a CaMV-specific P3 gene, and the actin gene used as a positive control for plants. Achieved limits of detection (LODs) were as follows: 1% for actin, 0.001% for P-35S, and 0.01% for both P3 and T-NOS [18]. A similar study was conducted in 2018 by Becker and Ulrich [19]. Additionally, there is a report from 2020 on the successful development of a qPCR detection method for the first commercialized genome-edited crop, a canola with a single base pair edit resulting in herbicide tolerance. The authors reported high sensitivity and specificity, with a quantification limit of 0.05% [20].
Besides qPCR, one of the most reliable techniques for GMO detection and quantification is digital PCR (dPCR), which, compared to qPCR, is less affected by reaction inhibitors and enables quantification without standard curves. The dPCR reaction mixture is split into many small reactions where each contains none, one, or more than one target copies. Concentration of target DNA is calculated based on Poisson statistics, from the portion of positive end-point reactions [21, 22]. The reaction partitions can be made on a chip with many chambers or small wells in a chamber-based dPCR (cdPCR) [23] or as droplets in emulsion/droplet-based dPCR (ddPCR) [24, 25]. The cdPCR was successfully used in the detection of the MON810 transgenic event from GM maize [23]. Bogožalec Košir et al. [22] developed the simplex and duplex ddPCR assays, which targeted the soybean lectin gene (Le1) and MON40-3–2 soybean transgenic event, while Demeke and Eng (2025) developed two multiplex ddPCR assays to detect 19 soybean GMO events, including element-specific (P-35S, tE9, T-NOS, Pat) and four event-specific (CV127, DP305423, MON87701, MON87751) targets.
Additionally, the ultra-fast PCR (UF-PCR) system can also be used for GM crop detection [26, 27]. The UF-PCR uses the same principle as real-time PCR but differs in the intercalating dye used for signal generation (Evagreen dye instead of SYBR green). It decreases the running time of PCR as well as reagent usage. The UF-PCR was used to detect GM events in rice—KMD1, Bt63, LLRice62, Kefeng6, and Kefeng8 [26], as well as two GM events in potato—EH92-527–1 and SPS-Y9 [27]. These methods use portable equipment and can be used for in-field GMO analyses. In another report on an ultra-fast PCR method, called DSPCR, authors describe a methodology for simultaneous detection of two GM elements (containing P-35S and T-NOS), in a total time of 10 min, achieved by combining Dual Super PCR (generating amplicons in 2.5 min) and a lateral flow biosensor containing gold nanoparticle-labelled antibodies [28].
Isothermal nucleic acid amplification methods for GMO screening
There is a significant discrepancy between the low efficiency of traditional DNA detection methods and the rapid growth of GMO diversity. The need for faster sample-to-result time has led to accelerated development of isothermal nucleic acid amplification techniques (isoNAATs), which are gaining traction in both research and regulatory settings. Among the advantages of isoNAATs, one that stands out is the applicability for in-field testing, enabled by the constant reaction temperature, thus eliminating the need for complex laboratory equipment. Numerous isothermal DNA amplification methodologies have been developed, but some are still in the proof-of-concept early stages (e.g., exponential amplification reaction (EXPAR), hybridization chain reaction (HCR), single primer isothermal amplification (SPIA), silicon film–mediated recombinase polymerase amplification (SMART)).
For example, a group of scientists from China has recently developed SPIA methodology for fast and visual real-time detection of GM crops in-field using SYBR Green II. The developed assay is specific to the CP4-EPSPS gene (the exogenous epsps gene from the Agrobacterium tumefaciens strain CP4 for CP4-EPSPS protein). They have determined the assay LOD to be 4 copies/μL, making this methodology more sensitive than the real-time PCR method (20 copies/μL) [29].
On the other hand, some methodologies are already in the commercialization phase: loop-mediated isothermal amplification (LAMP), rolling circle amplification (RCA), recombinase polymerase amplification (RPA), helicase-dependent amplification (HDA), strand displacement amplification (SDA), nucleic acid sequence–based amplification (NASBA).
Loop-mediated isothermal amplification
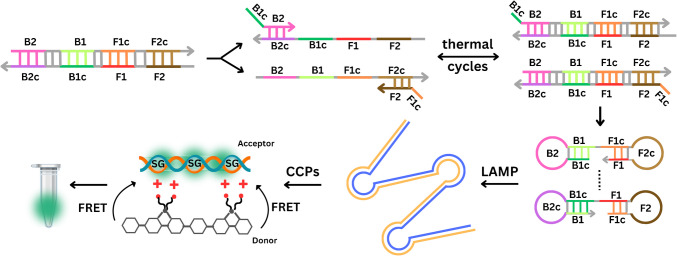
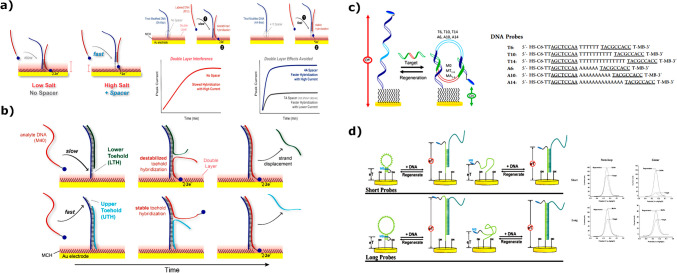
LAMP is the most studied isothermal amplification methodology and has been in use since its development in 2000 [30]. The reaction is highly specific, owing to the use of four or six primers which bind to six or eight regions on the target sequence. Amplification is carried out using a Bst polymerase with a high strand displacement activity, and time to result can be as fast as 15 min in some cases, but overall, amplification takes no longer than 60 min, generating between 109–1010 gene copies (Fig. 3). In terms of signal readout, there are two different options: real-time fluorescence-based quantitative analysis, using intercalating dyes (SYBR Green II), or a colorimetric qualitative analysis, using pH-based dyes such as phenol red. Scientists suggest that LAMP will become a gold standard in molecular diagnostics, next to PCR. Both methods hold several advantages over the others, but an additional advantage of LAMP in terms of PON testing lies in the fact that it is affected by higher concentrations of common inhibitors in comparison to PCR [31]. This holds great significance in the context of GMO screening in agriculture, since plant extracts often contain various inhibitors, such as polysaccharides, herbal metabolites, and tannic acid. In 2022, a LAMP reaction was developed for the detection of GM in maize by targeting T-NOS and P-35S sequences. In this study, LAMP was coupled with BART technology for rapid results using simple equipment [32]. In another study, LAMP was designed for visual detection of GM material in plant-made food samples, targeting a set of eight components including universal elements, marker genes, and exogenous target genes (CaMV P-35S, P-FMV 35S, T-NOS, bar gene, cry1 Ac gene, CP4 epsps gene, pat gene, and NptII gene) [33]. Overall, multiple papers describe LAMP assays for the detection of these elements individually or in different combinations [34–37]. Other targets detected with LAMP for GM screening include T-pinII terminator sequence [38]; transgenic soybean events such as MON87701, MON87705, MON87708, and MON87769 [39]; living modified cotton events such as MON88701, MON531, MON15985, MON88913, and COT102 [40]; or selectable marker genes such as pmi, hpt, and gus in GM rice [41]. An interesting approach to LAMP utilization in GM maize detection with high sensitivity was described recently. This LAMP reaction uses only two primers (FIP and BIP) which specifically recognize the four regions of the target gene (F1, F2, B1, and B2). During the first step of the two primer-induced cascade exponential amplification reaction, FIP and BIP primers produce multiple double-strand DNA (dsDNA) through repeated thermal cycles following the same principle as PCR. The products of the first step amplification can self-hybridize on both ends in a double stem-loop DNA, enabling the second step LAMP reaction. The two primer-induced cascade exponential amplification reaction was combined with cationic conjugated polymer (CCP)–based visual detection when exposed to UV light [42].
Fig. 3.
Water-soluble cationic conjugated polymers (CCPs) have a delocalized π-conjugated backbone that transfers excitation energy to an acceptor fluorophore via fluorescence resonance energy transfer (FRET), amplifying the fluorescence signal by about ten times. Sybr Green I (SG), a dsDNA-specific dye, also acts as an energy acceptor for efficient FRET with CCPs as the donor. This enables easy visual detection of GM crops, making CCPs ideal for field testing and screening. Reprinted and modified from [42], with permission from Elsevier B.V. Copyright © 2024
Rolling circle amplification
RCA methodology was invented in 1998 by Lizardi et al. [43], as a method that can rapidly (within a few minutes) produce hundreds of tandemly linked copies of target DNA using a circularizable probe. The probe binds on both ends to the target DNA, leaving a small gap that can be filled either by a small oligonucleotide ligated by DNA ligase or by adding nucleotides using DNA polymerase. In both cases, the probe becomes closed, and reaction starts with binding of a primer complementary to the circularizable probe and proceeds at a single temperature by activity of DNA polymerase with strand displacement activity. This results in a single strand of tandem repeats of a circular probe. A second (reverse) primer binds to each tandem repeat, forming dsDNA branches, enabling further reaction proliferation. Reaction time is variable and depends on the type of template, the concentration of reagents, the temperature at which the reaction is carried out, and the desired amplification yield. The reaction can be monitored in real time using DNA intercalating dyes (such as SYBR Green), or the RCA product can be detected using labeled probes or labeled dNTPs. A study from 2022 explored the feasibility of RCA for visual detection of the CaMV35S promoter. The detection limit was 3 × 10−14 mol/L in optimal experimental conditions [44].
Recombinase polymerase amplification
Another widely used methodology, developed in 2006 by Piepenburg et al. [45], is RPA. A reaction mechanism is based on the formation of complexes of recombinase proteins with forward and reverse primers. Recombinase helps primers bind homologous sequences in the target DNA by displacing the template’s strands. The displaced template strands are stabilized by single-strand DNA (ssDNA) binding proteins. Strand-displacing polymerase replaces recombinase, binds to each primer, and starts DNA elongation. Depending on the polymerase, the reaction temperature varies between 37 and 42 °C. In 2020, an RPA-based lateral flow strip was designed for the detection of CaMV P–35S and T-NOS elements, which have > 70% coverage in all authorized GMO species worldwide, as indicators of genetic modifications in various crops. In this study, RPA primers were labeled with fluorophores at the 5′ end and showed room temperature detection of 50 copies and 100 copies of GM genes [46]. Moreover, Wang et al. reported an RPA-based lateral flow strip for sensitive detection of CP4-EPSPS and Cry1 Ab/Ac genes in GM crops, applicable for on-site detection [47].
Helicase-dependent amplification
Helicase-dependent amplification (HDA) is an isoNAAT with high sensitivity that uses a helicase enzyme to achieve exponential amplification. Helicase is responsible for DNA unwinding and strand displacement. Besides helicase, the reaction also requires two target-specific primers and a DNA polymerase [48]. The method was used to detect GM maize lines NK603, MON810, and Bt11 and for the development of electrochemical-based genosensors for GMO detection [48, 49].
Strand displacement amplification
Additional but less used methodology for GMO detection is strand displacement amplification (SDA). The SDA uses primers with two functional parts, a target recognition sequence, and a restriction endonuclease recognition sequence. It also requires a polymerase with strand-displacement activity. This methodology failed to detect GM maize lines NK603, MON810, and Bt11 by Zahradnik et al. [48]. However, a proximity extension-mediated multiple cascade SDA (PE-MC/SDA) system was later successfully combined with CRISPR/Cpf1 for highly sensitive detection of GMO by Liu et al. [50].
Multifunction-integrated linear oligonucleotide probe sensing approach
In addition to previously described isoNAAT methods, Yan et al. [51] developed multifunction-integrated linear oligonucleotide probe (MI-LOP)-based sensing approach to detect edited regions of the genome in GMOs. MI-LOP enables label-free fluorescent detection of GMOs. It contains a region complementary to the target DNA included in the formation of the polymerization primer–linked G-quadruplex (PP-G-quadruplex). The PP-G-quadruplex, target DNA, and products of target analog formed by polymerase and nickase (endonuclease) activity are reused multiple times inducing a multiplex signal amplification, i.e., multiplication of stable G-quadruplex. The stable G-quadruplexes are further combined with N-methyl mesoporphyrin IX (NMM), increasing the detection fluorescence signal. The MI-LOP approach is highly sensitive and highly specific under isothermal conditions. However, the overall procedure is laboratory intensive, lasts more than 3 h, and is not suited for PON applications.
Despite obvious advantages, each isoNAAT has its own set of challenges that can impact its efficacy and suitability for specific tasks (Table 1).
Table 1.
Disadvantages of selected isothermal amplification techniques (adapted from Oliveira et al. [52] and Srivastava and Prasad [53])
| LAMP | RCA | RPA | HDA | SDA |
|---|---|---|---|---|
| Not ideal for amplifying short DNA sequences | Ability to amplify only circular DNA templates | Strict reaction conditions | Non-specific priming due to the lack of stringent temperature requirements | Initial template denaturation needed |
| Non-specific amplification | Complicated RNA amplification | Amplifications cannot be easily detected by electrophoresis | False positive result | Non-specific amplification |
| Demanding primer design (4–6 primers) | Linear targets require ligation reactions | Expensive commercial kits | Low sensibility and selectivity | Limitation to small targets |
| Fine-tuning the reaction conditions are often required to achieve reliable results | Linear amplification profile—yields lower amplification efficiency | / | Single-proprietary commercialization | Requires sample preparation |
LAMP loop-mediated isothermal amplification, RPA recombinase polymerase amplification, RCA rolling circle amplification, HDA helicase-dependent amplification, SDA strand displacement amplification
CRISPR/Cas
A clustered regularly interspaced short palindromic repeat (CRISPR) and CRISPR-associated protein (CRISPR/Cas) is a natural component of the adaptive immune system of bacteria, protecting them against plasmids or viral DNA and ensuring optimal bacterial cell functioning. When the system recognizes invading genetic material, fragments of it are incorporated into the bacterial DNA in the form of spacers. Thus, if a second interaction with the same or similar invading genetic material occurs, the CRISPR/Cas system recognizes and initiates destruction of the inviting genetic material. Since its discovery, researchers have been exploring its capabilities for various applications, including detection of pathogens, genetic mutations, and other biomarkers associated with diseases [54].
The CRISPR/Cas system relies on Cas enzymes and a guide RNA (gRNA) to cleave target molecules, which can be ssDNA and dsDNA, as well as single-stranded RNA (ssRNA). The most widely studied systems include CRISPR/Cas 9, CRISPR/Cas12, and CRISPR/Cas14 (now referred to as Cas12f), and CRISPR/Cas13. The different Cas enzymes vary in specific activities and the structure of the guide RNA they require. CRISPR/Cas9 is specific for dsDNA, CRISPR/Cas12a targets both ssDNA and dsDNA, CRISPR/Cas13 acts on ssRNA, and CRISPR/Cas14 focuses on ssDNA [55]. CRISPR/Cas systems are often combined with isothermal amplification methods like RPA and LAMP, which enhance their sensitivity. They are also employed to detect proteins, analytes, and hormones [56].
Recently, a CRISPR/Cas12a assay was coupled with RPA (named RPA-Cas12a-GM) to detect genes for herbicide and insect resistance (CP4-EPSPS and Cry1 Ab/Ac genes) in GM crops. Their approach enabled detection of only 45 copies/μL of the standard plasmid, with the results visualized in the form of band appearance by the generation of a fluorescence signal on a lateral flow strip within 45 min [57]. Moreover, a recent study reported a lateral flow assay for detection of CP4-EPSPS and Cry1 Ab/Ac in GM crops, based on duplex RPA coupled with CRISPR/Cas12a [58]. Similarly, a recent report describes a sensitive and specific LAMP-CRISPR/Cas12a lateral flow assay uses fluorescence and a test strip system for detection of CP4-EPSPS and Cry1 Ab/Ac genes in-field [59]. This assay was developed by Pataer et al. By monitoring fluorescent signal release, their assay enables visual differentiation of 0.5% genetic modifications in maize via the cleavage of reporter probes by the Cas12a, in isothermal conditions. In this study, multiple pairs of stem-loop primers are used to increase the formation of double stem-loop DNA and thus enhance the LAMP efficiency. By using universal primers simultaneously to detect multiple genes, the detection sensitivity is increased which enables amplification of target genes in concentrations as low as 100 aM [60].
Moreover, one tube CRISPR/Cas system coupled with RCA was developed to detect GM crops (Bt11 and MON89034). In this assay, a primer of RCA was used as the cleavage substrate of Cas12a/gRNA. Additionally, the authors proposed the developed assay to be used to target different gene sequences by simply changing gRNAs [61].
High-throughput sequencing in GMO detection
Although previously described methods are suitable for GM crop screening, identification of transgenes, insertion sites, and flanking sequencing in transgenic plants can be achieved using nucleic acid sequencing approaches. High-throughput sequencing technologies including both next-generation sequencing (NGS) and third-generation sequencing (TGS) revolutionized GMO characterization by enabling identification and description of all (including non-authorized) transgenic events present in the genome [62]. Most of the NGS systems are based on the concept of “sequencing by synthesis” (SBS) with sequential detection of nucleotide incorporation using an engineered DNA polymerase [63]. For characterization of GM plants, different NGS approaches such as whole genome sequencing (WGS) and targeted sequence enrichment are used (e.g. [64]—maize; [65] —soybean; [66]— rice; [67]—multiple crops; [68]—multiple plants and their food products). Targeted sequence enrichment includes a set of technologies designed to isolate specific genomic regions for subsequent NGS, providing an enriched pool of target sequences and enabling higher sequence coverage for each targeted region [69].
The main drawback of NGS approaches is short sequence reads. Having short read length, NGS approaches have limited potential to identify complex genomic sequences, all insertion sites and flanking sequences for rearrangement of transgene events, or complex modifications of exogenous fragments. Third-generation, long-read sequencing technologies overcome these challenges [62]. The TGS technologies are developed as real-time single-molecule sequencing (SMS). They include single-molecule real-time (SMRT) sequencing developed by Pacific Biosciences (PacBio) and nanopore sequencing developed by Oxford Nanopore Technologies (ONT). The most important features of these technologies are the absence of a PCR amplification step, real-time sequencing process, and the production of long reads [70].
Zhang et al. [71] developed a universal Large Integrated DNA Fragments Enrichment strategy (LIFE-Seq) for the identification of transgenes in GMOs based on PacBio sequencing. LIFE-Seq was tested using four crop species (soybean, maize, rice, and canola) and six GM events (MON810, GTS 40–3-2, TT51-1, NK603, RT73, and Rf2). The method achieved better data integrity and accuracy as well as greater universality compared to NGS-based WGS. Additionally, it is suitable for transgenic crops with complex structures of inserted DNA. Regarding nanopore sequencing, it is often used in combination with PCR and/or NGS [72, 73] for GM plant characterization since a higher error rate requires result confirmation.
The significance of high-throughput sequencing in GM plant characterization is indisputable, but its application for in-field experiments is still very limited. Apart from the MinION (ONT), a compact, lightweight sequencing instrument (< 100 g) compatible with desktop PCs or laptops, there are no sequencing instruments that can operate outside the laboratory. However, even the MinION implementation for on-site molecular characterization of GMOs is still challenging [62].
Methods for in-field DNA extractions
An important step in the development of PON DNA sensors, except for amplification and detection methods, is DNA extraction. Over the years, different rapid DNA extraction methods suitable for in-field conditions have been developed. However, many of these techniques result in crude DNA extracts containing cell debris and potential amplification inhibitors and, thus, have lower DNA quality compared to standard methodologies (cetyltrimethylammonium bromide (CTAB), sodium dodecyl sulfate (SDS), commercial DNA extraction kits, etc.). Although the presence of inhibitors can affect the performance of standard PCR methodologies, isoNAATs are less sensitive.
Zhu et al. [74] combined LAMP reaction with fast DNA extraction method from plant tissue of nine GM crops using lysis buffer containing 0.5 M guanidine hydrochloride (GuHCl) which destroys cell membranes, denature proteins, and reduce DNA degradation; 4 M urea responsible for protein denaturation and stability of GuHCl in aqueous solution; 0.1 M Tris–HCl (pH 8.0) as buffer; and 0.05 M ethylenediamine tetraacetic acid disodium (EDTA-2 Na), a metal chelating agent that can form stable complexes with metal ions. Plant tissue is ground with a handheld grinder for 3–5 min in the presence of the described lysis buffer. After grinding, it was left for 2 min to settle down before LAMP amplification. Further, for specific, in-field detection of the MON863, Wang et al. [75] performed NaOH-based method for preparation of crude cell lysate coupled with real-time RPA. First, the samples were ground into powder, transferred to a centrifuge tube (0.01 g), and dissolved in a lysis buffer (0.5 M NaOH, 10 mM Na2EDTA, pH 8.0). The tube was strongly mixed for 5–10 s and incubated for 1 min at room temperature. Finally, 1 μL of tenfold supernatant dilution was directly added into the real-time RPA system as the DNA template. Xiao et al. [76] further simplified the described methodology for application in a microfluidic LAMP system. Additionally, a method with a strong potential to be adapted for field applications is the alkaline polyethylene glycol (PEG) extraction method. The extraction using alkaline PEG lysis buffer (20 mM of NaOH in 6% PEG 200 solution) was specifically developed as a rapid and simple method for genotyping plant species using direct-PCR, i.e., PCR from crude plant extract [77]. Plant leaf tissues were cut into small pieces (1 mm) and soaked in 50 ul of freshly prepared alkaline PEG lysis buffer, briefly vortexed, spun down, and incubated at room temperature for 1 min for cell lysis. The method proved successful in direct-PCR amplification of transgenes in rice, tobacco, rape, potato arabidopsis, and chrysanthemum. Since most of the rapid DNA extraction methods suffer from lower DNA quality in comparison to standard DNA isolation methods, Wang et al. [78] adapted magnetic bead method, known for high quality of extracted DNA, for rapid on site applications. The magnetic bead method is based on magnetic beads coated with a substance that has a strong affinity for DNA. After DNA is freed from cells, it is mixed with magnetic beads; magnetic beads are then immobilized using a magnetic field, and contaminating factors are washed away. This extraction method requires a centrifuge and a magnetic field to absorb the magnetic beads and can take up to 1 h. Wang et al. [78] shorten the process to 5 min and use only a bar magnet and a plastic cover making it easy to apply in the field. However, the simplified procedure resulted in decreased DNA yield and purity, although DNA amplification using PCR and RPA was not affected.
Current point-of-need solutions for in-field GMO detection
For diagnostic purposes, such as in GM screening, applicability in-field is of high value. Simplified presentation and interpretation of results are essential for decentralized laboratory testing in resource-constrained settings. Moreover, in order to make GMO screening accessible to end users with no prior knowledge of diagnostic testing, molecular detection methods must be combined with an efficient and easy-to-perform sample preparation methodology without specialized equipment [79]. These requirements are unified through PON assays and devices, which enable fast, simple, specific, and sensitive laboratory-independent testing. The increasing cultivation and commercialization of GMOs, particularly their use in food and feed, raises concerns about consumer awareness and food labeling. Therefore, developing rapid, reliable, cost-effective, and portable methods for GMO detection is essential [80].
Biosensors for GMO detection based on isothermal amplification methods offer a promising solution for the efficient identification of GMOs in various food and agricultural products. These methods, such as LAMP, utilize specific primers to amplify target DNA sequences under constant temperature conditions, eliminating the need for expensive thermal cycling equipment. When combined with sensors, such as electrochemical, optical, or lateral flow sensors, these amplification techniques enable the real-time detection of GMO markers, such as the Cauliflower Mosaic Virus 35S promoter or other transgene sequences [36, 46]. The main advantages of isothermal amplification-based sensors include their applicability in field settings without large-scale equipment, their affordability, and their rapid processing time, often providing results in under an hour [81]. These sensors are highly sensitive, capable of detecting even trace amounts of GMO DNA, and can be easily adapted for various applications, including food safety monitoring, environmental control, and agricultural testing. The development of such sensors addresses the growing demand for reliable, on-site GMO detection methods that ensure proper labeling and consumer transparency [15].
Considering that LAMP reaction enables a fast time-to-result while retaining high specificity and sensitivity, there are a lot of reports on LAMP-based PON devices for GMO screening [35–38]. The study conducted by Ahmed et al. [82] introduces, for the first time, a rapid, cost-effective, and efficient electrochemical genosensor for detecting GM maize (CBH 351). The system integrates LAMP with electrochemical detection using a DNA stick (DS) containing a disposable electrochemical printed (DEP) chip. Unlike conventional methods, this approach enables direct analysis of unpurified LAMP amplicons, eliminating the need for probe immobilization and reducing cross-contamination risks. Detection is based on the interaction between DNA and the redox-active molecule Hoechst 33,258 (H33258), which binds to the minor groove of DNA, causing a significant decrease in peak current intensity during linear sweep voltammetry (LSV) analysis. The optimized sensor demonstrated a detection limit of approximately 3 × 102 copies per reaction and could identify GMOs within 20 min [82]. Several years later, Moura-Melo et al. [49] published a study on an electrochemical genosensor that combines HDA and sequence-specific detection of a commonly present CaMV P-35S element in GM plants. HDA is an isothermal DNA amplification method that mimics bacterial replication, and it is similar to PCR. However, it often lacks selectivity due to nonspecific amplification. To address this, an electrochemical platform was developed to target specific sequences in the amplification products. The system employs a binary monolayer on a gold film, which, upon hybridization with amplification products, triggers enzyme labeling and electrochemical detection. This approach improves selectivity and enhances sensitivity by up to 10⁶-fold, enabling the detection of GMOs with a LOD of approximately 30 copies of the CaMV P-35S sequence. The method, which requires minimal equipment (a heating block and potentiostat), is portable, low-cost, and suitable for in-field applications [49].
When it comes to PON biosensors, a portable and efficient on-site detection method for stacked GM soybean (DP305423 × GTS 40–3-2) was introduced in 2017, using event-specific tag-labeled multiplex LAMP (TM-LAMP) combined with a DNAzyme-enhanced lateral flow biosensor (DLFB). A trident-like lateral flow biosensor was developed to enable simultaneous visualization of amplified products while minimizing cross-contamination. Additionally, the incorporation of DNAzyme technology significantly enhanced detection sensitivity. Three newly designed primer sets, targeting both event-specific and species-specific sequences, ensured precise identification. The optimized assay demonstrated a detection limit of approximately 0.1% (w/w), aligning with international regulatory thresholds for GM content. The entire detection process was completed within 120 min, without requiring large-scale laboratory equipment, making it highly suitable for in-field applications [83]. One year later, Kaygusuz et al. [84] took a step further and presented DaimonDNA, an affordable, lightweight, and user-friendly biosensor for GMO detection based on LAMP. The device allows real-time, naked-eye visualization of amplification using hydroxynaphthol blue within 30 min. DaimonDNA is constructed from off-the-shelf electronic components and 3D-printed materials, making it highly cost-effective (< 25 euros), compact (6 × 6 × 3 cm), and portable (108 g). The system was validated for detecting the lectin gene of soybean as a species control and the CaMV P-35S element. It demonstrated specificity with genomic DNA of RoundUp Ready (RRS) and MON89788 soybean, achieving a detection limit of 0.1% in a 10% GM soybean sample. Performance benchmarking against a thermocycler confirmed that DaimonDNA’s amplification and detection efficiency is comparable to other isothermal amplification methods. Due to its portability and affordability, DaimonDNA has potential applications beyond laboratory settings. This platform overcomes the limitations of existing LAMP-based detection systems, making it a practical tool for resource-limited environments [84]. A recent report by Xiao et al. [76] demonstrated a hand-held microfluidic chip based on LAMP. To meet the requirements of PON testing, they integrated the microfluidic chip with (1) a rapid DNA extraction methodology for in-field qualitative detection of GM maize or soybean samples and (2) an analyzer and Android App to enable equipment-free analysis of the LAMP reaction. Moreover, a 2024 study described a similar way to design an integrated PON test, by combining a 5-min DNA preparation step with an easy automatic readout system on a smartphone, enabling a rapid LAMP-based assay for the detection of GM crops. With this approach, the sample-to-result time was lowered to only 25 min [74]. In a study conducted by Wang et al. [79], a platform for on-site detection of genetically modified crops was developed, with a time-to-result of 40 min without laboratory settings. Their system integrated sample pre-treatment modules into a microfluidic chip and performed LAMP-based DNA amplification via a battery-powered portable kit, and the results were detectable by naked eye observation of color change.
In addition to the LAMP method, other isothermal amplification methods, coupled with biosensors, have also found applications in GMO detection. Therefore, the study by Wang et al. [78] presents an integrated system based on duplex recombinase polymerase amplification (DRPA) and a lateral flow biosensor for fast and reliable detection of GM crops in the field. The system combines three key steps: rapid DNA extraction (5 min), amplification of target sequences, and visualization of results using a lateral flow biosensor. Through an optimized DRPA assay, the universal screening elements for GM crops (CaMV P-35S and T-NOS) were amplified with high specificity and sensitivity. The detection limit was approximately 10 copies of GM soybean DNA and 100 ng of DNA from 0.1% GM soybean. The entire detection process is completed within 20–30 min, does not require expensive laboratory equipment, and minimizes the risk of contamination. The system was successfully validated for detecting GM rice, demonstrating its effectiveness for field applications [78]. Moreover, RPA was also utilized for a fluorescence-based detection of MON863 in maize within 10 min, using a portable device for fluorescence readout [75]. Numerous studies outline different ways of integration of RPA methodology for on-site GM detection [46, 47, 58].
Concerning multiplex RPA, an interesting study was published on a novel isothermal paper-based biosensor for the multiplex detection of GM maize. The system integrates single universal primer recombinase polymerase amplification (SUP-RPA) with an LFB to enable rapid, event-specific detection. The SUP-RPA method employs primers with a universal sequence at the 5′ end, enhancing amplification efficiency and ensuring consistency across multiple targets. Additionally, biotin-labeled deoxycytidine triphosphate (dCTP) improves signal detection by increasing the binding of gold nanoparticles (AuNPs) to target DNA. The lateral flow biosensor visually identifies amplification products via dual hybridization, producing a characteristic red band for detection. This approach demonstrated high sensitivity, with a detection limit as low as 50 copies, allowing simultaneous identification of GM maize events MON863, MON810, and MON89034. The entire process is completed within 30 min without requiring complex laboratory equipment, making it a cost-effective and portable solution for PON GMO screening [85].
Biosensors based on isothermal amplification techniques offer numerous advantages, such as simplicity and suitability for field use, yet face significant limitations that affect their reliability, scalability, and practical application (Table 2).
Table 2.
Disadvantages and limitations of biosensors utilizing isothermal amplification techniques. Summarized from Oliveira et al. [52], Srivastava and Prasad [53], Li and Macdonald [86], Giuffrida and Spoto [87], Becherer et al. [88], Glökler et al. [89], and Cao et. al. [90]
| Non-specific amplification and background noise | Isothermal amplification methods, especially those based on exponential amplification, often generate unwanted, non-specific products that can lead to false-positive results. This makes it harder to differentiate between true signals and background noise, reducing the assay’s specificity. The ability to produce multiple unintended amplification products can complicate accurate target detection, thus undermining the overall reliability of the biosensor. |
| Primer design complexity | Methods like LAMP require the design of multiple primers—usually 4 to 6 for each assay. This complexity significantly increases the time required for assay development and optimization. The risk of errors during primer design also rises, as incorrect primers can result in inefficient amplification or nonspecific binding, further complicating the diagnostic process. |
| Sensitivity to reaction conditions | One of the main challenges with isothermal amplification techniques is their high sensitivity to the reaction conditions. Precise control of temperature, pH, ionic strength, and reagent concentrations is essential to ensure optimal amplification efficiency. For systems like DNAzyme-based amplification, these conditions become even more demanding, as they rely on the specific cation concentrations, which can vary widely and may not be compatible with all systems, further complicating the process. |
| Limited multiplexing capability | Isothermal amplification techniques generally face challenges when it comes to detecting multiple targets in a single reaction. The complexity of primer design for each target increases the risk of cross-reactivity, where primers may bind to unintended sequences. This significantly limits the ability to run multiplex assays that could detect multiple pathogens or biomarkers simultaneously, making these methods less effective for comprehensive diagnostic applications. |
| Difficulty in quantification | Most isothermal amplification methods are primarily qualitative, meaning they are designed to confirm the presence or absence of a target rather than measure its quantity. To achieve accurate quantification, additional steps or strategies are often required, such as digital methods or real-time monitoring systems. These supplementary technologies complicate the process and add cost, making the quantification aspect of isothermal amplification less accessible and less reliable in certain applications. |
| Contamination risk | Isothermal amplification systems, by virtue of their high sensitivity, are particularly prone to contamination. Even the smallest traces of amplified product from previous reactions can serve as templates in subsequent runs, leading to false positives. This risk is heightened in systems that require manual handling or during multiplexed assays, where contamination from one reaction can quickly spread across multiple targets. |
| Operational complexity and low automation | Many biosensor systems based on isothermal amplification still require manual intervention at various stages, such as reagent handling, mixing, or temperature adjustments. This introduces variability into the results, reducing the reproducibility and robustness of the assay. Full automation, necessary for consistent and reliable operation, remains a challenge, as many systems require intricate handling steps that are not easily integrated into automated platforms. |
| Challenges in miniaturization and point-of-need applications | While there have been advancements in miniaturizing isothermal amplification methods, fully integrating these systems into portable, PON devices is still a significant challenge. Achieving miniaturization while maintaining the sensitivity and accuracy of the assay is difficult due to the need for precise thermal control and reagent management, which are challenging to execute in small, portable formats. |
| High reagent costs and stability issues | The reagents required for isothermal amplification, such as specialized enzymes (e.g., recombinases, helicases) and modified nucleotides, are often expensive. These enzymes are crucial for the amplification process but tend to have limited shelf lives and may require cold-chain logistics, further adding to operational costs. The requirement for costly reagents and the logistical hurdles of ensuring reagent stability under field conditions make these systems less practical for widespread use. |
| Detection dependent on specialized equipment | Some isothermal amplification methods rely on fluorescence-based or sequencing-coupled detection systems, which require expensive, often bulky equipment to read results. Although efforts are being made to develop smartphone-based solutions, these detection methods are not yet universally accessible, limiting the usability of these isothermal amplification systems to well-equipped laboratories or specialized settings. |
| Low technology readiness level (TRL) | Many of the novel isothermal amplification biosensor systems are still in the early stages of development, typically below TRL 4. This means that these platforms are not yet ready for widespread commercial deployment. Issues such as system integration, scalability, and long-term stability need to be resolved before these technologies can be considered for practical, large-scale use in clinical or field settings. |
| Patent and licensing barriers | A significant challenge for the commercialization of isothermal amplification methods is the existence of numerous patents and intellectual property protections surrounding key amplification enzymes and reagents. These patent barriers increase the cost of production and limit the accessibility of the technology to researchers and companies, which can delay innovation and reduce the affordability of the final biosensor products. |
Given the specificity that a CRISPR/Cas system adds to a DNA detection assay, its usage has expanded significantly. Therefore, there are several recent reports of CRISPR/Cas-based PON tests [57, 58, 91], for example, an immunoassay strip for sensitive on-site detection of CaMV P-35S and T-NOS by combining CRISPR/Cas12a with RPA. The authors of this paper determined that the assay can be completed within 40 min at 37 °C, and the result can be interpreted by naked eye observation [91]. Furthermore, Duan et al. [92] reported a method for on‐site detection of GM crops, based on CRISPR/Cas12a in a single‐tube detection vessel, thus preventing contamination and reducing false positives. They reported detection of 0.01% GM tissue in a mixed sample of GM and non‐GM tissues within 40 min, without specialized equipment. Finally, Liu et al. [50] demonstrated an ultrasensitive fluorescent biosensor for detecting the CaMV P-35S element in GMOs. The biosensor utilizes a proximity extension-mediated multiple cascade strand displacement amplification (PE-MC/SDA) system combined with CRISPR/Cpf1 for highly sensitive detection. The detection mechanism begins with the recognition of CaMV 35S by adjacent primer probes, triggering a proximity extension reaction. This initiates a multiple cascade strand displacement amplification (MC/SDA), generating a large quantity of single-stranded DNA (ssDNA). These products activate the trans-cleavage function of CRISPR/Cpf1, which then degrades nearby ssDNA-FQ reporters, producing a strong fluorescent signal. The strategic three-way junction design minimizes background noise, while the combined MC/SDA and CRISPR/Cpf1 amplification significantly enhances detection sensitivity. The biosensor demonstrated a wide linear detection range (50 fM–10 pM and 10–500 pM), with an exceptionally low LOD of 14.4 fM. Additionally, it exhibited high specificity and accuracy in real sample analysis, making it a promising tool for GMO detection [50].
The aforementioned biosensors offer cost-effective, rapid, and sensitive solutions for GMO detection, suitable for on-site screening and regulatory compliance. The biosensors are adaptable for a variety of applications, including food and feed safety, environmental monitoring, and clinical diagnostics, enabling multiplex detection of different GMO events. They also improve GMO detection in food and enhance transparency for consumers, supporting regulatory compliance and better food safety standards. On the other hand, further optimization is needed to enhance biosensor sensitivity, expand target detection, and improve accessibility, with the potential for integration into digital platforms.
Electrode materials for electrochemical and FET-based DNA biosensors
The performance of all biosensors relies heavily on the materials used for electrode fabrication, which facilitate the electrochemical detection of DNA interactions. Electrodes in electrochemical biosensors serve as the interface for the transfer of electrons between the biological system (such as an enzyme or receptor) and the external circuit [93]. Besides good conductivity, the electrode material should be compatible with biological samples to prevent interference with the biochemical processes. An important feature is stability, because the electrode material must maintain its structural integrity and performance over time, especially under varying environmental conditions (pH, temperature). Electrocatalytic activity is another important property, since the material should facilitate efficient electron transfer during the biochemical reaction, enhancing sensitivity and response time. And one of the crucial parameters related to electrode materials is surface area. A high surface area allows for more interaction sites for the analyte or biomolecules, improving sensor performance and sensitivity. Common materials for electrode fabrication include metals, carbon-based materials, and conducting polymers, since good conductivity is the most important property. Recent developments pushed forward 2D nanomaterials such as graphene, reduced graphene oxide (rGO), MXenes, and transition metal dichalcogenides (TMDS) as alternatives to commonly used materials [94].
Overview of materials used in electrochemical electrode systems and FET-based devices
Noble metal materials
Noble metals like gold, platinum, and silver are the most common choice due to their high conductivity, biocompatibility, and ease of surface functionalization. Gold is favored for its ability to form self-assembled monolayers (SAMs) with thiol-based DNA probes [95], which can significantly enhance the sensor’s specificity and stability. However, the surface conditions of gold are crucial in determining the performance of the biosensor. Surface roughness is a key factor because it directly influences the surface area available for DNA probe immobilization. A rougher surface increases the active area on the electrode, allowing for a higher density of DNA probes to be attached, which can improve the sensor’s sensitivity by increasing the number of available binding sites. In addition, rough surfaces can also enhance the electrochemical signal by providing more localized sites for electron transfer, which can lead to stronger signal responses [96]. However, there is a balance to be struck, as excessively rough surfaces could result in non-uniform DNA immobilization or unwanted side reactions that may interfere with the biosensor’s performance. Nanoporous metals, such as gold, silver, and platinum, have gained significant attention as electrode materials for DNA biosensors [97]. The high surface area provided by the nanoporous structure allows for a greater density of DNA probes to be immobilized, which can improve the sensor’s sensitivity and detection limits. Additionally, the tunable pore size and morphology of nanoporous metals offer flexibility in optimizing the electrode for specific biosensing applications. Furthermore, nanoporous metals can be easily functionalized with various biorecognition elements, such as DNA probes, to ensure specific interactions with target DNA sequences. These features make nanoporous metals ideal candidates for DNA biosensors, where high sensitivity, fast response times, and reliable performance are critical. Surface thickness also plays an important role in the sensor’s functionality. Thin gold films are commonly used in DNA biosensors because they provide good conductivity while being compatible with microfabrication techniques. The thickness of the gold layer affects the mechanical flexibility and stability of the electrode, and it also influences the electrochemical properties, such as the efficiency of electron transfer and the formation of SAMs. Thicker gold films can be more durable but may lead to less efficient electron transfer if not carefully engineered. Conversely, thinner films might offer improved response times but can be more susceptible to surface degradation over time. Finally, the functional groups on the gold surface are critical for the specific and stable attachment of DNA probes. Functionalization typically involves introducing thiol groups (-SH) to form stable thiol-gold bonds, creating a SAM on the electrode surface. This surface modification is essential for attaching DNA strands in a well-organized manner, ensuring that the probes are oriented properly for hybridization with complementary DNA sequences. The presence of additional functional groups, such as amines or carboxyls, can further optimize the biosensor’s performance by enhancing the binding interactions between the DNA probes and the electrode surface or by enabling additional bioactive molecules to be immobilized. Surface functionalization also helps to minimize non-specific adsorption of biomolecules, which can reduce background noise and improve the selectivity of the sensor.
Carbon-based nanomaterials
Carbon-based materials, such as glassy carbon (GC) and graphite, and nanomaterials like carbon nanotubes (CNTs), graphene, and graphene oxide (GO), have gained significant attention in the development of DNA biosensors due to their excellent conductivity, large surface area, and high mechanical strength. Graphene, a two-dimensional material, offers unique properties that make it an ideal candidate for biosensing applications. Its high surface-to-volume ratio allows for more DNA immobilization, and its conductivity enhances the sensor’s sensitivity. CNTs also exhibit high conductivity and mechanical strength, while their small diameter and large surface area provide a favorable environment for DNA hybridization, improving the sensor’s response. GO exhibits a strong affinity for ssDNA, making it a valuable material in biosensor applications [98]. This interaction primarily involves π–π stacking between the aromatic bases of ssDNA and the conjugated π-system of GO, as well as hydrogen bonding between the nucleobases and the oxygen-containing functional groups on the GO surface [99]. These interactions facilitate the adsorption of ssDNA onto GO, enabling its use in various sensing platforms [100, 101]. The affinity between ssDNA and GO is influenced by several factors, including the length of the DNA strand. Studies have shown that longer ssDNA sequences bind more strongly to GO than shorter ones. The difference in binding affinity is attributed to the increased number of nucleobases available for interaction with the GO surface in longer ssDNA molecules [98]. Understanding the mechanisms of ssDNA adsorption on GO is crucial for designing effective biosensors. However, one of the interestingly published research articles indicates that the desorption of ssDNA from GO can be achieved by disrupting hydrogen bonding, such as through the addition of urea [102]. This finding suggests that the interaction between ssDNA and GO is primarily driven by hydrogen bonding, with π–π stacking playing a secondary role. In summary, the strong affinity of GO for ssDNA, driven by π–π stacking and hydrogen bonding, is a key factor in its effectiveness as a material for DNA biosensors. The length-dependent binding affinity and the reversible nature of the interaction further enhance its utility in various biosensing applications. Excellent performance of nitrogen-doped graphene nanosheets decorated with Au nanoparticles in the detection of genetically modified maize was recently reported by Liu et al. [103]. The DNA biosensor has also been shown to exhibit high selectivity, good stability, and reproducibility in fabrication. It has been successfully used to detect MIR162 in real samples, highlighting its potential as a powerful tool for GM crop analysis.
2D nanomaterials beyond carbon-based nanomaterials
Other types of 2D nanomaterials, such as MXenes and TMDCs, are rapidly gaining attention in the scientific community due to their remarkable properties and versatile applications, particularly in electrochemical biosensing [104, 105]. MXenes, known for their conductive nature, tunable surface chemistry, and high surface area, are proving valuable in enhancing the sensitivity and selectivity of biosensors. Among the various types of MXenes, the most promising performers for DNA biosensing are Ti-, Nb-, and V-based families of these nanomaterials. Ti₃C₂Tₓ is one of the most studied MXenes for DNA biosensing. It has excellent electrical conductivity, a large surface area, and surface terminations (e.g., –OH, –O, –F) that can be easily functionalized with biomolecules, such as DNA probes [106–109]. The high conductivity of Ti₃C₂Tₓ allows for efficient electron transfer, which enhances the sensitivity of DNA detection. The COVID-19 pandemic accelerated research related to DNA biosensors based on MXenes [110, 111]. The nanocomposite, based on Ti3C2NH2 MXene with Au nanoparticles, demonstrated rapid detection of hepatitis B virus-deoxyribonucleic acid (HBV-DNA) [112]. An interesting utilization of specific properties of these 2D nanomaterials was reported by Yadav et al., where nanopores in MXene sheets were used for DNA detection [113]. Such an approach can further shift the final frontier in science and technology, leading the scientific community to a higher technological ground. In another interesting study, MXenes with Au nanoparticles were used as support for biomimetic bilayer lipid membrane, in order to achieve zeptomolar detection of the breast cancer BRCA1 gene [114]. Nb₂C MXenes have also been explored for optical DNA biosensing with the surface plasmon resonance (SPR) technique [115]. They are particularly attractive because of their relatively easy functionalization and ability to form stable composites with other materials like selenium nanoparticles [116], further enhancing their biosensing capabilities. Nb MXenes were applied in the development of an aptasensor for the selective detection of lead in water resources [117]. V₂C MXenes are emerging as potential candidates for DNA biosensing, as they offer good conductivity [118]. Their surface chemistry allows for functionalization with various biomolecular recognition elements, improving their performance in DNA hybridization detection. Similarly, TMDCs, with their semiconducting properties and layered structure, have shown great potential in the detection of biomolecules [119], offering improved performance in terms of signal transduction and stability. The combination of these materials’ unique electronic properties and large surface-to-volume ratio positions them as promising candidates for next-generation biosensing platforms, with applications spanning from disease diagnosis to environmental monitoring [120, 121]. Their ability to integrate with various biomolecular recognition elements further bolsters their potential in achieving highly sensitive and efficient electrochemical biosensors. For DNA biosensing applications, several TMDCs have shown significant promise due to their unique properties, such as high surface area, excellent electrical conductivity, and tunable band gaps. MoS₂ is one of the most widely studied TMDCs for biosensing due to its high surface area, good conductivity, and ease of functionalization [122, 123]. Its layered structure allows for efficient interaction with biomolecules like DNA, and its properties can be tuned through functionalization with different groups (e.g., –NH₂, –COOH) to enhance DNA hybridization detection [124, 125]. MoS₂ also exhibits excellent electrocatalytic activity, which is beneficial for electrochemical DNA biosensing. In a recent study [126], the authors introduce an advanced electrochemical biosensor that utilizes MoS2@CNT nanocomposite as an electrode material combined with a specific DNA probe to detect Salmonella typhi in food samples. Extreme sensitivity was showcased for a FET-based biosensor with DNA functionalized MoS2, for the detection of PSA markers [127]. Like MoS₂, WS₂ shares similar properties, including high surface area and conductivity. WS₂ is also known for its good biocompatibility and stability in aqueous environments, which makes it ideal for biosensing and nanomedicine applications [128]. It can be functionalized to increase its interaction with DNA strands, improving the sensitivity and selectivity of the biosensor [129]. MoSe₂ has similar characteristics to MoS₂ but with a slightly different band gap, making it a good candidate for DNA biosensing as well. Combining MoS₂ and WS₂ in heterostructures can enhance the properties of each material, improving their sensitivity and performance in biosensing [130]. These heterostructures offer advantages such as increased surface area, improved charge transfer, and enhanced electrochemical properties, making them ideal for efficient DNA detection.
Polymer-based materials
Conducting polymers, like polyaniline, polypyrrole, and poly(3,4-ethylenedioxythiophene) (PEDOT), are another promising class of materials for DNA biosensor electrodes [131, 132]. These polymers can be easily synthesized, modified, and doped to tune their electrical properties. Their ability to form stable, conductive films on electrode surfaces makes them suitable for DNA immobilization and electron transfer in biosensor systems. The combination of these materials, or hybrid materials, is often used to enhance the electrode’s performance in terms of stability, sensitivity, and reproducibility in DNA detection. Interesting research was published by Gu et al. [133], where an innovative direction in the application of these polymers was showcased. The authors reported quantification of DNA by a thermal-durable electrode modified with PEDOT polymer and demonstrated the electrode reusability by heating denaturation and re-hybridization. While conducting polymers offer a range of advantages for DNA biosensors, such as flexibility, ease of modification, and tunable electrical properties, they also come with several downsides that can limit their effectiveness as electrode materials. These challenges must be addressed to optimize their performance in biosensing applications. One of the primary downsides of using conducting polymers is their poor long-term stability. Many conducting polymers, such as polypyrrole and polyaniline, tend to degrade over time due to environmental factors like exposure to light, humidity, or temperature changes [134]. This degradation can lead to a reduction in the polymer’s conductivity and a loss of electrochemical performance, which is a critical issue for biosensor applications that require long-term reliability. Functionalization and stability of immobilized biomolecules on polymer surfaces can be challenging. Although conducting polymers can provide a surface for DNA probe immobilization, ensuring that the probes remain stably attached during the sensor’s operation is not always straightforward.
Electrode fabrication techniques
The fabrication of electrodes for DNA biosensors involves a variety of techniques, ranging from traditional methods to more advanced, modern approaches. Classic techniques such as physical vapor deposition (PVD) and electrochemical deposition have been widely used for fabricating metal-based electrodes. PVD, for instance, allows for the deposition of thin metal layers like gold, platinum, or silver onto substrates, creating electrodes with high conductivity and durability [135]. Electrochemical deposition, on the other hand, involves the reduction of metal ions from a solution onto an electrode surface, which can provide precise control over the thickness and morphology of the metal layer. It was demonstrated by Partanen et al. that this technique, in combination with 3D printing, can yield electrodes for biosensors that can be applied for the detection of heavy metal ions at drinking water concentration thresholds [136]. Screen printing is one of the most commonly used techniques for the fabrication of commercial electrodes, because it is a cost-efficient and relatively fast method that can easily be upscaled for mass production. However, electrodes produced by screen-printing often lack repeatability and reproducibility, and their quality varies from batch to batch. These classic techniques have been crucial in providing cost-effective electrode materials for biosensor applications, particularly in microfabrication processes for integrated circuits and lab-on-chip devices.
In contrast, modern fabrication technologies have revolutionized electrode design, offering greater precision and functionality for DNA biosensors. Nanoimprint lithography (NIL) and focused ion beam (FIB) milling are examples of advanced techniques that allow for the creation of highly structured, nanoscale electrodes. NIL enables the patterning of electrodes with intricate nanoscale features, allowing for improved control over surface area and enhancing the electrochemical performance of biosensors [137]. FIB milling can be used to carve nanoscale structures into electrode surfaces, which can significantly improve the surface properties for electrical conductivity [138]. 3D printing is also gaining traction in electrode fabrication, offering the ability to create flexible, custom-designed electrodes with complex geometries [139]. These modern techniques provide a higher degree of control over electrode characteristics, such as surface roughness, porosity, and electrode shape, allowing for the design of electrodes that are highly optimized for specific biosensing applications, including the detection of DNA hybridization events.
Biosensor preparation—synergy of nanomaterials and bioreceptors
DNA probe immobilization on various nanomaterials through diverse approaches
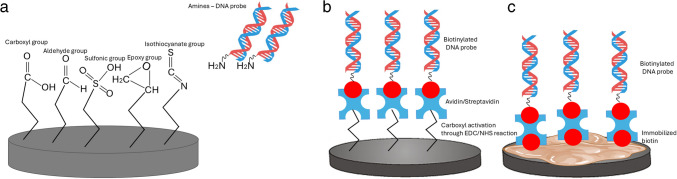
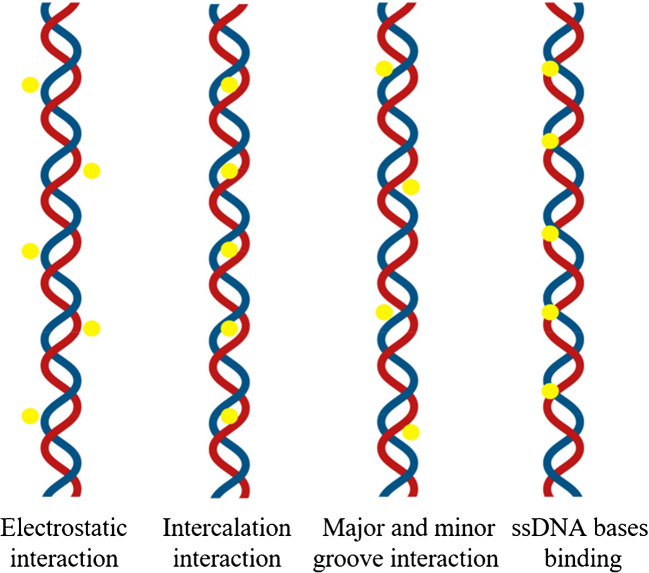
In electrochemical DNA biosensors, immobilizing DNA probes onto the working electrode is essential for detecting complementary DNA targets. Proper immobilization enhances probe reactivity by improving binding efficiency and ensuring its correct orientation for hybridization with the target DNA. Common techniques for DNA probe immobilization include physical and chemical adsorption [140], covalent bonding method [141], and affinity method—avidin–biotin interactions [142]. To attest that DNA probes are immobilized on the surface of working electrode in a manner which provides directionality, high activity, and stability, it is essential to regulate the immobilization process as well as the coverage of the electrode surface. The DNA molecule consists of two complementary chains that curl and encircle, forming a double helix. This shields the nitrogenous bases while exposing the anionic phosphate backbone [143]. Free phosphate groups can be further functionalized [144] or bonded to positively charged amino groups via electrostatic interactions, coordination chemistry, or the formation of ionic bridges on electrode surfaces or nanomaterials [145]. Additionally, they can covalently bind to amino [146], thiol [147], or epoxy functional groups [148], facilitating further hydrogen bonding with surface hydroxyl groups on nanomaterials such as ZnO [149] and TiO₂ [150]. Moreover, interactions with carbonyl groups on functionalized polymers or nanomaterials are also possible [151].
The large surface area of nanoparticles offers numerous sites for DNA to be bound, resulting in high efficiency in loading. Additionally, NPs protect nucleic acids from nuclease degradation [152]. The rapid and robust adsorption of DNA onto NPs facilitates the development of low-cost, convenient biosensors by eliminating the need for complex synthesis and time-consuming separation processes [153]. Furthermore, the functionality of DNA is preserved upon adsorption to nanomaterials, allowing for controlled and specific interactions between DNA and NPs [143]. Understanding DNA-nanoparticle interactions is crucial for optimizing biosensor performance and enhancing sensor platform designs. Despite recent research in this area, knowledge is still limited. This section of the review will cover the fundamentals of DNA-electrode material interfaces, focusing on metal nanoparticles, carbon-based materials, and metal oxide nanoparticles. It will also address various immobilization strategies, including covalent binding, adsorption methods (both physical and chemical), and avidin–biotin (i.e., affinity) interactions.
Physical and chemical adsorption
The most straightforward method for immobilizing DNA probes on the surface of the working electrode, requiring no chemical reagents or modifications to the probe, is adsorption [154]. DNA probes electrostatically adhere to the positively charged modified electrode surface via interactions with their negatively charged phosphate groups. Usually, an ssDNA capture probe is immobilized by electrostatic adsorption, but this method has a high risk of desorption from the electrode surface. Additionally, the ssDNA capture probes can be oriented randomly, which can alter the hybridization efficiency with complementary target sequences [155]. DNA adsorption onto nanoparticles is influenced by nucleotide properties, including charge variability, as nucleobases are neutral between pH 4.0 and 9.0. Hydrophobic variations affect DNA folding, exposing hydrophilic phosphate backbones. Additionally, DNA can interact with metal ions through chemisorption, mainly via amino and nitrogen groups, while aromatic bases enable π-π stacking with surfaces such as graphene [143].
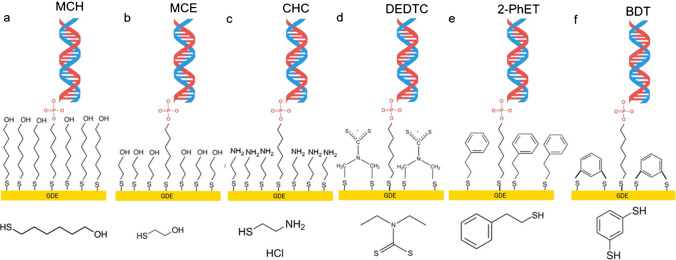
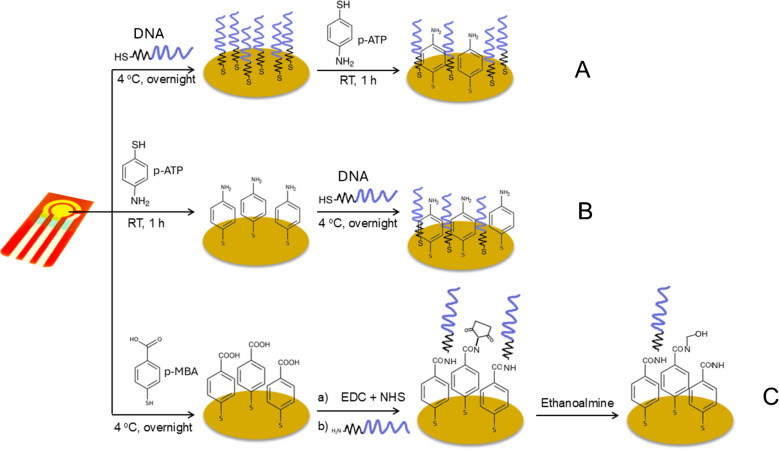
The most common chemical adsorption method is the self-adsorption of sulfhydryl-labeled DNA probes on the surfaces of platinum, palladium, gold, and silver electrodes via gold-sulfur links, resulting in a thick and well-organized monolayer [154]. However, nonspecific DNA adsorption on metal surfaces lowers hybridization effectiveness. To prevent this, nonspecific interactions are blocked using vertically aligned DNA probes or inserting alkane thiols with short chain, such as 6-mercaptohexanol [156]. These techniques allow for robust DNA immobilization on the electrode while keeping its structure. Non-thiolated DNA primarily interacts with metal nanoparticles through nucleobase adsorption at imine and ketonic oxygen sites, major binding sites for such chemisorption, while the anionic phosphate backbone remains uninvolved in surface binding [157]. In contrast, 5′-phosphorothioate (5′-PS) modification significantly enhances DNA attachment by forming two covalent bonds between oxygen and sulfur atoms and the metal nanoparticle surface [158]. Additionally, amine-functionalized 3′-terminal ssDNA can adsorb onto metal surfaces via electrostatic interactions; however, this approach typically results in less ordered structures compared to thiol-modified DNA. Nonetheless, the chemisorption principle is frequently used in the development of electrochemical DNA biosensors due to its high binding strength, simplicity, robustness, and repeatability of the DNA probe monolayer.
Cui et al. [159] developed a label-free electrochemical impedance genosensor integrated with recombinase polymerase amplification (RPA) for detecting GMO maize. A nanocomposite film was formed on a screen-printed carbon electrode (SPCE) by depositing an immobilization mixture containing gold carbon dots (GCDs) and 1.0% chitosan. A capture probe functionalized with thiol (ssDNA) was then immobilized via Au–thiol interactions. Mobed et al. [160] developed an innovative DNA-based genosensor using a gold nanostructure supported by cysteamine (CysA/AuNPs) on a gold electrode. The CysA/AuNP interface provides a large surface area for efficient ssDNA immobilization while ensuring the stability and bioactivity of the attached probe DNA. Because of the strong Au–S affinity, CysA/AuNPs facilitate the immobilization of thiolated DNA probes on the surface of the electrode. Additionally, cysteamine and AuNPs interact through covalent bonding between the sulfur group of CysA and AuNPs. The immobilized thiolated probe was then used in a cDNA hybridization assay.
Anionic metal nanoparticles (MNPs) can interact with dsDNA through hydrogen bonding in the DNA grooves or intercalation [161]. When MNPs are modified with cationic groups like amines, electrostatic attraction drives their adsorption. This binding can induce partial conformational changes in the dsDNAs, influenced by the MNPs’ surface charge and the DNA/MNPs ratio [143]. Considering both DNAs and MNPs are negatively charged, an appropriate countercharge is needed to facilitate ssDNA adsorption. Lowering pH and increasing salt concentration can enhance DNA surface density on MNPs [143]. Briefly raising the reaction temperature immediately after AuNP synthesis further strengthens oligonucleotide binding through enhanced hydrophobic interactions. Multivalent and larger-diameter cations effectively screen the negative charge of AuNPs, while monovalent and smaller-diameter cations reduce DNA-DNA repulsion [162]. Although higher DNA coverage improves signal response, excessive probe density can hinder hybridization efficiency due to steric crowding.
Metal-oxide nanoparticles (MONPs) that possess hydrated water molecules can dissociate into surface hydroxides, which deprotonate at higher pH, creating an anionic surface [163]. In acidic conditions, protonation leads to a positively charged surface [164]. Although most MONPs are negatively charged at physiological pH, exceptions like NiO, CoO, and ZnO still facilitate strong DNA adsorption. This occurs primarily through phosphate backbone coordination with unsaturated metal sites and electrostatic interactions, highlighting phosphate adsorption as the dominant mechanism [165]. For cationic MONPs, surface hydroxyl protonation generates positively charged –OH₂⁺ groups, enhancing electrostatic interactions with DNA [143]. These attractions involve the phosphate group’s oxygen atoms, π-electrons in aromatic rings, and non-bonding electron pairs on N and O atoms. Liu et al. [166] ranked DNA adsorption capability in the order ZnO ≈ CoO > NiO > Cr₂O₃ > Fe₂O₃ > Fe₃O₄ > TiO₂ > CeO₂, where ZnO and NiO showed higher resistance to protein desorption, most likely because of their better DNA binding affinity. Singhal et al. [167] developed an electrochemical genosensor that utilizes zinc oxide/platinum-palladium modified fluorine-doped tin oxide glass plate. ZnO/Pt–Pd nanocomposites were drop-deposited onto cleaned FTO electrodes, and such modified electrodes served as a matrix for attaching NH2-modified PDNA through cross-linking with chitosan and 2.5% glutaraldehyde.
Some previous studies reported that using cationic polymeric films made of carbon nanomaterials plays multiple roles in electrochemical sensor design due to their diverse physicochemical and electrical properties. Among their functions, they act as compounds for the development of surfaces, carriers, nanocatalysts, and platforms abundant in functional groups for bioreceptor immobilization [168]. Nanomaterial layers or their composites more often function as an interphase immobilized onto a transducer, where their function is to increase the surface area of the electrode, facilitate the immobilization of DNA receptors, and improve the conductivity. Acid treatment of partially oxidized carbon nanomaterials introduces abundant carboxyl and epoxy groups, enabling bioreceptor immobilization [103]. Then, DNA probes can be synthesized and functionalized with linkers containing amino or thiol groups to enhance bioreceptor stability [169]. Uncharged graphene is typically oxidized to graphene oxide to enhance its dispersion in water and interaction with DNA. Because of their benzene-like structure, DNA strand binding affinities on the surface of uncharged graphene fluctuate due to competition between nucleobase-graphene and nucleobase-nucleobase stacking. A balance of π-π stacking and hydrogen bonding between nucleotides and GO’s functional groups is involved in the interaction between GO and ssDNAs. A preference for oxidized versus unoxidized regions results from ssDNAs’ initial hydrogen bonding to the oxidized GO surface, followed by relaxation to favor π-π stacking [143]. Non-covalent functionalization of graphene materials and TMDCs commonly employs linker molecules such as 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE) [170]. At one end, this molecule contains an aromatic pyrene group that binds to the graphene lattice via π-π stacking, while the other end contains an ester group that undergoes covalent bonding to the amine groups via nucleophilic substitution. This approach requires probe modification with the NH2 group. Other strategies for non-covalent functionalization include 1-pyrenebutyric acid stacking and later activation by EDC/NHS chemistry, or direct modification of the probe by a pyrene group [171], which simplifies the functionalization protocol. Significant scientific interest has focused on DNA adsorption by CNTs, driven by the potential of DNA-CNT hybrid systems in optical and electrochemical biosensing. CNTs are hollow cylindrical structures formed by rolling single or multiple layers of graphene, which can be functionalized with carboxyl groups. Semiconducting SWCNTs are preferable for optical sensing, while MWCNTs are better suited for electrochemical applications due to their uniform electrical conductivity and lower cost [172]. Interactions such as van der Waals as well as π–π are crucial in the wrapping of ssDNA around CNTs, with only a portion of the DNA strand encircling the CNT while the rest extends outward. MWCNTs differ morphologically from SWCNTs, which may affect DNA adsorption and elastic energy during this process. The structural diversity of MWCNTs also influences the DNA-MWCNT hybrid’s surface properties and application potential. Attaching chemical groups like amine and poly (ethylene glycol) (PEG) can shield the negative charge on bare CNTs or create electrostatic attraction, facilitating DNA adsorption without prolonged salt aging [173]. CNTs demonstrate significantly greater binding strength compared to flat graphene, due to their similar curved structure to ssDNA [174].
Some previous studies reported that using cationic polymeric films made from chitosan for DNA probe adsorption aims to ensure good biocompatibility and a high density of positive charges [175]. Chitosan possesses reactive hydroxyl, carboxyl, and amino groups in its backbone, and because of that, it can be physically or chemically modified [176]. These functional groups can be altered without affecting the chitosan backbone. This cationic and biodegradable polymer interacts with hydrophilic molecules through electrostatic forces and hydrogen bonds, forming complexes with negatively charged DNA while functioning as a metal ion chelator [177].
Covalent bonding
In the preparation of DNA biosensors, covalent attachment of ssDNA to electrode surfaces has been extensively utilized [141]. This method is favored due to its strong binding stability and favorable vertical alignment, which can enhance hybridization efficiency with complementary target sequences [141]. Furthermore, carbodiimide bonding chemically attaches modified DNA to biosensors with oxygen groups through amide reactions [178]. Researchers commonly use NH2-terminated DNA probes for covalent immobilization onto modified electrodes, which present various surface functional groups (carboxyl, aldehyde, sulfonic, epoxy, and isothiocyanate), as illustrated in Fig. 4a. Carbodiimide reagents (NHS and EDC) facilitate covalent bonding between carboxyl groups and amine-terminated DNA probes by actuating carboxyl groups into O-acylisourea intermediates, promptly reacting with amines. For instance, graphene can be covalently functionalized using azide-modified probes, even though it is chemically inert [179]. Such groups can bind covalently to the graphene lattice, modifying carbon orbital hybridization from sp2 to sp3, which can significantly influence the electronic properties of graphene. Similarly, graphene oxide and rGO can be functionalized via both covalent and non-covalent strategies, leveraging their surface oxide groups for direct probe immobilization. On the other hand, the TMD surface can be activated by 11-mercaptoundecanoic acid (MUA) using the X vacancy of MX2 by covalent bonding [180]. Then, MUA is activated via EDC/NHS chemistry to enable reaction with the amino group of the bioreceptor.
Fig. 4.
DNA probe immobilization strategies: a Covalent immobilization of amine-terminated DNA probes on electrodes with various functional groups; b avidin/streptavidin-functionalized electrodes through carboxyl groups; c biotin/avidin (streptavidin)/biotin sandwiches technique. Reprinted and modified from [175]. Licensed under Creative Commons Attribution (CC BY-NC-ND 4.0), Copyright © 2017 The Authors. Published by Elsevier B.V
Jin et al. [181] developed a highly sensitive, label-free biosensor for dopamine (DA) detection. A GCE, employed as the working electrode, underwent surface modification via simple drop-coating with graphene oxide and Nile blue (NB). Subsequently, gold nanoparticles were electrodeposited onto this modified electrode (GO/NB/GCE) using HAuCl₄ as the precursor. Simultaneously, GO was reduced to rGO. The rGO/NB/AuNPs/GCE was coupled with a 5′-SH-terminated DA aptamer via Au–S bonding to create the DNA-rGO/NB/AuNPs/GCE system, while the remaining active sites were filled with 6-mercapto-1-hexanol. In this manner, a DA was effectively combined with its DNA sequence, which was modified on the surface of rGO/NB/AuNPs/GCE leading to the formation of a curled and binding complex between DA and the DNA.
Another method for creating electrochemical DNA biosensors was put out by Moustakim et al. [141]. It involved covalently immobilizing a non-modified ssDNA capture probe from its 5′-terminal phosphate group. As an electrochemical platform, a pencil graphite electrode (PGE) modified with AuNPs and carbon black (CB) was employed. After being submerged in an imidazole buffer solution containing 0.1 M EDC, the cysteamine/AuNPs/CB-modified PGE was left to react with the unmodified ssDNA. Imidazole and EDC combine to generate phosphorylimidazolide, a highly reactive diester intermediate that activates the ssDNA capture probe’s 5′-terminal phosphate group. A phosphoramidate bond was formed between the primary amine of cysteamine and the 5′-terminal phosphate of ssDNA, enabling the immobilization of non-modified ssDNA onto cysteamine-functionalized AuNPs/CB–PGE. To prevent nonspecific adsorption on the modified electrode surface, 1% BSA solution was utilized. The decreasing peak current of methylene blue (MB), which showed a linear association with the number of microRNA-21 target sequences, dropped as the hybridization with a complementary probe progressed. These results imply that phosphoramidate-bonding-based covalent immobilization of ssDNA is an economical and adaptable technique for creating DNA biosensors.
Gao et al. [182] developed a label-free electrochemical impedimetric biosensor for detecting genetically modified (GM) soybean, utilizing disposable SPCE modified with gold carbon dots (GCDs). The GCDs serve a dual function: directly binding single-stranded DNA (ssDNA) probes via Au-thiol interactions and enhancing the sensor’s electrical conductivity. Liu et al. [162] designed an electrochemical biosensor utilizing nitrogen-doped graphene nanosheets and gold nanoparticles (Au/N-G) for the precise detection of genetically modified maize. The incorporation of AuNPs was essential due to their high surface area, biocompatibility, and ability to optimize DNA probe orientation, facilitating efficient electron transfer. For sensor fabrication, a GCE was modified with an Au/N-G suspension, prepared by dispersing Au/N-G powder in DMF. A DNA capture probe (CP) functionalized with a thiol group was immobilized onto the Au/N-G-modified electrode via Au-thiol interactions.
In the article by Zhu et al. [183], an electrochemiluminescence (ECL) biosensor for detecting transgenic maize MON810 was developed. The gold electrode was cleaned and incubated overnight at 4 °C with 10 μl of a 1 μM DNA tetrahedron solution, forming Au–S covalent bonds. After washing the electrode with PBS buffer (0.1 M KCl, pH 7.4), it was incubated for 2 h with a 2.5 μM entropy-driven strand displacement reaction solution. The electrode was then treated with a 1.5 μM signal probe solution modified with Ru (bpy)32+ signal molecules for 45 min. This modified biosensor was subsequently used to detect various concentrations of target DNA standard solutions.
Tan et al. [184] reported a voltammetric genosensor incorporating a silica-gold nanoparticle composite for detecting the CaMV35S gene in genetically modified soybeans. To enhance electron transfer, gold nanoparticles were drop-cast onto a SPCE, facilitating the intercalation of anthraquinone-2-sulfonic acid monohydrate sodium salt (AQMS) into immobilized double-stranded DNA (dsDNA). Amine-functionalized silica nanospheres were then attached to the gold layer, forming a SiO2-Au composite electrode (SiO2-Au-SPCE). This approach enabled a higher density of DNA probe immobilization, leading to a tenfold increase in sensor sensitivity compared to AuNP-based DNA biosensors lacking silica nanospheres. Covalent attachment of amine-modified DNA probes to the SiO2-Au-SPCE was achieved using glutaric dialdehyde as a crosslinker, providing stronger bonding and enhancing calibration sensitivity for cDNA by a factor of ten relative to direct thiolated DNA adsorption onto AuNPs through thiol chemistry.
Chou et al. [185] proposed a genosensor utilizing a thiolated ssDNA probe complementary to a gene fragment associated with the CaMV 35S promoter (P-35S) to detect genetically modified soybeans (GMS). The electrode is created by sputtering a gold film onto the substrate, followed by the sequential deposition of 1,6-hexanedithiol and sulfur-functionalized gold nanoparticles. A target DNA extracted from genetically modified soybeans is immobilized on the electrode through a thiol-Au interaction.
Although covalent binding ensures a strong and stable attachment, enhancing sensor sensitivity and selectivity, it often requires more complex conditions than chemical or physical adsorption and can potentially damage DNA base pairs. To prevent interference with DNA hybridization, these reactions must be carefully controlled. Despite these challenges, the durability of covalent bonds provides long-term stability, allowing the removal of weakly bound molecules and improving the reliability of the detection process [186].
Avidin–biotin affinity interaction
Affinity binding is one of the most useful techniques for successfully immobilizing DNA on electrodes. Even though there are numerous affinity methods for non-covalent immobilization of DNA probes on electrode surfaces, the biotin-streptavidin system is one of the most widely used. Biotin has a high affinity (Ka = 1 × 10−15 M) for the avidin/streptavidin binding site, indicating covalent interaction [187]. As a result, avidin/streptavidin and biotin-binding are extremely stable, fast, and specific; have high affinity; and are unaffected by extreme temperatures, pH levels, organic solvents, and denaturing detergents [188]. Avidin/streptavidin is a big protein with four biotin binding sites that can be used to immobilize DNA probes on electrodes [189]. This is accomplished by introducing biotin to the 3′ or 5′ ends of DNA probes and placing them on the avidin/streptavidin-modified electrode [190]. Several approaches have been devised to functionalize electrode surfaces for the immobilization of biotinylated DNA probes in electrochemical biosensors, with EDC/NHC coupling being the most popular. Thus, this affinity method is becoming increasingly popular among researchers due to its distinct advantages. However, in comparison to other approaches, the electrochemical DNA biosensor established by this technology has a limited LOD.
Ouyang et al. [191] created an electrochemical DNA biosensor utilizing biotin and its receptor, streptavidin. B1-DNA, modified with a sulfhydryl group at the 5′ end, self-assembles on an Au electrode via Au-thiol bonds, while the 3′ end is modified with a biotin molecule. The gold electrode is then passivated with MCH to eliminate non-specifically adsorbed DNA. This DNA binds selectively to streptavidin (SA), forming M1-Biotin-SA DNA, which is subsequently modified with methylene blue positioned close to the electrode’s surface, generating a significant electrochemical signal.
Bage and Kar [192] conducted an electrochemical sensor for detecting biotin-avidin interactions using a gold electrode modified with silver nanoparticles. To create a self-assembled monolayer, the cleaned gold electrode was incubated overnight in a 1-mL ethanolic solution of 3-mercaptopropanoic acid (MPA), followed by thorough rinsing with ethanol and water to eliminate excess MPA. The formation of the MPA layer was attributed to sulfur chemisorption, aligning with the hexagonal gold atomic structure [193]. The electrode surface was then treated with EDC and NHS to activate the carboxyl (-COOH) groups of MPA, facilitating amide bond formation with amine (-NH2) groups present in biotin-, avidin-, or cysteamine-functionalized silver nanoparticles (AgNPs) [194]. The functionalized electrode was subsequently immersed in a solution containing synthesized AgNPs and either biotin or avidin at different ratios and left overnight. Acting as an electron mediator, AgNPs improved electron transfer across the organic layer. However, the peak current was lower for the avidin-modified electrode than for the biotin-modified one, likely due to avidin’s larger size, which caused greater insulation and charge transfer disruption.
A novel polymer, scopoletin (7-hydroxy-6-methoxy-coumarin), designed by Gajovic-Eichelmann et al. [195], immobilized streptavidin onto gold and carbon electrode surfaces for single base mutation detection via fluorescence after hybridization (Fig. 4b). A two-step method for directed nucleic acid immobilization on micro-sized ultramicroelectrodes is presented. Streptavidin was immobilized within an electrodeposited scopoletin polymer, while in the second part, biotinylated nucleic acids/proteins are bound. This offered advantages over polypyrrole/polyphenol methods due to the hydrophilic polymer, enabling high streptavidin incorporation and biotin binding, even if the film is non-conductive. This method is useful for microelectrode DNA chips and high-resolution biomolecule immobilization.
In some studies, sandwich strategies were used to prepare an avidin layer for the immobilization of biotinylated DNA probes (Fig. 4c). Owing to its four biotin-binding sites, the avidin layer functioned as a bridge linking the biotin-modified electrode to biotinylated DNA probes [196].
Blocking step of electrode surface
In biosensing, blocking vacant surface spaces is crucial to prevent non-specific interactions, which can bind untargeted molecules and cause high background signals, thereby interfering with detection [197]. The selection of an appropriate blocking agent, such as dithiothreitol, bovine serum albumin, 3-mercaptopropionic acid (MPA), 6-mercaptohexanol (MCH), or PEG, is contingent upon the substrate and serves to inhibit non-specific molecular binding [198]. These can also serve as spacers, aiding in the proper orientation of DNA probes during immobilization. The accessibility of the probes for target binding during immobilization depends on controlling their density and orientation, which are interdependent factors [199]. At lower probe densities, DNA probes spread farther apart and often lie horizontally on the electrode surface due to nonspecific adsorption, which makes them inaccessible for target binding. Conversely, higher probe densities cause them to stand away from the surface due to the repulsion between the negatively charged DNA probe backbones. Nonetheless, if probe density is overly high, steric hindrance and electrostatic forces can inhibit target binding, leading to weak or absent detection signals [200].
Szymczyk et al. [201] examined the impact of various electrode blocking agents (EBAs) on the performance of DNA biosensors based on gold surfaces. These biosensors utilize a binary receptor layer composed of a hairpin DNA probe with a covalently attached methylene blue redox marker, alongside a co-immobilized EBA. The goal was to evaluate parameters such as signal stabilization, hybridization efficiency, current intensity, detection limits, and repeatability, with a particular focus on comparing alternative EBAs to the traditional MCH approach. The EBAs examined included classical mercapto-derivatives of alkanols such as 2-mercaptoethanol (MCE) and MCH which carry a partial negative charge due to their –OH groups; cysteamine hydrochloride with a positively charged head group; sodium diethyldithiocarbamate (DEDTC), a neutral molecule with 2 resonance structures; and two aromatic diluents with distinct orientations on the electrode 2-phenylethanethiol (2-PhET), which is oriented perpendicular to the surface, and 1,3-benzenedithiol (BDT), which is significantly tilted toward the surface (Fig. 5a, b, c, d, e, f).
Fig. 5.
Schemes of each EBA molecule example: a 6-mercapto-1-hexanol (MCH); b 2-mercaptoethanol (MCE); c cysteamine hydrochloride (CHC); d sodium diethyldithiocarbamate (DEDTC); e 2-phenylethanethiol (2-PhET); f 1,3-benzenedithiol (BDT). Reprinted and modified from [201], with permission from Elsevier B.V. Copyright © 2022
The results disclosed that short-chain molecules (CHC and MCE) tend to form monolayers with multiplex defects, as they failed to produce clear SWV peaks for the methylene blue marker. This was particularly evident for MCE, where the absence of resonance structures led to no significant increase in faradaic current at higher SWV frequencies. Additionally, the choice of solvent for EBA deposition markedly influenced the electrochemical properties of the monolayer. Notably, the orientation of the aromatic rings in the blocking agents played a crucial role in overall biosensor performance and could cause readout inconsistencies due to increased nonspecific protein adsorption. Among the tested EBAs, DEDTC exhibited notably higher currents in SWV measurements and significantly reduced nonspecific protein adsorption, indicating its potential as a substitute for MCH. To further improve receptor layer stability and prevent undesired gold-catalyzed reactions at low potentials, such as oxygen reduction, the authors suggest future research should investigate ternary monolayers consisting of the molecular beacon probe, DEDTC, and MCH.
The following study was focused on exploring the use of SAMs of aromatic thiols for DNA sensing applications, offering an alternative to the traditional backfilling method with alkanethiols. The researchers found that conventional backfilling leads to a heterogeneous distribution of probes on the surface, negatively impacting hybridization efficiency and surface stability. By utilizing p-mercaptobenzoic acid (p-MBA) and p-aminothiophenol (p-ATP) monolayers, they achieved improved probe distribution, higher DNA surface coverage, and enhanced analytical performance. Electrochemical modification of p-ATP monolayers through potential cycling at low pH allowed for the insertion of thiolated capture probes, resulting in low background signals and better detectability compared to traditional backfilling. Meanwhile, p-MBA monolayers enabled covalent attachment of amino-terminated capture probes, leading to improved hybridization efficiency, particularly for long RNA targets, due to improved availability [202].
The authors explored different approaches to create DNA sensing platforms on gold surfaces using thioaromatic-based SAMs for improved stability and hybridization efficiency. In approach A (traditional backfilling), a thiolated DNA probe (HS-CP) and p-ATP are serially adsorbed via chemisorption onto Au electrode, replacing the typical MCH (Fig. 6A). Approach B (inserting) reverses the order by first passivating the gold with p-ATP, then inserting the HS-CP between the aromatic molecules, aiming for a more uniform probe distribution driven by intermolecular interactions (Fig. 6B). Finally, approach C involves covalently attaching amine-derivatized DNA probes to a shaped p-MBA monolayer via carbodiimide chemistry, which is expected to enhance packing efficiency and blocking capability (Fig. 6C).
Fig. 6.
Three DNA sensing platform approaches: A traditional backfilling; B insertion; C covalent DNA binding to a pure SAM. Reprinted and modified from [202], with permission from Elsevier B.V. Copyright © 2017
This study highlights the advantages of aromatic thiol-based SAMs for DNA sensing, emphasizing their role in achieving better probe distribution, enhanced hybridization, and improved sensor stability.
Electrochemical systems for nucleic acid hybridization detection
Electrochemical detection techniques
Electrochemical biosensors detect and analyze DNA by translating the signals from DNA hybridization into measurable electrochemical outputs. Typically, single-stranded DNA probes are immobilized on transducer surfaces-such as gold electrodes, indium tin oxide, or glassy carbon electrodes. Various electrochemical detection strategies exist, including direct DNA electrochemistry, electrochemistry on polymer-modified electrodes, the use of DNA-specific redox indicators, electrochemical amplification with nanoparticles, and systems that rely on DNA-mediated charge transfer. All these methods hinge on specialized electrochemical techniques to control what happens at the working electrode and capture the resulting chemical signals. This approach facilitates both the identification and quantification of DNA in the sample.
Few review articles on DNA electrochemical detection focus on the electrochemical techniques employed in DNA sensors. Similarly, in experimental studies on DNA detection, most authors typically mention the specific electrochemical techniques and parameters used in their work, but they often fail to provide comparisons or explanations as to why one detection method is superior to another.
Electrochemical techniques are among the most direct and information-rich methods for studying redox transformations and their kinetics in chemistry. These techniques can be seen as the “software” or “detection logic” of the device, controlling the sensor through electronic components that deliver an input signal to the sensing element at the working electrode. They then read out specific parameters, such as potential and current over time, resistance, conductance, or capacitance. Commonly employed techniques in sensor operation are briefly introduced in Introduction. Numerous new hybrid techniques are also under development and testing. Depending on the system and the detection mechanism at the working electrode surface, some techniques yield better electrochemical responses than others. Therefore, it is essential to test multiple techniques with a given molecular detection system and optimize the electrochemical parameters of each technique to achieve the best detection signal. The goal is to achieve the highest possible signal difference between the baseline (typically an empty buffer solution without DNA, or with random sequence amplified ssDNA) and the target DNA molecules. The stability of the baseline is crucial for the effectiveness of any electrochemical technique used.
CA applies the simplest voltage program for studying DNA electrochemical biosensors by measuring current over time after discrete voltage steps. There are notable technical differences in how many and how long the voltage pulses are applied in constant-voltage amperometry, intermittent-pulse amperometry, and stepped-voltage CA [203]. Because the voltage program is non-transient, amperometric measurements produce straightforward signal outputs that can be employed to determine the kinetic parameters of target-binding events. The simplicity of this voltage program also permits probing bioelectronic interfaces at millisecond frequencies, making it well suited for tracking transient, rapid, and dynamic processes. The amperometric readout of DNA-based biosensors varies depending on whether the redox reporter is freely dispersed in solution or anchored to the electrode surface [204].
CV characterizes DNA-based sensor interfaces by applying a sweeping potential and measuring the corresponding current. As a foundational type of voltammetry, CV monitors current while the potential is varied at a set scan rate, producing voltammograms that reflect both ionic and faradaic processes as peaks or waves. Since these processes depend on the interface’s organization and composition, CV provides a powerful tool for electrochemical characterization of DNA-based biosensors [205]. By analyzing different scanning rates, CV can reveal the surface concentration of double- and single-stranded DNA, gauge the coverage and homogeneity of passivating monolayers, and extract crucial electrochemical parameters, such as the diffusion coefficient or standard electron transfer rate, of the redox reporter. CV is also frequently employed for directly examining DNA-based biosensors, although the signal-to-noise ratio can be limited when using surface-tethered reporters. This limitation arises from the gradual charging of the electrical double layer during linear polarization, which appears as a charging current in the voltammogram.
Because most DNA target concentrations fall within the sub-nM range, the resulting changes in the signaling current output of DNA-based biosensors are quite minimal and can be easily disrupted by charging currents [206]. Methods that reduce the effect of double-layer charging are therefore highly beneficial. Pulsed voltammetric techniques accomplish this by using periodic voltage perturbations (e.g., voltage steps in SWV, DPV, and sinusoidal functions in ACV), rather than the linear perturbations typically employed in cyclic voltammetry and chronoamperometry [207]. By applying periodic perturbations, parameters such as pulse width, frequency, and amplitude can be adjusted to suppress charging currents while boosting the signal stemming from reversible redox events, such as the oxidation/reduction of a redox reporter. Consequently, these methods predominantly measure faradaic currents, leading to improved signal-to-noise ratios and lower detection limits.
ACV combines elements of CV and EIS. Similar to CV, it applies a DC voltage scan between two vertex potentials, but it also superimposes a small sinusoidal signal, akin to EIS (Fig. 7C). Owing to its capacity to minimize non-Faradaic currents, ACV is highly effective for both sensor characterization and target detection [208]. However, even though ACV is a powerful method, E-DNA biosensors tend to operate as “signal-off” biosensors, independent of the AC frequency [209]. “Signal-off” biosensors can be less desirable because their maximum possible signal suppression (SS) is 100%, leaving them vulnerable to false positives. For example, decreases in redox current may stem from issues like monolayer degradation or nonspecific adsorption. While one way to transform “signal-off” biosensors into “signal-on” biosensors is to redesign them, a more practical solution involves identifying an electrochemical technique that shifts the sensor’s performance from “signal-off” to “signal-on” by tweaking certain parameters. With square-wave voltammetry (Fig. 7B), for instance, both types of biosensors can be turned into “signal-on” simply by adjusting the operating frequency [210]. In the case of differential pulse voltammetry (Fig. 7A), modifying the pulse width yields similar effects [209]. When the pulse width is set to 1 ms, biosensors exhibit “signal-off” behavior with roughly 80% signal suppression upon exposure to 1.0 μM of fully complementary target DNA, comparable to ACV at optimal frequencies. However, increasing the pulse width to around 100 ms switches the sensor’s behavior to “signal-on,” resulting in approximately 700% signal enhancement.
Fig. 7.
Interrogation of DNA-based biosensors via pulsed-voltage methods: A In DPV, a voltage pulse of a certain width and amplitude is applied to the electrode. The current is measured immediately before and at the end of the pulse, and the difference between the two currents is used to create a voltammogram; B IN SWV, a square waveform is superimposed on a voltage staircase. The current is measured at the end of each square pulse, producing forward (If) and reverse currents (Ir). The absolute values of both currents are subtracted to obtain a differential voltammogram; C in ACV, a sinusoidal waveform is superimposed on a linear ramp, and the current is measured at a regular frequency. The reference parameter values shown in panels A-C were estimated from reported DNA-based biosensors. Reprinted and modified from [211]. Licensed under Creative Commons Attribution License (CC BY 4.0) Copyright © 2019, The Authors. Published by ECS
SWV, a specialized variant of the widely used DPV, is among the most advanced pulse voltammetric methods, offering high sensitivity and rapid analysis. Its growing use in exploring the mechanisms and kinetics of electrochemical processes highlights its adaptability and sophistication. Like DPV and other pulse voltammetric techniques, SWV effectively filters out charging currents. However, at solid electrodes, the main challenge arises from background currents linked to residual charge transfer processes rather than from the charging of the electric double layer [212]. In such cases, DPV can sometimes outperform SWV analytically by achieving lower background currents through careful adjustments to the ratio of potential step size and pulse duration [213]. On the other hand, DPV typically provides less mechanistic insight than SWV. Consequently, a hybrid method that combines the strengths of both techniques is ideal. Differential square-wave voltammetry (DSWV) serves this purpose by merging features of conventional DPV and SWV. DSWV not only supports advanced analytical applications but also facilitates mechanistic and kinetic studies. Through the incorporation of potential steps and square-wave modulation, DSWV enables mechanistic analysis of electrode reactions, accurately measures electrode kinetics for both rapid and sluggish processes, and enhances the electrochemical reversibility of slow systems, making it particularly advantageous for mechanistic and electrokinetic investigations [214].
EIS stands out as one of the most information-rich methods for examining electrochemical systems, offering the option to create DNA-based biosensors either with or without redox reporters. Its sensitivity to surface-based phenomena—especially changes in interfacial double-layer capacitance (Cdl) and electron transfer resistance (Ret)—makes EIS a powerful tool [215]. By applying a low-amplitude sinusoidal signal, usually around 10 mV, and measuring the resulting AC in real time over a frequency range spanning from mHz to MHz, EIS produces Nyquist and Bode plots (Fig. 8). Particularly sensitive to double-layer processes, EIS can detect targets even in the absence of redox reporters by tracking how target binding modifies double-layer capacitance through non-faradaic impedance spectroscopy [216]. Mechanistically, when immobilized DNA probes bind to target DNA, they alter water and ion distribution at the sensor interface, affecting double-layer thickness and interfacial capacitance—changes that correlate with the amount of target DNA. However, expanding this strategy to include signal amplification has proven challenging, partly because capacitance measurements can respond to any biomolecules or cells that contact the sensor surface. To enhance specificity and enable signal amplification, EIS can use redox reporters, either by covalently attaching them to ssDNA probes in a “labeled” strategy or by solvating and adding them externally in a “label-free” approach [205].
Fig. 8.
Interrogation of DNA-based biosensors via electrochemical impedance spectroscopy: A A sinusoidal voltage is applied (solid line) at a fixed frequency, generating the corresponding current (dashed line). This is repeated for a wide range of frequencies; B the imaginary part of the impedance is plotted against the real part for each sampled frequency to obtain the Nyquist plot. The value of the impedance and its phase angle at a certain frequency can be obtained from the module and the angle of the vector, respectively; C-D bode plots provide another way of representing the data obtained in an EIS measurement. In this case, the impedance and the phase angle are plotted against the applied frequency, offering a straightforward manner to view the impedance at a certain frequency value; B-D spectra obtained for a non-faradaic (dashed line) and a faradaic (solid line) system. Reprinted from [211]. Licensed under Creative Commons Attribution License (CC BY 4.0) Copyright © 2019, The Authors. Published by ECS
Each electrochemical technique provides unique benefits depending on the measurement requirements. CA is best suited for capturing sub-second data. CV excels at analyzing the sensor interface, though it may struggle to distinguish between charging and faradaic currents. Pulsed-voltage methods eliminate charging effects and offer high detection limits. In DPV, the longer pulse duration (around 100 ms) leads to a “signal-on” behavior in DNA biosensors, significantly boosting the signal. EIS is ideal for reporter-free sensing, especially when the reporter limits the temporal resolution of measurements.
Redox-active indicators in electrochemical nucleic acid biosensors
A widely used approach for electrochemical DNA hybridization detection relies on redox-active indicators that bind to DNA and modify the electrochemical signal upon target interaction. This technique is simple, sensitive, and cost-effective. Notably, these redox indicators are capable of clearly differentiating between single-stranded (ssDNA and (dsDNA) DNA.
Redox-active molecules can interact with DNA in several ways: they may attach via electrostatic forces, intercalate between the base pairs, bind within the grooves, or exhibit high specificity for certain DNA sequences (Fig. 9) [175].
Fig. 9.
Schematic depiction of redox-active molecule interactions with DNA molecules. Reprinted and modified from [175]. Licensed under Creative Commons Attribution License (CC BY-NC-ND 4.0). Copyright © 2017, The Authors. Published by Elsevier B.V
Over time, various redox indicators have been developed, differing in their binding mechanisms to DNA and their electrochemical behavior. The most important groups of these molecules include organic dyes, metal complexes, antitumor drugs, and redox indicators that bind to DNA grooves.
Organic dyes
Erdem et al. [217] showed that MB can serve as an effective redox indicator for DNA hybridization. They found that when carbon paste electrodes are modified with ssDNA, the MB current is significantly higher than when the electrodes are modified with dsDNA. This difference is due to MB’s strong affinity for the free guanine bases in ssDNA, which are more accessible. In contrast, once the DNA forms a duplex, the guanine bases become less accessible, leading to a lower MB current response.
Castro et al. [218] showed that ethidium bromide, when used as a hybridization indicator, delivers a distinct voltammetric response that can differentiate between mismatched and complementary DNA. Initially, no DPV peak current was detected for non-complementary DNA, as nearly no ethidium bromide bound to the ssDNA-functionalized pyrolytic graphite electrode. However, upon the introduction of complementary DNA, the current rose markedly due to increased ethidium bromide intercalation into the G-C base pairs of the hybridized DNA.
Metal complexes
[Co(phen)₃]3⁺ (cobalt-phenanthroline complex) and Os(bpy)₂Cl₂ (osmium-bipyridyl complex) are metal-based redox indicators with a high affinity for double-stranded DNA, as they intercalate between G-C base pairs [219, 220]. According to Liu et al. [219], reducing the concentration of the complementary DNA target led to a corresponding decrease in the response current of Co(phen)₃3⁺.
The electrochemical signal from Ru(NH₃)₆3⁺ (ruthenium-hexammonium complex) serves as an indicator of how many DNA strands are present on the electrode. For example, Guo et al. observed that as the concentration of the DNA target increased, the Ru(NH₃)₆3⁺ response also rose gradually. This occurs because hybridization introduces more phosphate groups on the DNA, which attract and bind Ru(NH₃)₆3⁺ through electrostatic interactions [221].
[Fe(CN)₆]3⁻/4⁻ (Ferrocyanide) is used as a redox indicator based on electrostatic repulsion. Its current response is high at a bare electrode but decreases after DNA hybridization occurs. This is because the negatively charged phosphate groups on the DNA repel the Fe(CN)₆3⁻/4⁻ ions, resulting in a lower current [222].
Anti-tumor drugs as redox indicators
Daunomycin and doxorubicin are anticancer drugs that are used as redox-active indicators in electrochemical DNA biosensors. They work by intercalating between the G–C base pairs of double-stranded DNA, which gives them a high affinity for the hybridized DNA structure [223, 224].
Redox indicators that bind to DNA grooves
Certain redox indicators, such as Hoechst 33,258 and ferrocene, bind specifically to the minor and major grooves of double-stranded DNA, respectively, making them useful components in electrochemical DNA biosensors [225].
Types of DNA probes
Biosensor design is crucial for bioanalysis, yet it often struggles because targets are not readily accessible at the bio-interface. DNA nanostructures in one, two, and three dimensions have gained attention, offering benefits like flexibility, precise control, high-yield synthesis, and biocompatibility. They enhance molecular recognition by modulating surface functionality, making them valuable for bio-surface engineering [226].
One-dimensional DNA
1D DNA probes, which are ssDNA molecules of at least 15 nucleotides, are frequently employed in DNA biosensors because they lack secondary structure. Through thiol-gold chemistry, these probes self-assemble onto gold surfaces, typically creating monolayers at densities ranging from 1011 to 1013 molecules/cm2 [227]. Ideally, these probes should adopt an upright orientation for efficient target hybridization, but interactions such as electrostatic repulsion, Au–N binding, and inter-strand entanglement create a more disordered structure [228]. Levicky et al. [229] developed a two-step assembly procedure incorporating MCH to enhance probe orientation. MCH fills any leftover space, displaces nonspecifically adsorbed DNA, and encourages a more upright probe conformation, ultimately improving biosensor performance (Fig. 10a). Neutron reflectivity measurements of DNA-layer thickness, combined with scattering length density modeling and comparison to the expected full DNA length, revealed DNA orientation.
Fig. 10.
a Schematics illustrating various binding states of ssDNA probes on a gold surface (i), representation of a MCH monolayer that inhibits direct interactions between the DNA backbone and the substrate (ii). Reprinted and modified from [226]. Licensed under Creative Commons Attribution License (CC BY-NC-ND 4.0). Copyright © 2018, The Authors. Published by De Gruyter Open Access. b Gold electrode-bound hairpin-structured DNA probe: target hybridization displaces the ferrocene label, altering the redox current. Reprinted and modified with permission from [230]. Copyright © 2003, The National Academy of Sciences. c Comparison of linear vs. stem–loop (hairpin) E-DNA biosensor. Reprinted and modified with permission from [231]. Copyright © 2011, American Chemical Society. d Schematic illustration of 3D DNA probe architectures for an electrochemical DNA (E-DNA) sensor: tetrahedral DNA probe A. Reprinted and modified from [226]. Licensed under Creative Commons Attribution License (CC BY-NC-ND 4.0). Copyright © 2018, The Authors. Published by De Gruyter Open Access
Two-dimensional DNA
2D structured DNA probes take advantage of the ability of ssDNA to form secondary structures through internal base pairing. These structured probes are more rigid and ordered than linear ssDNA, reducing inter-strand entanglement and improving probe organization on surfaces, which enhances their potential for bioanalysis [226].
In 2003, Fan et al. [230] introduced an electrochemical DNA (E-DNA) sensor by immobilizing hairpin-structured DNA probes on gold electrodes. These probes, tagged with thiol at one end and electroactive ferrocene at the other, generate an electrochemical signal based on target-induced conformational changes. In the absence of a target, ferrocene remains close to the electrode, producing a redox current. Upon hybridization, the stem-loop structure unfolds, increasing the distance between ferrocene and the surface, which alters electron transfer and provides a measurable electrochemical response. Unlike conventional electrochemical methods, this approach enables sensitive DNA detection without additional reagents, making it cost-effective and reusable, with promising applications in different fields (Fig. 10b). DNA orientation, designed using a stem-loop structure, was indirectly confirmed by electrochemical detection of the altered electron transfer efficiency.
Yang and Lai [231] conducted a thorough evaluation of 1D (linear) and 2D (stem-loop) DNA probe configurations. Their work examined the performance of stem-loop (SLP) and linear (LP) electrochemical DNA biosensors via ACV and CV, aiming to establish each design’s operational conditions and to clarify their signaling mechanisms. Although the LP sensor displayed a slightly higher percentage signal suppression (% SS) when hybridized with a matching target at 10 Hz, it only achieved optimal suppression within a narrow AC frequency range. CV measurements also confirmed that the LP sensor functioned optimally over a more limited range of scan rates than the SLP sensor-particularly when analyzing integrated charge instead of peak current. Temperature-dependent experiments revealed that the more flexible LP sensor was more sensitive to temperature fluctuations, leading to diminished % SS at higher temperatures despite faster hybridization kinetics. Additionally, electron-transfer kinetics data showed that the SLP sensor’s performance hinges largely on hybridization-induced structural changes in the redox-labeled probe, whereas the LP sensor’s behavior is governed primarily by changes in probe dynamics before and after hybridization. Overall, both sensor architectures have specific optimum ranges for frequency, scan rate, and temperature, but the SLP sensors’ operational parameters are less restrictive, making it a more robust platform for electrochemical DNA sensing (Fig. 10c). ACV and CV measurements revealed DNA orientation based on electron transfer rates between stem-loop and linear probes. Faster rates, indicative of closer ferrocene proximity, suggested a folded (stem-loop) conformation. Conversely, slower rates, reflecting greater ferrocene distance, implied an extended conformation (after hybridization or in linear probes). Quantitative electrochemical kinetic data provided strong indirect evidence regarding molecular orientation.
Three-dimensional DNA
Although 2D probes deliver improved biosensing functionality over 1D probes, they often lack the robustness required for dense surface environments. Consequently, precisely managing the surface density and orientation of grafted DNA probes is critical for high-performance biosensors (Fig. 10d). A promising approach to address this issue involves 3D DNA nanostructures [226]. In 2010, Pei et al. [232] presented the first 3D DNA-based electrochemical sensor featuring tetrahedron-structured DNA probes (TSPs). Formed by three thiolated 55-base oligonucleotides and one 80-base oligonucleotide, these TSPs adopt a tetrahedral configuration. The assembly process is rapid and achieves an approximately 85% yield, with TSPs firmly anchoring to gold surfaces via thiol-gold interactions and leaving a protruding probe at the tetrahedron’s top vertex. This self-assembly occurs in just 2 min, and real-time monitoring confirms a surface density of around 4.8 × 1012 TSPs/cm2, with probe-to-probe spacing of around 4 nm. Pei et al. used this TSP platform for an E-DNA sensor employing a sandwich hybridization approach, detecting biotinylated target DNA. The sensor demonstrated sensitivity about 250 times higher than that of 1D and 2D probe-based biosensors, detecting concentrations as low as 1 pM. Additionally, it could distinguish single-based mismatches 25–100 times more effectively than ssDNA probe-based biosensors. Electrochemical data supported the observation that electron transfer rates validated the impact of nanostructure rigidity on DNA orientation; rigid nanostructures preserved a constant redox label-surface distance.
Specific influences of redox-active molecules in electrochemical nucleic acid hybridization detection
Impact of DNA probe density and mismatches on electrochemical detection
Xiao et al. [233] show that the density of DNA probes on the electrode surface plays a critical role in determining the overall performance of E-DNA biosensors. They found that the greatest signal suppression occurs at the highest probe densities, even though hybridization efficiency decreases as probe density increases. While higher probe densities improve signal suppression, the sensor’s equilibration time steadily increases with density. Notably, changes in probe density do not significantly impact the specificity of hybridization. Optimal probe densities are reported to be in the range of 30 pmol/cm2 to 3 nmol/cm2. These values were determined under the assumption that all methylene blue redox tags are ideally positioned for electron transfer, which is unlikely to be the case in practice. Nevertheless, using relative probe density as a metric provides a useful basis for defining and optimizing probe density in these systems.
These findings offer a more profound understanding of the E-DNA signaling mechanism. Previously, the “electron tunneling effect” along the DNA double helix was thought to be responsible for signal suppression in E-DNA biosensors. However, this study proposes an alternative mechanism: hybridization alters the rate at which redox molecules collide with the electrode surface. At low probe densities, the hybridized DNA probe occasionally contacts the electrode, allowing electron transfer and limiting signal suppression. In contrast, at high probe densities, steric crowding of the densely packed DNA probes prevents these collisions, resulting in greater signal suppression, even though hybridization efficiency is likely lower (Fig. 11a) [234].
Fig. 11.
a Schematic diagram demonstrating how the density of DNA probes attached to an electrode impacts electrochemical detection: (i) high density, (ii) low density. Reprinted and modified with permission from [234]. Copyright © 2007, American Chemical Society. b Schematic representation of (i) A DNA-modified electrode with methylene blue as a redox molecule illustrating DNA-mediated and direct reduction pathways, (ii) the effect of a single base mismatch on the electrochemical signal. Reprinted and modified with permission from [235]. Copyright © 2012, American Chemical Society. c Schematic representation of (i) a well-formed and poorly formed DNA duplex monolayer, (ii) well-assembled DNA duplexes mediated charge transfer via redox-active probes, with a single CA mismatch significantly reducing the MB signal, in contrast to non-specific electron transfer in disordered monolayers. Reprinted and modified with permission from [236]. Licensed under Creative Commons Attribution License (CC BY-NC-ND 4.0). Copyright © 2021, The Authors. Published by American Chemical Society
In their work, Pheeney and Barton [235] described the mechanism of electron transfer during the hybridization process. MB, tethered to DNA via a flexible C12 alkyl linker, can be reduced through two distinct mechanisms: DNA base pair stack–mediated charge transport (CT) and direct electron transfer at the electrode surface. The relative contribution of each mechanism depends on assembly conditions, buffer composition, and the density of the DNA film factors that can be tuned to enhance mismatch sensitivity. Under conditions favoring DNA CT, single base mismatches can attenuate the electrochemical signal to as low as 7 ± 2% of that observed for perfectly matched DNA, underscoring the potential for high specificity. However, when direct surface reduction predominates, duplex formation has less impact on signal suppression, as MB remains accessible to the electrode. A thorough understanding of these parallel reduction pathways is thus essential for designing DNA-based biosensors with optimal sensitivity and mismatch discrimination (Fig. 11b).
Nano et al. [236] also investigated the charge-transfer pathways through DNA molecules, showing how DNA-modified electrodes exploit the natural capacity of base-paired DNA to mediate CT, thereby providing a powerful means to detect disruptions in the helix-such as base mismatches, lesions, and bound proteins. By monitoring redox-active probes attached to DNA, researchers can discern whether electron transfer proceeds through the well-ordered DNA duplex or via alternative, non-specific pathways. Consequently, proper assembly and characterization of the DNA monolayer are critical to ensure that observed electrochemical signals accurately reflect the integrity of the DNA base-pair stack. The authors further demonstrated that when DNA is hybridized and oriented upright, charge transport is effective, and any structural perturbations are readily detectable. Conversely, poorly formed monolayers—where single-stranded DNA may lie flat or fold unfavorably—permit alternate electron-transfer routes that mask or dilute DNA-mediated signals. Crucially, they showed that all-AT DNA sequences can still exhibit DNA-mediated electron transfer, challenging the claim that GC base steps are mandatory. Introducing a single CA mismatch almost abolished the MB signal—an unmistakable hallmark of DNA-mediated transfer—whereas simply adsorbing MB-labeled single-stranded DNA onto gold (without stabilizing a duplex) resulted in minimal signal differences after adding complementary strands, indicating direct MB-electrode contact rather than DNA-based charge transport. This underscores the importance of carefully controlling how DNA and MB are assembled on electrodes to reliably distinguish true DNA-mediated electron transfer from non-specific surface interactions (Fig. 11c).
According to Nano et al. [236], these electrochemical assays have deepened our understanding of DNA hybridization on surfaces and the influence of duplex integrity on electron transfer. In their study, they demonstrated that very low DNA densities—only a few pmol/cm2—allow for higher hybridization efficiencies, since higher densities create a strong negative barrier that impedes target strand binding. For more reliable results, the authors recommend first self-assembling thiolated DNA duplexes and then dehybridizing them to form single-stranded monolayers for subsequent target capture. Moreover, their experiments using intercalated redox probes such as daunomycin have shown that a single-base mismatch or any π-stack disruption leads to a linear decrease in the electrochemical signal—a phenomenon not observed with RuHex, which binds electrostatically. These findings clearly indicate that efficient electron transfer occurs through a well-formed, upright DNA duplex, underscoring the necessity of a robust DNA monolayer for sensitive, DNA-mediated electrochemistry.
Influence of redox molecule selection and positioning on electrochemical detection
Silva et al. [237] explored how the position of a MB redox label on a DNA probe influences both charge transfer at the DNA interface and hybridization efficiency. Typically, electrochemical DNA biosensors tether a redox-modified single-stranded DNA to an electrode and monitor changes in the redox signal upon target binding. However, conflicting reports in the literature have raised questions about how redox label placement affects charge transfer. To address this, the authors used a dual-label strategy: MB was attached at different positions along the probe strand, while a ferrocene molecule was fixed on the target DNA such that, upon hybridization, it resided at the distal end of the DNA monolayer. Correlating the reduction in MB signaling with the emergence of ferrocene signaling, they demonstrated that the location of the MB label can significantly influence hybridization efficiency, likely by altering MB’s accessibility to the electrode after duplex formation, as well as by affecting DNA-base interactions that govern binding thermodynamics. Notably, they found that placing the MB label at the distal end of the DNA strand (surface D) provides both the highest electrochemical signal and the best hybridization efficiency (Fig. 12a).
Fig. 12.
a Schematic illustration of the Influence of redox molecule placement on electrochemical detection. Reprinted and modified with permission from [237]. Copyright © 2018 Wiley–VCH Verlag GmbH & Co. KGaA, Weinheim. b Schematic illustration of dual-signaling DNA biosensor: redox markers at the distal end yield higher currents than proximal markers due to enhanced ion accessibility. Reprinted and modified with permission from [238]. Copyright © 2018, American Chemical Society
Building on this understanding of redox positioning, a second study employed a dual signaling approach to evaluate how label location in DNA-modified electrodes impacts electron transfer. Here, a redox marker-modified DNA probe is again tethered to an electrode, and target binding shifts the distance between the marker and the electrode [238]. Traditionally, the redox marker is placed at the distal end of the probe. The authors observed higher currents when the marker was located at the distal rather than the proximal end, attributing the reduced current in the proximal configuration to restricted ion accessibility near the electrode [238]. This finding highlights the significance of redox label placement in achieving efficient electron transfer through a well-formed DNA duplex (Fig. 12b).
Kang et al. [239] compared the performance of electrochemical DNA biosensors employing two different redox tags—ferrocene (Fc) and MB—to explore how the choice of redox label influences signal quality and device stability. Both Fc- and MB-based biosensors produced strong electrochemical signals, with Fc showing marginally higher signal gain and target affinity. However, these advantages were offset by poorer stability in Fc-based biosensors: over extended storage, repeated electrochemical interrogations, and multiple sensing/regeneration cycles (particularly in complex media), Fc biosensors suffered a pronounced loss in signal. By contrast, MB-based biosensors displayed comparable electron transfer efficiency yet maintained superior stability and reproducibility over time. This underscores the importance of selecting an appropriate redox label by balancing the desired signal enhancement with practical considerations of long-term sensor performance.
Influence of ionic strength and various probe design-related characteristics on electrochemical detection
Khuda et al. [240] demonstrated that ionic strength significantly influences the kinetics of DNA hybridization in electrochemical biosensors. At low ionic strength, a thicker electric double layer forms, restricting ion access to the electrode surface and slowing the hybridization process. By increasing ionic strength, the double layer’s thickness is reduced, thereby improving ion accessibility and enhancing both reaction rates and overall hybridization yields. This underscores the importance of optimizing salt concentration to achieve more efficient and sensitive biosensing performance.
Batistuti et al. [241] further emphasized that the assembly of DNA strands on gold electrodes is critical for electrochemical genosensor performance. They demonstrated that impedimetric DNA hybridization biosensors, which detect changes in negative charges due to target binding, require a well-organized DNA sensing interface for optimal functionality. By comparing different DNA probe preparation and immobilization protocols, they showed that the solution environment—especially the ionic strength—profoundly influences both the formation of the SAM and the subsequent hybridization efficiency. Specifically, higher ionic strength buffers help neutralize the negative charge of the DNA strands, thus boosting probe immobilization and enhancing target hybridization, as evidenced by stable and reproducible impedance spectroscopy measurements over a wide range of target concentrations. Moreover, the authors proposed an alternative protocol using only TE buffer, which improved the reproducibility of the DNA sensing interface by optimizing the molecular organization of the SAM. Their findings indicate that by carefully managing parameters such as ionic strength and DNA assembly, it is possible to substantially increase the sensitivity and stability of electrochemical DNA biosensors.
Khuda et al. [240] also examine the impact of nucleotide spacers and toehold positioning on electrochemical detection in their work (Fig. 13a). The authors emphasize that nucleotide spacers primarily function to position the DNA hybridization site at an optimal distance from the electrode surface, thereby attenuating the disruptive influence of the electric double layer on short-segment (10 bp) hybridizations. They found that omitting a spacer (0 nt) places the target binding region too close to the electrode, leading to restricted ion accessibility and slower hybridization kinetics. In contrast, longer spacer lengths (6–7 nt) position the methylene blue reporter outside of the most efficient electron-transfer range, reducing the faradaic current. Through systematic experimentation, the authors identified a 4–5 nt “sweet spot” that balances high hybridization efficiency with robust electrochemical signaling. As a result, they conclude that careful spacer design is essential for achieving both rapid and sensitive DNA detection near electrode interfaces.
Fig. 13.
a Schematic illustration of the influence of ionic strength and nucleotide spacer on electrochemical DNA detection. Reprinted and modified with permission from [240]. Copyright © 2023, American Chemical Society. b Schematic illustration showing how the toehold position impacts electrochemical DNA detection. Reprinted and modified with permission from [240]. Copyright © 2023, American Chemical Society. c Schematic design and signaling mechanism of an E-DNA sensor, emphasizing the critical role of internal spacers. Reprinted and modified with permission from [242]. Copyright © 2014, American Chemical Society. d Schematic comparison of E-DNA biosensors: linear biosensors with longer probes (proximal redox tag) boost signal intensity at the expense of specificity, while stem-loop biosensors exhibit smaller signal changes, offering improved sequence discrimination. Reprinted and modified with permission from [243]. Copyright © 2009, American Chemical Society
Also, by positioning the toehold further from the electrode (UTH), the reaction proceeds more rapidly, largely because the electric double layer near the surface is less restrictive. Conversely, toeholds placed near the electrode (LTH) experience slower kinetics due to electrostatic hindrance (Fig. 13b). These findings underscore the importance of carefully controlling the spatial arrangement of the toehold—particularly in electrochemical biosensors relying on strand displacement—so as to minimize double-layer effects and optimize both speed and efficiency of target binding [240].
Building on the role of nucleotide spacers in optimizing DNA hybridization kinetics, the flexibility and structural arrangement of both the DNA probe and target strand further influence electrochemical sensor performance.
Batistuti et al. [241] highlighted the significance of ionic strength and DNA monolayer organization, while Khuda et al. [240] demonstrated that carefully positioned nucleotide spacers enhance hybridization by mitigating double-layer interference. Expanding on this concept, recent studies have investigated how internal spacers within the DNA probe and target sequence contribute to sensor efficiency. Wu and Lai [242] evaluated the effects of both probe and target flexibility on the performance of a “signal-on” electrochemical DNA sensor (Fig. 13c). By systematically varying the length and identity of a non-target binding spacer, they compared six nearly identical DNA probes that differed only in the spacer connecting their two target binding domains—using lengths of 6, 10, or 14 bases composed of either thymine (T) or adenine (A). The MB-modified probe exists in a linear, unstructured state in the absence of the target, but upon hybridization, it folds into a “closed” conformation that increases the MB current. Their results showed that probes with a 14-base spacer, particularly the T14 and A14 sensors, produced the largest signal increase, highlighting the critical role of probe flexibility in optimizing target capture. Additionally, testing with 13 different target sequences revealed that targets containing a 2- or 3-base spacer yielded the most effective hybridization without introducing adverse steric or crowding effects, with targets containing oligo-A spacers further enhancing the signal through stacking interactions. Despite differences in spacer composition, all sensor designs could reliably distinguish between perfect match and mismatch targets, and the biosensors were regenerable and effective even in complex media such as diluted saliva. These findings underscore the importance of carefully tuning DNA probe and target flexibility for the development of highly sensitive, POC electrochemical DNA biosensors.
Probe length is an important design parameter in the development of E-DNA biosensors, as it can influence both signal intensity and target discrimination. Lubin et al. [243] described that increasing the probe length in linear E-DNA biosensors enhances the electrochemical signal when the redox tag is placed proximally, although this heightened sensitivity tends to compromise sequence specificity (Fig. 13d). In contrast, stem-loop probes yield smaller signal changes with longer lengths, suggesting that the benefits of increased probe length are less pronounced in these architectures, which in turn may offer improved sequence discrimination.
Electrochemical mechanisms of nucleic acid hybridization detection
Recent advances in electrochemical DNA biosensors have enabled the development of diverse detection strategies, incorporating both labeled and label-free approaches as well as signal-on and signal-off systems. These methods utilize various mechanisms—including enzyme amplification, strand displacement, target recycling, and hairpin conformational changes—to transduce DNA hybridization events into measurable electrochemical signals. By tailoring probe immobilization, redox tag positioning, and signal amplification techniques, researchers have achieved remarkable improvements in sensitivity and specificity, making these biosensors promising tools for rapid and accurate DNA analysis.
Electrochemical “signal‐off” mechanisms
Signal-off electrochemical DNA biosensors operate by exhibiting a reduction in the baseline electrochemical signal upon target DNA binding. This decrease in the signal occurs as the hybridization event disrupts electron transfer pathways, serving as a measurable indicator of DNA presence.
In their work, Xiao et al. [244] report that the E-DNA sensor operates using a self-complementary DNA probe that adopts a stem-loop conformation, enabling efficient electron transfer and generating a clear electrochemical signal (Fig. 14a). One terminus of this probe is anchored to a gold electrode via a thiol-based self-assembled monolayer, while the other terminus is covalently modified with a redox reporter (methylene blue). In the absence of a target, the stem-loop configuration holds the reporter close to the electrode surface, promoting efficient electron transfer and generating a relatively high Faradaic current. Upon hybridization with a complementary DNA target, the stem-loop “opens” into a more rigid, double-stranded structure, distancing the reporter from the electrode and reducing electron transfer rates. This decrease in Faradaic current provides a clear electrochemical signal indicating the presence of the target. Moreover, the E-DNA sensor can be regenerated by a brief rinse with deionized water, which restores the stem-loop conformation and permits repeated use.
Fig. 14.
a Schematic representation of a typical thiolated, MB-modified DNA probe used in constructing an E-DNA sensor. Reprinted and modified from [244], with permission from Springer Nature. Copyright © 2007. b Schematic of an electrochemical DNA biosensor employing a “Y” junction design combined with a restriction endonuclease-mediated target recycling strategy. Reprinted and modified from [245], with permission from the Royal Society of Chemistry. Copyright © 2012. c Schematic illustration of the electrochemical DNA biosensor based on circular strand-replacement polymerization (CSRP). Reprinted and modified from [246], with permission from Elsevier B.V. Copyright © 2015. d Schematic of an electrochemical DNA biosensor using a DNA origami sandwich assay. Reprinted and modified with permission from [247]. Licensed under Creative Commons Attribution License (CC BY 4.0). Copyright © 2023, The Authors. Published by American Chemical Society
Transitioning to a different strategy, Wang et al. [245] developed a universal, signal-off electrochemical DNA biosensor using a “Y” junction structure combined with a restriction endonuclease-aided target recycling strategy (Fig. 14b). In this system, a MB-labeled capture probe is self-assembled on a gold electrode, and an assistant probe is used alongside the target DNA to form a stable Y junction. This configuration isolates the enzyme’s cleavage site on the assistant/capture probe duplex rather than on the target, allowing the restriction endonuclease HaeIII to specifically cleave the capture probe. As a result, the MB-labeled fragment detaches from the electrode while the intact target is released and recycled for further hybridization cycles. This repeated process leads to a significant reduction in the MB peak current, measured by alternating current voltammetry, which serves as the detection signal. Notably, the biosensor’s design can be adapted for various target sequences simply by altering the capture and assistant strands.
Further exploring amplification strategies, another study describes an ultrasensitive electrochemical DNA sensor employing isothermal strand-displacement polymerization (ISDPR) with target recycling [246]. In this system, MB-labeled hairpin probes are self-assembled on a gold electrode, ensuring the MB molecules remain nearby for efficient electron transfer (Fig. 14c). When the target DNA is present, it hybridizes with the hairpin probes, causing them to open and expose a primer binding region. DNA polymerase then extends the primer, displacing the target DNA so it can trigger additional cycles of amplification. This repeated process moves a large number of MB molecules away from the electrode surface, leading to a significant decrease in the measured oxidation current via DPV. The change in current is directly related to the target concentration, allowing for detection over a wide linear range, all achieved without the need for thermal cycling or complex instrumentation.
Expanding on innovative signal amplification strategies, additional approaches have emerged that harness the unique properties of DNA nanostructures to further enhance detection sensitivity and selectivity. In the work presented [247], a novel amplification strategy is introduced that leverages DNA origami to construct a sandwich assay, thereby significantly boosting the charge transfer resistance associated with target binding (Fig. 14d). By employing this approach, the detection limit is improved by two orders of magnitude compared to conventional label-free designs, enabling linear quantification of target DNA without the need for probe labeling or enzymatic assistance. The enhanced electrochemical mechanism relies on the precise assembly of a DNA origami nanostructure that cross-links a target oligonucleotide to a functionalized electrode, thereby altering surface interfacial properties to generate a stronger signal. Moreover, this design demonstrates excellent selectivity even in complex, nucleic acid-rich environments.
Electrochemical “signal‐on” mechanisms
Electrochemical “signal‐on” mechanisms are sensing strategies in which the binding or hybridization of target DNA directly increases the measurable electrochemical signal.
In the work of Immoos et al. [248], a single-stranded oligodeoxynucleotide modified with a PEG triblock polymer is immobilized on a gold electrode. According to their findings, when target DNA is present, it simultaneously hybridizes with both the top and bottom regions of the immobilized probe, forcing it into a loop conformation. This looping brings a terminally linked ferrocene redox tag into proximity with the electrode surface, significantly enhancing electron transfer and increasing the oxidation current by 600% (Fig. 15a).
Fig. 15.
a Schematic representation of electrochemical nucleic acid detection using a DNA wrap assay. Reprinted and modified with permission from [248]. Copyright © 2004, American Chemical Society. b Schematic illustration of a signal-on E-DNA sensor based on target-induced unfolding of an electrode-bound, MB-modified DNA pseudoknot. Reprinted and modified with permission from [233]. Copyright © 2007, American Chemical Society. c Schematic diagram of triple-helix molecular switch electrochemical DNA biosensor that uses target-induced disassembly to decrease the ferrocene signal and increase the MB signal. Reprinted and modified with permission from [250]. Copyright © 2017, American Chemical Society. d Schematic depiction of an E-DNA sensor employing a target-catalyzed hairpin assembly strategy. Reprinted and modified from [251], with permission from Elsevier B.V. Copyright © 2015
Building on this concept of exploiting conformational changes for signal enhancement, another sensor design employs target-induced strand displacement to reposition the redox tag closer to the electrode, further amplifying the electrochemical signal. Initially, a capture probe is immobilized on a gold electrode via its 5′ terminus. Subsequently, a signaling probe—modified at its 5′ end with MB and complementary to both the 3′ and 5′ regions of the capture probe—is hybridized to the immobilized strand. In the absence of the target, the rigid double-stranded structure holds the MB tag away from the electrode surface, resulting in a low redox current. When target DNA is present, it displaces the 5′ end of the signaling probe, generating a short, flexible single-stranded segment that brings the MB tag closer to the electrode. This conformational change significantly enhances electron transfer, increasing the oxidation current by up to 700%. This performance ranks among the most sensitive methods available for single-step electrochemical DNA sensing. One limitation is that the sensor cannot be regenerated without adding a new signaling probe, because the hybridization between the signaling and capture probes breaks down under the conditions needed to remove the target. Consequently, the sensor is not stable enough for complex samples like blood serum [249].
Xiao et al. [233] have developed a signal-on E-DNA sensor in which the DNA forms a pseudoknot structure that normally holds a redox-active label (MB) away from the electrode, resulting in a low baseline signal (Fig. 15b). When target DNA binds, it causes the pseudoknot to unfold, which brings the redox label closer to the electrode and increases electron transfer, producing a “signal-on” response. When target DNA binds, the pseudoknot unfolds, converting the probe into a flexible single-stranded element that brings the methylene blue close to the electrode surface. This conformational change significantly enhances electron transfer, increasing the oxidation current. The sensor is straightforward to fabricate, can be readily regenerated, and is selective enough for direct use in complex matrices like blood serum, while also exhibiting exceptional sequence specificity. Despite these advantages, the overall signal gain is limited to 100%.
A sensor system was developed using a triple-helix molecular switch to achieve dual signaling in an E-DNA biosensor, ultimately enabling sensitive target detection [250]. In this setup, a hairpin capture probe labeled with MB (MB-DNA) is immobilized on a gold electrode via Au–S bonds and subsequently passivated with MCH (Fig. 15c). A second DNA strand, modified with two Fc groups (Fc-DNA), hybridizes with the MB-DNA to form a stable triple helix structure. Upon the addition of target DNA, the Fc-DNA preferentially binds with the target, causing the triple-helix to disassemble. This event allows the MB-DNA to revert to its original hairpin conformation, while the Fc tags diffuse away from the electrode surface. As a result, the oxidation current from ferrocene decreases, and that from methylene blue increases. This strategy offers a selective, reusable, and simple platform for electrochemical DNA detection.
Qian et al. [251] designed a signal-on E-DNA sensor utilizing a target-catalyzed hairpin assembly strategy for sensitive detection (Fig. 15d). In this configuration, a thiolated stem-loop probe (MB1) is immobilized on a gold electrode via Au–S bonds, while a Fc-labeled stem-loop probe (MB2) interacts with MB1. When target DNA is introduced, it hybridizes with MB1, opening its stem-loop structure. MB2 then binds to the unhybridized region of MB1 and displaces the target, which is released to trigger further cycles of hairpin opening and MB2 binding. This cyclic process accumulates multiple Fc labels near the electrode, enhancing electron transfer and leading to a significant increase in the oxidation current measured by DPV. In the absence of target DNA, MB1 and MB2 maintain their stable hairpin conformations, resulting in a low background signal. This approach enables highly sensitive and selective DNA detection, offering a simple and cost-effective method for signal amplification.
In this study, the authors developed a novel E-DNA biosensor based on an enzyme-amplified mechanism [252]. The process begins with a thiolated single-stranded DNA capture probe immobilized on a gold-modified screen-printed electrode via S–Au bonds (Fig. 16a). After blocking unreacted sites with bovine serum albumin (BSA), the electrode is incubated with a biotinylated target DNA solution, allowing hybridization to occur under controlled conditions. Unbound oligonucleotides are then washed off with DEA buffer. Next, the biotinylated hybrid is labeled with a streptavidin–alkaline phosphatase conjugate. For detection, the enzyme converts 4-aminophenyl phosphate into the electroactive product 4-aminophenol, whose oxidation signal is measured by DPV. Meanwhile, EIS was performed using 10 mM K3Fe(CN)6/10 mM K4Fe(CN)6 containing 0.01 M KCl as the electrolyte to monitor the changes in charge transfer resistance caused by DNA immobilization and hybridization, providing a label-free method to verify the process. This stepwise electrochemical detection approach enhances the sensor’s sensitivity and selectivity, making it a promising tool for rapid DNA analysis.
Fig. 16.
a Schematic of an enzyme-amplified electrochemical DNA biosensor featuring a gold electrode, target hybridization, enzymatic labeling and detection. Reprinted and modified from [252], with permission from Elsevier B.V. Copyright © 2018. b Schematic representation of a label-free electrochemical DNA biosensor on a gold film electrode using a sandwich hybridization assay, peroxidase labeling, and CA detection after TMB oxidation. Reprinted and modified with permission from [49]. Copyright © 2015, American Chemical Society. c Schematic of a label-free E-DNA biosensor where target hybridization frees the capture probe for gold nanoparticle binding, enhancing electron transfer. Reprinted and modified from [254], with permission from Elsevier B.V. Copyright © 2021. d Schematic illustration of the fabrication process for a DNA biosensor employing in-situ assembled AuNPs-Mel-Cu2⁺ as the signal tag. Reprinted and modified from [255]. Licensed under Creative Commons Attribution License (CC BY 4.0). Copyright © 2016, The Authors. Published by Springer Nature
The sensor was built on a gold film electrode, where a monolayer was formed by immobilizing a thiolated capture probe via Au–S bonds, then passivated with p-aminothiophenol to optimize orientation and block nonspecific adsorption (Fig. 16b). For detection, a two-step sandwich hybridization assay was employed. First, a diluted target sample (or amplified sample) was mixed with a FITC-labeled signaling probe and BSA to promote homogeneous hybridization. This mixture was then applied onto the sensing surface, where hybridization occurred over 120 min, forming a ternary duplex that binds the target specifically. After washing with a casein blocking buffer, the surface-bound target was enzymatically labeled using an anti-FITC Fab conjugated with peroxidase (POD). The key electrochemical detection step involved immersing the electrode in a substrate solution containing TMB and H₂O₂. The POD enzyme catalyzed the oxidation of TMB, and this electroactive product was quantified by chronoamperometry at 0 V after 30 s. The measured oxidation current is directly related to the amount of target DNA present, thereby providing a sensitive and selective detection method based on changes in electron transfer at the modified electrode interface [49].
A similar approach was demonstrated by Moura-Melo et al. [253]. Both methods share a common foundation in sensor fabrication, starting with a gold film electrode modified by immobilizing a thiolated capture probe and passivated with p-aminothiophenol to create a stable, low-background surface. However, while the first sensor employs a two-step sandwich hybridization assay with an anti-FITC Fab-POD conjugate to label and detect the target DNA via chronoamperometry, the second approach adapts this strategy for GMO analysis. In the latter, the sensor fabrication is integrated with an extraction and PCR amplification step of the target sequence (the CaMV 35S promoter) prior to hybridization with a FITC-labeled signaling probe. Furthermore, an anti-FITC-HRP conjugate is used, and the electrochemical signal is generated by reducing oxidized TMB at 0 V. These modifications extend the method’s applicability to complex food samples while preserving the core principle of sensitive, enzyme-catalyzed DNA detection.
A label-free electrochemical DNA biosensor was developed using AuNP–mediated electron transfer. First, a thiolated ssDNA (ssDNA1) is immobilized on a gold electrode via Au–S bonds and then blocked with MCH to prevent nonspecific adsorption (Fig. 16c). When a complementary strand (ssDNA2) hybridizes with ssDNA1, a triple-helix DNA structure forms that repels negatively charged AuNPs due to electrostatic forces, maintaining a high electron transfer (ET) resistance. However, upon introducing target DNA, hybridization with ssDNA2 occurs, leaving ssDNA1 free to capture AuNPs. This capture enhances ET efficiency between the redox species and the electrode, and the resulting change is measured using direct impedimetric detection. This simple yet effective approach enables sensitive, label-free DNA detection over a wide range, while minimizing complex fabrication and pre-treatment steps [254].
Wang et al. [255] introduced a novel, ultrasensitive DNA biosensor that overcomes the limitations of traditional hairpin probes (Fig. 16d). Instead of relying solely on a conformational change to reposition a redox tag, their method immobilizes a 3′-thiolated hairpin probe on a gold electrode via Au–S bonds. AuNPs are then attached to the free 5′ end of the probe through the specific affinity between gold and -NH₂ groups, serving as a high surface area and conductive platform. Subsequently, a melamine-Cu2⁺ complex is assembled onto the AuNPs via Au–N and Cu2⁺-N bonds. The combination of increased loading and improved electron transfer kinetics, thanks to the electrocatalytic properties of AuNPs, results in a significantly enhanced electrochemical signal. This stepwise in situ labeling process is simpler and more cost-effective than conventional homogeneous methods while still providing high selectivity, a wide linear range, and a low detection limit.
Field-effect transistor-based biosensors for nucleic acid detection
FETs represent a class of semiconductor devices in which an electric field is utilized to regulate the flow of current within the transistor channel, which is, traditionally, composed of a semiconductor material. However, advancements in micro- and nanofabrication technologies, coupled with innovations in material science, have significantly enhanced the properties of the channel and the overall FET performance in recent years [256]. A typical FET comprises three terminals, i.e., the source, drain, and gate, which facilitate its integration into an electrical circuit. Conventionally, the source electrode serves as the entry point for charge carriers, while the drain electrode marks their exit from the channel. The gate electrode, on the other hand, modulates the current flow through the channel by the application of an external voltage.
Recent advancements in 2D nanomaterials, attributed to their exceptional electrical properties, have led to the development of a novel class of FET devices known as 2D-FETs. These devices differ from conventional silicon-based FETs in terms of channel configuration and terminal organization. In 2D-FETs, the channel is fabricated from 2D nanomaterials positioned above an insulating oxide layer, requiring additional insulation for the gate electrode or its relocation to the backside of the transistor [257]. The 2D materials employed in FETs include carbon-based nanomaterials, such as graphene [258] (monolayer graphene, GO, and rGO), [259] TMDCs with the general formula MX₂ (where M represents a transition metal from group IVB or VIB, and X represents a chalcogenide), and MXenes [260] (transition metal nitrides and carbides). Owing to their high channel sensitivity to (bio)chemical or physical interactions, 2D-FET devices have found extensive applications in (bio)sensing technologies. These devices are particularly prominent in detecting biomarkers in (bio)medicine [261, 262], monitoring heavy metal ions [263], and ensuring safety in agricultural, environmental, and food protection domains [264, 265].
Principles of 2D-FET operation in biosensing
2D-FET sensing configuration
2D-FET devices are electrically characterized by three curves, i.e., output (IDS vs VDS), transfer (IDS vs VG), and time series (IDS vs t), of which the transfer curve provides the most information about the FET [266]. Such information is given by the parameters like threshold voltage (Vth), on/off ratio (Ion/Ioff), subthreshold slope (SS), transconductance (gm), and field-effect mobility (μFE). Vth denotes the minimum voltage that initiates carrier flow through the channel, above which the transistor operates in the linear and saturation regions. Field-effect mobility is the average mobility of charge carriers per unit electric field in a semiconducting channel, and it can be estimated from the linear region of the transfer curve as [266]:
where L and W mark the length and width of the channel, Ctot is the total gate capacitance, and is the transconductance, commonly denoted as gm. Transconductance mathematically represents the slope of the linear part and physically reflects the FET’s ability to convert a small change in voltage into a large change in current. On/off ratio describes the switching abilities of the FET, and it is the ratio of the highest and lowest drain current during a gate voltage sweep at a specific VDS. SS is a parameter that describes how fast a FET device can switch on. Based on the characteristic parameters, a good FET is characterized by low Vth, high μFE, high on/off ratio, and low SS.
The 2D-FETs operate in two primary configurations, distinguished by the position of the gate electrode relative to the channel layer: back-gate (or solid-gate) and top-gate (or liquid-gate) configurations (Fig. 17a) [266, 267]. In the back-gated configuration, the current flow is modulated through a highly doped silicon substrate, separated from the channel by a thin oxide layer, typically up to 1 μm thick. In the top-gated configuration, the gate electrode is positioned above or co-planar with the channel and separated by an electrolyte. When a gate bias is applied, the channel is electrically coupled to the gate via an electric double layer (EDL), formed by the redistribution of ions at the solid–liquid interface. Biosensors generally utilize the top-gate configuration due to its compatibility with liquid samples and the superior resolution of the EDL compared to the oxide dielectric layer in the back-gate configuration [267]. In the back-gate setup, gate capacitance is determined by the oxide dielectric and is inversely proportional to its thickness (t), that is , where εox is the electric permittivity of the dielectric. Conversely, in the top-gate configuration, the insulating layer is formed by the EDL, whose thickness ranges from angstroms to several nanometers, depending on the ionic concentration. This results in a significantly larger capacitance, often comparable to the quantum capacitance of the channel material. Consequently, the gate capacitance in the top-gate configuration is governed by the series combination of EDL (CEDL) and quantum capacitance (CQ) [267]:
Fig. 17.
Schematic representation of a 2D-FET operation principles. a Two operational configurations—back-gate (solid gate) and top-gate (liquid gate) used in (bio)sensing. b Signal development in 2D-FET biosensors: Dirac point shift in ambipolar channel materials (top left) or threshold voltage shift in semiconductor channels (down left), change in drain current at fixed gate voltage (top middle) and respective time-dependent change of ID due to transfer curve shift (down middle), transconductance change of a transfer curve (change in slope of the linear part) (top right), and respective time-dependent change of ID due to transfer curve slope change (down right). c Four types of sensing mechanisms in 2D-FETs: electrostatic gating effect between ssDNA probe and graphene channel inducing p-type doping (top left), charge transfer effect between adsorbed ssDNA on graphene channel inducing n-type doping (top right), Donnan potential effect explained by the semi-permeable layer formed by a polymer that adds a Donnan capacitance (CDonn) to the total capacitance (down left), and charge scattering effect induced by the DNA probe, which reduces carrier mobility and consequently transconductance (down right). d Schematic description of the screening effect in liquid-gated FET using PBS as an electrolyte example
The applied gate voltage required to achieve linear transfer curves in the top-gate configuration can be more than two orders of magnitude lower than that in the back-gate configuration (± 1 V vs. ± 100 V).
Signal development in FET-based biosensors
The signal interpretation of 2D-FET biosensors can be categorized based on the electrical output collected. Voltage-based outputs are the most common, with specific parameters indicating the FET state during a biosensing event. For instance, in ambipolar materials like graphene, the transfer curve exhibits a parabolic shape with a minimum, referred to as the charge neutrality point (CNP) or Dirac point (DP), VDP, instead of typical Vth [266]. Biosensing events can induce charge doping, resulting in a DP shift, calculated as , where VDPi and VDPf represent the gate voltages at DP before and after the event, respectively [170, 267]. In semiconducting materials such as TMDs, which are exclusively p- or n-type, the transfer curve is typical for semiconductor-based FETs, which is characterized by Vth, and such value changes similarly upon biosensing event: [267]. Besides voltage shifts, current-based outputs are also used at DP, with the drain current shift being a key metric, albeit one influenced by multiple mechanisms near the FET channel marking it unreliable. Therefore, another drain current change may be used, usually at a fixed gate voltage value on the linear part of the n- or p-branch [267]. Transconductance is also used as a signal parameter; it is affected by the carrier mobility, which in turn depends on charge scattering centers, meaning that gm changes upon charged impurity change rather than the doping mechanism [266]. Typically, maximum transconductance is used to determine the gate voltage of interest for current-based signal determination since it marks the highest linearity of the transfer curve. Additionally, 2D-FET biosensors are often used for time-series measurements, where the drain current is monitored continuously under constant bias (VG, VD = const.). The change in drain current over time is defined as , where IDi and IDf are the averaged drain currents before and after the biosensing event, respectively. Depending on the sample composition as well as FET type, such a change can occur faster or slower, which is defined by the response time (time needed for the drain current to stabilize/saturate after the (bio)interaction happens). The use of time-series measurement is often combined with transfer characterization in order to determine the nature of the signal [267]. In Fig. 17b, all types of signal development are depicted as discussed.
On the other hand, 2D-FETs can be utilized in an extended-gate configuration, where the gate electrode is exposed to the analyte, while the channel is being isolated from it [266]. Since channel does not interact with the electrolyte and external charges, the change in carrier density and electrical characteristics of the 2D-FET is a consequence of charge redistribution at the gate/electrolyte interface and change in the gate potential. This change is expressed as , where VGi denotes applied gate potential before the biosensing event and ΔVadd is additional positive or negative voltage added after the biosensing event [266]. This effective gate potential is responsible for the transfer curve shift and drain current.
Sensing mechanisms of 2D-FET biosensors
The liquid-gated configuration of 2D-FET biosensors is analogous to the electrochemical three-electrode system, where the top-gate electrode serves as the reference electrode. However, the signal generation mechanism in 2D-FETs is unlike electrochemical principles which require electrochemical labels to convert chemical interactions into electrical signals. In electrical biosensing with 2D-FETs, sensing mechanisms are based on electrostatic gating effect, charge transfer effect, Donnan potential effect, and carrier scattering effect, which are schematically presented in Fig. 17c [170]. In the electrostatic gating effect, biomolecule-induced charges can dope the sensing channel material, altering its electrical properties. The net charge of a bioreceptor (positive or negative) attracts opposite charges in the 2D material, modulating the carrier density and shifting the Fermi level, thereby affecting the electrical response of the transistor. For example, a DNA probe (with a negatively charged phosphate backbone) as a bioreceptor may induce such a kind of doping, leading to p-type doping effect that is recorded as a right-shift of DP in graphene FETs [268]. Another mechanism is the charge transfer effect, where direct charge transfer occurs upon biomolecule adsorption to the channel material, inducing changes in the carrier density of the channel. This is exemplified by interactions between aromatic ring bases of aptamers and graphene, where electron transfer from the negatively charged backbone increases graphene’s electron concentration, leading to the left-shift of the DP (n-doping) [269]. Additionally, the detection of large biomolecules, such as proteins or bacteria, beyond the Debye length constraints is explained by the Donnan potential effect. As an example, a ribonucleoprotein (RNP), such as CRISPR/Cas9, is immobilized at the surface of a sensing material that specifically interacts with negatively charged DNA, creating an ion-permeable layer due to the accumulation of counter-ions. Difference in the concentration of ions between the bulk solution and the immobilized ion-permeable layer creates a Donnan potential, which alters the electric field surrounding the channel and modulates the channel current [270]. Lastly, charged biomolecules on the channel surface can hinder carrier transport via the local carrier scattering effect, reducing the material’s conductivity. This can be explained by the interaction of receptor-target interaction of opposite charges, which can lead to the formation of a neutral ligand; however, adsorbed biomolecules may reduce overall carrier mobility, thus reducing the transconductance of the channel [271].
For nucleic acid detection, 2D-FET biosensors typically use DNA/RNA single-stranded probes complementary to the target oligonucleotide, with the (bio)interaction detected through hybridization into double-stranded chains [272]. Advanced probes, such as tetrahedral [273] or hairpin-shaped nucleic acids [274], are also employed. Emerging approaches include neutral charge probes, such as peptide nucleic acids (PNAs) [275] or morpholino antisense nucleotides (PMOs) [276], which avoid electrostatic repulsion with negatively charged targets. Additionally, CRISPR/Cas systems, or RNP, are being explored for detecting double-stranded nucleic acids, bypassing denaturation steps required for single-stranded target detection [277]
Electrical double layer
A critical physical property affecting the sensitivity of 2D-FET biosensors operating in the liquid-gated configuration is charge screening. When a gate potential is applied and the EDL forms, electrolyte ions redistribute at the channel-electrolyte interface as well as at the gate-electrolyte interface. Charged molecules in solution are screened by mobile counterions, resulting in the exponential attenuation of their electric potential with distance, which is determined by the Debye length (λD). The Debye length is given by [266]:
where ε is the permittivity of the electrolyte, kB is the Boltzmann constant, T is the temperature, NA is Avogadro’s number, e is the elementary charge, and I is the ionic strength of the electrolyte. For aqueous electrolytes, such as phosphate-buffered saline (PBS), the Debye length can be approximated as [266]:
where I is the ionic strength in mol/L. For example, a buffer with an ionic strength of 0.154 mol/L, e.g., 1 × PBS, results in a Debye length of approximately 0.7 nm, posing challenges for biosensor sensitivity in such media, see Fig. 17d. One approach to mitigate charge screening effects is to reduce the ionic strength of the electrolyte, thereby increasing the Debye length to several nanometers (0.1 × PBS – λD ≈ 2.3 nm, 0.01 × PBS—λD ≈ 7.4 nm), enhancing biosensor performance. However, too low ion concentration reduces the buffering properties of the solutions and poses a DNA hybridized structure to be more unstable [266]. Therefore, researchers proposed other strategies to improve the sensitivity of a biosensor beyond the Debye length. An introduction of self-assembled layer of PEG to the graphene surface increases the effective Debye length due to the bio-permeable nature of PEG and, additionally, inhibits non-specific adsorption on the graphene surface [278]. A study reported by Hwang et al. suggested that the morphological alteration of the channel surface can overcome Debye length limitations [279]. Bending graphene to hill-and-valley relief reduces the charge shielding effect in the nanoscale deformation region, especially in the valley region. Strategies to circumvent the screening effect of ions may be realized using emerging CRISPR/Cas technology in DNA detection, owing to the CRISPR/Cas target-enabled cleaving of the reported probe immobilized on the channel surface [277]. The formation of a molecular electromechanical system that is comprised of a tetrahedral dsDNA and flexible ssDNA cantilever linked to the tetrahedral structure enabled detection of target-receptor interaction within the Debye length [280].
Field-effect transistors for the detection of nucleic acid amplification products
Enzymatic nucleic acid amplification
FET-based biosensors employ various strategies to detect nucleic acid amplification (NAA) outcomes. One approach involves anchoring a specific DNA probe onto the FET channel surface, which is fully complementary to the ssDNA/RNA target sequence present in amplicons. For instance, Purwidyantri et al. [281] developed a portable GFET array chip to identify grape varieties in wine by detecting grapevine DNA in laboratory and real samples. The GFET chip, functionalized with a fully complementary DNA (FC DNA) probe, achieved a detection limit as low as 0.19 aM in hybridization buffer, with a wide detection range (10−18 to 10−12 M) following a dose–response curve. The biosensor also demonstrated the ability to distinguish single-nucleotide polymorphisms (SNPs) from FC DNA, showing detectable trends for 1 SNP, 2 SNPs, and 3 SNPs, attributed to mismatch positions near the DNA sequence end, which permitted near-complementary hybridization. Furthermore, the biosensor was validated using real grapevine and wine DNA samples amplified by PCR, showing discrimination between FC DNA and SNP-containing DNA in terms of sensitivity, while non-complementary DNA (NC DNA) exhibited weak signals. Weak discrimination between FC DNA and SNPs could be resolved by probe optimization aiming for the lower probability of probe-SNP hybridization detectable by the GFET biosensor.
An alternative detection strategy involves monitoring pH changes during NAA reactions using FET biosensors. Han et al. demonstrated this approach by detecting pH changes associated with LAMP of lambda phage genomic DNA [282]. A chamber-supported GFET monitored the release of protons during the LAMP reaction, leading to a decrease in pH and a corresponding DP shift, as shown in Fig. 18a. After optimizing the LAMP reaction time and buffer concentration, real-time LAMP detection was performed by measuring transfer curves every 5 min. Two signals were extracted: threshold time at ΔVDP = 0.05 V and DP shift after 40 min of reaction. The biosensor achieved a limit of detection (LOD) of 2 × 102 copies/µL of viral DNA, demonstrating high sensitivity.
Fig. 18.
a Detection of lambda phage genomic DNA LAMP reaction using chamber-supported GFET biosensor; the release of protons enables doping of graphene channel, and DP shift is recorded every 5 min, enabling detection of only 2 × 102 copies/μL of viral DNA. Reprinted and modified from [282], with permission from Elsevier B.V. Copyright © 2017. b POC device in the form of a crumpled graphene FET for the detection of SARS-CoV-2 DNA amplified by RT-LAMP using primer consumption strategy; crumpled graphene ensures higher DP shift upon ssDNA adsorption, with higher shift for no SARS-CoV-2 DNA (negative samples) and shift decrease for increased virus presence in positive patients. Reprinted and modified with permission from [284]. Copyright © 2021, American Chemical Society. c Innovative functionalization of graphene utilizing tetrahedral dsDNA structure with ssDNA (H1) probe for highly sensitive miRNA-21 detection; CHA is applied to amplify the signal from target RNA reaching an LOD of 5.67 × 10−19 M in buffer, while GFET biosensor was able to detect target RNA in different cell lines in complex medium. Reprinted and modified with permission from [273]. Copyright © 2023, American Chemical Society
Recent advancements in crumpled graphene have further enhanced FET-based detection of oligonucleotides. Ganguli et al. developed a crumpled graphene FET (cGFET) device for ultrasensitive detection of bacterial DNA amplified by LAMP [283]. An ssDNA strongly adsorbs onto graphene via π − π interactions between the graphene lattice and the unpaired aromatic bases of nucleobases, while dsDNA adsorbs less effectively. Moreover, the crumpled graphene surface alters the Debye length, increasing the region of unscreened ions [279]. Since primers are ssDNA, while LAMP products are dsDNA, the detection of primers by GFETs poses an efficient strategy for LAMP amplification biosensing. Using this primer consumption strategy, the authors detected as low as 8 × 10−21 M of target E. coli dsDNA, with clear discrimination between amplified and unamplified samples. This approach was further advanced by Park et al., who utilized cGFETs for point-of-care (POC) detection of SARS-CoV-2 viral RNA (Fig. 18b) [284]. The crumpled graphene FET, with its enhanced sensitivity to ssDNA (LAMP primers), demonstrated reduced charge screening and graphene bandgap opening, leading to significant changes in carrier density and larger DP shifts upon DNA adsorption. The biosensor achieved a detection limit of just 10 copies/µL after 30 min of LAMP reaction. Furthermore, for POC validation, 10 positive and 10 negative clinical samples were analyzed, showing a significant difference between positive (low DP shift) and negative (high DP shift) samples. While promising for POC diagnostics, primer consumption detection by cGFETs may face limitations, as it cannot discriminate between different samples on a single chip due to the detection mechanism relying solely on ssDNA adsorption onto the graphene surface.
Non-enzymatic nucleic acid amplification
Chen et al. developed a graphene FET (GFET)-based biosensor for detecting the VEGF165 biomarker using a hybridization chain reaction (HCR) strategy [285]. In this approach, a specific anti-VEGF165 aptamer (P1) serves as the primary recognition element, while two single-stranded DNAs (T1 and T2) facilitate targeted hybridization at the graphene surface. To amplify the signal, two hairpin-structured ssDNAs (H1 and H2) are introduced following successful hybridization. Initially, the T1 probe is immobilized onto the graphene channel using a PBASE linker. In the presence of the target molecule and the P1-T2 duplex, P1 binds to the target, enabling T1-T2 hybridization. Subsequently, the addition of H1 and H2 triggers the HCR process, significantly enhancing the GFET signal (observed as a leftward shift of the DP) compared to detection without HCR amplification. This approach enabled the detection of VEGF165 across a wide range of concentrations (10 pg/mL to 800 ng/mL) with a detection limit as low as 3.24 pg/mL (S/N = 3). A CHA methodology of DNA amplification is utilized for ultrasensitive detection of miRNA-21 in complex biological samples [273]. Here, a specific tetrahedral DNA nanostructure (TDN) containing hairpin DNA probe 1 (H1) is anchored on the graphene surface using PBASE immobilization strategy to enable antifouling properties of H1 probe with higher CHA probability, Fig. 18c. The biosensor exhibited the detection of a broad range of miRNA-21 concentrations from 5 × 10−19 to 5 × 10−15 M (dynamic range), with an LOD of 5.67 × 10−19 M according to the GFET left shift of DP. Finally, the authors tested the biosensor towards RNA extracts from three cell lines, showing promising results for clinical use of the device.
Going beyond nucleic acid amplification methods with field-effect transistors
Biosensing solutions are a hot topic for fast, reliable, on-demand, and in-field detection, compared to time-consuming laboratory standard methods. A combination of NAA methods with biosensors offers a bridge between the two detection principles and can even be integrated to lower the time and costs of diagnostics. Recently, nucleic acid detection by biosensors can be realized without standard molecular DNA/RNA amplification, thus avoiding the step of NAA and reducing the detection time even more. Table 1 summarizes significant achievements in the detection of nucleic acids using both amplified and non-amplified samples. The synergy between 2D-FETs and NAA methods is not well exploited, and mostly DNA hybridization is studied, giving promising results in terms of biosensor performances.
Graphene FET-based biosensors
Detection of single-stranded DNA (ssDNA) using hybridization with fully complementary DNA probes on GFETs exhibits significant potential due to the unique properties of graphene. The hexagonal lattice of graphene enables non-covalent π-π interactions with the aromatic nucleobases of ssDNA, and in physiological conditions, the negatively charged DNA induces direct charge transfer and n-doping of graphene. However, some researchers argue that negatively charged ssDNA induces p-doping due to electrostatic gating when a negatively charged phosphate backbone induces more opposite-charged carriers (holes) in graphene. The interaction can be monitored through the DP shift in GFETs. However, the oligonucleotide composition may also play a role in the degree of physical adsorption, and most probably, the recorded DP shift is a result of a competition of the two mechanisms. Campos et al. developed a GFET biosensor with a large-area gate electrode surrounding the graphene channel to enhance DNA hybridization detection [286]. The sensor demonstrated a detection limit of 25 aM using arbitrary probe-target DNA sequences, though signal saturation at 100 fM was attributed to limited probe DNA density on the graphene channel. Still, the authors hypothesized that it is a result of equilibrium between hybridization and electrostatic repulsion between probe DNA and target DNA. Similarly, a GFET-based biosensor for Staphylococcus aureus-specific DNA detection was developed by Zheng et al., employing an alternative functionalization strategy [287]. Here, the co-planar gold gate electrode was functionalized with probe DNA, while the remaining surface was blocked with MCH to prevent non-specific adsorption, as shown in Fig. 19a. The hybridization-induced potential drop at the graphene-electrolyte interface resulted in a DP shift, achieving detection across a broad range (10−16 to 10−8 M) with a detection limit below 10−17 M. This biosensor also enabled real-time detection within 27 min and demonstrated high selectivity for 9- and 3-mismatched DNA and cross-specificity with different bacterial species. As a POC device, the biosensor was integrated with smartphone data recording, showcasing promising results for portable diagnostics.
Fig. 19.
a Development of a POC device based on GFET biosensor for the ultrasensitive detection of S. Aureus DNA; gold gate electrode was functionalized with specific ssDNA probe and blocked by MCH to enable a broad range of detected DNA from 10−16 to 10−8 M using real-time measurements; they integrated the POC device with a smartphone using portable reader to showcase in-field possibilities of their biosensor. Reprinted and modified from [287], with permission from Elsevier B.V. Copyright © 2024. b Groundbreaking detection of DNA using crumpled GFET device with zeptomolar detection limit; they studied two types of probes, DNA and PNA, to reach LOD of 600 zM in 1 × PBS, owing to the crumpled morphology of graphene surface altering EDL structure. Reprinted and modified from Hwang et al. [279]. Licensed under Creative Commons Attribution License (CC BY). Copyright © 2020, Hwang et al. c An extended-gate FET POC device for the detection of E. coli O157:H7 DNA sequences using portable MOSFET configuration; they adopted DNA fragmentation by sonication to prove detection of the specific intimin gene in human serum samples with performances that are comparable to test laboratory samples. Reprinted and modified with permission from Paulose et al. [289]. Copyright © 2023, American Chemical Society. d Integration of CRISPR/Cas12a system with GFET for HPV-16 and E. coli detection; upon binding of the target, Cas12a cleaves the bystanding polyA or polyC probes on graphene, inducing DP shift, the GFET-CRISPR chip was able to recognize ssDNA from HPV-16 and dsDNA from E. coli with LODs of 1 and 10 aM, respectively. Reprinted and modified with permission from Weng et al. [277]. Copyright © 2023, American Chemical Society
Crumpled graphene FET-based biosensors
Crumpled graphene FETs (cGFETs) exhibit enhanced performance in DNA detection due to their unique surface topology. Hwang et al. developed a cGFET biosensor for zeptomolar detection of DNA in buffer solutions using PNA probes [279]. When compared to flat graphene, cGFETs demonstrated superior sensitivity, achieving detection limits of 20 aM (~ 600 molecules in 50 μL) for DNA-DNA hybridization and 600 zM (~ 18 molecules) for DNA-PNA hybridization (Fig. 19b). The authors attributed this enhanced performance to reduced charge screening and increased doping sensitivity on the concave surfaces of crumpled graphene. Additionally, the surface configuration induced a bandgap in graphene, further enhanced by DNA adsorption, as confirmed by molecular dynamics simulations and GW computations. The biosensor also demonstrated attomolar detection of miRNA in complex human serum samples, highlighting its potential for high-performance biomolecular detection.
Transition metal dichalcogenide FET-based biosensors
TMDC-based FETs, such as those using MoS2 or WS2, offer another promising platform for DNA hybridization biosensing. A few-layer MoS2 FET was developed using PMO probes immobilized via PBASE linkers [276]. This approach enabled detection of target DNA across a range of 10 fM to 1 nM, with a detection limit of 6 fM (S/N = 3), and excellent selectivity toward mismatched DNA targets. In contrast, Bahri et al. utilized a novel strategy for WS2 FETs by immobilizing a C15 DNA homopolymer probe through van der Waals forces [288]. DNA probe consisted of recognition complementary strand at 5′ end and C15 block at 3′ end is immobilized on the WS2 channel for the ultrasensitive detection of target DNA in the dynamic range of 100 aM to 1 nM, with a LOD of only 3 aM (S/N ratio of 3) based on the current change at threshold voltage. The authors claimed that the biosensor's ultra-sensitivity comes from the C15 blocking step, removing false-positive signals, and probe DNA displacement into the upright conformation that is more favorable to the hybridization with the target DNA. Additionally, their biosensor showed excellent selectivity toward mismatched DNA targets.
Other types of FET-based biosensors
Extended gate FETs (EG-FETs) have garnered interest due to their fabrication simplicity, as only the functionalized gate electrode interacts with the sample, and the FET body is usually a commercial MOSFET configuration. Paulose et al. developed a POC EG-FET for detecting E. coli DNA in serum samples [289]. Using a portable MOSFET readout and disposable sensor sticks, as presented in Fig. 19c, DNA hybridization of E. coli O157:H7 sequences was detected in the range of 1 fM to 1 pM. Moreover, a 1.2-kb dsDNA from the intimin gene was fragmented using pulsed sonication to prepare more realistic samples in TE buffer and human serum samples; the biosensor sensitivity was comparable to the results with artificial DNA strands. Hu et al. proposed a novel DNA probe design with partially neutralized phosphate-methylated backbones to optimize hybridization conditions [290]. Namely, using Si-nanowire FETs, the authors investigated the probe DNA neutralization and influence of ion concentration on hybridization, revealing that partially neutralized DNA probe can enhance the signal, yet retaining a partial electrostatic repulsion between target and probe, thus, reducing non-specific binding. The effect of ion concentration on DNA hybridization is such that higher ion concentration promotes hybridization, but at the cost of FET sensitivity due to low Debye length and charge screening. The authors proposed using a low ionic-strength bis–tris propane buffer (BTP) that can effectively increase the Debye length, promoting better sensitivity with neutralized DNA probes. As a proof-of-concept, the authors demonstrated the capability of Si-NWFET biosensing with partially neutralized DNA probes, detecting as low as 0.1 fM of target DNA.
Application of CRISPR/Cas systems with FET-based biosensors
CRISPR/Cas-based DNA detection without amplification has emerged as a highly promising and effective approach. The combination of CRISPR/Cas and FET platforms (CRISPR-FETs) is already well exploited primarily in biomedical applications in search of highly sensitive, fast, and reliable POC devices for the recent COVID-19 pandemic [291]. CRISPR-FETs can operate based on two mechanisms of CRISPR/Cas recognition and hydrolysis of nucleic acid strands, called cis-cleavage and trans-cleavage. In cis cleavage (used by Cas9 protein), a guide RNA bound to the enzyme recognizes target DNA and hybridizes with it, initiating hydrolysis of the target DNA. On a contrary, trans-cleavage (used by Cas12, Cas13, and Cas14) follows one more step, in which after successful hybridization of target DNA/RNA with guide RNA, Cas enzymes undergo structural reconfiguration revealing new catalytic site and triggering non-specific RNAse (or DNAse) activity of any DNA/RNA strand nearby. A pioneering work by Haijan et al. describes the use of a CRISPR-FET system utilizing CRISPR/Cas9 immobilization to the graphene channel of the FET chip [270]. Real-time response of GFET drain current is monitored to detect as low as 2.3 fM of bfp gene of HEK293 T cells with high specificity, fast time response (within 5 min), and without PCR amplification. Going further, authors provided a proof-of-concept clinical application of their device for the detection of Duchenne muscular dystrophy (DMD)-associated mutations targeting exons 3 and 51 of the human dystrophin gene. The biosensor was able to discriminate between healthy and DMD patients with an LOD of 1.7 fM genomic material (S/N = 3). The authors highlighted the advantages of CRISPR-FET biosensing: it requires only three steps (calibration, incubation, and rinsing) and does not require genomic sample fragmentation, along with no need for sample amplification. Recently, Weng et al. exploited the same strategy for the ultrasensitive detection of HPV-16 and non-pathogenic E. coli DNA [277]. They applied a CRISPR/Cas12a system that contains specific hairpin crRNA to detect target DNA and enable abovementioned trans-cleavage of a bystander ssDNA (poly-C or poly-A), Fig. 19d. The system demonstrated exceptional sensitivity and specificity, achieving detection of HPV-16 target ssDNA concentrations ranging from 1 aM to 100 pM, with a limit of detection (LOD) of 1 aM. Detection was based on a leftward shift of the DP caused by cleavage of a poly-A bystander ssDNA probe. Additionally, a CRISPR assay was developed for the detection of non-pathogenic E. coli by designing a target-specific crRNA complementary to the 16S rRNA gene of E. coli. The CRISPR/Cas12a combined with the GFET platform achieved detection of target dsDNA concentrations as low as 10 aM, employing a poly-C ssDNA probe as the signal transducer.
Comparative analysis of electrochemical and FET-based biosensing modalities
The electrochemical DNA sensor detection process involves the hybridization of a target nucleic acid with a complementary probe DNA/RNA immobilized on an electrode surface. The nature of the electrode nanomaterials and the roughness of the electrode area may influence the sensitivity of DNA detection a lot. Gold nanoparticles are frequently combined with other nanomaterials in composites to maximize their synergistic effects. To detect low concentrations of nucleic acids, amplification techniques are often required, such as enzymatic amplification (e.g., HRP, ALP), nanomaterial enhancement (e.g., gold nanoparticles, CNTs, graphene), electrochemical redox indicators (e.g., MB, Fc), and the isothermal nucleic acid amplification (LAMP, RCA, HCR). For example, the LAMP amplification method offers higher specificity than PCR due to the use of multiple primers (typically 4 to 6), but it produces more complex DNA structures as a result. Modern electrochemical genosensors rely on a handful of core techniques that have dominated in the past decade. DPV is one of the most widely used methods for DNA sensing, prized for its low background current and high sensitivity. In fact, nearly all recent electrochemical DNA biosensors employ DPV for quantifying target nucleic acids due to its superior signal discrimination. SWV, a related pulsed technique, is also popular; it offers a simple peak shape and high sensitivity, useful for kinetic studies and quantitative analysis. SWV’s frequency-dependent measurements make it versatile, though DPV tends to yield better signal-to-noise for trace DNA detection. Another prominent technique is EIS, often used in label-free genosensors. EIS measures changes in the interfacial charge transfer resistance or capacitance when target DNA hybridizes on the electrode surface. Because it operates over a range of AC frequencies, EIS can provide rich information about the interface and binding events, beyond what a single-potential method can. Its sensitivity is high for detecting surface changes (such as DNA duplex formation), making it ideal for direct, reagent-free GMO detection schemes. CA (amperometric detection) has been utilized, typically by holding the electrode at a fixed potential and measuring the current over time after target binding or substrate addition. CA can be very sensitive (high signal-to-noise ratio), especially when coupled to enzymatic reactions, but it sometimes suffers from lower reproducibility and a narrower linear response range. This is because any drift or slight variation in the steady-state current can affect quantitation, so it is less common to use it as the primary readout in DNA sensors. DPV and EIS have emerged as the top choices for ultrasensitive nucleic acid detection in the last decade, with SWV and chronoamperometry serving as complementary methods in specific assays. Each technique offers trade-offs between sensitivity, information content, and practical simplicity, and often multiple techniques are combined to validate results.
Electrochemical genosensors generally produce one of two signal responses upon target binding: “signal-off” (signal decrease) or “signal-on” (signal increase), as Table 3 highlights. In a classic signal-off DNA sensor, the electrode is preloaded with a redox reporter that yields a strong baseline signal when the probe is single-stranded and flexible. Target hybridization often rigidifies or insulates the probe layer, causing a drop in the redox current or an increase in impedance. This approach can be effective, but it has an inherent limit; the signal can only decrease at most 100% from its initial value. Moreover, any nonspecific event that blocks the electrode (fouling, non-target binding) will also produce a signal decrease, potentially mimicking a positive detection and causing false positives. In contrast, signal-on sensors are designed such that the electrochemical signal is low initially and increases upon target binding. For example, target binding might bring a redox tag closer to the electrode or trigger the release of a reporter, producing a measurable current where there was little before. The key advantage is a greatly enhanced dynamic range; in theory, a signal-on sensor can produce an arbitrarily large increase (limited only by background approaching zero), leading to improved sensitivity. In practical terms, signal-on designs tend to be more reliable for real-world applications because the appearance of a signal is easier to distinguish from noise than the signal's disappearance. Additionally, signal-on sensors are less prone to false positives from non-biological fouling; if an electrode becomes dirty or partially inactive, a signal-on sensor will simply remain “off” (no signal), whereas a signal-off sensor might erroneously indicate a target due to the reduced current.
Table 3.
Overview of electrochemical and FET-based biosensors for nucleic acid detection
| EC reaction mechanism | EC technique | Electrode (E) and transducer (T) | DNA probe + redox molecule | Detection mechanism | DNA/signal amplification method | Target NA | Analytical performance of biosensor | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dynamical range | LOD | Assay time (min) | ||||||||
| Signal-on | DPV | PG (E) + CB-AuNPs (T) | ssDNA + MB | DNA hybridization | / | MicroRNA-21 | 10–500 nM | 1 nM | 35 | [141] |
| SPC (E) + SiO2-Au nanocomposite (T) | ssDNA + AQMS | DNA hybridization | PCR | GM soybean CaMV35S gene | 10 fM–10 nM | 2.37 fM | 60 | [184] | ||
| Gold (E) | ssDNA + MB | DNA hybridization | / | Arbitrary DNA | 2–100 nM | 2 nM | 60 | [233] | ||
| Gold (E) + HGN (T) | ssDNA + Co(phen)33+ | DNA hybridization | / | Arbitrary DNA | 1 pM–10 nM | 1 pM | 20 | [219] | ||
| GC (E) + MWCNT-ZrO2 NPs-Chitosan (T) | ssDNA + daunomycin | DNA hybridization | / | Arbitrary DNA | 149 pM–93.2 nM | 75 pM | 30 | [223] | ||
| Gold (E) | ssDNA and dispDNA + Fc | DNA hybridization | CHA | Arbitrary DNA | 1 fM–0.1 nM | 0.74 fM | 120 | [251] | ||
| GSP (E) | ssDNA + SAP | DNA hybridization | / | Ebola DNA (biotinylated) | 10–75 nM | 4.7 nM | 60 | [252] | ||
| Gold (E) + AuNPs (T) | Hairpin ssDNA + AuNPs + Mel-Cu2+ | DNA hybridization | / | Arbitrary DNA | 1 aM–1 pM | 120 zM | 150 | [255] | ||
| SWV | Gold (E) | ssDNA + MB | DNA hybridization | / | Arbitrary DNA | 1–40 nM | 0.3 nM | 30 | [240] | |
| AuNPs on Si (E) | ssDNA + [Ru(NH3)6]3+ | DNA hybridization | / | Arbitrary DNA | 1 fM–10 pM | 1–10 fM | 30 | [221] | ||
| ACV | Gold ball (E) | Probe DNA-PEG-capture DNA + Fc | DNA hybridization | / | Arbitrary DNA | / | ~ 200 pM | / | [248] | |
| Gold (E) | Capture DNA-signal DNA + MB | DNA hybridization | / | Arbitrary DNA | / | 400 fM | 300 | [249] | ||
| CA | Gold (E) | Tetrahedron DNA + TMB and avidin-HRP + reporter biotin-DNA | DNA hybridization | Enzyme-based | Arbitrary DNA | / | 1 pM | ~ 45 | [232] | |
| Gold wire (E) | Capture DNA-signal DNA + FITC + anti-FITC-HRP + TMB | DNA hybridization |
RT-PCR Enzyme-based |
GM maze MON810 and GM soy GTS40-3–2 DNA |
P-35S: 20–1000 copies in MON810 P-35S: 100–4000 copies in RRS |
MON810: 0.2% (20 copies) RRS: 0.25% (100 copies) |
> 120 | [253] | ||
| CV | SPC (E) + PANI-AuNPs (T) | ssDNA + HRP-TMB-H2O2 | DNA hybridization | Enzyme-based | 16S rRNA gene of E. Coli | 1 fM–1 nM | 0.5 fM | / | [142] | |
| ECL | Gold (E) | Tetrahedron DNA + CRISPR/Cas-12a + ssDNA-Ru (bpy)32+ | Strand-displacement DNA hybridization, CRISPR/Cas DNA cleavage | / | GM maize MON810 | 1 fM–100 pM | 3.3 fM | 195 | [183] | |
| Signal-off | SWV | Gold (E) + AuNPs-CysA (T) | ssDNA + TB | DNA hybridization | / | Mip gene DNA of Legionella pneumophila | 1 zM–1 μM | 1 zM | ~ 30 | [160] |
| Gold (E) | Hairpin DNA + MB | DNA hybridization | / | Arbitrary DNA |
MCH: 5–100 nM DEDTC: 0.05–50 nM 2-PhET: 0.025–25 nM |
MCH: 5 nM DEDTC: 0.05 nM 2PhET: < 0.025 nM |
30 | [201] | ||
| Gold (E) | Hairpin DNA + MB | DNA hybridization | ISDPR | mecA gene DNA of MRSA | 0.075–200 pM | 63 fM | 120 | [246] | ||
| DPV | Platinum (E) + PANI (T) | ssDNA + MB | DNA hybridization | / | E. coli | 0.0125–1 ng/μL | < 0.0125 ng/μL | 1–14 | [292] | |
| ACV | Gold (E) | Capture DNA-assistant DNA (Y junction) + MB | DNA hybridization and Hae III cleavage | / | Arbitrary DNA | 1 pM–10 nM | 14 pM | 60 | [245] | |
| Both | DPV | Gold (E) | Hairpin capt. DNA + MB and ssDNA + Fc | DNA hybridization | / | HIV-1 DNA | 0.5–80 pM | 0.12 pM | / | [250] |
| / | EIS | SPC (E) + GCD (T) | ssDNA | DNA hybridization | RPA | GM maize Ruifeng12-5 (GBW10140) | 0.1–5% | 0.1% | 45 | [159] |
| SPC (E) + GCD (T) | ssDNA | DNA hybridization | PCR | GM soybean SHZD32-1 | 0.1 pM–0.1 μM | 31 fM | 45 | [182] | ||
| Monolayer AuNP on Si (E) | ssDNA | DNA hybridization | PCR | GM soybean P-35S | 1–6% (1.792–19.22 ng/mL) | 1% (1.792 ng/mL) | / | [185] | ||
| Gold (E) | ssDNA | DNA hybridization | / | Arbitrary DNA | 100 pM–100 nM | 678 pM | 60 | [241] | ||
| Gold (E) + AuNPs (T) | ssDNA1-ssDNA2 (TH DNA hairpin) | Strand displacement and DNA hybridization | AuNP amplification | Arbitrary DNA | 10 pM–100 nM | 1.47 pM | 90 | [254] | ||
| EIS, DPV, SWV | Gold (E) | DNA origami-capture DNA | DNA hybridization | DNA tile amplification | blaOXA-1 β-lactamase gene sequence (115 nt OXA fragment) | 10 pM–1 nM | 8.86 pM | 60 | [247] | |
| LSV | PG (E) + AuNPs (T) | ssDNA | DNA hybridization | / | Arbitrary DNA | 10 nM–1 μM | 6.9 nM | 60 | [222] | |
DPV differential-pulse voltammetry, SWV square-wave voltammetry, ACV alternating current voltammetry, CA chronoamperometry, CV cyclic voltammetry, ECL electrochemiluminescence, EIS electrochemical impedance spectroscopy, LSV linear sweep voltammetry, PG pencil graphite (electrode), CB carbon black, AuNPs gold nanoparticles, SPC screen-printed carbon (electrode), HGN hollow gold nanospheres, SiO2-Au silicon-dioxide gold (nanocomposite), GC glassy carbon (electrode), MWCNT multi-walled carbon nanotubes, ZrO2 NPs zirconium-oxide nanoparticles, PANI polyaniline, CysA cysteamine, GCD gold carbon dots, ssDNA single-stranded DNA, MB methylene blue, AQMS anthraqinone-2-sulfonic acid monohydrate sodium salt, Co(phen)33+ tris(1,10-phenanthroline) cobalt ions, dispDNA displacement DNA, Fc ferrocene, SAP streptavidin–alkaline phosphatase, Mel-Cu2+ melamine-copper ion complex, [Ru(NH3)6]3+ hexaammineruthenium (III) chloride ions, PEG polyethylene glycol, TMB 3,3′,5,5′-tetramethylbenzidine, HRP horseradish peroxidase, FITC fluorescein isothiocyanate, H2O2 hydrogen peroxide, Ru (bpy)32+ tris(2,2′-bipyridyl)ruthenium ions, TH triple-helix (DNA)
FET-based biosensing of DNA or RNA hybridization can be broadly divided into two main categories: the first encompasses approaches that employ NAA strategies to generate amplified target sequences, which are then detected through various mechanisms such as DNA hybridization, pH variation, or DNA probe cleavage. The second category bypasses molecular amplification techniques, relying instead on the intrinsic ultrasensitive properties of nanomaterials integrated within FET devices or leveraging advanced signal amplification reactions such as HCR and CHA. Biosensors from both categories exhibit highly sensitive detection capabilities, including a wide dynamic range for target NA concentrations and low LOD. These features stem from the synergistic combination of the exceptional properties of 2D nanomaterials used as channels in FET configurations and the fundamental mechanism of signal generation via modulation of carrier density. For instance, the remarkable electrical properties of monolayer graphene, underpinned by its unique hexagonal lattice, make it particularly effective for the detection of ssDNA through weak π-π interactions, interactions that suffice to alter graphene’s electrical characteristics and provide a measurable signal. However, this detection mode can be challenged by issues of selectivity. Table 4 provides a comparative overview of DNA biosensing solutions utilizing 2D-FET devices, highlighting key performance metrics. Despite the rapid signal response of FET biosensors, the total assay duration is typically governed by the time required for hybridization or amplification steps, often extending the detection window from 30 to over 120 min. Graphene-based FET devices, arguably the most extensively studied systems in this domain, face a major limitation in “lab-to-fab” scaling up. Although CVD methods yield generally high-quality and uniform monolayer graphene or TMDs, subsequent processes such as the transfer and surface functionalization can impair device performance. One approach to circumvent this issue involves functionalizing the gate electrode in EG-FET setups, thereby preserving the pristine properties of graphene by avoiding direct functionalization. Other 2D nanomaterials, such as TMDs, demonstrate comparable biosensing capabilities to GFETs, yet also face challenges in large-scale synthesis and consistent functionalization. A persistent limitation in DNA biosensing using 2D-FET platforms is the prevalent reliance on “proof-of-concept” studies, as found in Table 4, where detection is commonly demonstrated with arbitrarily selected NA sequences. Transitioning these platforms to real-world sample analysis necessitates critical preparatory steps, as real biological matrices present complex environments that demand further biosensor optimization.
Table 4.
Overview of FET-based biosensors for DNA detection using amplification or amplification-free strategies in various applications
| Channel material | Probe type | Detection mechanism | DNA/signal amplification method | Target molecule | Biosensor performances | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Dynamic range | LOD | Assay time (min) | ||||||
| CVD graphene | CRISPR/Cas9 + ssDNA | DNA cleavage | / | bfp gene of HEK293 T cells and DMD-associated gene | 300–1800 ng genomic bfp |
2.3 fM 1.7 fM (DMD) |
~ 15 | [270] |
| Tetrahedron DNA nanostructure | DNA hybridization | CHA | miRNA-21 | 0.5 aM–0.5 pM | 0.567 aM | 120 | [273] | |
| ssDNA | DNA hybridization | HCR | Arbitrary DNA | 10 fM–10 pM | 5 fM | 60 | [274] | |
| ssPNA | DNA hybridization | / | Arbitrary DNA | 1 fM–100 pM | 10 fM | 60 | [275] | |
| CRISPR/Cas12a + PolyA or PolyC | DNA cleavage | / | HPV-16 ssDNA and E. coli dsDNA |
1 aM–100 pM / |
1 aM 10 aM |
45 | [277] | |
| ssDNA | DNA hybridization | PCR | Monovarietal grapevine DNA | 1 aM–1 pM | 0.19 aM | / | [281] | |
| / | pH-change monitoring | LAMP | Lambda phage DNA | 2 × 102–2 × 108 copies/μL | 2 × 102 copies/μL | 40 | [282] | |
|
Aptamer ssDNA |
DNA hybridization | HCR | VEGF165 | 10 pg/mL–800 ng/mL | 3.24 pg/mL | 60 | [285] | |
| ssDNA | DNA hybridization | / | Arbitrary DNA | 1 aM–100 fM | 10 aM | 40 | [286] | |
| ssDNA | DNA hybridization | / | nuc gene of S. Aureus DNA | 10 aM–10 nM | 10 aM | 27 | [287] | |
| ssDNA | DNA hybridization | / | Arbitrary DNA | 1 aM–10 pM | ~ 0.642 aM | 20–30 | [293] | |
| / | pH-change monitoring | LAMP | E and RdRP genes of SARS-CoV-2 | /*PoC | / | 30 | [294] | |
| CRISPR/Cas12a and ssDNA | DNA hybridization and DNA cleavage | / | N-gene SARS-CoV-2 RNA and hexon gene of HAdv-7 dsDNA | 75 | [295] | |||
| CVD graphene on sapphire | ssDNA | DNA hybridization | / | Arbitrary DNA | 100 fM–1 nM | 100 fM | / | [272] |
| CVD graphene and CQD | ssDNA | DNA hybridization | / | Arbitrary DNA | 1 aM–0.1 nM | 1 aM | 5.5–13 | [296] |
| CVD crumpled graphene | ssDNA/ssPNA | DNA hybridization | / | Let-7b DNA and RNA | / | 600 zM | 60 | [279] |
| / | ssDNA adsorption (LAMP primers consumption) | LAMP | E. coli DNA | / | 8 zM | 90 | [283] | |
| / | ssDNA adsorption (LAMP primers consumption) | RT-LAMP | SARS-CoV-2 N-gene RNA | /*PoC | / | 30–35 | [284] | |
| MoS2/graphene heterostructure | ssDNA | DNA hybridization | / | Arbitrary DNA | 10 aM–100 pM | 10 aM | 360 | [297] |
| 4-layer MoS2 | ssDNA | DNA hybridization | / | Arbitrary DNA | 10 fM–10 nM | 10 fM | / | [298] |
| Few-layer MoS2 | ssPMO | DNA hybridization | / | Arbitrary DNA | 10 fM–1 nM | 6 fM | 60 | [276] |
| CVD WS2 | ssDNA | DNA hybridization | / | Arbitrary DNA | 100 aM–1 nM | 3 aM | 120 | [288] |
| Gold EG-FET | ssDNA | DNA hybridization | / | Intimine gene of E. coli | 1 fM–1 pM | 1 fM | < 24 | [289] |
| / | pH-change monitoring | RT-LAMP | SARS-Cov-2 RNA | 10 fg/μL–10 ng/μL | 0.31 pg/μL | 30 | [299] | |
| ITO EG-FET | ssDNA | DNA hybridization | LAMP + CRISPR/Cas12a | HCV-viral RNA | 1–102 genomic copies/reaction | 1 gen. copy/reaction | 30–40 | [300] |
| Si-NWs | Neutralized ssDNA | DNA hybridization | / | Arbitrary DNA | / | 0.1 fM | 40 | [290] |
| AlGaN/GaN heterostructure | ssDNA | DNA hybridization | / | Arbitrary DNA | / | 1 fM | / | [301] |
| InGaZnO amorphous film | ssDNA | DNA hybridization | / | Arbitrary DNA | 0.1 pM–1 μM | 0.1 pM | 120 | [302] |
CVD chemical vapor deposition (monolayer graphene), CQD carbon quantum dots, MoS2 molybdenum disulfide, WS2 wolfram disulfide, EG-FET extended gate filed-effect transistor, ITO indium tin oxide, Si-NWs silicon nanowires, AlGaN aluminum gallium nitride, InGaZnO indium gallium zinc oxide, ssDNA single-stranded DNA, ssPNA single-stranded peptide nucleic acid, PolyA/C adenosine/cytosine polynucleotide, ssPMO single-stranded phosphorodiamidate Morpholino oligonucleotide, LAMP loop-mediated isothermal amplification, CHA catalytic hairpin assembly, HCR hybridization chain reaction, RT-LAMP reverse transcriptase LAMP, DMD Duchenne muscular dystrophy, HPV human papillomavirus, dsDNA double-stranded DNA, VEGF vascular endothelial growth factor, SARS-CoV-2 severe acute respiratory syndrome-coronavirus 2, *PoC point-of-care (biosensor)
Challenges and future perspectives in GMO detection
GMO biosensor sensitivity can be improved through several approaches, most of them already been discussed in previous sections. Among target preamplification strategies, in addition to PCR, isothermal nucleic acid amplification techniques are widely used. To reduce the matrix effect when biosensing is used for real samples (e.g., environmental and food), target enrichment strategies that enable selective capture of the analyte present in the complex sample and subsequent separation of the captured target for detection are used. It is also possible to use cascade signal amplification, which can improve biosensor sensitivity by linking multiple reaction steps, where the product of one reaction initiates the next. Additionally, the sensitivity of biosensors can be enhanced using novel functional materials and specially designed microfluidic devices. Even artificial intelligence has found its place in modern biosensor design, enabling effective data collection and further data manipulation [303].
Despite significant advancements in molecular detection technologies, several challenges hinder the widespread implementation of reliable, rapid, and cost-effective GMO detection methods. PCR-based techniques remain the gold standard due to their high specificity and sensitivity; however, their dependence on complex instrumentation, thermal cycling, and extended processing time limits their feasibility for on-site applications. Additionally, inhibitors present in plant matrices can interfere with amplification efficiency, leading to false-negative results. Isothermal amplification methods such as LAMP and RPA provide alternatives with faster turnaround times and simplified workflows, yet their sensitivity and specificity vary depending on reaction conditions, and their regulatory acceptance remains a barrier to broader adoption. High-throughput sequencing offers comprehensive characterization of GMOs but remains impractical for routine field applications due to high costs and sophisticated infrastructure requirements.
Future advancements in GMO detection aim to enhance speed, portability, and reliability, particularly through the development of PON diagnostic tools. The integration of CRISPR-based detection systems with isothermal amplification presents a promising avenue for highly specific, rapid, and cost-efficient GMO identification. Additionally, biosensor technologies hold potential for further miniaturization and automation, facilitating user-friendly platforms for field applications. The convergence of molecular detection methods with machine learning and automated data analysis could further streamline GMO monitoring by enabling real-time, high-throughput screening with minimal human intervention.
As the diversity of genetically modified and genome-edited organisms continues to expand, regulatory frameworks must evolve to ensure comprehensive and adaptive detection strategies. The future of GMO detection lies in the development of accessible, field-deployable technologies that provide robust, precise, and efficient identification across diverse agricultural and food production environments. By addressing current limitations and leveraging emerging biotechnological innovations, next-generation GMO detection platforms will enhance food safety, regulatory compliance, and consumer transparency.
The development of electrochemical biosensors for GMOs detection is an emerging field that promises significant advancements in food safety, environmental monitoring, and biotechnology. However, there are several challenges to overcome before these biosensors can be widely adopted in real-world applications. At the same time, there are exciting future perspectives for improving their performance and expanding their utility.
The main challenges in the field of GMO electrochemical biosensors are to achieve high sensitivity to detect very low concentrations of GMOs in complex samples (e.g., food, water, soil). Detecting trace amounts of GMOs without false negatives or false positives is crucial for regulatory compliance and consumer safety. The sensitivity of electrochemical biosensors depends on factors such as the efficiency of the probe-target interaction, the electrochemical techniques used, and the ability of redox molecules to generate measurable signals in response to small amounts of DNA or proteins. Electrochemical biosensors must be highly specific to differentiate between GMOs and non-GMO organisms, especially in cases where genetic modifications are very subtle or where the target sequences are very similar to those of non-GMOs. The detection often involves samples with complex matrices, such as processed food, agricultural products, or environmental samples. These matrices may contain inhibitors or interfering substances that affect the performance of electrochemical biosensors. Sample preparation techniques, such as DNA extraction, amplification, or purification, are needed to reduce matrix effects and ensure that the sensor can detect GMOs effectively in real-world samples. Multiplication DNA techniques may influence the electrochemical detection signal significantly by producing DNA molecules of various complexities.
Electrochemical biosensors, especially those based on biological components like DNA probes or antibodies, can suffer from stability issues. They may lose their functionality over time due to environmental factors (e.g., temperature, humidity) or degradation of the biological material. Ensuring long-term stability and reproducibility of electrochemical biosensors is a key challenge, particularly in field applications where the biosensors are exposed to various conditions. While electrochemical biosensors offer a low-cost alternative to traditional laboratory methods, the overall cost of developing high-performance biosensors with sufficient sensitivity and specificity can still be prohibitive. There are multiple methods for GMO detection, making it difficult to compare results across different biosensors or laboratories. Regulatory frameworks around GMO detection are evolving, and biosensors need to be validated according to international standards to be accepted for regulatory purposes.
Future perspectives in GMO electrochemical biosensors present advanced nanomaterials such as AuNPs, 2D-nanomaterials, CNTs, or graphene, which can be incorporated into electrochemical biosensors to improve their sensitivity and provide better signal amplification. These materials enhance electron transfer efficiency and increase the surface area of the sensor, allowing for more efficient binding of target molecules. The integration of CRISPR-based technologies with electrochemical biosensors holds great promise. CRISPR can be used to specifically recognize and cleave DNA sequences associated with GMOs, leading to signal generation that could be detected electrochemically. This technology could significantly improve both specificity and sensitivity in GMO detection. Additionally, advancements in nucleic acid amplification techniques (e.g., LAMP, PCR) can be coupled with electrochemical biosensors to enable even more sensitive detection of low-abundance GMO targets without the need for extensive laboratory infrastructure. Future GMO electrochemical biosensors could become portable, handheld devices that allow on-site detection. This would be particularly useful for field testing in agriculture, food production, and environmental monitoring. These devices could enable rapid screening of products at various stages of production, leading to quicker decisions and ensuring compliance with regulations. The miniaturization of biosensors and the incorporation of microfluidics or lab-on-a-chip technologies could allow for PON testing in locations without access to sophisticated laboratories. Future biosensors may be designed to simultaneously detect multiple GMO markers or different genetic modifications in a single test. Multiplexed detection could be achieved by integrating various DNA probes or using different redox reporters, enabling a more comprehensive assessment of the presence of multiple GMOs in a single sample. The integration of artificial intelligence (AI) and machine learning (ML) can help improve the analysis of electrochemical signals. By using algorithms to analyze sensor data, it would be possible to detect subtle variations in electrochemical responses and distinguish between genetically modified and non-modified organisms more effectively.
Electrochemical biosensors can be expanded to monitor environmental contamination by GMOs, for example, in soil, water, and air. These biosensors could provide valuable data on the spread of GMOs into the environment, ensuring that proper containment measures are in place. As food traceability becomes more important, electrochemical biosensors could be part of supply chain monitoring systems, enabling the tracing of GMO content in food products from farm to table.
Conclusion
While electrochemical biosensors for GMO detection face several challenges, including sensitivity, selectivity, stability, and cost, they hold significant potential for the future. With advances in materials science, molecular biology, electrochemical techniques, and data analysis techniques, these biosensors could become powerful tools for rapid, on-site, and cost-effective detection of GMOs in various contexts. In this review, electrochemical DNA-based sensing strategies are presented. Meanwhile, the main functionalization methods of DNA and an overview of nanomaterials and electrochemical techniques used for electrochemical sensing are summarized. The future of GMO electrochemical biosensors is promising, with developments in nanotechnology, CRISPR-based methods, and AI poised to overcome existing limitations and expand their capabilities.
Acknowledgements
This research was supported by the Science Fund of the Republic of Serbia, LABOUR project (Grant agreement no. 6710), the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grant agreement no. 451-03-136/2025-03/200358) and Horizon 2020 project ANTARES (Grant agreement no. 739570).
Author contributions
Conceptualization: Z.P., M.R., Lj.Š.Z., Lj.J.; Literature search: A.K., S.J., Z.N., M.P., T.K.; Writing—original draft preparation: A.K., S.J., Z.N., M.R., M.P., T.K., Lj.Š.Z., Z.P.; Writing—review and editing: Z.P., M.R., Lj.Š.Z., Lj.J. All authors have read and reviewed the final manuscript version.
Funding
This research received funding from the Science Fund of the Republic of Serbia, LABOUR project (GA no. 6710), the Ministry of Science, Technological Development and Innovations of the Republic of Serbia (GA no. 451–03-136/2025–03/200358), and partly funding from the Horizon 2020, ANTRES project (GA no. 739570).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Clinical trial number
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/8/2025
The online version of this article has been updated to include the copyright in the figure caption.
References
- 1.Peter R, Mojca J, Primož P (2011) Genetically modified organisms (GMOs). In: Encyclopedia of Environmental Health. pp. 879–888. Elsevier
- 2.Eriksson D (2019) The evolving EU regulatory framework for precision breeding. Theor Appl Genet 132:569–573. 10.1007/s00122-018-3200-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uslu T (2021) Advantages, risks and legal perspectives of GMOs in 2020s. Plant Biotechnol Rep 15:741–751. 10.1007/s11816-021-00714-0 [Google Scholar]
- 4.Zilberman D, Holland TG, Trilnick I (2018) Agricultural GMOs—what we know and where scientists disagree. Sustainability 10:1514. 10.3390/su10051514 [Google Scholar]
- 5.Teferra TF (2021) Should we still worry about the safety of GMO foods? Why and why not? A review Food Sci Nutr 9:5324–5331. 10.1002/fsn3.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raman R (2017) The impact of Genetically Modified (GM) crops in modern agriculture: a review. GM Crops Food 8:195–208. 10.1080/21645698.2017.1413522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Land statistics and indicators 2000–2021. FAO (2023)
- 8.(2018) EFSA: Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005. EFSA J 16. 10.2903/j.efsa.2018.5263 [DOI] [PMC free article] [PubMed]
- 9.García M, García-Benítez C, Ortego F, Farinós GP (2023) Monitoring insect resistance to Bt maize in the European Union: update, challenges, and future prospects. J Econ Entomol 116:275–288. 10.1093/jee/toac154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heap I, Duke SO (2018) Overview of glyphosate-resistant weeds worldwide. Pest Manag Sci 74:1040–1049. 10.1002/ps.4760 [DOI] [PubMed] [Google Scholar]
- 11.GAIN: Agricultural Biotechnology Annual Report (2018) https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Agricultural%20Biotechnology%20Annual_Paris_EU-28_12-14-2018.pdf. Accessed 22 Oct 2024
- 12.EFSA GMO Panel, Casacuberta J, Nogué F, Naegeli H, Birch AN, De Schrijver A, Gralak MA, Guerche P, Manachini B, Messéan A, Nielsen EE, Robaglia C, Rostoks N, Sweet J, Tebbe C, Visioli F, Wal J, Moxon S, Schneeberger K, Federici S, Ramon M, Papadopoulou N, Jones H (2018) Technical Note on the quality of DNA sequencing for the molecular characterisation of genetically modified plants. EFSA J 16. 10.2903/j.efsa.2018.5345 [DOI] [PMC free article] [PubMed]
- 13.Lukasiewicz JM, Van De Wiel CCM, Lotz LAP, Smulders MJM (2024) Consumer transparency in the production chain for plant varieties produced using new genomic techniques. aBIOTECH. 5:239–246. 10.1007/s42994-024-00142-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Y, Dong S (2017) Nucleic acid biosensors: Recent advances and perspectives. Anal Chem 89:189–215. 10.1021/acs.analchem.6b04190 [DOI] [PubMed] [Google Scholar]
- 15.Leonardo S, Toldrà A, Campàs M (2021) Biosensors based on isothermal DNA amplification for bacterial detection in food safety and environmental monitoring. Sensors 21:602. 10.3390/s21020602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Falco M, De Felice M, Rota F, Zappi D, Antonacci A, Scognamiglio V (2022) Next-generation diagnostics: augmented sensitivity in amplification-powered biosensing. TrAC Trends Anal Chem 148:116538. 10.1016/j.trac.2022.116538 [Google Scholar]
- 17.Lai RY (2017) Folding- and dynamics-based electrochemical DNA sensors. In: Thompson, R.B. and Fierke, C.A. (eds), Methods in Enzymology, vol. 589, Elsevier, Amsterdam, 221–252. 10.1016/bs.mie.2017.02.001 [DOI] [PubMed]
- 18.Bak A, Emerson JB (2019) Multiplex quantitative PCR for single-reaction genetically modified (GM) plant detection and identification of false-positive GM plants linked to Cauliflower mosaic virus (CaMV) infection. BMC Biotechnol 19:73. 10.1186/s12896-019-0571-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker R, Ulrich A (2018) Improved detection and quantification of cauliflower mosaic virus in food crops: assessing false positives in GMO screening based on the 35S promoter. Eur Food Res Technol 244:1861–1871. 10.1007/s00217-018-3099-z [Google Scholar]
- 20.Chhalliyil P, Ilves H, Kazakov S, Howard S, Johnston B, Fagan J (2020) A real-time quantitative PCR method specific for detection and quantification of the first commercialized genome-edited plant. Foods 9:1245. 10.3390/foods9091245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salisu IB, Shahid AA, Yaqoob A, Ali Q, Bajwa KS, Rao AQ, Husnain T (2017) Molecular approaches for high throughput detection and quantification of genetically modified crops: a review. Front Plant Sci 8:1670. 10.3389/fpls.2017.01670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogožalec Košir A, Demšar T, Štebih D, Žel J, Milavec M (2019) Digital PCR as an effective tool for GMO quantification in complex matrices. Food Chem 294:73–78. 10.1016/j.foodchem.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 23.Burns MJ, Burrell AM, Foy CA (2010) The applicability of digital PCR for the assessment of detection limits in GMO analysis. Eur Food Res Technol 231:353–362. 10.1007/s00217-010-1277-8 [Google Scholar]
- 24.Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW (2011) High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83:8604–8610. 10.1021/ac202028g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S, Emslie KR (2012) Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem 84:1003–1011. 10.1021/ac202578x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin MK, Jeon SM, Koo YE (2022) Development of a rapid detection method for genetically modified rice using the ultra-fast PCR system. Food Sci Biotechnol 31:175–182. 10.1007/s10068-021-01025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin MK, Jeon SM, Koo YE (2023) Detection method for genetically modified potato using an ultra-fast PCR system. Food Sci Biotechnol 32:1185–1191. 10.1007/s10068-023-01258-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao W, Tian J, Huang K, Yang Z, Xu W, Luo Y (2019) Ultrafast, universal and visual screening of dual genetically modified elements based on dual super PCR and a lateral flow biosensor. Food Chem 279:246–251. 10.1016/j.foodchem.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 29.Yang Q, Zhang Y, Xu H, Han D, Tan J, Liu R, Fang B, He J, Xu W, Zhang W (2024) Single primer isothermal amplification for real-time and visual on-site detection of genetically modified crops by recognizing CP4-EPSPS gene. J Food Compos Anal 126:105937. 10.1016/j.jfca.2023.105937 [Google Scholar]
- 30.Notomi T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:63e–663. 10.1093/nar/28.12.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nwe MK, Jangpromma N, Taemaitree L (2024) Evaluation of molecular inhibitors of loop-mediated isothermal amplification (LAMP). Sci Rep 14:5916. 10.1038/s41598-024-55241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardinge P (2022) Optimized loop-mediated isothermal amplification (LAMP) allows single copy detection using bioluminescent assay in real time (BART). In: Kim S-B (ed) Bioluminescence. Springer, US, New York, NY, pp 107–117 [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Li R, Quan S, Shen P, Zhang D, Shi J, Yang L (2015) GMO detection in food and feed through screening by visual loop-mediated isothermal amplification assays. Anal Bioanal Chem 407:4829–4834. 10.1007/s00216-015-8652-z [DOI] [PubMed] [Google Scholar]
- 34.Li R, Shi J, Liu B, Wang C, Zhang D, Zhao X, Yang L (2019) Inter-laboratory validation of visual loop-mediated isothermal amplification assays for GM contents screening. Food Chem 274:659–663. 10.1016/j.foodchem.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Zhang X, Wu B, Qian C, Zhang F, Wang L, Ye Z, Wu J (2020) Rapid on-site detection of genetically modified soybean products by real-time loop-mediated isothermal amplification coupled with a designed portable amplifier. Food Chem 323:126819. 10.1016/j.foodchem.2020.126819 [DOI] [PubMed] [Google Scholar]
- 36.Xing Y, Liang J, Dong F, Wu J, Shi J, Xu J, Wang J (2023) Rapid visual LAMP method for detection of genetically modified organisms. ACS Omega 8:29608–29614. 10.1021/acsomega.3c03567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh M, Pal D, Aminedi R, Singh AK (2024) Multiplex real-time loop-mediated isothermal amplification (LAMP) based on the annealing curve analysis: toward an on-site multiplex detection of transgenic sequences in seeds and food products. J Agric Food Chem 72:17658–17665. 10.1021/acs.jafc.4c01803 [DOI] [PubMed] [Google Scholar]
- 38.Singh M, Palit P, Kaur K, Aminedi R, Paliwal A, Randhawa G (2023) Loop-mediated isothermal amplification targeting proteinase inhibitor II terminator sequence: an efficient approach for screening of genetically modified crops. Eur Food Res Technol 249:2311–2319. 10.1007/s00217-023-04294-x [Google Scholar]
- 39.Long LK, Yan W, Li CC, Xia W, Xing ZJ, Liu N, Dong LM, Li FW (2019) Rapid visual detection of four specific transgenic events in GM soybean using loop-mediated isothermal amplification method. Russ J Plant Physiol 66:646–655. 10.1134/S1021443719040071 [Google Scholar]
- 40.Choi W, Yoon A-M, Lee J-W, Lim HS, Jung YJ, Lee JR (2024) Event-specific loop-mediated isothermal amplification for living modified cotton MON88701, MON531, MON15985, MON88913, and COT102. Biotechnol Bioprocess Eng 29:955–962. 10.1007/s12257-024-00137-y [Google Scholar]
- 41.Ding G, Jin Z, Zhang Y, Han Y, Li G, Jing Y, Li W (2020) Detection of genetically modified rice by loop-mediated isothermal amplification assays on a self-priming compartmentalization chip. Food Anal Methods 13:1454–1461. 10.1007/s12161-020-01766-8 [Google Scholar]
- 42.Pataer P, Gao K, Zhang P, Li Z (2024) Ultrasensitive and visual detection of genetically modified crops using two primers-induced cascade exponential amplification assay. Talanta 268:125282. 10.1016/j.talanta.2023.125282 [DOI] [PubMed] [Google Scholar]
- 43.Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC (1998) Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet 19:225–232. 10.1038/898 [DOI] [PubMed] [Google Scholar]
- 44.Pang Y-H, Wang Y-Y, Sun M-M, Shen X-F (2022) Visual detection of CaMV35S promoter via target-triggered rolling circle amplification of DNAzyme. J Food Compos Anal 106:104304. 10.1016/j.jfca.2021.104304 [Google Scholar]
- 45.Piepenburg O, Williams CH, Stemple DL, Armes NA (2006) DNA detection using recombination proteins. PLoS Biol 4:e204. 10.1371/journal.pbio.0040204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Wang J, Li P, Bai L, Jia J, Pan A, Long X, Cui W, Tang X (2020) Rapid detection of P–35S and T-nos in genetically modified organisms by recombinase polymerase amplification combined with a lateral flow strip. Food Control 107:106775. 10.1016/j.foodcont.2019.106775 [Google Scholar]
- 47.Wang J, Wang Y, Hu X, Chen Y, Jiang W, Liu X, Liu J, Zhu L, Zeng H, Liu H (2024) A dual-RPA based lateral flow strip for sensitive, on-site detection of CP4 - EPSPS and Cry1Ab / Ac genes in genetically modified crops. Food Sci Hum Wellness 13:183–190. 10.26599/FSHW.2022.9250015 [Google Scholar]
- 48.Zahradnik C, Kolm C, Martzy R, Mach RL, Krska R, Farnleitner AH, Brunner K (2014) Detection of the 35S promoter in transgenic maize via various isothermal amplification techniques: a practical approach. Anal Bioanal Chem 406:6835–6842. 10.1007/s00216-014-7889-2 [DOI] [PubMed] [Google Scholar]
- 49.Moura-Melo S, Miranda-Castro R, de-los-Santos-Álvarez N, Miranda-Ordieres AJ, Dos SantosJunior JR, Da SilvaFonseca RA, Lobo-Castañón MJ (2015) Targeting helicase-dependent amplification products with an electrochemical genosensor for reliable and sensitive screening of genetically modified organisms. Anal. Chem. 87:850–8554. 10.1021/acs.analchem.5b02271 [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Zhou S, Sun H, Dong J, Deng L, Qi N, Wang Y, Huo D, Hou C (2022) Ultrasensitive fluorescent biosensor for detecting CaMV 35S promoter with proximity extension mediated multiple cascade strand displacement amplification and CRISPR/Cpf 1. Anal Chim Acta 1215:339973. 10.1016/j.aca.2022.339973 [DOI] [PubMed] [Google Scholar]
- 51.Yan C, Xu J, Yao B, Yang L, Yao L, Liu G, Chen W (2022) Facile design of multifunction-integrated linear oligonucleotide probe with multiplex amplification effect for label-free and highly sensitive GMO biosensing. Talanta 236:122821. 10.1016/j.talanta.2021.122821 [DOI] [PubMed] [Google Scholar]
- 52.Oliveira BB, Veigas B, Baptista PV (2021) Isothermal amplification of nucleic acids: the race for the next “gold standard.” Front Sens 2:752600. 10.3389/fsens.2021.752600 [Google Scholar]
- 53.Srivastava P, Prasad D (2023) Isothermal nucleic acid amplification and its uses in modern diagnostic technologies. 3 Biotech. 13:200. 10.1007/s13205-023-03628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song R, Chen Z, Xiao H, Wang H (2024) The CRISPR-Cas system in molecular diagnostics. Clin Chim Acta 561:119820. 10.1016/j.cca.2024.119820 [DOI] [PubMed] [Google Scholar]
- 55.Aman R, Mahas A, Mahfouz M (2020) Nucleic acid detection using CRISPR/Cas biosensing technologies. ACS Synth Biol 9:1226–1233. 10.1021/acssynbio.9b00507 [DOI] [PubMed] [Google Scholar]
- 56.Bhardwaj P, Kant R, Behera SP, Dwivedi GR, Singh R (2022) Next-generation diagnostic with CRISPR/Cas: beyond nucleic acid detection. Int J Mol Sci 23:6052. 10.3390/ijms23116052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Hu X, Wang Y, Zeng H, Liu X, Liu H (2022) Rapid detection of genetically modified products based on CRISPR-Cas12a combined with recombinase polymerase amplification. Curr Res Food Sci 5:2281–2286. 10.1016/j.crfs.2022.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J, Luo J, Liu H, Xu D, Li Y, Liu X, Zeng H (2025) “Blue-red-purple” multicolored lateral flow immunoassay for simultaneous detection of GM crops utilizing RPA and CRISPR/Cas12a. Talanta 282:127010. 10.1016/j.talanta.2024.127010 [DOI] [PubMed] [Google Scholar]
- 59.Liu H, Hu X, Zeng H, He C, Cheng F, Tang X, Wang J (2023) A rapid and high-throughput system for the detection of transgenic products based on LAMP-CRISPR-Cas12a. Curr Res Food Sci 7:100605. 10.1016/j.crfs.2023.100605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pataer P, Gao K, Zhang P, Wang X, Li Z (2024) A simple, portable and multiplex LAMP-based CRISPR/Cas12a assay for visually screening genetically modified crops. Sens Actuators B Chem 403:135124. 10.1016/j.snb.2023.135124 [Google Scholar]
- 61.Zhu X, Yang H, Wang M, Wu M, Khan MR, Luo A, Deng S, Busquets R, He G, Deng R (2022) Label-free detection of transgenic crops using an isothermal amplification reporting CRISPR/Cas12 assay. ACS Synth Biol 11:317–324. 10.1021/acssynbio.1c00428 [DOI] [PubMed] [Google Scholar]
- 62.Liang Z, Ding L, Tang M, Wang X, Chen X, Xu J, Lu Y, Peng C (2024) Review on the evolution in DNA-based techniques for molecular characterization and authentication of GMOs. Microchem J 198:110176. 10.1016/j.microc.2024.110176 [Google Scholar]
- 63.Uhlen M, Quake SR (2023) Sequential sequencing by synthesis and the next-generation sequencing revolution. Trends Biotechnol 41:1565–1572. 10.1016/j.tibtech.2023.06.007 [DOI] [PubMed] [Google Scholar]
- 64.Zastrow‐Hayes GM, Lin H, Sigmund AL, Hoffman JL, Alarcon CM, Hayes KR, Richmond TA, Jeddeloh JA, May GD, Beatty M.K (2015) Southern‐by‐sequencing: a robust screening approach for molecular characterization of genetically modified crops. Plant Genome. 8, plantgenome2014.08.0037. 10.3835/plantgenome2014.08.0037 [DOI] [PubMed]
- 65.Guo B, Guo Y, Hong H, Qiu L-J (2016) Identification of genomic insertion and flanking sequence of G2-EPSPS and GAT transgenes in soybean using whole genome sequencing method. Front Plant Sci 7. 10.3389/fpls.2016.01009 [DOI] [PMC free article] [PubMed]
- 66.Xu W, Zhang H, Zhang Y, Shen P, Li X, Li R, Yang L (2022) A paired-end whole-genome sequencing approach enables comprehensive characterization of transgene integration in rice. Commun Biol 5:667. 10.1038/s42003-022-03608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reyes-Avila CS, Waldvogel D, Pradervand N, Aubry S, Croll D (2024) Detection of genetically modified organisms using highly multiplexed amplicon sequencing. Food Control 165:110670. 10.1016/j.foodcont.2024.110670 [Google Scholar]
- 68.Cottenet G, Blancpain C, Pruvost S (2025) An AmpliSeq™ HD approach for specific and sensitive identification of GMO by next generation sequencing. J Food Compos Anal 139:107038. 10.1016/j.jfca.2024.107038 [Google Scholar]
- 69.Mamanova L, Coffey AJ, Scott CE, Kozarewa I, Turner EH, Kumar A, Howard E, Shendure J, Turner DJ (2010) Target-enrichment strategies for next-generation sequencing. Nat Methods 7:111–118. 10.1038/nmeth.1419 [DOI] [PubMed] [Google Scholar]
- 70.Van Dijk EL, Jaszczyszyn Y, Naquin D, Thermes C (2018) The third revolution in sequencing technology. Trends Genet 34:666–681. 10.1016/j.tig.2018.05.008 [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Li R, Guo Y, Zhang Y, Zhang D, Yang L (2022) LIFE-Seq: a universal L arge I ntegrated DNA F ragment E nrichment Seq uencing strategy for deciphering the transgene integration of genetically modified organisms. Plant Biotechnol J 20:964–976. 10.1111/pbi.13776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Dong Y, Huang Y, Fan J, Yang M, Zhang J (2021) Whole-genome resequencing using next-generation and Nanopore sequencing for molecular characterization of T-DNA integration in transgenic poplar 741. BMC Genomics 22:329. 10.1186/s12864-021-07625-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fraiture M-A, Saltykova A, Hoffman S, Winand R, Deforce D, Vanneste K, De Keersmaecker SCJ, Roosens NHC (2018) Nanopore sequencing technology: a new route for the fast detection of unauthorized GMO. Sci Rep 8:7903. 10.1038/s41598-018-26259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu Y, Xu Y, Du J, Peng C, Wang X, Wu J, Zhou Q, Xu J (2024) Field detection of genetically modified crops: simple DNA extraction, rapid assay and automatic output on smartphone. Sens Actuators B Chem 418:136339. 10.1016/j.snb.2024.136339 [Google Scholar]
- 75.Wang X, Xie S, Chen X, Peng C, Xu X, Wei W, Ma T, Cai J, Xu J (2020) A rapid and convenient method for on-site detection of MON863 maize through real-time fluorescence recombinase polymerase amplification. Food Chem 324:126821. 10.1016/j.foodchem.2020.126821 [DOI] [PubMed] [Google Scholar]
- 76.Xiao B, Wang M, Zhang J, Wang N, Fu W, Chen H, Wang H, Li L, Pang X, Liu C, Huang F, Chen A (2024) Rapid DNA extraction and microfluidic LAMP system in portable equipment for GM crops detection. Sens Actuators B Chem 411:135716. 10.1016/j.snb.2024.135716 [Google Scholar]
- 77.Hwang H, Bae S-C, Lee S, Lee Y-H, Chang A (2013) A rapid and simple genotyping method for various plants by direct-PCR. Plant Breed Biotechnol 1:290–297. 10.9787/PBB.2013.1.3.290 [Google Scholar]
- 78.Wang X, Chen Y, Chen X, Peng C, Wang L, Xu X, Wu J, Wei W, Xu J (2020) A highly integrated system with rapid DNA extraction, recombinase polymerase amplification, and lateral flow biosensor for on-site detection of genetically modified crops. Anal Chim Acta 1109:158–168. 10.1016/j.aca.2020.02.044 [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Yang F, Fu Y, He X, Tian H, Yang L, Wu M, Cao J, Liu J (2024) A point-of-care testing platform for on-site identification of genetically modified crops. Lab Chip 24:2622–2632. 10.1039/D4LC00040D [DOI] [PubMed] [Google Scholar]
- 80.Li J, Li J, Lin S, Zhu L, Li X, Xu W (2022) Advanced technologies in on-site detection of genetically modified products. Agriculture 12:888. 10.3390/agriculture12060888 [Google Scholar]
- 81.Kaymaz SV, Elitas M (2021) Optimization of loop-mediated isothermal amplification (LAMP) reaction mixture for biosensor applications. MethodsX 8:101282. 10.1016/j.mex.2021.101282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed MU, Saito M, Hossain MM, Rao SR, Furui S, Hino A, Takamura Y, Takagi M, Tamiya E (2009) Electrochemical genosensor for the rapid detection of GMO using loop-mediated isothermal amplification. Analyst 134:966. 10.1039/b812569d [DOI] [PubMed] [Google Scholar]
- 83.Cheng N, Shang Y, Xu Y, Zhang L, Luo Y, Huang K, Xu W (2017) On-site detection of stacked genetically modified soybean based on event-specific TM-LAMP and a DNAzyme-lateral flow biosensor. Biosens Bioelectron 91:408–416. 10.1016/j.bios.2016.12.066 [DOI] [PubMed] [Google Scholar]
- 84.Kaygusuz D, Vural S, Aytekin AÖ, Lucas SJ, Elitas M (2019) DaimonDNA: A portable, low-cost loop-mediated isothermal amplification platform for naked-eye detection of genetically modified organisms in resource-limited settings. Biosens Bioelectron 141:111409. 10.1016/j.bios.2019.111409 [DOI] [PubMed] [Google Scholar]
- 85.Li K, Luo Y, Huang K, Yang Z, Wan Y, Xu W (2020) Single universal primer recombinase polymerase amplification-based lateral flow biosensor (SUP-RPA-LFB) for multiplex detection of genetically modified maize. Anal Chim Acta 1127:217–224. 10.1016/j.aca.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 86.Li J, Macdonald J (2015) Advances in isothermal amplification: novel strategies inspired by biological processes. Biosens Bioelectron 64:196–211. 10.1016/j.bios.2014.08.069 [DOI] [PubMed] [Google Scholar]
- 87.Giuffrida MC, Spoto G (2017) Integration of isothermal amplification methods in microfluidic devices: recent advances. Biosens Bioelectron 90:174–186. 10.1016/j.bios.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 88.Becherer L, Borst N, Bakheit M, Frischmann S, Zengerle R, Von Stetten F (2020) Loop-mediated isothermal amplification (LAMP) – review and classification of methods for sequence-specific detection. Anal Methods 12:717–746. 10.1039/C9AY02246E [Google Scholar]
- 89.Glökler J, Lim TS, Ida J, Frohme M (2021) Isothermal amplifications – a comprehensive review on current methods. Crit Rev Biochem Mol Biol 56:543–586. 10.1080/10409238.2021.1937927 [DOI] [PubMed] [Google Scholar]
- 90.Cao X, Chen C, Zhu Q (2023) Biosensors based on functional nucleic acids and isothermal amplification techniques. Talanta 253:123977. 10.1016/j.talanta.2022.123977 [DOI] [PubMed] [Google Scholar]
- 91.Wang J, Wang Y, Liu H, Hu X, Zhang M, Liu X, Ye H, Zeng H (2023) An ultra-sensitive test strip combining with RPA and CRISPR/Cas12a system for the rapid detection of GM crops. Food Control 144:109383. 10.1016/j.foodcont.2022.109383 [Google Scholar]
- 92.Duan Z, Yang X, Ji X, Chen Y, Niu X, Guo A, Zhu J, Li F, Lang Z, Zhao H (2022) Cas12a-based on-site, rapid detection of genetically modified crops. J Integr Plant Biol 64:1856–1859. 10.1111/jipb.13342 [DOI] [PubMed] [Google Scholar]
- 93.Saputra HA (2023) Electrochemical sensors: basic principles, engineering, and state of the art. Monatshefte Für Chem - Chem Mon 154:1083–1100. 10.1007/s00706-023-03113-z [Google Scholar]
- 94.Nisar S, Dastgeer G, Shazad ZM, Zulfiqar MW, Rasheed A, Iqbal MZ, Hussain K, Rabani I, Kim D, Irfan A, Chaudhry AR (2024) 2D materials in advanced electronic biosensors for point-of-care devices. Adv Sci 11:2401386. 10.1002/advs.202401386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grzędowski AJ, Ma T, Bizzotto D (2023) FRET imaging of nonuniformly distributed DNA SAMs on gold reveals the role played by the donor/acceptor ratio and the local environment in measuring the rate of hybridization. Chem Biomed Imaging 1:286–296. 10.1021/cbmi.3c00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dutta G, Fernandes FCB, Estrela P, Moschou D, Bueno PR (2021) Impact of surface roughness on the self-assembling of molecular films onto gold electrodes for label-free biosensing applications. Electrochim Acta 378:138137. 10.1016/j.electacta.2021.138137 [Google Scholar]
- 97.Yoon J, Conley BM, Shin M, Choi J-H, Bektas CK, Choi J-W, Lee K-B (2022) Ultrasensitive electrochemical detection of mutated viral RNAs with single-nucleotide resolution using a nanoporous electrode array (NPEA). ACS Nano 16:5764–5777. 10.1021/acsnano.1c10824 [DOI] [PubMed] [Google Scholar]
- 98.He Y, Jiao B, Tang H (2014) Interaction of single-stranded DNA with graphene oxide: fluorescence study and its application for S1 nuclease detection. RSC Adv 4:18294–18300. 10.1039/C4RA01102C [Google Scholar]
- 99.Xu Z, Lei X, Tu Y, Tan Z, Song B, Fang H (2017) Dynamic cooperation of hydrogen binding and π stacking in ssdna adsorption on graphene oxide. Chem Eur J 23:13100–13104. 10.1002/chem.201701733 [DOI] [PubMed] [Google Scholar]
- 100.Yuan D, Kong J, Fang X, Chen Q (2019) A graphene oxide-based paper chip integrated with the hybridization chain reaction for peanut and soybean allergen gene detection. Talanta 196:64–70. 10.1016/j.talanta.2018.12.036 [DOI] [PubMed] [Google Scholar]
- 101.Xu X, Pan H, Li W, Xu J, Chen X, Zheng C, Peng J, Mao X, Liu M, Yan H, Wang H (2024) Binding of single/double stranded ct-DNA with graphene oxide-silver nanocomposites in vitro: a multispectroscopic approach. Int J Biol Macromol 275:133715. 10.1016/j.ijbiomac.2024.133715 [DOI] [PubMed] [Google Scholar]
- 102.Park JS, Na H-K, Min D-H, Kim D-E (2013) Desorption of single-stranded nucleic acids from graphene oxide by disruption of hydrogen bonding. Analyst 138:1745. 10.1039/c3an36493c [DOI] [PubMed] [Google Scholar]
- 103.Liu F, Li K, Zhang Y, Ding J, Wen T, Pei X, Yan Y, Ji W, Liu J, Zhang X, Li L (2020) An electrochemical DNA biosensor based on nitrogen-doped graphene nanosheets decorated with gold nanoparticles for genetically modified maize detection. Microchim Acta 187:574. 10.1007/s00604-020-04511-4 [DOI] [PubMed] [Google Scholar]
- 104.Mitrevska K, Milosavljevic V, Gagic M, Richtera L, Adam V (2021) 2D transition metal dichalcogenide nanomaterial-based miRNA biosensors. Appl Mater Today 23:101043. 10.1016/j.apmt.2021.101043 [Google Scholar]
- 105.Alwarappan S, Nesakumar N, Sun D, Hu TY, Li C-Z (2022) 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens Bioelectron 205:113943. 10.1016/j.bios.2021.113943 [DOI] [PubMed] [Google Scholar]
- 106.Chen WY, Lin H, Barui AK, Gomez AMU, Wendt MK, Stanciu LA (2022) DNA-functionalized Ti3 C2 T x MXenes for selective and rapid detection of SARS-CoV-2 nucleocapsid gene. ACS Appl Nano Mater 5:1902–1910. 10.1021/acsanm.1c03520 [DOI] [PubMed] [Google Scholar]
- 107.Yu Y-S, Tan R-R, Ding H-M (2024) Effect of surface functionalization on DNA sequencing using MXene-based nanopores. RSC Adv 14:405–412. 10.1039/D3RA05432B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amani AM, Kamyab H, Vafa E, Jahanbin A, Abbasi M, Vaez A, Munuswamy-Ramanujam G, Ravindran B, Gnanasekaran L, Rocchio D, Yusuf M (2025) Multifunctional MXenes nanocomposite platforms for biosensing and wearable sensor technologies. Adv Compos Hybrid Mater 8:63. 10.1007/s42114-024-01118-8 [Google Scholar]
- 109.Yao B, Yao J, Fan Z, Zhao J, Zhang K, Huang W (2022) Rapid advances of versatile MXenes for electrochemical enzyme-based biosensors, immunosensors, and nucleic acid-based biosensors. ChemElectroChem 9:e202200103. 10.1002/celc.202200103 [Google Scholar]
- 110.Bolourinezhad M, Rezayi M, Meshkat Z, Soleimanpour S, Mojarrad M, Zibadi F, Aghaee-Bakhtiari SH, Taghdisi SM (2023) Design of a rapid electrochemical biosensor based on MXene/Pt/C nanocomposite and DNA/RNA hybridization for the detection of COVID-19. Talanta 265:124804. 10.1016/j.talanta.2023.124804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sezen S, Zarepour A, Zarrabi A, Iravani S (2023) MXene-based biosensors for selective detection of pathogenic viruses and bacteria. Microchem J 193:109258. 10.1016/j.microc.2023.109258 [Google Scholar]
- 112.Fan P, Ma E, Liu C, Zhao Y, Wen X, Wang L, Li L, Qu Q (2024) A new electrochemical DNA biosensor based on the density control strategy of Ti3C2NH2 MXene@Au nanocomposites for the detection of hepatitis B virus-DNA. Ionics 30:541–552. 10.1007/s11581-023-05251-0 [Google Scholar]
- 113.Yadav P, Cao Z, Barati Farimani A (2021) DNA Detection with single-layer Ti3 C2 MXene nanopore. ACS Nano 15:4861–4869. 10.1021/acsnano.0c09595 [DOI] [PubMed] [Google Scholar]
- 114.Divya KP, Keerthana S, Viswanathan C, Ponpandian N (2023) MXene supported biomimetic bilayer lipid membrane biosensor for zeptomole detection of BRCA1 gene. Microchim Acta 190:116. 10.1007/s00604-023-05694-2 [DOI] [PubMed] [Google Scholar]
- 115.Chen R, Kan L, Duan F, He L, Wang M, Cui J, Zhang Z, Zhang Z (2021) Surface plasmon resonance aptasensor based on niobium carbide MXene quantum dots for nucleocapsid of SARS-CoV-2 detection. Microchim Acta 188:316. 10.1007/s00604-021-04974-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prabisha KE, Neena PK, Ankitha M, Rasheed PA, Suneesh PV, Satheesh Babu TG (2025) Selenium nanoparticles modified niobium MXene for non-enzymatic detection of glucose. Sci Rep 15:1749. 10.1038/s41598-025-85748-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rasheed PA, Pandey RP, Jabbar KA, Mahmoud KA (2022) Nb4 C3 Tx (MXene)/Au/DNA aptasensor for the ultraselective electrochemical detection of lead in water samples. Electroanalysis 34:1540–1546. 10.1002/elan.202100685 [Google Scholar]
- 118.Khan R, Andreescu S (2020) MXenes-based bioanalytical sensors: design, characterization, and applications. Sensors 20:5434. 10.3390/s20185434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun H, Li D, Yue X, Hong R, Yang W, Liu C, Xu H, Lu J, Dong L, Wang G, Li D (2022) A review of transition metal dichalcogenides-based biosensors. Front Bioeng Biotechnol 10:941135. 10.3389/fbioe.2022.941135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mia AK, Meyyappan M, Giri PK (2023) Two-dimensional transition metal dichalcogenide based biosensors: from fundamentals to healthcare applications. Biosensors 13:169. 10.3390/bios13020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang X, Teng SY, Loy ACM, How BS, Leong WD, Tao X (2020) Transition metal dichalcogenides for the application of pollution reduction: a review. Nanomaterials 10:1012. 10.3390/nano10061012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mphuthi N, Sikhwivhilu L, Ray SS (2022) Functionalization of 2D MoS2 nanosheets with various metal and metal oxide nanostructures: their properties and application in electrochemical sensors. Biosensors 12:386. 10.3390/bios12060386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hostert L, Dias MS, De Aquino CB, De Santos FC, Marangoni VS, De Carvalho C Silva C, Seixas L, Maroneze CM (2024) Covalent surface functionalization of exfoliated MoS2 nanosheets for improved electrocatalysis. J Phys Chem C 128:20856–20865. 10.1021/acs.jpcc.4c05965 [Google Scholar]
- 124.Kim Y, Lee D, Kim S, Kang E, Kim C (2019) Nanocomposite synthesis of nanodiamond and molybdenum disulfide. Nanomaterials 9:927. 10.3390/nano9070927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gong L, Feng L, Zheng Y, Luo Y, Zhu D, Chao J, Su S, Wang L (2022) Molybdenum disulfide-based nanoprobes: preparation and sensing application. Biosensors 12:87. 10.3390/bios12020087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yan J, Liu W, Wang J, Liu H, Wang L, Li X, Li Y (2024) Enhancing public health safety: development and application of a MoS2@CNT-Chit electrochemical DNA biosensor for rapid and accurate detection of S. Typhi Biochem Eng J 210:109416. 10.1016/j.bej.2024.109416 [Google Scholar]
- 127.Zhang Y, Feng D, Xu Y, Yin Z, Dou W, Habiba UE, Pan C, Zhang Z, Mou H, Deng H, Mi X, Dai N (2021) DNA-based functionalization of two-dimensional MoS2 FET biosensor for ultrasensitive detection of PSA. Appl Surf Sci 548:149169. 10.1016/j.apsusc.2021.149169 [Google Scholar]
- 128.Bahri M, Yu D, Zhang CY, Chen Z, Yang C, Douadji L, Qin P (2024) Unleashing the potential of tungsten disulfide: current trends in biosensing and nanomedicine applications. Heliyon 10:e24427. 10.1016/j.heliyon.2024.e24427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Narang J, Mishra A, Pilloton R, Vv A, Wadhwa S, Pundir CS, Khanuja M (2018) Development of MoSe2 nano-urchins as a sensing platform for a selective bio-capturing of Escherichia coli Shiga toxin DNA. Biosensors 8:77. 10.3390/bios8030077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Sun M (2021) Two-dimensional WS2 /MoS2 heterostructures: properties and applications. Nanoscale 13:5594–5619. 10.1039/D1NR00455G [DOI] [PubMed] [Google Scholar]
- 131.Ramanavicius S, Ramanavicius A (2020) Conducting polymers in the design of biosensors and biofuel cells. Polymers 13:49. 10.3390/polym13010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Janardhanan JA, Yu H (2024) Recent advances in PEDOT/PProDOT-derived nano biosensors: engineering nano assemblies for fostering advanced detection platforms for biomolecule detection. Nanoscale 16:17202–17229. 10.1039/D4NR01449A [DOI] [PubMed] [Google Scholar]
- 133.Gu Y, Tseng P-Y, Bi X, Yang JHC (2018) Quantification of DNA by a thermal-durable biosensor modified with conductive poly(3,4-ethylenedioxythiophene). Sensors 18:3684. 10.3390/s18113684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stejskal J (2020) Interaction of conducting polymers, polyaniline and polypyrrole, with organic dyes: polymer morphology control, dye adsorption and photocatalytic decomposition. Chem Pap 74:1–54. 10.1007/s11696-019-00982-9 [Google Scholar]
- 135.Kumar S, Kumar A, Deepak KS, Bhaiyya M, Balapure A, Dubey SK, Goel S (2025) Electrode fabrication techniques. In: Goel S (ed) Micro Electromechanical Systems (MEMS). pp. 59–70. Wiley
- 136.Partanen K, Pei Y, Hillen P, Hassan M, McEleney K, Schatte G, Payne SJ, Oleschuk R, She Z (2022) Investigating electrochemical deposition of gold on commercial off-the-shelf 3-D printing materials towards developing sensing applications. RSC Adv 12:33440–33448. 10.1039/D2RA05455H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zanut A, Cian A, Cefarin N, Pozzato A, Tormen M (2020) Nanoelectrode arrays fabricated by thermal nanoimprint lithography for biosensing application. Biosensors 10:90. 10.3390/bios10080090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vanpeene V, Soucy P, Xiong J, Dupré N, Lestriez B, Roué L (2021) Sequential focused ion beam scanning electron microscopy analyses for monitoring cycled-induced morphological evolution in battery composite electrodes. Silicon-graphite electrode as exemplary case. J Power Sources. 498:229904. 10.1016/j.jpowsour.2021.229904 [Google Scholar]
- 139.Munir S, Ali B, Gul S (2024) The advancements of 3D-printed electrodes in electrochemistry. Discov Electrochem 1:14. 10.1007/s44373-024-00013-7 [Google Scholar]
- 140.Abedi R, Raoof JB, Hashkavayi AB, Asghari M, Azimi R, Hejazi MS (2022) A novel genosensor based on Fe3O4@SiO2/DABCO-modified screen-printed graphite electrode for detection of prostate cancer gene sequence hybridization. J Iran Chem Soc 19:2631–2640. 10.1007/s13738-021-02480-w [Google Scholar]
- 141.Moustakim H, Mohammadi H, Amine A (2022) Electrochemical DNA biosensor based on immobilization of a non-modified ssDNA using phosphoramidate-bonding strategy and pencil graphite electrode modified with AuNPs/CB and self-assembled cysteamine monolayer. Sensors 22:9420. 10.3390/s22239420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shoaie N, Forouzandeh M, Omidfar K (2018) Voltammetric determination of the Escherichia coli DNA using a screen-printed carbon electrode modified with polyaniline and gold nanoparticles. Microchim Acta 185:217. 10.1007/s00604-018-2749-y [DOI] [PubMed] [Google Scholar]
- 143.He Q, Wu Q, Feng X, Liao Z, Peng W, Liu Y, Peng D, Liu Z, Mo M (2020) Interfacing DNA with nanoparticles: surface science and its applications in biosensing. Int J Biol Macromol 151:757–780. 10.1016/j.ijbiomac.2020.02.217 [DOI] [PubMed] [Google Scholar]
- 144.An T, Tan Q, Jiang L, Liu L, Jiang X, Liu L, Chang X, Tian X, Deng Z, Gao S, Wang L, Chen S (2025) A DNA phosphorothioation pathway via adenylated intermediate modulates Tdp machinery. Nat Chem Biol. 10.1038/s41589-024-01832-w [DOI] [PubMed] [Google Scholar]
- 145.Gabryel-Skrodzka M, Nowak M, Teubert A, Jastrzab R (2022) Coordination chemistry of phosphate groups in systems including Copper(II) ions, phosphoethanolamine and pyrimidine nucleotides. Int J Mol Sci 23:13718. 10.3390/ijms232213718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yammouri G, Mohammadi H, Amine A (2019) A highly sensitive electrochemical biosensor based on carbon black and gold nanoparticles modified pencil graphite electrode for microRNA-21 detection. Chem Afr 2:291–300. 10.1007/s42250-019-00058-x [Google Scholar]
- 147.Hock N, Racaniello GF, Aspinall S, Denora N, Khutoryanskiy VV, Bernkop-Schnürch A (2022) Thiolated nanoparticles for biomedical applications: mimicking the workhorses of our body. Adv Sci 9:2102451. 10.1002/advs.202102451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lednický T, Bonyár A (2020) Large scale fabrication of ordered gold nanoparticle–epoxy surface nanocomposites and their application as label-free plasmonic DNA biosensors. ACS Appl Mater Interfaces 12:4804–4814. 10.1021/acsami.9b20907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumari I, Kaur N, Gupta S, Goel N (2019) Nucleotide conjugated (ZnO)3 cluster: interaction and optical characteristics using TDDFT. J Mol Graph Model 87:211–219. 10.1016/j.jmgm.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 150.Yang J, Su Q, Song C, Luo H, Jiang H, Ni M, Meng F (2024) A comprehensive adsorption and desorption study on the interaction of DNA oligonucleotides with TiO2 nanolayers. Phys Chem Chem Phys 26:22681–22695. 10.1039/D4CP02260B [DOI] [PubMed] [Google Scholar]
- 151.Zandieh M, Hagar BM, Liu J (2020) Interfacing DNA and polydopamine nanoparticles and its applications. Part Part Syst Charact 37:2000208. 10.1002/ppsc.202000208 [Google Scholar]
- 152.Lin M, Qi X (2023) Advances and challenges of stimuli-responsive nucleic acids delivery system in gene therapy. Pharmaceutics 15:1450. 10.3390/pharmaceutics15051450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Novakovic Z, Khalife M, Costache V, Camacho MJ, Cardoso S, Martins V, Gadjanski I, Radovic M, Vidic J (2024) Rapid detection and identification of vancomycin-sensitive bacteria using an electrochemical apta-sensor. ACS Omega 9:2841–2849. 10.1021/acsomega.3c08219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang J, Zhu J, Chao J (2023) Recent advances in DNA-based electrogenerated chemiluminescence biosensors. Sens Diagn 2:582–599. 10.1039/D3SD00027C [Google Scholar]
- 155.Shearer CJ, Yu L, Fenati R, Sibley AJ, Quinton JS, Gibson CT, Ellis AV, Andersson GG, Shapter JG (2017) Adsorption and desorption of single-stranded DNA from single-walled carbon nanotubes. Chem Asian J 12:1625–1634. 10.1002/asia.201700446 [DOI] [PubMed] [Google Scholar]
- 156.Lai Q, Liu Y, Ge L, Yang Y, Ji X, He Z (2021) Investigating the effect of 6-mercaptohexanol on the performance of a biosensor based on nanosurface energy transfer between gold nanoparticles and quantum dots. Anal Methods 13:2092–2098. 10.1039/D1AY00209K [DOI] [PubMed] [Google Scholar]
- 157.Carnerero JM, Jimenez-Ruiz A, Castillo PM, Prado-Gotor R (2017) Covalent and non-covalent DNA–gold-nanoparticle interactions: new avenues of research. ChemPhysChem 18:17–33. 10.1002/cphc.201601077 [DOI] [PubMed] [Google Scholar]
- 158.Ou J, Tan H, Chen X, Chen Z (2018) DNA-assisted assembly of gold nanostructures and their induced optical properties. Nanomaterials 8:994. 10.3390/nano8120994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cui D, Zhai S, Yang Y, Wu Y, Li J, Yan X, Shen P, Gao H, Wu G (2022) A label-free electrochemical impedance genosensor coupled with recombinase polymerase amplification for genetically modified maize detection. Agriculture 12:454. 10.3390/agriculture12040454 [Google Scholar]
- 160.Mobed A, Hasanzadeh M, Hassanpour S, Saadati A, Agazadeh M, Mokhtarzadeh A (2019) An innovative nucleic acid based biosensor toward detection of Legionella pneumophila using DNA immobilization and hybridization: a novel genosensor. Microchem J 148:708–716. 10.1016/j.microc.2019.05.027 [Google Scholar]
- 161.Zhang H, Gao H, Liu S, Ren X, Que L, Gu X, Rong S, Ma H, Ruan J, Miao M, Qi X, Chang D, Pan H (2024) Dual electrochemical signal “signal-on-off” sensor based on CHA-Td-HCR and CRISPR-Cas12a for MUC1 detection. Talanta 279:126665. 10.1016/j.talanta.2024.126665 [DOI] [PubMed] [Google Scholar]
- 162.Liu G, Zhong H, Ahmad Z, Yang X, Huo L (2020) Transport of engineered nanoparticles in porous media and its enhancement for remediation of contaminated groundwater. Crit Rev Environ Sci Technol 50:2301–2378. 10.1080/10643389.2019.1694823 [Google Scholar]
- 163.Liu B, Liu J (2019) Sensors and biosensors based on metal oxide nanomaterials. TrAC Trends Anal Chem 121:115690. 10.1016/j.trac.2019.115690 [Google Scholar]
- 164.Matter F, Luna AL, Niederberger M (2020) From colloidal dispersions to aerogels: how to master nanoparticle gelation. Nano Today 30:100827. 10.1016/j.nantod.2019.100827 [Google Scholar]
- 165.Liu B, Duan H, Liu Z, Liu Y, Chu H (2024) DNA-functionalized metal or metal-containing nanoparticles for biological applications. Dalton Trans 53:839–850. 10.1039/D3DT03614F [DOI] [PubMed] [Google Scholar]
- 166.Liu B, Ma L, Huang Z, Hu H, Wu P, Liu J (2018) Janus DNA orthogonal adsorption of graphene oxide and metal oxide nanoparticles enabling stable sensing in serum. Mater Horiz 5:65–69. 10.1039/C7MH00804J [Google Scholar]
- 167.Singhal C, Pundir CS, Narang J (2017) A genosensor for detection of consensus DNA sequence of Dengue virus using ZnO/Pt-Pd nanocomposites. Biosens Bioelectron 97:75–82. 10.1016/j.bios.2017.05.047 [DOI] [PubMed] [Google Scholar]
- 168.Dubourg G, Pavlović Z, Bajac B, Kukkar M, Finčur N, Novaković Z, Radović M (2024) Advancement of metal oxide nanomaterials on agri-food fronts. Sci Total Environ 928:172048. 10.1016/j.scitotenv.2024.172048 [DOI] [PubMed] [Google Scholar]
- 169.Szymczyk A, Ziółkowski R, Malinowska E (2023) Modern electrochemical biosensing based on nucleic acids and carbon nanomaterials. Sensors 23:3230. 10.3390/s23063230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen S, Sun Y, Fan X, Xu Y, Chen S, Zhang X, Man B, Yang C, Du J (2023) Review on two-dimensional material-based field-effect transistor biosensors: accomplishments, mechanisms, and perspectives. J Nanobiotechnology 21:144. 10.1186/s12951-023-01898-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Farid S, Meshik X, Choi M, Mukherjee S, Lan Y, Parikh D, Poduri S, Baterdene U, Huang C-E, Wang YY, Burke P, Dutta M, Stroscio MA (2015) Detection of Interferon gamma using graphene and aptamer based FET-like electrochemical biosensor. Biosens Bioelectron 71:294–299. 10.1016/j.bios.2015.04.047 [DOI] [PubMed] [Google Scholar]
- 172.Tardani F, Sarti S, Sennato S, Leo M, Filetici P, Casciardi S, Schiavi PG, Bordi F (2020) Experimental evidence of single-stranded DNA adsorption on multiwalled carbon nanotubes. J Phys Chem B 124:2514–2525. 10.1021/acs.jpcb.0c00882 [DOI] [PubMed] [Google Scholar]
- 173.Yadav R, Kumar K, Venkatesu P (2022) Covalent functionalization of carbon nanotube. In: Abraham J, Thomas S, Kalarikkal N (eds) Handbook of Carbon Nanotubes. Springer International Publishing, Cham, pp 393–420 [Google Scholar]
- 174.Kolahdouz M, Xu B, Nasiri AF, Fathollahzadeh M, Manian M, Aghababa H, Wu Y, Radamson HH (2022) Carbon-related materials: graphene and carbon nanotubes in semiconductor applications and design. Micromachines 13:1257. 10.3390/mi13081257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rashid JIA, Yusof NA (2017) The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: a review. Sens Bio-Sens Res 16:19–31. 10.1016/j.sbsr.2017.09.001 [Google Scholar]
- 176.Alli YA, Adewuyi S, Bada BS, Thomas S, Anuar H (2022) Quaternary trimethyl chitosan chloride capped bismuth nanoparticles with positive surface charges: catalytic and antibacterial activities. J Clust Sci 33:2311–2324. 10.1007/s10876-021-02156-8 [Google Scholar]
- 177.Bian J, Wang A, Sun Y, Zhu Q (2022) Adsorption of nitrate from water by core-shell chitosan wrinkled microspheres @LDH composite: electrostatic interaction, hydrogen bonding and surface complexation. Appl Clay Sci 225:106550. 10.1016/j.clay.2022.106550 [Google Scholar]
- 178.Yusoff NM, Yusoff HM, Asari A (2024) Optimisation on n-amidation reaction of cinnamic acid by. MJAS 28:1374–1385 [Google Scholar]
- 179.Nekrasov N, Kudriavtseva A, Orlov AV, Gadjanski I, Nikitin PI, Bobrinetskiy I, Knežević NŽ (2022) One-step photochemical immobilization of aptamer on graphene for label-free detection of NT-proBNP. Biosensors 12:1071. 10.3390/bios12051071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fathi-Hafshejani P, Azam N, Wang L, Kuroda MA, Hamilton MC, Hasim S, Mahjouri-Samani M (2021) Two-dimensional-material-based field-effect transistor biosensor for detecting COVID-19 virus (SARS-CoV-2). ACS Nano 15:11461–11469. 10.1021/acsnano.1c01188 [DOI] [PubMed] [Google Scholar]
- 181.Jin H, Zhao C, Gui R, Gao X, Wang Z (2018) Reduced graphene oxide/nile blue/gold nanoparticles complex-modified glassy carbon electrode used as a sensitive and label-free aptasensor for ratiometric electrochemical sensing of dopamine. Anal Chim Acta 1025:154–162. 10.1016/j.aca.2018.03.036 [DOI] [PubMed] [Google Scholar]
- 182.Gao H, Cui D, Zhai S, Yang Y, Wu Y, Yan X, Wu G (2022) A label-free electrochemical impedimetric DNA biosensor for genetically modified soybean detection based on gold carbon dots. Microchim Acta 189:216. 10.1007/s00604-022-05223-7 [DOI] [PubMed] [Google Scholar]
- 183.Zhu X, Zhang J, Pan R, Zhang K, Dai H (2024) CRISPR/Cas12a-mediated entropy-driven electrochemical biosensor for detection of genetically modified maize Mon810. Anal Chim Acta 1296:342290. 10.1016/j.aca.2024.342290 [DOI] [PubMed] [Google Scholar]
- 184.Tan LL, Futra D, Heng LY, Ulianas A, Kadir AAA, Ishak Z (2025) Voltammetric genosensor from silica nanocomposites for transgenic soybean analysis. J Food Compos Anal 140:107277. 10.1016/j.jfca.2025.107277 [Google Scholar]
- 185.Chou C-C, Lin Y-T, Kuznetsova I, Wang G-J (2022) Genetically modified soybean detection using a biosensor electrode with a self-assembled monolayer of gold nanoparticles. Biosensors 12:207. 10.3390/bios12040207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rahmawati I, Einaga Y, Ivandini TA, Fiorani A (2022) Enzymatic biosensors with electrochemiluminescence transduction. ChemElectroChem 9:e202200175. 10.1002/celc.202200175 [Google Scholar]
- 187.Balzer AHA, Whitehurst CB (2023) An analysis of the biotin–(strept)avidin system in immunoassays: interference and mitigation strategies. Curr Issues Mol Biol 45:8733–8754. 10.3390/cimb45110549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee SJ, Bong J-H, Jung J, Sung JS, Kang M-J, Jose J, Pyun J-C (2021) Screening of biotin-binding FV-antibodies from autodisplayed FV-library on E. coli outer membrane. Anal Chim Acta 1169:338627. 10.1016/j.aca.2021.338627 [DOI] [PubMed] [Google Scholar]
- 189.Hong D, Kim K, Jo E-J, Kim M-G (2021) Electrochemiluminescence-incorporated lateral flow immunosensors using Ru(bpy)32+ -labeled gold nanoparticles for the full-range detection of physiological C-reactive protein levels. Anal Chem 93:7925–7932. 10.1021/acs.analchem.1c00623 [DOI] [PubMed] [Google Scholar]
- 190.Mana T, Bhattacharya B, Lahiri H, Mukhopadhyay R (2022) XNAs: a troubleshooter for nucleic acid sensing. ACS Omega 7:15296–15307. 10.1021/acsomega.2c00581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ouyang P, Fang C, Han J, Zhang J, Yang Y, Qing Y, Chen Y, Shang W, Du J (2021) A DNA electrochemical sensor via terminal protection of small-molecule-linked DNA for highly sensitive protein detection. Biosensors 11:451. 10.3390/bios11110451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bage N, Kar P (2022) Electrochemical sensing of biotin-avidin interaction on gold electrode modified by silver nanoparticles through covalent co-assembling. Sens Int 3:100159. 10.1016/j.sintl.2022.100159 [Google Scholar]
- 193.Bage N, Kar P (2021) Ultrasensitive electrochemical sensing of biotin-avidin interaction on gold electrode bio-conjugated with silver nanoparticles. IEEE Sens J 21:10400–10408. 10.1109/JSEN.2021.3060776 [Google Scholar]
- 194.López-Pérez G, Prado-Gotor R, Fuentes-Rojas JA, Martin-Valero MJ (2020) Understanding gold nanoparticles interactions with chitosan: crosslinking agents as novel strategy for direct covalent immobilization of biomolecules on metallic surfaces. J Mol Liq 302:112381. 10.1016/j.molliq.2019.112381 [Google Scholar]
- 195.Gajovic-Eichelmann N, Ehrentreich-Förster E, Bier FF (2003) Directed immobilization of nucleic acids at ultramicroelectrodes using a novel electro-deposited polymer. Biosens Bioelectron 19:417–422. 10.1016/S0956-5663(03)00224-0 [DOI] [PubMed] [Google Scholar]
- 196.Li Y, Zhang Y, Jiang L, Chu PK, Dong Y, Wei Q (2016) A sandwich-type electrochemical immunosensor based on the biotin- streptavidin-biotin structure for detection of human immunoglobulin G. Sci Rep 6:22694. 10.1038/srep22694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang F, Xie Y, Zhu W, Wei T (2023) Recent advances in functionalization strategies for biosensor interfaces, especially the emerging electro-click: a review. Chemosensors 11:481. 10.3390/chemosensors11090481 [Google Scholar]
- 198.Campuzano S, Pedrero M, Yáñez-Sedeño P, Pingarrón JM (2019) Antifouling (Bio)materials for Electrochemical (Bio)sensing. Int J Mol Sci 20:423. 10.3390/ijms20020423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Barrias S, Ibáñez J, Fernandes JR, Martins-Lopes P (2024) The role of DNA-based biosensors in species identification for food authenticity assessment. Trends Food Sci Technol 145:104350. 10.1016/j.tifs.2024.104350 [Google Scholar]
- 200.Hua Y, Ma J, Li D, Wang R (2022) DNA-based biosensors for the biochemical analysis: a review. Biosensors 12:183. 10.3390/bios12030183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Szymczyk A, Soliwodzka K, Moskal M, Różanowski K, Ziółkowski R (2022) Further insight into the possible influence of electrode blocking agents on the stem-loop based electrochemical DNA sensor parameters. Sens Actuators B Chem 354:131086. 10.1016/j.snb.2021.131086 [Google Scholar]
- 202.Miranda-Castro R, Sánchez-Salcedo R, Suárez-Álvarez B, de-los-Santos-Álvarez N, Miranda-Ordieres AJ, Jesús Lobo-Castañón M (2017) Thioaromatic DNA monolayers for target-amplification-free electrochemical sensing of environmental pathogenic bacteria. Biosens Bioelectron 92:162–170. 10.1016/j.bios.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 203.Arroyo-Currás N, Dauphin-Ducharme P, Ortega G, Ploense KL, Kippin TE, Plaxco KW (2018) Subsecond-resolved molecular measurements in the living body using chronoamperometrically interrogated aptamer-based sensors. ACS Sens 3:360–366. 10.1021/acssensors.7b00787 [DOI] [PubMed] [Google Scholar]
- 204.Kim D, Koseoglu S, Manning BM, Meyer AF, Haynes CL (2011) Electroanalytical eavesdropping on single cell communication. Anal Chem 83:7242–7249. 10.1021/ac200666c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pilz FH, Kielb P (2023) Cyclic voltammetry, square wave voltammetry or electrochemical impedance spectroscopy? Interrogating electrochemical approaches for the determination of electron transfer rates of immobilized redox proteins. BBA Adv 4:100095. 10.1016/j.bbadva.2023.100095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ricci F, Vallée-Bélisle A, Simon AJ, Porchetta A, Plaxco KW (2016) Using nature’s “tricks” to rationally tune the binding properties of biomolecular receptors. Acc Chem Res 49:1884–1892. 10.1021/acs.accounts.6b00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wang X, Shan Y, Gong M, Jin X, Lv L, Jiang M, Xu J (2019) A novel electrochemical sensor for ochratoxin A based on the hairpin aptamer and double report DNA via multiple signal amplification strategy. Sens Actuators B Chem 281:595–601. 10.1016/j.snb.2018.10.148 [Google Scholar]
- 208.Lai R.Y (2017) Folding- and dynamics-based electrochemical DNA sensors. In: Methods in Enzymology. pp. 221–252. Elsevier [DOI] [PubMed]
- 209.Lai RY, Walker B, Stormberg K, Zaitouna AJ, Yang W (2013) Electrochemical techniques for characterization of stem-loop probe and linear probe-based DNA sensors. Methods 64:267–275. 10.1016/j.ymeth.2013.07.041 [DOI] [PubMed] [Google Scholar]
- 210.White RJ, Plaxco KW (2010) Exploiting binding-induced changes in probe flexibility for the optimization of electrochemical biosensors. Anal Chem 82:73–76. 10.1021/ac902595f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pellitero MA, Shaver A, Arroyo-Currás N (2020) Critical review—approaches for the electrochemical interrogation of DNA-based sensors: a critical review. J Electrochem Soc 167:037529. 10.1149/2.0292003JES [Google Scholar]
- 212.Molina Á, González J (2016) Pulse voltammetry in physical electrochemistry and electroanalysis: theory and applications. Springer International Publishing, Cham [Google Scholar]
- 213.Parveen KR (2016) General theory for pulse voltammetric techniques on rough and finite fractal electrodes for reversible redox system with unequal diffusivities. Electrochimica Acta. 194:283–291. 10.1016/j.electacta.2016.02.039 [Google Scholar]
- 214.Mirceski V, Guziejewski D, Stojanov L, Gulaboski R (2019) Differential square-wave voltammetry. Anal Chem 91:14904–14910. 10.1021/acs.analchem.9b03035 [DOI] [PubMed] [Google Scholar]
- 215.Paleček E, Bartošík M (2012) Electrochemistry of nucleic acids. Chem Rev 112:3427–3481. 10.1021/cr200303p [DOI] [PubMed] [Google Scholar]
- 216.Shekari Z, Zare HR, Falahati A (2017) Developing an impedimetric aptasensor for selective label–free detection of CEA as a cancer biomarker based on gold nanoparticles loaded in functionalized mesoporous silica films. J Electrochem Soc 164:B739–B745. 10.1149/2.1991713jes [Google Scholar]
- 217.Erdem A, Kerman K, Meric B, Akarca US, Ozsoz M (2000) Novel hybridization indicator methylene blue for the electrochemical detection of short DNA sequences related to the hepatitis B virus. Anal Chim Acta 422:139–149. 10.1016/S0003-2670(00)01058-8 [Google Scholar]
- 218.Castro DLD, Júnior JADR, Teixeira WLE, Silva VDA, Filho FPL (2014) ground-Penetrating radar imaging techniques applied in 3D environment: example in inactive dunes. Rev Bras Geofísica 32:273. 10.22564/rbgf.v32i2.482 [Google Scholar]
- 219.Liu S, Liu J, Han X, Cui Y, Wang W (2010) Electrochemical DNA biosensor fabrication with hollow gold nanospheres modified electrode and its enhancement in DNA immobilization and hybridization. Biosens Bioelectron 25:1640–1645. 10.1016/j.bios.2009.11.026 [DOI] [PubMed] [Google Scholar]
- 220.Mazzochette Z, Mugweru A (2014) Electrochemical DNA hybridization sensor using poly[vinylpyridine Os(bipyridine)2Cl]-co-ethylamine redox polymer. Chem Mater Res 6:55 [Google Scholar]
- 221.Guo Y, Su S, Wei X, Zhong Y, Su Y, Huang Q, Fan C, He Y (2013) A silicon-based electrochemical sensor for highly sensitive, specific, label-free and real-time DNA detection. Nanotechnology 24:444012. 10.1088/0957-4484/24/44/444012 [DOI] [PubMed] [Google Scholar]
- 222.Wu N, Gao W, He X, Chang Z, Xu M (2013) Direct electrochemical sensor for label-free DNA detection based on zero current potentiometry. Biosens Bioelectron 39:210–214. 10.1016/j.bios.2012.07.038 [DOI] [PubMed] [Google Scholar]
- 223.Yang Y, Wang Z, Yang M, Li J, Zheng F, Shen G, Yu R (2007) Electrical detection of deoxyribonucleic acid hybridization based on carbon-nanotubes/nano zirconium dioxide/chitosan-modified electrodes. Anal Chim Acta 584:268–274. 10.1016/j.aca.2006.11.055 [DOI] [PubMed] [Google Scholar]
- 224.Hynek D, Krejcova L, Zitka O, Adam V, Trnkova L, Sochor J, Stiborova M, Eckschlager T, Hubalek J, Kizek R (2012) Electrochemical study of doxorubicin interaction with different sequences of single stranded oligonucleotides. Part I Int J Electrochem Sci 7:13–33. 10.1016/S1452-3981(23)13317-7 [Google Scholar]
- 225.Ribeiro Teles FR, França Dos Prazeres DM, De Lima-Filho JL (2007) Electrochemical detection of a dengue-related oligonucleotide sequence using ferrocenium as a hybridization indicator. Sensors 7:2510–2518. 10.3390/s7112510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang X, Lai W, Man T, Qu X, Li L, Chandrasekaran AR, Pei H (2018) Bio-surface engineering with DNA scaffolds for theranostic applications. Nanofabrication 4:1–16. 10.1515/nanofab-2018-0001 [Google Scholar]
- 227.Herne TM, Tarlov MJ (1997) Characterization of DNA probes immobilized on gold surfaces. J Am Chem Soc 119:8916–8920. 10.1021/ja9719586 [Google Scholar]
- 228.Kimura-Suda H, Petrovykh DY, Tarlov MJ, Whitman LJ (2003) Base-dependent competitive adsorption of single-stranded DNA on gold. J Am Chem Soc 125:9014–9015. 10.1021/ja035756n [DOI] [PubMed] [Google Scholar]
- 229.Levicky R, Herne TM, Tarlov MJ, Satija SK (1998) Using self-assembly to control the structure of DNA monolayers on gold: a neutron reflectivity study. J Am Chem Soc 120:9787–9792. 10.1021/ja981897r [Google Scholar]
- 230.Fan C, Plaxco KW, Heeger AJ (2003) Electrochemical interrogation of conformational changes as a reagentless method for the sequence-specific detection of DNA. Proc Natl Acad Sci 100:9134–9137. 10.1073/pnas.1633515100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yang W, Lai RY (2011) Comparison of the stem-loop and linear probe-based electrochemical DNA sensors by alternating current voltammetry and cyclic voltammetry. Langmuir 27:14669–14677. 10.1021/la203015v [DOI] [PubMed] [Google Scholar]
- 232.Pei H, Lu N, Wen Y, Song S, Liu Y, Yan H, Fan C (2010) A DNA nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv Mater 22:4754–4758. 10.1002/adma.201002767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xiao Y, Qu X, Plaxco KW, Heeger AJ (2007) Label-free electrochemical detection of DNA in blood serum via target-induced resolution of an electrode-bound DNA pseudoknot. J Am Chem Soc 129:11896–11897. 10.1021/ja074218y [DOI] [PubMed] [Google Scholar]
- 234.Ricci F, Lai RY, Heeger AJ, Plaxco KW, Sumner JJ (2007) Effect of molecular crowding on the response of an electrochemical DNA sensor. Langmuir 23:6827–6834. 10.1021/la700328r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pheeney CG, Barton JK (2012) DNA electrochemistry with tethered methylene blue. Langmuir 28:7063–7070. 10.1021/la300566x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nano A, Furst AL, Hill MG, Barton JK (2021) DNA electrochemistry: charge-transport pathways through DNA films on gold. J Am Chem Soc 143:11631–11640. 10.1021/jacs.1c04713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Silva SM, Hoque S, Gonçales VR, Gooding JJ (2018) The impact of the position of the redox label on charge transfer and hybridization efficiency at DNA interfaces. Electroanalysis 30:1529–1535. 10.1002/elan.201800197 [Google Scholar]
- 238.Silva SM, Tavallaie R, Gonçales VR, Utama RH, Kashi MB, Hibbert DB, Tilley RD, Gooding JJ (2018) Dual signaling DNA electrochemistry: an approach to understand DNA interfaces. Langmuir 34:1249–1255. 10.1021/acs.langmuir.7b02787 [DOI] [PubMed] [Google Scholar]
- 239.Kang D, Zuo X, Yang R, Xia F, Plaxco KW, White RJ (2009) Comparing the properties of electrochemical-based DNA sensors employing different redox tags. Anal Chem 81:9109–9113. 10.1021/ac901811n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Khuda N, Somasundaram S, Urgunde AB, Easley CJ (2023) Ionic strength and hybridization position near gold electrodes can significantly improve kinetics in DNA-based electrochemical sensors. ACS Appl Mater Interfaces 15:5019–5027. 10.1021/acsami.2c22741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Batistuti MR, Bueno PR, Mulato M (2020) The importance of the assembling of DNA strands on the performance of electrochemical genosensors. Microchem J 159:105358. 10.1016/j.microc.2020.105358 [Google Scholar]
- 242.Wu Y, Lai RY (2014) Effects of DNA probe and target flexibility on the performance of a “signal-on” electrochemical DNA sensor. Anal Chem 86:8888–8895. 10.1021/ac5027226 [DOI] [PubMed] [Google Scholar]
- 243.Lubin AA, Vander Stoep Hunt B, White RJ, Plaxco KW (2009) Effects of probe length, probe geometry, and redox-tag placement on the performance of the electrochemical E-DNA sensor. Anal Chem 81:2150–2158. 10.1021/ac802317k [DOI] [PubMed] [Google Scholar]
- 244.Xiao Y, Lai RY, Plaxco KW (2007) Preparation of electrode-immobilized, redox-modified oligonucleotides for electrochemical DNA and aptamer-based sensing. Nat Protoc 2:2875–2880. 10.1038/nprot.2007.413 [DOI] [PubMed] [Google Scholar]
- 245.Wang Q, Yang L, Yang X, Wang K, He L, Zhu J, Su T (2012) An electrochemical DNA biosensor based on the “Y” junction structure and restriction endonuclease-aided target recycling strategy. Chem Commun 48:2982. 10.1039/c2cc17679c [DOI] [PubMed] [Google Scholar]
- 246.Wang T, Zhang Z, Li Y, Xie G (2015) Amplified electrochemical detection of mecA gene in methicillin-resistant Staphylococcus aureus based on target recycling amplification and isothermal strand-displacement polymerization reaction. Sens Actuators B Chem 221:148–154. 10.1016/j.snb.2015.06.057 [Google Scholar]
- 247.Williamson P, Piskunen P, Ijäs H, Butterworth A, Linko V, Corrigan DK (2023) Signal amplification in electrochemical DNA biosensors using target-capturing DNA origami tiles. ACS Sens 8:1471–1480. 10.1021/acssensors.2c02469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Immoos CE, Lee SJ, Grinstaff MW (2004) DNA-PEG-DNA triblock macromolecules for reagentless DNA detection. J Am Chem Soc 126:10814–10815. 10.1021/ja046634d [DOI] [PubMed] [Google Scholar]
- 249.Xiao Y, Lubin AA, Baker BR, Plaxco KW, Heeger AJ (2006) Single-step electronic detection of femtomolar DNA by target-induced strand displacement in an electrode-bound duplex. Proc Natl Acad Sci 103:16677–16680. 10.1073/pnas.0607693103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Xiong E, Li Z, Zhang X, Zhou J, Yan X, Liu Y, Chen J (2017) Triple-helix molecular switch electrochemical ratiometric biosensor for ultrasensitive detection of nucleic acids. Anal Chem 89:8830–8835. 10.1021/acs.analchem.7b01251 [DOI] [PubMed] [Google Scholar]
- 251.Qian Y, Tang D, Du L, Zhang Y, Zhang L, Gao F (2015) A novel signal-on electrochemical DNA sensor based on target catalyzed hairpin assembly strategy. Biosens Bioelectron 64:177–181. 10.1016/j.bios.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 252.Ilkhani H, Farhad S (2018) A novel electrochemical DNA biosensor for Ebola virus detection. Anal Biochem 557:151–155. 10.1016/j.ab.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 253.Moura-Melo S, Miranda-Castro R, De-los-Santos-Álvarez N, Miranda-Ordieres A, Dos Santos Junior J, Da Silva Fonseca R, Lobo-Castañón M (2017) A quantitative PCR-electrochemical genosensor test for the screening of biotech crops. Sensors 17:881. 10.3390/s17040881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wang J, Zhou H, Liu J, He J, Liu J, Yang W (2021) Electrochemical detection of DNA by formation of efficient electron transfer pathways through adsorbing gold nanoparticles to DNA modified electrodes. Microchem J 169:106581. 10.1016/j.microc.2021.106581 [Google Scholar]
- 255.Wang Q, Gao F, Ni J, Liao X, Zhang X, Lin Z (2016) Facile construction of a highly sensitive DNA biosensor by in-situ assembly of electro-active tags on hairpin-structured probe fragment. Sci Rep 6:22441. 10.1038/srep22441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu Y, Duan X, Shin H-J, Park S, Huang Y, Duan X (2021) Promises and prospects of two-dimensional transistors. Nature 591:43–53. 10.1038/s41586-021-03339-z [DOI] [PubMed] [Google Scholar]
- 257.Ghasemi F, Salimi A (2023) Advances in 2d based field effect transistors as biosensing platforms: from principle to biomedical applications. Microchem J 187:108432. 10.1016/j.microc.2023.108432 [Google Scholar]
- 258.Sreejith S, Ajayan J, Radhika JM, Sivasankari B, Tayal S, Saravanan M (2023) A comprehensive review on graphene FET bio-sensors and their emerging application in DNA/RNA sensing & rapid Covid-19 detection. Measurement 206:112202. 10.1016/j.measurement.2022.112202 [Google Scholar]
- 259.Chen X, Liu C, Mao S (2020) Environmental analysis with 2D transition-metal dichalcogenide-based field-effect transistors. Nano-Micro Lett 12:95. 10.1007/s40820-020-00438-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rizzato S, Monteduro AG, Leo A, Todaro MT, Maruccio G (2023) From ion-sensitive field-effect transistor to 2D materials field-effect-transistor biosensors. Electrochem Sci Adv 3:e2200006. 10.1002/elsa.202200006 [Google Scholar]
- 261.Syedmoradi L, Ahmadi A, Norton ML, Omidfar K (2019) A review on nanomaterial-based field effect transistor technology for biomarker detection. Microchim Acta 186:739. 10.1007/s00604-019-3850-6 [DOI] [PubMed] [Google Scholar]
- 262.Zou J, Bai H, Zhang L, Shen Y, Yang C, Zhuang W, Hu J, Yao Y, Hu W (2024) (Walter): Ion-sensitive field effect transistor biosensors for biomarker detection: current progress and challenges. J Mater Chem B 12:8523–8542. 10.1039/D4TB00719K [DOI] [PubMed] [Google Scholar]
- 263.Du J, Cui C, Chen H, Liu H, Zhang X, Zhang W (2024) Graphene field-effect transistor biosensors for detection of heavy metal ions. J Food Meas Charact 18:5683–5694. 10.1007/s11694-024-02598-4 [Google Scholar]
- 264.Elli G, Hamed S, Petrelli M, Ibba P, Ciocca M, Lugli P, Petti L (2022) Field-Effect Transistor-based biosensors for environmental and agricultural monitoring. Sensors 22:4178. 10.3390/s22114178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Feng X, Li P, Xiao M, Li T, Chen B, Wang X, Wang L (2024) Recent advances in the detection of pathogenic microorganisms and toxins based on field-effect transistor biosensors. Crit Rev Food Sci Nutr 64:9161–9190. 10.1080/10408398.2023.2208677 [DOI] [PubMed] [Google Scholar]
- 266.Sun M, Zhang C, Lu S, Mahmood S, Wang J, Sun C, Pang J, Han L, Liu H (2024) Recent advances in graphene field-effect transistor toward biological detection. Adv Funct Mater 34:2405471. 10.1002/adfm.202405471 [Google Scholar]
- 267.Béraud A, Sauvage M, Bazán CM, Tie M, Bencherif A, Bouilly D (2021) Graphene field-effect transistors as bioanalytical sensors: design, operation and performance. Analyst 146:403–428. 10.1039/D0AN01661F [DOI] [PubMed] [Google Scholar]
- 268.Ping J, Vishnubhotla R, Vrudhula A, Johnson ATC (2016) Scalable production of high-sensitivity, label-free DNA biosensors based on back-gated graphene field effect transistors. ACS Nano 10:8700–8704. 10.1021/acsnano.6b04110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nekrasov N, Jaric S, Kireev D, Emelianov AV, Orlov AV, Gadjanski I, Nikitin PI, Akinwande D, Bobrinetskiy I (2022) Real-time detection of ochratoxin A in wine through insight of aptamer conformation in conjunction with graphene field-effect transistor. Biosens Bioelectron 200:113890. 10.1016/j.bios.2021.113890 [DOI] [PubMed] [Google Scholar]
- 270.Hajian R, Balderston S, Tran T, deBoer T, Etienne J, Sandhu M, Wauford NA, Chung J-Y, Nokes J, Athaiya M, Paredes J, Peytavi R, Goldsmith B, Murthy N, Conboy IM, Aran K (2019) Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nat Biomed Eng 3:427–437. 10.1038/s41551-019-0371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jarić S, Kudriavtseva A, Nekrasov N, Orlov AV, Komarov IA, Barsukov LA, Gadjanski I, Nikitin PI, Bobrinetskiy I (2024) Femtomolar detection of the heart failure biomarker NT-proBNP in artificial saliva using an immersible liquid-gated aptasensor with reduced graphene oxide. Microchem J 196:109611. 10.1016/j.microc.2023.109611 [Google Scholar]
- 272.Xu S, Jiang S, Zhang C, Yue W, Zou Y, Wang G, Liu H, Zhang X, Li M, Zhu Z, Wang J (2018) Ultrasensitive label-free detection of DNA hybridization by sapphire-based graphene field-effect transistor biosensor. Appl Surf Sci 427:1114–1119. 10.1016/j.apsusc.2017.09.113 [Google Scholar]
- 273.Yang Y, Kong D, Wu Y, Chen Y, Dai C, Chen C, Zhao J, Luo S, Liu W, Liu Y, Wei D (2023) Catalytic hairpin assembly-enhanced graphene transistor for ultrasensitive miRNA detection. Anal Chem 95:13281–13288. 10.1021/acs.analchem.3c02433 [DOI] [PubMed] [Google Scholar]
- 274.Gao Z, Xia H, Zauberman J, Tomaiuolo M, Ping J, Zhang Q, Ducos P, Ye H, Wang S, Yang X, Lubna F, Luo Z, Ren L, Johnson ATC (2018) Detection of sub-fM DNA with target recycling and self-assembly amplification on graphene field-effect biosensors. Nano Lett 18:3509–3515. 10.1021/acs.nanolett.8b00572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zheng C, Huang L, Zhang H, Sun Z, Zhang Z, Zhang G-J (2015) Fabrication of ultrasensitive field-effect transistor DNA biosensors by a directional transfer technique based on CVD-grown graphene. ACS Appl Mater Interfaces 7:16953–16959. 10.1021/acsami.5b03941 [DOI] [PubMed] [Google Scholar]
- 276.Mei J, Li Y-T, Zhang H, Xiao M-M, Ning Y, Zhang Z-Y, Zhang G-J (2018) Molybdenum disulfide field-effect transistor biosensor for ultrasensitive detection of DNA by employing morpholino as probe. Biosens Bioelectron 110:71–77. 10.1016/j.bios.2018.03.043 [DOI] [PubMed] [Google Scholar]
- 277.Weng Z, You Z, Li H, Wu G, Song Y, Sun H, Fradlin A, Neal-Harris C, Lin M, Gao X, Zhang Y (2023) CRISPR-Cas12a biosensor array for ultrasensitive detection of unamplified DNA with single-nucleotide polymorphic discrimination. ACS Sens 8:1489–1499. 10.1021/acssensors.2c02495 [DOI] [PubMed] [Google Scholar]
- 278.Wang X, Zhu Y, Olsen TR, Sun N, Zhang W, Pei R, Lin Q (2018) A graphene aptasensor for biomarker detection in human serum. Electrochim Acta 290:356–363. 10.1016/j.electacta.2018.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hwang MT, Heiranian M, Kim Y, You S, Leem J, Taqieddin A, Faramarzi V, Jing Y, Park I, Van Der Zande AM, Nam S, Aluru NR, Bashir R (2020) Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nat Commun 11:1543. 10.1038/s41467-020-15330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang L, Wang X, Wu Y, Guo M, Gu C, Dai C, Kong D, Wang Y, Zhang C, Qu D, Fan C, Xie Y, Zhu Z, Liu Y, Wei D (2022) Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nat Biomed Eng 6:276–285. 10.1038/s41551-021-00833-7 [DOI] [PubMed] [Google Scholar]
- 281.Purwidyantri A, Azinheiro S, García Roldán A, Jaegerova T, Vilaça A, Machado R, Cerqueira MF, Borme J, Domingues T, Martins M, Alpuim P, Prado M (2023) Integrated approach from sample-to-answer for grapevine varietal identification on a portable graphene sensor chip. ACS Sens 8:640–654. 10.1021/acssensors.2c02090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Han D, Chand R, Kim Y-S (2017) Microscale loop-mediated isothermal amplification of viral DNA with real-time monitoring on solution-gated graphene FET microchip. Biosens Bioelectron 93:220–225. 10.1016/j.bios.2016.08.115 [DOI] [PubMed] [Google Scholar]
- 283.Ganguli A, Faramarzi V, Mostafa A, Hwang MT, You S, Bashir R (2020) High sensitivity graphene field effect transistor-based detection of DNA amplification. Adv Funct Mater 30:2001031. 10.1002/adfm.202001031 [Google Scholar]
- 284.Park I, Lim J, You S, Hwang MT, Kwon J, Koprowski K, Kim S, Heredia J, Stewart De Ramirez SA, Valera E, Bashir R (2021) Detection of SARS-CoV-2 virus amplification using a crumpled graphene field-effect transistor biosensor. ACS Sens. 6:4461–4470. 10.1021/acssensors.1c01937 [DOI] [PubMed] [Google Scholar]
- 285.Chen L, Li G, Yang A, Wu J, Yan F, Ju H (2022) A DNA-functionalized graphene field-effect transistor for quantitation of vascular endothelial growth factor. Sens Actuators B Chem 351:130964. 10.1016/j.snb.2021.130964 [Google Scholar]
- 286.Campos R, Borme J, Guerreiro JR, Machado G, Cerqueira MF, Petrovykh DY, Alpuim P (2019) Attomolar label-free detection of dna hybridization with electrolyte-gated graphene field-effect transistors. ACS Sens 4:286–293. 10.1021/acssensors.8b00344 [DOI] [PubMed] [Google Scholar]
- 287.Zheng J, Li J, Lin T, Ren Z, Wang F, Shi Z, Yu H, Jiang W, Tang W (2024) Amplification-free and label-free rapid detection of Staphylococcus aureus using solution-gated graphene transistor-based DNA biosensor with hybridization enhancement by interface engineering. Chem Eng J 495:153329. 10.1016/j.cej.2024.153329 [Google Scholar]
- 288.Bahri M, Shi B, Elaguech MA, Djebbi K, Zhou D, Liang L, Tlili C, Wang D (2022) Tungsten disulfide nanosheet-based field-effect transistor biosensor for DNA hybridization detection. ACS Appl Nano Mater 5:5035–5044. 10.1021/acsanm.2c00067 [Google Scholar]
- 289.Paulose AK, Hou Y-J, Huang Y-S, Chakkalaparambil Dileep N, Chiu C-L, Pal A, Kalaimani VM, Lin Z-H, Chang C-R, Chen C-P, Lin Y-C, Cheng C-Y, Cheng S-H, Cheng C-M, Wang Y-L (2023) Rapid Escherichia coli cloned DNA detection in serum using an electrical double layer-gated field-effect transistor-based DNA sensor. Anal Chem 95:6871–6878. 10.1021/acs.analchem.2c05719 [DOI] [PubMed] [Google Scholar]
- 290.Hu W-P, Tsai C-C, Yang Y-S, Chan HW-H, Chen W-Y (2018) Synergetic improvements of sensitivity and specificity of nanowire field effect transistor gene chip by designing neutralized DNA as probe. Sci Rep 8:12598. 10.1038/s41598-018-30996-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Guermonprez P, Nioche P, Renaud L, Battaglini N, Sanaur S, Krejci E, Piro B (2024) CRISPR–Cas systems associated with electrolyte-gated graphene-based transistors: how they work and how to combine them. Biosensors 14:541. 10.3390/bios14110541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Arora K, Prabhakar N, Chand S, Malhotra BD (2007) Escherichia coli genosensor based on polyaniline. Anal Chem 79:6152–6158. 10.1021/ac070403i [DOI] [PubMed] [Google Scholar]
- 293.Purwidyantri A, Ipatov A, Domingues T, Borme J, Martins M, Alpuim P, Prado M (2022) Programmable graphene-based microfluidic sensor for DNA detection. Sens Actuators B Chem 367:132044. 10.1016/j.snb.2022.132044 [Google Scholar]
- 294.Kim HE, Schuck A, Park H, Chung DR, Kang M, Kim Y-S (2024) Dual-mode graphene field-effect transistor biosensor with isothermal nucleic acid amplification. Biosensors 14:91. 10.3390/bios14020091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang Q, Hao Y, Zeng T, Shu W, Xue P, Li Y, Huang C, Ouyang L, Zou X, Zhao Z, Wang J, Yu X, Zhou W (2024) Modular fabrication of microfluidic graphene FET for nucleic acids biosensing. Adv Sci 11:2401796. 10.1002/advs.202401796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Deng M, Li J, Xiao B, Ren Z, Li Z, Yu H, Li J, Wang J, Chen Z, Wang X (2022) Ultrasensitive label-free DNA detection based on solution-gated graphene transistors functionalized with carbon quantum dots. Anal Chem 94:3320–3327. 10.1021/acs.analchem.1c05309 [DOI] [PubMed] [Google Scholar]
- 297.Chen S, Sun Y, Xia Y, Lv K, Man B, Yang C (2020) Donor effect dominated molybdenum disulfide/graphene nanostructure-based field-effect transistor for ultrasensitive DNA detection. Biosens Bioelectron 156:112128. 10.1016/j.bios.2020.112128 [DOI] [PubMed] [Google Scholar]
- 298.Lee D-W, Lee J, Sohn IY, Kim B-Y, Son YM, Bark H, Jung J, Choi M, Kim TH, Lee C, Lee N-E (2015) Field-effect transistor with a chemically synthesized MoS2 sensing channel for label-free and highly sensitive electrical detection of DNA hybridization. Nano Res 8:2340–2350. 10.1007/s12274-015-0744-8 [Google Scholar]
- 299.Alvarez-Serna BE, Ramírez-Chavarría RG, Castillo-Villanueva E, Carrillo-Reyes J, Ramírez-Zamora RM, Buitrón G, Alvarez-Icaza L (2023) Label-free and portable field-effect sensor for monitoring RT-LAMP products to detect SARS-CoV-2 in wastewater. Talanta 253:124060. 10.1016/j.talanta.2022.124060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ho H-Y, Kao W-S, Deval P, Dai C-Y, Chen Y-H, Yu M-L, Lin C-H, Yu L-S (2023) Rapid and sensitive LAMP/CRISPR-powered diagnostics to detect different hepatitis C virus genotypes using an ITO-based EG-FET biosensing platform. Sens Actuators B Chem 394:134278. 10.1016/j.snb.2023.134278 [Google Scholar]
- 301.Chen Y-W, Tai T-Y, Hsu C-P, Sarangadharan I, Pulikkathodi AK, Wang H-L, Sukesan R, Lee G-Y, Chyi J-I, Chen C-C, Lee G-B, Wang Y-L (2018) Direct detection of DNA using electrical double layer gated high electron mobility transistor in high ionic strength solution with high sensitivity and specificity. Sens Actuators B Chem 271:110–117. 10.1016/j.snb.2018.05.119 [Google Scholar]
- 302.Liu H, Chen J, Hu J, Song J, Lin P (2024) High-performance electrolyte-gated amorphous InGaZnO field-effect transistor for label-free DNA sensing. Bioelectrochemistry 160:108794. 10.1016/j.bioelechem.2024.108794 [DOI] [PubMed] [Google Scholar]
- 303.Gul I, Raheem MA, Reyad-ul-Ferdous Md, Yuan X, Chen Z, Lv C, Chen M, Ji J, Wu D, Zhao Q, Yan C, Yu D (2025) State-of-the-art signal amplification strategies for nucleic acid and non-nucleic acid biosensors. Sens Actuators Rep 9:100268. 10.1016/j.snr.2024.100268 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.