Abstract
Background
Constrictive pericarditis from tuberculosis is a rare but serious consequence of a tuberculosis infection, especially in nonendemic areas.
Case Summary
A 40-year-old man with a history of tuberculosis as a child that was treated with antituberculosis medications presented with heart failure. He was found to have severe calcific constrictive pericarditis from his remote tuberculosis infection, leading to biventricular failure and volume overload. The patient was optimized with guideline-directed medical therapy and intravenous diuresis, and he successfully underwent pericardiectomy with marked improvement in symptoms and quality of life.
Discussion
We present a rare case of severe calcific constrictive pericarditis that manifested clinically decades after the initial tuberculosis infection. Multimodality imaging, which includes echocardiography, cardiac computed tomography, and cardiac magnetic resonance, is valuable for diagnosing and guiding treatment for constrictive pericarditis.
Key Words: cardiac magnetic resonance, computed tomography, constrictive pericarditis, echocardiography, imaging
Graphical Abstract
History of Presentation and Past Medical History
A 40-year-old African-American man presented with rapidly progressive dyspnea on exertion for the past couple of weeks (NYHA functional class III-IV symptoms). His medical history was notable for his having contracted tuberculosis as a child (age 7 years) in the United States, at which time he was treated with antituberculosis medications. In addition, the patient had 15-year history of incarceration and approximately 5-year pack-year history of tobacco smoking. Despite his significant incarceration history, he tested negative for active and latent tuberculosis infection on serial acid-fast sputum and QuantiFERON testing, respectively. He had not required additional antituberculosis treatment since childhood. His physical examination was notable for an elevated jugular venous pressure, decreased lung sounds at the bases, distended abdomen, and +1 pitting edema on his lower extremities.
Take-Home Messages
-
•
There should be a high clinical index of suspicion for constriction in patients presenting with heart failure and any prior history of tuberculosis.
-
•
Consider constrictive pericarditis as part of the differential diagnosis in any patient with heart failure symptoms with any prior history of tuberculosis.
-
•
Multimodal imaging can aid the diagnosis of constrictive pericarditis, which can often be challenging to diagnose in the early stages of the disease.
Differential Diagnosis
The differential diagnosis includes infiltrative cardiomyopathy (eg, cardiac amyloidosis, cardiac sarcoidosis), nonischemic dilated cardiomyopathy, severe valvular regurgitation (especially tricuspid, mitral valve disease), constrictive pericarditis from tuberculosis, and pulmonary embolism.
Investigations
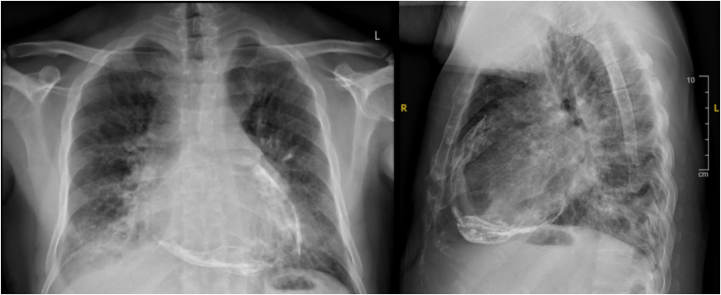
Electrocardiography and telemetry monitoring demonstrated paroxysmal atrial flutter. The patient’s QuantiFERON test and acid-fast sputum were negative. His chest X-ray demonstrated extensive pericardial calcifications (Figure 1). A cardiac computed tomography (CT) demonstrated the presence of severely calcified circumferential pericardium (Figure 2, Video 1), no evidence of coronary atherosclerosis or stenosis, dilated pulmonary artery measuring 3.5 cm consistent with pulmonary hypertension, and severely dilated inferior vena cava (measuring up to 5.1 cm). The pulmonary findings on the CT were notable for diffuse ground glass opacities in the bilateral lung fields and small punctate granulomas, likely from his prior tuberculosis infection.
Figure 1.
Chest X-Ray (Posteroanterior [Left] and Lateral [Right]) Showing Extensive Pericardial Calcifications
Figure 2.
Cardiac Computed Tomography Showing Extensive Severely Calcified Circumferential Pericardium
The upper abdomen images from the chest CT were notable for the cirrhotic appearance of the liver and small upper abdominal ascites, likely secondary to chronic congestive hepatopathy. Ultrasound of the liver confirmed the heterogenous/cirrhotic appearance of the liver with ascites. His renal function was normal (creatinine range: 0.9-1.1 mg/dL).
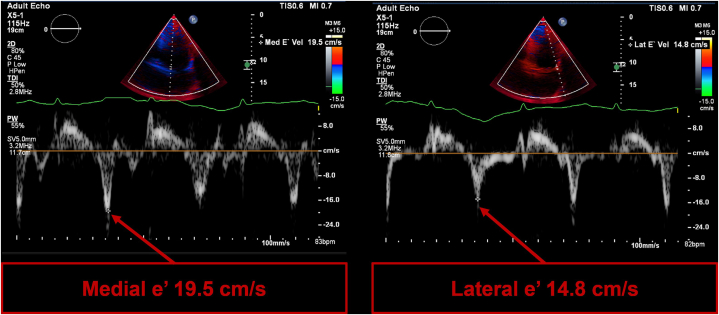
The transthoracic echocardiogram showed a reduced left ventricular ejection fraction (LVEF 40%) with abnormal septal motion consistent with constriction (Video 2). Furthermore, the diastolic assessment showed characteristic findings of constrictive physiology with paradoxically elevated mitral annular eʹ velocities despite a reduced ejection fraction, with a medial eʹ velocity of 19.5 cm/s (normal range: >7 cm/s) and a lateral eʹ velocity of 14.8 cm/s (normal range: >10 cm/s) (Figure 3).1
Figure 3.
Tissue Doppler Imaging of the Mitral Annulus
Tissue Doppler imaging of the mitral annulus for the diastolic assessment showing classic findings of constrictive physiology. Both the medial mitral eʹ velocity and the lateral eʹ velocity become significantly elevated with annulus reversus (reversal of normal mitral eʹ velocities in which the medial eʹ velocity becomes significantly higher than the lateral eʹ velocity).
It is important to note that young patients can have elevated mitral annular eʹ velocities. However, the presence annulus reversus (the reversal of the normal mitral velocities in which the medial eʹ velocity becomes higher the lateral eʹ velocity) makes constrictive physiology more likely (Figure 3).1
Cardiac magnetic resonance (CMR) demonstrated biventricular dysfunction (LVEF 38%; right ventricular ejection fraction 30%), with abnormal septal motion and biatrial enlargement (Video 3, Video 4, Video 5). On free-breathing imaging, there was evidence of ventricular interdependence with respiration due to constriction (Figure 4, Video 6). The pericardium was notable for chronic fibrosing constrictive pericarditis, characterized by significant asymmetric pericardial thickening and calcification (Figure 5, Video 7). The inferior vena cava was also severely dilated, measuring up to 5.5 cm (Videos 5 and 8).
Figure 4.
Cardiac Magnetic Resonance Demonstrating Ventricular Interdependence
(A) The septum shifts to the left during inspiration (diaphragm moves downward). (B) The septum shifts to the right during expiration (diaphragm moves upward).
Figure 5.
Cardiac Magnetic Resonance and Cardiac Computed Tomography Demonstrating Pericardial Calcification
(A) Magnetic resonance late gadolinium enhancement imaging showing asymmetric pericardial thickening with patchy areas of pericardial enhancement (blue arrow) suggestive of inflammation and low signal intensity regions consistent with pericardial calcification (white arrow). (B) Comparison with cardiac computed tomography demonstrating the calcified circumferential pericardium (white arrow). LV = left ventricle; RV = right ventricle.
Interestingly, CMR did not show evidence of intrinsic myocardial disease; late gadolinium enhancement showed no evidence of myocardial scar or necrosis, and parametric imaging showed no evidence of myocardial fibrosis (T1 time = 1,093 ms; extracellular volume fraction = 30%) or edema (T2 time = 46 ms). Finally, the invasive coronary angiogram showed normal coronary arteries. These findings are suggestive of cardiac dysfunction from a pure constrictive pathology.
Management and Follow-up
Patient was started on anticoagulation for his atrial flutter and underwent volume optimization with intravenous loop diuretics. Furthermore, the patient was started guideline-directed medical therapy for his heart failure with reduced ejection fraction, which included metoprolol, sacubitril-valsartan, spironolactone, and dapagliflozin. Once the patient had been medically optimized from a heart failure standpoint, he successfully underwent extended pericardiectomy, only preserving the pericardium posterior to both phrenic nerves to avoid injury.
The operative report described an extensive fibrous constricting sac of thick calcified pericardium with constriction involving all 4 chambers of the heart and all the great vessels. Furthermore, there was heavy involvement of the right ventricle as well as the diaphragmatic surface of the heart and the left ventricle. Large calcific thickened pericardium was sent for biopsy and microbiology, which showed fibrous tissue with calcification and negative fungal and acid-fast bacillus stains. The sternal lymph nodes showed reactive lymph nodes with non-necrotizing granulomas.
The patient recovered well from the surgery and was ultimately discharged on anticoagulation for his paroxysmal atrial flutter and guideline-directed medical therapy for his heart failure, as noted previously. During his postdischarge telehealth visit with the surgeon, the patient shared that he had experienced significant improvement in his symptoms and quality of life after his pericardiectomy.
Discussion
We present a rare case of severe calcific constrictive pericarditis from a remote tuberculosis infection that led to eventual biventricular heart failure and volume overload. This case highlights the importance of considering constrictive pericarditis as part of the differential diagnosis in any patient with heart failure symptoms with a prior history of tuberculosis, even decades after the initial infection was treated.
Constrictive pericarditis from tuberculosis is one of the most serious consequences of tuberculosis pericarditis, and it is a rare cause of constriction in nonendemic/developed countries.2 In most immunocompetent patients, when tuberculosis pericarditis is treated appropriately with the standard antituberculosis regimen, it is unlikely to progress to constrictive pericarditis.3 Furthermore, tuberculosis-associated constrictive pericarditis that is treated with the standard antituberculosis regimen has shown significant improvement (>80%) over a 6-month period.3 Despite the appropriate treatment of this patient’s tuberculosis, he likely had an ongoing subclinical pericardial inflammation, which progressed to severely calcific constriction and ultimately manifested as overt heart failure symptoms over 30 years after his initial tuberculosis infection.
The role of corticosteroids for management of tuberculosis-associated constrictive pericarditis remains unclear and requires clinical context. The IMPI (Investigation of the Management of Pericarditis) trial demonstrated that, in patients with tuberculosis pericarditis, treatment with prednisolone did not reduce the primary composite outcome of mortality, the incidence of constrictive pericarditis, or cardiac tamponade requiring intervention.4 However, when looking at the individual primary outcomes, there was an overall reduction in the incidence of constrictive pericarditis in patients treated with prednisolone. The World Health Organization recommends adding corticosteroids in tuberculosis pericarditis for patients with large effusions or high inflammatory burden. Though our patient did not recall having pericarditis, corticosteroid therapy during his initial infection may have reduced his risk of developing constrictive pericarditis.
In our patient’s case, his chest X-ray showed significantly advanced calcific constrictive disease, which made the diagnosis. The various multimodality imaging tests that followed the chest X-ray provided deeper understanding and insight into the pathophysiology and mechanism of this patient’s disease and ultimately helped guide his treatment. Echocardiography remains the cornerstone for evaluating for constriction because there are multiple hemodynamic parameters to assess constriction pathophysiology. However, in many cases the early signs of constriction can be challenging to diagnose without clinical suspicion, and CMR and cardiac CT play increased roles, in addition to echocardiography, in the diagnosis of constrictive pericarditis.5 CT can help define pericardial anatomy, and CMR can provide further insights into pericardial inflammation and tissue characterization.
Ferreira et al6 have shown that CMR can play an important role in both the diagnosis and monitoring of patients with constrictive pericarditis from tuberculosis. This can help provide insight into the effectiveness of an antituberculosis regimen and steroid therapy in the treatment of tuberculosis constrictive pericarditis. CMR has been shown to be a valuable tool in the assessment of the reversibility of constrictive pericarditis with medical therapy alone, especially early in the disease course.7 By assessing for treatment response, CMR can determine the subset of patients who do not response to conservative medical therapy alone and warrant early pericardiectomy for definitive treatment for constrictive pericarditis.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Cardiac computed tomography (transverse view) showing extensive severely calcified circumferential pericardium.
Transthoracic echocardiogram (apical 4-chamber view) showing reduced left ventricular ejection fraction (40%), abnormal septal bounce and biatrial enlargement.
Cardiac magnetic resonance (4-chamber view) showing reduced biventricular dysfunction with a (left ventricular ejection fraction 38%; right ventricular ejection fraction 30%), abnormal septal bounce, and biatrial enlargement.
Cardiac magnetic resonance (3-chamber view) showing reduced left ventricular ejection fraction, abnormal septal bounce, and significant left atrial enlargement.
Cardiac magnetic resonance (2-chamber view) showing reduced left ventricular ejection fraction and significantly dilated inferior vena cava (measuring up to 5.5 cm).
Free breathing cine on cardiac magnetic resonance (short axis view) showing evidence of increased ventricular interdependence with respiration.
Cardiac magnetic resonance showing significant asymmetric pericardial thickening and calcification.
Cardiac magnetic resonance showing a severely dilated inferior vena cava measuring up to 5.5 cm.
References
- 1.Geske J.B., Anavekar N.S., Nishimura R.A., Oh J.K., Gersh B.J. Differentiation of constriction and restriction. J Am Coll Cardiol. 2016;68:2329–2347. doi: 10.1016/j.jacc.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 2.Syed F.F., Schaff H.V., Oh J.K. Constrictive pericarditis—a curable diastolic heart failure. Nat Rev Cardiol. 2014;11:530–544. doi: 10.1038/nrcardio.2014.100. [DOI] [PubMed] [Google Scholar]
- 3.Kim M.S., Chang S.-A., Kim E.K., et al. The clinical course of tuberculous pericarditis in immunocompetent hosts based on serial echocardiography. Korean Circ J. 2020;50:599–609. doi: 10.4070/kcj.2019.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayosi B.M., Ntsekhe M., Bosch J., et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014;371:1121–1130. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Kazaz M., Klein A.L., Oh J.K., et al. Pericardial diseases and best practices for pericardiectomy: JACC state-of-the-art review. J Am Coll Cardiol. 2024;84:561–580. doi: 10.1016/j.jacc.2024.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira J., Voon V., Khanji M.Y. Tuberculous constrictive pericarditis: the role of cardiovascular magnetic resonance imaging in diagnosis and treatment monitoring. Eur Heart J - Cardiovasc Imaging. 2025;26:177. doi: 10.1093/ehjci/jeae252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng D., Glockner J., Kim K., et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy. Circulation. 2011;124:1830–1837. doi: 10.1161/CIRCULATIONAHA.111.026070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cardiac computed tomography (transverse view) showing extensive severely calcified circumferential pericardium.
Transthoracic echocardiogram (apical 4-chamber view) showing reduced left ventricular ejection fraction (40%), abnormal septal bounce and biatrial enlargement.
Cardiac magnetic resonance (4-chamber view) showing reduced biventricular dysfunction with a (left ventricular ejection fraction 38%; right ventricular ejection fraction 30%), abnormal septal bounce, and biatrial enlargement.
Cardiac magnetic resonance (3-chamber view) showing reduced left ventricular ejection fraction, abnormal septal bounce, and significant left atrial enlargement.
Cardiac magnetic resonance (2-chamber view) showing reduced left ventricular ejection fraction and significantly dilated inferior vena cava (measuring up to 5.5 cm).
Free breathing cine on cardiac magnetic resonance (short axis view) showing evidence of increased ventricular interdependence with respiration.
Cardiac magnetic resonance showing significant asymmetric pericardial thickening and calcification.
Cardiac magnetic resonance showing a severely dilated inferior vena cava measuring up to 5.5 cm.