Abstract
Background and Objectives
Ustekinumab (USTE) and vedolizumab (VEDO) are increasingly used in paediatric patients with inflammatory bowel diseases (pIBD). However, data on the usefulness of therapeutic drug monitoring (TDM) in children are scarce. The primary objective of this study was to evaluate the association between disease activity, measured by faecal calprotectin (F-CPT), and serum trough levels (TLs) of USTE and VEDO. Secondary outcomes were to explore factors potentially associated with the outcome and exposure, to determine the optimal USTE or VEDO dose that predicts remission (defined as F-CPT < 250 µg/g), to validate our hypothesis using a proof-of-concept cohort (POCC) and to assess the occurrence of serum antibodies to USTE and VEDO.
Methods
This was a prospective single-centre observational study performed at the University Hospital Motol, Prague, Czech Republic. Of the 87 patients (51 Crohn’s disease (CD), 30 ulcerative colitis (UC), and 6 IBD unclassified (IBD-U)), drug serum TLs and antibodies were measured in 282 observations (49 treatment courses) of USTE and 359 observations (38 courses) of VEDO. Serum and stool samples were collected before each study drug application during both the induction and maintenance phases of the treatment throughout the entire study period (January 2020 to June 2024). Clinical and laboratory data were obtained from the nationwide prospective registry CREdIT. Patients with perianal disease and those with previous major bowel surgery were not excluded from the study. As a POCC, we analysed a group of pIBD treated at our centre with anti-TNF agents—adalimumab or infliximab.
Results
In a linear multiple regression mixed model, an association was observed between logF-CPT levels and USTE treatment duration (β −0.0010, 95% confidence interval (CI) −0.0015 to −0.0006, p < 0.001) but not with USTE TLs (p = 0.12). VEDO TLs and logF-CPT levels were negatively associated both in the linear (β −0.0173, 95% CI −0.0292 to −0.0053, p = 0.005) and categorical models (p = 0.026), even after adjusting for time. A VEDO TL of 15.1 µg/mL showed the best, though still poor, combination of sensitivity (0.82) and specificity (0.32) to predict F-CPT < 250 µg/g (area under the curve (AUC) 0.56, 95% CI 0.49–0.63). Intensification, induction phase, undetectable TLs, and type of IBD (CD, UC, IBD-U) were not associated with logF-CPT. Slightly elevated anti-drug antibodies were detected in 5 USTE and 16 VEDO observations, with no clinical implications.
Conclusions
TDM of USTE does not appear to be useful in pIBD. TDM of VEDO may assist in therapeutic strategy decisions, although establishing clinically useful cut-offs remains challenging.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40272-025-00702-9.
Key Points
| Ustekinumab and vedolizumab are increasingly used in paediatric patients with inflammatory bowel diseases; however, data on the usefulness of therapeutic drug monitoring are scarce. |
| This is the largest prospective observational study evaluating the association between faecal calprotectin and serum trough levels of ustekinumab and vedolizumab. |
| Therapeutical drug monitoring of ustekinumab does not appear to be useful, but in vedolizumab it may assist in therapeutic strategy decisions, although establishing clinically useful cut-offs remains challenging. |
Introduction
Ustekinumab (USTE) and vedolizumab (VEDO) serve as subsequent lines of biologics for the treatment of paediatric patients with inflammatory bowel disease (pIBD) who do not achieve or maintain clinical remission on anti-TNF therapy, even with optimized dosing [1, 2]. Both biologics are approved for the treatment of adult patients with moderate-to-severe Crohn’s disease (CD) and moderate-to-severe ulcerative colitis (UC) [3]. In paediatric patients, USTE is approved for the treatment of psoriasis [4] and recently also for moderately-to-severely active CD in patients who weigh more than 40 kg [5]; however, VEDO is still not licensed for use in pIBD. Recent data, including systematic reviews, show the efficacy and safety of USTE in pIBD [6–11]. VEDO has been shown to be effective and safe in pIBD, particularly in those with UC [6, 12–18]. For IBD patients on infliximab (IFX) or adalimumab (ADA), early proactive therapeutic drug monitoring (TDM) and dosing optimization are recommended per evidence-based guidelines from the European Crohn’s and Colitis Organisation (ECCO) and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) [1, 2]. TDM is particularly important in the paediatric population, as available pharmacokinetic data suggest that most paediatric patients are underdosed [19]. Several mainly observational studies have suggested associations between disease activity in adult (and to a lesser extent in paediatric) patients with IBD and serum trough levels (TLs) of USTE, as well as VEDO. For VEDO, the association between TLs and clinical outcomes, including mucosal healing, is consistently reported. However, the evidence to support routine TDM is still missing, especially during the maintenance phase [20–28]. Data on the efficacy of TDM for USTE in paediatric patients with CD are still limited, and target TLs for USTE have not yet been firmly established. While real-world data and clinical studies suggest a potential relationship between exposure and efficacy for VEDO, there are currently no reliable target TLs for VEDO in the paediatric population. According to current guidelines, there is no recommendation to perform routine TDM in adult or paediatric patients with IBD treated by non-anti-TNF biologics [1, 2, 29, 30].
To address this, we conducted an analysis of prospectively collected data from our pIBD undergoing USTE or VEDO therapy to evaluate the association between drug TLs and disease activity represented by faecal calprotectin (F-CPT) and to clarify the importance of TDM in USTE/VEDO-treated paediatric patients.
Methods
Design of the Study, Objectives and Ethical Considerations
This unicentric prospective observational study was conducted at our University Hospital between January 2020 and June 2024. The study protocol was approved by the Ethical Committee of our University Hospital, and parents of all patients provided written informed consent before inclusion in the study.
The primary objective was to assess the association between serum USTE and VEDO TLs and F-CPT levels in pIBD. Secondary objectives were: (1) to explore factors potentially associated with the outcome and exposure, (2) to determine the optimal USTE or VEDO dose that predicts remission (defined as F-CPT < 250 µg/g [31]) with the highest sensitivity and specificity, (3) to validate our hypothesis using a proof-of-concept cohort of patients treated with anti-TNF and (4) to assess the occurrence of serum antibodies to USTE and VEDO in pIBD.
Patients
We prospectively included all paediatric patients with IBD treated at our gastroenterology department by USTE or VEDO (referred to as “study drugs” below). Inclusion criteria were: (1) age 2–19 years, (2) diagnosis of CD, UC or inflammatory bowel disease unclassified (IBD-U) based on revised Porto criteria [32], (3) treatment with USTE or VEDO based on usual clinical practice, and 4) signed written informed consent from parents/guardians. Exclusion criteria were: (1) no available TLs and/or antibodies to study drug and no available levels of F-CPT at the same visit, (2) patients not entered into the prospective nationwide registry of biological therapy (CREdIT), (3) refusal to sign written consent for the study, (4) diagnosis other than IBD, (5) pregnancy. Patients with perianal disease and those with previous major bowel surgery were not excluded from the study.
Collection of Laboratory Samples and Clinical Data
Study personnel were instructed to collect serum and stool samples before each study drug application during both the induction and maintenance phases of the treatment throughout the entire study period. At our centre, the prospective measurement of TLs and antibodies (therapeutic drug monitoring, TDM) as well as the collection of stool samples for F-CPT is part of the routine clinical practice for all patients with IBD receiving any biological treatment. However, proactive TDM is not performed for either USTE or VEDO. Both TLs (μg/mL) and antibodies (ng/mL) were measured in serum using the enzyme-linked immunosorbent assay (ELISA) method (ImmunoGuide®, AYBAYTECH Biyoteknoloji Ltd.) for both USTE (detection range 0.4–100 μg/mL) and VEDO (detection range 2–100 μg/mL). Positive anti-drug antibodies were defined as > 2 ng/mL both for USTE and VEDO. F-CPT was measured in stool using the fluorimetric enzyme-linked immunoassay (FEIA) method.
Clinical and phenotypic data are also prospectively recorded in our hospital information system as part of standard care and entered into the nationwide prospective registry of biological therapy (CREdIT) [9]. We collected the following data: phenotype of the disease (CD, UC, IBD-U and Paris classification), sex, age at diagnosis, age at start of the study drug treatment, time from diagnosis to start of the treatment, line of the treatment, treatment intensification, concomitant treatment, study drug TLs, presence of antibodies to study drug, F-CPT, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) level at the time of observation. For the VEDO group, information on the application route (intravenous (IV) versus subcutaneous (SC)) was also collected. All USTE patients received IV induction treatment (6mg/kg rounded up to every 130 mg) with subsequent SC maintenance treatment. All VEDO patients received both induction (10 mg/kg up to 300 mg, week 0, 2, and 6) and maintenance treatment IV (out of these, five were switched to SC (22 observations)). Treatment intensification in the maintenance phase was defined as increase of dose or shortening of the interval compared with standard treatment regimens (typically 90 mg SC every 8 weeks for USTE and 10 mg/kg up to 300 mg IV every 8 weeks for VEDO). Decision on treatment intensification was made by the treating physician after discussion with the patient and caregivers on the basis of clinical and laboratory markers of disease activity.
Statistics
All data were analysed using R statistical software (version 4.4.1; www.r-project.org). Continuous variables were described as medians and interquartile ranges (IQRs). Categorical variables were described as absolute frequencies and percentages. In consideration of the fact that measurements were available for all patients at subsequent time points, each combination of F-CPT and TLs was considered an observational case, and, thus, a regression mixed model was implemented. If information was missing at any time points, no imputation was performed, and the number of missing values was reported.
As a proof-of-concept cohort (POCC), we analysed the relationship between drug TLs and F-CPT adjusted for potentially confounding factors, in a group of pIBD treated at our centre with anti-TNF agents—ADA or IFX.
Results
Description of Cohorts
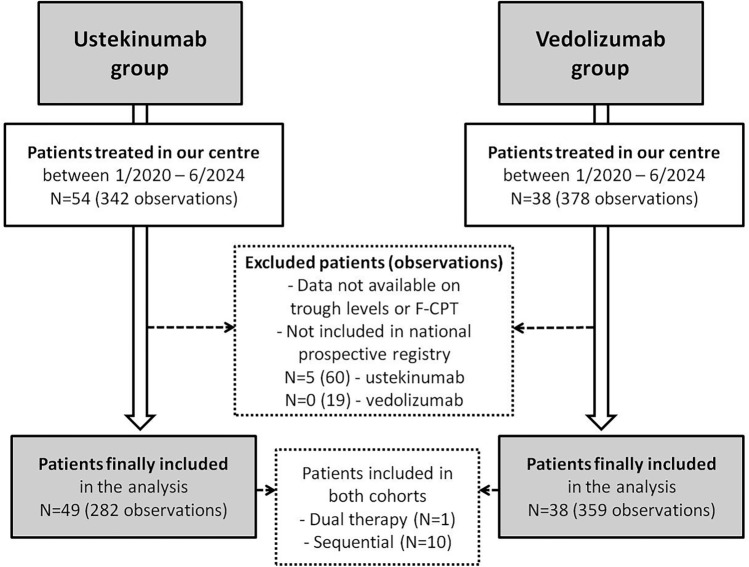
During the study period, a total of 54 patients received at least one dose of USTE, and 38 patients received at least one dose of VEDO at our IBD centre. After excluding patients who did not fulfil the inclusion criteria, we included 282 observations from 49 patients in the USTE group (5 median observations per patient (IQR 2–8); all anti-TNF-experienced) and 359 observations from 38 patients in the VEDO group (6 median observations per patient (IQR 2–14); 5 anti-TNF-naive). Ten patients are represented in both cohorts (initially receiving one of the study drugs and subsequently swapping to the other study drug). One patient received dual therapy (USTE + VEDO), and five patients initially started on IV VEDO and then switched to SC VEDO (Flowchart 1, Fig. 1). Basic characteristics of the study subjects and respective observations are reported in Tables 1 and 2.
Fig. 1.
Flowchart of patients included in the study
Table 1.
Characteristics of USTE and VEDO study groups
| USTE | VEDO | |
|---|---|---|
| Patients (N) | 49 | 38 |
| Basic characteristics | ||
| Age at diagnosis (y; median, IQR) | 9.8 (7.2–11.4) | 8.8 (5.5–12.4) |
| Sex (male, proportion) | 30 (0.61) | 25 (0.66) |
| Type of IBD (N, proportion) | ||
| CD | 42 (0.86) | 9 (0.24) |
| UC | 5 (0.1) | 25 (0.66) |
| IBD-U | 2 (0.04) | 4 (0.10) |
| Paris classification (CD; N, proportion) | ||
| L1 | 8 (0.16) | 1 (0.03) |
| L2 | 12 (0.25) | 5 (0.13) |
| L3 | 22 (0.45) | 3 (0.08) |
| L4a or L4b | 18 (0.37) | 2 (0.05) |
| B1 | 32 (0.65) | 8 (0.21) |
| B2 | 10 (0.2) | 1 (0.03) |
| B3 | 0 (0) | 0 (0) |
| B2 + B3 | 0 (0) | 0 (0) |
| Perianal disease | 10 (0.2) | 1 (0.03) |
| Growth impairment | 11 (0.22) | 4 (0.10) |
| Paris classification (UC, IBD-U; N, proportion) | ||
| E1 | 0 (0) | 2 (0.05) |
| E2 | 3 (0.06) | 5 (0.13) |
| E3 | 0 (0) | 1 (0.03) |
| E4 | 4 (0.08) | 21 (0.55) |
| S0 | 5 (0.1) | 19 (0.50) |
| S1 | 2 (0.04) | 10 (0.26) |
| Treatment | ||
| Time from diagnosis to the first dose (y; median, IQR) | 4.4 (2.4–6.2) | 2.6 (1.3–5.1) |
| Age at first induction dose (y; median, IQR) | 14.5 (12–16.2) | 13.3 (10.5–15.8) |
| Line of treatment (N, proportion) | ||
| First | 0 (0) | 5 (0.13) |
| Second | 6 (0.12) | 22 (0.58) |
| Third | 35 (0.71) | 8 (0.21) |
| Fourth | 8 (0.16) | 3 (0.08) |
| Missing data for all parameters listed above | 0 (0) | 0 (0) |
CD Crohn’s disease; IBD inflammatory bowel disease; IBD-U inflammatory bowel disease unclassified; IQR interquartile range; N number; UC ulcerative colitis; USTE ustekinumab; VEDO vedolizumab; y year
Table 2.
Characteristics of USTE and VEDO observations
| Observationsa | USTE (N = 282) | Unavailable observations N (proportion) ‡ | VEDO (N = 359) | Unavailable observations N (proportion) ‡ |
|---|---|---|---|---|
| Trough level (μg/mL; median, IQR) | 11.6 (5.9–17) | 0 ‡ | 25.9 (16.4–35.1) | 0‡ |
| Drug antibodies (AU/mL; median, IQR) | 0 (0–0.1) | 0 ‡ | 0 (0–0.2) | 2 (0.01)‡ |
| Drug antibodies positive (N, proportion) | 5 (0.02) | 0 | 16 (0.05) | 2 (0.01) |
| Intensified treatment (N, proportion) | 64 (0.23) | 116 (0.41) | 112 (0.31) | 75 (0.21) |
| Induction treatment (N, proportion) | 33 (0.12) | 0 | 81 (0.23) | 4 (0.01) |
| Length of the treatment (days; median, IQR) | 350 (165–678) | 0 ‡ | 307 (95–672) | 4 (0.01) ‡ |
| Subcutaneous application (N, proportion) | NA | 22 (0.06) | 75 (0.21) | |
| F-CPT (μg/g; median, IQR) | 398 (68.3–1400) | 0 ‡ | 257 (39–1050) | 0 ‡ |
| CRP (mg/L; median, IQR) | 1 (0.5–5.6) | 116 (0.41) ‡ | 0.85 (0.5–2.63) | 75 (0.21) ‡ |
| ESR (mm/h; median, IQR) | 10 (5–20) | 145 (0.51) ‡ | 12 (8–20) | 154 (0.43) ‡ |
| Concomitant treatment | ||||
| Azathioprine (N, proportion) | 31 (0.11) | 116 (0.41) | 119 (0.33) | 75 (0.21) |
| Methotrexate (N, proportion) | 3 (0.01) | 116 (0.41) | 54 (0.15) | 75 (0.21) |
| Steroids (N, proportion) | 6 (0.02) | 116 (0.41) | 20 (0.06) | 75 (0.21) |
| Antibiotics (N, proportion) | 1 (0) | 116 (0.41) | 24 (0.07) | 75 (0.21) |
| Exclusive enteral nutrition (N, proportion) | 0 (0) | 116 (0.41) | 1 (0) | 75 (0.21) |
CRP C-reactive protein; ESR erythrocyte sedimentation rate; F-CPT faecal calprotectin; IQR interquartile range; N number; NA not applicable; USTE ustekinumab; VEDO vedolizumab; y year
aMissing data during some observations are owing to the fact that not all drug applications were accompanied by full outpatient visit. Thus, in some observations, only trough levels, drug antibodies and faecal calprotectin levels were tested
‡ In case that variables are listed as (median, IQR), the number of observations with missing data for the respective variable is listed
In the POCC, we included 815 observations from 197 patients treated with ADA (4 median observations per patient (IQR 2–6) and 2197 observations from 231 patients treated with IFX (7 median observations per patient (IQR 2–16). Basic characteristics of these patients are listed in Supplementary Table 1.
USTE TLs and F-CPT
Analysis of All Observations
In a linear mixed model including all observations (N = 282), USTE TLs were not associated with logF-CPT levels (β −0.0133, 95% CI −0.0301–0.0033, p = 0.117). Neither induction phase, undetectable TLs, nor type of IBD (CD, UC, IBD-U) were associated with logF-CPT.
Analysis of Maintenance Observations Only
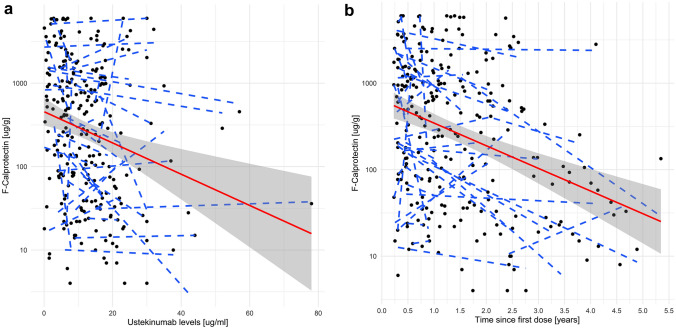
When considering only measurements taken during the maintenance period (N = 249) of the treatment, the relationship between USTE TLs and logF-CPT remained non-significant in the linear mixed model (β −0.0132, 95% CI −0.0304–0.0038, p = 0.13; Fig. 2a). However, in the categorical model, higher USTE maintenance TLs were significantly associated with F-CPT < 250 ug/g (odds ratio (OR) 1.024, 95% CI 1.022–1.026, p < 0.001) but not with F-CPT < 100 ug/g (OR 1.036, 95% CI 0.979–1.096, p = 0.219). Undetectable TLs of USTE were not associated with higher logF-CPT (β −0.0665, 95% CI −0.9054–0.7793, p = 0.877). Antibodies to USTE were not analysed owing to the low number of positive observations (five observations in four patients, all turned negative during subsequent measurements).
Fig. 2.
Relationship between USTE and F-CPT in the maintenance phase. a Relationship between USTE maintenance TLs and logF-CPT levels in the linear mixed model (β −0.0132, 95% CI −0.0304–0.0038, p = 0.13). b Relationship between length of USTE maintenance treatment (expressed in years) and logF-CPT levels in the linear mixed model (β −0.0010, 95% CI −0.0015 to −0.0006, p < 0.001). F-CPT faecal calprotectin; TLs trough levels; USTE ustekinumab
To analyse potential confounders, other factors were explored. First, we added time from the first USTE dose (length of treatment (LOT), expressed in days) into the model. When USTE TLs and LOT were adjusted for, only LOT was independently associated with lower F-CPT levels in both the linear (β −0.0010, 95% CI −0.0015 to −0.0006, p < 0.001; Fig. 2b) and categorical (OR 1.002, 95% CI 1–1.004, p = 0.004) models.
When we also added the age of the patient at the first USTE dose (tested in all maintenance observations, N = 249; final model, Table 3) and treatment intensification (higher dose and/or shorter interval; tested in observations where all these data were available, N =145; Supplementary Table 2a) into the model as independent factors, only the LOT remained strongly associated with lower logF-CPT (β −0.0010, 95% CI −0.0015 to −0.0006, p < 0.0001 and β −0.0010, 95% CI −0.0018 to −0.0003, p = 0.007, respectively). In other words, each additional week of USTE treatment duration was associated with a decrease of F-CPT levels by 5.6 ug/g (95% CI −10.8 to −0.7, p = 0.03; Supplementary Table 2b).
Table 3.
Final mixed model including USTE TLs, length of treatment and age of the patient at the first USTE dose
| Estimate for: logF-CPT | 95% CI | P-value | No. of observations | No. of patients | |
|---|---|---|---|---|---|
| Ustekinumab level (μg/mL) | −0.0096 | −0.0263–0.0069 | 0.26 | 249 | 43 |
| Length of the treatment (days) | −0.0010 | −0.0015 to −0.0006 | < 0.0001 | 249 | 43 |
| Age at the first ustekinumab dose | −0.0066 | −0.1573–0.144 | 0.93 | 249 | 43 |
CI confidence interval; F-CPT faecal calprotectin; TLs trough levels; USTE ustekinumab
VEDO TLs and F-CPT
Analysis of All Observations
In a linear mixed model including all observations (N = 359), VEDO TLs were associated with logF-CPT levels (β −0.0158, 95% CI −0.0267 to −0.0046, p = 0.005). Neither induction phase, undetectable TLs, nor type of IBD (CD, UC, IBD-U) were associated with logF-CPT.
Analysis of Maintenance Observations Only
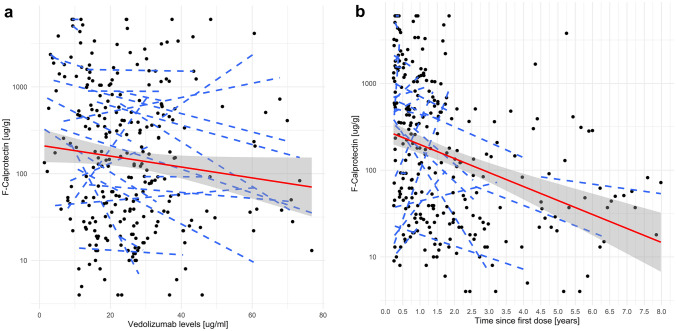
When considering only measurements taken during the maintenance period (N = 274) of the treatment, the relationship between VEDO TLs and logF-CPT remained significant in the linear mixed model (β −0.0173, 95% CI −0.0292 to −0.0053, p = 0.005; Fig. 3a) as well as in the categorical model with a cut-off of F-CPT < 250 ug/g (OR 1.03, 95% CI 1.005–1.057, p = 0.026) and with F-CPT < 100 ug/g (OR 1.03, 95% CI 1.005–1.057, p = 0.026). It was impossible to evaluate undetectable TLs of VEDO as no undetectable TLs were identified among our observations. Antibodies to VEDO were not analysed owing to the low number of positive observations (16 observations in 11 patients, all turned negative during subsequent measurements).
Fig. 3.
Relationship between VEDO and F-CPT in the maintenance phase. a Relationship between VEDO maintenance TLs and logF-CPT levels in the linear mixed model (β −0.0173, 95% CI −0.0292 to −0.0053, p = 0.005). b Relationship between length of VEDO maintenance treatment (expressed in years) and logF-CPT levels (β −0.0005, 95% CI −0.001–0, p = 0.066). F-CPT faecal calprotectin; TLs trough levels; VEDO vedolizumab
To analyse potential confounders, other factors were explored. First, we added LOT (expressed in days) into the model. When VEDO TLs and LOT were adjusted for, LOT was not independently associated with lower F-CPT levels in either linear (β −0.0005, 95% CI −0.001−0, p = 0.066; Fig. 3b) or categorical (OR 1.001, 95% CI 0.999–1.003, p = 0.205) models.
When we also added the age of the patient at the first VEDO dose (tested in all maintenance observations, N = 274; final model, Table 4) and treatment intensification into the model as independent factors (tested in observations where all these data were available, N =209; Supplementary Table 2c), only VEDO TLs remained significantly associated with lower logF-CPT (β −0.0156, 95% CI −0.0276 to −0.0033, p = 0.01 and β −0.0176, 95% CI −0.0307 to −0.0043, p = 0.01, respectively).
Table 4.
Final mixed model including VEDO TLs, length of treatment and age of the patient at the first VEDO dose
| VEDO dose | |||||
|---|---|---|---|---|---|
| Estimate for: logF-CPT | 95% CI | P-value | No. of observations | No. of patients | |
| Vedolizumab level (μg/mL) | −0.0156 | −0.0276 to −0.0033 | 0.01 | 274 | 33 |
| Length of the treatment (days) | −0.0005 | −0.001–0 | 0.06 | 274 | 33 |
| Age at the first vedolizumab dose | −0.0586 | −0.1857–0.0691 | 0.38 | 274 | 33 |
CI confidence interval; F-CPT faecal calprotectin; TLs trough levels; VEDO vedolizumab
To analyse whether there is an optimal VEDO TL that predicts F-CPT < 250 ug/g during the maintenance phase, a receiver operating characteristic (ROC) curve was constructed. A VEDO TL of 15.1 μg/mL appeared to have the best combination of sensitivity (0.82) and specificity (0.32). However, the overall predictive value was very poor (AUC 0.56, 95% CI 0.49–0.63; Supplementary Fig. 1a). Optimal VEDO TL predicting F-CPT < 100 ug/g during the maintenance phase was 22.1 μg/mL (sensitivity, 0.62; specificity, 0.51; AUC, 0.57; 95% CI 0.51–0.64; Supplementary Fig. 1b). Quartile analysis of VEDO TLs revealed no clear linear relationship with logF-CPT levels, and values across all quartiles greatly overlapped (Supplementary Fig. 2).
Disease Location and Immunomodulatory Treatment
As USTE was mainly used in CD and VEDO in patients with UC, we provide analysis limited to these phenotypes for respective treatments (owing to low number of observations in the other groups). For USTE, we evaluated L1 CD phenotype versus other locations and presence versus absence of perianal disease. Neither of these factors when added to the linear mixed model reached statistical significance. For VEDO we added analysis of the disease extension, where E1/E2 versus E3/E4 reached borderline significance when added to the linear mixed model being positively associated with higher F-CPT (β 1.915, 95% CI 0.1696–3.6621, p = 0.045). Immunomodulatory treatment did not show any significant association when added to the linear mixed model either for USTE or VEDO. Adding disease location or immunomodulatory treatment into the models did not change any previously identified relationships of USTE/VEDO TLs and log F-CPT mentioned above.
ADA and IFX—The POCC
To validate our conceptual framework and analytical methods for assessing the USTE and VEDO cohorts, we applied the same models to our cohort of patients treated with ADA and IFX. There was a strong association between ADA TLs and logF-CPT when tested in all observations (N = 815, β −0.0675, 95% CI −0.0975 to −0.0376, p < 0.0001); maintenance observations only (N = 410, β −0.0908, 95% CI −0.1346 to −0.0471, p < 0.0001; Supplementary Fig. 3a); and also in the categorical model with a cut-off for F-CPT < 250 ug/g (N = 410, OR 1.16, 95% CI 1.064–1.256, p = 0.001). The same association was found for IFX (N = 2197, β −0.0556, 95% CI −0.0701 to −0.0411, p < 0.0001; N = 1309, β −0.0805, 95% CI −0.0998 to −0.0612, p < 0.0001, Supplementary Fig. 4a; and N = 1309, OR 1.131, 95% CI 1.087−1.176, p < 0.001, respectively). The strong association remained in mixed models including LOT and age at the first drug dose (Supplementary Table 2d and 2e). Association of LOT and logF-CPT in the ADA and IFX cohort is shown in Supplementary Figs. 3b and 4b, respectively.
Discussion
This is the largest and most comprehensive prospective study to date describing the association between USTE and VEDO TLs and F-CPT in children as a primary endpoint. No paediatric study has shown a clear relationship of USTE serum levels and clinical or biochemical outcomes. Previously, only several small studies were performed in pIBD (mainly in paediatric patients with Crohn’s disease (pCD) showing that (1) substantial proportion of pIBD require USTE dose intensification, (2) intensified dosing is associated with greater TLs, (3) higher TLs are not clearly associated to objective markers of disease activity and (4) antibodies to USTE are very rarely detected [33–37]. The largest and most reliable USTE TDM data so far in pIBD were obtained within the Unistar trial—a phase 1, multicentre, 16-week, double-blind, induction dose-ranging RCT in 44 pCD who were randomized to low dose (130 mg IV, N = 23) versus high dose (390 mg IV, N = 21) induction. At week 8, all patients received a single SC USTE maintenance dose of 90 mg. Mean serum USTE concentrations were generally dose-proportional both in the lower and higher dose groups at several time points until week 8, and a greater proportion of patients were in clinical response in the higher USTE concentration group compared with the lower USTE concentration group at week 8. Similar trends were observed for median improvement from baseline in the Paediatric Crohn’s Disease Activity Index (PCDAI) score, but no correlation was observed between serum USTE concentration and clinical remission at week 8. No antibodies to USTE were detected throughout the study [38]. In the recently published long-term extension of the Unistar study, patients (N = 34) continued maintenance dosing every 8 weeks up to week 240. No relationships between serum USTE concentrations and clinical efficacy outcomes were observed at week 48. Although USTE concentrations tended to be lower in patients < 40 kg versus ≥ 40 kg, this difference did not appear to impact efficacy. Only descriptive results were presented, as the study was not designed to make any statistical comparisons [39]. Further data will be obtained from an ongoing phase 3 study evaluating the safety, efficacy and pharmacokinetics of USTE in pCD (ClinicalTrials.gov identifier: NCT04673357). In a CADMUS Jr phase III, open-label, single-arm, multicentre study of USTE in paediatric patients with moderate-to-severe plaque psoriasis, efficacy endpoints appeared to be associated with USTE TLs at week 40; however, detailed data are not presented [40]. Through week 52, the incidence of antibodies to USTE was 10%. A recently published multicentre retrospective study from the Paediatric IBD Porto Group of ESPGHAN evaluated USTE in 39 paediatric patients with UC and 19 with IBD-U (all had failed biologic therapies; 66% had failed two or more biologics). Corticosteroid-free clinical remission was observed in 47%, 57% and 64% children at 16, 26, and 52 weeks, respectively. Normalization of C-reactive protein (CRP) and F-CPT< 150 μg/g was achieved in 60% and 52%, respectively, by 52 weeks. Endoscopic and radiologic remissions were reached in 8% and 23%, respectively. USTE serum levels were not associated with disease activity. No antibodies to USTE were detected [41]. In our cohort with prospective multiple observations per patient, there was no independent association detected with F-CPT. Therefore, introducing TDM of USTE in pIBD does not seem reasonable, as it is not clinically useful and incurs additional costs. Neither measurement of antibodies to USTE is clinically useful, as the occurrence is consistently very low throughout all the published studies, and on top of that, recent case series failed to detect these antibodies even in patients with hypersensitivity reaction to IV USTE [42]. We have found a strong negative association of length of USTE treatment and F-CPT; however, this can be just a proxy marker of the phenomenon—that patients in remission on USTE tend to stay on the drug longer, without need of treatment discontinuation due to disease activity.
For VEDO, there are more data available. In the dose-ranging phase 2 HUBBLE trial evaluating pharmacokinetics, safety and efficacy of IV VEDO, pIBD (44 UC, 45 CD) were randomized to receive low- or high-dose vedolizumab (≥ 30 kg, 150 or 300 mg; < 30 kg, 100 or 200 mg) until week 14. The trough concentration was higher in each high-dose arm compared with the low-dose arms. At week 14, clinical responders with paediatric UC generally had higher TLs versus non-responders, while this trend was not observed in pCD. No analysis of VEDO TL association with F-CPT was performed. Anti-drug antibodies were detected in six patients (6.8%) [43]. Several observational studies on small numbers of pIBD show positive association between VEDO concentrations and favourable clinical, laboratory or endoscopic outcomes [44–47]. Despite efforts to establish a reasonable cut-off for optimal TLs, these cut-offs vary substantially between the studies, and no robust analysis of association of TLs and clinical or laboratory outcomes was routinely performed. Antibodies to VEDO were only rarely detected [16, 44–47]. On the contrary, in a recent study, VEDO TLs (198 samples of 50 pIBD) were neither associated with F-CPT levels both at 3 and 6 months (p = 0.188 and p = 0.859, respectively) nor with corticosteroid-free status of the patients [48]. In the so far largest paediatric prospective multicentre VEDOKIDS study to date (142 children; 65 CD), there was no association between VEDO serum levels and steroid-free and/or exclusive enteral nutrition-free clinical remission (SENFCR) at week 6 or 14. In children with a body weight of less than 30 kg, the optimal VEDO concentration associated with SENFCR at week 14 was 7 μg/mL (AUC 0.69, 95 % CI 0.41–0.98) [49]. In the follow-up of the VEDOKIDS cohort, serum samples for drug concentrations and antibodies were available for 73 (53%) children at the week 54 visit (median drug concentrations 12.1 μg/mL (IQR 5.6–20.7)). No association was shown between drug concentrations and sustained SENFCR. No antibodies to VEDO were detected [50]. A pharmacokinetic model on 312 serum VEDO concentrations from 129 children from the VEDOKIDS study has shown that increased baseline drug clearance was associated with lower deep biochemical remission rates at week 30 on the basis of F-CPT < 100 μg/g (OR 0.13, 95% CI 0.1–0.79) and < 250 μg/g (OR 0.15, 95% CI 0.04–0.5). Higher body weight and lower serum albumin were associated with increased clearance (p < 0.001) [51].Thus, despite the paucity of data and inconsistent results of studies concerning associations of VEDO TLs and disease outcomes, it seems that larger studies show some relationship, consistent with findings from our prospective observation. Despite the fact that in VEDO-treated pIBD the association of higher VEDO TLs with better disease outcomes seems to be more evident than in USTE, performing proactive TDM in VEDO-treated pIBD still remains questionable as optimal TLs during maintenance therapy cannot be clearly established at the moment and vary between studies, methods used and outcomes. The AUCs of the ROCs of VEDO TL that predict both F-CPT < 250 ug/g or F-CPT < 100 ug/g presented in our study are very low mainly due to the low specificity. This makes establishing the optimal cut-off value challenging. In addition, the saturation effect of VEDO might play a role in the relationship between VEDO dosing and clinical and/or laboratory effect [52, 53]. This supports the theory that the dose-response relationship is not linear.
The strengths of our study are inclusion of a high number of prospectively collected repeated measurements during both induction and maintenance therapy in pIBD, using F-CPT level as an objective marker of disease activity, collection of clinical data from a nationwide prospective registry and standardization of a clinical approach to our patients within a single pIBD centre. It is also noteworthy that our methodology has been validated in a substantial cohort of patients undergoing treatment with anti-TNF agents.
However, not all analyses could be performed in detail owing to missing data, especially from visits when only application of the drug was performed, without full clinical evaluation of the patient. As information about response or failure; active or inactive disease; erythrocyte sedimentation rate (ESR); and CRP was not available for all observations, exclusive evaluation of the association between TLs and F-CPT levels was used as primary outcome. We are aware that this approach may not fully represent the entire usual clinical scenario and decision-making process. The decision to change the therapy should be guided by a complex approach based on laboratory as well as clinical markers at the respective time point. In addition, the decision-sharing process between the treating physician, patient and caregivers should also be implemented with respect to patient-oriented outcomes.
Conclusions
USTE TLs do not appear to be associated with F-CPT levels, while VEDO shows stronger evidence of association, but it is difficult to define useful TLs cut-offs. However, in both drugs, it seems that, at this time, performing routine TDM is not justified. Anti-drug antibodies are rarely detected in both USTE and VEDO and probably do not play a significant clinical role. From this point of view, we consider regular monitoring of antibodies to either of the drugs as not relevant.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
Open access publishing supported by the institutions participating in the CzechELib Transformative Agreement. This work was supported by the Ministry of Health, Czech Republic, for conceptual development of research organizations [00064203/6001, University Hospital Motol, Prague, Czech Republic], and GAUK 227023.
Conflict of Interests
J.B.: lectures/congress fees/consultancy (outside submitted work)—MSD, AbbVie, Nutricia, Nestlé, Sanofi, Pfizer and Vitabalans; K.Z: lectures/congress fees/consultancy (outside submitted work)—Nestlé and ProMed; M.D.: congress fees (outside submitted work)—Nutricia; D.K.: lectures/workshop fee (outside submitted work)—Takeda and Vitabalans; M.K.: congress fees (outside submitted work)—Fresenius and Accord; T.L.: lectures/congress fees/consultancy (outside submitted work)—Accord, Nutricia, Biocodex and Ferring; K.M.: lectures/consultancy (outside submitted work)—Eli-Lilly and Takeda; E.V.: congress fees (outside submitted work)—Ewopharma; O.H.: lectures/congress fees/consultancy (outside submitted work)—MSD, AbbVie, Takeda, Sandoz, Lilly, Nutricia and Pfizer; I.C., J.Du., and J.Do. report no conflicts of interest.
Availability of Data and Material
Full data available on request.
Ethics Approval
The study was approved by the local ethics committee (EK, 662/23).
Consent for Participation and Publication of Data
Parents of all patients provided written informed consent before inclusion in the study and publication of the data.
Code Availability
Full statistical code available on request.
Author Contributions
J.B. (guarantor of the article)—study design, literature search, review of literature, data analysis, drafting of the manuscript and project supervision; O.H.—study design, acquisition of data, statistical analysis and revision of manuscript draft; K.Z., I.C., M.D., D.K., M.K., T.L., K.M., E.V., J.Du., J.Do.—study design, acquisition of data and revision of manuscript draft. All authors read and approved the final version of the manuscript.
References
- 1.Turner D, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257–91. [DOI] [PubMed] [Google Scholar]
- 2.van Rheenen PF, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2020;15(2):171–94. [DOI] [PubMed] [Google Scholar]
- 3.An overview of Stelara and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/stelara-epar-medicine-overview_en.pdf. An overview of Stelara and why it is authorised in the EU. https://www.ema.europa.eu/en/documents/overview/entyvio-epar-summary-public_en.pdf. Accessed 22 May 2025.
- 4.Cai XC, et al. Efficacy and safety of biological agents for the treatment of pediatric patients with psoriasis: a Bayesian analysis of six high-quality randomized controlled trials. Front Immunol. 2022;13: 896550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Commission approves STELARA® (ustekinumab) for the treatment of moderately to severely active Crohn’s disease in paediatric patients. https://innovativemedicine.jnj.com/emea/european-commission-approves-stelara-ustekinumab-for-the-treatment-of-moderately-to-severely-active-crohns-disease-in-paediatric-patients. Accessed 22 May 2025.
- 6.Classen M, Hoerning A. Current role of monoclonal antibody therapy in pediatric IBD: a special focus on therapeutic drug monitoring and treat-to-target strategies. Children (Basel). 2023;10(4):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dolinger MT, et al. Outcomes of children with inflammatory bowel disease who develop anti-tumour necrosis factor-induced skin reactions. J Crohns Colitis. 2022;16(9):1420–7. [DOI] [PubMed] [Google Scholar]
- 8.Fang S, et al. Effectiveness and safety of ustekinumab for pediatric inflammatory bowel disease: a systematic review. Paediatr Drugs. 2023;25(5):499–513. [DOI] [PubMed] [Google Scholar]
- 9.Hradsky O, et al. Sustainability of biologic treatment in paediatric patients with Crohn’s disease: population-based registry analysis. Pediatr Res. 2024;96(5):1283–91. [DOI] [PubMed] [Google Scholar]
- 10.Pujol-Muncunill G, et al. STEP-CD study: ustekinumab use in paediatric Crohn’s disease—a multicentre retrospective study from paediatric IBD Porto Group of ESPGHAN. Eur J Pediatr. 2024;183(8):3253–62. [DOI] [PubMed] [Google Scholar]
- 11.Yerushalmy-Feler A, et al. Safety and potential efficacy of escalating dose of ustekinumab in pediatric Crohn disease (the speed-up study): a multicenter study from the pediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2022;75(6):717–23. [DOI] [PubMed] [Google Scholar]
- 12.Atia O, et al. Children included in randomised controlled trials of biologics in inflammatory bowel diseases do not represent the real-world patient mix. Aliment Pharmacol Ther. 2022;56(5):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Romero R, et al. Safety and effectiveness of vedolizumab in paediatric patients with inflammatory bowel disease: an observational multicentre Spanish study. Eur J Pediatr. 2021;180(9):3029–38. [DOI] [PubMed] [Google Scholar]
- 14.Choi S, et al. Vedolizumab is safe and efficacious for the treatment of pediatric-onset inflammatory bowel disease patients who fail a primary biologic agent. J Korean Med Sci. 2022;37(37): e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakiuchi T, Yoshiura M. Vedolizumab as the first-line of biologicals for pediatric patients with ulcerative colitis. Clin Ther. 2022;44(7):1028–32. [DOI] [PubMed] [Google Scholar]
- 16.Patel H, Karam L, Kellermayer R. A single-center study of long-term effectiveness of vedolizumab in anti-tnf refractory pediatric inflammatory bowel disease. JPGN Rep. 2023;4(1): e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider AM, et al. Vedolizumab use after failure of TNF-alpha antagonists in children and adolescents with inflammatory bowel disease. BMC Gastroenterol. 2018;18(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokoyama K, et al. Safety and efficacy of vedolizumab in pediatric patients with ulcerative colitis: multicenter study in Japan. J Gastroenterol Hepatol. 2023;38(7):1107–15. [DOI] [PubMed] [Google Scholar]
- 19.Jongsma MME, et al. Infliximab in young paediatric IBD patients: it is all about the dosing. Eur J Pediatr. 2020;179(12):1935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsoud D, Vermeire S, Verstockt B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: hype or hope? Curr Opin Pharmacol. 2020;55:17–30. [DOI] [PubMed] [Google Scholar]
- 21.Carman N, Mack DR, Benchimol EI. Therapeutic drug monitoring in pediatric inflammatory bowel disease. Curr Gastroenterol Rep. 2018;20(5):18. [DOI] [PubMed] [Google Scholar]
- 22.Dutt K, Vasudevan A. Therapeutic drug monitoring for biologic and small-molecule therapies for inflammatory bowel disease. Medicina (Kaunas). 2024;60(2):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nassar IO, et al. Proposed pathway for therapeutic drug monitoring and dose escalation of vedolizumab. Frontline Gastroenterol. 2022;13(5):430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019;17(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasudevan A, et al. Systematic review and meta-analysis: the association between serum ustekinumab trough concentrations and treatment response in inflammatory bowel disease. Inflamm Bowel Dis. 2024;30(4):660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vootukuru N, Vasudevan A. Approach to loss of response to advanced therapies in inflammatory bowel disease. World J Gastroenterol. 2024;30(22):2902–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward MG, Sparrow MP, Roblin X. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Ther Adv Gastroenterol. 2018;11:1756284818772786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yacoub W, et al. Early vedolizumab trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther. 2018;47(7):906–12. [DOI] [PubMed] [Google Scholar]
- 29.Raine T, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17. [DOI] [PubMed] [Google Scholar]
- 30.Torres J, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4–22. [DOI] [PubMed] [Google Scholar]
- 31.Turner D, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. [DOI] [PubMed] [Google Scholar]
- 32.Levine A, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795–806. [DOI] [PubMed] [Google Scholar]
- 33.Bouhuys M, Mian P, van Rheenen PF. Ustekinumab trough levels in children with Crohn’s disease refractory to anti-tumor necrosis factor agents: a prospective case series of off-label use. Front Pharmacol. 2023;14:1180750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dayan JR, et al. Real world experience with ustekinumab in children and young adults at a tertiary care pediatric inflammatory bowel disease center. J Pediatr Gastroenterol Nutr. 2019;69(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhaliwal J, et al. One-year outcomes with ustekinumab therapy in infliximab-refractory paediatric ulcerative colitis: a multicentre prospective study. Aliment Pharmacol Ther. 2021;53(12):1300–8. [DOI] [PubMed] [Google Scholar]
- 36.Do P, et al. Augmented ustekinumab dosing is needed to achieve clinical response in patients with anti-TNF refractory pediatric Crohn’s disease: a retrospective chart review. F1000Res. 2020;9:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim FS, et al. Experience using ustekinumab in pediatric patients with medically refractory Crohn disease. J Pediatr Gastroenterol Nutr. 2021;73(5):610–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosh JR, et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: pharmacokinetics, safety, and efficacy results from UniStar, a phase 1 study. J Crohns Colitis. 2021;15(11):1931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner D, et al. Ustekinumab in paediatric patients with moderately to severely active Crohn’s disease: UniStar study long-term extension results. J Pediatr Gastroenterol Nutr. 2024;79(2):315–24. [DOI] [PubMed] [Google Scholar]
- 40.Philipp S, et al. Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥ 6 to < 12 years of age): efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS Jr study. Br J Dermatol. 2020;183(4):664–72. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S, et al. Effectiveness and safety of ustekinumab in pediatric ulcerative colitis: a multi-center retrospective study from the pediatric IBD Porto Group of ESPGHAN. Paediatr Drugs. 2024;26(5):609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunny J, et al. Hypersensitivity Reaction to ustekinumab in pediatric and young adult inflammatory bowel disease patients: a case series. JPGN Rep. 2022;3(2): e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyams JS, et al. Pharmacokinetics, safety and efficacy of intravenous vedolizumab in paediatric patients with ulcerative colitis or Crohn’s disease: results from the phase 2 HUBBLE study. J Crohns Colitis. 2022;16(8):1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aardoom MA, et al. Vedolizumab trough levels in children with anti-tumor necrosis factor refractory inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2020;71(4):501–7. [DOI] [PubMed] [Google Scholar]
- 45.Colman RJ, et al. Real world population pharmacokinetic study in children and young adults with inflammatory bowel disease discovers novel blood and stool microbial predictors of vedolizumab clearance. Aliment Pharmacol Ther. 2023;57(5):524–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowland P, et al. Proactive therapeutic drug monitoring and vedolizumab dose optimization in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2024;78(4):853–61. [DOI] [PubMed] [Google Scholar]
- 47.Ungaro RC, et al. Higher trough vedolizumab concentrations during maintenance therapy are associated with corticosteroid-free remission in inflammatory bowel disease. J Crohns Colitis. 2019;13(8):963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hemming-Harlo M, et al. Drug levels of VEDOLIZUMAB in patients with pediatric-onset inflammatory bowel disease in a real-life setting. Eur J Pediatr. 2024;183(1):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atia O, et al. Outcomes, dosing, and predictors of vedolizumab treatment in children with inflammatory bowel disease (VEDOKIDS): a prospective, multicentre cohort study. Lancet Gastroenterol Hepatol. 2023;8(1):31–42. [DOI] [PubMed] [Google Scholar]
- 50.Atia O, et al. Maintenance treatment with vedolizumab in paediatric inflammatory bowel disease (VEDOKIDS): 54-week outcomes of a multicentre, prospective, cohort study. Lancet Gastroenterol Hepatol. 2025;10(3):234–47. [DOI] [PubMed] [Google Scholar]
- 51.Stein R, et al. Baseline drug clearance predicts outcomes in children with inflammatory bowel disease treated with vedolizumab: results from the vedokids prospective multicentre study. Aliment Pharmacol Ther. 2025;61(6):1000–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Outtier A, et al. Effect of vedolizumab dose intensification on serum drug concentrations and regain of response in inflammatory bowel disease patients with secondary loss of response. GastroHep. 2021;3(2):63–71. [Google Scholar]
- 53.Ungar B, et al. Association of vedolizumab level, anti-drug antibodies, and alpha4beta7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(5):697-705e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.