Highlights
-
•
ACTB fragmentomics represents a reliable surrogate for tumor dynamics in first-line HR+ HER2- MBC
-
•
High ACTBshort levels negatively impact both progression-free survival and overall survival.
-
•
ACTBmedium fragments show a favorable impact on progression-free survival.
-
•
ACTB fragmentomics may represent a mutation-agnostic, early risk stratification tool for MBC monitoring.
Keywords: Metastatic breast cancer, Liquid biopsy, Fragmentomics, Circulating tumor DNA
Abstract
Background
In the context of hormone receptor positive, HER2 negative Metastatic breast cancer (MBC), CDK 4/6 inhibitors (CDK4/6i) combined with endocrine therapy represent the standard first-line treatment, improving Progression-Free Survival (PFS) and Overall Survival (OS). Despite these benefits, resistance to treatment develops, necessitating early risk classification to guide clinical management. This study explores the potential of cell-free DNA (cfDNA) fragmentomics, specifically ACTB fragments, in predicting tumor dynamics and treatment outcomes in luminal MBC, based on the principle that shorter DNA fragments are generally indicative of circulating tumor DNA (ctDNA) from tumor cells, while longer fragments are associated with leukocyte lysis.
Methods
In the MAGNETIC.1 study, 141 women with luminal-like MBC were enrolled between January 2018 and January 2023. Blood samples were collected at baseline (BL), and after 3 (T3) and 6 (T6) months of treatment. cfDNA was extracted and analyzed using droplet digital PCR (ddPCR) to quantify ACTB fragments (136 bp, 420 bp, and 2,000 bp). Continuous variables were compared using the Mann–Whitney test and Kruskall Wallis test depending on data distribution and number of groups. Categorical variables were compared using the Chi-square test or Fischer’s exact test whenever appropriate. Differences in survival were tested by log-rank test and uni- and multivariable Cox regression.
Results
By categorizing the values of actinic fragments into interquartiles (Q1, Q2, and Q3), ACTBshort Q3 at baseline was significantly associated with negative PR expression (RRR 0.27, P = 0.012) and a higher frequency of liver metastasis (RRR = 3.75, P = 0.009). In terms of clinical outcomes, regarding PFS a significant role was observed for baseline ACTBshort Q3 (HR 1.92, P = 0.041) and ACTBmedium Q3 (HR 0.47, P = 0.043), the latter maintaining significance in multivariable analysis (HR 0.33, 95 %, P = 0.012). For OS, ACTBshort Q3 demonstrated a significant impact in both univariable (HR 3.94, P = 0.003) and multivariable analyses (HR 3.25, P = 0.023).
Conclusions
This study demonstrates the feasibility of employing a fragmentomics mutation agnostic approach in luminal MBC. Baseline and longitudinal changes in ACTB fragments were significantly associated with clinical outcomes, suggesting their potential as non-invasive biomarkers for early risk classification and monitoring tumor dynamics.
Background
Metastatic breast cancer is the leading cause of cancer-related deaths among women, resulting in over 600,000 fatalities annually [1]. The luminal subtype accounts for more than 70 % of MBC diagnoses. In this context, the combination of CDK 4/6 inhibitors and endocrine therapy as a first-line treatment has been shown to significantly improve PFS and, in some cases, overall survival OS [2]. However, nearly all patients eventually develop resistance to treatment, with 10–30 % developing primary resistance, emphasizing the importance of early risk classification to guide clinical management [3,4].
Extensive efforts have been dedicated to understanding the mechanisms underlying this resistance, aiming to predict tumor dynamics and guide treatment choices in subsequent lines. Liquid biopsy is increasingly emerging as a crucial non-invasive tool for assessing tumor biology both statically and longitudinally, as well as for evaluating treatment responses. To date, the analysis of circulating tumor DNA (ctDNA) and Circulating Tumor Cells (CTCs) are the primary parameters explored for real-time early cancer detection, monitoring minimal residual disease, and longitudinally tracking clonal evolution in peripheral blood [5]. However, the challenge of identifying mutations that are not always detectable in plasma for ctDNA analysis, combined with the difficulties of analyzing CTCs due to their low abundance and associated costs, continues to make these approaches difficult to implement in all patients.
In this context, the study of cell-free DNA fragmentation patterns, known as fragmentomics, is progressively recognized as a crucial, mutation-agnostic tool for the longitudinal analysis of tumor evolution [6]. Our previous work has utilized a fragmentomics approach to assess the proportion of ctDNA by analyzing ACTB DNA fragments, categorized into lengths of 136 bp, 420 bp, and 2,000 bp and termed ACTBshort, ACTBmedium, and ACTBlong, respectively [7]. The underlying principle of this approach is that plasma DNA stems from distinct biological processes: short fragments are typically associated with ctDNA and reflect fragmentation patterns characteristic of tumor cell release, whereas medium and long fragments are generally attributed to genomic DNA (gDNA) released from non-tumoral cells, such as leukocytes, through cellular lysis [8,9]. This fragment-based stratification may therefore provide indirect yet informative insights into tumor burden and its dynamic changes during treatment.
In this study, we validated our ddPCR fragmentomics pathway and further explored it for the longitudinal profiling of first-line luminal MBC. By leveraging a more mature dataset, with a larger patient cohort and an extended follow-up period, this analysis enables a more comprehensive examination of the distribution and dynamics of ACTB fragments, uncovering their biological significance and their potential prognostic value in both PFS and overall survival OS.
Materials and methods
Study population and ethics statement
A cohort of 141 women was prospectively enrolled in the MAGNETIC.1 (NCT05814224) multicenter pragmatic study, between January 2018 and January 2023. All patients were diagnosed with luminal-like MBC and received either fulvestrant or aromatase inhibitors (Ais) with or without CDK4/6i as first-line ET according to the investigator’s choice. The two main exclusion criteria were a diagnosis of any secondary malignancy within the last three years and prior ET for MBC. Patients could have received both ET and chemotherapy in the adjuvant and neoadjuvant setting. Samples of 134 patients were collected before treatment start (BL) and after 3 and 6 months concomitantly with computed tomography (CT) scan restaging [first (T3) and second (T6) evaluation, respectively]. The study was approved by the ethics committee under the CEUR-2018-Sper-056-CRO protocol.
Extraction of circulating tumor DNA from plasma samples
Blood samples were collected using the PAXgene Blood ccfDNA Tubes (Qiagen) or the Cell-Free DNA BCT tubes (Streck). ctDNA was isolated from 4.8 ml of plasma with the QIAsymphony PAXgene Blood ccfDNA Kit (PreAnalytiX) through the QIAsymphony SP instrument (Qiagen) using the recommended Standard Protocol Line (STA) for small fragment enrichment and eluted in 60 μl of elution buffer (Qiagen). ctDNA concentration was estimated using the Qubit 1X dsDNA HS Assay Kit (Qiagen).
Droplet digital PCR
Circulating free DNA (cfDNA) samples were analyzed for cytolysis-derived contamination and ctDNA quantification by ddPCR though the detection of small (136 bp), medium (420 bp) and long (2000 bp) ACTB fragments. The presence of short ACTB is associated with ctDNA, while the medium and long fragments are associated with gDNA [5]. Based on this biological rationale, 5’ 6-FAM- conjugated short actin and 5’ HEX-conjugated medium actin assay (Bio-Rad) were used. Samples were run in duplicate and then merged for further analysis. Droplets were generated on the automated droplet generator QX200 AutoDG™ (Bio-Rad) and after PCR amplification, droplets were read on the QX200™ Droplet Reader (Bio-Rad). Data were analyzed using the QuantaSoft™ 1.7.4 Software (Bio-Rad). Based on FAM and HEX probe fluorophores, short actin is Ch1+Ch2- (FAM), medium actin is Ch1-Ch2+ (HEX) and long actin is Ch1+Ch2+ (positive for FAM and HEX) were considered for analysis.
Statistical analysis
Clinical and pathologic variables were reported using descriptive analyses. Categorical variables were reported as frequency distributions, whereas continuous variables were described through median and interquartile ranges (Qs). Continuous variables were compared using the Mann–Whitney test and Kruskall Wallis test depending on data distribution and the number of groups. Categorical variables were compared using the Chi-square test or Fischer’s exact test, as appropriate.
Variations over time of short actin fragments, medium actin fragments and long actin fragments were evaluated with generalized linear mixed-effects models (LME) for repeated measures. Multiple comparisons of parameters’ values across different follow-up periods (baseline, 3 months, and 6 months) were performed using the Friedman test for repeated measures, with p-values adjusted using the Bonferroni post-hoc test. A multivariate multinomial logistic regression model was adopted to assess the association between clinical factors (independent variables) and actin fragments (dependent variable, coded as, Q1,Q2 and Q3 considering Q2 as the reference category). The regression coefficients are expressed as relative risk ratio (RRR).
PFS was defined as the time from BL to progression (as determined by imaging) or death for any cause, whichever occurred first. OS was defined as the time from BL until death from any cause. Patients without an end point event at the last follow-up visit were censored. The statistical significance of associations between individual variables and survival was calculated using the log-rank test. Results are expressed as hazard ratios (HRs) and 95 % confidence intervals (95 % CIs).
Statistical analysis was conducted using StataCorp 2016 Stata Statistical Software: Release 15.1 (College Station, TX), R (version 3.3.1; The R foundation for Statistical Computing, Vienna, Austria) and JMP (version 14; SAS Institute, Cary, NC).
Results
A cohort of 134 patients with luminal-like MBC treated with first-line ET was prospectively enrolled between May 2018 and January 2023. Of the 134 patients, 24 % had progesterone receptor (PR)-negative MBC, while the proportion of HER2-negative and HER2-low disease was 35 % and 65 %, respectively. Bone was the most common metastatic site (70 %), followed by lymph nodes (54 %) and liver (28 %). Sixty-two percent of patients had a de novo diagnosis of MBC. The first-line ET regimen was mainly based on AIs (72 %) compared to fulvestrant, which was used in 28 % of cases. The proportion of patients who received a CDK 4/6 inhibitor was 96.26 %, with palbociclib being the most frequently administered (47.29 %), followed by ribociclib (38.76 %) and abemaciclib (13.95 %) (Table 1).
Table 1.
Cohort characteristics; missing data were not reported.
| N | % | ACTBshort | Chi2 | ACTBmedium | Chi2 | ACTBlong | Chi2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–25 | 25–75 | >75 | 0–25 | 25–75 | >75 | 0–25 | 25–75 | >75 | ||||||
| Histotype | ||||||||||||||
| IDC | 101 | 83.47 | 25 (26.04 %) | 49 (51.04 %) | 22 (22.92 %) | 0.515 | 24 (25 %) | 48 (50 %) | 24 (25 %) | 0.310 | 25 (26.04 %) | 47 (48.96 %) | 24 (25 %) | 0.566 |
| ILC | 20 | 16.53 | 6 (31.58 %) | 7 (36.84 %) | 6 (31.58 %) | 4 (21.05 %) | 7 (36.84 %) | 8 (42.11 %) | 4 (21.05 %) | 8 (42.11 %) | 7 (36.84 %) | |||
| PR | ||||||||||||||
| Negative | 31 | 23.48 | 6 (20 %) | 11 (36.67 %) | 12 (43.33 %) | 0.035 | 6 (20 %) | 15 (50 %) | 9 (30 %) | 0.891 | 3 (10 %) | 16 (53.33 %) | 11 (36.67 %) | 0.097 |
| Positive | 101 | 76.52 | 25 (26.04 %) | 52 (54.17 %) | 19 (19.79 %) | 23 (23.02 %) | 47 (48.96 %) | 26 (27.08 %) | 27 (28.12 %) | 46 (47.92 %) | 23 (23.96 %) | |||
| Lung | ||||||||||||||
| Yes | 32 | 24.06 | 5 (16.67 %) | 19 (63.33 %) | 6 (20 %) | 0.218 | 9 (30 %) | 14 (46.67 %) | 7 (23.33 %) | 0.616 | 6 (20 %) | 16 (53.33 %) | 8 (26.67 %) | 0.788 |
| No | 101 | 75.94 | 27 (27.84 %) | 44 (45.36 %) | 26 (26.80 %) | 21 (21.65 %) | 48 (49.48 %) | 28 (28.87 %) | 25 (25.77 %) | 46 (47.42 %) | 26 (26.80 %) | |||
| Liver | ||||||||||||||
| Yes | 37 | 27.82 | 11 (29.73 %) | 12 (32.43 %) | 14 (37.84 %) | 0.033 | 11 (29.73 %) | 19 (51.35 %) | 7 (18.92 %) | 0.315 | 12 (32.43 %) | 14 (37.84 %) | 11 (29.73 %) | 0.242 |
| No | 96 | 72.18 | 21 (23.33 %) | 51 (56.67 %) | 18 (20 %) |
19 (21.11 %) | 43 (47.78 %) | 28 (31.11 %) | 19 (21.11 %) | 48 (53.33 %) | 23 (25.56 %) | |||
| Bone | ||||||||||||||
| Yes | 93 | 69.92 | 24 (26.97 %) | 41 (46.07 %) | 24 (26.97 %) | 0.475 | 23 (25.84 %) | 39 (43.82 %) | 27 (30.34 %) | 0.226 | 24 (26.97 %) | 41 (46.07 %) | 24 (26.97 %) | 0.532 |
| No | 40 | 30.08 | 8 (21.05 %) | 22 (57.89 %) | 8 (21.05 %) | 7 (18.42 %) | 23 (60.53 %) | 8 (21.05 %) | 7 (18.42 %) | 21 (55.26 %) | 10 (26.32 %) | |||
| Node | ||||||||||||||
| Yes | 72 | 54.14 | 13 (18.57 %) | 37 (52.86 %) | 20 (28.57 %) | 0.153 | 17 (29.82 %) | 25 (43.86 %) | 15 (26.32 %) | 0.323 | 13 (18.57 %) | 35 (50 %) | 22 (31.43 %) | 0.175 |
| No | 61 | 45.86 | 19 (33.33 %) | 26 (45.61 %) | 12 (21.05 %) | 17 (29.82 %) | 25 (43.86 %) | 15 (26.32 %) | 18 (31.58 %) | 27 (47.37 %) | 12 (21.05 %) | |||
| CNS | ||||||||||||||
| Yes | 2 | 1.50 | 0 | 2 (100 %) | 0 | 0.356 | 0 | 1 (50 %) | 1(50 %) | 0.653 | 0 | 2 (100 %) | 0 | 0.345 |
| No | 131 | 98.50 | 32 (25.60 %) | 61 (48.80 %) | 32 (25.60 %) | 30 (24 %) | 61 (48.80 %) | 34 (27.20 %) | 31 (24.80 %) | 60 (48 %) | 34 (27.20 %) | |||
| De Novo | ||||||||||||||
| Yes | 60 | 61.86 | 7 (19.44 %) | 20 (55.66 %) | 9 (25 %) | 0.280 | 3 (8.33 %) | 20 (55.56 %) | 13 (36.11 %) | 0.163 | 5 (13.89 %) | 20 (55.56 %) | 11 (30.56 %) | 0.301 |
| No | 37 | 38.14 | 20 (33.90 %) | 29 (49.15 %) | 10 (16.95 %) | 14 (23.73 %) | 28 (47.46 %) | 17 (28.81 %) | 16 (27.12 %) | 26 (44.07 %) | 17 (28.81 %) | |||
| Type of CDK | ||||||||||||||
| Palbociclib | 61 | 47.29 | 21 (35.59 %) | 32 (54.24 %) | 6 (10.17 %) | <0.001 | 15 (25.42 %) | 35 (59.32 %) | 9 (15.25 %) | 0.035 | 14 (23.73 %) | 38 (64.41 %) | 7 (11.86 %) | <0.001 |
| Ribociclib | 50 | 38.76 | 5 (10.42 %) | 25 (52.08 %) | 18 (37.50 %) | 9 (18.75 %) | 19 (39.58 %) | 20 (41.67 %) | 8 (16.67 %) | 18 (37.50 %) | 22 (45.83 %) | |||
| Abemaciclib | 18 | 13.95 | 5 (31.25 %) | 25 (31.25 %) | 6 (37.50 %) | 5 (31.25 %) | 6 (37.50 %) | 5 (31.25 %) | 9 (56.25 %) | 3 (18.75 %) | 4 (25 %) | |||
| Endocrine companion | ||||||||||||||
| Fulvestrant | 38 | 28.15 | 11 (30.56 %) | 16 (44.44 %) | 9 (25 %) | 0.629 | 11 (30.56 %) | 18 (50 %) | 7 (19.44 %) | 0.368 | 11 (30.56 %) | 17 (47.22 %) | 8 (22.22 %) | 0.542 |
| AI | 97 | 71.85 | 21 (22.83 %) | 48 (52.17 %) | 23 (25 %) | 20 (21.74 %) | 44 (47.83 %) | 28 (30.43 %) | 20 (21.74 %) | 46 (50 %) | 26 (28.26 %) | |||
ACTB segments are associated with baseline clinical characteristics
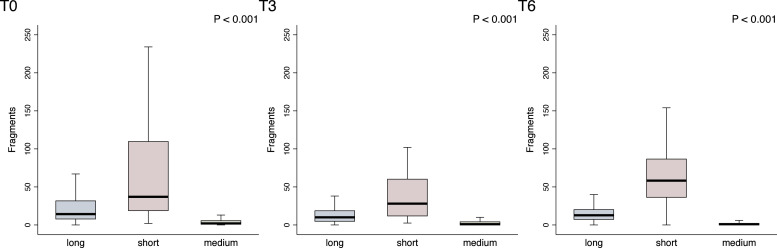
At baseline the median levels were 37 copies for ACTBshort (IQR 18.5–110), 2 (Q 1–6) for ACTBmedium and 14.2 (IQR 7.5–32) for ACTBlong (Supplementary Table 1). After 3 months (T3), median levels were 28 (IQR 11.5–60.5) for ACTBshort, 1 (IQR 0.5–4.5) for ACTBmedium, and 10 (IQR 4.5–19) for ACTBlong (Supplementary Table 1). At 6 months (T6), the median levels were 58.25 (IQR 36–87) for ACTBshort, 1 (IQR 0–2.5) for ACTBmedium, and 12.75 (IQR 7–20.5) for ACTBlong (Supplementary Table 1). Significant differences were observed among ACTBshort, ACTBmedium, and ACTBlong in the general population at baseline (P < 0.001), at T3 (P < 0.001), and T6 (P < 0.001) (Fig. 1).
Fig. 1.
ACTBlong(long) ACTBshort (short), ACTBmedium (medium) fragments distribution across the three investigated timepoints (T0, T3, T6). Median, interquartile range, and outliers are described for the overall biomarker distribution at each timepoint through box plots. Outliers were removed. Abbreviations: T0, baseline; T3, after 3 months; T6, after 6 months.
By categorizing the values of actinic fragments into interquartiles (0–25, 25–75, 75–100, respectively Q1, Q2, and Q3) and assessing their distribution in relation to clinical variables, a significant difference was observed for ACTBshort concerning PR status (P = 0.035) and liver metastases (P=0.033), and for ACTBshort, ACTBmedium, and ACTBlong with regards to the type of CDKi (P<0.001, P = 0.035, P < 0.001, respectively) (Table 1).
The association between clinical and molecular features across actin fragments Q was further explored through uni- and multi-variable multinomial logistic regression analyses. In the univariable analysis, concerning the intermediate group (Q2), ACTBshort Q3 was significantly associated with negative PR expression (Relative Risk Ratio [RRR]=0.31, P = 0.017) and a higher risk of liver metastasis (RRR 3.31, P = 0.013) (Supplementary Table 2). This association was also confirmed in the multivariable model (respectively RRR 0.27, P = 0.012 and RRR = 3.75, P = 0.009) (Table 2). No significant association was observed in relation to Actinmedium and Actinlong fragments (Supplementary Tables 3 and 4).
Table 2.
Multivariable multinomial logistic regression for Actinshort Q1 and Actinshort Q3. Actinshort Q2 was considered as base subgroup. Abbreviations: PR=Progesteron Receptor, Q= Interquartile Range.
| Relative Risk Ratio | 95 % Confidence interval | P | ||
|---|---|---|---|---|
| Q1 | ||||
| PR | 0.84 | 0.28 | 2.54 | 0.754 |
| Liver metastases | 2.04 | 0.76 | 5.47 | 0.154 |
| Q2 | ||||
| PR | 1.00 (Ref) | |||
| Liver metastases | 1.00 (Ref) | |||
| Q3 | ||||
| PR | 0.28 | 0.10 | 0.75 | 0.012 |
| Liver metastases | 3.65 | 1.38 | 9.69 | 0.009 |
ACTB dynamics across fragments subgroups after 3 and 6 months of treatment
We then analyzed the dynamics of actinic fragments across three timepoints. Median levels of ACTBshort significantly decreased between T3 and T0 (P < 0.001) and significantly increased between T6 and T3 (P < 0.001), while the reduction between T6 and T0 was not significant (P = 0.621) (Supplementary Fig. 1).
Median levels of ACTBmedium significantly decreased between T3 and T0 (P = 0.003) and T6 and T0 (P < 0.001), while the reduction between T6 and T3 was not significant (P = 0.220) (Supplementary Fig. 2).
Median levels of ACTBlong significantly decreased between T3 and T0 (P < 0.001), showed a non-significant increase between T6 and T3 (P = 0.645) and a non-significant reduction between T6 and T0 (P = 0.059) (Supplementary Fig. 3).
Sites of disease did not impact on the dynamics of ACTBshort, ACTBmedium and ACTBlong (Supplementary Tables 5–and 7).
Baseline levels of ACTBshort and ACTBmedium significantly impact on PFS and OS
The prognostic impact of ACTBshort, ACTBmedium, and ACTBlong, was then investigated in terms of PFS and OS after interquartile categorization, using the intermediate interquartile as reference.
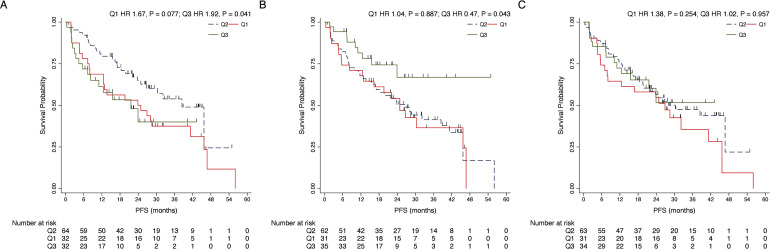
At baseline, across clinical variables, a significant impact on PFS emerged for lobular histotype (Hazard Ratio [HR] 2.23, 95 % Confidence Interval [CI] 1.19–4.17, P = 0.012) and the presence of liver metastases (HR 2.93, CI 1.79–4.78, P<0.001) (Table 3). Regarding ACTB fragments, an inverse significant role was observed for ACTBshort Q3 (HR 1.92, 95 % CI 1.03–3.59, P = 0.041) and ACTBmedium Q3 (HR 0.47, 95 % CI 0.23–0.97, P = 0.043) (Fig. 2, Table 3). ACTBshort Q1vs Q2 was associated with worse PFS but did not reach statistical significance (HR 1.67, CI 0.95–2.93 P = 0.077) (Fig. 2, Table 3). In multivariable analyses a significant impact on PFS was confirmed for lobular histotype (HR 3.04, CI 1.56–5.93, P = 0.001), liver metastases (HR 2.29, CI 1.33–3.97, P = 0.003) and ACTBmedium Q3 (HR 0.33, 95 % CI 0.12–0.76, P = 0.012) (Table 3). PFS at 12, 24 and 36 months for the respective interquartiles of ACTBshort, ACTBmedium and ACTBlong are reported in Table 5.
Table 3.
Univariate and Multivariate Cox regression analysis for Progression-Free Survival (PFS).
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p-value | HR | 95 % CI | p-value | |
| PR status | ||||||
| Negative | 1.00 (Ref) | |||||
| Positive | 0.61 | 0.35–1.06 | 0.081 | |||
| De novo MBC | ||||||
| Yes | 1.00 (Ref) | |||||
| No | 0.61 | 0.33–1.11 | 0.108 | |||
| Histotype | ||||||
| Ductal | 1.00 (Ref) | 1.00 (Ref) | ||||
| Lobular | 2.23 | 1.19- 4.17 | 0.012 | 3.04 | 1.56–5.93 | 0.001 |
| Liver Metastasis | ||||||
| No | 1.00 (Ref) | |||||
| Yes | 2.93 | 1.79–4.78 | <0.001 | 2.29 | 1.33–3.97 | 0.003 |
| Lung Metastasis | ||||||
| No | 1.00 (Ref) | |||||
| Yes | 0.57 | 0.30–1.08 | 0.085 | |||
| CNS Metastasis | ||||||
| No | not evaluable | |||||
| Yes | ||||||
| Bone Metastasis | ||||||
| No | 1.00 (Ref) | |||||
| Yes | 1.27 | 0.73–2.20 | 0.399 | |||
| Node Metastasis | ||||||
| No | 1.00 (Ref) | |||||
| Yes | 1.17 | 0.72–1.90 | 0.526 | |||
| ACTBshort | ||||||
| Q2 | 1.00 (Ref) | 1.00 (Ref) | ||||
| Q1 | 1.67 | 0.94–2.93 | 0.077 | 1.17 | 0.63–2.17 | 0.622 |
| Q3 | 1.92 | 1.03–3.59 | 0.041 | 1.97 | 0.93–4.19 | 0.079 |
| ACTBmedium | ||||||
| Q2 | 1.00 (Ref) | 1.00 (Ref) | ||||
| Q1 | 1.04 | 0.60–1.80 | 0.887 | 1.05 | 0.59–1.89 | 0.863 |
| Q3 | 0.46 | 0.23–0.97 | 0.043 | 0.33 | 0.14–0.78 | 0.012 |
| ACTBlong | ||||||
| Q2 | 1.00 (Ref) | |||||
| Q1 | 1.38 | 0.79–2.40 | 0.254 | |||
| Q3 | 1.01 | 0.53–1.95 | 0.957 | |||
Abbreviations: HR= Hazard Ratio, CI=Confidence Interval, PR=Progesteron Receptor, MBC= Metastatic Breast Cancer, ACTB=Actin fragments, Q= Interquartile Range.
Fig. 2.
Kaplan-Meier plots for the impact on PFS of different ACTBshort Qs (A), ACTBmedium Qs (B) and ACTBlong Qs (C). Relative to the intermediate quartile group (Q2), a significant prognostic impact was noted for ACTBshort Q3 (HR 1.92, P = 0.041) and ACTBmedium Q3 (HR 0.47, P = 0.043). Abbreviations: T0, baseline; T3, after 3 months; T6, after 6 months; Q, Interquartile range.
Table 5.
PFS at 12, 24 and 36 months for the respective interquartiles of ACTBshort, ACTBmedium and ACTBlong and the corresponding 95 % confidence intervals. Abbreviations: CI= 95 % Confidence intervals; Q= Interquartile Range; ACTB= Actin fragments.
| Progression free survival | 12 months | 24 months | 36 months |
|---|---|---|---|
| ACTBshort | |||
| Q1 | 71 % (CI 52 %–84 %) |
54 % (CI 35 %–70 %) |
37.5 % (CI 21 %–54 %) |
| Q2 | 79.5 % (CI 67 %–88 %) |
64.8 % (CI 56 %–71 %) |
53.8 % (CI 39 %–67 %) |
| Q3 | 61.4 % (CI 42 %–76 %) |
40 % (CI 19 %–60 %) |
40 % (CI 19 %–60 %) |
| ACTBmedium | |||
| Q1 | 67.7 % (CI 55 %–78 %) |
54.1 % (CI 35 %–69 %) |
36.6 % (CI 18 %–55 %) |
| Q2 | 67.7 % (CI 55 %–78 %) |
50.5 % (CI 37 %–62 %) |
41.3 % (CI 28 %–54 %) |
| Q3 | 81.4 % (CI 63 %–91 %) |
66.8 % (CI 43 %–82 %) |
66.8 % (CI 43 %–82 %) |
| ACTBlong | |||
| Q1 | 64.5 % (CI 45 %–79 %) |
54.7 % (CI 36 %–70 %) |
35.4 % (CI 17 %–55 %) |
| Q2 | 75.9 % (CI 63 %–85 %) |
56.5 % (CI 43 %–68 %) |
47.5 % (CI 34 %–60 %) |
| Q3 | 72.4 % (CI 54 %–85 %) |
51.6 % (CI 28 %–71 %) |
51.6 % (CI 28 %–71 %) |
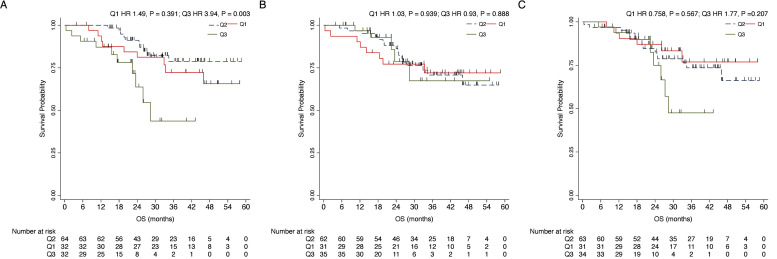
Univariable analysis at baseline in terms of OS showed a significant impact on prognosis for patients with PR positive status (HR 0.28, CI 0.13–0.58, P < 0.001), lobular histotype (HR 2.99, CI 1.35–6.65, P = 0.007), liver metastases (HR 2.21, CI 1.07–4.56, P = 0.031), node metastases (HR 2.27, CI 1.04–4.97, P = 0.040) and ACTBshort Q3 (HR 3.94, 95 % CI 1.60–9.68 P = 0.003) (Fig. 3,Table 4). In multivariable analyses, PR positive tumors were associated with lower risk of death (HR 0.22, CI 0.09–0.52, P=0.001), while lobular histotype (HR 3.54, CI 1.44–8.73, P = 0.006), presence of node metastases (HR 3.25, CI 1.27–8.32, P=0.014) and ACTBshort Q3 (HR 3.25, 95 % CI 1.27– 8.32, P = 0.023) were associated with worse prognosis (Table 4). OS at 12, 24 and 36 months for the respective interquartiles of ACTBshort, ACTBmedium and ACTBlong are reported in Table 6.
Fig. 3.
Kaplan-Meier plots for the impact on OS of different ACTBshort Qs (A), ACTBmedium Qs (B) and ACTBlong Qs (C). Relative to the intermediate quartile group (Q2), a significant prognostic impact was noted for ACTBshort Q3 (HR 3.94, 95 % CI 1.60–9.68 P = 0.003) when compared to the intermediate quartile group (Q2). Abbreviations: T0, baseline; T3, after 3 months; T6, after 6 months; Q, Interquartile range.
Table 4.
Univariate and Multivariate Cox Regression Analysis for Overall Survival.
| Univariate analysis |
Multivariate Analysis |
||||||
|---|---|---|---|---|---|---|---|
| HR | 95 % CI | p-value | HR | 95 % CI | p-value | ||
| PR status | |||||||
| Negative | 1.00 (Ref) | 1.00 (Ref) | |||||
| Positive | 0.28 | 0.13–0.58 | <0.001 | 0.22 | 0.09–0.52 | 0.001 | |
| De novo MBC | |||||||
| Yes | 1.00 (Ref) | ||||||
| No | 0.58 | 0.23– 1.48 | 0.258 | ||||
| Histotype | |||||||
| Ductal | 1.00 (Ref) | 1.00 (Ref) | |||||
| Lobular | 2.99 | 1.35–6.65 | 0.007 |
3.54 |
1.44–8.73 | 0.006 | |
| Liver Metastasis | |||||||
| No | 1.00 (Ref) | 1.00 (Ref) | |||||
| Yes | 2.21 | 1.07–4.56 | 0.031 |
1.97 |
0.90–4.33 | 0.091 | |
| Lung Metastasis | |||||||
| No | 1.00 (Ref) | ||||||
| Yes | 0.52 | 0.18–1.47 | 0.213 | ||||
| CNS Metastasis | |||||||
| No | |||||||
| Yes | not evaluable | ||||||
| Bone Metastasis | |||||||
| No | 1.00 (Ref) | ||||||
| Yes | 1.44 | 0.62–3.38 | 0.393 | ||||
| Node Metastasis | |||||||
| No | 1.00 (Ref) | 1.00 (Ref) | |||||
| Yes | 2.27 | 1.04–4.97 | 0.040 | 3.25 | 1.27–8.32 | 0.014 | |
| ACTBshort | |||||||
| Q2 | 1.00 (Ref) | ||||||
| Q1 | 1.49 | 0.60–3.68 | 0.391 | 1.64 | 0.61–4.37 | 0.326 | |
| Q3 | 3.94 | 1.60–9.68 | 0.003 | 3.74 | 1.20–11.67 | 0.023 | |
| ACTBmedium | |||||||
| Q2 | 1.00 (Ref) | ||||||
| Q1 | 1.03 | 0.44–2.42 | 0.939 | 1.81 | 0.72–4.54 | 0.207 | |
| Q3 | 0.93 | 0.33–2.57 | 0.888 | 0.31 | 0.08–1.24 | 0.098 | |
| ACTBlong | |||||||
| Q2 | 1.00 (Ref) | ||||||
| Q1 | 0.76 | 0.29–1.96 | 0.567 | ||||
| Q3 | 1.77 | 0.73–4.28 | 0.207 | ||||
Abbreviations: HR= Hazard Ratio, CI=Confidence Interval, PR=Progesteron receptor, MBC= Metastatic Breast Cancer, ACTB=Actin fragments, Q= Interquartile Range.
Table 6.
OS at 12, 24 and 36 months for the respective interquartiles of ACTBshort, ACTBmedium and ACTBlong and the corresponding 95 % confidence intervals. Abbreviations: CI= 95 % Confidence intervals; Q= Interquartile Range; ACTB=Actin fragments.
| Overall Survival | 12 months | 24 months | 36 months |
|---|---|---|---|
| ACTBshort | |||
| Q1 | 94 % (CI 77 %–98 %) |
81 % (CI 63 %–91 %) |
72 % (CI 51 %–85 %) |
| Q2 | 100 % | 91.2 % (CI 80 %–96 %) |
78.8 % (CI 63 %–88 %) |
| Q3 | 87 % (CI 69 %–95 %) |
64 % (CI 37 %–81 %) |
44 % (CI 17 %–68 %) |
| ACTBmedium | |||
| Q1 | 90.3 % (CI 73 %–97 %) |
77.2 % (CI 58 %–88 %) |
72.0 % (CI 51 %–85 %) |
| Q2 | 96.7 % (CI 88 %–99 %) |
86.3 % (CI 74 %–93 %) |
70.7 % (CI 56 %–82 %) |
| Q3 | 96.7 % (CI 79 %–99 %) |
78.6 % (CI 50 %–92 %) |
67.4 % (CI 34 %–86 %) |
| ACTBlong | |||
| Q1 | 93.6 % (CI 77 %–98 %) |
87.1 % (CI 69 %–95 %) |
76.9 % (CI 54 %–89 %) |
| Q2 | 96.8 % (CI 88 %–99 %) |
82.7 % (CI 70 %–90 %) |
73.5 % (CI 59 %–84 %) |
| Q3 | 93.8 % (CI 77 %–98 %) |
74.9 % (CI 47 %–90 %) |
47.6 % (CI 19 %–72 %) |
Discussion
In the context of luminal MBC, liquid biopsy features such as ctDNA, CTCs, epigenetics and exosomes are gaining traction as tools to investigate and monitor tumor evolution [10]. In this setting, fragmentomics is emerging as a new parameter capable of augmenting the insights obtainable through ctDNA [6].
At baseline, the distribution of ACTBshort changed significantly with respect to PR status and the presence of liver metastases. The highest interquartile group of ACTBshort was inversely associated with PR expression and directly associated with the presence of liver metastases. Given that both PR-negative status and the presence of liver metastases are acknowledged as unfavorable prognostic indicators in luminal-like MBC, these findings suggest these conditions are concurrently characterized by high tumor shedding, adding a further biological rationale to their aggressiveness.
Consistent with our previous work, where a >20 % increase between baseline and first evaluation of ACTBshort demonstrated a significant negative prognostic impact in PFS [7], the highest interquartile of baseline ACTBshort showed an unfavorable impact on both PFS and OS, confirming the association between increased tumor shedding and heightened aggressiveness, suggesting that patients at risk of faster progression could be identified not only through dynamic but also with baseline assessment.
Although not statistically significant, an intriguing trend towards an unfavorable prognostic effect was observed for the lowest interquartile of ACTBshort, hinting at the existence of a subgroup of non-shedding yet equally aggressive tumors. Considering that this subgroup was not significantly associated with the presence of brain metastases, which, although known as non-shedding sites, are associated with poorer outcomes, it is noteworthy that these patients, characterized by low shedding profile, require further clinical and biological analyses to be fully characterized. Future analyses through high sensitivity NGS methods may help identify potential concomitant mutations or associated epigenetic patterns, contributing to define a comprehensive biological landscape. Notably, for such cases, the detection of ctDNA during subsequent timepoints could serve as a crucial indicator of disease progression but further research will be necessary to delve into this hypothesis [11]
Interestingly, a favorable impact on PFS, but not on OS, was observed for the highest interquartile of ACTBmedium. This intermediate-length fragment, represented by ACTBmedium, is associated with neutrophils shedding. The impact on PFS alone may suggest a treatment-specific relationship, aligning with literature indicating an increased proportion of white blood cells during treatment with CDK4/6 inhibitors as a significant favorable prognostic factor [12].
To date, plasma ctDNA is gaining momentum as an emerging surrogate for tumor burden not only due to its prognostic value [[13], [14], [15]] but also for its predictive role [16,17]. However, most proposed approaches rely on either single-gene or gene panels, thereby restricting their monitoring sensitivity to the number of analyzed genes. Here, we demonstrate the feasibility of a droplet digital PCR-based agnostic approach focused on fragments size, which maximizes transferability and cost-effectiveness. While other fragmentomics approaches, such as nucleosomal patterns [18], end-fragment signatures [19], and epigenomic modifications [18,20,21], have been proposed as alternative methods, the evaluation of ACTB fragments currently emerges as one of the most scalable, cost-effective and robust strategies in the context of MBC.
Therefore, while traditional methods based on ddPCR and NGS technologies remain crucial for deciphering tumor clonal evolution and identifying potential target genes and resistance mechanisms, the assessment of ACTB fragments may serve as a complementary approach. This method, independent of the tumor’s mutational pattern, allows for both short- and long-term follow-up regardless of clonal and subclonal tumor evolution, potentially enabling the early identification of patient subgroups at risk of early progression. Alongside other features analyzable through ctDNA, analysis of progression hazard distribution peaks could facilitate the personalization of radiological monitoring modalities and schedules, adapting them to the varying baseline risk [[22], [23], [24]].
This study, however, has some limitations. Although it was a prospective study that enrolled patients receiving first-line treatment for luminal MBC, the relatively small sample size and the homogeneity of the study population necessitate validation of this approach on a larger scale with better representation of different subgroups, each potentially exhibiting specific shedding characteristics. Moreover, for some patients, follow-up is still in its early stages, potentially underestimating the prognostic impact of the biomarkers considered. The analysis of subsequent time points, particularly those preceding and concurrent with progression, will enable a better understanding of the biological behavior and dynamics of circulating actin fragments.
Conclusion
This study demonstrates the feasibility of employing a fragmentomics approach in cell free DNA based on the ACTB gene, in the context of first-line treatment of luminal-MBC patients. Although mutation-agnostic, this quantitative approach shows promise to being integrated into clinical practice, aiming to understand and predict the tumor dynamics.
Ethics approval and consent to participate
The study was approved by the ethics committee under the CEUR-2018-Sper-056-CRO protocol. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The study was performed in concordance with the Health Insurance Portability and Accountability Act and the Declaration of Helsinki.
Authors' contributions
LF, AF, GD, LG, FP conceived and supervised the study; LF, AF, EM, AD, DB, LC, SB, CN, SR,ER, SB, BP,SD, LA, MB, AM, BB, GD, LG, FP enrolled patients and collected follow-up patient information; AF, EM, BB, GD and LG performed molecular analysis; LG, AF, FG and LG performed the bioinformatics analysis; LG, AF, FG and LG performed the statistical analysis; EM, AD, DB, LC, SB, CN, SR,ER, SB, BP,SD, LA, MB, AM, BB, GD, LG, FP reviewed the paper and provided critical data interpretation; LF and AF wrote the manuscript with input from all authors. All of authors have read and approved the manuscript.
CRediT authorship contribution statement
Lorenzo Foffano: Writing – review & editing, Resources, Formal analysis, Visualization, Investigation, Conceptualization, Writing – original draft, Methodology, Data curation. Alessandra Franzoni: Visualization, Methodology, Formal analysis, Writing – original draft, Resources, Funding acquisition, Conceptualization, Writing – review & editing, Validation, Investigation, Data curation. Elisabetta Molteni: Writing – review & editing, Methodology, Validation, Writing – original draft. Fabiola Giudici: Writing – original draft, Investigation, Writing – review & editing, Methodology, Data curation, Software, Formal analysis. Arianna Dri: Writing – review & editing, Resources. Debora Basile: Writing – review & editing, Investigation, Resources. Linda Cucciniello: Resources, Writing – review & editing. Silvia Buriolla: Resources, Writing – review & editing. Claudia Noto: Resources, Writing – review & editing. Stefania Russo: Resources, Writing – review & editing, Data curation. Elena Nascimbeni: Resources, Writing – review & editing, Project administration. Silvia Bolzonello: Writing – review & editing, Resources. Brenno Pastò: Resources, Writing – review & editing. Serena Della Rossa: Resources, Writing – review & editing. Lorenzo Allegri: Resources, Writing – review & editing, Data curation. Marta Bonotto: Resources, Writing – review & editing, Methodology. Alessandro Marco Minisini: Writing – review & editing, Resources. Barbara Belletti: Writing – review & editing, Data curation, Resources. Giuseppe Damante: Writing – review & editing, Methodology, Resources. Lorenzo Gerratana: Writing – review & editing, Methodology, Formal analysis, Resources, Funding acquisition, Conceptualization, Writing – original draft, Investigation, Data curation. Fabio Puglisi: Writing – review & editing, Funding acquisition, Methodology, Conceptualization, Resources, Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
M.B. reports advisory/consultancy fee from AstraZeneca, Lilly, MSD, Novartis, Pfizer, SeaGen; travel grants from Lilly, Roche. A.M. reports advisory/consultancy fee from Novartis, MSD, BMS, Merk, Sunpharma, PierreFabre, Gilead, Seagen, Genomic Health; invited speech from Novartis, MSD, BMS, Merk, Sunpharma, Sanophi, PierreFabre, AstraZeneca, Daiichi Sankyo; travel grants from Gilead, PierreFabre; other familial: MSD, AstraZeneca, Pharmamar, GSK. L.G. reports advisory/consultancy fee from AstraZeneca, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Incyte, Novartis, Pfizer, Merck Sharp & Dohme, Menarini Stemline, Abbvie; research funding from Menarini Silicon Biosystems. F.P. reports honoraria for advisory boards, activities as a speaker, travel grants, research grants from Amgen, Astrazeneca, Daiichi Sankyo, Celgene, Eisai, Eli Lilly, Exact Sciences, Gilead, Ipsen, Italfarmaco, Menarini, MSD, Novartis,Pierre Fabre, Pfizer, Roche, Seagen, Takeda, Viatris; Research funding from Astrazeneca – Eisai – Roche
Funding
The present work was supported by the Italian Ministry of Health - Ricerca Corrente. This study was also supported by AstraZeneca and the Ministry of Health Ricerca Finalizzata grant (Grant Number: RF-2016-02362544) to FP; the CRO Aviano 5 × 1000 2014, redditi 2013 Cancer Specific Intramural Grant to LG; the Associazione Italiana per la Ricerca sul Cancro (AIRC) grant (IG#20061) and Ministry of Health Ricerca Finalizzata grant (Grant Number: RF-2021-12371961) to BB; The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2025.102456.
Appendix. Supplementary materials
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/CAAC.21660. [DOI] [PubMed] [Google Scholar]
- 2.Morrison L., Loibl S., Turner N.C. The CDK4/6 inhibitor revolution - a game-changing era for breast cancer treatment. Nat. Rev. Clin. Oncol. 2024;21:89–105. doi: 10.1038/S41571-023-00840-4. [DOI] [PubMed] [Google Scholar]
- 3.McCartney A., Migliaccio I., Bonechi M., Biagioni C., Romagnoli D., De Luca F., et al. Mechanisms of resistance to CDK4/6 inhibitors: potential implications and biomarkers for clinical practice. Front. Oncol. 2019;9 doi: 10.3389/FONC.2019.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migliaccio I., Bonechi M., McCartney A., Guarducci C., Benelli M., Biganzoli L., et al. CDK4/6 inhibitors: A focus on biomarkers of response and post-treatment therapeutic strategies in hormone receptor-positive HER2-negative breast cancer. Cancer Treat. Rev. 2021;93 doi: 10.1016/J.CTRV.2020.102136. [DOI] [PubMed] [Google Scholar]
- 5.Mazzitelli C., Santini D., Corradini A.G., Zamagni C., Trerè D., Montanaro L., et al. Liquid biopsy in the management of breast cancer patients: where are we now and where are we going. Diagnostics. 2023;13:1241. doi: 10.3390/DIAGNOSTICS13071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianni C., Palleschi M., Merloni F., Di Menna G., Sirico M., Sarti S., et al. Cell-free DNA fragmentomics: a promising biomarker for diagnosis, prognosis and prediction of response in breast cancer. Int. J. Mol. Sci. 2022;23 doi: 10.3390/IJMS232214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerratana L., Davis A.A., Zhang Q., Basile D., Rossi G., Strickland K., et al. Longitudinal dynamics of circulating tumor cells and circulating tumor DNA for treatment monitoring in metastatic breast cancer. JCO Precis. Oncol. 2021;5:943–952. doi: 10.1200/PO.20.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dessel L.F., Vitale S.R., Helmijr J.C.A., Wilting S.M., van der Vlugt-Daane M., Oomen-de Hoop E., et al. High-throughput isolation of circulating tumor DNA: a comparison of automated platforms. Mol. Oncol. 2019;13:392–402. doi: 10.1002/1878-0261.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dessel L.F., Beije N., Helmijr J.C.A., Vitale S.R., Kraan J., Look M.P., et al. Application of circulating tumor DNA in prospective clinical oncology trials – standardization of preanalytical conditions. Mol. Oncol. 2017;11:295–304. doi: 10.1002/1878-0261.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alimirzaie S., Bagherzadeh M., Akbari M.R. Liquid biopsy in breast cancer: a comprehensive review. Clin. Genet. 2019;95:643–660. doi: 10.1111/CGE.13514. [DOI] [PubMed] [Google Scholar]
- 11.Gouda M.A., Huang H.J., Piha-Paul S.A., Call S.G., Karp D.D., Fu S., et al. Longitudinal monitoring of circulating tumor DNA to predict treatment outcomes in advanced cancers. JCO Precis. Oncol. 2022 doi: 10.1200/PO.21.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zattarin E., Mariani L., Menichetti A., Leporati R., Provenzano L., Ligorio F., et al. Peripheral blood lymphocytes predict clinical outcomes in hormone receptor-positive HER2-negative advanced breast cancer patients treated with CDK4/6 inhibitors. Ther. Adv. Med. Oncol. 2023;15 doi: 10.1177/17588359231204857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerratana L., Davis A.A., Zhang Q., Basile D., Rossi G., Strickland K., et al. Longitudinal dynamics of circulating tumor cells and circulating tumor DNA for treatment monitoring in metastatic breast cancer. JCO Precis. Oncol. 2021;5:943–952. doi: 10.1200/PO.20.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson S.-J., Tsui D.W.Y., Murtaza M., Biggs H., Rueda O.M., Chin S.-F., et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 2013;368:1199–1209. doi: 10.1056/NEJMOA1213261. [DOI] [PubMed] [Google Scholar]
- 15.Stover D.G., Parsons H.A., Ha G., Freeman S.S., Barry W.T., Guo H., et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J. Clin. Oncol. 2018;36:543–553. doi: 10.1200/JCO.2017.76.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis A.A., Luo J., Zheng T., Dai C., Dong X., Tan L., et al. Genomic complexity predicts resistance to endocrine therapy and CDK4/6 inhibition in hormone receptor-positive (HR+)/HER2-negative metastatic breast cancer. Clin. Cancer Res. 2023;29:1719–1729. doi: 10.1158/1078-0432.CCR-22-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velimirovic M., Juric D., Niemierko A., Spring L., Vidula N., Wander S.A., et al. Rising circulating tumor DNA As a molecular biomarker of early disease progression in metastatic breast cancer. JCO Precis. Oncol. 2020;4:1246–1262. doi: 10.1200/PO.20.00117. [DOI] [PubMed] [Google Scholar]
- 18.Panagopoulou M., Karaglani M., Balgkouranidou I., Biziota E., Koukaki T., Karamitrousis E., et al. Circulating cell-free DNA in breast cancer: size profiling, levels, and methylation patterns lead to prognostic and predictive classifiers. Oncogene. 2019;38:3387–3401. doi: 10.1038/S41388-018-0660-Y. [DOI] [PubMed] [Google Scholar]
- 19.Budhraja K.K., McDonald B.R., Stephens M.D., Contente-Cuomo T., Markus H., Farooq M., et al. Analysis of fragment ends in plasma DNA from patients with cancer. MedRxiv. 2021 doi: 10.1101/2021.04.23.21255935. 2021.04.23.21255935. [DOI] [Google Scholar]
- 20.Fackler M.J., Bujanda Z.L., Umbricht C., Teo W.W., Cho S., Zhang Z., et al. Novel methylated biomarkers and a robust assay to detect circulating tumor DNA in metastatic breast cancer. Cancer Res. 2014;74:2160–2170. doi: 10.1158/0008-5472.CAN-13-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visvanathan K., Fackler M.S., Zhang Z., Lopez-Bujanda Z.A., Jeter S.C., Sokoll L.J., et al. Monitoring of serum DNA methylation as an early independent marker of response and survival in metastatic breast cancer: TBCRC 005 prospective biomarker study. J. Clin. Oncol. 2017;35:751–758. doi: 10.1200/JCO.2015.66.2080/ASSET/IMAGES/LARGE/JCO.2015.66.2080TA3.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.415P Patterns of time-to-progression intervals across clinical and liquid biopsy (LB) features in hormone receptor-positive (HR+) HER2-negative (HER2-) metastatic breast cancer (MBC) patients (pts) treated with first-line endocrine therapy (ET) - Annals of Oncology n.d. https://www.annalsofoncology.org/article/S0923-7534(23)01428-X/fulltext (accessed July 5, 2024).
- 23.Bonotto M., Basile D., Gerratana L., Pelizzari G., Bartoletti M., Vitale M.G., et al. Controversies in monitoring metastatic breast cancer during systemic treatment. Results of a GIM (Gruppo Italiano Mammella) survey. Breast. 2018;40:45–52. doi: 10.1016/J.BREAST.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Bonotto M., Basile D., Gerratana L., Bartoletti M., Lisanti C., Pelizzari G., et al. Clinico-radiological monitoring strategies in patients with metastatic breast cancer: a real-world study. Fut. Oncol. 2020;16:2059–2073. doi: 10.2217/FON-2020-0020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.