Abstract
Artificial light at night (ALAN) is an emergent yet already global form of sensory pollution. However, its effects on marine environments remain poorly understood compared to those on terrestrial ecosystems. Low-latitude ecosystems such as shallow coral reefs might be at greater risk as they experience little change in annual day length and reef organisms rely on moonlight illumination as a zeitgeber for critical biological processes. Moreover, many coral reef fish are demersal spawners, making them vulnerable to the effects of ALAN from early life. We performed a field experiment to determine whether artificial light affects the quality of fish embryos and newly hatched larvae by exposing wild nests of the orange-fin anemonefish (Amphiprion chrysopterus) to white light emitting diode (LED) light (22 ± 2.0 lx; 4000 K) throughout the 6-day embryonic development period. We also explored whether light pollution indirectly influences offspring traits by measuring parental care investment. Exposure to ALAN altered embryo quality, leading to a reduction in egg volume (2.40%) and yolk reserves (6.11%) alongside an increase in heart rate (7.42%) a few hours before hatching. These changes reflect higher metabolic demands of embryos developing under light-polluted conditions. As parental care investment was unaffected by light pollution, our results suggest that these effects are more likely the consequence of a direct effect of ALAN on embryogenesis. In contrast, there was no influence of artificial light on the larval morphology or swimming performance, suggesting that the direct effects of ALAN on fish embryos do not cascade onto the larval stage immediately after hatching. These results may suggest that embryos compensated for ALAN exposure to maintain their early post-hatching larval performance. Further studies are needed to investigate whether light pollution exposure during embryonic development has delayed effects on larval performance during the dispersal phase or on larval survival.
Keywords: ALAN, cascading effects, coral reef fish, early-life traits, ecological light pollution, offspring quality, parental care, Pomacentridae
Introduction
Ecological light pollution originates from the use of artificial light at night (ALAN) and is a pervasive pressure threatening the nighttime environment (Longcore and Rich, 2004; Gaston et al., 2023). Despite artificial lighting being a symbol of modernity, security and economic development, ALAN is now widely accepted as an emergent global anthropogenic threat and one of the main causes of the decline in worldwide biodiversity (Gaston et al., 2021). Organisms have adapted over evolutionary time scales to predictable natural light patterns, which serve as environmental drivers for a wide array of biological rhythms (Bradshaw and Holzapfel, 2007). However, disruption of these patterns can affect molecular up to ecosystem processes (Sanders et al., 2021). With an estimated global annual increase of 9.6% in nighttime sky brightness over the last decade (Kyba et al., 2023), no equivalent pollutant over any time period has shown such a high rate of increase and thus light pollution is expected to have growing ecological impacts in both terrestrial and aquatic environments (Gaston et al., 2015). Indeed, shallow-water marine ecosystems are highly exposed to ALAN (Davies et al., 2014) with recent estimates indicating that 1.9 million km2 of coastal areas are exposed to biologically relevant light at night to 1 m depth (Smyth et al., 2021). Light pollution in marine environments has multiple origins with towns, harbours, resorts and offshore structures representing permanent light pollution sources in urbanized coastlines while shipping, fishing and recreational vessels trigger temporary ALAN (Longcore and Rich, 2004; Davies et al., 2014). Alongside the increasing trend in the use of artificial lighting, the world is transitioning towards more energy-efficient technologies rich in short wavelengths such as Light Emitting Diodes (LEDs). These spectra are known to be more harmful to wildlife (Zapata et al., 2019) and penetrate deeper in the water column (Hochberg et al., 2020). Consequently, this global shift in lighting technologies has the potential to further exacerbate the intensity and spatial extent of the impacts of ALAN in coastal marine environments (Davies et al., 2016).
Many marine organisms are likely to be affected by the introduction of artificial light as they synchronize their circadian rhythms with the predictability and duration of day–night cycles and their circalunar rhythms with natural moonlight variation throughout the lunar cycle (Marangoni et al., 2022). There is growing evidence that this sensory pollutant can affect a wide array of marine taxa including seagrass (Dalle Carbonare et al., 2023), amphipods (Navarro-Barranco and Hughes, 2015), isopods (Bullough et al., 2023), molluscs (Gao et al., 2024), sea urchins (Bari, 2024), as well as scleractinian corals (Kramer et al., 2023) and fish (Bassi et al., 2022). Indeed, light pollution represents a direct threat to coral reefs (Aubrecht et al., 2008) as numerous species inhabiting these ecosystems are highly photosensitive and rely on the variation in moonlight illumination for the timing of many critical biological processes such as reproduction synchronization, larval development and larval settlement (Shima et al., 2022; Davies et al., 2023). Even the light intensity of a full moon under optimal conditions (Kyba et al., 2017) can easily be masked by ALAN, which is typically one or two orders of magnitude brighter (Davies et al., 2015). Among coral reef species, teleost fish are probably the most sensitive vertebrates to extremely low light levels (Grubisic et al., 2019) and studying how artificial lighting affects this taxa is therefore crucial. Although evidence shows that ALAN affects fish at every level of biological organization from gene expression (Khan et al., 2018), physiology (Brüning et al., 2018), adult behaviour (Pulgar et al., 2019), larval settlement (O’Connor et al., 2019), juvenile growth and survival (Schligler et al., 2021), to population abundance (Bolton et al., 2017) and community composition (Weschke et al., 2024), studies on coral reef fish remain surprisingly scarce. Furthermore, >3500 coral reef fish species are demersal spawners (Jan, 2000), making them potentially more vulnerable to ALAN as reproduction and nesting can occur in shallow light-polluted waters thus exposing their offspring to artificial light from the earliest stages of life.
Given their small size and rapid development, fish embryos are particularly sensitive to disruption by environmental perturbations especially during key developmental stages called ‘windows of sensitivity’ (Agathokleous, 2022). Environmental conditions during embryonic development can result in cascading effects later in life such as changes in post-hatching survival or growth (Gagliano et al., 2007). This is particularly pertinent in the context of ALAN, as fish embryos can detect environmental light in ovo (Tamai et al., 2004). Indeed, the key enzyme controlling melatonin production is expressed from the first day post-fertilization in teleost fish (Gothilf et al., 1999; Kazimi and Cahill, 1999), which induces a functional and light-reactive circadian clock in embryos (Dekens and Whitmore, 2008). However, the effects of light pollution on fish early-life stages have been scarcely documented. Several laboratory studies have investigated the effects of artificial light on fish hatching success, a crucial step in their life cycle triggered by the onset of darkness in many reef fish (Shima et al., 2022). Results have been mixed with no effect on hatching (Lucon-Xiccato et al., 2023), delayed hatching (Duncan et al., 2008), reduced hatching success (Fobert et al., 2021), or even complete suppression (Fobert et al., 2019). These contrasting observations may be the result of differences in methodologies (e.g. light intensity and/or spectrum used as ALAN treatment) or species-specific sensitivities to ALAN (Brüning et al., 2011). On the other hand, unpublished data of a field-based study found no effect of light pollution on hatching from wild nests (Schligler et al., in review). Regardless, it is likely that exposure over the course of embryonic development in the wild could affect embryos prior to hatching and/or newly hatched larvae. For example, fish larvae showed increased activity and impaired cognitive abilities (Lucon-Xiccato et al., 2023) as well as changes in pigmentation, morphology and locomotion (Üstündağ et al., 2019) when embryos were exposed to light at night. There is also evidence that coral reef fish embryos developing under ALAN in a laboratory were of lower quality prior to hatching (Fobert et al., 2021). However, as parents were exposed prior to spawning in the latter study, the deleterious effects of artificial light on embryo quality could have been the result of a direct effect on embryonic development, an indirect parental effect on offspring quality (Gagliano and McCormick, 2009) or a combination of both. Indeed, it is known that maternal condition and parental care influence fish offspring phenotypic traits (Gagliano and McCormick, 2007; Barbasch et al., 2020; Cortese et al., 2024). ALAN may thus indirectly influence early-life stages by affecting parental traits as there is already proof of altered activity during fish paternal care provisioning (Foster et al., 2016).
We investigated how exposure to ALAN during embryonic development affects fish early-life stages using wild nests from a natural population of orange-fin anemonefish (Amphiprion chrysopterus; Cuvier, 1830) in the lagoon of Mo’orea, French Polynesia. Anemonefish are model organisms for studies on coral reef fish (Laudet and Ravasi, 2022) and their reliance on lunar light cues for reproduction makes them particularly relevant in the context of ALAN studies. By implementing a multiple Before-After-Control-Impact design, we predicted that exposure to biologically relevant levels of ALAN using custom-built underwater white LEDs on wild A. chrysopterus embryos during their development would (1) reduce egg volume as well as yolk sac area and affect heart rate a few hours prior to hatching. Indeed, these three metrics provide a valuable estimation of an embryo’s normal development and survival potential (i.e. embryo quality) as they are, respectively, proxies of gas diffusion efficiency (Bonisławska et al., 2001), available energetic reserves (Jaworski and Kamler, 2002) and physiological fitness (Perrichon et al., 2017). Reduced egg volume and yolk reserves under ALAN would reflect a higher embryonic metabolism over their development, with the latter being a direct consequence of increased energy mobilization and the former enabling increased oxygen supply to meet higher metabolic needs. On the other hand, embryonic heart rate could either increase or decrease with light pollution, respectively, because of stress response or as a strategy to save energy. We then tested for cascading effects of this stressor on newly hatched larvae from the embryos that developed under ALAN in the wild by measuring larval morphology and swimming performance as indicators of larval quality since these measures are correlated with survival and dispersal distance (Peck et al., 2012; Nanninga and Manica, 2018; Downie et al., 2020). We hypothesized that ALAN would (2) impair newly hatched larval quality by modifying their overall morphology and reducing their swimming capacities. Finally, we measured parental care investment of breeding pairs as a potential indirect driving mechanism for the effects observed on their offspring. In anemonefish, parental care starts immediately after the eggs are laid and increases over the next 5 days of embryonic development, peaking a few hours before hatching. This increased care may help in synchronizing hatching (Barbasch et al., 2022). While there is no evidence of parental investment being directly related to the quality of their embryos, the existence of a correlation between parental care and the number of offspring produced (Barbasch et al., 2020) implicitly suggests such a relationship. Our prediction was that ALAN would (3) not affect the proportion of time breeding pairs spend taking care of their nest a few hours before hatching. In the absence of an effect of light pollution on parental care, any influence of ALAN on embryonic and/or larval traits would thus result from a direct effect of ALAN on fish embryonic development. Indeed, indirect effects of ALAN through altered maternal condition during oogenesis (Sopinka et al., 2014) have been avoided in our study design as adult fish were not exposed to light pollution prior to spawning.
Materials and Methods
Study site and spawning monitoring
We surveyed 19 wild breeding pairs of orange-fin anemonefish (A. chrysopterus) in the lagoon on the north coast of the island of Mo’orea, French Polynesia (17°32′19.8” S, 149°49′46.3” W), between January and March 2023. Mature female anemonefish lay benthic eggs, one to three times per lunar cycle, and hatching occurs after sunset on the sixth or seventh night after spawning (Beldade et al., 2022). Eggs are attached onto the substratum under the tentacles of the magnificent sea anemone, Radianthus magnifica (Quoy & Gaimard, 1833), with which anemonefish adults live in an obligate mutualism.
Breeding pairs were visited every day during the week preceding and following the full moon and every 2 days during the rest of the lunar cycle to monitor their spawning. Egg clutches were identified by adult parental care behaviours (Barbasch et al., 2022) and confirmed by gently raising the tentacles of the anemone to locate nests. When a nest was discovered, a trained observer (T.R.) aged the eggs directly in the field and a photograph was taken to confirm ageing (see Fig. 13.1 in Beldade et al., 2022). Egg ageing was used to determine when to deploy the treatments and presumed hatching date (see following ‘Experimental design’ and ‘Egg sampling in the wild and hatching in the laboratory’ sections).
Experimental design
A multiple Before-After-Control-Impact design was implemented on the 19 wild fish breeding pairs. They were randomly allocated to control (‘Ctrl’; n = 7) or light pollution (‘Alan non-naive’; n = 7) treatments (Fig. 1a). To account for a potential carryover effect since ‘Alan non-naive’ pairs were previously exposed to a light pollution treatment during a study in 2021 (Schligler et al., in review), we included five additional anemonefish pairs naively exposed to ALAN to the experiment, i.e. with no prior exposure to ALAN (‘Alan naive’; Fig. 1a).
Figure 1.

(a) Location of the anemonefish breeding pairs used for the study on the north coast of Mo’orea, French Polynesia. (b) Photograph of a custom-built underwater light system at night 1–1.5 m from an anemonefish nest (Frederic Zuberer). (c) Photograph of wild anemonefish embryos a few hours prior to hatching (Frederic Zuberer).
Regardless of the treatment, the first spawning of each breeding pair was exposed to a dummy light made from PVC pipes mimicking the structure of the underwater lights. Dummy lights were deployed attached to fence posts planted into the substrate at 1–1.5 m distance from the nest on the day of spawning when freshly spawned nests were discovered or as early as possible after spawning (Table S1). Installation of dummy lights never exceeded 10 min and any potential disturbance due to the deployment would have been the same across breeding pairs. Fish and their nest were then left undisturbed until the evening of predicted hatching when dummy lights were removed and eggs were collected for embryo quality assessment, hatchling in the lab and larval quality measurements. This first sampling represented the ‘Before’ period.
We continued to monitor breeding pairs until they spawned another time to expose this second nest to their allocated treatments (i.e. ‘After’ period) and assess offspring quality in the same conditions as the first sampling. Nests from ‘Ctrl’ pairs were exposed to the same previously described dummy light. ‘Alan non-naive’ and ‘Alan naive’ nests were exposed to light pollution by deploying custom-built underwater lights in the same way as dummy lights. Underwater lights were made from three white LED strips (4000 K) fixed to a PVC tube and supplied by a 12 V lead–acid battery, all contained within a waterproof custom-built housing (Fig. 1b). Lights were photosensitive and switched on and off automatically at dusk and dawn, respectively. They delivered a mean light intensity of 21.7 ± 1.98 lx (± SE) at 1 m distance and 4.6 ± 0.87 lx at 2 m (Table S2), which is in the range of light levels measured in near-shore light-polluted areas (5–21.6 lx, Davies et al., 2015; 25–68 lx, Szekeres et al., 2017). As anemonefish spawn one to three times per lunar cycle (Barbasch et al., 2020; Laudet and Ravasi, 2022), the exposure of the second nest (i.e. ‘After’ period) was carried out on the same spawning event within the lunar cycle as for the ‘Before’ period in order to control for potential variation between spawnings within the lunar cycle (e.g. if ‘Before’ exposure was during the first of two spawnings of the lunar cycle, the ‘After’ exposure was also during the first spawning of the subsequent lunar cycle). Permits for installations of underwater structures were given by ministerial decree N°2414 from the government of French Polynesia.
Egg sampling in the wild and hatching in the laboratory
Eggs were sampled under the same conditions for both spawnings (i.e. ‘Before’ and ‘After’ periods) for every breeding pair on the predicted evening of wild nest hatching day (Fig. 1c). Sampling occurred on SCUBA following the method described in SI-2 in Cortese et al. (2022). Attention was taken that samples never exceeded a third of the size of the nest to limit the impact on the wild population. Each bag containing eggs collected in the wild was placed in a 40-l black-covered aquarium previously set up at 28.5 ± 0.5°C. Bags were left for at least 30 min for temperature acclimation. Following this, a random subsample of 30 eggs was collected at 6:00 pm for embryo quality measures. The remaining eggs were transferred into a hatching chamber attached to a wall of the aquarium and left to hatch overnight (see SI-2 in Cortese et al. (2022) for hatching protocol). A 12L:12D light cycle was provided by LED aquaria lights (Marine White Aquaray by Tropical Marine Center, Hertfordshire, UK) with lights turning on and off at 6:30 am and 6:30 pm, respectively.
Embryo quality traits
From the subsample of 30 eggs, seven to eight were transferred into a glass petri dish filled with aquarium seawater and placed under a binocular microscope (Leica Microsystemes, Nanterre, France). Eggs showing a damaged envelope were discarded from the sample (Table S3). Using needles, eggs were gently positioned so that the embryo’s heart and yolk sac were facing upwards (Fig. S1a). Eggs were then videorecorded with a camera (Sony RX100 III) for 2 min under 30× magnification to measure embryonic heart rate. Embryos showing cardiac disturbances (e.g. irregular beating, heart stopping while filming) were not used for the analysis (Table S3). Afterwards, eggs were photographed under 12.5× magnification and pictures scaled using Leica’s software to measure egg volume and yolk sac area. The procedure was repeated until 15 embryos per nest were recorded with a regular heart rate and photographed.
The 2-min recordings were manually watched at half-speed by a single observer (T.R.) blind to treatment and period. The observer visually counted the number of heartbeats over the 2 min for each individual egg. Preliminary trials in 2020 showed no influence of the manipulation of the eggs on embryonic heart rate (t-test; t = −0.82, df = 12, P = 0.429). Photographs of the eggs were uploaded to ImageJ version 1.52 to measure egg height and width (Fig. S1a). Egg volume was then approximated as an ellipsoid (Coleman, 1991) using the formula 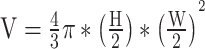 . V represents the volume of the egg in cubic millimetres, H the egg height in millimetres and W the egg width in millimetres. Yolk sac area was measured using the polygon selection tool on ImageJ. As every egg could not be oriented in the same position, egg rotation coded as ‘dorsal’ for dorsally positioned eggs (81.95% of total sampling; Fig. S1b), ‘tilted’ for dorso-laterally positioned eggs (9.75%; Fig. S1c) or ‘side’ for laterally positioned eggs (8.30%; Fig. S1d) was noted to correct for any effect of rotation on yolk sac area estimates.
. V represents the volume of the egg in cubic millimetres, H the egg height in millimetres and W the egg width in millimetres. Yolk sac area was measured using the polygon selection tool on ImageJ. As every egg could not be oriented in the same position, egg rotation coded as ‘dorsal’ for dorsally positioned eggs (81.95% of total sampling; Fig. S1b), ‘tilted’ for dorso-laterally positioned eggs (9.75%; Fig. S1c) or ‘side’ for laterally positioned eggs (8.30%; Fig. S1d) was noted to correct for any effect of rotation on yolk sac area estimates.
Larval quality traits
Larval swimming speed and morphology were measured as early as possible in the morning following the night when larvae hatched from wild-collected eggs in aquaria. We measured the maximum swimming speed (Umax) using a constant acceleration test (Farrell, 2008) of 8–10 newly hatched larvae per nest depending on daily logistical constraints (i.e. time limitation due to the number of nests to process). Swimming tests were performed in a Blažka-type swim tunnel immersed in a larger tank (Loligo System, Viborg, Denmark) kept at a constant temperature of 28.5 ± 0.25°C. The velocity of the water flowing through the swim tunnel against which larvae had to swim was regulated by a voltmeter and we used a calibrated correspondence table to convert voltage to velocity in centimetres per second. Each larva was gently transferred into the swim tunnel and, after a 10-min acclimation period at a velocity of 1 cm.s−1, the constant acceleration test started and flow speed was increased by 0.5 cm.s−1 every 30 s until the larva could no longer maintain its position in the flow for 10 s. Umax was calculated according to Brett (1964):  . Umax is the maximum swimming speed in centimetres per second, U the penultimate speed in centimetres per second, Ui the velocity increment (here 0.5 cm.s−1), t the amount of time the larva was able to swim within the final increment in seconds and ti the time increment (here 30 s).
. Umax is the maximum swimming speed in centimetres per second, U the penultimate speed in centimetres per second, Ui the velocity increment (here 0.5 cm.s−1), t the amount of time the larva was able to swim within the final increment in seconds and ti the time increment (here 30 s).
After the constant acceleration test, each larva was retrieved from the swim tunnel and placed in a glass petri dish under a binocular microscope (Leica Microsystemes, Nanterre, France) and anaesthetized using MS222 to take pictures of lateral and dorsal views at 20× magnification. Pictures were subsequently uploaded to ImageJ to measure total length, standard length, body depth and body width (Fig. S2).
Parental care investment
Prior to egg sampling in the wild, we deployed a stereo video system consisting of two GoPro HERO7 cameras permanently fixed to a metallic bar. Cameras were left for a minimum of 20 min and recordings were used to estimate parental care behaviour as well as the size of parents.
Only the recording from the camera providing the best point of view of the nest was used for the behavioural analysis. A single observer (L.V.E.) processed all the recordings blind to treatment and period. The first 7 min of each recording were considered as an acclimation period of the fish to the introduction of the cameras in their environment (Nanninga et al., 2017) and were therefore excluded from the analysis. Videos were then scored over 10 continuous minutes using BORIS software (Friard and Gamba, 2016). We defined six parental care behaviours to measure: fanning, mouthing, tending, chasing heterospecifics, chasing conspecifics and undetermined but on the nest (see Table S4 for definitions). The total amount of time spent displaying each of the six parental care behaviours was scored for both fish of each breeding pair. As males and females were not observed taking care of their offspring at the same time, we pooled together the durations of each parental care behaviour of each fish to obtain a total time of parental care investment per breeding pair. We expressed this amount of parental care investment as a proportion of watch time (i.e. 10 min).
To control for potential variation in egg and offspring traits due to natural differences in parental size (Green and McCormick, 2005), female and male total length of each breeding pair was estimated using the stereo video system. This required first filming a calibration panel, calculating the calibration parameters using VidSync software (Neuswanger et al., 2016) and then applying them to every recording. Using the same videos as for the behavioural analysis, total length of the female and male was measured and averaged three times from three different frames when fish were laterally positioned to account for measurement error. A ruler held by a snorkeler was filmed at the end of each recording, which was measured with VidSync to check the stereo video calibration. As female and male total length within pairs were significantly correlated (Pearson’s product–moment correlation; t = 4.34, df = 20, P < 0.001, estimate = 0.70) we decided to only use mean female total length as a covariate for statistical analyses (Table S5).
Statistical analyses
All statistical analyses were performed in R version 4.2.3 with an alpha threshold of 0.05. To determine the effect of ALAN exposure on embryo and larval quality, we implemented linear mixed-effects regression models (LMERs) using the lme4 package (Bates et al., 2015). Egg volume, yolk sac area, embryonic heart rate, larval morphology and larval maximum swimming speed were used as continuous response variables. As egg rotation had a significant effect on yolk sac area, a correction was applied on the raw values of the entire dataset according to the estimates of a linear model (Table S6). Embryonic heart rate was square root-transformed to improve linearity and normality of residuals. Larval morphology was calculated as a composite measure of total length, standard length and body depth given by the first principal component score of a Principal Component Analysis (PC1 explained 68.89% of overall variation; Table S7). Finally, Umax was standardized by larval total length and thus expressed in body length per second (BL.s−1) before being square root-transformed to improve linearity and normality of residuals.
We performed model selections for the three embryonic and the two larval quality traits using analyses of variance (ANOVAs) to compare LMERs, starting with the most complex model and sequentially dropping non-significant terms. Every model had ‘Treatment’ (categorical) in interaction with ‘Period’ (categorical) as fixed effects and site ID (i.e. breeding pair) as a random categorical effect to account for non-independence of data between periods. Depending on the response variable, mean female size (continuous) and/or egg volume (continuous) were included as fixed effects in the most complex model. Quality of each best-fit model was graphically explored using the ‘performance’ package (Lüdecke et al., 2021) and a correction for multiple comparison among five traits using False Discovery Rate was applied (Pike, 2011). To compare ‘Before’ periods between treatments, ‘Before’ and ‘After’ periods within treatments and ‘Before–After’ contrasts among treatments, we performed pairwise comparisons on Estimated Marginal Means (EMMs) using the lsmeans package (Lenth, 2020). First, we compared ‘Alan non-naive’ and ‘Alan naive’ data to test if fish offspring quality was affected by a carryover effect due to exposure to ALAN during a previous study in 2021 (Schligler et al., in review). As no statistical difference was detected in the ‘Before–After’ contrasts between ‘Alan non-naive’ and ‘Alan naive’ data for four (egg volume, yolk sac area, larval morphology and maximum swimming speed) out of the five early-life stage response variables (Tables S8–S13), we considered that fish offspring quality showed no carryover effect from being previously exposed. Thus, ‘Alan non-naive’ and ‘Alan naive’ data were combined into an ‘Alan’ group and compared to the ‘Ctrl’ using the same methods as described above (Tables S14–S19).
To explore the effect of ALAN exposure on parental care investment, we implemented beta regressions on the proportion of time breeding pairs spent at parental care using the betareg package (Cribari-Neto and Zeileis, 2010). Fixed effects included ‘Treatment’ (categorical) in interaction with ‘Period’ (categorical). We performed pairwise comparisons on EMMs, first between ‘Alan non-naive’ and ‘Alan naive’, and subsequently between ‘Alan’ (i.e. merged ‘Alan non-naive’ and ‘Alan naive’) and ‘Ctrl’ in the same way as described above (Tables S20 and S21). There was a single recording for which no parental care has been observed due to technical issues with the camera resulting in a shorter observation time (212 s against 600). As zeros are excluded from beta regression distribution, we arbitrary attributed a value of 1 s of total parental care investment for this video before standardizing it as a proportion of watch time.
Ethical declarations
Ethical approval for the study was granted from The Animal Ethics Committee, Centre National de la Recherche Scientifique (permit no. 006725).
Results
Embryo quality traits
No difference was found between ‘Before Ctrl’ and ‘Before Alan’ for the three embryo quality traits (Tables S15b, S16b and S17b), revealing a similar baseline for both treatment groups. There was a decrease in egg volume between the ‘Before’ and ‘After’ periods in both ‘Ctrl’ (EMM: estimate = 0.029 mm3, t = 3.877, P = 0.0001; Table S15b, Fig. 2a) and ‘Alan’ (EMM: estimate = 0.050 mm3, t = 8.713, P < 0.0001; Table S15b, Fig. 2a) treatments. This ‘Before–After’ contrast was, however, 72% greater in the ‘Alan’ treatment (EMM: estimate = 0.021 mm3, t = 2.260, P = 0.024; Table S15b) and taking into account the temporal variation estimated by the ‘Before–After’ contrast in the ‘Ctrl’ group, mean egg volume decreased by 2.40% in clutches exposed to light at night.
Figure 2.

Embryonic quality traits measured prior to hatching before (first spawning) and after (second spawning) exposure of embryos to control (Ctrl) or light pollution (Alan) treatments. (a) Egg volume, (b) yolk sac area, (c) embryonic heart rate. Large dots represent means and smaller dots represent individual measures. Significance codes for Before–After contrasts within treatments and their differences among treatments are *** for P-value <0.001, ** for P-value <0.01, * for P-value <0.05 and NS for P-value >0.05.
Similarly, yolk sac area was lower in the ‘After’ periods for both ‘Ctrl’ (EMM: estimate = 0.025 mm2, t = 3.254, P = 0.001, Table S16b, Fig. 2b) and ‘Alan’ (EMM: estimate = 0.044 mm2, t = 7.170, P < 0.0001; Table S16b, Fig. 2b) treatments. These contrasts were significantly different among treatments (EMM: estimate = 0.019 mm2, t = 2.052, P = 0.041; Table S16b) with ‘Before–After’ contrast being 76% greater in ‘Alan’ compared to ‘Ctrl’. As a result, mean yolk sac area was 6.11% lower for embryos exposed to artificial light at night when considering the ‘Before–After’ temporal variation estimated in the ‘Ctrl’ group.
There was no difference in embryonic heart rate between the ‘Before’ and ‘After’ periods in the ‘Ctrl’ group (EMM: estimate = −1.58 bpm, t = −0.385, P = 0.704; Table S17b, Fig. 2c) but heart rate was higher in the ‘After’ period compared to the ‘Before’ in the ‘Alan’ treatment (EMM: estimate = −14.80 bpm, t = −4.730, P = 0.0001; Table S17b, Fig. 2c). The comparison in ‘Before–After’ contrasts among treatments revealed that the contrast was 9.4 times greater in the ‘Alan’ group compared to the ‘Ctrl’ one (EMM: estimate = −13.2 bpm, t = 2.566, P = 0.018; Table S17b), revealing that mean embryonic heart rate was 7.42% greater in embryos exposed to light pollution.
Larval quality traits
No difference was found between ‘Before Ctrl’ and ‘Before Alan’ for both larval quality traits (Tables S18b and S19b), revealing a similar baseline for both treatment groups. Larvae were overall bigger in the ‘After’ period compared to the ‘Before’ period within the ‘Alan’ treatment (EMM: estimate = −0.490, t = −2.619, P = 0.009; Table S18b, Fig. 3a) but despite the absence of difference between periods within the ‘Ctrl’ group (EMM: estimate = −0.246, t = −0.986, P = 0.325; Table S18b, Fig. 3a), ‘Before–After’ contrasts were not different among treatments (EMM: estimate = −0.245, t = −0.786, P = 0.432; Table S18b).
Figure 3.

Larval quality traits measured at 0 days post-hatch before (first spawning) and after (second spawning) exposure of embryos to control (Ctrl) or light pollution (Alan) treatments. (a) Overall larval morphology expressed by the first principal component, (b) maximum swimming speed. Large dots represent means and smaller dots represent individual measures. Significance codes for Before–After contrasts within treatments and their differences among treatments are *** for P-value <0.001, ** for P-value <0.01, * for P-value <0.05 and NS for P-value >0.05.
Maximum swimming speed (Umax) was not affected by any main effect or combination of treatment and period as suggested by the lack of difference between ‘Before’ and ‘After’ periods in the ‘Ctrl’ (EMM: estimate = −2.95, t = −1.558, P = 0.133; Table S19b, Fig. 3b) and ‘Alan’ (EMM: estimate = 0.002, t = 0.001, P = 0.999; Table S19b, Fig. 3b) treatments and in ‘Before–After’ contrast comparison among treatments (EMM: estimate = 2.95, t = 1.249, P = 0.224; Table S19b).
Parental care
We found significant differences between sex regarding parental care investment with females spending 4 ± 1.5% (mean ± SE) of their time displaying parental care compared to 52 ± 4.4% for males, on average. Regardless, once pooled together the proportion of time breeding pairs spent providing care to their offspring was not affected by any treatment, period or the combination of these effects (beta regression: ‘Treatment:Period’, P = 0.911; Table S21a). Indeed, no differences were detected between ‘Before’ periods among treatments (EMM: estimate = 0.17, z = 1.416, P = 0.157; Table S21b) ‘Before’ and ‘After’ periods for both ‘Ctrl’ (EMM: estimate = 0.02, z = 0.179, P = 0.858; Table S21b, Fig. 4) and ‘Alan’ (EMM: estimate = 0.005, z = 0.048, P = 0.962; Table S21b, Fig. 4) treatments, or between ‘Before–After’ contrasts among the two treatments (EMM: estimate = −0.02, z = −0.110, P = 0.912; Table S21b).
Figure 4.

Mean proportion of time spent providing parental care for breeding pairs before (first spawning) and after (second spawning) exposure to control (Ctrl) or light pollution (Alan) treatments. Vertical error bars represent standard error of the mean, dots represent individual measures. Significance code for Before–After contrasts within treatments and their differences among treatments is NS for P-value >0.05.
Discussion
Fish early-life stages such as embryos and larvae show marked susceptibility to environmental stressors (Agathokleous, 2022). This study demonstrates that exposure to light pollution in the wild influences embryonic development of a coral reef fish. Our results show that embryos developing under ALAN had lower egg volume and yolk sac area as well as higher heart rate, suggesting higher energetic needs and a stress response. We propose that these observations are attributable to a direct effect of ALAN on embryogenesis. Indeed, we observed no influence of light pollution on parental care investment by both parents and nests were exposed only after spawning, ruling out any indirect effects of ALAN via altered parental care and/or maternal condition during oogenesis, respectively. However, further studies are needed to identify the mechanisms explaining the interaction of light pollution with embryogenesis (e.g. clock gene expression, hormone secretion). Despite the observed influence of light pollution on embryos, we surprisingly did not find any cascading effects of ALAN onto the morphology and swimming capacity of newly hatched larvae. A plausible explanation is that the ALAN exposure triggers an adaptative response in embryos, allowing them to cope with this stressor and maintain performance after hatching. However, it remains to be tested whether the metabolic costs of this response have mid- or long-term consequences such as reduced larval survival.
Altered embryo quality as a direct effect of ALAN on development
Our study found that eggs from clutches exposed to ALAN had reduced volume, a proxy of embryo quality, as reflected in offspring survival through the juvenile stage (Baynes and Howell, 1996). Egg volume overall was also lower during the second spawning in the control treatment, likely the result of reductions in food availability (Luckenbach et al., 2008). The observed ALAN treatment effect was independent of this change as the comparison of contrasts between periods among treatments accounts for such variation (Fisher et al., 2019). Other studies have reported the same observation of reduced egg size after exposure to light pollution such as for the pond snail Lymnaea stagnalis (Baz et al., 2022) and, more interestingly, another species of anemonefish (Amphiprion ocellaris; Fobert et al., 2021). However, in these two studies parents were exposed to ALAN prior to spawning and therefore the observed reduction in egg size could be the result of an indirect parental effect, a direct effect on embryonic development or a combination of both. As eggs were only exposed to ALAN conditions after laying and no effect of ALAN was found on parental care, the reduction in A. chrysopterus egg volume under light pollution treatment in the present study can only be explained by a direct influence of light pollution on embryogenesis. A reduction in egg volume would modify the egg surface-to-volume ratio, which dictates gas exchange efficiency via passive oxygen supply (Bonisławska et al., 2001). Here, reduced egg volume would result in higher surface-to-volume ratio, thereby enhancing embryos’ passive oxygen supply to meet their higher energetic requirements under ALAN as suggested by the reduction in yolk reserves and indicative of an adaptive response.
We observed a greater impact of embryonic exposure to ALAN on an embryo’s yolk reserves, with a reduction of 6.11% after taking into account the temporal variation detected in the control group. Interestingly, Fobert et al. (2021) found similar results in a laboratory experiment on A. ocellaris. Yolk is a critical component that provides embryos with the necessary free amino acids, proteins and fatty acids for their survival and development (Jaworski and Kamler, 2002; Kamler, 2008). Yolk reserves also play a vital role in an embryo’s immune function (Zhang et al., 2011) and developmental timing (Schwartz et al., 2021). The observed reduction in remaining yolk reserve under ALAN a few hours prior to hatching might be the consequence of higher energetic needs of embryos exposed to light pollution over their development. This increase in energetic requirements could be the result of a higher metabolism at night due to light pollution, thereby increasing overall energy needs during the 5 days of embryonic development. Even though it has not been demonstrated that teleost embryos exposed to ALAN show a higher metabolism at night, natural variation in photoperiod does influence the timing of their development (Villamizar et al., 2013) and in birds a longer photoperiod increases embryonic metabolic rate (Cooper et al., 2011). While reduced remaining yolk just before hatching might not be immediately deleterious for the embryos, it could potentially affect the performance of larvae after hatching as they rely on these reserves to supply energy until first exogenous feeding (Yúfera and Darias, 2007).
Furthermore, embryos developing under ALAN showed higher heart rate (7.42%). Heart rate is known to be an indicator of physiological fitness and energetic needs in fish embryos (Perrichon et al., 2017), with a lower heart rate suggesting an energy-saving strategy while a higher heart rate reflects increased energy use. This metric has commonly been associated with stress response (Atherton and McCormick, 2015) and used to assess sub-lethal effects of chemicals in fish embryos (Latif et al., 2001; Agathokleous, 2022). Heart rate is known to increase as embryos develop (Mousavi et al., 2021) and we only measured it during a particular time (i.e. just upon hatching), thereby providing only a snapshot of heart rate over the developmental period. However, this estimate should reflect an integrated response of embryos to light pollution as ALAN would have affected every critical step of embryogenesis. The only other study looking at the influence of ALAN on the heart rate of a marine organism, in adult Caribbean spiny lobster (Panulirus argus), did not find any effect (Steell et al., 2020) even though the light intensity used (1 lux) was considerably lower than the present study. In a similar way as noise pollution increases embryonic heart rate in other pomacentrid species (Jain-Schlaepfer et al., 2018; Fakan and McCormick, 2019), our results suggest that light pollution triggers a stress response in anemonefish embryos. This is consistent on the one hand with the observed reduction in remaining yolk reserves, as stress increases energetic requirements, and on the other hand, with the decrease in egg volume, which may facilitate passive oxygen diffusion to meet elevated metabolic demand.
We estimated parental care investment by both adults within breeding pairs to understand underlying mechanisms driving the effects of light pollution on embryos. Parental care plays a crucial role in shaping offspring traits in anemonefish (Barbasch et al., 2022). In this study, mature adults were also exposed to ALAN, which could have modified their circadian rhythm and influenced parental care investment. However, we found no effect of ALAN on the proportion of time both adults spent providing parental care. To our knowledge no other study has investigated the influence of light pollution specifically on parental care investment, only on fish activity. ALAN increases overall activity of nesting males in smallmouth bass Micropterus dolomieu (Foster et al., 2016) as well as in bluegill Lepomis macrochirus (Latchem et al., 2021) and rockfish Girella laevifrons (Pulgar et al., 2019). On the other hand, light pollution does not affect daytime fish activity at the group level despite modifying nocturnal behaviour in another pomacentrid species, Dascyllus aruanus (Georgiou et al., 2023). Regardless, as we observed a lack of effect of ALAN on the proportion of time breeding pairs spent providing parental care, it is unlikely that light pollution affected embryo traits indirectly by modifying parental care behaviour. By exposing embryos only after they were laid, we eliminated any indirect influence of light pollution on offspring traits through modified maternal condition during oogenesis (Sopinka et al., 2014). This means the observed effects of ALAN on embryos can only be attributed to a direct influence of light pollution on embryogenesis. Teleost embryos are photosensitive in ovo and express a functional and light-responsive circadian clock early after fertilization (Tamai et al., 2004; Dekens and Whitmore, 2008). ALAN modifies the natural day–night cycle and thus alters melatonin secretion as demonstrated in many taxa (Sanders et al., 2021), which may have triggered cascading physiological effects during embryonic development. Light pollution has been shown to affect fish hatching (Duncan et al., 2008; Fobert et al., 2019, 2021), which is controlled by a hatching enzyme (McAlary and McFarland, 1993), underscoring its potential to interfere with embryonic physiology. In our study, a disruption of the embryonic circadian rhythm could have interacted with the hypothalamus–pituitary–interrenal axis, which controls stress response in fish (Sánchez-Vázquez et al., 2019).
The absence of cascading effect of ALAN from embryos to larvae
Although we expected that altered embryo traits would cascade onto the larval stage, this did not occur. The lack of effect on larval morphology contrasts with the results of Üstündağ et al. (2019), who found that zebrafish (Danio rerio) larvae were smaller when embryos developed under ALAN, however their light treatment was an order of magnitude more intense (400 lux) than ecologically relevant light pollution (Davies et al., 2015; Szekeres et al., 2017). As egg size is often a good predictor of larval size in fishes (Pepin et al., 1997; Gisbert et al., 2000; Green et al., 2006), we expected a reduction in egg volume to result in smaller larvae. Given the effects of ALAN on embryo traits, the absence of differences in larval morphology suggests that fish embryos compensated for ALAN-induced stress by enhancing their energetic investment into growth and development, ensuring they reached a similar size at hatching. This finding is encouraging, as smaller larvae are known to have lower survival rates and reduced abilities for food acquisition and predator avoidance (Jenkins and King, 2006; Peck et al., 2012).
In the same vein, embryonic exposure to ALAN did not affect the swimming capabilities of newly hatched larvae. Swimming performance of teleost larvae has relevant implications for dispersal trajectories (Leis, 2007) and might be a good indicator of overall condition (Downie et al., 2020). As with larval morphology, the lack of influence of light pollution on larval swimming capacities suggests again that the direct effects of ALAN on embryos do not result in altered larval condition after hatching. It is worth noting that Umax includes both anaerobic and aerobic performances (Marras et al., 2010), the latter being correlated with heart rate (Clark et al., 2010). Therefore, the anaerobic component of Umax may have masked any effect of ALAN on the aerobic scope as suggested by its influence on embryonic heart rate.
Even though we found no immediate cascading effects of embryonic exposure to light pollution on early larval stage in anemonefish, evidence from other taxa suggests that delayed effects may still occur beyond hatching (Hicken et al., 2011). For example, toads exposed to ALAN during early life showed increased corticosterone concentration only at the juvenile stage, indicating a strongly delayed physiological response (Cope et al., 2020). Further studies are necessary to determine whether a similar mechanism exists in teleost fish. Additionally, measuring larval survival would provide a more holistic understanding of the cascading effects of embryonic exposure to ALAN. Given that embryos showed reduced yolk reserves it is reasonable to assume that larvae would hatch with diminished energy stores. A reduced yolk reserve shortens the critical time window for larvae to learn pre-capture skills (Yúfera and Darias, 2007) thus increasing its risk of starvation and potentially reducing larval survival, as recently demonstrated in moth (de Schoot et al., 2025).
Conclusions
This study assessed the effects of ecologically relevant exposure to ALAN in the wild on embryo and newly hatched larval quality in a coral reef fish. We revealed that light pollution reduced egg volume, decreased remaining yolk reserves and increased embryonic heart rate just before hatching. Since ALAN had no detectable effect on parental care and treatments were deployed immediately after spawning to prevent any indirect maternal effects, we propose that these changes in embryo traits result from a direct effect of nighttime light exposure on the photosensitive embryos during their development. On the other hand, the absence of cascading effects on larval traits suggests that embryos may have compensated for ALAN exposure to maintain their early post-hatching performance. Pomacentrids represent >400 species, many of which are strongly site-attached and provide parental care until hatching. These results may extend to a wide range of species commonly found in shallow coral reefs, with potential implications for population dynamics and recruitment. While this study was aimed at understanding how ALAN directly affects fish embryogenesis and its eventual cascading effects onto newly hatched larvae, in natural settings site-attached parents would also be exposed to ALAN before spawning. This prolonged exposure could influence offspring traits through altered parental gametogenesis, an aspect that warrants further investigation. We call for more research on the impact of ALAN in shallow-water marine environments, as this largely unregulated stressor is expected to expand and get more intense in coastal areas.
Supplementary Material
Acknowledgements
The authors thank Dean Chamberlain (University of Melbourne) for building and providing the underwater lights, Skye Instruments Ltd for donating the SpectroSense2+ underwater lux sensor to S.C.M. and Guillaume Iwankow, Clément Castrec and Tuiterai Salmon for their help during data collection. We are also grateful to the two anonymous reviewers for the valuable comments improving the manuscript. We want to acknowledge, pay respect and thank the indigenous people of French Polynesia.
Contributor Information
Thibaut Roost, UAR 3278 CRIOBE, BP 1013, PSL Université Paris: EPHE-UPVD-CNRS, 98729 Papetoai, Moorea, French Polynesia; National Centre for Coasts and Climate, School of Biosciences, University of Melbourne, Royal Parade, Parkville, VIC 3010, Australia.
Jade Hargous, UAR 3278 CRIOBE, BP 1013, PSL Université Paris: EPHE-UPVD-CNRS, 98729 Papetoai, Moorea, French Polynesia.
Lise Van Espen, Unité de Biologie des Organismes Marins et Biomimétisme, Université de Mons, 31 Boulevard Dolez, 7000 Mons, Hainaut, Belgium.
Jules Schligler, UAR 3278 CRIOBE, BP 1013, PSL Université Paris: EPHE-UPVD-CNRS, 98729 Papetoai, Moorea, French Polynesia.
Shaun S Killen, Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Graham Kerr Building, Science Way, Glasgow G12 8QQ, UK.
Ricardo Beldade, UAR 3278 CRIOBE, BP 1013, PSL Université Paris: EPHE-UPVD-CNRS, 98729 Papetoai, Moorea, French Polynesia; Facultad de Ciencias Biologicas, Pontificia Universidad Católica de Chile, Av Bernardo O’Higgins 340, Santiago, Chile.
Stephen E Swearer, National Centre for Coasts and Climate, School of Biosciences, University of Melbourne, Royal Parade, Parkville, VIC 3010, Australia; Oceans Institute, University of Western Australia, Fairway, Crawley 6009, WA, Australia.
Suzanne C Mills, UAR 3278 CRIOBE, BP 1013, PSL Université Paris: EPHE-UPVD-CNRS, 98729 Papetoai, Moorea, French Polynesia; Laboratoire d’Excellence ‘CORAIL’, France; Institut Universitaire de France, Ministère de l'Enseignement supérieur, de la Recherche et de l'Innovation, 1 rue Descartes, 75231 Paris, France.
Author contributions
Conceptualization: S.C.M., S.E.S., R.B., J.S. and T.R. Data curation: J.H., L.V.E. and T.R. Formal analysis: J.H., L.V.E. and T.R. Funding acquisition: S.C.M., S.E.S. and R.B. Investigation: J.H., J.S. and T.R. Methodology: S.C.M., S.E.S., R.B., J.S. and T.R. Project administration: S.C.M. and S.E.S. Resources: S.C.M. and S.E.S. Software: J.H., L.V.E. and T.R. Supervision: S.C.M. and S.E.S. Validation: S.C.M., S.E.S., R.B. and S.S.K. Visualization: T.R. Writing—original draft: T.R., J.H., S.C.M. and S.E.S. Writing—review and editing: All authors.
Conflicts of interest
The authors have no conflict of interest to declare.
Funding
This work was supported by a Graduate Research Projects Scheme scholarship between The University of Melbourne and The Centre National de la Recherche Scientifique provided to S.C.M. and S.E.S.; Pacific Funds BLEACHALAN grant given to S.C.M., S.E.S. and R.B.; ‘Recherche et Innovation Partenariat Public Privé pour Preuve de concept (RIP4): Raising Nemo’ funding [5637/MAF/REC] to S.C.M. and R.B.; High Commissioner of the Republic in French Polynesia grant ‘BLEACHALAN’ to S.C.M. [grant number HC/3041/DIE/BPT/cp]; and ‘Programme des Investissements d’Avenir EUR TULIP-GSR’ grant [ANR-18-EURE-0019] to J.H.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- Agathokleous E (2022) The hormetic response of heart rate of fish embryos to contaminants – implications for research and policy. Sci Total Environ 815: 152911. 10.1016/j.scitotenv.2021.152911. [DOI] [PubMed] [Google Scholar]
- Atherton JA, McCormick MI (2015) Active in the sac: damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim Behav 103: 1–6. 10.1016/j.anbehav.2015.01.033. [DOI] [Google Scholar]
- Aubrecht C, Elvidge CD, Longcore T, Rich C, Safran J, Strong AE, Eakin CM, Baugh KE, Tuttle BT, Howard AT et al. (2008) A global inventory of coral reef stressors based on satellite observed nighttime lights. Geocarto Int 23: 467–479. 10.1080/10106040802185940. [DOI] [Google Scholar]
- Barbasch TA, DeAngelis R, Rhodes J, Buston PM (2022) Parental care: patterns, proximate and ultimate causes, and consequences. In Laudet V, Ravasi T, eds, Evolution, Development and Ecology of Anemonefishes, First Edition. CRC Press, Boca Raton, pp. 159–166 [Google Scholar]
- Barbasch TA, Rueger T, Srinivasan M, Wong MYL, Jones GP, Buston PM (2020) Substantial plasticity of reproduction and parental care in response to local resource availability in a wild clownfish population. Oikos 129: 1844–1855. 10.1111/oik.07674. [DOI] [Google Scholar]
- Bari DD (2024) Effects of artificial light at night on the mobility of the sea urchin Paracentrotus lividus. Mar Fish Sci 37: 41–52. 10.47193/mafis.3712024010106. [DOI] [Google Scholar]
- Bassi A, Love OP, Cooke SJ, Warriner TR, Harris CM, Madliger CL (2022) Effects of artificial light at night on fishes: a synthesis with future research priorities. Fish Fish 23: 631–647. 10.1111/faf.12638. [DOI] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67: 1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Baynes SM, Howell BR (1996) The influence of egg size and incubation temperature on the condition of Solea solea (L.) larvae at hatching and first feeding. J Exp Mar Biol Ecol 199: 59–77. 10.1016/0022-0981(95)00189-1. [DOI] [Google Scholar]
- Baz E-S, Hussein AAA, Vreeker EMT, Soliman MFM, Tadros MM, El-Shenawy NS, Koene JM (2022) Consequences of artificial light at night on behavior, reproduction, and development of Lymnaea stagnalis. Environ Pollut 307: 119507. 10.1016/j.envpol.2022.119507. [DOI] [PubMed] [Google Scholar]
- Beldade R, Bernardi G, Mills SC (2022) Anemonefish behavior and reproduction. In Laudet V, Ravasi T, eds, Evolution, Development and Ecology of Anemonefishes, First Edition. CRC Press, Boca Raton, pp. 129–142 [Google Scholar]
- Bolton D, Mayer-Pinto M, Clark GF, Dafforn KA, Brassil WA, Becker A, Johnston EL (2017) Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci Total Environ 576: 1–9. 10.1016/j.scitotenv.2016.10.037. [DOI] [PubMed] [Google Scholar]
- Bonisławska M, Formicki K, Korzelecka-Orkisz A, Winnicki A (2001) Fish egg size variability: biological significance. Electron J Pol Agric Univ 4: 1–15. [Google Scholar]
- Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst 38: 1–25. 10.1146/annurev.ecolsys.37.091305.110115. [DOI] [Google Scholar]
- Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21: 1183–1226. 10.1139/f64-103. [DOI] [Google Scholar]
- Brüning A, Hölker F, Franke S, Kleiner W, Kloas W (2018) Influence of light intensity and spectral composition of artificial light at night on melatonin rhythm and mRNA expression of gonadotropins in roach Rutilus rutilus. Fish Physiol Biochem 44: 1–12. 10.1007/s10695-017-0408-6. [DOI] [PubMed] [Google Scholar]
- Brüning A, Hölker F, Wolter C (2011) Artificial light at night: implications for early life stages development in four temperate freshwater fish species. Aquat Sci 73: 143–152. 10.1007/s00027-010-0167-2. [DOI] [Google Scholar]
- Bullough K, Gaston KJ, Troscianko J (2023) Artificial light at night causes conflicting behavioural and morphological defence responses in a marine isopod. Proc R Soc B Biol Sci 290: 20230725. 10.1098/rspb.2023.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TD, Sandblom E, Hinch SG, Patterson DA, Frappell PB, Farrell AP (2010) Simultaneous biologging of heart rate and acceleration, and their relationships with energy expenditure in free-swimming sockeye salmon (Oncorhynchus nerka). J Comp Physiol B 180: 673–684. 10.1007/s00360-009-0442-5. [DOI] [PubMed] [Google Scholar]
- Coleman RM (1991) Measuring parental investment in nonspherical eggs. Copeia 1991: 1092–1098. 10.2307/1446105. [DOI] [Google Scholar]
- Cooper CB, Voss MA, Ardia DR, Austin SH, Robinson WD (2011) Light increases the rate of embryonic development: implications for latitudinal trends in incubation period. Funct Ecol 25: 769–776. 10.1111/j.1365-2435.2011.01847.x. [DOI] [Google Scholar]
- Cope KL, Schook MW, Benard MF (2020) Exposure to artificial light at night during the larval stage has delayed effects on juvenile corticosterone concentration in American toads, Anaxyrus americanus. Gen Comp Endocrinol 295: 113508. 10.1016/j.ygcen.2020.113508. [DOI] [PubMed] [Google Scholar]
- Cortese D, Crespel A, Mills SC, Norin T, Killen SS, Beldade R (2022) Adaptive effects of parental and developmental environments on offspring survival, growth and phenotype. Funct Ecol 36: 2983–2994. 10.1111/1365-2435.14202. [DOI] [Google Scholar]
- Cortese D, Diaz C, Beldade R, Killen SS, Scholz Z, Mills SC (2024) Environmental change mediates plasticity in offspring traits through maternal effects in a coral reef fish. Sci Total Environ 957: 177630. 10.1016/j.scitotenv.2024.177630. [DOI] [PubMed] [Google Scholar]
- Cribari-Neto F, Zeileis A (2010) Beta regression in R. J Stat Softw 34: 1–24. 10.18637/jss.v034.i02. [DOI] [Google Scholar]
- Dalle Carbonare L, Basile A, Rindi L, Bulleri F, Hamedeh H, Iacopino S, Shukla V, Weits DA, Lombardi L, Sbrana A et al. (2023) Dim artificial light at night alters gene expression rhythms and growth in a key seagrass species (Posidonia oceanica). Sci Rep 13: 10620. 10.1038/s41598-023-37261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TW, Coleman M, Griffith KM, Jenkins SR (2015) Night-time lighting alters the composition of marine epifaunal communities. Biol Lett 11: 20150080. 10.1098/rsbl.2015.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TW, Duffy JP, Bennie J, Gaston KJ (2014) The nature, extent, and ecological implications of marine light pollution. Front Ecol Environ 12: 347–355. 10.1890/130281. [DOI] [Google Scholar]
- Davies TW, Duffy JP, Bennie J, Gaston KJ (2016) Stemming the tide of light pollution encroaching into marine protected areas: light pollution in marine protected areas. Conserv Lett 9: 164–171. 10.1111/conl.12191. [DOI] [Google Scholar]
- Davies TW, Levy O, Tidau S, de Barros Marangoni LF, Wiedenmann J, D’Angelo C, Smyth T (2023) Global disruption of coral broadcast spawning associated with artificial light at night. Nat Commun 14: 2511. 10.1038/s41467-023-38070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekens MP, Whitmore D (2008) Autonomous onset of the circadian clock in the zebrafish embryo. EMBO J 27: 2757–2765. 10.1038/emboj.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie AT, Illing B, Faria AM, Rummer JL (2020) Swimming performance of marine fish larvae: review of a universal trait under ecological and environmental pressure. Rev Fish Biol Fish 30: 93–108. 10.1007/s11160-019-09592-w. [DOI] [Google Scholar]
- Duncan NJ, Ibarra-Castro L, Alvarez-Villaseñor R (2008) Effect of the dusk photoperiod change from light to dark on the incubation period of eggs of the spotted rose snapper, Lutjanus guttatus (Steindachner). Aquac Res 39: 427–433. 10.1111/j.1365-2109.2007.01851.x. [DOI] [Google Scholar]
- Fakan EP, McCormick MI (2019) Boat noise affects the early life history of two damselfishes. Mar Pollut Bull 141: 493–500. 10.1016/j.marpolbul.2019.02.054. [DOI] [PubMed] [Google Scholar]
- Farrell AP (2008) Comparisons of swimming performance in rainbow trout using constant acceleration and critical swimming speed tests. J Fish Biol 72: 693–710. 10.1111/j.1095-8649.2007.01759.x. [DOI] [Google Scholar]
- Fisher R, Shiell GR, Sadler RJ, Inostroza K, Shedrawi G, Holmes TH, McGree JM (2019) Epower: an r package for power analysis of before-after-control-impact (BACI) designs. Methods Ecol Evol 10: 1843–1853. 10.1111/2041-210X.13287. [DOI] [Google Scholar]
- Fobert EK, Burke da Silva K, Swearer SE (2019) Artificial light at night causes reproductive failure in clownfish. Biol Lett 15: 20190272. 10.1098/rsbl.2019.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fobert EK, Schubert KP, Burke da Silva K (2021) The influence of spectral composition of artificial light at night on clownfish reproductive success. J Exp Mar Biol Ecol 540: 151559. 10.1016/j.jembe.2021.151559. [DOI] [Google Scholar]
- Foster JG, Algera DA, Brownscombe JW, Zolderdo AJ, Cooke SJ (2016) Consequences of different types of littoral zone light pollution on the parental care behaviour of a freshwater teleost fish. Water Air Soil Pollut 227: 404. 10.1007/s11270-016-3106-6. [DOI] [Google Scholar]
- Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7: 1325–1330. 10.1111/2041-210X.12584. [DOI] [Google Scholar]
- Gagliano M, McCormick MI (2007) Maternal condition influences phenotypic selection on offspring. J Anim Ecol 76: 174–182. 10.1111/j.1365-2656.2006.01187.x. [DOI] [PubMed] [Google Scholar]
- Gagliano M, McCormick MI (2009) Hormonally mediated maternal effects shape offspring survival potential in stressful environments. Oecologia 160: 657–665. 10.1007/s00442-009-1335-8. [DOI] [PubMed] [Google Scholar]
- Gagliano M, McCormick MI, Meekan MG (2007) Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proc R Soc B Biol Sci 274: 1575–1582. 10.1098/rspb.2007.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang M, Luo Q, Lin S, Lyu M, Ke C (2024) Persistent exposure to artificial light at night (ALAN) accelerates metamorphosis and colonization in larvae of marine shellfish. Ecol Indic 158: 111472. 10.1016/j.ecolind.2023.111472. [DOI] [Google Scholar]
- Gaston KJ, Ackermann S, Bennie J, Cox DTC, Phillips BB, Sánchez de Miguel A, Sanders D (2021) Pervasiveness of biological impacts of artificial light at night. Integr Comp Biol 61: 1098–1110. 10.1093/icb/icab145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KJ, Gardner AS, Cox DTC (2023) Anthropogenic changes to the nighttime environment. Bioscience 73: 280–290. 10.1093/biosci/biad017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston KJ, Visser ME, Hölker F (2015) The biological impacts of artificial light at night: the research challenge. Philos Trans R Soc B 370: 20140133. 10.1098/rstb.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou D, Reeves SE, da Silva KB, Fobert EK (2023) Artificial light at night impacts night-time activity but not day-time behaviour in a diurnal coral reef fish. Basic Appl Ecol 74: 74–82. 10.1016/j.baae.2023.11.009. [DOI] [Google Scholar]
- Gisbert E, Williot P, Castelló-Orvay F (2000) Influence of egg size on growth and survival of early stages of Siberian sturgeon (Acipenser baeri) under small scale hatchery conditions. Aquaculture 183: 83–94. 10.1016/S0044-8486(99)00287-2. [DOI] [Google Scholar]
- Gothilf Y, Coon SL, Toyama R, Chitnis A, Namboodiri MAA, Klein DC (1999) Zebrafish serotonin N-Acetyltransferase-2: marker for development of pineal photoreceptors and circadian clock function. Endocrinology 140: 4895–4903. 10.1210/endo.140.10.6975. [DOI] [PubMed] [Google Scholar]
- Green B, McCormick M (2005) Maternal and paternal effects determine size, growth and performance in larvae of a tropical reef fish. Mar Ecol Prog Ser 289: 263–272. 10.3354/meps289263. [DOI] [Google Scholar]
- Green BS, Anthony KRN, McCormick MI (2006) Position of egg within a clutch is linked to size at hatching in a demersal tropical fish. J Exp Mar Biol Ecol 329: 144–152. 10.1016/j.jembe.2005.08.012. [DOI] [Google Scholar]
- Grubisic M, Haim A, Bhusal P, Dominoni DM, Gabriel KMA, Jechow A, Kupprat F, Lerner A, Marchant P, Riley W et al. (2019) Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 11: 6400. 10.3390/su11226400. [DOI] [Google Scholar]
- Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, Larsen M, Stekoll MS, Rice SD, Collier TK et al. (2011) Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. Proc Natl Acad Sci 108: 7086–7090. 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg EJ, Peltier SA, Maritorena S (2020) Trends and variability in spectral diffuse attenuation of coral reef waters. Coral Reefs 39: 1377–1389. 10.1007/s00338-020-01971-1. [DOI] [Google Scholar]
- Jain-Schlaepfer S, Fakan E, Rummer JL, Simpson SD, McCormick MI (2018) Impact of motorboats on fish embryos depends on engine type. Conserv Physiol 6: coy014. 10.1093/conphys/coy014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan R-Q (2000) Resource limitation underlying reproductive strategies of coral reef fishes: a hypothesis. Zool Stud 39: 266–274. [Google Scholar]
- Jaworski A, Kamler E (2002) Development of a bioenergetics model for fish embryos and larvae during the yolk feeding period. J Fish Biol 60: 785–809. 10.1111/j.1095-8649.2002.tb02410.x. [DOI] [Google Scholar]
- Jenkins GP, King D (2006) Variation in larval growth can predict the recruitment of a temperate, seagrass-associated fish. Oecologia 147: 641–649. 10.1007/s00442-005-0336-5. [DOI] [PubMed] [Google Scholar]
- Kamler E (2008) Resource allocation in yolk-feeding fish. Rev Fish Biol Fish 18: 143–200. 10.1007/s11160-007-9070-x. [DOI] [Google Scholar]
- Kazimi N, Cahill GM (1999) Development of a circadian melatonin rhythm in embryonic zebrafish. Dev Brain Res 117: 47–52. 10.1016/S0165-3806(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Khan ZA, Labala RK, Yumnamcha T, Devi SD, Mondal G, Sanjita Devi H, Rajiv C, Bharali R, Chattoraj A (2018) Artificial light at night (ALAN), an alarm to ovarian physiology: a study of possible chronodisruption on zebrafish (Danio rerio). Sci Total Environ 628-629: 1407–1421. 10.1016/j.scitotenv.2018.02.101. [DOI] [PubMed] [Google Scholar]
- Kramer N, Tamir R, Galindo-Martínez CT, Wangpraseurt D, Loya Y (2023) Light pollution alters the skeletal morphology of coral juveniles and impairs their light capture capacity. Mar Pollut Bull 193: 115212. 10.1016/j.marpolbul.2023.115212. [DOI] [PubMed] [Google Scholar]
- Kyba CCM, Altıntaş YÖ, Walker CE, Newhouse M (2023) Citizen scientists report global rapid reductions in the visibility of stars from 2011 to 2022. Science 379: 265–268. 10.1126/science.abq7781. [DOI] [PubMed] [Google Scholar]
- Kyba CCM, Mohar A, Posch T (2017) How bright is moonlight? Astron Geophys 58: 1.31-1.32. 10.1093/astrogeo/atx025. [DOI] [Google Scholar]
- Latchem E, Madliger CL, Abrams AEI, Cooke SJ (2021) Does artificial light at night alter the subsequent diurnal behavior of a teleost fish? Water Air Soil Pollut 232: 71. 10.1007/s11270-021-05023-4. [DOI] [Google Scholar]
- Latif MA, Bodaly RA, Johnston TA, Fudge RJP (2001) Effects of environmental and maternally derived methylmercury on the embryonic and larval stages of walleye (Stizostedion vitreum). Environ Pollut 111: 139–148. 10.1016/S0269-7491(99)00330-9. [DOI] [PubMed] [Google Scholar]
- Laudet V, Ravasi T (2022) Evolution, Development and Ecology of Anemonefishes: Model Organisms for Marine Science. CRC Press, Boca Raton, Florida, USA [Google Scholar]
- Leis JM (2007) Behaviour as input for modelling dispersal of fish larvae: behaviour, biogeography, hydrodynamics, ontogeny, physiology and phylogeny meet hydrography. Mar Ecol Prog Ser 347: 185–193. 10.3354/meps06977. [DOI] [Google Scholar]
- Lenth R (2020) emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package. https://cran.r-project.org/web/packages/emmeans/index.html [Google Scholar]
- Longcore T, Rich C (2004) Ecological light pollution. Front Ecol Environ 2: 191–198. 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2. [DOI] [Google Scholar]
- Luckenbach JA, Iliev DB, Goetz FW, Swanson P (2008) Identification of differentially expressed ovarian genes during primary and early secondary oocyte growth in coho salmon, Oncorhynchus kisutch. Reprod Biol Endocrinol 6: 2. 10.1186/1477-7827-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucon-Xiccato T, De Russi G, Cannicci S, Maggi E, Bertolucci C (2023) Embryonic exposure to artificial light at night impairs learning abilities and their covariance with behavioural traits in teleost fish. Biol Lett 19: 20230436. 10.1098/rsbl.2023.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D (2021) Performance: an R package for assessment, comparison and testing of statistical models. J Open Source Softw 6: 3139. 10.21105/joss.03139. [DOI] [Google Scholar]
- Marangoni LFB, Davies T, Smyth T, Rodríguez A, Hamann M, Duarte C, Pendoley K, Berge J, Maggi E, Levy O (2022) Impacts of artificial light at night in marine ecosystems—a review. Glob Change Biol 28: 5346–5367. 10.1111/gcb.16264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras S, Claireaux G, McKenzie DJ, Nelson JA (2010) Individual variation and repeatability in aerobic and anaerobic swimming performance of European sea bass, Dicentrarchus labrax. J Exp Biol 213: 26–32. 10.1242/jeb.032136. [DOI] [PubMed] [Google Scholar]
- McAlary FA, McFarland WN (1993) The effect of light and darkness on hatching in the pomacentrid Abudefduf saxatilis. Environ Biol Fishes 37: 237–244. 10.1007/BF00004631. [DOI] [Google Scholar]
- Mousavi SE, Purser GJ, Patil JG (2021) Embryonic onset of sexually dimorphic heart rates in the viviparous fish, Gambusia holbrooki. Biomedicines 9: 165. 10.3390/biomedicines9020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanninga GB, Côté IM, Beldade R, Mills SC (2017) Behavioural acclimation to cameras and observers in coral reef fishes. Ethology 123: 705–711. 10.1111/eth.12642. [DOI] [Google Scholar]
- Nanninga GB, Manica A (2018) Larval swimming capacities affect genetic differentiation and range size in demersal marine fishes. Mar Ecol Prog Ser 589: 1–12. 10.3354/meps12515. [DOI] [Google Scholar]
- Navarro-Barranco C, Hughes LE (2015) Effects of light pollution on the emergent fauna of shallow marine ecosystems: amphipods as a case study. Mar Pollut Bull 94: 235–240. 10.1016/j.marpolbul.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Neuswanger JR, Wipfli MS, Rosenberger AE, Hughes NF (2016) Measuring fish and their physical habitats: versatile 2D and 3D video techniques with user-friendly software. Can J Fish Aquat Sci 73: 1861–1873. 10.1139/cjfas-2016-0010. [DOI] [Google Scholar]
- O’Connor JJ, Fobert EK, Besson M, Jacob H, Lecchini D (2019) Live fast, die young: behavioural and physiological impacts of light pollution on a marine fish during larval recruitment. Mar Pollut Bull 146: 908–914. 10.1016/j.marpolbul.2019.05.038. [DOI] [PubMed] [Google Scholar]
- Peck MA, Huebert KB, Llopiz JK (2012) Chapter 3 - intrinsic and extrinsic factors driving match–mismatch dynamics during the early life history of marine fishes. In G Woodward, U Jacob, EJ O’Gorman, eds, Advances in Ecological Research. Academic Press, Cambridge, pp. 177–302 [Google Scholar]
- Pepin P, Orr DC, Anderson JT (1997) Time to hatch and larval size in relation to temperature and egg size in Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 54: 2–10. 10.1139/f96-154. [DOI] [Google Scholar]
- Perrichon P, Grosell M, Burggren WW (2017) Heart performance determination by visualization in larval fishes: influence of alternative models for heart shape and volume. Front Physiol 8: 464. 10.3389/fphys.2017.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike N (2011) Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol Evol 2: 278–282. 10.1111/j.2041-210X.2010.00061.x. [DOI] [Google Scholar]
- Pulgar J, Zeballos D, Vargas J, Aldana M, Manriquez PH, Manriquez K, Quijón PA, Widdicombe S, Anguita C, Quintanilla D et al. (2019) Endogenous cycles, activity patterns and energy expenditure of an intertidal fish is modified by artificial light pollution at night (ALAN). Environ Pollut 244: 361–366. 10.1016/j.envpol.2018.10.063. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vázquez FJ, López-Olmeda JF, Vera LM, Migaud H, López-Patiño MA, Míguez JM (2019) Environmental cycles, melatonin, and circadian control of stress response in fish. Front Endocrinol 10: 279. 10.3389/fendo.2019.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Frago E, Kehoe R, Patterson C, Gaston KJ (2021) A meta-analysis of biological impacts of artificial light at night. Nat Ecol Evol 5: 74–81. 10.1038/s41559-020-01322-x. [DOI] [PubMed] [Google Scholar]
- Schligler J, Cortese D, Beldade R, Swearer SE, Mills SC (2021) Long-term exposure to artificial light at night in the wild decreases survival and growth of a coral reef fish. Proc R Soc B Biol Sci 288: 20210454. 10.1098/rspb.2021.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Schoot EV, Wesselingh RA, Van Dyck H (2025) Artificial light at night reduces larval survival and constrains female body mass in a capital breeding moth. Basic Appl Ecol 85: 38–44. 10.1016/j.baae.2025.03.008. [DOI] [Google Scholar]
- Schwartz AV, Sant KE, Navarrete J, George UZ (2021) Mathematical modeling of the interaction between yolk utilization and fish growth in zebrafish, Danio rerio. Development 148: dev193508. 10.1242/dev.193508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima JS, Osenberg CW, Alonzo SH, Noonburg EG, Swearer SE (2022) How moonlight shapes environments, life histories, and ecological interactions on coral reefs. Emerg Top Life Sci 6: 45–56. 10.1042/ETLS20210237. [DOI] [PubMed] [Google Scholar]
- Smyth TJ, Wright AE, McKee D, Tidau S, Tamir R, Dubinsky Z, Iluz D, Davies TW (2021) A global atlas of artificial light at night under the sea. Elem Sci Anthr 9: 00049. 10.1525/elementa.2021.00049. [DOI] [Google Scholar]
- Sopinka NM, Hinch SG, Middleton CT, Hills JA, Patterson DA (2014) Mother knows best, even when stressed? Effects of maternal exposure to a stressor on offspring performance at different life stages in a wild semelparous fish. Oecologia 175: 493–500. 10.1007/s00442-014-2915-9. [DOI] [PubMed] [Google Scholar]
- Steell SC, Cooke SJ, Eliason EJ (2020) Artificial light at night does not alter heart rate or locomotor behaviour in Caribbean spiny lobster (Panulirus argus): insights into light pollution and physiological disturbance using biologgers. Conserv Physiol 8: coaa097. 10.1093/conphys/coaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres P, Wilson A, Haak C, Danylchuk A, Brownscombe J, Elvidge C, Shultz A, Birnie-Gauvin K, Cooke S (2017) Does coastal light pollution alter the nocturnal behavior and blood physiology of juvenile bonefish (Albula vulpes)? Bull Mar Sci 93: 491–505. 10.5343/bms.2016.1061. [DOI] [Google Scholar]
- Tamai TK, Vardhanabhuti V, Foulkes NS, Whitmore D (2004) Early embryonic light detection improves survival. Curr Biol 14: 446. 10.1016/j.cub.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Üstündağ ÜV, Çalıskan-Ak E, Ateş PS, Ünal İ, Eğilmezer G, Yiğitbaşı T, Ata Alturfan A, Emekli-Alturfan E (2019) White LED light exposure inhibits the development and xanthophore pigmentation of zebrafish embryo. Sci Rep 9: 10810. 10.1038/s41598-019-47163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamizar N, Blanco-Vives B, Oliveira C, Dinis M-T, Di Rosa V, Negrini P, Bertolucci C, Sánchez-Vázquez FJ (2013) Circadian rhythms of embryonic development and hatching in fish: a comparative study of zebrafish (diurnal), Senegalese sole (nocturnal), and Somalian cavefish (blind). Chronobiol Int 30: 889–900. 10.3109/07420528.2013.784772. [DOI] [PubMed] [Google Scholar]
- Weschke E, Schligler J, Hely I, Roost T, Schies J-A, Williams B, Dworzanski B, Mills SC, Beldade R, Simpson SD et al. (2024) Artificial light increases nighttime prevalence of predatory fishes, altering community composition on coral reefs. Glob Change Biol 30: e70002. 10.1111/gcb.70002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yúfera M, Darias MJ (2007) The onset of exogenous feeding in marine fish larvae. Aquaculture 268: 53–63. 10.1016/j.aquaculture.2007.04.050. [DOI] [Google Scholar]
- Zapata MJ, Sullivan SMP, Gray SM (2019) Artificial lighting at night in estuaries—implications from individuals to ecosystems. Estuaries Coasts 42: 309–330. 10.1007/s12237-018-0479-3. [DOI] [Google Scholar]
- Zhang S, Wang S, Li H, Li L (2011) Vitellogenin, a multivalent sensor and an antimicrobial effector. Int J Biochem Cell Biol 43: 303–305. 10.1016/j.biocel.2010.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


